- 1Department of Hematology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 2Central Laboratory, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan, China
- 3Institute of Cancer Research, Henan Academy of Innovations in Medical Science, Zhengzhou, China
Objective: To evaluate the clinical efficacy of maintenance therapy with sorafenib combined with interferon α-1b, interleukin-2, and thalidomide (the ITI regimen) in patients with FLT3-ITD-positive acute myeloid leukemia (AML).
Methods: 19 FLT3-ITD(+) AML patients were retrospectively analyzed, who received the sorafenib combined with ITI regimen as maintenance therapy after achieving remission at Affiliated Cancer Hospital of Zhengzhou University (January 2014–December 2024). Minimal residual disease (MRD) levels were monitored, and clinical outcomes, including survival duration, were assessed.
Results: This study included 19 patients (9 males, 10 females) with a median age at diagnosis of 59 years (range: 21–76). The median white blood cell count was 32.25×109/L (range: 0.7–254×109/L). Among them, 13 patients (68.4%) maintained sustained MRD negativity during sorafenib combined with TI regimen therapy, while 3 patients experienced morphological relapse and 3 had molecular relapse. Median overall survival (mOS) was not reached, with 12- and 24-month OS rates of 100% (19/19) and 94.7% (18/19), respectively. During maintenance therapy with sorafenib combined with ITI, median relapse-free survival (mRFS) was also not reached, with 12- and 24-month RFS rates of 73.7% (14/19) and 57.9% (11/19), respectively.
Conclusion: The sorafenib combined with ITI regimen is an effective maintenance therapy for FLT3-ITD (+) AML, significantly reducing relapse risk and prolonging survival.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous hematologic malignancy. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the optimal curative approach for intermediate- and adverse-risk AML patients (1, 2). However, for transplant-ineligible patients, effective minimal residual disease (MRD)-directed interventions to prevent relapse during post-remission therapy represent a critical unmet clinical need (3). Approximately 30% of AML patients harbor FMS-like tyrosine kinase 3 (FLT3) mutations, which are associated with high blast counts, low complete remission (CR) rates, reduced disease-free survival and overall survival (OS) (4, 5). Sorafenib, a small-molecule tyrosine kinase inhibitor targeting FLT3, exerts its antileukemic effects by competitively binding to the ATP-binding site, thereby inhibiting kinase activity, disrupting downstream signaling, and promoting leukemic cell differentiation and apoptosis (6–8). Multiple studies have demonstrated the efficacy of sorafenib in FLT3-ITD-mutated AML (9, 10). Our team previously reported promising results with the interferon-α1b, interleukin-2, and thalidomide (ITI) regimen, both in refractory/relapsed AML and MRD-positive AML (11, 12).
This study focuses on transplant-ineligible, FLT3-ITD(+) AML patients in remission post-chemotherapy, evaluating the combination of sorafenib and the ITI regimen as maintenance therapy, with close monitoring of MRD dynamics and survival outcomes, aiming to provide novel clinical insights and therapeutic strategies.
Materials and methods
Patients
19 FLT3-ITD-mutated AML patients treated at the Affiliated Cancer Hospital of Zhengzhou University (January 2014–December 2024). Diagnosis was confirmed based on clinical presentation, bone marrow morphology, flow cytometric immunophenotyping, cytogenetics, and molecular profiling. The sorafenib combined with ITI regimen was initiated as maintenance therapy upon completion of all planned consolidation chemotherapy cycles, while patients were in morphological CR. The patients were considered not to be candidates for allo-HSCT primarily due to (1): patient preference or financial reasons (2); significant comorbidities and/or poor performance status; or (3) lack of a suitable donor. The diagnostic criteria for AML were based on the relevant standards established by the World Health Organization (WHO) in 2016, while the classification followed the FAB (French-American-British) classification system. The patient’s risk stratification was assessed according to the European Leukemia Net (ELN) 2022 risk classification for acute myeloid leukemia. This study was approved by the Ethics Committee of Henan Cancer Hospital (No: 2022-548-001). This study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all guardians or patients.
Treatment protocol
Patients received sorafenib (400 mg orally twice daily) combined with the ITI regimen consisting of subcutaneous interferon α-1b (60 μg every other day), interleukin-2 (1 million IU every other day), and oral thalidomide (100 mg nightly) in 28-day cycles. For thromboprophylaxis in patients with platelet counts >50×109/L, compound Danshen tablets (3 tablets three times daily) were administered. Preemptive ibuprofen was administered orally 1 hour before interferon α-1b injections to prevent treatment-related fever.
Treatment response monitoring
Patients underwent monthly bone marrow evaluations during sorafenib plus ITI regimen therapy, including morphologic assessment (complete remission defined as <5% blasts) and MRD monitoring. MRD was assessed using EDTA-anticoagulated samples for quantitative PCR (fusion gene-positive cases) and heparinized samples for 10-color flow cytometry (FCM), with positivity defined as ≥ 0.01% blast cells. The FCM analysis utilized two comprehensive antibody panels (CD34/CD117/CD13/CD38/CD15/CD33/HLA-DR/CD45 and CD34/CD117/CD7/CD56/CD64/CD19/CD45) with ≥ 500, 000 acquired events. For patients with fusion genes (AML1-ETO), RNA was extracted from bone marrow samples and analyzed by real-time PCR using an ABI Prism 7500 system (Applied Biosystems; Foster City, CA, USA), with ABL as the endogenous control. The AML1-ETO assay employed specific primers. This sensitive molecular monitoring complemented the multiparameter flow cytometry approach, providing comprehensive MRD assessment.
Forward Primer: 5′-CACCTACCACAGAGCCATCAAA-3′;
Reverse Primer: 5′-ATCCACAGGTGAGTCTGGCATT-3′.
Safety profile assessment
Safety was evaluated each time before and after the treatment of each cycle. According to the criteria for adverse reaction assessment established by the World Health Organization, adverse reactions were graded from I to IV.
Follow-up
Patient follow-up was conducted through medical records and telephone interviews until May 31, 2025. OS was calculated from diagnosis to death or last follow-up, while relapse-free survival (RFS) was measured from sorafenib/ITI initiation to relapse (defined as bone marrow blasts >5% or MRD positivity [≥0.01%]) or last contact.
Statistical analysis
Statistical analysis was performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA). Continuous data are presented as median (range) and compared using the Mann-Whitney U test. Categorical variables between groups were compared using the Chi-square test. OS and RFS were analyzed with the Kaplan-Meier method, and survival curves were generated.
Results
Patient characteristics
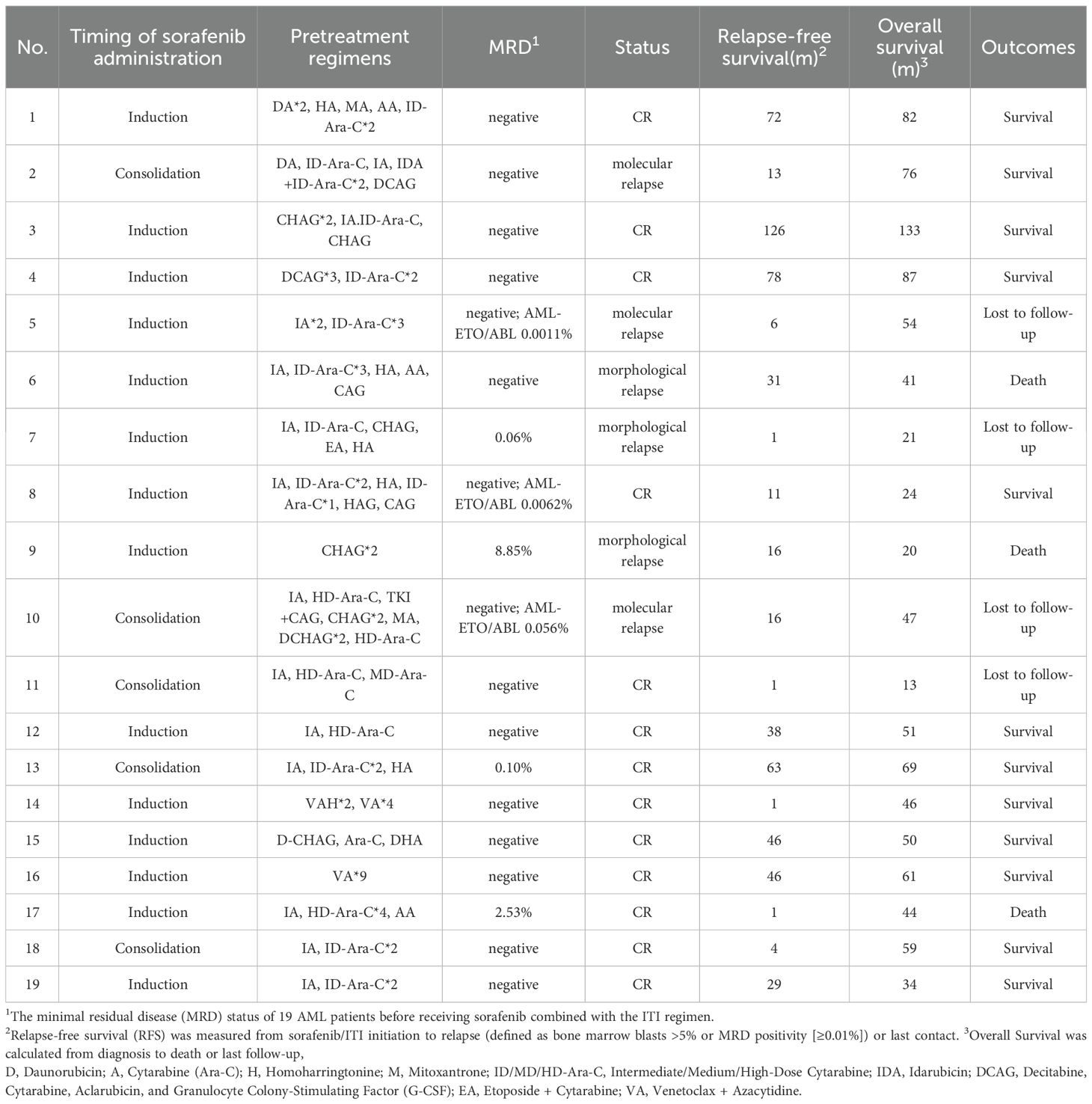
This retrospective study enrolled 19 consecutive FLT3-ITD-positive AML patients (9 males and 10 females) with a median age of 59 years (range: 21-76) at diagnosis. The cohort demonstrated a median presenting white white cell count of 32.25×109/L (range: 0.7-254×109/L). By FAB classification, cases included M1 (n=3, 15.8%), M2 (n=7, 36.8%), M4 (n=3, 15.8%), M5 (n=5, 26.3%), and M7 (n=1, 5.3%). According to the European LeukemiaNet (ELN) 2022 risk stratification criteria for acute myeloid leukemia, patients were categorized as follows: favorable risk in 3 cases (15.8%), intermediate risk in 9 cases (47.4%), and adverse risk in 7 cases (36.8%). All patients achieved complete morphological remission after ≤2 induction cycles. Fourteen patients (73.7%) received sorafenib (400mg twice daily) during both induction and consolidation phases, while 5 (26.3%) initiated sorafenib during consolidation only. Baseline patient characteristics are summarized in Table 1. Treatment details, including regimens prior to maintenance and MRD status immediately before initiating the sorafenib-ITI regimen, are detailed in Table 2.
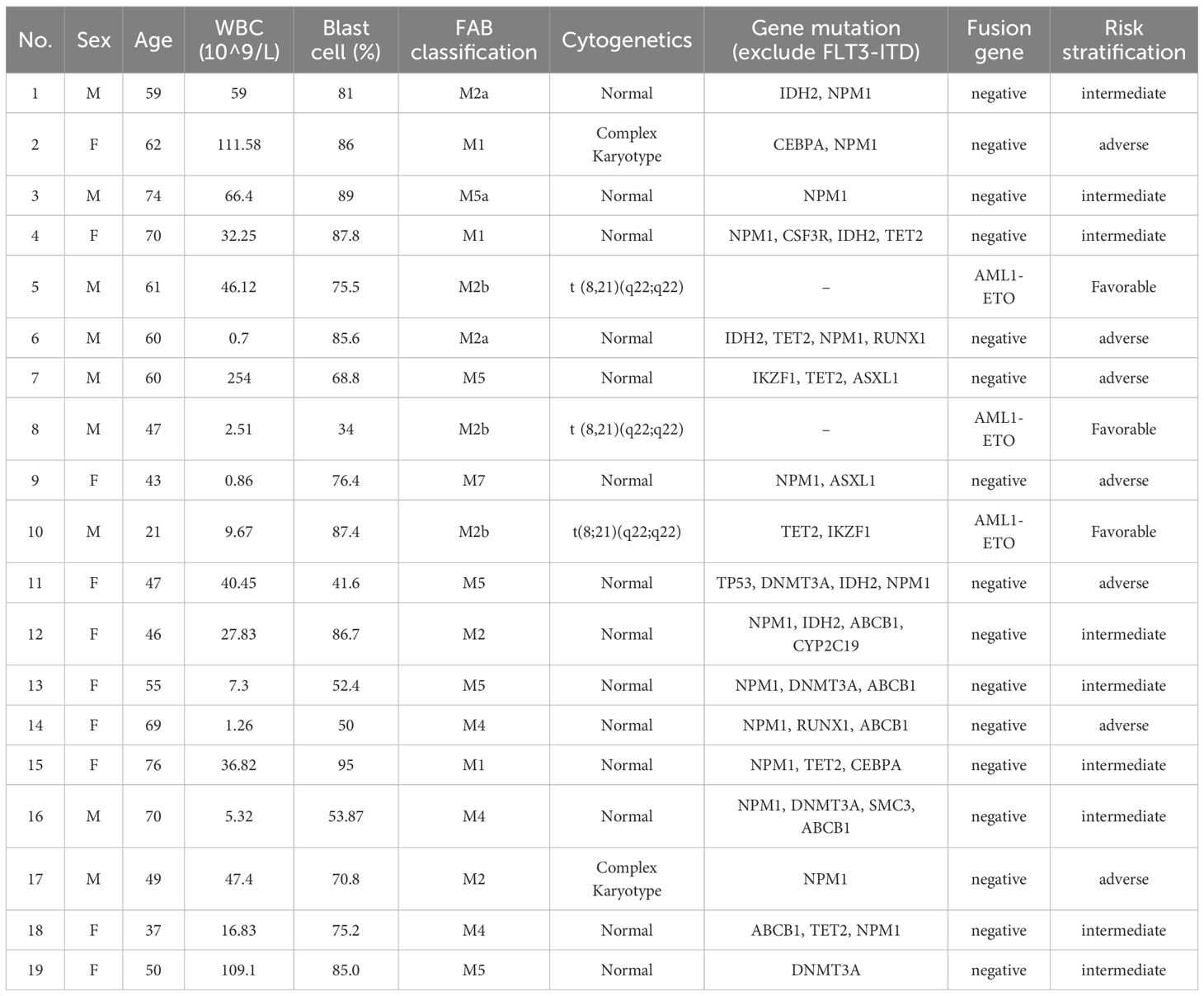
Table 1. Initial clinical data of 19 AML patients who received intervention therapy with Sorafenib and ITI regimen.
Treatment outcomes
During maintenance therapy with sorafenib combined with ITI regimen, 13 of 19 patients (68.4%) maintained sustained MRD negativity, while 3 (15.8%) developed molecular relapse and 3 (15.8%) experienced morphological relapse. Notably, among the four patients who were MRD-positive prior to maintenance therapy, Patient 13 achieved MRD negativity after one cycle of treatment and has maintained negative status to date. Patient 9 exhibited a progressive decrease in MRD levels during maintenance therapy and eventually achieved sustained negativity for over five months. Three patients (15.8%), who became eligible for allogeneic HSCT due to the subsequent availability of a matched donor and improved performance status, underwent the procedure after a median of one treatment cycle (range: 1-4) while in CR, despite initial ineligibility from donor unavailability and poor condition post-induction (Supplementary Table 1). With a median follow-up of 50 months (range: 13-133), median overall survival (mOS) was not reached (3 deaths, 15.8%), with 12- and 24-month OS rates of 100% and 94.7%, respectively. Similarly, median relapse-free survival (mRFS) was not reached (6 relapses, 31.6%), demonstrating 12- and 24-month RFS rates of 73.7% and 57.9% (Figure 1).
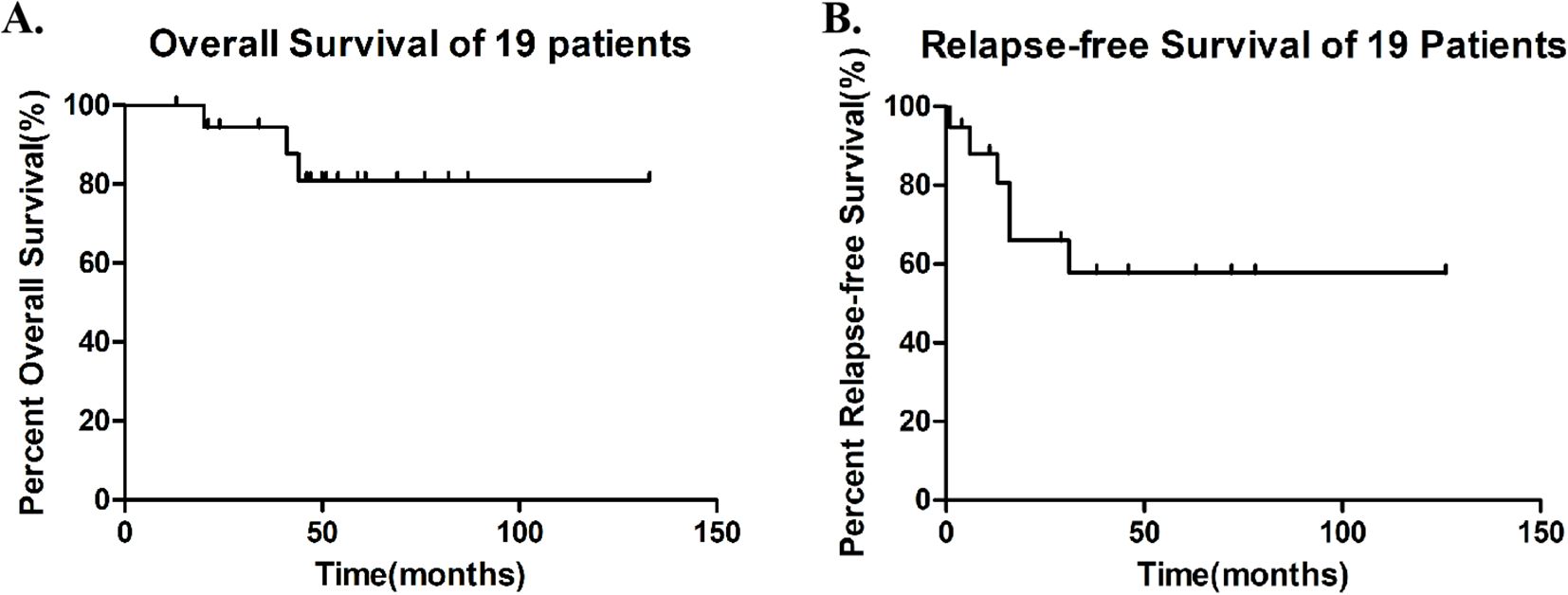
Figure 1. Survival outcomes in 19 patients (A) Overall Survival (OS), median OS was not reached, with 12- and 24-month OS rates of 100% and 94.7%. (B) Relapse-Free Survival (RFS) during Sorafenib-ITI maintenance, median RFS was not reached, with 12- and 24-month RFS rates of 73.7% and 57.9%.
Safety profile and adverse events
The sorafenib and ITI combination regimen exhibited characteristic yet manageable toxicities during maintenance therapy. Hematologic adverse events consisted primarily of grade I–II myelosuppression. Non-hematologic toxicities were predominantly interferon-related constitutional symptoms. Fever occurred in 89.5% of patients (Grade I–II: 84.2%; Grade III: 5.3%), typically lasting 1–3 days after interferon administration. Rhinorrhea (47.4%, all Grade I–II) and myalgia (31.6%, all Grade I–II) were also common, generally resolving within one week. Sorafenib-associated toxicities primarily involved the skin and gastrointestinal tract, including hand-foot skin reaction (36.8%, all Grade I–II), and diarrhea (31.6%, all Grade I–II). All observed toxicities were effectively managed with supportive measures and did not necessitate treatment interruption. The safety profile was consistent with the known effects of each individual agent, and no unexpected toxicities emerged with the combination. No grade IV adverse events or treatment-related mortality were observed.
Discussion
Current international guidelines, including NCCN and Chinese consensus for adult AML management, uniformly recognize FLT3-ITD mutations as an independent adverse prognostic factor (4, 5, 13). These genetically defined cases exhibit characteristic clinical presentations featuring hyperleukocytosis and elevated tumor burden at diagnosis. Depalmitoylation specifically modulates the biological function of the oncogenic FLT3-ITD mutant, driveing leukemogenesis by activating pathways including PI3K/AKT and RAS/MAPK signaling. This confers intrinsic chemoresistance to patients, manifested as lower complete remission rates and higher relapse incidence (4, 14). The therapeutic challenge is particularly pronounced in elderly patients (≥60 years), where FLT3-ITD co-occurs with age-related biological adversities: impaired organ function, frequent comorbidities, and heightened treatment-related mortality (15, 16). Consequently, only 20-30% of these patients qualify for allogeneic transplantation, the sole potentially curative option. This therapeutic dilemma underscores the critical need for novel maintenance strategies that can prolong remission duration and improve survival outcomes in transplant-ineligible patients while maintaining acceptable toxicity profiles.
Sorafenib, a small-molecule tyrosine kinase inhibitor, inhibits the autophosphorylation of FLT3, as well as the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) and signal transducer and activator of transcription 5 (Stat5) (17). Meanwhile, sorafenib synergizes with T cells by downregulating ATF4 expression, leading to the activation of the IRF7–IL-15 axis in leukemia cells. This activation subsequently induces metabolic reprogramming of leukemia-reactive T cells in vivo (18). Thus making it applicable for targeted therapy of AML. In the SORMAIN study, after a median follow-up of 41.8 months, the median RFS was not reached in the sorafenib group, whereas it was 30.9 months in the placebo group, representing a 75% reduction in the risk of relapse or death (9). In another phase III trial, the 2-year leukemia-free survival rate in the sorafenib group was 78.9%, which was higher than that in the placebo group (56.6%) (19). Based on the evidence that sorafenib as post-transplant maintenance therapy for FLT3-mutated AML can improve relapse-free survival and demonstrate good tolerability, the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT) recommends using sorafenib to optimize long-term disease control.
Thalidomide, rhIFNα-1b, and IL-2 all exhibit antileukemic activities. Our basic research has found that thalidomide can concentration-dependently inhibit the proliferation of the AML cell line Kasumi-1, and when combined with interferon, it can synergistically suppress cell proliferation and induce apoptosis in this cell line (20). As a lymphokine, IL-2 can promote the proliferation and activation of cytotoxic T cells, natural killer (NK) cells, and lymphokine-activated killer (LAK) cells. It also enhances antibody and IFN secretion by lymphocytes, exerting antiviral, antitumor, and immune-enhancing effects. In patients with core binding factor acute myeloid leukemia (CBF-AML), positive clinical outcomes were evidenced with IL-2 maintenance therapy. Moreover, the histamine dihydrochloride plus low-dose IL-2 regimen confers reduced relapse risk during the post-remission phase of AML through dual mechanisms (1): counteracting myeloid-derived immunosuppression (HDC component), and (2) activating anti-leukemic lymphocytes (including natural killer cells and cytotoxic T lymphocytes; IL-2 component) (21, 22). The three agents demonstrate synergistic effects in antitumor activity and immune regulation, which may make the combination of thalidomide, rhIFNα-1b, and IL-2 a novel effective therapeutic regimen for AML. Our clinical study has shown that the ITI regimen can reduce the level of MRD in AML patients and decrease the risk of relapse. Within our cohort, 20 patients achieving hematologic remission with persistent AML1-ETO fusion positivity received ITI regimen. The fusion transcript became undetectable or decreased in 18 patients, yielding an overall response rate of 90%. Concurrently, we initiated the regimen in 37 MRD-negative patients who had completed all planned consolidation chemotherapy without undergoing allo-HSCT). Maintenance therapy completion rates of 1, 2, 3 year were 75.7%, 59.5%, 40.5% (23). This strategy significantly reduced relapse rates in the treated cohort.
This study evaluated the efficacy of sorafenib combined with the ITI regimen as maintenance therapy in non-transplant eligible FLT3-ITD–positive AML patients in remission after chemotherapy. Among the 19 patients who received this combination, 13 maintained persistent MRD negativity. The longest follow-up was 133 months, and outcomes were encouraging. Additionally, we applied this regimen in three relapsed/refractory AML patients, observing certain therapeutic effects. Our patient cohort encompassed a wide age spectrum, which inherently introduced heterogeneity in the intensity of consolidation chemotherapy administered prior to maintenance, as clinical decisions are guided by individual tolerance and comorbidities. This variation in pre-maintenance treatment intensity is a potential confounding factor in our retrospective analysis. However, the consistent treatment benefit observed, particularly in older individuals often ineligible for intensive chemotherapy, underscores the potential of the sorafenib-ITI regimen. While this remains a study limitation, it also highlights the regimen’s potential applicability across a broad patient population. These findings suggest that this strategy may provide long-term survival benefits, though larger sample sizes and longer follow-up are needed for confirmation, along with mechanistic studies to elucidate underlying processes.
In conclusion, for non-transplant eligible intermediate- to adverse-risk FLT3-ITD–positive AML patients, maintenance therapy with sorafenib combined with the ITI regimen during post-chemotherapy remission effectively suppresses MRD conversion, prevents relapse, prolongs survival, and is associated with minimal adverse reactions and good tolerability. This approach warrants broader clinical application and promotion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Henan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CC: Conceptualization, Methodology, Formal Analysis, Resources, Data curation, Writing – original draft, Visualization. RM: Resources, Funding acquisition, Conceptualization, Writing – review & editing, Data curation. DL: Resources, Funding acquisition, Data curation, Methodology, Writing – review & editing. LC: Visualization, Writing – review & editing, Resources, Supervision, Data curation. XW: Supervision, Conceptualization, Funding acquisition, Writing – review & editing, Project administration, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The General Program of National Natural Science Foundation of China (82170151), Natural Science Foundation of Henan Province (No.242300421507), Basic Research Fund of Henan Province Academy of Medical Sciences (JBKY250306) and Investigator-initiated Clinical Trial and Transformation of Scientific Achievements, Henan Cancer Hospital (ITTA-2422) supported funding this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1698935/full#supplementary-material
References
1. Wang L, Zhang C, Fan S, Mo X, and Hu X. Treatment options for adult intermediate-risk AML patients in CR1: Allo-HSCT or chemotherapy? Innovation (Camb). (2023) 4:100461. doi: 10.1016/j.xinn.2023.100461
2. Senapati J, Kadia TM, and Ravandi F. Maintenance therapy in acute myeloid leukemia: advances and controversies. Haematologica. (2023) 108:2289–304. doi: 10.3324/haematol.2022.281810
3. Lachowiez CA and DiNardo CD. Mutation- and MRD-informed treatments for transplant-ineligible patients. Hematol Am Soc Hematol Educ Program. (2024) 2024:168–77. doi: 10.1182/hematology.2024000540
4. Daver N, Schlenk RF, Russell NH, and Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. (2019) 33:299–312. doi: 10.1038/s41375-018-0357-9
5. Daver N, Venugopal S, and Ravandi F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. (2021) 11:104. doi: 10.1038/s41408-021-00495-3
6. Perrone S, Ottone T, Zhdanovskaya N, and Molica M. How acute myeloid leukemia (AML) escapes from FMS-related tyrosine kinase 3 (FLT3) inhibitors? Still an overrated complication? Cancer Drug Resist. (2023) 6:223–38. doi: 10.20517/cdr.2022.130.
7. Zhang W, Konopleva M, Ruvolo VR, McQueen T, Evans RL, Bornmann WG, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. (2008) 22:808–18. doi: 10.1038/sj.leu.2405098.
8. Wang R, Xia L, Gabrilove J, Waxman S, and Jing Y. Sorafenib inhibition of mcl-1 accelerates ATRA-induced apoptosis in differentiation-responsive AML cells. Clin Cancer research: an Off J Am Assoc Cancer Res. (2016) 22:1211–21. doi: 10.1158/1078-0432.CCR-15-0663
9. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol Off J Am Soc Clin Oncol. (2020) 38:2993–3002. doi: 10.1200/JCO.19.03345
10. Loo S, Roberts AW, Anstee NS, Kennedy GA, He S, Schwarer AP, et al. Sorafenib plus intensive chemotherapy in newly diagnosed FLT3-ITD AML: a randomized, placebo-controlled study by the ALLG. Blood. (2023) 142:1960–71. doi: 10.1182/blood.2023020301
11. Mi R, Chen L, Yang H, Zhang Y, Liu J, Yin Q, et al. Combined use of interferon alpha-1b, interleukin-2, and thalidomide to reverse the AML1-ETO fusion gene in acute myeloid leukemia. Ann Hematol. (2021) 100:2593–601. doi: 10.1007/s00277-021-04621-w
12. Wei XD, Ai H, Mi RH, Chen L, Yuan FF, Hao QM, et al. Thalidomide combined with interferon and interleukin-2 in treatment of relapsed or refractory acute myelogenous leukemia. Zhonghua Nei Ke Za Zhi. (2016) 55:875–7. doi: 10.3760/cma.j.issn.0578-1426.2016.11.013
13. Bystrom R and Levis MJ. An update on FLT3 in acute myeloid leukemia: pathophysiology and therapeutic landscape. Curr Oncol Rep. (2023) 25:369–78. doi: 10.1007/s11912-023-01389-2
14. Lv K, Ren JG, Han X, Gui J, Gong C, and Tong W. Depalmitoylation rewires FLT3-ITD signaling and exacerbates leukemia progression. Blood. (2021) 138:2244–55. doi: 10.1182/blood.2021011582
15. Short NJ, Nguyen D, and Ravandi F. Treatment of older adults with FLT3-mutated AML: Emerging paradigms and the role of frontline FLT3 inhibitors. Blood Cancer J. (2023) 13:142. doi: 10.1038/s41408-023-00911-w
16. Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib combined with 5-azacytidine in older patients with untreated FLT3-ITD mutated acute myeloid leukemia. Am J Hematol. (2018) 93:1136–41. doi: 10.1002/ajh.25198
17. Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. (2007) 21:439–45. doi: 10.1038/sj.leu.2404508
18. Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. (2018) 24:282–91. doi: 10.1038/nm.4484
19. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. (2020) 21:1201–12. doi: 10.1016/S1470-2045(20)30455-1
20. Xu H, Mi R, Fan R, Yin Q, and Wei X. Effects of thalidomide combined with interferon on inhibiting kasumi-1 cell proliferation. Adv Clin Exp medicine: Off Organ Wroclaw Med University. (2016) 25:403–8. doi: 10.17219/acem/41048
21. Montesinos P, Buccisano F, Cluzeau T, Vennström L, and Heuser M. Relapse prevention in acute myeloid leukemia: the role of immunotherapy with histamine dihydrochloride and low-dose interleukin-2. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16101824
22. Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C, et al. Maintenance therapy with interleukin-2 for childhood AML: results of ELAM02 phase III randomized trial. HemaSphere. (2018) 2:e159. doi: 10.1097/HS9.0000000000000159
23. Chen L, Li GP, Mi RH, Yuan FF, Ai H, Wang Q, et al. Combination of interferon alpha-1b, interleukin-2 and thalidomide as maintenance therapy on acute myeloid leukemia patients with negative minimal residual disease. Zhonghua Xue Ye Xue Za Zhi. (2020) 41:766–9. doi: 10.3760/cma.j.issn.0253-2727.2020.09.011
Keywords: sorafenib, interferon α-1b, interleukin-2, thalidomide, FLT3-ITD, acute myeloid leukemia
Citation: Cheng C, Mi R, Li D, Chen L and Wei X (2025) Retrospective analysis of sorafenib combined with interferon α-1b, interleukin-2, and thalidomide as maintenance therapy in FLT3-ITD-positive acute myeloid leukemia. Front. Oncol. 15:1698935. doi: 10.3389/fonc.2025.1698935
Received: 04 September 2025; Accepted: 29 October 2025;
Published: 12 November 2025.
Edited by:
Massimo Martino, Bianchi Melacrino Morelli Great Metropolitan Hospital, ItalyReviewed by:
Yu Yu, Nanjing Medical University, ChinaCaterina Alati, Eugenio Morelli Hospital, Italy
Copyright © 2025 Cheng, Mi, Li, Chen and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Wei, eHVkb25nd2VpQHp6dS5lZHUuY24=
 Cheng Cheng
Cheng Cheng Ruihua Mi
Ruihua Mi Dongbei Li
Dongbei Li Lin Chen1
Lin Chen1 Xudong Wei
Xudong Wei