- 1School of Clinical Medicine, Shandong Second Medical University, Weifang, China
- 2Department of Hematology, Linyi People’s Hospital, Shandong Second Medical University, Linyi, Shandong, China
- 3Spine surgery, Linyi People’s Hospital, Shandong Second Medical University, Linyi, Shandong, China
- 4Department of Pathology, Linyi People’s Hospital, Shandong Second Medical University, Linyi, Shandong, China
- 5Pharmaceutical laboratory, Department of Pharmacy, Linyi People’s Hospital, Shandong Second Medical University, Linyi, Shandong, China
Background: Fluid overload-associated large B-cell lymphoma (FO-LBCL) is an exceptionally rare lymphoma characterized by predominant involvement of serous body cavities—such as the pleura, peritoneum, and pericardium—in the absence of a solid tumor mass. Its low incidence and nonspecific clinical presentation, which often includes symptoms like dyspnea due to effusion, contribute to diagnostic challenges in early stages. This study aims to address current gaps in the understanding of FO-LBCL by reporting two new cases and reviewing the clinical features, treatment regimens, and outcomes of 57 documented patients. Furthermore, through a detailed analysis of FO-LBCL characteristics, this work discusses relevant differential diagnoses and potential treatment strategies.
Methods: A literature search of PubMed and Web of Science was performed using the following search queries: (1) “Fluid overload-associated large B-cell lymphoma” OR “FO-LBC”; (2) “Human herpesvirus 8-unrelated” AND “effusion lymphoma”; (3) “HHV8-unrelated” AND “effusion lymphoma”.
Results: This study included a total of 57 patients. Fluid accumulation most commonly affected the pleural cavity (84.2%), followed by the pericardial (31.6%) and peritoneal (21.1%) cavities. The predominant clinical manifestation was dyspnea (55.8%). Chemotherapy was the primary treatment modality (56.1%), with the R-CHOP regimen representing the most commonly administered protocol. CD20 expression was the most significant favorable prognostic factor (P = 6×10-7). Other factors associated with improved survival included the absence of CD138 expression (P = 0.0009), age ≥ 65 years (P = 0.0015), LDH ≤ 500 U/L (P = 0.0064), the presence of pleural effusion (P = 0.0099), and CD79a expression (P = 0.0411). Treatment with rituximabcontaining chemotherapy regimens was also a significant favorable factor (P = 0.0036).
Conclusions: FO-LBCL often presents with dyspnea caused by fluid effusion. Routine laboratory tests typically show no significant abnormalities, making timely pathological examination essential for a definitive diagnosis. Clinicians should enhance their understanding of FO-LBCL characteristics to improve early diagnostic accuracy. It is crucial to select appropriate treatment strategies based on prognostic factors.
1 Introduction
Fluid overload–associated large B-cell lymphoma (FO-LBCL) is a rare subtype of large B-cell lymphoma characterized by selective involvement of serous body cavities (pleura, pericardium, and peritoneum) without formation of a palpable solid mass. Current epidemiological estimates indicate that FO-LBCL accounts for <0.1% of lymphoid malignancies; among reported cases, approximately 60% have been described in Japanese patients. FO-LBCL is not associated with Kaposi sarcoma–associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) infection. Although numerous HHV-8–unrelated effusion lymphomas were previously reported, they were not clearly defined in early lymphoma classifications. HHV-8–positive effusion lymphoma was historically designated primary effusion lymphoma (PEL), while KSHV/HHV-8–negative effusion lymphomas were variously described as PEL variants, PEL-like lymphomas, or KSHV/HHV-8–unrelated B-cell proliferative disorders. FO-LBCL was formally recognized as a distinct entity within large B-cell lymphomas in the 2022 WHO 5th Edition Classification of Tumours of Haematolymphoid Tissues. Clinically, patients with FO-LBCL commonly present with dyspnea secondary to serous effusion, while routine laboratory investigations are frequently nondiagnostic, which complicates and delays definitive diagnosis. Here, we report two cases presenting with severe dyspnea attributable to pericardial and pleural effusions, respectively, and we review the clinical features, treatment regimens, and outcomes of 57 FO-LBCL patients to characterize disease manifestations and inform differential diagnosis and management strategies.
2 Materials and methods
2.1 Data retrieval and methodology
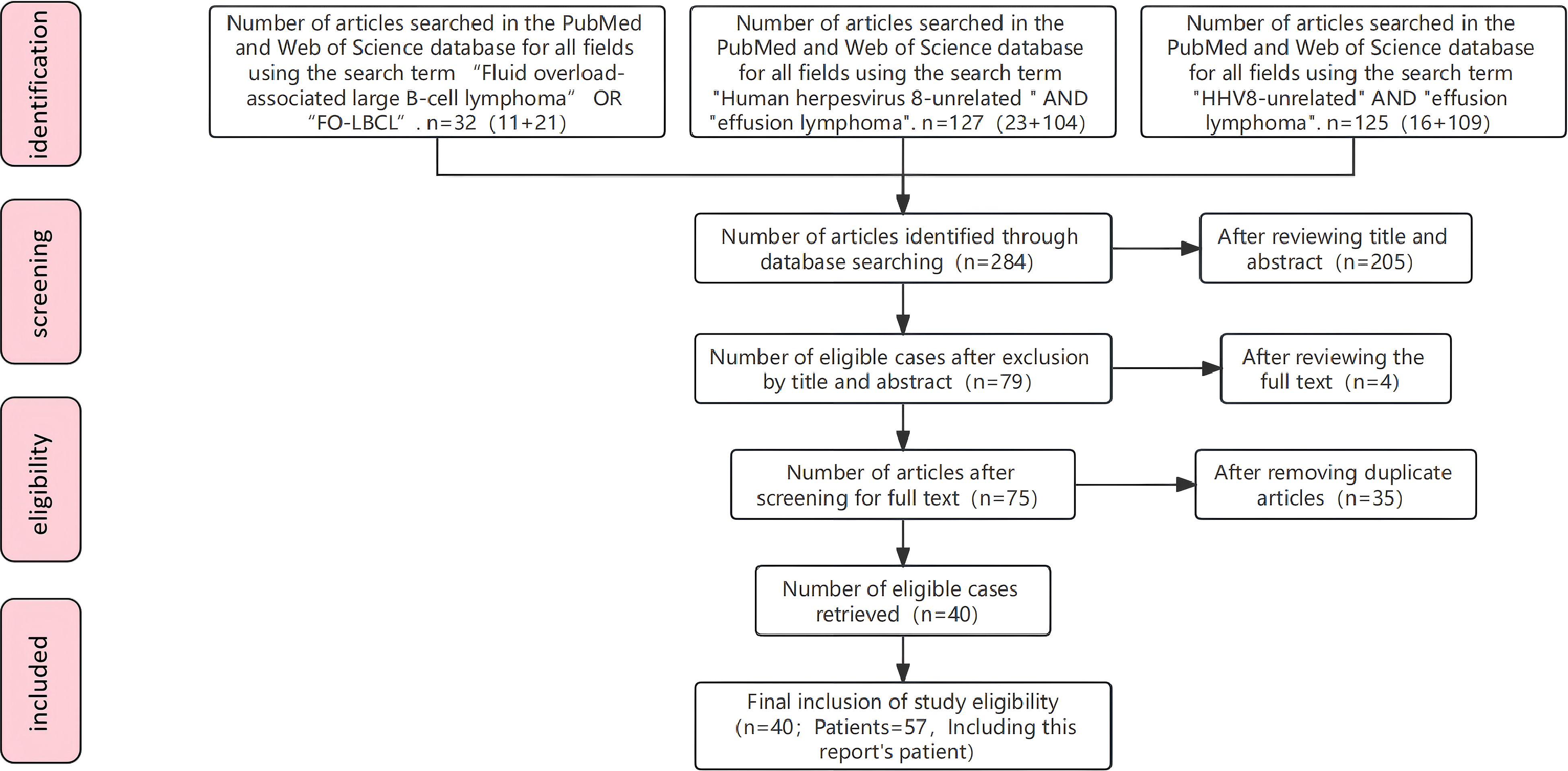
A literature search of PubMed and Web of Science was performed using the following search queries: (1) “Fluid overload-associated large B-cell lymphoma” OR “FO-LBC”; (2) “Human herpesvirus 8-unrelated” AND “effusion lymphoma”; (3) “HHV8-unrelated” AND “effusion lymphoma”. A total of 283 articles were retrieved. All titles, abstracts, and full texts were screened according to the “inclusion criteria” described below. Ultimately, 55 patients reported in 40 papers were included. Combined with the 2 patients reported in this study, a total of 57 patients were enrolled (Figure 1).
The inclusion criteria were as follows (1–3): (1) Patients in whom serous effusion (confirmed by paracentesis, thoracentesis, or pericardiocentesis) was the initial manifestation of disease, and who had no imaging or clinical evidence of lymphoma-related lymphadenopathy or concomitant solid tumors, were included; (2) The absence of HHV-8/KSHV infection can be confirmed through several methods, including immunohistochemistry for latent nuclear antigen-1, polymerase chain reaction (PCR) for viral DNA in effusion or tissue specimens, or in situ hybridization for viral genomic DNA within tumor cells. Alternatively, a negative status can be considered confirmed if explicitly stated in the original literature, even if the specific detection methodology is not detailed. (3) exclusion of alternative diagnoses, specifically primary effusion lymphoma, empyema-associated lymphoma, and diffuse large B-cell lymphoma presenting with malignant body-cavity effusion; (4) no restrictions on age or sex; and (5) for duplicated reports, only the earliest published case was included.
2.2 Statistical analysis
Statistical analyses were performed using SPSS version 27.0 and GraphPad Prism version 10.0. Continuous variables are reported as mean ± standard deviation or median (interquartile range), and categorical variables as counts and percentages. Overall survival (OS) was defined as the interval from diagnosis to death from any cause or to the date of last follow-up. For cases in which a definitive diagnosis at presentation was delayed because of insufficient diagnostic evidence, OS was calculated from the initiation of antitumor therapy. For patients who remained alive after multiple chemotherapy cycles but lacked a clearly recorded date of last follow-up, follow-up time was estimated from the number and type of chemotherapy cycles received. The estimation rules were: each cycle of rituximab monotherapy was counted as 1 week, and each cycle of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) or CHOP was counted as 3 weeks. Survival curves were generated using the Kaplan–Meier method and compared by the log-rank test. Cox proportional hazards regression was used to analyze overall survival (OS) and to identify independent prognostic factors.
3 Results
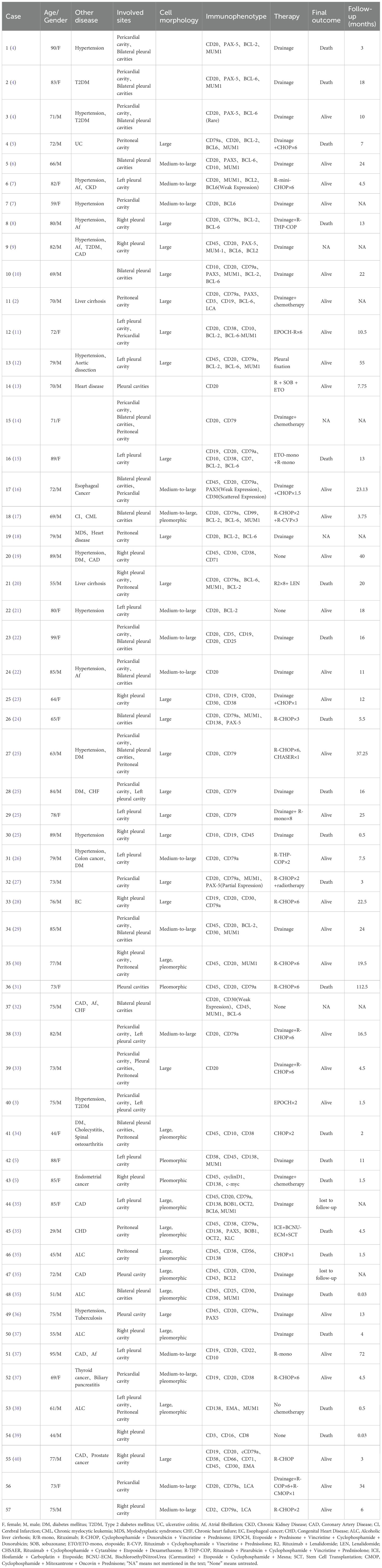
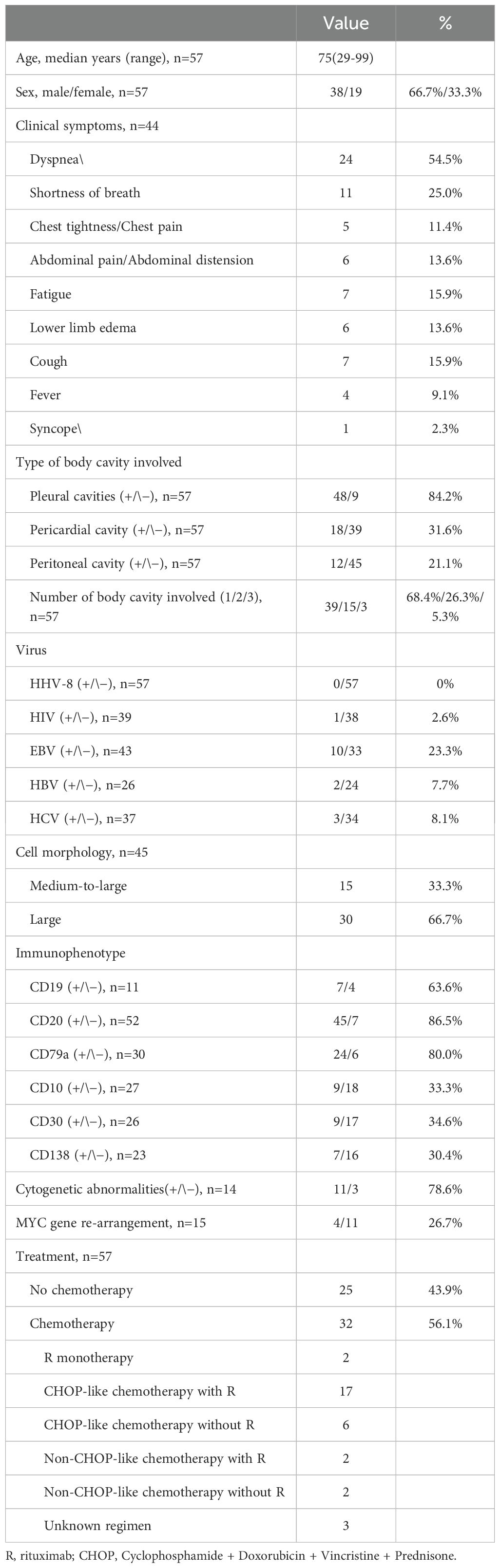
A total of 57 patients meeting the inclusion criteria was enrolled, with a male-to-female ratio of 2:1 (38 males, 66.7%; 19 females, 33.3%). The age range was 29 to 99 years, with a median age of 75. The pleural cavity was the most frequent site of fluid involvement (48/57, 84.2%), followed by the pericardial (18/57, 31.6%) and peritoneal (12/57, 21.1%) cavities. Consistent with the predominant pleural involvement, dyspnea was the most common presenting symptom, reported in 55.8% (24/43) of patients. Other reported symptoms included chest tightness, abdominal discomfort, fatigue, and lower limb edema, reflecting multisystem involvement. Most patients had comorbidities associated with chronic fluid overload, including liver cirrhosis, heart failure, and chronic kidney disease (Table 1). Consistent with prior literature, FO-LBCL occurred predominantly in HIV-negative individuals. The neoplastic cells typically expressed pan-B-cell markers and were negative for CD3, CD5, CD10, CD30, and CD138. Therapeutic data were available for all 57 patients. A total of 32 patients (56.1%) received chemotherapy, while the remaining 25 (43.9%) did not. Among the 32 patients who received chemotherapy, the majority (n=17) were treated with a rituximab-containing CHOP-like regimen. Other rituximab-based therapies were used in four patients (two with monotherapy and two with other regimens). The remaining eight patients received regimens without rituximab, comprising six who received a CHOP-like regimen and two on a non-CHOP-like regimen. Chemotherapy details were unavailable for three patients. Survival data were available for 49 patients, with a median overall survival of 10.5 months. The disease-related mortality rate was lower among patients who received chemotherapy (30.0%, 9/30) compared to those who did not (36.8%, 7/19) (Table 2).
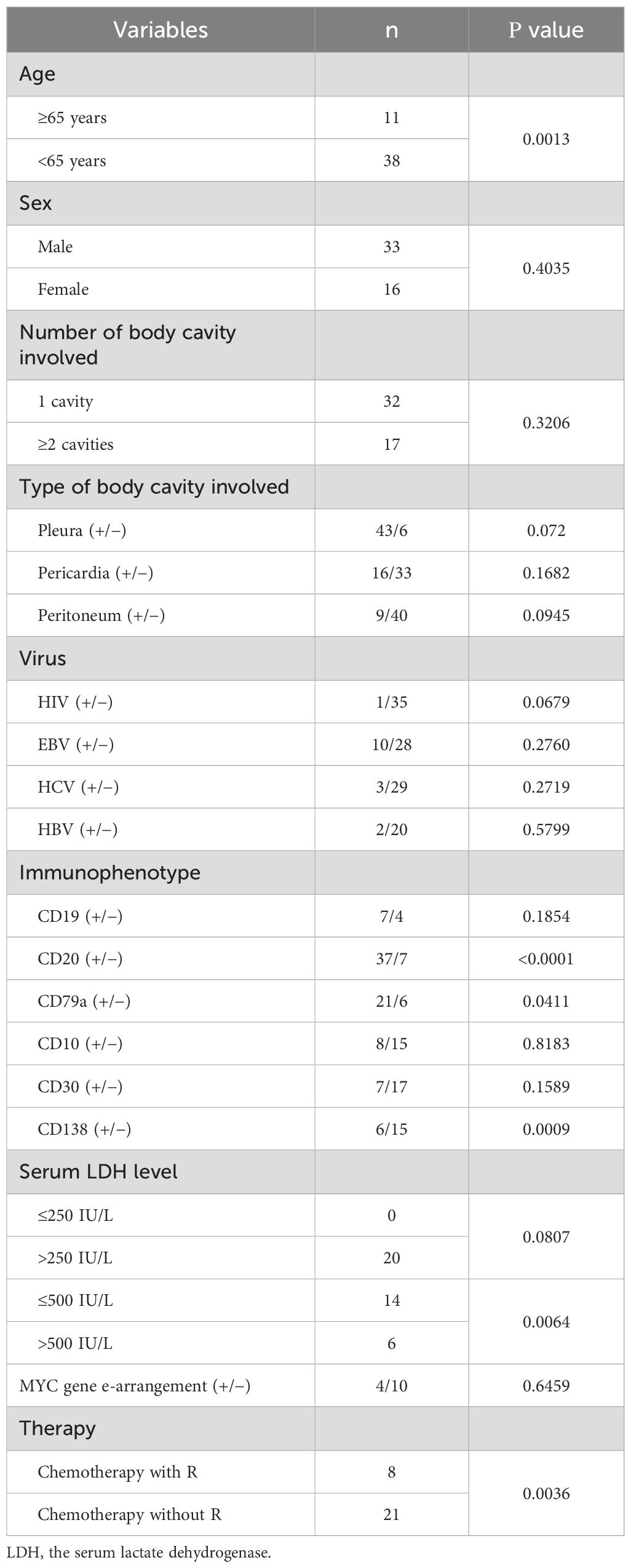
Univariate analysis of overall survival was performed on cases with complete prognostic and clinical data. CD20 expression was the most significant favorable prognostic factor (P = 6×10-7). Other factors associated with improved survival included the absence of CD138 expression (P = 0.0009), age ≥65 years (P = 0.0015), LDH ≤500 U/L (P = 0.0064), the presence of pleural effusion (P = 0.0099), and CD79a expression (P=0.0411). Treatment with rituximab-containing chemotherapy regimens was also a significant favorable factor (P=0.0036) (Table 3; Figure 2).
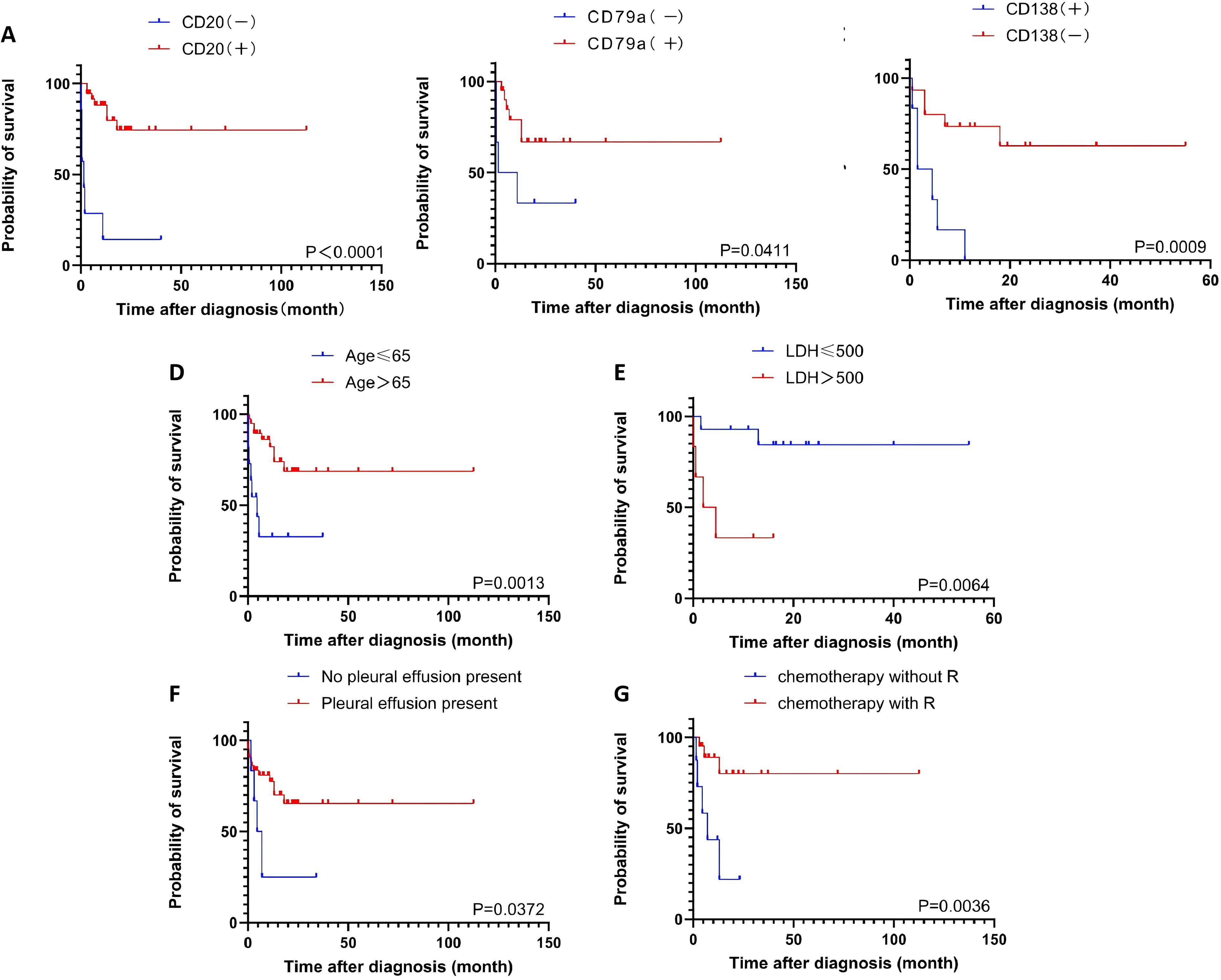
Figure 2. The overall survival according to the CD20 (A), the CD79a (B), the CD138 (C), the age (D), the LDH level (E), the pleural effusion (F), the chemotherapy regimen (G).
4 Case report
Case 1
A 73-year-old female presented in October 2022 with a ten-day history of exertional dyspnea. Her medical history was unremarkable. Laboratory investigations—including complete blood count, biochemistry, cardiac enzymes, coagulation studies, and serological tests for tuberculosis, EBV, cytomegalovirus, HHV-8, hepatitis B, hepatitis C, and HIV—were within normal limits. Cardiac ultrasonography revealed a moderate to massive pericardial effusion (Figure 3A). Pericardiocentesis was performed, draining approximately 900 ml of bloody fluid, and a cytological specimen was sent for pathological analysis. Microscopic examination revealed small, round tumor cells, suggestive of a hematolymphoid neoplasm of B-cell origin. Immunohistochemical analysis was positive for CD20, LCA, and CD79a, and negative for calretinin, Ber-EP4, cytokeratin (CK), CD3, and TTF-1. The Ki-67 (MIB-1) proliferation index was approximately 70% (Figure 4). A diagnosis of B-cell lymphoma was established, and the patient received one cycle of R-COP chemotherapy. A follow-up PET-CT scan on November 21, 2022, demonstrated diffusely heterogeneous increased FDG metabolism in the bone marrow (SUVmax 7.5), a finding suggestive of a myeloproliferative change, potentially treatment-related. No other foci of abnormal uptake indicative of lymphoma were identified. Following the initial cycle, the patient completed five additional cycles of R-COP chemotherapy, for a total of six cycles. Follow-up imaging with bilateral lung and whole-abdominal CT in October 2023 was unremarkable. A subsequent cardiac echocardiography and abdominal CT in June 2024 also showed no abnormalities (Figure 3B). Based on these findings, the patient was assessed as having achieved complete remission (CR).
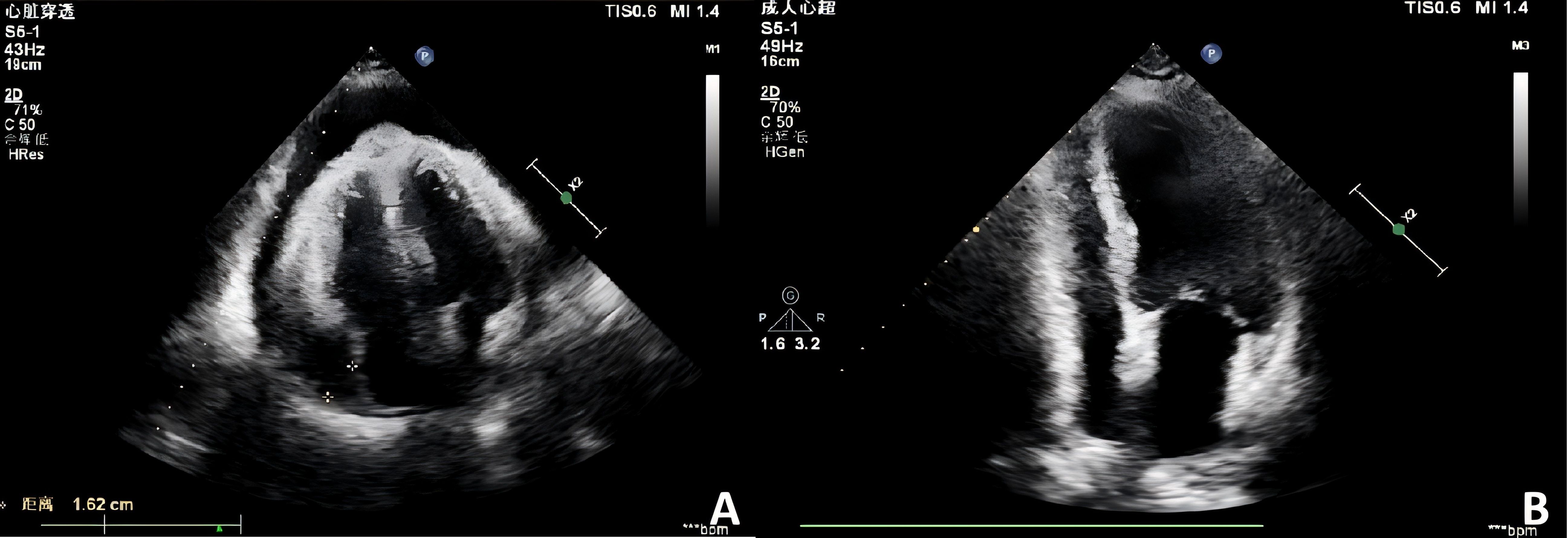
Figure 3. Echocardiographic assessment of pericardial effusion. (A) Initial echocardiogram (October 2022) reveals a moderate to severe pericardial effusion. (B) Follow-up study (June 2024) demonstrates complete resolution of the effusion.
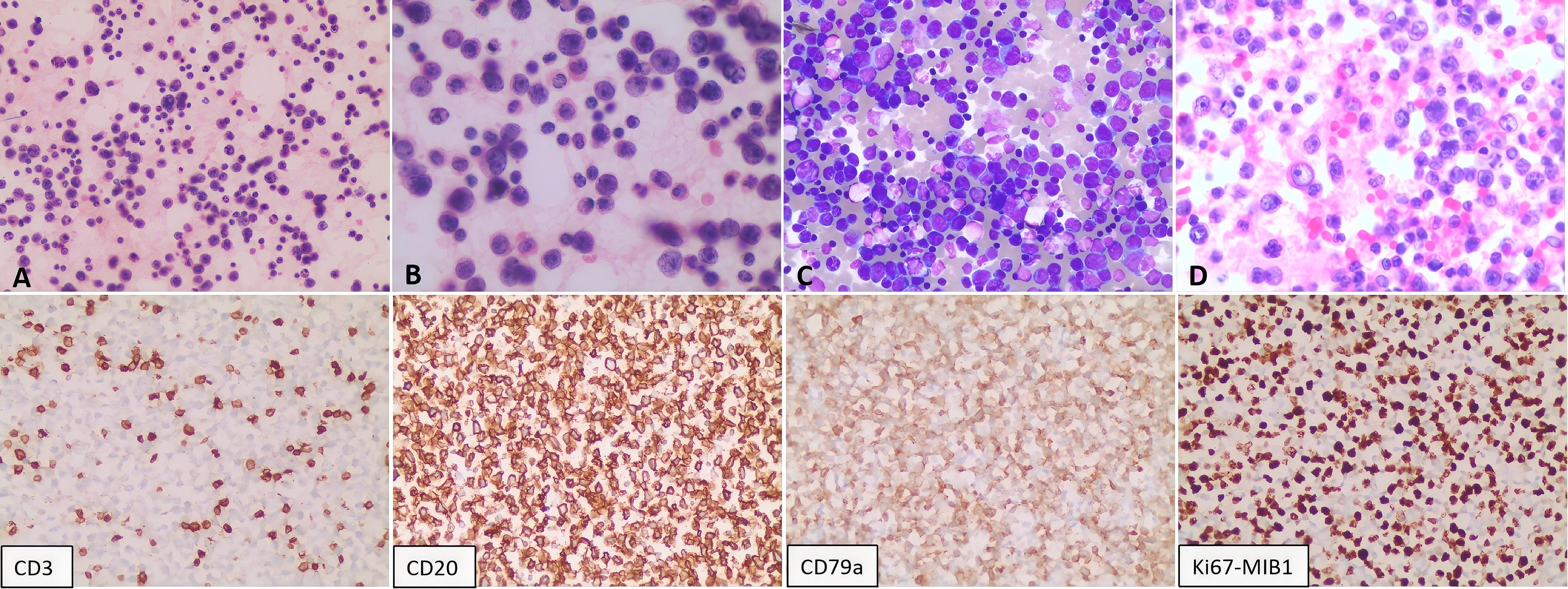
Figure 4. Pathological analysis of the pericardial effusion. Cytological smears show diffuse, scattered atypical lymphoid cells of moderate to large size. The cells exhibit round-to-oval nuclei, vacuolated chromatin, visible nucleoli, scant cytoplasm, and occasional mitotic figures. (A, B) Hematoxylin and eosin (H&E) staining at 200X and 400X magnification, respectively. (C) Giemsa stain (200X). (D) Cell block preparation from the sediment (H&E, 400X). Immunohistochemistry on the cell block specimen was positive for CD20 and CD79a, negative for CD3, and demonstrated a high proliferative index (Ki-67 approximately 70%).
However, in May 2025, the patient was re-admitted with a two-day history of dyspnea, accompanied by chest tightness, chest pain, and a productive cough with white sputum. She was febrile, with a maximum temperature of 38.0 °C. Laboratory investigations were notable only for mildly elevated inflammatory markers; complete blood count, biochemical panels, lactate dehydrogenase (LDH), and cardiac enzymes were otherwise within normal limits. An electrocardiogram revealed frequent ventricular premature beats in triplets, and cardiac echocardiography confirmed the recurrence of pericardial effusion. However, flow cytometry and histopathological analysis of the effusion were unremarkable. A follow-up PET-CT scan on May 29, 2025, was subsequently performed. Comparison with the scan from November 2022 demonstrated new pericardial thickening and effusion with associated increased FDG uptake showing peripheral enhancement (Figure 5). This new finding was highly suggestive of disease recurrence. No other foci indicative of lymphoma were identified. The initial pericardial effusion pathology specimen was subjected to an expert pathological consultation. The differential diagnosis included fluid overload-associated large B-cell lymphoma and diffuse large B-cell lymphoma, not otherwise specified (DLBCL-NOS). Immunohistochemical staining revealed a profile positive for PAX5, with partial positivity for BCL-2 and MUM1, and focal positivity for MYC and P53. The neoplastic cells were negative for CD5, CD10, CD138, and Cyclin D1. The disease was confined to the pericardial effusion, with no evidence of lymphoma involvement at other sites. A final diagnosis of FO-LBCL was established. Due to compromised cardiac function, the chemotherapy regimen was modified to R-CMOP. The patient has completed one cycle of this adjusted regimen and was alive and under ongoing follow-up as of August 2025.
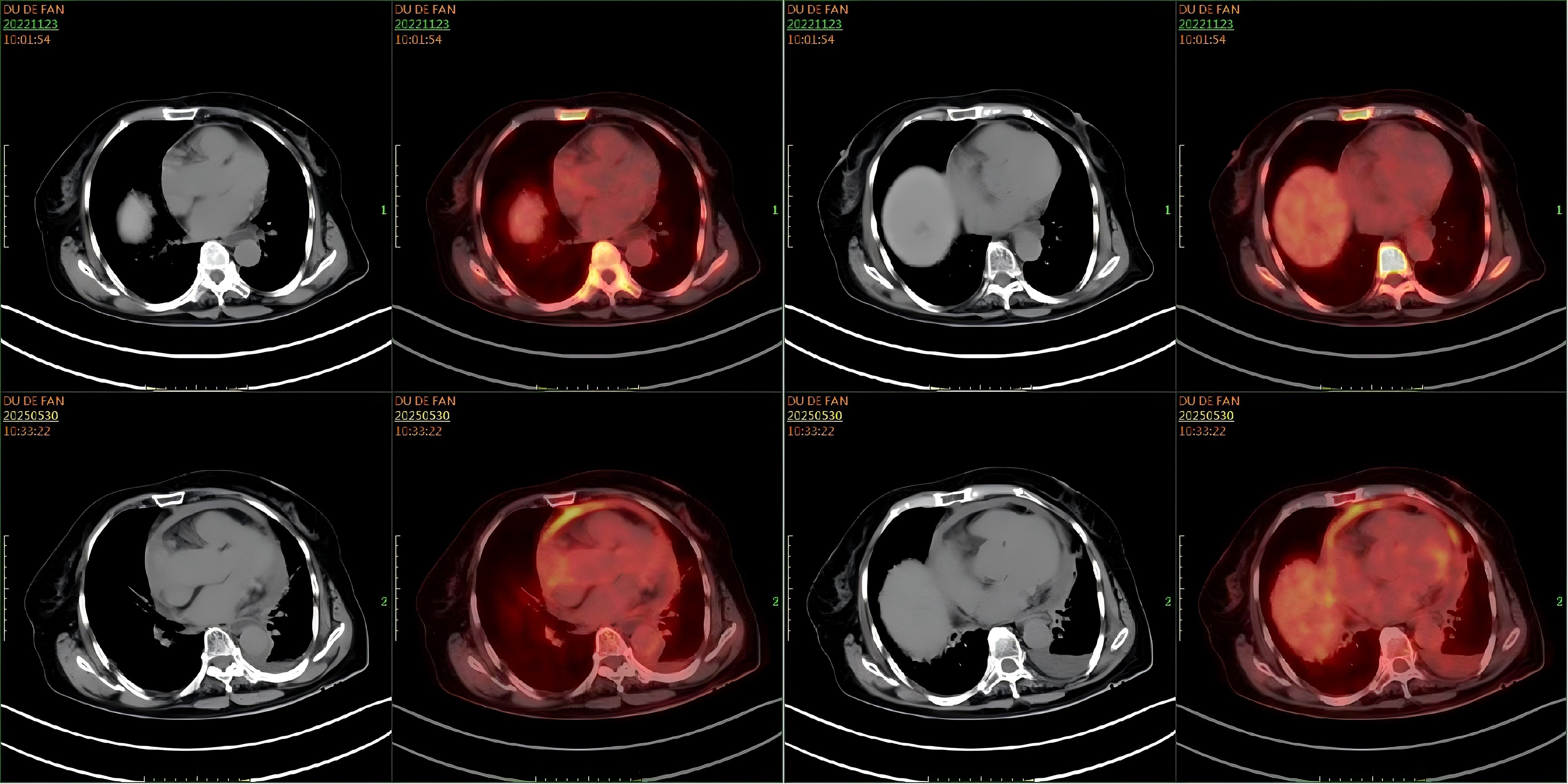
Figure 5. Comparison of PET-CT scans. A comparison of cardiac imaging from November 23, 2022, and May 29, 2025, reveals new findings of pericardial thickening with decreased fluid density, cardiac enlargement, and increased peripheral fluorodeoxyglucose (FDG) uptake.
Case 2
A 75-year-old male was admitted in February 2025 with a ten-day history of cough and dyspnea. The patient was otherwise healthy with no significant past medical history. Laboratory results, including complete blood count, biochemistry, cardiac enzymes, coagulation studies, tuberculin T-cell assay, and standard infection markers, were within normal limits. Serological tests for HCV, HBV, HIV, and HHV-8 were negative. Cardiac ultrasonography revealed no abnormalities. However, ultrasonography of the chest identified a massive right pleural effusion, with maximum vertical and horizontal diameters of 224 mm and 158 mm, respectively. Analysis of the pleural fluid demonstrated markedly elevated adenosine deaminase (ADA) at 204.7 U/L and lactate dehydrogenase (LDH) at 2086.1 U/L, while the carcinoembryonic antigen (CEA) level was within normal limits at 1.67 ng/mL. Microbiological and molecular studies for tuberculosis, including Mycobacterium tuberculosis DNA PCR, acid-fast bacillus (AFB) smear, and T-cell-based testing, were negative. Pathological examination of the pleural effusion was suggestive of a hematolymphoid neoplasm, likely of B-cell origin. Immunohistochemistry (Figure 6) demonstrated that the neoplastic cells were positive for LCA, CD20, and CD79a (mostly positive), with a high proliferation index (Ki-67 approximately 80%). The cells were negative for Ber-EP4, CEA, CD3, CD38, and CD138. Flow cytometric analysis confirmed a population of abnormal large cells, constituting 63.65% of nucleated cells, with an immunophenotype positive for CD20, CD22, CD81, and CD45, and negative for CD5, CD10, CD3, and CD38. These findings support a final diagnosis of large B-cell lymphoma with pleural involvement. Bone marrow examination, including cytology, biopsy, and flow cytometry, revealed no evidence of lymphomatous involvement. Similarly, a left axillary lymph node fine-needle aspiration biopsy was unremarkable. Based on the integrated clinical presentation and immunophenotypic profile, a definitive diagnosis of FO-LBCL was established. A follow-up bilateral chest CT scan on 6 March 2025 showed bilateral pleural effusions with adjacent pulmonary hyperexpansion and a minimal pericardial effusion (Figure 7B). The patient subsequently received two cycles of R-CHOP chemotherapy. However, a follow-up bilateral lung CT scan after the first cycle (Figure 7C) indicated a lack of significant therapeutic response, as evidenced by no appreciable reduction in the pericardial effusion compared to the scan from March 6, 2025. The patient was alive and under continued follow-up as of August 2025.
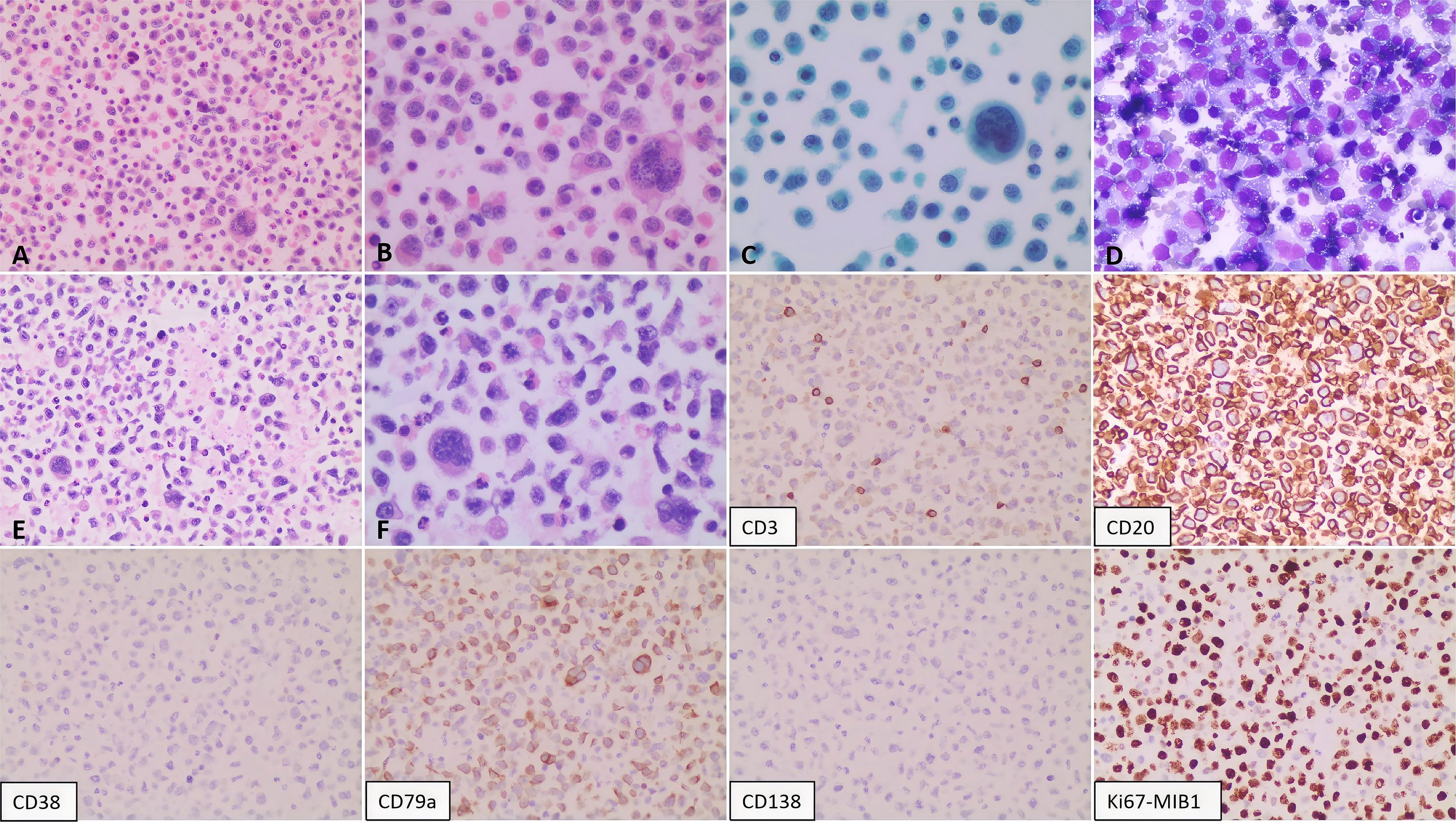
Figure 6. Pathological findings of the pleural effusion. The smear shows a diffuse population of scattered atypical lymphoid cells. These cells are moderately sized and characterized by round to oval nuclei with coarse, granular chromatin; prominent nucleoli are evident in a subset. The cytoplasm is abundant. Mitotic figures and multinucleated giant cells are present. Panels illustrate: (A, B) Cytology (H&E stain; 200X and 400X, respectively); (C) Cytology (Papanicolaou stain; 400X); (D) Cytology (routine stain; 200X); (E, F) Sediment cell block sections (H&E stain; 200X and 400X, respectively). Immunohistochemical analysis of the pericardial effusion reveals the following immunophenotype: CD2(+), CD79a (mostly positive), Ki-67 (approximately 80%), with negative staining for CD3, CD38, and CD138.
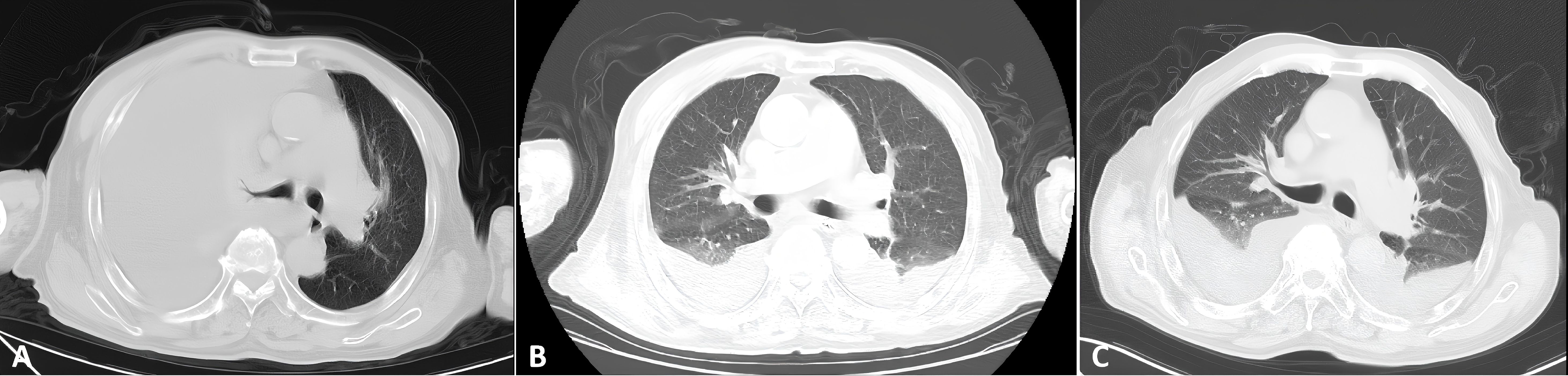
Figure 7. Serial bilateral chest CT imaging of pleural effusion. (A) February 2025: Image obtained at initial discovery. (B) March 2025: Pre-treatment follow-up scan. (C) April 2025: Scan obtained after one cycle of chemotherapy.
5 Discussion
FO-LBCL primarily affects elderly patients, with a median age of 70 years; over 95% of cases occur in individuals aged 60 or older. Most patients have no underlying immunodeficiency. The disease is frequently associated with conditions that cause fluid overload, including chronic heart failure, chronic renal failure, liver cirrhosis, and protein-losing enteropathy (36). EBV co-infection is present in only 9% to 29% of cases. Crucially, all cases are negative for KSHV/HHV-8, a key finding that helps differentiate FO-LBCL from PEL (24, 41). The clinical presentation is predominantly characterized by serous cavity effusions. The pleural cavity is most frequently involved (76%), followed by the pericardial (38%) and peritoneal (12%) cavities. A notable geographic variation exists, with Japanese patients demonstrating a significantly higher rate of pericardial involvement than non-Japanese patients (56% vs. 24%, p< 0.0001). Approximately 40% of patients present with effusions in multiple cavities simultaneously. The most common initial symptom is dyspnea, occurring in two-thirds of patients. This is often accompanied by lower extremity edema and, in some cases, B symptoms. Simultaneous involvement of multiple serous cavities is observed in approximately 40% of patients. The most common initial symptom is dyspnea, presenting in two-thirds of cases and often accompanied by lower limb edema. Less commonly, patients may experience B symptoms, including fever, night sweats, and weight loss (42, 43). This study’s analysis of 57 patients aligns with previous reports, confirming consistent patterns in the sites of involvement and initial clinical manifestations. Furthermore, neither of the two patients presented in this case series showed evidence of infection with KSHV/HHV-8 or EBV. The etiology of FO-LBCL remains unclear, though the retention of body cavity effusions is a proposed contributing factor (36); Additional studies suggest an association between FO-LBCL and HCV infection, with reported infection rates of 22% to 25% (24, 41), HCV may function as a persistent antigenic stimulus, driving the clonal expansion of peritoneal B-cells. This prolonged proliferation provides a context for the accumulation of additional genetic alterations, such as the t(8;22) translocation, ultimately culminating in the development of FO-LBCL (44, 45).
The tumor cells of FO-LBCL are characterized as medium to large lymphocytes with abundant, often vacuolated, basophilic cytoplasm. The nuclei are pleomorphic, exhibiting round, oval, or irregular contours, and contain prominent single or multiple eosinophilic nucleoli. The chromatin is loosely distributed and may appear vacuolated. Nuclear invaginations resulting in lobulated or binucleated forms are common. These cells display high mitotic activity and frequent apoptotic bodies, with morphological features that can range from centroblastic to immunoblastic or plasmablastic (24, 42, 43). The immunophenotype of FO-LBCL is characterized by consistent expression of pan-B-cell markers, including CD20 (98%), PAX5 (100%), and CD19. In contrast, markers such as CD138, CD45, and CD3 are typically absent. Molecular profiling most frequently identifies the non-germinal center B-cell subtype. Immunohistochemistry reveals high BCL2 expression (73%) and heterogeneous MYC expression (34%), with approximately 30% of cases demonstrating co-expression of MYC and BCL2 (43); Cases with unusual immunophenotypes have also been reported. These include instances lacking conventional pan-B-cell markers despite demonstrating clonal B-cell gene rearrangements (46), or those exhibiting an aberrant T-cell phenotype (47). Molecular genetic analyses revealed rearrangement rates of 19% (20/107) for MYC, 29% (16/55) for BCL6, and 11% (6/54) for BCL2. Furthermore, approximately 50% of cases displayed complex karyotype abnormalities, including the t(9;14) translocation (36, 43). The two reported cases exhibited consistent tumor morphology and immunophenotype. However, flow cytometry of the second patient’s pleural fluid revealed an absence of CD19 expression. This finding aligns with the broader literature, wherein this review identifies CD20 negativity in 13.7% of patients and CD138 positivity in 30.4%. These immunophenotypic variabilities underscore that a definitive diagnosis cannot rely on a limited panel of markers and must instead integrate comprehensive morphological, immunophenotypic, and clinical data to prevent misdiagnosis.
Serosal lesions in patients with FO-LBCL typically demonstrate intense fluorodeoxyglucose (18F-FDG) avidity on PET-CT. However, this hypermetabolism is non-specific and can be observed in other conditions involving the serosa, such as metastatic malignancies, other hematologic neoplasms, or inflammatory diseases. Consequently, a key utility of PET-CT in this context is to exclude other space-occupying pathologies within the serosal cavities. Furthermore, PET-CT offers superior delineation of diffuse serosal infiltration, which is often poorly defined on conventional CT, thereby enhancing its value for initial staging and post-therapeutic response assessment. Despite its higher cost and the currently limited clinical experience specific to FO-LBCL, we recommend PET-CT for suspected or confirmed cases to aid in comprehensive differential diagnosis, accurate staging, and subsequent monitoring of treatment efficacy.
PEL and FO-LBCL share highly similar clinical presentations, rendering them indistinguishable based solely on clinical features, routine laboratory tests, or bone marrow morphology. PEL is a distinct subtype of non-Hodgkin lymphoma defined by malignant effusions in body cavities. It predominantly affects middle-aged and young men (median age ~40 years), with a strong association with immunocompromised states; approximately 80% of cases occur in HIV-positive individuals (24). According to the WHO classification, PEL is defined as a large B-cell lymphoma that typically lacks expression of pan-B-cell markers (e.g., CD19, CD20, CD79a) although it often expresses CD45, CD138, MUM1, and EMA, and may aberrantly express T-cell markers such as CD3 and CD4 (48); A definitive diagnosis of PEL requires conclusive evidence of KSHV/HHV-8 infection, which is present in all cases; approximately 60%–80% of these are co-infected with EBV. In contrast to some other large B-cell lymphomas, PEL is not associated with c-myc gene rearrangements, a critical distinguishing feature (49, 50). Based on the absence of KSHV/HHV-8 infection and the presence of B-cell marker expression, the cases in this report were inconsistent with a PEL diagnosis (49, 51). Based on the absence of KSHV/HHV-8 infection and the presence of B-cell marker expression, the cases in this report were inconsistent with a PEL diagnosis.
Additionally, FO-LBCL must be differentiated from pyothorax-associated lymphoma (PAL) and diffuse large B-cell lymphoma with malignant effusion. ① PAL: Although both PAL and FO-LBCL are rare large B-cell lymphomas originating in body cavities, they are distinguished by key clinical and pathological features. PAL predominantly affects elderly patients with a long-term history of chronic empyema or pleurisy and is associated with EBV infection in approximately 85% of cases, usually in immunocompetent individuals. In contrast, FO-LBCL typically arises without this specific inflammatory history and shows no strong association with EBV. Pathologically, while both entities express B-cell markers such as CD20, aberrant expression of T-cell markers (e.g., CD2, CD3, CD4) can be found in approximately 30% of PAL cases. Furthermore, PAL is characterized radiologically by an eccentric, lens- or crescent-shaped soft tissue mass within the empyema cavity (50, 52, 53); ② Diffuse large B-cell lymphoma (DLBCL) with malignant effusion: Although the tumor cells of conventional DLBCL with malignant effusion and FO-LBCL can exhibit overlapping morphological and immunophenotypic features, their underlying etiologies are fundamentally distinct. Conventional DLBCL typically manifests with primary solid lesions, such as lymphadenopathy or extranodal masses; associated effusions are secondary phenomena resulting from advanced-stage dissemination or local infiltration. In contrast, FO-LBCL is primarily defined by the effusion itself, often in the absence of a dominant tumor mass. Consequently, the differential diagnosis critically relies on imaging techniques, particularly PET-CT, to evaluate the systemic disease burden and identify or rule out a primary solid lesion (54). Other lymphomas that may present with malignant body cavity effusions—including Burkitt lymphoma, mantle cell lymphoma, anaplastic large cell lymphoma, and peripheral T-cell lymphoma—must be considered in the differential diagnosis. These entities can be definitively distinguished from FO-LBCL based on their distinct clinical presentations, histopathological features, and immunophenotypic profiles.
Due to its rarity, no unified treatment standard has been established for FO-LBCL. Despite exhibiting the histopathological characteristics of a large B-cell lymphoma, FO-LBCL typically follows a more favorable clinical course than conventional DLBCL, with some cases demonstrating an indolent progression. The indolent nature of FO-LBCL is further evidenced by cases diagnosed only after six years of continuous monitoring for pericardial effusion (55), as well as by patients who have achieved prolonged, treatment-free remission—exceeding 18 months—even after experiencing recurrence and undergoing multi-drug salvage chemotherapy (56); A subset of patients can achieve complete remission with paracentesis alone, without systemic chemotherapy. In the present cohort, 43.9% of patients were managed without chemotherapy. Among those with available follow-up data, the survival rate for this non-chemotherapy group was only marginally lower than that of patients who received chemotherapy (61.2% vs. 70.0%). One hypothesis for this phenomenon is that paracentesis may lead to the release of tumor antigens, potentially stimulating a local anti-tumor immune response; however, this mechanism remains unconfirmed (22, 57, 58). Current evidence supports anthracycline-based chemotherapy as the first-line treatment for FO-LBCL, with studies indicating favorable outcomes. For instance, Kaji (42) et al. reported that among 56 Japanese patients treated with the CHOP or R-CHOP regimen, the overall response rate was 95%, with a complete remission rate of 73% and a 2-year overall survival rate of 84.7%. Furthermore, a larger analysis by Gisriel (43) et al. of 202 patients identified key prognostic factors. Residence in Japan was associated with a trend toward improved survival (hazard ratio [HR] = 0.475; 95% confidence interval [CI], 0.216–1.044; p = 0.064), while the presence of peritoneal effusion was an independent adverse prognostic factor (HR = 3.652; 95% CI, 1.763–7.565; p = 0.004).
These findings are supported by an independent review, which identified Japanese ethnicity, pericardial effusion, CD20 positivity, and rituximab-containing chemotherapy as favorable prognostic factors, while peritoneal effusion and CD20 negativity were unfavorable. Consistent with this, the present study also confirms CD20 positivity and rituximab-based chemotherapy as predictors of a favorable outcome. Furthermore, our analysis identified additional favorable prognostic factors, including CD79a positivity, CD138 negativity, age greater than 65 years, and a lactate dehydrogenase (LDH) level ≤ 500 U/L. In contrast to previous reports, our study identified pleural effusion as a favorable prognostic factor. This discrepancy is likely attributable to our study’s limited cohort size. Consequently, clinical prognosis should not be determined by the site of involvement alone but through a multifactorial assessment. Multivariate analysis confirmed that age >70 years and CD20 positivity were independent favorable prognostic factors. Notably, patients with CD20-positive FO-LBCL exhibited significantly improved outcomes following rituximab-containing therapy. These findings suggest that rituximab-based chemotherapy represents an appropriate first-line standard of care for this patient subgroup (59). For patients who are candidates for systemic chemotherapy, CHOP-based regimens constitute the cornerstone of treatment. However, for elderly patients or those with significant comorbidities that preclude chemotherapy, management may be limited to the drainage of effusions (43).
A 72-year-old male with FO-LBCL demonstrated only 10% CD20 positivity on ascites pathology. This low level of expression was directly associated with his resistance to CHOP chemotherapy and poor prognosis (5). The low expression or loss of CD20 is frequently observed in FO-LBCL and may arise from the intrinsic biology of the tumor cells or from clonal selection induced by therapeutic pressure. This phenomenon is documented in prior case reports; for instance, one described a patient with relapsed FO-LBCL whose immunophenotype shifted from CD20-positive at diagnosis to CD20-negative at relapse, with concomitant loss of other markers including CD79a and MUM1. These alterations in antigen expression significantly compromise the efficacy of R-CHOP-based chemotherapy (10).
This study demonstrates a 80.0% prevalence of CD79a expression in FO-LBCL. CD79a and CD79b form the heterodimeric signaling component of the B-cell receptor, with CD79b being nearly universally expressed in mature B-cell lymphomas and normal B cells (60). This expression profile makes CD79b a compelling therapeutic target. Polatuzumab vedotin, an antibody-drug conjugate directed against CD79b, received U.S. FDA approval in June 2019 in combination with bendamustine and rituximab for patients with relapsed/refractory DLBCL after at least two prior therapies (61). More recently, polatuzumab vedotin has shown superior efficacy to standard R-CHOP in frontline therapy. The phase III POLARIX trial (NCT03274492) compared Pola-R-CHP with R-CHOP in previously untreated, high-risk DLBCL patients, demonstrating a statistically significant improvement in progression-free survival (PFS) for the polatuzumab-containing regimen (2-year PFS 76.7% vs. 70.2%) and a significant reduction in the risk of disease progression, relapse, or death (62). Although FO-LBCL was not included in this trial, its typically aggressive clinical behavior and variable response to conventional chemotherapy parallel the high-risk profiles of the enrolled patients. Given the high frequency of CD79a/b complex expression in FO-LBCL, CD79b represents a rational therapeutic target for this subtype. We recommend the routine assessment of CD79b expression in FO-LBCL cases and advocate for future clinical trials to evaluate the efficacy of polatuzumab vedotin in this and other rare lymphoma entities.
In precision medicine, elucidating the molecular basis of FO-LBCL is critical for developing targeted therapies. Although FO-LBCL exhibits distinct clinical and immunophenotypic features, our study and prior literature suggest the absence of a unifying genomic driver. Instead, its clinical peculiarity may arise from epigenomic dysregulation. Epigenetic mechanisms are pivotal in germinal center B-cell differentiation and are frequently disrupted in aggressive lymphomas, leading to differentiation arrest, immune evasion, and therapy resistance. Supporting this, Pascual (7) et al. identified pathogenic or likely pathogenic mutations in the epigenetic regulators KMT2D and EP300 in FO-LBCL patients. Loss-of-function mutations in KMT2D, which encodes an H3K4 methyltransferase, diminish enhancer activity and repress differentiation-related genes, thereby maintaining tumor cells in an undifferentiated state. Similarly, dysfunction of the histone acetyltransferase EP300 impairs chromatin-mediated transcriptional activation and immune signaling (63). Given the demonstrated potential of epigenetic therapies in B-cell lymphomas, future research should leverage multi-omics approaches to define epigenetic subtypes of FO-LBCL. This strategy promises to uncover prognostic and predictive biomarkers, reveal novel targets for personalized therapy, and establish epigenetics as a cornerstone for future molecular classification and targeted treatment.
In summary, FO-LBCL is characterized by its primary manifestation as serous cavity effusion in the absence of solid masses, its occurrence in immunocompetent elderly patients with cardiorenal or hepatic comorbidities, and its distinct pathological profile, which is not associated with KSHV/HHV-8 infection. This review synthesizes the distinct clinicopathological features of FO-LBCL to aid clinicians in its diagnosis and differential diagnosis. A high index of suspicion is warranted when a patient’s serous cavity effusion is unexplained by known comorbidities, even in the setting of normal routine hematologic parameters. In such cases, early cytopathological and immunohistochemical analysis of the effusion is critical for a definitive diagnosis. Furthermore, this article outlines principles for prognostic assessment and treatment selection, emphasizing that a comprehensive evaluation is essential for staging, determining prognosis, and formulating an individualized management plan.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
LC: Formal Analysis, Investigation, Writing – original draft, Conceptualization, Visualization, Data curation, Writing – review & editing, Methodology. ZY: Validation, Methodology, Writing – review & editing, Supervision. CY: Investigation, Data curation, Writing – review & editing, Software. YW: Writing – review & editing, Supervision, Methodology, Data curation. DL: Data curation, Project administration, Writing – review & editing. HW: Data curation, Writing – review & editing, Methodology, Project administration. LZ: Writing – review & editing, Supervision, Visualization, Formal Analysis, Validation, Methodology. YZ: Visualization, Validation, Methodology, Supervision, Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Shandong Provincial Medical and Health Science and Technology Development Project (202403040460); Xuzhou Medical University Affiliated Hospital Development Fund Project (XYFM202427).
Acknowledgments
The work was supported by the Shandong Second Medical University and Department of Hematology of Linyi People’s Hospital, and I would like to show great gratitude to them all.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo LBDO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
2. Wang H, Zhang Q, Liu Q, Wu X, and Ma K. Fluid overload-associated large B-cell lymphoma with primary biliary cirrhosis: A case report. Front Oncol. (2023) 13:1145540. doi: 10.3389/fonc.2023.1145540
3. Isaac S, Richardson M, and Weil A. Fluid overload-associated large B cell lymphoma. Chest. (2024) 166:3775A–A. doi: 10.1016/j.chest.2024.06.2263
4. Sun S, Li T, Liu Y, Wu M, Zhao Q, Kang N, et al. Massive pericardial effusion as the initial and main manifestation of fluid overload-associated large B-cell lymphoma. JACC Case Rep. (2025) 30:104009. doi: 10.1016/j.jaccas.2025.104009
5. Nakabeppu S, Miyoshi H, Ishitsuka K, and Komohara Y. An aggressive case of Fluid overload-associated large B-cell lymphoma (FO-LBCL) with CD20 down-regulation. J Clin Exp Hematop. (2025) 65:77–80. doi: 10.3960/jslrt.24075
6. Chen W, Wang Z, Shi JN, and Zhang T. Fluid overload-associated large B-cell lymphoma: report of a case. Zhonghua Bing Li Xue Za Zhi. (2023) 52:949–51. doi: 10.3760/cma.j.cn112151-20230105-00008
7. Pascual LP, Crespo FJD, López BBA, de Los Ángeles Pérez Saénz M, Alonso RM, Pinilla SMR, et al. Large B-cell lymphoma associated with fluid overload: two new case reports with molecular insights. Virchows Arch. (2025) doi: 10.1007/s00428-025-04068-8
8. Liu Y, Shioya A, Shimizu R, Tsubata Y, Kumagai M, Okanemasa Y, et al. Fluid overload-associated large B-cell lymphoma with light chain restriction type plasma cell infiltration: A case report. Am J Case Rep. (2024) 25:e944268. doi: 10.12659/AJCR.944268
9. Bahmad HF, Gomez AS, Deb A, Safdie FM, and Sriganeshan V. Fluid overload-associated large B-cell lymphoma: A case report and review of literature. Hematol Rep. (2023) 15:411–20. doi: 10.3390/hematolrep15030042
10. Yan X, Chen B, Jing H, Yang Z, Zhang T, Lin Y, et al. A rare case of fluid overload-associated large B-cell lymphoma and antigen loss at relapse. J Hematop. (2023) 16:235–40. doi: 10.1007/s12308-023-00566-3
11. Liaskas A, Xanthopoulos V, Arapaki M, and Vassilakopoulos TP. Pleural fluid overload-associated large B-cell lymphoma with a formerly double-hit genotype. Lancet Haematol. (2023) 10:e306. doi: 10.1016/S2352-3026(23)00060-1
12. Wang T, Nava VE, Schechter GP, Lichy JH, and Liu ML. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: a patient successfully treated with pleurodesis. J Clin Oncol. (2011) 29:e747–750. doi: 10.1200/JCO.2011.35.7509
13. Toyoda K, Abe Y, Tsuda M, Haji S, Choi L, Suehiro Y, et al. Successful treatment with oral low-dose sobuzoxane and etoposide combined with rituximab in an elderly patient with HHV-8-negative primary effusion lymphoma-like lymphoma. Rinsho Ketsueki. (2014) 55:815–9.
14. Patil VV, Sideras P, and Machac J. PET/CT presentation of primary effusion lymphoma-like lymphoma unrelated to human herpes virus 8, a rare NHL subtype. Indian J Nucl Med. (2014) 29:182–4. doi: 10.4103/0972-3919.136586
15. Ogasawara Y, Machishima T, Shimada T, Takahashi H, Fukunaga M, Mizoroki F, et al. Human herpesvirus 8-negative primary effusion lymphoma-like lymphoma with t(8;14)(q24;q32). Rinsho Ketsueki. (2015) 56:1082–8. doi: 10.11406/rinketsu.56.1082
16. Lei JY, Chen L, Wang P, Kou MQ, and Li WS. HHV8-unrelated primary effusion lymphoma-like lymphoma: report of a case. Zhonghua Bing Li Xue Za Zhi. (2023) 52:84–6. doi: 10.3760/cma.j.cn112151-20220327-00226
17. Kojima M, Nakamura N, Amaki J, Numata H, Miyaoka M, Motoori T, et al. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma following tyrosine kinase inhibitor treatment for chronic myelogenous leukemia. J Clin Exp Hematop. (2017) 57:69–73. doi: 10.3960/jslrt.17020
18. Aota Y, Maki S, Moriyama M, Udagawa S, Saihara M, Watanabe T, et al. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma that developed during myelodysplastic syndrome. Nihon Ronen Igakkai Zasshi. (2021) 58:284–9. doi: 10.3143/geriatrics.58.284
19. Adiguzel C, Bozkurt SU, Kaygusuz I, Uzay A, Tecimer T, and Bayik M. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of the literature. APMIS. (2009) 117:222–9. doi: 10.1111/j.1600-0463.2008.00005.x
20. Liao PJ, Yuan H, and Wei XJ. HHV8-unrelated primary effusion lymphoma in a patient with HBV-related liver cirrhosis: A case successfully treated with rituximab and lenalidomide. Clin Case Rep. (2023) 11:e7411. doi: 10.1002/ccr3.7411
21. Kim KH, Lee JH, Jeong HC, and Bang SM. A case of human herpes virus-8 unrelated primary effusion lymphoma-like lymphoma presented as pleural effusion. Tuberc Respir Dis (Seoul). (2012) 73:336–41. doi: 10.4046/trd.2012.73.6.336
22. Terasaki Y, Yamamoto H, Kiyokawa H, Okumura H, Saito K, Ichinohasama R, et al. Disappearance of Malignant cells by effusion drainage alone in two patients with HHV-8-unrelated HIV-negative primary effusion lymphoma-like lymphoma. Int J Hematol. (2011) 94:279–84. doi: 10.1007/s12185-011-0906-8
23. Okada K, Asakura S, Yano T, and Kishimoto T. EBV-positive PEL-like lymphoma that developed in the course of antisynthetase syndrome treated with tacrolimus. Int J Hematol. (2018) 108:329–34. doi: 10.1007/s12185-018-2426-2
24. Wu W, Youm W, Rezk SA, and Zhao X. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma: report of a rare case and review of 54 cases in the literature. Am J Clin Pathol. (2013) 140:258–73. doi: 10.1309/AJCPHZ3CHO4HUWET
25. Zaimoku Y, Takahashi W, Iwaki N, Saito C, Yoshida A, Aoki G, et al. Human herpesvirus-8-unrelated primary effusion lymphoma of the elderly not associated with an increased serum lactate dehydrogenase level: A benign sub-group of effusion lymphoma without chemotherapy. Leuk Lymphoma. (2016) 57:1625–32. doi: 10.3109/10428194.2015.1088649
26. Kumode T, Ohyama Y, Kawauchi M, Yamaguchi T, Miyatake JI, Hoshida Y, et al. Clinical importance of human herpes virus-8 and human immunodeficiency virus infection in primary effusion lymphoma. Leuk Lymphoma. (2013) 54:1947–52. doi: 10.3109/10428194.2012.763122
27. Kim HJ, Lee K, Yoon CH, and Bang SM. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma presenting with cardiac tamponade: A case report. Med (Baltimore). (2017) 96:e8010. doi: 10.1097/MD.0000000000008010
28. Kashiwagi T, Minagawa K, Kawano H, Hirata T, Kashiwagi S, Nakagawa Y, et al. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma with genotypic infidelity and c-MYC expression. Ann Hematol. (2014) 93:1609–10. doi: 10.1007/s00277-013-1999-3
29. Nakamura H, Tsuta K, Nakagawa T, Hirai R, and Ota Y. Human herpes virus 8-unrelated primary effusion lymphoma-like lymphoma in the pericardium: A case with latency type III Epstein-Barr virus infection showing good prognosis without chemotherapy. Pathol Res Pract. (2015) 211:1010–3. doi: 10.1016/j.prp.2015.08.002
30. Shin J, Lee JO, Choe JY, Bang SM, and Lee JS. Human herpesvirus 8-unrelated primary effusion lymphoma-like lymphoma in an elderly korean patient with a good response to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer Res Treat. (2017) 49:274–8. doi: 10.4143/crt.2016.076
31. Zhang E, Cotton VE, Hidalgo-Bravo A, Huang Y, Bell AJ, Jarrett RF, et al. HHV-8-unrelated primary effusion-like lymphoma associated with clonal loss of inherited chromosomally-integrated human herpesvirus-6A from the telomere of chromosome 19q. Sci Rep. (2016) 6:22730. doi: 10.1038/srep22730
32. Raskin J, Slabbynck H, and Beel K. Human herpes virus 8 unrelated bilateral primary effusion lymphoma in a patient with chronic fluid overload. Arch Bronconeumol. (2016) 52:492–3. doi: 10.1016/j.arbres.2015.12.012
33. Takahashi T, Hangaishi A, Yamamoto G, Ichikawa M, Imai Y, and Kurokawa M. HIV-negative, HHV-8-unrelated primary effusion lymphoma-like lymphoma: report of two cases. Am J Hematol. (2010) 85:85–7. doi: 10.1002/ajh.21568
34. Cooper AR, Burack WR, and Allerton JP. A case of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8-unrelated but Epstein–Barr virus-positive primary effusion lymphoma-like lymphoma in the setting of human immunodeficiency virus and hepatitis C virus infection. Leukemia Lymphoma. (2010) 51:2303–5. doi: 10.3109/10428194.2010.520775
35. Alexanian S, Said J, Lones M, and Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. (2013) 37:241–9. doi: 10.1097/PAS.0b013e318267fabc
36. Alexanian S, Said J, Lones M, and Pullarkat ST. KSHV/HHV8-negative effusion-based lymphoma, a distinct entity associated with fluid overload states. Am J Surg Pathol. (2013) 37:241–9. doi: 10.1097/PAS.0b013e318267fabc
37. Xiao J, Selvaggi SM, Leith CP, Fitzgerald SA, and Stewart J. Kaposi sarcoma herpesvirus/human herpesvirus-8-negative effusion-based lymphoma: report of 3 cases and review of the literature. Cancer Cytopathol. (2013) 121:661–9. doi: 10.1002/cncy.21311
38. Chen BJ, Chen DYT, Kuo CC, and Chuang SS. EBV-associated but HHV8-unrelated double-hit effusion-based lymphoma. Diagn Cytopathol. (2017) 45:257–61. doi: 10.1002/dc.23638
39. Caliskan T, Ozgun A, Demirer E, Haholu A, Karagoz B, and Ciftci F. Aggressive and hiv-negative hhv8-unrelated primary effusion-like lymphoma with T-cell phenotype: A rare case report. Acta Med Mediterr. (2014) 30:581–4.
40. Tsagarakis NJ, Argyrou A, Gortzolidis G, Kentrou N, Papadhimitriou SI, Tzanetou K, et al. Report of an HIV and HHV-8 negative case of primary effusion lymphoma with idiopathic T4 lymphocytopenia. Int J Hematol. (2009) 90:94–8. doi: 10.1007/s12185-009-0343-0
41. Saini N, Hochberg EP, Linden EA, Jha S, Grohs HK, and Sohani AR. HHV8-negative primary effusion lymphoma of B-cell lineage: two cases and a comprehensive review of the literature. Case Rep Oncol Med. (2013) 2013:292301. doi: 10.1155/2013/292301
42. Kaji D, Ota Y, Sato Y, Nagafuji K, Ueda Y, Okamoto M, et al. Primary human herpesvirus 8-negative effusion-based lymphoma: a large B-cell lymphoma with favorable prognosis. Blood Adv. (2020) 4:4442–50. doi: 10.1182/bloodadvances.2020002293
43. Gisriel SD, Yuan J, Braunberger RC, Maracaja DLV, Chen XY, Wu XJ, et al. Human herpesvirus 8-negative effusion-based large B-cell lymphoma: a distinct entity with unique clinicopathologic characteristics. Mod Pathol. (2022) 35:1411–22. doi: 10.1038/s41379-022-01091-x
44. Ascoli V, Lo Coco F, Artini M, Levrero M, Fruscalzo A, and Mecucci C. Primary effusion Burkitt’s lymphoma with t(8;22) in a patient with hepatitis C virus-related cirrhosis. Hum Pathol. (1997) 28:101–4. doi: 10.1016/s0046-8177(97)90287-2
45. Paner GP, Jensen J, Foreman KE, and Reyes CV. HIV and HHV-8 negative primary effusion lymphoma in a patient with hepatitis C virus-related liver cirrhosis. Leuk Lymphoma. (2003) 44:1811–4. doi: 10.1080/1042819031000104015
46. Jenkins C, Sorour Y, Blake E, Elliot R, Al-Sabah AI, and Green J. Human-immunodeficiency-virus-negative, human-herpes-virus-8-negative abdominal cavity primary effusion lymphoma. Clin Oncol (R Coll Radiol). (2005) 17:636–8. doi: 10.1016/j.clon.2005.05.012
47. Yamamoto Y, Kitajima H, Sakihana H, Shigeki T, and Fukuhara S. CD3+CD4-CD8-TCR-alphabeta+ T-cell lymphoma with clinical features of primary effusion lymphoma: an autopsy case. Int J Hematol. (2001) 74:442–6. doi: 10.1007/BF02982089
48. Gathers DA, Galloway E, Kelemen K, Rosenthal A, Gibson SE, and Munoz J. Primary effusion lymphoma: A clinicopathologic perspective. Cancers (Basel). (2022) 14:722. doi: 10.3390/cancers14030722
49. Hu Z, Pan Z, Chen W, Shi Y, Wang W, Yuan J, et al. Primary effusion lymphoma: A clinicopathological study of 70 cases. Cancers (Basel). (2021) 13:878. doi: 10.3390/cancers13040878
50. Aozasa K, Takakuwa T, and Nakatsuka S. Pyothorax-associated lymphoma: a lymphoma developing in chronic inflammation. Adv Anat Pathol. (2005) 12:324–31. doi: 10.1097/01.pap.0000194627.50878.02
51. El-Fattah MA. Clinical characteristics and survival outcome of primary effusion lymphoma: A review of 105 patients. Hematol Oncol. (2017) 35:878–83. doi: 10.1002/hon.2372
52. Hu JY, Lee KH, and Yun G. Case 322: pyothorax-associated lymphoma. Radiology. (2024) 310:e223090. doi: 10.1148/radiol.223090
53. Ueda T, Andreas C, Itami J, Miyakawa K, Fujimoto H, Ito H, et al. Pyothorax-associated lymphoma: imaging findings. AJR Am J Roentgenol. (2010) 194:76–84. doi: 10.2214/AJR.09.2603
54. Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, López-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26:v116–125. doi: 10.1093/annonc/mdv304
55. Yamaoka O, Matsui T, Nishiyama K, Miyamoto A, Shiota H, Kawahara C, et al. Indolent primary effusion lymphoma-like lymphoma in the pericardium: A case report and review of the literature. J Cardiol Cases. (2019) 19:148–52. doi: 10.1016/j.jccase.2018.12.013
56. Inoue Y, Tsukasaki K, Nagai K, Soda H, and Tomonaga M. Durable remission by sobuzoxane in an HIV-seronegative patient with human herpesvirus 8-negative primary effusion lymphoma. Int J Hematol. (2004) 79:271–5. doi: 10.1532/ijh97.03107
57. Mohammad F, Siddique MN, Siddiqui F, Popalzai M, Asgari M, and Odaimi M. A unique case of Malignant pleuropericardial effusion: HHV-8-unrelated PEL-like lymphoma-A case report and review of the literature. Case Rep Oncol Med. (2014) 2014:436821. doi: 10.1155/2014/436821
58. Nakatsuka S, Kimura H, Nagano T, Fujita M, Kanda T, Iwata T, et al. Self-limited effusion large B-cell lymphoma: two cases of effusion lymphoma maintaining remission after drainage alone. Acta Haematol. (2013) 130:217–21. doi: 10.1159/000350482
59. Kubota T, Sasaki Y, Shiozawa E, Takimoto M, Hishima T, and Chong JM. Age and CD20 expression are significant prognostic factors in human herpes virus-8-negative effusion-based lymphoma. Am J Surg Pathol. (2018) 42:1607–16. doi: 10.1097/PAS.0000000000001168
60. Huse K, Bai B, Hilden VI, Bollum LK, Våtsveen TK, Munthe LA, et al. Mechanism of CD79A and CD79B support for igM+ B cell fitness through B cell receptor surface expression. J Immunol. (2022) 209:2042–53. doi: 10.4049/jimmunol.2200144
61. Deeks ED. Polatuzumab vedotin: first global approval. Drugs. (2019) 79:1467–75. doi: 10.1007/s40265-019-01175-0
62. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. (2022) 386:351–63. doi: 10.1056/NEJMoa2115304
Keywords: lymphoma, fluid overload-associated large B-cell lymphoma, HHV-8 infection, prognosis factor, primary effusion lymphoma
Citation: Chang L, Yang Z, Yang C, Wang Y, Li D, Wang H, Zang L and Zhang Y (2025) Fluid overload-associated large B-cell lymphoma: two case report and review of literature. Front. Oncol. 15:1724247. doi: 10.3389/fonc.2025.1724247
Received: 13 October 2025; Accepted: 10 November 2025; Revised: 06 November 2025;
Published: 26 November 2025.
Edited by:
Anna Maria Testi, Sapienza University of Rome, ItalyReviewed by:
Gianluca Gaidano, Università degli Studi del Piemonte Orientale, ItalyVineel Bhatlapenumarthi, Medical College of Wisconsin, United States
Copyright © 2025 Chang, Yang, Yang, Wang, Li, Wang, Zang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Zhang, ZG9jdG9yemhhbmd5eUAxNjMuY29t; Lanlan Zang, emFuZ3hpYW9sYW44OEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Yuanyuan Zhang, orcid.org/0009-0007-7675-5863
 Liudi Chang
Liudi Chang Zhaobo Yang3†
Zhaobo Yang3† Lanlan Zang
Lanlan Zang Yuanyuan Zhang
Yuanyuan Zhang