Abstract
Breast cancer has been the most frequent diagnosed cancer and the leading cause of cancer-related deaths among women worldwide, mainly due to delayed detection. Early diagnosis significantly improves prognosis and long-term survival rates. Various techniques, including imaging, sensors, and molecular biotechnology, have been developed to facilitate early detection. This review provides a comprehensive analysis of these diagnostic techniques, emphasizing precision, patient comfort, and cost-effectiveness. Additionally, it explores the emerging role of wearable technologies, such as smart bras and real-time monitoring devices, in revolutionizing breast cancer detection. The review concludes by discussing the limitations of current diagnostic methods and proposing future directions for enhancing early detection and improving patient outcomes.
1 Introduction
Breast cancer is the most diagnosed malignancy and one of the leading causes of cancer-related deaths among women worldwide (1). Breast cancer occurs when normal breast cells undergo genetic mutations, leading to uncontrolled growth, known as neoplasia. If not detected early, cancerous cells can invade surrounding tissues and spread to distant organs, complicating treatment and increasing the risk of fatality. To improve the prognosis and survival rates of breast cancer, early detection is essential. Early detection of breast cancer greatly improves long-term results by increasing the likelihood of successful treatment and breast-conserving surgery. Due to localized disease management and less aggressive treatment requirements, studies have demonstrated that patients diagnosed at earlier stages have higher survival rates than those diagnosed at advanced stages (1, 2). By reducing the need for extensive therapies like chemotherapy and radiation, early detection also lessens the financial and emotional burden on patients (2).This emphasizes how crucial it is to keep working to improve breast cancer screening tools and encourage routine examinations for early detection. Breast cancer is a significant global health concern, with rising incidence and mortality rates. From 2008 to 2017, new cases increased by 6%, reaching 11.7% in 2020, when approximately 685,000 women died, and 2.3 million new cases were reported. Early detection greatly enhances survival rates, with nearly 90% survival for early-stage diagnoses (2, 3). Given global population growth, experts estimate that by 2050, the number of new breast cancer cases will rise to approximately 3.2 million annually (4). Notably, breast cancer is increasingly affecting younger populations, raising concerns about its detection and management. Several risk factors, such as age, family history, lifestyle, unregulated use of medications like oral contraceptive pills (OCPs), and more, contribute to this trend. Recent studies indicate that prolonged use of hormone replacement therapy (HRT) for over 5–7 years increases breast cancer risk (3, 4). Similarly, Wang et al. presented that elderly woman, with higher BMI, have increased cancer risk compared to those with lower BMI (5). Additionally, alcohol can also increase the risk of estrogen-positive breast cancers (6).
Breast cancer poses a significant challenge due to the lack of early symptoms, often resulting in late-stage diagnoses. Factors like limited awareness, inadequate healthcare access, and infrequent screenings contribute to this issue. Early and accurate diagnosis is vital for survival. While traditional screening methods, such as mammography and clinical breast examinations, remain standard, they have limitations, including high false-positive rates and lower sensitivity for women with dense breast tissue. Breast cancer rates are notably higher in premenopausal women. While it is rare in those under 40, it has recently raised concerns. Due to the density of their breast tissue, premenopausal women are usually not included in screening programs or recommended to have mammograms (5–8).
Figure 1 presents a year-by-year analysis of research publications on breast cancer available in PubMed. Breast cancer in men is rare, accounting for less than 1% of all breast cancers worldwide and approximately 1% of all malignancies in men (9, 10). The most affected region in male breast cancer (MBC) is the nipple/areola area (11–15). Due to its rarity, early diagnosis remains a challenge, and there are limited therapeutic strategies and awareness programs specifically targeted at MBC. Consequently, treatment options for male breast cancer remain suboptimal (16). Male breast cancer (MBC) has distinct biological differences compared to female breast cancer. MBC is almost always hormone receptor-positive (HR+) and often associated with BRCA2 germline mutations, which increase the risk of aggressive breast cancer in men (17). Additionally, germline pathogenic variants (PVs) in the BRCA1/2 genes have been linked to an elevated risk of BC in both men and women. Multigene panel testing is increasingly used to assess breast cancer risk, allowing for the detection of pathogenic variants beyond BRCA1/2 (18).
Figure 1
Figure 2 shows the advances in breast cancer diagnosis: Figure 2A represents the basic process of the liquid biopsy procedure; 2B shows the sensors and embedded devices developed for the early detection of breast cancer, year by year and finally, 2C shows the flow process of breast cancer detection using AI. Mammography and clinical breast examination are the two most frequently used methods for breast screening (7, 8). To obtain a tissue sample for further histopathological diagnosis, a needle biopsy is essential. There are three methods of needle biopsy: vacuum-assisted breast biopsy (VABB), core needle biopsy (CNB), and fine-needle aspiration cytology (FNAC) (19). Although mammography is considered as the gold standard for diagnosing breast cancer, it has several limitations, including high false positive rates, limited effectiveness in cases of dense breasts, and the use of ionizing radiation (20). To address this issue, the integration of artificial intelligence (AI) with mammography is crucial for screening purposes. With thorough study and testing, these AI systems could potentially take over the role of radiologists in reading mammograms. However, adequate preparation and high-quality data are necessary for AI systems to function effectively. AI can be incorporated into regular screening procedures with the right investigation and validation (21). The proposed study explores screening methods that can help detect this deadly disease with minimal harm. Research on screening techniques should aim to reduce the number of undetected advanced cancers, as well as unnecessary biopsies and follow-up procedures. When discussing ultrasound, it has a sensitivity of 80%, but it is not suitable for imaging bony structures (9, 22). Thermography can be used to detect breast cancer in its early stages, potentially reducing the need for unnecessary biopsies in breast cancer screening (10, 11). However, a drawback of thermography is its inability to identify the specific cause of an increase in breast temperature. This is because mastitis, an inflammation of the breast tissue, can also lead to an increase in breast temperature. The risk of developing breast cancer increases by 2% for each x-ray exposure (12). Many publications are available for diagnosing BC, but few specify which approach is best for a certain subset of BC patients (13).
Figure 2
This review provides an in-depth analysis of the current and emerging breast cancer diagnostic techniques, with a focus on their advantages, limitations, and potential for improving early detection. We discuss various imaging modalities, including reflective optical imaging, microwave imaging, and ultrasound, as well as sensor-based detection techniques such as thermography, piezoresistive, near-infrared, and bioimpedance spectroscopy-based sensors. Additionally, we explore biosensor technologies, including optical biosensors (colorimetric, fluorescence, surface plasmon resonance imaging [SPRi], surface-enhanced Raman spectroscopy [SERS], and electrochemiluminescence [ECL] biosensors), electrochemical biosensors (field-effect transistor [FET], electrochemical impedance spectroscopy [EIS], and voltametric techniques), and other biosensors such as quartz crystal microbalance (QCM) and photoelectrochemical (PEC) biosensors. The review concludes by discussing the limitations of existing diagnostic techniques and potential future directions for improving breast cancer detection, with an emphasis on precision, accessibility, and patient-centric care.
2 Materials and methods
2.1 Goal of the review
The primary goal of this study is to enhance the understanding of breast cancer diagnosis by evaluating various early detection techniques, including biomarkers, biosensors, artificial intelligence (AI), sensors, and imaging technologies. This review explores the role of tumor markers, their detection methods, the advancements in biosensor technology, and the application of AI in improving diagnostic accuracy.
Our analysis provides a comprehensive assessment of past and emerging diagnostic approaches, emphasizing recent developments and breakthroughs in biosensors, AI, imaging, and sensor-based technologies. We present a balanced evaluation of each technique, discussing its advantages and limitations. Special attention is given to sensor-based methods, which offer affordable, accessible, and non-invasive breast cancer screening solutions. Additionally, figures and graphical illustrations are incorporated throughout the review to visually represent key findings and the relationships between different diagnostic methodologies.
2.2 Data sources
A systematic search strategy was employed to identify relevant research articles published between 2000 and 2024, with a primary focus on studies from 2015 to 2024. The databases searched included: PubMed; Springer; IEEE Xplore; ScienceDirect; Gray Literature, including Google Scholar. To ensure a comprehensive literature review, keyword searches were performed using the following search terms: “Early breast cancer detection”, “Early breast cancer detection through biomarkers”. “Early detection of breast cancer using sensors”, “Breast cancer screening techniques”, “Artificial intelligence techniques for early breast cancer detection”. The wildcard symbol (*) was used to retrieve variations of keywords, and Boolean operators (“AND” “OR,” “NOT”) were applied to refine the search.
2.3 Inclusion and exclusion criteria
Only peer-reviewed articles published in English were included.
Articles with similar findings and methodologies were excluded to avoid redundancy.
Non-English publications and studies without full-text access were omitted.
From an initial pool of 30,991 research articles retrieved from PubMed, filtering for free full-text availability and relevance to biosensor-based detection resulted in 98 selected papers. Similarly, filtering for sensor-based detection identified 94 studies, and the same method was applied for AI and imaging-based detection approaches.
Applying the same search strategy on Google Scholar, we identified a total of 238 eligible papers, all of which were available in PDF or free-text format and aligned with the scope of this review. The distribution of the shortlisted papers on early breast cancer detection from PubMed, IEEE, MDPI, ScienceDirect, and other databases in Figure 3.
Figure 3
2.3.1 Biomarkers
2.3.1.1 Tumor marker
A group of active compounds, called tumor markers, are formed when the body tissue or tumor interacts with them. These molecules can indicate the presence and progression of a tumor. Various factors like tumor size, mass, expression level, breakdown, excretion rates, blood supply, and resistance to medication can affect the concentration of tumor markers at different stages. Examples of commonly used indicators for breast tumors include human epidermal growth factor receptor 2 (HER2), progesterone, and estrogen receptor. Additionally, ongoing research is exploring the role and potential of newly developed biomarkers in the detection and management of breast cancer (14, 15). Table 1 explains the Overview of tumor markers used in breast cancer detection.
Table 1
| Tumor marker | How it works | Pictorial representation | Reference |
|---|---|---|---|
| Estrogen Receptors | Estrogen receptor (ER) binding in cells is essential for diagnosing metastatic breast cancer, prognosing, and assessing endocrine therapy suitability. A positive ER result indicates effective treatment, while a negative result does not. | 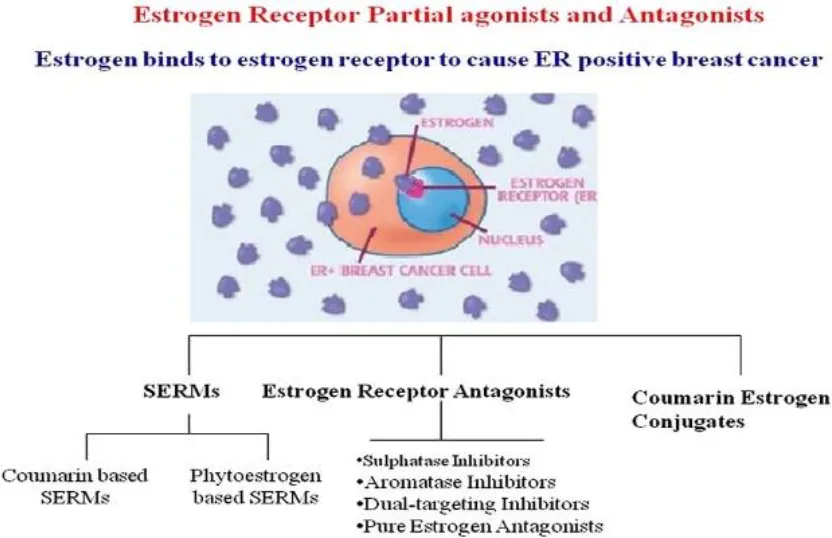 | (38) |
| Progesterone Receptors | PR, a hormone receptor, signals ER activity, affecting cellular gene regulation. PR-positive breast cancer patients account for 65-70%, necessitating re-detection to assess prognosis. |  | (39) |
| The Biomarker of Triple Negative Cancer (TNBC) | NBC, a subtype of breast cancer, has poor prognosis and lower survival rates, with biomarker surveys identifying VEGF as a marker for targeted therapy. |  | (40) |
| Human epidermal growth factor Receptor 2 | HER2 gene promotes tumor growth, affecting prognosis. HER2-targeted therapy is used in HER2-positive patients, with HER2 levels negatively correlated with ER and PR levels. | 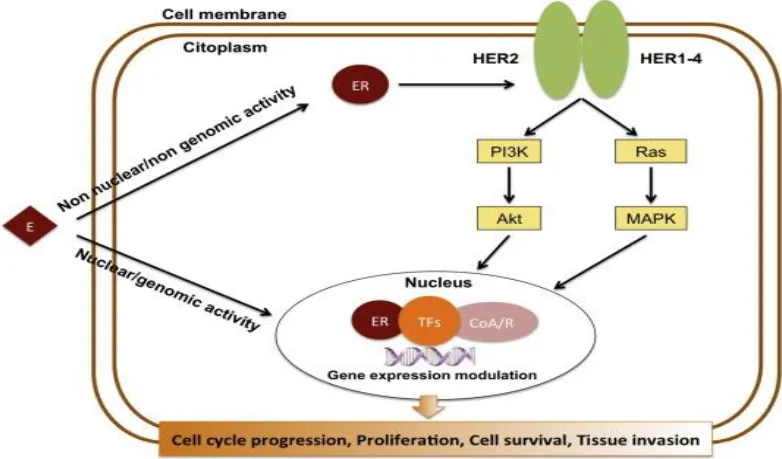 | (41) |
| Emerging Tumor Marker | Researchers are exploring new tumor indicators, including proteins, nucleic acids, cancer cells, and other types of cells, in addition to the three common clinical breast cancer markers. |  | (42) |
Overview of tumor markers used in breast cancer detection.
When determining if an organism or pathogenic process is normal or if therapeutic intervention is necessary, a biomarker provides an objective measurement (16). Stated differently, biomarkers are chemical indications of disease status that help distinguish between a normal tumor and a cancerous one (17, 18). Therefore, biomarkers provide insights into the onset and progression of cancer in the body. Body fluids such as blood, urine, and saliva can be used as analytes in sensor development because they contain biomarkers (23–25).
2.3.1.1.1 Estrogen receptor
A protein molecule called the estrogen receptor (ER) binds to estrogen in cells only (26). Cytoplasm, nucleus, or cell membrane can all have estrogen receptors. The conventional nuclear receptor is found in the nucleus, and following translation, its protein is momentarily translated into the cytoplasm, where it is detectable (27). As estrogen diffuses into the nucleus, it attaches to its nuclear receptor, activating a mechanism that controls gene regulation and the transcription of genes downstream. Estrogen receptor detection serves as a diagnostic tool for metastatic breast cancer, helps in prognostication, and assesses if a patient is appropriate for endocrine therapy. It does this by binding to the estrogen receptor in the patient. Consequently, if a patient has been shown to have estrogen receptors, this indicates that the patient may be a good candidate for endocrine therapy. For individuals who test positive for ER, endocrine treatment is an effective way to stop tumor progression (28). Patients who test negative for ER cannot benefit from the same treatment. Unmistakable data suggests that endocrine therapy is not beneficial for patients whose tumors do not express ER (29).
Upon estrogen binding (E2) or phosphorylation (P) by cellular kinases following growth factor (GF) receptor stimulation, ERα is activated and translocates into the nucleus. Once there, ERα can bind DNA directly or indirectly through estrogen-responsive elements (EREs) or by binding to other transcription factors such as AP1 or SP1, which bind DNA through serum-responsive elements (SREs). This genomic action of ERα regulates the transcription of target genes. Additionally, ERα can be anchored to the membrane and interact with G proteins (Ga) or GF receptors, leading to non-genomic activity such as the production of second messengers (cyclic adenosine monophosphate, cAMP) and stimulation of signaling pathways involving PI3K/AKT or Ras/MAPK. This non-genomic activity eventually leads to the activation of transcription factors (TFs) involved in the regulation of cell proliferation and survival (30).
2.3.1.1.2 Progesterone receptor
The progesterone receptor (PR) is a hormone receptor, like the ER. ER activates PR, and PR activation is a signal of ER activity (31). The interaction between PR and chromatin changes the binding position of ER and chromatin and then leads to a change in cellular gene regulation from proliferation to cell cycle arrest, apoptosis, and differentiation (27). PR-positive patients account for approximately 65–70% of breast cancer patients, and PR-positive patients are rarely concurrently ER-negative (31). Therefore, in strongly PR-positive and ER-negative patients, re-detection of ER is necessary to exclude the possibility of a false-negative result (28, 32). The main purpose of PR detection is to assess the prognosis of ER-positive patients (31).
2.3.1.1.3 Human epidermal growth factor receptor 2
The human epidermal growth factor receptor 2 (HER2) gene is one of the most studied breast cancer proto-oncogenes (15). HER2 promotes tumor growth by activating MAPK and PI3K/AKT signaling pathways, which in turn increase cell proliferation, invasion, and metastasis (27) In the absence of systemic therapy, HER2 gene amplification or protein expression is associated with a poor prognosis.HER2 levels were found to be negatively correlated with ER and PR levels (15). HER2-positive patients account for approximately 15–20% of breast cancer patients. In clinical practice, HER2-targeted therapy is used in HER2-positive patients and HER2 is used as a prognostic indicator. As with ER therapy, HER2- targeted therapy works only in HER2-positive patients but not in HER2-negative patients (27).
2.3.1.1.4 The biomarker of triple negative cancer
TNBC, a subtype of breast cancer that lacks ER, PR, and HER2 expression, accounts for 15-20% of patients. Triple-negative breast cancer (TNBC) has a worse prognosis and a lower survival rate. Currently, the most important treatment is cytotoxic chemotherapy. Further classification of TNBC is needed for more targeted therapy. A survey of biomarkers associated with TNBC has identified several biomarkers that can stratify patients for molecular therapy. VEGF, a key signaling factor, is highly expressed in 30-60% of TNBC patients and targeted anti-VEGF therapy improves treatment outcomes (33, 34). Binding Androgen in cell depends upon a hormone called as Androgen receptor(AR), this binds the transcription factor as well as control gene Expression. AR simulate proliferation as well dedifferentiation and induce cell death and apoptosis. The expression of AR is related to the biological behaviors of triple-negative breast cancer and plays a role in endocrine therapy and prognostic prediction (35, 36).
2.3.1.1.5 Emerging tumor marker
Researchers are now focusing on newly discovered tumor indicators in addition to the three typical clinical breast cancer tumor markers discussed earlier. These new indicators can be classified as proteins, nucleic acids, cancer cells, and other types of cells (37). One type of active material that can show the presence and progression of a tumor is a tumor marker. Finding tumor markers can be a useful tool in the diagnosis and management of breast cancer. Some of the drawbacks of the traditional tumor marker detection approaches are high equipment costs, labor-intensive procedures, and low sensitivity.
2.3.2 Biosensors
Depending on the detecting signal and detection technique, biosensors can be categorized as electrochemical, optical, or other types (43–47). Numerous biosensors for identifying breast tumor indicators have been created in recent years by researchers. This study reviews the advances made in the development of electrochemical biosensors, optical biosensors, and other forms of biosensors for breast tumor indicators. Figures 4–6 provides a brief overview of the types of electrochemical, optical and other biosensors utilized in breast cancer detection.
Figure 4
Figure 5
Figure 6
2.3.2.1 Electrochemical biosensor
Electrochemical biosensors rely on the detection of electrochemical processes occurring on electrode surfaces to determine target concentration. Thorough explanation of the types of electrochemical biosensors used to detect breast cancer (Table 2).
Table 2
| Name | Type | Target | Detection Limit | diagrams | Reference |
|---|---|---|---|---|---|
| Electrochemical Biosensor | CV | CA153 EGFR miRNA-155 | 0.64 U mL−1–1 pg mL−1 2×10^−2M |  | (48) (49) (50) |
| DPV | BRCA1 CA15-3 BRCA1 let-7a miRNA-21 | 0.0034 pM 3.34mUml^-1 3.01 × 10−16 M (let-7a) 8.2fM (miRNA-21) |  | (51) (52) (53) (54) | |
| SWV | MUC1 miRNA-21 miRNA-155 | 0.33 pM 39.6 aM 18.9 aM |  | (55) (56) | |
| LSV | HER2 HER2-ECD CD44 CD44 positive cells | 0.16 ng mL 1 4.4 ng mL 1 2.17 pg. mL−1–8 cells mL−1 | 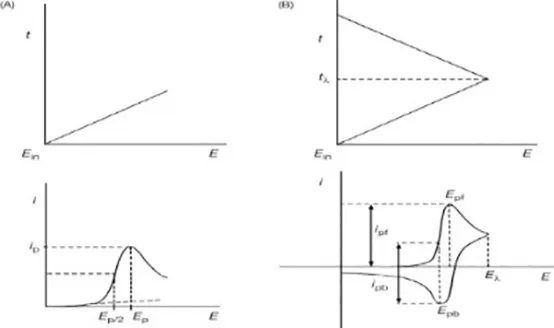 | (57) (58) (59) (60) | |
| EIS | HER 2 MCF-7 cell MUCI BRAC1 | 23 cells mL−1 2.7 nM |  | (61) (62) (63) | |
| FET | miRNA-155 CEA | 0.03 fM |  | (64) (65) (66) (67) |
Thorough explanation of the types of electrochemical biosensor used to detect breast cancer.
2.3.2.2 Optical biosensor
Refractive index, resonance, wavelength, intensity, and other optical changes on sensing layers are used by optical biosensors to identify targets.
Various optical sensors, such as colorimetric, fluorescence, SPRi, SERS, and ECL biosensors, have shown varying linear ranges and detection limits for distinct biomarkers. With a linear range of 0.03–6 ng mL−1, fluorescence biosensors have identified CEA at 7.9 pg mL−1 in water and 10.7 pg mL−1 in human serum samples. Using fluorescence, the detection limit of miRNA-21 is 0.03 fM, with a linear range of 0.1–125 fM (68, 69), The linear ranges of colorimetric biosensors are 10−12–10−18 M and 1 fM–100 pM, respectively, and they have detected BRCA1 at 10−18 M and 0.34 fM (70, 71), SPRi biosensors have identified HER2-positive EXO at 8280 exosomes µL−1, with a range of 8280–33,100 exosomes µL−1, and CEA at 0.12 ng mL−1, with a linear range of 0.40–20 ng mL−1. miR-K12-5-5p was discovered by SERS biosensors at 884 (72–76).
Because of their remarkable sensitivity to biomolecular interactions, PCF SPR (Photonic Crystal Fiber Surface Plasmon Resonance) biosensors have become promising instruments for the early detection of breast cancer. Ultra-low concentration detection is made possible by these biosensors, which pick up on minute changes in refractive index when breast cancer biomarkers adhere to metal surfaces. According to recent research, resonance shifts, and detection efficiency can be enhanced by optimized PCF designs that incorporate modifications to geometry and metal layering (77). In order to address the issue of environmental sensitivity, additional developments in nanomaterials and optical configurations improve signal stability and accuracy. PCF SPR biosensors are anticipated to transform real-time, non-invasive breast cancer detection as research advances (78).
2.3.2.3 Other types of biosensors
Finally, it should be noted that QCM and PEC can identify any kind of tumor marker. The primary purpose of the QCM biosensor’s signal amplification is to increase the mass change of the chip surface. The PEC biosensor uses signal amplification to increase the photovoltaic and photocurrent changes brought on by the target. While signal amplification techniques can be used to increase the detection limits of QCM and PEC sensors for a single target, these sensors struggle to detect many targets at once (79–83).
2.3.2.3.1 Innovative approaches for biosensor
The detection approach and the detecting device are the biggest obstacles for biosensors. Detection techniques typically find it challenging to handle biomolecules in challenging situations. For instance, the environment frequently affects the activity and shelf life of biomolecules. The majority of biosensors are not sufficiently integrated and compact, making it impossible for them to detect several targets at once at the device level. To overcome these obstacles, the coupling of microfluidic chips with biosensing and molecularly imprinted polymers (MIPs) holds considerable promise.
2.3.2.3.2 Summary of tumor marker and biosensors
Tumor indicators play an important role in breast cancer diagnosis and treatment, however no marker can effectively predict breast cancer before clinical symptoms appear. HER2-targeted biosensors allow for real-time detection of overexpressed HER2 proteins, often linked to aggressive types of breast cancer. This early detection enables quick initiation of HER2-specific therapies, improving treatment response and greatly increasing patient survival rates outcomes (84). Biosensor development has obstacles in detecting several targets at the same time, as most biosensors can only detect one target. Biosensors, such as antibodies, DNA probes, and aptamers, can give excellent sensitivity and specificity in laboratory settings. However, sensitivity in human serum samples declines due to chemical variables, which is especially critical for whole blood samples. To improve sensitivity and specificity, biosensors must improve their detecting technique and technology. The detection method seeks to improve both sensitivity and specificity, while the technology enables simultaneous detection of many objects. The usefulness of biosensors in practical applications for breast cancer detection has been shown by recent clinical validation studies. By tracking changes in refractive index as a result of antigen-antibody interactions, electrochemical and optical biosensors, such as PCF-SPR (Photonic Crystal Fiber Surface Plasmon Resonance) biosensors, have demonstrated high sensitivity in identifying breast cancer biomarkers. Repeated biopsies may not be necessary thanks to these devices’ potential for quick and non-invasive diagnosis (77). The stability and accuracy of biosensors have been further improved by developments in material science and nanotechnology, which have increased their clinical relevance. Recent advancements in biosensor technology have improved the detection of breast cancer due to their high sensitivity and real-time biomarker analysis. Although they don’t have constant monitoring, non-wearable biosensors offer remarkable accuracy in regulated settings. On the other hand, implantable biosensors provide real-time, in vivo tracking, which increases diagnostic accuracy. However, they pose challenges in terms of invasiveness and patient comfort. The requirement to balance accuracy and usability must continue to drive biosensor research in the future. Table 3 presents a detailed comparison of non-wearable and implantable biosensors for breast cancer detection, evaluating various clinical and operational parameters. Below, the table outlines a comparative study of the accuracy and patient outcomes concerning comfort for both biosensor types (77, 94).
Table 3
| Feature | Non-Wearable Biosensors | Implantable Biosensors | References |
|---|---|---|---|
| Placement | External (used in labs or diagnostic facilities) | Under the skin or internally placed through minimally invasive procedures | (86) |
| Monitoring Frequency | Periodic testing (e.g., blood samples, imaging visits) | Continuous real-time tracking of physiological or biochemical markers | (87) |
| Sample Type | Blood, serum, saliva, or tissue biopsies | Interstitial fluids, internal biomarker levels (e.g., HER2, pH, oxygen) | (88) |
| Detection Method | Electrochemical, optical (PCF_SPR), or immunoassays | Embedded microelectrodes, nanomaterial-based sensing elements, wireless signal transmitters | (89) |
| Invasiveness | Non-invasive or minimally invasive | Minimally invasive (requires implantation under skin or tissue) | (90) |
| Diagnostic Accuracy | High for certain biomarkers (e.g., HER2, CA 15-3); moderate for others | High specificity in pilot studies; early-stage detection potential | (86, 91) |
| Patient Comfort | Generally comfortable; requires clinic visits | Moderate; possible discomfort due to implantation or foreign body sensation | (92) |
| Power Supply | Externally powered (lab equipment) | Battery-powered or wirelessly powered via telemetry | (86) |
| Usage Context | Hospitals, diagnostic centers | Chronic condition monitoring, clinical research environments | (91) |
| Development Stage | Mature and widely adopted | Experimental, under preclinical or clinical testing | (93) |
A comparative analysis of non-wearable and implantable biosensors for breast cancer detection (85).
New nanomaterials, second antibodies, and indirect signal detection methods can help detect biomolecules. Sample pretreatment and MIPs can help with background interference and biomolecules’ trouble in hostile conditions. Combining microfluidic chips with biosensing can boost overall performance and multi-target detection. These new technologies can be marketed on a wide scale, altering the current detection paradigm and potentially leading to a shift. Biosensor technology must continue to advance in order to reach its full potential in the detection of breast cancer. This development calls for attention to be paid to both signal processing algorithms and biosensor materials. In terms of materials, research is still needed to create new materials with higher sensitivity so that biomarkers can be found at lower concentrations and an earlier diagnosis can be made. In order to reduce false positives, materials that are made to specifically target particular biomarkers and minimize interference from other substances are necessary for improved specificity. To guarantee accurate and consistent readings, biosensors must also show improved stability over time and in a variety of scenarios. Biocompatibility is another important factor for implantable sensors. It is crucial to develop complex signal processing algorithms concurrently with material advancements. These algorithms ought to be able to extract pertinent features from complex sensor data in order to identify subtle changes suggestive of cancer, effectively reduce noise in sensor signals, and adjust for sensor drift to preserve long-term reliability. The potential for further improving signal processing capabilities and raising the overall accuracy of breast cancer detection is high when artificial intelligence and machine learning techniques are combined.
2.3.3 Advanced computational and imaging techniques for breast cancer detection
2.3.3.1 Artificial intelligence for breast cancer detection
Since the advent of computer technology, researchers have developed automated analysis methods for medical imaging. Initially, low-level pixel processing (edge and line detector filters, region growing) and mathematical modeling (fitting lines, circles, and ellipses) were applied sequentially in medical image analysis from the 1970s to the 1990s in order to create compound rule-based systems that addressed specific tasks. Expert systems that had a lot of if-then-else statements, which were common in Artificial Intelligence (It refers to the capability of a computer to replicate human behavior, such as learning and taking action. AI developers teach computers to identify patterns in extensive datasets. After training, the program can independently analyze new data and make predictions) at the same time, can be compared to this. These expert systems, which resembled rule-based image processing systems, were frequently fragile and have been referred to as GOFAI (good old-fashioned artificial intelligence) (95). The clinical field is undergoing radical change as a result of the digital age, especially in the fields of radiology and pathology. In several fields, artificial intelligence techniques are being developed to address medical problems such diagnosis, prognosis, drug discovery, and testing (96–99). Artificial intelligence techniques have been applied specifically to breast cancer, where they have been used to diagnose (100) and prognosis, classify and quantify immunohistochemistry-stained images (101–103) and predict the pathological complete response (PCR) to neoadjuvant chemotherapy (104, 105). These applications have provided the opportunity for individualized care, increased therapy response rates, decreased adverse effects, and decreased costs of unnecessary treatment. AI has been utilized in radiology since the 1990s, initially with CADE tools in mammographic screening prompting readers to re-examine areas of concern in the image (106). With its ability to automate processes, extract minute information from photos, and provide predictive insights, artificial intelligence (AI) offers a viable solution to the problems that now exist (107–110).
In the field of diagnosis, medical images are first collected, then preprocessed, segmented, features extracted and eventually categorized. Image processing involves capturing digital images in a fixed format, usually a portable gray map. The next step is image preprocessing, which removes noise and enhances contrast using techniques like FPN, Bad pixels, temperature calibration, Vignetting, and Noise smoothing. Image segmentation divides an image into distinct sections, based on features, with the quality of the output largely reliant on measurement accuracy. Feature extraction converts input data into extracted features, such as spatial, transform, edge, color, shape, and texture features. These techniques are crucial in diagnosing disorders, particularly in distinguishing between natural and abnormal tissue features in breast masses or microcalcifications. The analysis of breast cancer sensor data has been transformed by contemporary In order to analyze and interpret the complex data produced by breast cancer detection technologies, artificial intelligence (AI) and machine learning are essential. These technologies have a number of significant benefits. Processing high-dimensional data from wearable sensors and imaging requires the ability to spot subtle anomalies and complex patterns in large datasets, which AI algorithms are better at than traditional analytical techniques. The analysis process can be streamlined by using AI to automatically extract the most relevant features from sensor data, such as subtle biomarker fluctuations or temperature variations. More precise diagnosis can be achieved by training machine learning models to categorize sensor data (e.g., differentiating between normal and abnormal tissue) and forecast the likelihood of cancer development. AI makes it possible to conduct customized analysis for each patient, taking into account their particular traits and risk factors to produce accurate and individualized evaluations. AI makes it easier to combine data from various sources, such as genetic information, imaging scans, and wearable sensors, to provide a comprehensive picture of the patient’s health. Table 4 explains all the terminologies used in paper related to Ai and ML and how they are related to breast cancer.
Table 4
| Algorithm | Definition | Relevance to Breast cancer | Reference |
|---|---|---|---|
| ANN (Artificial Neural Network) | a computer model that can recognize intricate patterns and is modeled after biological neural networks. | utilized for biomarker prediction, MRI tumor segmentation, and mammography classification. | (111) |
| LR (logistic Regression) | A statistical model that uses logistic functions to predict binary outcomes. | uses clinical information (such as tumor size and patient age) to predict the risk of malignancy. | (112) |
| KNN (K-Nearest Neighbors) | Labels are assigned by a non-parametric classifier using the nearest neighbors’ majority vote. | uses similarity to labeled tumor samples to classify histopathology images. | (113) |
| DT (Decision Tree) | Using feature thresholds, a tree-like model divides data into branches. | finds important diagnostic characteristics, such as the shape of the tumor and calcification patterns. | (114) |
| NB (Naïve Bayes) | A tree-like model separates data into branches based on feature thresholds. | uses biomarker information and patient demographics to forecast the recurrence of cancer. | (115) |
| SVM (Support Vector Machine) | A classifier looks for hyperplanes to divide data into classes. | high precision in classifying mammograms and differentiating between benign and malignant tumors. | (116) |
| RF (Random Forest) | a group approach that combines several decision trees. | Feature selection for tumor subtype classification and risk prediction. | (117) |
| K-Means | Data is divided into *k* groups by an unsupervised clustering algorithm. | separating tumor areas with unclear borders in MRI scans. | (118) |
| C-Means (Fuzzy C-Means) | Data points can be assigned to several weighted clusters through clustering. | using gene expression data to stratify breast cancer subtypes. | (119) |
| Hierarchical clustering | creates nested clusters using agglomerative/divisive proximity matrices. | modeling intricate distributions of biomarkers to detect cancer early. | (120) |
| GMM (Gaussian Mixture Model) | Data are represented as mixtures of Gaussian distributions in a probabilistic model. | Analyze complex Biomarker distributions | (121) |
AI and machine learning algorithms terminologies.
2.3.3.1.1 Machine learning algorithms for breast cancer prediction
Artificial Intelligence consists of a wide range of methods, such as machine learning, which is a subset of deep learning, of which CNNs are only one (122). Machine learning is an automated learning technique (123), with algorithms built to learn from previous datasets; we feed a mountain of data into a machine learning model, and it uses that data to anticipate what the future holds (103–106). Discuss the hierarchical classification of machine learning algorithms, which encompasses supervised, unsupervised, semi-supervised, and deep learning techniques (Figure 7).
Figure 7
Artificial Neural Network (ANN) (111) is a common data mining algorithm that consists of an input, hidden, and output layer. It is based on parallel processing (124), distributed memory (125), collective solution, and network architecture (115, 126, 127). Logistics regression (LR) (112) is a supervised learning algorithm that includes more dependent variables and provides continuous outcomes for specific data (115). K-Nearest Neighbor (KNN) (128) is used for pattern recognition and is effective for breast cancer prediction (115). Decision Tree (DT) (114) is a supervised learning algorithm that divides a dataset into smaller subsets for higher precision prediction (115). Naive Bayes Algorithm (NB) is a model used to make assumptions about a large training dataset and calculates probabilities using the Bayesian method. It is an analogy classifier that is used for comparing training datasets with training tuple (115). Support Vector Machine (SVM) is a supervised learning algorithm used for both classification and regression problems (116), providing the highest accuracy rate for large dataset predictions. Random Forest (RF) (117) is a building block of machine learning used for predicting new data based on previous datasets (115). K Mean Algorithm is a clustering algorithm that partitions data into small clusters based on similarity between data points (129). C Mean Algorithm is used for medical image segmentation and disease prediction (119). Hierarchical Algorithm evaluates raw data in the form of matrices, with each cluster separated by a probability model. Gaussian Mixture Algorithm is a popular unsupervised learning technique that computes the probability of different types of clustered data based on expectation maximization (121).
Machine learning techniques are only effective if the first input data has significant predictive characteristics. DL, a subset of ML, was created to use deep, multi-layered structures to enhance the performance of traditional ANNs. Among the various deep neural networks, CNNs rely on convolutional processes to transform unprocessed image data into intricate representations, eliminating the requirement for explicit feeding of features extracted from the image (130).
The use of AI and machine learning has led to substantial enhancements in breast cancer treatment outcomes. AI algorithms assess patient-specific data to predict treatment responses and identify the best treatment plans, thereby decreasing side effects and increasing effectiveness. AI-enhanced image analysis assists surgeons in accurately locating tumors and planning surgeries, ensuring precise tumor excision and better cosmetic results. Additionally, AI refines radiation therapy strategies for accurate tumor targeting, reducing harm to surrounding healthy tissues. Machine learning approaches evaluate patient data to forecast recurrence risk, allowing for timely interventions that boost survival rates. Notable examples include AI tools analyzing mammograms to predict breast cancer risk and customize screening schedules, as well as machine learning models that anticipate patient responses to chemotherapy. Table 5 explains the A Comprehensive Review of Major Machine Technique from 2015-2019 (For Breast Cancer Prediction).
Table 5
| Technique | Description | Merits | Limitations | Year/reference |
|---|---|---|---|---|
| Computer-Aided Diagnosis System (CAD) for predicting breast cancer. | Involved in the comparative analysis of Machine learning algorithms including random forest, gradient boosting and K nearest neighbors was conducted. | The random forest algorithm, which combines both regression as well as classification methods, achieved the highest accuracy. It involves multiple trained models to make predictions for various training classifiers. A method called the hybrid method designed to develop accurately compute the UCI online dataset, resulting in the most and precise accurate outcomes. | The expected probabilities of result (occurrence and non-occurrence are calculated via K-fold cross-validation, which is a more expensive task. The pre-processing stage of data acquired most of the time because of raw data conversion into scalable and valuable form. Additionally, the number of patients that were already mentioned in a list was not considered. | 2019 (136) |
| Comparison of classification algorithms through weka and spark | For the evaluation of tree types of data that contain DM, GE, and a mix of both, classification models that support vector machines, decision trees, and random forests were taken into consideration. | The support vector machine’s ability to examine several data sets simultaneously stems from its parallel computation foundation. It offers the best accuracy rate across Weka and Spark, two distinct tools. Compared to decision trees and random forests, SVM has a lower error rate and calculation time. | Gathering data on gene expression is a difficult undertaking. Many samples are needed for calculations in order to produce sensitive, accurate, and precise data. | 2019 (137) |
| Comparison of Nonlinear Machine Learning Algorithms. | MLP in contrast to non-linear machine learning techniques like K Nearest Neighbor, Support Vector Machine, CART, and Naïve Bayes. | Because MLP is composed of multiple layers, each of which carries out a distinct task independently, the calculation of this technique was sufficiently fast. When the datasets are linearly separable, it provides a respectable level of accuracy. | The user must specify the hidden layers of the MLP algorithm. There were times when setting a value resulted in overfitting and other times in underfitting outcomes. Without 10-fold cross-validation, it is difficult to predict the accuracy rate using train data models. | 2019 (138) |
| Comparing SVM and ANN for Breast Cancer Prediction | Metrics including accuracy, precision, recall, and ROC area were used to evaluate the performance of SVM and ANN. | Since SVM divides classes based on hyper lines and generates results with a better accuracy than ANN, it was found to be the most suitable technique for predicting breast cancer after comparison. | The predicted probability of occurrence and non-occurrence are calculated using K fold cross validation. This is a more expensive endeavor. | 2019 (139) |
| Optimizing algorithms using genetic programming techniques | To obtain the data, digital pictures were put through feature extraction and selection processes. | Then, specific characteristics were selected and several machine techniques were compared using the polynomial features operator. The additional tree classifier yielded the highest accuracy when compared to other techniques. | The processes for training and evaluating the model were excessively lengthy. The GP algorithm was used to solve the hyperparameter problem, but processing it was extremely time-consuming. | 2019 (140) |
| Comparative Analysis of Data Mining Classifiers for Cancer Prediction and Detection. | The classification algorithms random forest, bagging algorithm, random committee, simple CART, and IBK were investigated using k-fold cross-validation. | The adobe forest algorithm, which requires less effort, had the highest accuracy throughout the evaluation. Random forest algorithms don’t need data to be standardized or normalized, and they might be better at handling nonlinear data. | To detect malignancies, a new model was developed, however processing it took too long. Our iterative use of K-fold cross-validation led to an excessive amount of time spent on each iteration. | 2019 (141) |
| Prediction of breast cancer using Naive Bayes, KNN, and J48. | Training data and testing data were the two categories into which the dataset was divided. Tenfold cross validation was applied to the evaluation techniques. | The most effective way to predict cancer datasets was to group the data based on the degree of similarity between each incidence. Assign high accuracy to both training and testing data. | Testing is slow and takes a long time. Choosing a K value could be difficult. | 2019 (142) |
| Recursive Features Selection for Breast Cancer Detection | Several kernels, including linear, RBF, polynomial, and sigmoid, were used to examine the SVM algorithm. On linear kernels, SVM performed more accurately than alternative techniques. | The most accurate method for choosing relevant traits for breast cancer prediction is to use SVM linear kernels. High accuracy was achieved by developing the projected model and feature selection method for large datasets. | Calculation time arose when irrelevant information was extracted. Compared to other models, the SVM linear kernel computed more slowly and had higher error rates. | 2019 (143) |
| Breast cancer diagnoses through classification techniques. | Using linear discriminant analysis (LND) and dimension reduction methodology, machine learning approaches are compared. | Through feature selection and extraction, the classification model—which was developed using a training dataset—improves patient classification of benign or malignant tumors while requiring less data storage | Although the evaluation step takes a long time because of CFS, LDA, and PCA approaches, the solution uses the R programming language, which has fewer packages and requires less processing than other languages. | 2019 (144) |
| Breast Cancer risk prediction and Diagnosis. | Performance metrics evaluate the C4.5, SVM, NB, and KNN models’ sensitivity, accuracy, and precision. Out of all the models, SVM has the highest accuracy. | Each method is accurately evaluated by the ROC curve. The SVM algorithm increases the precision of accurately classifying events. The error rate value was lower for this algorithm. | The ROC curve provides an accurate evaluation of each algorithm. The SVM algorithm improves accuracy in predicting correctly classified occurrences. This algorithm had a reduced error rate value. | 2018 (145) |
| Most effective machine learning for predicting breast cancer. | The dataset was divided in two parts. Prior to the feature extraction and selection techniques, the K-fold validation methodology was employed. SVM improved the accuracy of predictions. | SVM provided an accuracy of 99.7% for the benign class and 94.6% for the malignant class when a predictive model was developed. Compared to other algorithms, SVM has a lower error rate and a quicker turnaround time. | It’s crucial to use a suitable approach when evaluating a machine learning algorithm. The conflict matrix was created with the intended class outcome in mind; it correctively predicted the occurrences, but the maximum prediction time was used. | 2018 (146) |
| Hyperparameter Optimization for the Prediction of Breast Cancer. | The HPO technique’s clustering method was used to identify the best prediction algorithm for breast cancer. | Hyperparameterization utilizing the clustering method yielded the highest accuracy. Hyperparameters performed better with continuous and categorical data types. | Certain features also gave some redundant data. There are too many steps in the BCOAP model, and each one takes too long to analyze breast cancer data. | 2018 (147) |
| A Neural Artificial Network for Breast Cancer. | ANN algorithms were employed for prediction using the backpropagation process. Each hidden layer provided a different level of accuracy during evaluation. | The arbitrary weight produced by the multi-layered neural network produced the Mean Square Error, whose rate is too low. By changing the weight, the feed-forward algorithm lowers error. | Demand a lot of processing power and time for a significant amount of data, which has an impact on the data’s overall correctness. For computations, a huge number of samples are required in order to attain good accuracy, precision, and sensitivity of data. | 2018 (148) |
| Comparing data mining techniques for the categorization of breast cancer | The fusion classifier, which combines many classifiers, was created to assess the algorithm using various data mining tools. | More accuracy was obtained from a single classification than from a fusion classification. When the confusion matrix was designed, the WPBC, WBC, and LBCD datasets offered the higher level of accuracy throughout the evaluation of various algorithms. | With the exception of the LBCD dataset, the Weka tool’s accuracy was subpar. However, it was the most accurate for the WPBC and WBC datasets. | 2017 (149) |
| Breast cancer prediction by the use of data mining methods. | For the purpose of comparing the classification and clustering algorithms, a confusion matrix was created. | Compared to the other algorithms, the classification algorithms C4.5 and SVM produced better results. Furthermore, EM created the most effective clustering method for breast cancer. | One of the hardest tasks is figuring out the effect algorithm that predicts the onset and recurrence of diseases. | 2017 (150) |
| Breast cancer using computer algorithms for diagnosis and prognosis. | The time build model was created to analyze the effectiveness of various classifiers. | After evaluating each classifier using a confusion matrix, SVM has a higher accuracy rate and lower error rate for breast cancer prognoses. | SVM was longer to process than KNN, however KNN was a lazy learner approach with poor accuracy results. | 2016 (151) |
| Breast Cancer Analysis Using Classification Algorithms | The classification algorithm’s performance was evaluated using accuracy, sensitivity, and precision metrics. | We compared categorization algorithms using weighted average values. The CART algorithm accurately predicts breast cancer outcomes in a short period of time. | The model compares the data mining decision tree algorithms J48, CART, and ADtree. The evaluation step took too much time. | 2016 (152) |
| Data mining categorization algorithms for risk prediction of breast cancer. | A performance matrix was used to compare Naive Bayes and J48 models. The J48 algorithm outperformed Naive Bayes in terms of accuracy rate. | The naive Bayes algorithm produced the lowest error rate throughout computing. Increasing the number of attributes and sample size resulted in improved accuracy. | The expression rule was intended to identify the best features for breast cancer prediction, but the review procedure was overly difficult. | 2015 (153) |
A comprehensive review of major machine technique from 2015-2019 (for breast cancer prediction).
2.3.3.1.2 Deep learning techniques for breast cancer prediction
An extension of artificial neural networks, or ANNs, is called deep learning. The architecture of deep learning algorithms is made up of numerous layers. These algorithms can recognize all of the data from various categories and are used to process a significant amount of natural data. When we have a large amount of unlabeled data, we typically use unsupervised deep learning algorithms (132). Autoencoders are neural networks that learn from large datasets by training their network to ignore irrelevant signals like noise (133, 134). Sparse auto-encoders learn from unlabeled data using a feed-forward and backpropagation algorithm, handling the sparsity regularizer (132–134). Stacked Sparse Auto Encoder (SSAE) (132) combines the basic layers to construct a stacked sparse, with hidden layers based on classifiers providing output (133, 134).
Convolutional neural networks (CNN) analyze cancer datasets using CovNet for data analysis and filters to capture different dimensions of images. CNN consists of pooling, convolutional, classification, and fully contacted layers (133, 135). Recurrent neural networks (RNNs) are a class of neural networks that consist of hidden states that use the output of previous states as input for the next state. While they can process a sequence of inputs using the same parameters at each layer, they cannot process a large number of inputs through ReLU and Tanh activation functions.
2.3.3.2 Imaging techniques
The landscape of breast cancer detection has evolved with a variety of imaging techniques, each offering its own capabilities for early diagnosis and monitoring. Artificial Neural Networks (ANN) have become an integral part of the field, applying sophisticated algorithms to analyze complex patterns in image data, thus improving diagnostic accuracy. Reflective optical imaging devices (ROIDs) provide high-resolution images by reflecting light that helps distinguish tissue types and identify potential malignancies. Microwave imaging (MI) and microwave-induced thermo acoustic imaging (MITI) represent state-of-the-art techniques that use microwave signals and thermo acoustic effects to reveal breast tissue abnormalities and provide a non-invasive method of detection. Automated Breast Ultrasound (ABUS) and Ultrasound Imaging Systems (UIS) assist the field by providing detailed, real-time images of breast tissue, making it easier to detect structural changes and abnormalities. In addition, infrared imaging technology (IIT) detects temperature fluctuations in the breast tissue, which can indicate pathological changes. Together, these imaging modalities provide a comprehensive toolkit to improve breast cancer detection, and each offers unique strengths to improve early diagnosis and treatment strategies. These all imaging techniques are explained in Figure 8 for the diagnosis of breast cancer.
Figure 8
Here, we provide an overview of popular imaging modalities used in breast cancer analysis and diagnosis. Studies have demonstrated that there are various imaging modalities, such as digital breast tomosynthesis, positron emission tomography, magnetic resonance imaging, ultrasound, histopathology, mammography, and combinations of these modalities (multimodalities). Table 6: Summary of various imaging modalities for screening of breast cancer.
Table 6
| Imaging Modalities | Principles | Diagnostic accuracy | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Mammography (first line tool for detecting breast cancer) | Detailed images can be found in low dose ionizing X-rays. | Sensitivity: 75–90% Specificity: 90–95% Spatial Resolution: 50 µm | most economical. Excellent reaction with a high level of sensitivity and specificity. portable gadget. | Ionizing radiation is used. As breast density increases, sensitivity falls. The accuracy of young ladies is low. Young women with thick breasts have a high rate of false-positive outcomes. In contrast to MRI, the contrast is poor. | (155–157) |
| Magnetic Resonance Imaging | obtains fine-grained images of the breast’s architecture using powerful magnets and low-energy radio waves. | Sensitivity: 75–100% Specificity: 83–98.4% Spatial Resolution: 25–100 µm | Capacity to identify breast cancers that frequently evade diagnosis by clinical, mammography, and ultrasound . | costly, and the test cannot be standardized. unnecessary breast biopsies as a result of the incapacity to differentiate between benign and malignant tumors. | (158–161) |
| Dynamic Contrast Enhanced MRI (DCE-MRI) | Several MRI scans performed after an intravenous contrast agent injection | Sensitivity: 89–99% Specificity: 37–86% Spatial Resolution: 25–100 µm | performs well in tracking reaction after treatment. | Artifacts based on tumor shape and hemorrhage caused false-negative results. | (162–164) |
| Diffusion-Weighted Imaging | creates contrast by using the diffusion of water molecules. | Sensitivity: 83% Specificity: 84% Spatial Resolution: 25–100 µm | Non-radioactive imaging technique | High apparent diffusion coefficients make it difficult to identify cancerous tumors with a high water content. | (165) |
| MR Elastography (MRE) | dynamic elasticity imaging method that blends low frequency with MRI imaging. evaluates tissue stiffness by producing an elastogram using mechanical waves. | Sensitivity: 90–100% Specificity: 37–80% Spatial Resolution: 25–100 µm | Non-invasive, non-ionizing and cross-sectional imaging modality | inability to detect small focal lesions and lack of spatial resolution. | (166, 167) |
| Positron Emission Tomography conjugated with computed Tomography (PET-CT) | Combines nuclear medicine technique and computed tomography resulting in high detailed images. | Sensitivity: 90–100% Specificity: 75–90% Spatial Resolution: 2–10 mm | not invasive. offers twice as many diagnostic advantages (intricate images of tissues and organs by CT scan, and elevated activity within the body detected by PET scan). | High-cost. Unable to detect tumors less than 8 mm. | (166) |
| Sentinel lymph node biopsy (SLNB) | Surgical procedure to detect spreading of cancer in lymphatic system. | Sensitivity: 90.5% Specificity: 85.7% Spatial Resolution: Not Applicable | Significantly reduces post-operative complications | Patients with inflammatory breast cancer and locally progressed tumors will not benefit from this treatment. | (167, 168), |
| Breast Specific Gamma Imaging | Employs use of a radiotracer. Image captured using a special camera. | Sensitivity: 90–96% Specificity: 71–80% Spatial Resolution: ≥7 mm | Able to identify smaller lesions (<1 cm) | High radiation dose. Not suited for routine tumor screening. | (169–171) |
| Ultrasound | Employs sound waves to image breast tissues | Sensitivity: 80–89% Specificity: 34–88% Spatial Resolution: 50–500 µm | Accessible, real-time lesion visualization, cost-effective, patient compliant. | Not helpful for those with inflammatory breast cancer and locally advanced tumors. | (172) |
Summary of imaging modalities for screening of breast cancer.
2.3.3.2.1 Digital breast tomosynthesis
Due to the limitations of two-dimensional mammography (DM), digital breast tomosynthesis (DBT) has been developed and clinically introduced over the last two decades. DBT is an advanced imaging technique that creates 3d images of the breast and makes it easy to detect lesions and abnormalities because it reduces the chance of overlapping tissue (173). DBT detects 15-30% more cancers than mammography and reduces false positivity rate by 15-20% (174). Studies have shown that DBT increases cancer detection and can lower the recall rate depending on the baseline recall rate for DM. A review on digital breast tomosynthesis has included results of different studies on why DBT should be used in regular screenings and what its limitations are. Among the merits of dbt, it can analyze overlapping breast structures more clearly which helps radiologists distinguish normal and abnormal shadows and helps lower the number of false positive recalls (175–178). Figure 9 clearly illustrates that Digital Breast Tomosynthesis (DBT) provides a clearer image compared to traditional mammography.
Figure 9
A study in 2019 aiming to compare the results of dbt over multiple years to digital mammography concluded that dbt outperformed digital mammography in detecting invasive cancers, reducing false negative rates and higher sensitivity. The findings supported the use of dbt in breast cancer screening, despite longer follow-ups and more data to support such claims (179). Another research in 2024 comparing dbt and dm (digital mammography) concluded that dbt improved cancer detection rates especially those at early stages. Findings from the paper highlight dbts potential in screening practices globally, but further long-term studies are needed to evaluate its impact on screening outcomes (180).
However, one drawback is that interpreting DBT images takes about twice as long as reading DM images due to the higher number of images. To introduce DBT into large-scale screening programs, methods to reduce reading time need to be developed. Automated interpretation methods could play a significant role in this by enabling faster image navigation and reducing variability in interpretation, potentially improving the impact of DBT on recall rate at screening. The demerits of dbt include higher radiation exposure, increased cost, longer reading times, data storage, and changes to diagnostic practice (181). Dbt also has potential for overdiagnosis which raises concern for its incorporation in daily screening (174). Although DBT has better results than mammography, it still requires extensive research to be used as a proper screening tool. This is because DBT has a higher radiation dose and longer reading time (182) Table 7 shows the Comparison of several Digital Breast Tomosynthesis (DBT) methods, emphasizing the advantages and disadvantages of each for the identification of breast cancer.
Table 7
| Techniques | Advantages | Disadvantages | References |
|---|---|---|---|
| Artificial Neural Networks (ANN) | High accuracy in detecting BC Can be integrated with Computer- aided Diagnosis (CAD) systems Can be used in thermal imaging, mammography, ultrasound, MRI, and other imaging modalities. | Computationally intensive Requires large amount of data for training | (184) |
| Reflective Optical Imaging Device (ROID) | Offer real-time, high-resolution imaging, making potential anomalies easier to see. ROIDs are typically less expensive. More comfortable diagnostic technique to patients | Penetration limit of approximately 15 cm ROID procedures can be complex and time-consuming. | (185) |
| Microwave Imaging (MWI) | Non-invasive and non-ionizing Better differentiation in dense tissues Designed to be portable | Complexity of Interpretation More thorough clinical trials are required to confirm its efficacy. | (186, 187) |
| Automated Breast Ultrasound (ABUS) | Enhanced Visualization Automation streamlines and speeds up picture registration and segmentation. | Dependence on Fiducial Markers Limited Lesion Visibility | (188) |
| Ultrasound Imaging System (UIS) | Cost-Effective Non-Contact and Painless | Complex Setup Maintaining a constant temperature is crucial for accurate imaging | (188) |
| Microwave-Induced Thermos Acoustic Imaging (MITI) | Detects very small tumors (radius of 0.25 cm) early. Higher temperature and pressure in tumor area for distinguishability. Combines microwave and ultrasound imaging benefits. | Complex multi-physics modeling and simulations. Harder detection in glandular tissues. | (189) |
| Infrared Imaging Technology (IIT) | Noninvasive and radiation-free imaging. Potential for early detection of breast cancer. | Sensitivity and specificity remain less than optimal. | (190, 191) |
Comparison of several Digital Breast Tomosynthesis (DBT) methods, emphasizing the advantages and disadvantages of each for the identification of breast cancer.
2.3.3.2.2 Reflective optical imaging device
Accurate vein identification and early breast cancer detection are critical in modern medicine. Vein location can be challenging, especially in children, obese patients, and those with difficult venous access, causing patient discomfort and complications during blood collection. Meanwhile, breast cancer remains a leading cause of cancer death globally. Traditional diagnostic methods like mammography, MRI, and CT scans have limitations such as high cost and long scan times. The BKA-06 device was developed to improve the accuracy and efficiency of detecting blood vessels and breast tumors using red to near-infrared light-emitting diodes, providing a non-invasive, cost-effective solution for real-time imaging in clinical settings (185).
The BKA-06 is an advanced medical imaging device using red to near-infrared LEDs to capture real-time images of blood vessels and breast tumors. It offers a non-invasive and cost-effective alternative for breast cancer detection, providing high-resolution images of breast tissue for early tumor detection. With a maximum light intensity of 98,592 lux, it enables thorough examinations and quick visible results. It is more affordable than MR and CT scans, making it a more accessible option for many patients. However, extensive clinical validation is needed to ensure accuracy, particularly in detecting deeper tumors. Continuous research and development are crucial to enhance its capabilities for better health outcomes and more accessible medical care.
2.3.3.2.3 Microwave Imaging
MBI (microwave breast imaging system) is another imaging technique that is non-invasive, cost-effective and nonionizing which makes it safe for patients (192). Microwave Imaging (MWI) is a promising method for detecting breast cancer using non-invasive electromagnetic waves in the microwave frequency range. Tumors with higher water content than normal tissues have distinct dielectric properties that MWI can detect. Microwave antennas such as monopole antennas provide the simplest design among different antennas in MBI (microwave breast imaging systems) systems. Monopole antennas are easily fabricated into pcb, which makes it cost-effective. Slot antennas are also low-cost and offer wideband performance antennas are used in wearable systems but require improvements in radiation, bandwidth, and gain (193). Different countries have conducted research on their mbi-based prototypes. A study in UK (Bristol) claimed to achieve a sensitivity of 76% by clinical trials on 225 patients, using mbi prototype MARIA (194). Mammowave a prototype developed in Italy, gave a sensitivity of 78% in clinical trials on 58 patients (195).
The SAFE device is a noninvasive, painless, and non-invasive microwave imaging system designed for early detection of breast cancer. It uses harmless electromagnetic waves and does not require breast compression, making it a safer alternative to traditional X-rays. The device’s sensitivity varies by breast size, suggesting potential for improved detection (186, 189). Another study in the medical imaging department of Italy validated their mbi-based prototype called Wavelia on 24 subjects and achieved an accuracy of 88.5% by successfully differentiating between benign and malignant lesions (196). New technologies such as MTM (metamaterial antenna), MTS (meta surface antenna), AMC(artificial magnetic conductor antenna) are recently used by researchers. For example, the MTM microstrip patch antenna was developed in 2022 with AMC to enhance gain (197). Another MIMO (multiple input multiple output) UWB antenna was developed to improve detection accuracy to be successfully used in breast imaging devices (198). The first radar-based system was developed in 1997 for breast cancer (199). Their system was able to detect size and tumor inside the breast. Radar-based microwave imaging techniques use electromagnetic signals to create high-resolution breast images. These techniques include CMI, TSAR, MIST, MSA, and TDDA. TSAR analyzes signals that penetrate tissue, while CMI concentrates microwaves for subsurface imaging. While MSA employs several radar pairs for screening, TDDA uses algorithms to evaluate time-domain data for imaging, and MIST creates 3D images from multiple radar signals (193).
Various MWI techniques, such as microwave tomography and radar-based imaging, have shown promising results, but challenges such as variation in performance due to breast size and the need for better resolution remain. Future research will focus on overcoming these challenges and integrating machine learning to enhance MWI’s clinical applications. Overall, MWI has significant potential as a stand-alone or adjunctive tool in breast cancer screening, but further research and development are needed to fully integrate it into clinical practice (189).
2.3.3.2.4 Ultrasound Imaging System
Ultrasound is becoming popular because this imaging technique is suitable for dense breasts, unlike mammography (200). Ultrasound is inexpensive and suitable for those who are not eligible for mammography. It can also prove beneficial for those who cannot tolerate breast MRI (201). Many systems have been developed for analyzing ultrasound images which are computer-aided (202). The main point highlighted in them is the need for improvement of the resolution of images (203). Figure 10 demonstrates that Ultrasound scans of breast in different conditions. Recent studies have revealed the increased sensitivity of ultrasound for dense breasts because mammography has reduced effectiveness for that kind of breast. Mammography has the potential for false negative results, leading to the masking of abnormalities. When used as an adjunct therapy, ultrasound can identify malignancies that are often missed by mammography. This means women with dense breasts have a reduced chance of missing malignancies because of the increased sensitivity of ultrasound (22, 204–209).
Figure 10
Ultrasound offers real time imaging which helps in identifying minor irregularities and lesion features. This real time feature enables ultrasound guided biopsies, reducing need for more invasive procedures for breast cancer diagnosis and improved tissue sample accuracy (211–214). Recent studies suggest that ultrasound is effective for identifying tumors in young females. Because mammography is not recommended particularly for younger women and may not be as effective. Ultrasound detects lesions in a broader group of people without sacrificing its sensitivity. Thus, it allows for personalized treatment depending on every person’s risk factors (215, 216). Although there are advantages to using ultrasound as a diagnostic tool for breast cancer diagnosis it also has significant drawbacks. Ultrasound has difficulties while assessing thick breast tissues. This reduces sensitivity of ultrasound. Mammography because of its capacity to penetrate is useful while accessing thick breasts. RI offers a better diagnosis in terms of thick breast tissue (206, 217). Ultrasound is operator-dependent, and its results depend on the skills of operator compared to MRI and mammography which are regarded as more objective and do not rely on operator for the interpretation of images (218–220). Ultrasound has the ability to be used as independent screening tool for breasts, but More research and rigorous clinical trials are needed to assess the efficacy and limitations of utilizing it as the primary screening method (221). Ultrasound is dependent on the operator. To overcome this problem automated breast ultrasounds can give more fruitful results (222).
2.3.3.2.4.1 The inherent limitations and biases of particular imaging techniques
Although imaging methods are essential for detecting breast cancer, it is important to recognize their inherent drawbacks and possible biases. Ionizing radiation is used in mammography, for instance, and although the dosage is usually low, repeated exposure over time, especially during long-term follow-up, increases the risk of radiation-induced cancer. Furthermore, because tumors and dense tissue can appear similar on mammograms, abnormalities may be obscured, reducing the sensitivity of mammography in women with dense breast tissue. Some women may be discouraged from getting screened for breast cancer on a regular basis due to the discomfort of breast compression during the procedure. Another popular imaging technique, ultrasound, has limited specificity and is operator-dependent, which means that different people may interpret it differently. Additional biopsies are often necessary to distinguish between benign and malignant lesions. Although magnetic resonance imaging (MRI) provides good soft tissue contrast, it is more costly and less widely available than other methods. Additionally, it has a higher risk of false positives, which could result in needless procedures. Additionally, patients who have certain metallic implants should not have an MRI. Lastly, compared to mammography, other imaging methods such as CT and PET scans require much larger doses of ionizing radiation, which raises concerns about radiation exposure. Additionally, PET scans may have limited spatial resolution. Digital breast tomosynthesis (DBT), contrast-enhanced mammography, and the creation of artificial intelligence (AI) algorithms to help with image interpretation and boost diagnostic accuracy are some of the innovations being researched to help overcome these constraints.
2.3.4 Sensors
Sensors offer painless and non-invasive diagnosis of breast cancer (223, 224). In comparison to other modalities, it reduces safety threats, allowing women to receive routine breast cancer screening (225). The work done on sensors and sensor-based devices between 2015 and 2024 is explained in the following section.
Table 8 outlines the sensors used in experimental configurations, including the models of the sensors, how they are oriented during testing, and the technology used to identify breast abnormalities.
Table 8
| Name of sensor | Model | Orientation of sensors in experiment | Name of technology used | Reference | Pictures of devices: |
|---|---|---|---|---|---|
| Digital temperature sensor | ADT7420 | Biometric patch | Thermography | (239) | 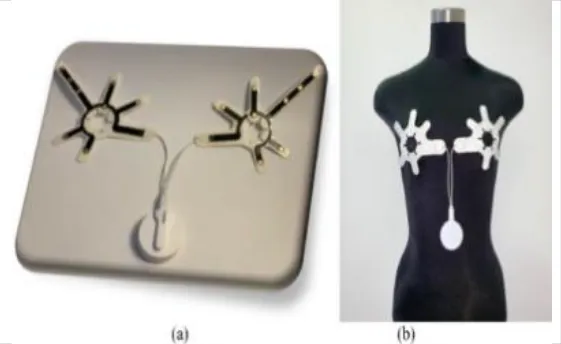 |
| Piezoresistive sensor | Not mentioned | Vertically/perpendicularly | Not mentioned | (231) |  |
| IR theomorphic sensor | FLIR A 300 | Not mentioned | Thermography | (243) |  |
| IR imaging sensor | AMG 8833 | Sensor embedded in bra cup | Thermography | (229) | 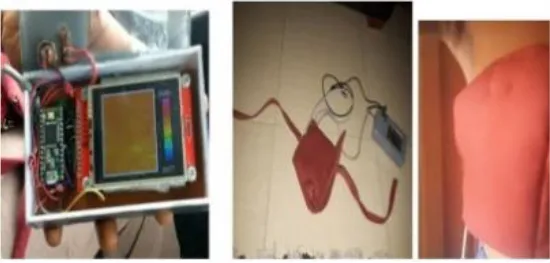 |
| Multiple leads | Not mentioned | In form of array | NIRS | (244) |  |
| Bioimpedance sensor | Not mentioned | Not mentioned | BIS | (244) |  |
| Thermal Sensor | LM35 | In quarterly order | Thermography | (228) |  |
| Antenna sensor | Not mentioned | Circular/Rectangular | Microwaves | (242) |  |
| Textile sensor | Not mentioned | Circular | Microwaves | (225) |  |
An outline of the sensors used in experimental configurations.
2.3.4.1 Thermography based sensors
Thermography is a commonly used method for the detection of Breast Cancer. In essence, thermography uses imaging technologies such as cameras and sensors to map the variations in breast temperature. The idea behind this procedure is that when the breasts experience abnormalities, the blood flow pattern to them is altered, which causes significant temperature variations (226). When using the current detection technologies, such as mammography, women with higher breast density levels frequently receive the wrong diagnosis. For women with higher breast densities, thermography is a useful approach (227). Figure 11 demonstrates the Normal vs Abnormal breast thermogram.
Figure 11
Sensors integrated with breast thermography make an excellent combination because they are small, cost effective and easily available. A 2018 study created a Breast Cancer detecting device by correlating the output from a thermographic camera and a thermal sensor. According to the study, the thermal sensor and camera produced almost identical results, suggesting that this gadget could be useful for BC screening. The sensor LM35, FLIR C2 thermal camera, microcontroller, heater (which was imitating a tumor), and breast phantom were the materials used. On the bra pad, eight heat sensors were arranged quarterly (228).
Many studies have been carried out in which sensors based on the concept of thermography are being attached to some kind of patches or brassiere with proper skin contact (227). For example, a 2020 study that used infrared thermography to create a smart bra. Irt sensor AMG8833, microprocessor, and image acquisition system were among the materials employed. By gathering radiation from the breast region, the infrared sensor was able to produce a thermal gram based on variations in temperature. The sensors were created for both the left and right breast. When conducting self-examinations at home, this can be more helpful than potentially dangerous alternative diagnostic techniques (229). Another study from the same year used thermal microsensors as a 3*3 matrix. CMS sensors, a PCB with embedded microsensors, a processing unit, a Raspberry Pi web server, and a Wi-Fi system for temperature data transmission made up the system. CMS sensors have an operational temperature range of 0 to 50 degrees Celsius and are small, sensitive, and have low energy consumption. The future aim of the paper was to increase the number of microsensors in order to improve the quality of thermal photographs (230).
2.3.4.1.1 Piezoresistive sensors
A critical component of early cancer detection is clinical breast examination. When a Piezoresistive sensor comes into contact with a lump, it changes its electrical resistance. In 2020, an automated probe that can take the role of CBE was introduced. This probe made use of a microprocessor and Piezoresistive sensor based on electro graphite. According to research on this idea, which is allegedly still ongoing, the probe can detect the position, depth, and size of the mass but is unable to distinguish between normal and pathological breast masses. Future developments in this field will focus on improving sensor calibration, streamlining the production process, and creating real-time smartphone applications (231).
In another study, a piezoresistive sensor was used in an Intelligent Breast Exam device (iBE) to detect breast stiffness. This non-invasive, low-cost method was used for screening. The device was part of a comparative study with clinical breast exams and mammography. While iBE and CBE missed some small lesions, they can be utilized in resource-limited areas for early detection (232). In 2021, a piezoresistive fabric sensor was utilized to enhance clinical breast exam simulators. The sensor performance was compared to existing sensor maps of the simulator. The sensors did not interfere with the clinical examination and also improved the measurement capabilities of the simulator (233).
2.3.4.2 Near infrared and bio-impedance spectroscopy-based sensors
Another radiation-free near-infrared sensor was utilized in a paper published in 2016. The beer lambert theory forms the basis of this tumor detection technology. An array sensor produces light in the infrared spectrum. Technology can distinguish between a healthy breast and an unhealthy breast based on the amount of light absorbed by breast tissue. The development of breast phantom models with various scattering and absorption coefficients was the main goal of the project. For the others, the breast with the normal absorption coefficient and representation was used as a guide. Breast phantoms were made with India ink to resemble tumors. The suggested method of determining the ink concentration in sick breasts worked well (234). In 2018 near-infrared spectroscopy and bioimpedance-based sensors were utilized in the same device. Bioimpedance has been used to differentiate healthy tissues from cancerous tissues (235). The control unit received the results from both sensors. In recent years bioimpedance is increasingly used in bioengineering and many products have been launched in the market (236). The PCB was used to implement the entire device. The gadget and app were connected via a Bluetooth module as well. BIS intended to measure output impedance after stimulating breast tumors with low current. In contrast, NIRS sought to employ various LEDs with various wavelengths. The photo detector OPT101 is used to measure the NIRS output. The suggested device is known to have a 99.3% accuracy rate in addition to its 80mW low power consumption (237). Bioimpedance spectroscopy has a bright future in terms of human health applications (238).
2.3.4.3 Digital temperature sensors
In 2020, a circadian device with digital temperature sensors was created with the goal of enhancing breast cancer detection in conjunction with other modalities. Temperature sensors ADT7420 were integrated into patches designed for both breasts, with eight sensors per patch. For a full day, the device recorded data. This sensor-based artificial intelligence device was claimed to have a 78% accuracy rate in differentiating between malignant and benign tumors. The device’s accuracy is similar to that of mammography. However, some temperature data values were missing as a result of the sensor’s inadequate skin contact (239).
2.3.4.4 Microwave based sensors
Diagnostic techniques like mammography, x-rays, ultrasounds, and CT scans have been used for ages to identify cancer early still more advanced techniques are required (193). It has been demonstrated that recent developments in microwave imaging and sensing is offering ease in the detection of breast cancer. The promising results of using antennas to deliver microwaves have attracted the attention of numerous researchers. Antenna has shapes from simple to spirals (240). An attempt was made in 2016 to incorporate a microwave-based antenna array for screening purposes in a bra (241). It has also been demonstrated that circularly polarized microwave sensors, which have an axial ratio that makes them more efficient than linearly polarized ones, are safe to employ in brassieres (225). The addition of microwaves to an imaging system can improve its capabilities for breast cancer diagnosis. A study in 2016 with a compact micro strip antenna proved to be sensitive and effective on detecting breast tumors when the antenna was in contact with the skin. This antenna could easily be implemented in UWB microwave imaging system for more enhanced diagnosis. The experimentation on textile monopole sensors in 2021 did simulations of rectangle shaped monopoles with and without tumor (242). A more enhanced version of this work came out in 2023, where rectangle and circular antennas were used. Computer simulations as well as experimentation on breast models were conducted. The study claims antennas to be effective on tumors greater than 5mm only (225, 242). If we are considering using microwave imaging for diagnosis, we should employ sensor arrays while working with microwave sensors, and we should also examine their sensitivity since this will directly affect the quality of the images. Uneven spacing between sensor arrays and approaches like compressed sensing in signal and image processing should be taken into consideration if image quality improvement is to be achieved at a reasonable cost (224).
2.3.5 Wearable technology for breast cancer detection
Novel, non-invasive techniques for the early detection of breast cancer have been made possible by recent developments in wearable technology. New developments in wearable technology provide a continuous, non-invasive, and patient-friendly method of screening for breast cancer. In order to identify physiological and molecular alterations suggestive of cancer, these devices combine biosensors, imaging elements, and artificial intelligence (AI) algorithms. With an emphasis on smart bras and other cutting-edge gadgets, this section examines the creation, clinical validation, engineering difficulties, and socioeconomic effects of wearable technologies. These devices are a promising replacement or addition to conventional diagnostic methods like mammography and ultrasound because they provide real-time data analysis, enhanced comfort, and continuous monitoring.
Wearable technology uses biosensors to continuously track physiological biomarkers like blood flow, tissue elasticity, electrical impedance, and temperature. Among these, smart bras with optical, piezoresistive, and electrochemical sensors have shown encouraging outcomes in identifying abnormalities in breast tissue early on. These tools have the potential to increase early detection, especially in women with dense breast tissue, which lowers the sensitivity of mammography.
2.3.5.1 Working principles of wearable technologies
Wearable devices incorporate sophisticated engineering principles to facilitate continuous, non- invasive monitoring of physiological and biochemical signals. Below, we detail their fundamental operational mechanisms:
2.3.5.1.1 Flexible electronics and sensor integration
Contemporary wearables utilize flexible materials (such as polymers, graphene, or elastomers) to adapt to the skin’ s contours, promoting comfort and ensuring signal accuracy. These materials house microsensors (like strain gauges and temperature sensors) and biosensors (such as electrochemical and optical sensors) to monitor biomarkers (like glucose, lactate, or pH) and physiological parameters (such as heart rate or movement). For instance, stretchable circuits printed on polydimethylsiloxane (PDMS) allow for real- time tracking of joint movements during rehabilitation (245).
2.3.5.1.2 Biosensing mechanisms
Optical Biosensors: Leverage light- matter interactions (e.g., photoplethysmography (PPG) used in smartwatches) to gauge blood oxygen levels (SpO 2) or pulse rates. They also use techniques such as surface plasmon resonance (SPR) and near- infrared spectroscopy (NIRS) for biomarker identification.
Electrochemical Biosensors: Identify analytes through redox reactions (for example, glucose oxidase- based sensors utilized in diabetes management patches). These systems typically employ enzyme- coated electrodes to transform biochemical signals into electrical outputs.
2.3.5.1.3 Energy harvesting and power management
Wearables adopt energy- efficient designs to enhance their operational lifespan:
Battery- Powered Systems: Most commercial devices, such as fitness trackers, are powered by miniaturized lithium- ion batteries.
Energy Harvesting: Innovative solutions like triboelectric nanogenerators (which convert mechanical energy from movement) and solar cells (which harness light energy) aim to create self- sustaining devices.
2.3.5.1.4 Wireless data transmission
Low- power transmission protocols, including Bluetooth Low Energy (BLE) or ZigBee, facilitate the transfer of data to smartphones or cloud services. Edge computing minimizes latency by processing data locally before it is sent.
2.3.5.1.5 Signal processing and machine learning
Raw sensor data undergo filtering (for instance, noise reduction through Kalman filters) and are analyzed with algorithms (like neural networks) to derive actionable insights (such as detecting arrhythmias from ECG signals).
2.3.5.2 Smart bras
A leading wearable technology for breast cancer detection is the smart bra. These bras are equipped with various biosensors that monitor physiological indicators such as blood flow, tissue stiffness, and temperature, each potentially signaling the early stages of tumor development. By employing IoT-enabled sensors, including ultrasound transducers and thermal sensors, smart bras facilitate early detection by consistently observing breast tissue. They can identify irregularities like temperature fluctuations, changes in density, or concerning lumps (246).
2.3.5.2.1 Thermosensors
Because tumors typically have higher metabolic activity, heat is produced locally. Thermal sensor-equipped smart bras, like Cyrcadia Health’s iTBra, continuously measure the temperature of the breast skin to identify anomalies. Clinical studies showed sensitivity rates that were on par with mammography, especially in dense breast tissue where conventional imaging is ineffective.
2.3.5.2.2 Piezoresistive sensors
These sensors identify variations in the elasticity of breast tissue, which may indicate the existence of tumors. Research has indicated that modifications in mechanical characteristics frequently precede detectable morphological changes.
A pilot clinical study evaluating a thermal-based smart bra reported promising sensitivity in detecting asymmetric heat patterns associated with malignancies, supporting its potential in early detection strategies.
2.3.5.3 Wearable ultrasound patch
The creation of a wearable ultrasound patch by Massachusetts Institute of Technology (MIT) researchers is a noteworthy development in wearable technology for breast cancer. This cutting-edge tool allows users to perform breast imaging at home by attaching to a bra. This patch’s technology uses a miniature ultrasound scanner that creates high-resolution images of breast tissue using piezoelectric materials. Users can obtain a level of detail similar to that of conventional ultrasound probes used in medical imaging centers by moving a tracker along the patch to image the breast from various perspectives (247). This scanner’s initial tests have shown that it can identify cysts as small as 0.3 cm in diameter, which is in line with early-stage tumors.4. By enabling more frequent screening, especially for high-risk individuals who may develop cancer in between routine mammograms, this capability holds great promise for increasing the overall survival rate for patients with breast cancer (247). Additionally, this technology may make breast cancer screening more accessible in rural areas or in less developed nations. Even though there are still efforts to reduce the size of the imaging system and integrate artificial intelligence for image analysis, the device is a big step in the direction of more accessible and individualized breast cancer detection.
2.3.5.4 Biosensors for biomarker detection
Wearable biosensors target specific biomarkers present in bodily fluids, such as blood, sweat, or interstitial fluid, providing early biochemical evidence of malignancy.
2.3.5.4.1 Photonic Crystal Fiber Surface Plasmon Resonance biosensors
PCF-SPR biosensors are highly sensitive to molecular interactions, making them ideal for detecting breast cancer biomarkers like HER2 and CA15-3. Recent advancements in PCF-SPR technology have demonstrated high sensitivity and selectivity for breast cancer detection at low biomarker concentrations. Another study explored SPR biosensors for early cancer detection, noting their potential for non-invasive and real-time monitoring.
2.3.5.4.2 Electrochemical and optical biosensors
These devices measure electrical and optical signals when interacting with specific cancer biomarkers. They offer the advantage of being cost-effective and suitable for at-home use.
Additionally, scientists are working to create customized wearable technology that uses biosensors to analyze bodily fluids non-invasively to find cancer biomarkers. These cutting-edge gadgets, which make use of thin and flexible sensor arrays, can be made into a variety of form factors, including contact lenses, wristbands, mouthguards, and headbands (248). These wearables with biosensors are primarily designed to allow for continuous and real-time monitoring of molecular markers that may indicate the occurrence or recurrence of breast cancer. Early detection, treatment efficacy monitoring, and even the identification of interval cancers—cancers that might arise in between planned screenings—are all possible with this strategy (248). Even though this technology is still mostly in the early stages of development, it is a major step forward for non-invasive diagnostics and could lessen the need for frequent, invasive procedures.
2.3.5.5 Wearables for real-time imaging
Continuous breast tissue monitoring is now possible without the need for expensive imaging facilities thanks to advancements in wearable technology.
2.3.5.5.1 Microwave Imaging
Wearable MWI devices identify variations in breast tissue’s dielectric characteristics. Because malignant tissues contain more water, they can be identified.
2.3.5.5.2 DICOM analysis integration
Continuous, high-resolution imaging and automated anomaly detection outside of clinical settings are made possible by DICOM (Digital Imaging and Communications in Medicine) analysis, which is transforming wearable technologies for breast cancer detection. Its incorporation into smart bras and chest-worn devices, which have historically been used for hospital imaging, offers real-time monitoring, early detection, and enhanced accuracy, especially in dense breast tissues. These devices leverage machine learning algorithms to classify lesions and transmit data remotely, enhancing accessibility through telehealth platforms. Recent studies, such as those employing DICOM-based CT chest imaging (249), show comparable sensitivity to conventional methods with better patient compliance. However, challenges in data management, privacy, and regulatory approval remain. These obstacles should be overcome by developments in edge computing and secure data protocols, making DICOM-integrated wearables a game-changing instrument in breast cancer screening.
2.3.5.6 Artificial intelligence in wearable technologies
2.3.5.6.1 Convolutional Neural Networks
CNNs are used in smart bras and other wearable devices to distinguish between benign and malignant tissue patterns; these models increase sensitivity while lowering false positives.
2.3.5.6.2 Machine learning for risk stratification
Machine learning algorithms evaluate patient-specific data, such as age, genetic factors, and hormonal profiles, to predict breast cancer risk and recommend customized screening intervals.
2.3.3.6.2.1 Challenges in integrating AI with biosensors and wearable technologies
There are many obstacles to overcome when integrating AI-powered imaging with data from wearables and biosensors, especially when it comes to data privacy, effective data transfer, and the requirement for real-time, on-the-fly processing. The complexity is increased by the need for complex fusion algorithms due to the varied nature of the data produced by these technologies (such as photos and time-series sensor readings) (250). Since biosensors and wearable technology gather private health data, data privacy is crucial. Strong encryption and access control systems are essential for ensuring compliance with laws like HIPAA (in the US) and GDPR (in Europe) (251). Another challenge is the effective transfer of data from biosensors and wearable technology to processing units. High-bandwidth communication channels and optimized data compression techniques are necessary to prevent bottlenecks and delays due to the sheer volume of data, particularly when combined with imaging data (252). Additionally, real-time applications like instant feedback or alerts frequently call for on-the-fly processing. This calls for strong computer resources and effective algorithms that can process and analyze data almost instantly, which is computationally demanding and difficult for wearable technology with constrained processing power (253).
2.3.3.6.2.2 Challenges in implementing wearable technologies
Wearable technologies designed for breast cancer detection show great potential, but several hurdles need to be overcome to achieve widespread use in clinical settings.
Accuracy and reliability: While advancements in technology are promising, the issues of false positives and false negatives persist. Over-detection can cause unnecessary worry and lead to invasive procedures, whereas missed diagnoses may delay crucial treatment. To enhance diagnostic accuracy, ongoing sensor calibration and the creation of sophisticated signal processing algorithms are vital.
Battery life and material durability: For continuous, long-term monitoring, devices must be made of robust materials with efficient power management. Products like smart bras and biosensors need to perform reliably even after extended use. Researchers are investigating flexible electronics and energy-efficient circuits to improve both battery life and the durability of materials.
Cost and accessibility: The steep production costs and the complexity of advanced sensor technology can make these wearable devices too expensive for many, especially in low-resource environments. Strategies like government subsidies, collaborations with healthcare organizations, and scalable manufacturing processes are essential for lowering costs and advancing global adoption.
Tackling these challenges is essential for wearable technologies to evolve from experimental tools into dependable clinical instruments, significantly enhancing early breast cancer detection and improving patient outcomes.
Accessibility and affordability are key factors in the broad adoption of wearable breast cancer detection technologies. These devices could become more economically feasible for mass production if production costs are lowered by developments in flexible electronics, material science, and 3D printing (254). Government policies and non-governmental organization (NGO) initiatives are also essential in bridging the gap between public health needs and technological innovation. These life-saving technologies can reach low-income populations with the help of subsidies and healthcare funding, especially in settings with limited resources where traditional screening methods are less accessible. Additionally, by enabling remote screening and follow-up care, the combination of wearable technology and telehealth services offers a revolutionary approach that lowers logistical and geographic barriers to healthcare. Wearable technology holds the key to solving infrastructure and economic issues.
Wearable technology has a lot of promises for detecting breast cancer, but there are still a number of practical issues. Because false positives or negatives might cause needless concern or missed diagnosis, accuracy and dependability are critical issues. Long-term use also depends on battery life and material durability, particularly for devices that are intended for continuous monitoring. Widespread accessibility is nevertheless hampered by high expenses, especially in environments with limited resources. Beyond technical constraints, moral considerations are becoming more and more important. These include preventing AI biases that could impair the accuracy of diagnoses in a variety of populations, guaranteeing informed consent, and protecting private patient data gathered by wearables. To achieve fair, secure, and clinically successful deployment, addressing these issues calls for strong data governance frameworks, inclusive algorithm training, and supportive public health policies.
Table 9 shows the Weaknesses of Wearable Technologies for Breast Cancer Detection and Potential Solutions.
Table 9
| Weakness | Description | Impact on Detection | Potential Solutions | References |
|---|---|---|---|---|
| False Positives/Negatives | The presence (false positive) or absence (false negative) of breast cancer may be misidentified by wearable technology. | False positive results lead to worry, pointless testing, and higher expenses. False negative results can worsen outcomes by delaying diagnosis and treatment. | Enhanced sensitivity and specificity of the sensor. sophisticated algorithms to distinguish between signals that are benign and those that are malignant. | (255) |
| Reliability of Continuous Monitoring | Wearable sensor accuracy can change over time and be impacted by motion artifacts, skin contact, and sensor drift. | Inaccurate or inconsistent data limits the device’s clinical utility and undermines confidence in its readings. | methods for calibrating sensors to account for drift. sturdy designs to reduce the effects of environmental influences and motion. | (256, 257) |
| Clinical Utility | Wearable technology’s place in standard clinical practice is still developing, and there are still issues with data interpretation and integration. | ambiguity regarding the incorporation of wearable data into decisions regarding diagnosis and treatment. absence of established procedures for interpreting data. | extensive clinical studies to confirm efficacy. Rules for clinical decision-making and data interpretation. | (258, 259) |
| Data Interpretation | It can be challenging to distinguish between signs of cancer and normal physiological changes; multimodal analysis and sophisticated algorithms are needed. | may lead to either a failure to recognize actual problems or an overabundance of data analysis and unnecessary actions. | Machine learning algorithms uncover subtle patterns that could be signs of cancer. A more comprehensive analysis can be accomplished by combining different data streams. | (260, 261) |
Weaknesses of wearable technologies for breast cancer detection and potential solutions.
2.3.3.6.2.3 Wearable technology’s cost-benefit analysis for breast cancer screening
To assess the clinical and financial feasibility of these novel methods, a thorough cost-benefit analysis contrasting wearable technology with traditional breast cancer screening is necessary. Both direct costs, like the equipment, personnel and Maintenance, and indirect costs, like lost productivity and travel/logistics, must be taken into account in this analysis. Furthermore, it is important to carefully evaluate how wearable technology might enhance scalability and lessen the strain on healthcare systems. This section offers a preliminary summary of the main cost and benefit factors involved, but a definitive analysis necessitates more investigation and long-term data. To qualitatively assess traditional breast cancer screening techniques, such as mammography and MRI, against emerging wearable technology methods, the authors created a conceptual cost-benefit framework illustrated in Table 10. Conventional screening techniques usually entail significant initial equipment and facility expenses, alongside ongoing costs for clinical appointments, technician labor, and maintenance. Conversely, while still in clinical development, wearable devices have the potential to lower operational costs due to continuous home monitoring and a diminished necessity for frequent hospital visits. Our analysis indicates that the long-term costs of wearable screening might be more advantageous, particularly in minimizing expenses related to delayed diagnoses and treatments. The authors’ analytical review of cost estimates, clinical workflow needs, and diagnostic features from diverse academic literature, healthcare technology assessments, and market analysis served as the foundation for the data presented, which was not derived from a single empirical source. It’s essential to note that most wearable breast cancer detection technologies remain in pre-commercial or experimental phases and have yet to secure full clinical approval for routine diagnostic use. Therefore, this comparison aims to explore how wearable systems may complement traditional modalities, highlighting possible benefits and drawbacks. The figures provided do not indicate precise financial modeling or direct clinical equivalency; they are instead illustrative, intended to facilitate forward-thinking discussions about cost-effectiveness, patient experience, and healthcare access Table 10.
Table 10
| Cost/Benefit Category | Conventional Screening (e.g., Mammography) | Wearable Technology-Based Screening |
|---|---|---|
| Direct Costs | ||
| Equipment | MRI, Mammography machines are highly costly(approx; mammography: $ 150,000 – $500,000, MRI: $1,000,000 – $3,000,000+) | Less than the cost of an MRI, mammography |
| Personnel | Radiologists, technicians. or any staff member required | Minimal staff required, mostly automated |
| Maintenance | 10,000-50,000/year for machine maintenance | It’s less than the conventional, we have to check the software and sensors just |
| Indirect Costs | ||
| Lost Productivity | Workdays missed for appointments | Home-based monitoring |
| Travel/logistics | Transportation cost if a person travels for screening, and this also includes the cost for the transporting machines to rural areas etc | none |
| Benefits | ||
| Early detection | Detect tumors mostly less than 5mm (85% sensitivity) | It can detect early because of biomarker based detection |
| Patients comfort | Moderate; if a person is claustrophobic in MRI or the pain during compression in Mammography | High; radiation free |
| Cost savings | If detected earlier, than saves the cost of late treatment | It can give us long term savings for long-term real-time monitoring |
| Challenges | ||
| False positive | ~10% false positives | This is an emerging technology so it has a higher risk of false positives |
| Accessibility | In a low-resource region, it is difficult for the availability of such equipment | Requires internet, data security |
| Regulatory hurdles | Approvals from FDA | Its novel device so requires more approval |
Cost-benefit analysis framework for breast cancer screening methods.
Wearable technology has the potential to change cost distributions, as shown by the data in Table. For instance, frequent clinical visits may result in lower costs, but it is necessary to account for the cost of wearable technology and the infrastructure that supports it. Furthermore, even though they are hard to measure in monetary terms, wearable technology’s increased convenience and decreased patient anxiety represent substantial potential advantages that ought to be taken into account in addition to the financial considerations. Opportunities for long-term cost savings and better health outcomes are also presented by wearable technologies’ scalability, particularly in remote areas, and their potential for personalized screening. To provide a more conclusive cost-benefit analysis, however, more research is required, including extensive clinical trials and health economic studies.
2.3.6 Smart implants in breast cancer detection
Smart implants are cutting-edge, miniaturized devices specifically engineered to be seamlessly implanted within breast tissue, where they play a crucial role in monitoring vital physiological changes and identifying biomarkers that may signal the early onset of cancer. Smart implants employ microfluidic channels and biosensing electrodes to measure biomarker concentrations in interstitial fluids. When they identify cancer-specific biomarkers, such as HER2 or estrogen receptors, these implants wirelessly transmit data to external monitors for analysis. This continuous real-time monitoring system serves as an early warning mechanism for tumor progression.
Now we will explain the smart implants working in the following workflow:
➢ Integrated sensing technology
These sophisticated implants are equipped with advanced sensors that continuously gather real-time data on various parameters, such as temperature fluctuations, pH levels, and other biochemical markers that can indicate cancerous activity (262). Implanted microsensors identify physical alterations (such as tissue density or impedance) or tumor-associated biomarkers (such as pH changes, hypoxia, or HER2 proteins) connected to the advancement of cancer (263).
➢ Wireless data transmission
The information collected by the implants is transmitted wirelessly to external devices—like smartphones or specialized monitoring systems enabling healthcare professionals to analyze the data with remarkable accuracy. This innovative approach not only allows for the continuous assessment of changes within the body but also acts as a proactive health management tool.
• Power Management and Miniaturization:
Smart implants are engineered with advanced energy-efficient components that prioritize long-term viability. Some utilize innovative wireless energy harvesting technologies, drawing power from ambient sources or employing cutting-edge miniature battery systems. This thoughtful integration allows for prolonged functionality within the human body, significantly reducing the need for surgical replacements or interventions, thereby lessening the burden on patients and healthcare systems alike.
Clinical Relevance:
These smart implants facilitate continuous, high-precision monitoring of breast tissue, employing sophisticated sensors to detect minute changes in tissue composition and density. This capability enhances the potential for early detection of abnormalities, empowering healthcare providers to initiate timely interventions. As a result, the application of smart implants is poised to significantly improve treatment outcomes for breast cancer patients, ultimately contributing to better rates of survival and quality of life.
3 Limitations
Breast cancer diagnosis has improved over the years, but there are still some gaps that require further investigation. The barrier remained in creating unified diagnostic protocol where multiple imaging (including biosensors, sensor arrays and soon AI) can be merged. Our current continuing challenge is to increase the sensitivity and specificity of mammography, particularly in dense breast tissues as well developing more accurate image quality and diagnostic precision for MRI and US. The sensors currently used for diagnosis have low penetration depth, higher degree of which can give high performance. Besides, the sensors may be integrated to detect over all breast areas for superior results or fabricated and movable in circular axis. They are not very suitable for this diagnosis, like LM35 sensors and mostly they are tested on the breast phantoms. In addition, the development of new biomarkers and multiplex biosensors for multiple detection is required to provide more precise and personalized diagnosis. In AI and deep learning algorithms our challenges would be image variance as well at morphological changes, that needs very efficient in coding the models that can withstand images out of variety of datasets. It’s also critical to investigate wearable technology for continuous monitoring, lessen the need for invasive procedures, and advance non-invasive ways for early detection. Furthermore, it is critical to develop affordable, user-friendly diagnostic techniques and to enhance patient comfort and accessibility, particularly in environments with limited resources. The need for creative and comprehensive methods to enhance breast cancer diagnosis and patient care is highlighted by these research gaps.
4 Conclusions and future perspectives
This review has provided a comprehensive analysis of the standard and emerging techniques used for breast cancer (BC) diagnosis. Early detection remains crucial for improving treatment outcomes and survival rates. The study has explored various diagnostic approaches, including advanced imaging techniques such as reflective optical imaging devices (ROIDs), microwave imaging (MWI), automated breast ultrasound (ABUS), and infrared imaging technology (IIT). Additionally, we have highlighted innovative biosensors, including piezoelectric sensors, near-infrared sensors, and digital temperature sensors, each offering unique advantages such as non-invasiveness, enhanced sensitivity, and improved detection accuracy. Despite these advancements, several challenges persist, including the need to enhance diagnostic accuracy, patient comfort, and cost-effectiveness. Many existing technologies, while promising, still require further validation, optimization, and accessibility improvements to ensure widespread clinical adoption. By improving diagnostic precision and reducing false positives, artificial intelligence (AI) has the potential to completely transform the early detection of breast cancer. A subset of deep learning algorithms called Convolutional Neural Networks (CNNs) have shown remarkable performance in image analysis, more accurately detecting tumors in mammograms and ultrasounds than conventional techniques. Large datasets can be processed by AI-driven models to find subtle patterns that humans might miss, resulting in earlier and more accurate diagnoses. Additionally, combining AI with wearable technology—like biosensors and smart bras—allows for continuous data collection and real-time monitoring, which improves patient outcomes and provides individualized risk assessments. Future developments in these technologies will probably concentrate on improving AI algorithms for increased breast cancer screening sensitivity and specificity.
As breast cancer detection methods continue to evolve, wearable and integrated technologies are expected to play a transformative role in early diagnosis and continuous monitoring. One notable innovation in development is the smart bra insert equipped with ultrasound sensors, designed to provide real-time, continuous monitoring of breast tissue. This device detects abnormalities, such as lumps or irregularities, using embedded ultrasound technology, potentially reducing reliance on traditional hospital-based screenings. By integrating these sensors into a comfortable and user-friendly bra design, this technology aims to improve accessibility, encourage proactive health monitoring, and facilitate early detection. Devices like the iTBra have undergone clinical trials to compare performance with standard mammography. Results showed that smart bras could detect anomalies earlier in high-risk populations. Such wearable innovations could significantly reduce late-stage diagnoses and enhance patient outcomes by offering convenient, at-home breast health tracking.
The future of breast cancer detection will likely involve a multi-modal approach, combining AI-driven imaging, advanced biosensors, and wearable technologies to create a more efficient, non-invasive, and personalized diagnostic framework. As research progresses, optimizing these technologies and seamlessly integrating them into routine healthcare practices will be critical in the ongoing fight against breast cancer. Decentralized, continuous breast cancer care with increased precision and privacy may be made possible by emerging technologies like federated AI frameworks, smart fabrics, and microneedle-based biosensors. According to current research, improved mechanical flexibility, multifunctionality, and wireless communication capabilities are anticipated in next-generation biosensing platforms—essential characteristics for a smooth transition into wearable technology and remote healthcare ecosystems (264). Continued interdisciplinary collaboration, clinical trials, and technological refinements will be essential to enhancing early detection methods and ultimately improving patient survival rates worldwide.
Statements
Author contributions
AK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MTo: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. SR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. MTa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. EJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. SA: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI tool named ChatGPT was used to improve the writing. We verify and take full responsibility.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, Body mass index; HRT, Hormone replacement therapy; OCP, Oral contraceptive pills; MBC, Male breast cancer; VABB, Vacuum-assisted breast biopsy; CNB, Core needle biopsy; FNAC, Fine needle aspiration cytology; BC, Breast cancer; QCM, Quartz crystal microbalance; PEC, Photoelectrochemical; FET, Field effect transistor; EIS, Electrochemical impedance spectroscopy; LSV, Linear sweep voltammetry; CV, Cyclic voltammetry; DPV, Differential pulse voltammetry; SWV, Square wave voltammetry; HERC, Human epidermal growth factor receptor; ER, Estrogen receptor; PR, Progesterone receptor; MAPK, Mitogen-activated protein kinase; VEGF, Vascular endothelial growth factor; CBE, Clinical breast examination; UWB, Ultra-wide band.
References
1
SiegelRLMillerKDFuchsHEJemalA. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
2
SiegelRLMillerKDFuchsHEJemalA. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
3
NarodSA. Hormone replacement therapy and the risk of breast cancer. (2011). doi: 10.1038/nrclinonc.2011.110
4
VinogradovaYCouplandCHippisley-CoxJ. Use of hormone replacement therapy and risk of breast cancer: Nested case-control studies using the QResearch and CPRD databases. BMJ. (2020) 371. doi: 10.1136/bmj.m3873
5
LiZJShenGShiMZhengYGuanYXinYet al. Association between high body mass index and prognosis of patients with early-stage breast cancer: A systematic review and meta-analysis. Chin Med Association. (2023) 1:205–15. doi: 10.1016/j.cpt.2023.03.002
6
ZeinomarNKnightJAGenkingerJMPhillipsKADalyMBMilneRLet al. Alcohol consumption, cigarette smoking, and familial breast cancer risk: Findings from the Prospective Family Study Cohort (ProF-SC). Breast Cancer Res. (2019) 21. doi: 10.1186/s13058-019-1213-1
7
TakkarNKochharSGargPPandeyADalalUHandaU. Screening methods(clinical breast examination and mammography) to detect breast cancer in women aged 40–49 years. J Midlife Health. (2017) 8:2–10. doi: 10.4103/jmh.JMH_26_16
8
OeffingerKCFonthamETHEtzioniRHerzigAMichaelsonJSShihYCTet al. Breast cancer screening for women at average risk: 2015 Guideline update from the American cancer society. Am Med Association. (2015) 314:1599–614. doi: 10.1001/jama.2015.12783
9
AutierPBoniolM. Mammography screening: A major issue in medicine. Eur J Cancer. (2018) 90:34–62. doi: 10.1016/J.EJCA.2017.11.002
10
FigueiredoAAAFernandesHCGuimaraesG. Experimental approach for breast cancer center estimation using infrared thermography. Infrared Phys Technol. (2018) 95:100–12. doi: 10.1016/J.INFRARED.2018.10.027
11
DengZSLiuJ. Mathematical modeling of temperature mapping over skin surface and its implementation in thermal disease diagnostics. Comput Biol Med. (2004) 34:495–521. doi: 10.1016/S0010-4825(03)00086-6
12
ArabiPMMuttanSSujiRJ. (2010). 2010 International Conference on Computing, Communication and Networking Technologies (ICCCNT). in: International Conference on Computing, Communication and Networking Technologies (ICCCNT). Piscataway, NJ, USA: IEEE.
13
HeZChenZTanMElingaramiSLiuYLiTet al. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. (2020) 53(7):e12822. doi: 10.1111/cpr.12822
14
DuffyMJHarbeckNNapMMolinaRNicoliniASenkusEet al. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer. (2017) 75:284–98. doi: 10.1016/j.ejca.2017.01.017
15
HongRSunHLiDYangWFanKLiuCet al. A review of biosensors for detecting tumor markers in breast cancer. MDPI. (2022) 12. doi: 10.3390/life12030342
16
García-GutiérrezMSNavarreteFSalaFGasparyanAAustrich-OlivaresAManzanaresJ. Biomarkers in psychiatry: concept, definition, types and relevance to the clinical reality. Front Media S.A. (2020) 11. doi: 10.3389/fpsyt.2020.00432
17
SerhanMJackemeyerDLongMSprowlsMDiez PerezIMaretWet al. (2019). Total iron measurement in human serum with a smartphone, in: AIChE Annual Meeting, Conference Proceedings. New York, NY, USA (American Institute of Chemical Engineers headquarters): American Institute of Chemical Engineers. doi: 10.1039/x0xx00000x
18
KhanmohammadiAAghaieAVahediEQazviniAGhaneiMAfkhamiAet al. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta. (2020) 206:120251. doi: 10.1016/J.TALANTA.2019.120251
19
DonaldsonARMcCarthyCGorayaSPedersonHJSturgisCDGrobmyerSRet al. Breast cancer risk associated with atypical hyperplasia and lobular carcinoma in situ initially diagnosed on core-needle biopsy. Cancer. (2018) 124:459–65. doi: 10.1002/cncr.31061
20
ResminiRFaria da SilvaLMedeirosPRTAraujoASMuchaluat-SaadeDCConciA. A hybrid methodology for breast screening and cancer diagnosis using thermography. Comput Biol Med. (2021) 135:104553. doi: 10.1016/J.COMPBIOMED.2021.104553
21
DíazORodríguez-RuízASechopoulosI. Artificial Intelligence for breast cancer detection: Technology, challenges, and prospects. Eur J Radiol. (2024) 175:111457. doi: 10.1016/J.EJRAD.2024.111457
22
SoodRRositchAFShakoorDAmbinderEPoolKLPollackEet al. Ultrasound for breast cancer detection globally: A systematic review and meta-analysis. J Global Oncol. (2019) 5:1–17. doi: 10.1200/JGO.19
23
BrozaYYZhouXYuanMQuDZhengYVishinkinRet al. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Am Chem Soc. (2019) 119:11761–89. doi: 10.1021/acs.chemrev.9b00437
24
StecklAJRayP. Stress biomarkers in biological fluids and their point-of-use detection. ACS Sens. (2018) 3:2025–44. doi: 10.1021/acssensors.8b00726
25
MaurizE. Low-fouling substrates for plasmonic sensing of circulating biomarkers in biological fluids. MDPI. (2020) 10. doi: 10.3390/BIOS10060063
26
NzegwuMUzoigweJOmotowoBUgochukwuAOzumbaBSuleEet al. Predictive and prognostic relevance of immunohistochemical testing of estrogen and progesterone receptors in breast cancer in South East Nigeria: A review of 417 cases. Rare Tumors. (2021). doi: 10.1177/20363613211006338
27
NicoliniAFerrariPDuffyMJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. (2018) 52:56–73. doi: 10.1016/j.semcancer.2017.08.010
28
AllisonKHHammondMEDowsettMMcKerninSECareyLAFitzgibbonsPLet al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. (2020) 38:1346–66. doi: 10.1200/JCO.19
29
WeigelMTDowsettM. Current and emerging biomarkers in breast cancer: Prognosis and prediction. Endocr Relat Cancer. (2010). doi: 10.1677/ERC-10-0136
30
Breast Cancer TrialistsEGroupC. Relevance of breast cancer hormone receptors and other factors to the effi cacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. (2011) 378:771–84. doi: 10.1016/S0140
31
ClusanLFerrièreFFlouriotGPakdelF. A basic review on estrogen receptor signaling pathways in breast cancer. Multidiscip Digital Publishing Institute (MDPI). (2023) 24. doi: 10.3390/ijms24076834
32
DaiXXiangLLiTBaiZ. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. (2016) 7(10):1281–94. doi: 10.7150/jca.13141
33
CrownJO’ShaughnessyJGulloG. Emerging targeted therapies in triple-negative breast cancer. Ann Oncol. (2012) 23. doi: 10.1093/annonc/mds196
34
da SilvaJLCardoso NunesNCIzettiPde MesquitaGGde MeloAC. Triple negative breast cancer: A thorough review of biomarkers. Crit Rev Oncol Hematol. (2020) 145. doi: 10.1016/j.critrevonc.2019.102855
35
GiovannelliPDi DonatoMGalassoGDi ZazzoEBilancioAMigliaccioA. The androgen receptor in breast cancer. Front Endocrinol (Lausanne). (2018) 9. doi: 10.3389/fendo.2018.00492
36
AsanoYKashiwagiSGotoWTanakaSMorisakiTTakashimaTet alExpression and clinical significance of androgen receptor in triple-negative breast cancer. Ann Oncol. (2014) 25:v69. doi: 10.1093/annonc/mdu435.103
37
Tellez-GabrielMKnutsenEPeranderM. Current status of circulating tumor cells, circulating tumor DNA, and exosomes in breast cancer liquid biopsies. MDPI AG. (2020) 21. doi: 10.3390/ijms21249457
38
Jameera BegamAJubieSNanjanMJ. Estrogen receptor agonists/antagonists in breast cancer therapy: A critical review. Bioorg Chem. (2017) 71:257–74. doi: 10.1016/J.BIOORG.2017.02.011
39
HorwitzKBSartoriusCA. Progesterone and progesterone receptors in breast cancer: Past, present, future. J Mol Endocrinol. (2020) 65(1):T49–63. doi: 10.1530/JME-20-0104
40
KumarHGuptaNVJainRMadhunapantulaSVBabuCSKesharwaniSSet al. A review of biological targets and therapeutic approaches in the management of triple-negative breast cancer. J Adv Res. (2023) 54:271–92. doi: 10.1016/J.JARE.2023.02.005
41
SchettiniFBuonoGCardalesiCDesideriIDe PlacidoSDel MastroL. Hormone Receptor/Human Epidermal Growth Factor Receptor 2-positive breast cancer: Where we are now and where we are going. Cancer Treat Rev. (2016) 46:20–6. doi: 10.1016/J.CTRV.2016.03.012
42
SidranskyD. Emerging molecular markers of cancer. Eur Assoc Cardio-Thoracic Surg. (2002) 2:210–9. doi: 10.1038/nrc755
43
RanjanPPariharAJainSKumarNDhandCMuraliSet al. Biosensor-based diagnostic approaches for various cellular biomarkers of breast cancer: A comprehensive review. Anal Biochem. (2020) 610. doi: 10.1016/j.ab.2020.113996
44
RoointanAMirTAWaniSIRehmanMHussainKKAhmedBet al. Early detection of lung cancer biomarkers through biosensor technology: A review. J Pharm Biomed Anal. (2019) 164:93–103. doi: 10.1016/j.jpba.2018.10.017
45
PiroozmandFMohammadipanahFFaridbodF. Emerging biosensors in detection of natural products. Synth Syst Biotechnol. (2020) 5(4):293–303. doi: 10.1016/j.synbio.2020.08.002
46
MehrotraP. Biosensors and their applications - A review. J Oral Biol Craniofac Res. (2016) 6(2):153–59. doi: 10.1016/j.jobcr.2015.12.002
47
Kal-KoshvandiAT. Recent advances in optical biosensors for the detection of cancer biomarker α-fetoprotein (AFP). TrAC Trends Analytical Chem. (2020) 128:115920. doi: 10.1016/J.TRAC.2020.115920
48
HongCYuanRChaiYZhuoY. Ferrocenyl-doped silica nanoparticles as an immobilized affinity support for electrochemical immunoassay of cancer antigen 15-3. Anal Chim Acta. (2009) 633:244–9. doi: 10.1016/j.aca.2008.11.068
49
VasudevAKaushikABhansaliS. Electrochemical immunosensor for label free epidermal growth factor receptor (EGFR) detection. Biosens Bioelectron. (2013) 39:300–5. doi: 10.1016/j.bios.2012.06.012
50
HakimianFGhourchianH. Ultrasensitive electrochemical biosensor for detection of microRNA-155 as a breast cancer risk factor. Anal Chim Acta. (2020) 1136:1–8. doi: 10.1016/j.aca.2020.08.039
51
WangJWangDHuiN. A low fouling electrochemical biosensor based on the zwitterionic polypeptide doped conducting polymer PEDOT for breast cancer marker BRCA1 detection. Bioelectrochemistry. (2020) 136:107595. doi: 10.1016/J.BIOELECHEM.2020.107595
52
HanRWangGXXuZZhangLLiQHanYet al. Designed antifouling peptides planted in conducting polymers through controlled partial doping for electrochemical detection of biomarkers in human serum. Biosens Bioelectron. (2020) 164:112317. doi: 10.1016/J.BIOS.2020.112317
53
XiaYMLiMYChenCLXiaMZhangWGaoWW. Employing label-free electrochemical biosensor based on 3D-reduced graphene oxide and polyaniline nanofibers for ultrasensitive detection of breast cancer BRCA1 biomarker. Electroanalysis. (2020) 32:2045–55. doi: 10.1002/elan.202060039
54
ChangJWangXWangJLiHLiF. Nucleic acid-functionalized metal-organic framework-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal Chem. (2019) 91:3604–10. doi: 10.1021/acs.analchem.8b05599
55
WangHSunJLuLYangXXiaJZhangFet al. Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin1. Anal Chim Acta. (2020) 1094:18–25. doi: 10.1016/j.aca.2019.10.003
56
XuSChangYWuZLiYYuanRChaiY. One DNA circle capture probe with multiple target recognition domains for simultaneous electrochemical detection of miRNA-21 and miRNA-155. Biosens Bioelectron. (2020) 149. doi: 10.1016/j.bios.2019.111848
57
MarquesRCBViswanathanSNouwsHPADelerue-MatosCGonzález-GarcíaMB. Electrochemical immunosensor for the analysis of the breast cancer biomarker HER2 ECD. Talanta. (2014) 129:594–9. doi: 10.1016/j.talanta.2014.06.035
58
FreitasMNouwsHPADelerue-MatosC. Electrochemical sensing platforms for HER2-ECD breast cancer biomarker detection. Electroanalysis. (2019) 31:121–8. doi: 10.1002/elan.201800537
59
ZhaoJTangYCaoYChenTChenXMaoXet al. Amplified electrochemical detection of surface biomarker in breast cancer stem cell using self-assembled supramolecular nanocomposites. Electrochim Acta. (2018) 283:1072–8. doi: 10.1016/j.electacta.2018.07.002
60
CesewskiEJohnsonBN. Electrochemical biosensors for pathogen detection. Biosens Bioelectron. (2020) 159. doi: 10.1016/j.bios.2020.112214
61
GuCGuoCLiZWangMZhouNHeLet al. Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: Ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens Bioelectron. (2019) 134:8–15. doi: 10.1016/j.bios.2019.03.043
62
PaimardGShahlaeiMMoradipourPKaramaliVArkanE. Impedimetric aptamer based determination of the tumor marker MUC1 by using electrospun core-shell nanofibers. Microchimica Acta. (2020) 187. doi: 10.1007/s00604-019-3955-y
63
LazanasACProdromidisMI. Electrochemical impedance spectroscopy─A tutorial. Am Chem Society. (2023) 3:97–113. doi: 10.1021/acsmeasuresciau.2c00070
64
ShahrokhianSSalimianR. Ultrasensitive detection of cancer biomarkers using conducting polymer/electrochemically reduced graphene oxide-based biosensor: Application toward BRCA1 sensing. Sens Actuators B Chem. (2018) 266:160–9. doi: 10.1016/j.snb.2018.03.120
65
MajdSMSalimiAGhasemiF. An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosens Bioelectron. (2018) 105:6–13. doi: 10.1016/J.BIOS.2018.01.009
66
BaoZSunJZhaoXLiZCuiSMengQet al. Top-down nanofabrication of silicon nanoribbon field effect transistor (Si-NR FET) for carcinoembryonic antigen detection. Int J Nanomed. (2017) 12:4623–31. doi: 10.2147/IJN.S135985
67
NguyenTTHNguyenCMHuynhMAVuHHNguyenTKNguyenNT. Field effect transistor based wearable biosensors for healthcare monitoring. BioMed Cent Ltd. (2023) 21. doi: 10.1186/s12951-023-02153-1
68
MohammadiSMohammadiSSalimiA. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells. Talanta. (2021) 224:121895. doi: 10.1016/J.TALANTA.2020.121895
69
WangYWeiZLuoXWanQQiuRWangS. An ultrasensitive homogeneous aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Talanta. (2019) 195:33–9. doi: 10.1016/J.TALANTA.2018.11.011
70
BaiYLiHXuJHuangYZhangXWengJet al. Ultrasensitive colorimetric biosensor for BRCA1 mutation based on multiple signal amplification strategy. Biosens Bioelectron. (2020) 166:112424. doi: 10.1016/J.BIOS.2020.112424
71
ChoiJHLimJShinMPaekSHChoiJW. CRISPR-cas12a-based nucleic acid amplification-free DNA biosensor via au nanoparticle-assisted metal-enhanced fluorescence and colorimetric analysis. Nano Lett. (2021) 21:693–9. doi: 10.1021/acs.nanolett.0c04303
72
SzymanskaBLukaszewskiZHermanowicz-SzamatowiczKGorodkiewiczE. An immunosensor for the determination of carcinoembryonic antigen by Surface Plasmon Resonance imaging. Anal Biochem. (2020) 609:113964. doi: 10.1016/J.AB.2020.113964
73
SinaAAIVaidyanathanRWuethrichACarrascosaLGTrauM. Label-free detection of exosomes using a surface plasmon resonance biosensor. Anal Bioanal Chem. (2019) 411:1311–8. doi: 10.1007/s00216-019-01608-5
74
WangQZouLYangXLiuXNieWZhengYet al. Direct quantification of cancerous exosomes via surface plasmon resonance with dual gold nanoparticle-assisted signal amplification. Biosens Bioelectron. (2019) 135:129–36. doi: 10.1016/J.BIOS.2019.04.013
75
HaYQiangLGaoYGaoJHeQLiuHet al. Large-area surface-enhanced Raman spectroscopy substrate by hybrid porous GaN with Au/Ag for breast cancer miRNA detection. Appl Surf Sci. (2021) 541:148456. doi: 10.1016/J.APSUSC.2020.148456
76
WangHMWangAJYuanPXFengJJ. Flower-like metal-organic framework microsphere as a novel enhanced ECL luminophore to construct the coreactant-free biosensor for ultrasensitive detection of breast cancer 1 gene. Sens Actuators B Chem. (2020) 320:128395. doi: 10.1016/J.SNB.2020.128395
77
RamolaAMarwahaASinghS. Design and investigation of a dedicated PCF SPR biosensor for CANCER exposure employing external sensing. Appl Phys A Mater Sci Process. (2021) 127. doi: 10.1007/s00339-021-04785-2
78
ShakyaAKRamolaASinghSVanV. Design of an ultra-sensitive bimetallic anisotropic PCF SPR biosensor for liquid analytes sensing. Opt Express. (2022) 30:9233. doi: 10.1364/oe.432263
79
YangXZhouRHaoYYangP. A CD44-biosensor for evaluating metastatic potential of breast cancer cells based on quartz crystal microbalance. Sci Bull (Beijing). (2017) 62:923–30. doi: 10.1016/J.SCIB.2017.05.022
80
LinSWangJLinYWangX. Biosensitivity of molybdenum disulfide for monitoring breast cancer marker CA15–3 using quartz crystal microbalance. Int J Electrochem Sci. (2021) 16:150712. doi: 10.20964/2021.01.02
81
BakhshpourMPiskinAKYavuzHDenizliA. Quartz crystal microbalance biosensor for label-free MDA MB 231 cancer cell detection via notch-4 receptor. Talanta. (2019) 204:840–5. doi: 10.1016/J.TALANTA.2019.06.060
82
GuoXLiuSYangMDuHQuF. Dual signal amplification photoelectrochemical biosensor for highly sensitive human epidermal growth factor receptor-2 detection. Biosens Bioelectron. (2019) 139:111312. doi: 10.1016/J.BIOS.2019.05.017
83
FuYZouKLiuMZhangXDuCChenJ. Highly selective and sensitive photoelectrochemical sensing platform for VEGF165 assay based on the switching of photocurrent polarity of cdS QDs by porous cu2O-cuO flower. Anal Chem. (2020) 92:1189–96. doi: 10.1021/acs.analchem.9b04319
84
A layered signalling network (2001). Available online at: www.nature.com/reviews/molcellbio (Accessed October 23, 2024).
85
XinHShaXJiangXZhangWChenLFangX. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. (2012) 33:8167–76. doi: 10.1016/J.BIOMATERIALS.2012.07.046
86
LuTJiSJinWYangQLuoQRenTL. Biocompatible and long-term monitoring strategies of wearable, ingestible and implantable biosensors: reform the next generation healthcare. MDPI. (2023) 23. doi: 10.3390/s23062991
87
ParlakOKeeneSTMaraisACurtoVFSalleoA. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing(2018). Available online at: https://www.science.org (Accessed October 23, 2024).
88
SinhIFogleRLGulfidanGStanleyAEWalterVHollenbeakCSet al. Potential early markers for breast cancer: A proteomic approach comparing saliva and serum samples in a pilot study. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24044164
89
JafariMHasanzadehM. Cell-specific frequency as a new hallmark to early detection of cancer and efficient therapy: Recording of cancer voice as a new horizon. Biomed Pharmacother. (2020) 122. doi: 10.1016/j.biopha.2019.109770
90
LiPLeeGHKimSYKwonSYKimHRParkS. From diagnosis to treatment: recent advances in patient-friendly biosensors and implantable devices. Am Chem Soc. (2021) 15:1960–2004. doi: 10.1021/acsnano.0c06688
91
IkegwuonuTHaddowGTaitJMurrayAFKunklerIH. Horizon scanning implanted biosensors in personalising breast cancer management: First pilot study of breast cancer patients views. Health Sci Rep. (2018) 1. doi: 10.1002/hsr2.30
92
GrayMEMeehanJBlairEOWardCLangdonSPMorrisonLRet al. Biocompatibility of common implantable sensor materials in a tumor xenograft model. J BioMed Mater Res B Appl Biomater. (2019) 107:1620–33. doi: 10.1002/jbm.b.34254
93
ChandraPTanYNSinghSP. Next generation point-of-care biomedical sensors technologies for cancer diagnosis. Singapore: Springer (2017). doi: 10.1007/978-981-10-4726-8
94
ChowdhurySAbdulrazakLFAkhtar MituSAAhmedKBuiFMSmiraniLKet al. A highly sensitive multi-channel SPR-PCF based biosensor with deep learning prediction approach. Alexandria Eng J. (2023) 77:189–203. doi: 10.1016/J.AEJ.2023.06.093
95
LitjensGKooiTEhteshami BejnordiBSetioAACiompiFGhafoorianMet al. A survey on deep learning in medical image analysis. Med Image Anal. (2017) 42:60–88. doi: 10.1016/j.media.2017.07.005
96
ChilamkurthySGhoshRTanamalaSBivijiMCampeauNGVenugopalVKet al. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet. (2018) 392:2388–96. doi: 10.1016/S0140-6736(18)31645-3
97
BurbidgeRTrotterMBuxtonBHoldenS. Drug design by machine learning: support vector machines for pharmaceutical data analysis (2001). Available online at: www.elsevier.com/locate/compchem (Accessed October 23, 2024).
98
HwangEJParkSJinKNKimJIChoiSYLeeJHet al. Development and validation of a deep learning-based automated detection algorithm for major thoracic diseases on chest radiographs. JAMA Netw Open. (2019) 2:e191095. doi: 10.1001/jamanetworkopen.2019.1095
99
GeraKJet al. High-resolution breast cancer screening with multi-view deep convolutional neural networks(2017). Available online at: http://arxiv.org/abs/1703.07047 (Accessed October 23, 2024).
100
SadoughiFKazemyZHamedanFOwjiLRahmanikatigariMAzadboniTT. Artificial intelligence methods for the diagnosis of breast cancer by image processing: A review. Breast Cancer Targets Ther. (2018) 10:219–30. doi: 10.2147/BCTT.S175311
101
MazoCOrue-EtxebarriaEZabalzaIVivancoMDMKyptaRMBeristainA. In silico approach for immunohistochemical evaluation of a cytoplasmic marker in breast cancer. Cancers (Basel). (2018) 10. doi: 10.3390/cancers10120517
102
.
103
ShiPZhongJHongJHuangRWangKChenY. Automated ki-67 quantification of immunohistochemical staining image of human nasopharyngeal carcinoma xenografts. Sci Rep. (2016) 6. doi: 10.1038/srep32127
104
MazoCBarronSMooneyCGallagherWM. Multi-gene prognostic signatures and prediction of pathological complete response of ER-Positive HER2-negative breast cancer patients to neo-adjuvant chemotherapy. Ann Oncol. (2019) 30:v86. doi: 10.1093/ANNONC/MDZ240.081
105
MazoCBarronSMooneyCGallagherWM. Multi-gene prognostic signatures and prediction of pathological complete response to neoadjuvant chemotherapy in er-positive, her2-negative breast cancer patients. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12051133
106
CupplesTE. Single reading with computer-aided detection for screening mammography. Breast Diseases: A Year Book Q. (2009) 20:161–2. doi: 10.1016/s1043-321x(09)79258-6
107
RajpurkarPChenEBanerjeeOTopolEJ. AI in health and medicine. Nat Res. (2022) 28:31–38. doi: 10.1038/s41591-021-01614-0
108
HickmanSEBaxterGCGilbertFJ. Adoption of artificial intelligence in breast imaging: evaluation, ethical constraints and limitations. Nat Med. (2021) 28:31–8. doi: 10.1038/s41416-021-01333-w
109
KannBHHosnyAAertsHJWL. Artificial intelligence for clinical oncology. Cancer Cell. (2021) 397:916–27. doi: 10.1016/j.ccell.2021.04.002
110
NiaziMKKParwaniAVGurcanMN. Digital pathology and artificial intelligence. Digit Pathol Artif Intell. (2019) 20:E253–61. doi: 10.1016/S1470-2045(19)30154-8
111
UzunYTezelG. RULE LEARNING WITH MACHINE LEARNING ALGORITHMS AND ARTIFICIAL NEURAL NETWORKS. J Selcuk Univ Nat Appl Sci. (2012) 1:54–65.
112
Joanne PengC-YLida LeeKIngersollGM. An introduction to logistic regression analysis. J Educ Res. (2002) 96:3–14. doi: 10.1080/00220670209598786
113
DaiQWangT. Comparison study on k-word statistical measures for protein: From sequence to ‘sequence space,’. BMC Bioinf. (2008) 9. doi: 10.1186/1471-2105-9-394
114
KumarSSharmaH. A survey on decision tree algorithms of classification in data mining(2016). Available online at: www.ijsr.net (Accessed November 4, 2024).
115
TranH. Survey of machine learning and data mining techniques used in multimedia system A SURVEY OF MACHINE LEARNING AND DATA MINING TECHNIQUES USED IN MULTIMEDIA SYSTEM A PREPRINT. Preprint. (2019). doi: 10.13140/RG.2.2.20395.49446/1
116
EvgeniouTPontilM. Support Vector Machines: Theory and Applications. (2001). Center for Biological and Computational Learning, and Artificial Intelligence Laboratory, MIT, E25-201, Cambridge, MA 02139, USA.
117
BreimanL. Random Forests. (2001). Statistics Department, University of California, Berkeley, CA.
118
JainAK. Data clustering: 50 years beyond K-means. Pattern Recognit Lett. (2010) 31:651–66. doi: 10.1016/J.PATREC.2009.09.011
119
BezdekJCEhrlichRFullW. FCM: The fuzzy c-means clustering algorithm. Comput Geosci. (1984) 10:191–203. doi: 10.1016/0098-3004(84)90020-7
120
MurtaghFLegendreP. Ward’s hierarchical agglomerative clustering method: which algorithms implement ward’s criterion? J Classif. (2014) 3:274–95. doi: 10.1007/s00357-014-9161-z
121
ChenGLvZWangPSunZLiYLiuJet al. (2016). 2016 8th International Conference on Information Technology in Medicine and Education (ITME). in: 2016 8th International Conference on Information Technology in Medicine and Education (ITME). Piscataway, NJ, USA (IEEE headquarters): IEEE.
122
SechopoulosITeuwenJMannR. Artificial intelligence for breast cancer detection in mammography and digital breast tomosynthesis: State of the art. Semin Cancer Biol. (2021) 72:214–25. doi: 10.1016/j.semcancer.2020.06.002
123
TuggenerLAmirianMRombachKLörwaldSVarletAWestermannCet al. (2019). Automated machine learning in practice: state of the art and recent results, in: Proceedings - 6th Swiss Conference on Data Science, SDS 2019, . pp. 31–6. Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers Inc. doi: 10.1109/SDS.2019.00-11
124
QingDXuSHXinL. (2009). Parallel process neural networks and its application in the predication of sunspot number series, in: Proceedings of the 5th International Conference on Natural Computation (ICNC 2009). Los Alamitos, California, USA VLDBA Villanova University Library Open Library: IEEE Computer Society DBLP +1 ACM Digital Library. pp. 237–41. doi: 10.1109/ICNC.2009.335
125
TsaiWKParlosAFernandezB. ASDM-A novel neural network model based on sparse distributed memory. In Proceedings of the 1990 IJCNN International Joint Conference on Neural Network (1990) 1:771–6. doi: 10.1109/IJCNN.1990.137662
126
UtomoCPKardianaAYuliwulandariR. Breast Cancer Diagnosis using Artificial Neural Networks with Extreme Learning Techniques(2014). Available online at: www.ijarai.thesai.org (Accessed November 4, 2024).
127
BordeSDJoshiKR. Enhanced signal detection slgorithm using trained neural network for cognitive radio receiver. Int J Electrical Comput Eng (IJECE). (2019) 9:323. doi: 10.11591/ijece.v9i1.pp323-331
128
BolandraftarMBafandehSAndI. Application of K-nearest neighbor (KNN) approach for predicting economic events theoretical background. Available online at: www.ijera.com (Accessed November 4, 2024).
129
LiYWuH. A clustering method based on K-means algorithm. Phys Proc. (2012) 25:1104–9. doi: 10.1016/j.phpro.2012.03.206
130
SchmidhuberJ. Deep Learning in neural networks: An overview. Neural Netw. (2015) 61:85–117. doi: 10.1016/j.neunet.2014.09.003
131
NassifABShahinIAttiliIAzzehMShaalanK. Speech recognition using deep neural networks: A systematic review. IEEE Access. (2019) 7:19143–65. doi: 10.1109/ACCESS.2019.2896880
132
HuZTangJWangZZhangKZhangLSunQ. Deep learning for image-based cancer detection and diagnosis – A survey. Pattern Recognit. (2018) 83:134–49. doi: 10.1016/J.PATCOG.2018.05.014
133
SelvathiDAarthy PoornilaA. Deep learning techniques for breast cancer detection using medical image analysis. In: Lecture Notes in Computational Vision and Biomechanics, vol. 25. Springer, Netherlands (2018). p. 159–86. doi: 10.1007/978-3-319-61316-1_8
134
MunirKElahiHAyubAFrezzaFRizziA. Cancer diagnosis using deep learning: A bibliographic review. Cancers (Basel). (2019) 11. doi: 10.3390/cancers11091235
135
FakoorRNaziAHuberM. Using deep learning to enhance cancer diagnosis and classification (2013). Available online at: https://www.researchgate.net/publication/281857285 (Accessed November 5, 2024).
136
KishoreVSMahaGSGomathiRMVaniSMadhusudhana RaoTVet al. (2019). Proceedings of the International Conference on Trends in Electronics and Informatics (ICOEI 2019) : 23-25, April 2019. Piscataway, NJ, USA: IEEE.
137
AlghunaimSAl-BaityHH. On the scalability of machine-learning algorithms for breast cancer prediction in big data context. IEEE Access. (2019) 7:91535–46. doi: 10.1109/ACCESS.2019.2927080
138
Al BatainehA. A comparative analysis of nonlinear machine learning algorithms for breast cancer detection. Int J Mach Learn Comput. (2019) 9:248–54. doi: 10.18178/ijmlc.2019.9.3.794
139
TurgutSDagtekinMEnsariT. (2018). Microarray breast cancer data classification using machine learning methods, in: 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting, EBBT 2018, . pp. 1–3. Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers Inc. doi: 10.1109/EBBT.2018.8391468
140
DhahriHAl MaghayrehEMahmoodAElkilaniWNagiMF. Automated breast cancer diagnosis based on machine learning algorithms. J Healthc Eng. (2019) 1–11. doi: 10.1155/2019/4253641
141
Kaya KeleşM. Breast cancer prediction and detection using data mining classification algorithms: A comparative study. Tehnicki Vjesnik. (2019) 26:149–55. doi: 10.17559/TV-20180417102943
142
Khan MalihaSAhmedHRahman EmaRRafsun Jony MollickMKumar GhoshSIslamT. Cancer disease prediction using naive bayes,K-nearest neighbor and J48 algorithm. In Proceedings of the 1990 IJCNN International Joint Conference on Neural Network. doi: 10.1109/ICCCNT45670.2019.8944686
143
MemonMHLiJPHaqAUMemonMHZhouWLacuestaR. Breast cancer detection in the IOT health environment using modified recursive feature selection. Wirel Commun Mob Comput. (2019) 2019. doi: 10.1155/2019/5176705
144
OmondiagbeDAVeeramaniSSidhuAS. (2019). Machine learning classification techniques for breast cancer diagnosis, in: IOP Conference Series: Materials Science and Engineering. Piscataway, NJ, USA: Institute of Physics Publishing. doi: 10.1088/1757-899X/495/1/012033
145
BharatAAnishka ReddyR. Using Machine Learning algorithms for breast cancer risk prediction and diagnosis. Available online at: http://archive.ics.uci.edu/ml (Accessed November 5, 2024).
146
SchambersAEavis-O’QuinnMERobergeVTarbouchiMAllaireFEl HammoutiIet al. (2018). 2018 4th International Conference on Optimization and Applications (ICOA) in: ICOA : 2018 4th International Conference on Optimization and Applications : 26–27 April 2018, Mohammedia, Morocco. Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers.
147
SaidAAAbd-ElmegidLAKholeifSProfessorAGaberAA. Classification based on Clustering Model for Predicting Main Outcomes of Breast Cancer using Hyper-Parameters Optimization(2018). Available online at: www.ijacsa.thesai.org (Accessed November 6, 2024).
148
ShreyasCNSrivatsaVPitaleVAnandaMKumarMMiniSet al. (2018)., in: Proceedings of the International conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC 2018) : 30-31, August 2018, . IEEE.
149
IbrahimAAHashadAIShawkyNEM. (2017), 636–51. doi: 10.4018/978-1-5225-2229-4.ch027
150
BansalASinghalA. (2017)., in: Proceedings of the 7th International Conference Confluence 2017 on Cloud Computing, Data Science and Engineering : 12th-13th January 2017, Amity University, Noida, Uttar Pradesh, India. Piscataway, NJ, USA: IEEE.
151
AsriHMousannifHAl MoatassimeHNoelT. Using machine learning algorithms for breast cancer risk prediction and diagnosis. Proc Comput Sci. (2016) 83:1064–9. doi: 10.1016/J.PROCS.2016.04.224
152
PadmapriyaBVelmuruganTInVC. Classification algorithm based analysis of breast cancer data. IIR) Int J Data Min Techniques Appl. (2016) 5:43–9. doi: 10.20894/IJDMTA.102.005.001.010
153
WilliamsKAdebayo IdowuPAdemola BalogunJIshola OluwarantiA. BREAST CANCER RISK PREDICTION USING DATA MINING CLASSIFICATION TECHNIQUES. Trans Networks Commun. (2015) 3. doi: 10.14738/tnc.32.662
154
IranmakaniSMortezazadehTSajadianFGhazianiMFGhafariAKhezerlooDet al. A review of various modalities in breast imaging: technical aspects and clinical outcomes. Egypt J Radiol Nucl Med. (2020) 51. doi: 10.1186/s43055-020-00175-5
155
ZeeshanMSalamBKhalidQSBAlamSSayaniR. Diagnostic accuracy of digital mammography in the detection of breast cancer. Cureus. (2018) 10. doi: 10.7759/cureus.2448
156
Von Euler-ChelpinMLillholmMVejborgINielsenMLyngeE. Sensitivity of screening mammography by density and texture: A cohort study from a population-based screening program in Denmark. Breast Cancer Res. (2019) 21. doi: 10.1186/s13058-019-1203-3
157
ProczSRoqueGAvilaCRacedoJRuedaRSantosIet al. Investigation of cdTe, gaAs, se and si as sensor materials for mammography. IEEE Trans Med Imaging. (2020) 39:3766–78. doi: 10.1109/TMI.2020.3004648
158
MannRMKuhlCKMoyL. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. (2019) 50:377–90. doi: 10.1002/jmri.26654
159
HuJFengWHuaJJiangQXuanYLiTet al. A high spatial resolution in vivo 1H magnetic resonance spectroscopic imaging technique for the human breast at 3 T. Med Phys. (2009) 36:4870–7. doi: 10.1118/1.3213087
160
FardaneshRMarinoMAAvendanoDLeithnerDPinkerKThakurSB. Proton MR spectroscopy in the breast: Technical innovations and clinical applications. J Magn Reson Imaging. (2019) 50:1033–46. doi: 10.1002/jmri.26700
161
LaiPHHsuSSDingSWKoCWFuJHWengMJet al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging in intracranial cystic mass lesions. Surg Neurol. (2007) 68:S25–36. doi: 10.1016/J.SURNEU.2007.07.080
162
CaiHLiuLPengYWuYLiL. Diagnostic assessment by dynamic contrast-enhanced and diffusion-weighted magnetic resonance in differentiation of breast lesions under different imaging protocols. BMC Cancer. (2014) 14. doi: 10.1186/1471-2407-14-366
163
JansenSAFanXKarczmarGSAbeHSchmidtRAGigerMGet al. DCEMRI of breast lesions: Is kinetic analysis equally effective for both mass and nonmass-like enhancement? Med Phys. (2008) 35:3102–9. doi: 10.1118/1.2936220
164
TaoWHuCBaiGZhuYZhuY. Correlation between the dynamic contrast-enhanced MRI features and prognostic factors in breast cancer A retrospective case-control study. Med (United States). (2018) 97. doi: 10.1097/MD.0000000000011530
165
PereiraNPCuriCOsorioCATMarquesEFMakdissiFBPinkerKet al. Diffusion-weighted magnetic resonance imaging of patients with breast cancer following neoadjuvant chemotherapy provides early prediction of pathological response – A prospective study. Sci Rep. (2019) 9. doi: 10.1038/s41598-019-52785-3
166
NarayananDBergWA. Dedicated breast gamma camera imaging and breast PET: current status and future directions. (2018). doi: 10.1016/j.cpet.2018.02.008
167
FerrucciMFranceschiniGDouekM. New techniques for sentinel node biopsy in breast cancer. Transl Cancer Res. (2018) 7:405–17. doi: 10.21037/tcr.2018.02.07
168
NanduVVChaudhariMS. Efficacy of sentinel lymph node biopsy in detecting axillary metastasis in breast cancer using methylene blue. Indian J Surg Oncol. (2017) 8:109–12. doi: 10.1007/s13193-016-0616-z
169
BremRFRudaRCYangJLCoffeyCMRapelyeaJA. Breast-specific γ-imaging for the detection of mammographically occult breast cancer in women at increased risk. J Nuclear Med. (2016) 57:678–84. doi: 10.2967/jnumed.115.168385
170
HolbrookANewellMS. Alternative screening for women with dense breasts: Breast-specific gamma imaging (molecular breast imaging). AJR Am J Roentgenol. (2015) 204:252–6. doi: 10.2214/AJR.14.13525
171
LiuHZhanHSunDZhangY. Comparison of BSGI, MRI, mammography, and ultrasound for the diagnosis of breast lesions and their correlations with specific molecular subtypes in Chinese women. BMC Med Imaging. (2020) 20. doi: 10.1186/s12880-020-00497-w
172
GøtzschePCJørgensenKJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. (2013). doi: 10.1002/14651858.CD001877.pub5
173
BaeMSSeoBK. Breast cancer screening with digital breast tomosynthesis improves performance of mammography screening. Radiology. (2023) 307. doi: 10.1148/RADIOL.230306
174
GilbertFJTuckerLYoungKC. Digital breast tomosynthesis (DBT): A review of the evidence for use as a screening tool. Clin Radiol. (2016) 71:141–50. doi: 10.1016/j.crad.2015.11.008
175
GurDAbramsGSChoughDMGanottMAHakimCMPerrinRLet al. Digital breast tomosynthesis: Observer performance study. Am J Roentgenol. (2009) 193:586–91. doi: 10.2214/AJR.08.2031
176
RaffertyEAParkJMPhilpottsLEPoplackSPSumkinJHHalpernEFet al. assessing radiologist Performance Using combined Digital Mammography and Breast Tomosynthesis compared with Digital Mammography alone: Results of a Multicenter, Multireader Trial 1. Radiology. (2013) 266. doi: 10.1148/radiol.12120674/-/DC1
177
SkaanePBandosAIGullienREbenEBEksethUHaakenaasenUet al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a populationbased screening program. Radiology. (2013) 267:47–56. doi: 10.1148/radiol.12121373
178
CaumoFBernardiDCiattoSMacaskillPPellegriniMBrunelliSet al. Incremental effect from integrating 3D-mammography (tomosynthesis) with 2D-mammography: Increased breast cancer detection evident for screening centres in a population-based trial. Breast. (2014) 23:76–80. doi: 10.1016/j.breast.2013.11.006
179
ConanEFZuckermanSPMcDonaldESWeinsteinSPKorhonenKEBirnbaumJAet al. Five consecutive years of screening with digital breast tomosynthesis: Outcomes by screening year and round. Radiology. (2020) 295:285–93. doi: 10.1148/radiol.2020191751
180
PhilpottsLEGrewalJKHorvathLJGiwercMYStaibLEtesamiM. Breast cancers detected during a decade of screening with digital breast tomosynthesis: comparison with digital mammography. Radiology. (2024) 312. doi: 10.1148/radiol.232841
181
BernardiDCiattoSPellegriniMAnesiVBurlonSCauliEet al. Application of breast tomosynthesis in screening: Incremental effect on mammography acquisition and reading time. Br J Radiol. (2012) 85. doi: 10.1259/bjr/19385909
182
GiampietroRRCabralMVGLimaSAMWeberSATdos Santos Nunes-NogueiraV. Accuracy and effectiveness of mammography versus mammography and tomosynthesis for population-based breast cancer screening: A systematic review and meta-analysis. Sci Rep. (2020) 10. doi: 10.1038/s41598-020-64802-x
183
SteyerovaPBurgetovaA. Current imaging techniques and impact on diagnosis and survival —a narrative review. Ann Breast Surg. (2022) 6:25–5. doi: 10.21037/abs-21-22
184
MehdyMMNgPYShairEFSalehNIMGomesC. Artificial neural networks in image processing for early detection of breast cancer. Comput Math Methods Med. (2017). doi: 10.1155/2017/2610628
185
MaiHTNgoDQNguyenHPTLaDD. Fabrication of a reflective optical imaging device for early detection of breast cancer. Bioengineering. (2023) 10. doi: 10.3390/bioengineering10111272
186
AlsawaftahNEl-AbedSDhouSZakariaA. Microwave imaging for early breast cancer detection: current state, challenges, and future directions. J Imaging. (2022) 8. doi: 10.3390/jimaging8050123
187
JanjicACayorenMAkdumanIYilmazTOnemliEBugdayciOet al. Safe: A novel microwave imaging system design for breast cancer screening and early detection—clinical evaluation. Diagnostics. (2021) 11. doi: 10.3390/diagnostics11030533
188
LiuCXueCZhangBZhangGHeC. The application of an ultrasound tomography algorithm in a novel ring 3D ultrasound imaging system. Sensors (Switzerland). (2018) 18. doi: 10.3390/s18051332
189
RahpeimaRSoltaniMMoradi KashkooliF. Numerical study of microwave induced thermoacoustic imaging for initial detection of cancer of breast on anatomically realistic breast phantom. Comput Methods Programs BioMed. (2020) 196:105606. doi: 10.1016/J.CMPB.2020.105606
190
SinghDSinghAK. Role of image thermography in early breast cancer detection- Past, present and future. Comput Methods Programs BioMed. (2020) 183:105074. doi: 10.1016/J.CMPB.2019.105074
191
KandlikaSGPerez-RayaIRaghupathiPAGonzalez-HernandezJLDabydeenDMedeirosLet al. Infrared imaging technology for breast cancer detection – Current status, protocols and new directions. Int J Heat Mass Transf. (2017) 108:2303–20. doi: 10.1016/J.IJHEATMASSTRANSFER.2017.01.086
192
JanjicAAkdumanICayorenMBugdayciOAribalME. Gradient-boosting algorithm for microwave breast lesion classification—SAFE clinical investigation. Diagnostics. (2022) 12. doi: 10.3390/diagnostics12123151
193
WangL. Microwave imaging and sensing techniques for breast cancer detection. Micromachines. (2023) 14. doi: 10.3390/mi14071462
194
ShereMLyburnISidebottomRMasseyHGillettCJonesL. MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur J Radiol. (2019) 116:61–7. doi: 10.1016/J.EJRAD.2019.04.017
195
SaniLVispaALoretoniRDurantiMGhavamiNAlvarez Sanchez-BayuelaDet al. Breast lesion detection through MammoWave device: Empirical detection capability assessment of microwave images’ parameters. PloS One. (2021) 16. doi: 10.1371/journal.pone.0250005
196
FasoulaADuchesneLCanoJDGMoloneyBMElwahabSMAKerinMJ. Automated breast lesion detection and characterization with the wavelia microwave breast imaging system: Methodological proof-of-concept on first-in-human patient data. Appl Sci (Switzerland). (2021) 11. doi: 10.3390/app11219998
197
HamzaMNAbdulkarimYISaeedSRAltintasOMahmudRHAppasaniBet al. Low-cost antenna-array-based metamaterials for non-invasive early-stage breast tumor detection in the human body. Biosensors (Basel). (2022) 12. doi: 10.3390/bios12100828
198
AlhawariARHAlmawganiAHMHindiATAlghamdiHSaeidiT. Metamaterial-based wearable flexible elliptical UWB antenna for WBAN and breast imaging applications. AIP Adv. (2021) 11. doi: 10.1063/5.0037232
199
.
200
SaulsberryLPaceLEKeatingNL. The impact of breast density notification laws on supplemental breast imaging and breast biopsy. J Gen Intern Med. (2019) 34:1441–51. doi: 10.1007/s11606-019-05026-2
201
VeguntaSBhattAAChoudherySAPruthiSKaurAS. Identifying women with increased risk of breast cancer and implementing risk-reducing strategies and supplemental imaging. Breast Cancer. (2022) 29:19–29. doi: 10.1007/s12282-021-01298-x
202
ChengHDShanJJuWGuoYZhangL. Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recognit. (2010) 43:299–317. doi: 10.1016/j.patcog.2009.05.012
203
OuyangYTsuiPHWuSWuWZhouZ. Classification of benign and Malignant breast tumors using h-scan ultrasound imaging. Diagnostics. (2019) 9. doi: 10.3390/diagnostics9040182
204
AklilSBainCBansilPde SanjoseSDunstanJACastilloVet al. Evaluation of diagnostic ultrasound use in a breast cancer detection strategy in Northern Peru. PloS One. (2021) 16. doi: 10.1371/journal.pone.0252902
205
PereiraROLuzLAChagasDCAmorimJRNery-JuniorEJAlvesACBRet al. Evaluation of the accuracy of mammography, ultrasound and magnetic resonance imaging in suspect breast lesions. Clinics. (2020) 75:e1805. doi: 10.6061/CLINICS/2020/E1805
206
ThigpenDKapplerABremR. The role of ultrasound in screening dense breasts - A review of the literature and practical solutions for implementation. Diagnostics. (2018) 8. doi: 10.3390/diagnostics8010020
207
BisqueraOCValparaisoAPEspirituNTAyusteECPaloyoSR. Diagnostic validity of point-of-care breast ultrasound for females with palpable breast masses. Clin Breast Cancer. (2023) 23:e189–93. doi: 10.1016/J.CLBC.2023.02.003
208
JafariSHSaadatpourZSalmaninejadAMomeniFMokhtariMSadri NahandJet al. Breast cancer diagnosis: Imaging techniques and biochemical markers. J Cell Physiol. (2018) 233:5200–13. doi: 10.1002/jcp.26379
209
WangYLiYSongYChenCWangZLiLet al. Comparison of ultrasound and mammography for early diagnosis of breast cancer among Chinese women with suspected breast lesions: A prospective trial. Thorac Cancer. (2022) 13:3145–51. doi: 10.1111/1759-7714.14666
210
AbhishekaBBiswasSKPurkayasthaB. A comprehensive review on breast cancer detection, classification and segmentation using deep learning. Arch Comput Methods Eng. (2023) 30:5023–52. doi: 10.1007/s11831-023-09968-z
211
JiXLiDGaoDLvXFengYZhangDet al. Value of ultrasound-guided biopsy in evaluating internal mammary lymph node metastases in breast cancer. Clin Breast Cancer. (2021) 21:532–8. doi: 10.1016/J.CLBC.2021.04.016
212
IacobRManolescuDLStoicescuERFabianAMalitaDOanceaC. Breast cancer—How can imaging help? MDPI. (2022) 10. doi: 10.3390/healthcare10071159
213
BhattAAWhaleyDHLeeCU. Ultrasound-guided breast biopsies: basic and new techniques. J Ultrasound Med. (2021) 40:1427–43. doi: 10.1002/jum.15517
214
ShengXWangYYangFLinYXuSYinWet al. Ultrasound-guided breast biopsy: improved accuracy of 10-G cable-free elite compared with 14-G CCNB. J Surg Res. (2020) 247:172–9. doi: 10.1016/J.JSS.2019.10.025
215
LeeJMAraoRFSpragueBLet al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Intern Med. (2019) 179:658–67. doi: 10.1001/jamainternmed.2018.8372
216
RedmondCEHealyGMMurphyCFO’DohertyAFosterA. The use of ultrasonography and digital mammography in women under 40 years with symptomatic breast cancer: a 7-year Irish experience. Ir J Med Sci. (2017) 186:63–7. doi: 10.1007/s11845-016-1472-0
217
VourtsisABergWA. Breast density implications and supplemental screening. Eur Radiol. (2019) 29:1762–77. doi: 10.1007/s00330-018-5668-8
218
FrijaGBlažićIFrushDPHierathMKawooyaMDonoso-BachLet al. How to improve access to medical imaging in low- and middle-income countries? EClinicalMedicine. (2021) 38:101034. doi: 10.1016/J.ECLINM.2021.101034
219
SivarajahRTBrownKChetlenA. ’I can see clearly now.’ fundamentals of breast ultrasound optimization. Clin Imaging. (2020) 64:124–35. doi: 10.1016/J.CLINIMAG.2020.03.012
220
AfrinHLarsonNBFatemiMAlizadA. Deep learning in different ultrasound methods for breast cancer, from diagnosis to prognosis: current trends, challenges, and an analysis. Cancers. (2023) 15. doi: 10.3390/cancers15123139
221
IacobRIacobERStoicescuERGhenciuDMCocoleaDConstantinescuAet al. Evaluating the role of breast ultrasound in early detection of breast cancer in low- and middle-income countries: A comprehensive narrative review. Bioengineering. (2024) 11. doi: 10.3390/bioengineering11030262
222
ZanotelMBednarovaILonderoVLindaALorenzonMGiromettiRet al. Automated breast ultrasound: basic principles and emerging clinical applications. Radiol Med. (2018) 123:1–12. doi: 10.1007/s11547-017-0805-z
223
LehmannTRossaCSlobodaRUsmaniNTavakoliM. Needle path control during insertion in soft tissue using a force-sensor-based deflection estimator. In Proceedings of the IEEE International Conference on Advanced Intelligent Mechatronics (AIM). (2016) 1174–9. doi: 10.1109/AIM.2016.7576929
224
TosiDMacchiEGCigadaA. Fiber-optic temperature and pressure sensors applied to radiofrequency thermal ablation in liver phantom: Methodology and experimental measurements. J Sens. (2015) 2015:909012. doi: 10.1155/2015/909012
225
ElsheakhDNElgendyYKElsayedMEEldamakAR. Circularly polarized textile sensors for microwave-based smart bra monitoring system. Micromachines (Basel). (2023) 14. doi: 10.3390/mi14030586
226
KetfiRAl MasryZZerhouniNGayCDevallandC. Breast cancer detection using smart wearable devices with thermal sensors. Available online at: https://orcid.org/0009-0003-9224-786X (Accessed November 28, 2024).
227
WahabAASalimMIMAhamatMAManafNAYunusJLaiKW. Thermal distribution analysis of three-dimensional tumor-embedded breast models with different breast density compositions. Med Biol Eng Comput. (2016) 54:1363–73. doi: 10.1007/s11517-015-1403-7
228
(2018)., in: ICBAPS : 2nd International Conference on BioSignal Analysis, Processing and Systems (ICBAPS 2018) : 24–26 July 2018, Kuching, Sarawak, Malaysia. Piscataway, NJ, USA: IEEE.
229
NwoyeEOFidelisOPOgunsoluO. Wearable infra-red pre-screening smartbra for early detection of breast cancer. Nigerian J Technol. (2020) 39:506–13. doi: 10.4314/njt.v39i2.21
230
ElouerghiABellarbiLAfyfATalbiT. (2020). A novel approach for early breast cancer detection based on embedded micro-bioHeat ultrasensitive sensors: ioT technology, in: 2020 International Conference on Electrical and Information Technologies, ICEIT 2020. Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers Inc.. doi: 10.1109/ICEIT48248.2020.9113180
231
ClarkeCJZegeyeEF. An affordable and portable palpable system for sensing breast tissue abnormalities. Bioengineered. (2020) 7. doi: 10.1115/SMASIS2020-2273
232
ClanahanJMReddySBroachRBRositchAFAndersonBOWileytoEPet al. Clinical utility of a hand-held scanner for breast cancer early detection and patient triage. JCO Global Oncol. (2020) 6:27–34. doi: 10.1200/JGO.19
233
LaufeSRasskeKStopferLKurzynskiCAbbottTPlatnerMet al. Fabric force sensors for the clinical breast examination simulator. Stud Health Technol Inform. (2016) 220:193–8. doi: 10.3233/978-1-61499-625-5-193
234
LiuDLiuXZhangYWangQLuJ. Tissue phantom-based breast cancer detection using continuous near-infrared sensor. Bioengineered. (2016) 7:321–6. doi: 10.1080/21655979.2016.1197747
235
AbdulSBrownBHMilnesPTidyJA. The use of electrical impedance spectroscopy in the detection of cervical intraepithelial neoplasia. Int J Gynecological Cancer. (2006) 16:1823–32. doi: 10.1111/j.1525-1438.2006.00651.x
236
KassanosP. Bioimpedance sensors: A tutorial. IEEE Sens J. (2021) 21:22190–219. doi: 10.1109/JSEN.2021.3110283
237
ZhuHZhaoPChanYPKangHLeeDL. Breast cancer early detection with time series classification, in: Proceedings of the 31st ACM International Conference on Information and Knowledge Management (CIKM 2022), (2020) New York, NY, USA: Association for Computing Machinery (ACM). pp. 3735–45. doi: 10.1145/3511808.3557107
238
AbasiSAggasJRGarayar-LeyvaGGWaltherBKGuiseppi-ElieA. Bioelectrical impedance spectroscopy for monitoring mammalian cells and tissues under different frequency domains: A review. ACS Meas Sci Au. (2022) 2:495–516. doi: 10.1021/acsmeasuresciau.2c00033
239
VinithaSSRoyeaRBuckmanKJBenardisMHolmesJFletcherRLet al. An introduction to the Cyrcadia Breast Monitor: A wearable breast health monitoring device. Comput Methods Programs BioMed. (2020) 19. doi: 10.1016/j.cmpb.2020.105758
240
BahramiabarghoueiHPorterESantorelliAGosselinBPopovícMRuschLA. Flexible 16 antenna array for microwave breast cancer detection. IEEE Trans BioMed Eng. (2015) 62:2516–25. doi: 10.1109/TBME.2015.2434956
241
PorterEBahramiHSantorelliAGosselinBRuschLAPopovicM. A wearable microwave antenna array for time-domain breast tumor screening. IEEE Trans Med Imaging. (2016) 35:1501–9. doi: 10.1109/TMI.2016.2518489
242
ElsheakhDMAlsherifSAEldamakAR. Textile monopole sensors for breast cancer detection. Telecommun Syst. (2023) 82:363–79. doi: 10.1007/s11235-023-00990-x
243
Garduño-RamónMAVega-MancillaSGMorales-HenándezLAOsornio-RiosRA. Supportive noninvasive tool for the diagnosis of breast cancer using a thermographic camera as sensor. Sensors (Switzerland). (2017) 17. doi: 10.3390/s17030497
244
FaragOMohamedMAbd El GhanyMHofmannK. (2018). Integrated sensors for early breast cancer diagnostics, in: Proceedings - 21st IEEE International Symposium on Design and Diagnostics of Electronic Circuits and Systems, DDECS 2018, . pp. 153–7. Piscataway, NJ, USA: Institute of Electrical and Electronics Engineers Inc. doi: 10.1109/DDECS.2018.00034
245
ElsheakhDNFahmyOMFaroukMEzzatKEldamakAR. An early breast cancer detection by using wearable flexible sensors and artificial intelligent. IEEE Access. (2024) 12:48511–29. doi: 10.1109/ACCESS.2024.3380453
246
AyalewAMOussalahMAbuhayiBMBezabhYA. Smart breast cancer detection: enhancing early breast cancer diagnosis-transitioning from convolutional neural network to involutional neural network and using smart wearable devices. Sens Imaging. (2025) 26. doi: 10.1007/s11220-025-00561-1
247
W.Det al. Conformable ultrasound breast patch for deep tissue scanning and imaging(2023). Available online at: https://www.science.org (Accessed December 2, 2024).
248
ChowRDrkulecHImJHTsaiJNafeesAKumarSet al. The use of wearable devices in oncology patients: A systematic review. Oncologist. (2024) 29:e419–30. doi: 10.1093/oncolo/oyad305
249
ShakyaAKVidyarthiA. Comprehensive study of compression and texture integration for digital imaging and communications in medicine data analysis. Technologies. (2024) 12. doi: 10.3390/technologies12020017
250
WubinehBZDeribaFGWoldeyohannisMM. Exploring the opportunities and challenges of implementing artificial intelligence in healthcare: A systematic literature review. Urologic Oncol: Semin Original Investigations. (2024) 42:48–56. doi: 10.1016/J.UROLONC.2023.11.019
251
TheodosKSittigS. HEALTH INFORMATION PRIVACY LAWS IN THE DIGITAL AGE: HIPAA DOESN’T APPLY. Perspect Health Inf Manag. (2020)
252
NassraICapellaJV. Data compression techniques in IoT-enabled wireless body sensor networks: A systematic literature review and research trends for QoS improvement. Internet Things. (2023) 23:100806. doi: 10.1016/J.IOT.2023.100806
253
AminSUHossainMS. Edge intelligence and internet of things in healthcare: A survey. IEEE Access. (2021) 9:45–59. doi: 10.1109/ACCESS.2020.3045115
254
ShenG. Recent advances of flexible sensors for biomedical applications. Prog Natural Sci: Materials Int. (2021) 3:872–82. doi: 10.1016/J.PNSC.2021.10.005
255
Corrigendum: Dermatologist-level classification of skin cancer with deep neural networks. Nature. (2017) 542:115–8. doi: 10.1038/nature22985
256
BanaeeHAhmedMULoutfiA. Data mining for wearable sensors in health monitoring systems: A review of recent trends and challenges. Sensors. (2013) 13. doi: 10.3390/s131217472
257
VandendriesscheBGodfreyAIzmailovaES. Multimodal biometric monitoring technologies drive the development of clinical assessments in the home environment.Maturitas. (2021) 151:41–7. doi: 10.1016/j.maturitas.2021.06.009
258
TopolEJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. (2019) 25:44–56. doi: 10.1038/s41591-018-0300-7
259
SteinhublSRMuseEDTopolEJ. The emerging field of mobile health. Available online at: www.ScienceTranslationalMedicine.org (Accessed December 2, 2024).
260
RajkomarADeanJKohaneI. Machine learning in medicine. New Engl J Med. (2019) 14:1347–58. doi: 10.1056/nejmra1814259
261
BeamALKohaneIS. Big data and machine learning in health care. JAMA. (2018) 319:1317–18. doi: 10.1001/jama.2017.18391
262
MaduraiveeranGSasidharanMGanesanV. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens Bioelectron. (2018) 103:113–29. doi: 10.1016/J.BIOS.2017.12.031
263
ZhaoLYangHZhengXLiJJianLFengWet al. Dual signal amplification by polysaccharide and eATRP for ultrasensitive detection of CYFRA 21–1 DNA. Biosens Bioelectron. (2020) 150:111895. doi: 10.1016/J.BIOS.2019.111895
264
VoDKTrinhKTL. Advances in wearable biosensors for healthcare: current trends, applications, and future perspectives. Biosensors. (2024) 14. doi: 10.3390/bios14110560
Summary
Keywords
breast cancer, imaging, biosensors, wearable technologies, artificial intelligence
Citation
Khan AQ, Touseeq M, Rehman S, Tahir M, Ashfaq M, Jaffar E and Abbasi SF (2025) Advances in breast cancer diagnosis: a comprehensive review of imaging, biosensors, and emerging wearable technologies. Front. Oncol. 15:1587517. doi: 10.3389/fonc.2025.1587517
Received
04 March 2025
Accepted
15 May 2025
Published
18 June 2025
Volume
15 - 2025
Edited by
Bhishma Karki, National Research Council Nepal, Nepal
Reviewed by
Santosh Kumar, K L University, India
Amit Shakya, Sant Longowal Institute of Engineering and Technology, India
Hussein Elaibi, Dumlupinar University, Türkiye
Updates
Copyright
© 2025 Khan, Touseeq, Rehman, Tahir, Ashfaq, Jaffar and Abbasi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saadullah Farooq Abbasi, s.f.abbasi@bham.ac.uk
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.