- 1Department of Medical Oncology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
- 2Department of General Medicine, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
- 3Department of Radiation Oncology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
- 4Department of Radiodiagnosis and Imaging, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
- 5Department of Pathology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, India
Background: Follicular dendritic cell sarcoma (FDCS) is a rare mesenchymal malignant tumor derived from follicular dendritic cells. FDCS arises mainly from lymph nodes and rarely are extranodal. Diagnostic dilemma occurs due to the same micromorphology as other sarcomas and lymphomas. Curative radical resection is the standard therapy, and adjuvant treatment is not defined. For unresectable disease, chemotherapy and radiotherapy are indicated with variable response rates. Due to its rarity, a standard treatment is not yet defined.
Objective: This study aims to analyze the clinicopathological features, treatment patterns, and survival outcome of FDCS cases in our institution.
Methodology: The study was conducted in the Department of Medical Oncology State Cancer Institute, Sher I Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Jammu and Kashmir, India. Biopsy-proven FDCS patients were identified through the hospital-based registry from January 1, 2020 to December 31, 2023.
Results: A total of six patients were diagnosed during the study period. The median age was 28 years (range, 21–51 years). There were four male and two female patients, with male-to-female ratio of 2:1. Common symptoms were abdominal pain (50%) and cough and dyspnea (33.3%). Four patients (66.6%) had nodal involvement with retroperitoneum and mediastinum in two cases each. Three patients had extranodal involvement, with the colon in two and with the liver in one. Five patients were initially misdiagnosed as non-Hodgkin’s lymphoma, soft tissue sarcoma, neurogenic tumor, and carcinoma. The treatments offered were surgery, chemotherapy, targeted therapy, radiotherapy, and observation. Four patients were alive at a median follow-up of 12 months, with three patients having no evidence of disease and one case living with the disease. Two patients had succumbed to the disease.
Conclusion: The study described the clinicopathological characteristics, diagnostic challenges, and management difficulties in FDCS patients. Due to the rarity of this disease, high expertise is needed to diagnose FDCS; otherwise, the diagnosis usually gets delayed.
Introduction
Follicular dendritic cell ѕаrcоmа (FDCS) is a rare and slowly growing tumor that was first recognized by Monda and Rosai in 1986 (1). It originates from mesenchymal dendritic cells (2). Dendritic cells are immune cells that specialize in antigen presentation and endocytosis, found primarily in peripheral lymphoid organs (3). They are classified into three major subtypes: follicular dendritic cells (FDCs), interdigitating dendritic cells (IDCs), and fibroblastic reticular cells (FRCs). FDCs play a central role in primary and secondary lymphoid follicles by presenting antigens to B cells. Both FDCs and FRCs are derived from mesenchymal tissue, while IDCs originate from hematopoietic cells (4).
FDCS is classified as a type of sarcoma with only a few hundred cases reported to date, with an incidence rate of <0.4% (5). This typically affects young adults in their fifth decade and is rare in children, with no sex predilection (6). FDCS commonly present as a slow-growing localized tumor and rarely as a widespread disease (7). It involves nodal and extranodal sites with varying frequencies, and in some cases, it has mixed involvement (4, 8–11). The commonly involved nodal sites are the neck, mediastinum, axilla, and abdomen, while the liver, lung, and spleen are common extranodal sites (4, 7).
Diagnosing FDCS is challenging due to its histopathological similarities to various other conditions, including non-Hodgkin lymphoma, sarcoma, melanoma, undifferentiated carcinomas, various inflammatory conditions like inflammatory myofibroblastic tumor (IMT), reactive follicular hyperplasia, and other dendritic and histiocytic cell disorders (8, 12, 13). Morphologically, FDCS is characterized by spindled to ovoid cells that form fascicles, whorls, diffuse sheets, or nodules, often with lymphoplasmacytic infiltration. Tumor cells generally express markers associated with follicular dendritic cell differentiation, such as CD21, CD23, and CD35 (14–16).
Due to its rarity, there are no established treatment protocols. Radical surgical resection is the primary treatment for localized disease, while adjuvant radiotherapy has not shown a significant impact on survival (6, 11). Chemotherapy is recommended for unresectable or widespread disease, though an optimal regimen has yet to be identified (6, 16, 17). To date, several hundred cases have been reported worldwide. Here we present our experience of six (6) FDCS patients diagnosed and treated at our oncology center.
Materials and methods
This retrospective study was conducted in the Department of Medical Oncology, State Cancer Institute at Sher I Kashmir Institute of Medical Sciences (SKIMS), Deemed Medical University, Srinagar, Jammu and Kashmir, India. All biopsy-proven FDCS patients were identified through the hospital-based cancer registry over a 4-year period from January 1, 2020 to December 31, 2023. A total of six cases were diagnosed during the study period. The details about demographics, clinical presentation, radiology, histopathology, treatment offered, and outcome were collected from the patients’ case record files from the Medical Record Section of the State Cancer Institute, SKIMS, and was documented on a designed proforma. Immunohistochemistry was performed in all of the cases on formalin-fixed paraffin-embedded tissues by using a fully automated machine (Ventana Benchmark XT) using monoclonal antibodies to CD117 (Cellmarque−1:100, YR145), CD68 (Cellmarque−1:50 PG−M1), CD34 (DAKO−1:50, QBEnd10), CD45 (Thermo−1:100, RA/RO), CD43 (DAKO−1:50, DF−T1), Desmin (BioGenex−1:30, D33), multiple myeloma 1 (MUM1) (Cellmarque−1:100, MRQ43), CD2 (Cellmarque−1:100, MRQ11), CD3 (DAKO−1:50 F7.2.38), CD10 (Ventana−RTU, SP67), CD23 (Cellmarque−1:50, MRQ12), CD21 (Cellmarque−1:50, MRQ32), CD20 (Cellmarque−1:100, L26), CD79a (Cellmarque−1:100, JCB117), CD1a (BioGenex−1:30, O10), epithelial membrane antigen (EMA) (Cellmarque−1:100, E29), CD19 (Cellmarque−1:50, MRQ36), synaptophysin (Thermo−1:50, SP11), chromogranin A (Cellmarque−1:100, LKH110), human melanoma black−45 (HMB 45) (Cellmarque−1:50, HMB45), and anaplastic lymphoma kinase−1 (ALK−1) (Thermo−1:50, 5A4). Descriptive statistics were analyzed by using the appropriate statistical software SPSS V24. Ethical clearance was granted by IEC SKIMS.
Results
Clinical features
In the present study, six patients of FDCS were analysed, with four male and two female patients, respectively (Table 1). The median age was 28 years (range, 21–51 years). The common presenting symptoms included abdominal pain and discomfort, cough, breathlessness, and lower back pain. The sites of involvement were the mediastinum (2/6), retroperitoneum (2/6), and cecum (2/6). One patient with mediastinal FDCS had superior vena cava syndrome (case no. 1), and another had been treated for Hodgkin’s lymphoma 8 years back and had concomitant myasthenia gravis with FDCS (case no. 4). De novo metastasis to the liver was present in one patient (case no. 3) with retroperitoneal FDCS (Figure 1).
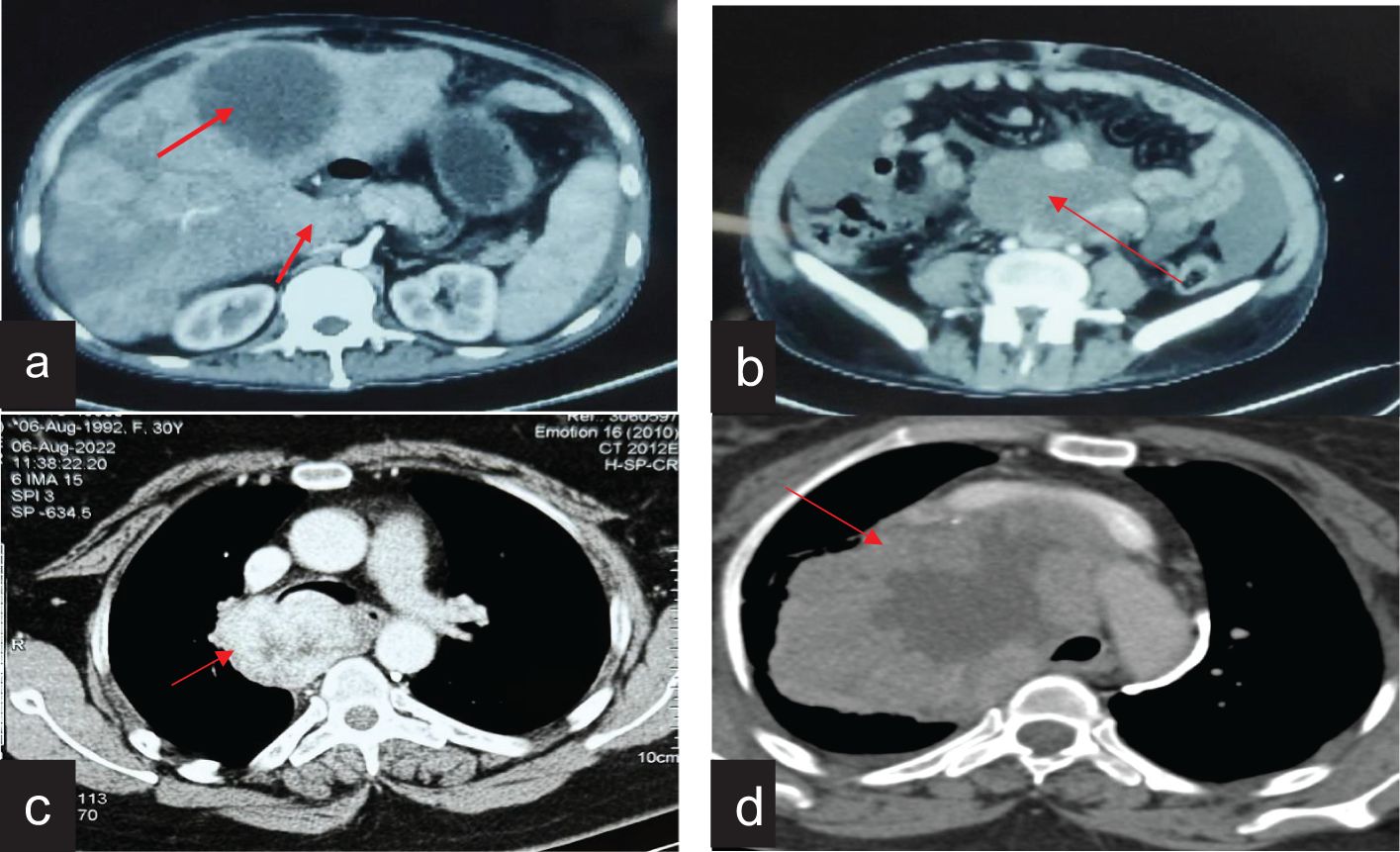
Figure 1. Radiological features of FDCS cases on contrast-enhanced computed tomography. (a) CT of the abdomen showing retroperitoneal FDCS with liver metastasis. (b) CT of the abdomen showing a large retroperitoneal FDCS. (c) CT of the chest showing mediastenial FDCS extending from the lower paratracheal region and abutting the trachea and esophagus. (d) CT of the chest showing a bulky mediastinal FDCS causing bronchial compression and SVC syndrome.
Pathologic features
On gross examination, the tumor was well circumscribed and soft to firm with necrosis, and the hemorrhagic areas had sizes ranging from 6 to 11 cm. Microscopically, the tumor was composed of round to spindle cells arranged in sheets in a fascicular and whorled pattern. Lymphoid cells were interspersed in one case and also had giant cells and mitotic figures. On immunohistochemistry, the tumor cells demonstrated positive staining for the follicular dendritic cell markers CD21 (100%), CD23 (100%), and CD35 (50%). Vimentin and D2–40 were done in one patient, who was immunoreactive (Figure 2).
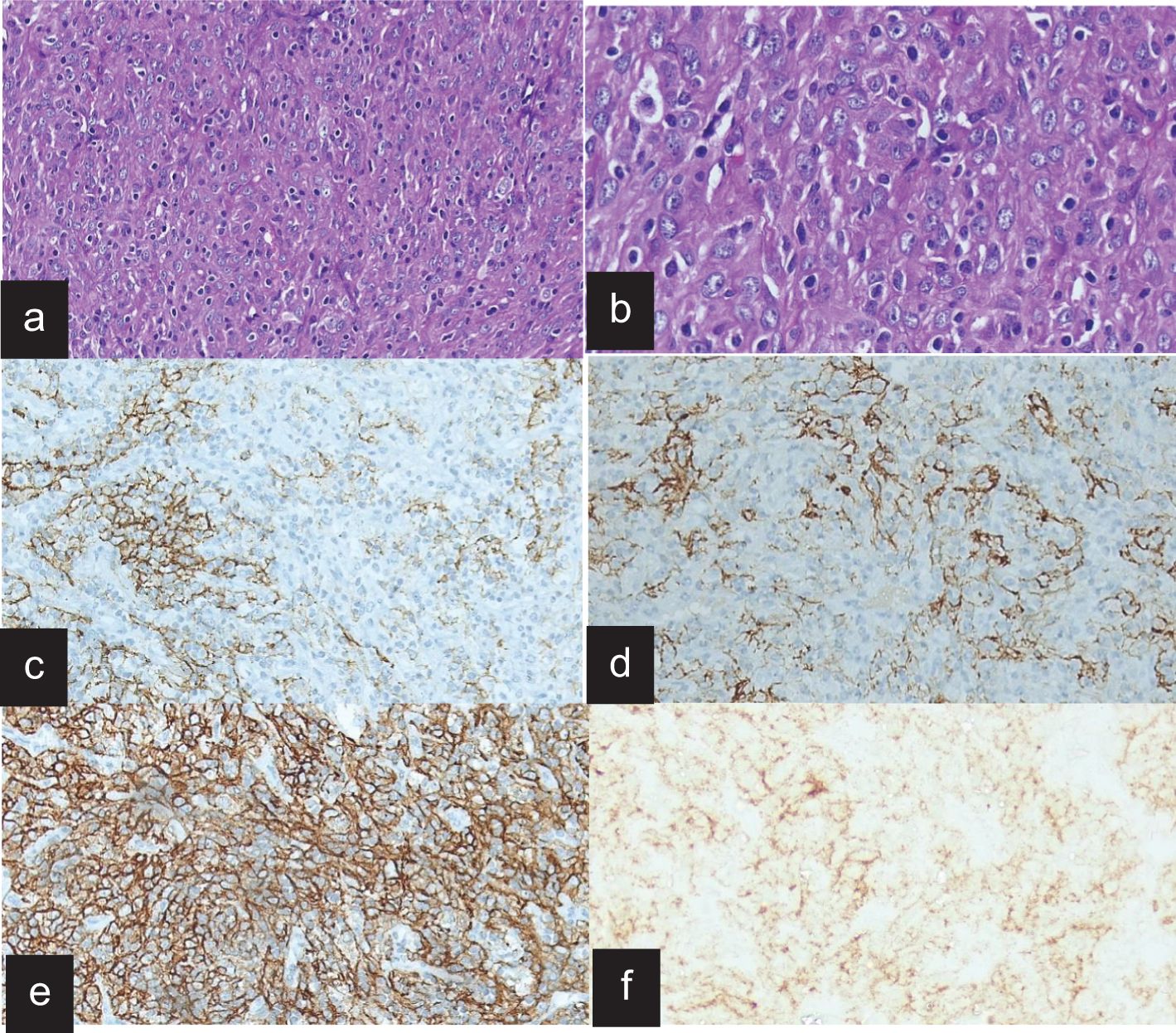
Figure 2. Histopathology and immunohistochemistry of FDCS. (a) H&E stain on low power, showing fascicle whorls of spindle cells in a fibro−collagenious stroma. (b) H&E stain on high power shows prominent giant cells and mitotic figures and scattered small lymphocytes. (c) The tumor cells are immunoreactive for CD21. (d) The tumor cells are immunoreactive for CD35. (e) The tumor cells are immunoreactive for D2-40 diffusely. (f) The tumor cells are immune negative for EMA.
Diagnostic dilemma
Only one patient in our study had upfront diagnosis as FDCS. The rest of the patients (5/6) had an initial diagnosis as either diffuse large B cell lymphoma (1/5), pleomorphic sarcoma (2/5), neurogenic tumor (1/5), or poorly differentiated adenocarcinoma (1/5).
Treatment and outcome
In three patients (two colon cases and one retroperitoneum case), upfront surgery was done (case numbers 2, 5, and 6). Among the two colonic FDCS, one case received 6 cycles of adjuvant chemotherapy. The patient who was initially diagnosed as DLBCL received 6 cycles of R-CHOP and local radiotherapy, but due to non-responsive disease, he was re-evaluated for an alternative diagnosis. The differentials in such case could be lymphoblastic lymphoma, Hodgkin’s lymphoma, thymoma, small cell carcinoma, extra-gonadal germ cell tumor, etc. A repeat biopsy along with a review of the initial biopsy confirmed FDCS. His mediastinal mass was unresectable. He was started on combination chemotherapy with gemcitabine and docetaxel. After 4 cycles, the reassessment showed a significant reduction in the tumor. He was subjected to R0 resection followed by two more cycles of the same chemotherapy. One patient with retroperitoneal FDCS had liver metastasis at presentation. He received oral pazopanib 400 mg BD for 1 year and upon progression was started on gemcitabine and docetaxel but died after 2 cycles due to disease progression. The patient who had been treated 8 years back for Hodgkin’s lymphoma and now had mediastinal FDCS denied any treatment and opted for observation. One patient in our study received radiotherapy. Two patients (2/6) died due to disease progression. Four patients (4/6) are alive at the time of the last follow-up.
Discussion
Follicular dendritic cell sarcoma (FDCS) is a very rare mesenchymal tumor of follicular dendritic cell origin (1). FDCS has been a diagnostic challenge since its description as most of the cases are initially labeled as lymphoma, sarcoma, or carcinoma. There is limited literature on this entity. Most of the literature on FDCS are from case reports and case series. A pooled analysis of 462 patients by Saygin C et al. documented a median age of 50 years (4). In contrast, our patient group was generally younger, with a median age of 28 years (ranging from 21 to 51 years). Two-thirds of the patients had abdominal symptoms, which is consistent with other studies on FDCS (4, 15). A diagnostic challenge was encountered in five patients, with initial misdiagnosis including non-Hodgkin’s lymphoma, pleomorphic sarcoma, thymic carcinoma, and poorly differentiated adenocarcinoma. Similar diagnostic discrepancies have been reported in other studies, with rates as high as 58% (4, 10, 18). Immunohistochemistry was the primary method used for confirmation, with all patients (6/6) testing positive for CD21, CD23, and CD35. Typically, follicular dendritic cell (FDC) markers, such as CD21, CD23, CD35, D2-40, clusterin, CXCL13, FDC-secreted protein, and serglycin, are positive in FDCS. In contrast, cytokeratin, CD1a, langerin, lysozyme, and myeloperoxidase stains are negative. Markers like EMA, S100, and CD68 may show variable staining patterns. However, it is not uncommon for some markers to be lost in FDCS cases (7, 19).
Interestingly, “one” patient was treated for Hodgkin’s lymphoma and presented with a mediastinal mass and myasthenia gravis. Gounder M et al. reported that 35% patients had solid tumors, and 3% were reported as lymphoma previously. Their paper suggested that immunosuppression may be a risk factor for FDCS (6). This probability of immunosuppression as a risk factor might be valid as our patient was on prolonged chemotherapy followed by hematopoietic stem cell transplant. The inflammatory variant of follicular dendritic cell sarcoma is aggressive and presents in the liver and spleen. If surgically not amenable, the patients with this variant are more likely to receive systemic therapy (20).
Most of our patients had a localized disease, in line with other studies showing that 85% of FDCS patients present with localized tumors (3). However, despite being localized, the majority of our cases were locally advanced. The patients who underwent surgery remain disease-free, while the others, except for two, are living with the disease. According to a pooled analysis, the 2-year survival rates for early-stage, locally advanced, and metastatic disease are 82.4%, 80%, and 42.8%, respectively (4). In our series, the locally advanced patients also demonstrated good 1-year survival rates, though they experienced significant morbidities. The low proliferation index, indicating a slower tumor growth rate, may explain this outcome. Previous studies have shown that adjuvant chemotherapy or radiotherapy does not improve survival, so no adjuvant treatment was generally recommended for the patients who underwent surgery (4).
The systemic therapies used in various case series and case reports are CHOP, EPOCH, ICE, CVP, gemictabine and docetaxol, methyprednisolone, various VEGF inhibitors, and TKIs with different response rates. The systemic therapies administered to our patients included R-CHOP, gemcitabine and docetaxel, and pazopanib. One patient, initially diagnosed with DLBCL, was treated with R-CHOP and radiotherapy but showed no improvement. Two other patients received gemcitabine and docetaxel upon disease progression. In a large, single-center study by Jain et al., the overall response rate to gemcitabine and docetaxel was 80%, while the CHOP regimen resulted in only a partial response in 60% (21). In our study, the patient who received pazopanib experienced a stable disease for 1 year but eventually progressed. Preclinical studies suggest that vascular endothelial growth factor (VEGF) inhibits the maturation and activation of dendritic cells, which could explain why pazopanib, with its anti-VEGFR properties, was effective. FDCS is also linked to BRAF mutations, EGFR overexpression, PDL1/2 overexpression, and potential involvement of the mTOR pathway. Radiotherapy is primarily used for palliation of symptoms. Targeted agents against these pathways could be explored in clinical trials (6).
Strengths and weaknesses
FDCS is a rare condition with diagnostic and therapeutic challenges. This study has tried to highlight the diagnostic dilemma and challenges in treating these patients. However, the sample size of this study is small in view of the rarity of the condition.
Conclusion
Our study described the clinicopathological characteristics and management challenges in FDCS. Due to the rarity of this disease, high expertise is needed for the diagnosis of FDCS; otherwise, it will lead to a delay in diagnosis. For resectable disease, surgery remains the standard of care. The role of the adjuvant and the choice of chemotherapy are challenging with only limited results, and radiotherapy is used in palliative settings only. Further research into the pathogenesis and management of FDCS is required for better outcomes of this rare entity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee IEC SKIMS Srinagar. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AI: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FB: Formal Analysis, Funding acquisition, Validation, Writing – original draft, Writing – review & editing. MS: Conceptualization, Funding acquisition, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. AK: Data curation, Formal Analysis, Funding acquisition, Resources, Validation, Writing – review & editing. SW: Data curation, Formal Analysis, Funding acquisition, Resources, Validation, Writing – review & editing. SR: Data curation, Formal Analysis, Funding acquisition, Visualization, Writing – review & editing. MB: Data curation, Funding acquisition, Resources, Validation, Visualization, Writing – review & editing. NS: Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing. FG: Formal Analysis, Funding acquisition, Resources, Visualization, Writing – review & editing. UW: Funding acquisition, Resources, Visualization, Writing – review & editing. SQ: Funding acquisition, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Monda L, Warnke R, and Rosai J. A primary lymph node Malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. (1986) 122:562–72.
2. Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. (2016) 127:2672–81. doi: 10.1182/blood-2016-01-690636
3. Sasaki M, Izumi H, Yokoyama T, Kojima M, and Hosono A. Follicular dendritic cell sarcoma treated with a variety of chemotherapy. Hematol Oncol. (2017) 35:905–8. doi: 10.1002/hon.v35.4
4. Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, and Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. (2013) 88:253–71. doi: 10.1016/j.critrevonc.2013.05.006
5. Perkins SM and Shinohara ET. Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. Am J Clin Oncol. (2013) 36:395–8. doi: 10.1097/COC.0b013e31824be22b
6. Gounder M, Desai V, Kuk D, Agaram N, Arcila M, Durham B, et al. Impact of surgery, radiation and systemic therapy on the outcomes of patients with dendritic cell and histiocytic sarcomas. Eur J Cancer. (2015) 51:2413–22. doi: 10.1016/j.ejca.2015.06.109
7. Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. (2002) 41:1–29. doi: 10.1046/j.1365-2559.2002.01418.x
8. Wu A and Pullarkat S. Follicular dendritic cell sarcoma. Arch Pathol Lab Med. (2016) 140:186–90. doi: 10.5858/arpa.2014-0374-RS
9. Chen T and Gopal P. Follicular dendritic cell sarcoma. Arch Pathol Lab Med. (2017) 141:596–9. doi: 10.5858/arpa.2016-0126-RS
10. Duan GJ, Wu F, Zhu J, Guo DY, Zhang R, Shen LL, et al. Extranodal follicular dendritic cell sarcoma of the pharyngeal region: a potential diagnostic pitfall, with literature review. Am J Clin Pathol. (2010) 133:49–58. doi: 10.1309/AJCP7U8YISBUAVNW
11. Perez-Ordoñez B and Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol. (1998) 15:144–54.
12. Perez-Ordonez B, Erlandson RA, and Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol. (1996) 20:944–55. doi: 10.1097/00000478-199608000-00003
13. Facchetti F and Lorenzi L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol. (2016) 33:159–66. doi: 10.1053/j.semdp.2016.05.002
14. Dalia S, Shao H, Sagatys E, Cualing H, and Sokol L. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Control. (2014) 21:290–300. doi: 10.1177/107327481402100405
15. Dalia S, Jaglal M, Chervenick P, Cualing H, and Sokol L. Clinicopathologic characteristics and outcomes of histiocytic and dendritic cell neoplasms: the moffitt cancer center experience over the last twenty five years. Cancers. (2014) 6:2275–95. doi: 10.3390/cancers6042275
16. Caces DB, Daniel S, Paredes-Tejada JM, and Smith S. Spontaneous regression of follicular dendritic cell sarcoma. J Clin Oncol. (2012) 30:e24–6. doi: 10.1200/JCO.2011.38.4164
17. Chen S, Sun Y, Sun W, Dan M, and Jiang Y. Survival analysis in patients with follicular dendritic cell sarcoma: a population-based study. Hematology. (2023) 28:2260975. doi: 10.1080/16078454.2023.2260975
18. Shia J, Chen W, Tang LH, Carlson DL, Qin J, Guillem JG, et al. Extranodal follicular dendritic cell sarcoma: clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. (2006) 449:148–58. doi: 10.1007/s00428-006-0231-4
19. Lorenzi L, Doring C, Rausch T, Benes V, Lonardi S, Bugatti M, et al. Identification of novel follicular dendritic cell sarcoma markers, FDCSP and SRGN, by whole transcriptome sequencing. Oncotarget. (2017) 8:16463–72. doi: 10.18632/oncotarget.14864
20. Ge R, Liu C, Yin X, Chen J, Zhou X, Huang C, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol. (2014) 7:2421–9.
Keywords: dendritic cells, follicular dendritic cell sarcoma, mimicker, histopathology, immunohistochemistry
Citation: Ibrahim A, Mir MH, Bagdadi FS, Sofi MA, Khanday AR, Wani SA, Regmi SK, Bhat MH, Syed NA, Guru FR, Wani UA and Qadri SK (2025) Follicular dendritic cell sarcoma: a great mimicker with unpredictable clinical course—experience from a tertiary care cancer center in India. Front. Oncol. 15:1544803. doi: 10.3389/fonc.2025.1544803
Received: 13 December 2024; Accepted: 12 May 2025;
Published: 10 June 2025.
Edited by:
Pier Paolo Piccaluga, University of Bologna, ItalyReviewed by:
Liqiong Liu, The 6th Affiliated Hospital of Shenzhen University Health Science Center, ChinaKashif Ali Sarwar, National University of Medical Sciences (NUMS), Pakistan
Copyright © 2025 Ibrahim, Mir, Bagdadi, Sofi, Khanday, Wani, Regmi, Bhat, Syed, Guru, Wani and Qadri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohmad Hussain Mir, bWh1c3NhaW5taXJAZ21haWwuY29t
 Ajas Ibrahim
Ajas Ibrahim Mohmad Hussain Mir
Mohmad Hussain Mir Farhana Siraj Bagdadi
Farhana Siraj Bagdadi Mushtaq Ahmad Sofi
Mushtaq Ahmad Sofi Abrar Rasool Khanday
Abrar Rasool Khanday Suhail Ahmad Wani1
Suhail Ahmad Wani1 Sunil K. Regmi
Sunil K. Regmi Nisar Ahmad Syed
Nisar Ahmad Syed