- 1Department of Pediatric Oncology, Children’s Cancer Hospital (57357), Cairo, Egypt
- 2Department of Pediatric Oncology, National Cancer Institute, Cairo, Egypt
- 3Department of Clinical Oncology, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt
- 4Department of Surgical Pathology, Children’s Cancer Hospital (57357), Cairo, Egypt
- 5Department of Surgical Pathology, National Cancer Institute (NCI), Cairo University, Cairo, Egypt
- 6Department of Clinical Pathology, Children’s Cancer Hospital (57357), Cairo, Egypt
- 7Department of Clinical Pathology, National Cancer Institute (NCI), Cairo University, Cairo, Egypt
- 8Department of Radio-diagnosis, Children’s Cancer Hospital (57357), Cairo, Egypt
- 9Department of Clinical Research, Children’s Cancer Hospital (57357), Cairo, Egypt
- 10Department of Pediatric Oncology, Dana- Farber Cancer Institute, Boston, MA, United States
Background: Anaplastic large cell lymphoma (ALCL) constitutes 10-15% of childhood non-Hodgkin lymphoma. EFS is 70% and currently 80% with the additional of targeted agents such as CD30 directed conjugated monoclonal antibody brentuximab or ALK inhibitors such as crizotinib. Expression of CD3, a T-cell marker, can be lost or diminished in some ALCL cases. The literature is conflicting on whether CD3 expression affects prognosis, and it has been analyzed mostly in the relapse setting. The purpose of this study was to determine the effect of CD3 expression on survival and its relation to the other prognostic variables in newly diagnosed patients with pediatric ALCL treated at a single large pediatric oncology center.
Methods: A retrospective study was done on 89 newly diagnosed pediatric ALCL patients (under 18 years old) treated at Children’s Cancer Hospital Egypt (CCHE-57357) from July 2007 to December 2019. Immunohistochemistry was utilized to confirm the diagnosis and determine CD3 expression in tumor cells. The impact of CD3 expression on event-free survival (EFS), relapse-free survival (RFS) and overall survival (OS)) was analyzed.
Results: The median age was 10.7 years with male to female ratio 1.8:1. The majority of patients (85.4%) were ALK positive. CD3 was positive in 31 (34.8%) of patients. The median follow-up period was 60 months. The five-year OS, EFS, and RFS rates for the entire group were 84.3%, 73.1%, and 81.5%, respectively. CD3 positivity was associated with a higher incidence of CNS involvement (p=0.03) but did not significantly impact other patient outcomes (EFS, RFS and OS). However, stage, B symptoms, and skin involvement were linked to a shorter relapse-free survival.
Conclusion: This study indicates that CD3 expression may not be a major factor predicting survival in newly diagnosed pediatric ALCL. Additional research is needed to understand its association with CNS positive disease.
Introduction
Anaplastic large cell lymphoma (ALCL) accounts for 10% to 15% of all childhood non-Hodgkin lymphomas (1). This tumor is characterized by strong expression of CD30. The majority of pediatric cases also demonstrate overexpression of anaplastic lymphoma kinase protein (ALK) (2).
Since the addition of novel agents to standard chemotherapy has improved outcome. Also targeted monotherapy or combination targeted therapy has shown efficacy in relapsed ALCL. EFS is 70% and currently 80% with the additional of targeted agents such as CD30 directed conjugated monoclonal antibody brentuximab or ALK inhibitors such as crizotinib (2).
CD3 is a T-cell marker that can be detected by immunohistochemistry (IHC) on tissue specimens or flow cytometry on peripheral blood samples (3). It is often expressed in ALCL but in some cases CD3 expression is lost or diminished. It has been postulated that this may have an impact on outcome (4).
The incidence and relevance of CD3 expression in pediatric ALCL are not firmly established. Some studies have found CD3 expression to be more prevalent in ALK-negative versus ALK-positive ALCL which may confound the association with a worse outcome (4).
A recent clinical trial (ALCL-Relapse) investigated the role of stem cell transplantation (SCT) versus vinblastine monotherapy for relapsed or refractory pediatric ALCL. The trial stratified patients based on the time of relapse and CD3 expression to prospectively test different reinduction approaches combined with consolidation via allogeneic or autologous SCT as compared to vinblastine monotherapy (5). The study reported CD3 expression in 28% of patients with ALK-positive ALCL and 67% of patients with ALK-negative ALCL. CD3 expression was associated with a higher risk of relapse after SCT, but not with overall survival. The trial also demonstrated that vinblastine monotherapy was effective and well tolerated as maintenance therapy after SCT (5).
This study’s objectives were to assess the impact of CD3 expression on the outcomes in the setting of newly diagnosed ALCL in the pediatric age group, and evaluate the relationship between CD3 expression and other known prognostic factors
Patients and methods
This retrospective study comprised 89 newly diagnosed patients under age 18 who were treated at Children’s Cancer Hospital Egypt (CCHE) between July 2007 and December 2019. Patients with primary cutaneous T cell lymphoma, an underlying immunodeficiency or whose paraffin blocks were unavailable were excluded.
Retrospective review of medical records included history, physical examination, full blood count, bone marrow (BM) aspirate and bilateral bone marrow biopsy, and cerebrospinal fluid cytology. Plain X-rays, ultrasonography, computed tomography, PET CT scan (if available), and magnetic resonance imaging were used for radiologic staging. Patients were staged using the Murphy staging system (6), and chemotherapy was administered according to the Children’s Oncology Group ANHL 0131 protocol, Vinblastine arm (7).
Paraffin blocks were collected from the Pathology Department’s archives. The pathologist reviewed H&E stained and immunostained slides to confirm the diagnosis and examine the immunohistochemical expression of CD3, CD30, and ALK in tumor cells.
Immunostaining was performed with the BenchMark Ventana Autostainer. Positive CD3 expression was defined as a membrane/cytoplasmic response in neoplastic cells regardless of the strength of staining or percent of positive cells. Univariant analysis was used to determine the correlation between CD3 expression in ALCL cells and overall (OS), event-free survival (EFS), and clinical characteristics such as age, disease stage, and treatment response.
Definitions
Complete remission (CR): Defined as disappearance of all evidence of disease
Complete response, unconfirmed (Cru): Defined as a residual lymph node mass > 1.5 cm that regressed by >75% in the sum of the products of the greatest perpendicular diameters (SPD) or any residual lesions in organs that decreased by >75% with a negative gallium scan and/or PET scan.
Partial response (PR): defined as a ≥ 50% decrease in the SPD of the lesions with no new lesion
Progressive Disease (PD): defined as the appearance of any new lesion or any evidence of primary tumor progression (6).
Statistical analysis
We summarized patient characteristics using frequencies and percentages for categorical data and mean (standard deviations) or median (IQR) for continuous variables.
The chi-squared or Fisher’s exact tests were used to examine the relationship between CD3 status and other clinical factors. The EFS and OS were analyzed using Kaplan-Meier, and differences between CD3 expression statuses were examined using the Log-rank test. The Cox regression method was used to determine the difference in survival rates between CD3 status and other confounding variables. All of these data were examined with SPSS version 25, and a 95% confidence interval was employed in all analyses.
Results
Patient characteristics
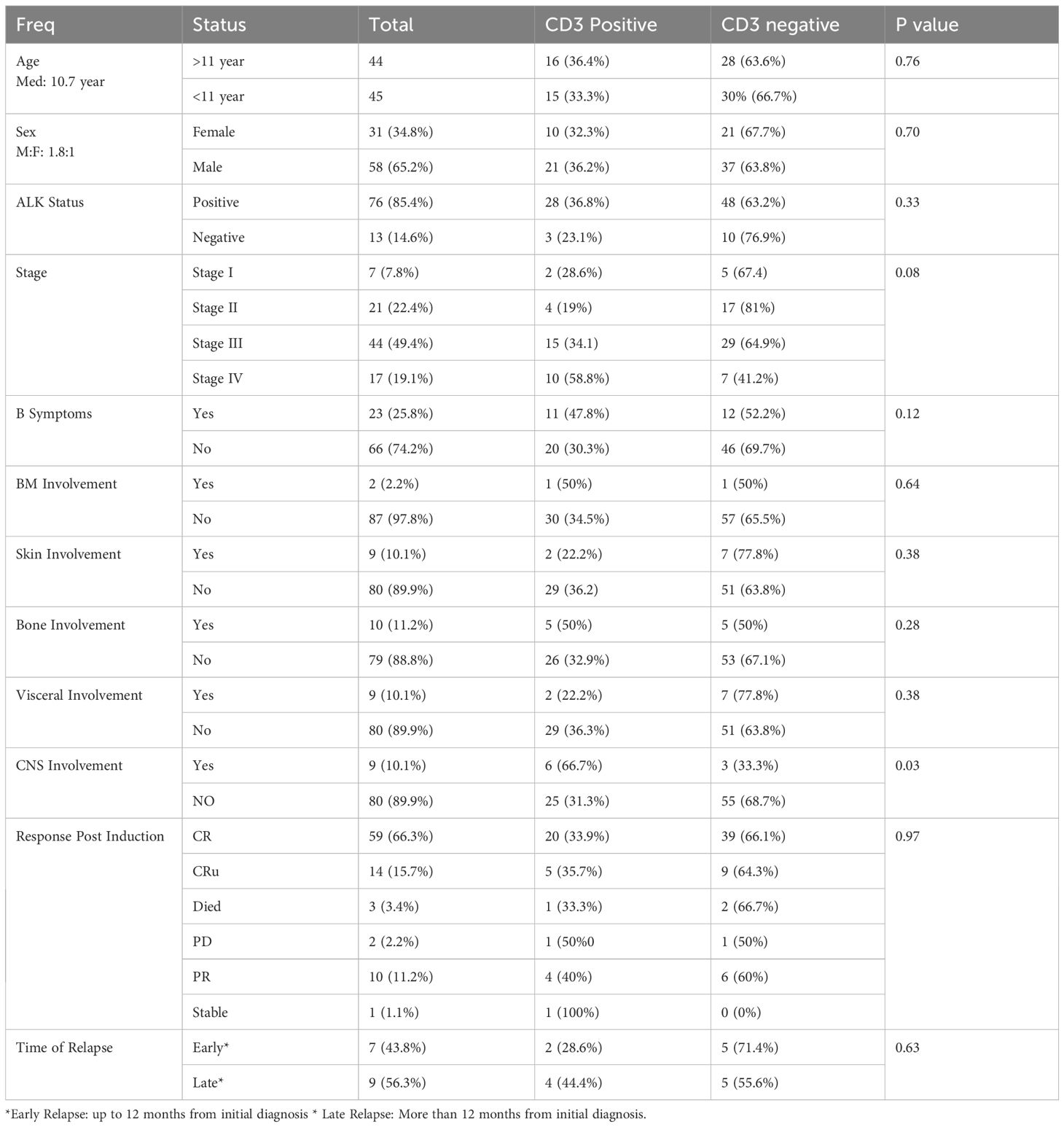
The median age of the 89 patients was 10.7 years (range: 1–18 years), with 58 (65.2%) being male and a male-to-female ratio of 1.8:1. 76 (85.4%) had ALK-positive ALCL and 13 (14.6%) had ALK-negative ALCL. 44 patients (49.4%) were stage III, whereas 17 (19.1%) were stage IV (Table 1).
B symptoms were detected in 23 individuals (25.8%) and involvement was assessed in bone marrow (BM), skin, bone, visceral organs, and central nervous system (CNS). Two patients (2.2%) had BM involvement, nine (10.1%) had skin involvement, ten (11.2%) had bone involvement, nine (10.1%) had visceral involvement, and nine (10.1%) had CNS involvement (Table 1). A univariate analysis of the association between CD 3 expression and other known prognostic factors was done.
Survival outcomes
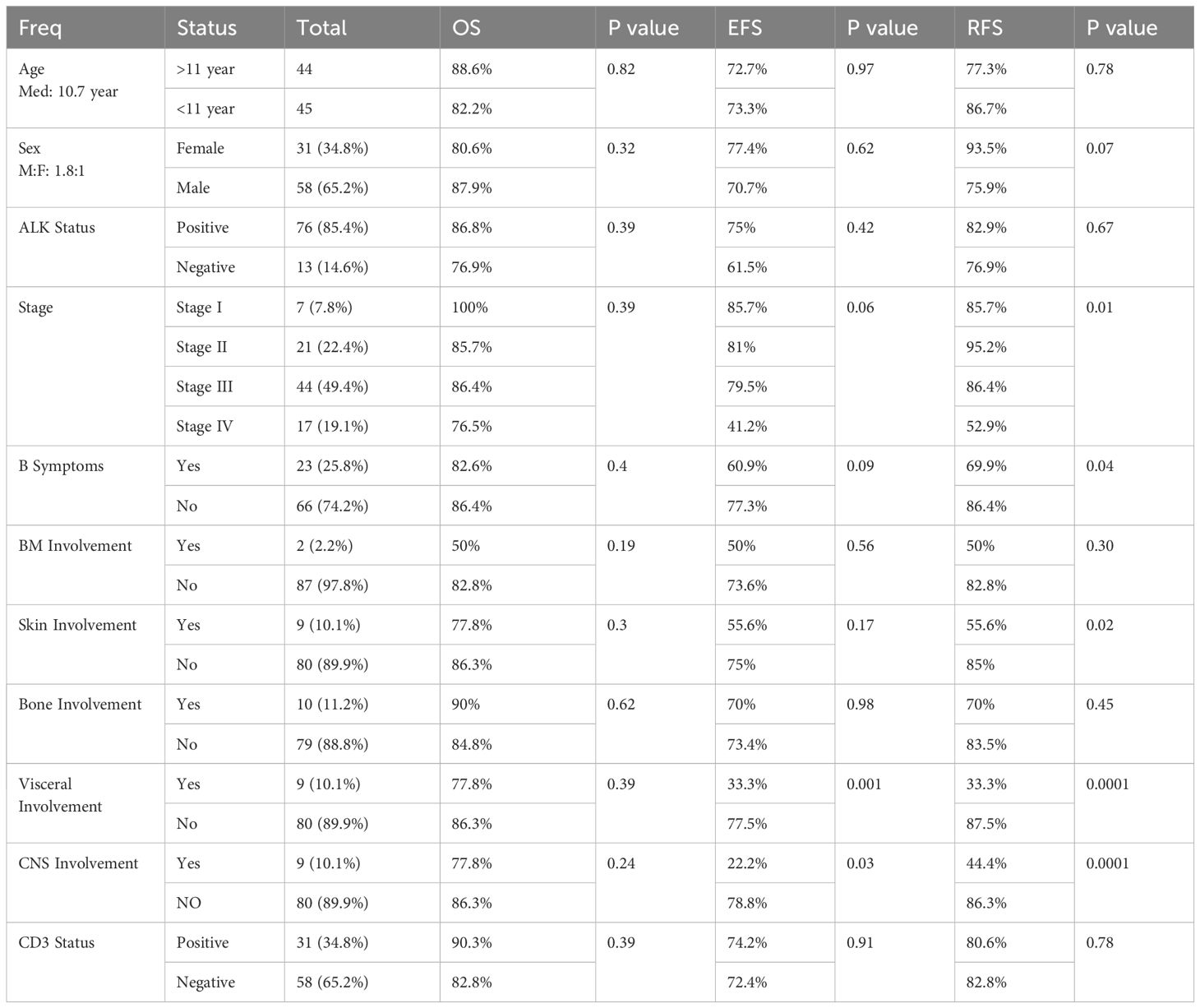
The median follow-up period was 60 months. The five-year OS, EFS, and RFS rates for the entire group were 84.3%, 73.1%, and 81.5%, respectively.
There were no significant differences in OS, EFS, or RFS based on patients’ characteristics (Table 2). However, there was a significant difference in RFS based on stage, B symptoms and skin involvement with (Table 2).
Furthermore, there was a significant difference in EFS and RFS based on visceral and CNS involvement, with lower rates in individuals with visceral or CNS involvement compared to those without (Table 2).
CD3 status
Univariant analysis showed no significant difference in OS, EFS, or RFS according to CD3 status (positive vs negative) (Table 2). There were no statistically significant association between CD3 status and other patient characteristics including age, sex, ALK status, stage, B symptoms, BM involvement, skin involvement, bone involvement, and visceral involvement. However, the relationship between CD3-positive cases and CNS involvement was statistically significant (p = 0.03).
Discussion
Survival of ALCL has been improved over the last decade. The addition of Brentuximab resulted in a 2-year event-free survival (EFS) was 79.1%. and 76.8% with the addition of Crizotinib OS was 95-97% (8, 9).
There has been a desire to identify biological risk factors to predict outcome and tailor upfront therapy. CD3 expression is one of the risk factors that has been identified although its impact has not been completely defined.
In this study, we looked at the characteristics and survival rates of 89 patients with ALCL who were treated at Children Cancer Hospital Egypt.
The median age of 10.7 years and the predominance of male gender and ALK positivity in the study are consistent with previous reports (10, 11).
The most common stage at diagnosis in our population was stage III (49.4%), followed by stage IV (19.1%). Thus the majority of our patients had advanced disease upon presentation, which could imply a delay in diagnosis or a lack of access to early detection and treatment. Previous investigations have found varying frequencies of advanced-stage ALCL, ranging from 25% to 60% (9). The prevalence of B symptoms in our sample was 25.8%, which is lower than the frequency reported in some studies (up to 50%) (11) but greater than the frequency reported in others (around 10%) (12). This may be due to discrepancies in the definition and assessment of B symptoms.
We investigated how numerous factors affected survival. We demonstrate a 5-year OS, EFS, and RFS rates for the entire group of 84.3%, 73.1%, and 81.5%, respectively. These percentages are consistent with those reported in previous investigations of pediatric and adolescent ALCL patients (10). In our study, there was no significant difference in OS, EFS, or RFS based on age, gender, ALK status, or CD3 status. These findings are in contradiction to some recent studies finding a better prognosis in younger patients (13), female patients (13), ALK-positive patients, and CD3-negative patients (14). However, some investigations have found no significant correlation between these parameters and survival outcomes (10). The disparities between studies could be attributed to changes in sample size, patient selection, treatment regimens, follow-up duration, and statistical methodologies.
There was a substantial difference in RFS depending on stage, B symptoms, and skin involvement. Patients with stage I or II disease lacking B symptoms and skin involvement had a longer RFS than those with stage III or IV disease, B symptoms, and skin involvement. These findings are consistent with prior research, which identified advanced-stage disease (11), B symptoms (9), and skin involvement (11) as poor prognostic factors.
Furthermore, we discovered a substantial variation in EFS and RFS based on visceral and CNS involvement. Patients with visceral or CNS involvement exhibited poorer EFS and RFS compared to those without. These findings are consistent with earlier research suggesting an inferior prognosis for patients with visceral or CNS involvement (11). This extranodal involvement may indicate a more aggressive biology that is less susceptible to traditional treatment.
In our study only 9 patients had CNS involvement, 6 of which had CD3 positive tumors. Prior studies have linked CD3 expression with outcome. The high incidence of CD3 positive tumors among patients with CNS involvement in our study may support these previous studies. However, the results have to be interpreted with caution because of the small number of patients.
Our study has several limitations, which should be noted. First, it is a retrospective study using data from a single center, which may restrict its generalizability and add potential biases. Second, the small sample size may limit the reproducibility of the findings. Therefore, further work in a larger cohort is needed to investigate the of CD3 positivity in relation to the occurrence of CNS involvement in pediatric ALCL.
Conclusion
This study indicates that CD3 expression may not be a major factor predicting survival in newly diagnosed pediatric ALCL. Additional research is needed to understand its association with CNS disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by CCHE-IRB, Children’s Cancer Hospital 57357- Egypt. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective study.
Author contributions
AS: Writing – review & editing, Writing – original draft. SSe: Writing – review & editing. HH: Supervision, Writing – review & editing. EN: Writing – review & editing. SSo: Validation, Writing – review & editing. IZ: Writing – review & editing. SM: Software, Writing – review & editing. LL: Supervision, Writing – review & editing. AE: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1569370/full#supplementary-material
References
1. Wright D, McKeever P, and Carter R. Childhood non-Hodgkin lymphomas in the United Kingdom: findings from the UK Children’s Cancer Study Group. J Clin Pathol. (1997) 50:128–34. doi: 10.1136/jcp.50.2.128
2. Mussolin L, Le Deley MC, Carraro E, Damm-Welk C, Attarbaschi A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: long term results of the international ALCL99 trial. Cancers (Basel). (2020) 12:2747. doi: 10.3390/cancers12102747
3. Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A children’s oncology group study. J Clin Oncol. (2017) 35:3215–21. doi: 10.1200/JCO.2017.73.4830
4. Hapgood G and Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. (2015) 126:17–25. doi: 10.1182/blood-2014-10-567461
5. Knörr F, Brugières L, Pillon M, Zimmermann M, Ruf S, Attarbaschi A, et al. Stem cell transplantation and vinblastine monotherapy for relapsed pediatric anaplastic large cell lymphoma: results of the international, prospective ALCL-relapse trial. J Clin Oncol. (2020) 38:3999–4009. doi: 10.1200/JCO.20.00157
6. Murphy SB. Childhood non-Hodgkin's lymphoma. N Engl J Med. (1978) 299(26):1446–8. doi: 10.1056/NEJM197812282992606
7. Alexander S, Kraveka JM, Weitzman S, Lowe E, Smith L, Lynch JC, et al. Advanced stage anaplastic large cell lymphoma in children and adolescents: results of ANHL0131, a randomized phase III trial of APO versus a modified regimen with vinblastine: a report from the children’s oncology group. Pediatr Blood Cancer. (2014) 61:2236–42. doi: 10.1002/pbc.25187
8. Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, et al. Brentuximab vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: results of COG trial ANHL12P1. Blood. (2021) 137:3595–603. doi: 10.1182/blood.2020009806
9. Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, et al. Crizotinib in combination with chemotherapy for pediatric patients with ALK+ Anaplastic large-cell lymphoma: the results of children’s oncology group trial ANHL12P1. J Clin Oncol. (2023) 41:2043–53. doi: 10.1200/JCO.22.00272
10. Brugières L, Le Deley MC, and Rosolen A. Impact of the ALK status on outcome of patients with systemic anaplastic large cell lymphoma treated within the European intergroup trial on childhood non-Hodgkin lymphoma (EICNHL) trials. Pediatr Blood Cancer. (2014) 61:2218–21. doi: 10.1002/pbc.25186
11. Kerry JS, Nancy LH, Julie MV, Fred U, Elaine SJ, Joseph M, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. (2008) 111:5496–504. doi: 10.1182/blood-2008-01-134270
12. Ilske O, Birgit B, Catherine C-C, Emanuel SA, Ulrika H, Konnie H, et al. Diagnosis and immunophenotype of 188 pediatric lymphomas working group of the Berlin-Frankfurt-Munster study group for pediatric lymphomas. Am J Clin Pathol. (2010) 134:822–9. doi: 10.1309/AJCPZQX7YQZVXWZL
13. Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. (2010) 116:3418–25. doi: 10.1182/blood-2010-02-270785
Keywords: CD3, survival, pediatric anaplastic large cell lymphoma, ALK, outcome
Citation: Salah A, Hafez H, Semary S, Nageb E, Soliman S, Zaky I, Shahin SM, Lehmann L and ElHaddad A (2025) Impact of CD3 expression on outcome in pediatric anaplastic large cell lymphoma. Front. Oncol. 15:1569370. doi: 10.3389/fonc.2025.1569370
Received: 03 February 2025; Accepted: 22 April 2025;
Published: 15 May 2025.
Edited by:
Jeffrey J. Pu, Upstate Medical University, United StatesReviewed by:
Zeinab Afify, The University of Utah, United StatesRussell Gollard, Optum, United States
Copyright © 2025 Salah, Hafez, Semary, Nageb, Soliman, Zaky, Shahin, Lehmann and ElHaddad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed Salah, YWhtZWQub2JlaWRANTczNTcub3Jn; Samah Semary, U2FtYWguU2VtYXJ5QDU3MzU3Lm9yZw==
†ORCID: Samah Semary, orcid.org/0000-0002-5394-3307
 Ahmed Salah
Ahmed Salah Hanafy Hafez
Hanafy Hafez Samah Semary
Samah Semary Eman Nageb4,5
Eman Nageb4,5 Shahenda M. Shahin
Shahenda M. Shahin Leslie Lehmann
Leslie Lehmann Alaa ElHaddad
Alaa ElHaddad