- 1Department of Biomedical Sciences, Humanitas University, Milan, Italy
- 2IRCCS Humanitas Research Hospital, Humanitas Cancer Center, Milan, Italy
The use of immune checkpoint inhibitors (ICIs) for treating several types of cancer is increasing, but they may be associated with immune-related adverse events (irAEs). Pancreatitis is a rare irAE, mostly responsive to steroid treatment. There are no published data on the management of steroid-refractory ICI-induced pancreatitis. Rituximab has shown efficacy in the setting of relapsing non-ICI-induced autoimmune pancreatitis. However, its use has not been tested for treating immunotherapy-related pancreatitis. Here, we present the case of a patient with steroid-refractory immune-related pancreatitis successfully treated with rituximab as a potential strategy for irAE management.
1 Introduction
The use of immune checkpoint inhibitors (ICIs), including cytotoxic T-lymphocyte-associated protein 4 (CTLA4)-directed and programmed cell death protein 1 (PD-1) and its ligand 1 (PD-L1)-targeting monoclonal antibodies, is increasing thanks to the efficacy shown in treating various cancer types (1). However, their use can lead to immune-related adverse events (irAEs), resulting from excessive self-immune responses against normal organs (1–3). ICI-related toxicity incidence and prevalence remain unclear. The most common and well-documented irAEs are gastrointestinal, dermatological, pulmonary, and endocrine toxicities (1–5). A less common irAE is represented by pancreatic toxicity reported in 0.9%–3% with anti-CTLA-4, 0.5%–1.6% with anti-PD-L1, and 1.2%–2.1% with combined anti-CTLA-4 and anti-PD-L1, respectively (6). The clinical presentation varies from asymptomatic amylase and/or lipase elevations to, more rarely, acute pancreatitis (7–10). Two meta-analyses reported a higher risk of pancreatic toxicity with ICIs than with chemotherapy or placebo. However, both studies were limited by the lack of clinical and radiologic data (11, 12). The National Comprehensive Cancer Network and the American Society of Clinical Oncology developed therapeutic algorithms to aid the diagnosis and treatment of these adverse events, consisting of corticosteroids for the standard treatment of most high-grade irAEs (4, 13). However, only the National Comprehensive Cancer Network guidelines reported indications for the first-line management of immune-related pancreatitis but not for steroid-refractory patients.
On the other hand, according to the international consensus for the treatment of non-ICI-induced autoimmune pancreatitis (AIP), steroid treatment is the first line for remission induction in patients with active untreated AIP (14). However, when glucocorticoid monotherapy fails to induce remission or control the disease, rituximab is suggested as a second-line treatment. In particular, the Mayo Clinic Experience reported a complete remission rate of 83% with rituximab in a series of patients with steroid-resistant AIP; furthermore, no relapses were observed while on maintenance therapy (15).
In the present case report, a 53-year-old woman was diagnosed with atypical metastatic carcinoid of the lung in November 2020. Staging by whole-body computed tomography (CT) scan and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) showed bilateral involvement of the lungs and adrenal glands and multiple lesions in the thyroid gland. An ultrasound scan displayed suspicious nodules in the scalp and right parotid gland. PET with gallium-68 was negative. The diagnosis was carried out with a transthoracic biopsy. Histological examination revealed Ki67 at 30% and PD-L1 at 30%. Fine-needle aspiration confirmed thyroid metastasis. Given the histopathological characteristics, the aggressive onset, and the disease behavior, we decided to treat our patient with six 21-day courses of carboplatin (area under the curve of 5 mg/ml/min) and etoposide (100 mg/mq) combined with atezolizumab (1,200 mg) from December 2020 to April 2021 (16). Two weeks after the last cycle of induction chemotherapy, the patient presented to the emergency department with grade 3 (G3) abdominal pain, elevated serum lipase and amylase levels (both G1), and inhomogeneous pancreas on an abdominal ultrasound scan.
Here, we report on rituximab treatment to address the patient’s steroid-refractory and relapsing ICI-related pancreatitis. This is the first reported case of a patient receiving rituximab with a positive clinical outcome.
2 Case presentation
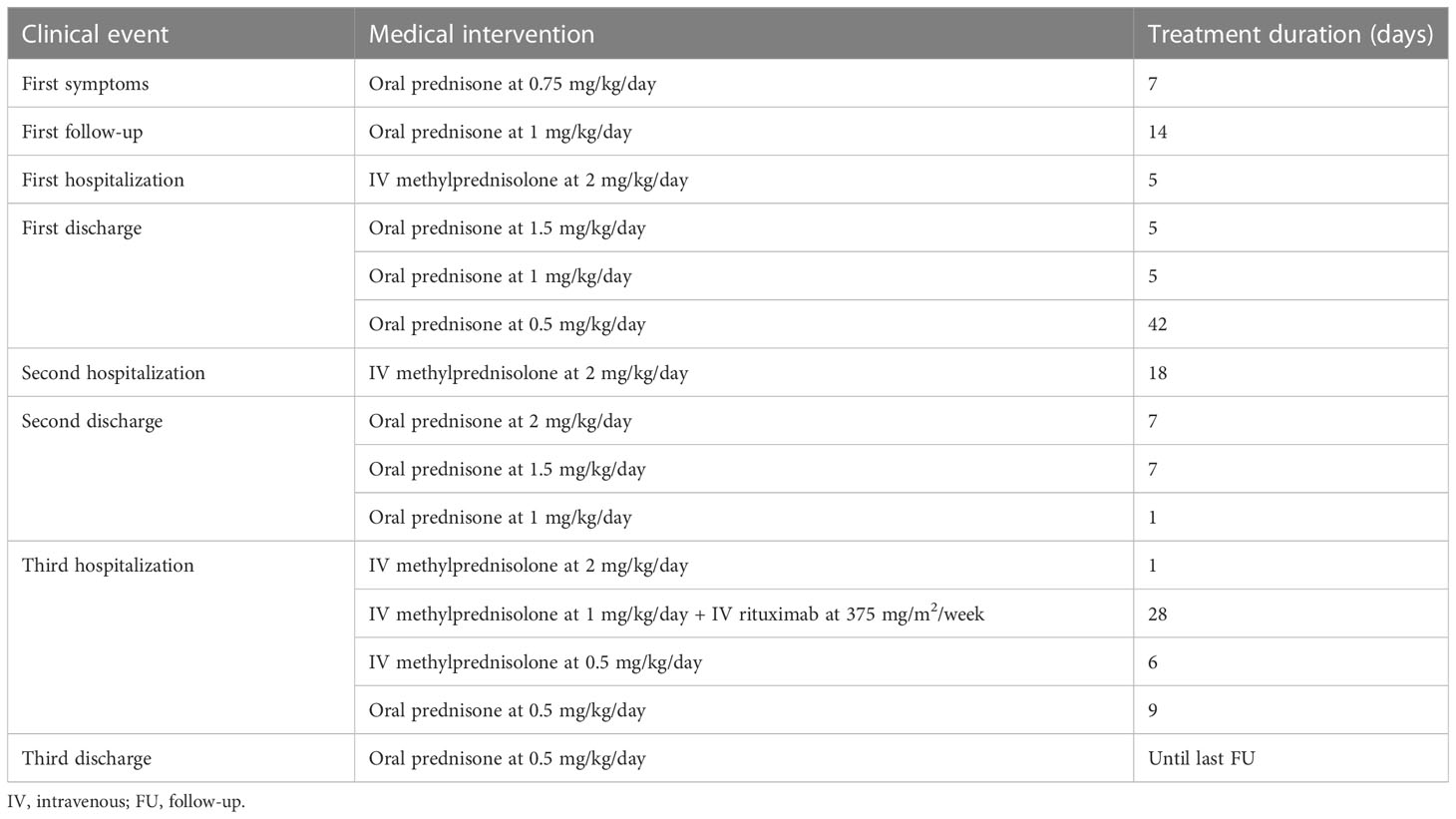
The clinical events and the type and duration of interventions from the emergency department admission onward are reported in Table 1 and Figure 1. Atezolizumab was permanently discontinued, and oral administration of prednisone at 0.75 mg/kg daily was given for 7 days. On subsequent re-evaluation, the patient’s symptoms and pancreatic enzyme elevation persisted despite continued treatment with analgesics and steroids. The CT scan revealed imaging features of pancreatitis. Therefore, steroid treatment was increased to 1 mg/kg/day. The patient reported pain improvement 2 weeks later, but concomitantly G3–G4 elevated serum amylase and lipase levels were detected. A new CT scan showed the first signs of pancreatitis resolution compared with the previous scan. The patient was hospitalized, and treatment was delivered, including fasting, parenteral nutrition, steroid agents (methylprednisolone 1 mg/kg intravenous twice a day), and opioids for pain management, with clinical benefit. At discharge, oral prednisone at 1.5 mg/kg/day was started and tapered. After 2 months, despite maintenance treatment with glucocorticoids (0.5 mg/kg/day), the patient presented again to the emergency department with severe abdominal pain and increased G3–G4 amylase and lipase, requiring hospitalization. A gastroenterologist monitored the patient during admission. A CT scan evaluation confirmed radiologic features of pancreatitis. MRI and magnetic resonance cholangiopancreatography identified a fluid collection in the pancreatic tail involving the spleen associated with restricted diffusion-weighted imaging and diffuse stenosis of the main pancreatic duct, suggestive of AIP (17, 18). The same steroid regimen was established, but this time steroid responsiveness was not as expected, as the radiologic findings of pancreatitis on CT improved only slightly. Therefore, the patient was discharged with high-dose oral steroid therapy (prednisone at 2 mg/kg/day). During this hospitalization, new-onset diabetes was diagnosed, and insulin was initiated. After 2 weeks from the start of steroid tapering, there was a relapse with severe abdominal pain and elevation of serum pancreatic markers, together with C-reactive protein levels. A CT scan showed worsening of the known pancreatic fluid collection with hemorrhagic development (Figure 2). In the course of this third hospital admission, we started a new regimen made of piperacillin/tazobactam, methylprednisolone, and morphine intravenous to achieve pain control and treat undergoing covered infections. Abdominal MRI was repeated, describing several new fluids and hemorrhagic collections with far worse stenosis of the main pancreatic duct, confirming the poor responsiveness to steroid treatment associated with clinical deterioration; no increase in carbohydrate antigen 19-9 (CA 19-9) was observed.
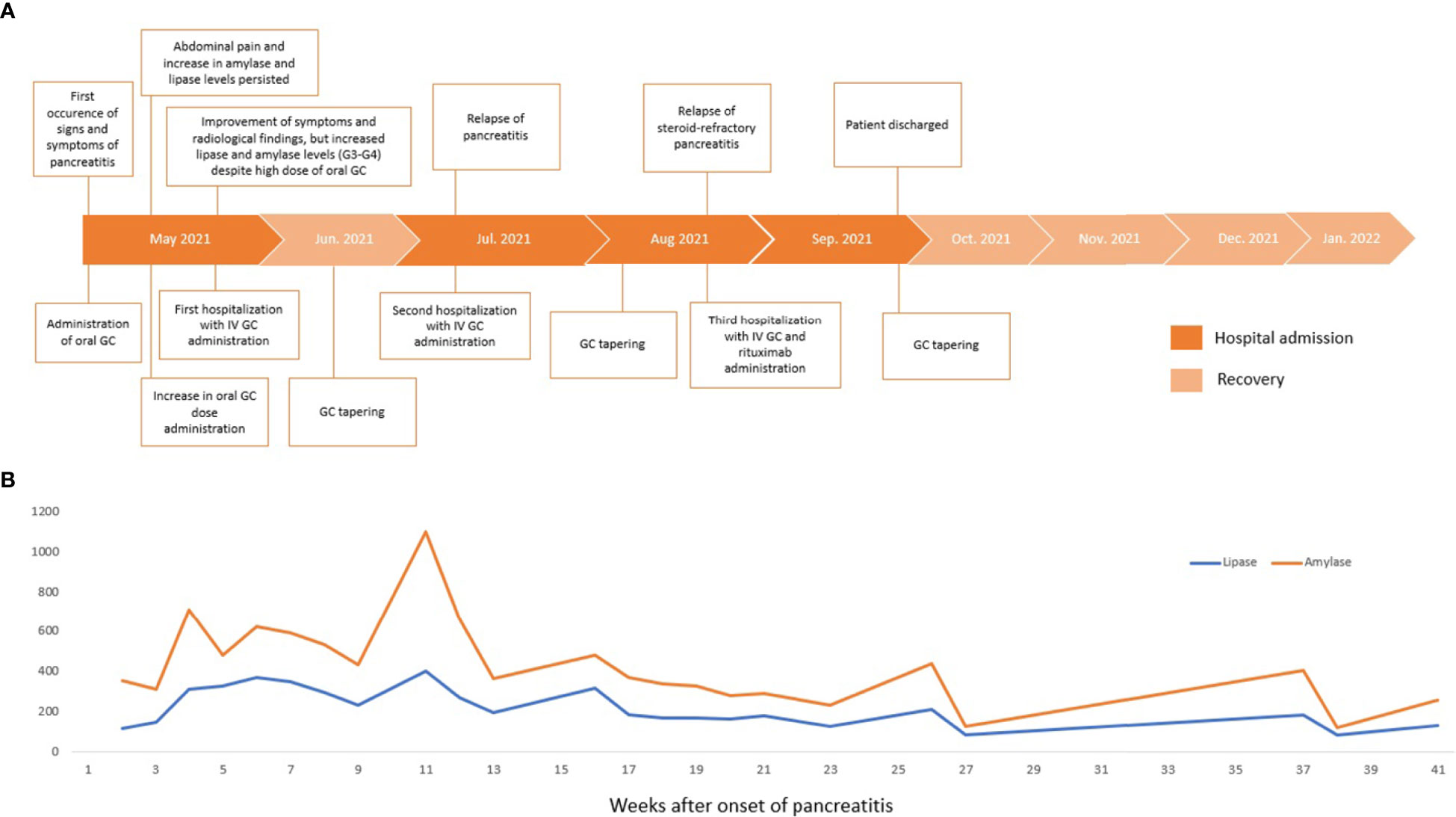
Figure 1 Timeline of clinical presentation, interventions, and outcomes (A). Serum lipase and amylase levels trend during clinical management (B). GC, glucocorticoids; IV, intravenous.
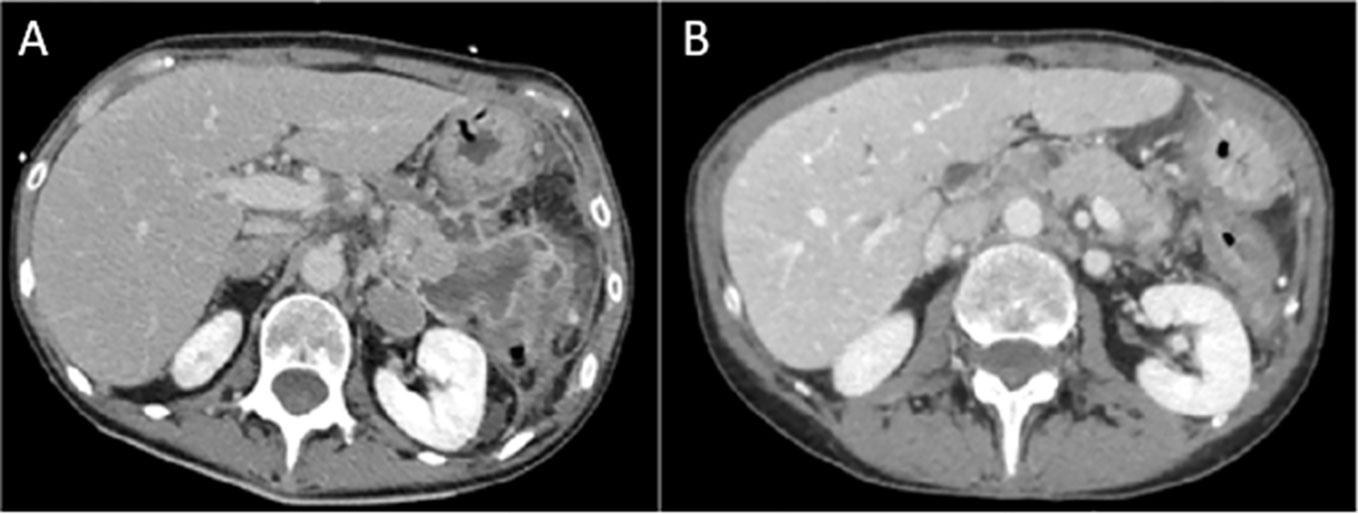
Figure 2 The baseline abdominal CT scan (A) shows findings of pancreatitis and a multiloculated fluid and hemorrhagic collection in the pancreatic tail, with a maximum size of 6.5 cm × 5 cm. After 16 weeks since the last dose of rituximab, CT scan (B) shows complete resolution of pancreatitis, with homogeneous pancreatic parenchyma and fluid collection.
According to Hart and colleagues’ reported data in the Mayo Clinic experience in non-ICI-induced steroid-refractory AIP treatment (15), we decided to administer rituximab (MabThera, Roche) at 375 mg/m2 intravenous weekly for 4 weeks in combination with methylprednisolone (1 mg/kg/day), resulting in good clinical and radiologic control. Before rituximab was administered, a blood screening was performed with an immune panel including serum immunoglobulin G4 (IgG4), rheumatoid factor, antinuclear antibodies, anti-mitochondrial antibodies, anti-smooth muscle antibodies, antineutrophil cytoplasmic antibodies, and anti-thyroglobulin antibodies levels, with negative results. Antimicrobic prophylaxis was modified by adding sulfamethoxazole/trimethoprim and acyclovir. Between the third and fourth courses of rituximab, a CT scan described the occurrence of a pancreatic pseudocyst and a dramatic reduction in the peripancreatic fluid. The pseudocyst was treated with the placement of a transgastric prosthesis through endoscopic ultrasound. Serum amylase and lipase levels were recorded during the whole clinical course, and no correlation was shown with the severity of pancreatitis (Figure 1). On the following admissions, no finding of pancreatitis was detected on the CT scan (Figure 2).
One month after the last rituximab administration, the patient was admitted for fever and a new elevation of inflammation markers, such as serum C-reactive protein and procalcitonin, under antimicrobic prophylaxis. Blood and urine cultures were negative. However, empiric treatment with piperacillin/tazobactam was initiated with a remarkable clinical and laboratory markers’ response. No further episodes followed, and antimicrobic prophylaxis was continued until one year after the last dose of rituximab. On follow-up, there was no clinical or laboratory evidence of pancreatitis. In January 2023, disease progression (PD) was detected in multiple sites (brain, lymph nodes), and second-line chemotherapy was initiated. The brain metastases were treated with whole-brain radiotherapy (WBRT). The patient died of PD in March 2023.
3 Discussion
To our knowledge, there are no data on successful steroid-refractory and relapsing ICI-induced pancreatitis treatments. This brief report presents the first case of a patient with atezolizumab-related pancreatitis refractory to steroids successfully treated with rituximab.
IrAEs can affect any organ. Gastrointestinal irAEs, including diarrhea, colitis, and hepatitis, are well documented and studied (1, 2, 19), while cases of pancreatitis after ICIs are reported only in clinical trials and case series (7–10). According to available algorithms, steroids are the mainstay of treatment of most high-grade irAEs. For steroid-refractory cases, recommendations for using immune-modulating agents are extrapolated from the evidence for treating autoimmune diseases (2–4). Rituximab was used to treat steroid-refractory irAEs, such as encephalitis, autoimmune cytopenias, or severe bullous skin disease, with a good clinical and laboratory response (20–22). Considering the similarities with AIP (17), we decided to administer rituximab based on efficacy results from the study of Hart et al. and the international consensus recommendations for AIP treatment (14, 15). The Mayo Clinic experience showed that patients with normal serum IgG4 levels might receive rituximab to manage AIP (15). Furthermore, IgG4 levels were reported to be normal in ICI-related pancreatitis (23) and there were no data on the diagnostic value of IgG4 in ICI-related pancreatitis. Based on this evidence, we were motivated to start a rituximab regimen even though our patient’s serum IgG4 levels were negative. As a result, rituximab showed a surprising clinical and radiologic improvement only 2 weeks after the first infusion, and no relapses were detected during follow-up. Serum amylase and lipase levels were not associated with the clinical trend and did not decrease after steroid therapy, as opposed to previous steroid-responsive ICI-related pancreatitis findings (7, 9, 10). Although elevations in pancreatic enzymes may be helpful in the diagnosis of acute pancreatitis (14, 17, 23), they are not indicated in monitoring response to immunosuppressive treatment based on the resolution of clinical symptoms and radiological abnormalities (17). Serum IgG4 have been described as biomarkers of AIP activity and as predictive markers of relapse (14); however, they were negative in our case. Therefore, we could not identify a possible biomarker for steroid-refractory or relapsed ICI-induced pancreatitis.
Immunosuppression for treating irAEs may increase the risk of opportunistic infections, such as Aspergillus fumigatus, Pneumocystis jirovecii pneumonia, and cytomegalovirus (2). Therefore, antibiotic prophylaxis should be considered according to available recommendations (2). In our case, sulfamethoxazole/trimethoprim and acyclovir were administered during steroid and rituximab treatment. Only one admission occurred because of a suspected infection. Patients with ongoing steroid therapy should be strictly monitored for the high risk of developing hyperglycemia and/or diabetes (4). Moreover, ICI may cause endocrine pancreatic toxicity (4). Our patient developed diabetes, but we cannot demonstrate whether it is associated with glucocorticoid administration or immune-related adverse events.
The specific mechanism underlying irAEs is still unknown. Atezolizumab is a humanized monoclonal antibody that inhibits PD-L1, a transmembrane glycoprotein expressed on antigen-presenting cells (APCs), including B cells, T cells, macrophages, and dendritic cells (24–26), as well as on nonimmune cells, such as pancreatic islet cells (27). PD-L1 inhibition stimulates the activation of T cells, especially CD8+ cytotoxic T lymphocytes, which are considered the effectors of the anticancer effect of ICIs and are responsible for immune-related toxicities (25, 28). Although the role of B cells and antibodies in irAEs is unclear, their involvement in endocrine disorders, including ICI-related thyroiditis and diabetes, appears to be more evident (25). Considering the onset of diabetes in our patient during immune-mediated pancreatitis, we may assume that there was an immune-mediated injury involving both the exocrine parenchyma and the pancreatic islets, and the efficacy observed with rituximab administration, an anti-CD20 monoclonal antibody acting on B-cell depletion, may indicate an underlying mechanism mainly involving B-cell activity, such as the production of autoantibodies and B- and T-cell interactions (29). Yet, our patient had a negative immune panel screening. Agrawal et al. reported a case of autoimmune pancreatitis and cholangitis induced by nivolumab with normal serum IgG4 levels; the patient underwent surgical resection due to biliary stricture, with IgG4 predominant plasmacytic cell infiltrates on histological examination (30). Considering AIP, its subtypes can be seronegative (17), which may be prevalent in ICI-related pancreatitis (23). Some reasons to explore might be low levels of circulating autoantibodies or the presence of unknown autoantibodies whose mechanism of action has not been described yet.
4 Conclusion
Pancreatitis is a rare irAE that responds to steroid therapy in most cases, but data on the management of steroid-refractory pancreatitis are unavailable. We reported the first successful treatment case with rituximab, which can be considered a good and safe option for patients with steroid-refractory, relapsed pancreatitis caused by ICIs and maybe other steroid-resistant IrAEs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study conception and design: AS and SM. Collection and interpretation of data: AS, SM, and FV. Statistical analysis: N/A. Manuscript drafting: AS, SM, MT, RC, and FV. Manuscript editing: AS, SM, MT, RC, and FV. All authors contributed to the article and approved the submitted version.
Funding
Editorial and linguistic assistance was supported with internal funds. Editorial and linguistic assistance was partially supported by “Ricerca Corrente” funding from the Italian Ministry of Health to IRCCS Humanitas Research Hospital.
Acknowledgments
The authors wish to acknowledge Fabio Perversi, Valentina Attanasio, and Aashni Shah (Polistudium Srl, Milan, Italy) for their editorial and linguistic assistance.
Conflict of interest
AS participated in the advisory boards and speaker’s bureau of Bristol–Myers–Squibb, Servier, Pfizer, Eisai, Bayer, Merck Sharp & Dohme, Takeda, Roche, Abb-Vie, Amgen, Celgene, Gilead, Servier, AstraZeneca, Pfizer, Lilly, Sandoz, and Novartis. AS received consultancy fees from Sanofi and Incyte.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6:38. doi: 10.1038/s41572-020-0160-6
2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378:158–68. doi: 10.1056/NEJMra1703481
3. Spain L, Diem S, Larkin J. Management of toxicities of immune inhibitors. Cancer Treat Rev (2016) 44:51–60. doi: 10.1016/j.ctrv.2016.02.001
4. Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Version 1.2022 Management of Immunotherapy-Related Toxicities NCCN Guidelines Panel Disclosures Continue NCCN (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.
5. Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol (2021) 22:1265–74. doi: 10.1016/S1470-2045(21)00333-8
6. Zhang Y, Fang Y, Wu J, Huang G, Bin J, Liao Y, et al. Pancreatic adverse eventsassociated with immune checkpoint inhibitors: a large-scale analysis. Front Pharmacol (2022) 13:817662. doi: 10.3389/fphar.2022.817662
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Canc (2016) 99:148–50. doi: 10.1016/j.lungcan.2016.07.001
8. Jiang R, Xu L, Huang Y, Fang C, Guo H, Li S, et al. Anti-PD-1 drug (nivolumab) may induce acute and life-threatening pancreatitis in lung cancer patient: A case report. Pancreas. (2018) 47:e53–4. doi: 10.1097/MPA.0000000000001107
9. Yamamoto K, Oka K, Son R, Honda H, Sakae H, Hasegawa K, et al. Acute pancreatitis without abdominal pain induced by administration of nivolumab and ipilimumab. Mod Rheumatol Case Rep (2021) 5:425–30. doi: 10.1080/24725625.2021.1899444
10. Ofuji K, Hiramatsu K, Nosaka T, Naito T, Takahashi K, Matsuda H, et al. Pembrolizumab-induced autoimmune side effects of colon and pancreas in a patient with lung cancer. Clin J Gastroenterol (2021) 14:1692–9. doi: 10.1007/s12328-021-01499-z
11. Su Q, Zhang XC, Zhang CG, Hou YL, Yao YX, Cao BW. Risk of immune-related pancreatitis in patients with solid tumors treated with immune checkpoint inhibitors: systematic assessment with meta-analysis. J Immunol Res (2018) 2018:1027323. doi: 10.1155/2018/1027323
12. George J, Bajaj D, Sankaramangalam K, Yoo JW, Joshi NS, Gettinger S, et al. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatology. (2019) 19:587–94. doi: 10.1016/j.pan.2019.04.015
13. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
14. Okazaki K, Chari ST, Frulloni L, Lerch MM, Kamisawa T, Kawa S, et al. International consensus for the treatment of autoimmune pancreatitis. Pancreatology. (2017) 17:1–6. doi: 10.1016/j.pan.2016.12.003
15. Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. (2013) 62:1607–15. doi: 10.1136/gutjnl-2012-302886
16. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
17. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. (2011) 40:352–8. doi: 10.1097/MPA.0b013e3182142fd2
18. Widmann G, Nguyen VA, Plaickner J, Jaschke W. Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy. Curr Radiol Rep (2016) 5:59. doi: 10.1007/s40134-017-0256-2
19. Cramer P, Bresalier RS. Gastrointestinal and hepatic complications of immune checkpoint inhibitors. Curr Gastroenterol Rep (2017) 19:3. doi: 10.1007/s11894-017-0540-6
20. Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol (2017) 153(6):603–5. doi: 10.1001/jamadermatol.2017.0091
21. Williams TJ, Benavides DR, Patrice KA, Dalmau JO, de Ávila AL, Le DT, et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol (2016) 73(8):928–33. doi: 10.1001/jamaneurol.2016.1399
22. Khan U, Ali F, Khurram MS, Zaka A, Hadid T. Immunotherapy-associated autoimmune hemolytic anemia. J Immunother Canc (2017) 5:15. doi: 10.1186/s40425-017-0214-9
23. Nakano R, Shiomi H, Fujiwara A, Yoshihara K, Yoshioka R, Kawata S, et al. Clinical characteristics of ICI-related pancreatitis and cholangitis including radiographic and endoscopic findings. Healthcare (2022) 10:763. doi: 10.3390/healthcare10050763
24. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563–7. doi: 10.1038/nature14011
25. Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatol (Oxford) (2019) 58:vii59–67. doi: 10.1093/rheumatology/kez308
26. Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol (2007) 19:813–24. doi: 10.1093/intimm/dxm057
27. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med (2016) 375:1767–78. doi: 10.1056/NEJMra1514296
28. Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. (2021) 184:1575–88. doi: 10.1016/j.cell.2021.02.011
29. Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease–the immune complex decoy hypothesis. Nat Clin Pract Rheumatol (2007) 3:86–95. doi: 10.1038/ncprheum0424
Keywords: rituximab, steroid-refractory pancreatitis, immune-related adverse event, atezolizumab, immune checkpoint inhibitors
Citation: Santoro A, Masini S, Cavina R, Tronconi MC and De Vincenzo F (2023) Rituximab in steroid-refractory immune-related pancreatitis: a case report. Front. Oncol. 13:1205720. doi: 10.3389/fonc.2023.1205720
Received: 14 April 2023; Accepted: 03 July 2023;
Published: 31 July 2023.
Edited by:
Guanghua Rong, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Muhammad Salman Faisal, University at Buffalo, United StatesXiaoxiang Zhou, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Santoro, Masini, Cavina, Tronconi and De Vincenzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Armando Santoro, YXJtYW5kby5zYW50b3JvQGh1bWFuaXRhcy5pdA==
†These authors have contributed equally to this work
 Armando Santoro
Armando Santoro Silvia Masini
Silvia Masini Raffaele Cavina2
Raffaele Cavina2 Fabio De Vincenzo
Fabio De Vincenzo