- 1Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Nuclear Medicine, Sanmenxia Central Hospital, Henan, China
- 3Department of Nuclear Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany
Currently, the incidence of prostate cancer is increasing, and it has become a great threat to men’s health. The detection, staging, and follow-up of prostate cancer patients are inseparable from morphology or magnetic resonance imaging (MRI). However, these do not fully meet the needs of diagnosis and patient management. In particular, owing to the late diagnosis, metastatic castration-resistant prostate cancer (mCRPC) patients usually have poor survival and few options for further effective treatment. Prostate-specific membrane antigen (PSMA), because of its overexpression on prostate cancer cells, has gained interest due to its application in the imaging and theranostics field. Several PSMA radioligands have been developed for imaging and treating prostate cancer. Many clinical trials have assessed the efficacy and safety profiles of these radionuclide agents and show promise in patients who have exhausted other standard treatment options. To date, several small compounds for targeting PSMA have been developed, and 68Ga-PSMA-11 and 18F-DCFPyL have been approved by the United States (US) Food and Drug Administration (FDA) for imaging of prostate cancer. 111In- or 99mTc-labeled PSMA-ligand can guide surgeons searching for radioactive metastatic lymph nodes, and 177Lu- or 225Ac-labeled PSMA-ligand can be used for internal radiotherapy. Moreover, some molecules for therapeutic application are undergoing different stages of clinical trials. In this review, we present current perspectives on the use of PSMA-targeted imaging and theranostics in prostate cancer. As PSMA-targeted imaging and therapeutics are becoming the standard of care for prostate cancer patients, we emphasize the importance of integrating nuclear medicine physicians into multidisciplinary oncology teams.
Introduction
Prostate cancer (PCa) ranks third among the most common cancers diagnosed in men worldwide. With approximately 2.3 million new cases by 2040, PCa is becoming one of the most prevalent neoplasms in men worldwide (1, 2). Thus, accurate diagnosis, staging, and effective personalized treatment methods are of vital importance.
Based on anatomy information, conventional imaging strategies such as computed tomography (CT) and magnetic resonance imaging (MRI) have limitations in lesion detection, especially in metastatic patients, while bone scans do not offer anatomical details. Thus, novel molecular imaging biomarkers are urgently needed. Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein encoded by folate hydrolase 1 gene (FOLH1) (3–5). It is weakly expressed in healthy prostate tissue but is overexpressed in PSMA-positive PCa cells (3). Furthermore, increasing expression of PSMA expression is observed in androgen deficiency, hormone refractory and metastatic diseases, higher PSA levels, and a higher International Society of Urologic Pathologists (ISUP) grade at diagnosis (6, 7). Primary PCa lesions are PSMA-negative proximately in 5%–10% of cases (8). Moreover, PSMA is not specific to the prostate, and the expression in the neovasculature of other solid malignancies (such as renal cell, bladder transitional cell, and colon cell) was observed (3). Moreover, in numerous normal tissues, for example, endometrial glands, testis, bladder, kidney tubules, pancreas islets, heart, and ganglion cells in the gastrointestinal tract and brain, weak-to-moderate levels of PSMA expression have been detected (9). Although it is reported that certain benign diseases such as lymphadenitis and malignant diseases such as lung adenocarcinoma may also exhibit PSMA uptake (10), its remarkable sensitivity and specificity for PCa continue to establish it as a dedicated imaging modality for patients with PCa. Thus, PSMA-positron emission tomography (PET) is still considered to be a rather sensitive and highly specific tool for PCa diagnosis despite its expression by subsets of various types of other tissues (9, 11, 12).
In the field of PSMA-related therapy, PSMA-ligand imaging can help improve the prognosis. Felix et al. combined PSMA-PET and MRI-guided single-component stereotactic radiotherapy (SBRT) for local recurrence of PCa, and the findings are encouraging (13). Furthermore, several clinic trials such as VISION trial (14), TheraP trial (15), and LuPSMA trial (16) have demonstrated promising results of PSMA-targeted therapy in PCa patients. Moreover, Horn et al. found that PCa patients with recurrence at a single anatomical interval of location experienced significantly longer biochemical recurrence-free survival (bRFS) and treatment-free survival interval through PSMA radioguided surgery (RGS) (17).
This review will provide an overview of the current clinical applications of PSMA-targeted imaging and therapy.
PSMA-ligand imaging
PSMA-ligand PET/CT or MRI is mainly used in the following clinical indications: primary staging of cancers, biochemical recurrence (BCR), and detecting and monitoring of advanced disease.
Primary staging
A precise staging, local extent, and extra-prostatic metastasis are crucial in intermediate-risk and high-risk PCa patients for further treatment method decisions. These include radical prostatectomy (RP) with standard nodal dissection or extended pelvic lymph node dissection (ePLND) in PCa, radiotherapeutic treatment, or multimodal therapy. A prospective comparison of primary staging before and after PSMA-ligand PET/CT was conducted. Intermediate- and high-risk PCa patients (n = 108) have indicated that PSMA-ligand PET/CT upstaged 6.4% of patients from M0 to M1. Moreover, compared with conventional imaging, 14% of N0M0 patients became N1M0, and 2.8% of M1 patients ended up with M0. A change in management of the disease occurred in 21% of patients (18).
The additional molecular imaging information has increased the detection rate of PSMA-ligand PET/CT. As evidence increases, PSMA-ligand PET/CT is more effective in initial staging than other imaging modalities. Compared with traditional imaging modalities such as bone scans and CT, several studies have shown 68Ga-PSMA-11 PET improved detection rates in malignant lesions (19). Several retrospective studies have shown that 68Ga-PSMA-11 PET/CT is superior to standard imaging and has high sensitivity and specificity (94% and 99%, respectively) for lesion detection (12, 20). A recent prospective trial, proPSMA, demonstrated that the diagnostic accuracy of PET/CT was 27% greater than conventional imaging (p < 0.001) (21).
The high specificity and high sensitivity of PSMA-ligand PET in lesion detection of primary PCa patients have been proven. A retrospective study of 1,253 patients (high-risk, 47.6%), metastatic disease was detected by 68Ga-PSMA-11 PET in 12.1% of the cohort. Nearly half of the detected lymph node metastases were outside the boundaries of an ePLND (Figure 1). Skeletal metastases were found in 59 men (4.7%). PCa metastases were identified in 5.2% of intermediate-risk patients, compared with 19.9% of high-risk patients. The study highly demonstrated the potential of using PSMA-PET for primary staging (22). Another study comparing 340 segments from 68Ga-PSMA-11 PET and multiparametric MRI with histopathology has revealed that 68Ga-PSMA-11 PET/CT significantly outperformed multiparametric MRI and even better results were achieved when both methods were combined (23).
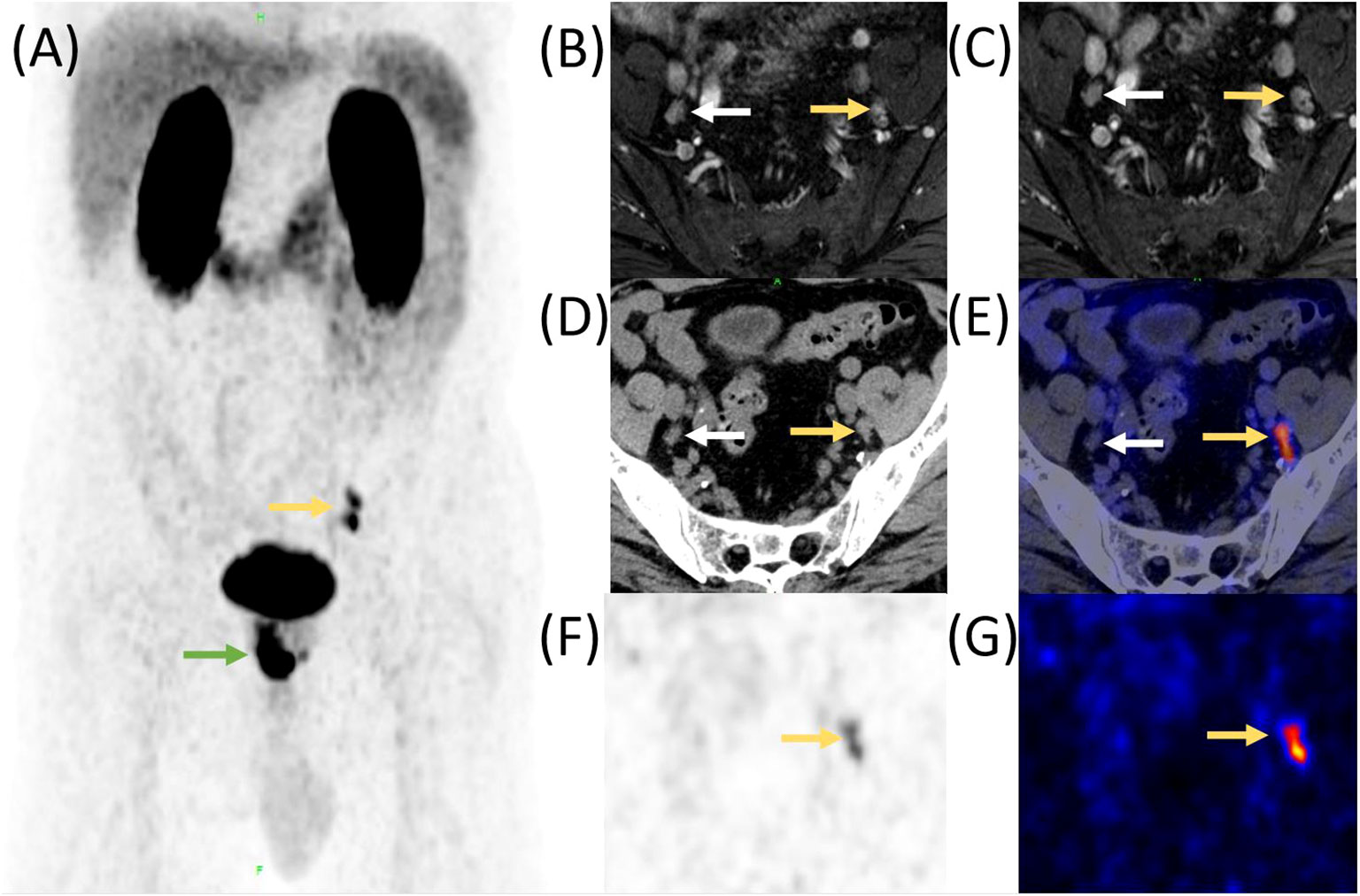
Figure 1 Comparison of enhanced MR, CT, and 68Ga-PSMA-11 PET/CT in a PCa patient with lymph node metastasis before RP. A 55-year-old man was diagnosed with prostate cancer;, MR and 68Ga-PSMA-11 PET/CT was performed before RP. High 68Ga-PSMA-11 uptake was observed in the lower left abdomen and pelvis (A, yellow and green arrow). The preoperative MR identified several lymph nodes with a maximum of 5 mm in short diameter, adjacent to the internal iliac vessels bilaterally (B, axial enhanced T2-weighted phase 1 MR image; (C), axial enhanced T2-weighted phase 2 MR image, yellow and white arrow). CT also revealed those lymph nodes (D, yellow and white arrow), but PET/CT fusion image (E), PET image (F), and “hot iron” pseudocolor PET image (G) demonstrated that only the lymph nodes on left side had high 68Ga-PSMA-11 uptake. Postoperative histopathology confirmed that the left lymph nodes were metastasis while the right one was reactive hyperplasia.
Furthermore, for detection of bony lesions in PCa, PSMA-ligand PET clearly outperformed standard imaging (CT, MRI, and bone scanning) in several studies (24, 25). Janssen et al. have reported a superior sensitivity and specificity of 68Ga-PSMA-11 PET/CT compared with 99mTc-3,3-diphospho-1,2-propanodicarboxylic acid (DPD), single-photon emission computed tomography (SPECT). In the region-based analysis, the sensitivity and specificity of 68Ga-PSMA-11 PET/CT were 97.7% and 100%, respectively, and those of 99mTc-DPD-SPECT were 69.4% and 98.3%, respectively (p < 0.05) (25). A study including 75 patients and 410 bone regions has demonstrated similar results. It has been proven that 68Ga-PSMA-11 PET outperformed planar 99mTc bone scintigraphy to detect affected bone regions (sensitivities and specificities for PET vs. 99mTc bone scintigraphy [patient-based analysis]: 98.7%–100% vs. 86.7%–89.3%; 88.2%–100% vs. 60.8%–96.1%; [region-based analysis]: 98.8%–99% vs. 82.4%–86.6%; 98.9%–100% vs. 91.6%–97.9%; p < 0.001, respectively) (24). Interestingly, according to a systematic review of 12 studies and 322 patients who underwent 68Ga-PSMA-11 PET scanning for primary stage disease, methodology and outcomes differed greatly. The median sensitivity and specificity were 33%–92%, 82%–100% per lesion and 82%–100%, 67%–99% per patient, respectively.
Several studies indicated that in most tumor diseases, the diagnostic performance of PET/MRI is similar to or even better than that of PET/CT (26). PET/MRI is widely utilized in PCa patients, primarily for its advantages in combining PET and MRI, providing high-resolution multiparametric imaging (27). PET/MRI is employed to delineate radiation therapy target areas (28) and diagnose BCRs (29), and for the preoperative assessment of high-risk patients (30). This technology has the potential to enhance the management and treatment decision-making for PCa patients, especially in complex cases, but further research is needed to validate its advantages.
Therefore, PSMA-ligand PET/CT or PET/MRI can be used to determine the extent of local tumors, lymph nodes, bone, and organ metastases, and, more importantly, to plan effective treatment and improve prognosis. However, published data on mechanism experiments and heterogeneous uptake of PSMA-ligand are still limited (31). Recent studies have demonstrated this using PSMA immunohistochemistry; nonetheless, PSMA ligands and antibodies have different sizes and binding positions, which may introduce methodological limitations. Further studies are needed before reliable conclusions can be drawn.
Biochemical recurrence detection
In approximately 30% to 40% of PCa cases, disease will recur after the primary treatment. Elevating levels of prostate-specific antigen (PSA) always indicate the recurrence (11). Because of the implications for future disease management, the location of the lesions must be determined once BCR has been detected. However, localizing lesions is a difficult task. There has been evidence that salvage radiotherapy in patients with BCR after RP is most effective while serum PSA <0.5 ng/mL (32, 33). Radiotherapy timing is crucial to these patients. Monitoring PSA cannot easily provide enough information.
Regarding conventional imaging modalities, CT can detect only 11%–14% of lesions in patients with BCR after RP (34). Compared with bone scans, choline-based PET imaging has higher pooled specificity [0.82, 95% confidence interval (CI) 0.78, 0.85; 0.99, 95% CI 0.93, 1.00, respectively] and reports fewer false-positive lesions (35). Moreover, the PSA level, kinetics, and morphology have significantly affected the sensitivity of choline PET (36–38).
However, PSMA-ligand PET shows a much higher detection rate, especially in low PSA levels. Afshar-Oromieh et al. assessed a large cohort including 319 patients with recurrent PCa who underwent 68Ga-PSMA-11 PET (median serum PSA value of 4.59 ng/mL, range: 0.01–41395 ng/mL). They reported detection rates of 47% for serum PSA values ≤ 0.2 ng/mL, rising to 100% for serum PSA values > 20 ng/mL (39). Similar results from other studies have further proven that 68Ga-PSMA-11 PET has higher detection rate for BCR with low PSA levels (40, 41). In addition, data from other research groups that studied 18F-DCFBC, 18F-DCFPyL, and 18F-rhPSMA-7 have also indicated rather high detection rates with low serum PSA levels (Figure 2) (42–44).
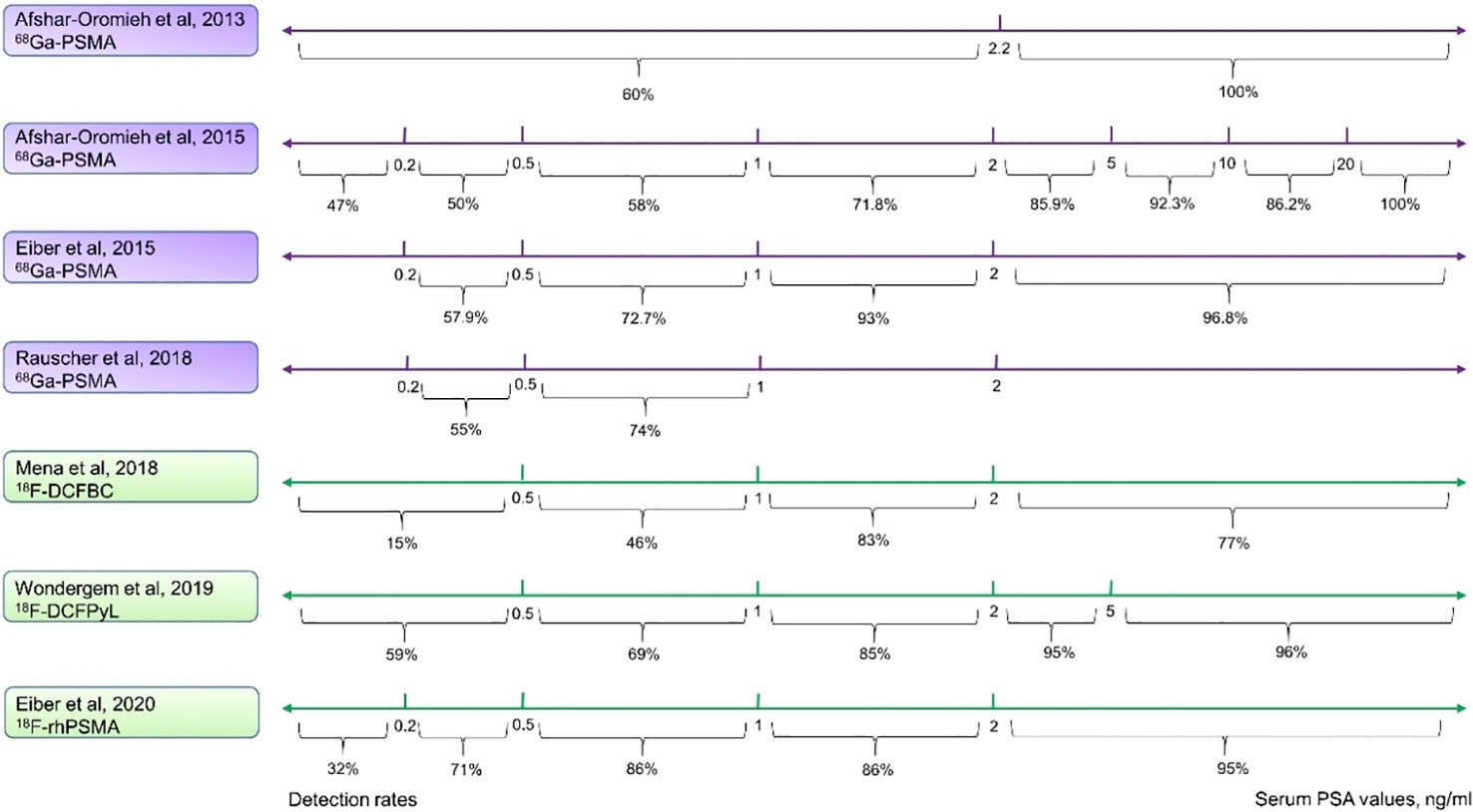
Figure 2 Comparison of detection rates of 68Ga and 18F labeled PSMA-ligand. Purple lines indicate the 68Ga-PSMA-11, and green lines show 18F-PSMA-ligands. The 68Ga-PSMA-11 PET has a modestly high detection rate even at low serum PSA levels. 18F-DCFPyL has a higher detection rate in all the various PSA levels as compared with 18F-DCFBC and is comparable with 68Ga-PSMA-11. 18F-rhPSMA performs the best among these 18F-labeled ligands and is similar to 68Ga-PSMA-11.
However, these results should be treated with caution because there was no systematic histological confirmation in most of the patients, and follow-up imaging was incomplete in the entire cohort. Current guidelines do not make recommendation for a single radiopharmaceutical. The comparison among different PSMA-targeted tracers are missing; thus, the term “PSMA-ligand” refers to a number of different tracers (45). It is necessary to perform further clinical studies with standardized follow-up protocols and histological validation before comparing outcomes associated with the use of these tracers.
Advanced disease monitoring
Androgen deprivation therapy (ADT) is the mainstay of treatment for PCa patients with recurrent or advanced disease (46, 47). However, in the first 5 years following ADT, approximately 10%–20% of patients will develop castration-resistant PCa (CRPC) (48). Once the metastatic castration-resistant prostate cancer (mCRPC) is detected, the overall survival (OS) ranges from 2 to 3 years (46, 47).
Monitoring the serum PSA level provides insight into both the disease burden and the biology of the disease; however, some mCRPC patients may have a low PSA level, because the tumor is less dependent on androgen receptor signaling. Moreover, PSA levels can stay low while visceral metastases are developing (49). The relationship between increasing PSA values and disease progression has been confirmed, but decreasing PSA values are not always associated with response to therapy. Using serum PSA alone to monitor disease development or therapy response in mCRPC patients is not entirely reliable (47, 50).
For mCRPC patients, some studies have suggested the choline PET/CT may be used in disease monitoring (51, 52). However, another prospective study has reported that there was no significant correlation between 11C-choline PET/CT image findings and chemotherapy treatment response (53). In summary, the application of choline PET/CT appears to be limited to the routine monitoring of mCRPC patients.
It has been demonstrated that new tracers, such as 68Ga-PSMA-11 PET/CT (54), can be used for monitoring disease in addition to choline, although these are not yet recommended in the mCRPC patient guidelines (45). Gafita et al. have reported a case of an mCRPC patient who underwent 177Lu-PSMA therapy, and the 68Ga-PSMA PET has clearly reflected the lesion alteration during the therapy (55). Thus, further studies in mCRPC patients are urgently needed to confirm the potential clinical application of PSMA-ligand in disease monitoring.
PSMA-targeted radiation therapy
Several therapies have been approved for mCRPC patients; however, the survival benefit in patients is usually less than 6 months (56). Novel therapies are needed for clinical application, and PSMA has emerged as a promising therapeutic molecular target. There are mainly two types of radioisotopes: alpha-emitting and beta-emitting.
Beta-emitting PSMA-targeted radioligand therapies
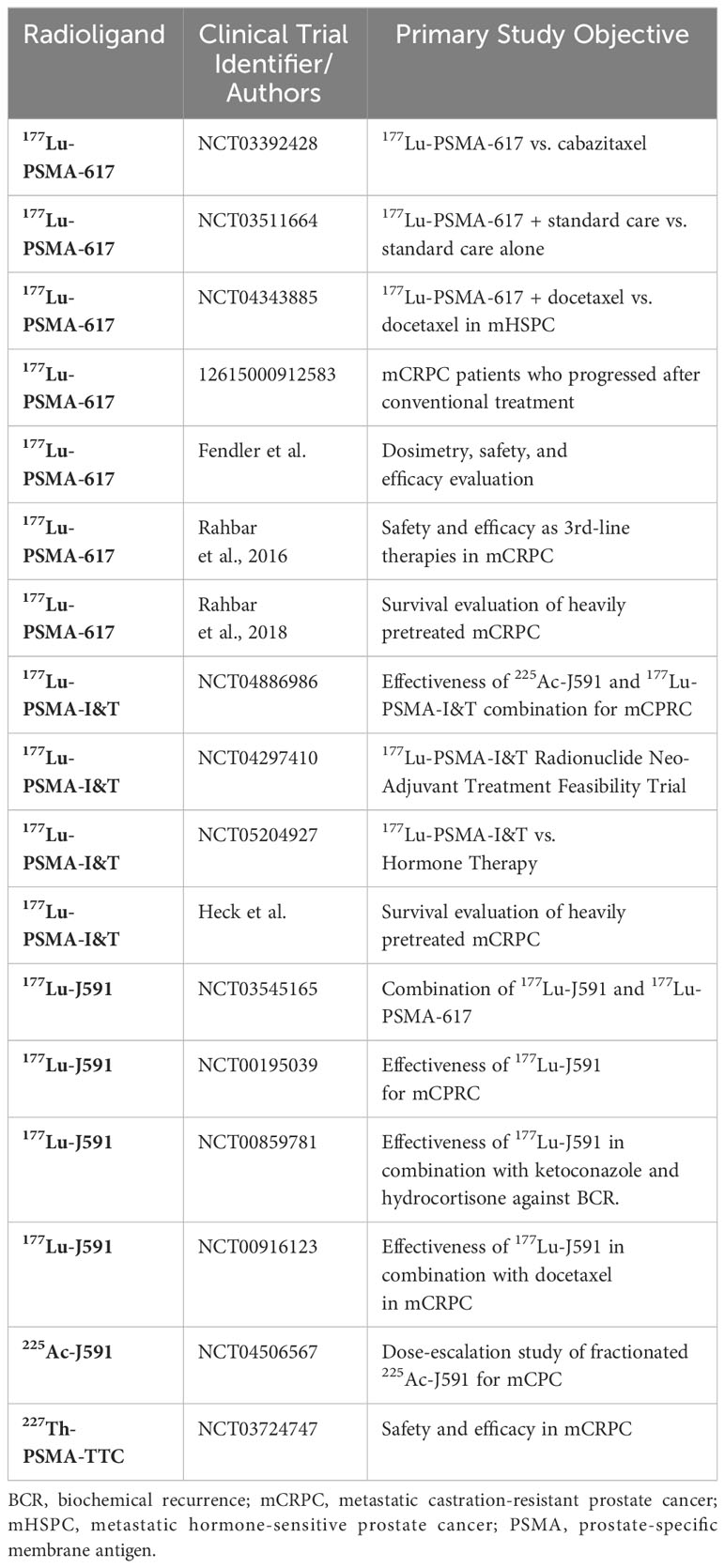
177Lu-PSMA-617 is the most used beta-therapy molecule. The TheraP phase 2 trial reported that 177Lu-PSMA-617 led to a higher PSA response rate (65/98 vs. 37/85) and fewer adverse events (32/98 vs. 45/85) as compared with cabazitaxel (15). Furthermore, the VISION trial has reported that compared with standard care, 177Lu-PSMA-617 plus standard care significantly prolonged both imaging-based progression-free survival (median, 8.7 vs. 3.4 months; hazard ratio for progression or death, 0.40; 95% CI, 0.29, 0.57; p < 0.001) and OS (median, 15.3 vs. 11.3 months; hazard ratio for death, 0.62; 95% CI, 0.52 to 0.74; p < 0.001). Additionally, the quality of life was not affected by the adverse events caused by 177Lu-PSMA-617 therapy (14). Another trial using LuPSMA reported that of 30 patients with mCRPC who had progressed after conventional treatment, 17 achieved a PSA decline of 50% or more. Fewer toxic effects and a reduction in pain were observed (16).
The other commonly used PSMA-ligand is 177Lu-PSMA-I&T, which also shows promising results (57). In a study of 100 patients where 177Lu-PSMA-I&T was used, a good response to treatment and low toxicity were observed (PSA decline ≥ 50%: 38 patients had a median clinical progression-free survival of 4.1 months and median OS was 12.9 months) (58). Several studies (59–61) have shown similar results (summarized in Table 1).
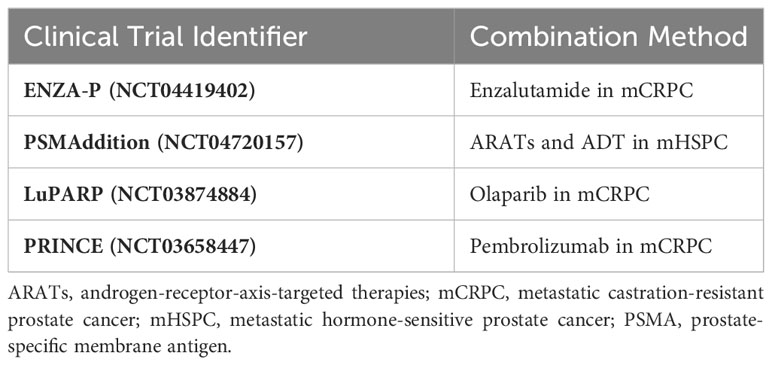
In addition, 177Lu-PSMA-617 has been used in several types of combination therapies. In CRPC patients, the combination of androgen-receptor-axis-targeted therapies (ARATs) may lead to improved tumor control. The DNA damage repair (DDR) pathway has been proven to participate closely during the radioligand therapy (RLT) (62); hence, the combination with PARP inhibitors such as olaparib and rucaparib have been under analysis. Moreover, immune checkpoint inhibitors such as anti-PD-1 or anti-CTLA-4 monoclonal antibodies are an important class of cancer therapies. Owing to the abscopal effect, combination therapy is under evaluation. Table 2 summarizes representative clinical trials of combination therapies.
There are other notable PSMA-targeting radionuclide therapy agents. 131I-MIP-1095 is one of the first agents used in radiopharmaceutical therapy against PCa (63). A study of 34 mCRPC patients who received 131I-MIP-1095 found that the first dose of RLT presented with the best therapeutic effect compared with the second and the third doses (64). 177Lu-PSMA-R2 is a urea-based PSMA-targeting small-molecule inhibitor (63). It has been shown to present a rapid tumor uptake and elimination through the urinary system in preclinical studies (65). A phase 1/2 clinical trial to evaluate 177Lu-PSMA-R2 in mCRPC is currently under way (NCT03490838).
Alpha-emitting PSMA-targeted radioligand therapies
Utilizing alpha emitters for treatment offers two distinct advantages compared to conventional radioligand therapies. The short distance between cells within human tissue (<0.1 mm) allows the selective destruction of target cancer cells while preserving the surrounding healthy tissue (66). The two most commonly used alpha-emitting radioactive isotopes currently are 225Ac and its daughter nuclide 213Bi, with half-lives of 9.9 days and 46 min, respectively.
Recent studies have demonstrated the remarkable efficacy of 225Ac-PSMA-617 in the treatment of mCRPC, particularly in patients who have experienced disease progression after 177Lu-PSMA therapy (67). There is also a clinical case report of 213Bi-PSMA-617, which is both an alpha and beta emitter, that achieved a drop in PSA from 237 μg/L to 43 μg/L in the patient with mCRPC (68). The study by Sathekge et al. (69) involved a relatively small cohort of 21 patients, and confirmed that 225Ac-PSMA-617 can lead to a significant reduction of pre-treatment PSA levels by more than 50% in the majority of hormone-sensitive prostate carcinoma (mHSPC) patients.
However, the use of high-activity 225Ac/213Bi generators for preparing therapeutic doses in the gigabecquerel (GBq) range remains prohibitively expensive, and the global supply of 225Ac produced from 229Th is severely limited (66). In terms of treatment, due to its highly cytotoxic nature, alpha-emitting isotopes like 225Ac may be associated with more serious side effects compared with PSMA-targeted β-radiation, including conditions like xerostomia (70). Furthermore, 225Ac produces a substantial number of recoiling daughter nuclei during its decay process, making this radioisotope challenging to control as a targeted alpha therapy agent (71). Nonetheless, this form of treatment offers hope for mCRPC and mHSPC patients and underscores the significant potential that targeted alpha therapy may have in the treatment of other cancers.
PSMA-targeted immunotherapies
There are several anti-PSMA radioimmunotherapies under evaluation, with a notable example being the J591 antibody. 177Lu-J591, 255Ac-J591, and 227Th-PSMA-TTC are three promising antibodies also under evaluation. The preliminary results using J591 show high efficacy and few side effects (72). The related clinical trials are listed in Table 1.
PSMA-targeted bispecific T-cell engager (BiTE) immunotherapy has been developed in recent years. Pasotuxizumab (AMG 212) binds to CD3 on T cells and PSMA on the tumor cells. A phase I clinical trial (NCT 01723475) has shown that a PSA decrease ≥ 50% was observed in 3 out of 16 patients and that BiTE immune therapy could be efficacious in solid tumors (73).
PSMA radioguided surgery
In patients with PCa, metastatic or recurrent lymph nodes may be in atypical locations and/or small in morphology, hindering accurate identification before and at the time of potential surgery (74).
Owing to the high detection rate for lymph node metastases, PSMA-ligand imaging combined with lymphadenectomy and salvage lymph node resection are increasingly used. PSMA-RGS allows the detection of PSMA-positive PCa cells and metastatic lymph nodes during the operation (75). RGS is a radionuclide-based radiomic model to measure intra-operative γ-emission using 99mTc- or 111In-labeled PSMA-ligand and provide acoustic feedback with a gamma probe (31). Schottelius et al. first reported the possibility of 111In-PSMA-I&T as an RGS radionuclide probe in 2015 (76). After that, Rauscher et al. conducted a comparative study in which the sensitivity, specificity, and accuracy of 111In-labeled PSMA RGS were 92.3%, 93.5%, and 93.1% respectively (77). 111In-PSMA-I&T-RGS facilitated intra-operative resection of sub-centimeter metastatic lymph nodes. The smallest resected metastatic lesion was 2 mm in size (75). In contrast, the 99mTc, known as the most widely applied and available radioisotope, was introduced as a PSMA-targeted RGS probe due to its medium-energy γ-radiation, low cost, short half-life, and high stability in vivo in 2016 (78). In the past few years, 99mTc-labeled PSMA-I&S gradually replaced the application of 111In-labeled PSMA-I&T in RGS (79, 80). In a retrospective analysis of 132 surgical specimens in 31 patients with BCR after primary RP who underwent 99mTc-PSMA-RGS, Maurer et al. reported that this novel approach had a sensitivity of 83.6% (95% CI 70.9%, 91.5%), a specificity of 100%, and an accuracy of 93.0% (95% CI 85.8%, 96.7%) (74).
Moreover, the robot-assisted PSMA-ligand surgery has gradually become a hot spot in RGS recently. Yılmaz et al. conducted 99mTc-PSMA-targeted robot-assisted RGS in 15 intermediate- or high-risk score (D’Amico risk stratification) patients; the sensitivity, specificity, accuracy, and negative and positive predictive value were 100%, respectively (81). Thus, patients with PCa profit from RGS because suspicious metastatic lymph nodes are accurately resected, which could correlate with better prognosis.
This technique has been used in PSMA-positive PCa patients; however, the correlation between PSMA IHC and PSMA uptake using gamma probe during surgery is still unclear. The activity gathered by the gamma probe during surgery is dependent not only on the specific PSMA-ligand accumulation in cells, but also on the distance of the probe to the lesions and the size of the tumor deposit. Therefore, clear correlations between the signal from the gamma probe and the PSMA expression on preclinical and clinical experiments are difficult to achieve and have not been reported so far in literature. Thus, there are no guidelines and threshold values of gamma probe counts for metastatic lesion detection.
Conclusions
PSMA-targeted imaging and therapy represent a rapidly emerging strategy in PCa management. PSMA-ligand imaging provides accurate staging of primary PCa and high diagnostic efficacy in recurrent lesions compared with conventional imaging, allowing early therapeutic intervention. Moreover, there is evidence that the results may contribute to a change in the risk classification, and impact clinical practice. Standardized interpretation (e.g,. miTNM) of PSMA-ligand PET and its potential for predicting prognosis are evolving. Several institutions have adopted these in clinical practice. Furthermore, PSMA-targeted radioligand therapy is a very promising approach for the treatment of advanced PCa, especially mCRPC, with recent evidence showing a survival benefit. Several prospective trials are ongoing and will hopefully provide evidence for supporting approval for marketing. We believe that PSMA-targeted theranostics will soon become significant strategies in the standard of care for PCa management. Nuclear medicine physicians should be integrated into multidisciplinary teams, along with medical oncologists, radiation oncologists, urologists, and radiologists, to optimize patient care.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by HW, GL, JZ, and ME. The first draft of the manuscript was written by HW and GL. The manuscript was reviewed by RT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADT, androgen deprivation therapy; ARATs, androgen-receptor-axis-targeted therapies; BCR, biochemical recurrence; BiTE, bispecific T-cell engager; bRFS, biochemical recurrence-free survival; CI, confidence interval; CRPC, castration-resistant prostate cancer; CT, computed tomography; DDR, DNA damage repair; DPD, 3,3-diphospho-1,2-propanodicarboxylic acid; ePLND, extended pelvic lymph node dissection; FDA, Food and Drug Administration; FOLH1, folate hydrolase 1 gene; GCPII, carboxypeptidase II; mCRPC, metastatic castration-resistant prostate cancer; mHSPC, majority of hormone-sensitive prostate carcinoma; MRI, magnetic resonance imaging; OS, overall survival; PCa, prostate cancer; PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; RGS, radioguided surgery; rhPSMA, radio-hybrid PSMA; RLT, radioligand therapy; RP, radical prostatectomy; SBRT, single-component stereotactic radiotherapy; SPECT, single-photon emission computed tomography; US, United States.
References
1. Ferlay J, Laversanne M, Ervik M, Lam F, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer (2020). Available at: https://gco.iarc.fr/tomorrow.
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and Malignant human tissues. Clin Cancer Res (1997) 3(1):81–5.
4. Cimadamore A, Cheng M, Santoni M, Lopez-Beltran A, Battelli N, Massari F, et al. New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol (2018) 8:653. doi: 10.3389/fonc.2018.00653
5. Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem (2004) 91(3):528–39. doi: 10.1002/jcb.10661
6. Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate (2011) 71(3):281–8. doi: 10.1002/pros.21241
7. Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res (2009) 15(2):167–72. doi: 10.1007/s12253-008-9104-2
8. Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical validation of PSMA expression measured by (68)Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med (2018) 59(2):238–43. doi: 10.2967/jnumed.117.195172
9. Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology (2007) 50(4):472–83. doi: 10.1111/j.1365-2559.2007.02635.x
10. Ozcan PP, Serdengectı M, Koc ZP, Balcı Y, Tek M, Bozlu M, et al. Cancers and benign processes on (68) Ga PSMA PET-CT imaging other than prostate cancer. World J Nucl Med (2022) 21(2):106–11. doi: 10.1055/s-0042-1750331
11. Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med (2017) 58(10):1545–52. doi: 10.2967/jnumed.117.191031
12. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol (2016) 195(5):1436–43. doi: 10.1016/j.juro.2015.12.025
13. Ehret F, Hofmann T, Fürweger C, Kufeld M, Staehler M, Muacevic A, et al. Single-fraction PSMA-PET- and multiparametric MRI-guided SBRT for prostate cancer local recurrences. BJU Int (2022) 131(1):101–8. doi: 10.1111/bju.15894
14. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med (2021) 385(12):1091–103. doi: 10.1056/NEJMoa2107322
15. Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet (2021) 397(10276):797–804. doi: 10.1016/s0140-6736(21)00237-3
16. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol (2018) 19(6):825–33. doi: 10.1016/s1470-2045(18)30198-0
17. Horn T, Krönke M, Rauscher I, Haller B, Robu S, Wester HJ, et al. Single lesion on prostate-specific membrane antigen-ligand positron emission tomography and low prostate-specific antigen are prognostic factors for a favorable biochemical response to prostate-specific membrane antigen-targeted radioguided surgery in recurrent prostate cancer. Eur urol (2019) 76(4):517–23. doi: 10.1016/j.eururo.2019.03.045
18. Hruby G, Eade T, Emmett L, Ho B, Hsiao E, Schembri G, et al. (68) Ga-PSMA-PET/CT staging prior to definitive radiation treatment for prostate cancer. Asia Pac J Clin Oncol (2018) 14(4):343–6. doi: 10.1111/ajco.12872
19. Corfield J, Perera M, Bolton D, Lawrentschuk N. 68 Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol (2018) 36(4):519–27. doi: 10.1007/s00345-018-2182-1
20. Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate (2015) 75(16):1934–40. doi: 10.1002/pros.23091
21. Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet (2020) 395(10231):1208–16. doi: 10.1016/S0140-6736(20)30314-7
22. Yaxley JW, Raveenthiran S, Nouhaud FX, Samaratunga H, Yaxley WJ, Coughlin G, et al. Risk of metastatic disease on (68) gallium-prostate-specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU Int (2019) 124(3):401–7. doi: 10.1111/bju.14828
23. Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics (2017) 7(1):228–37. doi: 10.7150/thno.16638
24. Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging (2016) 43(12):2114–21. doi: 10.1007/s00259-016-3435-0
25. Janssen JC, Meißner S, Woythal N, Prasad V, Brenner W, Diederichs G, et al. Comparison of hybrid (68)Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. Eur Radiol (2018) 28(2):610–9. doi: 10.1007/s00330-017-4994-6
26. Riola-Parada C, García-Cañamaque L, Pérez-Dueñas V, Garcerant-Tafur M, Carreras-Delgado JL. Simultaneous PET/MRI vs PET/CT in oncology. A systematic review. Rev espanola medicina Nucl e imagen molecular (2016) 35(5):306–12. doi: 10.1016/j.remn.2016.06.001
27. Eiber M, Martinez-Möller A, Souvatzoglou M, Holzapfel K, Pickhard A, Löffelbein D, et al. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging (2011) 38(9):1691–701. doi: 10.1007/s00259-011-1842-9
28. Marinescu IM, Spohn SKB, Kiefer S, Bronsert P, Ceci L, Holzschuh J, et al. Intraindividual comparison between [(18)F] PSMA-1007 PET/CT and multiparametric MRI for radiotherapy planning in primary prostate cancer patients. Front Oncol (2022) 12:880042. doi: 10.3389/fonc.2022.880042
29. Glemser PA, Rotkopf LT, Ziener CH, Beuthien-Baumann B, Weru V, Kopp-Schneider A, et al. Hybrid imaging with [(68)Ga]PSMA-11 PET-CT and PET-MRI in biochemically recurrent prostate cancer. Cancer Imaging (2022) 22(1):53. doi: 10.1186/s40644-022-00489-9
30. Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur urol (2016) 69(3):393–6. doi: 10.1016/j.eururo.2015.06.010
31. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol (2016) 13(4):226–35. doi: 10.1038/nrurol.2016.26
32. King CR. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int J Radiat Oncol Biol Phys (2012) 84(1):104–11. doi: 10.1016/j.ijrobp.2011.10.069
33. Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur urol (2014) 65(6):1034–43. doi: 10.1016/j.eururo.2013.08.013
34. Beresford MJ, Gillatt D, Benson RJ, Ajithkumar T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin Oncol (R Coll Radiol) (2010) 22(1):46–55. doi: 10.1016/j.clon.2009.10.015
35. Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol (2014) 43(11):1503–13. doi: 10.1007/s00256-014-1903-9
36. Brogsitter C, Zöphel K, Kotzerke J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur J Nucl Med Mol Imaging (2013) 40 Suppl 1:S18–27. doi: 10.1007/s00259-013-2358-2
37. Treglia G, Ceriani L, Sadeghi R, Giovacchini G, Giovanella L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: a meta-analysis. Clin Chem Lab Med (2014) 52(5):725–33. doi: 10.1515/cclm-2013-0675
38. Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J Nucl Med (2014) 55(9):1424–9. doi: 10.2967/jnumed.114.138313
39. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging (2015) 42(2):197–209. doi: 10.1007/s00259-014-2949-6
40. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med (2015) 56(5):668–74. doi: 10.2967/jnumed.115.154153
41. Rauscher I, Düwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur urol (2018) 73(5):656–61. doi: 10.1016/j.eururo.2018.01.006
42. Eiber M, Kroenke M, Wurzer A, Ulbrich L, Jooß L, Maurer T, et al. 18F-rhPSMA-7 PET for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med (2020) 61(5):696–701. doi: 10.2967/jnumed.119.234914
43. Wondergem M, Jansen BHE, van der Zant FM, van der Sluis TM, Knol RJJ, van Kalmthout LWM, et al. Early lesion detection with (18)F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging (2019) 46(9):1911–8. doi: 10.1007/s00259-019-04385-6
44. Mena E, Lindenberg ML, Shih JH, Adler S, Harmon S, Bergvall E, et al. Clinical impact of PSMA-based (18)F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging (2018) 45(1):4–11. doi: 10.1007/s00259-017-3818-x
45. Mottet N, van den Bergh RCN, Briers E, Cornford P, De Santis M, Fanti S, et al. EAU - ESTRO - ESUR - SIOG guidelines on prostate cancer 2020. In: European Association of Urology Guidelines. 2020 Edition. Arnhem, The Netherlands: European Association of Urology Guidelines Office; (2020).
46. Davies A, Conteduca V, Zoubeidi A, Beltran H. Biological evolution of castration-resistant prostate cancer. Eur Urol Focus (2019) 5(2):147–54. doi: 10.1016/j.euf.2019.01.016
47. Ceci F, Castellucci P, Nanni C, Fanti S. PET/CT imaging for evaluating response to therapy in castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging (2016) 43(12):2103–4. doi: 10.1007/s00259-016-3493-3
48. Alpajaro SIR, Harris JAK, Evans CP. Non-metastatic castration resistant prostate cancer: a review of current and emerging medical therapies. Prostate Cancer Prostatic Dis (2019) 22(1):16–23. doi: 10.1038/s41391-018-0078-1
49. Pezaro C, Omlin A, Lorente D, Rodrigues DN, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur urol (2014) 65(2):270–3. doi: 10.1016/j.eururo.2013.10.055
50. Payne H, Cornford P. Prostate-specific antigen: an evolving role in diagnosis, monitoring, and treatment evaluation in prostate cancer. Urol Oncol (2011) 29(6):593–601. doi: 10.1016/j.urolonc.2009.11.003
51. De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget (2014) 5(23):12448–58. doi: 10.18632/oncotarget.2558
52. De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. (18)F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging (2015) 42(8):1276–83. doi: 10.1007/s00259-015-3042-5
53. Schwarzenböck SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, et al. Prospective evaluation of [11C]Choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging (2016) 43(12):2105–13. doi: 10.1007/s00259-016-3439-9
54. Plouznikoff N, Artigas C, Sideris S, Martinez Chanza N, Gil T, Peltier A, et al. Evaluation of PSMA expression changes on PET/CT before and after initiation of novel antiandrogen drugs (enzalutamide or abiraterone) in metastatic castration-resistant prostate cancer patients. Ann Nucl Med (2019) 33(12):945–54. doi: 10.1007/s12149-019-01404-2
55. Gafita A, Wang H, Tauber R, D’Alessandria C, Weber WA, Eiber M. Exceptional 4-year response to 177 Lu-PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol imaging (2019) 46(10):2212–3. doi: 10.1007/s00259-019-04410-8
56. Jones W, Griffiths K, Barata PC, Paller CJ. PSMA theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers (Basel) (2020) 12(6):1367. doi: 10.3390/cancers12061367
57. Heck MM, Retz M, D'Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with (177)Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol (2016) 196(2):382–91. doi: 10.1016/j.juro.2016.02.2969
58. Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur urol (2019) 75(6):920–6. doi: 10.1016/j.eururo.2018.11.016
59. Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget (2017) 8(2):3581–90. doi: 10.18632/oncotarget.12240
60. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med (2017) 58(1):85–90. doi: 10.2967/jnumed.116.183194
61. Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging (2018) 45(1):12–9. doi: 10.1007/s00259-017-3848-4
62. Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med (2016) 375:443–53. doi: 10.1056/NEJMoa1603144
63. Zhang H, Koumna S, Pouliot F, Beauregard JM, Kolinsky M. PSMA theranostics: current landscape and future outlook. Cancers (Basel) (2021) 13(16):4023. doi: 10.3390/cancers13164023
64. Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with (131)I-MIP-1095. Eur J Nucl Med Mol Imaging (2017) 44(6):950–9. doi: 10.1007/s00259-017-3665-9
65. Muzio V, Ravasi L, Sacchetti L, Fugazza L, Bacot S, Debiossat M, et al. Biodistribution of PSMA-R2 in mice bearing prostate cancer. EANM (2019).
66. Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An overview of targeted alpha therapy with (225)Actinium and (213)Bismuth. Curr Radiopharm (2018) 11(3):200–8. doi: 10.2174/1874471011666180502104524
67. Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur urol (2021) 79(3):343–50. doi: 10.1016/j.eururo.2020.11.013
68. Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. 213 Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol imaging (2017) 44(6):1099–100. doi: 10.1007/s00259-017-3657-9
69. Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Mokoala K, Reed J, et al. (225)Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): preliminary clinical findings. Eur J Nucl Med Mol Imaging (2023) 50(7):2210–8. doi: 10.1007/s00259-023-06165-9
70. Zacherl MJ, Gildehaus FJ, Mittlmeier L, Böning G, Gosewisch A, Wenter V, et al. First clinical results for PSMA-targeted α-therapy using (225)Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med (2021) 62(5):669–74. doi: 10.2967/jnumed.120.251017
71. Filippi L, Chiaravalloti A, Schillaci O, Bagni O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev Anticancer Ther (2020) 20(10):823–9. doi: 10.1080/14737140.2020.1814151
72. Tagawa ST, Osborne J, Fernandez E, Thomas C, Niaz MJ, Ciriaco A, et al. Phase I dose-escalation study of PSMA-targeted alpha emitter 225Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). Am Soc Clin Oncol (2020).
73. Hummel H-D, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler M-E, et al. Phase I study of pasotuxizumab (AMG 212/BAY 2010112), a PSMA-targeting BiTE (Bispecific T-cell Engager) immune therapy for metastatic castration-resistant prostate cancer (mCRPC). Am Soc Clin Oncol (2020).
74. Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, Van Den Berg N, et al. 99mTechnetium-based prostate-specific membrane antigen-radioguided surgery in recurrent prostate cancer. Eur Urol (2018) 75(4):659–66. doi: 10.1016/j.eururo.2018.03.013
75. Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur urol (2015) 68(3):530–4. doi: 10.1016/j.eururo.2015.04.034
76. Schottelius M, Wirtz M, Eiber M, Maurer T, Wester HJ. [(111)In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res (2015) 5(1):68. doi: 10.1186/s13550-015-0147-6
77. Rauscher I, Eiber M, Maurer T. [PSMA-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer]. Aktuelle Urol (2017) 48(2):148–52. doi: 10.1055/s-0042-120455
78. Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M, et al. Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med (2017) 58(2):235–42. doi: 10.2967/jnumed.116.178939
79. Rauscher I, Maurer T, Souvatzoglou M, Beer AJ, Vag T, Wirtz M, et al. Intrapatient comparison of 111In-PSMA I&T SPECT/CT and hybrid 68Ga-HBED-CC PSMA PET in patients with early recurrent prostate cancer. Clin Nucl Med (2016) 41(9):e397–402. doi: 10.1097/rlu.0000000000001273
80. Fallahi B, Khademi N, Karamzade-Ziarati N, Fard-Esfahani A, Emami-Ardekani A, Farzanefar S, et al. 99mTc-PSMA SPECT/CT versus 68Ga-PSMA PET/CT in the evaluation of metastatic prostate cancer. Clin Nucl Med (2021) 46(2):e68–74. doi: 10.1097/rlu.0000000000003410
Keywords: PSMA, PET, prostate cancer, theranostics, radioguided surgery
Citation: Wang H, Li G, Zhao J, Eiber M and Tian R (2024) Current status of PSMA-targeted imaging and therapy. Front. Oncol. 13:1230251. doi: 10.3389/fonc.2023.1230251
Received: 28 May 2023; Accepted: 23 November 2023;
Published: 09 January 2024.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Luca Filippi, Policlinico Tor Vergata, ItalyIsmaheel Lawal, Emory University, United States
Copyright © 2024 Wang, Li, Zhao, Eiber and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Tian, cm9uZ3RpYW5udWNsZWFyQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Hui Wang1†
Hui Wang1† GuanNan Li
GuanNan Li Matthias Eiber
Matthias Eiber Rong Tian
Rong Tian