- 1Department of Haematology, Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, Henan, China
- 2Shandong Qilu Stem Cell Engineering Co. LTD., High-tech Development Zone, Jinan, Shandong, China
- 3Department of Haematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 4Department of Oncology, Anyang People’s Hospital, Anyang, China
Introduction: Pure red cell aplasia (PRCA) is one of the complications after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Its main pathogenesis is immune dysfunction leading to erythrocytes destruction. Currently, there is no gold standard for PRCA after allo-HSCT. Umbilical cord blood (UCB) and mesenchymal stem cells (MSCs) have been widely used in hematological and immune system diseases due to their hematopoietic reconstitution and immunomodulatory functions. However, few studies about using UCB and MSCs to treat PRCA after allo-HSCT have been reported.
Case presentation: In this report, different cell therapy regimens of UCB and MSCs were used in 3 acute myeloid leukemia (AML) patients diagnosed with PRCA after allo-HSCT. Results showed that all patients achieved significant progress without adverse reactions or complications. Furthermore, Case 1 treated with UCB combined with umbilical cord MSCs (UC-MSCs), and Case 2 treated with 3 doses of UCB mononuclear cells (UCB-MNC) achieved earlier RBC transfusion independence (2 months and 2 weeks after cell therapy, respectively) than Case 3 treated with one unit of UCB (3 months after cell therapy).
Conclusion: This report provides cell therapy strategies using UCB/UCB-MNC and UC-MSCs to treat PRCA after allo-HSCT. Our study demonstrates the safety and efficacy of 3 doses of UCB-MNC regimen and UCB combined with UC-MSCs regimen, providing a new treatment option for patients with PRCA after allo-HSCT.
Introduction
Pure red cell aplasia (PRCA) is one of the complications in allogeneic hematopoietic stem cell transplantation (allo-HSCT), especially in patients undergoing ABO blood group-incompatible HSCT, and the incidence of PRCA after allo-HSCT is approximately 7%-30% (1–4). PRCA could cause delayed recovery of erythropoiesis and many complications, such as blood transfusion dependence, iron overload, and secondary infection (5, 6).
PRCA after allo-HSCT was characterized by anemia, low reticulocyte (RET) counts (<1%) in peripheral blood for more than 60 days after allo-HSCT, and a lack of erythroid precursors in bone marrow (7). The pathogenesis is still unclear, mainly caused by the interaction between donor-derived red blood cells and residual or persistent allogenic antibodies in the recipient, anti-donor isohemagglutinins (IH) were produced in the patient, which could mediate immune abnormalities and lead to the destruction of red blood cells and erythroid precursor cells in donors (3).
There is currently no “gold standard” to treat PRCA after HSCT (8). Many therapies have been used to treat PRCA, including erythropoietin (EPO), desensitization apheresis (plasma exchange, rituximab, and etc.), immunosuppression, and donor leukocyte infusion (DLI), but the efficacy is different and unsatisfactory (2, 9, 10). With the development of cell therapy, UCB and MSCs have been increasingly used in hematological and immune system diseases due to their hematopoietic supporting and immunomodulatory functions. Moreover, UCB has the advantage of a lower probability of causing PRCA after allo-HSCT (1, 10–12). However, studies about using UCB and MSCs to treat PRCA are exceedingly infrequent, especially in PRCA after allo-HSCT (10, 13–15).
In this study, we reported three cell therapy regimens in PRCA after allo-HSCT, including UCB combined with MSCs, repeated doses of UCB-MNC, and one unit of UCB. The protocol and results would provide data support for the choice of treatment regimens for PRCA after allo-HSCT.
Case presentation
Case 1
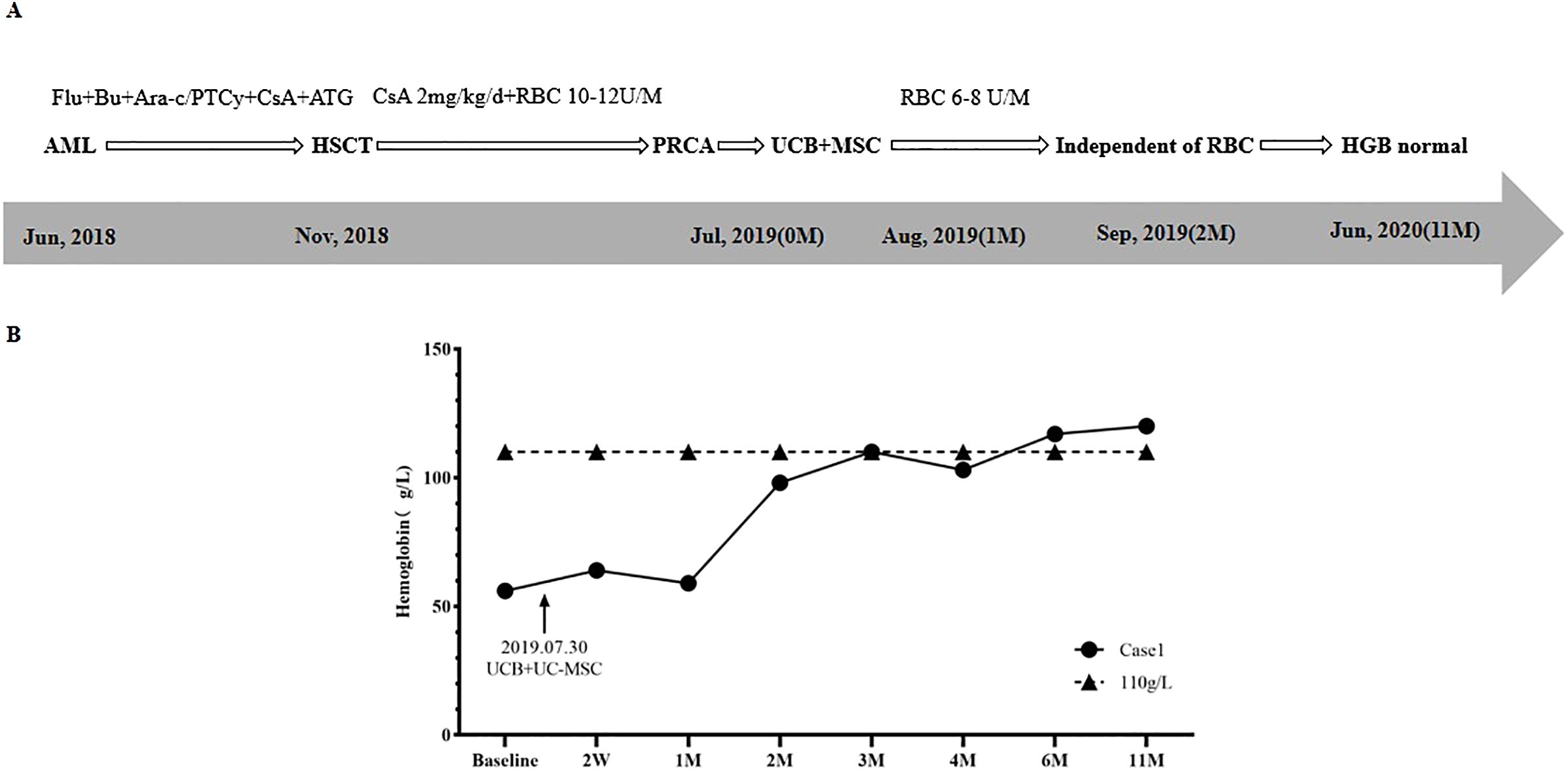
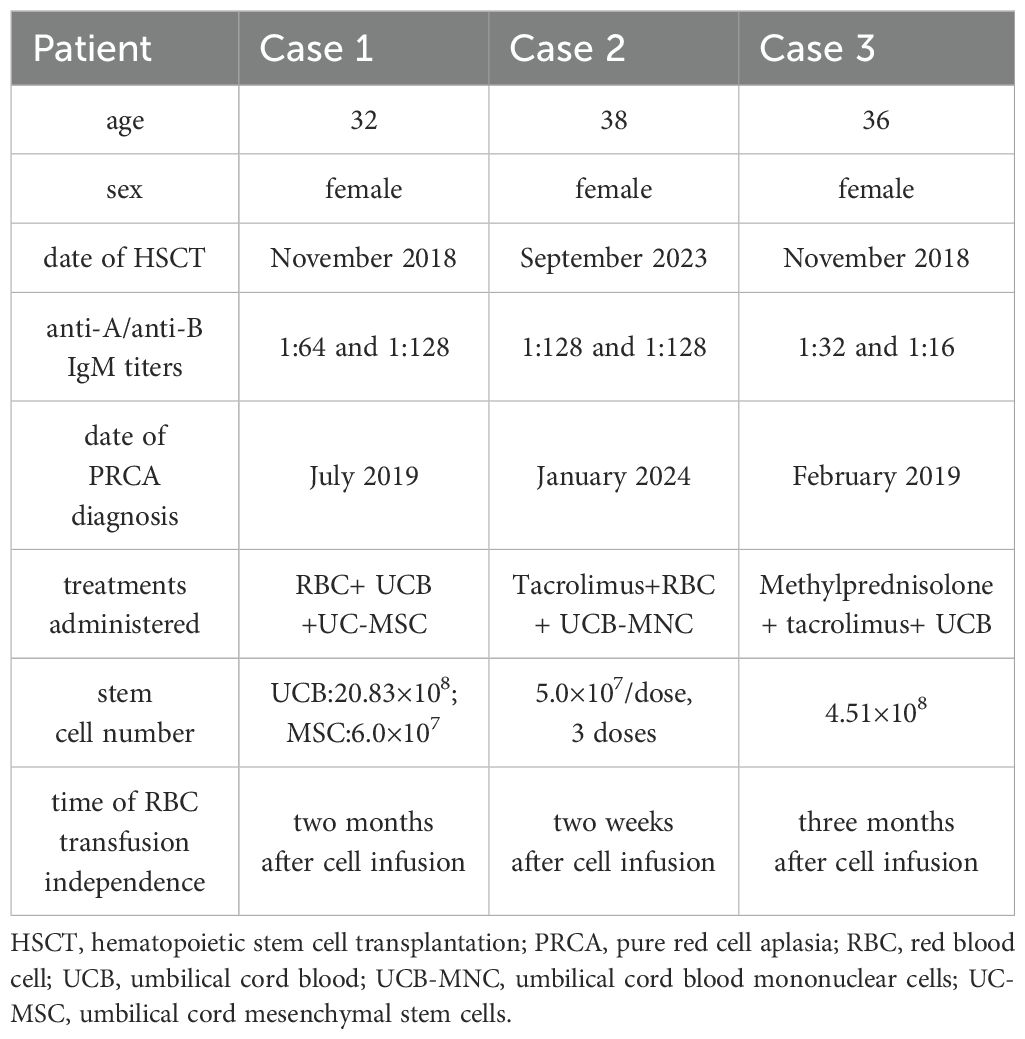
A 32-year-old female was diagnosed with acute myeloid leukemia (AML) in June 2018. As shown in Figure 1, in November 2018, the patient underwent HLA-identical sibling peripheral blood hematopoietic stem cell transplantation (HSCT). The blood types of the patient and donor were O/Rh+ and B/Rh+, respectively. Anti-A IgM and anti-B IgM titers were 1:64 and 1:128, respectively. A conditioning regimen of Fludarabine (FLU) + Busulfan (BU) + Cytosine Arabinoside (Ara-C) was used. A total of 11.56×108/Kg mononuclear cells (MNC) (including 8.9×106/Kg CD34+ cells) were infused. The Graft-versus-host disease (GvHD) prevention regimen was Posttransplant cyclophosphamide (PTCy) combined with anti-thymocyte globulin (ATG) and Cyclosporine A (CsA). On the 11th and 12th days after transplantation, the patient achieved megakaryocyte and granulocyte lineages recovery.
One month after HSCT, the patient’s physical examination (with an anemic face) and blood routine test (BRT) showed anemia, with HGB of 65 g/L and RET of 1.2×109/L (0.1%), so she received intermittent red blood cell (RBC) transfusion therapy. Results of bone marrow aspirate smear (BMA) showed that granulocyte cells and erythroid cells accounted for 88.6% and 0.0%, respectively. Short tandem repeat (STR) chimerism analysis showed that donor cells accounted for 99.23%. No infection of Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Human parvovirus B19 (HPV B19), or hemorrhagic cystitis (HC) and GvHD occurred. The patient was considered to be PRCA. Supportive therapy was taken for 6 months, including 2mg/kg/d CsA and 10U-14U/month of RBC. However, the RET remained lower than 1% (0.1%-0.2%), and the patient was dependent on RBC transfusion (HGB < 60g/L).
In July 2019, BRT showed that WBC was 2.95×109/L, HGB was 56g/L, and RET was 0.15%. BMA results displayed that granulocyte cells accounted for 80.0%, erythroid cells accounted for 0.0%, and megakaryocytes could be seen. The patient was diagnosed with PRCA after allo-HSCT.
The treatment regimen and change of HGB after the diagnosis of PRCA are displayed in Figure 1. On July 30th, 2019, one unit of UCB (with 6/10 HLA compatibility by high resolution) and 100mL UC-MSCs were infused. The UCB unit was from Shandong Qilu Stem Cell Engineering Co., LTD, of which total nucleated cells (TNC) was 20.83×108 (including 4.20×106 CD34+ cells), and UC-MSCs was 6.0×107. As shown in Figure 1B, 2 weeks (2W) after cell infusion, HGB increased to 64g/L; one month (1M) after cell infusion, HGB remained stable (59 g/L), but the RBC transfused decreased from 10U-14U/month to 6U-8U/month. Two months (2M) after cell infusion, HGB rose to 98g/L, and the patient became independent of RBC transfusion.
Four months after cell infusion, the results of BMA showed that the proliferation of nucleated cells was significantly active, with 59.6% of granulocyte cells and 6% of erythroid cells, implying the erythroid cells were gradually recovering. Six months after cell infusion, BMA results showed granulocyte cells accounted for 36.4%, and erythroid cells accounted for 43.8%, indicating erythroid cells recovered to normal. Eleven months after cell infusion, HGB returned to 120g/L. Five years after HSCT (June 2024), HGB remained normal, and the patient’s primary disease was completely relieved.
Case 2
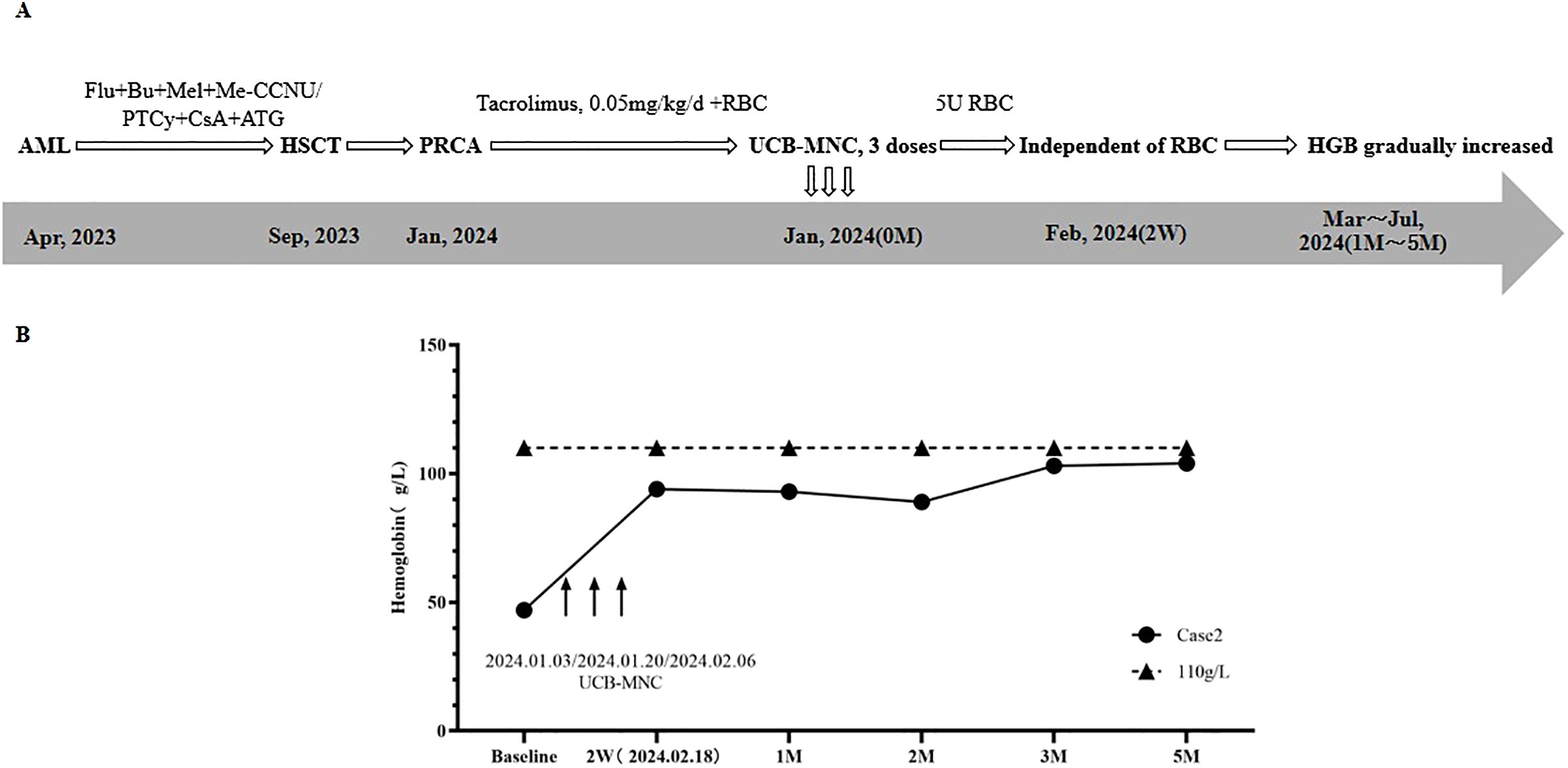
A 38-year-old female was diagnosed with AML in April 2023. Treatment regimens are shown in Figure 2A. In September 2023, HLA-identical sibling peripheral blood HSCT was performed. The patient’s blood type was O/Rh+, while the donor’s blood type was A/Rh+. Anti-A IgM and Anti-B IgM titers were both 1:128. The conditioning regimen was FLU+BU+melphalan (Mel) + Semustine (Me-CCNU), and a total of 8.28×108/Kg MNC (5.88×106/Kg CD34+ cells) were transfused. GvHD prevention regimen was PTCy combined with ATG and CsA.
Twelve days after HSCT, granulocyte and megakaryocyte lineages recovered. One month after HSCT, STR chimerism analysis showed that donor cells accounted for 98.56%. BRT showed that HGB was 52g/L, and RET was 0.8×109/L (0.1%). Supportive therapy with intermittent RBC transfusion was used to improve anemia. Two months after HSCT, RET continued to be lower than 1%, and more RBCs were transfused. Three months after HSCT, BMA results displayed that granulocyte cells and erythroid cells accounted for 91.6%, and 3.2%, respectively. No infection of EBV, CMV, HPV B19, or HC and GvHD occurred. Based on the above results of examinations, the patient was diagnosed with PRCA after allo-HSCT.
In January 2024, the patient was admitted to the hospital due to severe anemia. The treatment regimen and change of HGB after the diagnosis of PRCA are shown in Figure 2. At first, Tacrolimus (0.05mg/kg/d) and supportive RBC transfusion were used, but there was no obvious improvement in HGB, and the amount of RBC infused per month gradually increased. From January 3rd to February 6th, 2024, 3 doses of UCB-MNC were infused, with an interval of 17 days. During this period, 5U RBCs were also transfused. UCB-MNC was from Shandong Qilu Stem Cell Engineering Co., LTD, and the cell number was 5.0×107 per dose.
As shown in Figure 2B, two weeks after the last UCB-MNC infusion, the patient’s HGB rose to 94 g/L, and the patient achieved RBC transfusion independence. One month after cell infusion, HGB was stable at 93 g/L. BMA results showed that granulocyte cells and erythrocyte cells accounted for 30.8% and 60.4%, respectively. The results of following-up from 2 months to 5 months after cell infusion showed that HGB gradually increased to 104 g/L and the primary disease was completely relieved. No GvHD, or EBV and CMV infection occurred.
Case 3
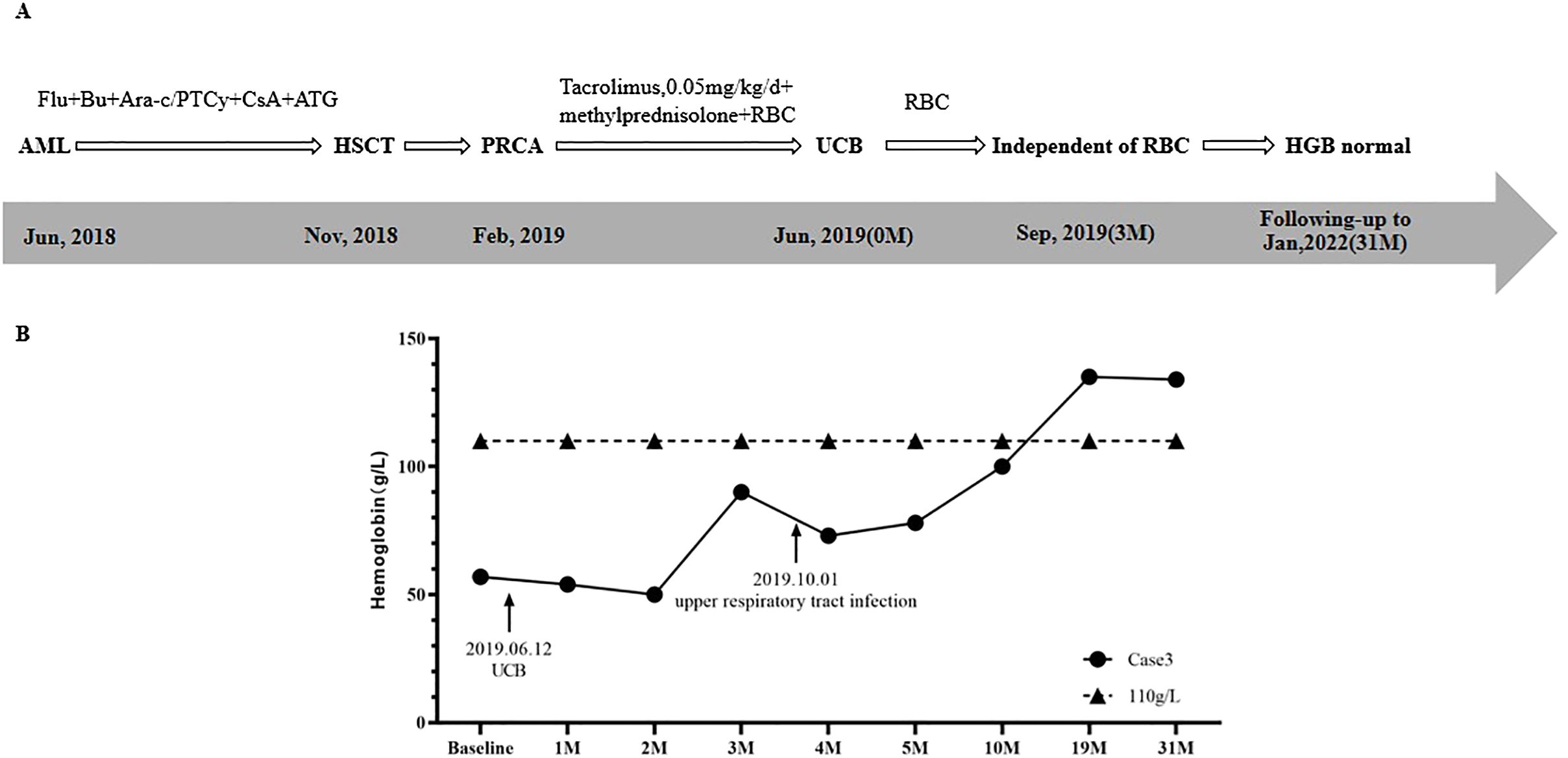
A 36-year-old female was diagnosed with AML in June 2018. Treatment regimens are shown in Figure 3A. In November 2018, HLA-identical sibling peripheral blood stem cell transplantation was performed. The patient’s blood type was O/Rh+, and the donor’s blood type was B/Rh+. The results of anti-donor IH titers of anti-A IgM and anti-B IgM were 1:32 and 1:16, respectively.
The conditioning regimen was FLU+BU+Ara-c, and a total of 26.60×108/kg MNC with 8.3×106/kg CD34+ cells were transfused. GvHD prevention regimen was PTCy combined with ATG and CsA. The granulocyte and megakaryocyte lineages were reconstructed on the 15th day and 17th day after transplantation, respectively. STR chimerism analysis showed donor cells accounted for 99.33%.
Three months after HSCT, in February 2019, the patient was admitted to hospital due to fatigue. Physical examination showed that the patient had an anemic face, and others were normal. BRT showed HGB was 49 g/L and RET was 0.3%. Results of BMA showed that granulocytes cells and erythroid cells accounted for 71.2% and 2%, respectively, while megakaryocytes were rarely found. A diagnosis of PRCA after allo-HSCT was made.
Interventions and changes of HGB are shown in Figure 3. After the diagnosis of PRCA, a supportive RBC transfusion was given. With CsA discontinued, methylprednisolone (8mg/time, 2 times/day) and tacrolimus (0.05mg/kg/day) were used to treat PRCA and prevent GvHD. However, after 4 months of treatment, the effect was not obvious. On June 12th, 2019, one unit of UCB (with 4/6 HLA compatibility by low resolution) was infused, of which TNC was 4.51×108, and CD34+ cells was 2.56×106. As shown in Figure 3B, HGBs from 1 month to 2 months after cell infusion were 54.0 g/L and 50.0 g/L, respectively. RBC transfusion was still needed to treat anemia. Three months after cell infusion, HGB recovered to 90.0 g/L, and the patient achieved RBC transfusion independence. On October 1st, the patient had upper respiratory tract infection, and HGB decreased slightly. After symptomatic treatment, HGB gradually increased. Ten months after cell infusion, HGB increased to 100.0 g/L. Nineteen months after cell infusion, HGB recovered to 135.0 g/L. BMA results showed that granulocyte cells and erythroid cells accounted for 54.2% and 25.0%, respectively. At 31 months after cell infusion, HGB was 134 g/L, and the patient’s condition continued to be stable.
Discussion
Several risk factors have been found for PRCA after HSCT, mainly including ABO blood group incompatibility (especially in blood group A donor to blood group O recipient, with IH titer higher than 1:16) and sibling donor (4, 11, 16, 17). In our study, all the patients were blood group O, with IH titers above 1:16, while the blood group of donors were 1 case of A and 2 cases of B. And all the 3 cases received sibling HSCT. In terms of risk factors for PRCA occurrence, our study is consistent with previous reports.
Regarding the treatment of PRCA after HSCT, with supportive RBC transfusion, there is a high probability of spontaneous remission after 1∼2 months or so. But beyond 2 months, other treatments would be required (3, 9, 18, 19). Immunosuppressive therapy, such as CsA is currently the first-line choice, with an effective rate of 65%-87% (19–22). For patients unresponsive to CsA, other regimens such as tacrolimus, rituximab, plasma exchange, and DLI have been reported, but the efficacy was controversial among centers (10, 12). In our study, all three cases were treated with immunosuppressive therapy and RBC transfusion after the diagnosis of PRCA, but the result was not significantly effective, patients are still dependent on RBC transfusion. So we tried new treatments of cell therapy.
To our knowledge, several studies of using UCB and MSCs to treat acquired PRCA have been reported, however, cases used in PRCA after HSCT are rare (13, 15, 23–25). Two cases were reported using MSCs derived from umbilical cord and adipose to treat PRCA after ABO-mismatched allo-HSCT, and patients reached rapid recovery without any side effects (24, 25). One study used UCB to treat 4 patients with PRCA after allo-HSCT, but the efficacy was not ideal, which might because immunosuppressive agents were not allowed from 2 weeks before to 2 months after UCB infusion (23). In our study, with conventional therapy ineffective, we explored three regimens of UCB and MSCs in combination with immunosuppressive agents and RBC transfusion to treat PRCA after HSCT, all patients achieved significant progress and RBC transfusion independence. This suggests that the combination of cell infusion can effectively improve the therapeutic effect of PRCA after HSCT when conventional treatment is ineffective.
RBC transfusion independence is a key indicator of red blood cell engraftment (8, 10), therefore, we discussed the mechanism of HSCT combined with cell therapy based on this indicator in our study and previous literatures. As shown in Table 1, the time of RBC transfusion independence was 2 weeks (Case 2, 3 doses of UCB-MNC), 2 months (Case 1, UCB combined with MSC), and 3 months (Case 3, UCB), respectively. That might related to cell source and dose, which are vital to the efficacy of cell therapy (26). In Case 1, HSCT combined with UCB and MSCs might play a synergistic role in hematopoietic supporting and immune regulation, which displayed a better effect than in Case 3. There are hematopoietic stem cells (HSC), MSCs, other progenitor cells, and immune cells in UCB, which could perform hematopoiesis support and immune regulation function (27, 28). A recent study showed that after UCB infusion in AML patients, immune functions were enhanced by promoting cell proliferation and cytokine secretion of CD8+T cells and natural killer cells and mitigating the immunosuppressive effects of CD14+ monocytes (29). MSCs play a remarkable immunomodulation role by releasing cytokines, growth factors, chemokines, and exosomes through paracrine actions, such as vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) (30, 31). MSCs could regulate the immune function by modulating the activation, expansion, and differentiation of natural killer cells, dendritic cells, B lymphocytes, macrophages, and T lymphocytes (32–34). Multiple doses of UCB-MNC infusion have been proven to enhance the effect of stem cells and improve disease outcomes (35–40). In our report, 3 doses of UCB-MNC were more effective than one unit of UCB, which conformed to previous studies.
However, our study has some limitations, which are mainly from the inherent shortcomings of small-scale sample cases and retrospective studies. A well-designed prospective research with controls and more studies are needed to confirm our results and the exact mechanism of cell therapy. This report would provide supportive data for PRCA treatment, reduce patients’ blood transfusion dependence, and improve the quality of life. Further studies on different cell combinations and doses of UCB, UCB-MNC, and MSCs are deserved to evaluate the efficacy of different treatment regimens.
Conclusion
This report provides three cell therapy strategies using UCB and UC-MSCs for the treatment of PRCA after allo-HSCT. Our study demonstrates the safety and efficacy of 3 doses of UCB-MNC regimen and UCB combined with UC-MSCs regimen, providing a new treatment option for patients with PRCA after allo-HSCT.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of He’nan Cancer Hospital (ethical review number: 2019287). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ZL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SZ: Conceptualization, Writing – original draft, Writing – review & editing. RG: Data curation, Resources, Writing – original draft. BZ: Data curation, Resources, Writing – review & editing. WZ: Data curation, Resources, Writing – original draft. JW: Data curation, Resources, Writing – original draft. YiZ: Data curation, Resources, Writing – original draft. FY: Data curation, Resources, Writing – original draft. XX: Data curation, Resources, Writing – original draft. YL: Data curation, Resources, Writing – original draft. YaZ: Data curation, Resources, Writing – original draft. BF: Supervision, Writing – original draft. FY: Data curation, Resources, Writing – original draft. HZ: Data curation, Resources, Writing – original draft. WL: Data curation, Resources, Writing – original draft. YS: Writing – review & editing. JZ: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Author SZ was employed by the company Shandong Qilu Stem Cell Engineering Co. LTD, a provider of stem cells.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu P, Wu Y, Cui D, Shi J, Yu J, Zhao Y, et al. Prevalence of pure red cell aplasia following major ABO-incompatible hematopoietic stem cell transplantation. Front Immunol. (2022) 13:829670. doi: 10.3389/fimmu.2022.829670
2. Means RT Jr. Pure red cell aplasia: The second hundred years. Am J Med Sci. (2023) 366:160–6. doi: 10.1016/j.amjms.2023.06.009
3. Worel N. ABO-mismatched allogeneic hematopoietic stem cell transplantation. Transfus Med Hemother. (2016) 43:3–12. doi: 10.1159/000441507
4. Aung FM, Lichtiger B, Bassett R, Liu P, Alousi A, Bashier Q, et al. Incidence and natural history of pure red cell aplasia in major ABO-mismatched haematopoietic cell transplantation. Br J Haematol. (2013) 160:798–805. doi: 10.1111/bjh.2013.160.issue-6
5. Bolan CD, Leitman SF, Griffith LM, Wesley RA, Procter JL, Stroncek DF, et al. Delayed donor red cell chimerism and pure red cell aplasia following major ABO-incompatible nonmyeloablative hematopoietic stem cell transplantation. Blood. (2001) 98:1687–94. doi: 10.1182/blood.V98.6.1687
6. Tomac G, Bojanić I, Mazić S, Vidović I, Raos M, Ćepulić BG, et al. Haemolysis, pure red cell aplasia and red cell antibody formation associated with major and bidirectional ABO incompatible haematopoietic stem cell transplantation. Blood Transfus. (2018) 16:397–404. doi: 10.2450/2017.0322-16
7. Helbig G, Stella-Holowiecka B, Wojnar J, Krawczyk M, Krzemien S, Wojciechowska-Sadus M, et al. Pure red-cell aplasia following major and bi-directional ABO-incompatible allogeneic stem-cell transplantation: recovery of donor-derived erythropoiesis after long-term treatment using different therapeutic strategies. Ann Hematol. (2007) 86:677–83. doi: 10.1007/s00277-007-0304-8
8. Booth GS, Savani BN, and Langston AA. Pure red blood cell aplasia: patient management pitfalls in major ABO-incompatible haematopoietic cell transplantation. Br J Haematol. (2021) 193:701–2. doi: 10.1111/bjh.v193.4
9. Mangla A and Hamad H. Pure Red Cell Aplasia. 2024 Feb 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. (2024).
10. Longval T, Galimard JE, Leprêtre AC, Suarez F, Amiranoff D, Cazaux M, et al. Treatment for pure red cell aplasia after major ABO-incompatible allogeneic stem cell transplantation: a multicentre study. Br J Haematol. (2021) 193:814–26. doi: 10.1111/bjh.v193.4
11. Wada S, Asano-Mori Y, Yamamoto H, Yuasa M, Kageyama K, Kaji D, et al. No post-transplant pure red cell aplasia development in 106 major ABO incompatible cord blood transplantation. Bone Marrow Transplant. (2019) 54:765–8. doi: 10.1038/s41409-018-0375-2
12. Metafuni E, Busnego Barreto MT, Valentini CG, Giammarco S, Limongiello MA, Sorà F, et al. Pure red cell aplasia among ABO mismatched hematopoietic stem cell transplant recipients: a 13-years retrospective study and literature review. Front Oncol. (2024) 14:1386670. doi: 10.3389/fonc.2024.1386670
13. Halkes C, de Wreede LC, Knol C, Simand C, Aljurf M, Tbakhi A, et al. Allogeneic stem cell transplantation for acquired pure red cell aplasia. Am J Hematol. (2019) 94:E294–6. doi: 10.1002/ajh.v94.11
14. Tseng SB, Lin SF, Chang CS, Liu TC, Hsiao HH, Liu YC, et al. Successful treatment of acquired pure red cell aplasia (PRCA) by allogeneic peripheral blood stem cell transplantation. Am J Hematol. (2003) 74:273–5. doi: 10.1002/ajh.10421
15. Zhu WW, Zhuang S, Yu Z, Li X, Han TJ, Ma Y, et al. Umbilical cord blood and UC-MSCs combined with low-dose immunosuppressant in the treatment of elderly patients with pure red cell aplastic: A case series. Curr Stem Cell Res Ther. (2024) 20:350–5. doi: 10.2174/011574888X290378240424075002
16. Varela Gomez R, Vázquez Vázquez G, Noriega Concepción V, Galego García A, and Andón Saavedra C. Successful treatment of pure red cell aplasia with high-dose dexamethasone after ABO-incompatible allogeneic hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. (2018) 11:44–6. doi: 10.1016/j.hemonc.2017.08.004
17. Schetelig J, Breitschaft A, Kröger N, Zabelina T, Ebell W, Bornhäuser M, et al. After major ABO-mismatched allogeneic hematopoietic progenitor cell transplantation, erythroid engraftment occurs later in patients with donor blood group A than donor blood group B. Transfusion. (2005) 45:779–87. doi: 10.1111/j.1537-2995.2005.04236.x
18. Marco-Ayala J, Gómez-Seguí I, Sanz G, and Solves P. Pure red cell aplasia after major or bidirectional ABO incompatible hematopoietic stem cell transplantation: to treat or not to treat, that is the question. Bone Marrow Transplant. (2021) 56:769–78. doi: 10.1038/s41409-020-01124-6
19. Red Blood Cell Disease Group, C.S.o.H.C.M.A. Chinese expert consensus on the diagnosis and treatment of acquired pure red cell aplasia (2020). Zhonghua Xue Ye Xue Za Zhi. (2020) 41:177–84. doi: 10.3760/cma.j.issn.0253-2727.2020.03.001
20. Gurnari C and Maciejewski JP. How I manage acquired pure red cell aplasia in adults. Blood. (2021) 137:2001–9. doi: 10.1182/blood.2021010898
21. Fu R, Zhang T, Liu B, Song J, Wang G, Li L, et al. The clinical characteristics and therapy response of patients with acquired pure red cell aplasia. Hematology. (2018) 23:639–45. doi: 10.1080/10245332.2018.1470068
23. Hu QL, Han B, He WH, Yang C, and Chen M. Allogeneic unrelated non HLA matched umbilical cord blood transfusion for refractory immune cytopenia: results of a phase I clinical trial. Zhonghua Xue Ye Xue Za Zhi. (2023) 44:431–5. doi: 10.3760/cma.j.issn.0253-2727.2023.05.014
24. Sergeevicheva V, Kruchkova I, Chernykh E, Shevela E, Kulagin A, Gilevich A, et al. Rapid Recovery from Chronic PRCA by MSC Infusion in Patient after Major ABO-Mismatched alloSCT. Case Rep Med. (2012) p:862721. doi: 10.1155/2012/862721
25. Fang B, Song Y, Li N, Li J, Han Q, and Zhao RC. Mesenchymal stem cells for the treatment of refractory pure red cell aplasia after major ABO-incompatible hematopoietic stem cell transplantation. Ann Hematol. (2009) 88:261–6. doi: 10.1007/s00277-008-0599-0
26. Yu H, Lu K, Zhu J, and Wang J. Stem cell therapy for ischemic heart diseases. Br Med Bull. (2017) 121:135–54. doi: 10.1093/bmb/ldw059
27. Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. (2005) 7:209–18. doi: 10.1080/14653240510027190
28. Mayani H. Umbilical cord blood hematopoietic cells: from biology to hematopoietic transplants and cellular therapies. Arch Med Res. (2024) 55:103042. doi: 10.1016/j.arcmed.2024.103042
29. Wang J, Li X, Liu P, Dai Y, Zhu H, Zhang Y, et al. A phase 2 pilot study of umbilical cord blood infusion as an adjuvant consolidation therapy in elderly patients with acute myeloid leukemia. Signal Transduct Target Ther. (2024) 9:358. doi: 10.1038/s41392-024-02065-y
30. Nauta AJ and Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. (2007) 110:3499–506. doi: 10.1182/blood-2007-02-069716
31. Mabotuwana NS, Rech L, Lim J, Hardy SA, Murtha LA, Rainer PP, et al. Paracrine factors released by stem cells of mesenchymal origin and their effects in cardiovascular disease: A systematic review of pre-clinical studies. Stem Cell Rev Rep. (2022) 18:2606–28. doi: 10.1007/s12015-022-10429-6
32. Cruz-Barrera M, Flórez-Zapata N, Lemus-Diaz N, Medina C, Galindo CC, González-Acero LX, et al. Integrated analysis of transcriptome and secretome from umbilical cord mesenchymal stromal cells reveal new mechanisms for the modulation of inflammation and immune activation. Front Immunol. (2020) 11:575488. doi: 10.3389/fimmu.2020.575488
33. Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, and Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. (2017) 59:87–95. doi: 10.1016/j.placenta.2017.04.003
34. Shen Z, Huang W, Liu J, Tian J, Wang S, and Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. (2021) 12:749192. doi: 10.3389/fimmu.2021.749192
35. Willing AE, Garbuzova-Davis SN, Zayko O, Derasari HM, Rawls AE, James CR, et al. Repeated administrations of human umbilical cord blood cells improve disease outcomes in a mouse model of Sanfilippo syndrome type III B. Cell Transplant. (2014) 23:1613–30. doi: 10.3727/096368914X676916
36. Penny TR, Pham Y, Sutherland AE, Mihelakis JG, Lee J, Jenkin G, et al. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. (2020) 1746:147001. doi: 10.1016/j.brainres.2020.147001
37. Darlington D, Deng J, Giunta B, Hou H, Sanberg CD, Kuzmin-Nichols N, et al. Multiple low-dose infusions of human umbilical cord blood cells improve cognitive impairments and reduce amyloid-beta-associated neuropathology in Alzheimer mice. Stem Cells Dev. (2013) 22:412–21. doi: 10.1089/scd.2012.0345
38. Garbuzova-Davis S, Rodrigues MC, Mirtyl S, Turner S, Mitha S, Sodhi J, et al. Multiple intravenous administrations of human umbilical cord blood cells benefit in a mouse model of ALS. PloS One. (2012) 7:e31254. doi: 10.1371/journal.pone.0031254
39. Yuan C, Yu G, Yang T, Li W, Ai Q, and Deng L. Enhanced therapeutic effects on acute myocardial infarction with multiple intravenous transplantation of human cord blood mononuclear cells. Int J Cardiol. (2013) 168:2767–73. doi: 10.1016/j.ijcard.2013.03.131
Keywords: pure red cell aplasia, allogeneic hematopoietic stem cell transplantation, cord blood, mesenchymal stem cells, cell therapy
Citation: Li Z, Zhuang S, Gui R, Zhang B, Zhang W, Wang J, Zu Y, Yang F, Xin X, Liu Y, Zhang Y, Fang B, Yu F, Zhao H, Li W, Song Y and Zhou J (2025) Cord blood therapy for pure red cell aplasia after allogeneic hematopoietic stem cell transplantation: case series and review. Front. Oncol. 15:1585088. doi: 10.3389/fonc.2025.1585088
Received: 28 February 2025; Accepted: 22 April 2025;
Published: 03 June 2025.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Fatin Fazrina Roslan, Cryocord Sdn Bhd, MalaysiaPeter Odutola, Harvard University, United States
Copyright © 2025 Li, Zhuang, Gui, Zhang, Zhang, Wang, Zu, Yang, Xin, Liu, Zhang, Fang, Yu, Zhao, Li, Song and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Zhou, emhvdWppYW5kb2N0b3JAMTYzLmNvbQ==
 Zhen Li
Zhen Li Sujing Zhuang
Sujing Zhuang Ruirui Gui
Ruirui Gui Binglei Zhang
Binglei Zhang Wenli Zhang
Wenli Zhang Juan Wang
Juan Wang Yingling Zu
Yingling Zu Fei Yang1,4
Fei Yang1,4 Xiangke Xin
Xiangke Xin Yanli Zhang
Yanli Zhang Fengkuan Yu
Fengkuan Yu Huifang Zhao
Huifang Zhao Wei Li
Wei Li Yongping Song
Yongping Song Jian Zhou
Jian Zhou