- Department of Clinical Pharmacology, College of Basic Medical Sciences, China Medical University, Shenyang, China
A commentary on
In “The link between brain energy homeostasis and neuronal activity” two papers discuss the importance of optimum energy metabolism for neuronal spike activity in brain slices incubated in glucose-containing media, with one demonstrating benefits of lactate supplementation. A third demonstrates effects of succinate and γ-hydroxybutyrate on ATP-mediated [Ca2+]i gradients in astrocytes, and a fourth discusses whether lactate is the glycolytic end product and exerts neuroprotection. This commentary discusses the quantitative importance of oxidative metabolism in astrocytes, importance of their [Ca2+]i, and role(s) of lactate.
Metabolic brain slice studies were initiated by Warburg et al. (1924). During the 1930s several such studies showed lactate release to incubation media and stimulation of respiration by high K+ concentrations, initially by ∼65% (Ashford and Dixon, 1935; Dickens and Greville, 1935). Electrical stimulation acted similarly (McIlwain, 1951, 1955). Glutamate caused neuronal depolarization (Gibson and McIlwain, 1965), and slices displayed synaptic activity (Yamamoto and McIlwain, 1966). Hertz and Schou (1962) and Weiss et al. (1972), using Warburg equipment with rapidly oscillating tissue chambers or an oxygen electrode inserted into intensely aerated flasks, reported O2 uptake rates similar to Ivanov and Zilberter’s (2011) and Ca2+-dependence and procaine-inhibition of the K+-mediated stimulation. The center and both surfaces of slices showed marked cell swelling under all conditions, but especially at high extracellular K+ concentrations (Møller et al., 1974). Elevated K+ increased (Franck, 1970; Lund-Andersen and Hertz, 1970), and electrical stimulation decreased (Cummins and McIlwain, 1961) intracellular K+ content. Electrical pulses evoked transition from a more oxidized to a more reduced phase in NAD(P)H and cytochromes, blockable by tetrodotoxin, whereas elevated extracellular K+ caused a more oxidized redox state (Cummins and Bull, 1971; Galeffi et al., 2011). In 13C-NMR studies, using labeled glucose and the astrocyte-specific substrate acetate, Badar-Goffer et al. (1992) concluded that the high K+-mediated increase in O2 consumption occurred in glial cells. This may reflect a normally occurring active astrocytic uptake of K+ released from neurons (Somjen et al., 2008; Hertz, 2011) and depolarization-induced increase in [Ca2+]i, stimulating astrocytic metabolism. Electrical stimulation of brain slices also increase astrocytic [Ca2+]i (Filosa et al., 2004).
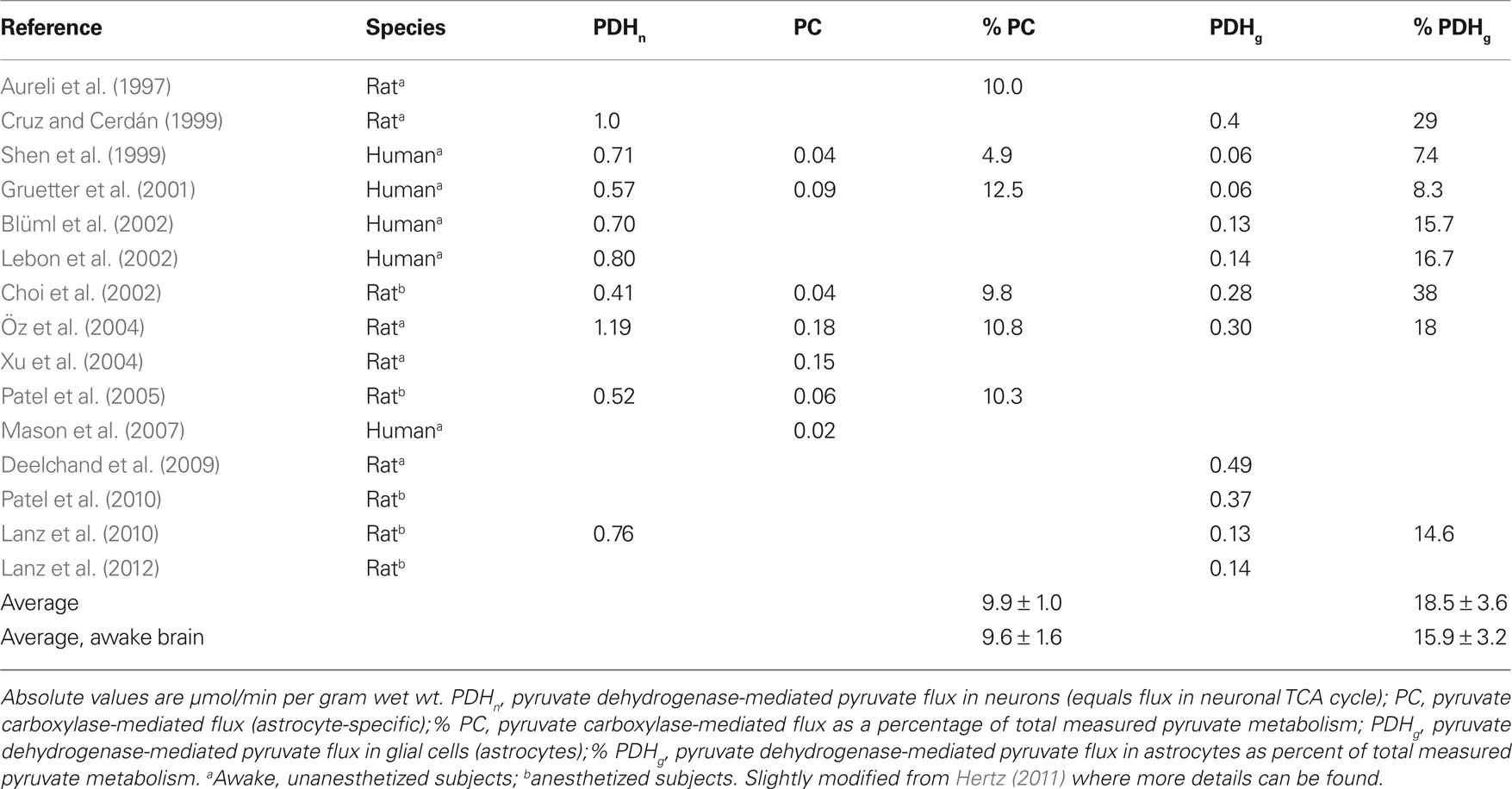
Recently, several groups have measured tricarboxylic acid (TCA) cycle activity in the living, functioning brain in humans and rats using 13C-NMR (reviewed by Hertz, 2011) and tabulated in Table 1. In awake rats total pyruvate fluxes after glycolytic conversion of glucose to pyruvate followed by pyruvate dehydrogenase (PDH-) mediated) entry into the TCA cycle (in both neurons and astrocytes) together with flux mediated by the astrocyte-specific pyruvate carboxylase (PC) amount to ∼1.67 μmol/min/g wet wt (Öz et al., 2004; Table 1). With a pyruvate/O2 ratio of 3.0, this equals 300 μmol of O2/h/g wet wt, close to the upper limit cited by Ivanov and Zilberter (2011). As noted by them, ourselves, and Okada and Lipton (2007), this rate is substantially higher than that of oxygen uptake in brain slices. However, under anesthesia in vivo, respiration becomes more comparable to that in brain slices (see Choi et al., 2002; Table 1). Thus, the enhanced rates of oxygen consumption in slices during neuronal stimulation shown by Ivanov and Zilberter (2011), discussed by Kann (2011), and quantitated by Galeffi et al. (2011), are functionally the most meaningful. Moreover, determination of average metabolic rates in neurons (PDHn) and astrocytes (PFHg + PC) separately (lower two lines of Table 1) shows that astrocytic O2 consumption equals one quarter of total brain energy metabolism in vivo, indicating that per volume astrocytes consume O2 at least at the same rate as neurons. Additional 13C-NMR studies in brain slices during different types of neuronal activation would be useful to evaluate neuronal and astrocytic responses.
Astrocytes are the topic of the non-metabolic study by Molnár et al. (2011) It describes astrocytic [Ca2+]i responses to ATP and modulation of a subset of astrocytic ATP receptors by succinate and γ-aminobutyrate. Besides illustrating the high density of functioning ATP receptors, even in the young astrocytes studied, and the localization of the succinate-affected receptors to vascular-associated astrocyte processes, the study emphasizes important effects of succinate beyond its role as a TCA cycle constituent. Succinate is present in serum and its concentration is increased in diabetes, which may be of considerable importance in diabetic nephropathy (Deen and Robben, 2011), and raises the possibility of involvement of succinate and astrocytes in diabetic effects on the brain. The Molnar paper is also of interest in connection with that by Zilberter (2011), and it supports that the roles of astrocytes in brain metabolism may be underestimated in the Venkateswaran et al. (2012) paper.
Observations in brain slices by Takagaki and Tsukada (1957) that lactate sustains similar rates of oxygen consumption as glucose have been repeatedly confirmed. The Schurr and Gozal (2011) paper suggests important physiological (mitochondrial lactate oxidation) and pathological (neuroprotection) roles of lactate. However, most authors agree that lactate dehydrogenase activity in mitochondria is unlikely (Sahlin et al., 2002; Yoshida et al., 2007), and lactate cannot prevent anoxic depolarization in rat hippocampal slices, when glycolysis is completely inhibited (Allen et al., 2005). Techniques used during preparation of slices are important for subsequent metabolic effects of glucose and lactate (Dienel and Hertz, 2005; Okada and Lipton, 2007; Dienel, 2011). Lactate serves as a partial substrate for brain metabolism during intense exercise (when its blood concentration is increased), but this does not indicate any need for lactate in addition to glucose in brain function, including ongoing activity, since during rest there is a small lactate exit from brain (Quistorff et al., 2008). Nevertheless, if serum lactate is increased, lactate is preferentially oxidized (van Hall et al., 2009). In brain slices the question is complex, because of simultaneous lactate release. Could simple replacement of this lactate restore optimum glucose metabolism? Can results in astrocyte cultures (Sotelo-Hitschfeld et al., 2012) be similarly explained?
In conclusion, a considerable part of oxidative glucose metabolism in brain is astrocytic, exogenous lactate is not a necessary brain fuel in vivo, and past history of metabolic brain slice experiments may inspire future studies.
References
Allen, N. J., Káradóttir, R., and Attwell, D. (2005). A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J. Neurosci. 25, 848–859.
Ashford, C. A., and Dixon, K. C. (1935). The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochem. J. 29, 157–168.
Aureli, T., Di Cocco, M. E., Calvani, M., and Conti, F. (1997). The entry of [1-13C]glucose into biochemical pathways reveals a complex compartmentation and metabolite trafficking between glia and neurons: a study by 13C-NMR spectroscopy. Brain Res. 765, 218–227.
Badar-Goffer, R. S., Ben-Yoseph, O., Bachelard, H. S., and Morris, P. G. (1992). Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem. J. 282(Pt 1), 225–230.
Blüml, S., Moreno-Torres, A., Shic, F., Nguy, C. H., and Ross, B. D. (2002). Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed. 15, 1–5.
Choi, I. Y., Lei, H., and Gruetter, R. (2002). Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J. Cereb. Blood Flow Metab. 22, 1343–1351.
Cruz, F., and Cerdán, S. (1999). Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed. 12, 451–462.
Cummins, J. T., and Bull, R. (1971). Spectrophotometric measurements of metabolic responses in isolated rat brain cortex. Biochim. Biophys. Acta 253, 29–38.
Cummins, J. T., and McIlwain, H. (1961). Electrical pulses and the potassium and other ions of isolated cerebral tissues. Biochem. J. 79, 330–341.
Deelchand, D. K., Shestov, A. A., Koski, D. M., Uğgurbil, K., and Henry, P. G. (2009). Acetate transport and utilization in the rat brain. J. Neurochem. 109(Suppl. 1), 46–54.
Deen, P. M., and Robben, J. H. (2011). Succinate receptors in the kidney. J. Am. Soc. Nephrol. 22, 1416–1422.
Dickens, F., and Greville, G. D. (1935). The metabolism of normal and tumour tissue: neutral salt effects. Biochem. J. 29, 1468–1483.
Dienel, G. A. (2011). Brain lactate metabolism: the discoveries and the controversies. J. Cereb. Blood Flow Metab. doi: 10.1038/jcbfm.2011.175. [Epub ahead of print]
Dienel, G. A., and Hertz, L. (2005). Astrocytic contributions to bioenergetics of cerebral ischemia. Glia 50, 362–388.
Filosa, J. A., Bonev, A. D., and Nelson, M. T. (2004). Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 95, e73–e81.
Franck, G. (1970). Echanges cationiques au niveau des neurones et des cellules gliales du cerveau. Arch. Int. Physiol. Biochim. 78, 613–866.
Galeffi, F., Somjen, G. G., Foster, K. A., and Turner, D. A. (2011). Simultaneous monitoring of tissue PO2 and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices. J. Cereb. Blood Flow Metab. 31, 626–639.
Gibson, I. M., and McIlwain, H. (1965). Continuous recordings of changes in membrane potential in mammalian cerebral tissues in vitro; recovery after depolarization by added substrates. J. Physiol. 176, 261–283.
Gruetter, R., Seaquist, E. R., and Uğgurbil, K. (2001). A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am. J. Physiol. Endocrinol. Metab. 281, E100–E112.
Hertz, L. (2011). Astrocytic energy metabolism and glutamate formation – relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn. Reson. Imaging 29, 1319–1329.
Hertz, L., and Schou, M. (1962). Univalent cations and the respiration of brain-cortex slices. Biochem. J. 85, 93–104.
Ivanov, A., and Zilberter, Y. (2011). Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front. Neuroeng. 3:9. doi: 10.3389/fnene. 00009
Kann, O. (2011). The energy demand of fast neuronal network oscillations: insights from brain slice preparations. Front. Pharmacol. 2:90. doi: 10.3389/fphar. 00090
Lanz, B., Uffmann, K. T., Wyss, M., Weber, B., Buck, A., and Gruetter, R. (2012). A two-compartment mathematical model of neuroglial metabolism using [1-11C] acetate. J. Cereb. Blood Flow Metab. doi: 10.1038/jcbfm.2011.162.
Lanz, B., Xin, L., Wyss, M. T., Weber, B., Buck, A., and Gruetter, R. (2010). Measurements of glial metabolic fluxes with 11C-acetate using positron emission and 1H[13C] NMR spectroscopy. Abstracts, 9th International Conference on Brain Energy Metabolism, Budapest, 25.
Lebon, V., Petersen, K. F., Cline, G. W., Shen, J., Mason, G. F., Dufour, S., Behar, K. L., Shulman, G. I., and Rothman, D. L. (2002). Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy, elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J. Neurosci. 22, 1523–1531.
Lund-Andersen, H., and Hertz, L. (1970). Effects of potassium content in brain-cortex slices from adult rats. Exp. Brain Res. 11, 199–212.
Mason, G. F., Petersen, K. F., de Graaf, R. A., Shulman, G. I., and Rothman, D. L. (2007). Measurements of the anaplerotic rate in the human cerebral cortex using 13C magnetic resonance spectroscopy and [1-13C] and [2-13C] glucose. J. Neurochem. 100, 73–86.
McIlwain, H. (1951). Metabolic response in vitro to electrical stimulation of sections of mammalian brain. Biochem. J. 49, 382–393.
McIlwain, H. (1955). A transitory, rapid, production of lactate in electrically excited cerebral tissues. Biochem. J. 60, xxxi.
Møller, M., Møllgård, K., Lund-Andersen, H., and Hertz, L. (1974). Concordance between morphological and biochemical estimates of fluid spaces in rat brain cortex slices. Exp. Brain Res. 21, 299–314.
Molnár, T., Héja, L., Emri, Z., Simon, A., Nyitrai, G., Pál, I., and Kardos, J. (2011). Activation of astroglial calcium signaling by endogenous metabolites succinate and gamma-hydroxybutyrate in the nucleus accumbens. Front. Neuroeng. 3:7. doi: 10.3389/fnene. 00007
Okada, Y., and Lipton, P. (2007). “Glucose, oxidative metabolism and neural function in brain slices – Glycolysis plays a key role in neural activity,” in Handbook of Neurochemistry and Molecular Neurobiology, Vol. 2, Brain Energetics, Integration of Molecular and Cellular Processes, eds G. E. Gibson, and G. A. Dienel (New York/Heidelberg: Springer), 18–39.
Öz, G., Berkich, D. A., Henry, P. G., Xu, Y., LaNoue, K., Hutson, S. M., and Gruetter, R. (2004). Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J. Neurosci. 24, 11273–11279.
Patel, A. B., Chowdhury, G. M., de Graaf, R. A., Rothman, D. L., Shulman, R. G., and Behar, K. L. (2005). Cerebral pyruvate carboxylase flux is unaltered during bicuculline-seizures. J. Neurosci. Res. 79, 128–138.
Patel, A. B., de Graaf, R. A., Rothman, D. L., Behar, K. L., and Mason, G. F. (2010). Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J. Cereb. Blood Flow Metab. 30, 1200–1213.
Quistorff, B., Secher, N. H., and Van Lieshout, J. J. (2008). Lactate fuels the human brain during exercise. FASEB J. 22, 3443–3449.
Sahlin, K., FernstrÖm, M., Svensson, M., and Tonkonogi, M. (2002). No evidence of an intracellular lactate shuttle in rat skeletal muscle. J. Physiol. 541, 569–574.
Schurr, A., and Gozal, E. (2011). Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2:96. doi: 10.3389/fphar. 00096
Shen, J., Petersen, K. F., Behar, K. L., Brown, P., Nixon, T. W., Mason, G. F., Petroff, O. A., Shulman, G. I., Shulman, R. G., and Rothman, D. L. (1999). Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc. Natl. Acad. Sci. U.S.A. 96, 8235–8240.
Somjen, G. G., Kager, H., and Wadman, W. J. (2008). Computer simulations of neuron-glia interactions mediated by ion flux. J. Comput. Neurosci. 25, 349–365.
Sotelo-Hitschfeld, T., Fernández-Moncada, I., and Barros, L. F. (2012). Acute feedback control of astrocytic glycolysis by lactate. Glia 60, 674–680.
Takagaki, G., and Tsukada, Y. (1957). The effect of some inorganic ions on brain slices metabolizing glucose or pyruvate. J. Neurochem. 2, 21–29.
van Hall, G., Strømstad, M., Rasmussen, P., Jans, O., Zaar, M., Gam, C., Quistorff, B., Secher, N. H., and Nielsen, H. B. (2009). Blood lactate is an important energy source for the human brain. J. Cereb. Blood Flow Metab. 29, 1121–1129.
Venkateswaran, N., Sekhar, S., Thirupatchur Sanjayasarathy, T., Krishnan, S. N., Kabaleeswaran, D. K., Ramanathan, S., Narayanasamy, N., Jagathrakshakan, S. S., and Vignesh, S. R. (2012). Energetics based spike generation of a single neuron: simulation results and analysis. Front. Neuroeng. 4:2. doi: 10.3389/fnene. 00002
Warburg, O., Posener, K., and Negelein, E. (1924). Ueber den Stoffwechsel der tumoren. Biochem. Z. 152, 319–344.
Weiss, G. B., Hertz, L., and Goodman, F. R. (1972). Drug-induced alterations in respiration of rat brain cortex and striatum slices in a carbon dioxide-bicarbonate-buffered medium. Biochem. Pharmacol. 21, 625–634.
Xu, Y., Öz, G., LaNoue, K. F., Keiger, C. J., Berkich, D. A., Gruetter, R., and Hutson, S. H. (2004). Whole-brain glutamate metabolism evaluated by steady-state kinetics using a double-isotope procedure: effects of gabapentin. J. Neurochem. 90, 1104–1116.
Yamamoto, C., and McIlwain, H. (1966). Electrical activities in thin sections from the mammalian brain maintained in chemically-defined media in vitro. J. Neurochem. 13, 1333–1343.
Yoshida, Y., Holloway, G. P., Ljubicic, V., Hatta, H., Spriet, L. L., Hood, D. A., and Bonen, A. (2007). Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J. Physiol. (Lond.) 582, 1317–1335.
Citation: Hertz L (2012) Metabolic studies in brain slices – past, present, and future. Front. Pharmacol. 3:26. doi: 10.3389/fphar.2012.00026
Received: 30 January 2012; Accepted: 12 February 2012;
Published online: 09 March 2012.
Copyright: © 2012 Hertz. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence:bGVpZmhlcnR6QHhwbG9ybmV0LmNh