Since the discovery of the gene that causes Cystic Fibrosis, our knowledge of how mutations in this gene cause the varied pathophysiological manifestations of this disease has increased substantially. This knowledge has led to the possibility of new therapeutic approaches aimed at the basic defect. Class I mutations of CFTR include premature termination codons (PTCs) or stop codons. In the last 10 years there has been a concerted international effort to utilize the concept of read-through of the stop codon producing full length functioning CFTR protein. This author considers that this approach will result in clinical trials in CF patients carrying these mutations.
Class I mutations include PTCs or nonsense codons. A nonsense mutation is a single point alteration in DNA that results in the inappropriate presence of a UAA, UAG, or UGA stop codon in the protein-coding region of the corresponding messenger RNA (mRNA) transcript. Such a stop codon causes premature cessation of translation, with protein truncation leading to loss of function and consequent disease. Nonsense mutations are responsible for about 10% of cystic fibrosis cases worldwide. However, in Israel, nonsense mutations are the cause of cystic fibrosis in most patients (Kerem et al., 1997). As such mutations produce little functional CFTR, these patients usually have a phenotype of CF with exocrine pancreatic insufficiency.
The increased understanding of ribosomal function, the process of translation, and small molecules that change the interaction between the ribosome and mRNA have led to the identification of several agents that are capable of suppressing PTCs. This has resulted in a novel strategy to treat CF and other genetic disorders caused by PTCs by restoring full length protein.
Aminoglycoside antibiotics were the first drugs demonstrated to suppress PTCs in disease-causing mutations, allowing the translation of full length proteins (Hermann, 2007). Aminoglycosides are antibacterial agents, their mode of action is interfering with normal translation via binding to the bacteria 16S rRNA. There is reduced discrimination between cognate and near-cognate tRNA hence reducing translational fidelity. Eventually, there is accumulation of truncated and non-functioning proteins resulting in bacterial cell death.
Gorini and Kataja (1964) demonstrated that aminoglycosides may suppress PTCs and lead to full length translation in E. coli. Aminoglycosides may also bind to human 18S rRNA subunit reducing discrimination of near-cognate tRNAs. This interaction is less stable than in bacteria but may be sufficient to lead to an insertion of a near-cognate aminoacyl-tRNA into the ribosomal A site that is subsequently incorporated into the polypeptide chain.
Howard et al. (1996) described PTC suppression by the synthetic aminoglycoside geneticin (G418) to restore function in HeLa cells expressing nonsense codons in 1996. This pivotal work was extended to four nonsense mutations of cftr who were expressed by the human airway cell line IB3-1.In this study, the commonly used aminoglycoside, gentamicin, was incubated with these cells and full length protein was produced (Bedwell et al., 1997).
Animal Models
Two mouse models have been developed that contain PTCs including the mdx mouse model of Duchenne Muscular Dystrophy and the G542X-hCFTR mouse which is a transgenic model of CF. Barton-Davis et al. (1999) reported suppression of PTC in the dystrophin gene of the mdx mouse by gentamicin. Intra-peritoneal injection of gentamicin restored the full length dystrophin protein in both skeletal and cardiac muscle. Similar studies in the G542X-hCFTR mouse model with gentamicin injections caused full length functional CFTR protein in intestinal tissues. There was also a tendency to increased survival in these mice (Du et al., 2002).
Clinical Trials
The preclinical studies mentioned above have led to a number of clinical trials designed to test both proof of principle and efficacy in patients with genetic diseases caused by PTCs. As stated earlier, about 60% of CF patient in Israel carry PTCs or Class I mutations. An initial open label pilot study showed a significant improvement of Nasal Potential Difference measurements (NPD) after the instillation of gentamicin nose drops (Wilschanski et al., 2001). This was followed by a double-blind, placebo-controlled study on 24 patients which included NPD measurement and membrane localization by immuno-fluorescent staining utilizing an anti-body directed against the C-terminus of CFTR (Wilschanski et al., 2003). These studies utilized nasal gentamicin administered for 2 weeks which resulted in significant improvements of basal PD and chloride secretion representing CFTR function in the treatment arms compared with placebo. Together with this immuno-fluorescent staining was positive in the treatment group. These results were specific for patients with Class I mutations with no effect in the control group of patients homozygous for the Delta F508 mutation. In both studies, the vast majority of patients with PTCs expressed at least one copy of the W1282X CFTR mutation which is highly prevalent in CF patients of Ashkenazi Jewish descent. In a study performed in the USA, intravenous administration of gentamicin administered for 1 week also resulted in NPD improvement representing CFTR function in four out of five patients with Class I CF mutations (Clancy et al., 2001). Sermet-Gaudilus et al. (2007) reported similar results following 15 days of systemic gentamicin treatment in six out of nine CF patients carrying the Y122X mutation. In all these studies there was a variability of response with some patients not responding to gentamicin. Linde et al. showed that this NMD variability may be related to nonsense-mediated mRNA decay (NMD) – the major machinery evolved to protect against harmful products of nonsense mutations. This is a post-transcriptional translation-dependent surveillance mechanism that prevents the synthesis of proteins carrying PTCs. NMD has been shown to degrade transcripts carrying disease-causing nonsense or frameshift mutations. It is the efficiency of NMD which affect the level of transcripts carrying PTCs, which govern the response to read-through treatment. Response to gentamicin was found only in patients with a higher level of transcripts (Linde et al., 2007). Down regulation of NMD in cells carrying the W1282X mutation increased the level of CFTR nonsense transcripts and enhanced the CFTR chloride channel activity in response to gentamicin. This may have a critical clinical correlation in the read-through of PTCs in various diseases. However, the inconvenience of parenteral administration and the potential for serious toxic effects preclude long-term systemic use of gentamicin for suppression of nonsense mutations.
Recently a novel agent PTC124 or Ataluren was developed through an extensive high throughput screening program using a luciferase based system (Welch et al., 2007). The molecule is a 1,2,4-oxadiazole benzoic acid and is reported to interact with mammalian ribosomes in a manner distinct from aminoglycosides. Ataluren does not have antibiotic activity and is orally bioavailable. Studies in myocytes isolated from the mdx mouse defined target doses and exposures to rescue dystrophin function. After treatment with, full length dystrophin was localized in skeletal and cardiac tissue. In the G542X-hCFTR mouse oral and intra-peritoneal administration led to detectable full length CFTR localization at the apical cell membrane of intestinal glandular cells by immuno-fluorescent staining together with improved chloride conductance as assayed by trans-epithelial ion transport (Du et al., 2008). Correction of CFTR chloride transport was incomplete. Less than 30% of the short-circuit current that was observed in wild-type mice occurred in the CF mice. This suggests that potential clinical benefit would only need partial restoration of protein function.
Phase I studies in healthy volunteers established the initial safety profile for Ataluren, and defined dosing regimens to achieve target trough plasma concentrations (of 2–10 μg/mL) that are known to be active in preclinical models.
Our group reported a phase II clinical trial of PTC124 in 23 patients with cystic fibrosis (Kerem et al., 2008). This open label study included two consecutive 28-day cycles, each of 14 days of treatment followed by 14 days of washout. In the first cycle, patients received daily postprandial doses of 4, 4, and 8 mg/kg. The doses were increased in the second cycle to 10, 10, and 20 mg/kg. Convincing changes in NPD were observed in more than half the patients in the first cycle. Interestingly, this effect was seen in only about a third of the patients in the second cycle. Coupled to this finding, modest but statistically significant improvements in lung function and bodyweight were observed after the first cycle which, in general, persisted to the end of the second cycle. Following this study, 19 of these patients were enrolled in a 12 week open label extension study. NPD improvements were reported over time in both the higher and lower dose treatment groups including four patients who did not respond to PTC124 in the 2 week study. This was accompanied by modest improvements in pulmonary function and a significant reduction in quantitative cough assessment (Wilschanski et al., 2011; Figure 1). A similar phase 2 study was performed on adults in the United States which did not reach statistical significance in nasal potential difference measurements. This may be due to the multitude of sites performing the trial each having relatively few patients and the different mutations carried by the patients.
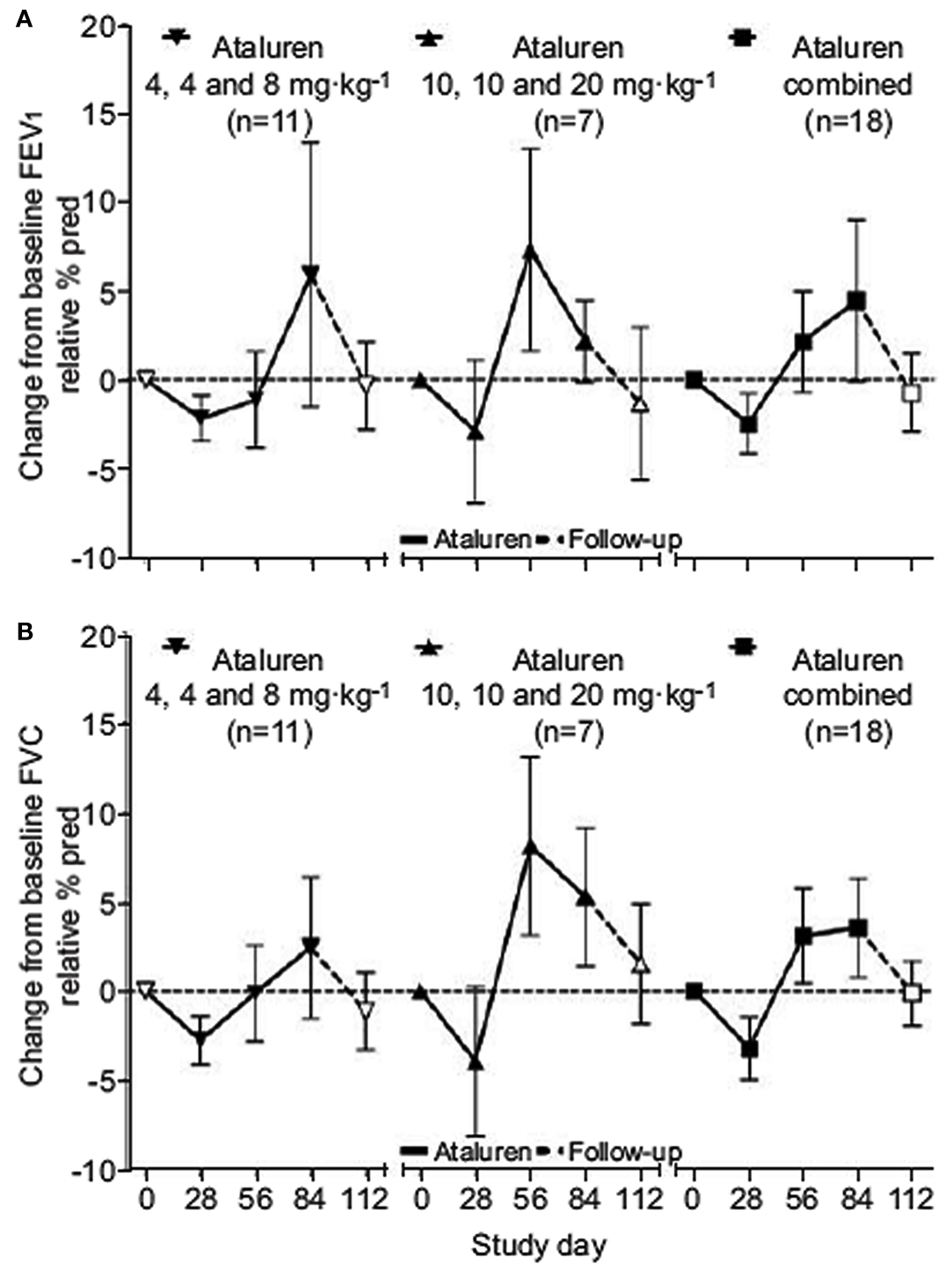
Figure 1. Change in mean pulmonary function tests. Data are presented as mean ± SEM. (A) FEV1, forced expiratory volume in 1 s. (B) FVC, forced vital capacity.
A similar phase 2a study was carried out in children in France and Belgium. Twenty-two children aged 6–18 years of age completed a dose-ranging crossover study. There was significant improvement in NPD and nasal epithelial CFTR protein by immunofluorescence (Sermet-Gaudelus et al., 2010).
The development of agents that suppress premature stop codons, such as Ataluren, is not without theoretical risk, because there are at least two potential concerns about its mode of action. First, Ataluren might lead to erroneous suppression of native stop codons, and second, Ataluren might disrupt NMD. Encouragingly, Ataluren seems to be remarkably selective for premature, rather than native, stop codons, and it seems to restrict its action to those ribosomes that are involved in productive translation of proteins rather than those that are involved in NMD. These preclinical findings were supported by the observation that CFTR mRNA levels are largely unaffected by Ataluren treatment (unlike after gentamicin administration).
Suppression of PTCs with small molecules is emerging as a rational approach to treat a variety of genetic disorders including CF. Following these positive findings, a multinational Phase 3 placebo-controlled efficacy trials is currently underway. These studies provide hope that a treatment strategy could be applied to the basic defect rather than downstream manifestations of the disease.
References
Barton-Davis, E. R., Cordier, L., Shoturma, D. I., Leland, S. E., and Sweeney, H. L. (1999). Aminoglycoside antibiotics restore dystrophin function to skeletal muscle of mdx mice. J. Clin. Invest. 104, 3745–3781.
Bedwell, D. M., Kaenjak, A., Benos, D. J., Bebok, Z., Bubien, J. K., Hong, J., Tousson, A., Clancy, J. P., and Sorscher, E. J. (1997). Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat. Med. 3, 1280–1284.
Clancy, J. P., Bebok, Z., Ruiz, F., King, C., Jones, J., Walker, L., Greer, H., Hong, J., Wing, L., Macaluso, M., Lyrene, R., Sorscher, E. J., and Bedwell, D. M. (2001). Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 163, 1683–1692.
Du, M., Jones, J. R., Lanier, J., Keeling, K. M., Lindsey, J. R., Tousson, A., Bebök, Z., Whitsett, J. A., Dey, C. R., Colledge, W. H., Evans, M. J., Sorscher, E. J., and Bedwell, D. M. (2002). Aminoglycoside suppression of a premature stop mutation in a Cftr-/- mouse carrying CFTR-G542X transgene. J. Mol. Med. 80, 595–604.
Du, M., Liu, X., Welch, E. M., Hirawat, S., Peltz, S. W., and Bedwell, D. M. (2008). PTC124is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc. Natl. Acad. Sci. U.S.A. 105, 2064–2069.
Gorini, L., and Kataja, E. (1964). Phenotypic repair by streptomycin of defective genmotypes in E. coli. Proc. Natl. Acad. Sci. U.S.A. 51, 487–493.
Hermann, T. (2007). Aminoglycoside antibiotics: old drugs and new therapeutic approaches. Cell. Mol. Life Sci. 64, 1841–1852.
Howard, M., Frizzel, R. A., and Bedwell, D. M. (1996). Aminoglycoside antibiotics restore CccFTR function by overcoming premature stop mutations. Nat. Med. 2, 467–469.
Kerem, B., Chiba-Falek, O., and Kerem, E. (1997). Cystic fibrosis in Jews: frequency and mutation distribution. Genet. Test. 1, 35–39.
Kerem, E., Hirawat, S., Armoni, S., Yaakov, Y., Shoseyov, D., Cohen, M., Nissim-Rafinia, M., Blau, H., Rivlin, J., Aviram, M., Elfring, G. L., Northcutt, V. J., Miller, L. L., Kerem, B., and Wilschanski, M. (2008). Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet 372, 719–727.
Linde, L., Boelz, S., Nissim-Rafinia, M., Oren, Y. S., Wilschanski, M., Yaacov, Y., Virgilis, D., Neu-Yilik, G., Kulozik, A. E., Kerem, E., and Kerem, B. (2007). Ninsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. J. Clin. Invest. 117, 683–692.
Sermet-Gaudelus, I., De Boeck, K., Casimir, G., Vermeulen, F., Leal, T., Mogenet, A., Roussel, D., Fritsch, J., Hanssens, L., Hirawat, S., Miller, N. L., Constantine, S., Reha, A., Ajayi, T., Elfring, G. L., and Miller, L. L. (2010). Ataluren (PTC124) induces CFTR protein expression and activity in children with nonsense-mutation cystic fibrosis. Am. J. Respir. Crit. Care Med. 182, 1262–1272.
Sermet-Gaudilus, I., Renouil, M., Fajac, A., Bidou, L., Parbaille, B., Pierrot, S., Davy, N., Bismuth, E., Reinert, P., Lenoir, G., Lesure, J. F., Rousset, J. P., and Edelman, A. (2007). In vitro prediction of stop codon suppression by intravenous gentamicin in patients with cystic fibrosis: a pilot study. BMC Med. 5, 5. doi: 10.1186/1741-7015-5-5
Welch, E., Barton, E. R., Zhuo, J., Tomizawa, Y., Friesen, W. J., Trifillis, P., Paushkin, S., Patel, M., Trotta, C. R., Hwang, S., Wilde, R. G., Karp, G., Takasugi, J., Chen, G., Jones, S., Ren, H., Moon, Y. C., Corson, D., Turpoff, A. A., Campbell, J. A., Conn, M. M., Khan, A., Almstead, N. G., Hedrick, J., Mollin, A., Risher, N., Weetall, M., Yeh, S., Branstrom, A. A., Colacino, J. M., Babiak, J., Ju, W. D., Hirawat, S., Northcutt, V. J., Miller, L. L., Spatrick, P., He, F., Kawana, M., Feng, H., Jacobson, A., Peltz, S. W., and Sweeney, H. L. (2007). PTC124 targets genetic disorders caused by nonsense mutations. Nature 447, 87–91.
Wilschanski, M., Famini, C., Blau, H., Rivlin, J., Augarten, A., Avital, A., Kerem, B., and Kerem, E. (2001). A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am. J. Respir. Crit. Care Med. 163, 1683–1692.
Wilschanski, M., Miller, L. L., Shoseyov, D., Blau, H., Rivlin, J., Aviram, M., Cohen, M., Armoni, S., Yaakov, Y., Pugatsch, T., Cohen-Cymberknoh, M., Miller, N. L., Reha, A., Northcutt, V. J., Hirawat, S., Donnelly, K., Elfring, G. L., Ajayi, T., and Kerem, E. (2011). Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur. Respir. J. 38, 59–69.
Citation: Wilschanski M (2012) Class 1 CF mutations. Front. Pharmacol. 3:117. doi: 10.3389/fphar.2012.00117
Received: 14 May 2012; Accepted: 30 May 2012;
Published online: 20 June 2012.
Copyright: © 2012 Wilschanski. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence:bWljaGFlbHdpbEBoYWRhc3NhaC5vcmcuaWw=
