- 1Department of Hematology-Oncology, Hôtel Dieu de France University Hospital, Faculty of Medicine-Saint Joseph University of Beirut, Beirut, Lebanon
- 2Department of Medical Oncology, Institut Gustave Roussy, Villejuif, France
Trimodality therapy (TMT), including transurethral resection (TUR), chemotherapy (CT), and radiotherapy (RT), offers the bladder-preserving treatment option for patients with muscle-invasive bladder cancer (MIBC). TMT, once indicated, has demonstrated effective and favorable local tumor control in MIBC, with complete response rates ranging between 50% and 80%. However, residual tumor is identified on follow-up TUR in approximately 20–30% of patients, and tumor recurrence occurs in a similar proportion. In both situations, the prognosis becomes unfavorable. This manuscript reviews the current evidence regarding recurrence patterns after TMT, differentiating between non–muscle-invasive (NMIBC) and muscle-invasive (MIBC) relapses. NMIBC recurrences after TMT are often manageable with conservative treatments like repeat TURBT and intravesical BCG, without negatively impacting survival. In contrast, MIBC recurrences typically require salvage cystectomy in fit patients, offering outcomes similar to primary surgery. For those unfit for or who continue to decline cystectomy, treatment remains uncertain due to the absence of clear guidelines, and systemic therapies used in metastatic urothelial carcinoma seem commonly applied by extrapolation.
Introduction
Bladder cancer is a relatively common malignancy, with an estimated 82,290 new cases in 2023, resulting in approximately 16,710 deaths (12,160 in men and 4,550 in women) (1). In Lebanon, bladder cancer ranks among the most prevalent cancers, accounting for 9% of all new diagnoses. It is the second most common cancer in men and the ninth in women (2). Diagnosis is typically established via transurethral resection of bladder tumor (TURBT). The predominant histologic subtype is transitional cell carcinoma (urothelial carcinoma) (3), with smoking recognized as the primary risk factor. Other contributing factors include occupational exposures, chronic indwelling catheters, recurrent bladder infections, and schistosomiasis in endemic regions.
Muscle-invasive bladder cancer (MIBC) is both prevalent and life-threatening, though it remains potentially curable with timely intervention. The prognosis is generally poor, with a 5-year overall survival (OS) rate of approximately 50%. Staging of MIBC involves abdominal and pelvic CT scans, chest imaging, and bone scans when clinically indicated. Although FDG-PET imaging can be employed to detect distant metastases, its utility in evaluating primary bladder tumors and local nodal disease is limited due to urinary excretion of FDG (4).
The primary therapeutic objective in MIBC is curative intent through a multimodal approach, which typically includes neoadjuvant chemotherapy, followed by radical cystectomy or bladder-preserving treatment (5). Bladder preservation, commonly referred to as trimodality therapy (TMT), is a viable option in carefully selected patients, particularly those unfit for or unwilling to undergo cystectomy. TMT consists of maximal TURBT followed by chemoradiotherapy (6–9). Chemoradiotherapy regimens commonly include gemcitabine, cisplatin, or 5-fluorouracil, with gemcitabine often preferred due to its favorable toxicity profile and relatively low incidence of adverse effects. Preclinical studies have shown that gemcitabine can radiosensitize a wide range of tumor cells, even at non-cytotoxic doses, thereby enhancing the effectiveness of RT (10). Radiation therapy is typically delivered with curative intent at a total dose of 64–66 Gy to the bladder over 6.5 to 7 weeks, using daily fractions of 1.8–2 Gy, 5 days per week. An alternative hypofractionated schedule could be used, such as 55 Gy in 20 fractions over 4 weeks (2.75 Gy/fraction). Elective pelvic lymph node irradiation may be included in select high-risk patients, usually to 45–50.4 Gy in 25–28 fractions (10). TMT has demonstrated favorable local tumor control, with complete response rates ranging from 50% to 80% in these MIBC patients (11).
Materials and methods
Search strategy
A comprehensive literature search was conducted using PubMed, Scopus, and Google Scholar databases for articles published up to May 2025. The search terms included combinations of the following keywords: “trimodality therapy,” “TMT,” “bladder preservation,” “muscle-invasive bladder cancer,” “recurrence,” “non–muscle-invasive bladder cancer,” “NMIBC,” “salvage cystectomy,” and “systemic therapy.” Boolean operators (AND, OR) were used to broaden or refine the search. Reference lists of the retrieved articles and relevant reviews were manually screened to identify additional studies.
Eligibility criteria
Studies were eligible if:
-included patients with muscle-invasive bladder cancer treated with trimodality therapy
-reported recurrence patterns (NMIBC or MIBC) after TMT, and
-described management approaches and oncological outcomes.
Eligible study types included clinical trials, retrospective series, prospective observational studies, and meta-analyses. Case reports, editorials, conference abstracts without full data, non-English publications, and studies lacking specific recurrence outcomes were excluded.
Data synthesis and analysis
Due to heterogeneity in study design and outcome reporting, a narrative synthesis was undertaken rather than a meta-analysis. Data were summarized qualitatively to highlight recurrence patterns after TMT, risk factors associated with recurrence, and therapeutic strategies employed. Particular attention was given to differences in prognosis and management between NMIBC and MIBC relapses, as well as outcomes of salvage cystectomy versus conservative or systemic approaches.
Bladder outcome after TMT
Despite these promising percentages, bladder-sparing multimodal approaches are associated with a significant risk of residual or recurrent disease. Post-TMT surveillance involves urine cytology, cystoscopy, and thoracoabdominal-pelvic (TAP) CT every 3 months for the first 2 years, every 6 months up to 5 years, and annually thereafter.
Top of form
The follow-up TURs identify residual tumors in approximately 20–30% of patients, and local recurrence occurs in a similar proportion. The prognosis is particularly poor for patients with tumors that fail to respond to initial therapy or who develop recurrent disease (11). Local recurrent disease could be non-muscle invasive bladder cancer (NMIBC) or MIBC (12–14). Tumor location, multifocality, a history of multiple transurethral resections, and a neutrophil-to-lymphocyte ratio (NLR) ≥ 2.56 were all significantly associated with an increased risk of recurrence following TMT. In contrast, patients who received early TMT after the initial diagnosis exhibited a lower recurrence rate (15).
NMIBC recurrence approach after TMT
When recurrence is non-invasive, conservative management, similar to standard NMIBC protocols (resection with or without intravesical therapy), can be pursued. Such recurrences do not appear to impact overall prognosis or increase the risk of BCG (Bacillus Calmette-Guérin) related toxicity (16). Low-grade, noninvasive recurrences after TMT can be effectively managed with TURBT combined with intravesical therapies such as BCG or mitomycin. For NMIBC with higher risk features, such as high-grade lesions, carcinoma in situ (CIS), or T1 disease, treatment options include TURBT with BCG or early cystectomy, guided by clinical judgment. Of note, intravesical BCG remains effective after bladder-preserving chemoradiation, with 59% of patients with Ta grade 2/3, CIS, or T1 achieving no further recurrence. Moreover, BCG is generally well tolerated in this setting, with 68% completing induction therapy without interruption or significant toxicity (17). A study using the Princess Margaret Cancer Centre’s institutional database (University Health Network, University of Toronto), aimed to evaluate whether standard NMIBC therapies are effective for NMIBC recurrences following TMT (18). They identified 12 patients with NMIBC recurrence post-TMT between 2008 and 2019 and compared them to 60 matched controls with primary NMIBC, based on stage and grade (5:1 ratio). All TMT patients initially received weekly cisplatin (40 mg/m²), bladder-directed radiotherapy (64–66 Gy), and Lipiodol injections for localization. Recurrences were managed with conventional NMIBC treatments. Median age was higher in the TMT group (78 vs. 66 years), with median follow-up of 3.6 vs. 5.4 years. Recurrence stages in the TMT group included Ta (n=4), T1 (n=3), and CIS (n=5). The TMT group had fewer subsequent recurrences (17% vs. 63%, P = 0.004) and similar cystectomy rates (8% vs. 18%, P = 0.68). These findings suggest that NMIBC recurrence after TMT can be successfully managed using traditional intravesical and endoscopic therapies.
MIBC recurrence approach after TMT
Salvage cystectomy is indicated for patients who are suitable surgical candidates and have failed to achieve a complete response following bladder-preserving TMT. It is particularly recommended in cases of muscle-invasive recurrence at any time post-TMT (18). Post-TMT salvage cystectomy has been shown to be a safe and viable option, with a 90-day major complication rate (Clavien-Dindo grade ≥3) of approximately 16% and a 90-day mortality rate of around 2%, rates comparable to those seen with primary radical cystectomy in patients without prior radiation. Early postoperative complications are typically cardiovascular or hematologic, while late complications often involve wound healing, uretero-enteric anastomotic strictures, or stoma-related issues. Serious complications like rectal injury and the need for colostomy are rare (3% and 1%, respectively) when the surgery is performed in experienced centers (18).
In a large series of patients undergoing cystectomy after TMT, 265 patients were analyzed specifically for outcomes following cystectomy post-TMT. 21 patients received salvage cystectomy following local relapse and the 244 received primary cystectomy post TMT. While salvage cystectomy demonstrated similar rates of intraoperative and early complications compared with primary cystectomy, it was associated with an increased risk of long-term complications. In terms of survival, no significant differences in disease-specific or overall survival were observed, supporting salvage cystectomy as an effective and safe option for appropriately selected patients who relapse after bladder-preserving therapy (19).
In another study by Rödel et al., among 42 non-responders to initial chemoradiotherapy who underwent primary cystectomy, the 5- and 10-year cancer-specific survival (CSS) rates were 21% and 18%, respectively. This was significantly worse than complete responders (41 patients) who subsequently required salvage cystectomy for MIBC recurrence, whose 5- and 10-year cancer specific survival(CSS) rates were 50% and 45%, respectively. The reasons for this difference may include more aggressive tumor biology, the adverse effects of delayed cystectomy, treatment-induced changes in tumor behavior, or selection bias, as non-responders are more likely to have unfavorable pathological features (20).
The differences in CSS between the studies may be explained by the smaller overall number of patients and the limited number who underwent primary cystectomy in the first study. If the sample size were larger, outcomes might resemble those reported in the second study, where CSS was lower for patients undergoing salvage cystectomy after relapse compared with those receiving primary cystectomy.
If a patient with recurrent MIBC after TMT remains unfit for or continues to refuse salvage cystectomy, there is currently no high-level evidence or consensus guidelines specifically supporting one treatment approach over another in this setting. However, in clinical practice, these patients are typically managed similarly to those with metastatic urothelial carcinoma (mUC). Systemic therapy options include antibody-drug conjugates (ADCs), chemotherapy, immune checkpoint inhibitors (ICIs), used either as monotherapy or in combination. The preferred initial treatment is enfortumab vedotin plus pembrolizumab if available, otherwise platinum-based chemotherapy followed by maintenance immunotherapy. For cisplatin-eligible patients, gemcitabine, cisplatin and nivolumab followed by maintenance nivolumab could be another option. Cisplatin-ineligible patients could be also treated with gemcitabine plus carboplatin followed by Avelumab or single-agent pembrolizumab. Thus, these options are usually guided by cisplatinum eligibility and the history of short free-chemotherapy interval after neoadjuvant chemotherapy.
Moreover, one can postulate that efficacy of such therapies on irradiated bladder tissue could be reduced. Radiotherapy profoundly remodels the bladder tumor microenvironment, simultaneously exerting both stimulatory and suppressive influences that can undermine subsequent systemic therapies. It induces fibrosis and vascular damage that impede drug delivery, fosters hypoxia that contributes to chemoresistance, and depletes lymphocytes, thus compromising ICIs, while also recruiting immunosuppressive cells such as regulatory T cells that blunt antitumor immunity (21, 22). This altered environment, coupled with the “tumor bed effect” where radiation-primed stroma paradoxically promotes tumor regrowth, diminishes efficacy across chemotherapy, ICIs, and ADCs.
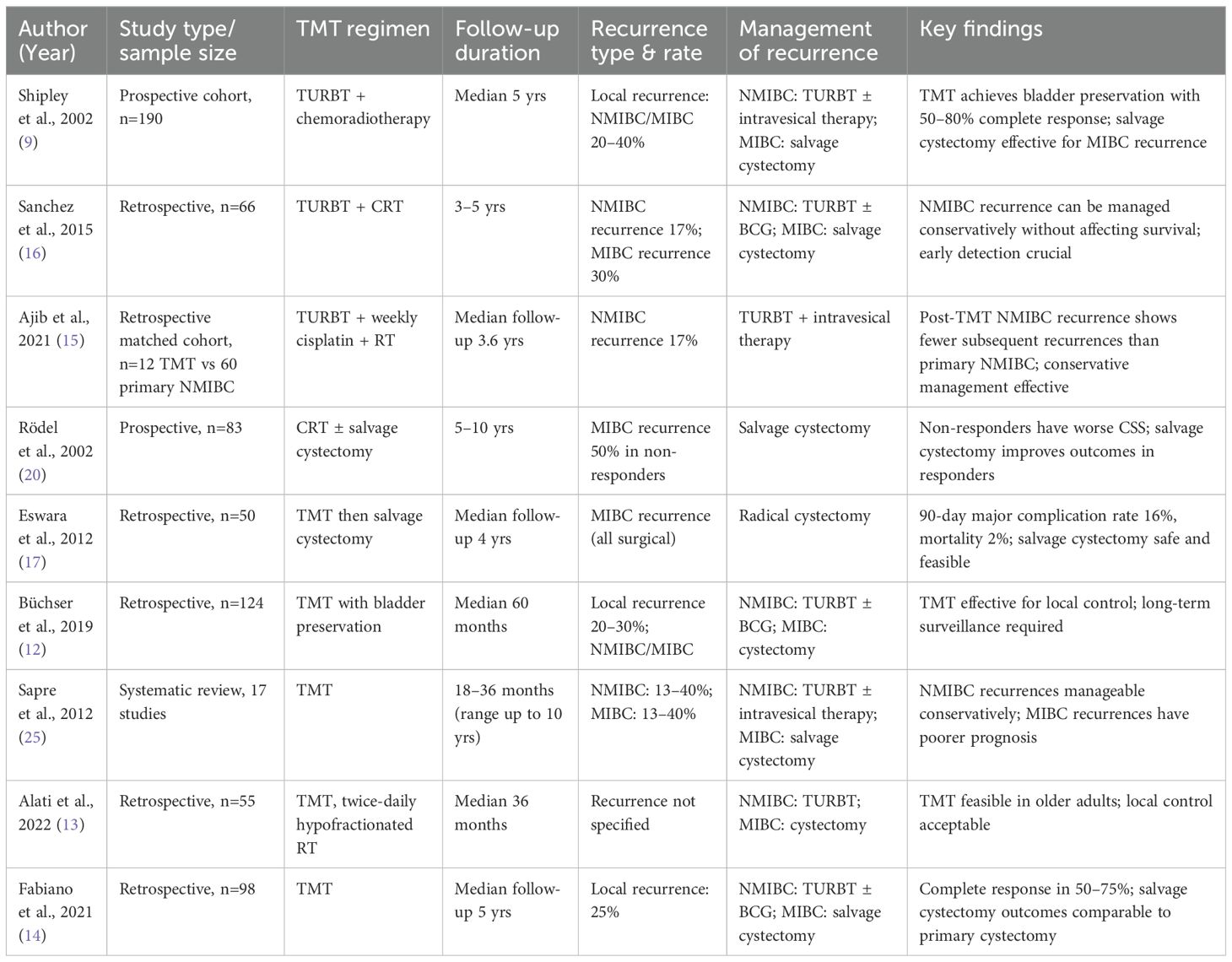
Sapre et al. (2012) conducted a systematic review of 17 studies evaluating local recurrences in patients with MIBC who initially achieved a complete response to bladder-sparing CRT. Complete response rates ranged from 56% to 100%, while local recurrence occurred in 13–40% of cases, with NMIBC and MIBC recurrences occurring at similar frequencies. Recurrences typically developed within 18–36 months after radiotherapy, though late relapses up to 10 years were reported. NMIBC recurrences were usually managed with TURBT with or without intravesical therapy, whereas salvage cystectomy was most often advocated for MIBC recurrences. Five-year cancer-specific survival was favorable for NMIBC recurrences (50–70%) but much lower for MIBC recurrences (16–40%). Importantly, patients with recurrence had a reduced likelihood of long-term bladder preservation compared to those without recurrence. Poor prognostic factors included advanced age, incomplete resection, higher T stage, and carcinoma in situ at the time of radiotherapy. The authors concluded that while NMIBC recurrence following CRT can be managed conservatively with survival comparable to non-recurrent cases, MIBC recurrence carries a poor prognosis with a high likelihood of distant metastases despite salvage cystectomy, underscoring the need for cystoscopic surveillance extending up to 10 years (23). Table 1 summarizes all the studies mentioned above.
Emerging evidence suggests that integrating immunotherapy with RT may enhance antitumor immunity in localized disease. The review by Zhang R et al. highlights strategies for combining immune modulation with radiotherapy, providing a framework for future clinical trials in the bladder preservation setting. While high-level evidence remains limited, these approaches may expand treatment options for patients who cannot undergo salvage cystectomy and support the development of tailored post-IO/RT management strategies (24).
Conclusion
TMT, a combination of transurethral resection, chemotherapy, and radiotherapy, offers a bladder-preserving alternative for selected patients with MIBC, achieving complete response rates in 50–80% of cases. However, up to 30% of patients may experience local recurrences, either as NMIBC or MIBC. NMIBC recurrences following TMT can often be effectively managed with standard conservative strategies such as TURBT and intravesical therapy, mainly BCG, without negatively impacting overall outcomes. In contrast, salvage cystectomy remains the standard of care for MIBC recurrence post-TMT in surgically fit patients, with perioperative risks comparable to primary cystectomy but poorer prognosis and lower CSS. Yet, for those who are unfit for or decline cystectomy, no evidence-based consensus exists to guide management. In such cases, patients could typically treated using systemic therapies employed in metastatic urothelial carcinoma. In such cases, the use of enfortumab vedotin plus pembrolizumab, platinum-based chemotherapy with or without immunotherapy, or alternative regimens are based on cisplatin eligibility and the free-chemotherapy interval after neo-adjuvant approach. This highlights the need for further research to define optimal treatment strategies, on previously irradiated territories, for this growing subgroup of patients.
Author contributions
ND: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. NN: Formal Analysis, Methodology, Writing – original draft. AS: Formal Analysis, Software, Writing – review & editing. CK: Formal Analysis, Supervision, Writing – review & editing. JK: Funding acquisition, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Cancer Society. (2023) Key statistics for bladder cancer. In: Bladder cancer in Lebanon: Incidence and Comparison to Regional and Western Countries. Thousand Oaks (CA): SAGE Publications. doi: 10.1177/1073274818789359
2. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. (2013) 63(2):234-241. doi: 10.1016/j.eururo.2012.07.033
3. Moch H, Humphrey PA, Ulbright TM, and Reuter V. International agency for research on cancer. 4th edition ed. Lyon, France: infiltrating urothelial carcinoma; (2016). In: Flaig TW, Spiess PE, and Chair V, editors. WHO classification of tumours of the urinary system and male genital organs. p. 81–98. Plymouth Meeting (PA): National Comprehensive Cancer Network
4. Gakis G, Efstathiou J, and Lerner SP. ICUD-EAU international consultation on bladder cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. (2013) 63:45–57. doi: 10.1016/j.eururo.2012.08.009
5. Kattan J, Kattan C, Aoun F, and Nemr E. The practical roadmap for peri-cystectomy approaches in muscle-invasive bladder cancer. Front Oncol. (2025) 15:1543837. doi: 10.3389/fonc.2025.1543837
6. Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, and Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. (2006) 176:486–492. doi: 10.1016/j.juro.2006.03.038
7. Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, and Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA. (2011) 306:737–45. doi: 10.1001/jama.2011.1142
8. Sangar VK, McBain CA, Lyons J, Ramani VA, Logue JP, Wylie JP, et al. Phase I study of conformal radiotherapy with concurrent gemcitabine in locally advanced bladder cancer. Int J Radiat Oncol Biol Phys. (2005) 61:420–5. doi: 10.1016/j.ijrobp.2004.05.074
9. Shipley WU, Kaufman DS, Zehr E, Heney NM, Lane SC, Thakral HK, et al. Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology. (2002) 60:62–7. doi: 10.1016/S0090-4295(02)01650-3
10. Solakhan M, Benlier N, Yıldırım Z, Seran AI, Kaya V, and Yıldırım M. (2021). doi: 10.1186/s12301-021-00199-x
11. Sanchez A, Wszolek MF, Niemierko A, Clayman RH, Drumm M, Rodríguez D, et al. Incidence, clinicopathological risk factors, management and outcomes of nonmuscle invasive recurrence after complete response to trimodality therapy for muscle invasive bladder cancer. J Urol. (2018) 199:407–15. doi: 10.1016/j.juro.2017.08.106
12. Büchser D, Zapatero A, Rogado J, Talaya M, Martín de Vidales C, Arellano R, et al. Long-term outcomes and patterns of failure following trimodality treatment with bladder preservation for invasive bladder cancer. Urology. (2019) 124:183–90. doi: 10.1016/j.urology.2018.07.058
13. Alati A, Fabiano E, Geiss R, Mareau A, Charles-Nelson A, Bibault JE, et al. Bladder preservation in older adults with muscle-invasive bladder cancer: a retrospective study with concurrent chemotherapy and twice-daily hypofractionated radiotherapy schedule. J Geriatr Oncol. (2022) 13:978–86. doi: 10.1016/j.jgo.2022.05.014
14. Fabiano E, Durdux C, Dufour B, Mejean A, Thiounn N, Chrétien Y, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer. Acta Oncol (Stockholm Sweden). (2021) 60:794–802. doi: 10.1080/0284186x.2021.1915498
15. Ajib K, Berjaoui MB, Herrera-Caceres J, Nason G, Tan GH, Tjong MC, et al. Characterization and management of NMIBC recurrences after TMT: a matched cohort analysis. Urol Oncol. (2021) 39:835. doi: 10.1016/j.urolonc.2021.05.008
16. Sanchez A, Wszolek MF, Clayman RH, Spiess PE, Pisters LL, Swanson DA, et al. Incidence and management of non-muscle invasive bladder cancer recurrences after complete response to combined-modality organ-preserving therapy for muscle invasive bladder cancer. J Urol. (2015) 193:e298. doi: 10.1016/j.juro.2015.02.1134
17. Eswara JR, Efstathiou JA, Heney NM, Niemierko A, Wu CL, Coen JJ, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle invasive bladder cancer. J Urol. (2012) 187:463. doi: 10.1016/j.juro.2011.09.159
18. Ramani VA, Maddineni SB, Grey BR, and Clarke NW. Differential complication rates following radical cystectomy in the irradiated and nonirradiated pelvis. Eur Urol. (2010) 57:1058. doi: 10.1016/j.eururo.2009.12.002
19. Pieretti A, Krasnow R, Drumm M, Jain RH, Howard SA, Preston MA, et al. Complications and outcomes of salvage cystectomy after trimodality therapy. J Urol. (2021) 206:29. doi: 10.1097/JU.0000000000001696
20. Rödel C, Grabenbauer GG, Kühn R, Papadopoulos T, Dunst J, Meyer M, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. (2002) 20:3061–71. doi: 10.1200/JCO.2002.11.027
21. Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. (2018) 11:117906441876163. doi: 10.1177/1179064418761639
22. Kuonen F, Secondini C, and Ruegg C. Molecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin Cancer Res. (2012) 18:5196–202. doi: 10.1158/1078-0432.CCR-11-1758
23. Sapre N, Anderson P, and Foroudi F. Management of local recurrences in the irradiated bladder: a systematic review. BJU Int. (2012) 110 Suppl 4:51–7. doi: 10.1111/j.1464-410X.2012.11476.x
24. Zhang R and Ke CX. Advances in bladder preservation therapy for muscle-invasive bladder cancer. Front Oncol. (2025) 15:1562260. doi: 10.3389/fonc.2025.1562260
Keywords: trimodality therapy, cystectomy, chemotherapy, radiotherapy, muscle invasive bladder cancer
Citation: Damaj N, Naim N, Saad A, Kattan C and Kattan J (2025) Management of bladder cancer recurrence following the trimodality therapy. Front. Oncol. 15:1672431. doi: 10.3389/fonc.2025.1672431
Received: 24 July 2025; Accepted: 17 September 2025;
Published: 01 October 2025.
Edited by:
Mohamed Saad Zaghloul, Cairo University, EgyptReviewed by:
Jure Murgic, Sisters of Charity Hospital, CroatiaCopyright © 2025 Damaj, Naim, Saad, Kattan and Kattan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nahed Damaj, bmFoZWQuZGFtYWpAb3V0bG9vay5jb20=
 Nahed Damaj
Nahed Damaj Nabih Naim1
Nabih Naim1 Joseph Kattan
Joseph Kattan