- Department of Hematology, Aerospace Center Hospital, Beijing, China
Background: Blinatumomab is a bispecific T-cell engager approved for the treatment of relapse/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL). Most studies evaluating blinatumomab were conducted in Caucasian populations, with limited data available in Chinese patients. This retrospective study aims to evaluate the efficacy and safety of blinatumomab in Chinese patients with R/R B-ALL.
Methods: A total of 19 patients (10 males, 9 females) with a median age of 49 years (range: 9-66) who received blinatumomab treatment at the Aerospace Center Hospital between November 2021 and November 2024 were included. Rates of complete remission (CR) and minimal residual disease (MRD) response, 1-year overall survival (OS) and relapse-free survival (RFS), and adverse events were analyzed.
Results: The median number of blinatumomab cycles administered was 1 (range: 1-6). Twelve (80.0%, 95% confidence interval [CI]: 51.9-95.7) of the 15 patients with overt marrow disease achieved CR, with 8 achieving MRD negativity. Four patients with < 5% blast but positive MRD all sustained CR and achieved MRD negativity. The overall MRD response rate was 63.2% (12/19, 95%CI: 38.4-83.7). The 1-year overall survival (OS) and relapse-free survival (RFS) rates were 64.2% ± 12.1% and 73.3% ± 11.4%, respectively. MRD responders had significantly better OS compared to MRD non-responders (log-rank test, P = 0.023). Of the 16 patients with CR, 62.5% proceeded to allogeneic hematopoietic stem cell transplantation (allo-HSCT). The most frequent adverse event was cytokine release syndrome, which occurred in 11 patients (10 with grade 1–2 and 1 with grade 3 severity).
Conclusion: Blinatumomab is both effective and well-tolerated in Chinese patients with R/R B-ALL, achieving high rates of CR and MRD negativity and facilitating more patients’ eligibility for allo-HSCT.
Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is the most common type of ALL, accounting for 70% of all cases (1–3). Most newly-diagnosed B-ALL patients achieve complete remission (CR) following induction chemotherapy (4). In pediatric patients, the 5-year overall survival (OS) rate approaches 90%, and the 10-year OS rate exceeds 85% (5). However, 15% to 20% of pediatric patients experience relapse or refractory disease (6). In contrast, adult patients have a significantly lower long-term survival rate (20%-40%), with more than half eventually relapsing or becoming refractory to chemotherapy (7, 8). Only 20% to 30% of relapse/refractory (R/R) B-ALL patients achieve CR after reinduction by conventional chemotherapy (9), resulting in a 5-year OS rate as low as 10% (10). Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative option for R/R B-ALL; however, only a small proportion of these patients are able to undergo this procedure due to failure to achieve CR after salvage chemotherapy or due to chemotherapy-related toxicities, which make them unsuitable for transplantation (11). Therefore, novel treatment strategies with high response rates and minimal toxicity will allow more R/R B-ALL patients to proceed to transplantation and improve long-term survival.
Blinatumomab, a bispecific T-cell engaging (BiTE) antibody, targets CD19+ B cells and CD3+ T cells, redirecting cytotoxic T cells to recognize and eliminate CD19+ B cells, including those in B-ALL (12, 13). A phase II, single-arm trial demonstrated that 43% of Philadelphia chromosome-negative (Ph-) R/R ALL patients treated with blinatumomab achieved a CR or CR with partial hematologic recovery (CRh) (14). A phase III, randomized controlled trial further showed that blinatumomab significantly improved CR rate (34% vs. 16%) and prolonged OS (median 7.7 months vs. 4.0 months) compared to chemotherapy (15). Additionally, 78% of B-ALL patients with minimal residual disease (MRD) positivity achieved a complete MRD response following blinatumomab treatment (16). Based on the results from clinical trials demonstrating its efficacy and safety (14–19), the Food and Drug Administration (FDA) approved blinatumomab for the treatment of Ph- R/R B-ALL in 2014, Ph+ R/R B-ALL in 2017, and MRD-positive ALL in 2018 (20).
Most studies evaluating blinatumomab have been conducted in Caucasian populations, with limited data available on its efficacy and safety in Chinese patients. This single-center, retrospective study aims to evaluate the efficacy and safety of blinatumomab in Chinese patients with R/R B-ALL.
Materials and methods
Participants
This single-center study retrospectively collected data from patients with R/R B-ALL who received blinatumomab treatment at the Department of Hematology, Aerospace Center Hospital, China between November 2021 and November 2024. B-ALL was diagnosed according to the 2016 version of the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues (21). Refractory B-ALL patients was defined by any of the following criteria: (1) failure to achieve CR after induction therapy; (2) relapse within 6 months after CR1; (3) relapse after 6 months of CR2 but failure to achieve CR following subsequent induction therapy; (4) two or more relapses; (5) extramedullary leukemia (22, 23). Relapse B-ALL was defined as reappearance of leukemic cells in peripheral blood, ≥ 5% blast in bone marrow aspirates, and/or extramedullary disease after documented CR (22, 23). Only patients who had completed at least one cycle of blinatumomab and underwent bone marrow assessment were included in the analysis. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Aerospace Center Hospital. Written informed consent was obtained from all patients or their guardians.
Blinatumomab treatment and data collection
For patients with a body weight ≥ 45 kg, blinatumomab was administered at a dose of 9 μg/day from days 1 to 7 and 28 μg/day from days 8 to 14 or 28. For patients weighting < 45 kg, the dose was adjusted according to body surface area (5 μg/m2/day from days 1 to 7, 15 μg/m2/day from days 8 to 14 or 21). Bone marrow assessments were conducted on day 8 of blinatumomab infusion and within two weeks after treatment. Demographic information, clinical, and laboratory data, as well as details of treatment and transplantation, were collected from the electronic medical records. Follow-up data were acquired through telephone contact and during each hospital visit for treatment.
Outcomes
The primary outcomes were the rates of CR and minimal residual disease (MRD) response. MRD response was defined as the conversion from MRD positivity (≥ 0.01%) to MRD negativity (< 0.01%) following blinatumomab treatment, as detected by multiparameter flow cytometry (MFC) with a sensitivity of 0.01% in bone marrow aspirates. Secondary outcomes included OS, RFS, and adverse events (AEs). OS was defined as the time interval from the start of blinatumomab infusion to death from any cause or the last follow-up. RFS was calculated from the initiation of blinatumomab to the date of relapse, death from any cause, or the last follow-up. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, and cytokine release syndrome (CRS) was graded based on the American Society for Transplantation and Cellular Therapy Consensus Grading (24).
Statistical analysis
Categorical variables are presented as counts and percentages and were compared using the chi-square test or Fisher’s exact test. Continuous variables are presented as medians with ranges. OS and RFS were estimated using the Kaplan-Meier method and compared by the log-rank test. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using STATA v16 (StataCorp, TX, USA).
Results
Patient characteristics
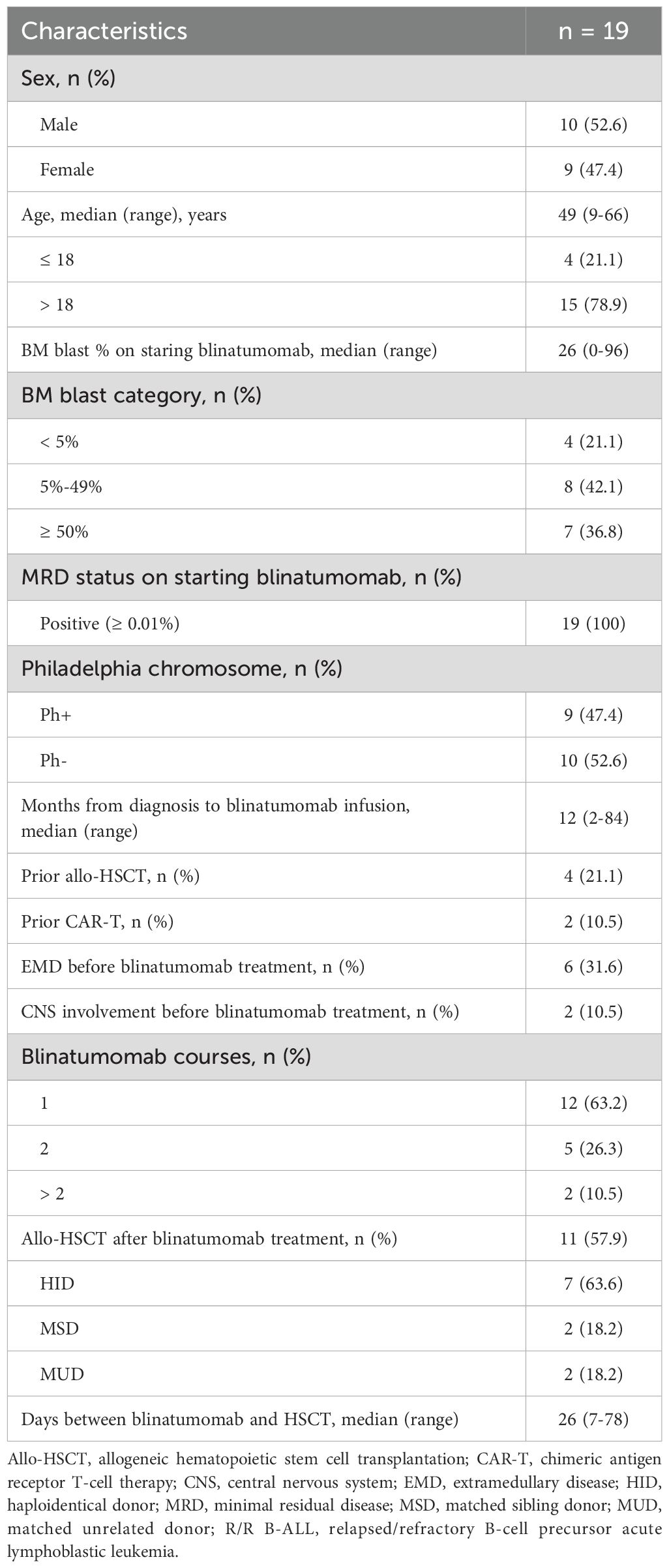
A total of 19 R/R B-ALL patients (10 males, 9 females) were included in the analysis (Table 1). The median age at the time of blinatumomab treatment was 49 years (range: 9-66). The median time from diagnosis to blinatumomab treatment was 12 months (range: 2-84). Nine patients were diagnosed with Philadelphia-positive (Ph+) B-ALL. Four patients had previously received allogeneic hematopoietic stem cell transplantation (allo-HSCT) and two had received chimeric antigen receptor T-cell therapy (CAR-T) before blinatumomab. At the start of blinatumomab infusion, four patients had < 5% bone marrow blasts, while 15 patients had overt marrow disease with ≥ 5% blast. All patients were MRD positive (≥ 0.01%). The median number of blinatumomab cycles was 1 (range: 1-6). Twelve patients (63.2%) received one cycle of blinatumomab infusion, and four (21.1%) underwent two cycles. Two patients underwent one cycle of blinatumomab re-induction followed by five cycles of consolidation.
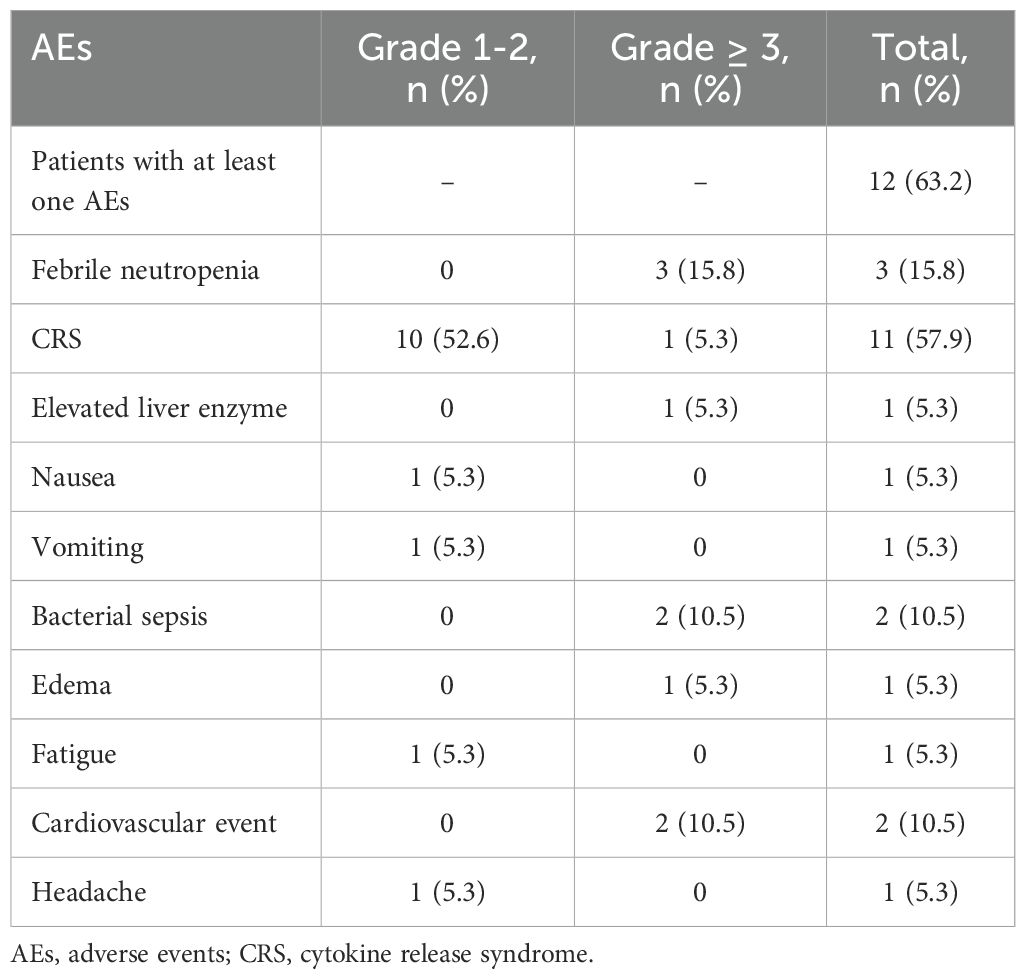
Adverse events
Twelve patients (63.2%) experienced at least one AEs during blinatumomab infusion (Table 2). The most common AEs was CRS, which occurred in 11 patients. Most cases of CRS were mild (grade 1-2), with only one patient experienced grade 3 CRS. Three patients developed grade 3 febrile neutropenia, and two experienced bacterial sepsis. Grade 3 cardiovascular events occurred in two patients (one with heart failure and one with cardiac insufficiency). One patient experienced a grade 1 headache. One patient temporarily interrupted treatment due to hepatotoxicity (grade 4 elevated liver enzymes), while another had treatment interruption due to grade 3 CRS; both resumed treatment after appropriate interventions. In one patient, the dose of blinatumomab was reduced from 28 μg/day (days 8-10) to 14 μg/day (days 11-28) due to AEs (grade 4 edema, grade 3 cardiac insufficiency). One patient discontinued treatment after 14 days of infusion due to multiple AEs, including febrile neutropenia, bacterial sepsis and heart failure.
Treatment response and post-blinatumomab treatments
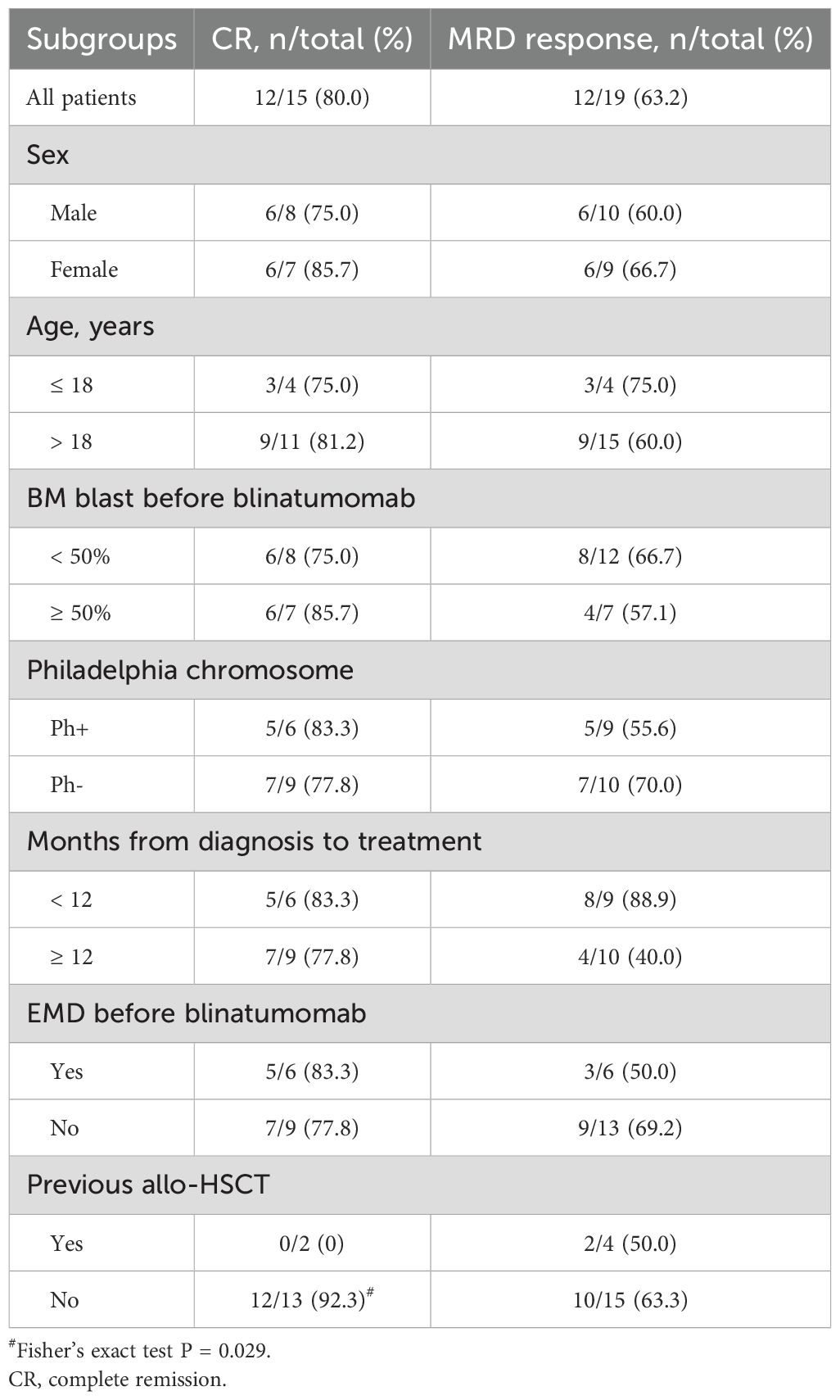
Among the four patients with < 5% blast and MRD positivity at the start of blinatumomab, all sustained CR and achieved MRD negativity after 1–2 cycles of treatment. Twelve (80.0%) of the 15 patients with ≥ 5% blast at the start achieved CR, with 8 achieving MRD negativity. The other three patients did not response to blinatumomab treatment. Therefore, the CR rate among patients with > 5% blast was 80.0% (12/15, 95% confidence interval [CI]: 51.9-95.7), and the MRD response rate of all patients was 63.2% (12/19, 95%CI: 38.4-83.7). As shown in Table 3, the rates of CR and MRD response did not significantly differ across patient subgroups based on sex (male vs. female), age (≤ 18 vs. > 18 years), BM blast percentage (< 50% vs. ≥ 50%), Philadelphia chromosome status (Ph+ vs. Ph-), time from diagnosis to treatment (< 12 months vs. ≥ 12 months), or the presence of extramedullary disease before treatment (yes vs. no). Among patients with overt marrow disease, two had received allo-HST previously but both did not obtain CR after blinatumomab treatment, whereas 12 of 13 patients without previous transplantation achieved CR after blinatumomab (Fisher’s exact test, P = 0.029). MRD response did not significantly differ between patients with or without previous transplantation.
A total of 11 patients underwent allo-HSCT during the follow-up period. The median time from blinatumomab treatment to HSCT was 26 days (range: 7-78). Of these, 9 patients with CR (7 MRD responders and 2 MRD non-responders) proceeded directly to HSCT without additional bridging therapy. One patient who was MRD non-responsive received imatinib prior to HSCT. All of these three MRD non-responders achieved MRD negativity post-transplant. One patient without MRD response who experienced early bone marrow relapse after one cycle of blinatumomab received azacitidine, ponatinib, and a CD22 monoclonal antibody before HSCT and subsequently achieved CR with MRD negativity. Two patients who did not achieve CR after blinatumomab treatment received further therapies followed by CAR-T.
Survival outcomes
At a median follow-up of 11.0 months (range: 1.7-28.5), 12 patients (63.2%) were still alive, all in CR with MRD negativity. Seven patients (36.8%) died and three patients (15.8%) experienced relapse during the follow-up period. Of the three patients who did not achieve CR after blinatumomab, two died from disease progression, and one died from severe infection during post-blinatumomab CAR-T. Another patient, who achieved CR and received allo-HSCT, also died from severe infection. One patient who experienced bone marrow and central marrow system relapse after one cycle of treatment received a second cycle of blinatumomab but eventually died from disease progression. Another patient, who had bone marrow relapse after allo-HSCT, achieved CR with MRD negativity after a second cycle of blinatumomab but later died from chronic graft-versus-host disease. One patient who had bone marrow relapse achieved CR again after multiple therapies and allo-HSCT, but later died from heart failure and renal failure.
The median OS and RFS were not reached. The 1-years OS rate for the entire cohort was 64.2% ± 12.1% (Figure 1a). There were no significant differences in OS between Ph+ and Ph- patients (P = 0.616, Figure 1b) or between patients with ≥ 50% blast vs. < 50% blast before blinatumomab treatment (P = 0.174, Figure 1c). However, MRD responders had significantly better OS compared to MRD non-responders (P = 0.023, Figure 1d). The 1-year RFS rate for patients with CR was 73.3% ± 11.4% (Figure 2a). No significant differences in RFS were observed between in Ph+ vs. Ph-, ≥ 50% blast vs. < 50% blast, or MRD responders vs. non-responders (Figures 2b–d). Among the 16 patients obtaining CR, there were no significant differences in OS (Figure 3a) or RFS (Figure 3b) between patients who did or did not undergo allo-HSCT.
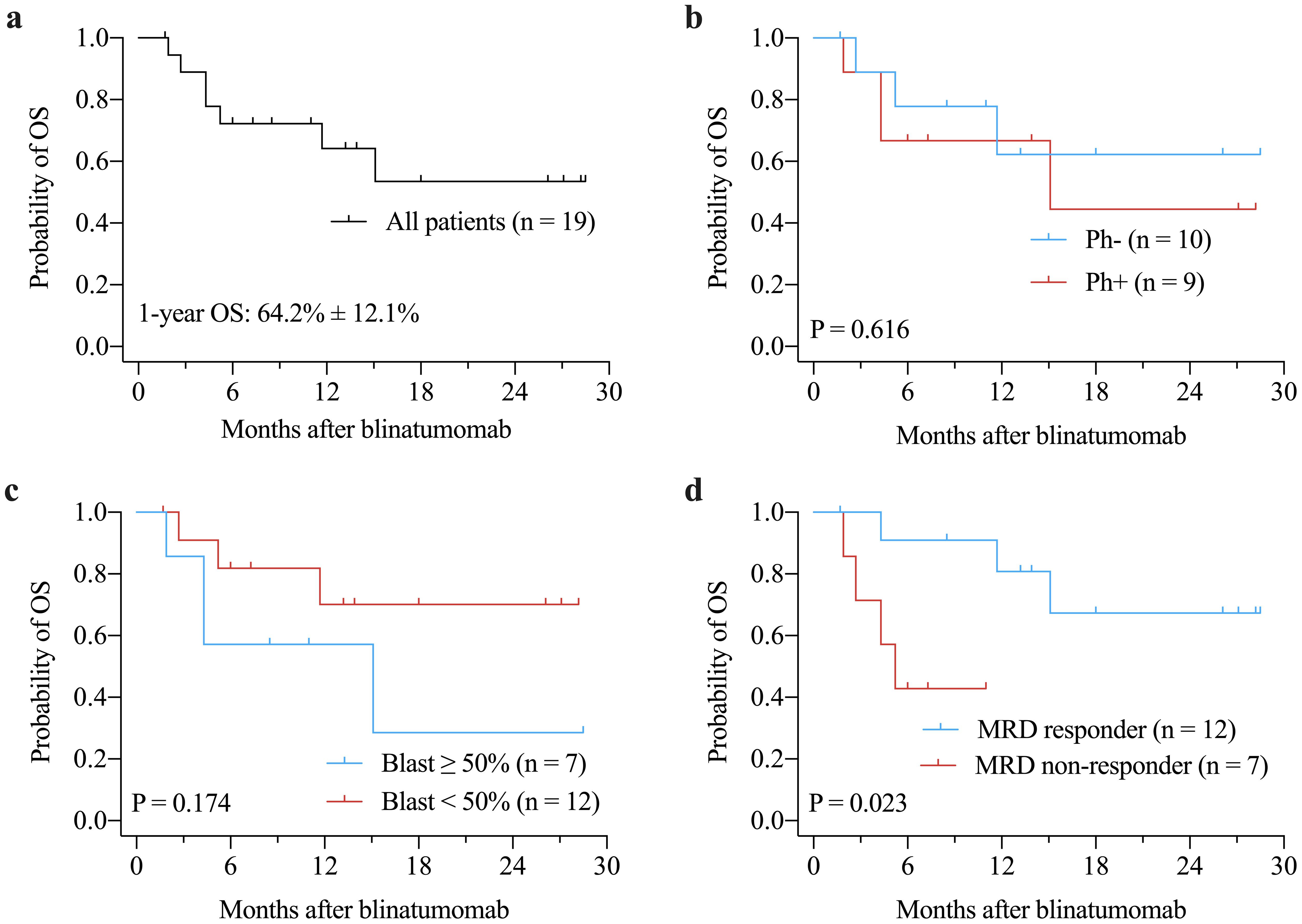
Figure 1. Kaplan-Meier curves of OS for the entire cohort (a), and for subgroups according to Philadelphia chromosome status (b), bone marrow blast prior to blinatumomab treatment (c), and MRD response (d). MRD, minimal residual disease; OS, overall survival.
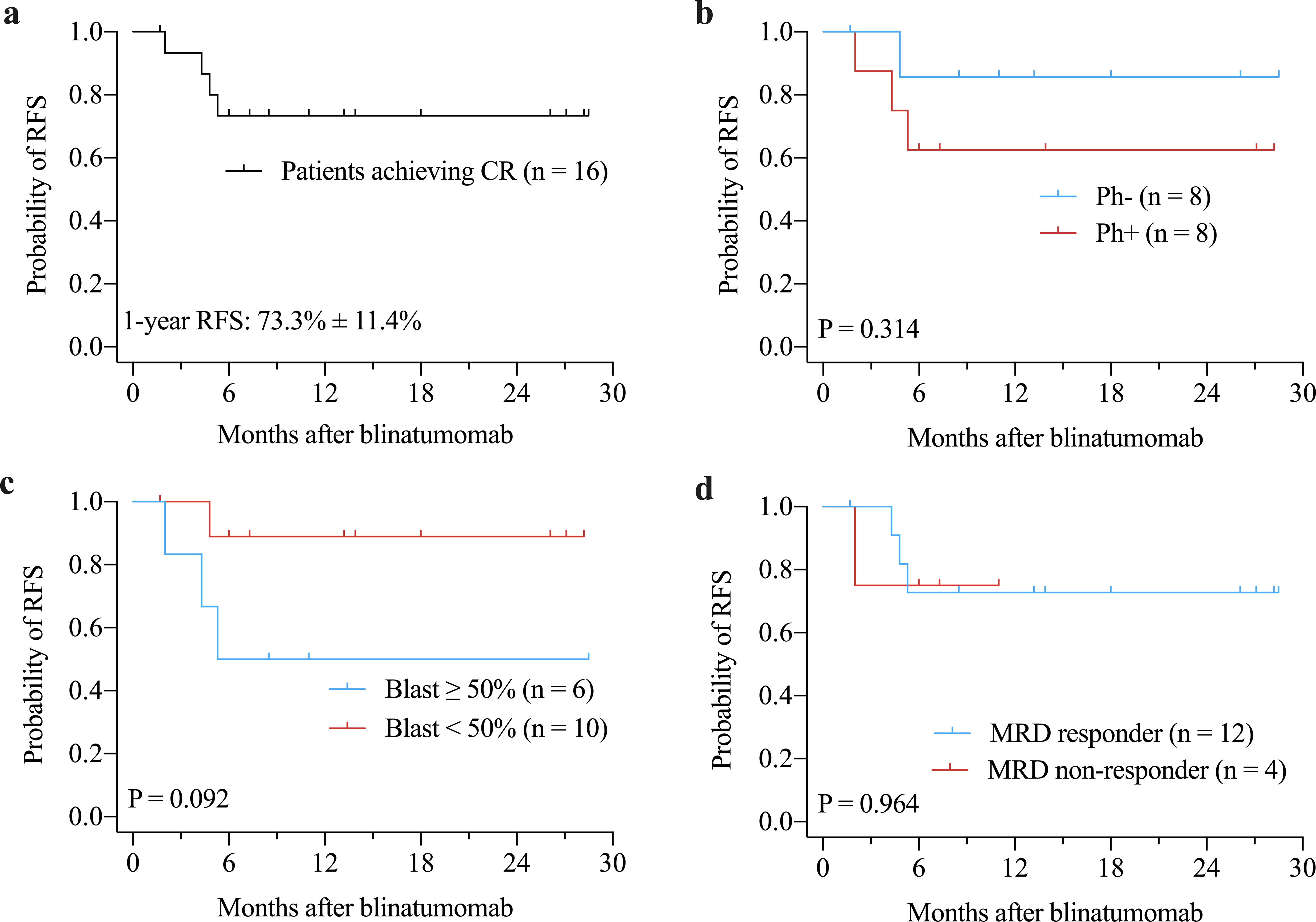
Figure 2. Kaplan-Meier curves of RFS for patients achieving CR (a), and for subgroups according to Philadelphia chromosome status (b), bone marrow blast prior to blinatumomab treatment (c), and MRD response (d). CR, complete remission; MRD, minimal residual disease; RFS, relapse-free survival.
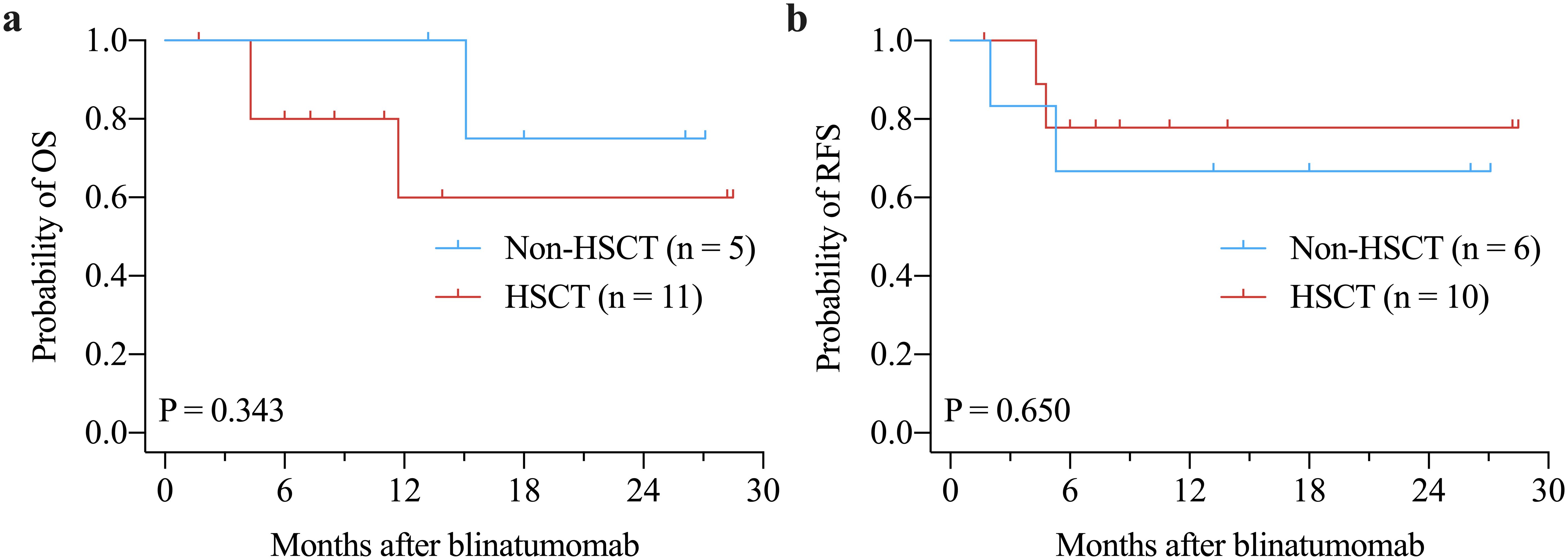
Figure 3. Kaplan-Meier curves of OS (a) and RFS (b) in patients achieving CR by allo-HSCT after blinatumomab treatment. allo-HSCT: allogeneic hematopoietic stem cell transplantation; CR: complete remission; OS: overall survival; RFS: relapse-free survival.
Discussion
Blinatumomab is the first CD19/CD3 bispecific T cell-engaging monoclonal antibody approved for the treatment of adult and pediatric patients with R/R B-ALL and MRD (25). CD19 is a surface marker of B lymphocytes and is widely expressed in leukemia and lymphoma derived from the B-cell lineage, while CD3 is a critical marker expressed on the surface of cytotoxic T lymphocytes (26). Blinatumomab facilitates the interaction between CD3 on T cells and CD19 on both benign and malignant B cells, promoting the transient cytolytic synapse formation between T cells and tumor cells (27). This process upregulates cell adhesion molecules, increases the production of proteolytic enzymes, enhances the release of inflammatory cytokines, and promotes T-cell proliferation, ultimately leading to the lysis of CD19+ cells (28, 29). Notably, normal cells lacking CD19 expression are not affected by CD19-targeted therapy (29). Due to this unique mechanism, blinatumomab has demonstrated highly efficacy with minimal toxicity.
Numerous clinical trials and real-world analyses have confirmed the efficacy and safety of blinatumomab, the majority of which were conducted in Western populations (15, 16, 30, 31). Recently, Zhou HS et al. conducted a multicenter, open-label, single-arm trial to evaluate the efficacy and safety of blinatumomab for the first time in Chinese patients with R/R B-ALL (32). The study reported a CR/CRh rate of 47.8%, a median OS of 9.2 months, and a median RFS of 4.3 months after blinatumomab treatment (32). Additionally, Zhou HF et al. performed a retrospective analysis of the treatment patterns, effectiveness, and safety of blinatumomab in Chinese patients with B-ALL in a real-word setting (33). In this study, newly-diagnosed B-ALL patients achieved a CR/CRi rate of 100% and a MRD negativity rate of 87.2%, while R/R patients who received blinatumomab re-induction achieved a CR/CRi rate of 50.0% and a MRD negativity rate of 64.2% (33). The 1-year event-free survival (EFS) rate was 90.8% for newly-diagnosed patients and 55.1% for R/R patients (33). In our study, the CR rate of 80.0% was higher than previously reported, while the MRD response rate of 63.2% that was consistent with earlier studies. This higher CR rate in our study could be explained by the small sample size that resulted in a wider confidence interval (95%CI: 51.9-95.7) and the low proportion of patients with high tumor burden. Previous trials have shown that patients with high tumor burden (≥ 50% bone marrow blast) had significantly lower CR/CRh rates compared with those with < 50% blast (14, 32). In these two trials that reported CR/CRh/CRi rates of 40-50%, over 75% of patients had ≥ 50% blast (32, 33). In contrast, only 36.8% of patients in our study had high tumor burden. Similar to our study, Shi et al. reported a CR rate of 75% in 8 patients with R/R B-ALL, of which only 4 patients had high tumor burden (34). However, our findings need to be confirmed in future studies with a larger sample size. Nonetheless, these results suggest that blinatumomab is highly effective in inducing CR and eradicating MRD in Chinese patients with R/R B-ALL.
Despite achieving a high hematological CR rate following intensive induction/consolidation chemotherapy, approximately 30% to 50% of adult and 10% to 20% of pediatric patients remain MRD-positive (35, 36). Persistent or relapsed MRD is a marker of resistance to standard chemotherapy and represents a key risk factor for relapse in ALL (37). In adult patients, the 5-year hematological relapse rate for MRD-positive individuals ranges from 56% to 100%, compared to 18% to 30% in MRD-negative patients (38). A meta-analysis of 39 studies involving 13,637 patients concluded that both pediatric and adult patients who achieved MRD negativity had significantly improved OS and EFS compared to those who did not (39). In the RIALTO trial, 52% of pediatric patients with R/R B-ALL achieved CR with MRD response within two cycles of blinatumomab, resulting in significantly prolonged OS compared to MRD non-responders (40). However, no difference in RFS between MRD responders and non-responders was observed in this study (40). Conversely, the phase I/II MT-103–205 study reported a longer median RFS in R/R patients who were MRD-negative compared to those who were MRD-positive following blinatumomab (7.0 months vs. 1.9 months) (19). A multi-center, real-world study also observed that MRD responders had superior OS and EFS compared to non-responders (31). In our study, R/R B-ALL patients who achieved MRD negativity had significantly improved OS compared to those who did not. These findings support the survival benefit of using blinatumomab to eradicate MRD in R/R B-ALL patients.
In this study, we further analyzed the survival benefit of allo-HSCT after blinatumomab. Among 16 patients who had CR after blinatumomab, no significant OS and RFS differences were found between patients who received HSCT and those who did not. However, several previous studies have reported OS benefit of allo-HSCT after blinatumomab. In RIALTO trial, there was a trend toward improved OS for patients who received allo-HSCT after blinatumomab as compared with those who did not (41). Another trial also reported longer OS in patients who received allo-HSCT compared with those who did not (42). This discrepancy may be caused by the inclusion of patients who did not response to blinatumomab treatment into the survival analysis. Patients who are not in CR after blinatumomab definitely have shorter OS and are less likely to proceed to HSCT. Therefore, the OS benefit of HSCT after blinatumomab may be overestimated due to the inclusion of more patients without CR into non-HSCT group in these trials (41, 42). This explanation is supported by the other two trials (30, 43). Jabbour et al. reported that post-blinatumomab HSCT conferred a survival advantage compared with no HSCT in the total study population (43). However, this advantage disappeared among patients who achieved CR/CRh/CRi, suggesting that the survival may be driven by response to blinatumomab but not HSCT (43). Similarly, Beneduce et al. found marginally significant OS benefit of allo-HSCT in the analysis of the overall study patients, whereas not OS benefit of allo-HSCT among patients who achieved CR after blinatumomab was observed (30). These findings suggest that blinatumomab may provide an alternative to HSCT for potential cure in some patients (43). However, these results may be influenced by confounding factors, such as small sample size, limited follow-up duration, transplant-related mortality, and donor matching. Notably, in our study, two patients who achieved CR and MRD negativity and subsequently proceeded to allo-HSCT experienced transplant-related death. Therefore, the survival benefit of post-blinatumomab HSCT, particularly in patients with CR after blinatumomab, needs further confirmation in future studies.
Conclusion
Our study demonstrates that blinatumomab is effective and well-tolerated in Chinese patients with R/R B-ALL, inducing high rates of CR and MRD negativity and allowing more patients to proceed to allo-HSCT. However, due to the retrospective nature, small sample size, and short follow-up period of this study, further validation in well-designed, prospective, randomized trials with larger sample sizes is required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Aerospace Center Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YW: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. CC: Data curation, Investigation, Writing – original draft, Writing – review & editing. ZW: Data curation, Investigation, Writing – original draft, Writing – review & editing. JZ: Data curation, Investigation, Writing – original draft, Writing – review & editing. JW: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. (2013) 381:1943–55. doi: 10.1016/S0140-6736(12)62187-4
2. Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. (2020) 395:1146–62. doi: 10.1016/S0140-6736(19)33018-1
3. Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. (2015) 33:2938–48. doi: 10.1200/JCO.2014.59.1636
4. Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. (2017) 7:e577. doi: 10.1038/bcj.2017.53
5. Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: a SEER analysis. Sci Rep. (2014) 4:4227. doi: 10.1038/srep04227
6. Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. (2013) 14:e205–17. doi: 10.1016/S1470-2045(12)70580-6
7. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
8. Sive JI, Buck G, Fielding A, Lazarus HM, Litzow MR, Luger S, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. (2012) 157:463–71. doi: 10.1111/j.1365-2141.2012.09095.x
9. Qian LR, Fu W, Shen JL. Agents for refractory/relapsed acute lymphocytic leukemia in adults. Eur Rev Med Pharmacol Sci. (2014) 18:2465–74.
10. Oriol A, Vives S, Hernández-Rivas JM, Tormo M, Heras I, Rivas C, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica. (2010) 95:589–96. doi: 10.3324/haematol.2009.014274
11. El Fakih R, Ahmed S, Alfraih F, Hanbali A. Hematopoietic cell transplantation for acute lymphoblastic leukemia in adult patients. Hematol Oncol Stem Cell Ther. (2017) 10:252–8. doi: 10.1016/j.hemonc.2017.05.015
12. Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. (2008) 321:974–7. doi: 10.1126/science.1158545
13. Löffler A, Gruen M, Wuchter C, Schriever F, Kufer P, Dreier T, et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia. (2003) 17:900–9. doi: 10.1038/sj.leu.2402890
14. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. (2015) 16:57–66. doi: 10.1016/S1470-2045(14)71170-2
15. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. (2017) 376:836–47. doi: 10.1056/NEJMoa1609783
16. Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. (2018) 131:1522–31. doi: 10.1182/blood-2017-08-798322
17. Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. (2017) 35:1795–802. doi: 10.1200/JCO.2016.69.3531
18. Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. (2012) 120:5185–7. doi: 10.1182/blood-2012-07-441030
19. von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. (2016) 34:4381–9. doi: 10.1200/JCO.2016.67.3301
20. Curran E, Stock W. Taking a “BiTE out of ALL”: blinatumomab approval for MRD-positive ALL. Blood. (2019) 133:1715–9. doi: 10.1182/blood-2018-12-852376
21. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
22. Hematology Oncology Committee CA-CA, Leukemia, Lymphoma Group CSoHCMA. Chinese guidelines for diagnosis and treatment of adult acute lymphoblastic leukemia (2021). Zhonghua Xue Ye Xue Za Zhi. (2012) 42:705–16. doi: 10.3760/cma.j.issn.0253-2727.2021.09.001
23. Stem Cell Application Group CSoHCMA, Chinese Pediatric Society CMA. Chinese expert consensus of allogeneic hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia (2022). Zhonghua Xue Ye Xue Za Zhi. (2022) 43:793–801. doi: 10.3760/cma.j.issn.0253-2727.2022.10.001
24. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
25. Ribera JM. Efficacy and safety of bispecific T-cell engager blinatumomab and the potential to improve leukemia-free survival in B-cell acute lymphoblastic leukemia. Expert Rev Hematol. (2017) 10:1057–67. doi: 10.1080/17474086.2017.1396890
26. Sigmund AM, Sahasrabudhe KD, Bhatnagar B. Evaluating blinatumomab for the treatment of relapsed/refractory ALL: design, development, and place in therapy. Blood Lymphat Cancer. (2020) 10:7–20. doi: 10.2147/BLCTT.S223894
27. Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. (2011) 317:1255–60. doi: 10.1016/j.yexcr.2011.03.010
28. Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. (2002) 100:690–7. doi: 10.1002/ijc.v100:6
29. Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. (2005) 115:98–104. doi: 10.1002/ijc.v115:1
30. Beneduce G, De Matteo A, Stellato P, Testi AM, Bertorello N, Colombini A, et al. Blinatumomab in Children and Adolescents with Relapsed/Refractory B Cell Precursor Acute Lymphoblastic Leukemia: A Real-Life Multicenter Retrospective Study in Seven AIEOP (Associazione Italiana di Ematologia e Oncologia Pediatrica) Centers. Cancers (Basel). (2022) 14:426. doi: 10.3390/cancers14020426
31. Leotta S, Markovic U, Duminuco A, Mulè A, Porretto F, Federico V, et al. Impact of minimal residual disease response and of status of disease on survival after blinatumomab in B-cell acute lymphoblastic leukemia: results from a real-life study. Ann Hematol. (2024) 103:3701–312. doi: 10.1007/s00277-024-05725-9
32. Zhou H, Yin Q, Jin J, Liu T, Cai Z, Jiang B, et al. Efficacy and safety of blinatumomab in Chinese adults with Ph-negative relapsed/refractory B-cell precursor acute lymphoblastic leukemia: A multicenter open-label single-arm China registrational study. Hematology. (2022) 27:917–27. doi: 10.1080/16078454.2022.2111992
33. Zhou H, Wu X, Yang Z, Lu S, Zhang X, Yang X, et al. Real-world evidence on treatment pattern, effectiveness, and safety of blinatumomab in Chinese patients with B-cell acute lymphoblastic leukemia. Invest New Drugs. (2024) 42:299–308. doi: 10.1007/s10637-024-01435-1
34. Shi Y, Han Y, Wang Y, Mao D, Zhang J, Xi R, et al. Analysis on the clinical efficacy and adverse reactions of blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi. (2023) 44:516–9. doi: 10.1016/j.bbmt.2018.12.758
35. Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. (2009) 113:4153–62. doi: 10.1182/blood-2008-11-185132
36. Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. (2012) 120:1868–76. doi: 10.1182/blood-2011-09-377713
37. Apel A, Ofran Y, Wolach O, Shimony S, Ram R, Levi I, et al. Safety and efficacy of blinatumomab: a real world data. Ann Hematol. (2020) 99:835–58. doi: 10.1007/s00277-019-03854-0
38. Beldjord K, Chevret S, Asnafi V, Huguet F, Boulland ML, Leguay T, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. (2014) 123:3739–49. doi: 10.1182/blood-2014-01-547695
39. Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol. (2017) 3:e170580. doi: 10.1001/jamaoncol.2017.0580
40. Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, et al. Blinatumomab in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia: RIALTO expanded access study final analysis. Blood Adv. (2022) 6:1004–14. doi: 10.1182/bloodadvances.2021005579
41. Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. (2020) 10:77. doi: 10.1038/s41408-020-00342-x
42. Gore L, Locatelli F, Zugmaier G, Handgretinger R, O’Brien MM, Bader P, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J. (2018) 8:80. doi: 10.1038/s41408-018-0117-0
Keywords: acute lymphoblastic leukemia, relapsed/refractory, blinatumomab, complete remission, minimal residual disease
Citation: Wang Y, Chang C, Wang Z, Zhao J and Wang J (2025) Efficacy and safety of blinatumomab in Chinese patients with relapsed/refractory B-cell acute lymphoblastic leukemia: a single-center retrospective study. Front. Oncol. 15:1587185. doi: 10.3389/fonc.2025.1587185
Received: 04 March 2025; Accepted: 22 April 2025;
Published: 12 May 2025.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Miao Miao, The First Affiliated Hospital of Soochow University, ChinaMichaela Seng, SingHealth, Singapore
Copyright © 2025 Wang, Chang, Wang, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingbo Wang, d2FuZ2ppbmdib0Bhc2NoLm5ldC5jbg==
 Yan Wang
Yan Wang Chun Chang
Chun Chang Jingbo Wang
Jingbo Wang