- 1Unidad de Investigacion en Enfermedades Infecciosas, Unidad Medica de Alta Especialidad (UMAE) Pediatria, Instituto Mexicano del Seguro Social, Ciudad de Mexico, Mexico
- 2Instituto de Investigação e Inovação, Universidade do Porto (i3S), Porto, Portugal
- 3Institute of Molecular Pathology and Immunology of the University of Porto (Ipatimup), Porto, Portugal
- 4Department of Oncology, Wayne State University, Porto, Portugal
- 5Department of Pathology, Wayne State University, Detroit, MI, United States
Editorial on the Research Topic
The role of Helicobacter pylori in gastric carcinogenesis
H. pylori (Hp) is the only bacterium recognized as a cancer-causing agent by the WHO and has drawn attention from several disciplines, including oncology, microbiology, immunology, cell biology, epidemiology, genomics, and even human evolution. Evidence suggests that Hp has colonized the human stomach since the origin of our species, and it is not surprising that this bacterium mirrors the race of its human host (1). This marked human-bacterium co-evolution implies a symbiotic relationship in which both benefit from sharing a highly specialized niche. Hp represents the best- documented member of the human microbiota that has co-evolved with humans. While more than 60% of the adult population remains colonized, less than 2% of those colonized will eventually develop gastric cancer (GC), strongly suggesting that GC is a rare event occurring only under as yet unknown conditions.
Colonization of the gastric mucosa by Hp elicits an inflammatory reaction, a natural dialogue between the two species, as seen with the gut microbiota. The chronic inflammation induced by Hp significantly alters the transcriptomic pattern of the gastric mucosal cells, which in turn alter the Hp transcriptome. When this relationship lasts for decades , as with human-Hp, accidents can happen, giving rise to a hostile relationship that threatens homeostasis. The challenge for science is to understand the natural history of this relationship.
In this Research Topic, five groups of researchers present results that add to our knowledge of the human-Hp dialog, particularly when things go wrong, and aim to understand the mechanisms behind tissue damage. He et al. exposed gastric cell lines to Hp lysates for 30 generations and showed that this prolonged exposure to Hp antigens leads to the promotion of cell proliferation, and inhibition of apoptosis and autophagy, probably via the Nod1-NF-kB/MAPK-ERK/FOXO4 pathway. Infection of gerbils with Hp for 90 weeks produced similar results. Thus, chronic exposure to Hp seems to modify the response of gastric epithelial cells in a way that favors the development of gastric preneoplastic lesions.
Jan et al. studied patients with GC and Hp infection and found that infection and cagA expression is associated with increased expression of IL-6, IL-10, and TGF-β, and with activation of the JAK/STAT pathway via inactivation of the promoter of SOCS1 by hypermethylation of its promoter region. The authors found that Hp is associated with unregulated inflammation even when patients have advanced GC, an observation that challenges the current notion that when GC develops, Hp is absent or has extremely low metabolic activity. If Hp keeps pushing boundaries at this late clinical-stage, then Hp eradication should be considered even in GC cases.
The histone demethylase JARID1B was investigated in GC tissues by Zheng et al., who found that increased expression has a significant correlation with poor survival, low immune score, low stromal score, and increased proliferation of GC cells, probably by regulating the expression of CCND1. Furthermore, in vitro and in vivo experimental in vitro in vivo models showed that Hp upregulates the expression of JARID1B by downregulation of miR-29c, a regulator of JARID1B. Histone demethylases have been shown to have a role in tumorigenesis in breast cancer, ovarian cancer, colorectal , and even lung cancers (2). This study offers another explanation for the role of Hp in causing dysregulated gene expression.
Angiogenesis is an essential component in all cancers to provide nutrients and oxygen to tissue with highly unregulated growth. Previous studies have documented the ability of Hp to activate factors that stimulate angiogenesis. Malespin-Bendaña et al. extended these studies by documenting the role of Angpt2, Vegf-A, and Tnf-A in angiogenesis using a mouse model. It is known that Hp activates NF-κβ, which induces the expression of TNF-A, which then upregulates the expression of Vegf-A, Angpt2, and Tie2. The mouse model demonstrates that Hp can also downregulate Angpt1. Thus, Hp can activate and sustain angiogenesis to feed unwanted cells.
The anatomic geography of Hp colonization has been under studied and may provide important clues to understanding how Hp lives in intimate contact with different cell types in the gastric mucosa. Beccaceci and Sigal reviewed the few studies that have addressed this issue and offered a challenging hypothesis about the consequences of Hp colonization along the gastric glands. Contrary to previous theories, Hp has evolved to live deep in the glands in close contact with stem cells, a condition that may help Hp thrive in the stomach for decades but also carries the risk of damaging the progenitor cell sanctuary.
Hp can modify the dialog between different regions of the gland from the tip to the base regions, including differentiated epithelial , progenitor, stem, and stromal cells, with consequences for cell cycle, differentiation, proliferation, and other functions necessary for homeostasis and the inflammatory-immune response. Most cells in the glands have a short life cycle, and it is difficult to assume that they play a major role in oncogenesis. In contrast, effects on stem cells can have long-lasting effects and are likely to be more important in oncogenesis.
In the 40 years since the discovery of Hp, much has been learned about its physiology, which is highly specialized for living in the human gastric mucosa, a niche with extremely harsh conditions for most other microorganisms. This exquisite adaptation is the result of human-Hp co-evolution since the beginning of our species. This VIP resident of the gastric mucosa has learned to offer health benefits to its host, as expected for key components of the human microbiota (3). Hp has also been recognized as the main risk factor for GC, a fact that might be related to changes in our ways of living. Early humans lived as nomads, rather isolated, whereas now we live in large cities. This social evolution (sanitation, diet, personal habits, medicine, etc.) has been faster than biological evolution, resulting in a much longer life expectancy and a longer human-Hp relationship, with unexpected circumstances that increase the chances of biological accidents leading to disease (Figure 1). Understanding the conditions under which these accidents happen will provide tools to prevent unwanted outcomes and preserve the benefits gained from human-Hp symbiosis.
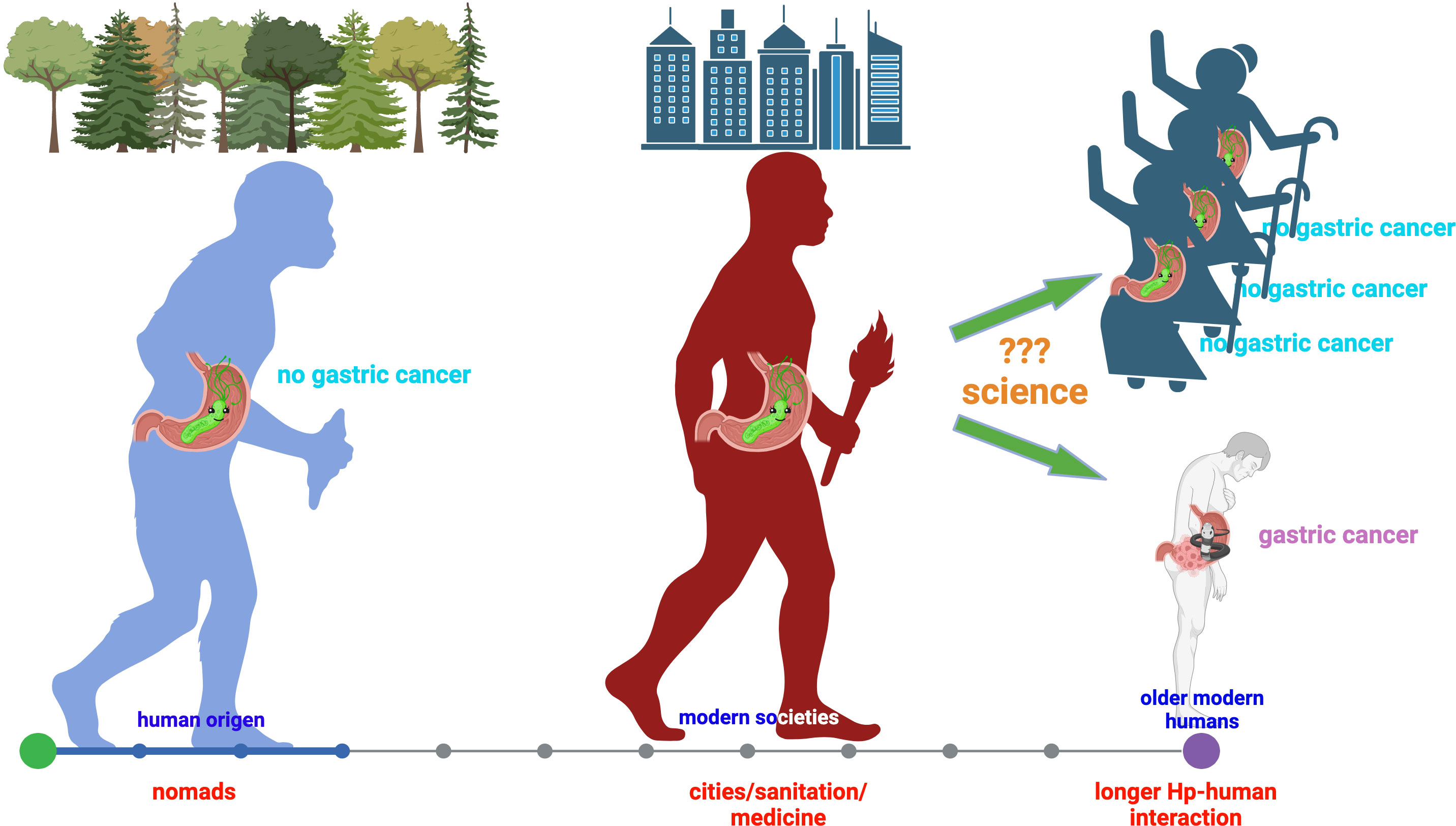
Figure 1 Human-H. pylori co-evolution, social evolution, and gastric cancer risk. This figure was designed using BioRender.
Author contributions
JT wrote the initial draft and reviewed the final manuscript. RF reviewed and edited the manuscript. IK reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science (2003) 299:1582–5. doi: 10.1126/science.1080857
2. Kim TD, Shin S, Berry WL, Oh S, Janknecht R. The JMJD2A demethylase regulates apoptosis and proliferation in colon cancer cells. J Cell Biochem (2012) 113(4):1368–76. doi: 10.1002/jcb.24009
Keywords: Helicobacter pylori, gastric cancer, oncogenesis, evolution, gastric mucosa
Citation: Torres J, Ferreira RM and Kato I (2023) Editorial: The role of Helicobacter pylori in gastric carcinogenesis. Front. Oncol. 13:1233890. doi: 10.3389/fonc.2023.1233890
Received: 02 June 2023; Accepted: 15 June 2023;
Published: 26 June 2023.
Edited and Reviewed by:
Luisa Lanfrancone, European Institute of Oncology (IEO), ItalyCopyright © 2023 Torres, Ferreira and Kato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier Torres, dWltZWlwQGdtYWlsLmNvbQ==
 Javier Torres
Javier Torres Rui M. Ferreira2,3
Rui M. Ferreira2,3 Ikuko Kato
Ikuko Kato