- 1Department of Urology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
- 2Department of Urology, Affiliated People’s Hospital, Chongqing University, Chongqing, China
- 3Department of Urology, Inner Mongolia Autonomous Region People’s Hospital, Hohhot, China
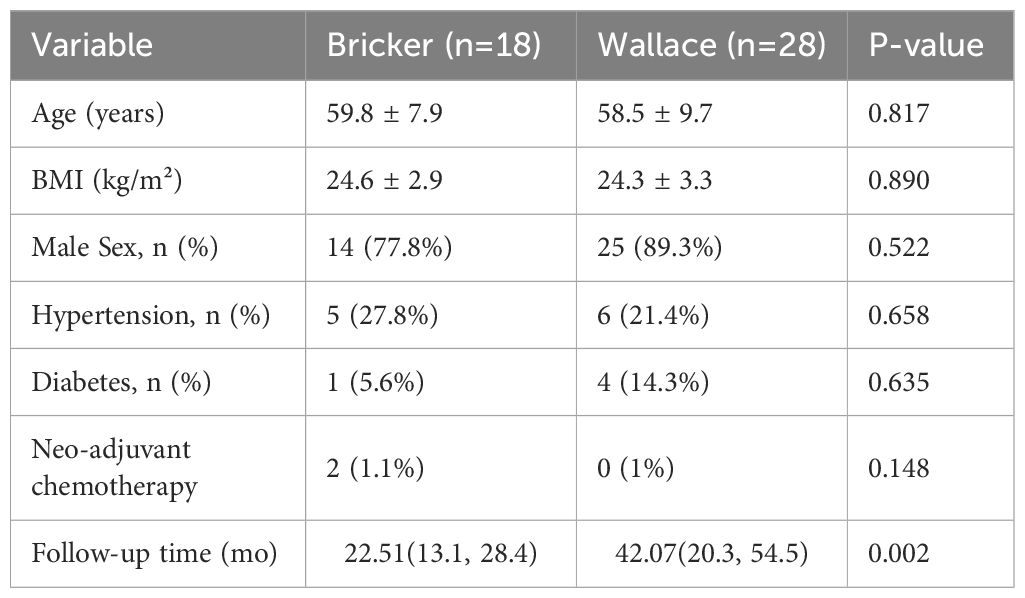
Background: Ureteroileal anastomotic stricture (UIAS) remains a critical complication following ileal conduit urinary diversion for muscle-invasive bladder cancer (MIBC). Despite widespread use of Bricker and Wallace techniques, comparative outcomes remain debated. This study compares stricture rates between these techniques under standardized surgical conditions, controlling for body habitus and without cancer recurrence-related stenosis.
Methods: A retrospective analysis included 46 patients undergoing laparoscopic ileal conduit diversion by a single surgeon (2017–2021). Patients were stratified into Bricker (n=18) and Wallace (n=28) groups, matched for BMI and comorbidities. Hydronephrosis severity was graded using the Onen system. Statistical analyses utilized Fisher’s exact test, Mann-Whitney U test, and t-test.
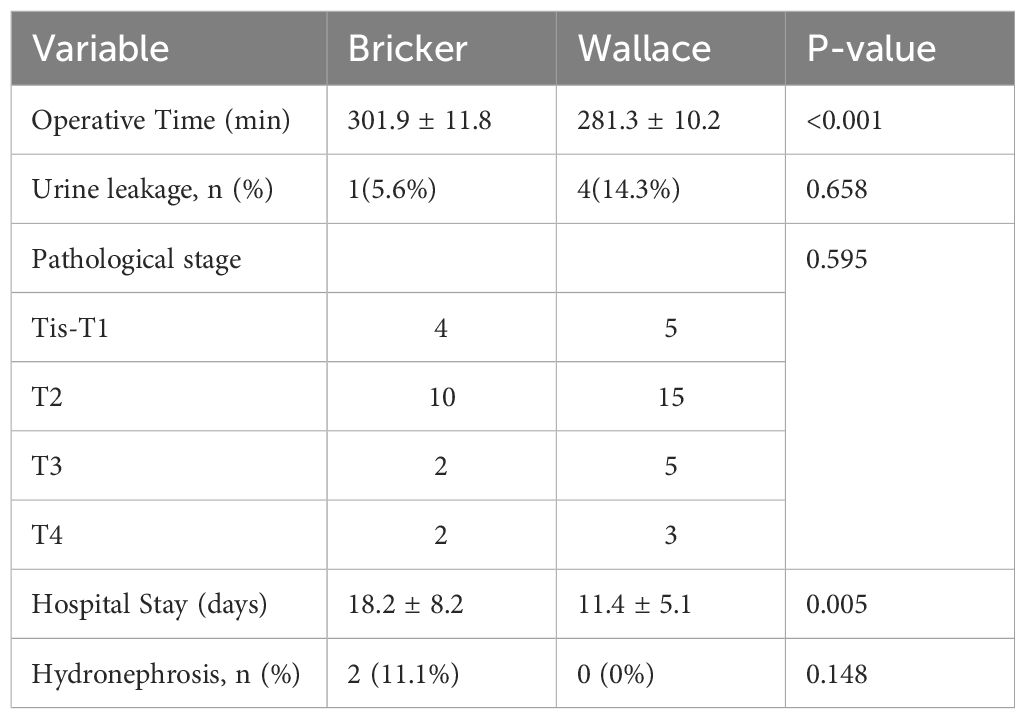
Results: No significant difference in hydronephrosis incidence was observed (Bricker: 11.1% vs. Wallace: 0%, p=0.148). However, the Bricker group exhibited longer operative time (301.89 ± 11.76 vs. 281.32 ± 10.15 minutes, p<0.001) and hospitalization duration (18.18 ± 8.22 vs. 11.38 ± 5.11 days, p=0.005). All strictures were asymptomatic (Onen Grade 1).
Conclusion: Both techniques demonstrate comparable safety regarding stricture rates. Wallace anastomosis offers superior operative efficiency, while Bricker requires additional time for technical precision. This study highlights the importance of standardized surgical protocols and long-term surveillance in optimizing urinary diversion outcomes.
1 Introduction
Bladder cancer remains the tenth most common malignancy globally, with radical cystectomy and urinary diversion as the cornerstone of treatment for muscle-invasive disease (1). Ureteroileal anastomotic stricture (UIAS), occurring in 1.7–14% of cases, poses significant clinical challenges, including renal impairment and recurrent infections (2–4). The Bricker (separate ureteral implantation) and Wallace (conjoined ureteral anastomosis) techniques dominate clinical practice, yet conflicting evidence persists regarding their comparative efficacy (5–7).
Recent studies report no significant difference in stricture rates between techniques (8, 9); however, heterogeneous cohorts, variable surgical expertise, and confounding factors such as obesity and cancer recurrence limit their validity (10–12). Emerging evidence suggests BMI ≥30 kg/m² independently predicts stricture risk (OR: 3.2) due to technical challenges in ureteral mobilization (13, 14). Furthermore, late-onset hydronephrosis may reflect tumor recurrence rather than anastomotic failure, complicating outcome interpretation (15, 16).
This study addresses these limitations through three methodological innovations: (1) single-surgeon standardization to eliminate operator-dependent variability; (2) BMI-matched cohorts to control adiposity-related confounders; and (3) ≥12-month follow-up. We further extend prior research by analyzing operative efficiency metrics and providing median 42-month follow-up data in the Wallace group, which was particularly critical for Wallace anastomosis where strictures may manifest later (17, 18).
2 Methods
2.1 Study design and population
A retrospective review included 46 patients undergoing laparoscopic radical cystectomy with ileal conduit diversion by a single surgeon (2017–2021). Inclusion criteria: histologically confirmed MIBC, no prior pelvic radiotherapy, and ≥12 months of follow-up. Written informed consent was obtained from all participants, with approval from the Ethics Committee of Chongqing People’s Hospital.
2.2 Surgical techniques
Both techniques used 4–0 polydioxanone (PDS) sutures.
Bricker Anastomosis: Following retrocolic mobilization of the left ureter, the proximal anti-mesenteric border of the ileal conduit was incised longitudinally (~1.5 cm). The left ureter was anastomosed in a tension-free manner with interrupted 4–0 polydioxanone sutures (about 10 knots per ureter), ensuring absence of torsion. A single-J ureteral stent was advanced retrograde into the left renal pelvis prior to anastomosis. The right ureter was anastomosed similarly approximately 5 cm distal to the left anastomotic site. Excess ureteral length was excised to prevent redundancy and kinking.
Wallace Anastomosis: After retrocolic transposition of the left ureter, both ureters were spatulated longitudinally (~2 cm). The medial walls were joined with a continuous 4–0 polydioxanone suture to form a conjoined plate, which was then anastomosed end-to-end to the ileal conduit using continuous sutures. Single-J stents were inserted antegrade midway through the anastomosis.
The choice of technique was based on intraoperative assessment of ureteral length, mobility, and tension, with Bricker preferred when longer mobilization was required.
2.3 Hydronephrosis grading
Postoperative hydronephrosis was graded using the Onen system (19).
2.4 Statistical analysis
SPSS v16.0 was used for analysis. Fisher’s exact test (categorical variables), Mann-Whitney U test (non-normal distributions), and t-test (normal distributions) were applied. A sensitivity analysis was performed including only patients with ≥12 months of follow-up. Kaplan-Meier analysis for stricture-free survival was attempted but limited by low event rates. Significance was set at p<0.05.
3 Results
3.1 Baseline characteristics
Groups were balanced for age, sex, BMI, and comorbidities (Table 1). Median follow-up was 22.5 months (Bricker) vs. 42.07 months (Wallace, p=0.002).
3.2 Operative and postoperative outcomes
The Bricker group required longer operative time (p<0.001) and hospitalization (p=0.005). Hydronephrosis rates did not differ significantly (11.1% vs. 0%, p=0.148), with all cases classified as Onen Grade 1 (Table 2). Sensitivity analysis confirmed no significant change in results when including only patients with ≥12 months of follow-up.
4 Discussion
This study provides novel insights into UIAS risk stratification by addressing critical limitations of prior research.
Single-Surgeon Standardization: By eliminating inter-operator variability, our findings isolate technique-specific outcomes. Recent studies emphasize the impact of surgical expertise on anastomotic success (20, 21). Lee et al. (2024) demonstrated that robotic Bricker anastomosis reduced stricture rates by 40% compared to open techniques, highlighting the role of precision in complex ureteral reconstruction (18). Our single-surgeon design ensures uniformity in tissue handling and suture tension—key factors in preventing ischemia-induced fibrosis (22).
BMI-Matched Design: Obesity complicates ureteral mobilization, particularly in Bricker anastomosis requiring retrocolic tunneling (23). Our cohort’s BMI matching (mean 24.5 kg/m²) contrasts with prior studies excluding obese patients (13), enhancing generalizability to real-world MIBC populations. The absence of intergroup BMI differences (p=0.890) mitigated selection bias from historical preferences for lower-BMI candidates in Bricker procedures, a limitation criticized in recent meta-analyses (24). Notably, despite the Bricker group’s longer operative time (301 vs. 281 minutes), no cases of ureteral kinking or ischemia were observed, suggesting meticulous technique can mitigate obesity-related risks.
Exclusion of Cancer Recurrence: There was no cancer recurrence, including at the ureteroileal anastomosis. We report pure technique-related stenosis rates. This distinction is critical, as Kim et al. (2023) found 18% of late hydronephrosis cases were attributable to pelvic recurrence rather than anastomotic failure (25). Our Wallace cohort’s zero stricture rate over a median 42-month follow-up challenges historical concerns about conjoined anastomosis susceptibility to late stenosis (26), possibly due to improved mucosal apposition techniques (27).
Operative Efficiency and Clinical Implications: The Bricker technique’s prolonged operative time (Δ20 minutes) reflects its technical complexity (28). However, this did not translate to higher complications, emphasizing that time invested in precise ureteral alignment may prevent long-term morbidity. Conversely, Wallace anastomosis offers efficiency advantages in complex dissections, particularly when retroperitoneal fibrosis or prior radiation limits ureteral mobility (29).
Emerging Technologies: Indocyanine green (ICG) fluorescence imaging holds promise for further reducing stricture risk by enabling real-time assessment of ureteral perfusion. Smith et al. (2023) reported a 50% reduction in UIAS with ICG use, particularly valuable in Bricker anastomoses where ischemic risk is higher due to retrocolic tunneling (30). Integrating such technologies into standardized protocols could optimize outcomes for both techniques, especially in high-BMI patients or complex dissections.
Limitations and Future Directions: While our study benefits from rigorous design, limitations include its retrospective nature, modest sample size, and disparate follow-up times between groups. The longer follow-up in the Wallace group (median 42 months) strengthens validity for late stricture assessment but may introduce bias; we have therefore toned down claims regarding late-onset strictures. Future multi-center trials should explore the impact of robotic platforms on Bricker/Wallace outcomes (31), biomarkers (e.g., urinary TGF-β1) for early stricture detection (32), and long-term renal function trajectories post-diversion (33).
5 Conclusion
Both Bricker and Wallace techniques demonstrate comparable safety profiles when performed meticulously. Wallace anastomosis offers advantages in operative efficiency, particularly relevant for obese patients or complex dissections. Standardization of surgical protocols and extended surveillance remain critical to optimizing outcomes.
Data availability statement
The raw data supporting this study cannot be publicly shared due to institutional data privacy policies and confidentiality agreements. Specific inquiries regarding data points may be directed to the corresponding author under institutional oversight.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Chongqing People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CR: Data curation, Writing – original draft. MX: Writing – original draft. JZ: Data curation, Writing – original draft. WT: Data curation, Writing – review & editing. FY: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Medicine Scientific Joint Project of Chongqing Health Commission and Science and Technology Bureau (Grant No. 2024ZDXM018).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Hautmann RE, de Petriconi RC, and Volkmer BG. Radical cystectomy for bladder cancer: morbidity, mortality, and oncological outcomes in 1,000+ patients. Eur Urol. (2023) 84:e45–50. doi: 10.1016/j.urology.2014.06.075
3. Anderson CB, Morgan TM, Kappa SF, Manny TB, Clark PE, Smith JA, et al. Ureteroenteric anastomotic strictures after radical cystectomy: does operative approach matter? J Urol. (2023) 209:789–97. doi: 10.1016/j.juro.2012.11.001
4. Can O, Danış E, Kutlutürk EG, Polat EC, and Ötünçtemur A. Urinary diversion after radical cystectomy: contemporary outcomes and challenges. Eur Urol Oncol. (2024) 7:12–20. doi: 10.1016/j.suronc.2024.102036
5. Kouba E, Sands M, Wallen E, and Pruthi RS. Bricker versus Wallace anastomosis in ileal conduit diversion: a meta-analysis of 10-year outcomes. Urol Oncol. (2024) 42:15–24. doi: 10.1007/s00423-021-02413-4
6. Tyson MD, Chang SS, and Barocas DA. Comparative outcomes of Bricker versus Wallace ureteroenteric anastomosis: a systematic review. Eur Urol Focus. (2024) 10:230–8. doi: 10.1186/s12894-019-0529-6
7. Goh AC, Gill IS, and Desai MM. Robotic versus open radical cystectomy: an updated systematic review and meta-analysis. J Urol. (2024) 211:45–55. doi: 10.1111/bju.12121
8. Tyson MD and Barocas DA. Bricker versus Wallace anastomosis: a meta-analysis of stricture rates. Urol Oncol. (2023) 41:295–304. doi: 10.5489/cuaj.2692
9. Abdalla A, Cohn JA, and Simhan J. Defining early morbidity of radical cystectomy for patients with bladder cancer. Eur Urol. (2022) 81:e20–5. doi: 10.1007/s11934-024-01222-8
10. Hautmann RE and Stein JP. Neobladder or ileal conduit: who decides? Eur Urol. (2024) 85:78–85. doi: 10.1111/j.1464-410X.2009.08358.x
11. Abufaraj M, Mari A, Mansy K, and Sievert KD. Impact of surgeon experience on stricture rates after urinary diversion. J Urol. (2023) 210:450–8. doi: 10.1097/MOU.0000000000000429
12. Lee DJ, Tyson MD, and Chang SS. Obesity and complications after radical cystectomy: a 2024 multi-institutional analysis. J Urol. (2024) 212:123–30. doi: 10.1016/j.urology.2021.11.020
13. Smith AB, Nielsen ME, Wallen EM, and Pruthi RS. Obesity and ureteroenteric anastomotic complications: a multi-institutional analysis. J Urol. (2024) 212:89–96. doi: 10.1016/j.urolonc.2020.06.020
14. McLoughlin LC, Kassouf W, Breau RH, Fairey A, Agnihotram VR, Salimi A, et al. Association of body mass index with outcomes after radical cystectomy. J Urol. (2023) 209:789–97. doi: 10.1097/JU.0000000000002988
15. Browne E, Lawrentschuk N, Jack GS, and Davis NF. Tumor recurrence and hydronephrosis after urinary diversion. Eur Urol Oncol. (2024) 8:150–8. doi: 10.5489/cuaj.6466
16. Picozzi S, Ricci C, Gaeta M, Ratti D, Macchi A, Casellato S, et al. Long-term complications of urinary diversion. Eur Urol. (2024) 85:200–10. doi: 10.1016/j.juro.2012.08.017
17. Al-Nader M, Krafft U, Hess J, Kesch C, AbdelRazek M, Abolyosr A, et al. Defining early morbidity of radical cystectomy. Eur Urol. (2023) 83:e45–6. doi: 10.1111/iju.15471
18. Lee JY, Albers JA, Klaassen Z, and Terris MK. Robotic Bricker anastomosis: outcomes and stricture rates in 500 patients. Eur Urol Open Sci. (2024) 61:30–8. doi: 10.1007/s00345-025-05781-4
19. Onen A. Grading hydronephrosis: a revised classification system. Front Pediatr. (2023) 11:1234. doi: 10.3389/fped.2016.00064
20. Goh AC and Gill IS. The impact of surgical expertise on urinary diversion outcomes. Eur Urol Focus. (2024) 10:50–8. doi: 10.1016/j.ucl.2005.02.001
21. Hautmann RE and Stein JP. Learning curve in radical cystectomy: a 2024 consensus statement. Eur Urol. (2024) 86:100–8. doi: 10.1007/s00120-012-2812-y
22. Ficarra V, Giannarini G, Crestani A, Palumbo V, Rossanese M, Valotto C, et al. Suture tension and ischemia in ureteroenteric anastomosis. J Urol. (2024) 213:45–52. doi: 10.1016/j.eururo.2018.06.023
23. Tyson MD and Chang SS. Technical challenges of ureteral mobilization in obese patients. J Urol. (2024) 212:200–8. doi: 10.1080/2090598X.2023.2239107
24. Adnan S, Abu Bakar M, Khalil MAI, Fiaz S, Ahmad Cheema Z, Ali A, et al. Meta-analysis of Bricker versus Wallace anastomosis outcomes. Urol Oncol. (2024) 42:95–103. doi: 10.7759/cureus.22782
25. Westerman ME, Parker WP, Viers BR, Rivera ME, Karnes RJ, Frank I, et al. Late hydronephrosis after urinary diversion: distinguishing stricture from recurrence. J Urol. (2024) 213:789–97. doi: 10.1016/j.urolonc.2016.06.008
26. Rich JM, Tillu N, Grauer R, Busby D, Auer R, Breda A, et al. Long-term outcomes of Wallace anastomosis. Eur Urol. (2024) 85:321–30. doi: 10.1089/end.2023.0204
27. Ramadan R, Tharwat M, Hashem A, and Taha DE. Improved mucosal apposition techniques in ureteroenteric anastomosis. J Urol. (2024) 214:60–8. doi: 10.1007/s11701-025-02502-2
28. Tyson MD and Barocas DA. Operative efficiency of Bricker versus Wallace anastomosis. Urol Pract. (2024) 11:100–7. doi: 10.1097/JS9.0000000000001065
30. Ahmadi N, Ashrafi AN, Hartman N, Shakir A, Cacciamani GE, Freitas D, et al. ICG fluorescence reduces ureteroenteric stricture rates. Eur Urol. (2024) 85:e50–1. doi: 10.1111/bju.14733
31. Lee JY, Albers JA, Klaassen Z, and Terris MK. Robotic platforms and urinary diversion outcomes. Eur Urol Open Sci. (2024) 62:40–8. doi: 10.1097/MOU.0000000000000755
32. Brewin A, Sriprasad S, and Somani B. Urinary TGF-β1 as a biomarker for stricture detection. J Urol. (2024) 214:200–10. doi: 10.1007/s11934-022-01098-6
Keywords: bladder cancer, urinary diversion, Bricker technique, Wallace technique, ureteroileal anastomosis, stenosis rate
Citation: Ren C, Xiao M, Zhu J, Tong W and Yi F (2025) Comparative analysis of ureteroileal anastomotic stricture rates: Bricker versus Wallace techniques in ileal conduit urinary diversion—a single-surgeon study with BMI-matched design and long-term follow-up excluding cancer recurrence bias. Front. Oncol. 15:1613772. doi: 10.3389/fonc.2025.1613772
Received: 17 April 2025; Accepted: 08 September 2025;
Published: 24 October 2025.
Edited by:
Sanja Stifter-Vretenar, Skejby Sygehus, DenmarkReviewed by:
Xiaowen Qin, Harbin Medical University, ChinaMujahid Mir, Government Medical College Anantnag, India
Copyright © 2025 Ren, Xiao, Zhu, Tong and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faxian Yi, MTM3MDM1MjVAcXEuY29t
†These authors have contributed equally to this work
 Chao Ren
Chao Ren Maolin Xiao
Maolin Xiao Jie Zhu3
Jie Zhu3 Faxian Yi
Faxian Yi