- 1Department of Medicine, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Medical Oncology, Dr. B.R. Ambedkar - Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Epstein-Barr virus (EBV) is a ubiquitous human gamma-herpesvirus causally linked to a diverse spectrum of lymphoid malignancies. This review provides a comprehensive overview of EBV-associated lymphomas, encompassing their global epidemiology, the intricate pathogenesis driven by viral latency proteins and complex host immune interactions, and the varied clinical presentations of distinct subtypes. We delve into the detailed pathological features, molecular characteristics, and diagnostic strategies for classic Hodgkin lymphoma (cHL), Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL), post-transplant lymphoproliferative disorder (PTLD), and extra-nodal NK/T-cell lymphoma, nasal type (ENKTL). Current subtype-specific treatment paradigms are critically evaluated, along with a thorough exploration of emerging therapeutic avenues, including novel immunotherapeutic approaches such as immune checkpoint inhibitors, adoptive cell therapies like EBV-specific cytotoxic T lymphocytes and chimeric antigen receptor T-cells (CAR-T), and targeted molecular therapies. Finally, we highlight the persistent challenges, critical knowledge gaps, and promising future prospects, including preventative and therapeutic vaccine strategies, aimed at optimizing diagnostic precision and improving long-term outcomes for patients afflicted with these heterogeneous and often aggressive diseases.
Introduction
Epstein-Barr virus (EBV), a ubiquitous human gamma-herpesvirus holds a well-established and profound role in the development of several lymphoid and epithelial cancers (1). First identified in Burkitt lymphoma cells in 1964 by Epstein, Achong, and Barr (2), EBV has since been recognized as one of the most successful human pathogens infecting over 90% of the adult population globally (3). Following primary infection, typically asymptomatic in early childhood, EBV establishes a lifelong latent infection primarily within memory B-lymphocytes, where it persists in a dormant state (4). However, primary EBV infection during adolescence or early adulthood can manifest as infectious mononucleosis characterized by fever, pharyngitis, and lymphadenopathy, representing a robust host immune response to viral replication and B-cell proliferation (5).
Crucially, EBV is etiologically linked to a wide spectrum of lymphomas, ranging from well-recognized entities to less common variants (1). These include classic Hodgkin lymphoma (cHL), Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL), post-transplant lymphoproliferative disorder (PTLD), and extra-nodal NK/T-cell lymphoma, nasal type (ENKTL) (1). These EBV-associated lymphomas can affect both immunocompetent and immunocompromised individuals, particularly in contexts of chronic immune suppression such as human immunodeficiency virus (HIV) infection, other primary or acquired immunodeficiencies, or post-transplantation (5). PTLD serves as a prime example of uncontrolled EBV-driven lymphocyte proliferation in the setting of impaired T-cell surveillance.
The oncogenic potential of EBV is mediated by a complex interplay of viral genes and host cellular pathways (6). During latency, EBV expresses a limited set of viral proteins including Epstein-Barr nuclear antigens (EBNAs), latent membrane proteins (LMPs), and non-coding ribonucleic acids (RNAs) such as EBV-encoded RNAs (EBERs) and micro-RNAs (miRNAs) (7). These viral products orchestrate a profound transformation of infected B-cells, promoting their proliferation, inhibiting apoptosis, and enabling immune evasion (8). Moreover, the specific type of latency program expressed; latency I, II, or III defines the pattern of viral gene expression and consequently dictates the precise type of lymphoma that develops (9). These distinct latency profiles not only drive the unique biological characteristics of each tumor but also significantly influence host immune surveillance mechanisms and ultimately the response to therapeutic interventions (9). Given the remarkable diversity in clinical behavior, histological presentation, molecular features, and treatment responses among EBV-associated lymphomas, there is a growing and urgent imperative for subtype-specific diagnostics, precise prognostication, and highly targeted therapeutic approaches. This comprehensive review aims to provide an in-depth overview of the pathobiology, detailed diagnostic methods, current treatment modalities, and prospective therapeutic strategies for EBV-associated lymphomas, highlighting key challenges and future directions in the field.
Epstein-Barr virus: overview
EBV, officially designated as human herpesvirus 4 (HHV-4), is a double-stranded DNA virus belonging to the Gammaherpes virinae subfamily of the Herpesviridae family. Its approximately 172-kilobase pair genome encodes over 80 genes. Following primary infection, which often occurs through saliva, EBV preferentially infects B-lymphocytes, establishing a lifelong latent infection characterized by its ability to immortalize these cells in vitro (10). This persistence is maintained within memory B-cells for the host’s lifetime, where the virus remains largely dormant but can periodically reactivate under certain physiological or immunosuppressive conditions, leading to lytic replication and viral shedding (10).
A hallmark of EBV’s interaction with the host cell is its ability to establish distinct latency programs, each characterized by a specific pattern of viral gene expression (Figure 1). These programs dictate the cellular tropism, oncogenic potential, and clinical manifestation of EBV-associated diseases.
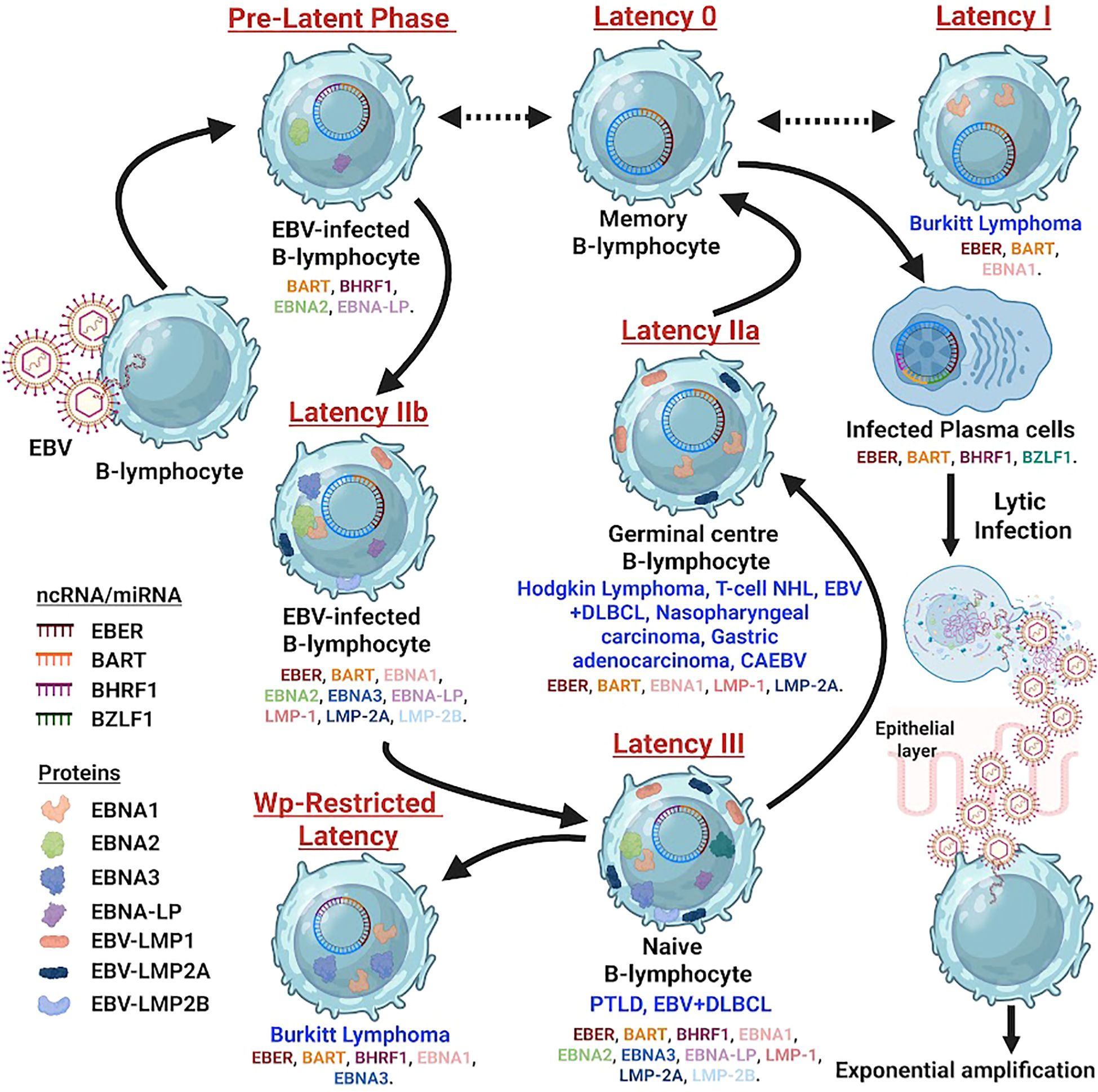
Figure 1. Schematic diagram depicting the EBV infection cycle in B-cells, beginning with viral entry through the oropharyngeal epithelium and progression from lytic replication to multiple latency states (0, I, IIa, IIb, III). Color-coded symbols represent EBV proteins and non-coding RNAs, including EBNA1–6, LMP1, LMP2A/2B, EBERs, BHRF1, BARTs, and EBV miRNAs. Arrows show movement of infected cells from naïve B-cells through germinal centers into memory or lymphoblast stages. Each latency phase is linked to specific diseases such as Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, CAEBV, PTLD, and EBV-positive DLBCL, with lytic reactivation shown during plasma cell differentiation.
Latency I (latency program): This is the most restricted form of latency, characterized by the expression of only Epstein-Barr nuclear antigen 1 (EBNA1) and often the non-coding EBERs and BamHI-A rightward transcripts (BARTs) miRNAs (9, 11). EBNA1 is essential for the replication and segregation of the viral episome during cell division, ensuring the persistence of the viral genome in daughter cells. This latency type is predominantly observed in endemic BL (9).
Latency II (default program): In addition to EBNA1, EBERs, and BARTs, latency II involves the expression of latent membrane proteins 1 (LMP1) and 2A/2B (LMP2A/2B) (9, 12). LMP1 is a potent oncogene that mimics a constitutively active CD40 receptor, activating critical signaling pathways like nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K)/AKT (8). LMP2A mimics the B-cell receptor, maintaining B-cell survival in the absence of exogenous stimulation (13). This latency type is characteristic of cHL, nasopharyngeal carcinoma, and ENKTL (9, 12).
Latency III (growth program): This is the most comprehensive latency program, expressing all six EBNAs (EBNA1, 2, 3A, 3B, 3C, LP), LMP1, LMP2A/2B, EBERs, and BARTs (9, 12). EBNA2 is a transcriptional activator crucial for driving the expression of other latent genes, including LMP1 and LMP2, and several host genes (14). This broad viral gene expression promotes the robust proliferation and transformation of B-cells. Latency III is typically observed in PTLD and some cases of DLBCL (9, 12).
Beyond the protein-coding genes, EBV also expresses non-coding RNAs, such as the abundant EBV-encoded RNAs (EBERs) and a complex repertoire of miRNAs derived from BARTs (7). EBERs are believed to contribute to immune evasion and cellular transformation, while BARTs miRNAs play a critical role in modulating host gene expression, impacting cellular proliferation, apoptosis, and immune surveillance (7, 15). Understanding these intricate latency programs and the functions of their associated viral products is fundamental to deciphering the diverse oncogenic mechanisms and clinical manifestations of EBV-associated lymphomas.
Pathogenesis of EBV-associated lymphomas
The pathogenesis of EBV-associated lymphomagenesis is a multifaceted process resulting from complex interactions between viral oncogenes, the host immune system, and contributing environmental cofactors. EBV’s ability to establish latent infection and express specific viral proteins is central to its oncogenic potential.
Latent protein activity
The latent proteins of EBV are key drivers of lymphomagenesis. LMP1 is arguably the most critical oncoprotein (8). It is a functional analogue of a constitutively active CD40 receptor, an important co-stimulatory molecule in B-cell activation. LMP1 activates several key signaling pathways, including NF-κB, activator protein 1 (AP-1), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and the PI3K/AKT pathway (8, 16). Activation of these pathways promotes cell proliferation, enhances survival by inhibiting apoptosis, and upregulates the expression of adhesion molecules and cytokines, thereby fostering an environment conducive to tumor growth. LMP2A mimics the signaling of a constitutively active B-cell receptor, contributing to cell survival and proliferation in the absence of antigen stimulation (13). It can also inhibit tyrosine kinase Lyn and suppress B-cell receptor-mediated signaling, potentially preventing infected B-cells from undergoing differentiation or apoptosis (17).
Immune evasion
EBV has evolved sophisticated mechanisms to evade host immune surveillance, which is critical for its persistence and for the survival of transformed cells. One significant mechanism involves the downregulation of major histocompatibility complex (MHC) class I and II molecules on the surface of infected cells (18). This prevents effective presentation of viral antigens to cytotoxic T lymphocytes (CTLs), thereby allowing EBV-infected cells to escape immune recognition and destruction. Furthermore, EBV produces a viral interleukin-10 (vIL-10) homolog, which suppresses T-cell responses and inhibits the production of pro-inflammatory cytokines, further contributing to local immunosuppression within the tumor microenvironment (18, 19). The expression of high levels of programmed death-ligand 1 (PD-L1) by EBV-infected tumor cells is another critical immune evasion strategy, leading to T-cell exhaustion and an inability of the immune system to clear the malignant cells (20, 21).
Genomic instability
While EBV itself does not directly cause gene mutations in the same way as some other oncogenic viruses, its chronic presence and the activity of its latent proteins can indirectly contribute to genomic instability. For instance, EBV infection can induce the expression of activation-induced cytidine deaminase (AID) in B-cells (22). AID is an enzyme crucial for somatic hypermutation and class switch recombination in normal B-cell development. However, dysregulated AID activity in EBV-infected cells can lead to off-target mutations and chromosomal translocations, such as the characteristic t(8;14) translocation that juxtaposes the MYC oncogene to immunoglobulin heavy chain (IgH) loci, leading to its overexpression in BL (22, 23). This highlights a mechanism by which chronic viral presence can subvert normal cellular processes to promote oncogenesis.
Microenvironmental factors
The tumor microenvironment plays a crucial role in the development and progression of EBV-associated lymphomas. EBV-infected tumor cells can recruit and reprogram various stromal and immune cells, creating an immunosuppressive milieu that supports tumor growth and progression (24). This includes the presence of regulatory T-cells (Tregs), which suppress anti-tumor immune responses and tumor-associated macrophages (TAMs), which can promote angiogenesis and tumor cell proliferation (24, 25). Cytokines and chemokines secreted by both tumor cells and surrounding stromal cells further contribute to this pro-tumorigenic and immunosuppressive environment, impairing the efficacy of endogenous anti-tumor immunity. For example, LMP1 can induce the production of various chemokines and cytokines, including IL-6 and TNF-α, which can contribute to the inflammatory and immunosuppressive microenvironment (16).
Classification and types of EBV-associated lymphomas
EBV is implicated in a diverse array of lymphoid malignancies, each characterized by distinct clinical, pathological, and molecular features. The classification of these lymphomas often considers the predominant cell type, anatomical site, and the specific EBV latency program expressed.
Classic Hodgkin lymphoma
EBV is detectable in 20–50% of cHL cases globally, with higher rates observed in pediatric, elderly, and HIV-infected patients, as well as in developing countries (26, 27). The characteristic malignant cells in cHL, Reed-Sternberg (RS) cells and their variants, are consistently of B-cell origin and express the EBV latency II program (9, 26). LMP1 plays a crucial role in the survival and proliferation of RS cells. EBV-positive cHL often presents with specific histological subtypes, notably mixed cellularity and lymphocyte-depleted HL, and may have a different clinical course compared to EBV-negative cases, potentially responding more favorably to certain immunotherapies (27, 28).
Burkitt lymphoma
BL is an aggressive B-cell non-Hodgkin lymphoma characterized by high proliferation and typically a MYC gene translocation (23). EBV association varies by epidemiological form:
Endemic BL: Highly prevalent in equatorial Africa and closely associated with Plasmodium falciparum malaria infection, which is thought to impair immune control of EBV (29). Endemic BL is almost universally (95–100%) EBV-positive and expresses the latency I program (9, 29).
Sporadic BL: Occurs worldwide, less common than endemic form, and shows EBV positivity in only 10–20% of cases (29).
Immunodeficiency-associated BL: Seen in immunocompromised individuals (e.g., HIV, post-transplant), with high rates of EBV positivity; often latency III (30). Regardless of EBV status, all forms of BL are characterized by the t(8;14) translocation or variants [t(2;8), t(8;22)], leading to constitutive activation of the MYC oncogene (23).
Diffuse large B-cell lymphoma
EBV-positive DLBCL, not otherwise specified (NOS), is recognized as a distinct entity in the World Health Organization (WHO) classification (31). It is predominantly observed in elderly individuals (typically >50 years) and those with immunosuppression, though it can occur in immunocompetent younger patients (32). These lymphomas frequently express either the latency II or III profile (32). EBV-positive DLBCL generally exhibits a poorer prognosis compared to EBV-negative cases, characterized by more aggressive clinical features, higher rates of central nervous system (CNS) involvement, and often resistance to standard rituximab-cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) chemotherapy (33, 34). The presence of EBV in these tumors suggests a unique biology that warrants specific therapeutic considerations.
Post-transplant lymphoproliferative disorder
PTLD represents a heterogeneous group of lymphoid proliferations that arise in the setting of iatrogenic immunosuppression following solid organ or hematopoietic stem cell transplantation (35). The vast majority (>80%) of PTLD cases are EBV-positive, particularly those occurring early after transplantation (35). The pathogenesis is driven by the uncontrolled proliferation of EBV-infected B-cells due to impaired T-cell immune surveillance (36). PTLD spans a broad spectrum of morphological presentations, from benign polyclonal plasmacytic hyperplasia to aggressive monomorphic lymphomas resembling DLBCL or BL. These lymphomas typically express the latency III profile (9, 35). Reduction of immunosuppression is often the first line of management, leading to remission in a significant proportion of cases through restoration of EBV-specific T-cell immunity (36).
In the 5th edition of the WHO classification of hemato-lymphoid neoplasms, a significant change has been introduced in the categorization of immunodeficiency-related lymphoproliferative disorders (LPDs) (37). In the 4th edition, these disorders were classified under four distinct headings; post-transplant, HIV-associated, primary immunodeficiency-related, and iatrogenic immunosuppression-related. The current classification consolidates these entities into a unified framework, presenting a three-part diagnostic approach that includes; histopathological features, associated viral agents, and underlying clinical context (37). Under the histological features, the three major categories are; hyperplasia, lymphoproliferative disorder of varied malignant potential, and lymphomas. Hyperplasia includes benign conditions like follicular hyperplasia, plasmacytic hyperplasia, infectious mononucleosis, HHV-associated Castleman disease, and other hyperplasia and involutions. The second category includes polymorphic LPDs, and muco-cutaneous ulcer, while the last category includes lymphomas as per the main classification. This three-tier diagnostic system includes all the entities and their related conditions, hence, will be helpful for clinical decision making and excluding unnecessary repetition of similar morphological entities of lymphomas or LPDs in different clinical conditions.
Extra-nodal NK/T-cell lymphoma, nasal type
ENKTL is a rare, aggressive lymphoma with a strong geographical predilection for East Asia, Latin America, and other regions, accounting for a significant proportion of lymphomas in these areas (38). It is virtually 100% associated with EBV, with tumor cells expressing the EBV latency II program (9, 38). Clinically, ENKTL often presents as a destructive lesion in the upper aerodigestive tract (nasal cavity, nasopharynx, palate), leading to symptoms like nasal obstruction, epistaxis, and facial swelling (39). However, extra-nasal involvement can occur, affecting the skin, gastrointestinal tract, or testis, and is associated with a poorer prognosis (39). ENKTL is known for its aggressive nature and resistance to conventional anthracycline-based chemotherapy regimens due to the high expression of p-glycoprotein (MDR1) (40).
Diagnosis and biomarkers
Accurate diagnosis and prognostication of EBV-associated lymphomas rely on a comprehensive approach integrating histopathology, immunophenotyping, and molecular techniques for EBV detection (Figures 2–4).
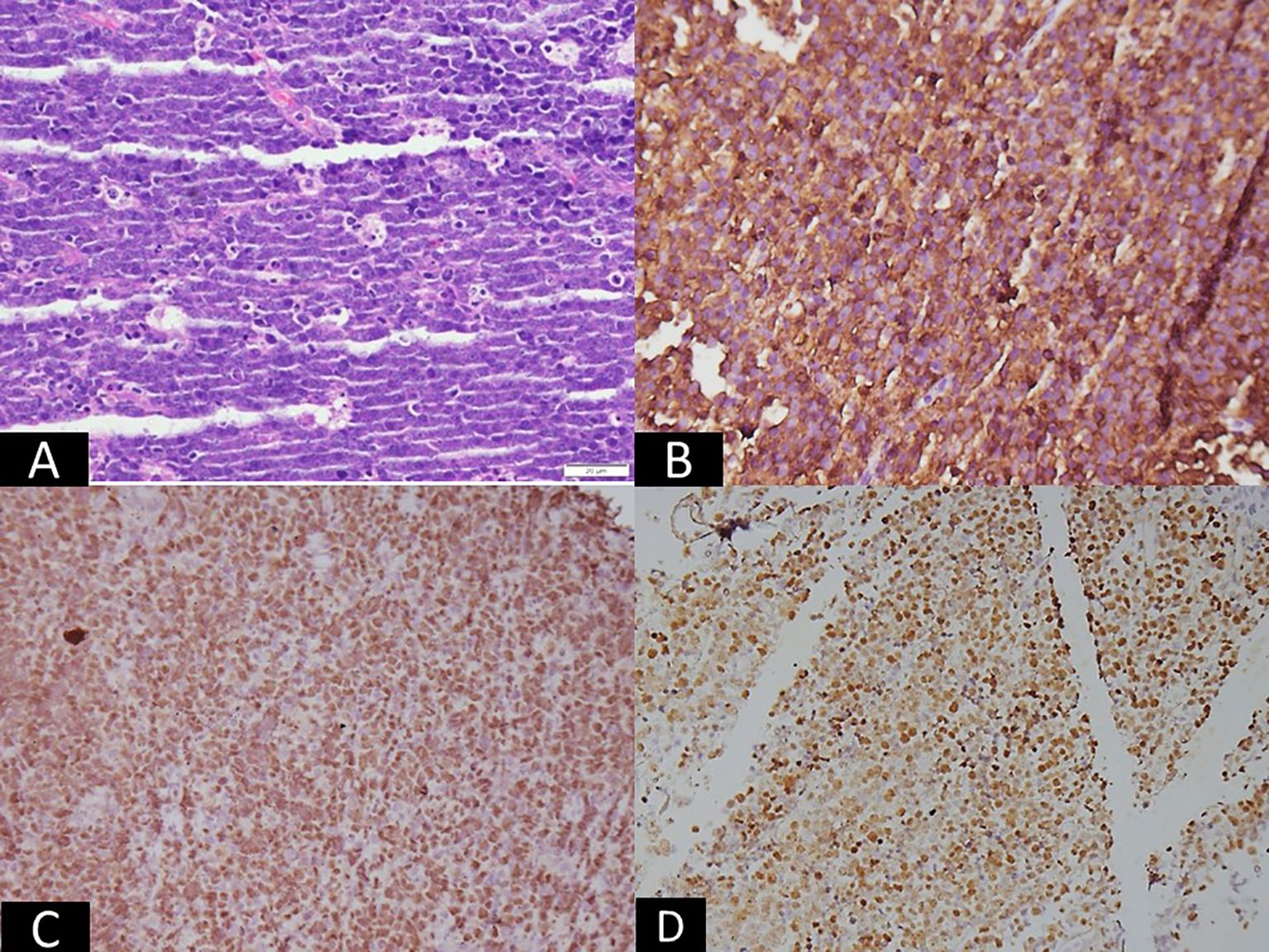
Figure 2. Microscopic panel showing intermediate-sized atypical lymphoid cells arranged in sheets with numerous tingible-body macrophages, creating a starry-sky pattern (H&E, ×200). Adjacent immunohistochemistry images show strong membranous staining for CD20 (×100) and nuclear positivity for c-MYC (×100). An in-situ hybridization image demonstrates strong nuclear EBER signals (×200). The combined panels visually indicate Burkitt lymphoma, corresponding to EBV latency I, characterized by monomorphic lymphoid cells and EBER positivity.
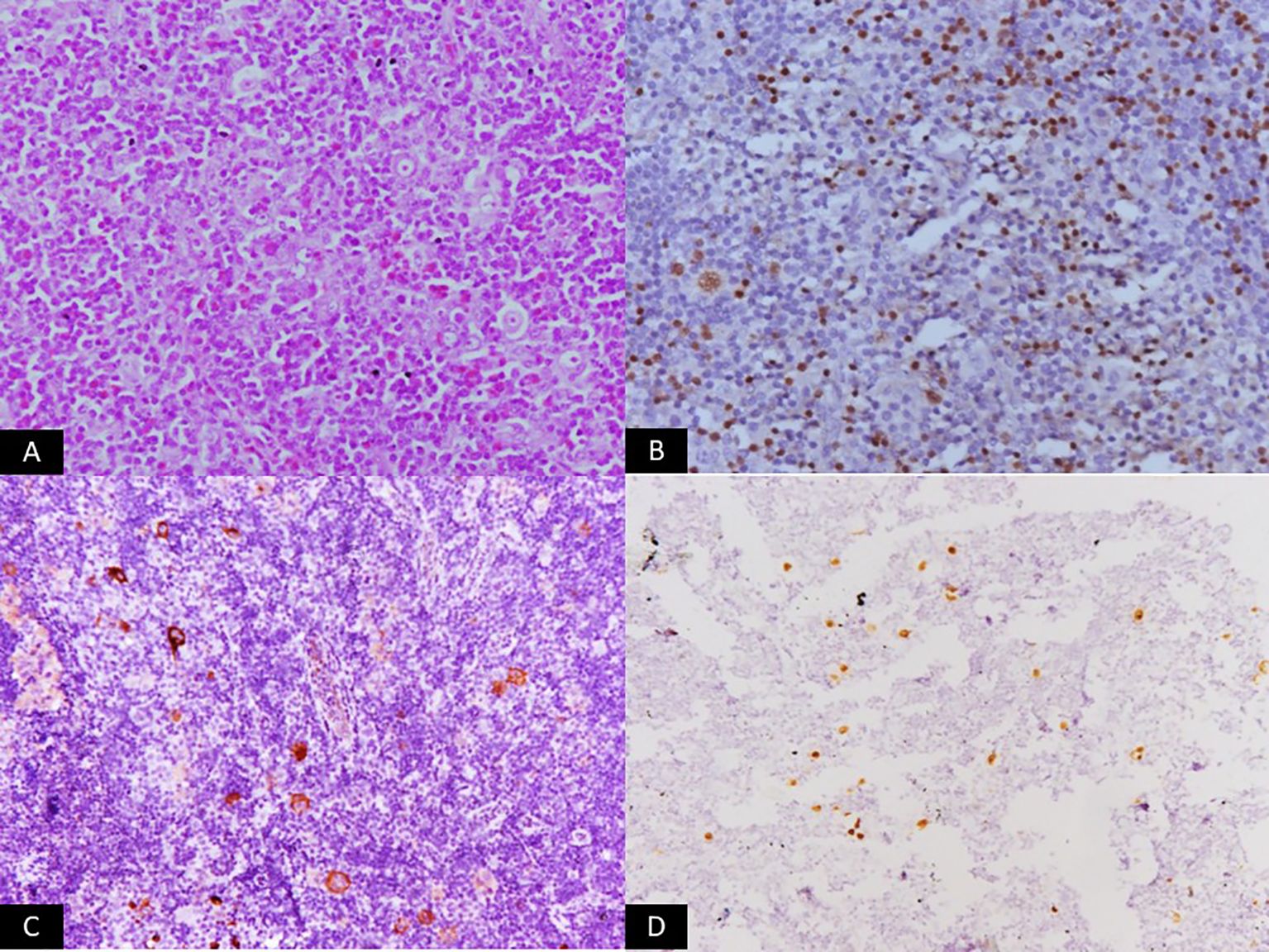
Figure 3. Microscopic panel showing a polymorphous infiltrate composed of small lymphocytes, eosinophils, histiocytes, and scattered classic Reed–Sternberg cells (H&E, ×200). Additional immunohistochemistry panels display dim nuclear staining for PAX5 (×200) and membranous/cytoplasmic positivity for EBV-LMP1 (×200). An in-situ hybridization panel shows strong nuclear EBER signals (×200). The set of images visually represents classic Hodgkin lymphoma associated with EBV latency II.
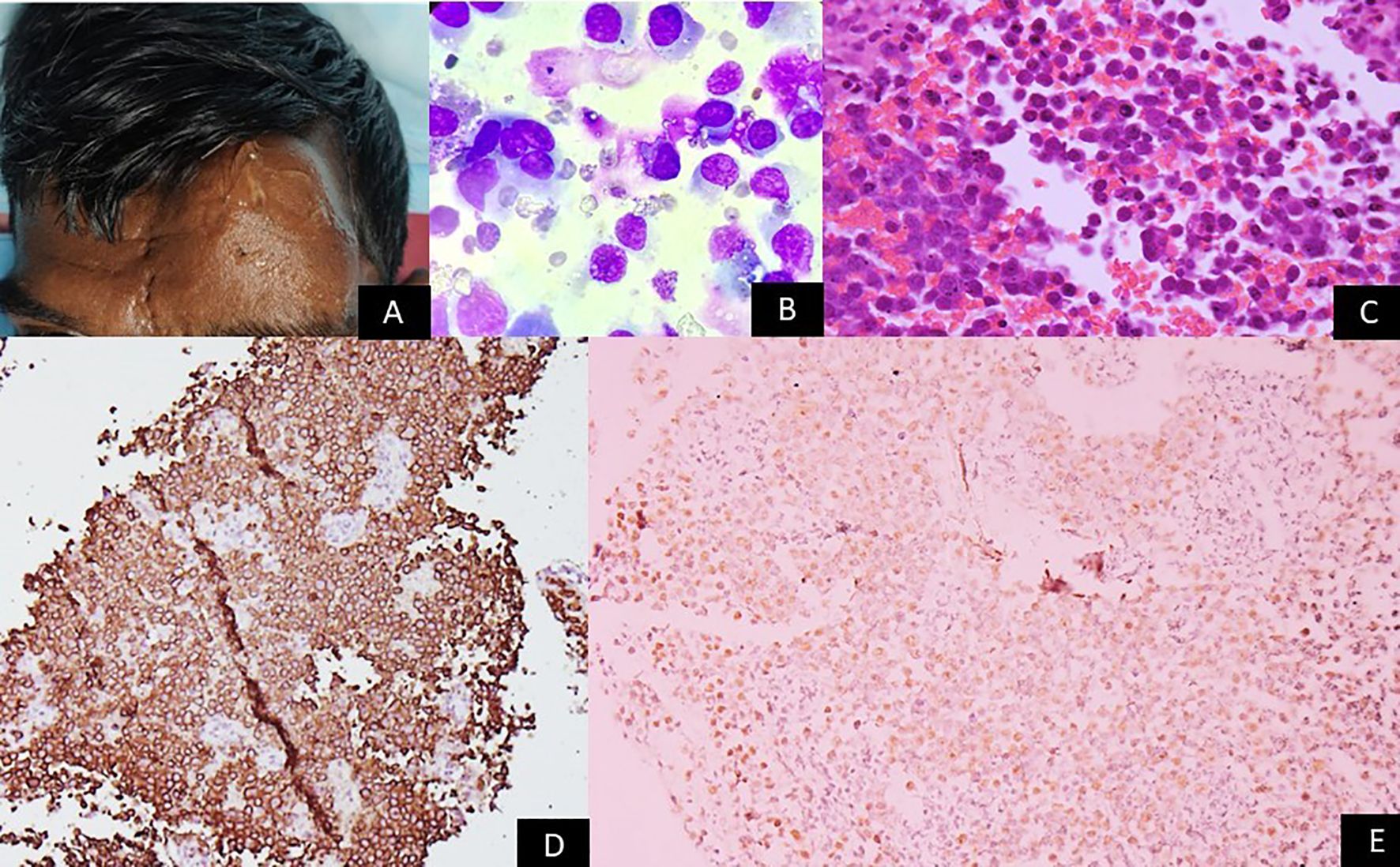
Figure 4. Gross image showing a person living with HIV with a large nodular swelling on the forehead. Microscopy panels show sheets of large atypical lymphoid cells with moderate cytoplasm (Giemsa ×200 and H&E ×200). Immunohistochemistry reveals strong CD138 membranous staining (×100), and in-situ hybridization shows nuclear EBER positivity (×200). The images collectively depict features consistent with plasmablastic lymphoma, corresponding to EBV latency III.
Histology and immunohistochemistry
The initial diagnosis of lymphoma is based on histological examination of tissue biopsy. Morphological features vary widely across different EBV-associated lymphoma subtypes. For instance, cHL is characterized by the presence of large, often binucleated RS cells, while BL exhibits a monotonous proliferation of medium-sized lymphoid cells with a ‘starry-sky’ pattern (23, 41). Immunophenotyping using IHC is crucial for lineage assignment and differentiation from other lymphoid neoplasms. Common markers include CD20 for B-cell lymphomas, CD3 for T-cell lymphomas, and CD30 and CD15 for cHL. For NK/T-cell lymphomas, markers like CD2, cytoplasmic CD3, CD56, and cytotoxic granules (granzyme B, perforin, TIA-1) are typically positive (39).
EBV detection methods
Direct detection of EBV within tumor cells is critical for establishing an EBV-associated lymphoma diagnosis.
EBV-encoded RNA (EBER) in-situ hybridization (ISH): EBER ISH is considered the gold standard for detecting EBV in tissue sections due to its high sensitivity and specificity (42). EBERs are small, non-coding RNAs expressed at high copy numbers in virtually all EBV latency programs, making them an excellent molecular marker for the presence of EBV-infected cells (42). A positive EBER ISH confirms the presence of EBV in the malignant cells.
IHC for latent proteins: IHC can detect the expression of specific EBV latent proteins, particularly LMP1 and EBNA2. LMP1 is commonly expressed in latency II (cHL, ENKTL) and latency III (PTLD), while EBNA2 is uniquely expressed in latency III (12). IHC for these proteins can provide insights into the specific EBV latency program, aiding in classification and understanding of pathogenesis.
Polymerase chain reaction (PCR) and quantitative PCR (qPCR) for EBV DNA: PCR-based methods can detect EBV DNA in tissue or circulating cell-free DNA (cfDNA) in plasma (43). qPCR allows for the quantification of EBV DNA load, which is particularly useful for diagnosis and monitoring disease activity in conditions like PTLD and ENKTL (43, 44). Elevated pre-treatment EBV DNA levels often correlate with increased tumor burden and poorer prognosis, and a reduction in viral load post-treatment can indicate therapeutic response (44).
Emerging biomarkers
Beyond EBV detection, several emerging biomarkers are being investigated for their prognostic or therapeutic implications.
Programmed death-ligand 1 (PD-L1): High expression of PD-L1 is frequently observed in EBV-associated lymphomas, particularly cHL and ENKTL, often driven by EBV-mediated signaling (e.g., LMP1 activation of JAK/STAT) (20, 21). PD-L1 expression can serve as a predictive biomarker for response to immune checkpoint inhibitors. PD-L1 expression in EBV-associated lymphomas is assessed on formalin-fixed paraffin-embedded tissue using IHC, with parallel EBV confirmation by EBER ISH. Tumor and immune cell membranous staining are evaluated, and expression quantified using tumor proportion score (TPS), combined positive score (CPS), or H-score. Results are correlated with EBV status, as EBV-positive lymphomas frequently exhibit PD-L1 overexpression. Appropriate antibody clones, validated platforms, and internal controls ensure reliable assessment for prognostic and therapeutic interpretation.
EBV-Encoded micro-RNAs (miRNAs): EBV expresses its own set of miRNAs (BARTs), which can be detected in tumor tissue and plasma. Their levels may serve as prognostic indicators or targets for novel therapies.
Host genetic signatures: Research is exploring host genetic alterations and gene expression profiles that interact with EBV infection to drive lymphomagenesis, potentially revealing new therapeutic targets.
Current treatment strategies
Treatment strategies for EBV-associated lymphomas are largely dictated by the specific lymphoma subtype, disease stage, patient’s performance status, and prior treatment history. While EBV positivity can influence prognosis in certain settings, its direct impact on first-line treatment choice varies.
cHL
For early-stage cHL, combined modality therapy involving chemotherapy (e.g., ABVD: doxorubicin, bleomycin, vinblastine, dacarbazine) followed by involved-site radiation therapy (ISRT) is standard. For advanced-stage disease, ABVD remains a common regimen, though dose-escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) is also used for high-risk patients (28, 45). While EBV status does not currently dictate initial treatment selection for cHL, EBV-positive cHL has been shown to exhibit a higher expression of PD-L1, making it more amenable to immune checkpoint blockade in relapsed or refractory settings (28, 46).
BL
This being an extremely aggressive lymphoma, requires rapid initiation of intensive, short-duration multi-agent chemotherapy regimens to achieve cure. Common regimens include CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine) or DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab) (47). Due to the high risk of CNS involvement, mandatory CNS prophylaxis with intrathecal chemotherapy is crucial. While EBV positivity is a defining feature of endemic BL, its presence does not generally alter the chemotherapy regimen for BL, as the underlying biology is driven by MYC deregulation regardless of EBV status (47).
EBV-positive DLBCL, NOS
For newly diagnosed cases, the standard first-line treatment is R-CHOP, similar to EBV-negative DLBCL. However, given its generally poorer prognosis, particularly in elderly patients, there is ongoing research into more intensified or novel approaches for this subgroup (33, 34). This includes exploring the addition of agents like bortezomib, lenalidomide, or immune checkpoint inhibitors in clinical trials, but these are not yet standard of care.
PTLD
Management of PTLD is highly individualized and depends on the type of transplant, disease extent, and specific PTLD subtype. The cornerstone of treatment for most EBV-positive PTLDs is reduction of immunosuppression (RIS) (36). RIS often leads to disease regression by restoring EBV-specific T-cell immunity against the proliferating B-cells. For patients who do not respond to RIS or have aggressive disease, further therapies include rituximab for CD20-positive B-cell PTLDs, and multi-agent chemotherapy regimens (e.g., CHOP or R-CHOP) particularly for monomorphic PTLDs resembling DLBCL (36).
ENKTL
Aggressive nature and inherent resistance to anthracyclines are bottlenecks in treatment of this lymphoma (40). Standard treatment often involves non-anthracycline-based regimens combined with radiation therapy, especially for localized disease. Regimens incorporating L-asparaginase (e.g., SMILE: dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide) have demonstrated superior outcomes (40). For advanced or relapsed/refractory ENKTL, novel agents and immunotherapies are actively being investigated, given the high expression of PD-L1 in these tumors (39, 46).
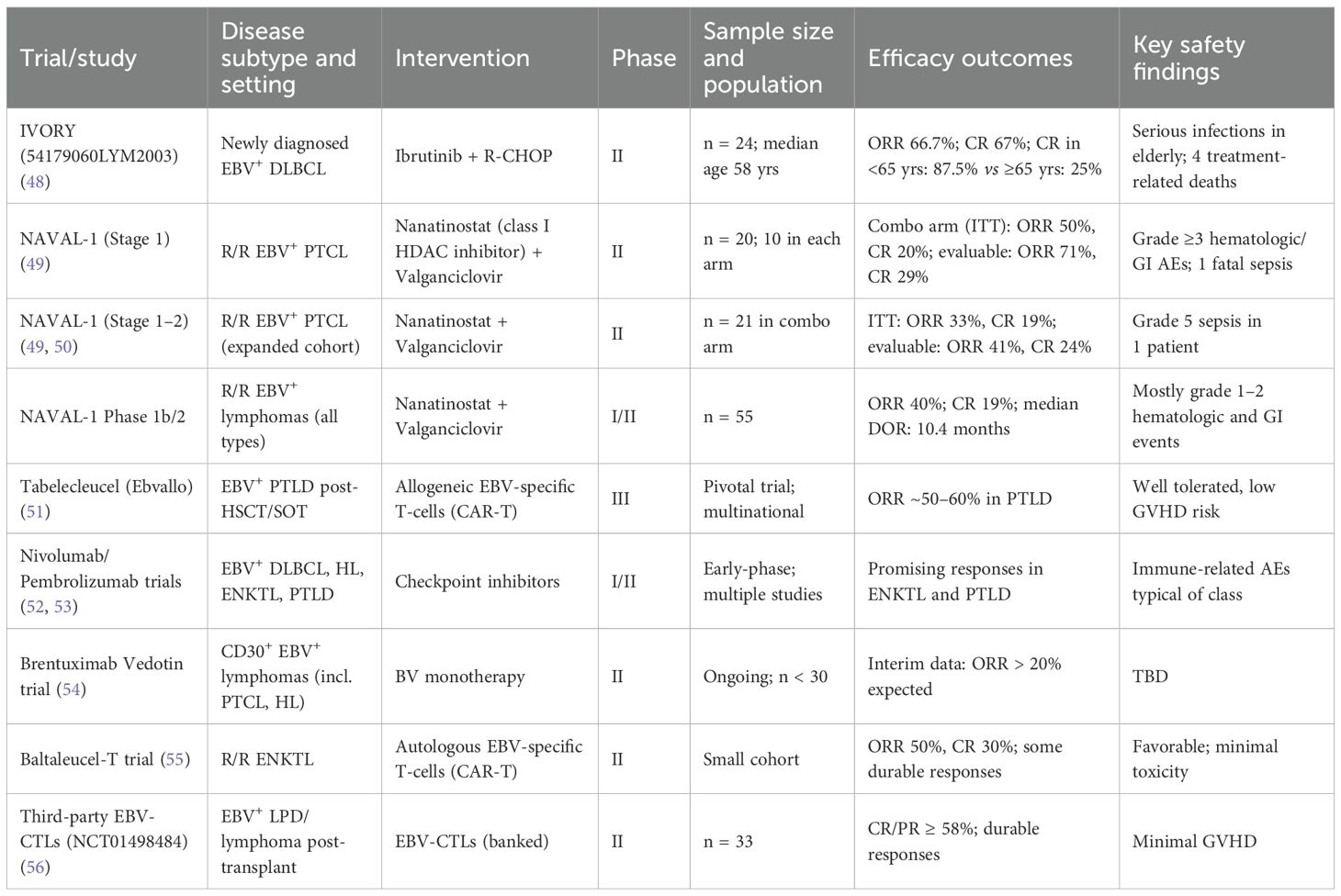
Table 1 summarizes key clinical trials evaluating treatment in EBV-associated lymphomas.
Emerging therapies and future prospects
The unique biology of EBV-associated lymphomas, particularly their dependence on viral proteins and interactions with the host immune system, offers distinct opportunities for novel therapeutic interventions.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs), particularly those targeting the PD-1/PD-L1 pathway, have transformed the therapeutic landscape of oncology by harnessing the host immune system to fight cancer. Their efficacy in Epstein–Barr virus (EBV)-associated lymphomas is increasingly recognized due to the virus’s intrinsic role in immune evasion. EBV-infected tumor cells, particularly in classical Hodgkin lymphoma (cHL) and extranodal NK/T-cell lymphoma (ENKTL), often show marked overexpression of PD-L1 on their surface, driven by EBV-encoded proteins such as LMP1 and EBNA2, as well as by genetic alterations in the PD-L1/PD-L2 locus (20, 21). This PD-L1 upregulation suppresses cytotoxic T-cell function by engaging PD-1 receptors on T-cells, leading to T-cell exhaustion and impaired anti-tumor surveillance.
Blocking this interaction with anti–PD-1 antibodies such as nivolumab and pembrolizumab reinvigorates exhausted T-cells, restoring cytokine production and cytotoxic activity against tumor cells. Clinical trials have demonstrated remarkable response rates, particularly in relapsed or refractory cHL and ENKTL, where durable remissions have been observed even after multiple prior therapies (46, 57, 58). Furthermore, ICIs have shown favorable safety profiles, making them suitable for heavily pretreated or frail patients. Current research is exploring their use in earlier lines of therapy, in combination with chemotherapy or radiotherapy, or as consolidation after remission, with the goal of achieving deeper and more sustained responses through synergistic immune activation.
EBV-specific CTLs
Adoptive transfer of ex vivo–expanded EBV-specific CTLs represents one of the most precise and biologically rational forms of immunotherapy in EBV-driven malignancies (59). This approach utilizes the natural immune defense against EBV by isolating T-cells capable of recognizing EBV antigens, expanding them outside the body, and then re-infusing them into the patient. The CTLs may be donor-derived, sourced from the transplant donor, third-party donors, or autologous (patient-derived) T-cells that are primed and selected for EBV antigen recognition.
These EBV-specific CTLs selectively target infected or transformed cells expressing latent viral antigens such as LMP1, LMP2, or EBNA proteins, thereby sparing healthy tissue. The therapy has achieved notable clinical success in PTLD, where it has induced durable and sometimes complete remissions (57, 60). The safety profile is also favorable, with minimal risk of graft-versus-host disease (GVHD) when appropriately matched.
Beyond PTLD, research is ongoing to extend this therapy to other EBV-associated cancers, including nasopharyngeal carcinoma and certain lymphomas with latency II or III expression profiles. However, certain challenges remain. tumors with restricted latency programs (e.g., expressing only EBNA1) may present few immunogenic targets, limiting CTL efficacy. Additionally, tumor microenvironmental suppression and immune escape mutations can diminish CTL persistence or function, underscoring the need for strategies to optimize antigen selection and enhance in vivo expansion of these cells.
CAR-T
CAR-T cell therapy involves genetically modifying a patient’s or donor’s T-cells to express a synthetic chimeric antigen receptor (CAR) that recognizes specific tumor-associated surface antigens. Once infused, these engineered T-cells can identify and destroy tumor cells in a major histocompatibility complex (MHC)-independent manner, overcoming one of the key immune evasion mechanisms of EBV-infected cells.
CD19-directed CAR-T cells have already revolutionized the treatment of B-cell lymphomas and acute lymphoblastic leukemia, and their role in EBV-associated B-cell lymphomas parallels that seen in EBV-negative settings, such as CD19-positive diffuse large B-cell lymphoma (DLBCL) (61). However, the therapeutic potential of CAR-T cells extends further in EBV-driven disease. Novel CAR constructs are being developed to specifically target EBV-related antigens such as LMP1 and LMP2, which are selectively expressed on the surface of EBV-transformed cells, allowing for more direct and virus-specific tumor killing (62, 63).
In classical Hodgkin lymphoma, CD30-directed CAR-T cells have demonstrated encouraging early results, reflecting the high CD30 expression characteristic of this disease. These EBV-specific or tumor-specific CAR-T cell designs hold the promise of achieving highly selective cytotoxicity while minimizing off-target effects. Ongoing preclinical and early clinical studies are investigating strategies to improve CAR-T cell persistence, trafficking into tumor sites, and resistance to the immunosuppressive tumor microenvironment that characterizes many EBV-driven lymphomas.
Epigenetic and molecular inhibitors
EBV latent proteins profoundly reprogram host cell signaling and epigenetic machinery, promoting survival, proliferation, and immune escape. Consequently, targeting these aberrant signaling cascades and epigenetic regulators provides a promising therapeutic avenue. One of the most critical pathways is NF-κB, which is constitutively activated by LMP1, leading to transcription of anti-apoptotic and proliferative genes. NF-κB inhibitors are therefore being explored to disrupt this axis and restore apoptotic sensitivity in EBV-driven tumors (64).
Other key survival pathways influenced by EBV include the PI3K/AKT/mTOR cascade, which promotes cell growth and metabolic adaptation. Inhibitors of these signaling components can suppress tumor cell proliferation and sensitize them to chemotherapy or immune-mediated killing. Epigenetic modifiers such as histone deacetylase (HDAC) inhibitors and EZH2 inhibitors are also under investigation, as they can reverse EBV-induced transcriptional silencing and restore expression of viral or tumor suppressor genes (65, 66).
Proteasome inhibitors like bortezomib may exert therapeutic benefit by indirectly inhibiting NF-κB activation and promoting accumulation of pro-apoptotic factors (67). These agents can also modulate antigen presentation, potentially enhancing tumor immunogenicity and complementing immunotherapeutic strategies. Together, these molecular inhibitors offer a multi-pronged approach to disrupt EBV-driven oncogenic signaling and restore normal cellular control mechanisms.
Vaccine strategies
The development of effective EBV vaccines is considered a cornerstone of long-term prevention and control of EBV-associated malignancies. Two major approaches are being pursued: prophylactic and therapeutic vaccination.
Prophylactic vaccines aim to prevent primary EBV infection or block the virus’s entry into B-cells and epithelial cells. Early candidates have focused on the envelope glycoprotein gp350, which mediates viral attachment to the B-cell receptor CD21 (68, 69). Vaccines targeting gp350 have demonstrated some success in preventing infectious mononucleosis, a common manifestation of primary EBV infection. However, completely preventing EBV persistence and subsequent tumorigenesis remains challenging, necessitating the inclusion of additional viral targets and adjuvants to induce durable humoral and cellular immunity.
Therapeutic vaccines, on the other hand, are designed to elicit potent cytotoxic T-cell responses against EBV-infected malignant cells. These typically target latent antigens such as LMP1 and LMP2, which are consistently expressed in many EBV-associated tumors (68, 69). The objective is to boost the host immune system’s ability to recognize and eradicate established tumors while minimizing immune tolerance. Early-phase clinical trials are evaluating recombinant viral vector or peptide-based vaccines incorporating these antigens, with encouraging evidence of immunogenicity and occasional clinical responses. Continued optimization of antigen selection, delivery platforms, and combination with immune checkpoint blockade may further enhance the efficacy of therapeutic EBV vaccines in the future.
The use of CAR-T and EBV vaccines are still highly experimental with limited clinical applicability,
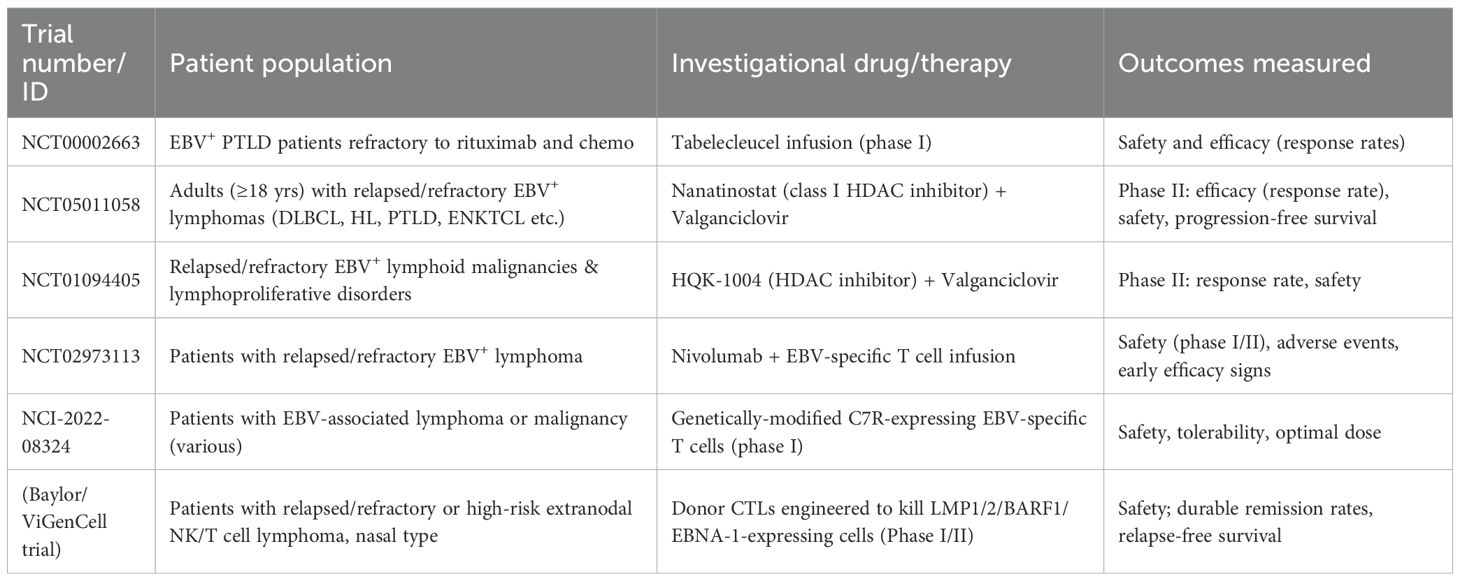
Table 2 summarizes ongoing trials on therapeutics of EBV-associated lymphoma.
Challenges and knowledge gaps
Despite significant progress in understanding EBV biology and its role in lymphomagenesis, several challenges and knowledge gaps persist, hindering optimal patient management and the development of more effective therapies. The vast clinical and biological heterogeneity of EBV-associated lymphomas presents a major challenge. Each subtype has distinct pathological features, latency programs, and clinical behaviors, requiring tailored diagnostic and therapeutic approaches (1). While advances have been made, comprehensive molecular profiling is not universally available, and the nuanced interplay between viral factors, host genetics, and microenvironment within each subtype is still being elucidated. This complexity makes it difficult to apply a single, unified therapeutic strategy. Currently, for many lymphoma types (e.g., cHL, BL), EBV status is not routinely used to guide first-line treatment decisions, despite its prognostic implications (28, 47). This is partly due to the fact that standard regimens are generally effective and the added benefit of EBV-specific approaches in the upfront setting is not yet fully established. Incorporating EBV status into treatment algorithms would require further robust clinical trial evidence demonstrating superior outcomes with EBV-guided therapies. Many clinical trials for lymphomas do not stratify patients based on EBV status or specifically enroll patients with EBV-associated subtypes. This results in a lack of high-level evidence for optimal management strategies for these distinct entities (33). There is a critical need for dedicated, prospective clinical trials focusing on specific EBV-associated lymphomas to evaluate novel agents and refine existing therapies. While quantitative PCR for EBV DNA is a valuable tool for diagnosis and monitoring, particularly in PTLD and ENKTL, there is a lack of widespread standardization across different laboratories regarding assay methodologies, cut-off values, and interpretation of results (43, 44). This limits the comparability of data across studies and clinical centers. Standardized assays and established guidelines for their use would significantly improve clinical utility. In many regions of the world, particularly in developing countries where the burden of certain EBV-associated lymphomas (e.g., endemic BL, ENKTL) is high, access to advanced diagnostic techniques (e.g., EBER ISH, quantitative EBV DNA PCR) and sophisticated treatments (e.g., ICIs, CAR-T) remains limited (29, 38). Addressing these disparities is crucial for improving outcomes globally.
Inborn errors of immunity and EBV-associated lymphomas
Recent advances in the field of inborn errors of immunity (IEIs) have greatly enhanced the understanding of the interplay between host immune defects and EBV-driven lymphomagenesis. Virus-associated neoplasia, including EBV-associated lymphomas, can often represent the first clinical manifestation of underlying IEIs, particularly in pediatric and young adult patients (70, 71). Among these, X-linked lymphoproliferative disease (XLP) types 1 and 2, caused by mutations in SH2D1A and XIAP respectively, are classical examples in which defective cytotoxic T-cell and NK-cell responses to EBV result in uncontrolled B-cell proliferation and life-threatening lymphoproliferative disease (71, 72). Similarly, activated PI3Kδ syndrome (APDS), due to PIK3CD or PIK3R1 mutations, and common variable immunodeficiency (CVID) have been associated with EBV-positive lymphomas, reflecting impaired immune regulation and defective viral clearance (73). Other disorders such as CTPS1 deficiency, MAGT1 deficiency, and CD27/CD70 axis defects further underscore how disruption of cytotoxic lymphocyte function predisposes to persistent EBV infection and malignant transformation (71, 74). Recognition of these conditions is crucial, as EBV-associated lymphoma arising at an unusually early age, within a family history of immune dysregulation, or accompanied by autoimmunity, hypogammaglobulinemia, or hemophagocytic episodes, should prompt evaluation for underlying IEI (75). Genetic diagnosis not only informs pathogenesis but also guides therapeutic decisions, since certain IEIs, notably XLP and other severe cytotoxic pathway defects, are amenable to curative hematopoietic stem cell transplantation (HSCT) (72, 75). Integrating systematic immunologic and genomic assessment into the diagnostic work-up of EBV-associated lymphomas therefore represents a critical step toward precision medicine and improved outcomes in these patients.
Discussion
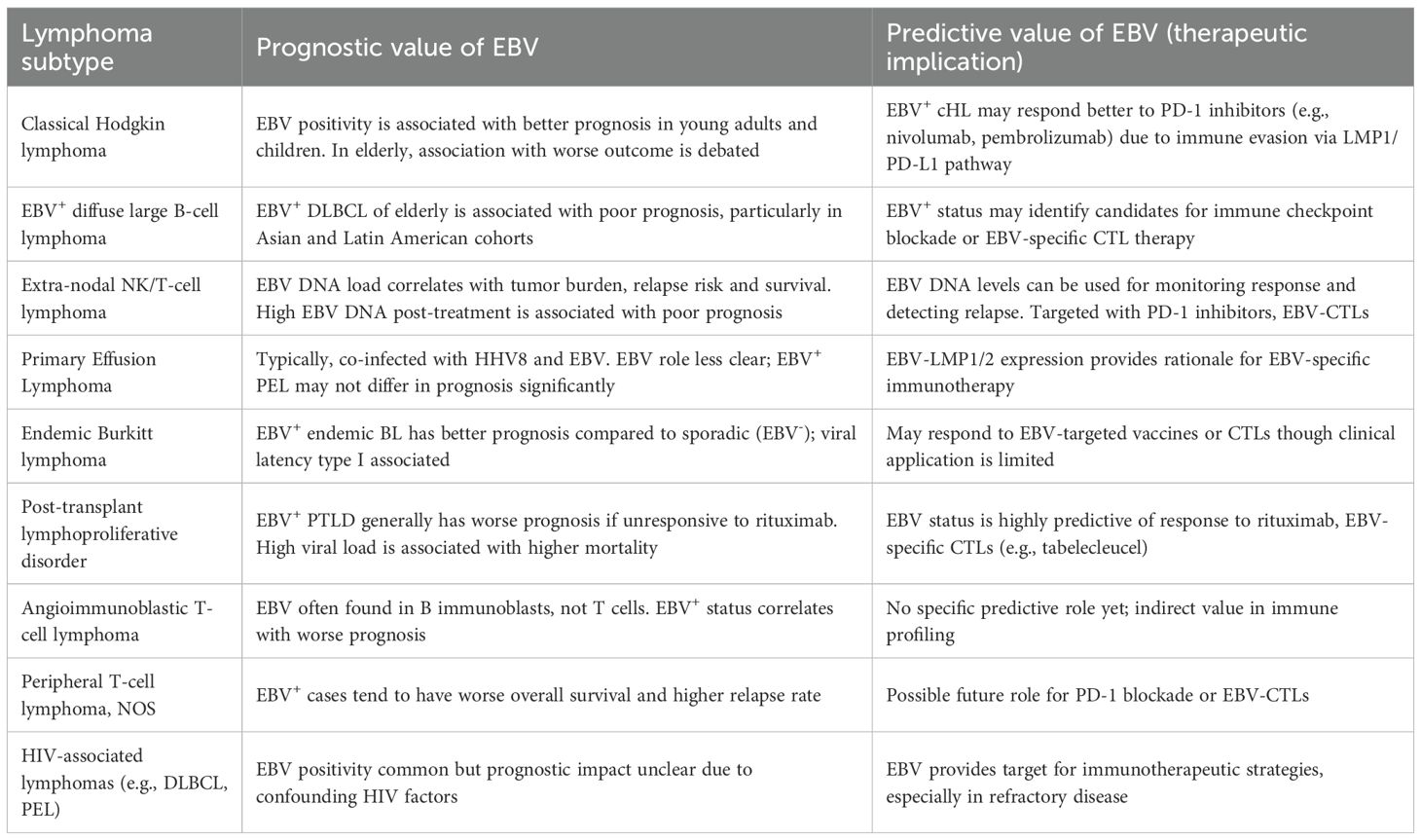
EBV-associated lymphomas represent a fascinating and challenging group of malignancies, underscoring the complex interplay between viral infection and host oncogenesis. The information presented herein highlights the significant diversity of these neoplasms, emphasizing how the specific viral latency programs, the host’s immune status, and co-factors like malaria or immunosuppression profoundly influence disease behavior and prognosis. While the histological subtype largely dictates current treatment paradigms, there is a rapidly growing recognition of the unique biological features conferred by EBV presence, which increasingly points towards the need for more targeted and personalized therapeutic approaches. Table 3 summarizes the prognostic and predictive value of EBV in various EBV-associated lymphoid malignancies.
The detailed understanding of viral oncogenes like LMP1 and their direct impact on host signaling pathways provides clear molecular targets for drug development. Similarly, the mechanisms by which EBV promotes immune evasion, such as through PD-L1 upregulation, have directly informed the successful application of immune checkpoint inhibitors in these diseases. The impressive responses seen with PD-1 blockade in cHL and ENKTL, for instance, validate the strategy of leveraging the unique immunobiology of EBV-driven tumors.
Furthermore, the success of adoptive immunotherapy with EBV-specific CTLs in PTLD offers a compelling paradigm for precision immune targeting that could potentially be extended to other EBV-associated malignancies. Challenges remain in translating these successes to all EBV-positive lymphomas, particularly those with more restricted latency programs or in immunocompetent hosts, where the tumor cells may be less immunogenic or the endogenous immune response more robust. Nevertheless, these advancements underscore the importance of leveraging the viral component as a distinct therapeutic vulnerability.
Future research efforts must focus on several key areas. Firstly, integrating comprehensive EBV characterization (latency type, viral load, specific oncogene expression) into routine diagnostic workups will be crucial for refined risk stratification and treatment selection. Secondly, dedicated, well-designed clinical trials specifically for EBV-associated lymphoma subtypes are essential to establish optimal, evidence-based treatment guidelines. This includes evaluating novel combinations of chemotherapy with targeted agents or immunotherapies, and exploring the potential of maintenance strategies or adjuvant therapies to prevent relapse. Thirdly, continued research into novel therapeutic targets, beyond the current ICIs, for instance, targeting upstream EBV-mediated signaling or epigenetic dysregulation, holds significant promise. Finally, the development of both prophylactic and therapeutic EBV vaccines remains a long-term goal that could dramatically alter the landscape of these diseases. Collaborative research efforts across various disciplines; virology, immunology, oncology, and epidemiology will be paramount to translating these scientific insights into improved clinical outcomes.
Conclusion
Epstein-Barr virus remains a pivotal and multifaceted factor in the pathogenesis of a significant proportion of lymphoid malignancies. The past few decades have witnessed remarkable advancements in our understanding of EBV biology, its intricate latency programs, and the diverse mechanisms by which it contributes to cellular transformation and immune evasion. These scientific insights have directly opened new avenues for both novel diagnostics and innovative immunotherapies. As the field continues to progress, the more precise incorporation of EBV-specific biomarkers into clinical practice, alongside the strategic deployment of targeted therapies that exploit the unique viral vulnerabilities, holds immense potential to significantly improve patient outcomes, particularly in high-risk and immunocompromised populations. The journey from initial discovery of EBV in lymphoma to current precision medicine approaches exemplifies the power of basic science research informing clinical translation. Continued collaborative efforts across epidemiology, virology, oncology, and immunology are not merely beneficial but essential to further unravel the complexities of EBV-associated lymphomagenesis and to ultimately translate these profound scientific insights into more effective and personalized clinical practice worldwide.
Author contributions
SD: Writing – original draft, Writing – review & editing. AG: Conceptualization, Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Young LS and Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. (2004) 4:757–68. doi: 10.1038/nrc1452
2. Epstein MA, Achong BG, and Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. (1964) 1:702–3. doi: 10.1016/S0140-6736(64)91524-7
3. Cohen JI. Epstein–Barr virus infection. N Engl J Med. (2000) 343:481–92. doi: 10.1056/NEJM200008173430707
4. Thorley-Lawson DA and Gross A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N Engl J Med. (2004) 350:1328–37. doi: 10.1056/NEJMra032015
5. Kieff E and Rickinson AB. Epstein–Barr virus and its replication. In: Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia (2007). p. 2603–54.
6. Young LS and Rickinson AB. Epstein-Barr virus: a human carcinogen. Nat Rev Cancer. (2010) 10:26–39. doi: 10.1038/nrc2846
7. Kung CP, Raab-Traub N, Dittmer DP, et al. EBV-encoded BART microRNAs target multiple pro-apoptotic pathways in epithelial cells. PloS Pathog. (2018) 14:e1007370. doi: 10.1371/journal.ppat.1007370.
8. Kilger E, Kieser A, Baumann M, et al. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1. EMBO J. (1998) 17:1700–9. doi: 10.1093/emboj/17.6.1700
9. Latza U, Kempkes B, Zimber-Strobl U, et al. Expression of LMP2A in EBV-associated lymphoproliferative disorders. Blood. (2001) 98:1187–94. doi: 10.1182/blood.V98.5.1187
10. Henle G and Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. (1966) 91:1248–56. doi: 10.1128/jb.91.3.1248-1256.1966
11. Strong MJ, Munger J, Coen DM, et al. The Epstein-Barr virus microRNA BHRF1–1 targets the pro-apoptotic protein BCL2. J Virol. (2016) 90:6118–29.
12. Cohen JI. Epstein-Barr virus biology and disease. In: Principles and Practice of Pediatric Oncology, 7th ed. Lippincott Williams & Wilkins, Philadelphia (2016). p. 448–60.
13. Mancao C, Kang MS, Li M, et al. Epstein-Barr virus latent membrane protein 2A (LMP2A) regulates B-cell survival by modulating the expression of the prosurvival protein Bcl-xL. J Virol. (2005) 79:13268–79. doi: 10.1128/JVI.79.20.13268-13279.2005
14. Lo AK, Lo YM, Chan LY, et al. Circulating Epstein-Barr virus DNA as a tumor marker for nasopharyngeal carcinoma. Clin Cancer Res. (2004) 10:4214–8. doi: 10.1158/1078-0432.CCR-04-0450
15. Dawson CW, Port RJ, and Young LS. Epstein-Barr virus-encoded LMP1: a paradigm for oncogene-mediated evasion of apoptosis. Adv Cancer Res. (2006) 95:115–41.
16. Mainou BA, Lee D, and Longnecker R. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB and IRF7 leads to induction of a large number of interferon-stimulated genes. J Virol. (2005) 79:14959–73. doi: 10.1128/JVI.79.23.14959-14973.2005
17. Longnecker R. Epstein-Barr virus latency: LMP2, a regulatory protein. Curr Top Microbiol Immunol. (2006) 303:127–42.
18. Ressing ME, Horst D, Wieland K, et al. Immune evasion by Epstein-Barr virus. Curr Top Microbiol Immunol. (2015) 391:355–81.
20. Green MR, Monti S, Hatzi K, et al. Integrative analysis reveals selective 9p24.1 amplification and increased PD-1 ligand expression in EBV+ lymphomas. Cancer Cell. (2010) 20:723–37. doi: 10.1016/j.ccr.2011.10.001
21. Ansell SM. PD-1 blockade in Hodgkin lymphoma. Blood. (2016) 128:1199–206. doi: 10.1182/blood-2016-06-718121
22. Poudel-Neupane N, Kouno T, Maejima Y, et al. Epstein-Barr virus-driven AID expression in gastric carcinoma. Oncotarget. (2018) 9:31649–62. doi: 10.18632/oncotarget.25783
23. Hecht JL and Aster JC. Molecular biology of Burkitt's lymphoma. J Clin Oncol. (2000) 18:3707–21. doi: 10.1200/JCO.2000.18.21.3707
24. Kelly GL and Rickinson AB. EBV latent genes, chromatin, and the host cell environment. J Virol. (2007) 81:9439–48. doi: 10.1128/JVI.00817-07
25. Komatsu M, Tadano M, Noguchi M, et al. Tumor-associated macrophages promote the growth of EBV-positive nasopharyngeal carcinoma. Cancer Res. (2012) 72:2418–29. doi: 10.1158/0008-5472.CAN-11-2346
26. Jarrett RF. Viruses and Hodgkin's lymphoma. Ann Oncol. (2002) 13:23–9. doi: 10.1093/annonc/13.S1.23
27. Hjalgrim H, Askling J, and Sørensen P. The aetiology of Hodgkin lymphoma. Br J Haematol. (2011) 154:13–22. doi: 10.1111/j.1365-2141.2011.08572.x
28. Ansell SM. Hodgkin lymphoma: 2023 update on diagnosis, risk stratification, and treatment. Am J Hematol. (2023) 98:123–35.
29. Mbulaiteye SM, Katongole-Mbidde E, and Mutalima N. Endemic Burkitt lymphoma: a disease of children in sub-Saharan Africa. WHO Bull. (2003) 81:594–601.
30. International Agency for Research on Cancer. Epstein-barr virus and kaposi's sarcoma herpesvirus/human herpesvirus 8. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 70. IARC Press, Lyon (1997).
31. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC (2017).
32. Oyama T, Ichimura K, Suzuki R, et al. EBV-associated diffuse large B-cell lymphoma of the elderly. Blood. (2003) 102:4191–8. doi: 10.1182/blood-2003-03-0869
33. Ok CY, Li S, and Medeiros LJ. EBV-positive diffuse large B-cell lymphoma of the elderly. Pathology. (2014) 46:189–98. doi: 10.1097/PAT.0000000000000021
34. Liu Y, Bodor C, Kansal R, et al. EBV-positive diffuse large B-cell lymphoma, NOS: a clinicopathologic study of 60 cases. Mod Pathol. (2016) 29:123–33. doi: 10.1038/modpathol.2015.127
35. Styczynski J, Van Der Velden W, Dührsen U, et al. Posttransplant lymphoproliferative disorder: pathogenesis, risk factors, and management. Transpl Infect Dis. (2020) 22:e13251. doi: 10.1111/tid.13251
36. Trappe R, Dierickx D, Zimmermann H, et al. International consensus recommendations on the management of post-transplant lymphoproliferative disorders. Blood. (2017) 130:2632–46. doi: 10.1182/blood-2017-07-794109
37. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
38. Tse E and Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. (2017) 10:85. doi: 10.1186/s13045-017-0452-9
39. Lee J, Suh C, Park K, et al. Clinical characteristics and treatment outcomes of extranodal natural killer/T-cell lymphoma, nasal type, in Korea: a retrospective analysis of 37 cases. J Korean Med Sci. (2005) 20:948–52. doi: 10.3346/jkms.2005.20.6.948
40. Kim SJ, Yoon SE, Kim YS, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline–based treatment: a multicentre, retrospective analysis. Lancet Oncol. (2016) 17:389–400. doi: 10.1016/S1470-2045(15)00533-1
41. Jaffe ES, Harris NL, and Stein H. Burkitt lymphoma. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. IARC, Lyon (2017). p. 306–11.
42. Chan JKC, Quintanilla-Martinez L, Ferry JA, et al. Epstein–Barr virus (EBV)-associated lymphoid proliferations: diagnostic approach and practical application. Pathology. (2020) 52:19–31. doi: 10.1016/j.pathol.2019.10.017
43. Au WY, Pang A, Choy C, et al. Quantification of circulating EBV DNA in the diagnosis and monitoring of NK/T-cell lymphomas. Br J Haematol. (2004) 124:463–9. doi: 10.1111/j.1365-2141.2004.04935.x
44. Lin N, Chen H, Yu X, et al. Prognostic significance of plasma EBV DNA load in extranodal NK/T-cell lymphoma, nasal type. Ann Oncol. (2009) 20:1232–7. doi: 10.1093/annonc/mdp367
45. National Comprehensive Cancer Network. NCCN Guidelines for Hodgkin Lymphoma (2023). Available online at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1432 (Accessed June 15, 2025).
46. Kwong YL, Kim SJ, Tang T, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma. J Clin Oncol. (2022) 40:632–40. doi: 10.1200/JCO.21.01477
47. Jacobson C, Lunning M, Yahalom J, et al. A randomized phase II study of DA-EPOCH-R versus standard chemotherapy for patients with newly diagnosed Burkitt lymphoma. Blood. (2017) 130:2013–9. doi: 10.1182/blood-2017-05-783682
48. Park YH, Ko YH, Jang KY, et al. A phase II study of ibrutinib in combination with R CHOP in Epstein-Barr virus-positive diffuse large B cell lymphoma (IVORY). Ann Hematol. (2021) 100:101–10. doi: 10.1007/s00277-020-04219-1
49. Navarro L, Chang H, Ferreri AJM, et al. Nanatinostat with or without valganciclovir in patients with relapsed/refractory EBV-positive PTCL (NAVAL-1 stage 1). Hematol Transfus Cell Ther. (2024) 46:S258.
50. Chang H, Navarro L, Kim SJ, et al. Global phase II expansion study of nanatinostat + valganciclovir in EBV+ relapsed/refractory peripheral T cell lymphomas (NAVAL 1 Stage 2). Hematol Transfus Cell Ther. (2024) 46:S259.
51. Prockop SE, Doubrovina E, Senechal B, et al. Tabelecleucel for posttransplant lymphoproliferative disorder after hematopoietic stem cell or solid organ transplant: results of two multicenter phase 3 studies. Blood. (2022) 140:904–15. doi: 10.1182/blood.2022017013
52. Kim SJ, Lim JQ, Laurensia Y, et al. Avelumab for relapsed or refractory extranodal NK/T-cell lymphoma: phase II study. J Clin Oncol. (2020) 38:3101–9. doi: 10.1200/JCO.19.02534
53. Khodadoust MS, Momtahen S, and Levy R. Nivolumab for EBV-positive lymphomas: early results from a phase 2 trial. Blood. (2017) 129:2292–300. doi: 10.1182/blood-2016-09-740918
54. Yoon SE, Kim SJ, Ko YH, et al. A phase II study of brentuximab vedotin in CD30-positive EBV-associated lymphomas. Haematologica. (2021) 106:3183–6. doi: 10.3324/haematol.2021.278353
55. Bollard CM, Aguilar L, Straathof KC, et al. EBV-specific cytotoxic T lymphocytes for relapsed extranodal NK/T-cell lymphoma: phase II results. Blood. (2021) 137:712–21. doi: 10.1182/blood.2020008505
56. Heslop HE, Slobod KS, Pule MA, et al. Long-term outcomes of EBV-specific T-cell therapy for posttransplant lymphoproliferative disease. Blood. (2010) 115:925–35. doi: 10.1182/blood-2009-08-239186
57. Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease. Blood. (2010) 115:925–35. doi: 10.1182/blood-2009-08-239186
58. Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of autologous stem-cell transplantation. N Engl J Med. (2016) 375:307–16. doi: 10.1056/NEJMoa1510436
59. Bollard CM, Rooney CM, and Heslop HE. Cytotoxic T lymphocyte therapy for Epstein–Barr virus+ lymphomas. J Clin Invest. (2004) 114:70–8. doi: 10.1172/JCI200420025
60. Prockop S, Doubrovina E, Senechal B, et al. EBV-specific cytotoxic T lymphocyte therapy for posttransplant lymphoproliferative disease. Clin Lymphoma Myeloma Leuk. (2014) 14:S53–7.
61. Neelapu SS, Locke FL, Ghobadi N, et al. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
62. Wang CM, Zhang X, Huang S, et al. CD30-directed CAR-T cells for relapsed Hodgkin lymphoma. J Hematol Oncol. (2020) 13:1–12. doi: 10.1186/s13045-020-00945-2
63. Pincus DW, Kochenderfer JN, and Gress RE. CD30-directed CAR T cells: a novel approach for Hodgkin lymphoma. Blood Adv. (2019) 3:2876–88. doi: 10.1182/bloodadvances.2019031063
64. Cai H, Wang Z, Wei H, et al. Inhibition of NF-κB enhances the effect of bortezomib in Epstein-Barr virus-positive nasopharyngeal carcinoma cells. J Cancer Res Clin Oncol. (2012) 138:145–53. doi: 10.1007/s00432-011-1072-6
65. Jardin F. The epigenetics of Epstein-Barr virus-associated lymphomas. Front Genet. (2020) 11:480. doi: 10.3389/fgene.2020.00480
66. Qi X, Xu X, Li X, et al. EZH2 inhibition downregulates Epstein-Barr virus lytic gene expression and reactivates virus in EBV-positive gastric carcinoma. Oncotarget. (2015) 6:20537–46. doi: 10.18632/oncotarget.3872
67. Feng WH, Quenelle D, Kappler K, et al. The proteasome inhibitor bortezomib sensitizes Epstein-Barr virus-associated tumor cells to chemotherapy. Cancer Res. (2007) 67:7397–404. doi: 10.1158/0008-5472.CAN-07-0372
68. Kanekiyo M and Cohen JI. Advances in EBV vaccine research: from animal models to human trials. Front Immunol. (2021) 12:677027. doi: 10.3389/fimmu.2021.677027
69. Sokal EM, Hoppenbrouwers M, Vandermeulen C, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial. J Infect Dis. (2007) 196:1310–18. doi: 10.1086/523813
70. Bosch B, Maccari ME, Volpi S, et al. Virus-driven lymphoproliferative disorders in primary immunodeficiencies and immune dysregulation: from pathogenesis to treatment. Front Immunol. (2022) 13:895312. doi: 10.3389/fimmu.2022.895312
71. Latour S and Winter S. Inherited immunodeficiencies with high predisposition to Epstein–Barr virus-driven lymphoproliferative diseases. Front Immunol. (2018) 9:1103. doi: 10.3389/fimmu.2018.01103
72. Booth C, Gilmour KC, Veys P, et al. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the clinical and immunologic phenotype. Blood. (2011) 117:53–62. doi: 10.1182/blood-2010-06-284935
73. Coulter TI, Chandra A, Bacon CM, et al. Clinical spectrum and features of activated PI3Kδ syndrome: a large patient cohort study. J Allergy Clin Immunol. (2017) 139:597–606.e4. doi: 10.1016/j.jaci.2016.06.021
74. Alkhairy OK, Perez-Becker R, Driessen GJ, et al. Novel mutations in MAGT1 cause X-linked immunodeficiency with EBV-associated lymphoproliferative disease (XMEN). J Allergy Clin Immunol. (2014) 134:1459–62. doi: 10.1016/j.jaci.2014.06.014
Keywords: Epstein-Barr virus, lymphoid malignancies, lymphomas, immune checkpoint inhibitors, CAR-T
Citation: Datta S, Gogia A and Mallick S (2025) Epstein-Barr virus-associated lymphomas: biology, molecular genomics and precision oncology. Front. Oncol. 15:1677060. doi: 10.3389/fonc.2025.1677060
Received: 31 July 2025; Accepted: 03 November 2025;
Published: 24 November 2025.
Edited by:
Pasquale Niscola, Sant’Eugenio Hospital of Rome, ItalyReviewed by:
Giorgio Costagliola, Azienda Ospedaliero Universitaria Pisana, ItalyFederica Re, University of Brescia, Italy
Pietro Tralongo, University of Messina, Italy
Copyright © 2025 Datta, Gogia and Mallick. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ajay Gogia, YWpheWdvZ2lhQGdtYWlsLmNvbQ==
†ORCID: Soumyadeep Datta, orcid.org/0009-0006-1075-2988
Ajay Gogia, orcid.org/0000-0001-6500-1061
Saumyaranjan Mallick, orcid.org/0000-0003-4366-5873
 Soumyadeep Datta
Soumyadeep Datta Ajay Gogia
Ajay Gogia Saumyaranjan Mallick
Saumyaranjan Mallick