- 1Medical Education, University of Michigan Medical School, Ann Arbor, MI, United States
- 2Internal Medicine Program, Mercyhealth Graduate Medical Education Consortium, Rockford, IL, United States
- 3Department of Radiation Oncology, Emory University School of Medicine, Atlanta, GA, United States
- 4Department of Otolaryngology – Head and Neck Surgery, Emory University School of Medicine, Atlanta, GA, United States
- 5Department of Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, GA, United States
As the prognosis for squamous cell carcinoma of the head and neck remains unsatisfactory when compared to other malignancies, novel therapies targeting specific biomarkers are a critical emerging area of great promise. One particular class of drugs that has been developed to impede tumor angiogenesis is vascular endothelial growth factor-tyrosine kinase inhibitors. As current data is primarily limited to preclinical and phase I/II trials, this review summarizes the current and future prospects of these agents in squamous cell carcinoma of the head and neck. In particular, the combination of these agents with immunotherapy is an exciting area that may be a promising option for patients with recurrent or metastatic disease, evidenced in recent trials such as the combination immune checkpoint inhibitors with lenvatinib and cabozantinib. In addition, the use of such combination therapy preoperatively in locally advanced disease is another area of interest.
1 Introduction
Prognosis of squamous cell carcinoma of the head and neck (SCCHN) has remained unsatisfactory when compared to other malignancies despite advancements in many treatment modalities (1). Five-year overall survival (OS) estimates of SCCHN range from 30-70% depending on staging and clinicopathologic characteristics (2). Mainstays of treatment have historically been a combination or sole approach of chemotherapy and/or radiation, with surgical approaches primarily restricted to locoregional disease (3). More recently, novel systemic therapies and immunotherapeutics promise change to this treatment paradigm (4) with certain treatments, such as T-cell associated therapeutics, specifically targeting human papillomavirus (HPV) related disease (5).
In addition to HPV-related disease, extensive research is aimed at developing targeted therapies against other biomarkers specific to SCCHN (6); there is also rising interest in the combination of these targeted therapies with immunotherapy (7). Vascular endothelial growth factor (VEGF) is one such pathway that has been recognized as a potential target due to its integral role in angiogenesis and tumor growth, as well as its relationship with immunosuppression (8). One particular class of drugs that has been developed to impede this classical pathway of tumor angiogenesis is VEGF-tyrosine kinase inhibitors (TKIs), which have been implemented in the treatment of solid tumors for over 20 years (9).
This review aims to summarize the literature regarding VEGF-TKIs in solid tumors, specifically in the management of SCCHN, with a broad focus on current and future prospects. With appropriate surveillance and management of on target toxicities, these agents have been shown to be well tolerated (10) and have shown modest benefit in other malignancies, such as renal cell carcinoma (RCC) (11). However, investigation and application to SCCHN particularly is an emerging area primarily restricted to preclinical and phase I/II trials. By reviewing the evidence to date, avenues for future study of VEGF-TKIs in SCCHN can be uncovered.
2 VEGF in SCCHN
Angiogenesis is a critical component in the growth and proliferation of many solid tumors and is thought to be essential for rapid tumor growth and metastasis. As tumors outgrow their blood supply and enter a state of relative hypoxia, cancer cells release a cascade of pro-angiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and angiopoietins (12). These factors collectively stimulate endothelial cells lining nearby blood vessels to undergo proliferation, migration, and invasion (13). This marks the initiation of angiogenesis, where endothelial cells degrade the basement membrane surrounding blood vessels, enabling them to sprout towards the tumor in a directed manner (14). As the new blood vessels infiltrate the tumor mass, they deliver oxygen and nutrients, allowing the tumor to grow rapidly and potentially gain the ability to metastasize; in SCCHN specifically, increased expression of VEGF is correlated with nodal and distant spread (15, 16). Of the many factors contributing to angiogenesis, VEGF is the most extensively studied, and its action at its various target receptor tyrosine kinase vascular endothelial growth factor receptors (VEGFR) has become a subject of interest as a therapeutic target.
Angiogenesis is a highly regulated phenomenon and contributes to the complexity of the tumor microenvironment, particularly through its relationship with immunosuppression. VEGF is thought to contribute to immunosuppression through several mechanisms, including the upregulation of immune checkpoints such as programmed death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (17). VEGF expression also influences T-lymphocyte maturation, inhibiting differentiation into CD8+ and CD4+ T cells from hematopoietic stem cells (18). VEGF has also been shown to inhibit natural killer (NK) cell cytotoxicity and dendritic cell (DC) maturation (19, 20). Together, these mechanisms contribute to the relative state of immunosuppression induced by cancer cells through overexpression of immune regulatory cells versus immune effector cells in the tumor microenvironment. The parallels between the mechanisms of angiogenic agents and those of other critical immunosuppressive pathways in various cancers present an argument for the use of angiogenic drugs in conjunction with immuno-oncologic (IO) agents, such as immune checkpoint inhibitors (ICIs) (21).
The role and clinical significance of VEGF and VEGFR expression in SCCHN is thus a topic of ongoing study. Multiple subtypes of VEGF signaling proteins, such as VEGF-A and VEGF-C, have been identified and shown to play specific roles in angiogenesis (22, 23). In a study analyzing 73 tumor samples via immunohistochemistry, the 5-year OS rate of patients with VEGF-C positive and negative oral SCC were found to be significantly different, at 51.7% and 94.2% respectively; VEGF-A, however, was found to be not significantly correlated with survival (24). A separate study utilizing immunohistochemistry in 166 oral dysplasia and tumor samples found increasing expression of VEGF with increasing grades of tumor dysplasia (25). A more recent analysis of 157 tumor samples found VEGF expression to be an independent negative predictor for locoregional control, metastasis-free survival, and OS (26). A meta-analysis of 12 studies found that VEGF overexpression was correlated with an 88% increased risk of death within 2 years, but it was not significantly correlated with nodal and distant spread (27). In contrast, a more recent meta-analysis of several different biomarkers in oral tongue SCC found that increased VEGF-C expression was actually associated with improved survival and VEGF-A and VEGF-C expression together were not significantly prognostic; however, when the single VEGF-C study was excluded from the meta-analysis VEGF-A expression alone demonstrated strong prognostic significance in OS in patients with tongue SCC (28). Overall, the relationship between subtypes of VEGF and their various receptors on pathologic disease progression and patient survival appears to hold some prognostic significance, though to what extent remains unclear and requires further study.
Of note, increased VEGFR2 expression in HPV-related SCCHN has been associated with worse prognosis, and marked differences in expression have been noted in tumor cells and tumor-supporting blood vessels when compared to HPV-negative disease (29). These differences in VEGF expression appear to be partly mediated by HPV-E5/E6 oncoproteins in HPV-related cervical cancer (30), though the mechanism in HPV-related SCCHN is currently less clear. The increasing incidence of HPV-related SCCHN (31) has led to further study into targeted therapies tailored to markers unique to HPV-related disease (5).
There are currently 14 FDA-approved antiangiogenic therapeutics that fall into several different broad classes; ligand directed antibodies, including bevacizumab, ziv-aflibercept, and dalantercept; receptor directed antibodies, of which ramucirumab is the first in class; and tyrosine kinase inhibitors, which are small molecules that exert their effects by binding a vast array of possible angiogenic targets (32, 33). Of these, VEGFR multikinase inhibitors exhibit their effects on VEGFR, PDGFR, and FGFRs, among other members of the VEGFR family, and have demonstrated robust inhibition of angiogenesis in the tumor microenvironment leading to clinical success in many solid tumors (9, 34). However, the application of VEGFR TKIs in the treatment of SCCHN has yet to be fully established.
2.1 VEGF-TKI monotherapy in solid tumors
There are currently no FDA-approved antiangiogenesis therapies for SCCHN. Although there was rising interest in using such therapies in the field, bevacizumab in particular, the results of a phase III trial (E1305) of chemotherapy with or without bevacizumab for first line treatment of patients with R/M SCCHN were discouraging. Results demonstrated the addition of bevacizumab improved response rate (RR) and progression free survival (PFS); however, OS was not significantly different between the bevacizumab (12.6 months) group versus with chemotherapy alone (11 months). In addition, there was increased toxicity in the bevacizumab group with 6.7% of patients experiencing grade 3 to 5 adverse bleeding events (35). Subsequent studies have focused on better tolerated agents with a focus on molecular biomarkers as described below.
The use of VEGF-TKIs in other solid organ tumors has been promising and may suggest further study for the use of anti-angiogenic therapies in SCCHN, either as monotherapy or in combination with immune checkpoint inhibitors, two of which are currently approved for use in SCCHN. Given the possible synergistic effects of PD-1 checkpoint inhibitors and anti-angiogenic agents (36), the pairing of ICIs and TKIs is an appealing strategy and has already shown benefit in many tumor types.
Sunitinib is a multikinase inhibitor (MKI) that inhibits VEGFR-1, -2, -3, PDGFR, and protooncogenes c-Kit and RET. It was FDA-approved for metastatic RCC after an international multicenter phase 3 trial demonstrated significant improvements in RR, PFS, and OS (following censoring) vs. IFN-α (37). Further studies elucidated optimal treatment schedules to manage its high toxicity profile (38). It is also approved for treatment of gastrointestinal stromal tumor (GIST) (39).
Sorafenib is an MKI that inhibits VEGFR-1, -2, -3, PDGFR, c-Kit, RET, and protooncogene Raf. It is approved as monotherapy in RCC, hepatocellular carcinoma (HCC), and radioactive iodine (RAI)-refractory differentiated thyroid cancer (40–42). It was initially FDA-approved for use as monotherapy in RCC following the TARGET trial which demonstrated increased PFS and increased likelihood of response or stable disease, though a trend in improvement in OS was initially deemed not statistically significant (40).
Lenvatinib is a MKI of VEGFR, FGFR, PDGFR, RET, and KIT. It was similarly approved for use as monotherapy in patients with RAI-refractory differentiated thyroid cancer after a randomized placebo controlled trial demonstrated improvements in PFS and RR (43). Following a study demonstrating robust improvement in OS, PFS, and overall response rate (ORR) after combination treatment of lenvatinib and everolimus versus either drug alone in advanced or metastatic RCC, it was approved as combination therapy (44).
Axitinib is a second generation TKI specific for VEGFR. Following two stage III trials, axitinib demonstrated improved PFS and ORR versus sorafenib in patients with recurrent metastatic RCC; however, neither trial demonstrated improvements in OS (45, 46). Therefore, the drug is not currently FDA-approved in the first line setting.
Cabozantinib is a recently developed MKI that inhibits VEGFR, as well as AXL, MET, KIT, and RET which are associated with resistance to VEGF inhibitors in RCC (47). Cabozantinib was granted FDA approval after a phase III trial demonstrated improved OS, PFS, and ORR vs everolimus in patients with advanced or metastatic RCC who exhibited disease progression following treatment with other VEGF inhibitors (48). It has also been approved as second line therapy in patients with locally advanced or metastatic RAI-refractory differentiated thyroid cancer who have progressed on first line VEGFR-targeted therapy (49). Given cabozantinib has an extended plasma half-life that leads to drug accumulation during initial dosing, zanzalintinib (XL092) was developed. XL092 retains the same target profile as cabozantinib, as an MKI that inhibits VEGFR, MET, and the TAM kinases (TYRO3, AXL, MER), but has a considerably shorter half-life (50). Currently there is an ongoing multicenter Phase 1b study (NCT05176483) involving dose escalation and cohort expansion of XL092 in combination with immuno-oncology agents. This study is being conducted in patients with unresectable advanced or metastatic solid tumors (51).
2.2 Combination of VEGF-TKIs with immunotherapy in solid tumors
The use of VEGF-TKIs and ICIs as combination therapy presents an exciting prospect for the treatment of many different tumor types. Though no combination therapy is currently approved for SCCHN, the combination of ICI and TKI has recently demonstrated benefit in other malignancies. Two initial studies evaluating this combination therapy in RCC were published simultaneously: the Javelin renal 101 and KEYNOTE 426 trials (52, 53). The Javelin renal 101 trial assigned 886 patients with previously untreated advanced RCC to receive avelumab (PD-L1 ICI) plus axitinib (442 patients) or sunitinib alone (444 patients). In patients with PD-L1 positive tumors, PFS was higher in the avelumab plus axitinib group (13.8 months) versus sunitinib alone (7.2 months). This was also true for the overall population with PFS for the avelumab plus axitinib group (13.8 months) versus sunitinib alone (8.4 months). However, OS was not significantly different in the overall population, at 11.6 months and 10.7 months after combination and monotherapy, respectively. Both groups had similar rates of adverse events, with 71.2% and 71.5% of patients experiencing grade 3 or higher events (52).
The KEYNOTE 426 trial treated 432 patients with previously untreated advanced RCC with axitinib plus pembrolizumab (PD-I ICI) and compared them to 429 patients treated with sunitinib alone. After a median follow-up of 12.8 months, OS in the combination therapy group was significantly higher at 89.9% versus 78.3%. Combination therapy also provided improved PFS (15.1 months versus 11.1 months) and ORR (59.3% versus 35.7%). These results were exhibited regardless of PD-L1 receptor status in tumors. Interestingly, though both groups experienced similar rates of adverse events at 75.8% vs 70.6%, only 10.7% of patients undergoing combination therapy discontinued treatment due to these adverse reactions, versus 49.0% of patients taking sunitinib alone (53). These encouraging results led to many more studies of combination TKI ICI therapy.
The CHECKMATE 9ER trial treated 323 patients with previously untreated advanced RCC with nivolumab (PD-1 ICI) plus cabozantinib and compared them to 328 patients treated with sunitinib alone. The combination therapy afforded improved PFS at 12.5 months compared to 8.3 in the combination and monotherapy groups respectively. Probability of survival at 12 months was 85.7% compared to 75.6%, and ORR was 55.7% compared to 27.1%. Again, grade 3 adverse events occurred at similar rates of 75.3 and 70.6% in the respective groups (54).
Finally, running simultaneously to the previous study, the CLEAR trial compared 3 groups among 1069 total patients: lenvatinib plus pembrolizumab (355 patients), lenvatinib plus everolimus (357 patients), and sunitinib alone (357 patients). PFS was 20.8, 14.7, and 9.2 months in the respective groups. When analyzing OS at 24 months, 79.2% of patients in the lenvatinib plus pembrolizumab group, 66.1% of patients in the lenvatinib plus everolimus group, and 70.4% of patients in the sunitinib group survived; the difference between lenvatinib plus pembrolizumab and sunitinib was significantly different, while the difference between lenvatinib plus everolimus and sunitinib was not. Respective ORR were 71%, 53.5%, and 36.1%. Toxicity was slightly higher in this study, most commonly diarrhea, with 82.4%, 83.1%, and 71.8% of patients experiencing one or more grade 3 adverse events in each of the respective groups (55). Overall, these results in RCC were extremely encouraging and redefined the landscape for first-line treatment. As such, further research about the use of VEGF-TKIs and ICIs combinations in SCCHN is an exciting emerging field of study that warrants further attention.
3 VEGF-TKIs in SCCHN
Although the use of VEGF-TKIs has been more extensively investigated in other solid tumors, their use in SCCHN is also a growing area of interest. As the landscape of SCCHN rapidly transforms with the use of immunotherapeutic agents and other novel targeted therapies (1), the use of anti-angiogenic agents in conjunction with these emerging therapies or alone is under much scrutiny. A multitude of preclinical and preliminary phase I/II trials exist or are ongoing as detailed below. However, careful selection criteria are warranted as anti-angiogenic agents are known to increase risk of hemorrhages from tumor sites, intracranially, or in microvasculature, as well as risk of venous and arterial thrombosis (56).
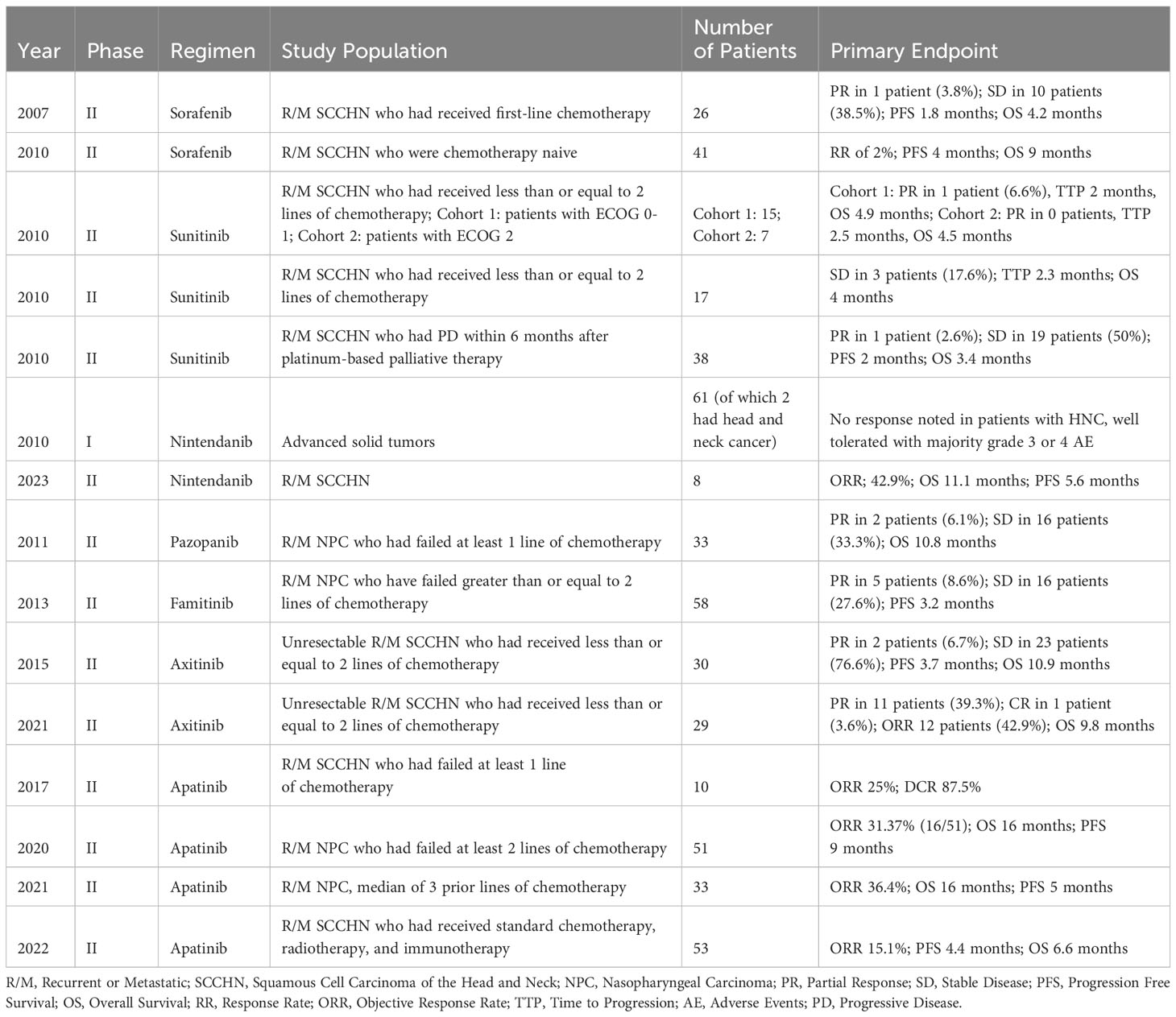
Sorafenib was one of the first VEGF-TKIs to be studied in the setting of SCCHN. A phase II trial was conducted in 2007 to assess its efficacy as monotherapy in 26 patients with recurrent or metastatic (R/M) SCCHN who had received first-line chemotherapy for R/M disease. Although the agent exhibited evidence of angiogenesis inhibition through matched immunohistochemistry, clinical responses were limited to a partial response in only 1 patient (3.8%), and the trial was not advanced to further stages. The most common adverse reactions were fatigue (79%), lymphopenia (42%), mucositis/stomatitis (42%), anemia (35%), hand-foot skin reaction (29%), and hypertension (28%). No grade 4 toxicities were observed, and the most common grade 3 toxicities included lymphopenia in 17% and fatigue in 7% of patients (57). A similar phase II trial subsequently investigated sorafenib in 41 patients with R/M SCCHN, although these patients were chemotherapy naive. The response was again minimal, with a single patient experiencing a partial response, yielding an estimated RR of 2%. There were two grade 4 thrombotic reactions during this trial, one asymptomatic pulmonary embolus, and one episode of cerebral ischemia. The most common grade 3 adverse events were hand-foot syndrome, anorexia, nausea, and stomatitis (58).
Three other trials were conducted in 2010 investigating sunitinib (59–61). This agent had previously shown promise in GIST and metastatic renal tumors (37, 39). Similar to sorafenib, however, all three studies showed minimal RR for patients with R/M SCCHN. Some toxicities noted in these trials included worsening of tumor skin ulceration and bleeding complications (61). Additional VEGF-TKIs that have previously been used in small samples of patients with SCCHN, but have not garnered much traction since, include cediranib and motesanib (62, 63).
Despite the limited efficacy of these TKIs, additional agents have continued to be developed and investigated, such as axitinib. Axitinib was first studied in patients with SCCHN in 2015 and administered to 30 patients with unresectable R/M disease. This initial study demonstrated a similarly poor RR compared to prior TKIs at 6.7%; however, results were more promising with an OS of 10.9 months and 76.7% of patients achieving stable disease (64). A follow-up expanded study was conducted by the same researchers on 29 additional patients using a different novel radiographic response criteria (65), which revealed an OS of 9.8 months, 11 patients with partial response, and 1 patient with complete response for an ORR of 42.9%; these results suggested clinical efficacy and the researchers concluded that further study both as monotherapy and in conjunction with immunotherapy is warranted (66). Of note, the combination of axitinib and pembrolizumab has demonstrated promise as a standard of care in advanced clear cell renal carcinoma in phase III trials such as KEYNOTE-426 (67).
Another more recently developed agent is nintendanib, an MKI that targets VEGF, PDGF, and FGF. Historically, it has been used to treat idiopathic pulmonary fibrosis with a more recent focus on applications for non-small cell lung cancer (68, 69). Preclinically, an in vitro study investigated the combination of various MKIs with cisplatin in SCCHN cell lines. Nintendanib was noted to exert the greatest synergistic effect with cisplatin chemotherapy (70). Phase I trials in patients with head and neck cancer showed adequate tolerance (71, 72). KCSG-TRIUMPH was a phase II umbrella trial involving patients with platinum-refractory SCCHN, and one arm specifically investigated nintendanib monotherapy in 8 patients with R/M SCCHN. Marked clinical benefit was demonstrated with ORR of 42.9%, OS of 11.1 months, and PFS of 5.6 months (73).
Apatinib is another TKI that has been explored in SCCHN and shown promising efficacy. Preclinical studies of monotherapy and combination with chemotherapy demonstrated efficacy in in vivo murine models with inhibition of tumor growth and improved overall survival (74). A phase II pilot study investigated apatinib monotherapy in patients with R/M SCCHN who had failed at least one prior chemotherapy regimen. Treatment was found to have moderate efficacy and tolerable toxicity, with an ORR of 25% (75). Apatinib monotherapy has been investigated in multiple subsequent studies on nasopharyngeal and non-nasopharyngeal SCCHN with encouraging results (76–78). Apatinib has also been shown to be efficacious in ameliorating radiation-induced brain injury amongst patients with head and neck cancer (79). Table 1 summarizes the aforementioned studies on VEGF-TKI monotherapy in SCCHN.
3.1 Combination of VEGF-TKIs with chemotherapy or EGFR inhibitors in SCCHN
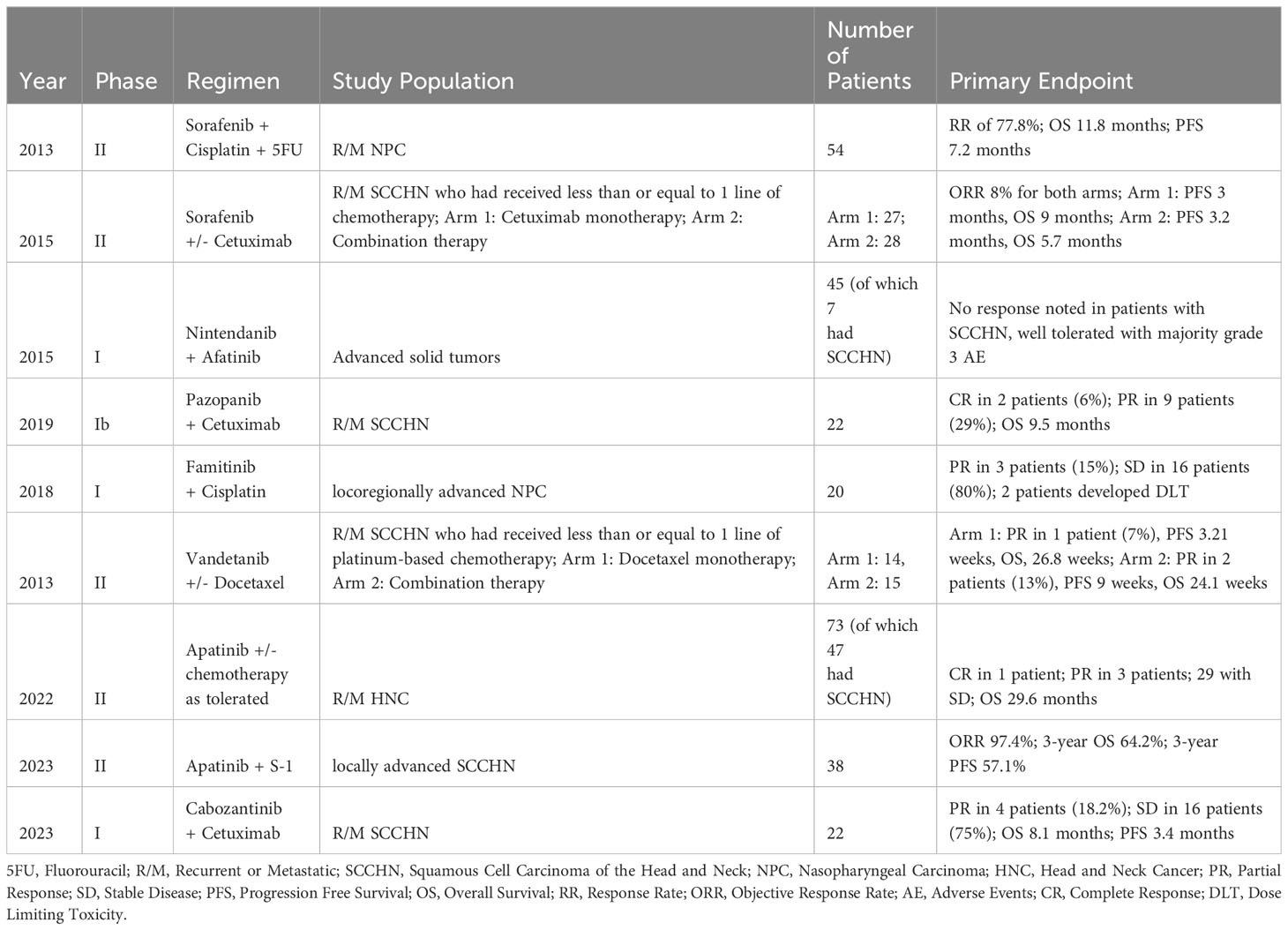
Results from previous studies on the combination of VEGF-TKIs and other therapeutic agents for patients with SCCHN have been mixed. Apatinib was studied in conjunction with various chemotherapy regimens in a phase II study enrolling patients with R/M head and neck cancer. 47 of the patients enrolled had SCCHN, and patients received axitinib in addition to S-1, capecitabine, nab-paclitaxel, or gemcitabine chemotherapy as tolerated. Preliminary results demonstrated a complete response in 1 patient, a partial response in 3 patients, and an OS of 29.6 months. Final results are forthcoming (80).
Apatinib has also been studied concurrently with S-1 oral chemotherapy as a novel induction therapy in patients with locally advanced SCCHN. 49 patients were enrolled in this single arm phase II trial. Although ultimately survival was minimally altered with a 3-year OS of 64.2%, the ORR was markedly high at 97.4%. Of note, p16 immunohistochemical testing revealed p16 positivity in 14 of 30 oropharyngeal cancer patients tested. Researchers stated one of the limitations of the study was that routine HPV testing had not been practiced in their institution until more recently, and future studies should focus on such testing given HPV has shown to be a prognostic marker (81).
Additional TKIs have been tested concurrently with chemotherapy. One randomized controlled trial investigated docetaxel with or without vandetanib. Although there was a minor trend indicating improved RR of 13% and PFS of 9 weeks in the combination therapy group versus RR of 7% and PFS of 3.2 weeks in the monotherapy group, these results were deemed not clinically meaningful (82). Another randomized controlled trial evaluating the addition of the epidermal growth factor (EGFR) inhibitor cetuximab to sorafenib was conducted as the researchers hypothesized that the two agents may have a synergistic anti-tumor effect; however, the study did not demonstrate significant clinical benefit, with similar RR of 8% and an OS of 9 months and 5.7 months without or with sorafenib respectively (83).
In contrast, a phase II single arm study evaluating the combination of sorafenib with cisplatin and 5-fluorouracil chemotherapy in 54 patients with metastatic nasopharyngeal carcinoma demonstrated clinically efficacious results. RR was 77.8%, with 1 complete response and 41 partial responses. OS was 11.8 months, PFS was 7.2 months, and the investigators ultimately concluded that further randomized controlled trials on the comparison of cisplatin and 5-fluorouracil with or without sorafenib are warranted (84).
Another TKI that has been studied as monotherapy or combined with other agents is pazopanib. Pazopanib is currently approved for the treatment of soft-tissue sarcomas (83) and has shown promise in metastatic RCC (85). Pazopanib was administered in a phase II trial as monotherapy in 33 patients with R/M nasopharyngeal carcinoma. Partial response was demonstrated in 2 patients, stable disease in 16 patients, with an OS of 10.8 months (86). Another phase Ib trial investigated pazopanib combined with cetuximab in 22 patients with R/M SCCHN. 2 patients had complete responses, 9 patients had partial responses, and OS was 9.5 months (87). Pazopanib was tolerated in both trials and showed promising clinical efficacy.
Famitinib is another agent currently under investigation for use in RCC, colorectal cancer, and breast cancer, amongst other malignancies (88–90). One phase II single arm trial of famitinib monotherapy in 58 patients with R/M nasopharyngeal carcinoma showed partial response in 5 patients, stable disease in 16 patients, and PFS of 3.2 months. Researchers concluded that famitinib exhibited substantial clinical benefit and mild-moderate adverse events (91). Phase I trials for famitinib in combination with concurrent chemotherapy also showed moderate clinical efficacy and adequate tolerance in patients with locoregionally advanced nasopharyngeal carcinoma (92). Table 2 summarizes the aforementioned studies on the combination of VEGF-TKIs with chemotherapy or EGFR inhibitors in SCCHN.
3.2 Combination of VEGF-TKIs with immunotherapy in SCCHN
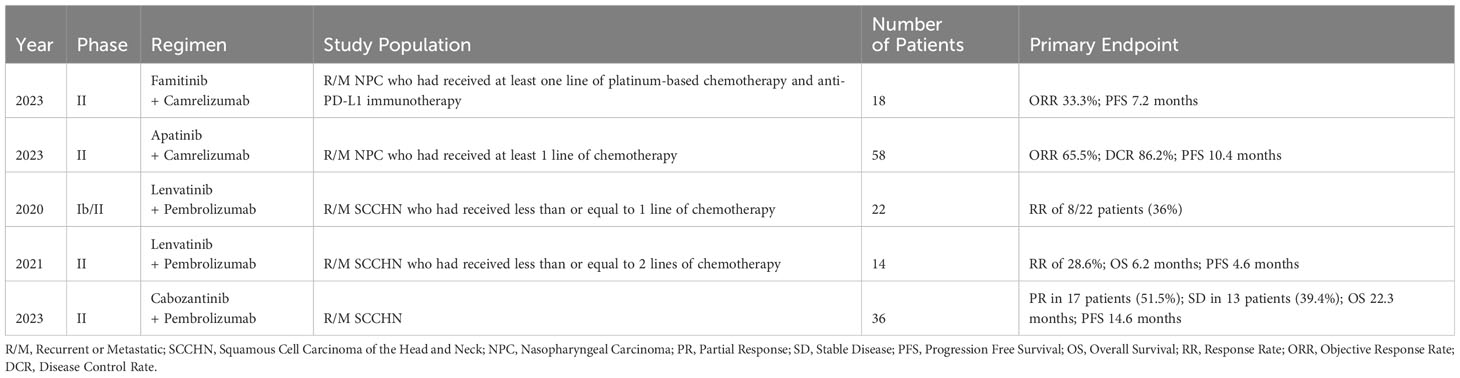
With VEGF-TKIs exhibiting modest benefit in SCCHN to date, either as monotherapy or in combination with chemotherapy or EGFR inhibitors, a more enticing area of interest is their use in combination with immunotherapy. The synergetic effects of combining VEGF-TKIs and ICIs has been noted in multiple clinical head and neck cancer studies (36, 93, 94). Besides the role of combination therapy as an initial approach, studies have suggested the addition of VEGF-TKIs may also play a role in clinical situations requiring immunotherapy retreatment. A recent trial of famitinib and camrelizumab (PD-1 ICI) was conducted in 18 patients with R/M nasopharyngeal carcinoma who had already progressed on one line of platinum-based chemotherapy and anti-PD-L1 immunotherapy. Results were encouraging, and ORR was 33.3% with a PFS of 7.2 months. These results supported the hypothesis that the addition of concurrent antiangiogenics during immunotherapy retreatment may increase efficacy (93). The same group had previously shown a similar synergistic effect when investigating the combination of apatinib and camrelizumab in patients with R/M nasopharyngeal carcinoma (94).
Concurrent use of lenvatinib and pembrolizumab has also been studied in two trials (95, 96). The phase Ib/II KEYNOTE-146 study investigated this combined regimen in 137 patients with various malignancies, including 22 patients with metastatic SCCHN. 8 patients achieved a response (36%) (96). The subsequent phase II study in 14 patients with heavily pretreated R/M SCCHN treated with lenvatinib and pembrolizumab demonstrated a similar RR of 28.6%, with OS of 6.2 months and PFS of 4.6 months (95). Additional investigations regarding lenvatinib use in SCCHN are ongoing. LEAP-009 (NCT04428151) is a randomized control trial investigating lenvatinib +/- pembrolizumab in comparison to standard-of-care chemotherapy in SCCHN (97). LEAP-010 (NCT04199104) was another randomized control trial comparing pembrolizumab monotherapy to its combination with lenvatinib as first line therapy for PD-L1 positive R/M SCCHN patients, though a recent press release indicated that the trial was closed based on lack of observed significant difference in the primary endpoint of overall survival. No further details were available at the time of this publication (98).
Regorafenib is currently approved in the United States for the treatment of HCC, GIST, and colorectal cancer, among others (99, 100). Addition of regorafenib has been shown to improve the efficacy of anti-PD-L1 immunotherapy in oral squamous cell carcinoma animal models (101). This agent has also shown some clinical efficacy in SCCHN patient derived xenografts (102). One ongoing trial (NCT04704154) is investigating the combination of nivolumab immunotherapy and regorafenib in patients with various solid tumors, including one cohort of those with R/M SCCHN who are immunotherapy naive (103).
Another TKI that has shown recent promise is cabozantinib. Overexpression of c-MET and AXL tyrosine kinase receptors has been demonstrated in cisplatin- and radiotherapy-resistant SCCHN, and cabozantinib targets both receptors. In preclinical trials, administration of cabozantinib was efficacious in mice, zebrafish, and specimens of human SCCHN (104). A follow-up phase I study investigated concurrent cabozantinib and cetuximab therapy in 22 patients with R/M SCCHN. 4 patients exhibited a partial response, of which 2 had prior cetuximab resistance. Stable disease was noted in 75% of the population, with an OS of 8.1 months and PFS of 3.4 months (105). Of note, a recent phase II single arm trial of cabozantinib and pembrolizumab was conducted in 36 R/M SCCHN patients. Partial response was demonstrated in 17 patients, stable disease in 13 patients, with an OS of 22.3 months and PFS of 14.6 months (106). These results further reinforced the enticing prospect of combining VEGF-TKIs and immunotherapy. A more recently promising agent is zanzalintinib (XL092). STELLAR-305 is a randomized, double-blind, phase 2/3 study that will evaluate the efficacy and safety of zanzalintinib plus pembrolizumab vs pembrolizumab and placebo in patients with previously untreated, PD-L1-positive, recurrent or metastatic SCCHN. Table 3 summarizes the aforementioned studies on the combination of VEGF-TKI with immunotherapy in SCCHN.
3.3 Data in the preoperative setting
Studies evaluating VEGF-TKIs in the preoperative setting for SCCHN are limited. In contrast, there has already been extensive research into the use of EGFR inhibitors, such as cetuximab, and single agent or double agent ICI preoperatively (107–110). Although the majority of studies detailed above are focused on R/M disease, one study to be highlighted that was previously described is the recent 2023 study on apatinib and S-1 in patients with locally advanced SCCHN (81). Typically, the current treatment guidelines for locally advanced disease recommend concurrent chemoradiation, although newer approaches such as induction chemotherapy prior to (chemo)radiation have complicated the picture (111–113). Regardless, lingering concerns regarding the known toxicities of concurrent chemoradiation after induction chemotherapy have engendered further research into newer investigational approaches using ICIs or EGFR inhibitors (114–117).
The use of induction chemotherapy to convert unresectable or borderline resectable disease to definitively resectable disease is also controversial, as results are mixed as to whether this strategy improves survival (118, 119). That being said, the use of apatinib and S-1 allowed for three patients with unresectable SCCHN to undergo curative-intent surgery post therapy (81). The use of VEGF-TKIs preoperatively and for downstaging prior to procedures has been investigated and shown promise in a wide variety of other malignancies (120–123). Cabozantinib in particular has shown promise in the preoperative setting in RCC (124). In addition, the combination of VEGF-TKIs and immunotherapy in the preoperative setting is also a growing area of study (125–127). Already, there exists precedent for using a combinatorial preoperative approach of VEGF-TKIs with ICIs in other malignancies, such as in RCC and HCC (125–127). The toxicity profile of such an approach in SCCHN may perhaps be desirable when compared to standard concurrent chemoradiation (128). Our knowledge is currently limited in this area, given that the use of VEGF-TKIs in the preoperative setting for SCCHN was only explicitly discussed in the one above mentioned clinical study.
4 Discussion
As outlined in this review, VEGF-TKIs are diverse in their targets, toxicities, and effectiveness. This makes it difficult to select which TKIs to study, particularly in the setting of SCCHN with early and non-definitive data in the field. The use of VEGF-TKIs in R/M RCC has demonstrated vastly varying effectiveness and toxicities even among agents with multiple overlapping targets; for example, axitinib and sorafenib were found to have similar effectiveness, although axitinib has a much more manageable toxicity profile (46). Combinations with ICIs further complicate the matter, with improved effectiveness at the cost of worsening toxicity (52). Additionally, agents such as sunitinib that demonstrate marked effectiveness in other malignancies such as RCC seem to have little to no effect in SCCHN but with worse toxicity (61). As such, much intentionality is required when choosing agents to study, whether selecting a new agent such as cabozantinib or an older agent with unremarkable results in other malignancies.
The toxicity profiles of VEGF-TKIs are diverse and require careful consideration. Intended selective inhibition of angiogenic tyrosine kinases, in an excessive manner, can lead to on target toxicities, such as skin reactions, hypertension, hypothyroidism and proteinuria (129). However, as VEGF-TKIs have been shown to have promiscuous activity (130), unintended inhibition of non-angiogenic tyrosine kinases can lead to off target toxicities. Other cardiotoxic and hepatotoxic side effects, particularly rare pancreatic enzyme elevations, fatal hepatotoxicity, hypoglycemia, and corrected QT prolongation, have been hypothesized to be partly or fully secondary to these off target effects (131). Additional cardiotoxic side effects, such as left ventricular systolic dysfunction, heart failure, and arterial thromboembolism, have been studied in more detail and are thought to be a result of both on target and off target inhibition (132, 133). The most common side effects of VEGF-TKIs include fatigue, diarrhea, skin toxicity, bleeding and vascular complications, and a variety of laboratory abnormalities (134, 135). These vary greatly among TKIs; for example, sorafenib and regorafinib were found to be the worst offenders for increasing risk for hemorrhagic events at odds ratios of 3.31 and 2.92 respectively (136). With regards to hypertension, a meta-analysis across multiple malignancies found axitinib and sunitinib to increase systolic and diastolic blood pressure the most during treatment, while cabozantinib and sorafenib had the smallest effect (10). With many anti-angiogenic agents to choose from, and even more immuno-oncologic agents to pair them with, studying these agents in a novel setting provides a challenging yet unique opportunity to tailor therapy for SCCHN to a very high degree to individual settings and patients.
It would be of relevance to decipher the characteristics of each of the VEGF-TKIs, namely their immunomodulatory effects and possible benefits or advantages of certain agents over others, especially in the setting of combinatorial effects with immunotherapeutic agents such as PD-1 inhibitors. Along those lines, it is of interest that lenvatinib, despite its clinical activity in combination with pembrolizumab, did not lead to an improvement in OS in a phase III randomized trial (98). Cabozantinib does have other immunomodulatory targets besides VEGF (TAM, MER, cMET) and could possibly result in a stronger combinatorial effect with PD-1 inhibitors in a disease such as SCCHN (47). Despite the encouraging results of cabozantinib in combination with pembrolizumab in recurrent metastatic SCCHN (106), this possible advantage to cabozantinib remains to be speculative at this stage and will require confirmation in a randomized clinical trial. Furthermore, pre-clinical studies using combination of these agents with PD-1 inhibitors in cell lines or syngeneic transplantable mouse oral cancer models (MOC 1 and 2) may help shed some light as to the advantages of one versus the other agent.
Currently, evaluation of patient biomarker expression is not routinely performed prior to treatment of SCCHN with VEGF-TKIs. Precedent for biomarker selection in this patient population exists for treatment with checkpoint inhibitors such as pembrolizumab, where patients whose tumors express PD-L1 have been shown to clinically benefit from treatment (137–139). However, these results are limited by small sample sizes, inconsistent assays, and subjective scoring systems (140). Further, no biomarkers beyond PD-L1 combined positive score (CPS), established in the first line setting based on KEYNOTE 048, have resulted in a practice-changing recommendation. However, two more recent analyses of these pivotal early studies continue to demonstrate significant associations between PD-L1 expression, tumor mutational burden (TMB), and response to ICI treatment; both studies also demonstrate a consistent effect regardless of tumor HPV status (141, 142). However, in our recent study, TMB did not appear to correlate with likelihood of response to treatment with pembrolizumab and cabozantinib (106). As such, with respect to anti-angiogenic treatment, it remains unclear if biomarker selection is worthwhile, considering all tumors rely on angiogenesis to some extent for growth and metastasis. The relationship between variation in treatment response following VEGF-TKIs and respective biomarker expression is still largely unknown and has not been typically evaluated prior to treatment in the various clinical studies outlined above. Given the known correlations between levels of VEGF expression and tumor dysplasia (25) and lowered rates of survival (24), testing patients’ tumors for VEGF expression prior to treatment may be a plausible method to estimate possible effectiveness of anti-angiogenic treatment. However, this would require a viable assay for measuring expression of VEGF and its various receptors, which could prove difficult (143, 144). In addition, most VEGF-TKIs have multiple and varied targets, for which the relationship between expression and treatment effectiveness is largely unexplored. Finally, given the known relationship between angiogenesis and immunosuppression in the tumor microenvironment (21, 145), the potential effects of anti-angiogenic agents may be underestimated without also accounting for tumor expression of related biomarkers such as PD-1/PD-L1 and the effects VEGF-TKIs may have on these pathways, or accounting for combination therapy. As such, biomarker selection may be redundant or unnecessary in the setting of multi-target anti-angiogenic therapy with or without combination ICI therapy, though more understanding is still required regarding the relationship between biomarkers and VEGF inhibitors.
In addition to current uncertainty regarding VEGF-TKI use in SCCHN, ambiguity regarding their use in HPV-related disease further complicates the picture. Just as angiogenesis plays a major role in influencing the tumor microenvironment, HPV-positive disease has also been shown to have an entirely distinct microenvironment when compared to HPV-negative SCCHN (146, 147). It is unclear how this may impact response to VEGF-TKI therapy, given that HPV-related disease engenders marked differences in antigen presentation based on viral oncoproteins and increased activation of immune infiltrates (148, 149). Despite this, there is no clear evidence that these combinatorial approaches are less effective in one versus the other disease, making them more attractive. The added lack of a treatment related biomarker for patient selection may also be an advantage as it adds to the ease and simplicity of using these combinatorial approaches.
As we look towards the future of care for SCCHN, studies on VEGF-TKIs are encouraging for broadening use case scenarios in a disease that still has limited therapeutic options. Recent studies on the combination of VEGF-TKIs and immunotherapy indicate that such an approach may be promising; in addition to the clinical benefit of both classes of agents, the lack of additive side effects with ICIs, and the broad applicability given no required biomarker or patient selection, all point to a high potential for a promising strategy in treating SCCHN and potentially other SCCs. As cancer care and research increasingly move towards targeted therapies and personalized care, VEGF-TKIs in combination with immune checkpoint inhibitors are an important class of therapeutics to focus on in SCCHN and other diseases.
Author contributions
PP: Conceptualization, Writing – original draft, Writing – review & editing. SP: Conceptualization, Writing – original draft, Writing – review & editing. JB: Writing – review & editing. JG: Writing – review & editing. WS: Writing – review & editing. NS: Writing – review & editing. CS: Writing – review & editing. YT: Writing – review & editing. NS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge Anthea Hammond, PhD for her editorial feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet (2021) 398(10318):2289–99. doi: 10.1016/S0140-6736(21)01550-6
2. Guo K, Xiao W, Chen X, Zhao Z, Lin Y, Chen G. Epidemiological trends of head and neck cancer: A population-based study. BioMed Res Int (2021) 2021:e1738932. doi: 10.1155/2021/1738932
3. Cramer JD, Burtness B, Le QT, Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol (2019) 16(11):669–83. doi: 10.1038/s41571-019-0227-z
4. Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: A 2023 update. Drugs (2023) 83(3):217–48. doi: 10.1007/s40265-023-01835-2
5. Saba NF, Pamulapati S, Patel B, Mody M, Strojan P, Takes R, et al. Novel immunotherapeutic approaches to treating HPV-related head and neck cancer. Cancers (2023) 15(7):1959. doi: 10.3390/cancers15071959
6. Li Q, Tie Y, Alu A, Ma X, Shi H. Targeted therapy for head and neck cancer: signaling pathways and clinical studies. Signal Transduct Target Ther (2023) 8(1):1–28. doi: 10.1038/s41392-022-01297-0
7. Fasano M, Corte CMD, Liello RD, Viscardi G, Sparano F, Iacovino ML, et al. Immunotherapy for head and neck cancer: Present and future. Crit Rev Oncol Hematol (2022) 174:103679. doi: 10.1016/j.critrevonc.2022.103679
8. Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular mechanisms and future implications of VEGF/VEGFR in cancer therapy. Clin Cancer Res Off J Am Assoc Cancer Res (2023) 29(1):30–9. doi: 10.1158/1078-0432.CCR-22-1366
9. Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J Hematol OncolJ Hematol Oncol (2020) 13(1):143. doi: 10.1186/s13045-020-00977-0
10. Gomez JA. Vascular endothelial growth factor-tyrosine kinase inhibitors: Novel mechanisms, predictors of hypertension and management strategies. Am Heart J Plus Cardiol Res Pract (2022) 17:100144. doi: 10.1016/j.ahjo.2022.100144
11. Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol (2020) 12:1758835920907504. doi: 10.1177/1758835920907504
12. Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol (2003) 9(6):1144–55. doi: 10.3748/wjg.v9.i6.1144
13. Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci (1997) 22(7):251–6. doi: 10.1016/S0968-0004(97)01074-8
14. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature (2011) 473(7347):298–307. doi: 10.1038/nature10144
15. Beasley NJP, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res (2002) 62(5):1315–20.
16. Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res (2003) 63(8):1920–6.
17. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med (2015) 212(2):139–48. doi: 10.1084/jem.20140559
18. Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer (2012) 130(4):857–64. doi: 10.1002/ijc.26094
19. Lee JY, Park S, Min WS, Kim HJ. Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett (2014) 354(2):281–9. doi: 10.1016/j.canlet.2014.08.027
20. Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, et al. Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2-RhoA-cofilin1 pathway. Cancer Sci (2019) 110(8):2357–67. doi: 10.1111/cas.14091
21. Saba NF, Vijayvargiya P, Vermorken JB, Rodrigo JP, Willems SM, Zidar N, et al. Targeting angiogenesis in squamous cell carcinoma of the head and neck: opportunities in the immunotherapy era. Cancers (2022) 14(5):1202. doi: 10.3390/cancers14051202
22. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J (1999) 13(1):9–22. doi: 10.1096/fasebj.13.1.9
23. Hoeben A, Landuyt B, Highley MS, Wildiers H, Oosterom ATV, Bruijn EAD. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev (2004) 56(4):549–80. doi: 10.1124/pr.56.4.3
24. Tanigaki Y, Nagashima Y, Kitamura Y, Matsuda H, Mikami Y, Tsukuda M. The expression of vascular endothelial growth factor-A and -C, and receptors 1 and 3: correlation with lymph node metastasis and prognosis in tongue squamous cell carcinoma. Int J Mol Med (2004) 14(3):389–95. doi: 10.3892/ijmm.14.3.389
25. Cheng SJ, Lee JJ, Kok SH, Chou CH, Chang HH, Yang H, et al. Expression of vascular endothelial growth factor is significantly associated with progression and prognosis of oral squamous cell carcinomas in Taiwan. J Formos Med Assoc Taiwan Yi Zhi (2011) 110(1):50–7. doi: 10.1016/S0929-6646(11)60008-9
26. Seibold ND, Schild SE, Bruchhage KL, Gebhard MP, Noack F, Rades D. Prognostic impact of VEGF and FLT-1 receptor expression in patients with locally advanced squamous cell carcinoma of the head and neck. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al (2013) 189(8):639–46. doi: 10.1007/s00066-013-0341-2
27. Kyzas PA, Cunha IW, Ioannidis JPA. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res Off J Am Assoc Cancer Res (2005) 11(4):1434–40. doi: 10.1158/1078-0432.CCR-04-1870
28. Almangush A, Heikkinen I, Mäkitie AA, Coletta RD, Läärä E, Leivo I, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer (2017) 117(6):856–66. doi: 10.1038/bjc.2017.244
29. Uzun S, Korkmaz Y, Wuerdemann N, Arolt C, Puladi B, Siefer OG, et al. Comprehensive analysis of VEGFR2 expression in HPV-positive and -negative OPSCC reveals differing VEGFR2 expression patterns. Cancers (2021) 13(20):5221. doi: 10.3390/cancers13205221
30. Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell Mol Life Sci CMLS (2006) 63(7–8):930–8. doi: 10.1007/s00018-005-5561-x
31. Chaturvedi AK, Graubard BI, Broutian T, Xiao W, Pickard RKL, Kahle L, et al. Prevalence of oral HPV infection in unvaccinated men and women in the United States, 2009-2016. JAMA (2019) 322(10):977–9. doi: 10.1001/jama.2019.10508
32. Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med (2012) 2(10):a006577. doi: 10.1101/cshperspect.a006577
33. Jászai J, Schmidt MHH. Trends and challenges in tumor anti-angiogenic therapies. Cells (2019) 8(9):1102. doi: 10.3390/cells8091102
34. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol OncolJ Hematol Oncol (2019) 12(1):27. doi: 10.1186/s13045-019-0718-5
35. Argiris A, Li S, Savvides P, Ohr JP, Gilbert J, Levine MA, et al. Phase III randomized trial of chemotherapy with or without bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37(34):3266–74. doi: 10.1200/JCO.19.00555
36. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med (2020) 52(9):1475–85. doi: 10.1038/s12276-020-00500-y
37. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med (2007) 356(2):115–24. doi: 10.1056/NEJMoa065044
38. Deng H, Li M, Wu Q, Wang L, Hong Z, Yi F, et al. A 2/1 sunitinib dosing schedule provides superior antitumor effectiveness and less toxicity than a 4/2 schedule for metastatic renal cell carcinoma: A systematic review and meta-analysis. Front Oncol (2020) 10:313. doi: 10.3389/fonc.2020.00313
39. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet (2006) 368(9544):1329–38. doi: 10.1016/S0140-6736(06)69446-4
40. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med (2007) 356(2):125–34. doi: 10.1056/NEJMoa060655
41. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in locally advanced or metastatic, radioactive iodine-refractory, differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet (2014) 384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9
42. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857
43. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med (2015) 372(7):621–30. doi: 10.1056/NEJMoa1406470
44. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol (2015) 16(15):1473–82. doi: 10.1016/S1470-2045(15)00290-9
45. Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol (2013) 14(13):1287–94. doi: 10.1016/S1470-2045(13)70465-0
46. Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol (2013) 14(6):552–62. doi: 10.1016/S1470-2045(13)70093-7
47. Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther (2011) 10(12):2298–308. doi: 10.1158/1535-7163.MCT-11-0264
48. Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373(19):1814–23. doi: 10.1056/NEJMoa1510016
49. Brose MS, Robinson BG, Sherman SI, Jarzab B, Lin CC, Vaisman F, et al. Cabozantinib for previously treated radioiodine-refractory differentiated thyroid cancer: Updated results from the phase 3 COSMIC-311 trial. Cancer (2022) 128(24):4203–12. doi: 10.1002/cncr.34493
50. Hsu J, Chong C, Goon L, Balayan J, Wu S, Johnson E, et al. XL092, a multi-targeted inhibitor of MET, VEGFR2, AXL and MER with an optimized pharmacokinetic profile. Eur J Cancer (2020) 138:S16. doi: 10.1016/S0959-8049(20)31107-2
51. Choueiri TK, McGregor BA, Shah NJ, Bajaj A, Chahoud J, O’Neil B, et al. A phase 1b study (STELLAR-002) of XL092 administered in combination with nivolumab (NIVO) with or without ipilimumab (IPI) or bempegaldesleukin (BEMPEG) in patients (pts) with advanced solid tumors. J Clin Oncol (2022) 40(16_suppl):TPS4600–TPS4600. doi: 10.1200/JCO.2022.40.16_suppl.TPS4600
52. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047
53. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714
54. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
55. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med (2021) 384(14):1289–300. doi: 10.1056/NEJMoa2035716
56. Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies–focus on hypertension and arterial thrombotic events. J Am Soc Hypertens (2018) 12(6):409–25. doi: 10.1016/j.jash.2018.03.008
57. Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol (2007) 25(24):3766–73. doi: 10.1200/JCO.2006.10.2871
58. Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: southwest oncology group study S0420. J Clin Oncol (2010) 28(20):3330–5. doi: 10.1200/JCO.2009.25.6834
59. Choong NW, Kozloff M, Taber D, Hu HS, Wade J, Ivy P, et al. Phase II study of sunitinib malate in head and neck squamous cell carcinoma. Invest New Drugs (2010) 28(5):677–83. doi: 10.1007/s10637-009-9296-7
60. Fountzilas G, Fragkoulidi A, Kalogera-Fountzila A, Nikolaidou M, Bobos M, Calderaro J, et al. A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol (2010) 65(4):649–60. doi: 10.1007/s00280-009-1070-1
61. Machiels JPH, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol (2010) 28(1):21–8. doi: 10.1200/JCO.2009.23.8584
62. Hong DS, Kurzrock R, Mulay M, Rasmussen E, Wu BM, Bass MB, et al. A phase 1b, open-label study of trebananib plus bevacizumab or motesanib in patients with solid tumours. Oncotarget (2014) 5(22):11154–67. doi: 10.18632/oncotarget.2568
63. Saura C, Baselga J, Herbst R, del Campo J, Marotti M, Tessier J, et al. Antitumor activity of cediranib in patients with metastatic or recurrent head and neck cancer (HNC) or recurrent non-small cell lung cancer (NSCLC): An open-label exploratory study. J Clin Oncol (2009) 27(15_suppl):6023–3. doi: 10.1200/jco.2009.27.15_suppl.6023
64. Swiecicki PL, Zhao L, Belile E, Sacco AG, Chepeha DB, Dobrosotskaya I, et al. A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Invest New Drugs (2015) 33(6):1248–56. doi: 10.1007/s10637-015-0293-8
65. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol Off J Am Soc Clin Oncol (2007) 25(13):1753–9. doi: 10.1200/JCO.2006.07.3049
66. Swiecicki PL, Bellile EL, Brummel CV, Brenner JC, Worden FP. Efficacy of axitinib in metastatic head and neck cancer with novel radiographic response criteria. Cancer (2021) 127(2):219–28. doi: 10.1002/cncr.33226
67. Plimack ER, Powles T, Stus V, Gafanov R, Nosov D, Waddell T, et al. Pembrolizumab plus axitinib versus sunitinib as first-line treatment of advanced renal cell carcinoma: 43-month follow-up of the phase 3 KEYNOTE-426 study. Eur Urol (2023) 84(5):449–54. doi: 10.1016/j.eururo.2023.06.006
68. Yan S, Xue S, Wang T, Gao R, Zeng H, Wang Q, et al. Efficacy and safety of nintedanib in patients with non-small cell lung cancer, and novel insights in radiation-induced lung toxicity. Front Oncol (2023) 13:1086214. doi: 10.3389/fonc.2023.1086214
69. Reck M, Kaiser R, Eschbach C, Stefanic M, Love J, Gatzemeier U, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol (2011) 22(6):1374–81. doi: 10.1093/annonc/mdq618
70. Brands RC, De Donno F, Knierim ML, Steinacker V, Hartmann S, Seher A, et al. Multi-kinase inhibitors and cisplatin for head and neck cancer treatment in vitro. Oncol Lett (2019) 18(3):2220–31. doi: 10.3892/ol.2019.10541
71. Bahleda R, Hollebecque A, Varga A, Gazzah A, Massard C, Deutsch E, et al. Phase I study of afatinib combined with nintedanib in patients with advanced solid tumours. Br J Cancer (2015) 113(10):1413–20. doi: 10.1038/bjc.2015.374
72. Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res (2010) 16(1):311–9. doi: 10.1158/1078-0432.CCR-09-0694
73. Kim KH, Lim SM, Ahn HK, Lee YG, Lee KW, Ahn MJ, et al. A phase II trial of nintedanib in patients with metastatic or recurrent head and neck squamous cell carcinoma (HNSCC): in-depth analysis of nintendanib arm from the KCSG HN 15-16 TRIUMPH trial. Cancer Res Treat (2023). doi: 10.4143/crt.2023.433
74. Peng QX, Han YW, Zhang YL, Hu J, Fan J, Fu SZ, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget (2017) 8(32):52813–22. doi: 10.18632/oncotarget.17264
75. Gui L, He X. A pilot study of apatinib in heavily pretreated metastatic adenocarcinoma of the head and neck. Ann Oncol (2017) 28:v386–7. doi: 10.1093/annonc/mdx374.037
76. Li L, Kong F, Zhang L, Li X, Fu X, Wang X, et al. Apatinib, a novel VEGFR-2 tyrosine kinase inhibitor, for relapsed and refractory nasopharyngeal carcinoma: data from an open-label, single-arm, exploratory study. Invest New Drugs (2020) 38(6):1847–53. doi: 10.1007/s10637-020-00925-2
77. Liu Z, Zheng Z, Dong L, Guo X, Jia X, Wang J, et al. Efficacy and safety of apatinib in patients with recurrent or metastatic head and neck squamous cell carcinoma: a retrospective multi-center study. Sci Rep (2022) 12:18327. doi: 10.1038/s41598-022-20272-x
78. Ruan X, Liang JH, Pan Y, Cai R, Zhang RJ, He Z, et al. Apatinib for the treatment of metastatic or locoregionally recurrent nasopharyngeal carcinoma after failure of chemotherapy: A multicenter, single-arm, prospective phase 2 study. Cancer (2021) 127(17):3163–71. doi: 10.1002/cncr.33626
79. He L, Pi Y, Li Y, Wu Y, Jiang J, Rong X, et al. Efficacy and safety of apatinib for radiation-induced brain injury among patients with head and neck cancer: an open-label, single-arm, phase 2 study. Int J Radiat Oncol Biol Phys (2022) 113(4):796–804. doi: 10.1016/j.ijrobp.2022.03.027
80. Wu Q, Liu J, Li S, Wang J, Zhong Y. Apatinib in recurrent or metastatic head and neck cancer patients. J Clin Oncol (2022) 40(16_suppl):e18010–0. doi: 10.1200/JCO.2022.40.16_suppl.e18010
81. Jiang W, Li R, Zhang L, Dou S, Ye L, Shao Z, et al. Efficacy and feasibility of Apatinib and S-1 as a novel oral induction therapy in locally advanced head and neck squamous cell carcinoma: an exploratory phase 2 open-label, single-arm trial. Front Oncol (2023) 13:1072538. doi: 10.3389/fonc.2023.1072538
82. Limaye S, Riley S, Zhao S, O’Neill A, Posner M, Adkins D, et al. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN). Oral Oncol (2013) 49(8):835–41. doi: 10.1016/j.oraloncology.2013.04.010
83. Gilbert J, Schell MJ, Zhao X, Murphy B, Tanvetyanon T, Leon ME, et al. A randomized phase II efficacy and correlative studies of cetuximab with or without sorafenib in recurrent and/or metastatic head and neck squamous cell carcinoma. Oral Oncol (2015) 51(4):376–82. doi: 10.1016/j.oraloncology.2014.12.011
84. Xue C, Huang Y, Huang PY, Yu QT, Pan JJ, Liu LZ, et al. Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol Off J Eur Soc Med Oncol (2013) 24(4):1055–61. doi: 10.1093/annonc/mds581
85. Nguyen DT, Shayahi S. Pazopanib: approval for soft-tissue sarcoma. J Adv Pract Oncol (2013) 4(1):53–7. doi: 10.6004/jadpro.2013.4.1.6
86. Wang B, Song JW, Chen HQ. First-line pazopanib treatment in metastatic renal cell carcinoma: real-world data from a single chinese center. Front Pharmacol (2020) 11:517672. doi: 10.3389/fphar.2020.517672
87. Adkins D, Mehan P, Ley J, Siegel MJ, Siegel BA, Dehdashti F, et al. Pazopanib plus cetuximab in recurrent or metastatic head and neck squamous cell carcinoma: an open-label, phase 1b and expansion study. Lancet Oncol (2018) 19(8):1082–93. doi: 10.1016/S1470-2045(18)30350-4
88. Chen L, Jiang YZ, Wu SY, Wu J, Di GH, Liu GY, et al. Famitinib with camrelizumab and nab-paclitaxel for advanced immunomodulatory triple-negative breast cancer (FUTURE-C-plus): an open-label, single-arm, phase II trial. Clin Cancer Res (2022) 28(13):2807–17. doi: 10.1158/1078-0432.CCR-21-4313
89. Qu YY, Zhang HL, Guo H, Luo H, Zou Q, Xing N, et al. Camrelizumab plus famitinib in patients with advanced or metastatic renal cell carcinoma: data from an open-label, multicenter phase II basket study. Clin Cancer Res (2021) 27(21):5838–46. doi: 10.1158/1078-0432.CCR-21-1698
90. Xu RH, Shen L, Wang KM, Wu G, Shi CM, Ding KF, et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer (2017) 36(1):97. doi: 10.1186/s40880-017-0263-y
91. Huang Y, Zhang L, Pan JJ, Hu G, Gang W, Xiong JP, et al. A phase II, multicenter, open-label, single-arm trial of famitinib in patients with advanced recurrent and/or metastatic nasopharyngeal carcinoma (NPC) after two previous treatment regimens. J Clin Oncol (2013) 31(15_suppl):6026–6. doi: 10.1200/jco.2013.31.15_suppl.6026
92. Chen Q, Tang L, Liu N, Han F, Guo L, Guo S, et al. Famitinib in combination with concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 1, open-label, dose-escalation Study. Cancer Commun (2018) 38(1):66. doi: 10.1186/s40880-018-0330-z
93. Ding X, Hua YJ, Zou X, Chen XZ, Zhang XM, Xu B, et al. Camrelizumab plus famitinib in patients with recurrent or metastatic nasopharyngeal carcinoma treated with PD-1 blockade: data from a multicohort phase 2 study. eClinicalMedicine (2023) 61:102043. doi: 10.1016/j.eclinm.2023.102043
94. Ding X, Zhang WJ, You R, Zou X, Wang ZQ, Ouyang YF, et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: an open-label, single-arm, phase II study. J Clin Oncol (2023) 41(14):2571–82. doi: 10.1200/JCO.22.01450
95. Chen TH, Chang PMH, Yang MH. Combination of pembrolizumab and lenvatinib is a potential treatment option for heavily pretreated recurrent and metastatic head and neck cancer. J Chin Med Assoc JCMA (2021) 84(4):361–7. doi: 10.1097/JCMA.0000000000000497
96. Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(11):1154–63. doi: 10.1200/JCO.19.01598
97. Harrington K, Cohen E, Siu L, Rischin D, Licitra L, Vermorken J, et al. Phase 2 LEAP-009: Lenvatinib (Lenva) With or Without Pembrolizumab (Pembro) vs. Chemotherapy (Chemo) for Recurrent or Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC) That has Progressed on Platinum and Immunotherapy. Int J Radiat Oncol Biol Phys (2022) 112(5):e43–4. doi: 10.1016/j.ijrobp.2021.12.101
98. Merck Sharp & Dohme LLC. A phase 3, randomized, placebo-controlled, double-blind clinical study of pembrolizumab (MK-3475) with or without lenvatinib (E7080/MK-7902) to evaluate the safety and efficacy of pembrolizumab and lenvatinib as 1L intervention in a PD-L1 selected population of participants with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) (LEAP-010) (2022). Available at: https://clinicaltrials.gov/study/NCT04199104.
99. Cytryn SL, Moy RH, Cowzer D, Shah RH, Chou JF, Joshi SS, et al. First-line regorafenib with nivolumab and chemotherapy in advanced oesophageal, gastric, or gastro-oesophageal junction cancer in the USA: a single-arm, single-centre, phase 2 trial. Lancet Oncol (2023) 0(10):1073–82. doi: 10.1016/S1470-2045(23)00358-3
100. Grothey A, Cutsem EV, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (2013) 381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X
101. Chiang IT, Lee YH, Tan ZL, Hsu FT, Tu HF. Regorafenib enhances antitumor immune efficacy of anti-PD-L1 immunotherapy on oral squamous cell carcinoma. BioMed Pharmacother (2022) 147:112661. doi: 10.1016/j.biopha.2022.112661
102. Klinghammer KF, Raguse JD, Albers A, Wulf-Goldenberg A, Zopf D, Hoffmann J, et al. Employing head and neck cancer patient derived xenografts to inform clinical trial design: Results from combining regorafenib with everolimus. J Clin Oncol (2015) 33(15_suppl):e17034–4. doi: 10.1200/jco.2015.33.15_suppl.e17034
103. Bayer. A multi-indication, single-treatment arm, open-label phase 2 study of regorafenib and nivolumab in combination in patients with recurrent or metastatic solid tumors (2023). Available at: https://clinicaltrials.gov/study/NCT04704154.
104. Hagege A, Saada-Bouzid E, Ambrosetti D, Rastoin O, Boyer J, He X, et al. Targeting of c-MET and AXL by cabozantinib is a potential therapeutic strategy for patients with head and neck cell carcinoma. Cell Rep Med (2022) 3(9):100659. doi: 10.1016/j.xcrm.2022.100659
105. Memorial Sloan Kettering Cancer Center. A phase 1 study of concurrent cabozantinib and cetuximab in recurrent or metastatic head and neck squamous cell cancer (2022). Available at: https://clinicaltrials.gov/study/NCT03667482.
106. Saba NF, Steuer CE, Ekpenyong A, McCook-Veal A, Magliocca K, Patel M, et al. Pembrolizumab and cabozantinib in recurrent metastatic head and neck squamous cell carcinoma: a phase 2 trial. Nat Med (2023) 29(4):880–7. doi: 10.1038/s41591-023-02275-x
107. Machiels JP, Bossi P, Menis J, Lia M, Fortpied C, Liu Y, et al. Activity and safety of afatinib in a window preoperative EORTC study in patients with squamous cell carcinoma of the head and neck (SCCHN). Ann Oncol (2018) 29(4):985–91. doi: 10.1093/annonc/mdy013
108. Schmitz S, Hamoir M, Reychler H, Magremanne M, Weynand B, Lhommel R, et al. Tumour response and safety of cetuximab in a window pre-operative study in patients with squamous cell carcinoma of the head and neck. Ann Oncol Off J Eur Soc Med Oncol (2013) 24(9):2261–6. doi: 10.1093/annonc/mdt180
109. Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-unrelated head and neck cancer: A multicenter, phase II trial. Clin Cancer Res Off J Am Assoc Cancer Res (2020) 26(19):5140–52. doi: 10.1158/1078-0432.CCR-20-1695
110. Zuur CL, Elbers JBW, Vos JL, van der Leun A, Qiao X, Karakullukcu B, et al. Feasibility and toxicity of neoadjuvant nivolumab with or without ipilimumab prior to extensive (salvage) surgery in patients with advanced head and neck cancer (the IMCISION trial, NCT03003637). J Clin Oncol (2019) 37(15_suppl):2575–5. doi: 10.1200/JCO.2019.37.15_suppl.2575
111. Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol J Eur Soc Ther Radiol Oncol (2021) 156:281–93. doi: 10.1016/j.radonc.2021.01.013
112. Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codecà C, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol Off J Eur Soc Med Oncol (2017) 28(9):2206–12. doi: 10.1093/annonc/mdx299
113. Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol (2013) 14(3):257–64. doi: 10.1016/S1470-2045(13)70011-1
114. Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol Off J Am Soc Clin Oncol (2014) 32(27):2940–50. doi: 10.1200/JCO.2013.53.5633
115. Caudell JJ, Torres-Saavedra PA, Rosenthal DI, Axelrod RS, Nguyen-Tan PF, Sherman EJ, et al. Long-term update of NRG/RTOG 0522: A randomized phase 3 trial of concurrent radiation and cisplatin with or without cetuximab in locoregionally advanced head and neck cancer. Int J Radiat Oncol Biol Phys (2023) 116(3):533–43. doi: 10.1016/j.ijrobp.2022.12.015
116. Powell SF, Gold KA, Gitau MM, Sumey CJ, Lohr MM, McGraw SC, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: A phase IB study. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(21):2427–37. doi: 10.1200/JCO.19.03156
117. Tao Y, Biau J, Sun XS, Sire C, Martin L, Alfonsi M, et al. Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015-01 PembroRad): a multicenter, randomized, phase II trial. Ann Oncol Off J Eur Soc Med Oncol (2023) 34(1):101–10. doi: 10.1016/j.annonc.2022.10.006
118. Patil VM, Prabhash K, Noronha V, Joshi A, Muddu V, Dhumal S, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol (2014) 50(10):1000–4. doi: 10.1016/j.oraloncology.2014.07.015
119. Gangopadhyay A, Bhatt S, Nandy K, Rai S, Rathod P, Puj KS. Survival impact of surgical resection in locally advanced T4b oral squamous cell carcinoma. Laryngoscope (2021) 131(7):E2266–74. doi: 10.1002/lary.29394
120. Toda S, Iwasaki H, Murayama D, Nakayama H, Suganuma N, Masudo K. Invasive procedures in patients undergoing treatment with lenvatinib for thyroid cancer. Mol Clin Oncol (2021) 14(4):1–5. doi: 10.3892/mco.2021.2243
121. de Velasco G, González B, Alonso T, Pinto A, Rodriguez-Moreno JF, Sepúlveda JM, et al. CABOPRE: Phase II study of cabozantinib prior to cytoreductive nephrectomy (CN) in locally advanced and/or metastatic renal cell carcinoma (mRCC). J Clin Oncol (2023) 36(15):TPS4603–TPS4603. doi: 10.1200/JCO.2018.36.15_suppl.TPS4603
122. Zhang Y, Deng X, Ding Z, Kang J, Wu B, Guo B, et al. Preoperative neoadjuvant targeted therapy with apatinib for inoperable differentiated thyroid cancer: A case report. Med (Baltimore) (2021) 100(12):e25191. doi: 10.1097/MD.0000000000025191
123. Tran NH, Muñoz S, Thompson S, Hallemeier CL, Bruix J. Hepatocellular carcinoma downstaging for liver transplantation in the era of systemic combined therapy with anti-VEGF/TKI and immunotherapy. Hepatology (2022) 76(4):1203–18. doi: 10.1002/hep.32613
124. Bilen MA, Liu Y, Nazha B, Brown JT, Osunkoya AO, Williams S, et al. Phase 2 study of neoadjuvant cabozantinib in patients with locally advanced non-metastatic clear cell renal cell carcinoma. J Clin Oncol (2022) 40(6_suppl):340–0. doi: 10.1200/JCO.2022.40.6_suppl.340
125. Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer (2022) 10(4):e004656. doi: 10.1136/jitc-2022-004656
126. Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: A pilot study of ten patients. Front Oncol (2021) 11:747950. doi: 10.3389/fonc.2021.747950
127. Schwacha-Eipper B, Minciuna I, Banz V, Dufour JF. Immunotherapy as a downstaging therapy for liver transplantation. Hepatol Baltim Md (2020) 72(4):1488–90. doi: 10.1002/hep.31234
128. Iqbal MS, Chaw C, Kovarik J, Aslam S, Jackson A, Kelly J, et al. Primary concurrent chemoradiation in head and neck cancers with weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol (2017) 21(2):171–7. doi: 10.1055/s-0036-1594020
129. Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf (2013) 36(6):413–26. doi: 10.1007/s40264-013-0050-x
130. Miljković F, Bajorath J. Computational analysis of kinase inhibitors identifies promiscuity cliffs across the human kinome. ACS Omega (2018) 3(12):17295–308. doi: 10.1021/acsomega.8b02998
131. Grossman M, Adler E. Protein kinase inhibitors - selectivity or toxicity? In: Kumar Singh R, editor. Biochemistry. Rijeka, Croatia: IntechOpen (2021). Available at: https://www.intechopen.com/books/protein-kinases-promising-targets-for-anticancer-drug-research/protein-kinase-inhibitors-selectivity-or-toxicity-.
132. Vallerio P, Orenti A, Tosi F, Maistrello M, Palazzini M, Cingarlini S, et al. Major adverse cardiovascular events associated with VEGF-targeted anticancer tyrosine kinase inhibitors: a real-life study and proposed algorithm for proactive management. ESMO Open (2021) 7(1):100338. doi: 10.1016/j.esmoop.2021.100338
133. Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN. Cardiotoxic effects of angiogenesis inhibitors. Clin Sci (2021) 135(1):71–100. doi: 10.1042/CS20200305
134. Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl (2013) 11(2):172–91. doi: 10.1016/j.ejcsup.2013.07.016
135. Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther (2023) 8(1):1–27. doi: 10.1038/s41392-023-01469-6
136. Das A, Mahapatra S, Bandyopadhyay D, Samanta S, Chakraborty S, Philpotts LL, et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: A network meta-analysis. Crit Rev Oncol Hematol (2021) 157:103186. doi: 10.1016/j.critrevonc.2020.103186
137. Cohen EEW, Machiels JPH, Harrington KJ, Burtness B, Shin SW, Gause CK, et al. KEYNOTE-040: A phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J Clin Oncol (2015) 33(15_suppl):TPS6084–TPS6084. doi: 10.1200/jco.2015.33.15_suppl.tps6084
138. Haddad R, Seiwert T, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab after progression on platinum and cetuximab in head and neck squamous cell carcinoma (HNSCC): results from KEYNOTE-055. Ann Oncol (2016) 27:vi330. doi: 10.1093/annonc/mdw376.09
139. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(14):1542–9. doi: 10.1200/JCO.2016.70.1524
140. Alsahafi E, Begg K, Amelio I, Raulf N, Lucarelli P, Sauter T, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis (2019) 10(8):1–17. doi: 10.1038/s41419-019-1769-9
141. Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer (2022) 10(2):e003026. doi: 10.1136/jitc-2021-003026
142. Pfister DG, Haddad RI, Worden FP, Weiss J, Mehra R, Chow LQM, et al. Biomarkers predictive of response to pembrolizumab in head and neck cancer. Cancer Med (2023) 12(6):6603–14. doi: 10.1002/cam4.5434
143. Basheeth N, Patil N. Biomarkers in head and neck cancer an update. Indian J Otolaryngol Head Neck Surg (2019) 71(Suppl 1):1002–11. doi: 10.1007/s12070-019-01683-1
144. Siemert J, Wald T, Kolb M, Pettinella I, Böhm U, Pirlich M, et al. Pre-therapeutic VEGF level in plasma is a prognostic bio-marker in head and neck squamous cell carcinoma (HNSCC). Cancers (2021) 13(15):3781. doi: 10.3390/cancers13153781
145. Zhu C, Gu L, Liu Z, Li J, Yao M, Fang C. Correlation between vascular endothelial growth factor pathway and immune microenvironment in head and neck squamous cell carcinoma. BMC Cancer (2021) 21:836. doi: 10.1186/s12885-021-08547-4
146. Campo MS, Graham SV, Cortese MS, Ashrafi GH, Araibi EH, Dornan ES, et al. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology (2010) 407(1):137–42. doi: 10.1016/j.virol.2010.07.044
147. de Freitas AC, de Oliveira THA, Barros MR, Venuti A. hrHPV E5 oncoprotein: immune evasion and related immunotherapies. J Exp Clin Cancer Res CR (2017) 36(1):71. doi: 10.1186/s13046-017-0541-1
148. Solomon B, Young RJ, Bressel M, Urban D, Hendry S, Thai A, et al. Prognostic significance of PD-L1+ and CD8+ Immune cells in HPV+ Oropharyngeal squamous cell carcinoma. Cancer Immunol Res (2018) 6(3):295–304. doi: 10.1158/2326-6066.CIR-17-0299
Keywords: squamous cell carcinoma of the head and neck, vascular endothelial growth factor, tyrosine kinase inhibitor, immune checkpoint inhibitors, human papillomavirus
Citation: Puttagunta P, Pamulapati SV, Bates JE, Gross JH, Stokes WA, Schmitt NC, Steuer C, Teng Y and Saba NF (2023) Critical review of the current and future prospects of VEGF-TKIs in the management of squamous cell carcinoma of head and neck. Front. Oncol. 13:1310106. doi: 10.3389/fonc.2023.1310106
Received: 09 October 2023; Accepted: 28 November 2023;
Published: 13 December 2023.
Edited by:
Fujun Han, The First Hospital of Jilin University, ChinaReviewed by:
Silvia-Mezi, Sapienza University of Rome, ItalyMaria Cossu Rocca, European Institute of Oncology (IEO), Italy
Copyright © 2023 Puttagunta, Pamulapati, Bates, Gross, Stokes, Schmitt, Steuer, Teng and Saba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nabil F. Saba, bmZzYWJhQGVtb3J5LmVkdQ==
 Prashant Puttagunta
Prashant Puttagunta Saagar V. Pamulapati
Saagar V. Pamulapati James E. Bates
James E. Bates Jennifer H. Gross4
Jennifer H. Gross4 William A. Stokes
William A. Stokes Nicole C. Schmitt
Nicole C. Schmitt Conor Steuer
Conor Steuer Yong Teng
Yong Teng Nabil F. Saba
Nabil F. Saba