- 1Department of Thoracic Surgery, Affiliated Zhongshan Hospital of Dalian University, Dalian, Liaoning, China
- 2Department of Thoracic Surgery, The Second Hospital of Dalian Medical University, Dalian, Liaoning, China
- 3School of Software Technology, Dalian University of Technology, Dalian, Liaoning, China
Objective: This study aimed to explore the relationship between EGFR mutations, ALK positivity, and demographic, tumor, radiological, and pathological characteristics in lung adenocarcinoma patients.
Methods: This study included 626 patients with early-stage lung adenocarcinoma who underwent surgical resection between October 2017 and December 2023.EGFR and ALK mutations were analyzed postoperatively. Clinical, pathological, and imaging features such as gender, age, smoking status, and tumor characteristics were assessed. Patients were categorized based on their mutation status, and comparisons were made regarding their clinical and imaging features.
Results: Results indicated that EGFR-positive patients were predominantly female, younger, and had a higher frequency of non-smokers compared to the wild-type (WT) group. EGFR mutations, particularly the exon 19 deletions and L858R mutations, were more common in patients with moderate differentiation and lepidic or acinar predominant histological subtypes. CT imaging revealed that EGFR-positive tumors were smaller in size and had fewer solid components compared to WT tumors. Additionally, certain CT features such as the spicule sign and air bronchogram were significantly associated with EGFR mutations. For ALK mutations, the analysis showed that patients with ALK-positive tumors had distinct radiological features, including a higher occurrence in the lower lobes and fewer ground glass nodules compared to the WT group.
Conclusions: The study concluded that specific radiological and pathological characteristics, along with EGFR and ALK mutation statuses, could be used to guide the treatment and diagnosis of lung adenocarcinoma.
Introduction
Lung cancer is the leading cause of cancer-related death globally (1), with 733,300 diagnoses and 610,200 deaths in China in 2015 (2, 3). Lung adenocarcinoma is the most common type (4). In recent years, molecular-targeted therapies have transformed treatment, offering better control and less toxicity than traditional chemotherapy (5). Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are the most common druggable targets in lung adenocarcinoma. EGFR mutations affect about 20% of patients, particularly in females, non-smokers, and Asians, with exon 19 deletions and exon 21 mutations being the most common (6, 7). ALK rearrangements occur in about 5% of non-small cell lung cancer (NSCLC) cases, especially in younger, light, or non-smokers, and are commonly found in adenocarcinomas (8–10).
Clinical trials (11) have shown that patients with EGFR mutations and ALK rearrangements treated with targeted therapies experience longer progression-free survival and higher response rates compared to chemotherapy. EGFR mutations includes three types (point mutation, multinucleotide in-frame deletion,and in-frame insertion) which have been documented in exon 18 through 21, highlighting that deletion mutation in exon 19 (45%) and point mutation in exon 21 (40–45%) are two most common mutations, accounting for about 90% EGFR mutations in lung adenocarcinoma (12).
The IASLC/ATS/ERS classification system for lung adenocarcinoma was proposed in 2011 (13),Though several groups had analyzed association between the IASLC/ATS/ERS classification scheme and survival (14–17), but the relationship between gene mutations and this new classification remains unclear, highlighting the need to understand correlations between gene mutations and pathological subtypes.
CT imaging plays a key role in diagnosing and assessing response in NSCLC (1, 18–25). Some studies (18–23) suggest CT features such as small lesion size and the presence of air bronchograms may be associated with EGFR mutations, while ALK mutations are linked to larger, solid masses (1, 24, 25).
However, the relationship between these mutations and the clinicopathological,imaging features features of lung adenocarcinoma has not been fully elucidated. Additionally, obtaining tissue for gene detection is difficult in advanced-stage or surgically ineligible patients, and biopsies often yield false negatives due to tumor heterogeneity and low cell counts. This has led to growing interest in identifying clinical, pathological, and radiological features associated with EGFR and ALK mutations.
Materials and methods
Patient selection
This retrospective study was approved by our institutional review board, which waived the need for informed consent. From October 2017 to December 2023, we identified 626 early-stage lung adenocarcinoma patients who underwent EGFR mutation and ALK rearrangement retesting after surgery at the Second Hospital of Dalian Medical University.
Inclusion criteria included pathologically confirmed lung adenocarcinoma by surgical resection, available EGFR and ALK mutation results, clinical data (age, sex, smoking history, hypertension, diabetes, tumor biomarkers), and CT scans performed within a month prior to surgery. Exclusion criteria included missing CT scans, preoperative treatments (Neoadjuvant chemotherapy, radiotherapy, targeted therapy and immunotherapy), incomplete data, severe comorbidities, or a history of lung cancer.
Histologic evaluation and molecular analysis
Histological specimens were formalin-fixed and stained with hematoxylin-eosin. Two experienced pathologists reviewed the samples and recorded the subtype and stage according to the 8th edition TNM system and IASLC/ATS/ERS classification. EGFR mutations (exons 18-21) and ALK mutations were analyzed using next-generation sequencing (NGS). The detection is based on the Illumina sequencing platform and employs the target region probe capture technology. It examines at least 9 genes that are highly relevant to personalized treatment of non-small cell lung cancer. These genes must include EGFR, ALK, ERBB2 (HER2), BRAF, KRAS, MET, RET, ROS1, and NTRK1/2/3.ALK positivity was further confirmed by FISH testing.
CT imaging data
High-resolution CT scans (1 mm slice thickness) were reviewed by two chest subspecialists who were blind to the genetic classification. Tumor features assessed included size, location, margins (Figure 1), and density (pure or mixed ground-glass nodules, subsolid, or solid).
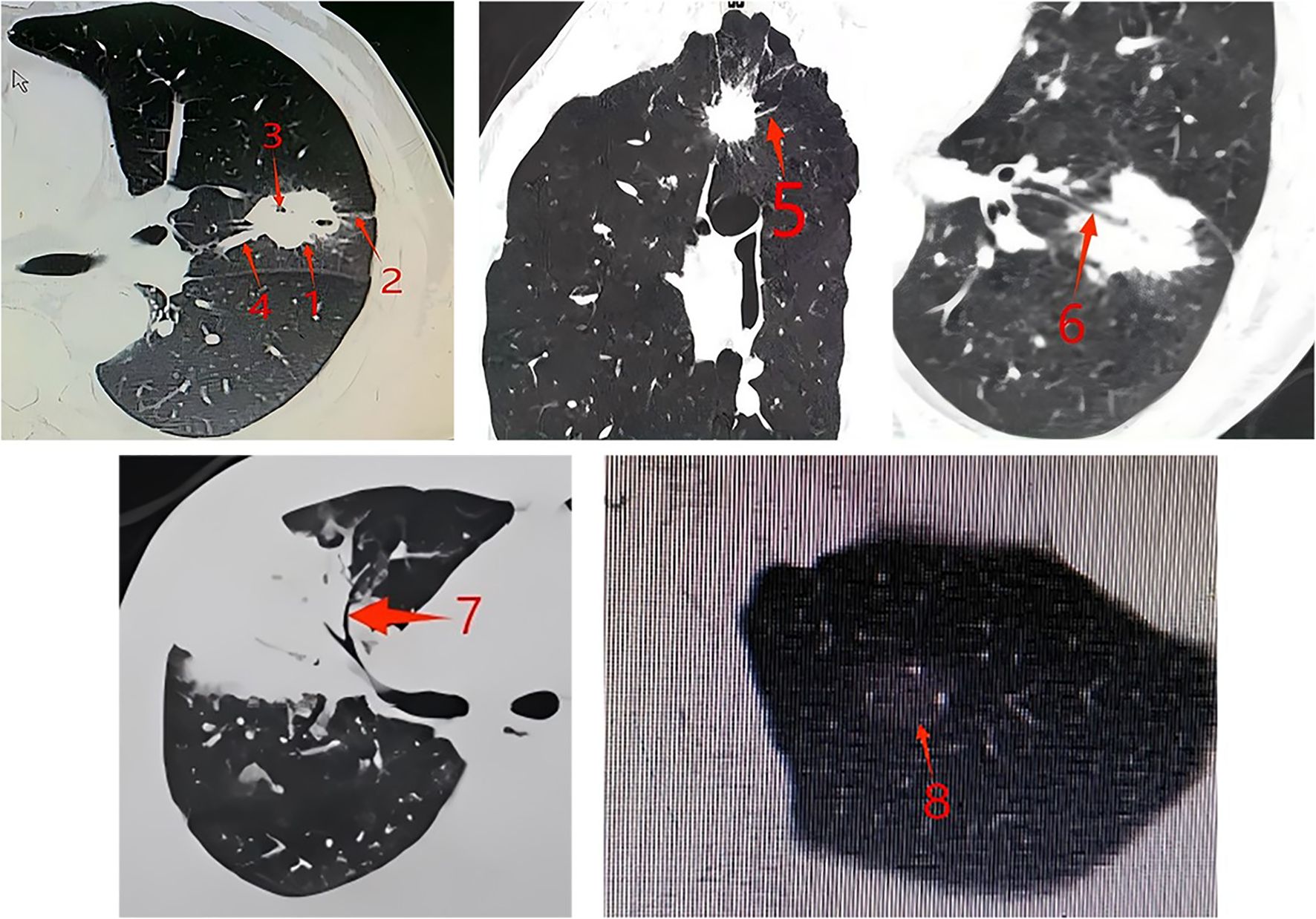
Figure 1. CT features of tumors. Arrow 1: Lobulation sign: The surface of the nodule is uneven, with multiple arc-shaped depressions visible on it, resembling the combined appearance of multiple nodules. Arrow 2: Pleural indentation sign: The distal pleura of the tumor is pulled by the tumor, forming a linear high-density shadow. The corresponding concave area shows a trumpet-shaped change. Arrow 3: Vacuole sign: Round, transparent shadows with a diameter of less than 5mm (mostly 1-2mm) can be seen within the lesion, either singly or in multiple clusters, and they can be located anywhere within the lesion. Arrow 4: Vessel convergence sign: Around the tumor, there is one or more blood vessels that converge towards the tumor. It can also be manifested as the blood vessels stopping or being completely surrounded and destroyed when they reach the edge of the tumor. Arrow 5: Spicule sign: The tumor margin extends outward, the base is slightly thick and gradually becomes thinner as it spreads outward, presenting a straight and forceful linear shadow. Arrow 6: Bronchial cutoff sign: The bronchus is interrupted at the edge of the lesion or as it enters the lesion. The terminal end of the lumen may be straight, blunt, or conical narrowed. The bronchial wall is locally thickened and asymmetric, gradually thinning towards the center of the lesion. Arrow 7: Air bronchogram: There are long, branched or tubular low-density shadows within the lesion, which may be accompanied by blood vessels. When perpendicular to the scanning plane, they can appear as continuous circular low-density shadows on the upper and lower layers. Arrow 8: Ground-glass nodules: The tumor nodules have a relatively low density in part or the entire area, presenting as ground-glass opacity, without obscuring the pulmonary vascular patterns, and the lesion boundaries are clear.
Statistical analysis
Statistical analyses were conducted using SPSS version 21.0. An independent-sample Student’s t-test compared continuous variables, and chi-square tests compared categorical variables. Multivariate regression analysis was performed after addressing multicollinearity. A prediction tool for EGFR and ALK mutations was developed using principal component analysis, and receiver operating characteristic (ROC) curves were generated to calculate the area under the curve (AUC). Furthermore, univariate and multivariate subgroup analyses were conducted for EGFR exon 19 and exon 21 mutation sites.
Results
Patient characteristics and EGFR, ALK mutation status
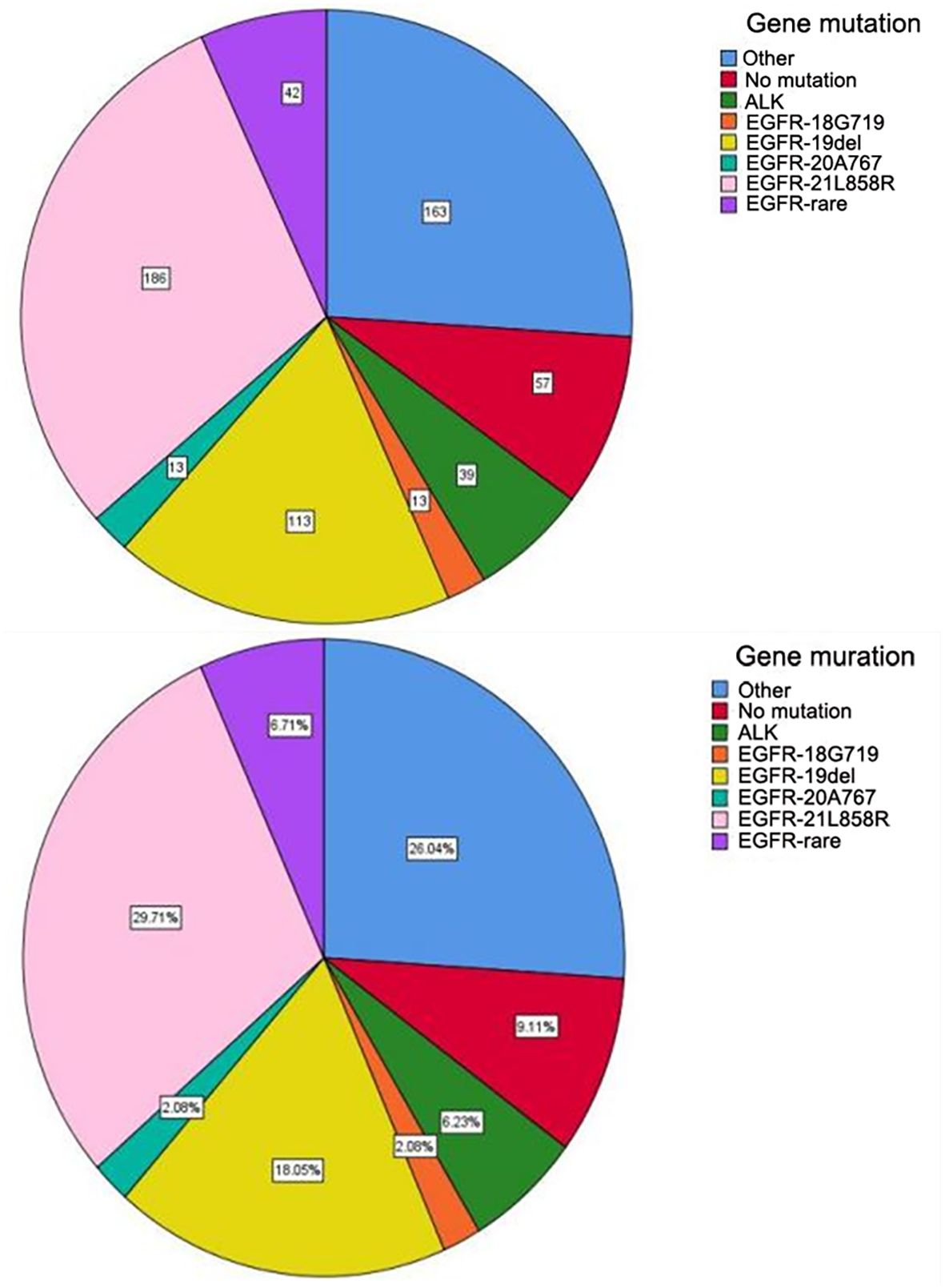
A total of 626 patients underwent surgery between October 2017 and December 2023, with EGFR mutation and ALK rearrangement retesting conducted post-surgery. The cohort consisted of 234 men (37.4%) and 392 women (62.6%), with a median age of 60.8 years (range 24–80). Of the patients, 452 (72.2%) were non-smokers, with 163 male and 11 female smokers. All cases were invasive lung adenocarcinomas. Among the 626 patients, 367 had EGFR mutations and 39 had EML4-ALK fusion mutations, EGFR mutations included 113 (30.8%) exon 19 deletions, 186 (50.7%) exon 21 mutations, and 68 cases (18.5%) of rare mutations (Figure 2).
Correlations of EGFR mutations and ALK status with clinical, pathological and CT features
The clinical characteristics of the patients are summarized in Table 1. EGFR-positive patients had significantly lower mean CYFRA21–1 levels (2.358 vs. 2.796 ng/ml; P = 0.003). A higher proportion of women (66%) and non-smokers (65%) were found in the EGFR-positive group compared to the EGFR-negative group (46% and 41%, respectively; P = 0.000 for both). There were few ALK-positive patients, and no significant differences in clinical features were observed for this group.
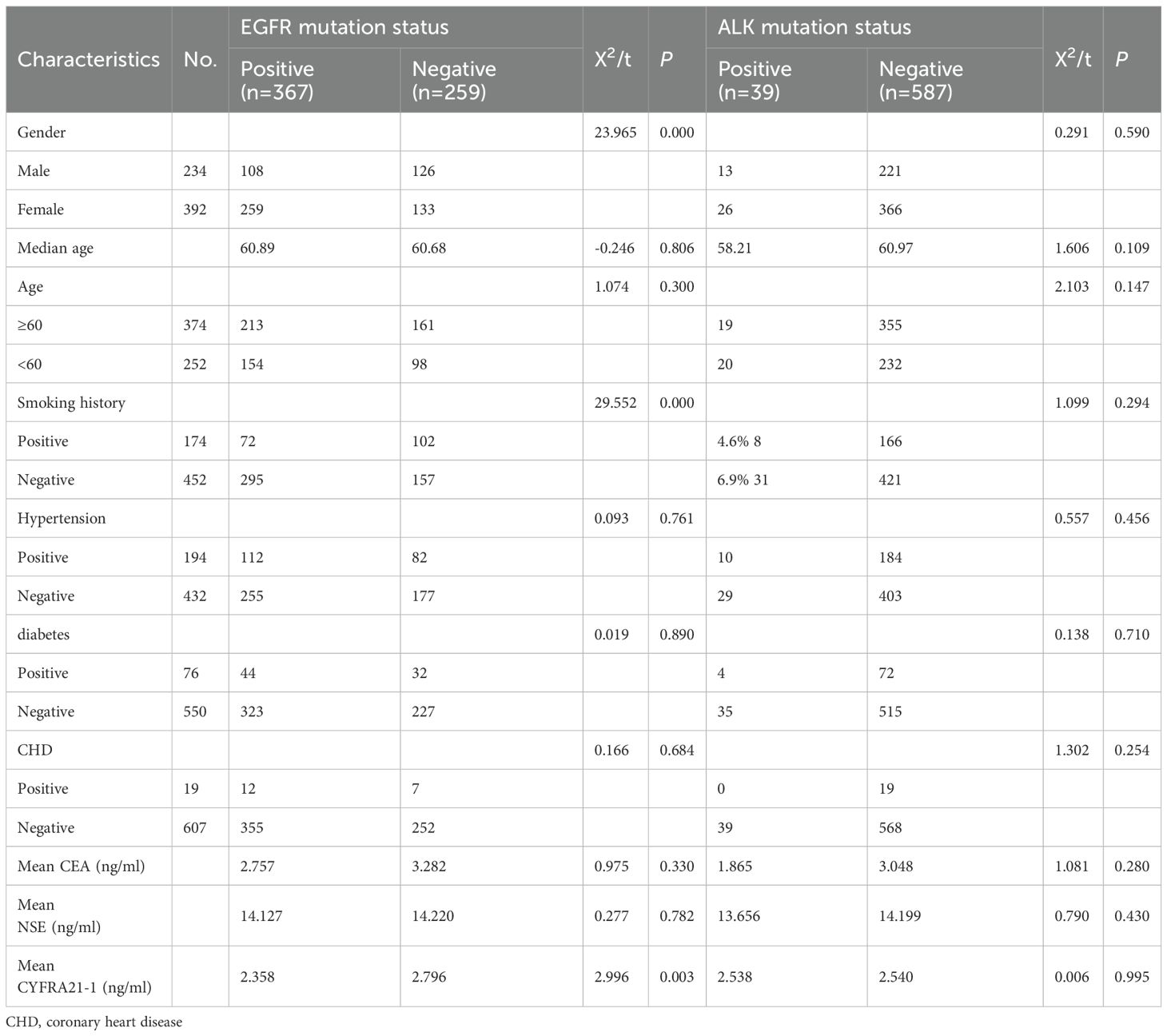
Table 1. Univariate analysis of the effects of EGFR gene mutation and ALK gene mutation on clinical features of invasive lung adenocarcinoma.
The pathological features of surgically resected cases are shown in Table 2. EGFR-positive cases were more frequently lepidic and acinar (69% vs. 51% and 68% vs. 50%, respectively; P < 0.05), and less frequently solid and mucinous (37% vs. 60% and 11% vs. 62%, respectively; P < 0.05). EGFR-positive patients were more likely to be moderately differentiated (P = 0.000) and less likely to be poorly differentiated (P = 0.000). No significant pathological differences were found between ALK-positive and wild-type (WT) patients.
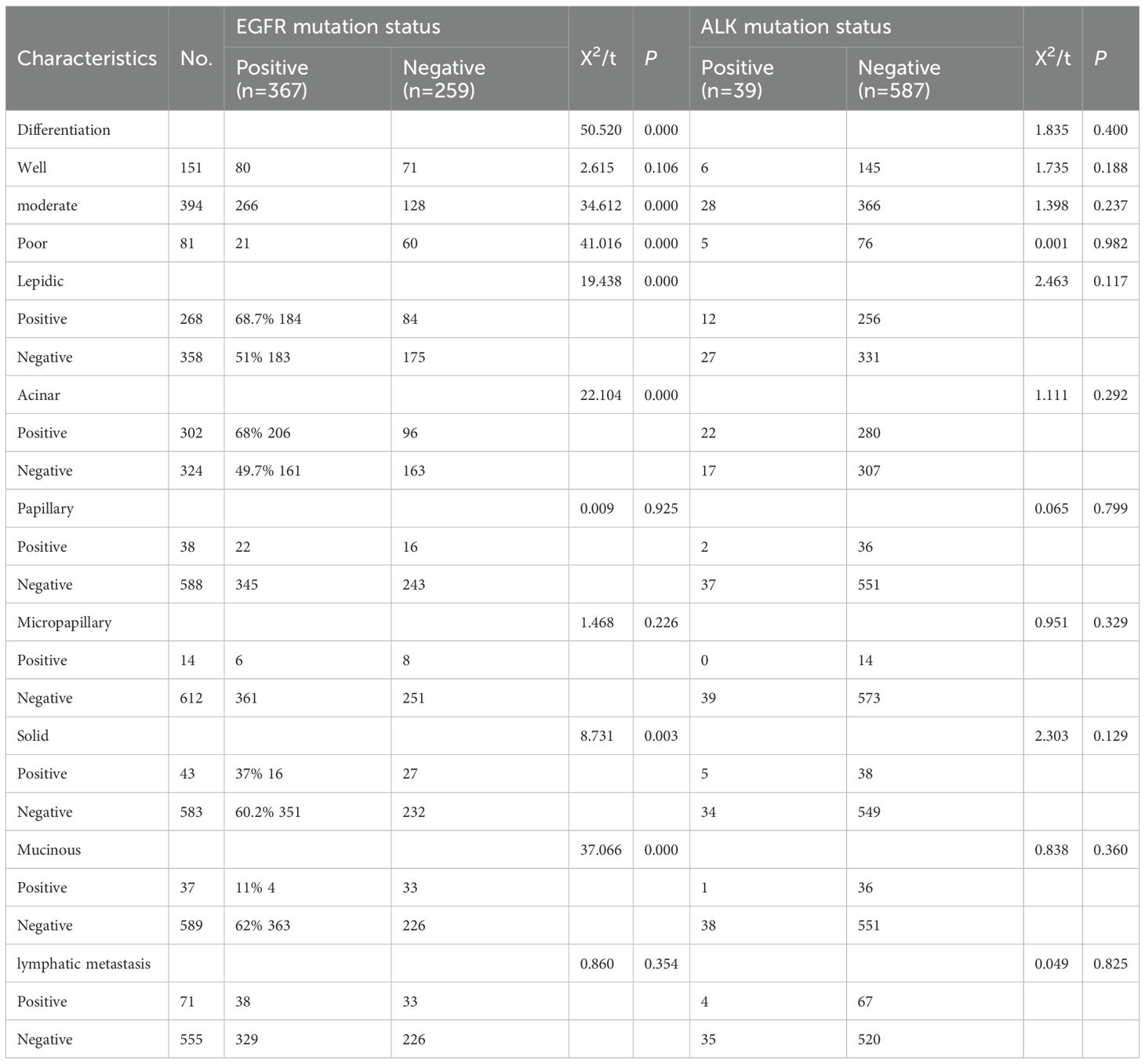
Table 2. Univariate analysis of the effects of EGFR gene mutation and ALK gene mutation on pathological features of invasive lung adenocarcinoma.
The CT features of surgically resected cases are shown in Table 3. EGFR mutations were significantly associated with nodule location (P = 0.013), particularly in the upper lobe of the left lung (68% vs. 56%; P = 0.011) and middle lobe of the right lung (48% vs. 60%; P = 0.035). EGFR-positive tumors had smaller solid components (1.073 vs. 1.380 cm; P = 0.003), with a higher mutation rate in ground glass nodules with 25-50% solid components (P = 0.002) and a lower rate in those with 75-100% solid components (P = 0.000) or pure solid nodules (P = 0.001). CT features such as the spicule sign (P = 0.001) and air bronchogram (P = 0.034) were more common in EGFR mutations. For ALK mutations, the mutation rate was higher in the left lower lobe (11% vs. 5%; P = 0.031), with less frequent co-occurrence of pure ground glass nodules (2.7% vs. 7.3%; P = 0.035) and vessel convergence signs (1.8% vs. 7.2%; P = 0.030).
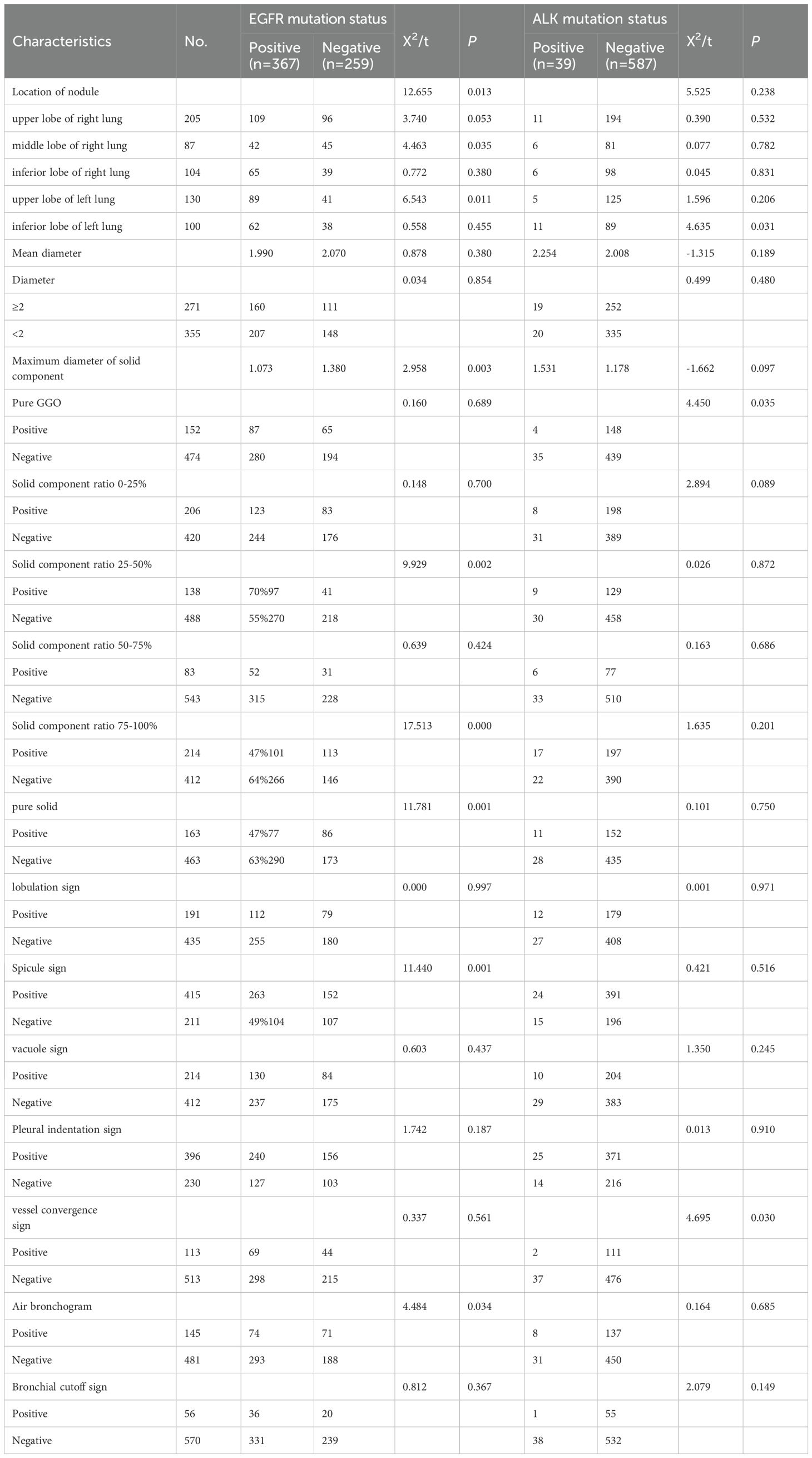
Table 3. Univariate analysis of the effects of EGFR gene mutation and ALK gene mutation on radiological features of invasive lung adenocarcinoma.
Differences in clinical, pathologica and CT features between 19 deletion and L858R EGFR mutations
Table 4 shows clinical differences between EGFR exon 19 deletions and L858R mutations. Both mutations were more common in females and non-smokers compared to WT EGFR (P < 0.05). EGFR exon 19 deletions occurred more frequently in patients under 60 years of age (58.38 vs. 62.22 years; P = 0.002) and had lower mean CYFRA21–1 levels compared to WT EGFR (2.217 vs. 2.796 ng/ml; P = 0.012). Additionally, EGFR exon 19 deletions were more common in younger patients compared to L858R mutations.
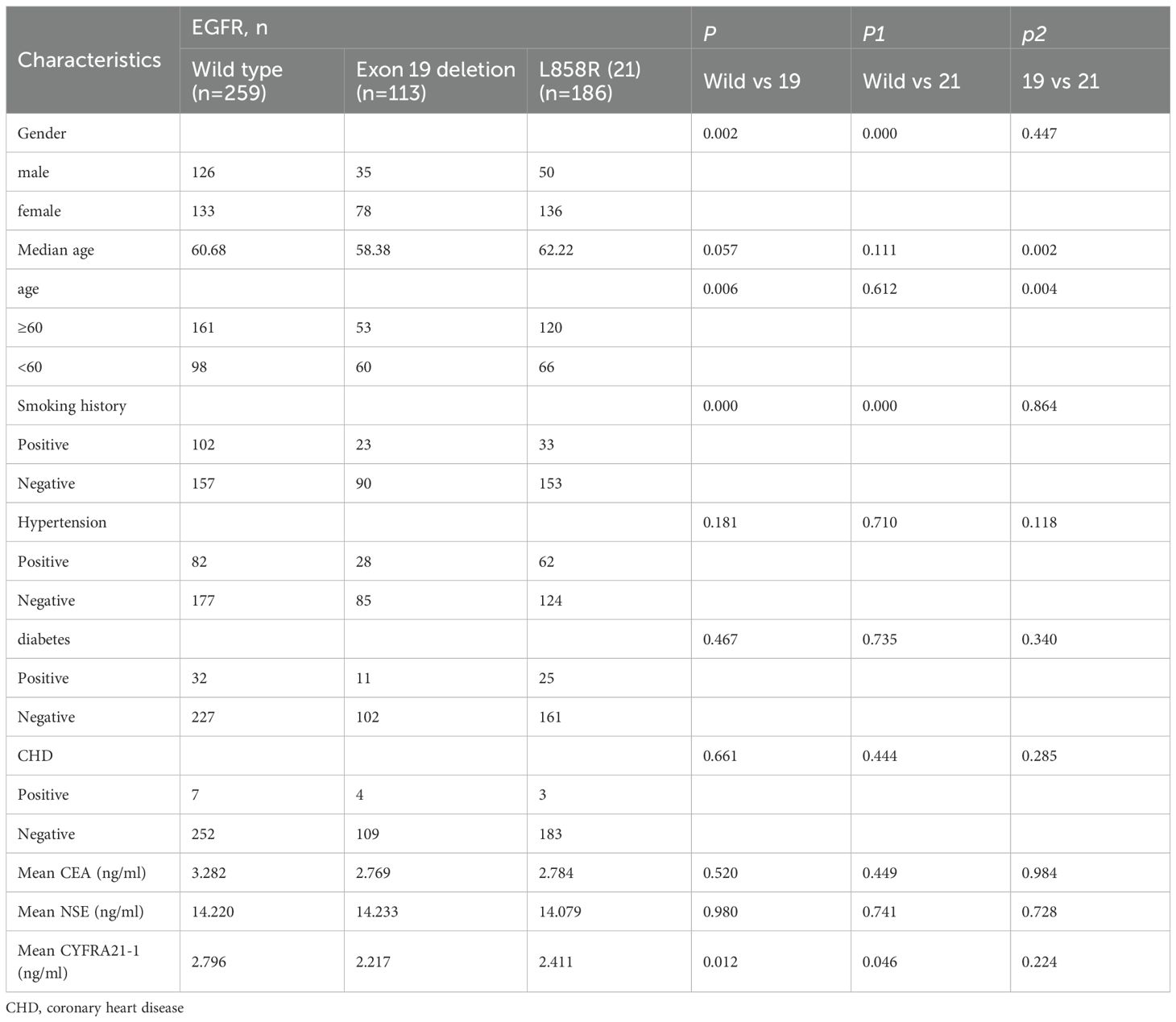
Table 4. Univariate analysis of the effects of EGFR 19, 21 mutation on clinical features of invasive lung adenocarcinoma.
Table 5 shows the pathological features of EGFR 19 deletion and L858R mutations. Both mutations were more commonly associated with moderate differentiation (P = 0.000) and high frequencies of lepidic and acinar subtypes, while being rare in mucinous subtypes. EGFR 21 mutations were also rare in solid pathological subtypes (16% vs. 41%; P = 0.002).
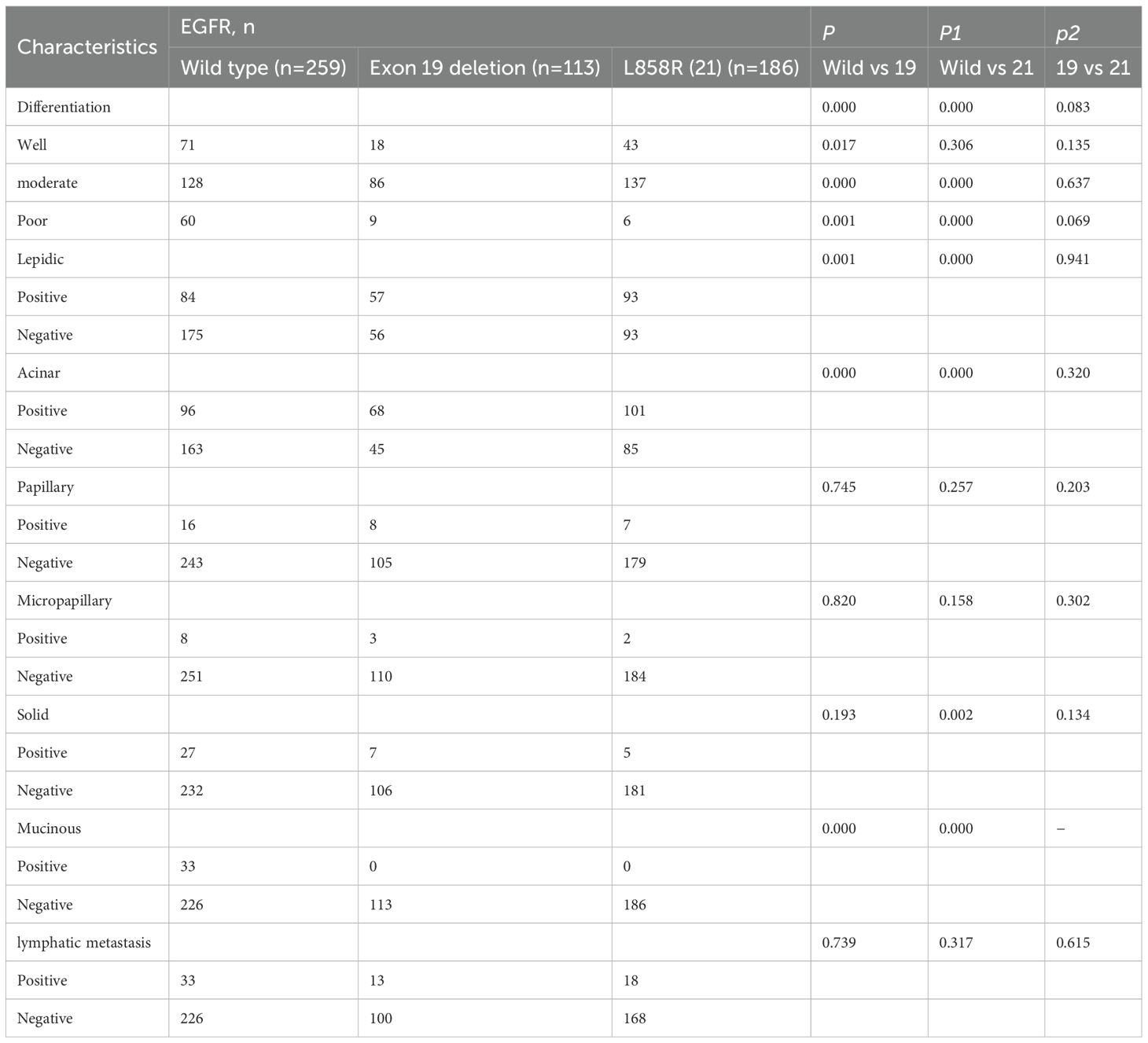
Table 5. Univariate analysis of the effects of EGFR 19, 21 mutation on pathological features of invasive lung adenocarcinoma.
Table 6 shows the CT features of EGFR 19 deletion and L858R mutations. Compared to WT EGFR, EGFR 21 mutations were more frequent in the upper lobe of the left lung (P = 0.038) and rare in the middle lobe of the right lung. EGFR L858R mutations had smaller solid components (1.030 vs. 1.380 cm; P = 0.007), with higher mutation rates in ground glass nodules with 25-50% solid components (P = 0.001). Both EGFR 19 and L858R mutations were rare in ground glass nodules with 75-100% solid components or pure solid nodules. EGFR 19 mutations were more likely to occur in ground glass nodules with 50-75% solid components. The CT characteristics that significantly differed between EGFR mutations and WT EGFR were the spicule sign (P = 0.011, P = 0.003). EGFR 21 mutations were more likely to show a bronchial cutoff sign (P = 0.034).
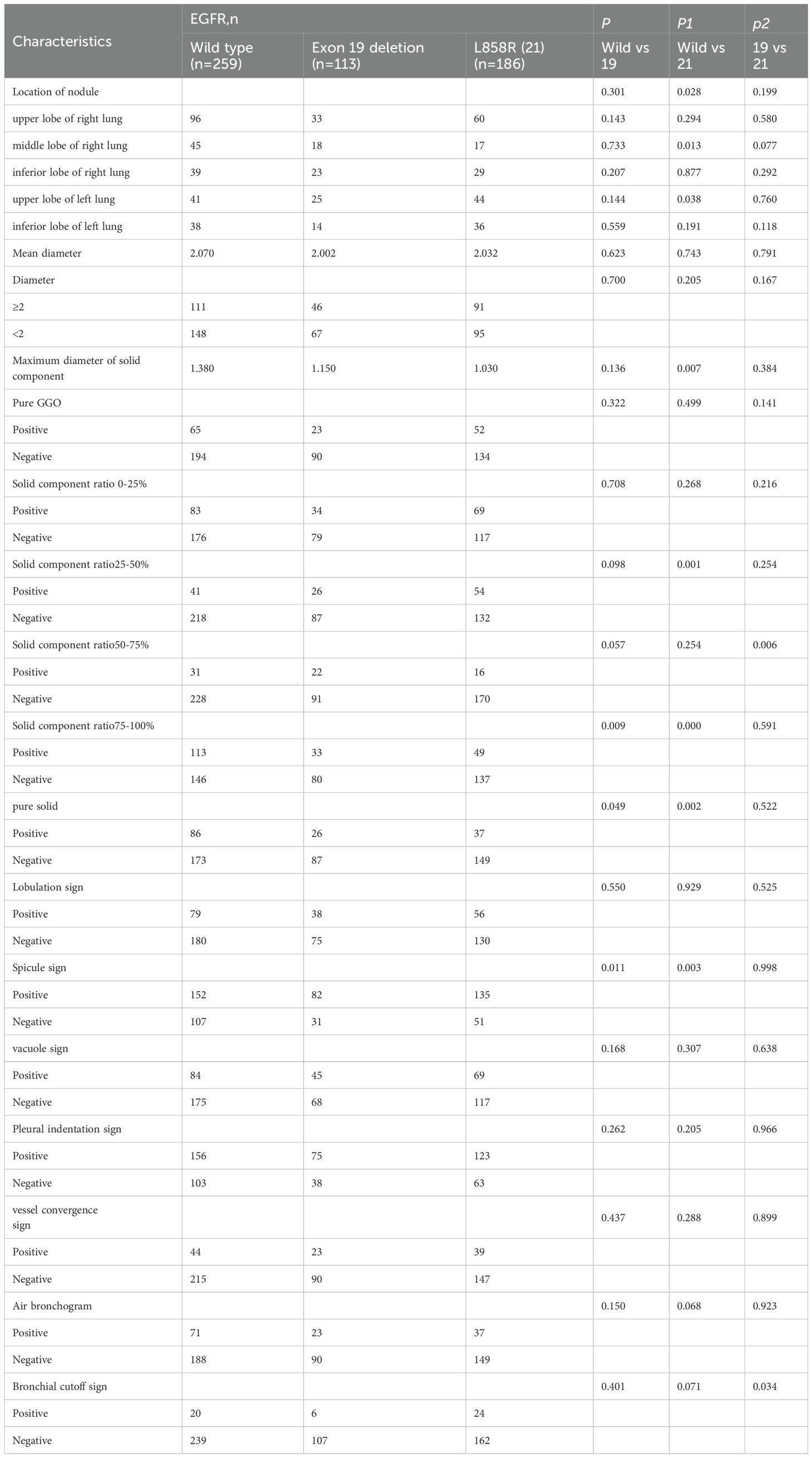
Table 6. Univariate analysis of the effects of EGFR 19, 21 mutation on radiological features of invasive lung adenocarcinoma.
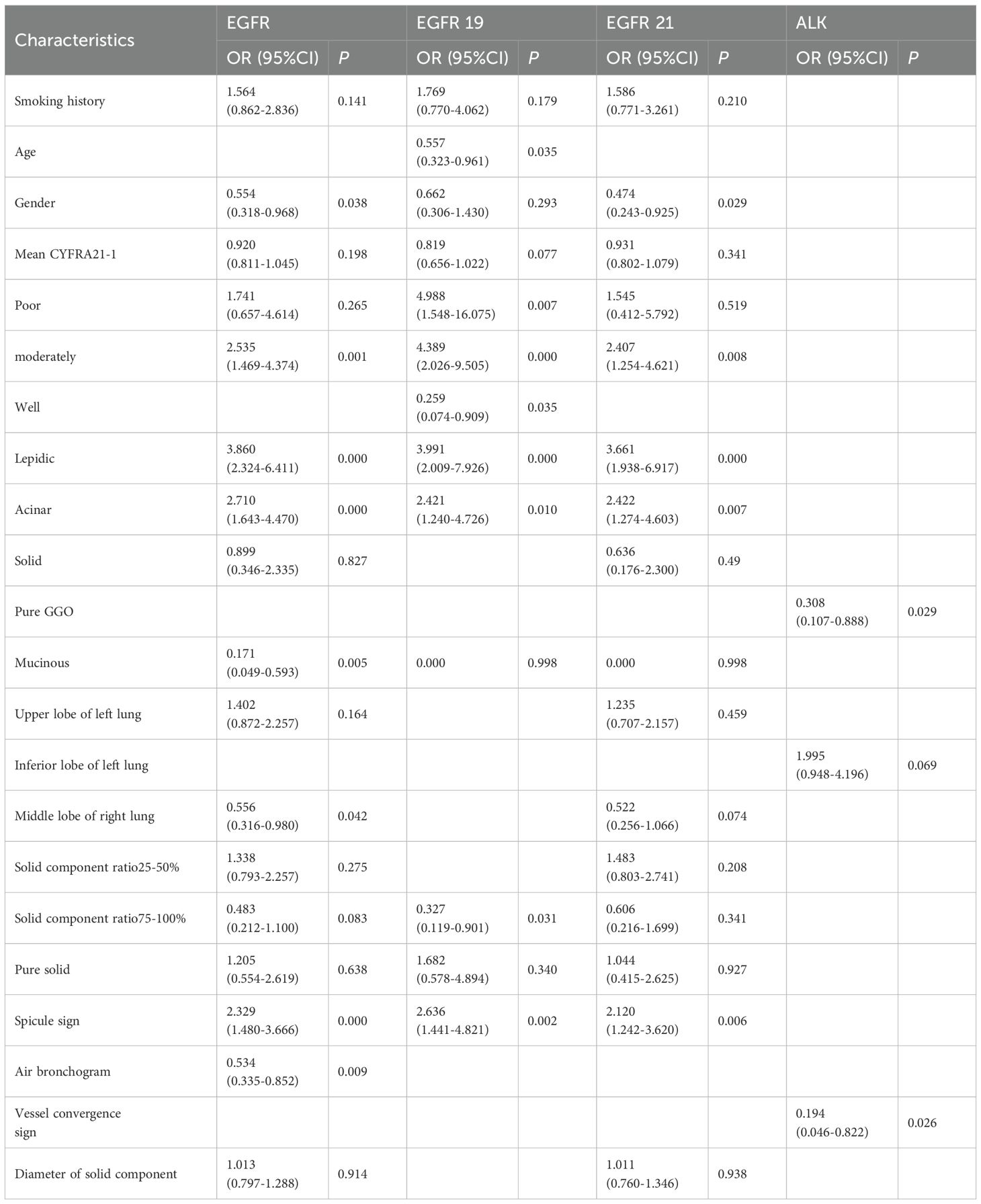
Multivariable analyses of prognostic factors for EGFR, EGFR19 and 21 mutation and ALK mutation and receiver operating characteristic curve analysis
Multivariate analysis (Table 7) identified several independent predictors of EGFR mutations: gender, moderate differentiation, lepidic, acinar, and mucinous subtypes, middle lobe of the right lung, spicule sign, and air bronchogram (P < 0.05). For EGFR exon 19 mutations, independent predictors included age, degree of differentiation, lepidic, acinar subtypes, 75-100% solid component ratio, and spicule sign (P < 0.05). For EGFR exon 21 mutations, gender, moderate differentiation, lepidic, acinar subtypes, and spicule sign were significant (P < 0.05). Pure GGO and vessel convergence sign were independent predictors of ALK gene fusion (P < 0.05).
ROC
To validate the logistic regression model for predicting EGFR, EGFR19, EGFR21, and ALK mutation status, ROC curves were generated using the regression equations. The ROC for EGFR showed an AUC of 0.782 (P = 0.000, 95% CI: 0.745-0.819), for EGFR19 an AUC of 0.809 (P = 0.000, 95% CI: 0.764-0.855), for EGFR21 an AUC of 0.791 (P = 0.000, 95% CI: 0.750-0.832), and for ALK an AUC of 0.675 (P = 0.000, 95% CI: 0.595-0.755) (Figure 3).
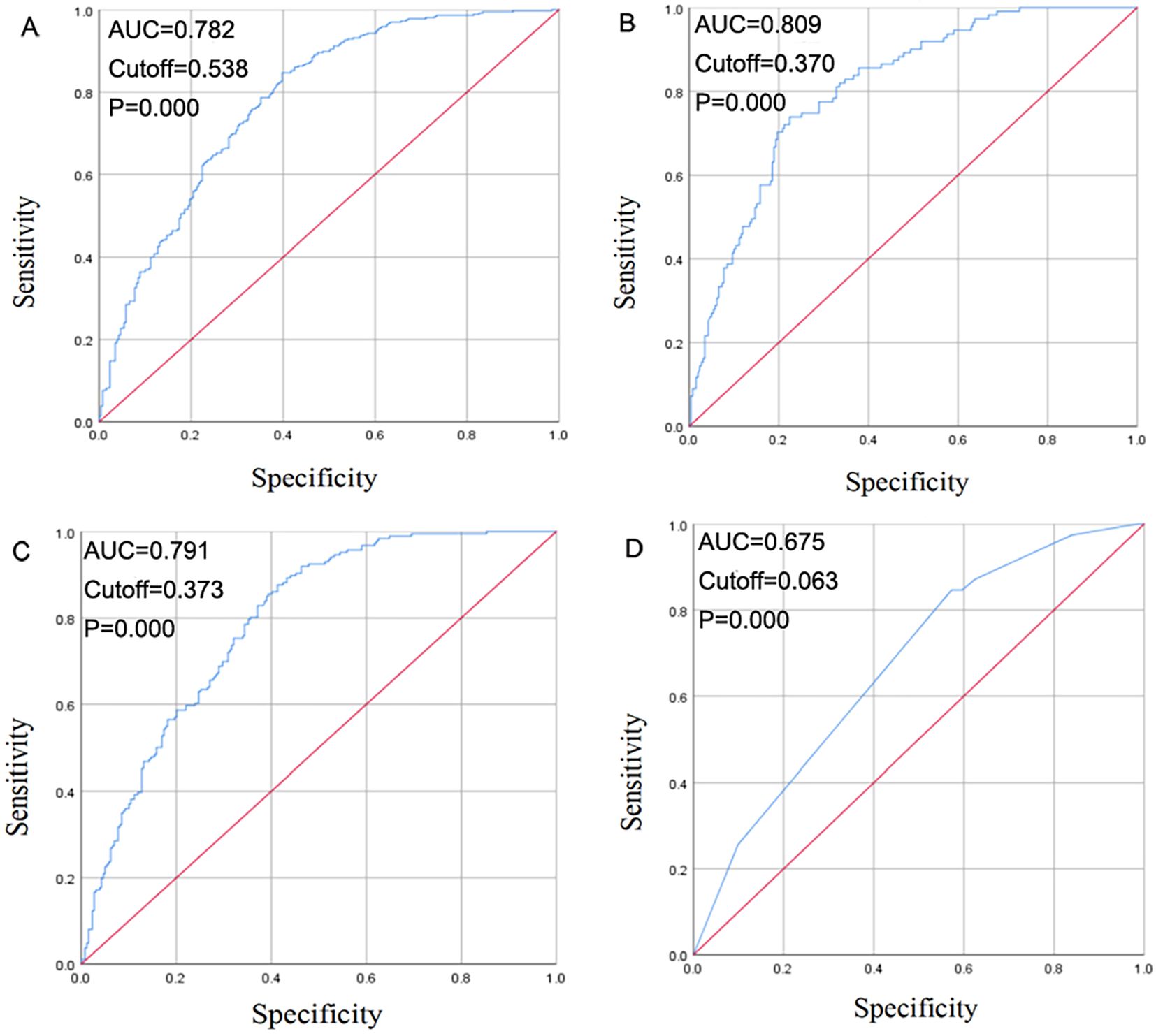
Figure 3. Receiver operating curve (ROC) curve for EGFR mutation (A), EGFR 19 mutation (B), EGFR 21 mutation (C), ALK positivity (D) prediction.
Discussion
EGFR and ALK mutations are recognized as critical drivers in the development of NSCLC, especially lung adenocarcinoma, and serve as important targets for clinical therapy. These mutations play a pivotal role in diagnosis and treatment planning (26). This study aims to explore the correlation between EGFR mutation status, ALK positivity, and various demographic, radiological, and pathological features of lung adenocarcinoma.
This study included 626 lung adenocarcinoma patients, with EGFR mutations found in 58.63% (367/626) and ALK gene rearrangements in 6.23% (39/626) of cases. These rates were consistent with previous studies (27). Univariate and multivariate analyses were conducted, and ROC curves were plotted to evaluate the diagnostic value of clinical, radiological, and pathological features in predicting EGFR and ALK mutation statuses. Subgroup analysis was performed for different EGFR mutation sites, focusing on exon 19 and exon 21 mutations, to assess the impact of specific mutation types.
Clinical characteristics of EGFR and ALK mutations
The study reaffirmed that EGFR mutations are more prevalent in non-smoking women, which aligns with prior studies by Paez et al. (28) and Lynch et al. (29). The PIONEER study (6) also demonstrated a high EGFR mutation rate (51.4%) in Asian women and non-smokers with lung adenocarcinoma. In this study, smoking history did not emerge as an independent predictor of EGFR mutations in multivariate analysis, suggesting that smoking may not play a direct role in EGFR mutation status in this cohort, likely due to sample bias from the single-center study.
The results of this study showed that there was no correlation between the mean age or the 60-year age stratification and EGFR mutation status, which is consistent with previous literature (30, 31). Although some studies have suggested that diabetes may influence tumor biology and EGFR signaling pathways, thus participating in tumor development (32–34), no association between diabetes and EGFR mutation rate was found in this study. Hypertension and coronary heart disease also did not show a significant correlation with EGFR mutation.
Regarding tumor markers, although some studies have reported that elevated CEA levels are associated with a higher EGFR mutation rate (35–37), its independence as a risk factor remains controversial. NSE levels are not significantly correlated with EGFR mutation (38), but it may serve as a predictor of EGFR-TKI treatment response and a prognostic indicator for patients with EGFR mutations (39). In this study, the mean CYFRA21–1 level in the mutation group was lower than in the WT group (2.358 vs. 2.796 ng/ml, P = 0.003), but multivariate regression analysis did not identify it as an independent predictor of EGFR mutation. This is consistent with the retrospective study by Takeuchi et al. (40).
In terms of EGFR mutation subtypes, this study found that EGFR exon 19 and 21 mutations were more common in women and patients without a smoking history, which is in line with the conclusions of Cao et al. (41). However, after multivariate analysis, smoking was not an independent risk factor. Female gender was an independent predictor for EGFR exon 21 mutation, and EGFR exon 19 mutations were more common in patients under 60 years of age compared to those with wild-type EGFR, which was an independent predictor after multivariate analysis. The CYFRA21–1 level was lower in patients with EGFR exon 19 mutations but was not an independent predictor after regression analysis. Patients with EGFR exon 19 mutations were younger than those with exon 21 mutations (58.38 vs 62.22 years, P = 0.002).
Compared to EGFR gene mutations, ALK rearrangement is more likely to occur in younger, non-smoking or light-smoking lung adenocarcinoma patients (42). In this study, it was also found that the average age of patients with ALK mutations was younger, and the mutation rate was significantly higher in those under 60 years old. The mutation rate in patients without a smoking history was higher than in those with a smoking history. However, due to the small number of ALK fusion cases, no statistical difference was found. The research on the proportion of male and female ALK fusion mutation-positive patients is still controversial (43, 44). In this study, the ALK mutation rate was slightly higher in female patients, but no significant statistical difference was observed. There were no significant differences in tumor markers, hypertension, diabetes, or coronary heart disease.
Differences in pathological characteristics between EGFR and ALK mutations
Regarding tumor differentiation, this study found that EGFR mutations were more common in moderately differentiated adenocarcinomas and rare in poorly differentiated tumors, which slightly differs from previous research suggesting a lower mutation rate in poorly differentiated cases (45). In general, better tumor differentiation correlates with a better prognosis, supporting that EGFR mutation detection aligns with tumor differentiation in determining lung cancer prognosis.
Histopathological subtypes of lung adenocarcinoma show varying sensitivities to molecular-targeted drugs. Studies have indicated that certain subtypes, such as papillary and lepidic, have higher EGFR mutation rates, although the exact relationship remains unclear (46, 47). In this study, the mutation rate was higher in lepidic and acinar dominant subtypes, consistent with previous reports (21, 48–50). Previous studies (51) also showed that lepidic as the main subtype manifested as GGO on CT.The more ground glass components of GGO, the higher the EGFR mutation rate, which was consistent with the above results.Conversely, solid and mucinous subtypes had a lower EGFR mutation rate, with mucinous subtypes showing rare mutations, aligning with Inamura et al.’s findings (52).
Further analysis of EGFR mutations at exon 19/21 sites showed higher mutation rates in moderately differentiated adenocarcinomas, particularly in lepidic and acinar subtypes, and low mutation rates in mucinous subtypes. Solid subtypes also had a low incidence of EGFR21 mutations, though multivariate analysis did not identify this as an independent predictor.
Regarding ALK mutations, Wang et al. reported an association with solid dominant subtypes, which was also observed in this study, though the difference was not statistically significant (53). Several studies suggest ALK mutations are linked to lymph node metastasis, indicating poor prognosis (54–56). However, this study found no significant correlation between ALK mutations and lymph node metastasis, likely due to the inclusion of early-stage tumors and no significant differences in tumor differentiation or histological subtypes.
Differences in Imaging Characteristics Between EGFR and ALK Mutation Populations
Regarding lesion location, previous studies have shown no correlation between lesion location and EGFR mutation status (23, 57). However, some studies suggest that EGFR mutations are more common in the upper lung lobes, possibly due to a complex interaction between genetic and environmental factors (58). In this study, univariate analysis found a higher EGFR mutation rate in tumors located in the upper lobe of the left lung and a lower rate in the middle lobe of the right lung. Multivariate regression analysis identified the middle lobe of the right lung as an independent risk factor for EGFR mutations, indicating the need for further research to determine whether lobe distribution in peripheral small lung adenocarcinomas can predict EGFR mutations.
In terms of CT features, early lung adenocarcinomas often present as pure ground glass opacity (pGGO), partial solid nodules, or solid nodules. Previous studies have suggested a relationship between EGFR mutations and solid components of GGO (59, 60), although findings are inconsistent. Most studies report that EGFR mutations correlate with a higher proportion of GGO (61, 62), suggesting that GGO could predict EGFR mutations. However, other studies (63, 64) found that EGFR mutations were more common in lesions with more than 50% solid components. This study showed that EGFR mutation patients had smaller maximum solid component diameters, with a higher mutation rate in tumors with 25-50% solid components and a lower rate in tumors with 75-100% solid components. In pure solid nodules, the mutation rate was also low, supporting a positive correlation between EGFR mutations and GGO components. The presence of solid components in GGO may indicate increased tumor aggression and pathological grade (60).
CT features like the air bronchogram, spicule sign, and tumor size have been linked to EGFR mutations. Some studies suggest a positive correlation between air bronchogram and EGFR mutation (58, 65), while others report a negative correlation (66). In this study, EGFR mutations were positively correlated with the spicule sign and negatively correlated with the air bronchogram, with no significant correlation found with other features. Multivariate analysis identified spicule sign and air bronchogram as independent predictors of EGFR mutations.
Further analysis of EGFR mutation subtypes, specifically exon 19/21 mutations, found that EGFR21 mutations were more common in the upper lobe of the left lung and rare in the middle lobe of the right lung, consistent with Tengeng et al.’s study (58). However, multivariate regression showed no correlation between lesion location and EGFR mutation. Solid components of GGO in EGFR21 patients were smaller than in EGFR19 patients, and EGFR21 mutations were more frequent in patients with 25-50% solid components, consistent with Lee et al.’s findings (18). The mutation rate increased with the proportion of GGO. Both EGFR19 and EGFR21 mutations had lower mutation rates in pure solid nodules or GGO with 75-100% solid content and higher rates with spicule signs. There was no significant difference in the air bronchogram between the two mutation types.
In terms of ALK mutations, previous studies have shown that ALK gene fusion is associated with larger tumor volumes and higher solid component proportions (55). This study found the same trend, but no statistical significance due to the small number of ALK fusion cases. ALK mutations were more common in the left inferior lobe, but this was not an independent predictor. Studies have shown that ALK mutations are associated with larger tumor diameters and higher solid component proportions, which was confirmed in this study, although no significant statistical differences were observed due to the small sample size. Pure GGO nodules had a very low ALK mutation rate (P<0.05), with mutation rates increasing as the solid component proportion increased.
Other studies (67–71) have shown that ALK-positive tumors are less likely to exhibit cavity signs and air bronchograms, and are associated with larger tumor size and a higher proportion of solid components. In this study, ALK mutations were less common in patients with vessel convergence signs, with no statistical differences found in other imaging features. This may be related to the fact that the lung cancer patients in this study were early-stage and had fewer lymph node or distant metastases.
Multi-factor analysis of EGFR and ALK mutations
Multivariate analysis identified several independent predictors for EGFR mutations: female gender, moderate differentiation, lepidic and acinar subtypes, mucinous subtype, middle lobe of the right lung, spicule sign, and air bronchogram. Subgroup analysis revealed that age under 60, degree of differentiation, lepidic and acinar subtypes, 75-100% GGO solid component, and spicule sign were independent predictors for EGFR19 mutation. For EGFR21 mutation, female gender, moderate differentiation, lepidic and acinar subtypes, and spicule sign were independent predictors. Pure GGO and vessel convergence sign were independent predictors for ALK mutation.
An ROC curve analysis showed the diagnostic value of clinical, pathological, and HRCT models for predicting EGFR, EGFR19, EGFR21, and ALK mutations. The AUC values were 0.782 for EGFR, 0.809 for EGFR19, 0.791 for EGFR21, and 0.675 for ALK, indicating that the combined radiomic and clinical feature models can effectively predict gene mutation status.
Limitations: (1) The study was a single-center, retrospective analysis with 100% Asian patients, which may introduce selection bias. (2) The low ALK fusion rate resulted in fewer positive cases, and differences from previous studies may be due to the early-stage tumors. Further research with a broader patient population and extended follow-up is needed to assess the relationship between gene mutations, clinicopathological features, and prognosis. (3) Only early-stage lung adenocarcinomas were included, so it remains unclear whether the findings apply to advanced stages.
Conclusion
This study found a correlation between EGFR mutation status, ALK positivity, and demographic, tumor, radiological, and pathological features in lung adenocarcinoma patients. Although CT imaging and pathological features cannot replace the role of molecular testing, to a certain extent, they can assist in predicting the genetic mutation status and further determine which patients may be suitable for receiving EGFR-TKIs or ALK inhibitors treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study has been approved by the ethics committee of Affiliated Zhongshan Hospital of Dalian University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XFZ: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. XZ: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the “1+X” program for Clinical Competency enhancement-Clinical Research Incubation Project, The Second Hospital of Dalian Medical University under Grant 2022LCYJYB09.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Halpenny DF, Riely GJ, Hayes S, Yu H, Zheng J, Moskowitz CS, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements. Lung Cancer. (2014) 86:190–4. doi: 10.1016/j.lungcan.2014.09.007
2. Rongshou Z, Hongmei Z, Siwei Z, and Wangqing C. Estimates of cancer Incidence and mortality in China,2013. Chin J Cancer. (2017) 36:66. doi: 10.1186/s40880-017-0234-3
3. Chen W, Zheng RS, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Kuhn E, Morbini P, Cancellieri A, Damiani S, Cavazza A, and Comin CE. Adenocarcinoma classification: patterns and prognosis. Pathologica. (2018) 110:5–11.
5. Ozkan E, West A, Dedelow JA, Chu BF, Zhao W, Yildiz VO, et al. CT Gray-level texture analysis as a quantitative imaging biomarker of epidermal growth factor receptor mutation status in adenocarcinoma of the lung. Am J Roentgenol. (2015) 205:1016–25. doi: 10.2214/AJR.14.14147
6. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. (2014) 9:154–62. doi: 10.1097/JTO.0000000000000033
7. Lo H-W. Nuclear mode of the EGFR signaling network: biology, prognostic value, and therapeutic implications. Discov Med. (2010) 10:44–51.
8. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
9. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
10. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. (2010) 11:121–8. doi: 10.1016/S1470-2045(09)70364-X
11. Jean-Charles S, Tony SM, Cappuzzo F, and Pasi AJ. EGFR-mutated oncogene-addicted non-small cell lung cancer: current trends and future prospects. Cancer Treat Rev. (2012) 38:416–30. doi: 10.1016/j.ctrv.2011.10.003
12. Jinyong K, Sehhoon P, Bo MK, and Myung-Ju A. Updates on the treatment of epidermal growth factor receptor-mutant non-small cell lung cancer. Cancer. (2025) 15:131. doi: 10.1002/cncr.35778
13. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. (2011) 6:244–85. doi: 10.1097/JTO.0b013e318206a221
14. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. (2011) 24:653–64. doi: 10.1038/modpathol.2010.232
15. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, and Williams RA. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. (2011) 6:1496–504. doi: 10.1097/JTO.0b013e318221f701
16. Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. (2012) 30:1438–46. doi: 10.1200/JCO.2011.37.2185
17. Gu J, Lu CL, Guo J, Chen LL, Chu Y, Ji Y, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients – a single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol. (2013) 107:474–80. doi: 10.1002/jso.23259
18. Lee HJ, Kim YT, Kang CH, Zhao B, Tan Y, Schwartz LH, et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology. (2013) 268:254–64. doi: 10.1148/radiol.13112553
19. Glynn C, Zakowski MF, and Ginsberg MS. Are there imaging characteristics associated with epidermal growth factor receptor and KRAS mutations in patients with adenocarcinoma of the lung with bronchioloalveolar features. J Thorac Oncol. (2010) 5:344–8. doi: 10.1097/JTO.0b013e3181ce9a7a
20. Sugano M, Shimizu K, Nakano T, Kakegawa S, Miyamae Y, Kaira K, et al. Correlation between computed tomography findings and epidermal growth factor receptor and KRAS gene mutations in patients with pulmonary adenocarcinoma. Oncol Rep. (2011) 26:1205–11. doi: 10.3892/or.2011.1412
21. Liu Y, Kim J, Qu F, Liu S, Wang H, Balagurunathan Y, et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology. (2016) 280:271–80. doi: 10.1148/radiol.2016151455
22. Hsu KH, Chen KC, Yang TY, Yeh YC, Chou TY, Chen HY, et al. Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol. (2011) 6:1066–72. doi: 10.1097/JTO.0b013e31821667b0
23. Rizzo S, Petrella F, Buscarino V, De Maria F, Raimondi S, Barberis M, et al. CT Radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol. (2016) 26:32–42. doi: 10.1007/s00330-015-3814-0
24. Zhou JY, Zheng J, Xiao WB, Xiao WB, Zhao J, Sun K, et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol. (2015) 25:1257–66. doi: 10.1007/s00330-014-3516-z
25. Choi CM, Kim MY, Hwang HJ, Lee JB, and Kim WS. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology. (2015) 275:272–9. doi: 10.1148/radiol.14140848
26. Nakajima N, Yoshizawa A, Rokutan-Kurata M, Noguchi M, Teramoto Y, Sumiyoshi S, et al. Prognostic significance of cribriform adenocarcinoma of the lung:validation analysis of 1,057 Japanese patients with resected lung adenocarcinoma and a review of the literature. Transl Lung Cancer Res. (2021) 10:117–27. doi: 10.21037/tlcr-20-612
27. Liu Y, Wu A, Li X, Wang S, Fang S, and Mo Y. A retrospective analysis of eleven gene mutations, PD - L1 expression and clinicopathological characteristics in non - small cell lung cancer patients. Asian J Surg. (2022) 45:367–75. doi: 10.1016/j.asjsur.2021.06.030
28. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Sci (New York NY). (2004) 304:1497–500. doi: 10.1126/science.1099314
29. Lynch TJ, Bell DW, Sordella R, Kakegawa S, Miyamae Y, Kaira K, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Engl J Med. (2004) 350:2129–39. doi: 10.1056/NEJMoa040938
30. Liu WS, Zhao LJ, Pang QS, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Prognostic value of epidermal growth factor receptor mutations in resected lung adenocarcinomas. Med Oncol. (2014) 31:771. doi: 10.1007/s12032-013-0771-9
31. Lian W and Ouyang Y. CT-guided aspiration lung biopsy for EGFR and ALK gene mutation analysis of lung cancer. Oncol Lett. (2017) 13:3415–22. doi: 10.3892/ol.2017.5921
32. Yang X, Liu Y, Mani H, Olson J, Clawson G, Caruso C, et al. Biologic evaluation of diabetes and local recurrence in non-small cell lung cancer. Pathol Oncol Res. (2017) 23:73–7. doi: 10.1007/s12253-016-0086-1
33. Benter IF, Sarkhou F, Al-Khaldi AT, Chandrasekhar B, Attur S, Dhaunsi GS, et al. The dual targeting of EGFR and ErbB2 with the inhibitor Lapatinib corrects high glucose-induced apoptosis and vascular dysfunction by opposingmultiple diabetes-induced signaling changes. J Drug Targeting. (2015) 23:506–18. doi: 10.3109/1061186X.2015.1057150
34. Rajaram P, Chandra P, Ticku S, Pallavi BK, Rudresh KB, and Mansabdar P. Epidermal growth factor receptor: Role in human cancer. Indian J Dental Res. (2017) 28:687–94. doi: 10.4103/ijdr.IJDR_534_16
35. Wang S, Ma P, Ma G, Lv Z, Wu F, Guo M, et al. Value of serum tumor markers for predicting EGFR mutations and positive ALK expression in 1089 Chinese non-small-cell lung cancer patients:a retrospective analysis. Eur J Cancer. (2020) 124:1–14. doi: 10.1016/j.ejca.2019.10.005
36. Wen L, Wang S, Xu W, Xu X, Li M, Zhang Y, et al. Value of serum tumor markers for predicting EGFR mutations in non-small cell lung cancer patients. Ann Diagn Pathol. (2020) 49:151633. doi: 10.1016/j.anndiagpath.2020.151633
37. Gu J, Xu S, Huang L, Li S, Wu J, Xu J, et al. Value of combining serum carcinoembryonic antigen and PET/CT in predicting EGFR mutation in non-small cell lung cancer. J Thorac Dis. (2018) 10:723–31. doi: 10.21037/jtd.2017.12.143
38. Jiang R, Wang X, and Li K. Predictive and prognostic value of preoperative serum tumor markers is EGFR mutation-specific in resectable non-small-cell lung cancer. Oncotarget. (2016) 7:26823–36. doi: 10.18632/oncotarget.8662
39. Suh KJ, Keam B, Kim M, Park YS, Kim TM, Jeon YK, et al. Serum neuron-specific enolase levels predict the efficacy of first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring EGFR mutations. Clin Lung cancer. (2016) 17:245–52. doi: 10.1016/j.cllc.2015.11.012
40. Takeuchi A, Oguri T, Sone K, Ito K, Kitamura Y, Inoue Y, et al. Predictive and prognostic value of CYFRA 21–1 for advanced non-small cell lung cancer treated with EGFR-TKI. Anticancer Res. (2017) 37:5771–6. doi: 10.21873/anticanres.12018
41. Cao Y, Xu H, Liao M, Qu Y, Xu L, Zhu D, et al. Associations between clinical data and computed tomography features in patients with epidermal growth factor receptor mutations in lung adenocarcinoma. Int J Clin Oncol. (2018) 23:249–57. doi: 10.1007/s10147-017-1197-8
42. Fjaellegaard K, Koefod PJ, Andersen G, Biagini M, Bhatnagar R, Laursen CB, et al. The prevalence of tumour markers in Malignant pleural effusions associated with primary pulmonary adenocarcinoma:a retrospective study. Eur Clin Respir J. (2021) 8:1984375. doi: 10.1080/20018525.2021.1984375
43. Mendozad P, Lin JJ, Rooney MM, Chen T, Sequist LV, Shaw AT, et al. Imaging features and metastatic patterns of advanced ALK -rearranged non-small cell lung cancer. Am J Roentgenol. (2020) 214:766–74. doi: 10.2214/AJR.19.21982
44. Christopoulos P, Endris V, Bozorgmehr F, Elsayed M, Kirchner M, Ristau J, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer. (2018) 142:2589–98. doi: 10.1002/ijc.31275
45. Ullas B, Bivas B, Kumar P, and Vamshi Krishna M. Differential clinicopathological features, treatments and outcomes in patients with Exon 19 deletion and Exon 21 L858R EGFR mutation-positive adenocarcinoma non-small-cell lung cancer. BMJ Open Respir Res. (2023) 10:e001492. doi: 10.1136/bmjresp-2022-001492
46. Gerber DE, Gandhi L, and Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am Soc Clin Oncol Educ Book. (2014) 34:e353–65. doi: 10.14694/EdBook_AM.2014.34.e353
47. Santarpia M, Menis J, Chaib I, Gonzalez Cao M, and Rosell R. Dacomitinib for the first-line treatment of patients with EGFR-mutated metastatic non-small cell lung cancer. Expert Rev Clin Pharmacol. (2019) 12:831–40. doi: 10.1080/17512433.2019.1649136
48. Gallo M, Luca AD, Frezzetti D, Passaro V, Maiello MR, and Normanno N. The potential of monitoring treatment response in non-small cell lung cancer using circulating tumour cells. Expert Rev Mol Diagn. (2019) 19:683–94. doi: 10.1080/14737159.2019.1640606
49. Gaikwad R, Bobde Y, Ganesh R, Patel T, Rathore A, Ghosh B, et al. 2-Phenylindole derivatives as anticancer agents:synthesis and screening against murine melanoma,human lung and breast cancer cell lines. Synthetic Commun. (2019) 49:2258–69. doi: 10.1080/00397911.2019.1620282
50. Zhang Y, Sun Y, Pan Y, Li C, Shen L, Li Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res. (2012) 18:1947–53. doi: 10.1158/1078-0432.CCR-11-2511
51. Honda T, Kondo T, Murakami S, Saito H, Oshita F, Ito H, et al. Radiographic and pathological analysis of small lung adenocarcinoma using the new IASLC classification. Clin Radiol. (2013) 68:e21–6. doi: 10.1016/j.crad.2012.09.002
52. Inamurak, Ninomiya H, Ishikawa Y, and Matsubara O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch Pathol Lab Med. (2010) 134:66–72. doi: 10.1088/1361-6560/ab6f98
53. Wang H, Schabath MB, Liu Y, Han Y, Li Q, Gillies RJ, et al. Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol. (2016) 85:1934–40. doi: 10.1016/j.ejrad.2016.08.023
54. Li P, Gao Q, Jiang X, Zhan Z, Yan Q, Li Z, et al. Comparison of clinicopathological features and prognosis between ALK rearrangements and EGFR mutations in surgically resected early-stage lung adenocarcinoma. J Cancer. (2019) 10:61–71. doi: 10.7150/jca.26947
55. Kim TH, Woo S, Yoon SH, Halpenny DF, Han S, and Suh CH. CT characteristics of non-small cell lung cancer with anaplastic lymphoma kinase rearrangement:a systematic review and meta-analysis. Am J Roentgenol. (2019) 213:1059–72. doi: 10.2214/AJR.19.21485
56. Shin SH, Lee H, Jeong BH, Choi YS, Shin MH, Kim S, et al. Anaplastic lymphoma kinase rearrangement in surgically resected stage Ia lung adenocarcinoma. J Thorac Dis. (2018) 10:3460–7. doi: 10.21037/jtd.2018.05.131
57. Dai J, Shi J, Soodeen-Lalloo AK, Zhang P, Yang Y, Wu C, et al. Air bronchogram:A potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer. (2016) 98:22–8. doi: 10.1016/j.lungcan.2016.05.009
58. Tseng CH, Chen KC, Hsu KH, Tseng JS, Ho CC, Hsia TC, et al. EGFR mutation and lobar location of lung Adenocarcinoma. Carcinogenesis. (2016) 37:157–62. doi: 10.1093/carcin/bgv168
59. Choi Y, Kim KH, Jeong BH, Lee KJ, Kim H, Kwon OJ, et al. Clinicoradiopathological features and prognosis according to genomic alterations in patients with resected lung adenocarcinoma. J Thorac Dis. (2020) 12:5357–68. doi: 10.21037/jtd-20-1716
60. Zou J, Lv T, Zhu S, Lu Z, Shen Q, Xia L, et al. Computed tomography and clinical features associated with epidermal growth factor receptor mutation status in stage I/II lung adenocarcinoma. Thorac Cancer. (2017) 8:260–70. doi: 10.1111/1759-7714.12436
61. Sabri A, Batool M, Xu Z, Bethune D, Abdolell M, and Manos D. Predicting EGFR mutation status in lung cancer:Proposal for a scoring model using imaging and demographic characteristics. Eur Radiol. (2016) 26:4141–7. doi: 10.1007/s00330-016-4252-3
62. Yano M, Sasaki H, Kobayashi Y, Yukiue H, Haneda H, Suzuki E, et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol. (2006) 1:413–6. doi: 10.1097/01243894-200606000-00006
63. Chung JH, Choe G, Jheon S, Sung SW, Kim TJ, Lee KW, et al. Epidermal growth factor receptor mutation and pathologic-radiologic correlation between multiple lung nodules with ground-glass opacity differentiates multicentric origin from intrapulmonary spread. J Thorac Oncol. (2009) 4:1490–5. doi: 10.1097/JTO.0b013e3181bc9731
64. Wang T, Zhang T, Han X, Liu XI, Zhou N, and Liu Y. Impact of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of stage IA adenocarcinoma of the lung: Correlation between computed tomography images and EGFR and KRAS gene mutations. Exp Ther Med. (2015) 9:2095–103. doi: 10.3892/etm.2015.2422
65. Sacconi B, Anzidei M, Leonardi A, Boni F, Saba L, Scipione R, et al. Analysis of CT features and quantitative texture analysis in patients with lung adenocarcinoma:a correlation with EGFR mutations and survival rates. Clin Radiol. (2017) 72:443–50. doi: 10.1016/j.crad.2017.01.015
66. Algharras A, Kovacina B, Tian Z, Alexander JW, Semionov A, van Kempen LC, et al. Imaging-based surrogate markers of epidermal growth factor receptor mutation in lung adenocarcinoma:a local perspective. Can Assoc Radiol J. (2020) 71:208–16. doi: 10.1177/0846537119888387
67. Harrison P, Vyse S, and Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. (2020) 61:167–79. doi: 10.1016/j.semcancer.2019.09.015
68. Kim TJ, Lee CT, Jheon SH, Park JS, and Chung JH. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann Thorac Surg. (2016) 101:473–80. doi: 10.1016/j.athoracsur.2015.07.062
69. Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, Hatooka S, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer. (2012) 77:319–25. doi: 10.1016/j.lungcan.2012.03.013
70. Chang C, Sun X, Wang G, Yu H, Zhao W, Ge Y, et al. A machine learning model based on PET/CT radiomics and clinical characteristics predicts ALK rearrangement status in lung adenocarcinoma. Front Oncol. (2021) 11:603882. doi: 10.3389/fonc.2021.603882
71. Mendozad P, Stowell J, Muzikansky A, Shepard JO, Shaw AT, and Digumarthy SR. Computed tomography imaging characteristics of non-small cell lung cancer with anaplastic lymphoma kinase rearrangements: a systematic review and meta-analysis. Clin Lung Cancer. (2019) 20:339–49. doi: 10.1016/j.cllc.2019.05.006
Keywords: lung adenocarcinomas, EGFR gene mutation, ALK positivity, demographic data, tumor biomarkers, imaging, pathology
Citation: Zhang XF, Zhang X, Zhao L and Zhao ZL (2025) Correlation analysis between EGFR gene mutation status, ALK positivity and demographic data, tumor biomarkers, radiological and pathological features in patients with lung adenocarcinoma. Front. Oncol. 15:1627019. doi: 10.3389/fonc.2025.1627019
Received: 12 May 2025; Accepted: 07 November 2025; Revised: 30 October 2025;
Published: 25 November 2025.
Edited by:
Xi Yang, Affiliated Central Hospital Huzhou University, ChinaCopyright © 2025 Zhang, Zhang, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Long Zhao, enpsaG9zcGl0YWxfbG5AMTYzLmNvbQ==; Liang Zhao, bGlhbmd6aGFvQGRsdXQuZWR1LmNu
†These authors share first authorship
 Xue Fei Zhang1,2†
Xue Fei Zhang1,2† Liang Zhao
Liang Zhao Zhi Long Zhao
Zhi Long Zhao