- 1Section of Gerontology and Geriatrics, Department of Medicine, University of Perugia, Perugia, Italy
- 2Geriatrics Department, Medical Faculty, University of Cologne, Cologne, Germany
Several chemical substances belonging to classes of natural dietary origin display protective properties against some age-related diseases including neurodegenerative ones, particularly Alzheimer’s disease (AD). These compounds, known as nutraceuticals, differ structurally, act therefore at different biochemical and metabolic levels and have shown different types of neuroprotective properties. The aim of this review is to summarize data from observational studies, clinical trials, and randomized clinical trials (RCTs) in humans on the effects of selected nutraceuticals against age-related cognitive impairment and dementia. We report results from studies on flavonoids, some vitamins and other natural substances that have been studied in AD and that might be beneficial for the maintenance of a good cognitive performance. Due to the substantial lack of high-level evidence studies there is no possibility for recommendation of nutraceuticals in dementia-related therapeutic guidelines. Nevertheless, the strong potential for their neuroprotective action warrants further studies in the field.
Introduction
Advanced age is often characterized by a decline in a large spectrum of cognitive abilities including reasoning, memory, perceptual speed, and language. The impairment of more of these activities, when lasting long enough and being associated to functional loss is referred to as dementia. Alzheimer’s disease (AD) is the most common and feared form of dementia representing circa 70% of all dementia cases and displaying a dramatic epidemics due to the enormous growth of the aged population worldwide. Incidence of dementia has increased over the recent years although recent epidemiological studies seem to show a decline that needs to be confirmed in the future (Larson et al., 2013). The number of cases is expected to approach a million people per year in USA by 2050 (Alzheimer’s Association, 2009). AD impacts dramatically on everyday life of older adults, being one of the main causes of disability in the old age. There has been an increasing interest in the past decades about interventions that may help to improve cognitive performance in older age or, at least, delay the onset of dementia. Due to the absence of a cure against dementia and AD, the public health priority has focused more recently on prevention of cognitive decline. It is still unclear which factors lead to the molecular cascade of neurodegeneration in AD, but along with genetic and environmental factors vascular pathology and risk factors have been recently shown to play a crucial role in AD pathogenesis (Polidori et al., 2012). Therefore, lifestyle strategies with beneficial effects on neurodegeneration and vascularity – including natural nutrition and nutritional supplementation, cognitive and social activity, physical exercise – have been identified as possible target options for AD prevention (Brown et al., 2010; Polidori et al., 2012; Polidori and Schulz, 2014).
The effect of a correct diet on human health has been reported in many epidemiological studies and randomized controlled trials (RCTs; Everitt et al., 2006). There is clear evidence that a diet rich in specific nutritional food groups (fruit, fish, vegetables) can reduce the incidence and prevalence of some of the main clinical outcomes, such as neurodegenerative disorders, cardiovascular diseases, diabetes, cancer (Grant, 1999; Panza et al., 2004; Sofi et al., 2008; Frisardi et al., 2010). These specific nutritional food groups are rich in micronutrients and vitamins that, for their characteristic of being of nutritional nature and beneficial for health (like pharmaceuticals) at the same time, have been defined as nutraceuticals by De Felice in 1989 (Kalra, 2003). Among different types of diet, the Mediterranean pattern obtained a considerable amount of interest in the past recent decades due to the results of large epidemiological and bench studies showing its high content in nutraceuticals. The Mediterranean diet is characterized by a high consumption of plant foods, fish, olive oil as primary sources of monounsaturated fat and moderate intake of wine. This kind of food intake pattern might be particularly healthy due to synergistic actions of its components. Synergistic mechanisms between food components are responsible particularly for the neuroprotective effects displayed by certain nutrients and nutraceuticals. In addition, cardiovascular diseases such as diabetes mellitus, hypertension, and lipid disorders, as well as white substance lesions are highly susceptible to micronutrient changes. This might be particularly important in light of the recently shown major pathophysiological role of vascular pathology and risk factors in both AD and its prodromal phase, mild cognitive impairment (MCI; De la Torre, 2010; Polidori et al., 2012; Féart et al., 2013). Independent of the mechanism through which the Mediterranean dietary pattern exerts its beneficial effects, a large amount of studies have recently shown its protective activity against MCI and AD (systematically reviewed and meta-analyzed by Singh et al., 2013 and Psaltopoulou et al., 2013).
The aim of this review was to present evidence on a particular group of plant and food components, the so-called nutraceuticals, which have displayed over the years the ability or a strong potential to act as neuroprotectans and/or delay cognitive impairment.
Practically, no official definition exists for the term “nutraceutical,” though it is often used to describe a broad list of products sold under the premise of food components with an expressed intent of treatment or prevention of disease and for enhancing the health and wellbeing of an individual (Hardy et al., 2003). In other words, nutraceuticals are foods, or food components, that provide medical or health benefits, including prevention and treatment of several diseases (Calabrese et al., 2009).
In this review, we focused our attention on a group of substances proposed to prevent or treat cognitive impairment or dementia, with a particular attention on AD. Nutraceuticals evaluated in humans in epidemiological, observational, or clinical studies as well as in RCTs were selected. Only molecules with stronger evidence for neuroprotection as well as those more widely studied were included. To avoid redundancies, we refer the reader to our previous studies for the role and use of vitamin E family (Mangialasche et al., 2010, 2012, 2013; Mecocci and Polidori, 2012; Mecocci et al., 2013) and to recent reviews for the role and use of vitamin C (Heo et al., 2013; Harrison et al., 2014) and of omega-3 fatty acids (Lin et al., 2012; Sydenham et al., 2012; Dacks et al., 2013; Denis et al., 2013).
Flavonoids
Flavonoids are a group of poliphenolic compounds that are very common in the daily human diet. They are found in most plants, including fruits, vegetables, and several types of natural drinks such as tea, cocoa and wine (Manach et al., 2004; Spencer et al., 2008).
On the basis of their chemical structure they can be divided into six subgroups (Table 1).
Flavonoids and their metabolites modulate several neurological processes as shown by studies in which an interaction with neuronal-glial signaling pathways involved in neuronal survival and function was observed (Spencer, 2010; Williams and Spencer, 2012). In addition, flavonoids induce changes in cerebral blood flow (Francis et al., 2006; Fisher et al., 2006; Williams and Spencer, 2012), upregulate antioxidant enzymes and proteins involved in synaptic plasticity and neuronal repair (Mann et al., 2007; Eggler et al., 2008; Spencer, 2009) and inhibit neuropathological processes in brain regions typically involved in AD pathogenesis (Wang et al., 2008a).
Flavanols: Catechin, Epicatechin, Epigallocatechin, Epigallocathechin Gallate
Flavanols are a main flavonoid group and are found in cocoa and chocolate as well as in black and green tea and in grapes. Research over the past decade has identified flavanols as showing diverse beneficial physiologic and antioxidant effects, particularly in the context of vascular function (Francis et al., 2006).
Catechin and epicatechin are the most abundant flavanols in grape seeds and grape juice. A study on supplementation of grape juice from a variety of Vitis vinifera called Koshu demonstrated an inhibition of glutamate excitotoxicity (Narita et al., 2011). In humans, few clinical trials with grape juice have shown that short- and moderate-term supplementation produces benefit in individuals with cerebrovascular diseases including increased serum antioxidant capacity and reduced LDL oxidation, improvement of endothelial function and reduction of platelet aggregation (Krikorian et al., 2010a).
Daily EGCG treatment in rats has been demonstrated to reduce the progressive increase of oxidative stress induced by hypertension. EGCG also decreases the concentration of reactive oxygen species on hippocampus. Based on these results a therapeutic effect of EGCG in treating vascular-induced learning and memory impairment has been proposed (Wang et al., 2012). Black and green teas have a high content of catechins, being EGCG the most abundant. There are several studies that show the protective effect of catechins in cellular and animal models of AD. Despite the lack of clinical trials with tea polyphenols in neurodegenerative diseases, epidemiological observations in US and Finnish populations showed a reduced risk of Parkinson’s disease in high consumers of tea (Checkoway et al., 2002; Hu et al., 2007) and a reduced risk of cognitive impairment in a Japanese population drinking green tea (Kuriyama et al., 2006).
Consumption of cocoa flavanols has been previously shown to influence cerebral hemodynamics. It has been suggested that one consequence of the effect of cocoa flavanols on cerebral blood flow might be to improve performance on visual and cognitive tasks as shown after drinking of cocoa beverages (Scholey et al., 2010; Field et al., 2011).
Flavonols: Quercetin, Kaempferol
Quercetin, one of the most studied bioflavonoids, is found in many common foods, such as capers, apples, onions and green tea (Kelsey et al., 2010). Its primary activity is to prevent endothelial apoptosis caused by oxidants, thanks to a highly potent antioxidant activity and cytoprotective effects (Dong et al., 2012). There are several in vitro studies about quercetin effects demonstrating that this molecule increases cell survival in neurotoxic conditions as in the presence of hydrogen peroxide (Heo and Lee, 2004), linoleic acid hydroperoxide (Sasaki et al., 2003; Kelsey et al., 2010), interleukin-1β (Sharma et al., 2007; Kelsey et al., 2010). In vivo studies have demonstrated that quercetin could have a role in vascular dementia by decreasing the size of ischemic lesions (Dajas et al., 2003; Kelsey et al., 2010). Quercetin improves memory and hippocampal synaptic plasticity in models of impairment induced by chronic lead exposure and it could have a role in neuronal repairing, as shown in spinal cord injury models (Schültke et al., 2003).
Kaempferol is widely distributed in the human daily diet such as fruits, beverages, tea, and vegetables (Aherne and O’Brien, 2002). Kaempferol protects PC12 cells against the oxidative stress induced by H2O2 (Hong et al., 2009) and improves cognitive learning and memory capability in mice (Lei et al., 2012). It was reported that the intake of flavonols including quercetin, kaempferol, and myricetin has favorable effects on cognitive performance (Spencer, 2009).
Ginkgo biloba (Gb) is a plant whose herbal extracts (mainly EGb761) are often used as an alternative treatment to improve cognitive function. Extracts of Gb include several components, such as the flavonols quercetin and kaempferol as well as terpenoid lactones that are considered to be responsible for the neuroprotective functions of Gb (Rendeiro et al., 2012). Standardized extracts of Gb leaves are studied for their potential to improve memory and cognitive function in general. Hemodynamic, neurotransmitter, and free-radical scavenging effects of Gb have been shown in several studies. All of these biological functions may be relevant to aging and age-related disorders (Maclennan et al., 2002; Brown et al., 2010). For this reason, several in vivo studies from humans have been performed and beneficial effects of Gb in prevention and treatment of neurodegenerative disorders like AD have been shown. Improvement of cognitive performance (Le Bars et al., 1997; Kanowski and Hoerr, 2003), memory (Kanowski and Hoerr, 2003), and attention (Le Bars, 2003; Chan et al., 2007) were consistently observed. Two large RCTs on the use of Gb extracts (the GEM and the GuidAge study) did not show less cognitive decline over time in older adults with normal cognition or MCI taking Gb than those assuming placebo (Snitz et al., 2009; Vellas et al., 2012). Also, Gb showed no effects in reducing either the overall incidence rate of dementia or AD in old age individuals with normal cognition or MCI (De Kosky et al., 2008). The latest published Cochrane review including 36 RCTs could not report a significant evidence for a predictable clinical benefit of Gb for people with dementia or cognitive impairment (Birks and Grimley Evans, 2009).
Flavones: Luteolin, Apigenin
Luteolin is the most abundant flavonoid in plants such as rosemary, celery, and parsley (Chowdhury et al., 2002; El Omri et al., 2012). It has been shown that luteolin has several pharmacological properties including a protective role of DNA against hydrogen peroxide-induced toxicity and anti-inflammatory actions (Cheng et al., 2010; El Omri et al., 2012).
Apigenin was shown to protect neurons against Aβ-mediated toxicity induced by copper, to increase neuronal viability as well as to relieve mitochondrial membrane dissipation and neuronal nuclear condensation (Zhao et al., 2013). Apigenin also modulates GABAergic and glutamatergic transmission in cultured cortical neurons (Losi et al., 2004). Up to now no clinical studies have been performed with luteolin and apigenin in cognitive impairment or AD.
Isoflavones: Soy - Genistein, Daidzein, Glycitin
Soybean (soy) is a rich source of phytoestrogens, especially isoflavones. These are not the only constituents of soy; in fact, soybean contains also several minerals, fibers, proteins and oligosaccharides. The isoflavones from soybean are considered agonists of estrogen receptors. The presence of soy isoflavones may be responsible for the observed memory-improving effects of soybean supplementation. Isoflavones appear to improve cognitive function by mimicking the effects of estrogen, in particular through estrogen receptor β in the brain (Lee et al., 2004a). Estrogen replacement can improve cholinergic function by increasing choline uptake and potassium-stimulated acetylcholine release (Gabor et al., 2003; Bansal and Parle, 2010). Former studies revealed that soy isoflavones improve visual spatial memory and learning ability as well as memory of male and female animals (Duncan et al., 2003; Lee et al., 2004b) and humans (File et al., 2001; Zhao and Brinton, 2007; Bansal and Parle, 2010). Furthermore, soy isoflavones can influence the brain cholinergic system and reduce age-related neuronal loss and cognition decline in male rats (Lee et al., 2004b). A study on young and mature mice (Bansal and Parle, 2010) demonstrated that chronic dietary supplementation with soybean improves cognitive performance, decreases thiobarbituric acid reactive substances (TBARs) and increases plasma glutathione peroxidase levels, suggesting that soy isoflavones have antioxidant properties (Djuric et al., 2001; Lee et al., 2004b).
Few RCTs have been performed with soy supplementation, with controversial results. A long-term supplementation of soy in women had no effect on global cognition but improved visual memory after thirty months (Henderson et al., 2012), while in men, treated for twelve weeks, only spatial working memory improved compared to the placebo group (Thorp et al., 2009). A previous study in postmenopausal women who received soy protein for twelve months had no benefit in cognitive performance (Kreijkamp-Kaspers et al., 2004).
Anthocyanidins: Pelargonidin, Cyanidine, Malvidin
Blueberry, bilberry, cranberry, elderberry, raspberry seeds, and strawberry are sources of natural anthocyanin antioxidants. Proanthocyanidins extracted from grape seeds (the bark of the Chinese Scutellaria baicalensis herb) exert potent anti-inflammatory, antioxidant, antinociceptive, and vasodilatative effects and may show antidepressant properties (Ogle et al., 2013). Berry anthocyanins also improve neuronal and cognitive brain function, ocular health as well as protect genomic DNA integrity (Zafra-Stone et al., 2007). However, blueberries also contain significant amounts of flavanols, flavonols, and other phenolics which may justify their role in increasing their beneficial effects (Harnly et al., 2006).
After blueberries feeding, anthocyanidins are found in specific cerebral sites, including hippocampus and neocortex (Andres-Lacueva et al., 2005). Neurogenesis acting on hippocampus may represent one mechanism by which blueberry flavonoids improve memory. There is strong evidence suggesting that blueberry can improve memory and learning in aged animals. These improvements seem linked to the modulation of important structural and synaptic plasticity markers (Rendeiro et al., 2012). One of the role of anthocyanins in neuroprotection could be mediated through phospholipase A2 inhibition (Frisardi et al., 2010), which is negatively involved in a complex network of signaling pathways linking receptor agonists, oxidants, and proinflammatory cytokines to the release of arachidonic acid and eicosanoid synthesis (Sun et al., 2004).
Memory performance has been demonstrated to be linked to the modulation of the expression of particular proteins like CREB (cAMP-response element-binding protein), which is a pathway known to be activated in response to Aβ and brain-derived neurotrophic factor (BDNF). Changes in CREB and BDNF in berry-feed animals were accompanied by increases in the phosphorylation state of the protein factor ERK, very important for synaptic plasticity and memory formation (Williams et al., 2008). Furthermore, blueberry seems to have a more significant effect on short-term memory than long-term memory, as demonstrated by improved performance in several memory maze tasks (Ramirez et al., 2005; Williams et al., 2008; Rendeiro et al., 2012). Another study (Fuentealba et al., 2011) that underlines the role of berries-extract against Aβ shows how these extracts could partially antagonize two newly found effects of Aβ: the decrease in intracellular Ca2+ activity, an important element in neurodegenerative processes and ATP leakage, an effect of aggregated Aβ (Petrozzi et al., 2007). Short-time blueberry diet might produce benefits on memory in aged rats (Malin et al., 2011) by a suggested alteration of ROS signaling through CREB and MAP-kinase (Brewer et al., 2010). Inflammation pathways and modulation of the expression of inflammatory genes might also be involved (Shukitt-Hale et al., 2008). Finally, there is evidence that anthocyanins have insulin-like and glitazone-like properties which may contribute to improve metabolic function and lipid lowering effects (Kalt et al., 2008; Tsuda, 2008; Krikorian et al., 2010b) as well as to improve memory and reduce depressive symptoms (Krikorian et al., 2010b).
In humans, a prospective evaluation with a food frequency questionnaire showed that a greater intake of blueberries and strawberries is associated with slower rates of cognitive decline in subjects older than seventy years, suggesting the potential protective role of berries on different cognitive functions (Devore et al., 2012).
Non-Flavonoid Polyphenols: Resveratrol and Curcumin
There is evidence that both resveratrol and curcumin, non-flavonoid polyphenols, show beneficial effects in cell culture and in vivo models of neurotoxicity and neurodegeneration.
Resveratrol is a polyphenol found enriched in seeds and skin of several fruits, including grapes used for red wine. It is well known mostly for its cardiovascular effects (Bertelli and Das, 2009; Kelsey et al., 2010). Recent evidence has shown that resveratrol can increase 5-HT activity, which could explain its antidepressant properties (Ogle et al., 2013). In animal models it has been shown that resveratrol inhibits noradrenalin and 5-HT reuptake in rats, with increasing hippocampal serotonin (Xu et al., 2010). Resveratrol is also able to reduce inflammation and protect neurons from death, as shown by in vivo experiments on animal models of oxidation-induced neuronal toxicity (Alvira et al., 2007; Kelsey et al., 2010). Protection of organotypic hippocampal slices from hydroperoxide insults has been also observed (Karlsson et al., 2000; Kelsey et al., 2010). Other possible mechanisms for the neuroprotective action displayed by resveratrol are related to its antioxidant properties and its capacity of modulation of Aβ processing and up-regulation of the longevity-linked gene sirtuin 1 (Pocernich et al., 2011).
Several studies in humans have shown a lower risk of dementia in subjects drinking moderate amounts of red wine when compared to abstainers (Orgogozo et al., 1997; Truelsen et al., 2002). Furthermore, a small clinical trial in healthy adults showed an increase of cerebral blood flow during cognitive tasks in subjects treated with resveratrol compared to placebo (Kennedy et al., 2010).
Curcumin is the most active element of tumeric (Curcuma longa), an herb of the ginger family. It has been a staple of oriental medicine for thousands of years (Ogle et al., 2013). Since the prevalence of AD in Indian countries is much lower than in US, it has been suggested that a diet rich in curcumin may reduce the risk of AD (Ganguli et al., 2000). Curcumin seems to act with different mechanisms including antioxidant, anti-inflammatory and anti-amylodogenic ones. For example, curcumin enhances cell viability by decreasing ROS and inhibiting pro-apoptotic signals in mouse models of encephalitis (Dutta et al., 2009; Kelsey et al., 2010). It also reduces Aβ-related cerebral burden and inflammation in transgenic AD mice (Lim et al., 2001). It has been proposed that curcumin exerts most of its effects by inhibiting monoamine oxidase levels, thereby reducing depression also because it modulates serotonergic, dopaminergic, and noradrenergic transmission (Xu et al., 2012; Ogle et al., 2013).
A 6-month RCT performed in patients with AD showed no beneficial results in cognitive performances (Baum et al., 2008). More recently, a randomized clinical trial with Curcumin C3 Complex -consisting of 95% curcuminoids with 70–80% comprised by curcumin, 15–25% demethoxycurcumin, and 2.5–6.5% bisdemethoxycurcumin-was conducted in mild to moderate AD with no evident benefit on cognitive functions (Ringman et al., 2012).
Carotenoids
Over 700 different members of the carotenoid family have been identified and about 40 are found in human blood and tissue. Major carotenoids in human organism are lycopene, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, and the most prominent carotenoid, β-carotene. Depending on dietary habits, blood levels vary between 0.01 and 1 mol/L but they can be considerably increased upon supplementation with single compounds or mixtures (Stahl et al., 1992). For structural reasons and based on experimental data carotenoids have been assigned as antioxidants (Krinsky and Johnson, 2005). Carotenoids as natural fat-soluble pigments are found mostly in vegetables and fruits that are red, orange, and deep yellow in color. More recently, astaxanthin, a carotenoid mainly present in seafood, has been extensively studied in in vitro and in vivo models, showing antioxidant and anti-inflammatory properties as well as protective functions in microcirculation and in mitochondrial functions (Kidd, 2011) suggesting a potential efficacy in several neurodegenerative diseases (Barros et al., 2014). High plasma carotene concentrations associated with lower mortality from all causes were shown by the SENECA investigators (Buijsse et al., 2005), although conflicting data from intervention studies with β-carotene to prevent cancers and cardiovascular disorders have challenged the concept (Polidori and Stahl, 2009). Like other important carotenoids of the antioxidant network, lutein and zeaxanthin, the predominant carotenoids of the macula lutea, are suggested to act as photoprotectants preventing age-related degeneration of the macula lutea (Sabour-Pickett et al., 2012). Astaxanthin showed a protective effect for visual problems, such as blurred vision or reduced visual acuity, and also improved muscle strength and endurance in runners and in soccer players (Kidd, 2011).
Patients with moderate to severe AD showed lower plasma levels of two major carotenoids, lutein, and β-carotene, compared to patients with mild AD or controls (Wang et al., 2008b). Among AD patients a lower MMSE score (Mini Mental State Examination, a measure of cognitive performance) was associated with lower lutein and β-carotene levels in this study (Wang et al., 2008b). Lycopene displays not only sun protective effects (Stahl et al., 2006) but also beneficial effects against development of prostate cancer (Seren et al., 2008). Among six carotenoids tested, lycopene was the only carotenoid inversely associated with quality of cognitive performance as assessed by both MMSE, DemTect (Dementia Detection Test) and Clock Drawing Test in healthy subjects from 45 to 102 years of age (Polidori et al., 2009).
A randomized trial with beta carotene supplementation in men participating to the Physicians’ Health Study showed, after a mean treatment duration of 18 years, better cognitive performances in the treated compared to the placebo group. The same effect was not observed in newly randomized subjects after one year treatment, suggesting that efficacy of beta carotene can be obtained only after a long term supplementation (Grodstein et al., 2007). In a multicenter trial in old age subjects suffering from MCI, a dietary supplement of astaxanthin, phosphatidylserine, and vitamin E improved memory skills after sixty days of treatment, suggesting a significant potential role of his kind of supplementation in counteracting age-related cognitive decline (Zanotta et al., 2014).
Crocin – Saffron
Crocin is the main chemical compound identified in saffron, whose scientific name is Crocus sativus. It has been used over the years in folk medicine as an antispasmodic, gingival sedative, nerve sedative, carminative, and expectorant stimulant (Akhondzadeh and Abbasi, 2006; Akhondzadeh et al., 2010). It has been reported that saffron active constituents have anticonvulsant, antidepressant, and anti-inflammatory effects, and improve learning and memory (Schmidt et al., 2007). Crocin is the actual active component involved in both the improvement of learning and memory and preventing effect of long term potentiation blocked by ethanol (Akhondzadeh and Abbasi, 2006) and its potential in the treatment of neurodegenerative diseases like AD (Khalili and Hamzeh, 2010). In animal models, the effectiveness of crocin has been shown in antagonizing the cognitive deficits caused by neurotoxic agents like streptozocin (Naghizadeh et al., 2013).
Crocin improved cognition as evaluated by means of ADAS-Cog and CDR-SB in subjects with mild to moderate AD (Akhondzadeh et al., 2010). In a recent in vivo study, it has been demonstrated that crocin significantly modulate the levels of oxidative markers in the hippocampus, abolishing the deleterious effects of chronic stress on learning and memory (Ghadrdoost et al., 2011).
B-Vitamins: Folate, Cobalamin, Pyridoxin
Folate (vitamin B9), cobalamin (vitamin B12), and pyridoxin (vitamin B6) are the most studied B-vitamins in the field of cognitive decline and dementia (Brown et al., 2010). They are essential for maintaining the integrity of the nervous and hematopoietic systems (Malouf and Areosa Sastre, 2009).
Folate is absorbed from the diet and its decrease in blood is highly dependent on a poor diet, malabsorption, and alcoholism; cobalamin deficiency is also associated with malabsorption due to digestive disorders occurring in older adults, and can result in irreversible neurological disorders such as peripheral neuropathy (Rébeillé et al., 2007; Clarke, 2008). Vitamin B6 - comprising three chemically distinct compounds, pyridoxal, pyridoxamine, and pyridoxine - is involved in the regulation of mental function and mood. It is also an essential homocysteine re-methylation cofactor, and its deficiency is associated with an increase in blood homocysteine levels (Malouf and Grimley Evans, 2003). Poor vitamin B6 status is common among older people. Folate and cobalamine deficiencies cause accumulation of homocysteine. Many studies have found cross-sectional associations between low circulating folate levels or hyperhomocysteinemia and low MMSE scores (Morris and Jacques, 2009; Morris et al., 2012). Vitamin B12 is involved in the methylation of homocysteine to methionine for the synthesis of methyl acceptors such as membrane phospholipids, myelin and neurotransmitters. Homocysteine is potentially toxic to neurons, its levels have been associated with atrophic changes in the brain and are negatively correlated with neuropsychological tests scores; it is also considered a marker for low serum vitamin B12 and folate (Ellinson et al., 2004).
A prospective study over a 4, 5-year period found that homocysteine is a risk factor for dementia or cognitive impairment (Haan et al., 2007). In this study, plasma cobalamin levels were associated with reduced risk (Haan et al., 2007). A recent clinical cohort trial found that plasma homocysteine levels are inversely related to cognitive performance, but no evidence of a significant protection of high plasma folate against dementia was found (Morris et al., 2012). However, B12- and B6-vitamin treatment has been demonstrated to stabilize performance on the CLOX test (Royall et al., 1998) as well as on executive and planning function (De Jager et al., 2012). Kado et al. (2005) and colleagues demonstrated that folate, but not cobalamin levels, are independently predictive of cognitive decline over a 7-year period in high functioning old people.
These heterogeneous and contradictory results are reported in the last Cochrane reviews, in which a significant effect of B-vitamin treatment in cognitive function could overall not be reported (Malouf and Grimley Evans, 2008; Malouf and Areosa Sastre, 2009).
Supplementation with B vitamins including vitamin B6 has been shown to reduce blood homocysteine levels. In addition, B6 vitamin concentration has been associated with better global cognition scores, especially with better attention, and executive function scores (Moorthy et al., 2012). Although vitamin B6 did not succeed in reducing atherosclerotic manifestations in hyperhomocysteinemic patients (Cacciapuoti, 2013), neuropsychiatric disorders - seizures, migraine, chronic pain, and depression - have been linked to vitamin B6 deficiency. However, there is no evidence that short-term treatment with vitamin B6 improves mood (depression, fatigue, and tension symptoms) or cognition (Malouf and Grimley Evans, 2003).
Recent RCTs on the effects of folate, vitamin B12, and vitamin B6 supplementation have been performed in subjects with mild to moderate AD. These failed in showing any effect of supplementation in slowing cognitive decline (Sun et al., 2007; Aisen et al., 2008; Malouf and Grimley Evans, 2008; Malouf and Areosa Sastre, 2009). A review on supplementation of vitamins B12, B6, and folic acid alone or in combination showed that results from nineteen RCTs did not appear to improve cognitive function in individuals with or without existing cognitive impairment (Ford and Almeida, 2012). So, it remains to be established if prolonged treatment with B-vitamins can reduce the risk of dementia in later life. However, in a recent study high-dose B-vitamin treatment (folic acid, vitamin B6, and vitamin B12) not only slowed shrinkage of the whole brain volume over 2 years but it reduced, by as much as sevenfold, the cerebral atrophy in those gray matter regions specifically vulnerable to the AD process, including the medial temporal lobe (Douaud et al., 2013).
Diterpenes: Carnosic Acid, and Rosmarinic Acid
Carnosic and rosmarinic acids are two of the most important antioxidant compounds in rosemary. They are parts of the bigger family of phenolic acids and diterpenes, that have been extensively studied (Yang et al., 2001; Kayashima and Matsubara, 2012). Both carnosic and rosmarinic acid showed a neuroprotective action both in in vitro models of neuronal death and in in vivo models of neurodegeneration. They scavenge reactive nitrogen species (Qiao et al., 2005; Kelsey et al., 2010) and protect neuroblastoma cells from hydrogen peroxide-induced oxidative stress (Lee et al., 2008; Kelsey et al., 2010).
Diterpenes also significantly alleviate memory impairment associated with Aβ neurotoxicity in AD and significantly delay the onset of the disease (Kelsey et al., 2010; Shimojo et al., 2010). Recently it has been found that carnosic acid has antiangiogenic effects (Kayashima and Matsubara, 2012). The neuroprotective effects of this substance, therefore, might be explained on the basis of the recently identified role of angiogenesis in the formation of β-amyloid plaques and the consequent neurotoxicity (Vagnucci and Li, 2003). The exact mechanism by which carnosinic acid inhibits angiogenesis is not clear; however, its antioxidant activity seems to play an important antiangiogenic role.
Conclusion and Future Perspective
When placed in the context of a healthy lifestyle behavior, age-related changes in nutrition may play an important role in brain functioning as well as in major organ functioning of old people. Susceptibility of elderly population to specific nutrient deficits may exacerbate processes of cognitive decline. Indeed, there is large evidence regarding benefits of several nutrients in the common diet towards cognitive impairment and other diseases. There is great public and scientific interest about the potential of nutritional supplementation to prevent age-related diseases in general and cognitive decline in particular by counteracting deleterious neurodegenerative and pathological processes. We reviewed several components of common diets and several phytochemicals that have been shown to have benefits on these diseases and cognitive impairment. Unfortunately, there is a substantial lack of well conducted studies to be included in comparison analyses; scientific literature is still poor of RCTs (a summary is reported in Table 2) for effects of some of these molecules, and lots of studies are conducted on either in vitro models or animal models. A very few studies testing the effects of a combination of two substances or antioxidant mixtures also display little benefit against AD onset or progression as well as against the transition of MCI to AD. All critical components of studies on nutraceuticals in cognitive impairment, from sample size to cell types, to dosage to cognitive measures used, are not comparable. For instance, diagnostic criteria of MCI and AD as well as inclusion criteria and outcomes are not homogeneous. Start and end of the intervention with a particular nutraceutical in MCI or AD have been set for logistic reasons mostly so that intervention begin occurs too late during the course of the disease and study end delimits a far too short intervention period. Finally, specific compound-related issues might be responsible for the lack of clear success of intervention, including dosage-, kinetic-, bioavailability-, metabolic, and genetic-related issues that have been repeatedly discussed (http://lpi.oregonstate.edu/infocenter/cognition.html; Polidori and Schulz, 2014). Despite this, it seems difficult to uniform trial design and method in AD nutritional studies.
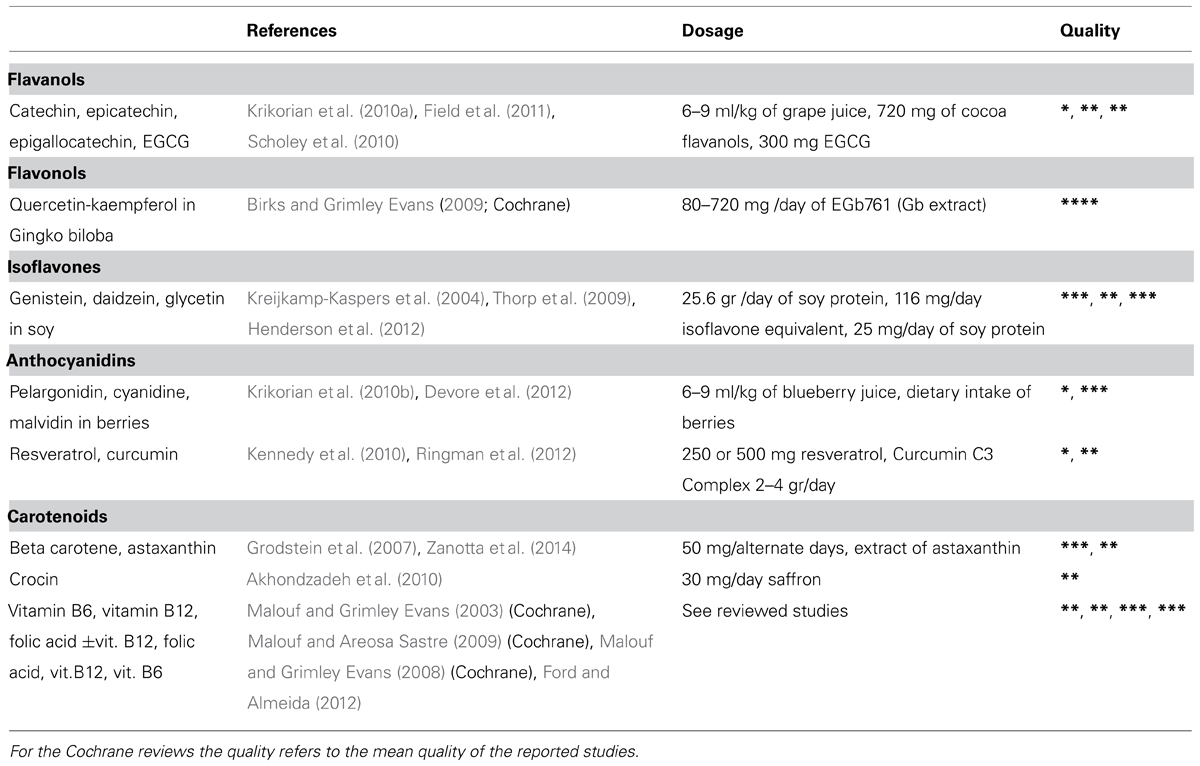
TABLE 2. Nutraceuticals in clinical trials: reference, dosage, and study quality (*low; **moderate; ***high; ****very high).
Theoretically, phytochemical-based treatments for geriatric cognitive decline and depression could be moved into a stronger clinical trial level on humans, especially due to their low toxicity and high bioavailability. Future studies addressing whether short-term or long-term dietary intake of nutraceuticals can reduce the severity and incidence of neurodegenerative and other age-related diseases appear critical and important for the future.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aherne, S. A., and O’Brien, N. M. (2002). Dietary flavonols: chemistry, food content, and metabolism. Nutrition 18, 75–81. doi: 10.1016/S0899-9007(01)00695-5
Aisen, P. S., Schneider, L. S., Sano, M., Diaz-Arrastia, R., van Dyck, C. H., Weiner, M. F., et al. (2008). High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 300, 1774–1783. doi: 10.1001/jama.300.15.1774
Akhondzadeh, S., and Abbasi, S. H. (2006). Herbal medicine in the treatment of Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 21, 113–118. doi: 10.1177/153331750602100211
Akhondzadeh, S., Sabet, M. S., Harirchian, M. H., Togha, M., Cheraghmakani, H., Razeghi, S., et al. (2010). Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 35, 581–588. doi: 10.1111/j.1365-2710.2009.01133.x
Alvira, D., Yeste-Velasco, M., Folch, J., Verdaguer, E., Canudas, A. M., Pallàs, M., et al. (2007). Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience 147, 746–756. doi: 10.1016/j.neuroscience.2007.04.029
Alzheimer’s Association, (2009). Available at: http://www.alz.org/alzheimers_disease_facts_and_figures.asp
Andres-Lacueva, C., Shukitt-Hale, B., Galli, R. L., Jauregui, O., Lamuela-Raventos, R. M., and Joseph, J. A. (2005). Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 8, 111–120. doi: 10.1080/10284150500078117
Bansal, N., and Parle, M. (2010). Soybean supplementation helps reverse age- and scopolamine-induced memory deficits in mice. J. Med. Food 13, 1293–1300. doi: 10.1089/jmf.2010.1132
Barros, M. P., Poppe, S. C., and Bondan, E. F. (2014). Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 6, 1293–1317. doi: 10.3390/nu6031293
Baum, L., Lam, C. W., Cheung, S. K., Kwok, T., Lui, V., Tsoh, J., et al. (2008). Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin inpatients with Alzheimer disease. J. Clin. Psychopharmacol. 28, 110–113. doi: 10.1097/jcp.0b013e318160862c
Bertelli, A. A., and Das, D. K. (2009). Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 54, 468–476. doi: 10.1097/FJC.0b013e3181bfaff3
Birks, J., and Grimley Evans, J. (2009). Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst. Rev. CD003120. doi: 10.1002/14651858.CD003120.pub3
Brewer, G. J., Torricelli, J. R., Lindsey, A. L., Kunz, E. Z., Neuman, A., Fisher, D. R., et al. (2010). Age-related toxicity of amyloid-beta associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. J. Nutr. Biochem. 21, 991–998. doi: 10.1016/j.jnutbio.2009.08.005
Brown, L. A., Riby, L. M., and Reay, J. L. (2010). Supplementing cognitive aging: a selective review of the effects of ginkgo biloba and a number of everyday nutritional substances. Exp. Aging Res. 36, 105–122. doi: 10.1080/03610730903417960
Buijsse, B., Feskens, E. J., Schlettwein-Gsell, D., Ferry, M., Kok, F. J., Kromhout, D., et al. (2005). Plasma carotene and alpha-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am. J. Clin. Nutr. 82, 879–886.
Cacciapuoti, F. (2013). Lowering homocysteine levels with folic acid and B-vitamins do not reduce early atherosclerosis, but could interfere with cognitive decline and Alzheimer’s disease. J. Thromb. Thrombolysis 36, 258–262. doi: 10.1007/s11239-012-0856-x
Calabrese, V., Cornelius, C., Mancuso, C., Barone, E., Calafato, S., Bates, T., et al. (2009). Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front. Biosci. 14:376–397. doi: 10.2741/3250
Chan, P. C., Xia, Q., and Fu, P. P. (2007). Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 25, 211–244. doi: 10.1080/10590500701569414
Checkoway, H., Powers, K., Smith-Weller, T., Franklin, G. M., Longstreth, W. T. Jr., and Swanson, P. D. (2002). Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 155, 732–738. doi: 10.1093/aje/155.8.732
Cheng, H. Y., Hsieh, M. T., Tsai, F. S., Wu, C. R., Chiu, C. S., Lee, M. M., et al. (2010). Neuroprotective effect of luteolin on amyloid beta protein (25-35)-induced toxicity in cultured rat cortical neurons. Phytother. Res. 24(Suppl 1), S102–S108. doi: 10.1002/ptr.2940
Chowdhury, A. R., Sharma, S., Mandal, S., Goswami, A., Mukhopadhyay, S., and Majumder, H. K. (2002). Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 366, 653–661. doi: 10.1042/BJ20020098
Clarke, R. (2008). B-vitamins and prevention of dementia. Proc. Nutr. Soc. 67, 75–81. doi: 10.1017/S0029665108006046
Dajas, F., Rivera-Megret, F., Blasina, F., Arredondo, F., Abin-Carriquiry, J. A., Costa, G., et al. (2003). Neuroprotection by flavonoids. Braz. J. Med. Biol. Res. 36, 1613–1620. doi: 10.1590/S0100-879X2003001200002
Dacks, P. A., Shineman, D. W., and Fillit, H. M. (2013). Current evidence for the clinical use of long-chain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer’s disease. J. Nutr. Health Aging 17, 240–251. doi: 10.1007/s12603-012-0431-3
De Jager, C. A., Oulhaj, A., Jacoby, R., Refsum, H., and Smith, A. D. (2012). Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int. J. Geriatr. Psychiatry 27, 592–600. doi: 10.1002/gps.2758
De Kosky, S. T., Williamson, J. D., Fitzpatrick, A. L., Kronmal, R. A., Ives, D. G., Saxton, J. A., et al. (2008). Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA 300, 2253–2262. doi: 10.1001/jama.2008.683
De la Torre, J. C. (2010). Vascular risk factor detection and control may prevent Alzheimer’s disease. Ageing Res. Rev. 9, 218–25. doi: 10.1016/j.arr.2010.04.002
Denis, I., Potier, B., Vancassel, S., Heberden, C., and Lavialle, M. (2013). Omega-3 fatty acids and brain resistance to ageing and stress: body of evidence and possible mechanisms. Ageing Res. Rev. 12, 579–594. doi: 10.1016/j.arr.2013.01.007
Devore, E. E., Kang, J. H., Breteler, M. M., and Grodstein, F. (2012). Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 72, 135–143. doi: 10.1002/ana.23594
Djuric, Z., Chen, G., Doerge, D. R., Heilbrun, L. K., and Kucuk, O. (2001). Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 172, 1–6. doi: 10.1016/S0304-3835(01)00627-9
Dong, Z. H., Zhang, C. Y., and Pu, B. H. (2012). Effects of ginkgo biloba tablet in treating mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi 32, 1208–1211.
Douaud, G., Refsum, H., de Jager, C. A., Jacoby, R., Nichols, T. E., Smith, S. M., et al. (2013). Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. U.S.A. 110, 9523–9528. doi: 10.1073/pnas.1301816110
Duncan, A. M., Phipps, W. R., and Kurzer, M. S. (2003). Phyto-oestrogens. Best Pract. Res. Clin. Endocrinol. Metab. 17, 253–271. doi: 10.1016/S1521-690X(02)00103-3
Dutta, K., Ghosh, D., and Basu, A. (2009). Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J. Neuroimmune Pharmacol. 4, 328–337. doi: 10.1007/s11481-009-9158-2
Eggler, A. L., Gay, K. A., and Mesecar, A. D. (2008). Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 52(Suppl. 1), S84–S94. doi: 10.1002/mnfr.200700249
El Omri, A., Han, J., Kawada, K., Ben, A. M., and Isoda, H. (2012). Luteolin enhances cholinergic activities in PC12 cells through ERK1/2 and PI3K/Akt pathways. Brain Res. 1437, 16–25. doi: 10.1016/j.brainres.2011.12.019
Ellinson, M., Thomas, J., and Patterson, A. (2004). A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J. Hum. Nutr. Diet. 17, 371–383. doi: 10.1111/j.1365-277X.2004.00532.x
Everitt, A. V., Hilmer, S. N., Brand-Miller, J. C., Jamieson, H. A., Truswell, A. S., Sharma, A. P., et al. (2006). Dietary approaches that delay age-related diseases. Clin. Interv. Aging 1, 11–31. doi: 10.2147/ciia.2006.1.1.11
Féart, C., Samieri, C., Allès, B., and Barberger-Gateau, P. (2013). Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc. Nutr. Soc. 72, 140–152. doi: 10.1017/S0029665112002959
Field, D. T., Williams, C. M., and Butler, L. T. (2011). Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 103, 255–260. doi: 10.1016/j.physbeh.2011.02.013
File, S. E., Jarrett, N., Fluck, E., Duffy, R., Casey, K., and Wiseman, H. (2001). Eating soya improves human memory. Psychopharmacology (Berl). 157, 430–436. doi: 10.1007/s002130100845
Fisher, N. D., Sorond, F. A., and Hollenberg, N. K. (2006). Cocoa flavanols and brain perfusion. J. Cardiovasc. Pharmacol. 47(Suppl. 2), S210–S214. doi: 10.1097/00005344-200606001-00017
Ford, A. H., and Almeida, O. P. (2012). Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J. Alzheimers Dis. 29, 133–149. doi: 10.3233/JAD-2012-111739
Francis, S. T., Head, K., Morris, P. G., and Macdonald, I. A. (2006). The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 47(Suppl. 2), S215–S220. doi: 10.1097/00005344-200606001-00018
Frisardi, V., Panza, F., Solfrizzi, V., Seripa, D., and Pilotto, A. (2010). Plasma lipid disturbances and cognitive decline. J. Am. Geriatr. Soc. 58, 2429–2430. doi: 10.1111/j.1532-5415.2010.03164.x
Fuentealba, J., Dibarrart, A. J., Fuentes-Fuentes, M. C., Saez-Orellana, F., Quinones, K., Guzman, L., et al. (2011). Synaptic failure and adenosine triphosphate imbalance induced by amyloid-β aggregates are prevented by blueberry-enriched polyphenols extract. J. Neurosci. Res. 89, 1499–1508. doi: 10.1002/jnr.22679
Gabor, R., Nagle, R., Johnson, D. A., and Gibbs, R. B. (2003). Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res. 962, 244–247. doi: 10.1016/S0006-8993(02)04053-2
Ganguli, M., Chandra, V., Kamboh, M. I., Johnston, J. M., Dodge, H. H., Thelma, B. K., et al. (2000). Apolipoprotein E polymorphism and Alzheimer disease: The Indo-US Cross-National Dementia Study. Arch. Neurol. 57, 824–830. doi: 10.1001/archneur.57.6.824
Ghadrdoost, B., Vafaei, A. A., Rashidy-Pour, A., Hajisoltani, R., Bandegi, A. R., Motamedi, F., et al. (2011). Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 667, 222–229. doi: 10.1016/j.ejphar.2011.05.012
Grant, W. B. (1999). Dietary links to Alzheimer’s disease: 1999 update. J. Alzheimers Dis. 1, 197–201.
Grodstein, F., Kang, J. H., Glynn, R. J., Cook, N. R., and Gaziano, J. M. (2007). A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians’ Health Study II. Arch. Intern. Med. 167, 2184–2190. doi: 10.1001/archinte.167.20.2184
Haan, M. N., Miller, J. W., Aiello, A. E., Whitmer, R. A., Jagust, W. J., Mungas, D. M., et al. (2007). Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 85, 511–517.
Hardy, G., Hardy, I., and Ball, P. A. (2003). Nutraceuticals – a pharmaceutical viewpoint: part II. Curr. Opin. Clin. Nutr. Metab. Care 6, 661–671. doi: 10.1097/00075197-200311000-00010
Harnly, J. M., Doherty, R. F., Beecher, G. R., Holden, J. M., Haytowitz, D. B., Bhagwat, S., et al. (2006). Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 54, 9966–9977. doi: 10.1021/jf061478a
Harrison, F. E., Bowman, G. L., and Polidori, M. C. (2014). Ascorbic acid and the brain: rationale for the use against cognitive impairment. Nutrients 6, 1752–1781. doi: 10.3390/nu6041752
Henderson, V. W., St John, J. A., Hodis, H. N., Kono, N., McCleary, C. A., Franke, A. A., et al. (2012). Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology 78, 1841–1848. doi: 10.1212/WNL.0b013e318258f822
Heo, H. J., and Lee, C. Y. (2004). Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 52, 7514–7517. doi: 10.1021/jf049243r
Heo, J. H., Hyon-Lee, and Lee, K. M. (2013). The possible role of antioxidant vitamin C in Alzheimer’s disease treatment and prevention. Am. J. Alzheimers Dis. Other Demen. 28, 120–125. doi: 10.1177/1533317512473193
Hong, J. T., Yen, J. H., Wang, L., Lo, Y. H., Chen, Z. T., and Wu, M. J. (2009). Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells. Toxicol. Appl. Pharmacol. 237, 59–68. doi: 10.1016/j.taap.2009.02.014
Hu, G., Bidel, S., Jousilahti, P., Antikainen, R., and Tuomilehto, J. (2007). Coffee and tea consumption and the risk of Parkinson’s disease. Mov. Disord. 22, 2242–2248. doi: 10.1002/mds.21706
Kado, D. M., Karlamangla, A. S., Huang, M. H., Troen, A., Rowe, J. W., Selhub, J., et al. (2005). Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am. J. Med. 118, 161–167. doi: 10.1016/j.amjmed.2004.08.019
Kalra, E. K. (2003). Nutraceutical – definition and introduction. AAPS Pharm. Sci. 5, E25. doi: 10.1208/ps050325
Kalt, W., Foote, K., Fillmore, S. A., Lyon, M., Van Lunen, T. A., and McRae, K. B. (2008). Effect of blueberry feeding on plasma lipids in pigs. Br. J. Nutr. 100, 70–78. doi: 10.1017/S0007114507877658
Kanowski, S., and Hoerr, R. (2003). Ginkgo biloba extract EGb 761 in dementia: intent-to-treat analyses of a 24-week, multi-center, double-blind, placebo-controlled, randomized trial. Pharmacopsychiatry 36, 297–303. doi: 10.1055/s-2003-45117
Karlsson, J., Emgard, M., Brundin, P., and Burkitt, M. J. (2000). Trans-resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. J. Neurochem. 75, 141–150. doi: 10.1046/j.1471-4159.2000.0750141.x
Kayashima, T., and Matsubara, K. (2012). Antiangiogenic effect of carnosic acid and carnosol, neuroprotective compounds in rosemary leaves. Biosci. Biotechnol. Biochem. 76, 115–119. doi: 10.1271/bbb.110584
Kelsey, N. A., Wilkins, H. M., and Linseman, D. A. (2010). Nutraceutical antioxidants as novel neuroprotective agents. Molecules 15, 7792–7814. doi: 10.3390/molecules15117792
Kennedy, D. O., Wightman, E. L., Reay, J. L., Lietz, G., Okello, E. J., Wilde, A., et al. (2010). Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 91, 1590–1597. doi: 10.3945/ajcn.2009.28641
Khalili, M., and Hamzeh, F. (2010). Effects of active constituents of Crocus sativus L., crocin on streptozocin-induced model of sporadic Alzheimer’s disease in male rats. Iran Biomed. J. 14, 59–65.
Kidd, P. (2011). Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 16, 355–364.
Kreijkamp-Kaspers, S., Kok, L., Grobbee, D. E., de Haan, E. H., Aleman, A., Lampe, J. W., et al. (2004). Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 292, 65–74. doi: 10.1001/jama.292.1.65
Krikorian, R., Nash, T. A., Shidler, M. D., Shukitt-Hale, B., and Joseph, J. A. (2010a). Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 103, 730–734. doi: 10.1017/S0007114509992364
Krikorian, R., Shidler, M. D., Nash, T. A., Kalt, W., Vinqvist-Tymchuk, M. R., Shukitt-Hale, B., et al. (2010b). Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 58, 3996–4000. doi: 10.1021/jf9029332
Krinsky, N. I., and Johnson, E. J. (2005) Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26, 459–516. doi: 10.1016/j.mam.2005.10.001
Kuriyama, S., Hozawa, A., Ohmori, K., Shimazu, T., Matsui, T., Ebihara, S., et al. (2006). Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 83, 355–361.
Larson, E. B., Yaffe, K., and Langa, K. M. (2013). New insights into the dementia epidemic. New Engl. J Med. 369, 2275–2277. doi: 10.1056/NEJMp1311405
Le Bars, P. L. (2003). Magnitude of effect and special approach to Ginkgo biloba extract EGb 761 in cognitive disorders. Pharmacopsychiatry 36(Suppl. 1), S44–S49. doi: 10.1055/s-2003-40458
Le Bars, P. L., Katz, M. M., Berman, N., Itil, T. M., Freedman, A. M., and Schatzberg, A. F. (1997). A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 278, 1327–1332. doi: 10.1001/jama.1997.03550160047037
Lee, H., Kim, H. J., Kim, J. M., and Chang, N. (2004a). Effects of dietary folic acid supplementation on cerebrovascular endothelial dysfunction in rats with induced hyperhomocysteinemia. Brain Res. 996, 139–147. doi: 10.1016/j.brainres.2003.10.027
Lee, Y. B., Lee, H. J., Won, M. H., Hwang, I. K., Kang, T. C., Lee, J. Y., et al. (2004b). Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J. Nutr. 134, 1827–1831.
Lee, H. J., Cho, H. S., Park, E., Kim, S., Lee, S. Y., Kim, C. S., et al. (2008). Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 250, 109–15. doi: 10.1016/j.tox.2008.06.010
Lei, Y., Chen, J., Zhang, W., Fu, W., Wu, G., Wei, H., et al. (2012). In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 135, 2702–2707. doi: 10.1016/j.foodchem.2012.07.043
Lim, G. P., Chu, T., Yang, F., Beech, W., Frautschy, S. A., and Cole, G. M. (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21, 8370–8377.
Lin, P. Y., Chiu, C. C., Huang, S. Y., and Su, K. P. (2012). A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J. Clin. Psychiatry 73, 1245–1254. doi: 10.4088/JCP.11r07546
Losi, G., Puia, G., Garzon, G., de Vuono, M. C., and Baraldi, M. (2004). Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur. J. Pharmacol. 502, 41–46. doi: 10.1016/j.ejphar.2004.08.043
Maclennan, K. M., Darlington, C. L., and Smith, P. E. (2002). The CNS effects of ginkgo biloba extracts and ginkgolide B. Progr. Neurobiol. 67, 235–257. doi: 10.1016/S0301-0082(02)00015-1
Malin, D. H., Lee, D. R., Goyarzu, P., Chang, Y. H., Ennis, L. J., Beckett, E., et al. (2011). Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition 27, 338–342. doi: 10.1016/j.nut.2010.05.001
Malouf, R., and Areosa Sastre, A. (2009). Vitamin B12 for cognition. Cochrane Database Syst. Rev. CD004326.
Malouf, R., and Grimley Evans, J. (2003). The effect of vitamin B6 on cognition. Cochrane Database Syst. Rev. CD004393.
Malouf, R., and Grimley Evans, J. (2008). Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. CD004514. doi: 10.1002/14651858.CD004514.pub2
Manach, C., Scalbert, A., Morand, C., Rémésy, C., and Jiménez, L. (2004). Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79, 727–747.
Mangialasche, F., Kivipelto, M., Mecocci, P., Rizzuto, D., Palmer, K., Winblad, B., et al. (2010). High plasma levels of vitamin E forms and reduced Alzheimer’s disease risk in advanced age. J. Alzheimers Dis. 20, 1029–1037. doi: 10.3233/JAD-2010-091450
Mangialasche, F., Solomon, A., Kåreholt, I., Hooshmand, B., Cecchetti, R., Fratiglioni, L., et al. (2013). Serum levels of vitamin E forms and risk of cognitive impairment in a Finnish cohort of older adults. Exp. Gerontol. 48, 1428–1435. doi: 10.1016/j.exger.2013.09.006
Mangialasche, F., Xu, W., Kivipelto, M., Costanzi, E., Ercolani, S., Pigliautile, M., et al. (2012). Tocopherols and Tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. Aging 33, 2282–2290. doi: 10.1016/j.neurobiolaging.2011.11.019
Mann, G. E., Rowlands, D. J., Li, F. Y., de Winter, P., and Siow, R. C. (2007). Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 75, 261–274. doi: 10.1016/j.cardiores.2007.04.004
Mecocci, P., and Polidori, M. C. (2012). Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta 1822, 631–638. doi: 10.1016/j.bbadis.2011.10.006
Mecocci, P., Polidori, M. C., and Praticò, D. (2013). “Antioxidant clinical trials in mild cognitive impairment and alzheimer’s disease,” in Studies on Alzheimer’s Disease, eds D. Praticò and P. Mecocci (New York: Springer), 223–232.
Morris, M. S., and Jacques, P. F. (2009). “Folate and neurological function: Epidemiological perspective,” in Folate in Health and Disease, ed. L. B. Bailey, Vol. 2 (Boca Raton, FL: CRC Press), 325–353.
Morris, M. S., Selhub, J., and Jacques, P. F. (2012). Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the Framingham heart study. J. Am. Geriatr. Soc. 60, 1457–1464. doi: 10.1111/j.1532-5415.2012.04076.x
Moorthy, D., Peter, I., Scott, T. M., Parnell, L. D., Lai, C. Q., Crott, J. W., et al. (2012). Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J. Nutr. 142, 1554–1560. doi: 10.3945/jn.112.161828
Naghizadeh, B., Mansouri, M. T., Ghorbanzadeh, B., Farbood, Y., and Sarkaki, A. (2013). Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine 20, 537–542. doi: 10.1016/j.phymed.2012.12.019
Narita, K., Hisamoto, M., Okuda, T., and Takeda, S. (2011). Differential neuroprotective activity of two different grape seed extracts. PLoS ONE 6:e14575. doi: 10.1371/journal.pone.0014575
Ogle, W. O., Speisman, R. B., and Ormerod, B. K. (2013). Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology 59, 23–31. doi: 10.1159/000342208
Orgogozo, J. M., Dartigues, J. F., Lafont, S., Letenneur, L., Commenges, D., Salamon, R., et al. (1997). Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev. Neurol. 153, 185–192.
Panza, F., Solfrizzi, V., Colacicco, A. M., D’Introno, A., Capurso, C., Torres, F., et al. (2004). Mediterranean diet and cognitive decline. Public Health Nutr. 7, 959–963. doi: 10.1079/PHN2004561
Petrozzi, L., Ricci, G., Giglioli, N. J., Siciliano, G., and Mancuso, M. (2007). Mitochondria and neurodegeneration. Biosci. Rep. 27, 87–104. doi: 10.1007/s10540-007-9038-z
Pocernich, C. B., Lange, M. L., Sultana, R., and Butterfield, D. A. (2011). Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 8, 452–469. doi: 10.2174/156720511796391908
Polidori, M. C., Pientka, L., and Mecocci, P. (2012). A review of the major vascular risk factors related to Alzheimer’s disease. J. Alzheimers Dis. 32, 521–530. doi: 10.3233/JAD-2012-120871
Polidori, M. C., Praticó, D., Mangialasche, F., Mariani, E., Aust, O., Anlasik, T., et al. (2009). High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J. Alzheimers Dis. 17, 921–927. doi: 10.3233/JAD-2009-1114
Polidori, M. C., and Schulz, R. J. (2014). Nutritional contributions to dementia prevention: main issues on antioxidant micronutrients. Genes Nutr. 9, 382. doi: 10.1007/s12263-013-0382-2
Polidori, M. C., and Stahl, W. (2009). “Carotenoids and vitamin A,” in Chemoprevention of Cancer and DNA Damage by Dietary Factors, eds I. Johnson, D. De Marini, C. Gerhäuser, and S. Knasmüller (Weinheim: Wiley International), 23.
Psaltopoulou, T., Sergentanis, T. N., Panagiotakos, D. B., Sergentanis, I. N., Kosti, R., and Scarmeas, N. (2013). Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 74, 580–591. doi: 10.1002/ana.23944
Qiao, S., Li, W., Tsubouchi, R., Haneda, M., Murakami, K., Takeuchi, F., et al. (2005). Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 9, 995–1003. doi: 10.1080/10715760500231836
Ramirez, M. R., Izquierdo, I., do Carmo Bassols Raseira, M., Zuanazzi, J. A., Barros, D., and Henriques, A. T. (2005). Effect of lyophilised Vaccinium berries on memory, anxiety and locomotion in adult rats. Pharmacol. Res. 52, 457–462. doi: 10.1016/j.phrs.2005.07.003
Rébeillé, F., Ravanel, S., Marquet, A., Mendel, R. R., Webb, M. E., Smith, A. G., et al. (2007). Roles of vitamins B5, B8, B9, B12 and molybdenum cofactor at cellular and organismal levels. Nat. Prod. Rep. 24, 949–962. doi: 10.1039/b703104c
Rendeiro, C., Guerreiro, J. D., Williams, C. M., and Spencer, J. P. (2012). Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects. Proc. Nutr. Soc. 71, 246–262. doi: 10.1017/S0029665112000146
Ringman, J. M., Frautschy, S. A., Teng, E., Begum, A. N., Bardens, J., Beigi, M., et al. (2012). Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer Res. Therapy 4, 43. doi: 10.1186/alzrt146
Royall, D. R., Cordes, J. A., and Polk, M. (1998). CLOX: an executive clock drawing task. J. Neurol. Neurosurg. Psychiatry 64, 588–594. doi: 10.1136/jnnp.64.5.588
Sabour-Pickett, S., Nolan, J. M., Loughman, J., and Beatty, S. (2012). A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol. Nutr. Food Res. 56, 270–286. doi: 10.1002/mnfr.201100219
Sasaki, N., Toda, T., Kaneko, T., Baba, N., and Matsuo, M. (2003). Protective effects of flavonoids on the cytotoxicity of linoleic acid hydroperoxide toward rat pheochromocytoma PC12 cells. Chem. Biol. Interact. 145, 101–116. doi: 10.1016/S0009-2797(02)00248-X
Schmidt, M., Betti, G., and Hensel, A. (2007). Saffron in phytotherapy: pharmacology and clinical uses. Wien Med. Wochenschr. 157, 315–319. doi: 10.1007/s10354-007-0428-4
Scholey, A. B., French, S. J., Morris, P. J., Kennedy, D. O., Milne, A. L., and Haskell, C. F. (2010). Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 24, 1505–1514. doi: 10.1177/0269881109106923
Schültke, E., Kendall, E., Kamencic, H., Ghong, Z., Griebel, R. W., and Juurlink, B. H. (2003). Quercetin promotes functional recovery following acute spinal cord injury. J. Neurotrauma 20, 583–591. doi: 10.1089/089771503767168500
Seren, S., Lieberman, R., Bayraktar, U. D., Heath, E., Sahin, K., Andic, F., et al. (2008). Lycopene in cancer prevention and treatment. Am. J. Ther. 15, 66–81. doi: 10.1097/MJT.0b013e31804c7120
Sharma, V., Mishra, M., Ghosh, S., Tewari, R., Basu, A., Seth, P., et al. (2007). Modulation ofinterleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res. Bull. 73, 55–63. doi: 10.1016/j.brainresbull.2007.01.016
Shimojo, Y., Kosaka, K., Noda, Y., Shimizu, T., and Shirasawa, T. (2010). Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J. Neurosci. Res. 88, 896–904. doi: 10.1002/jnr.22242
Shukitt-Hale, B., Lau, F. C., Carey, A. N., Galli, R. L., Spangler, E. L., Ingram, D. K., et al. (2008). Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 11, 172–82. doi: 10.1179/147683008X301487
Singh, B., Parsaik, A. K., Mielke, M. M., Erwinc, P. J., Knopman, D. S., Petersen, R. C., et al. (2013). Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 39, 271–282. doi: 10.3233/JAD-130830
Snitz, B. E., O’Meara, E. S., Carlson, M. C., Arnold, A. M., Ives, D. G., Rapp, S. R., et al. (2009). Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA 302, 2663–2670. doi: 10.1001/jama.2009.1913
Sofi, F., Cesari, F., Abbate, R., Gensini, G. F., and Casini, A. (2008). Adherence to Mediterranean diet and health status: meta-analysis. Br. Med. J. 337, a1344aq. doi: 10.1136/bmj.a1344
Spencer, J. P. (2009). The impact of flavonoids on memory: physiological and molecular considerations. Chem. Soc. Rev. 38, 1152–1161. doi: 10.1039/b800422f
Spencer, J. P. (2010). Beyond antioxidants: the cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc. Nutr. Soc. 69, 244–260. doi: 10.1017/S0029665110000054
Spencer, J. P., Abd El Mohsen, M. M., Minihane, A. M., and Mathers, J. C. (2008). Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 99, 12–22. doi: 10.1017/S0007114507798938
Stahl, W., Heinrich, U., Aust, O., Tronnier, H., and Sies, H. (2006). Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 5, 238–242. doi: 10.1039/b505312a
Stahl, W., Schwarz, W., Sundquist, A. R., and Sies, H. (1992). cis-trans Isomers of lycopene and b-carotene in human serum and tissues. Arch. Biochem. Biophys. 294, 173–177. doi: 10.1016/0003-9861(92)90153-N
Sun, G. Y., Xu, J., Jensen, M. D., and Simonyi, A. (2004). Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 45, 205–213. doi: 10.1194/jlr.R300016-JLR200
Sun, Y., L, C. J., Chien, K. L., Chen, S. T., and Chen, R. C. (2007). Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 29, 2204–2214. doi: 10.1016/j.clinthera.2007.10.012
Sydenham, E., Dangour, A. D., and Lim, W. S. (2012). Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database. Syst. Rev. 6, D005379. doi: 10.1002/14651858.CD005379.pub3
Thorp, A. A., Sinn, N., Buckley, J. D., Coates, A. M., and Howe, P. R. (2009). Soya isoflavone supplementation enhances spatial working memory in men. Br. J. Nutr. 102, 1348–1354. doi: 10.1017/S0007114509990201
Truelsen, T., Thudium, D., Grønbaek, M., and Copenhagen City Heart Study. (2002). Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology 59, 1313–1319. doi: 10.1212/01.WNL.0000031421.0369.E7
Tsuda, T. (2008). Regulation of adipocyte function by anthocyanins; possibility of preventing the metabolic syndrome. J. Agric. Food. Chem. 56, 642–646. doi: 10.1021/jf073113b
Vagnucci, A. H. Jr., and Li, W. W. (2003). Alzheimer’s disease and angiogenesis. Lancet 361, 605–608. doi: 10.1016/S0140-6736(03)12521-4
Vellas, B., Coley, N., Ousset, P. J., Berrut, G., Dartigues, J. F., Dubois, B., et al. (2012). Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 11, 851–859. doi: 10.1016/S1474-4422(12)70206-5
Wang, J., Ho, L., Zhao, W., Ono, K., Rosensweig, C., Chen, L., et al. (2008a). Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 28, 6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008
Wang, W., Shinto, L., Connor, W. E., and Quinn, J. F. (2008b). Nutritional biomarkers in Alzheimer’s disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J. Alzheimers Dis. 13, 31–38.
Wang, M. H., Chang, W. J., Soung, H. S., and Chang, K. C. (2012). (-)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behav. Pharmacol. 23, 771–780. doi: 10.1097/FBP.0b013e32835a3bc8
Williams, C. M., El Mohsen, M. A., Vauzour, D., Rendeiro, C., Butler, L. T., Ellis, J. A., et al. (2008). Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 45, 295–305. doi: 10.1016/j.freeradbiomed.2008.04.008
Williams, R. J., and Spencer, J. P. (2012). Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 52, 35–45. doi: 10.1016/j.freeradbiomed.2011.09.010
Xu, Y., Barish, P. A., Pan, J., Ogle, W. O., and O’Donnell, J. M. (2012). Animal models of depression and neuroplasticity: assessing drug action in relation to behavior and neurogenesis. Methods Mol. Biol. 829, 103–124. doi: 10.1007/978-1-61779-458-2_6
Xu, Y., Wang, Z., You, W., Zhang, X., Li, S., Barish, P. A., et al. (2010). Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 20, 405–413. doi: 10.1016/j.euroneuro.2010.02.013
Yang, C. S., Landau, J. M., Huang, M. T., and Newmark, H. L. (2001). Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 21, 381–406. doi: 10.1146/annurev.nutr.21.1.381
Zafra-Stone, S., Yasmin, T., Bagchi, M., Chatterjee, A., Vinson, J. A., and Bagchi, D. (2007). Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 51, 675–683. doi: 10.1002/mnfr.200700002
Zanotta, D., Puricelli, S, and Bonoldi, G. (2014). Cognitive effects of a dietary supplement made from extract of Bacopa monnieri, astaxanthin, phosphatidylserine, and vitamin E in subjects with mild cognitive impairment: a noncomparative, exploratory clinical study. Neuropsychiatr. Dis. Treat. 10, 225–230. doi: 10.2147/NDT.S51092
Zhao, L., and Brinton, R. D. (2007). WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert. Rev. Neurother. 7, 1549–1564. doi: 10.1586/14737175.7.11.1549
Keywords: cognitive impairment, dementia, Alzheimer’s disease, dietary natural substances, neurodegeneration, nutraceuticals, neuroprotection
Citation: Mecocci P, Tinarelli C, Schulz RJ and Polidori MC (2014) Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 5:147. doi: 10.3389/fphar.2014.00147
Received: 18 March 2014; Accepted: 03 June 2014;
Published online: 23 June 2014.
Edited by:
Cesare Mancuso, Catholic University School of Medicine, ItalyReviewed by:
Nicolas Blondeau, Centre National de la Recherche Scientifique, FranceGeorge Anthony Oyler, Synaptic Research, USA
Copyright © 2014 Mecocci, Tinarelli, Schulz and Polidori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: P. Mecocci, Section of Gerontology and Geriatrics, Department of Medicine, University of Perugia, Santa Maria della Misericordia Hospital, Block C Floor 4, S. Andrea delle Fratte, 06156 Perugia, Italy e-mail:cGF0cml6aWEubWVjb2NjaUB1bmlwZy5pdA==
 P. Mecocci
P. Mecocci C. Tinarelli1
C. Tinarelli1