- Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Angioimmunoblastic T cell lymphoma (AITL) is classified as a nodal T-cell lymphoma from T follicular helper(Tfh) cells. Dysfunctional Tfh cells can lead to abnormal B cell regulation and plasma cell differentiation in AITL. However, the coexistence of AITL with plasma cell tumors is exceedingly rare. Here, we report three patients diagnosed with AITL accompanied by monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), and multiple myeloma (MM). The clinicopathologic findings, together with a review of previously reported cases, provide insight into the possible biological relationship between AITL and plasma-cell neoplasms. Epstein–Barr virus (EBV) infection was detected in all cases, suggesting a potential—though unproven—association between EBV-driven immune dysregulation and plasma-cell proliferation. Case analysis showed that some monoclonal plasma cells originated from the interior of AITL, while others originated from the bone marrow. This indicated that the evolution of plasma cell tumors may arise from either the lymphoma microenvironment or the systemic immune status. In cases of coexistence of AITL and plasma cell tumors, the treatment containing anti-MM drugs such as lenalidomide was usually recommended and might bring therapeutic benefits. Careful identification of monoclonal plasma-cell populations is therefore important for accurate diagnosis and individualized management of AITL.
Introduction
Angioimmunoblastic T cell lymphoma (AITL) is classified as a nodal T-cell lymphoma with follicular helper T-cell (Tfh) phenotype, which is a unique subtype of peripheral T cell lymphoma(PTCL). AITL accounted for 15-20% of PTCL (1). This disease mainly affects the middle-aged and older population, with a median diagnosis age of about 65 years old. More than 75% of AITL patients were diagnosed in advanced stages and had a poor prognosis (2). AITL patients often tested positive for autoimmunity and presented a general increase in immunoglobulins (3).
The cell of origin for AITL lymphoma was the Tfh, which coincided with the autoimmune features and B-cell proliferation in AITL tissue. Recent studies have emphasized the close relationship between the AITL tumor microenvironment and the abnormal proliferation of Tfh cells. The characteristics of the AITL tumor microenvironment included the proliferation of high endothelial venules (HEVs), complex network structures of follicular dendritic cells (FDCs), and infiltration of various reactive cell types, including fibroblasts, germinal center B cells, and dendritic cells. The intense interaction between Tfh cells and germinal center B cells might lead to dysregulation of B cells, resulting in immune disorders such as autoimmune hemolytic anemia, plasma cell proliferation, or polyclonal hypergammaglobulinemia (4). The increased expression levels of inflammatory/anti-inflammatory cytokines and the characteristic vascular proliferation reflected the AITL enriching inflammation signature (5).
Chronic inflammation plays a crucial role in the development of tumors. In the AITL tumor microenvironment, the dysfunction of Tfh cells and the imbalance of cytokines led to the proliferation of B cells and plasma cells (4). Infiltrated B cells and plasma cells might develop into monoclonal B cells and plasma cells under DNA damage and successfully grow into tumors through immune escape mechanisms. The Epstein-Barr virus(EBV)-positive B cells were detected in 66–86% of patients with AITL, and a strong correlation between EBV-infected B cells and AITL pathogenesis was established (6). Previous reports showed 19 cases of B-cell non-Hodgkin lymphoma among 161 cases of AITL diagnosed between 1996 and 2005 (7). Cases of AITL with coexisting plasma cell tumors were rarely reported. The behavior of plasma cells, which ranged from reactive plasmacytosis to striking clonal proliferation, has not yet been deciphered in AITL (8).
We report 3 cases of AITL with coexisting plasma cell tumors, including Monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), and multiple myeloma (MM). These cases reflected the evolution of plasma cell clones and the possible association with EBV virus infection during the development of AITL. We completed a narrow literature review to gain a deeper understanding of the behavior of plasma cells in AITL.
Case presentation
Case 1
A 54-year-old male patient was admitted to the hematology department of our hospital (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology)because of relapsing fever and bilateral lower limb skin rash for three months in October 2021. The patient denied bone pain, night sweats, and significant weight loss. His past medical history included cervical lymphadenitis, which was cured after anti-infection treatment in April 2021. Physical examination revealed cervical, axillary, and inguinal lymphadenopathy, splenomegaly, and a bilateral lower extremity rash. The laboratory data showed anemia [91g/L (normal: 115–150g/L)], normal renal function with proteinuria, high serum lactic dehydrogenase [278 U/L, (normal: 120–250 U/L)], low serum albumin [28g/L, (normal: 40–55g/L)], abnormal erythrocyte sedimentation rate [25mm/H (normal: 0–20mm/H)]. The overall autoimmune workup revealed a high titer of antinuclear antibodies and low complement C3. Traditional detection of pathogenic microorganisms in the blood was negative, except for Epstein-Barr virus (EBV). Serological tests for EBV showed positive for viral capsid antigen (VCA) immunoglobulin G (IgG) and viral nuclear antigen (VNA) IgG antibodies, while negative for VCA-IgM. PCR detection showed an increase in the copy number of EBV in plasma and peripheral blood mononuclear cells (PBMC), and EBV sorting PCR showed EBV mainly infected T and B cells (Table 1). Monoclonal gamma globulin identification showed IgG-λ M protein. The quantitative results from serum protein electrophoresis showed that the absolute value of serum M protein was 2.2g/L (normal: 0g/L). The quantitative results from serum immunofixation electrophoresis showed normal levels of IgA [3.91g/L, (normal: 0.82–4.53g/L)], IgG [15.3g/L, (normal: 7.51–15.6g/L)], IgM [1.66g/L, (normal: 0.46–3.04g/L)] and abnormal levels of serum free light chain κ [138.6mg/L, (normal: 3.30–19.40mg/L)], serum free light chain λ[155.2mg/L, (normal: 5.71–26.30mg/L)], κ/λ[0.098, (normal: 0.26–1.65)]. The level of beta-2 microglobulin [10.83mg/L (normal: 0.8–2.2mg/L)] increased significantly. Then, bone marrow tests were carried out. The flow cytometric analysis of bone marrow revealed 0.88% mature T cells with abnormal phenotypes (CD45+、CD2+、CD5+、CD45RO+、CD200+、PD1hi+、CD4dim+、CD3-、CD7-、CD56-、CD8-、CD45RA-、CD57-、CD30-、TCRab- 、TCRrd- 、CD103-、CD25-、TRBC1-、CD10-、CD26-、Ki67 LI (about 25.6%). The flow cytometric analysis of bone marrow revealed a normal plasma cell phenotype. The bone marrow PCR assay, as described in the European BIOMED−2 collaborative study (9), indicated the presence of clonal rearrangements of TCR instead of Ig.
After anti-infective treatment, his fever remained. Positron emission tomography-computed tomography (PET-CT) was performed. PET-CT showed multiple enlarged hypermetabolic lymph nodes in bilateral neck (SUVmax, 3.6), bilateral supraclavicular region (SUVmax, 5.4), bilateral axillary (SUVmax, 5.0), mediastinum (SUVmax, 4.8), retroperitoneum (SUVmax, 4.4), bilateral iliac vessels (SUVmax, 3.7) and splenomegaly (SUVmax, 3.9). We performed a needle biopsy of the enlarged lymph node on the right supraclavicular region. The immunohistochemistry results showed CD3+、PD1+、BCL-6+、CD4+、CD8+、irregular proliferation of follicular dendritic cell (FDC) network defined by CD21/35 staining、CD30 (VENTANA)(+, positive cells/total cell count of approximately 5%, positive control+)、Ki67 LI:about 60%、EBER (CISH) (slightly+) (Figure 1). EBER(CISH) is intended to detect EBV RNA by chromogenic in situ hybridization. The tumor samples’ PCR assay (9) indicated the presence of clonal rearrangements of TCR. The pathological diagnosis was AITL. The patient was eventually diagnosed with AITL and Non-IgM MGUS based on diagnostic criteria (10) on 7 November 2021, and the patient received CHOP therapy. After two CHOP therapy courses, PCR detection showed that the copy number of EBV in plasma turned negative. Subsequently, the patient completed six cycles of CHOP therapy. The patient was followed up regularly every 1–3 months via outpatient clinic visits. Laboratory tests including serum M protein levels, EBV viral load, and imaging studies were performed at each visit. After six cycles of CHOP chemotherapy, the patient’s M protein level dropped to 0 g/L, and EBV DNA in plasma became undetectable. However, six months post-therapy, PET-CT revealed lymphadenopathy suggestive of relapse. The patient received GEMOX+selinexor regimen with disease progression, followed by PD-1 inhibitor combined with SMILE chemotherapy. Due to poor tolerance, the patient transitioned to traditional Chinese medicine and monthly PD-1 inhibitor therapy. As of December 2024, the patient remains under stable observation with signs of partial lymphoma shrinkage.
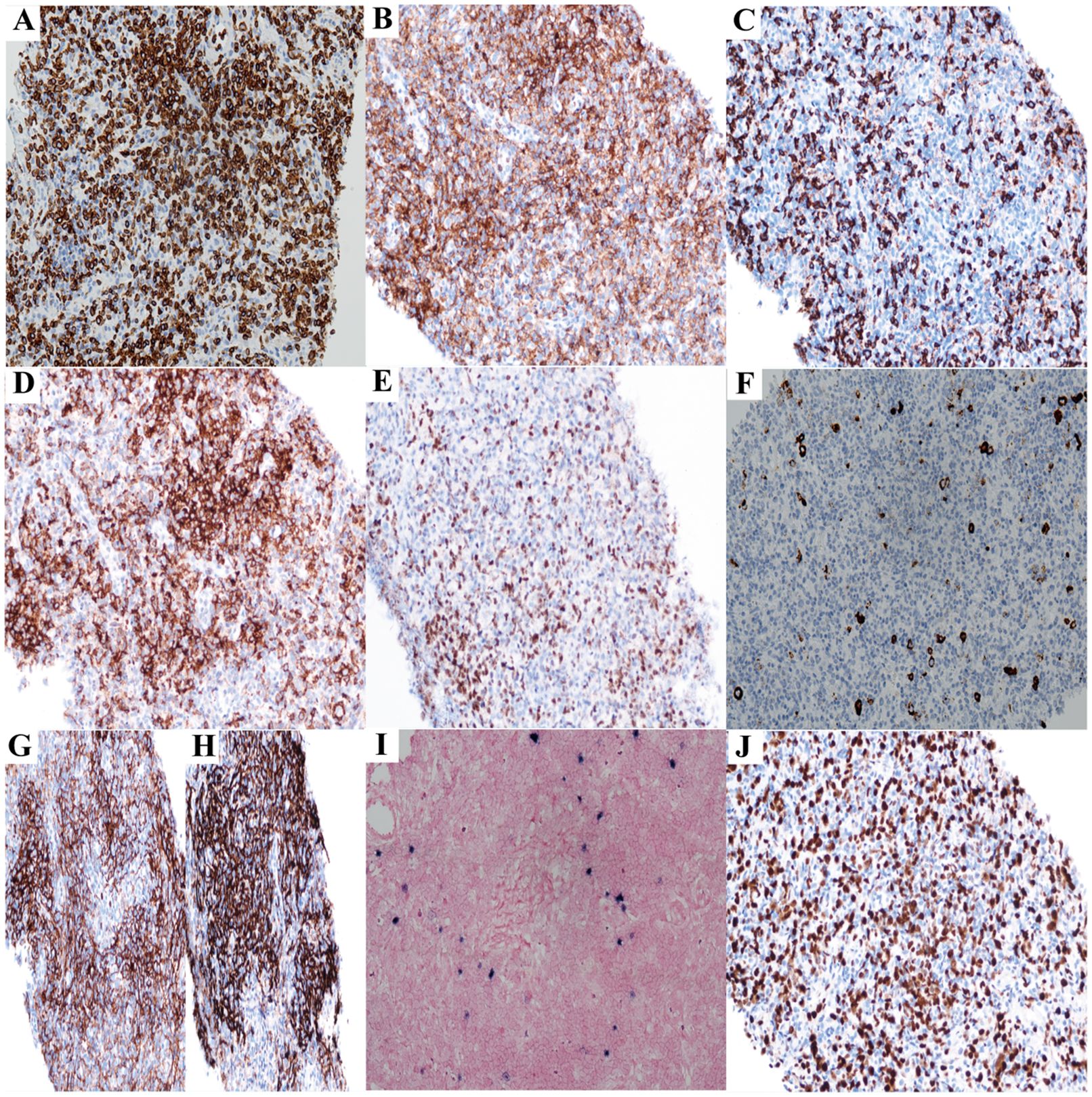
Figure 1. The immunohistochemistry results of lymph node biopsy (Case1) (All images original magnification: ×200). (A-E) Tumor cells show extensive positive expression of CD3 (A), CD4 (B), and PD1 (D); scattered cells express CD8 (C) and Bcl6 (E). (F) CD30-positive cells account for 5% of the total cells. (G, H) CD21 (H) and CD35 (G) reveal irregular proliferation of the FDC network. (I) EBER is expressed in a small number of cells. (J) The Ki67 proliferation rate is approximately 60%.
Case 2
A 60-year-old female patient was admitted to the otolaryngology department of our hospital(Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) because of a right cervical lymph node enlargement for two months in May 2023. The patient denied bone pain, fever, night sweats, and significant weight loss. Her past medical history included left upper lobe bronchiectasis, which was ineffective after anti-infection treatment in May 2021. The physical examination showed swollen lymph nodes in the right neck. The blood biochemistry and routine blood test showed normal, except for anemia [102g/L (normal: 115–150g/L)], increased globulin[63g/L(20-35g/L)] and decreased albumin[30.6g/L (40-55g/L)]. The overall autoimmune workup revealed normal results, except for the high titer of antinuclear antibodies. Serological tests for EBV showed positive for VCA-IgG and VNA-IgG antibodies while negative for VCA-IgM. EBV PCR detection showed an increase in the copy number of EBV in plasma and PBMC (Table 1). The overall autoimmune workup revealed a high titer of antinuclear antibodies. Monoclonal gamma globulin identification showed IgG-λ M protein. The quantitative results from serum protein electrophoresis showed that the absolute value of serum M protein was 43.8g/L (normal: 0g/L). The quantitative results from serum immunofixation electrophoresis showed normal levels of IgA [1.44g/L, (normal: 0.82–4.53g/L)], IgM [1.61g/L, (normal: 0.46–3.04g/L)], abnormal levels of IgG [44.3g/L, (normal: 7.51–15.6g/L)] and abnormal levels of serum free light chain κ [47.14mg/L, (normal: 3.30–19.40mg/L)], serum free light chain λ[272.97mg/L, (normal: 5.71–26.30mg/L)], κ/λ[0.173, (normal: 0.26–1.65)]. The level of beta-2 microglobulin [6.80mg/L (normal: 0.8–2.2mg/L)] increased significantly. Then, bone marrow tests were carried out. The flow cytometric analysis of bone marrow did not detect any abnormal T or plasma cell clones.
Chest and abdominal CT scans showed enlarged lymph nodes in the bilateral cervical, axillary, and mediastinum (Supplementary Figures 3A-D). We performed a needle biopsy of the enlarged lymph node on the right supraclavicular region. The immunohistochemistry results showed tumor cells CD3(+)、CD5(+)、CD43(+)、BCL2(Partial+)、BCL6(scattered weak+)、CD10 (–)、irregular proliferation of follicular dendritic cell (FDC) network defined by CD21/35 staining、CD20(-)、CD20(positive control+)、CD19(-)、CD22(-)、CD79a(-)、PCK(-)、P53 (scattered+, wild-type)、 CyclinD1(-)、IgD(-)、Ki-67 LI:50% in hot spot、plasma cells CD38(+)、CD138(+)、MUM1(+)、λ(+)>>κ(+, monoclonality)、EBER CISH(individual moderate cells+) (Figure 2). The tumor samples’ PCR assay (9) indicated the presence of clonal rearrangements of IgH and TCR. The pathological diagnosis was AITL and clonal plasma cell proliferation.
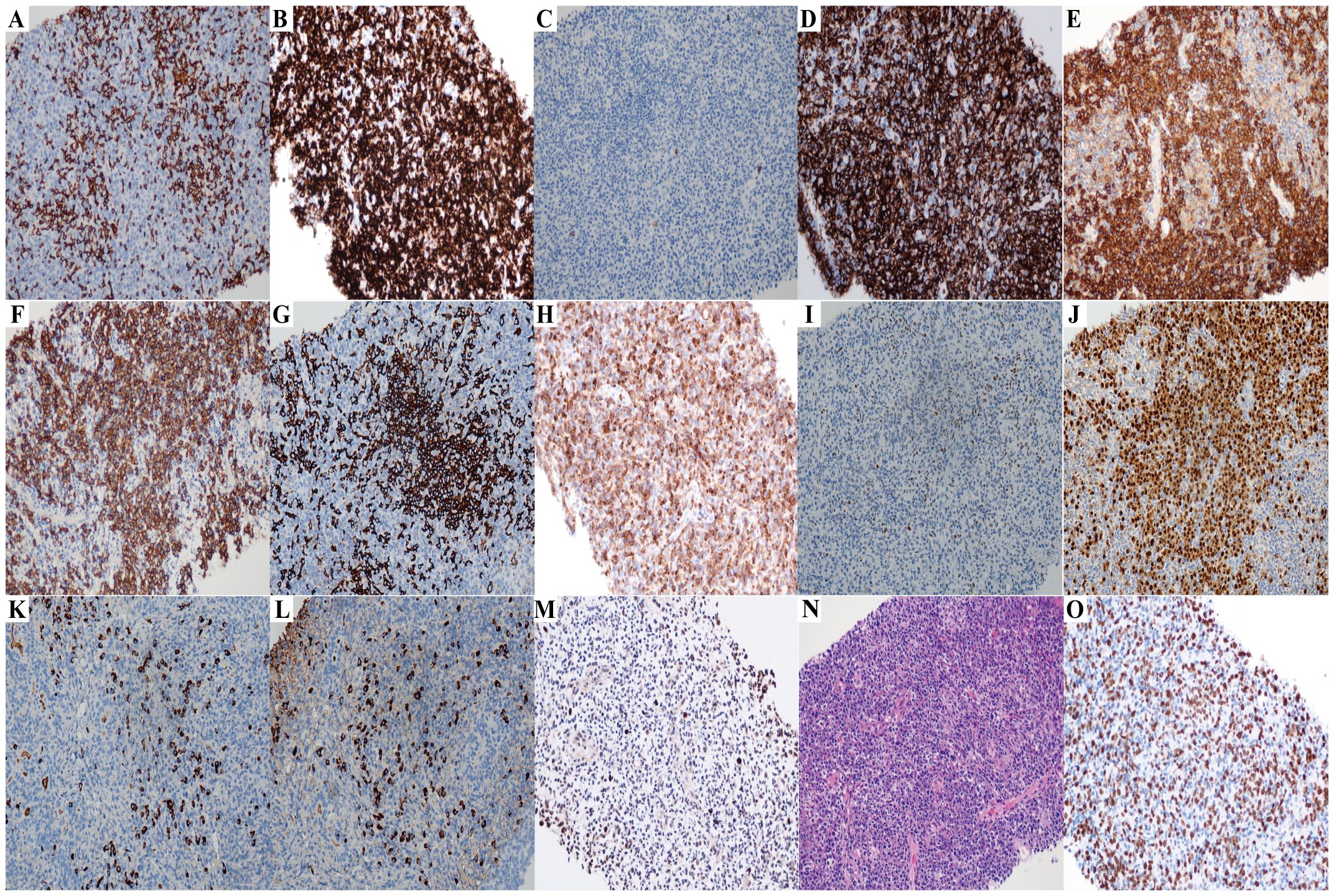
Figure 2. The immunohistochemistry results of lymph node biopsy (Case2) (All images original magnification: ×200). (A-D,G-I) Tumor cells commonly express CD3 (A), CD5 (B) and CD43 (D), while some also express BCL2 (H) and a few BCL6 (I); CD10 (C) and CD20 (G) are negative. (E, F, J) Plasma cell immunostaining: CD38 (E), CD138 (F), and MUM1 (J) are positive. (K, L) λ light chain (L) is significantly more abundant than κ light chain (K), indicating λ light chain restriction. (M) EBER is expressed in a small number of cells. (N) Hematoxylin and eosin staining reveals histological features of AITL. The lymph node architecture is effaced by a polymorphic infiltrate, with the prominent proliferation of arborizing high endothelial venules (HEVs), and perivascular aggregation of neoplastic clear cells is observed. There is a small amount of plasma cell distribution in it. (O) The Ki67 proliferation rate is approximately 50%.
The patient was eventually diagnosed with AITL and SMM based on diagnostic criteria (10) on 20 May 2023, and the patient received lenalidomide+COP therapy. She was lost to follow-up after one cycle of lenalidomide+COP therapy due to personal reasons. This precluded assessment of long-term treatment response, M-protein dynamics, and imaging changes. This represents a significant limitation in evaluating the efficacy of lenalidomide-based regimens in this rare composite lymphoma.
Case 3
A 75-year-old female patient was admitted to the otolaryngology department of our hospital(Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) because of a cervicalmass of one-week duration in September 2021. The patient denied bone pain, fever, night sweats, and significant weight loss. Her past medical history included an appendectomy due to appendicitis. The physical examination showed the cervical swollen lymph nodes and the right nasopharyngeal wall mass. The blood biochemistry and routine blood test showed normal. The patient underwent a nasopharyngeal mass biopsy under nasal endoscopy. The pathological diagnosis was atypical lymphocytic proliferation. One month later, the patient underwent cervical mass resection surgery again in the otolaryngology department due to enlargement of the cervical mass. The immunohistochemistry results showed tumor cells CD3(+)、CD5(+)、CD43(+)、LCA(+)、PD1(scattered weak+)、BCL6(scattered weak+)、CD4(scattered +)、C-MYC(+)、BCL2(+)、CD8 (–), plasma cells MUM(+)、λ(a little +)、κ(a little +), CD30 (approximately 5% moderate intensity+, positive control+)、GATA3 (slight+)、 P53 (scattered+, indicating wild-type)、 CD21 and CD23 (residual FDC network+)、CD35 (irregular proliferation of small focal FDC network+)、 CD20 (-, positive control+)、CD19(-)、PAX5(-)、CD10(-)、CyclinD1(-)、 PCK (-) Ki -67(LI about80%), EBER CISH (individual+, positive control+) (Figure 3). The tumor samples’ PCR assay [9] indicated the presence of clonal rearrangements of TCR. The patient was eventually diagnosed with AITL and admitted to the hematology department. The blood biochemistry and routine blood test showed normal, except for anemia [90g/L (normal: 115–150g/L)]. EBV PCR detection showed an increase in the copy number of EBV in PBMC, and EBV sorting PCR showed that EBV mainly infected B cells (Table 1). Monoclonal gamma globulin identification showed IgA-λ M protein. The quantitative results from serum protein electrophoresis showed that the absolute value of serum M protein was 11.8g/L (normal: 0g/L). The quantitative results from serum immunofixation electrophoresis showed elevated levels of IgA [14.9g/L, (normal: 0.82–4.53g/L)], decreased levels of IgM [0.43g/L, (normal: 0.46–3.04g/L)] and IgG [44.3g/L, (normal: 7.51–15.6g/L)], normal levels of serum free light chain κ [10.2 mg/L, (normal: 3.30–19.40mg/L)], serum free light chain λ[24.1mg/L, (normal: 5.71–26.30mg/L)], κ/λ[0.43, (normal: 0.26–1.65)]. Then, bone marrow tests were carried out. Bone marrow aspiration revealed 21% immature plasma cells. The flow cytometric analysis of bone marrow revealed abnormal plasma cells with phenotypic abnormalities (CD38dim+, CD138+, clambda +, CD45-, CD20-, CD19-, CD56-, ckappa-). The flow cytometric analysis of bone marrow also detected 0.02% abnormal T cell clones with phenotypic abnormalities (CD45+, CD4+, CD5bri+, CD45RO+, PD1bri, CD26bri, Ki69 9.4%, CD3-, CD7-, CD8-, CD45RA-, CD200-, CD57-, CD30-, TCRab-, TCRrd-, CD103-, CD25-, TRBC1-, CD10-) (Supplementary File 2).
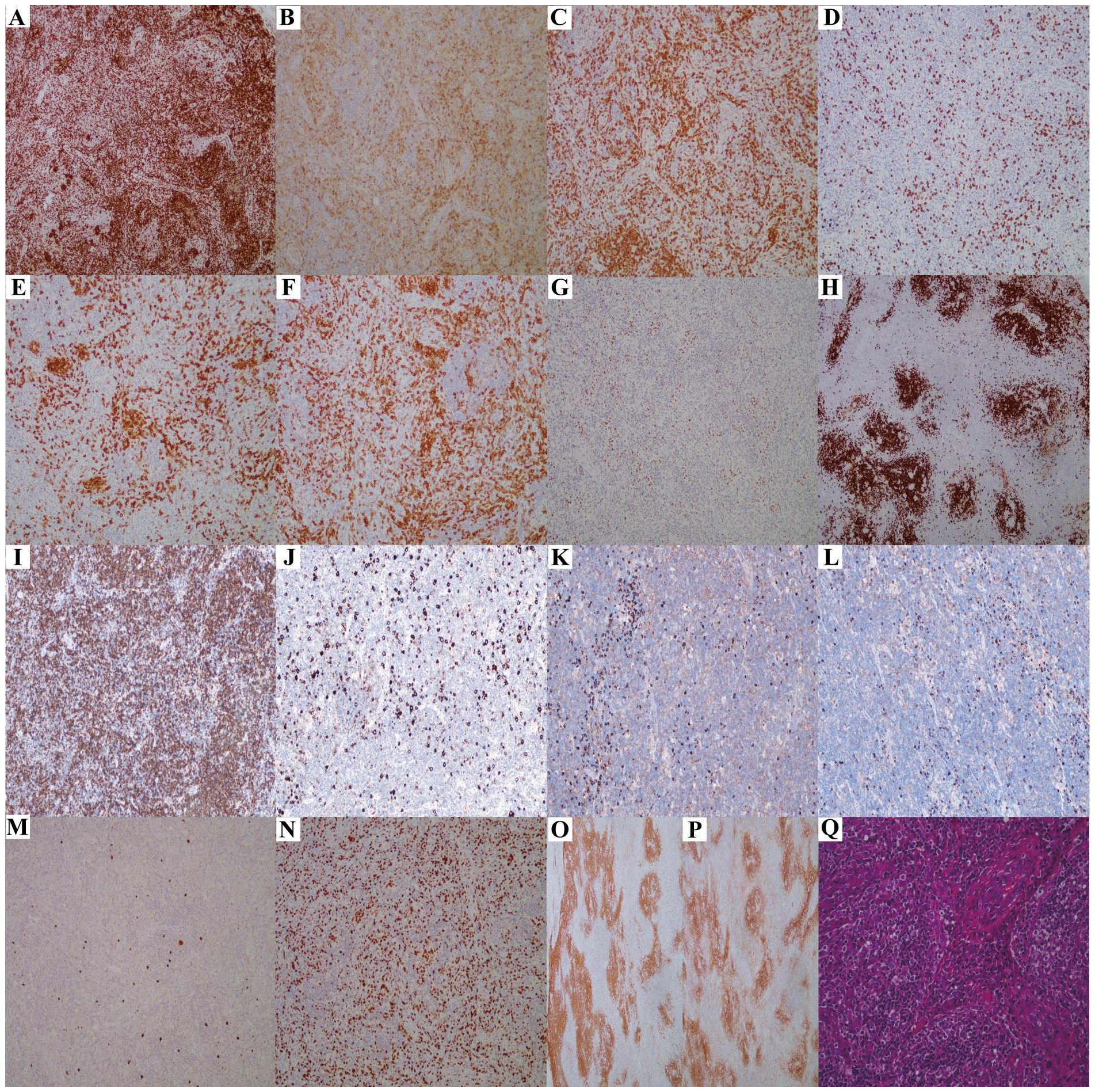
Figure 3. The immunohistochemistry results of lymph node biopsy (Case3). (A-I) Tumor cells show widespread expression of CD3 (A), CD5 (C), and Bcl2 (I); scattered positivity for CD4 (B), PD1 (F), and Bcl6 (G); and negativity for CD8 (D), CD10 (E), and CD20 (H) (×100 magnification). (J) CD30-positive cells represent 5% of the total cells(×100 magnification). (K, L) Plasma cells show slight positivity for lambda (K) and kappa (L), with no light chain restriction(×100 magnification). (M) EBER positivity is noted in individual cells(×100 magnification). (N) Ki67 indicates a high rate of cell proliferation, approximately 80%(×100 magnification). (O, P) CD21 (O) and CD35 (P) reveal irregular proliferation of the FDC network (×100 magnification). (Q) Hematoxylin and eosin staining reveals the histological characteristics of AITL, with a significant number of high endothelial venules (HEVs) proliferating (×200 magnification).
The results of bone marrow fluorescence in situ hybridization (FISH) showed that the patient had 1q21 amplification, IGH gene amplification, CCND gene amplification, and +17 chromosome (Supplementary Figure 2). The patient was eventually diagnosed with AITL and MM (10) on 19 November 2021. The patient received 5 courses of therapy (lenalidomide+CHOP). PET evaluation in April 2022 showed regression of lymphoma tumor. The quantitative results from serum protein electrophoresis showed the absolute value of serum M protein was 7.4g/L (normal: 0g/L). From May 2022, the patient received five courses of therapy (VRD). Bone marrow cytology and flow cytometry did not detect any abnormal T or plasma cell clones. The quantitative results from serum protein electrophoresis showed the absolute value of serum M protein was 0 g/L (normal: 0g/L). The results from serum immunofixation electrophoresis were normal. Patients took oral lenalidomide for maintenance treatment. As of December 2024, the patient is still alive.
Discussion
AITL is the nodal T-cell lymphoma originating from Tfh. The Tfh are a subset of CD4+ T cells specialized to regulate antibody responses. Tfh cells help B cells form germinal centers (GC) to differentiate into memory cells and plasma cells (antibody-secreting cells) as inflammatory responses (11). AITL tumor microenvironment might include abundant B cells and plasma cells (12), but plasma cells were usually polyclonal rather than monoclonal in a few cases (13). So, cases of AITL with coexisting plasma cell tumors were rarely reported.
MM is a common hematologic malignancy involving malignant plasma cells that can occur not only in the bone marrow but also in extramedullary tissue. MM is universally preceded by precursor stages clinically termed as MGUS and SMM (10). We reported three cases of AITL with coexisting plasma cell tumors. These cases were AITL combined with MGUS, AITL combined with SMM, and AITL combined with MM. All three cases were diagnosed almost simultaneously, and none of them used chemotherapeutic drugs before diagnosis. For case 1 of AITL with coexisting MGUS, there were no monoclonal plasma cells in the bone marrow and AITL samples, except for the M protein. MGUS did not receive more attention because of slow progression to MM and undamaged organs. The patient received CHOP regimens, which did not include common drugs for treating MM, such as bortezomib and lenalidomide. After treatment, M protein could be cleared. For case 2 of AITL with coexisting SMM, there were monoclonal plasma cells in AITL samples and M protein, but there were no monoclonal plasma cells in the bone marrow. The level of M protein far exceeded the diagnostic criteria for SMM (10). The patient was treated with a regimen containing lenalidomide. For case 3 of AITL with coexisting MM, there were 21% monoclonal plasma cells in the bone marrow and M protein, but there were no monoclonal plasma cells in AITL samples. The bone marrow fluorescence in situ hybridization (FISH) results showed that the patient had 1q21 amplification, IGH gene amplification, CCND gene amplification, and +17 chromosome (Supplementary Figure 2). The patient was also treated with a regimen containing lenalidomide and continued to receive lenalidomide maintenance after treatment.
EBV was associated with AITL in more than 80% of cases and was thought to promote immune escape and survival of infected AITL cells (14). EBV predominantly latently infects activated B cells in AITL tissues and exhibits latent and clonal proliferation (15). EBV latent membrane proteins (LMPs) may play pivotal roles in shaping the tumor microenvironment of AITL. LMP1 functions as a constitutive mimic of CD40 signaling, activating the NF-κB and JAK/STAT pathways, while LMP2A acts as a surrogate B-cell receptor (BCR) that provides chronic survival signals even in the absence of antigenic stimulation (16). Through these mechanisms, LMP2A simultaneously down-regulates MHC class II expression, enabling EBV-infected B cells to evade immune recognition (17). Together, LMP1 and LMP2A drive the secretion of IL-10 and other immunomodulatory cytokines, fostering an immunosuppressive milieu that supports plasma-cell differentiation and survival (18, 19). Persistent expression of these latent genes is closely associated with genomic instability and epigenetic dysregulation. EBV-encoded proteins can induce DNA-damage responses, impair DNA-repair pathways, and alter chromatin remodeling, thereby predisposing infected B cells to malignant transformation (19–21). Within the AITL microenvironment, EBV-positive plasma cells may thus represent a latency-driven proliferative compartment characterized by cumulative genetic and epigenetic insults. Importantly, EBV-encoded proteins further contribute to tumorigenesis by inducing mutations, disrupting cell-cycle regulation, and establishing aberrant methylation patterns that promote plasma-cell survival and expansion (20, 22, 23). The TET2 and DNMT3A gene, as common epigenetic regulators, often underwent mutations in both AITL and clonal hematopoiesis (CH) in the hematopoietic system, and TET2/DNMT3A mutations can also be detected in MM, albeit rarely (24–26). The same TET2 mutation was also detected in bystander B cells of AITL patients as in AITL tumor T cells, suggesting a possible shared clonal origin or cross-lineage evolution (27). Taken together, these findings suggest that EBV-driven genomic instability cooperates with pre-existing TET2/DNMT3A mutations to facilitate the emergence of monoclonal plasma-cell populations within AITL.
In addition to their intrinsic survival advantage, these EBV-positive B cells can present viral antigens to tumor Tfh cells via MHC class II molecules and provide co-stimulatory signals, thereby activating tumor T cells. Simultaneously, EBV-induced cytokine alterations and surface molecular changes inhibit CD8+ cytotoxic T cells, impairing the immune surveillance of EBV-positive B cells, allowing them to evade immune clearance and proliferate abnormally, often in a polyclonal manner (28). Through this reciprocal interaction, EBV-positive B cells and neoplastic T cells form a symbiotic interaction: EBV provides proliferation stimulation for B cells, while B cells provide antigens and co-stimulatory signals to TFH tumor cells, promoting tumor cell survival and proliferation. Over time, this bidirectional signaling may promote progressive immune dysregulation and chronic antigenic stimulation, setting the stage for monoclonal plasma-cell expansion and eventual plasma-cell neoplasm development. Indeed, different EBV subtypes appeared to be associated with post-diagnosis survival (15), suggesting biological heterogeneity in EBV-driven transformation pathways.
In the rare case of AITL combined with plasma cell tumors, EBV may influence the behavior of plasma cell clones indirectly. High levels of pro-inflammatory cytokines (e.g., IL-6, IL-21, etc.) can occur in AITL patients due to EBV-associated immune dysregulation (29). Among them, IL-6 from dendritic cells and macrophages serves as a pivotal factor in promoting plasma cell proliferation within the AITL microenvironment. The IL-6 not only stimulates the proliferation of Tfh tumor cells but also imparts pro-survival and anti-apoptotic signals to adjacent plasma cells, thereby promoting the clonal expansion of plasma cells (29). Mechanistically, IL-6 activates the STAT3 pathway, leading to upregulation of anti-apoptotic proteins such as BCL-xL and BCL-2 in neighboring plasma cells (30, 31). In addition, persistent IL-6/STAT3 signaling can aberrantly reactivate BCL6 expression in IL-6–dependent myeloma cells, fostering uncontrolled proliferation and treatment resistance (32, 33). Consequently, dysregulated IL-6/STAT3/BCL6 signaling—further amplified by EBV-induced inflammation—may represent a biological link between AITL and coexisting plasma-cell neoplasms, providing a rationale for the emergence of plasma-cell clones both within AITL lesions and in the bone marrow compartment.
Clinically, EBV involvement was evident in our cohort: there were not only positive EBER CISH in AITL samples but also positive EBV DNA load in the PBMC. In two cases of EBV sorting PCR test, B cells were the main cells infected by EBV. This suggested that the evolution of plasma cell tumors in the AITL environment may be driven by B cell-mediated mechanisms. However, our ability to assess the dynamic role of EBV infection was limited by the available data. EBV sorting PCR was performed only at initial diagnosis, and post-treatment B/T-cell infection ratios were not available because archived samples were insufficient for repeat analysis. This limitation underscores the need for future studies incorporating serial EBV quantification and cell-type–specific analyses to better clarify the temporal relationship between EBV reactivation and plasma-cell evolution in AITL. Notably, plasma EBV DNA was detectable in Case 1 but absent in Case 3. Case 1 relapsed after six months of CHOP chemotherapy and had multiple relapses, while Case 3 presented with persistent remission of AITL. These contrasting outcomes are consistent with prior studies reporting that elevated EBV DNA load may reflect chronic active infection and portend poorer prognosis in peripheral T-cell lymphomas (34). Moreover, in the study of the relationship between mature B cell neoplasms and the infection, Bosseboeuf A found that EBV was the most frequent initiating factor of plasma cell neoplasms (MGUS, SMM, MM) and EBV was more involved in the development of MGUS and SMM rather than MM (35, 36). Together, these molecular and clinical findings support a model in which EBV infection, through combined genetic, epigenetic, and immunologic mechanisms, contributes to plasma-cell tumor evolution within the AITL microenvironment. A comprehensive literature search was conducted using the PubMed database (from inception to December 2024) with the following search strategy: (“angioimmunoblastic T-cell lymphoma” OR “AITL”) AND (“plasma cell” OR “multiple myeloma” OR “MGUS” OR “plasmacytosis” OR “monoclonal gammopathy”). Reports written in English describing concurrent or sequential AITL and plasma-cell disorders were included. Through searching literature from the PubMed, we retrieved 10 cases of AITL with coexisting plasma cell tumors (37–45) (Table 2). All cases tested positive for EBERs, which supported the involvement of EBV in the MM evolution in AITL cases. Only 3 cases were diagnosed with MM combined with AITL (2 cases of MM, 1 case of SMM). The 7 cases were still on the way to MM. There were 4 cases of clonal plasma cell proliferation diagnosed in AITL tissues. The other three cases showed M protein with or without corresponding organ damage (MGUS, MGRS, POEMS). We analyzed these 10 retrieved cases in conjunction with the 3 cases in the text for commonalities and differences, The expression of CD138 was consistent in most cases; high percentage of light chain restriction of lambda chain and IgG type M proteins; EBERs were positive in some cases, indicating that EBV may play a certain role in pathological progression. EBV-positive patients may show more immune escape characteristics, such as increased expression of Bcl6 and PD1, indicating a limitation in the immune system’s ability to recognize and eliminate tumor cells. However, the clinical manifestations of these patients vary widely, such as the presence of B symptoms and concomitant skin rashes. The treatment strategies employed for the patients differed, and accordingly, their therapeutic responses exhibited variation. It might be related to the fact that the type of monoclonal plasma cells (e.g., IgG, IgA, IgM) and the expression of immunophenotypic markers (CD56, PD1, etc.) have an important impact on the clinical prognosis of the patients.
The literature search results showed numerous case reports of AITL accompanied by polyclonal plasmacytosis, and most of these cases tested negative for M protein. Two cases of AITL presenting with polyclonal plasmacytosis tested positive for clonal rearrangements of Ig in AITL samples (13, 46). We compared the retrieved case data with patient data from our own center (Supplementary Table 1), the PPCP group consisted of 12 cases from previous case reports (47–53). The median age of the overall patients was 69 years old, and the CPCP group showed a generally older age of the patients (median age of 70 years, range of 53–87 years old); In addition, the CPCP group had a lower LDH, with a median LDH of 310 IU/L (160–780 IU/L); The CPCP group showed significant light chain restriction (P<0.001), which is a typical hallmark of monoclonal proliferation, and leukocytosis (P = 0.005) were less frequent in the CPCP group as compared to the PPCP group; Although thrombocytopenia appeared more common in the PPCP group (75.0%) than in the CPCP group (25.0%), the difference did not reach statistical significance (p > 0.05), possibly due to the small sample size.
In terms of other clinical characteristics, the rates of gender, rash, hypoalbuminemia, bone marrow infiltration, and splenomegaly were relatively balanced between the two groups and did not show a significant differences.
The immunohistochemical expression heat map of these 25 patients is shown in (Supplementary Figure 1). Data analysis indicated that, when compared to the polyclonal plasma cell proliferation (PPCP) group, the clonal plasma cell proliferation (CPCP) group exhibited an older median age and a lower lactate dehydrogenase(LDH) level; however, these differences were not statistically significant (Supplementary Table 1). The PPCP group exhibited a higher incidence of thrombocytopenia (P>0.05), and leukocytosis (P = 0.005) compared to the CPCP group (Supplementary Table 1). This phenomenon appeared to be explained by the humoral immune abnormalities observed in AITL patients, which were attributed to polyclonal proliferation of plasma cells. In terms of other clinical characteristics, the rates of gender, rash, hypoalbuminemia, bone marrow infiltration, and splenomegaly were relatively balanced between the two groups and did not show differences. The analysis results revealed that light chain restriction constituted a significant differentiating characteristic between the clonal plasma cell proliferation (CPCP) group and the polyclonal plasma cell proliferation (PPCP) group (Supplementary Table 1). The restriction of light chains serves as a typical hallmark of monoclonal proliferation and also functions as a crucial indicator for identifying neoplastic plasma cells. Although the immunohistochemical analysis did not reveal any notable discrepancies, the findings suggested a tendency towards increased expression of CD56 and BCL6 in the CPCP group, when compared to the PPCP group. The immunohistochemical expression heat map of these 25 patients is shown in (Supplementary Figure 1). Early literature suggested that BCL6 was highly expressed in B cells, yet underwent a significant downregulation during the differentiation of normal B cells into plasma cells (54). In MM, BCL6 might underwent abnormal reactivation, particularly in specific subtypes such as those with low CD138 expression or IL-6-dependent cells, where heightened expression was intimately linked to tumor cell proliferation, survival, and resistance to treatment (32, 55, 56). Early studies further revealed that normal plasma cells did not express CD56, whereas malignant plasma cells demonstrate high expression of CD56, and this characteristic possessed diagnostic significance (57, 58). Our literature analysis indicated that, in addition to light chain restriction, the expression of BCL6 and CD56 also had certain significance in identifying the plasma cell characteristics within AITL tissues.
The tumor microenvironment should be an important factor in tumor immune escape and tumor occurrence. The tumor microenvironment of AITL might be an important factor leading to the malignant clonal transformation of plasma cells in AITL. Analyzing MM cases from our center and literature, we found two cases were diagnosed with MM because of monoclonal plasma cells inside the AITL. In comparison, the other three cases were diagnosed with MM because monoclonal plasma cells were detected inside the bone marrow instead of AITL. This indicated that the evolution of plasma cell tumors was not only related to the AITL microenvironment but also to the overall immune status of AITL patients.
Lenalidomide had multiple complex anti-tumor mechanisms, including anti-tumor cell proliferation, anti-angiogenesis, and immunomodulatory effects. Lenalidomide was not only the basic drug for plasma cell tumors but was also used for B-cell and T-cell lymphoma (59). Given the coexistence of AITL and plasma-cell neoplasms in our cases, lenalidomide was selected to leverage its dual therapeutic potential. Clinical data support the feasibility and activity of lenalidomide-containing regimens in AITL.
In a phase II trial of lenalidomide combined with CHOP in newly diagnosed AITL, the complete metabolic response (CMR) rate was 41% with a 2-year progression-free survival (PFS) of 42% (60). Likewise, a phase II study of CHOEP plus lenalidomide in PTCL, reported an overall response rate (ORR) of 69% and a 2-year PFS of 67% among AITL patients (61). In relapsed or refractory disease, single-agent lenalidomide demonstrated clinical activity with an ORR of about 30% (62, 63).
In contrast, bortezomib, a proteasome inhibitor, has been explored as a potential therapeutic addition to CHOP in PTCL.A multicenter phase II trial reported high initial response rates (ORR ~76%, complete remission ~65%),but long-term efficacy was limited, with a 3-year PFS of only approximately 35% (64). These findings suggest that while bortezomib-CHOP can induce rapid remissions, durability is suboptimal—likely because proteasome inhibition alone does not address the underlying IL-6/STAT3–driven survival pathways within the AITL microenvironment. In this context, lenalidomide’s established efficacy in plasma-cell disorders, together with its ability to modulate immune signaling, makes it a rational choice for composite disease. In our Case 3, lenalidomide plus CHOP achieved sustained remission, supporting this approach(Note: Case 2 data are limited by loss to follow-up). We acknowledge a theoretical risk that lenalidomide’s immune-stimulatory effects—through T-cell activation, restoration of immune-synapse function, and cytokine induction (e.g., IL-21)—could enhance Tfh-cell activity or modify the tumor microenvironment (65, 66). However, clinical data do not indicate disease acceleration in AITL, and no evidence supports that lenalidomide directly promotes Tfh-driven proliferation. Given this theoretical concern, careful monitoring of immune and viral parameters (e.g., EBV DNA load, serum M protein, and plasma-cell clonality) is recommended during treatment.
Although both lenalidomide- and bortezomib-based regimens may be considered depending on the dominant tumor component, lenalidomide-containing therapy currently appears the more balanced approach for concurrent AITL and plasma-cell neoplasms. Future studies integrating proteasome inhibitors or next-generation myeloma agents (e.g., anti-CD38 antibodies, bispecifics) may further optimize outcomes in such rare composite cases.
High-dose chemotherapy followed by hematopoietic stem-cell transplantation (HCT) remains a standard consolidation strategy for both AITL and MM in eligible patients; however, no specific guidelines exist for composite cases. In PTCL, retrospective studies support consolidative autologous HCT(ASCT) in first remission, demonstrating improved disease-free and overall survival compared with chemotherapy alone (67). Allogeneic HCT (allo-HCT)is generally reserved for relapsed or refractory cases due to its graft-versus-lymphoma potential, although it carries significant non-relapse mortality (67, 68). Similarly, in MM, ASCT remains the standard consolidation for fit patients, while allo-HCT is limited to clinical trials for high-risk or refractory disease.
For composite AITL with plasma-cell neoplasms, transplant decisions should be individualized. ASCT may be considered for eligible patients achieving remission of both components, while allo-HCT should be reserved for relapse or high-risk transformation after multidisciplinary evaluation. Early stem-cell collection is advisable, particularly in those exposed to lenalidomide, to avoid mobilization failure. Given the rarity of this dual pathology, there is a clear gap in standardized guidance. Prospective case reporting and registry studies are urgently needed to clarify optimal transplant timing and outcomes. In our cohort, none of the patients underwent HCT due to age or comorbidities, underscoring the unmet clinical need for evidence-based recommendations in this setting.
Conclusion
In conclusion, we report 3 cases of coexistence of AITL with plasma cell tumors, including MGUS, SMM, and MM. These cases, combined with literature, demonstrated the development process of plasma cell tumors during the pathological development of AITL. Our study highlights the importance of monoclonal identification of plasma cells for accurate diagnosis and treatment planning. Moreover, immunohistochemical profiling revealed distinct expression patterns—such as elevated PD1 and CD56, and altered BCL6 expression—in clonal cases, which may serve as useful biomarkers for early recognition of plasma cell neoplasm transformation in AITL patients.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Mac, version 29.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as medians with interquartile ranges (IQR) due to non-normal distribution. Categorical variables were expressed as counts and percentages. Comparisons between two independent groups were performed using the Mann–Whitney U test for continuous variables, and the Chi-square test or Fisher’s exact test for categorical variables, as appropriate. All P-values were two-sided, and statistical significance was defined as P < 0.05. The statistical test used for each comparison is specified in the corresponding table and figure legends.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethical Review Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YY: Data curation, Validation, Writing – original draft, Writing – review & editing. XB: Data curation, Writing – original draft. WH: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors thank the patient and his family for consenting to report the detailed information. The authors thank Professor Chunrui Li and Professor Donghua Zhang for treating these patients. The authors thank Professor Qilin Ao and Dong Kuang from the Department of Pathology for providing us with assistance.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1705496/full#supplementary-material
References
1. Rüdiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, et al. Peripheral T-cell lymphoma (Excluding anaplastic large-cell lymphoma): results from the non-hodgkin’s lymphoma classification project. Ann Oncol. (2002) 13:140–9. doi: 10.1093/annonc/mdf033
2. Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the groupe D’etude des lymphomes de L’adulte (Gela) trials. Blood. (2008) 111:4463–70. doi: 10.1182/blood-2007-08-105759
3. Lunning MA and Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. (2017) 129:1095–102. doi: 10.1182/blood-2016-09-692541
4. Mhaidly R, Krug A, Gaulard P, Lemonnier F, Ricci JE, and Verhoeyen E. New preclinical models for angioimmunoblastic T-cell lymphoma: filling the gap. Oncogenesis. (2020) 9:73. doi: 10.1038/s41389-020-00259-x
5. Carbone A, Tripodo C, Carlo-Stella C, Santoro A, and Gloghini A. The role of inflammation in lymphoma. Adv Exp Med Biol. (2014) 816:315–33. doi: 10.1007/978-3-0348-0837-8_12
6. Tokunaga T, Shimada K, Yamamoto K, Chihara D, Ichihashi T, Oshima R, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: A multicenter cooperative study in Japan. Blood. (2012) 119:2837–43. doi: 10.1182/blood-2011-08-374371
7. Willenbrock K, Bräuninger A, and Hansmann ML. Frequent occurrence of B-cell lymphomas in angioimmunoblastic T-cell lymphoma and proliferation of epstein-barr virus-infected cells in early cases. Br J Haematol. (2007) 138:733–9. doi: 10.1111/j.1365-2141.2007.06725.x
8. Gaulard P and de Leval L. The microenvironment in T-cell lymphomas: emerging themes. Semin Cancer Biol. (2014) 24:49–60. doi: 10.1016/j.semcancer.2013.11.004
9. van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of pcr primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the biomed-2 concerted action bmh4-ct98-3936. Leukemia. (2003) 17:2257–317. doi: 10.1038/sj.leu.2403202
10. Schmidt T and Callander N. Diagnosis and management of monoclonal gammopathy and smoldering multiple myeloma. J Natl Compr Canc Netw. (2020) 18:1720–9. doi: 10.6004/jnccn.2020.7660
11. Eivazi S, Bagheri S, Hashemzadeh MS, Ghalavand M, Qamsari ES, Dorostkar R, et al. Development of T follicular helper cells and their role in disease and immune system. BioMed Pharmacother. (2016) 84:1668–78. doi: 10.1016/j.biopha.2016.10.083
12. Marques-Piubelli ML, Amador C, and Vega F. Pathologic and molecular insights in nodal T-follicular helper cell lymphomas. Front Oncol. (2023) 13:1105651. doi: 10.3389/fonc.2023.1105651
13. Huppmann AR, Roullet MR, Raffeld M, and Jaffe ES. Angioimmunoblastic T-cell lymphoma partially obscured by an epstein-barr virus-negative clonal plasma cell proliferation. J Clin Oncol. (2013) 31:e28–30. doi: 10.1200/jco.2012.43.3797
14. Bayda N, Tilloy V, Chaunavel A, Bahri R, Halabi MA, Feuillard J, et al. Comprehensive epstein-barr virus transcriptome by rna-sequencing in angioimmunoblastic T cell lymphoma (Aitl) and other lymphomas. Cancers (Basel). Cancers (2021) 13:610. doi: 10.3390/cancers13040610
15. Bahri R, Boyer F, Halabi MA, Chaunavel A, Feuillard J, Jaccard A, et al. Epstein-barr virus (Ebv) is mostly latent and clonal in angioimmunoblastic T cell lymphoma (Aitl). Cancers (Basel). (2022) 14:2899. doi: 10.3390/cancers14122899
16. Šimičić P, Batović M, Stojanović Marković A, and Židovec-Lepej S. Deciphering the role of epstein-Barr virus latent membrane protein 1 in immune modulation: A multifaced signalling perspective. Viruses. (2024) 16:564. doi: 10.3390/v16040564
17. Lin JH, Lin JY, Chou YC, Chen MR, Yeh TH, Lin CW, et al. Epstein-barr virus lmp2a suppresses mhc class ii expression by regulating the B-cell transcription factors E47 and pu. 1. Blood. (2015) 125:2228–38. doi: 10.1182/blood-2014-08-594689
18. Incrocci R, Barse L, Stone A, Vagvala S, Montesano M, Subramaniam V, et al. Epstein-barr virus latent membrane protein 2a (Lmp2a) enhances il-10 production through the activation of bruton’s tyrosine kinase and stat3. Virology. (2017) 500:96–102. doi: 10.1016/j.virol.2016.10.015
19. Lambert SL and Martinez OM. Latent membrane protein 1 of ebv activates phosphatidylinositol 3-kinase to induce production of il-10. J Immunol. (2007) 179:8225–34. doi: 10.4049/jimmunol.179.12.8225
20. Chen YR, Liu MT, Chang YT, Wu CC, Hu CY, and Chen JY. Epstein-barr virus latent membrane protein 1 represses DNA repair through the pi3k/akt/foxo3a pathway in human epithelial cells. J Virol. (2008) 82:8124–37. doi: 10.1128/jvi.00430-08
21. Dawson CW, Tramountanis G, Eliopoulos AG, and Young LS. Epstein-barr virus latent membrane protein 1 (Lmp1) activates the phosphatidylinositol 3-kinase/akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem. (2003) 278:3694–704. doi: 10.1074/jbc.M209840200
22. Parker GA, Touitou R, and Allday MJ. Epstein-barr virus ebna3c can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. (2000) 19:700–9. doi: 10.1038/sj.onc.1203327
23. Scott RS. Epstein-barr virus: A master epigenetic manipulator. Curr Opin Virol. (2017) 26:74–80. doi: 10.1016/j.coviro.2017.07.017
24. Jeon K, Kim HJ, Kim M, and Lee YK. A rare episode of extensive bone marrow necrosis in a chemotherapy-naïve patient with multiple myeloma exhibiting tp53 and tet2 mutations. Ann Clin Lab Sci. (2020) 50:136–9.
25. Chen Y, Zhang FH, Zhou M, Hou CX, Xu CL, Wang Q, et al. Effective treatment with venetoclax and hypomethylating agents for the coexistence of multiple myeloma and acute myeloid leukemia: A case report and literature review. Ann Clin Lab Sci. (2024) 54:553–7.
26. Lewis NE, Petrova-Drus K, Huet S, Epstein-Peterson ZD, Gao Q, Sigler AE, et al. Clonal hematopoiesis in angioimmunoblastic T-cell lymphoma with divergent evolution to myeloid neoplasms. Blood Adv. (2020) 4:2261–71. doi: 10.1182/bloodadvances.2020001636
27. Hu L, Zhang X, Li H, Lin S, and Zang S. Targeting tet2 as a therapeutic approach for angioimmunoblastic T cell lymphoma. Cancers (Basel). (2022) 14:5699. doi: 10.3390/cancers14225699
28. Li Y, Gao X, Kong LZ, and Li J. Misdiagnosis of angioimmunoblastic T−Cell lymphoma: A case report. Oncol Lett. (2023) 25:250. doi: 10.3892/ol.2023.13836
29. Lage L, Culler HF, Reichert CO, da Siqueira SAC, and Pereira J. Angioimmunoblastic T-cell lymphoma and correlated neoplasms with T-cell follicular helper phenotype: from molecular mechanisms to therapeutic advances. Front Oncol. (2023) 13:1177590. doi: 10.3389/fonc.2023.1177590
30. Chong PSY, Zhou J, Lim JSL, Hee YT, Chooi JY, Chung TH, et al. Il6 promotes a stat3-prl3 feedforward loop via shp2 repression in multiple myeloma. Cancer Res. (2019) 79:4679–88. doi: 10.1158/0008-5472.Can-19-0343
31. Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, Henze C, Löffler D, Koczan D, et al. Interleukin-6-dependent gene expression profiles in multiple myeloma ina-6 cells reveal a bcl-2 family-independent survival pathway closely associated with stat3 activation. Blood. (2004) 103:242–51. doi: 10.1182/blood-2003-04-1048
32. Tsuyama N, Danjoh I, Otsuyama K, Obata M, Tahara H, Ohta T, et al. Il-6-induced bcl6 variant 2 supports il-6-dependent myeloma cell proliferation and survival through stat3. Biochem Biophys Res Commun. (2005) 337:201–8. doi: 10.1016/j.bbrc.2005.09.036
33. Forster S, Radpour R, and Ochsenbein AF. Molecular and immunological mechanisms of clonal evolution in multiple myeloma. Front Immunol. (2023) 14:1243997. doi: 10.3389/fimmu.2023.1243997
34. Chen J, Zhou J, Cheng F, Chen D, Guan F, Zhang E, et al. Role of plasma ebv-DNA load and eber status on newly diagnosed peripheral T-cell lymphoma. J Cancer Res Clin Oncol. (2024) 150:181. doi: 10.1007/s00432-024-05702-9
35. Bosseboeuf A, Feron D, Tallet A, Rossi C, Charlier C, Garderet L, et al. Monoclonal igg in mgus and multiple myeloma targets infectious pathogens. JCI Insight. (2017) 2:e95367. doi: 10.1172/jci.insight.95367
36. Bosseboeuf A, Mennesson N, Allain-Maillet S, Tallet A, Piver E, Decaux O, et al. Characteristics of mgus and multiple myeloma according to the target of monoclonal immunoglobulins, glucosylsphingosine, or epstein-barr virus ebna-1. Cancers (Basel). (2020) 12:1254. doi: 10.3390/cancers12051254
37. Chan TSY, Ip AHW, Au-Yeung R, Pang AWK, and Kwong YL. Unique evolution of angioimmunoblastic T cell lymphoma to epstein-barr virus-positive plasma cell myeloma. Ann Hematol. (2020) 99:2949–52. doi: 10.1007/s00277-020-04110-6
38. Xu J, Tang Y, Zhao S, Zhang W, Xiu Y, Liu T, et al. Angioimmunoblastic T-cell lymphoma with coexisting plasma cell myeloma: A case report and review of the literature. Tohoku J Exp Med. (2015) 235:283–8. doi: 10.1620/tjem.235.283
39. Kishimoto W, Takiuchi Y, Nakae Y, Tabata S, Fukunaga A, Matsuzaki N, et al. A case of aitl complicated by ebv-positive B cell and monoclonal plasma cell proliferation and effectively treated with lenalidomide. Int J Hematol. (2019) 109:499–504. doi: 10.1007/s12185-018-02587-6
40. Morita K, Matsumura Y, Kudo H, Fujii K, Tachibana T, Ohta K, et al. An autopsy case of angioimmunoblastic T-cell lymphoma with a high content of epithelioid cells in the lymph node: immunohistochemical and genomic analyses. J Dermatol. (1997) 24:642–8. doi: 10.1111/j.1346-8138.1997.tb02309.x
41. Zou F, Li Z, Ma JA, Qiu Z, Tang YF, and Zheng JY. T-cell lymphoma with poems syndrome. Oncol Lett. (2015) 9:1313–6. doi: 10.3892/ol.2014.2810
42. Miura N, Suzuki K, Yoshino M, Kitagawa W, Yamada H, Ohtani H, et al. Acute renal failure due to igm-lambda glomerular thrombi and mpgn-like lesions in a patient with angioimmunoblastic T-cell lymphoma. Am J Kidney Dis. (2006) 48:e3–9. doi: 10.1053/j.ajkd.2006.03.084
43. Suárez AE, Artiga MJ, Santonja C, Montes-Moreno S, De Pablo P, Requena L, et al. Angioimmunoblastic T-cell lymphoma with a clonal plasma cell proliferation that underwent immunoglobulin isotype switch in the skin, coinciding with cutaneous disease progression. J Cutan Pathol. (2016) 43:1203–10. doi: 10.1111/cup.12814
44. Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T, et al. Epstein-barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. (2002) 117:368–79. doi: 10.1309/6utx-gvc0-12nd-jjeu
45. Balagué O, Martínez A, Colomo L, Roselló E, Garcia A, Martínez-Bernal M, et al. Epstein-barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: A phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. (2007) 31:1310–22. doi: 10.1097/PAS.0b013e3180339f18
46. Adachi Y, Hino T, Ohsawa M, Ueki K, Murao T, Li M, et al. A case of cd10-negative angioimmunoblastic T cell lymphoma with leukemic change and increased plasma cells mimicking plasma cell leukemia: A case report. Oncol Lett. (2015) 10:1555–60. doi: 10.3892/ol.2015.3490
47. Sachdev R, Goel S, Gautam D, and Sood N. Angioimmunoblastic T-cell lymphoma presenting with extensive marrow plasmacytosis and hypergammaglobulinaemia: A diagnostic challenge. Pathology. (2018) 50:665–8. doi: 10.1016/j.pathol.2018.03.014
48. Mejia Saldarriaga M, Alhomoud M, Roboz G, Allan JN, Ruan J, Ouseph MM, et al. Angioimmunoblastic T-cell lymphoma presenting with severe plasmacytosis mimicking plasma cell leukemia. Am J Hematol. (2023) 98:1119–26. doi: 10.1002/ajh.26878
49. Ahsanuddin AN, Brynes RK, and Li S. Peripheral blood polyclonal plasmacytosis mimicking plasma cell leukemia in patients with angioimmunoblastic T-cell lymphoma: report of 3 cases and review of the literature. Int J Clin Exp Pathol. (2011) 4:416–20.
50. Yamane A, Awaya N, Shimizu T, Ikeda Y, and Okamoto S. Angioimmunoblastic T-cell lymphoma with polyclonal proliferation of plasma cells in peripheral blood and marrow. Acta Haematol. (2007) 117:74–7. doi: 10.1159/000096894
51. Sakai H, Tanaka H, Katsurada T, Yoshida Y, Okamoto E, and Ohno H. Angioimmunoblastic T-cell lymphoma initially presenting with replacement of bone marrow and peripheral plasmacytosis. Intern Med. (2007) 46:419–24. doi: 10.2169/internalmedicine.46.6121
52. Nagoshi H, Kuroda J, Kobayashi T, Maegawa S, Chinen Y, Kiyota M, et al. Clinical manifestation of angioimmunoblastic T-cell lymphoma with exuberant plasmacytosis. Int J Hematol. (2013) 98:366–74. doi: 10.1007/s12185-013-1411-z
53. Wang C, Mao X, Liu S, He C, Wang Y, Zhu L, et al. The early diagnostic dilemma in angioimmunoblastic T cell lymphoma with excessive plasma cells proliferation. Case Rep Med. (2021) 2021:9951122. doi: 10.1155/2021/9951122
54. Mirlekar B, Wang Y, Li S, Zhou M, Entwistle S, De Buysscher T, et al. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep Med. (2022) 3:100744. doi: 10.1016/j.xcrm.2022.100744
55. Kawano Y, Fujiwara S, Wada N, Izaki M, Yuki H, Okuno Y, et al. Multiple myeloma cells expressing low levels of cd138 have an immature phenotype and reduced sensitivity to lenalidomide. Int J Oncol. (2012) 41:876–84. doi: 10.3892/ijo.2012.1545
56. Tahara K, Takizawa M, Yamane A, Osaki Y, Ishizaki T, Mitsui T, et al. Overexpression of B-cell lymphoma 6 alters gene expression profile in a myeloma cell line and is associated with decreased DNA damage response. Cancer Sci. (2017) 108:1556–64. doi: 10.1111/cas.13283
57. Seegmiller AC, Xu Y, McKenna RW, and Karandikar NJ. Immunophenotypic differentiation between neoplastic plasma cells in mature B-cell lymphoma vs plasma cell myeloma. Am J Clin Pathol. (2007) 127:176–81. doi: 10.1309/5el22bh45phupm8p
58. Kaiser U, Auerbach B, and Oldenburg M. The neural cell adhesion molecule ncam in multiple myeloma. Leuk Lymphoma. (1996) 20:389–95. doi: 10.3109/10428199609052420
59. Tilly H, Morschhauser F, Casasnovas O, Molina TJ, Feugier P, Gouill SL, et al. Lenalidomide in combination with R-chop (R2-chop) as first-line treatment of patients with high tumour burden follicular lymphoma: A single-arm, open-label, phase 2 study. Lancet Haematol. (2018) 5:e403–e10. doi: 10.1016/s2352-3026(18)30131-5
60. Lemonnier F, Safar V, Beldi-Ferchiou A, Cottereau AS, Bachy E, Cartron G, et al. Integrative analysis of a phase 2 trial combining lenalidomide with chop in angioimmunoblastic T-cell lymphoma. Blood Adv. (2021) 5:539–48. doi: 10.1182/bloodadvances.2020003081
61. Stuver R, Horwitz SM, Advani RH, Vose JM, Lee HJ, Mehta-Shah N, et al. Final results of a phase ii study of choep plus lenalidomide as initial therapy for patients with stage ii-iv peripheral T-cell lymphoma. Br J Haematol. (2023) 202:525–9. doi: 10.1111/bjh.18885
62. Chaoui D, Bouallegue S, Arakelyan N, Genet P, Aljijakli A, and Sutton L. Bortezomib, lenalidomide and dexamethasone (Vrd) combination as salvage therapy in refractory angioimmunoblastic T cell lymphoma. Br J Haematol. (2014) 164:750–2. doi: 10.1111/bjh.12678
63. Morschhauser F, Fitoussi O, Haioun C, Thieblemont C, Quach H, Delarue R, et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-hodgkin lymphoma: the expect trial. Eur J Cancer. (2013) 49:2869–76. doi: 10.1016/j.ejca.2013.04.029
64. Kim SJ, Yoon DH, Kang HJ, Kim JS, Park SK, Kim HJ, et al. Bortezomib in combination with chop as first-line treatment for patients with stage iii/iv peripheral T-cell lymphomas: A multicentre, single-arm, phase 2 trial. Eur J Cancer. (2012) 48:3223–31. doi: 10.1016/j.ejca.2012.06.003
65. Ménard C, Rossille D, Dulong J, Nguyen TT, Papa I, Latour M, et al. Lenalidomide triggers T-cell effector functions in vivo in patients with follicular lymphoma. Blood Adv. (2021) 5:2063–74. doi: 10.1182/bloodadvances.2020003774
66. Browning RL, Byrd WH, Gupta N, Jones J, Mo X, Hertlein E, et al. Lenalidomide induces interleukin-21 production by T cells and enhances il21-mediated cytotoxicity in chronic lymphocytic leukemia B cells. Cancer Immunol Res. (2016) 4:698–707. doi: 10.1158/2326-6066.Cir-15-0291
67. Dreger P and Schmitz N. The role of stem cell transplant (Auto and allo) in ptcl and ctcl. Hematol Am Soc Hematol Educ Program. (2024) 2024:69–77. doi: 10.1182/hematology.2024000670
Keywords: angioimmunoblastic T cell lymphoma, plasma cell tumors, EBV, case report, lenalidomide
Citation: Yang Y, Bao X and Huang W (2025) Case Report: Angioimmunoblastic T-cell lymphoma with coexisting plasma cell tumors: three cases and review of the literature. Front. Oncol. 15:1705496. doi: 10.3389/fonc.2025.1705496
Received: 15 September 2025; Accepted: 06 November 2025;
Published: 21 November 2025.
Edited by:
Erel Joffe, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Fengbo Huang, Zhejiang University, ChinaGil Fridberg, Tel Aviv Sourasky Medical Center, Israel
Copyright © 2025 Yang, Bao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, Mjg5NjYwNzA1MUBxcS5jb20=
‡ORCID: Xingshuo Bao, orcid.org/0009-0003-3418-0804
 Yulin Yang
Yulin Yang Xingshuo Bao‡
Xingshuo Bao‡ Wei Huang
Wei Huang