- 1Department of Medical and Surgical Sciences (DIMEC), Alma Mater Studiorum University of Bologna, Bologna, Italy
- 2Radiation Oncology Unit, Gemelli Molise Hospital - Università Cattolica del Sacro Cuore, Campobasso, Italy
- 3Radiation Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 4Radiotherapy Unit, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy
- 5Nuclear Medicine, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 6Division of Urology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 7Medical Physics Unit, Gemelli Molise Hospital, Campobasso, Italy
- 8Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Rome, Italy
- 9Department of Medical Physics, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 10Istituto di Radiologia, Università Cattolica del Sacro Cuore, Roma, Italy
Background: The objective of this study was to assess the impact of age and other patient and treatment characteristics on toxicity in prostate cancer patients receiving adjuvant radiotherapy (RT).
Materials and methods: This observational study (ICAROS-1) evaluated both acute (RTOG) and late (RTOG/EORTC) toxicity. Patient- (age; Charlson’s comorbidity index) and treatment-related characteristics (nodal irradiation; previous TURP; use, type, and duration of ADT, RT fractionation and technique, image-guidance systems, EQD2 delivered to the prostate bed and pelvic nodes) were recorded and analyzed.
Results: A total of 381 patients were enrolled. The median EQD2 to the prostate bed (α/β=1.5) was 71.4 Gy. The majority of patients (75.4%) were treated with intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT). Acute G3 gastrointestinal (GI) and genitourinary (GU) toxicity rates were 0.5% and 1.3%, respectively. No patients experienced >G3 acute toxicity. The multivariable analysis of acute toxicity (binomial logistic regression) showed a statistically significant association between older age (> 65) and decreased odds of G≥2 GI acute toxicity (OR: 0.569; 95%CI: 0.329-0.973; p: 0.040) and decreased odds of G≥2 GU acute toxicity (OR: 0.956; 95%CI: 0.918-0.996; p: 0.031). The 5-year late toxicity-free survival rates for G≥3 GI and GU toxicity were 98.1% and 94.5%, respectively. The only significant correlation found (Cox’s regression model) was a reduced risk of late GI toxicity in patients undergoing hypofractionation (HR: 0.38; 95% CI: 0.18-0.78; p: 0.008).
Conclusions: The unexpected results of this analysis could be explained by a “response shift bias” concerning the protective effect of older age and by treatment in later periods (using IMRT/VMAT) concerning the favorable effect of hypofractionation. However, overall, the study suggests that age should not be a reason to avoid adjuvant RT and that the latter is well-tolerated even with moderately hypofractionated regimens.
Introduction
Prostate cancer (PCa) is a significant health concern, ranking second in terms of incidence and fifth in terms of mortality among male populations (1). Radical prostatectomy (RP) is a commonly employed treatment option for PCa. However, the five-year biochemical relapse-free survival (bRFS) rate after RP is approximately 50% of patients with high-risk features at pathological evaluation (2–4).
Postoperative radiotherapy (RT) has been investigated as an adjunctive treatment following RP, and the results of four randomized studies (2–5) have demonstrated improved bRFS rates (around 25% at five years) compared to RP alone. Moreover, one of these studies has shown a significantly reduced risk of metastasis and improved overall survival (OS) with postoperative RT (6).
Consequently, international guidelines, such as those from the European Association of Urology1 (EAU 2022) and the National Comprehensive Cancer Network2 (NCCN 2022), recommend postoperative RT as an adjuvant therapy for selected PCa patients. Specifically, EAU guidelines recommend adjuvant RT for high-risk patients (pN0) with at least two of the following high-risk features: International Society of Urological Pathology (ISUP) grade group 4–5, pT3 stage, and positive surgical margins.
Nevertheless, recent randomized trials (7–9) and a meta-analysis (10) have demonstrated that early salvage RT can achieve biochemical and clinical outcomes comparable to those of adjuvant RT, while significantly reducing the number of patients requiring pelvic RT and improving overall treatment tolerability. These findings highlight the importance of careful patient selection for adjuvant RT, considering the cost/benefit ratio.
In this regard, it is crucial to consider both factors that predict greater benefit from adjuvant RT, such as seminal vesicle involvement (11) and positive surgical margins (12) as well as factors that indicate a higher risk of side effects. However, the available evidence on the latter topic is limited and often derived from small studies that have analyzed only specific patient and/or treatment characteristics (13–17).
Therefore, the aim of this study is to analyze multiple patient- and treatment-related factors in a large multicenter series of PCa patients who underwent adjuvant RT, with the goal of identifying predictors of increased toxicity, and in particular to evaluate whether older age is associated with a greater risk of radiation-induced side effects.
Material and methods
Study design and endpoints
This sub-analysis is part of a multicenter observational study (311/2019/Oss/AOUBo, ICAROS-1 study) focusing specifically on patients with PCa who underwent postoperative adjuvant RT. The study endpoints encompass both acute and late gastrointestinal (GI) and genitourinary (GU) toxicities.
Inclusion criteria
The inclusion criteria were as follows: 1) patients diagnosed with PCa who underwent RP with negative or microscopically positive margins (R0-1) and no distant metastases, and 2) RT delivered using external beam techniques with photon beams. Exclusion criteria were: 1) presence of macroscopic (R2) residual disease after RP, 2) postoperative PSA level exceeding 0.2 ng/ml, and 3) postoperative RT delivered more than one year after RP.
Evaluated parameters
The recorded and evaluated patient-related characteristics included age and Charlson’s comorbidity index. Age was analyzed both as a continuous variable and as a dichotomous variable using a cut-off at the median value. The analyzed treatment characteristics encompassed the delivery of prophylactic lymph node irradiation (PNI), previous transurethral resection of the prostate (TURP), use and type of adjuvant androgen deprivation therapy (ADT) (LH-RH analogues or high-dose bicalutamide) and its duration, RT fractionation and technique (including the type of image-guidance systems employed), as well as the Equivalent Dose in 2 Gy per fraction (EQD2) delivered to the prostate bed and pelvic lymph nodes. Acute toxicity was monitored with weekly visits during treatment and with a follow-up visit 2 months after the end of treatment. Late toxicity was evaluated with a first follow-up visit 6 months after the end of treatment and then with further visits every 6 months up to 24 months after treatment, followed by annual assessments up to 10 years. Gastrointestinal toxicity was evaluated by patient interviews and proctoscopy, if necessary. Genitourinary toxicity was assessed through patient interviews and urine analysis during follow-up.
Statistical analysis
Statistical computations were performed using IBM SPSS Version 22.0 software package (IBM Corp, Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant. Acute toxicity was evaluated using the RTOG scale, while late toxicity was assessed using the RTOG/EORTC scale (18). The chi-squared test with Yates’ continuity correction and Fisher’s exact test were employed in univariate logistic regression to examine the correlation between the analyzed variables and acute toxicity. Additionally, a binomial logistic stepwise regression was used to estimate the likelihood of acute toxicity based on the aforementioned variables. Late toxicity-free survival estimates were calculated using the Kaplan-Meier product-limit method (19) and compared using the log-rank test (20). Variables with a p-value less than 0.05 or showing a trend (p < 0.1) in the univariate analysis were included in a multivariate Cox regression model (21).
Ethical considerations
The study received approval from the local institutional review board, and participation in the analysis was limited to patients who provided written informed consent.
Results
Patients, tumors, and treatment characteristics
A total of 381 patients were included in this analysis, with a median age of 65 years (range: 43-79 years). Table 1 presents the patients, tumor, and treatment characteristics. The median delivered EQD2 to the prostate bed, calculated using α/β ratios of 1.5 Gy, 3 Gy, and 10 Gy, was 71.4 (range: 66.2-78.0), 68.7 (range: 67.0-78.0), and 68.2 (range: 65.1-78.0), respectively. Among the patients, 127 (33.3%) were treated with standard fractionation, while 254 (66.7%) received a hypofractionated regimen. EQD2α/β=3 was significantly higher in patients treated with hypofractionated regimens compared to standard fractionation protocols (mean: 71.3 Gy versus 69.7 Gy; p<0.001). RT was delivered using either 3D-conformal RT (94 patients, 24.7%) or modulated techniques such as intensity modulated arc therapy (IMRT) or volumetric modulated arc therapy (VMAT) (287 patients, 75.3%). The dose to the prostatic bed ranged between 65 and 78 Gy (median: 66 Gy). Moreover, of 254 patients treated with hypofractionation, the dose per fraction was 2.2 Gy in 100 patients, 2.5 Gy in 142 patients, and 2.6 Gy in 12 patients. Furthermore, of 127 patients treated with standard fractionation, the dose per fraction was 1.8 Gy in 42 patients and 2 Gy in 85 patients. Additionally, out of 254 patients treated with hypofractionation, 239 (94.1%) were treated with IMRT/VMAT and 15 (5.9%) with 3D-CRT. Finally, out of 297 patients receiving nodal irradiation, 250 (84.2%) were treated with IMRT/VMAT, while 47 (15.8%) were treated with 3D-CRT. Daily on-line set-up corrections were performed using an electronic portal imaging device (351 patients, 92.1%) or an on-board cone-beam CT (30 patients, 7.9%), as previously described (22).
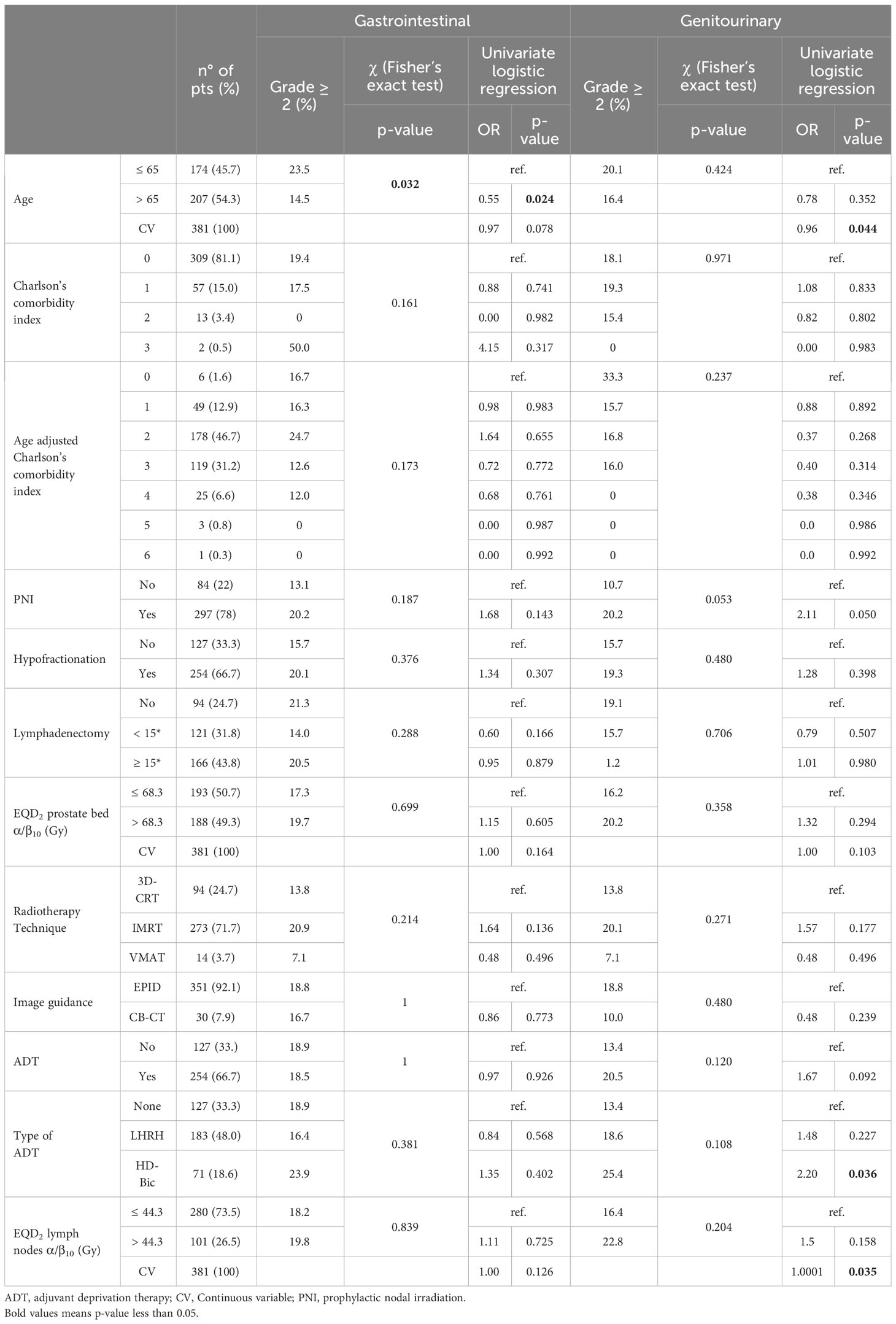
Table 1 Patients and treatment characteristics and results of univariate analysis on acute toxicity.
Acute and late toxicity
Table 1 provides the results in terms of acute toxicity. None of the patients experienced acute toxicity greater than Grade 3, and the rates of Grade 3 gastrointestinal (GI) and genitourinary (GU) toxicity were 0.5% and 1.3%, respectively. The actuarial 5-year rates of Grade ≥ 2 GI and GU late toxicity-free survival were 90.4% and 83.5%, respectively. The actuarial 5-year rates of Grade ≥ 3 GI and GU late toxicity-free survival were 98.1% and 94.5%, respectively.
Univariate analysis
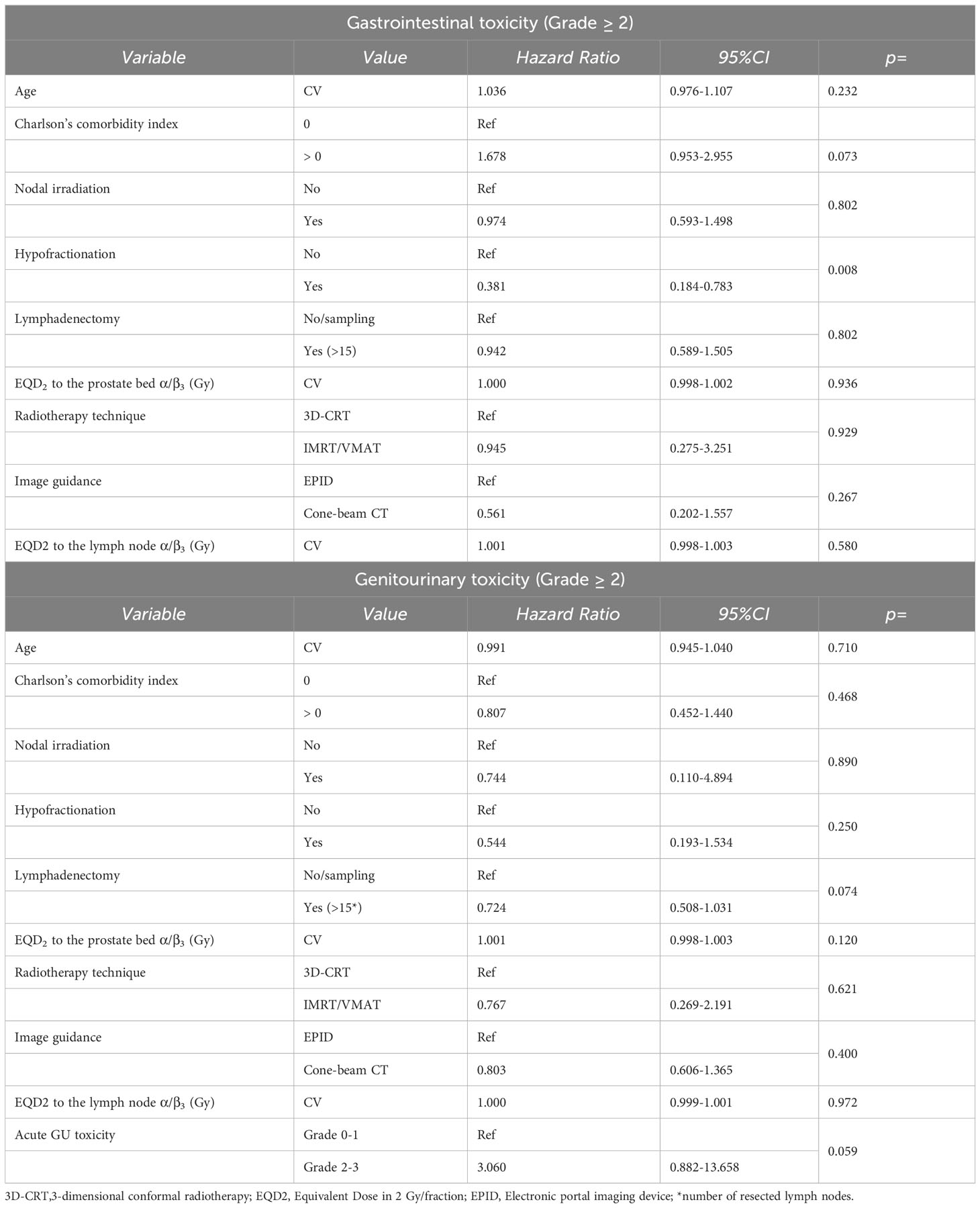
Univariate analysis revealed that acute Grade ≥ 3 GI and GU toxicity rates were not significantly correlated with any of the analyzed parameters. However, the delivery of PNI showed a trend for correlation with higher rates of Grade ≥ 2 acute GU toxicity (Table 1).
The actuarial 5-year late Grade ≥ 2 GI toxicity was significantly lower in patients treated with hypofractionation (dose per fraction > 2 Gy compared to ≤ 2 Gy; 93.6% vs 84.0%; p: 0.006), IMRT or VMAT techniques (compared to 3D-conformal therapy; 93.2-100.0% vs 82.6%; p: 0.027), and PNI (compared to irradiation of the prostate bed only; 92.9% vs 80.2%; p: 0.009). Moreover, actuarial 5-year late Grade ≥ 2 GU toxicity did not show any significant correlation with the analyzed parameters. Furthermore, actuarial 5-year late Grade ≥ 3 GI toxicity was significantly lower in patients treated with hypofractionation (dose per fraction > 2 Gy compared to ≤ 2 Gy; 99.2% vs 96.1%; p-value: 0.033) and IMRT or VMAT techniques (compared to 3D-conformal therapy; 100.0% vs 93.5%; p-value: 0.022). Late Grade ≥ 3 GU toxicity did not exhibit any significant correlation with the analyzed parameters (Table 2).

Table 2 Actuarial 5-year gastrointestinal and genitourinary late toxicity-free survival rates (Grade ≥ 2 and Grade ≥ 3; Kaplan-Meier) and results of univariate analysis (log-rank).
Multivariate analysis
The multivariable analysis of acute toxicity, conducted using binomial logistic regression, revealed a statistically significant association between older age and a reduced risk of Grade ≥ 2 GI acute toxicity (age analyzed as a dichotomous variable: OR: 0.569; 95% confidence interval [95%CI]: 0.329-0.973; p: 0.040). Apart from age, no other variable fitted in the multivariable logistic model for GI Grade ≥ 2 toxicity. Moreover, older age was significantly associated to a lower risk of Grade ≥ 2 GU acute toxicity (age analyzed as a continuous variable: OR: 0.956; 95%CI: 0.918-0.996; p: 0.031). Regarding dichotomous variable GU Grade ≥ 2 acute toxicity, three variables were included in the multivariable model, including age as a continuous variable, ADT, and EQD2 α/β=10 to the prostate bed. While ADT and EQD2 enhanced the predictive model, they were not statistically significant (ADT: OR: 1.730, 95%CI: 0.966-3.234, p: 0.073; EQD2: OR: 1.0005, 95%CI: 0.9999-1.0010, p = 0.075). In contrast, the age variable remained statistically significant, with age showing an inverse association with toxicity (OR: 0.956, 95% CI: 0.918-0.996, p: 0.031). The multivariable analysis of late toxicity confirmed only a lower risk of Grade ≥ 2 GI toxicity in patients undergoing hypofractionation (OR: 0.38; 95%CI: 0.18-0.78; p: 0.008). (Table 3).
Discussion
Adjuvant RT has been associated with an increased risk of side effects compared to surgery alone (4) and early salvage RT (7–9). However, it is important to note that, in selected high-risk PCa patients, adjuvant RT offers a higher chance of cure compared to surgery alone. Our multicenter observational study confirms that severe acute toxicity is rare in this setting. The rates of acute Grade ≥ 3 GI and GU toxicity were only 0.5% and 1.3%, respectively, and the 5-year actuarial cumulative incidence of late Grade ≥ 3 GI and GU toxicity rates were 1.9% and 5.5%, respectively.
Furthermore, our analysis demonstrated lower rates of GI acute toxicity in older patients. This unexpected result may arise from the fact that elderly patients may be more likely to have pre-existing symptoms or discomfort due to age-related health issues or comorbidities. As a result, they might be less inclined to report or attribute certain side effects to RT, especially if these side effects are mild or non-serious. The phenomenon of underreporting or downplaying side effects in elderly patients is known as “response shift” or “response shift bias” (23).
Other studies have reported an increased risk of GI early adverse effects in patients with higher mean rectal dose (16) or larger irradiated bowel volumes (24), those receiving PNI (25, 26), individuals with previous abdominal surgery (24), and those under anticoagulant or antiplatelet therapy (16). Additionally, Fiorino et al. observed reduced toxicity rates in patients receiving IMRT (24), although this effect was not observed in our cohort or in the study by Flores-Balcazar et al. (27).
Furthermore, our study demonstrated a reduced risk of GU acute toxicity in older patients, while Martinez-Arribas et al. reported higher GU acute toxicity rates in patients with urinary symptoms before RT. Additionally, similar to our findings, Flores-Balcazar et al. (27) and Deville et al. (26) did not observe a significant impact of IMRT/VMAT and PNI, respectively.
Moreover, our analysis revealed a reduced risk of GI late toxicity in patients treated with hypofractionated RT. Another study observed a higher risk of late GI adverse effects in subjects with higher body mass index values and those treated with higher RT doses (17). Furthermore, Flores-Balcazar et al. did not find a significant impact of IMRT/VMAT, in line with our findings, while Goenka et al. reported significantly reduced toxicity in patients treated with IMRT (28). Similarly, Deville et al. did not find different toxicity rates in subjects treated with PNI (26). **.
In our analysis, no parameter was significantly correlated with late GU toxicity. However, other studies have reported a significant correlation between higher toxicity rates and older age and receiving > 70 Gy to larger bladder volumes (17), hypofractionated RT (15), and Grade > 2 acute GU toxicity (13, 15). Interestingly, IMRT did not show an impact on late GU toxicity in two studies (27, 28), consistent with our analysis. Waldstein et al. reported increased toxicity rates in patients treated with PNI (25), while Deville et al. did not observe this correlation (26), similar to our series.
In conclusion, the results of available evidence conflict regarding: i) the impact of modulated RT techniques on acute GU toxicity and late GI side effects, and ii) the impact of PNI on late GU toxicity. Moreover, there is limited evidence available regarding parameters predicting acute GU side effects.
The use of hypofractionation in the adjuvant RT setting of PCa remains a controversial topic. Moderately hypofractionated regimens are considered preferable in patients undergoing exclusive RT (NCCN 2022) but not in the adjuvant setting. According to the NCCN guidelines, the recommended standard fractionation dose for adjuvant/salvage RT is 64-72 Gy (NCCN 2022). However, the data available on this topic are very heterogeneous. For instance, a systematic review on hypofractionated postoperative RT reported rates of Grade ≥ 2 late GU toxicity ranging between 0% and 66% (29).
The results of our analysis did not indicate a worse toxicity profile in patients undergoing hypofractionated RT. Furthermore, the multivariable analysis revealed a reduced rate of late GI toxicity after RT delivered with > 2 Gy per fraction. In contrast, Cozzarini et al. reported a significant increase in the rate of Grade ≥ 3 GU toxicity in patients receiving hypofractionated regimens compared to conventional fractionation (5-year risk: 18.1% versus 6.9%). This difference can be explained by comparing the equivalent doses delivered in our study and Cozzarini’s et al. study. Assuming an α/β ratio of 3 Gy for late effects, patients undergoing hypofractionation in our study received a median dose of 68.7 Gy, while in Cozzarini’s et al. study, the range was 68.4-80.8 Gy. Moreover, in Cozzarini’s et al. study, the EQD2 was > 70 Gy in 79.8% of patients and > 79 Gy in 32.4% of subjects. Additionally, the EQD2 for PNI was 43.2 Gy in our series and 50.2 Gy in Cozzarini’s et al. series. Even when using an α/β ratio of 5, as done by Cozzarini et al., our median EQD2 (67.0 Gy) was lower compared to their analysis (median: 70.4 Gy, IQR: 70.4-79.2 Gy).
Taken together, the results from the two studies suggest a possible association between dose and late urological toxicity in this setting, highlighting the need for further investigation. It is also worth noting that the safety of hypofractionation observed in our data is consistent with recent analyses (30–32). Probably, the lower incidence of late toxicity recorded in patients treated with hypofractionation in our study, despite a significantly higher EQD2α/β=3 value, may derive from the delivery of RT in more recent times, and therefore with more precise techniques.
The paradoxical result of our analysis, of reduced late gastrointestinal toxicity in patients undergoing PNI, remains to be explained. The only interpretation we can propose is that patients with better general conditions and fewer comorbidities (particularly at the intestinal level) were more frequently referred to PNI.
Our study has certain limitations. The scales used to score acute and late toxicity are outdated, and an assessment of the treatment impact on quality of life is lacking. Furthermore, despite efforts to include as many parameters as possible in the analysis, some were missing from our database. Among these, several factors have shown a significant impact on toxicity rates in previous studies, such as baseline symptoms (16) drug therapy during RT (16), planning dose/volume indices (14, 17), body mass index (17), and tobacco history (17).
On the other hand, the strengths of this study lie in the large number of cases analyzed and the comprehensive inclusion of numerous parameters related to both patients and treatments, as well as RT techniques in the analysis.
In conclusion, the results of our analysis demonstrate that although adjuvant RT significantly increases the overall rate of adverse events in PCa patients, the risk of severe toxicity is low. Additionally, acute toxicity rates were higher in younger patients, while a protective effect of hypofractionation was observed in terms of late GI toxicity.
To minimize the negative impact of adjuvant RT, further studies are warranted. These analyses should aim to: i) develop predictive models of toxicity combined with the risk of recurrence based on a comprehensive range of clinical, genetic-molecular, and treatment-related parameters, to guide the careful selection of patients for immediate adjuvant RT; ii) analyze toxicity rates in patients undergoing tailored/intensified adjuvant RT. For example, studies have shown that biochemical relapse-free survival can be improved by modulating postoperative RT, such as adjusting the dose based on surgical margin status, delivering PNI in selected cases, and administering ADT based on the risk of treatment failure (33–36); iii) clarify the impact of hypofractionation on late GU toxicity, given the conflicting evidence in the literature (29).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of IRCCS Azienda Ospedaliero-Universitaria di Bologna (311/2019/Oss/AOUBo, ICAROS-1 study). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MB: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. GM: Conceptualization, Writing – review & editing. LC: Data curation, Investigation, Writing – review & editing. AC: Data curation, Investigation, Writing – review & editing. CM: Formal analysis, Writing – review & editing. LB: Writing – review & editing. MN: Writing – review & editing. AA: Data curation, Formal analysis, Writing – review & editing. IC: Data curation, Investigation, Writing – review & editing. EN: Writing – review & editing. SCi: Formal analysis, Writing – review & editing. FC: Writing – review & editing. LT: Writing – review & editing. LS: Writing – review & editing. SCa: Data curation, Writing – review & editing. RS: Writing – review & editing. EB: Writing – review & editing. AGM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. FD: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Cinzia Giacometti for her invaluable help in data management.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ https://uroweb.org/guidelines/prostate-cancer/chapter/treatment.
- ^ http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA (2006) 296(19):2329–35. doi: 10.1001/jama.296.19.2329
3. Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol (2009) 27(18):2924–30. doi: 10.1200/JCO.2008.18.9563
4. Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet (2012) 380(9858):2018–27. doi: 10.1016/S0140-6736(12)61253-7
5. Hackman G, Taari K, Tammela TL, Matikainen M, Kouri M, Joensuu T, et al. Randomised trial of adjuvant radiotherapy following radical prostatectomy versus radical prostatectomy alone in prostate cancer patients with positive margins or extracapsular extension. Eur Urol (2019) 76(5):586–95. doi: 10.1016/j.eururo.2019.07.001
6. Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol (2009) 181(3):956–62. doi: 10.1016/j.juro.2008.11.032
7. Parker CC, Clarke NW, Cook AD, Kynaston HG, Petersen PM, Catton C, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet (2020) 396(10260):1413–21. doi: 10.1016/S0140-6736(20)31553-1
8. Sargos P, Chabaud S, Latorzeff I, Magné N, Benyoucef A, Supiot S, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol (2020) 21(10):1341–52. doi: 10.1016/S1470-2045(20)30454-X
9. Kneebone A, Fraser-Browne C, Duchesne GM, Fisher R, Frydenberg M, Herschtal A, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol (2020) 21(10):1331–40. doi: 10.1016/S1470-2045(20)30456-3
10. Vale CL, Fisher D, Kneebone A, Parker C, Pearse M, Richaud P, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet (2020) 396(10260):1422–31. doi: 10.1016/S0140-6736(20)31952-8
11. Swanson GP, Goldman B, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: data from Southwest Oncology Group 8794. J Urol (2008) 180(6):2453–7; discussion 2458. doi: 10.1016/j.juro.2008.08.037
12. Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, et al. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol (2007) 25(27):4178–86. doi: 10.1200/JCO.2006.10.4067
13. Choo R, Pearse M, Danjoux C, Gardner S, Morton G, Szumacher E, et al. Analysis of gastrointestinal and genitourinary morbidity of postoperative radiotherapy for pathologic T3 disease or positive surgical margins after radical prostatectomy using national cancer institute expanded common toxicity criteria. Int J Radiat Oncol Biol Phys (2008) 72(4):989–95. doi: 10.1016/j.ijrobp.2008.02.044
14. Perna L, Alongi F, Fiorino C, Broggi S, Cattaneo Giovanni M, Cozzarini C, et al. Predictors of acute bowel toxicity in patients treated with IMRT whole pelvis irradiation after prostatectomy. Radiother Oncol (2010) 97(1):71–5. doi: 10.1016/j.radonc.2010.02.025
15. Cozzarini C, Fiorino C, Deantoni C, Briganti A, Fodor A, La Macchia M, et al. Higher-than-expected severe (Grade 3-4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: a single-institution analysis of 1176 patients. Eur Urol (2014) 66(6):1024–30. doi: 10.1016/j.eururo.2014.06.012
16. Martínez-Arribas CM, González-San Segundo C, Cuesta-Álvaro P, Calvo-Manuel FA. Predictors of urinary and rectal toxicity after external conformed radiation therapy in prostate cancer: Correlation between clinical, tumour and dosimetric parameters and radical and postoperative radiation therapy. Actas Urol Esp. (2017) 41(10):615–23. doi: 10.1016/j.acuro.2017.03.010
17. Akthar AS, Liao C, Eggener SE, Liauw SL. Patient-reported outcomes and late toxicity after postprostatectomy intensity-modulated radiation therapy. Eur Urol (2019) 76(5):686–92. doi: 10.1016/j.eururo.2019.05.011
18. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C
19. Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. In: Kotz S, Johnson NL, editors. Breakthroughs in Statistics: Methodology and Distribution. New York, NY: Springer (1992). p. 319–37. doi: 10.1007/978-1-4612-4380-9_25
20. Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A (General) (1972) 135(2):185–207. doi: 10.2307/2344317
21. Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodological) (1972) 34(2):187–220. doi: 10.1111/j.2517-6161.1972.tb00899.x
22. Deodato F, Cilla S, Massaccesi M, Macchia G, Ippolito E, Caravatta L, et al. Daily on-line set-up correction in 3D-conformal radiotherapy: is it feasible? Tumori (2012) 98(4):441–4. doi: 10.1177/030089161209800407
23. Ilie G, Bradfield J, Moodie L, Lawen T, Ilie A, Lawen Z, et al. The role of response-shift in studies assessing quality of life outcomes among cancer patients: A systematic review. Front Oncol (2019) 9:783. doi: 10.3389/fonc.2019.00783
24. Fiorino C, Alongi F, Perna L, Broggi S, Cattaneo GM, Cozzarini C, et al. Dose–volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat OncologyBiologyPhysics (2009) 75(1):29–35. doi: 10.1016/j.ijrobp.2008.10.086
25. Waldstein C, Dörr W, Pötter R, Widder J, Goldner G. Postoperative radiotherapy for prostate cancer: Morbidity of local-only or local-plus-pelvic radiotherapy. Strahlenther Onkol (2018) 194(1):23–30. doi: 10.1007/s00066-017-1215-9
26. Deville C, Vapiwala N, Hwang WT, Lin H, Ad VB, Tochner Z, et al. Comparative toxicity and dosimetric profile of whole-pelvis versus prostate bed-only intensity-modulated radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys (2012) 82(4):1389–96. doi: 10.1016/j.ijrobp.2011.04.041
27. Flores-Balcázar CH, Urías-Arce DM, Bourlon MT, Gabilondo-Navarro F, Pérez-Álvarez SI, Ramos-Prudencio R, et al. Transitioning from conformal radiotherapy to intensity-modulated radiotherapy after radical prostatectomy: Clinical benefit, oncologic outcomes and incidence of gastrointestinal and urinary toxicities. Rep Pract Oncol Radiother (2020) 25(4):568–73. doi: 10.1016/j.rpor.2020.04.018
28. Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol (2011) 60(6):1142–8. doi: 10.1016/j.eururo.2011.08.006
29. Siepe G, Buwenge M, Nguyen NP, Macchia G, Deodato F, Cilla S, et al. Postoperative hypofractionated radiation therapy in prostate carcinoma: A systematic review. Anticancer Res (2018) 38(3):1221–30. doi: 10.21873/anticanres.12343
30. Tramacere F, Arcangeli S, Pignatelli A, Bracci S, Vinella M, Portaluri M. Postoperative hypofractionated radiotherapy for prostate cancer. Anticancer Res (2018) 38(5):2951–6. doi: 10.21873/anticanres.12544
31. Mahase S, Nagar H. Hypofractionated postoperative radiotherapy for prostate cancer: is the field ready yet? Eur Urol Open Sci (2020) 22:9–16. doi: 10.1016/j.euros.2020.10.001
32. Moll M, D’Andrea D, Zaharie A, Grubmüller B, Paschen C, Zehetmayer S, et al. Comparative effectiveness of moderate hypofractionation with volumetric modulated arc therapy versus conventional 3D-radiotherapy after radical prostatectomy. Strahlenther Onkol (2022) 198(8):719–26. doi: 10.1007/s00066-022-01909-2
33. Mantini G, Siepe G, Alitto AR, Buwenge M, Nguyen NP, Farioli A, et al. Tailored postoperative treatment of prostate cancer: final results of a phase I/II trial. Prostate Cancer Prostatic Dis (2018) 21(4):564–72. doi: 10.1038/s41391-018-0064-7
34. Cozzarini C, Montorsi F, Fiorino C, Alongi F, Bolognesi A, Da Pozzo LF, et al. Need for high radiation dose (>or=70 gy) in early postoperative irradiation after radical prostatectomy: a single-institution analysis of 334 high-risk, node-negative patients. Int J Radiat Oncol Biol Phys (2009) 75(4):966–74. doi: 10.1016/j.ijrobp.2008.12.059
35. Ost P, Cozzarini C, De Meerleer G, Fiorino C, De Potter B, Briganti A, et al. High-dose adjuvant radiotherapy after radical prostatectomy with or without androgen deprivation therapy. Int J Radiat Oncol Biol Phys (2012) 83(3):960–5. doi: 10.1016/j.ijrobp.2011.09.007
Keywords: prostate neoplasms, observational study, toxicity, predictive factors, radiotherapy, adjuvant therapy
Citation: Buwenge M, Macchia G, Cavallini L, Cortesi A, Malizia C, Bianchi L, Ntreta M, Arcelli A, Capocaccia I, Natoli E, Cilla S, Cellini F, Tagliaferri L, Strigari L, Cammelli S, Schiavina R, Brunocilla E, Morganti AG and Deodato F (2023) Unraveling the safety of adjuvant radiotherapy in prostate cancer: impact of older age and hypofractionated regimens on acute and late toxicity - a multicenter comprehensive analysis. Front. Oncol. 13:1281432. doi: 10.3389/fonc.2023.1281432
Received: 22 August 2023; Accepted: 28 November 2023;
Published: 13 December 2023.
Edited by:
Nam Phong Nguyen, International Geriatric Radiotherapy Group, United StatesReviewed by:
David Yang, Dana–Farber Cancer Institute, United StatesRaphael Pfeffer, Assuta Medical Center, Israel
Copyright © 2023 Buwenge, Macchia, Cavallini, Cortesi, Malizia, Bianchi, Ntreta, Arcelli, Capocaccia, Natoli, Cilla, Cellini, Tagliaferri, Strigari, Cammelli, Schiavina, Brunocilla, Morganti and Deodato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Letizia Cavallini, bGV0aXppYWNhdmFsbGluaTkxQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Milly Buwenge
Milly Buwenge Gabriella Macchia
Gabriella Macchia Letizia Cavallini
Letizia Cavallini Annalisa Cortesi
Annalisa Cortesi Claudio Malizia5
Claudio Malizia5 Lorenzo Bianchi
Lorenzo Bianchi Alessandra Arcelli
Alessandra Arcelli Elena Natoli
Elena Natoli Savino Cilla
Savino Cilla Francesco Cellini
Francesco Cellini Luca Tagliaferri
Luca Tagliaferri Lidia Strigari
Lidia Strigari Silvia Cammelli
Silvia Cammelli Riccardo Schiavina
Riccardo Schiavina Eugenio Brunocilla
Eugenio Brunocilla Alessio Giuseppe Morganti
Alessio Giuseppe Morganti Francesco Deodato
Francesco Deodato