- Department of Pediatrics, Floating Hospital for Children at Tufts Medical Center, Boston, MA, USA
Introduction: As more infants are surviving at younger gestational ages, bronchopulmonary dysplasia (BPD) remains as a frequent neonatal complication occurring after preterm birth. The multifactorial nature of the disease process makes BPD a challenging condition to treat. While multiple pharmacologic therapies have been investigated over the past two decades, there have been limited advances in the field. Often multiple therapies are used concurrently without clear evidence of efficacy, with potential for significant side effects from drug-drug interactions.
Methods: Systematic literature review.
Conclusion: Although there is physiologic rationale for the use of many of these therapies, none of them has single-handedly altered the incidence, severity, or progression of BPD. Future research should focus on developing clinically significant end-points (short and long term respiratory assessments), investigating biomarkers that accurately predict risk and progression of disease, and creating appropriate stratification models of BPD severity. Applying a multi-modal approach to the study of new and existing drugs should be the most effective way of establishing the optimal prevention and treatment regimens for BPD.
Introduction
Bronchopulmonary dysplasia (BPD) has been traditionally defined as a chronic form of lung disease in neonates treated with oxygen and positive pressure ventilation for a primary lung disorder. As more neonates at the threshold of viability are surviving, BPD continues to be a persistent and prevalent NICU morbidity with approximately 15,000 neonates diagnosed in the United States each year. BPD also carries a higher risk of neurodevelopmental morbidity and (Ehrenkranz et al., 2005) mortality, making it an extremely important complication of neonatal intensive care.
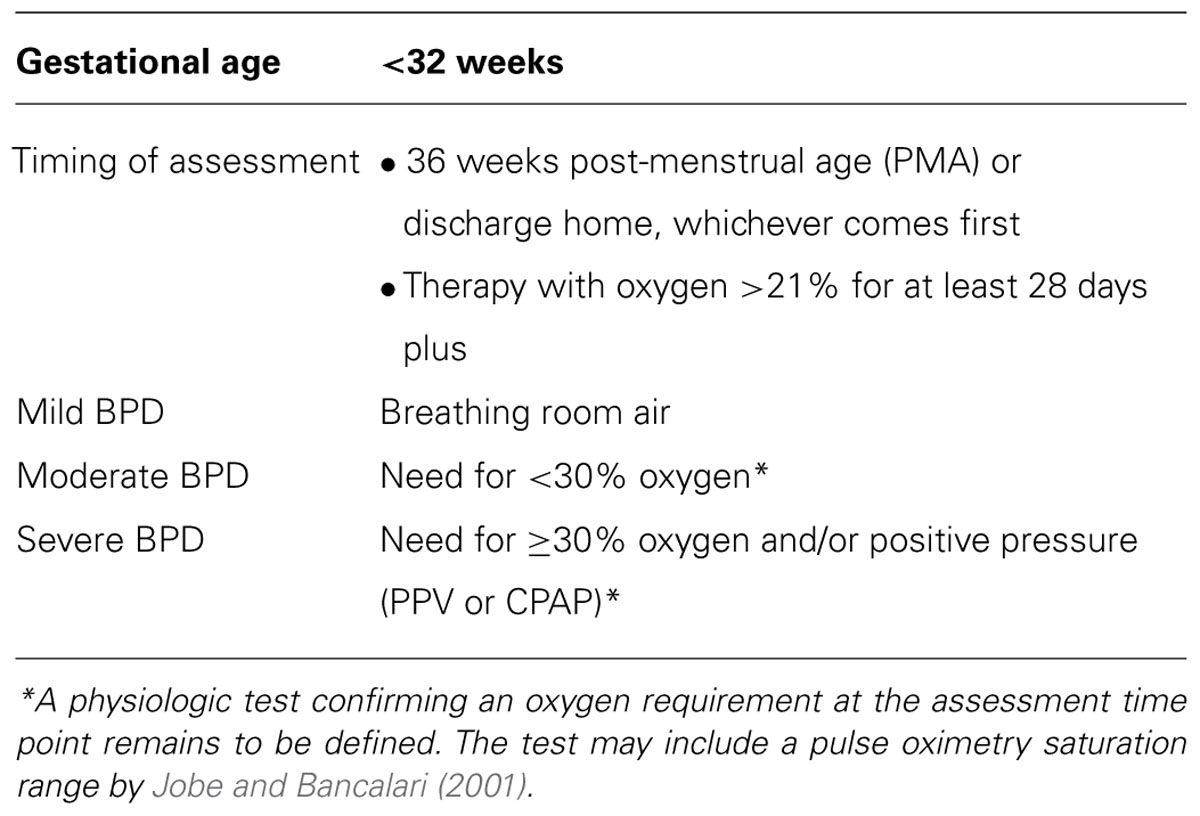
Bronchopulmonary dysplasia was first described by Northway et al. (1967) as chronic lung disease that developed in premature infants who were ventilated with high pressures and concentrations of oxygen. The histological appearance was characterized by parenchymal fibrosis, inflammation, and smooth muscle hypertrophy resulting in diffuse airway damage (O’Brodovich and Mellins, 1985). However, the nature of BPD has evolved into a “new” form of BPD typically seen in neonates surviving at the threshold of viability and characterized primarily by arrest of alveolar and vascular development (Husain et al., 1998; Jobe, 1999; Jobe and Bancalari, 2001; Baraldi and Filippone, 2007). The definition of BPD has also changed over time, with an NIH consensus conference capturing criteria from previous definitions and incorporating a stratification system based on clinical severity (Jobe and Bancalari, 2001; Table 1). Since the pathogenesis of BPD is multifactorial (e.g., mechanical ventilation, oxygen exposure, nutritional deficits, fetal growth restriction, and genetic susceptibility), a multidisciplinary approach is necessary for effective treatment. This article will discuss current pharmacologic approaches, which have not changed dramatically in the last 20 years. Often, many of these therapies are used concurrently with inadequate studies to define efficacy as well as the potential for significant side effects (especially drug-drug interactions). Finally, experimental agents that are being investigated for the prevention of BPD and associated complications of this common neonatal disease will be introduced.
Methods
Approximately 100 articles, including animal studies, human pilot studies, randomized controlled trials (RCTs), meta-analyses, and systematic reviews published on the PubMed database were evaluated for inclusion in this article. Additionally, ClinicalTrials.gov was queried for ongoing studies investigating pharmacologic therapies for BPD.
Diuretics
Large volumes of intravenous fluids are often administered to premature neonates to provide adequate hydration and nutrition. Excessive fluid administration can be associated with pulmonary edema (especially with acute lung injury) and lead to increased respiratory support and ultimately BPD (Oh et al., 2005). Furosemide acts on the ascending loop of Henle and blocks chloride transport. Additionally, furosemide decreases interstitial edema and pulmonary vascular resistance and increases plasma oncotic pressure and lymphatic flow. It is the treatment of choice for fluid overload in BPD. Several studies have demonstrated alternate-day, daily, and even aerosolized furosemide improve clinical respiratory status, pulmonary mechanics, oxygenation, and facilitate weaning from mechanical ventilation. However, long-term benefits have not been established in infants with BPD (Rush et al., 1990; Sahni and Phelps, 2011; Stewart and Brion, 2011; Segar, 2012). Thiazides affect the renal tubular excretion of electrolytes but are less potent than loop diuretics. Potassium and bicarbonate excretion also occur which has prompted the use of thiazides in conjunction with spironolactone, a competitive inhibitor of aldosterone. This weak potassium-sparing diuretic facilitates sodium, chloride, and water excretion. A small number of controlled trials examining the use of thiazide diuretics and spironolactone in BPD have generated mixed results with urine output increasing, but not always accompanied by improvements in pulmonary mechanics (Engelhardt et al., 1989; Hoffman et al., 2000). The use of spironolactone does appear to offer any substantial benefit and is not recommended. Overall, diuretics offer short-term improvements in pulmonary mechanics but are associated with a number of side effects that may limit longer-term use (e.g., ototoxicity, electrolyte disturbances, azotemia, etc.). Furthermore, there are limited data demonstrating significant benefits of these agents when more meaningful outcome measures are analyzed such as reduction in the duration of mechanical ventilation and hospitalization or improved long-term clinical outcomes (less asthma, pulmonary infections, etc.). Additional longer-term studies are needed to establish optimal treatment regimens in infants with established BPD.
Bronchodilators
Albuterol (also known as ‘Salbutamol’) is an inhaled β2-agonist that is the recommended for the treatment of BPD with a strong component of reversible bronchospasm (Davis and Rosenfeld, 2005). It has been associated with short-term improvements in pulmonary resistance and lung compliance secondary to bronchial smooth muscle relaxation (Wilkie and Bryan, 1987). While a Cochrane review examining the role of albuterol was unable to find sufficient evidence of efficacy in the prevention of BPD, other studies have shown improvement in pulmonary mechanics following treatment (Robin et al., 2004; Ng et al., 2012). In summary, long-term efficacy has not been established and tolerance may develop with prolonged use.
Ipratropium bromide is a muscarinic antagonist that produces bronchodilation in chronically ventilated infants with BPD. Significant improvements in airway resistance and compliance has been shown in its isolated use or combined with a β2-agonist (Brundage et al., 1990). However, clinical trials have not demonstrated changes in the natural progression of BPD or long-term clinical respiratory status (De Boeck et al., 1998; Pantalitschka and Poets, 2006). Despite these findings, infants with BPD who develop wheezing may warrant a trial with albuterol initially with the addition of ipratropium bromide if significant side effects occur or clinical improvement isn’t seen with a β2-agonist alone.
Vitamin A
Vitamin A (i.e., retinol) is important in maintaining cell integrity and promoting tissue repair with deficiencies producing significant changes in the tracheobronchial tree (Anzano et al., 1980). Multiple studies have demonstrated that very low birth weight infants are deficient in Vitamin A and at a propensity to develop BPD (Shenai et al., 1990; Darlow and Graham, 2011). A landmark, multicenter Neonatal Research Network (NRN) trial investigated the benefits of vitamin A supplementation in improving survival without BPD in 807 neonates weighing <1000 g at birth. Intramuscular doses of 5000 IU of Vitamin A given three times a week for 4 weeks demonstrated a small (9%), but significant reduction in survival without chronic lung disease at 36 weeks post-menstrual age (PMA; Tyson et al., 1999). No increased toxicity was seen with the higher dosing regimen compared to placebo. However, long-term follow-up of these infants at 18–22 months could not demonstrate any improvement in mortality, neurodevelopmental impairment, or respiratory outcomes from treatment with Vitamin A (Ambalavanan et al., 2005). However, this study was not powered for demonstrating differences in these longer-term outcomes, so many centers still administer vitamin A routinely in infants at high risk for developing BPD.
Methylxanthines
Caffeine treatment for the prevention of apnea of prematurity and BPD is currently the standard of care in most neonatal intensive care units (Ghanta et al., 2013). It has been shown to increase respiratory drive, diaphragm contractility, and pulmonary compliance while reducing airway resistance (Davis et al., 1989; Aranda et al., 2010). These effects are of particular importance in chronically ventilated neonates who can develop skeletal muscle and diaphragmatic atrophy and fatigue. The improved muscle contractility may stabilize the chest wall and improve functional residual capacity facilitating successful extubation (Davis and Rosenfeld, 2005). Schmidt et al. (2006) conducted a large, multicenter RCT investigating the effects of caffeine on apnea of prematurity in a cohort of infants weighing 500–1250 g at birth. While infants in the treatment group had significantly less apnea of prematurity, they were also noted to have less BPD (defined as need for supplemental oxygen at 36 weeks PMA), patent ductus arteriosus (PDA), and cerebral palsy when followed out to 18–21 months corrected gestational age (Schmidt et al., 2007). However, these outcomes did not translate into longer-term benefits when this same cohort of infants was examined at 5 years of age (Schmidt et al., 2012). Despite these findings, caffeine therapy remains a standard medical approach to the prevention and treatmentof BPD.
Pentoxifylline is a methylxanthine derivative and phosphodiesterase inhibitor with immunomodulatory and anti-fibrotic properties (Almario et al., 2012; Ghanta et al., 2013). It has been proposed to have a therapeutic role in attenuating tissue injury associated with sepsis (Harris et al., 2000; Michetti et al., 2003). In a hyperoxia-induced lung injury model of BPD, pentoxifylline reduced lung edema and inflammatory cell infiltration while improving antioxidant activity, vascular development, and overall survival (Almario et al., 2012). A RCT in 150 very low birth weight infants demonstrated a reduction in BPD in infants receiving pentoxifylline compared to placebo (Ruszard et al., 2006). However, there is still insufficient evidence to support widespread usage and further safety and efficacy data is needed.
Corticosteroids
Marked inflammation in the lung appears to play an important role in the pathogenesis of BPD, unifying many factors into a single common pathway. Therefore, it is reasonable to consider the use of corticosteroids in treating BPD. The use of corticosteroids can be further delineated based on route of administration.
Systemic
Historically, reviews of the use of systemic corticosteroids have investigated the effects of dexamethasone on BPD (treatment of existing lung injury as well as prevention) when administered during different time periods: early (<96 h after birth), moderately early (7–14 days after birth), or late (>3 weeks after birth; Halliday et al., 2003a,b,c). However, more recent reviews have classified trials as early (<7 days of life) or late (≥7 days after birth) based on timing of dexamethasone administration (Doyle et al., 2014a,b). All of these reviews have shown that dexamethasone facilitates extubation, reduces the combined endpoint of death or BPD at 28 days or 36 weeks PMA, and also reduces the incidence of PDA and ROP. However, the meta-analyses investigating trials where glucocorticoids have been used early in life have also found a significant increase in adverse long-term neurologic outcomes, specifically cerebral palsy (Doyle et al., 2014a). In contrast, there has been no significant increase in long-term neurologic outcomes detected with regard to moderately early and late administration of glucocorticoids (Halliday et al., 2003a,c; Doyle et al., 2014a). Nevertheless, the trend toward an increase in the incidence of abnormal neurologic findings in trials of late administration of glucocorticoids have prompted the American Academy of Pediatrics to issue a policy statement advising against the early use of dexamethasone and strongly recommending caution with the routine use of dexamethasone after 7 days of life (Watterberg, 2010). However, data from 16 RCTs extracted by Onland et al. (2009) demonstrated that moderately early administration of dexamethasone (7–14 days after birth) did not significantly increase the combined outcome of death or cerebral palsy and actually showed a dose dependent decrease (6.2%) in cerebral palsy with each incremental mg/kg increase in cumulative dexamethasone dose. Interestingly enough, this promising dose-dependent effect on neurodevelopmental outcome was not demonstrated in the delayed (>3 weeks) glucocorticoid treatment trials. These data illustrate the potential time-sensitive effects of dexamethasone and the need for clinicians to balance the known impact on neurodevelopmental outcome associated with prolonged mechanical ventilation and the development of BPD with the risks/benefits of systemic glucocorticoid treatment.
Other investigators have suggested that a primary cortisol deficiency in preterm infants increases the risk of BPD which may be amenable to early treatment with a less potent corticosteroid such as hydrocortisone (Watterberg, 2007). A meta-analysis from Doyle et al. (2010) evaluated eight RCT investigating the clinical effects of postnatal hydrocortisone given in the first week of life to VLBW infants. Infants who received hydrocortisone did not demonstrate a significant reduction in mortality, BPD, or cerebral palsy and actually had a significant increase in the incidence of gastrointestinal perforation (although this occurred more often when indomethacin was given concurrently). Indeed there has been emerging literature indicating that prolonged hydrocortisone exposure can negatively impact language and motor skills in the first years of life (Patra et al., 2014). Ongoing randomized, controlled clinical trials will no doubt help generate data on the appropriate dose and timing of hydrocortisone treatment for the prevention of BPD1 (Onland et al., 2011).
Inhaled
Inhaled steroids have been examined as a therapeutic approach to the treatment of BPD in order to promote respiratory benefits while minimizing systemic side effects. Studies examining the benefits of inhaled corticosteroids administered early or late have not been able to demonstrate any impact of inhaled corticosteroids on short-term respiratory outcomes (e.g., death or BPD at 36 weeks PMA) or longer-term clinical respiratory status (Onland et al., 2012; Shah et al., 2012b). Additionally, inhaled corticosteroids appear to offer no clinical advantage over systemic steroid therapy (Shah et al., 2012a). The potential for systemic absorption of inhaled steroids and subsequent side effects (e.g., growth, adrenal suppression, etc.) warrants careful consideration before initiation of this treatment approach. Further research is needed to evaluate the type of inhaled steroid, timing, formulation, dosage, and method of administration that is most appropriate for the prevention and treatment of BPD.
Pulmonary Vasodilators
Inhaled
It is well-recognized that infants with BPD can experience intermittent episodes of hypoxia which can promote secondary pulmonary vasoconstriction and pulmonary hypertension, adding to the complexity of BPD (Khemani et al., 2007; Steinhorn, 2013). This has resulted in much interest in the selective pulmonary vasodilator nitric oxide (NO) as alterations in NO signaling, vascular growth, and reactivity appear to play a role in the development of BPD (MacRitchie et al., 2001; Afshar et al., 2003). In animal models of BPD, inhaled NO promotes pulmonary angiogenesis, reduces inflammation, and decreases apoptosis and oxidant damage (Gutierrez et al., 1996; Balasubramaniam et al., 2006; Tang et al., 2007). Three large randomized trials have been conducted to evaluate the effect of inhaled NO on survival without BPD in VLBW infants (Ballard et al., 2006; Kinsella et al., 2006; Mercier et al., 2010). Only one study was able to demonstrate a modest but statistically significant benefit in survival without BPD at 36 weeks PMA (Ballard et al., 2006). Furthermore, evidence from Van Meurs et al. (2005) indicate a higher rate of mortality and intraventricular hemorrhage (IVH) in infants weighing <1000 g at birth who received inhaled NO. Large meta-analyses have since been unable to find consistent long-term improvement in mortality or the incidence and severity of BPD when using inhaled NO in preterm infants as a prevention or rescue therapy (Askie et al., 2011; Donahue et al., 2011). Currently, there remains insufficient evidence to recommend the use of inhaled NO therapy in preterm infants who have respiratory failure for the purpose of preventing or improving BPD, even in infants who have developed pulmonary hypertension (Kumar and Committee on Fetus and Newborn, 2014).
Systemic
Sildenafil is a selective phosphodiesterase inhibitor that increases concentrations of cyclic guanosine monophosphate (GMP) and thus promotes pulmonary vasodilation. Animal studies of sildenafil have shown that it promotes alveolar growth, mitigates lung inflammation, and reduces pulmonary hypertension in hyperoxia-induced lung injury models (Ladha et al., 2005; De Visser et al., 2009). Small pilot studies have shown that sildenafil reduces pulmonary vascular pressures in infants with severe BPD with no additional side effects (Baquero et al., 2006; Mourani et al., 2009). Concerns do remain in recommending widespread use in high risk preterm neonates as an increase in mortality was found in studies of older children receiving higher doses of sildenafil (Wardle and Tulloh, 2013). However, it remains a promising therapy and further studies are needed to elucidate appropriate dose, formulation, and timing of administration in neonates with BPD (especially those with secondary pulmonary hypertension).
Late Surfactant
Historically, surfactant administration has been administered shortly after birth for the prevention and treatment of respiratory distress syndrome (RDS). While early surfactant administration has not been shown to significantly impact the development of BPD, alterations in surfactant function have been reported in older patients with a variety of chronic lung disorders, suggesting a possible benefit to late surfactant administration in the treatment of BPD (Gunther et al., 2002; Bahadue and Soll, 2012). Analysis of surfactant samples of chronically ventilated neonates suggests that this may be due to a deficiency of surfactant proteins (SP) B and C (Merrill et al., 2004). Multiple pilot trials have demonstrated an increase in tracheal SP-B concentrations and a transient improvement in oxygenation with no short-term side effects following late administration of exogenous surfactant (Merrill et al., 2011; Keller et al., 2012). A large, multicenter, blinded, RCT is currently underway in an extremely low gestational age (ELGAN) cohort examining the effects of late surfactant therapy on surfactant function and survival without BPD2.
Prevention Strategies
Antioxidants
Oxygen has a unique molecular structure that is capable of accepting free electrons generated by oxidative metabolism into its outer ring. Hyperoxia, reperfusion, infection, ventilator-associated inflammation, and inadequate antioxidant defenses can produce reactive oxygen species (ROS) which are toxic to living tissues. Clinical studies suggest that ROS are involved in the pathogenesis of BPD. Plasma concentrations of ROS (allantoin, expired pentane, protein carbonyls, and 3-nitro tyrosine molecules) have been shown to be significantly elevated in the first week of life in infants developing BPD compared to infants who recover without the development of significant chronic lung disease (Ballard et al., 2008; Poggi and Dani, 2014). A strategy for antioxidant enzyme replacement was investigated by Davis et al. (1997)in high risk VLBW infants. Intratracheal administration of recombinant human CuZn superoxide dismutase (rhSOD) was associated with increased SOD levels (lung, serum, urine) and lower levels of biomarkers of acute lung injury (Rosenfield et al., 1996; Davis et al., 1997). Limited follow up data in this initial cohort did not demonstrate any difference in death, BPD, days of mechanical ventilation, oxygen requirement, or neurodevelopmental outcome (Davis et al., 2000). However, a larger trial in 302 VLBW infants followed out to 1 year corrected gestational age demonstrated a significant reduction in pulmonary morbidity (e.g., respiratory illness, emergency room visits, hospital readmissions) in the rhSOD-treatment versus the placebo group, suggesting that a reduction in early oxidant injury may still impact longer-term pulmonary outcomes (Davis et al., 2003).
Club (Clara) Cell Protein (CC10)
CC10 is a 10-kilodalton protein secreted by non-ciliated bronchiolar epithelial cells (club cells) and is one of the most abundant proteins within the fluid lining the lung epithelium (Greenough, 2008). CC10 has extensive anti-inflammatory properties and has been shown to be significantly lower in tracheal aspirates of premature infants who subsequently died or developed BPD (Jorens et al., 1995; Broeckaert et al., 2000; Schrama et al., 2008). Animal studies have demonstrated that administration of recombinant human CC10 (rhCC10) upregulates SP and vascular endothelial growth factor (VEGF) expression while improving respiratory mechanics (Miller et al., 2007; Wolfson et al., 2008). A pilot trial conducted in 22 VLBW infants by Levine et al. (2005) demonstrated that intratracheal administration of rhCC10 was well-tolerated and had significant anti-inflammatory effects in the lung. No infant followed out to 6 months corrected gestational age had any significant respiratory illness following treatment with rhCC10 compared to 50% in the control group. This promising treatment is being further investigated in a multi-center randomized, blinded trial evaluating survival without long-term pulmonary morbidity (chronic respiratory morbidity at 1 year corrected age) as the primary outcome3.
Conclusion
Decades after initially being described by Northway et al. (1967), BPD still remains a very important complication of neonatal intensive care. BPD is a complicated multisystem disease that carries a significant physical, social, and economic burden for the survivors and their families. While multiple therapies are used routinely either alone or in combination (potentially increasing drug–drug interactions and associated side effects), there is insufficient evidence supporting short and longer-term use of many of these agents. In fact, no single therapy has been shown to have a significant impact on the incidence or severity of BPD. Targeting single mechanisms is unlikely to significantly influence BPD since it is multifactorial in nature. Future research should be focused on establishing better biomarkers predictive of BPD and associated longer-term chronic respiratory morbidity, developing stratification models to identify high-risk infants early on, and applying a multimodal approach when studying various pharmacologic interventions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^ ClinicalTrials.gov PREMILOC trial to prevent bronchopulmonary dysplasia in very preterm neonates. Clinical.Trials.Gov identifier: NCT00623740.
- ^ ClinicalTrials.gov TOLSURF trial of late surfactant for prevention of bronchopulmonary dysplasia. Clinical.Trials.gov Identifier: NCT01022580.
- ^ ClinicalTrials.gov Efficacy of recombinant human Clara cell 10 protein (rhCC10) administered to premature neonates with respiratory distress syndrome. Clinical.Trials.gov Identifier: NCT01941745.
References
Afshar, S., Gibson, L. L., Yuhanna, I. S., Sherman, T. S., Kerecman, J. D., Grubb, P. H.,et al. (2003). Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L749–L758.
Almario, B., Wu, S., Peng, J., Alapati, D., Chen, S., and Sosenko, I. R. (2012). Pentoxifylline and prevention of hyperoxia-induced lung injury in neonatal rats. Pediatr. Res. 71, 583–589. doi: 10.1038/pr.2012.14
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ambalavanan, N., Tyson, J. E., Kennedy, K. A., Hansen, N. I., Vohr, B. R., Wright, L. L.,et al. (2005). Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics 115, e249–e254. doi: 10.1542/peds.2004-1812
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Anzano, M. A., Olson, J. A., and Lamb, A. J. (1980). Morphologic alterations in the trachea and the salivary gland following the induction of rapid synchronous vitamin A deficiency in rats. Am. J. Pathol. 98, 717–732.
Aranda, J. V., Beharry, K., Valencia, G. B., Natarajan, G., and Davis, J. (2010). Caffeine impact on neonatal morbidities. J. Matern. Fetal Neonatal Med. 3, 20–23. doi: 10.3109/14767058.2010.517704
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Askie, L. M., Ballard, R. M., Cutter, G. R., Dani, C., Elbourne, D., Field, D.,et al. (2011). Inhaled nitric oxide in preterm infants: an individual patient-data meta-analysis of randomized trials. Pediatrics 128, 729–739. doi: 10.1542/peds.2010-2725
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bahadue, F. L., and Soll, R. (2012). Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 11:CD001456.
Balasubramaniam, V., Maxey, A. M., Morgan, D. B., Markham, N. E., and Abman, S. H. (2006). Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L119–L127. doi: 10.1152/ajplung.00395.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ballard, P. L., Truog, W. E., Merrill, J. D., Gow, A., Posencheg, M., Golombek, S. G.,et al. (2008). Plasma biomarkers of oxidative stress: relationship to lung disease and inhaled nitric oxide therapy in premature infants. Pediatrics 121, 555–561. doi: 10.1542/peds.2007-2479
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ballard, R. A., Truog, W. E., Cnaan, A., Martin, R. J., Ballard, P. L., Merrill, J. D.,et al. (2006). NO CLD Study Group. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N. Engl. J. Med. 355, 343–353. doi: 10.1056/NEJMoa061088
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baquero, H., Soliz, A., Neira, F., Venegas, M. E., and Sola, A. (2006). Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics 117, 1077–1083. doi: 10.1542/peds.2005-0523
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baraldi, E., and Filippone, M. (2007). Chronic lung disease after premature birth. N. Engl. J. Med. 357, 1946–1955. doi: 10.1056/NEJMra067279
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Broeckaert, F., Clippe, A., Knoops, B., Hermans, C., and Bernard, A. (2000). Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann. N. Y. Acad. Sci. 923, 68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x
Brundage, K. L., Mohsini, K. G., Froese, A. B., and Fisher, J. T. (1990). Bronchodilator response to ipratropium bromide in infants with bronchopulmonary dysplasia. Am. Rev. Respir. Dis. 142, 1137–1142. doi: 10.1164/ajrccm/142.5.1137
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Darlow, B. A., and Graham, P. J. (2011). Vitamin A supplementation to prevent mortality and short-and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 10:CD000501. doi: 10.1002/14651858.CD000501.pub3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, J. M., Bhutani, V. K., Stefano, J. L., Fox, W. W., and Spitzer, A. R. (1989). Changes in pulmonary mechanics following caffeine administration in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 6, 49–52. doi: 10.1002/ppul.1950060112
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, J. M., Parad, B. R., Michele, T., Allred, E., Price, A., Rosenfeld, W.,et al. (2003). Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 111, 469–476. doi: 10.1542/peds.111.3.469
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, J. M., Richter, S. E., Biswas, S., Rosenfeld, W. N., Parton, L., Gewolb, I. H.,et al. (2000). Long-term follow-up of premature infants treated with prophylactic, intratracheal recombinant human CuZn superoxide dismutase. J. Perinatol. 20, 213–216. doi: 10.1038/sj.jp.7200363
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Davis, J., and Rosenfeld, W. N. (2005). “Bronchopulmonary dysplasia,” in Neonatology, eds G. Avery, M. Fletcher, and M. MacDonald (Philadelphia, PA: JB Lippincott Co), 578–582.
Davis, J. M., Rosenfield, W. N., Richter, S. E., Parad, M. R., Gewolb, I. H., Spitzer, A. R.,et al. (1997). Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 100, 24–30. doi: 10.1542/peds.100.1.24
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Boeck, K., Smith, J., Van Lierde, S., and Devlieger, H. (1998). Response to bronchodilators in clinically stable 1-year-old patients with bronchopulmonary dysplasia. Eur. J. Pediatr. 157, 75–79. doi: 10.1007/s004310050771
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Visser, Y. P., Walther, F. J., Laghmani el, H., Boersma, H., van der Laarse, A., and Wagenaar, G. T. (2009). Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir. Res. 10, 30. doi: 10.1186/1465-9921-10-30
Donahue, P. K., Gilmore, M. M., Cristofalo, E., Wilson, R. F., Weiner, J. Z., Lau, B. D.,et al. (2011). Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics 127, e414–e422. doi: 10.1542/peds.2010-3428
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doyle, L. W., Ehrenkranz, R. A., and Halliday, H. L. (2010). Postnatal hydrocortisone for preventing or treating bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 98, 111–117. doi: 10.1159/000279992
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doyle, L. W., Ehrenkranz, R. A., and Halliday, H. L. (2014a). Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 5:CD001146. doi: 10.1002/14651858.CD001146.pub4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doyle, L. W., Ehrenkranz, R. A., and Halliday, H. L. (2014b). Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 5:CD001145. doi: 10.1002/14651858.CD001145.pub2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ehrenkranz, R. A., Walsh, M. C., Vohr, B. R., Jobe, A. H., Wright, L. L., Fanaroff, A. A.,et al. (2005). Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics 116, 1353–1360. doi: 10.1542/peds.2005-0249
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Engelhardt, B., Blalock, W. A., DonLevy, S., Rush, M., and Hazinski, T. A. (1989). Effect of spironolactone-hydrochlorothiazide on lung function in infants with chronic bronchopulmonary dysplasia. J. Pediatr. 114, 619–624. doi: 10.1016/S0022-3476(89)80708-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ghanta, S., Leeman, K. T., and Christou, H. (2013). An update on pharmacologic approaches to bronchopulmonary dysplasia. Semin. Perinatol. 37, 115–123. doi: 10.1053/j.semperi.2013.01.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Greenough, A. (2008). Clara cell secretory protein and bronchopulmonary dysplasia in prematurely born infants. Eur. J. Pediatr. 167, 1347–1348. doi: 10.1007/s00431-008-0746-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gunther, A., Schmidt, R., Harodt, J., Schmehl, T., Walmrath, D., Ruppert, C.,et al. (2002). Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: impact on biophysical and biochemical surfactant proteins. Eur. Respir. J. 19, 797–804. doi: 10.1183/09031936.02.00243302
Gutierrez, H. H., Neives, B., Chumley, P., Rivera, A., and Freeman, B. A. (1996). Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic. Biol. Med. 21, 43–52. doi: 10.1016/0891-5849(95)02226-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Halliday, H. L., Ehrenkranz, R. A., and Doyle, L. W. (2003a). Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 1:CD001145.
Halliday, H. L., Ehrenkranz, R. A., and Doyle, L. W. (2003b). Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 1:CD001146.
Halliday, H. L., Ehrenkranz, R. A., and Doyle, L. W. (2003c). Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 1:CD001144.
Harris, E., Schulzke, S. M., and Patole, S. K. (2000). Pentoxifylline in preterm neonates: a systematic review. Paediatr. Drugs 12, 301–311. doi: 10.2165/11532600-000000000-00000
Hoffman, D. J., Gerdes, J. S., and Abbasi, S. (2000). Pulmonary function and electrolyte balance following spironolactone treatment in preterm infants with chronic lung disease: a double-blind, placebo-controlled, randomized trial. J. Perinatol. 20, 41–45. doi: 10.1038/sj.jp.7200307
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Husain, A. N., Siddiqui, N. H., and Stocker, J. T. (1998). Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum. Pathol. 29, 710–717. doi: 10.1016/S0046-8177(98)90280-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jobe, A. H., and Bancalari, E. (2001). Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729. doi: 10.1164/ajrccm.163.7.2011060
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jobe, A. J. (1999). The new BPD: an arrest of lung development. Pediatr. Res. 46, 641–643. doi: 10.1203/00006450-199912000-00007
Jorens, P. G., Sibille, Y., Goulding, N. J., van Overveld, F. J., Herman, A. G., Bossaert, L.,et al. (1995). Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur. Respir. J. 8, 1647–1653. doi: 10.1183/09031936.95.08101647
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keller, R. L., Merrill, J. D., Black, D. M., Steinhorn, R. H., Eichenwald, E. C., Durand, D. J.,et al. (2012). Late administration of surfactant replacement therapy increases surfactant protein-B content: a randomized pilot study. Pediatr. Res. 72, 613–619. doi: 10.1038/pr.2012.136
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Khemani, E., McElhinney, D. B., Rhein, L., Andrade, O., Lacro, R. V., Thomas, K. C.,et al. (2007). Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120, 1260–1269. doi: 10.1542/peds.2007-0971
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kinsella, J. P., Cutter, G. R., Walsh, W. F., Gerstmann, D. R., Bose, C. L., Hart, C.,et al. (2006). Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N. Engl. J. Med. 335, 354–364. doi: 10.1056/NEJMoa060442
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kumar, P. K., and Committee on Fetus and Newborn. (2014). Use of inhaled nitric oxide in preterm infants. Pediatrics 133, 164–170. doi: 10.1542/peds.2013-3444
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ladha, F., Bonnet, S., Eaton, F., Hashimoto, K., Korbutt, G., and Thebaud, B. (2005). Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 750–756. doi: 10.1164/rccm.200503-510OC
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Levine, C. R., Gewolb, I. H., Allen, K., Welch, R. W., Melby, J. M., Pollack, S.,et al. (2005). The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr. Res. 58, 15–21. doi: 10.1203/01.PDR.0000156371.89952.35
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
MacRitchie, A. N., Albertine, K. H., Sun, J., Lei, P. S., Jensen, S. C., Freestone, A. A.,et al. (2001). Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1011–L1020.
Mercier, J. C., Hummler, H., Durrmeyer, X., Sanchez-Luna, M., Carnielli, V., Field, D.,et al. (2010). EUNO Study Group. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet 376, 346–354. doi: 10.1016/S0140-6736(10)60664-2
Merrill, J. D., Ballard, P. L., Courtney, S. E., Durand, D. J., Hamvas, A., Hibbs, A. M.,et al. (2011). Pilot trial of late booster doses of surfactant for ventilated premature infants. J. Perinatol. 31, 599–606. doi: 10.1038/jp.2010.210
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Merrill, J. D., Ballard, R. A., Avital, C., Hibbs, A. M., Godinez, R. I., Godinez, M. H.,et al. (2004). Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr. Res. 56, 918–926. doi: 10.1203/01.PDR.0000145565.45490.D9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Michetti, C., Coimbra, R., Hoyt, D. B., Loomis, W., Junger, W., and Wolf, P. (2003). Pentoxifylline reduces acute lung injury in chronic endotoxemia. J. Surg. Res. 115, 92–99. doi: 10.1016/S0022-4804(03)00219-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miller, T. L., Shashikant, B. N., Pilon, A. L., Pierce, R. A., Shaffer, T. H., and Wolfson, M. R. (2007). Effects of recombinant Clara cell secretory protein (rhCC10) on inflammatory-related matrix metalloproteinase activity in a preterm lamb model of neonatal respiratory distress. Pediatr. Crit. Care Med. 8, 40–46. doi: 10.1097/01.PCC.0000253022.10607.61
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mourani, P. M., Sontaq, M. K., Ivy, D. D., and Abman, S. H. (2009). Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J. Pediatr. 154, 379–384. doi: 10.1016/j.jpeds.2008.09.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ng, G., Da Silva, O., and Ohlsson, A. (2012). Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 6:CD003214. doi: 10.1002/14651858.CD003214.pub2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Northway, W. H. Jr., Rosan, R. C., and Porter, D. Y. (1967). Pulmonary disease following respiratory therapy of hyaline-membrane disease. N. Engl. J. Med. 276, 357–368. doi: 10.1056/NEJM196702162760701
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
O’Brodovich, H. M., and Mellins, R. B. (1985). Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. Am. Rev. Respir. Dis. 132, 694–709.
Oh, W., Poindexter, B. B., Perritt, R., Lemons, J. A., Bauer, C. R., Ehrenkranz, R. A.,et al. (2005). Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 147, 786–790. doi: 10.1016/j.jpeds.2005.06.039
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Onland, W., Offringa, M., Cools, F., De Jaegere, A. P., Rademaker, K., Blom, H.,et al. (2011). Systemic hydrocortisone to prevent bronchopulmonary dysplasia in preterm infants (the SToP-BPD study); a multicenter randomized placebo controlled trial. BMC Pediatr. 11:102. doi: 10.1186/1471-2431-11-102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Onland, W., Offringa, M., De Jaegere, A. P., and van Kaam, A. H. (2009). Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics 123, 367–377. doi: 10.1542/peds.2008-0016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Onland, W., Offringa, M., and van Kaam, A. (2012). Late (≥7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 4:CD002311.
Pantalitschka, T., and Poets, C. F. (2006). Inhaled drugs for the prevention and treatment of bronchopulmonary dysplasia. Pediatr. Pulmonol. 41, 703–708. doi: 10.1002/ppul.20467
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patra, K., Greene, M. M., and Silvestri, J. M. (2014). Neurodevelopmental impact of hydrocortisone exposure in extremely low birth weight infants: outcomes at 1 and 2 years. J. Perinatol. 35, 77–81. doi: 10.1038/jp.2014.133
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Poggi, C., and Dani, C. (2014). Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxid. Med. Cell. Longev. 2014:721043. doi: 10.1155/2014/721043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robin, B., Kim, Y. J., Huth, J., Klocksieben, J., Torres, M., Tepper, R. S.,et al. (2004). Pulmonary function in bronchopulmonary dysplasia. Pediatr. Pulmonol. 37, 236–242. doi: 10.1002/ppul.10424
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rosenfield, W. N., Davis, J. M., Parton, L., Richter, S. E., Price, A., Flaster, E.,et al. (1996). Safety and pharmacokinetics of recombinant human superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 6, 811–817.
Rush, M. G., Engelhardt, B., Parker, R. A., and Hazinski, T. A. (1990). Double-blind, placebo-controlled trial of alternate-day furosemide therapy in infants with chronic bronchopulmonary dysplasia. J. Pediatr. 117, 112–118. doi: 10.1016/S0022-3476(05)82458-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ruszard, L., Szymura-Oleksiak, J., Pawlik, D., Warchol, J., Lisowska-Miszcqyk, I., and Rytlewski, K. (2006). Nebulized pentoxifylline for prevention of bronchopulmonary dysplasia in very low birth weight infants: a pilot clinical study. J. Matern. Fetal Neonatal Med. 19, 433–438. doi: 10.1080/14767050600736754
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sahni, J., and Phelps, S. J. (2011). Nebulized furosemide in the treatment of bronchopulmonary dysplasia in preterm infants. J. Pediatr. Pharmacol. Ther. 16, 14–22. doi: 10.1002/14651858.CD001453
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schmidt, B., Anderson, P. J., Doyle, L. W., Dewey, D., Grunau, R. E., Asztalos, E. V.,et al. (2012). Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307, 275–282. doi: 10.1001/jama.2011.2024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schmidt, B., Roberts, R. S., Davis, P., Doyle, L. W., Barrington, K. J., Ohlsson, A.,et al. (2006). Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 354, 2112–2121. doi: 10.1056/NEJMoa054065
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schmidt, B., Roberts, R. S., Davis, P., Doyle, L. W., Barrington, K. J., Ohlsson, A.,et al. (2007). Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 357, 1893–1902. doi: 10.1056/NEJMoa073679
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schrama, A. J., Bernard, A., Poorthuis, B. J., Zwinderman, A. H., Berge, H. M., and Walther, F. J. (2008). Cord blood Clara cell protein CC16 predicts development of bronchopulmonary dysplasia. Eur. J. Pediatr. 167, 1305–1312. doi: 10.1007/s00431-008-0713-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Segar, J. L. (2012). Neonatal diuretic therapy: furosemide, thiazides, and spironolactone. Clin. Perinatol. 39, 209–220. doi: 10.1016/j.clp.2011.12.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shah, S. S., Ohlsson, A., Halliday, H. L., and Shah, V. S. (2012a). Inhaled versus systemic corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst. Rev. 5:CD002058. doi: 10.1002/14651858.CD002058.pub2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shah, V. S., Ohlsson, A., Halliday, H. L., and Dunn, M. (2012b). Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst. Rev. 5:CD001969. doi: 10.1002/14651858.CD001969.pub3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shenai, J. P., Rush, M. G., Stahlman, M. T., and Chytil, F. (1990). Plasma retinol-binding protein response to vitamin A administration in infants susceptible to bronchopulmonary dysplasia. J. Pediatr. 116, 607–614. doi: 10.1016/S0022-3476(05)81614-2
Steinhorn, R. H. (2013). Diagnosis and treatment of pulmonary hypertension in infancy. Early Hum. Dev. 89, 865–874. doi: 10.1016/j.earlhumdev.2013.09.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stewart, A., and Brion, L. P. (2011). Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst. Rev. 9:CD001453. doi: 10.1002/14651858.CD001453.pub2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tang, J. R., Seedorf, G., Balasubramaniam, V., Maxey, A., Markham, N. E., and Abman, S. H. (2007). Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1271–L1280. doi: 10.1152/ajplung.00224.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tyson, J. E., Wright, L. L., Oh, W., Kennedy, K. A., Mele, L., Ehrenkranz, R. A.,et al. (1999). Vitamin A supplementation for extremely low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N. Engl. J. Med. 340, 1962–1968. doi: 10.1056/NEJM199906243402505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Meurs, K. P., Wright, L. L., Ehrenkranz, R. A., Lemons, J. A., Ball, M. B., Poole, W. K.,et al. (2005). Preemie inhaled nitric oxide study. Inhaled nitric oxide for premature infants with severe respiratory failure. N. Engl. J. Med. 353, 13–22. doi: 10.1056/NEJMoa043927
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wardle, A. J., and Tulloh, R. M. (2013). Paediatric pulmonary hypertension and sildenafil: current practice and controversies. Arch. Dis. Child Educ. Pract. Ed. 98, 141–147. doi: 10.1136/archdischild-2013-303981
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Watterberg, K. L. (2007). Postnatal steroids for bronchopulmonary dysplasia: where are we now? J. Pediatrics 50, 327–328. doi: 10.1016/j.jpeds.2006.12.041
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Watterberg, K. L. (2010). American academy of pediatrics. Committee on Fetus and Newborn. Policy statement-postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 126, 800–808. doi: 10.1542/peds.2010-1534
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wilkie, R. A., and Bryan, M. H. (1987). Effect of bronchodilators on airway resistance in ventilator-dependent neonates with chronic lung disease. J. Pediatr. 111, 278–282. doi: 10.1016/S0022-3476(87)80087-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wolfson, M. R., Funanage, V. L., Kirwin, S. M., Pilon, A. L., Shashikant, B. N., Miller, T. L.,et al. (2008). Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. Am. J. Perinatol. 25, 637–645. doi: 10.1055/s-0028-1090587
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: bronchopulmonary dysplasia, bronchodilators, corticosteroids, diuretics, lung injury
Citation: Iyengar A and Davis JM (2015) Drug therapy for the prevention and treatment of bronchopulmonary dysplasia. Front. Pharmacol. 6:12. doi:10.3389/fphar.2015.00012
Received: 23 October 2014; Paper pending published: 24 November 2014;
Accepted: 13 January 2015; Published online: 16 February 2015.
Edited by:
George Giacoia, The Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health, USAReviewed by:
Michael John Rieder, University of Western Ontario, CanadaSunil K. Jain, University of Texas Medical Branch, USA
Copyright © 2015 Iyengar and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjali Iyengar, Department of Pediatrics, Floating Hospital for Children at Tufts Medical Center, TMC Box 44, 800 Washington Street, Boston, MA 02111, USA e-mail:YWl5ZW5nYXJAdHVmdHNtZWRpY2FsY2VudGVyLm9yZw==
 Anjali Iyengar
Anjali Iyengar Jonathan M. Davis
Jonathan M. Davis