- 1School of Medicine and Medical Science, University College Dublin, Dublin, Ireland
- 2Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, UK
- 3Radcliffe Department of Medicine, University of Oxford, Oxford, UK
- 4Translational Gastroenterology Unit, University of Oxford, Oxford, UK
Liver fibrosis reflects sustained liver injury often from multiple, simultaneous factors. Whilst the presence of mild fibrosis on biopsy can be a reassuring finding, the identification of advanced fibrosis is critical to the management of patients with chronic liver disease. This necessity has lead to a reliance on liver biopsy which itself is an imperfect test and poorly accepted by patients. The development of robust tools to non-invasively assess liver fibrosis has dramatically enhanced clinical decision making in patients with chronic liver disease, allowing a rapid and informed judgment of disease stage and prognosis. Should a liver biopsy be required, the appropriateness is clearer and the diagnostic yield is greater with the use of these adjuncts. While a number of non-invasive liver fibrosis markers are now used in routine practice, a steady stream of innovative approaches exists. With improvement in the reliability, reproducibility and feasibility of these markers, their potential role in disease management is increasing. Moreover, their adoption into clinical trials as outcome measures reflects their validity and dynamic nature. This review will summarize and appraise the current and novel non-invasive markers of liver fibrosis, both blood and imaging based, and look at their prospective application in everyday clinical care.
Introduction
As the gateway to the systemic circulation from the gut, the liver performs several critical functions, coordinating substrate metabolism, and detoxification as well as responding to immune stimuli. Its unique position and vascular supply also leaves it constantly exposed to potential injury, and liver damage, induced by hepatotoxins such as viral hepatitis, alcohol or excess fat, leads to a cycle inflammation and fibrosis. This process involves the deposition of extracellular matrix (ECM), which if degraded results in repair and regeneration; however should the source of liver injury persist, fibrosis may progress, and ultimately evolve to cirrhosis or end-stage liver disease.
Historically, the role of liver biopsy was both for diagnostic and staging purposes. Given the ability to diagnose the majority of liver conditions with blood testing alone, liver biopsy now primarily serves to quantify fibrosis in order to stage disease. This informs the clinician and patient regarding the urgency of initiating therapy (where possible) and prognosis. Indeed, recent longitudinal studies have demonstrated the presence and severity of fibrosis on biopsy as the single most important predictor of outcome in NAFLD, the commonest cause of liver disease in the Western world, highlighting the need for disease staging for an individual patient (Angulo et al., 2015; Ekstedt et al., 2015).
Liver biopsy represents the “gold standard” for the assessment and quantification of liver fibrosis. However, its invasiveness engenders pain and significant potential complications, leading to poor patient acceptance (Cadranel et al., 2000). Other disadvantages include considerable cost (Pasha et al., 1998), and its inherent shortcomings related to sampling error and sample quality from needle biopsy specimens (Sherlock, 1962; Villeneuve et al., 1996; Bedossa et al., 2003; Merriman et al., 2006) have lead to questions regarding its suitability as the reference standard for liver fibrosis (Standish et al., 2006; Bedossa and Carrat, 2009). These factors, along with an acknowledgement of the need to stage disease without reliance on liver biopsy, have lead to an exponential interest in the identification and use of non-invasive markers of liver fibrosis.
The performance of a non-invasive marker of fibrosis is measured using liver biopsy staging of fibrosis as the reference standard. This is typically reported by sensitivity and sensitivity measurements, as well as an area under the receiver operating curve (AUROC) value, which indicates the ability of the marker (at different cut-offs) to correctly classify a patient into a particular category of fibrosis. Values of < 0.7, 0.7−0.8, 0.8−0.9, and >0.9 indicate poor, fair, good, and excellent accuracy, respectively. Serum non-invasive tests can potentially achieve a perfect AUROC value, having been developed and calibrated using liver biopsy as a reference. The same is not true for imaging-based modalities, who are unable to achieve excellent diagnostic accuracy for fibrosis, an important point to consider when interpreting studies (Tsochatzis et al., 2011a). Another issue relates to “spectrum bias,” where different stages of liver fibrosis may be represented unequally within a candidate study, leading to potentially misleading results, highlighting the importance of independent validation of markers.
Non-invasive markers of fibrosis are being incorporated into the routine clinical care of patients with liver disease, although the field continues to evolve. The importance of these markers comes with the stark realization that standard liver biochemistry is of little value in determining the severity of liver fibrosis in primary care (Armstrong et al., 2012). Instead, simple scores based on blood test results can enable a tier system whereby primary care physicians can determine the need for specialist referral for patients (Sheron et al., 2012). With the availability of accurate non-invasive tests, the ability to screen large cohorts for significant liver disease is now becoming a possibility, allowing the assessment of the true burden of liver disease in the general population (Koehler et al., 2016). Moreover, as novel anti-fibrosis therapies enter clinical trials, robust non-invasive markers are crucial to allow effective trial design and obviate the need for multiple, invasive liver biopsies to assess efficacy.
In this review, the types and application of several blood-based non-invasive markers of liver fibrosis are described, followed by a thorough description of current ultrasound based elastography methods and their utility in staging of liver disease. Finally, magnetic resonance imaging techniques to improve staging of liver fibrosis and cirrhosis are considered.
Non-Invasive Serological Markers
Whilst there have been many non-invasive biomarkers investigated and proposed, an ideal serological test for liver fibrosis which reduces the need to perform invasive investigations such as liver biopsy is still awaited. This ideal serological test would be quick to perform and analyse as well as inexpensive and reproducible. Furthermore, an ideal test would be able to distinguish between distinct entities in liver pathology such as inflammation and fibrosis, be unaffected by impairment in liver function, as well as being able to predict and track disease progression or regression.
When evaluating potential biomarkers, there is a need to ensure consistency in assessment and evaluation, and the related need for simple and robust classification systems (Veidal et al., 2010). This is important as the various serological biomarkers for liver fibrosis described in the medical literature to date have been appraised and validated in differing liver disease contexts, and so in different cohorts of patients. The majority of studies published have been performed in patients with hepatitis C (HCV), with fewer studies performed in patients with hepatitis B (HBV), non-alcoholic fatty liver disease (NAFLD), and alcoholic liver disease (ALD), with fewer still reported in rarer conditions such as in autoimmune liver diseases and hereditary haemochromatosis. As one may expect, these biomarkers perform differently in these differing liver disease contexts. As such, care must be taken to ensure that use of a specific biomarker test at individual patient level has both diagnostic and clinical validity in the context of the patient's illness (Balistreri, 2014). It is equally important to note that most of the biomarkers described in the literature work best in predicting established or advanced fibrosis when compared to the gold standard of liver biopsy, and that biomarkers generally do not perform well in indeterminate stages of liver disease.
To help aid understanding, non-invasive biomarkers of fibrosis may be broadly divided into two classes. Class I markers are direct serum markers reflecting ECM turnover and/or fibrogenic changes at cellular level in the liver. Class II markers of fibrosis are indirect serum markers based on algorithms and mathematical model of varying complexity and derived from changes that occur in liver function (Gressner et al., 2007; Veidal et al., 2010).
Class I (Direct) Biomarkers of Liver Fibrosis
Class I biomarkers are direct markers of fibrosis and reflect the molecular pathogenesis and turnover of liver ECM. The main source of fibrosis in the liver is derived from hepatic stellate cells (HSCs) and myofibroblasts (Korner et al., 1996; Friedman, 2000; Rockey and Friedman, 2006; Gressner et al., 2007; Henderson et al., 2013). Injury to the liver leads to the production of cytokines and to the activation of HSCs initially causing liver inflammation, and then to potentially irreversible change and scarring within the ECM tissue architecture.
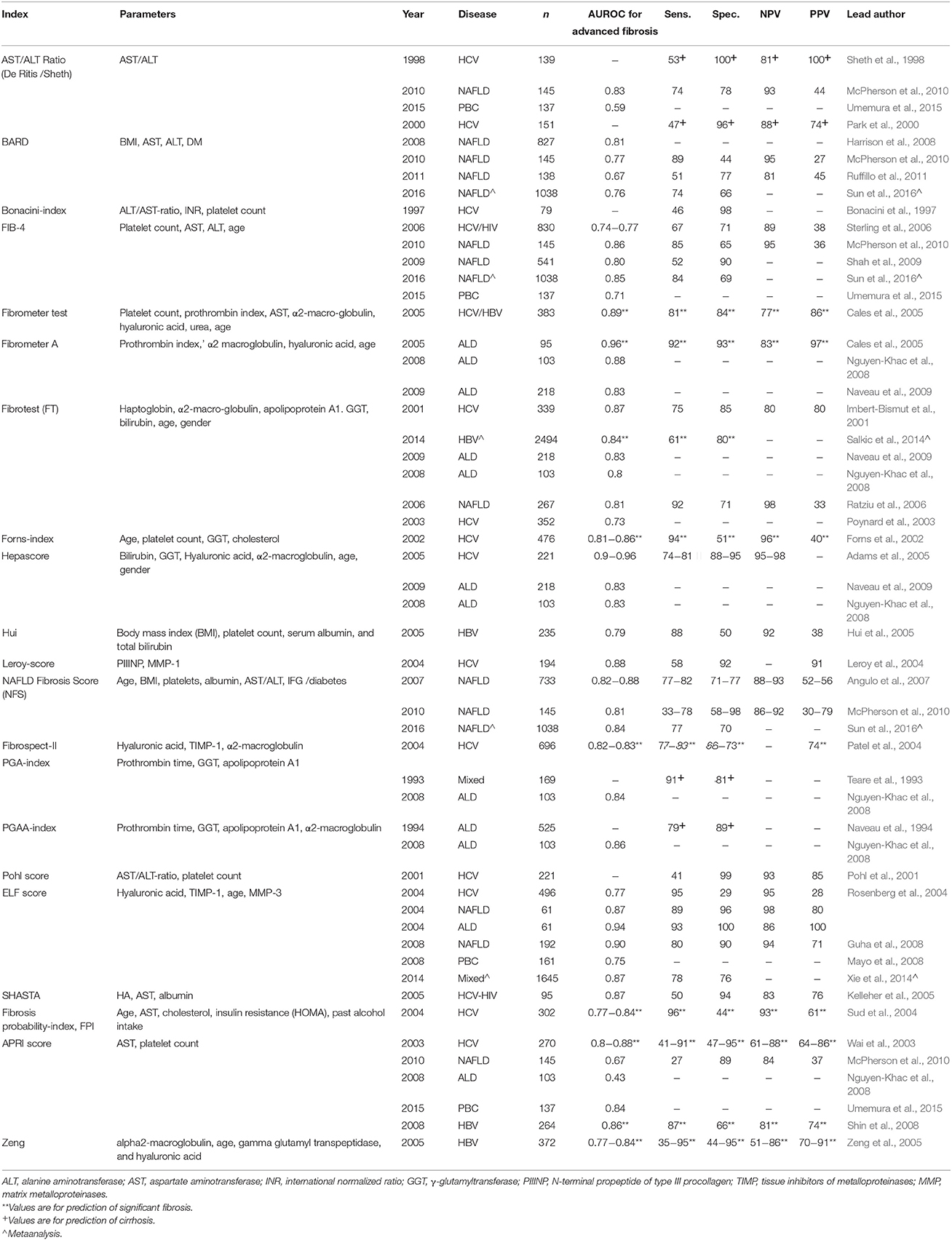
Class I markers may be subcategorized into enzymatic markers, collagen (and related) markers, Glycoproteins and matrix-metalloproteinase markers, and Glycosaminoglycan markers (Table 1; Guechot et al., 1996; Murawaki et al., 1999; Walsh et al., 1999; McHutchison et al., 2000; Gressner et al., 2007). Of these various classes, collagen derived markers and glycosaminoglycans (hyaluronic acid) have proven to be the most widely utilized in the development of new fibrosis biomarkers. Few class I markers are used in routine clinical practice as they largely do not directly correlate with whole tissue or body function and so provide data of limited clinical value. As such, their turnaround time is higher, availability is limited to mainly research and specialist settings and investigational costs are generally increased when compared to class II markers. Another limitation is that class I markers are neither all liver derived nor liver specific and so serum measurements do not always directly relate to liver activity. For example, during infection, where acute systemic inflammation co-exists, these markers may also be affected and their absolute values can reflect both fibrinolytic as well as fibrogenic activity.
Class II (Indirect) Biomarkers of Liver Fibrosis
Class II biomarkers provide an indirect reflection of liver ECM activity and fibrosis through the measurement of liver function or injury. As these tests reflect alterations in hepatic function and often correlate with signs and symptoms of illness, they are well established and used routinely in clinical practice to both diagnose and to monitor liver disease.
The broad range of activities the liver performs includes thermostasis, energy balance, carbohydrate, fat and protein metabolism, haemocoagulation, iron and hemoglobin metabolism, immune balance, production of bile acids and hormones, and clearance of toxins (Pratt and Kaplan, 2000; Limdi and Hyde, 2003; Tan et al., 2012; Neuman et al., 2014). Therefore, the number of class II biomarkers that may be tested is extensive.
In daily clinical practice, serological testing for liver function may be broadly categorized into the following categories:
− Liver enzymes, including alanine amino transferase (ALT), aspartate amino transferase (AST), alkaline phosphatase (ALP), and gamma glutamyl transferase (GGT)
− Markers of synthetic function, such as prothrombin time (PT/INR), bilirubin, haptoglobin, albumin, Apolipoprotein A1, α2-macroglobulin, ceruloplasmin, transferrin and hepcidin
− Other indirectly measured markers, such as platelet count, α1-antitrypsin, ferritin, and certain adipokines (adiponectin and leptin; Ding et al., 2005).
As one may expect, although class II markers are more readily available and generally more cost-effective to employ, as proxy markers for fibrosis, their results are not as reliable or accurate as class I markers (Adams et al., 2005). Serum levels of these markers may be affected by a wide range of factors. It is well recognized that ALT levels, commonly thought to indicate the severity of liver disease, are wholly unreliable as a predictor of fibrosis (McCormick et al., 1996) and often within normal range in individuals with advanced disease (Mofrad et al., 2003). Indeed, one recent paper demonstrated a 56% prevalence of NAFLD in 103 volunteers with type 2 diabetes who had normal plasma aminotransferase levels (Portillo-Sanchez et al., 2015).
Combination or Panel Non-invasive Biomarkers
Whilst individual biomarkers have value as described, the combination of various biomarkers—with varying degrees of complexity—can greatly improve the sensitivity and specificity of these tests. Simple combination tests broadly employ class II biomarkers to indirectly assess liver fibrosis. The simplest of these is the AST/ALT ratio. Afdhal and Nunes summarized a number of studies that assessed its value, with most of these studies undertaken in patients with HCV reporting sensitivities between 53–78% and specificities between 90 and 100% for detecting liver fibrosis (Afdhal and Nunes, 2004). Another simple test is the AST to platelet ratio index (APRI), which uses the platelet count in addition to AST to predict fibrosis, first described in 2003 by Wai and colleagues in patients with HCV (Wai et al., 2003). The APRI score has also been shown to have some value in patients with HBV and PBC, although not in ALD, probably due to the confounding effects of alcohol on platelet suppression (Lieber et al., 2006; Loaeza-del-Castillo et al., 2008; Umemura et al., 2015; Xiao et al., 2015). Other simple indirect combination markers include the PGA (prothrombin time, γ-glutamyl transferase and apolipoprotein A1) and PGAA (PGA plus α-2 macroglobulin) indices, Pohl score and Forn's Index (Imbert-Bismut et al., 2001; Pohl et al., 2001; Forns et al., 2002). Another simple combination panel is the NAFLD fibrosis score (NFS), an easily accessible online score which can be used by general practitioners to triage patients with suspected NAFLD in order to determine the need for referral for specialist assessment (Angulo et al., 2007). The AUROC values, sensitivities, specificities, and predictive values of these tests where available for liver fibrosis are provided in Table 2.
More complex combination biomarkers involve a panel of tests or complex algorithms, some of which are patented in the assessment liver fibrosis. These include the Fibrotest/Fibrosure®, Fibrometer®, and Hepascore® (Imbert-Bismut et al., 2001; Adams et al., 2005; Cales et al., 2005). As these tests generally include some class I markers as well as class II markers (Table 2), they have enhanced diagnostic accuracy, however this comes at an increased cost and reduced test availability in some cases, while also demanding greater physician time and effort to calculate and subsequently to interpret. Nevertheless, as these panels continue to improve, they are gaining increased acceptance in clinical practice, and large longitudinal studies have demonstrated their ability to predict prognosis and liver-related outcomes. For example, the Fibrometer® and FIB-4 showed good ability to predict significant liver-related events [AUROC values of 0.88 (0.83–0.91) and 0.87 (0.81–0.92)] in 373 patients with HCV over a median 9.5 year follow up, better than that predicted by fibrosis grade (Boursier et al., 2014). The Enhanced Liver Fibrosis (ELF) test similarly demonstrated prognostic capabilities in 457 patients with chronic liver disease followed a median over 7 years to predict death and clinical outcomes (Parkes et al., 2010). Moreover, a number of studies have now shown the value of the simpler investigations such as the NFS, the FIB-4 and APRI not only in the assessment of liver fibrosis, but also in estimating prognosis (Vallet-Pichard et al., 2007; Treeprasertsuk et al., 2013; Vergniol et al., 2014; Xiao et al., 2015). These findings are important considerations for the application of non-invasive markers with well-validated diagnostic and prognostic ability as outcome measures in clinical trials, obviating the need for repeated liver biopsies thereby enhancing recruitment and study efficiency. In routine clinical practice, the use of these scores alone or in combination can also significantly reduce the need for a liver biopsy, increasing the appropriateness and diagnostic yield from the remaining biopsies that are performed. For instance, Sebastiani and co-workers found that the combination of the APRI test with the Fibrotest-Fibrosure in 2035 patients with chronic HCV infection reduced the need for liver biopsy in 50–80% of cases, with high (>90%) diagnostic accuracy for significant fibrosis and cirrhosis (Sebastiani et al., 2009).
In summary, the quest to find an ideal non-invasive serological biomarker or combination of markers to diagnose and predict the outcome of liver fibrosis is ongoing, with considerable progress made over the past decade. Nevertheless, these investigations remain imperfect, particularly for the detection of intermediate fibrosis grades, and require judicious use tailored to the individual case, and detailed understanding to ensure their use is valid. As these investigations continue to be refined and improved, the need for staging liver biopsy will continue to decline over time.
Non-Invasive Ultrasound Elastography
Ultrasound Based Elastography for the Assessment of Liver Fibrosis
Ultrasound based elastography has revolutionized the assessment of liver fibrosis over the last decade. The concept of elastography for the assessment of fibrosis is simple and stems from applying an external force to soft tissue, compressing the tissue, and thereby generating a shear wave which can be measured (Ophir et al., 1991). The speed of the shear wave propagating through the tissue corresponds to the tissue elasticity based on Young's modulus and hence, the amount of tissue fibrosis (Sandrin et al., 2003).
To date, a number of different types of ultrasound based elastography methods have been developed to assess liver fibrosis and diagnose cirrhosis (Thiele et al., 2015). One can broadly distinguish these elastography methods into two main groups: one-dimensional ultrasound elastography—transient elastography (TE); or two-dimensional (or B-mode) ultrasound which utilizes conventional ultrasound imaging - acoustic radiation force impulse (ARFI) imaging or point shear wave elastography (pSWE), real-time elastography (RTE), and real-time 2D shear wave elastography (2D-SWE). It is likely that some of these ultrasound-based elastography instruments will improve in time with the advent of novel technology.
Elastography measures tissue stiffness. Assessing liver fibrosis with ultrasound-based elastography only measures a small component of tissue stiffness. Many other factors can affect liver tissue stiffness and have been shown to affect the accuracy of assessing liver fibrosis with elastography. These factors were initially studied in TE and include: Inflammation from acute hepatitis (Arena et al., 2008a; Oliveri et al., 2008; Sagir et al., 2008); cholestasis from biliary tract obstruction (Millonig et al., 2008); blood congestion due to hepatic outflow obstruction and portal hypertension(Millonig et al., 2010; Shi et al., 2013); and food intake (Mederacke et al., 2009; Arena et al., 2013; Berzigotti et al., 2013) Another example that elastography measures more than just tissue fibrosis is the utilization of elastography to measure spleen stiffness in patients with cirrhosis; where spleen stiffness correlates with hepatic venous pressure gradient (HVPG; Colecchia et al., 2012; Sharma et al., 2013; Takuma et al., 2013) and reduces after liver transplantation (Chin et al., 2015). Although not studied extensively in other ultrasound-based elastography methods, all of these factors are thought to apply when interpreting liver stiffness measurements obtained with newer techniques (Bota et al., 2013a; Popescu et al., 2013).
Transient Elastography
Transient elastography (Fibroscan®, Echosens, Paris) is the first ultrasound-based elastography developed and is now a well-established non-invasive method of diagnosing and staging hepatic fibrosis (Sandrin et al., 2003). TE utilizes a mono-dimensional ultrasound to determine liver stiffness by measuring the velocity of low frequency elastic shear waves propagating through the liver. TE can be performed in a short time (typically less than 5 min) and has excellent intra- and inter- observer variability (Fraquelli et al., 2007; Boursier et al., 2008).
The Fibroscan® probe consists of a 3.5 MHz ultrasound transducer installed on the axis of a low amplitude vibrator (frequency of 50 Hz and amplitude of 2 mm peak-to-peak). To obtain liver stiffness measurements, the tip of the ultrasound transducer is placed in the right intercostal area, at the level of the right lobe of liver. When activated, the vibrator generates an elastic shear wave to the liver while the ultrasound transducer performed a series of ultrasound acquisitions (transmission/ reception) with a repeat frequency of 4 kHz. A median value of at least 10 successful measurements with an Interquartile range (IQR) of ≤ 30% from the median and success rate of ≥60%, were considered as reflective of the liver stiffness or shear modulus of the liver. This value is expressed in kilopascals (kPa).
TE has been extensively studied for the assessment of fibrosis in many chronic liver diseases, particularly viral hepatitis. The two index studies on TE correlated liver stiffness with the degree of fibrosis (METAVIR staging) on liver biopsy due to chronic HCV infection (Castéra et al., 2005; Ziol et al., 2005). Since then, a number of comparable studies in HCV validated this finding (Coco et al., 2007; Arena et al., 2008b; Lupsor et al., 2009; Wang et al., 2009; Degos et al., 2010; Zarski et al., 2012; Afdhal et al., 2015), and a meta-analysis had reported excellent diagnostic accuracy for HCV-related cirrhosis with an AUROC of 0.95 (0.87–0.99; Shaheen et al., 2007). Similar studies were performed for patients with chronic HBV (Oliveri et al., 2008; Chan et al., 2009; Kim et al., 2009; Marcellin et al., 2009; Wang et al., 2009; Degos et al., 2010; Sporea et al., 2010; Cardoso et al., 2012; Goyal et al., 2013; Afdhal et al., 2015) and co-infection with HCV/HIV (de Lédinghen et al., 2006; Vergara et al., 2007; Kirk et al., 2009; Sánchez-Conde et al., 2010; Castera et al., 2014), with good correlation between liver stiffness and fibrosis. Furthermore, liver stiffness by TE has been shown to predict liver fibrosis in ALD (Nahon et al., 2008; Nguyen-Khac et al., 2008), NAFLD (Nobili et al., 2008; Yoneda et al., 2008; Lupsor et al., 2010; Wong et al., 2010a; Petta et al., 2011) and chronic cholangitides (notably, PBC and PSC; Corpechot et al., 2006, 2012). The diagnostic performance of TE have been confirmed by several meta-analyses to show excellent diagnostic accuracy for cirrhosis of (>90%), but only good accuracy for fibrosis (>80%; Shaheen et al., 2007; Friedrich-Rust et al., 2008; Stebbing et al., 2010; Tsochatzis et al., 2011b; Chon et al., 2012; Table 3).
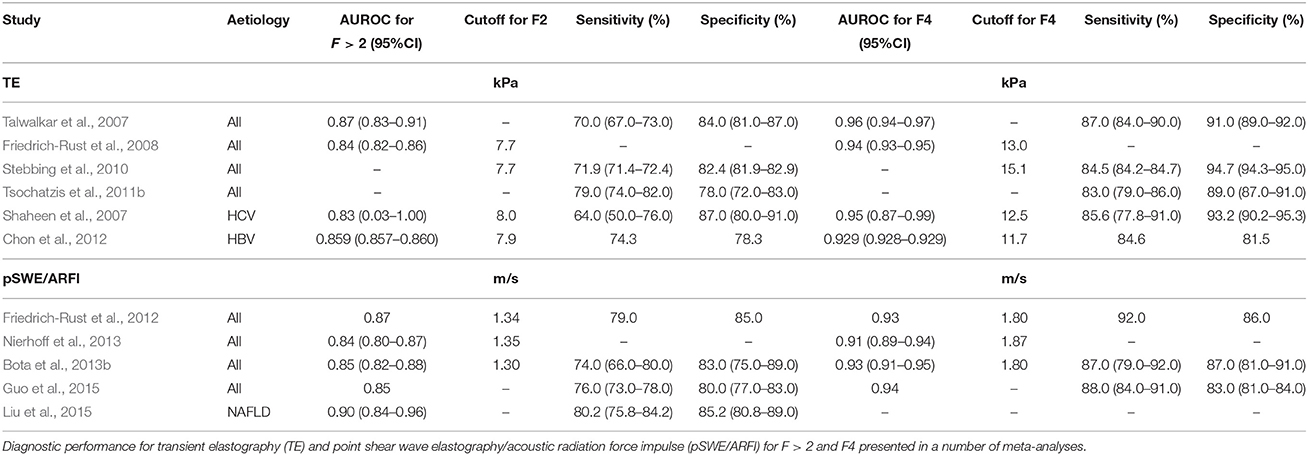
Table 3. Diagnostic performance for ultrasound-based elastography methods for the detection of liver fibrosis.
Point Shear Wave Elastography/Acoustic Radiation Force Impulse Imaging
pSWE (Elastography point quantification, ElastPQ™, Phillips) and ARFI imaging (Virtual touch tissue quantification™, Siemens) are ultrasound-based elastography techniques which utilize B-mode ultrasound. This elastography technique can be incorporated into conventional ultrasound platforms, such as the iU22xMATRIX by Philips (Amsterdam, Netherlands) and Acuson S2000/3000 by Siemens Healthcare (Erlangen, Germany). Hitachi Aloka (Tokyo, Japan) also recently announced their pSWE method known as Shear Wave Measurement in the Radiological Society of North America Meeting in November 2015. pSWE/ARFI measures the speed of shear waves generated by acoustic pulses that lead to localized displacements of liver tissue (Nightingale et al., 2002). Liver stiffness measurement with pSWE/ARFI is performed at the right lobe of the liver, through the intercostal space, at the same site as TE. After selecting a region of interest, at a depth of 2–8 cm with B-mode ultrasound, the shear-wave velocity is measured within the defined region by using ultrasound tracking beams laterally adjacent to the single push beam (Friedrich-Rust et al., 2009). Similar to TE, pSWE/ARFI have good intra- and inter-observer agreement, with an intra-class correlation coefficient of between 0.84 and 0.87 (Boursier and Cales, 2010; Bota et al., 2012).
pSWE/ARFI has been described in many studies to be comparable to TE (Friedrich-Rust et al., 2009; Lupsor et al., 2009; Yoneda et al., 2010; Ebinuma et al., 2011; Piscaglia et al., 2011; Rifai et al., 2011; Sporea et al., 2012). Like TE, pSWE/ARFI is able to diagnose cirrhosis more accurately than significant fibrosis. Several meta-analyses of pSWE/ARFI confirmed good diagnostic accuracy for significant fibrosis, with mean AUROC of 0.84 to 0.87 and excellent diagnostic accuracy for cirrhosis of 0.91–0.94 (Friedrich-Rust et al., 2012; Bota et al., 2013a; Nierhoff et al., 2013; Guo et al., 2015). Based on data from these meta-analyses, the suggested cut-off values for the diagnosis of significant fibrosis were 1.30–1.35 m/s and for cirrhosis, the values were 1.80–1.87 m/s. A recent meta-analysis of pSWE/ARFI in NAFLD reported good diagnostic accuracy of pSWE/ARFI in assessing significant fibrosis in patients with NAFLD (AUROC of 0.898; Liu et al., 2015; Table 3).
Real-Time Elastography
RTE is a qualitative assessment of liver stiffness. For RTE, strain elastography is provided either by the operator applying manual compression (Friedrich-Rust et al., 2008) or compression is applied by the transmitted heartbeat (Koizumi et al., 2011). The reflected ultrasound echoes between the states of compression can be computed to measure displacement within each location of the tissue. Hence, the harder the tissue, the lower the amount of displacement of reflected ultrasound echoes before and under compression. A number of quantitative methods for RTE have been described to improve the comparability of this elastography technique, which include the elastic ratio, elastic index, elasticity score, and Liver Fibrosis Index (Friedrich-Rust et al., 2007; Kanamoto et al., 2009; Tatsumi et al., 2010; Koizumi et al., 2011; Colombo et al., 2012; Ferraioli et al., 2012a; Fujimoto et al., 2013; Yada et al., 2013). The basis of these quantitative methods for RTE is to provide a ratio of stiffness or elasticity. When comparing RTE to other ultrasound elastography methods, a recent meta-analysis reported lower diagnostic accuracy with RTE for significant liver fibrosis and cirrhosis, compared to TE or ARFI (Kobayashi et al., 2015). The marked heterogeneity of RTE studies was reported as one of the main reasons for lower diagnostic performance of RTE.
Real-Time 2D Shear Wave Elastography
Real time 2D-SWE is a relatively new ultrasound elastography method, which combines the initiation of a radiation force in tissue with focused ultrasonic beams and acquisition of transiently propagating resultant shear waves in real-time with a high-frequency ultrasound imaging sequence (Muller et al., 2009). In 2D-SWE, a large color coded elastography map is generated by combining several shear waves over time with rapid ultrasound acquisition (Thiele et al., 2016). 2D-SWE is performed quite similarly to pSWE/ARFI. Both the size and location of the region of interest can be chosen by the operator. Although different to pSWE/ARFI in technology, 2D-SWE is similar to pSWE/ARFI for the clinical user as it is implemented on a commercial B-mode ultrasound platform (Aixplorer®, Supersonic Imaging, Aix en Provence, France).
Liver stiffness measured by 2D-SWE has been shown to correlate with the stages of hepatic fibrosis on liver biopsy in chronic HCV (Ferraioli et al., 2012b), chronic HBV (Leung et al., 2013; Zeng et al., 2014), and alcohol-related liver disease (Thiele et al., 2016). In mixed cohorts of patients with chronic liver disease, several studies have shown similar results (Cassinotto et al., 2014; Jeong et al., 2014; Bota et al., 2015; Deffieux et al., 2015; Gerber et al., 2015; Grgurevic et al., 2015; Samir et al., 2015). There is now increasing evidence that 2D-SWE is a comparable elastography method to TE and pSWE/ARFI (Bavu et al., 2011; Ferraioli et al., 2012a; Leung et al., 2013; Cassinotto et al., 2014; Bota et al., 2015; Gerber et al., 2015; Thiele et al., 2016), although no published meta-analysis confirming these findings exists, as yet.
Comparative Performance of Ultrasound Based Elastography
Based on data from published literature on elastography, the diagnostic performance for significant fibrosis and cirrhosis for TE, pSWE/ARFI and probably 2D-SWE are equivalent. In contrast, RTE has a slightly lower diagnostic accuracy and remains limited to centers with experience with this method (Kobayashi et al., 2015). The diagnostic accuracy (AUROC values) for significant fibrosis using TE, pSWE/ARFI and 2D-SWE ranges between 0.65–0.97, 0.77–0.94, and 0.82–0.99 respectively, with excellent results consistently seen for cirrhosis, ranging between 0.80–0.99, 0.87–0.99, and 0.87–0.99, respectively. One advantage of TE is that it can be performed at the point of care, whereas second-generation elastrography techniques tend to require operators trained in ultrasound, reducing accessibility.
The failure rate of TE has been reported to range from 2.7 to 3.1% in obtaining any measurement, and 11.6 to 15.8% in acquiring unreliable results (Castéra et al., 2010; Wong et al., 2011). For pSWE/ARFI (Bota et al., 2013b) and 2D-SWE (Leung et al., 2013; Poynard et al., 2013; Elkrief et al., 2015), the failure rate for acquiring reliable results has been reported to be significantly lower than TE. Liver stiffness measured with 2D-SWE can be expressed either in kPa at a wide range of values (2–150 kPa) or in the form of shear wave velocity, in m/s. One of the main limitations of ARFI is the narrow range of shear wave velocity measurements of 0.5–4.4 m/s, in contrast to the range of TE of 2.5–75 kPa. This is thought to limit the ability of ARFI to define discriminative cut-off values for certain stages of fibrosis.
Quality criteria for liver stiffness measurements have previously been emphasized for TE to provide consistent and reproducible results. These criteria are less clear for pSWE/ARFI and 2D-SWE. The manufacturer of TE recommends that at least 10 successful liver stiffness measurements are acquired with an IQR of ≤ 30% from the median, and a success rate of ≥60%. The median value is taken as representative of liver stiffness. For pSWE/ARFI, most studies emulate the quality criteria of TE, where 10 valid measurements are performed and the median value deemed as the representative measure. Unreliable results for pSWE/ARFI were defined as an IQR/liver stiffness of greater than 30%. In both TE and pSWE/ARFI, a single shear wave is emitted temporarily at a single frequency for each measure. Hence, maintaining these quality criteria is fundamental to consistent measure of liver stiffness.
For 2D-SWE, these quality criteria do not apply. In 2D-SWE, the ultrasound transducer emits a plurality of pulse waves at increasing depth, using a very wide frequency band ranging from 60 to 600 Hz (Bercoff et al., 2004; Muller et al., 2009). The 2D-SWE transducer synchronously evaluate the velocity of several shear wave fronts over a wide frequency range. A real-time color coded map of elasticity is generated and is superimposed on the standard B-mode image. By selecting a region of interest in the center of the color map, the calculated value is the average of many values within the area (Cassinotto et al., 2014). Therefore, the quality criteria for 2D-SWE are not identical. Although not defined, a number of 2D-SWE studies have suggested the following: standard deviation/median ratio < 0.1–0.3 and depth of measurement < 5.6 cm; stable viscoelasticity map for at least 3 s with a homogenous color in the region of interest of at least 15 mm; and 3 measurements with the mean calculated as representative or using the median when >6 measurements are acquired (Sporea et al., 2013; Yoon et al., 2014; Procopet et al., 2015; Thiele et al., 2016).
Similar to serum biomarkers, ultrasound-based elastography is excellent for the non-invasive diagnosis of cirrhosis but only modestly good for significant fibrosis. When comparing TE with serum biomarkers, a number of studies have shown that both TE and serum biomarkers have equivalent performance for detecting significant fibrosis in HCV (Castéra et al., 2005; Degos et al., 2010; Zarski et al., 2012). Conversely, TE performs better than serum biomarkers for detecting cirrhosis due to HCV, if TE measurement is possible (lower applicability of TE compared to serum biomarkers, 80 vs. 95%; Degos et al., 2010; Zarski et al., 2012). While algorithms combining TE with serum biomarkers improve diagnostic accuracy in assessing fibrosis, particularly in HCV patients (Wong et al., 2010b; Boursier et al., 2011, 2012; Zarski et al., 2012), this strategy do not increase the overall diagnostic accuracy for detecting cirrhosis (Castéra et al., 2009; Boursier et al., 2011; Zarski et al., 2012). Furthermore, the combination of TE with serum fibrosis biomarkers is often advocated to better inform the clinician, allowing an informed judgment regarding the need for liver biopsy, for example where concordance exists in extremes of fibrosis, or discordance in intermediate stages.
Several studies have demonstrated that liver stiffness measured by ultrasound based elastography could predict clinical hepatic decompensation, liver related complication and prognosis (de Lédinghen et al., 2006; Robic et al., 2011; Vergniol et al., 2011; Corpechot et al., 2012; Merchante et al., 2012; Pang et al., 2014). Although a number of reports have described good correlation between liver stiffness and HVPG (Vizzutti et al., 2007; Bureau et al., 2008; Lemoine et al., 2008), the correlation between clinically significant portal hypertension and liver stiffness becomes less significant for HVPG values of >12 mmHg (Vizzutti et al., 2007). One potential explanation is that as cirrhosis progresses, the mechanism for portal hypertension is thought to be less dependent on intrahepatic resistance, and more dependent of the resultant hyperdynamic circulation and splanchnic vasodilatation (Reiberger et al., 2012). At present, ultrasound based elastography cannot replace HVPG for evaluation of portal hypertension or upper gastrointestinal endoscopy for detecting oesophageal varices. However, TE is an invaluable tool to risk stratify patients with clinically significant portal hypertension and identify patients at risk of developing HCC (Latinoamericana, 2015).
Non-Invasive Magnetic Resonance Imaging
Magnetic Resonance Techniques for Liver Fibrosis Assessment
Magnetic resonance (MR) imaging techniques are attractive tools for liver fibrosis assessment. In contrast to ultrasound techniques, MR allows deep penetration into tissues, and unlike computed tomography avoids ionizing radiation. Repeated assessments can therefore be performed, without safety concerns. Furthermore, MR can assess the whole liver, eliminating sampling errors and can provide additional information on anatomy. There is now extensive experience in the use of MR for the evaluation of diffuse liver disease due to liver fat or iron deposition. Both magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) were found to be more accurate than histological assessment by a pathologist or by morphometry. (Roldan-Valadez et al., 2010; Raptis et al., 2012) MR has been used to study the epidemiology of NAFLD (Browning et al., 2004; Szczepaniak et al., 2005; Wong et al., 2012) and more recently it has been used as a surrogate end-point in two NAFLD clinical trials (Le et al., 2012; Loomba et al., 2015). MR techniques are also regarded as the gold standard for quantification of liver iron as they have shown an excellent inverse association with biochemically quantified hepatic iron concentration (Gandon et al., 2004; St Pierre et al., 2005). More recently, MR techniques for the assessment of liver fibrosis have been developed and have produced some promising results.
Magnetic Resonance Elastography
Similar to ultrasound based elastography techniques, magnetic resonance elastography (MRE) can determine liver stiffness by analysis of mechanical waves propagating through the liver. The technique requires the positioning of an external passive driver on the patient's body adjacent to the liver. The passive driver is connected to a pneumatic active driver placed outside the scanner room which generates the mechanical waves that are propagated through the liver.
MRE for Liver Fibrosis Evaluation
In a large prospective single center study of 96 patients with mixed liver disease aetiologies (63% HCV; 8% NAFLD), MRE had excellent diagnostic accuracy for the differentiation of all stages of fibrosis, with an area under the receiver operating characteristic curve (AUROC) for ≥F3 and F4 of 0.99 and 1.00 respectively. (Huwart et al., 2008) Similar results have been obtained in patients with chronic HBV and HCV, (Ichikawa et al., 2012; Venkatesh et al., 2014a) and MRE was also shown to be closely related to morphometric quantification of liver fibrosis (r = 0.78; p < 0.001; Venkatesh et al., 2014b). In a meta-analysis of 697 individual patient data from 12 studies, MRE was found to have a good diagnostic performance for all stages of liver disease (with excellent accuracy in advanced disease; AUROC for ≥F3 and F4 of 0.93 and 0.92, respectively; Singh et al., 2015). A separate meta-analysis of 13 studies containing 989 patients (but not individual patient data) found pooled AUROC of 0.96 and 0.98 for the diagnosis of fibrosis stage ≥F3 and F4 respectively (Su et al., 2014). Despite these promising results, some concerns still exist regarding the true diagnostic accuracy of MRE, as some of the studies suffer from spectrum bias due to underrepresentation of patients with intermediate stages of fibrosis. For example, the distribution of fibrosis stages in one study was: F0 (n = 28), F1 (n = 12), F2 (n = 6), F3 (n = 6), F4 (n = 20; Wang et al., 2011).
MRE for the Evaluation of NALFD
MRE was found to be useful in the assessment of fibrosis in patients with NAFLD, with AUROC 0.92 and 0.89 for the diagnosis of fibrosis stage ≥F3 and F4 respectively (Loomba et al., 2014). Mixed results have been reported for use of MRE in the diagnosis of non-alcoholic steatohepatitis (NASH). Animal and retrospective human studies (Salameh et al., 2009; Chen et al., 2011), have shown some promise, although in a prospective study, MRE only had a modest diagnostic accuracy for NASH (AUROC of 0.73; Loomba et al., 2014).
Predictive Value of MRE in Patients with Cirrhosis
A recent study examined the value of MRE in predicting clinical outcomes in 430 patients with cirrhosis, with follow up data on 167 patients whose cirrhosis decompensated during the study. The authors showed that liver stiffness (LS) measured by MRE was independently associated with the presence of decompensation at baseline. Furthermore, a liver stiffness ≥5.8 kPa was a significant risk for decompensation (hazard ratio 4.96; 95%CI 1.4-17.0) in patients who had compensated liver disease at baseline (Asrani et al., 2014).
Diffusion Weighted Imaging
Diffusion weighted imaging (DWI) is a magnetic resonance technique that quantifies the diffusion of water molecules in tissues, and this is quantified as the apparent diffusion coefficient (ADC). The rationale for using this technique to assess liver fibrosis is that the deposition of collagen fibers in the liver would inhibit water diffusion, therefore leading to a decrease in the ADC. There is now considerable experience with this imaging technique, and a meta-analysis of 10 studies reporting the performance of DWI compared to histology, reported pooled AUROCs of 0.86, 0.83, and 0.86 for the diagnosis of any fibrosis (F1-4), significant fibrosis (F2-4) and bridging fibrosis (F3-4), respectively (Wang et al., 2012). This technique has not seen widespread application as it has been demonstrated that confounding factors like steatosis and perfusion also affect the ADC (Luciani et al., 2008; Leitao et al., 2013). Furthermore, when compared with other MR biomarkers of liver fibrosis, DWI was inferior to MRE (Wang et al., 2012) and T1 mapping (Cassinotto et al., 2015), and only equivalent to transient elastography and serum based biomarkers (Lewin et al., 2007).
T1 Relaxometry
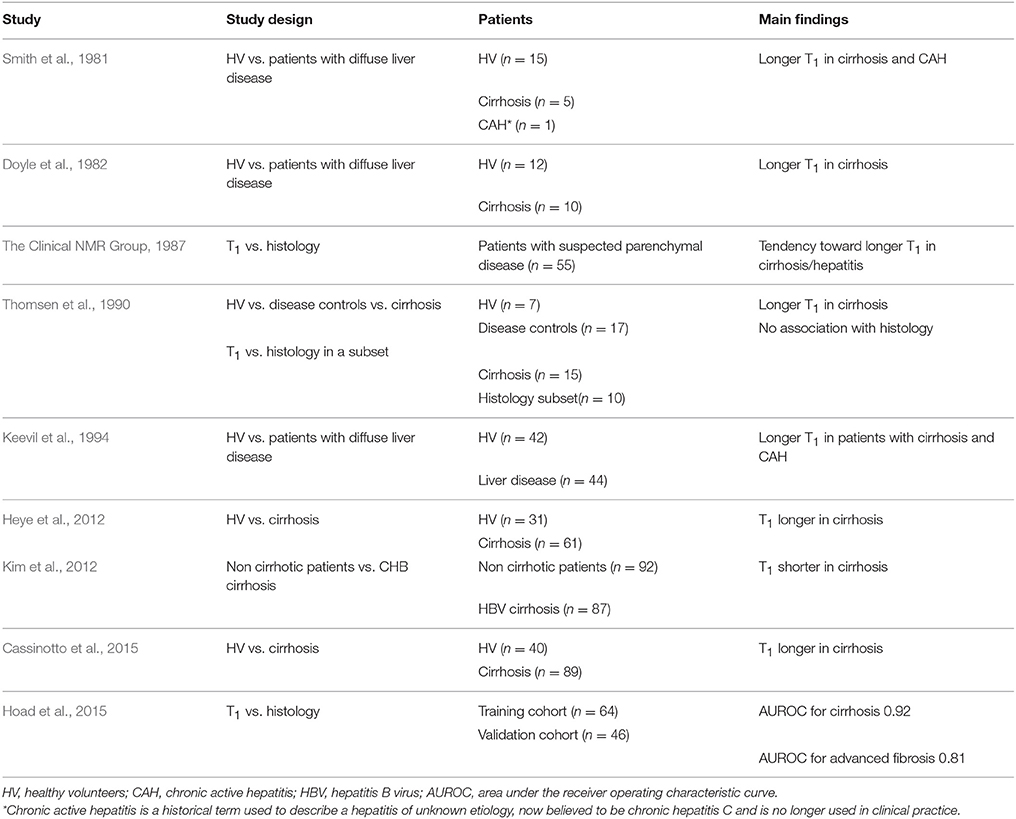
T1 relaxation time is a physical property of atoms that varies according to their electrochemical environment. The T1 relaxation time of hydrogen atoms in water molecules is longer that the T1 relaxation time of hydrogen atoms in long hydrocarbon chains like fatty acids. Therefore measuring T1 can provide information about tissue composition. The observation that T1 differed between healthy and diseased livers was made in very early studies of the clinical applicability of MR in visceral organs (Smith et al., 1981; Doyle et al., 1982). Despite this initial promise and subsequent studies in humans that showed some accuracy in the diagnosis of cirrhosis, T1 imaging was largely developed for the anatomical assessment of the liver and particularly for the evaluation of liver tumors.
The confounding effects of iron (The Clinical NMR Group, 1987; Hoad et al., 2015) and inflammation / oedema (Chamuleau et al., 1988) on liver T1 measures have limited the application of this technique until recently. The current impetus to develop non-invasive assessments of liver disease, combined with enhanced MRI methodologies, have resulted in improvements in the performance of liver T1 as a biomarker of fibrosis, with some exciting results. Examples of studies examining the role of T1 relaxometry for the assessment of liver fibrosis are outlined in Table 4.
Combination of T1 Relaxation Time with Other Non-invasive Biomarkers
In the study by Hoad et al. (2015) liver T1 is proposed as a biomarker of liver inflammation, which together with liver T2* for liver iron quantification and the ELF panel for fibrosis (William et al., 2004), are combined in a decision tree to determine who should be referred for liver biopsy. This approach resulted in a sensitivity of 90%, specificity of 71%, positive predictive value of 72% and negative predictive value of 89% in identifying those with significant fibrosis or inflammation.
Dynamic Contrast Enhanced MRI
Dynamic contrast enhanced (DCE) MRI requires the intravenous injection of hepatocyte specific gadolinium contrast agents like gadoxetic acid (Gd-EOB-DTPA; Primovist©; Bayer, Berlin, Germany). This technique gives an estimate of liver function, and has been shown to differentiate between healthy controls and patients with cirrhosis, and between the cirrhosis severity assessed by Child-Pugh stage or MELD score (Haimerl et al., 2013, 2014; Nilsson et al., 2013; Verloh et al., 2014; Zhao et al., 2015). Retrospective studies have also shown good accuracy for the diagnosis of NASH (AUROC 0.85; Bastati et al., 2014), and for the assessment of fibrosis (Feier et al., 2013; Ding et al., 2015). Furthermore, in a study by Feier et al. fibrosis was the only independent histological predictor of the relative T1 change on DCE MRI (Feier et al., 2013).
Multi-Parametric MR Protocols
Combinations of several techniques for liver fibrosis assessment have also been examined. In a retrospective study of patients who had undergone liver MR, the combination of parameters derived from DWI, DCE MRI and susceptibility weighed imaging resulted in improved diagnostic accuracy for the classification of fibrosis (AUROC for F0 vs. F1-4: 0.95; F0-1 vs. F2-4: 0.95; F0-2 vs. F3-4: 0.90; F0-3 vs. F4: 0.93; Feier et al., 2016). Incorporation of MR texture analysis techniques with other MR modalities has also produced some promising early results (House et al., 2015; Wu et al., 2015).
Iron Corrected T1
Liver iron is one of the major confounders in the use of liver T1 as a biomarker of liver fibrosis, as discussed above. One innovation that has led to improved diagnostic accuracy for liver T1 was the development of the liver “iron corrected T1” (cT1), which removes the confounding effect of iron from the T1 measurements making it much more widely applicable (Tunnicliffe et al., 2014). In a prospectively recruited cohort of patients undergoing liver biopsy for the assessment of fibrosis, cT1 showed excellent diagnostic accuracy against the Ishak staging system of fibrosis with an AUROC of 0.94 for the diagnosis of patients with any degree of fibrosis (F0 vs. F≥1; Banerjee et al., 2014). Furthermore, liver cT1 was used to derive the liver inflammation and fibrosis (LIF) score, a standardized continuous (0–4) score recently shown to predict liver related clinical outcomes, and is thus of potential use in predicting prognosis in patients with chronic liver disease (Pavlides et al., 2016).
Molecular MR Imaging
Molecular MR imaging represents a unique implementation of MR technology to visualize biological processes at the cellular and molecular level. Studies examining these technologies are still restricted to animal models and it remains to be seen whether they are safe and clinically applicable in humans.
Type I collagen is a major constituent of liver fibrosis. As it deposited in the extracellular space, it is an ideal target for molecular MR imaging. A molecular probe (EP-3533) to type I collagen has been developed, and is the most extensively studied probe in molecular MR imaging of liver fibrosis. When injected, this probe leads to shortening of the T1 relaxation time. In a feasibility study (Polasek et al., 2012), animals with fibrosis were found to have greater signal intensity compared to controls (0.55 vs. 0.39; p < 0.05) when imaged after EP-3533 injection. Furthermore, EP-3533 content measured in the sacrificed animals had a strong correlation with Ishak staging (r = 0.79 to 0.84 depending on the animal model). A subsequent study demonstrated that EP-3533 was superior to other MR biomarkers of fibrosis (T1 relaxation time, T1ρ, DWI and magnetisation transfer techniques; Fuchs et al., 2013). In their latest study, the group used a clinical scanner and showed that the technique can be useful in monitoring therapeutic effects using the bile duct ligation animal model of fibrosis (Farrar et al., 2015). Control animals, or animals that responded to rapamycin developed less fibrosis and had lower enhancement after EP-3533 compared to animals that did not receive or did not respond to treatment.
Conclusion
In summary, this review serves to provide a reference for the application of both serum and imaging based non-invasive markers of liver fibrosis. The use of these important clinical adjuncts has been discussed in detail, although given the plethora of non-invasive markers reported in the literature, the authors have specifically focused on the most well-known and available tools. While serum based algorithms and established elastography methods are being validated in large clinical cohorts of different liver diseases and demonstrate important predictive power in detecting liver related outcomes, imaging methodologies evolve apace, with promising novel approaches giving simultaneous diagnostic, staging as well as prognostic information entering the non-invasive arena.
Author Contributions
JR devised, wrote and revised the manuscript, provided funding for open access. JC, MP, and AM wrote sections of the manuscript and critically reviewed the entire paper.
Funding
Oxford Health Services Research Committee (OHSRC) grant (Ref: EC/1163).
Conflict of Interest Statement
MP is a shareholder of Perspectum Diagnostics, an Oxford University spin-out company.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adams, L. A., Bulsara, M., Rossi, E., DeBoer, B., Speers, D., George, J., et al. (2005). Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin. Chem. 51, 1867–1873. doi: 10.1373/clinchem.2005.048389
Afdhal, N. H., and Nunes, D. (2004). Evaluation of liver fibrosis: a concise review. Am. J. Gastroenterol. 99, 1160–1174. doi: 10.1111/j.1572-0241.2004.30110.x
Afdhal, N. H., Bacon, B. R., Patel, K., Lawitz, E. J., Gordon, S. C., Nelson, D. R., et al. (2015). Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin. Gastroenterol. Hepatol. 13, 772–773. doi: 10.1016/j.cgh.2014.12.014
Angulo, P., Hui, J. M., Marchesini, G., Bugianesi, E., George, J., Farrell, G. C., et al. (2007). The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854. doi: 10.1002/hep.21496
Angulo, P., Kleiner, D. E., Dam-Larsen, S., Adams, L. A., Bjornsson, E. S., Charatcharoenwitthaya, P., et al. (2015). Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–97.e10. doi: 10.1053/j.gastro.2015.04.043
Arena, U., Lupsor Platon, M., Stasi, C., Moscarella, S., Assarat, A., Bedogni, G., et al. (2013). Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology 58, 65–72. doi: 10.1002/hep.26343
Arena, U., Vizzutti, F., Abraldes, J. G., Corti, G., Stasi, C., Moscarella, S., et al. (2008b). Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 57, 1288–1293. doi: 10.1136/gut.2008.149708
Arena, U., Vizzutti, F., Corti, G., Ambu, S., Stasi, C., Bresci, S., et al. (2008a). Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 47, 380–384. doi: 10.1002/hep.22007
Armstrong, M. J., Houlihan, D. D., Bentham, L., Shaw, J. C., Cramb, R., Olliff, S., et al. (2012). Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J. Hepatol. 56, 234–240. doi: 10.1016/j.jhep.2011.03.020
Asrani, S. K., Talwalkar, J. A., Kamath, P. S., Shah, V. H., Saracino, G., Jennings, L., et al. (2014). Role of magnetic resonance elastography in compensated and decompensated liver disease. J. Hepatol. 60, 934–939. doi: 10.1016/j.jhep.2013.12.016
Balistreri, W. F. (2014, December 02). Noninvasive alternatives to assess liver fibrosis: ready for prime time? Medscape.
Banerjee, R., Pavlides, M., Tunnicliffe, E. M., Piechnik, S. K., Sarania, N., Philips, R., et al. (2014). Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J. Hepatol. 60, 69–77. doi: 10.1016/j.jhep.2013.09.002
Bastati, N., Feier, D., Wibmer, A., Traussnigg, S., Balassy, C., Tamandl, D., et al. (2014). Noninvasive differentiation of simple steatosis and steatohepatitis by using gadoxetic acid-enhanced mr imaging in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology 271, 739–747. doi: 10.1148/radiol.14131890
Bavu, E., Gennisson, J.-L., Couade, M., Bercoff, J., Mallet, V., Fink, M., et al. (2011). Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med. Biol. 37, 1361–1373. doi: 10.1016/j.ultrasmedbio.2011.05.016
Bedossa, P., and Carrat, F. (2009). Liver biopsy: the best, not the gold standard. J. Hepatol. 50, 1–3. doi: 10.1016/j.jhep.2008.10.014
Bedossa, P., Dargere, D., and Paradis, V. (2003). Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 38, 1449–1457. doi: 10.1053/jhep.2003.09022
Bercoff, J., Tanter, M., and Fink, M. (2004). Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 51, 396–409. doi: 10.1109/TUFFC.2004.1295425
Berres, M. L., Papen, S., Pauels, K., Schmitz, P., Zaldivar, M. M., Hellerbrand, C., et al. (2009). A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J. Hepatol. 50, 370–376. doi: 10.1016/j.jhep.2008.09.016
Berzigotti, A., De Gottardi, A., Vukotic, R., Siramolpiwat, S., Abraldes, J. G., García-Pagan, J. C., et al. (2013). Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. PLoS ONE 8:e58742. doi: 10.1371/journal.pone.0058742
Bonacini, M., Hadi, G., Govindarajan, S., and Lindsay, K. L. (1997). Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 92, 1302–1304.
Bota, S., Herkner, H., Sporea, I., Salzl, P., Sirli, R., Neghina, A. M., et al. (2013b). Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 33, 1138–1147. doi: 10.1111/liv.12240
Bota, S., Paternostro, R., Etschmaier, A., Schwarzer, R., Salzl, P., Mandorfer, M., et al. (2015). Performance of 2-D shear wave elastography in liver fibrosis assessment compared with serologic tests and transient elastography in clinical routine. Ultrasound Med. Biol. 41, 2340–2349. doi: 10.1016/j.ultrasmedbio.2015.04.013
Bota, S., Sporea, I., Peck-Radosavljevic, M., Sirli, R., Tanaka, H., Iijima, H., et al. (2013a). The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig. Liver Dis. 45, 762–768. doi: 10.1016/j.dld.2013.02.008
Bota, S., Sporea, I., Sirli, R., Popescu, A., Danila, M., and Costachescu, D. (2012). Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography–preliminary results. Ultrasound Med. Biol. 38, 1103–1108. doi: 10.1016/j.ultrasmedbio.2012.02.032
Boursier, J., and Cales, P. (2010). Clinical interpretation of Fibroscan(R) results: a real challenge. Liver Int. 30, 1400–1402. doi: 10.1111/j.1478-3231.2010.02355.x
Boursier, J., Brochard, C., Bertrais, S., Michalak, S., Gallois, Y., Fouchard-Hubert, I., et al. (2014). Combination of blood tests for significant fibrosis and cirrhosis improves the assessment of liver-prognosis in chronic hepatitis C. Aliment. Pharmacol. Ther. 40, 178–188. doi: 10.1111/apt.12813
Boursier, J., de Ledinghen, V., Zarski, J. P., Fouchard-Hubert, I., Gallois, Y., Oberti, F., et al. (2012). Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive. Hepatology 55, 58–67. doi: 10.1002/hep.24654
Boursier, J., de Ledinghen, V., Zarski, J. P., Rousselet, M. C., Sturm, N., Foucher, J., et al. (2011). A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am. J. Gastroenterol. 106, 1255–1263. doi: 10.1038/ajg.2011.100
Boursier, J., Konaté, A., Gorea, G., Reaud, S., Quemener, E., Oberti, F., et al. (2008). Reproducibility of liver stiffness measurement by ultrasonographic elastometry. Clin. Gastroenterol. Hepatol. 6, 1263–1269. doi: 10.1016/j.cgh.2008.07.006
Browning, J. D., Szczepaniak, L. S., Dobbins, R., Nuremberg, P., Horton, J. D., Cohen, J. C., et al. (2004). Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40, 1387–1395. doi: 10.1002/hep.20466
Bureau, C., Metivier, S., Peron, J. M., Selves, J., Robic, M. A., Gourraud, P. A., et al. (2008). Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment. Pharmacol. Ther. 27, 1261–1268. doi: 10.1111/j.1365-2036.2008.03701.x
Cadranel, J. F., Rufat, P., and Degos, F. (2000). Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32, 477–481. doi: 10.1053/jhep.2000.16602
Cales, P., Oberti, F., Michalak, S., Hubert-Fouchard, I., Rousselet, M. C., Konate, A., et al. (2005). A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 42, 1373–1381. doi: 10.1002/hep.20935
Cardoso, A.-C., Carvalho-Filho, R. J., Stern, C., Dipumpo, A., Giuily, N., Ripault, M.-P., et al. (2012). Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 32, 612–621. doi: 10.1111/j.1478-3231.2011.02660.x
Cassinotto, C., Feldis, M., Vergniol, J., Mouries, A., Cochet, H., Lapuyade, B., et al. (2015). MR relaxometry in chronic liver diseases: comparison of T1 mapping, T2 mapping, and diffusion-weighted imaging for assessing cirrhosis diagnosis and severity. Eur. J. Radiol. 84, 1459–1465. doi: 10.1016/j.ejrad.2015.05.019
Cassinotto, C., Lapuyade, B., Mouries, A., Hiriart, J.-B., Vergniol, J., Gaye, D., et al. (2014). Non-invasive assessment of liver fibrosis with impulse elastography: comparison of Supersonic Shear Imaging with ARFI and FibroScan®. J. Hepatol. 61, 550–557. doi: 10.1016/j.jhep.2014.04.044
Castéra, L., Foucher, J., Bernard, P.-H., Carvalho, F., Allaix, D., Merrouche, W., et al. (2010). Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51, 828–835. doi: 10.1002/hep.23425
Castéra, L., Le Bail, B., Roudot-Thoraval, F., Bernard, P.-H., Foucher, J., Merrouche, W., et al. (2009). Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J. Hepatol. 50, 59–68. doi: 10.1016/j.jhep.2008.08.018
Castéra, L., Vergniol, J., Foucher, J., Le Bail, B., Chanteloup, E., Haaser, M., et al. (2005). Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128, 343–350. doi: 10.1053/j.gastro.2004.11.018
Castera, L., Winnock, M., Pambrun, E., Paradis, V., Perez, P., Loko, M.-A., et al. (2014). Comparison of transient elastography (FibroScan), FibroTest, APRI and two algorithms combining these non-invasive tests for liver fibrosis staging in HIV/HCV coinfected patients: ANRS CO13 HEPAVIH and FIBROSTIC collaboration. HIV Med. 15, 30–39. doi: 10.1111/hiv.12082
Chamuleau, R. A., Creyghton, J. H., De Nie, I., Moerland, M. A., Van der Lende, O. R., and Smidt, J. (1988). Is the magnetic resonance imaging proton spin-lattice relaxation time a reliable noninvasive parameter of developing liver fibrosis? Hepatology 8, 217–221. doi: 10.1002/hep.1840080204
Chan, H. L.-Y., Wong, G. L.-H., Choi, P. C.-L., Chan, A. W.-H., Chim, A. M.-L., Yiu, K. K.-L., et al. (2009). Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J. Viral Hepat. 16, 36–44. doi: 10.1111/j.1365-2893.2008.01037.x
Chen, J., Talwalkar, J. A., Yin, M., Glaser, K. J., Sanderson, S. O., and Ehman, R. L. (2011). Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 259, 749–756. doi: 10.1148/radiol.11101942
Chin, J. L., Chan, G., Ryan, J. D., and McCormick, P. A. (2015). Spleen stiffness can non-invasively assess resolution of portal hypertension after liver transplantation. Liver Int. 35, 518–523. doi: 10.1111/liv.12647
Chon, Y. E., Choi, E. H., Song, K. J., Park, J. Y., Kim, D. Y., Han, K.-H., et al. (2012). Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS ONE 7:e44930. doi: 10.1371/journal.pone.0044930
Coco, B., Oliveri, F., Maina, A. M., Ciccorossi, P., Sacco, R., Colombatto, P., et al. (2007). Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J. Viral Hepat. 14, 360–369. doi: 10.1111/j.1365-2893.2006.00811.x
Colecchia, A., Montrone, L., Scaioli, E., Bacchi-Reggiani, M. L., Colli, A., Casazza, G., et al. (2012). Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 143, 646–654. doi: 10.1053/j.gastro.2012.05.035
Colombo, S., Buonocore, M., Del Poggio, A., Jamoletti, C., Elia, S., Mattiello, M., et al. (2012). Head-to-head comparison of transient elastography (TE), real-time tissue elastography (RTE), and acoustic radiation force impulse (ARFI) imaging in the diagnosis of liver fibrosis. J. Gastroenterol. 47, 461–469. doi: 10.1007/s00535-011-0509-4
Corpechot, C., Carrat, F., Poujol-Robert, A., Gaouar, F., Wendum, D., Chazouillères, O., et al. (2012). Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 56, 198–208. doi: 10.1002/hep.25599
Corpechot, C., El Naggar, A., Poujol-Robert, A., Ziol, M., Wendum, D., Chazouillères, O., et al. (2006). Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology 43, 1118–1124. doi: 10.1002/hep.21151
de Lédinghen, V., Douvin, C., Kettaneh, A., Ziol, M., Roulot, D., Marcellin, P., et al. (2006). Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J. Acquir. Immune Defic. Syndr. 41, 175–179. doi: 10.1097/01.qai.0000194238.15831.c7
Deffieux, T., Gennisson, J.-L., Bousquet, L., Corouge, M., Cosconea, S., Amroun, D., et al. (2015). Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J. Hepatol. 62, 317–324. doi: 10.1016/j.jhep.2014.09.020
Degos, F., Perez, P., Roche, B., Mahmoudi, A., Asselineau, J., Voitot, H., et al. (2010). Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J. Hepatol. 53, 1013–1021. doi: 10.1016/j.jhep.2010.05.035
Ding, X., Saxena, N. K., Lin, S., Xu, A., Srinivasan, S., and Anania, F. A. (2005). The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am. J. Pathol. 166, 1655–1669. doi: 10.1016/S0002-9440(10)62476-5
Ding, Y., Rao, S.-X., Zhu, T., Chen, C.-Z., Li, R.-C., and Zeng, M.-S. (2015). Liver fibrosis staging using T1 mapping on gadoxetic acid-enhanced MRI compared with DW imaging. Clin. Radiol. 70, 1096–1103. doi: 10.1016/j.crad.2015.04.014
Doyle, F., Pennock, J., Banks, L., McDonnell, M., Bydder, G., Steiner, R., et al. (1982). Nuclear magnetic resonance imaging of the liver: initial experience. Am. J. Roentgenol. 138, 193–200. doi: 10.2214/ajr.138.2.193
Ebinuma, H., Saito, H., Komuta, M., Ojiro, K., Wakabayashi, K., Usui, S., et al. (2011). Evaluation of liver fibrosis by transient elastography using acoustic radiation force impulse: comparison with Fibroscan(®). J. Gastroenterol. 46, 1238–1248. doi: 10.1007/s00535-011-0437-3
Ekstedt, M., Hagstrom, H., Nasr, P., Fredrikson, M., Stal, P., Kechagias, S., et al. (2015). Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61, 1547–1554. doi: 10.1002/hep.27368
Elkrief, L., Rautou, P.-E., Ronot, M., Lambert, S., Dioguardi Burgio, M., Francoz, C., et al. (2015). Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology 275, 589–598. doi: 10.1148/radiol.14141210
Farrar, C. T., DePeralta, D. K., Day, H., Rietz, T. A., Wei, L., Lauwers, G. Y., et al. (2015). 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J. Hepatol. 63, 689–696. doi: 10.1016/j.jhep.2015.04.029
Feier, D., Balassy, C., Bastati, N., Fragner, R., Wrba, F., and Ba-Ssalamah, A. (2016). The diagnostic efficacy of quantitative liver MR imaging with diffusion-weighted, SWI, and hepato-specific contrast-enhanced sequences in staging liver fibrosis—a multiparametric approach. Eur. Radiol. 26, 539–546. doi: 10.1007/s00330-015-3830-0
Feier, D., Balassy, C., Bastati, N., Stift, J., Badea, R., and Ba-Ssalamah, A. (2013). Liver fibrosis: histopathologic and biochemical influences on diagnostic efficacy of hepatobiliary contrast-enhanced MR imaging in staging. Radiology 269, 460–468. doi: 10.1148/radiol.13122482
Ferraioli, G., Tinelli, C., Dal Bello, B., Zicchetti, M., Filice, G., and Filice, C. (2012b). Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology 56, 2125–2133. doi: 10.1002/hep.25936
Ferraioli, G., Tinelli, C., Malfitano, A., Dal Bello, B., Filice, G., Filice, C., et al. (2012a). Performance of real-time strain elastography, transient elastography, and aspartate-to-platelet ratio index in the assessment of fibrosis in chronic hepatitis C. AJR Am. J. Roentgenol. 199, 19–25. doi: 10.2214/AJR.11.7517
Flisiak, R., Maxwell, P., Prokopowicz, D., Timms, P. M., and Panasiuk, A. (2002). Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta 1–possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepatogastroenterology 49, 1369–1372.
Forns, X., Ampurdanes, S., Llovet, J. M., Aponte, J., Quinto, L., Martinez-Bauer, E., et al. (2002). Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 36(4 Pt 1), 986–992. doi: 10.1053/jhep.2002.36128
Fraquelli, M., Rigamonti, C., Casazza, G., Conte, D., Donato, M. F., Ronchi, G., et al. (2007). Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 56, 968–973. doi: 10.1136/gut.2006.111302
Friedman, S. L. (2000). Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275, 2247–2250. doi: 10.1074/jbc.275.4.2247
Friedrich-Rust, M., Nierhoff, J., Lupsor, M., Sporea, I., Fierbinteanu-Braticevici, C., Strobel, D., et al. (2012). Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J. Viral Hepat. 19, e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x
Friedrich-Rust, M., Ong, M.-F., Herrmann, E., Dries, V., Samaras, P., Zeuzem, S., et al. (2007). Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am. J. Roentgenol. 188, 758–764. doi: 10.2214/AJR.06.0322
Friedrich-Rust, M., Ong, M.-F., Martens, S., Sarrazin, C., Bojunga, J., Zeuzem, S., et al. (2008). Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134, 960–974. doi: 10.1053/j.gastro.2008.01.034
Friedrich-Rust, M., Wunder, K., Kriener, S., Sotoudeh, F., Richter, S., Bojunga, J., et al. (2009). Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252, 595–604. doi: 10.1148/radiol.2523081928
Fuchs, B. C., Wang, H., Yang, Y., Wei, L., Polasek, M., Schuhle, D. T., et al. (2013). Molecular MRI of collagen to diagnose and stage liver fibrosis. J. Hepatol. 59, 992–998. doi: 10.1016/j.jhep.2013.06.026
Fujimoto, K., Kato, M., Kudo, M., Yada, N., Shiina, T., Ueshima, K., et al. (2013). Novel image analysis method using ultrasound elastography for noninvasive evaluation of hepatic fibrosis in patients with chronic hepatitis C. Oncology 84(Suppl. 1), 3–12. doi: 10.1159/000345883
Gandon, Y., Olivie, D., Guyader, D., Aube, C., Oberti, F., Sebille, V., et al. (2004). Non-invasive assessment of hepatic iron stores by MRI. Lancet 363, 357–362. doi: 10.1016/S0140-6736(04)15436-6
George, D. K., Ramm, G. A., Walker, N. I., Powell, L. W., and Crawford, D. H. (1999). Elevated serum type IV collagen: a sensitive indicator of the presence of cirrhosis in haemochromatosis. J. Hepatol. 31, 47–52. doi: 10.1016/S0168-8278(99)80162-7
Gerber, L., Kasper, D., Fitting, D., Knop, V., Vermehren, A., Sprinzl, K., et al. (2015). Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med. Biol. 41, 2350–2359. doi: 10.1016/j.ultrasmedbio.2015.04.014
Goyal, R., Mallick, S. R., Mahanta, M., Kedia, S., Shalimar, Dhingra, R., et al. (2013). Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J. Gastroenterol. Hepatol. 28, 1738–1745. doi: 10.1111/jgh.12318
Gressner, O. A., Weiskirchen, R., and Gressner, A. M. (2007). Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta 381, 107–113. doi: 10.1016/j.cca.2007.02.038
Grgurevic, I., Puljiz, Z., Brnic, D., Bokun, T., Heinzl, R., Lukic, A., et al. (2015). Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur. Radiol. 25, 3214–3221. doi: 10.1007/s00330-015-3728-x
Guechot, J., Laudat, A., Loria, A., Serfaty, L., Poupon, R., and Giboudeau, J. (1996). Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 42, 558–563.
Guha, I. N., Parkes, J., Roderick, P., Chattopadhyay, D., Cross, R., Harris, S., et al. (2008). Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 47, 455–460. doi: 10.1002/hep.21984
Guo, Y., Parthasarathy, S., Goyal, P., McCarthy, R. J., Larson, A. C., and Miller, F. H. (2015). Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdom. Imaging 40, 818–834. doi: 10.1007/s00261-014-0137-6
Haimerl, M., Verloh, N., Fellner, C., Zeman, F., Teufel, A., Fichtner- Feigl, S., et al. (2014). MRI-based estimation of liver function: Gd-EOB-DTPA-enhanced T1 relaxometry of 3T vs. the MELD score. Sci. Rep. 4:5621. doi: 10.1038/srep05621
Haimerl, M., Verloh, N., Zeman, F., Fellner, C., Muller-Wille, R., Schreyer, A. G., et al. (2013). Assessment of clinical signs of liver cirrhosis using T1 mapping on Gd-EOB-DTPA-enhanced 3T MRI. PLoS ONE 8:e85658. doi: 10.1371/journal.pone.0085658
Harrison, S. A., Oliver, D., Arnold, H. L., Gogia, S., and Neuschwander-Tetri, B. A. (2008). Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 57, 1441–1447. doi: 10.1136/gut.2007.146019
Henderson, N. C., Arnold, T. D., Katamura, Y., Giacomini, M. M., Rodriguez, J. D., McCarty, J. H., et al. (2013). Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624. doi: 10.1038/nm.3282
Heye, T., Yang, S. R., Bock, M., Brost, S., Weigand, K., Longerich, T., et al. (2012). MR relaxometry of the liver: significant elevation of T1 relaxation time in patients with liver cirrhosis. Eur. Radiol. 22, 1224–1232. doi: 10.1007/s00330-012-2378-5
Hoad, C. L., Palaniyappan, N., Kaye, P., Chernova, Y., James, M. W., Costigan, C., et al. (2015). A study of T(1) relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 28, 706–714. doi: 10.1002/nbm.3299
House, M. J., Bangma, S. J., Thomas, M., Gan, E. K., Ayonrinde, O. T., Adams, L. A., et al. (2015). Texture-based classification of liver fibrosis using MRI. J. Magn. Reson. Imaging 41, 322–328. doi: 10.1002/jmri.24536
Hui, A. Y., Chan, H. L., Wong, V. W., Liew, C. T., Chim, A. M., Chan, F. K., et al. (2005). Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am. J. Gastroenterol. 100, 616–623. doi: 10.1111/j.1572-0241.2005.41289.x
Huwart, L., Sempoux, C., Vicaut, E., Salameh, N., Annet, L., Danse, E., et al. (2008). Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 135, 32–40. doi: 10.1053/j.gastro.2008.03.076
Ichikawa, S., Motosugi, U., Ichikawa, T., Sano, K., Morisaka, H., Enomoto, N., et al. (2012). Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magn. Reson. Med. Sci. 11, 291–297. doi: 10.2463/mrms.11.291
Imbert-Bismut, F., Ratziu, V., Pieroni, L., Charlotte, F., Benhamou, Y., Poynard, T., et al. (2001). Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357, 1069–1075. doi: 10.1016/S0140-6736(00)04258-6
Jeong, J. Y., Kim, T. Y., Sohn, J. H., Kim, Y., Jeong, W. K., Oh, Y.-H., et al. (2014). Real time shear wave elastography in chronic liver diseases: accuracy for predicting liver fibrosis, in comparison with serum markers. World J. Gastroenterol. 20, 13920–13929. doi: 10.3748/wjg.v20.i38.13920
Kanamoto, M., Shimada, M., Ikegami, T., Uchiyama, H., Imura, S., Morine, Y., et al. (2009). Real time elastography for noninvasive diagnosis of liver fibrosis. J. Hepatobiliary Pancreat. Surg. 16, 463–467. doi: 10.1007/s00534-009-0075-9
Keevil, S. F., Alstead, E. M., Dolke, G., Brooks, A. P., Armstrong, P., and Farthing, M. J. (1994). Non-invasive assessment of diffuse liver disease by in vivo measurement of proton nuclear magnetic resonance relaxation times at 0.08 T. Br. J. Radiol. 67, 1083–1087. doi: 10.1259/0007-1285-67-803-1083
Kelleher, T. B., Mehta, S. H., Bhaskar, R., Sulkowski, M., Astemborski, J., Thomas, D. L., et al. (2005). Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J. Hepatol. 43, 78–84. doi: 10.1016/j.jhep.2005.02.025
Kim, C., Nan, B., Kong, S., and Harlow, S. (2012). Changes in iron measures over menopause and associations with insulin resistance. J. Womens Health 21, 872–877. doi: 10.1089/jwh.2012.3549
Kim, D. Y., Kim, S. U., Ahn, S. H., Park, J. Y., Lee, J. M., Park, Y. N., et al. (2009). Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig. Dis. Sci. 54, 1758–1763. doi: 10.1007/s10620-008-0541-2
Kirk, G. D., Astemborski, J., Mehta, S. H., Spoler, C., Fisher, C., Allen, D., et al. (2009). Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin. Infectious Dis. 48, 963–972. doi: 10.1086/597350
Kobayashi, K., Nakao, H., Nishiyama, T., Lin, Y., Kikuchi, S., Kobayashi, Y., et al. (2015). Diagnostic accuracy of real-time tissue elastography for the staging of liver fibrosis: a meta-analysis. Eur. Radiol. 25, 230–238. doi: 10.1007/s00330-014-3364-x
Koehler, E. M., Plompen, E. P., Schouten, J. N., Hansen, B. E., Darwish Murad, S., Taimr, P., et al. (2016). Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 63, 138–147. doi: 10.1002/hep.27981
Koizumi, Y., Hirooka, M., Kisaka, Y., Konishi, I., Abe, M., Murakami, H., et al. (2011). Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography–establishment of the method for measurement. Radiology 258, 610–617. doi: 10.1148/radiol.10100319
Korner, T., Kropf, J., and Gressner, A. M. (1996). Serum laminin and hyaluronan in liver cirrhosis: markers of progression with high prognostic value. J. Hepatol. 25, 684–688. doi: 10.1016/S0168-8278(96)80239-X
Latinoamericana, A. (2015). EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63, 237–264. doi: 10.1016/j.jhep.2015.04.006
Le, T. A., Chen, J., Changchien, C., Peterson, M. R., Kono, Y., Patton, H., et al. (2012). Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56, 922–932. doi: 10.1002/hep.25731
Leitao, H. S., Doblas, S., d'Assignies, G., Garteiser, P., Daire, J. L., Paradis, V., et al. (2013). Fat deposition decreases diffusion parameters at MRI: a study in phantoms and patients with liver steatosis. Eur. Radiol. 23, 461–467. doi: 10.1007/s00330-012-2626-8
Lemoine, M., Katsahian, S., Ziol, M., Nahon, P., Ganne-Carrie, N., Kazemi, F., et al. (2008). Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment. Pharmacol. Ther. 28, 1102–1110. doi: 10.1111/j.1365-2036.2008.03825.x
Leroy, V., Monier, F., Bottari, S., Trocme, C., Sturm, N., Hilleret, M. N., et al. (2004). Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am. J. Gastroenterol. 99, 271–279. doi: 10.1111/j.1572-0241.2004.04055.x
Leung, V. Y, Shen, J., Wong, V. W., Abrigo, J., Wong, G. L., Chim, A. M., et al. (2013). Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology 269, 910–918. doi: 10.1148/radiol.13130128
Lewin, M., Poujol-Robert, A., Boelle, P. Y., Wendum, D., Lasnier, E., Viallon, M., et al. (2007). Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology 46, 658–665. doi: 10.1002/hep.21747
Lieber, C. S., Weiss, D. G., Morgan, T. R., and Paronetto, F. (2006). Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am. J. Gastroenterol. 101, 1500–1508. doi: 10.1111/j.1572-0241.2006.00610.x
Limdi, J. K., and Hyde, G. M. (2003). Evaluation of abnormal liver function tests. Postgrad. Med. J. 79, 307–312. doi: 10.1136/pmj.79.932.307
Liu, H., Fu, J., Hong, R., Liu, L., and Li, F. (2015). Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: a systematic review & meta-analysis. PLoS ONE 10:e0127782. doi: 10.1371/journal.pone.0127782
Loaeza-del-Castillo, A., Paz-Pineda, F., Oviedo-Cardenas, E., Sanchez-Avila, F., and Vargas-Vorackova, F. (2008). AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann. Hepatol. 7(4):350–357.
Loomba, R., Sirlin, C. B., Ang, B., Bettencourt, R., Jain, R., Salotti, J., et al. (2015). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 61, 1239–1250. doi: 10.1002/hep.27647
Loomba, R., Wolfson, T., Ang, B., Hooker, J., Behling, C., Peterson, M., et al. (2014). Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 60, 1920–1928. doi: 10.1002/hep.27362
Luciani, A., Vignaud, A., Cavet, M., Nhieu, J. T., Mallat, A., Ruel, L., et al. (2008). Liver cirrhosis: intravoxel incoherent motion MR imaging–pilot study. Radiology 249, 891–899. doi: 10.1148/radiol.2493080080
Lupsor, M., Badea, R., Stefanescu, H., Grigorescu, M., Serban, A., Radu, C., et al. (2010). Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J. Gastrointestin. Liver Dis. 19, 53–60.
Lupsor, M., Badea, R., Stefanescu, H., Sparchez, Z., Branda, H., Serban, A., et al. (2009). Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J. Gastrointestin. Liver Dis. 18, 303–310.
Marcellin, P., Ziol, M., Bedossa, P., Douvin, C., Poupon, R., de Lédinghen, V., et al. (2009). Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 29, 242–247. doi: 10.1111/j.1478-3231.2008.01802.x
Mayo, M. J., Parkes, J., Adams-Huet, B., Combes, B., Mills, A. S., Markin, R. S., et al. (2008). Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 48, 1549–1557. doi: 10.1002/hep.22517
McCormick, S. E., Goodman, Z. D., Maydonovitch, C. L., and Sjogren, M. H. (1996). Evaluation of liver histology, ALT elevation, and HCV RNA titer in patients with chronic hepatitis C. Am. J. Gastroenterol. 91, 1516–1522.
McHutchison, J. G., Blatt, L. M., de Medina, M., Craig, J. R., Conrad, A., Schiff, E. R., et al. (2000). Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J. Gastroenterol. Hepatol. 15, 945–951. doi: 10.1046/j.1440-1746.2000.02233.x
McPherson, S., Stewart, S. F., Henderson, E., Burt, A. D., and Day, C. P. (2010). Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 59, 1265–1269. doi: 10.1136/gut.2010.216077
Mederacke, I., Wursthorn, K., Kirschner, J., Rifai, K., Manns, M. P., Wedemeyer, H., et al. (2009). Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 29, 1500–1506. doi: 10.1111/j.1478-3231.2009.02100.x
Merchante, N., Rivero-Juárez, A., Téllez, F., Merino, D., José Ríos-Villegas, M., Márquez-Solero, M., et al. (2012). Liver stiffness predicts clinical outcome in human immunodeficiency virus/hepatitis C virus-coinfected patients with compensated liver cirrhosis. Hepatology 56, 228–238. doi: 10.1002/hep.25616
Merriman, R. B., Ferrell, L. D., Patti, M. G., Weston, S. R., Pabst, M. S., Aouizerat, B. E., et al. (2006). Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology 44, 874–880. doi: 10.1002/hep.21346
Millonig, G., Friedrich, S., Adolf, S., Fonouni, H., Golriz, M., Mehrabi, A., et al. (2010). Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 52, 206–210. doi: 10.1016/j.jhep.2009.11.018
Millonig, G., Reimann, F. M., Friedrich, S., Fonouni, H., Mehrabi, A., Buchler, M. W., et al. (2008). Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 48, 1718–1723. doi: 10.1002/hep.22577
Mofrad, P., Contos, M. J., Haque, M., Sargeant, C., Fisher, R. A., Luketic, V. A., et al. (2003). Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 37, 1286–1292. doi: 10.1053/jhep.2003.50229
Molleken, C., Sitek, B., Henkel, C., Poschmann, G., Sipos, B., Wiese, S., et al. (2009). Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology 49, 1257–1266. doi: 10.1002/hep.22764
Muller, M., Gennisson, J.-L., Deffieux, T., Tanter, M., and Fink, M. (2009). Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: preliminary in vivo feasibility study. Ultrasound Med. Biol. 35, 219–229. doi: 10.1016/j.ultrasmedbio.2008.08.018
Murawaki, Y., Ikuta, Y., Idobe, Y., and Kawasaki, H. (1999). Serum matrix metalloproteinase-1 in patients with chronic viral hepatitis. J. Gastroenterol. Hepatol. 14, 138–145. doi: 10.1046/j.1440-1746.1999.01821.x
Nahon, P., Kettaneh, A., Tengher-Barna, I., Ziol, M., de Lédinghen, V., Douvin, C., et al. (2008). Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J. Hepatol. 49, 1062–1068. doi: 10.1016/j.jhep.2008.08.011
Naveau, S., Gaude, G., Asnacios, A., Agostini, H., Abella, A., Barri-Ova, N., et al. (2009). Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 49, 97–105. doi: 10.1002/hep.22576
Naveau, S., Poynard, T., Benattar, C., Bedossa, P., and Chaput, J. C. (1994). Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig. Dis. Sci. 39, 2426–2432. doi: 10.1007/BF02087661
Neuman, M. G., Cohen, L. B., and Nanau, R. M. (2014). Biomarkers in nonalcoholic fatty liver disease. Can. J. Gastroenterol. Hepatol. 28, 607–618. doi: 10.1155/2014/757929
Nguyen-Khac, E., Chatelain, D., Tramier, B., Decrombecque, C., Robert, B., Joly, J.-P., et al. (2008). Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment. Pharmacol. Ther. 28, 1188–1198. doi: 10.1111/j.1365-2036.2008.03831.x
Nierhoff, J., Chávez Ortiz, A. A., Herrmann, E., Zeuzem, S., and Friedrich-Rust, M. (2013). The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur. Radiol. 23, 3040–3053. doi: 10.1007/s00330-013-2927-6
Nightingale, K., Soo, M. S., Nightingale, R., and Trahey, G. (2002). Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med. Biol. 28, 227–235. doi: 10.1016/S0301-5629(01)00499-9
Nilsson, H., Blomqvist, L., Douglas, L., Nordell, A., Janczewska, I., Naslund, E., et al. (2013). Gd-EOB-DTPA-enhanced MRI for the assessment of liver function and volume in liver cirrhosis. Br. J. Radiol. 86:20120653. doi: 10.1259/bjr.20120653
Nobili, V., Vizzutti, F., Arena, U., Abraldes, J. G., Marra, F., Pietrobattista, A., et al. (2008). Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology 48, 442–448. doi: 10.1002/hep.22376
Oliveri, F., Coco, B., Ciccorossi, P., Colombatto, P., Romagnoli, V., Cherubini, B., et al. (2008). Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J. Gastroenterol. 14, 6154–6162. doi: 10.3748/wjg.14.6154
Ophir, J., Céspedes, I., Ponnekanti, H., Yazdi, Y., and Li, X. (1991). Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 13, 111–134. doi: 10.1177/016173469101300201
Pang, J. X. Q., Zimmer, S., Niu, S., Crotty, P., Tracey, J., Pradhan, F., et al. (2014). Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS ONE 9:e95776. doi: 10.1371/journal.pone.0095776
Park, G. J., Lin, B. P., Ngu, M. C., Jones, D. B., and Katelaris, P. H. (2000). Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J. Gastroenterol. Hepatol. 15, 386–390. doi: 10.1046/j.1440-1746.2000.02172.x
Parkes, J., Roderick, P., Harris, S., Day, C., Mutimer, D., Collier, J., et al. (2010). Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59, 1245–1251. doi: 10.1136/gut.2009.203166
Pasha, T., Gabriel, S., Therneau, T., Dickson, E. R., and Lindor, K. D. (1998). Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 27, 1220–1226. doi: 10.1002/hep.510270506
Patel, K., Gordon, S. C., Jacobson, I., Hezode, C., Oh, E., Smith, K. M., et al. (2004). Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J. Hepatol. 41, 935–942. doi: 10.1016/j.jhep.2004.08.008
Pavlides, M., Banerjee, R., Sellwood, J., Kelly, C. J., Robson, M. D., Booth, J. C., et al. (2016). Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J. Hepatol. 64, 308–315. doi: 10.1016/j.jhep.2015.10.009
Petta, S., Di Marco, V., Cammà, C., Butera, G., Cabibi, D., and Craxì, A. (2011). Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: the effects of body mass index. Aliment. Pharmacol. Ther. 33, 1350–1360. doi: 10.1111/j.1365-2036.2011.04668.x
Piscaglia, F., Salvatore, V., Di Donato, R., D'Onofrio, M., Gualandi, S., Gallotti, A., et al. (2011). Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 32, 167–175. doi: 10.1055/s-0029-1245948
Pohl, A., Behling, C., Oliver, D., Kilani, M., Monson, P., and Hassanein, T. (2001). Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am. J. Gastroenterol. 96, 3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x
Polasek, M., Fuchs, B. C., Uppal, R., Schuhle, D. T., Alford, J. K., Loving, G. S., et al. (2012). Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J. Hepatol. 57, 549–555. doi: 10.1016/j.jhep.2012.04.035
Popescu, A., Bota, S., Sporea, I., Sirli, R., Danila, M., Racean, S., et al. (2013). The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med. Biol. 39, 579–584. doi: 10.1016/j.ultrasmedbio.2012.11.013
Portillo-Sanchez, P., Bril, F., Maximos, M., Lomonaco, R., Biernacki, D., Orsak, B., et al. (2015). High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J. Clin. Endocrinol. Metab. 100, 2231–2238. doi: 10.1210/jc.2015-1966
Poynard, T., McHutchison, J., Manns, M., Myers, R. P., and Albrecht, J. (2003). Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology 38, 481–492. doi: 10.1053/jhep.2003.50319
Poynard, T., Munteanu, M., Luckina, E., Perazzo, H., Ngo, Y., Royer, L., et al. (2013). Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J. Hepatol. 58, 928–935. doi: 10.1016/j.jhep.2012.12.021
Pratt, D. S., and Kaplan, M. M. (2000). Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 342, 1266–1271. doi: 10.1056/NEJM200004273421707
Procopet, B., Berzigotti, A., Abraldes, J. G., Turon, F., Hernandez-Gea, V., García-Pagán, J. C., et al. (2015). Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J. Hepatol. 62, 1068–1075. doi: 10.1016/j.jhep.2014.12.007
Raptis, D. A., Fischer, M. A., Graf, R., Nanz, D., Weber, A., Moritz, W., et al. (2012). MRI: the new reference standard in quantifying hepatic steatosis? Gut 61, 117–127. doi: 10.1136/gutjnl-2011-300155
Ratziu, V., Massard, J., Charlotte, F., Messous, D., Imbert-Bismut, F., Bonyhay, L., et al. (2006). Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 6:6. doi: 10.1186/1471-230X-6-6
Reiberger, T., Ferlitsch, A., Payer, B. A., Pinter, M., Homoncik, M., and Peck-Radosavljevic, M. (2012). Non-selective β-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J. Gastroenterol. 47, 561–568. doi: 10.1007/s00535-011-0517-4
Rifai, K., Cornberg, J., Mederacke, I., Bahr, M. J., Wedemeyer, H., Malinski, P., et al. (2011). Clinical feasibility of liver elastography by acoustic radiation force impulse imaging (ARFI). Dig. Liver Dis. 43, 491–497. doi: 10.1016/j.dld.2011.02.011
Robic, M. A., Procopet, B., Métivier, S., Péron, J. M., Selves, J., Vinel, J. P., et al. (2011). Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J. Hepatol. 55, 1017–1024. doi: 10.1016/j.jhep.2011.01.051
Rockey, D. C. F., and Friedman, S. L. (2006). “Hepatic fibrosis and cirrhosis,” in Zakim and Boyer's Hepatology A Textbook of Liver Disease, 5th Edn., eds T. D. Boyer, T. L. Wright, and M. P. Manns (New York, NY: Elsevier Inc.), 87–109.
Roldan-Valadez, E., Favila, R., Martinez-Lopez, M., Uribe, M., Rios, C., and Mendez-Sanchez, N. (2010). In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: correlation with biochemical method and morphometry. J. Hepatol. 53, 732–737. doi: 10.1016/j.jhep.2010.04.018
Rosenberg, W. M., Voelker, M., Thiel, R., Becka, M., Burt, A., Schuppan, D., et al. (2004). Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127, 1704–1713. doi: 10.1053/j.gastro.2004.08.052
Ruffillo, G., Fassio, E., Alvarez, E., Landeira, G., Longo, C., Dominguez, N., et al. (2011). Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 54, 160–163. doi: 10.1016/j.jhep.2010.06.028
Sagir, A., Erhardt, A., Schmitt, M., and Haussinger, D. (2008). Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 47, 592–595. doi: 10.1002/hep.22056
Salameh, N., Larrat, B., Abarca-Quinones, J., Pallu, S., Dorvillius, M., Leclercq, I., et al. (2009). Early detection of steatohepatitis in fatty rat liver by using MR elastography. Radiology 253, 90–97. doi: 10.1148/radiol.2523081817
Salkic, N. N., Jovanovic, P., Hauser, G., and Brcic, M. (2014). FibroTest/Fibrosure for significant liver fibrosis and cirrhosis in chronic hepatitis B: a meta-analysis. Am. J. Gastroenterol. 109, 796–809. doi: 10.1038/ajg.2014.21
Samir, A. E., Dhyani, M., Vij, A., Bhan, A. K., Halpern, E. F., Méndez-Navarro, J., et al. (2015). Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology 274, 888–896. doi: 10.1148/radiol.14140839
Sánchez-Conde, M., Montes-Ramírez, M. L., Miralles, P., Alvarez, J. M. C., Bellón, J. M., Ramírez, M., et al. (2010). Comparison of transient elastography and liver biopsy for the assessment of liver fibrosis in HIV/hepatitis C virus-coinfected patients and correlation with noninvasive serum markers. J. Viral Hepat. 17, 280–286. doi: 10.1111/j.1365-2893.2009.01180.x
Sandrin, L., Fourquet, B., Hasquenoph, J. M., Yon, S., Fournier, C., Mal, F., et al. (2003). Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001
Sebastiani, G., Halfon, P., Castera, L., Pol, S., Thomas, D. L., Mangia, A., et al. (2009). SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 49, 1821–1827. doi: 10.1002/hep.22859
Shah, A. G., Lydecker, A., Murray, K., Tetri, B. N., Contos, M. J., Sanyal, A. J., et al. (2009). Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1112. doi: 10.1016/j.cgh.2009.05.033
Shaheen, A. A. M., Wan, A. F., and Myers, R. P. (2007). FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am. J. Gastroenterol. 102, 2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x
Shahin, M., Schuppan, D., Waldherr, R., Risteli, J., Risteli, L., Savolainen, E. R., et al. (1992). Serum procollagen peptides and collagen type VI for the assessment of activity and degree of hepatic fibrosis in schistosomiasis and alcoholic liver disease. Hepatology 15, 637–644. doi: 10.1002/hep.1840150414
Sharma, P., Kirnake, V., Tyagi, P., Bansal, N., Singla, V., Kumar, A., et al. (2013). Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am. J. Gastroenterol. 108, 1101–1107. doi: 10.1038/ajg.2013.119
Sherlock, S. (1962). Needle biopsy of the liver: a review. J. Clin. Pathol. 15, 291–304. doi: 10.1136/jcp.15.4.291
Sheron, N., Moore, M., Ansett, S., Parsons, C., and Bateman, A. (2012). Developing a 'traffic light' test with potential for rational early diagnosis of liver fibrosis and cirrhosis in the community. Br. J. Gen. Pract. 62, e616–e624. doi: 10.3399/bjgp12X654588
Sheth, S. G., Flamm, S. L., Gordon, F. D., and Chopra, S. (1998). AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 93, 44–48. doi: 10.1111/j.1572-0241.1998.044_c.x
Shi, K.-Q., Fan, Y.-C., Pan, Z.-Z., Lin, X.-F., Liu, W.-Y., Chen, Y.-P., et al. (2013). Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 33, 62–71. doi: 10.1210/jc.2015-1966
Shin, W. G., Park, S. H., Jang, M. K., Hahn, T. H., Kim, J. B., Lee, M. S., et al. (2008). Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig. Liver Dis. 40, 267–274. doi: 10.1016/j.dld.2007.10.011
Singh, S., Venkatesh, S. K., Wang, Z., Miller, F. H., Motosugi, U., Low, R. N., et al. (2015). Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin. Gastroenterol. Hepatol. 13, 440-51.e6. doi: 10.1016/j.cgh.2014.09.046
Smith, F. W., Mallard, J. R., Reid, A., and Hutchison, J. M. (1981). Nuclear magnetic resonance tomographic imaging in liver disease. Lancet 1, 963–966. doi: 10.1016/S0140-6736(81)91731-1
Sporea, I., Bota, S., Peck-Radosavljevic, M., Sirli, R., Tanaka, H., Iijima, H., et al. (2012). Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur. J. Radiol. 81, 4112–4118. doi: 10.1016/j.ejrad.2012.08.018
Sporea, I., Grădinaru-Taşcãu, O., Bota, S., Popescu, A., Şirli, R., Jurchiş, A., et al. (2013). How many measurements are needed for liver stiffness assessment by 2D-Shear Wave Elastography (2D-SWE) and which value should be used: the mean or median? Med. Ultrason. 15, 268–272. doi: 10.11152/mu.2013.2066.154.isp2
Sporea, I., Sirli, R., Deleanu, A., Tudora, A., Popescu, A., Curescu, M., et al. (2010). Liver stiffness measurements in patients with HBV vs HCV chronic hepatitis: a comparative study. World J. Gastroenterol. 16, 4832–4837. doi: 10.3748/wjg.v16.i38.4832
St Pierre, T. G., Clark, P. R., Chua-anusorn, W., Fleming, A. J., Jeffrey, G. P., Olynyk, J. K., et al. (2005). Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105, 855–861. doi: 10.1182/blood-2004-01-0177
Standish, R. A., Cholongitas, E., Dhillon, A., Burroughs, A. K., and Dhillon, A. P. (2006). An appraisal of the histopathological assessment of liver fibrosis. Gut 55, 569–578. doi: 10.1136/gut.2005.084475
Stebbing, J., Farouk, L., Panos, G., Anderson, M., Jiao, L. R., Mandalia, S., et al. (2010). A meta-analysis of transient elastography for the detection of hepatic fibrosis. J. Clin. Gastroenterol. 44, 214–219. doi: 10.1097/MCG.0b013e3181b4af1f
Sterling, R. K., Lissen, E., Clumeck, N., Sola, R., Correa, M. C., Montaner, J., et al. (2006). Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. doi: 10.1002/hep.21178
Su, L. N., Guo, S. L., Li, B. X., and Yang, P. (2014). Diagnostic value of magnetic resonance elastography for detecting and staging of hepatic fibrosis: a meta-analysis. Clin. Radiol. 69, e545–e552. doi: 10.1016/j.crad.2014.09.001
Sud, A., Hui, J. M., Farrell, G. C., Bandara, P., Kench, J. G., Fung, C., et al. (2004). Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology 39, 1239–1247. doi: 10.1002/hep.20207
Sun, W., Cui, H., Li, N., Wei, Y., Lai, S., Yang, Y., et al. (2016). Comparison of FIB4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol. Res. doi: 10.1111/hepr.12647. [Epub ahead of print].
Szczepaniak, L. S., Nurenberg, P., Leonard, D., Browning, J. D., Reingold, J. S., Grundy, S., et al. (2005). Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 288, E462–E468. doi: 10.1152/ajpendo.00064.2004
Takuma, Y., Nouso, K., Morimoto, Y., Tomokuni, J., Sahara, A., Toshikuni, N., et al. (2013). Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 144, 92–101. doi: 10.1053/j.gastro.2012.09.049
Talwalkar, J. A., Kurtz, D. M., Schoenleber, S. J., West, C. P., and Montori, V. M. (2007). Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 5, 1214–1220. doi: 10.1016/j.cgh.2007.07.020
Tan, T. C., Crawford, D. H., Franklin, M. E., Jaskowski, L. A., Macdonald, G. A., Jonsson, J. R., et al. (2012). The serum hepcidin:ferritin ratio is a potential biomarker for cirrhosis. Liver Int. 32, 1391–1399. doi: 10.1111/j.1478-3231.2012.02828.x
Tatsumi, C., Kudo, M., Ueshima, K., Kitai, S., Ishikawa, E., Yada, N., et al. (2010). Non-invasive evaluation of hepatic fibrosis for type C chronic hepatitis. Intervirology 53, 76–81. doi: 10.1159/000252789
Teare, J. P., Sherman, D., Greenfield, S. M., Simpson, J., Bray, G., Catterall, A. P., et al. (1993). Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 342, 895–898. doi: 10.1016/0140-6736(93)91946-J
The Clinical NMR Group (1987). Magnetic resonance imaging of parenchymal liver disease: a comparison with ultrasound, radionuclide scintigraphy and X-ray computed tomography. Clinical NMR Group Clin. Radiol. 38, 495–502. doi: 10.1016/S0009-9260(87)80131-9
Thiele, M., Detlefsen, S., Sevelsted Møller, L., Madsen, B. S., Fuglsang Hansen, J., Fialla, A. D., et al. (2016). Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology 150, 123–133. doi: 10.1053/j.gastro.2015.09.040
Thiele, M., Kjærgaard, M., Thielsen, P., and Krag, A. (2015). Contemporary use of elastography in liver fibrosis and portal hypertension. Clin. Physiol. Funct. Imaging. doi: 10.1111/cpf.12297. [Epub ahead of print].
Thomsen, C., Christoffersen, P., Henriksen, O., and Juhl, E. (1990). Prolonged T1 in patients with liver cirrhosis: an in vivo MRI study. Magn. Reson. Imaging 8, 599–604. doi: 10.1016/0730-725X(90)90137-Q
Treeprasertsuk, S., Bjornsson, E., Enders, F., Suwanwalaikorn, S., and Lindor, K. D. (2013). NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 19, 1219–1229. doi: 10.3748/wjg.v19.i8.1219
Tsochatzis, E. A., Germani, G., Hall, A., Anousou, P. M., Dhillon, A. P., and Burroughs, A. K. (2011a). Noninvasive assessment of liver fibrosis: the need for better validation. Hepatology. 53, 1781–1782; author reply 2-3. doi: 10.1002/hep.24271
Tsochatzis, E. A., Gurusamy, K. S., Ntaoula, S., Cholongitas, E., Davidson, B. R., and Burroughs, A. K. (2011b). Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J. Hepatol. 54, 650–659. doi: 10.1016/j.jhep.2010.07.033
Tunnicliffe, E., Robson, M., and Banerjee, R. (2014). Medical Imaging. Application number PCT/GB2014/050815.
Umemura, T., Joshita, S., Sekiguchi, T., Usami, Y., Shibata, S., Kimura, T., et al. (2015). Serum wisteria floribunda agglutinin-positive Mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am. J. Gastroenterol. 110, 857–864. doi: 10.1038/ajg.2015.118
Vallet-Pichard, A., Mallet, V., Nalpas, B., Verkarre, V., Nalpas, A., Dhalluin-Venier, V., et al. (2007). FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46, 32–36. doi: 10.1002/hep.21669
Veidal, S. S., Bay-Jensen, A. C., Tougas, G., Karsdal, M. A., and Vainer, B. (2010). Serum markers of liver fibrosis: combining the BIPED classification and the neo-epitope approach in the development of new biomarkers. Dis. Markers 28, 15–28. doi: 10.1155/2010/548263
Venkatesh, S. K., Wang, G., Lim, S. G., and Wee, A. (2014a). Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur. Radiol. 24, 70–78. doi: 10.1007/s00330-013-2978-8
Venkatesh, S. K., Xu, S., Tai, D., Yu, H., and Wee, A. (2014b). Correlation of MR elastography with morphometric quantification of liver fibrosis (Fibro-C-Index) in chronic hepatitis B. Magn. Reson. Med. 72, 1123–1129. doi: 10.1002/mrm.25002
Vergara, S., Macías, J., Rivero, A., Gutiérrez-Valencia, A., González-Serrano, M., Merino, D., et al. (2007). The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin. Infectious Dis. 45, 969–974. doi: 10.1086/521857
Vergniol, J., Boursier, J., Coutzac, C., Bertrais, S., Foucher, J., Angel, C., et al. (2014). Evolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis C. Hepatology 60, 65–76. doi: 10.1002/hep.27069
Vergniol, J., Foucher, J., Terrebonne, E., Bernard, P. H., le Bail, B., Merrouche, W., et al. (2011). Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 140, 1970–9, 1979.e1–3. doi: 10.1053/j.gastro.2011.02.058
Verloh, N., Haimerl, M., Zeman, F., Schlabeck, M., Barreiros, A., Loss, M., et al. (2014). Assessing liver function by liver enhancement during the hepatobiliary phase with Gd-EOB-DTPA-enhanced MRI at 3 Tesla. Eur. Radiol. 24, 1013–1019. doi: 10.1007/s00330-014-3108-y
Villeneuve, J. P., Bilodeau, M., Lepage, R., Cote, J., and Lefebvre, M. (1996). Variability in hepatic iron concentration measurement from needle-biopsy specimens. J. Hepatol. 25, 172–177. doi: 10.1016/S0168-8278(96)80070-5
Vizzutti, F., Arena, U., Romanelli, R. G., Rega, L., Foschi, M., Colagrande, S., et al. (2007). Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 45, 1290–1297. doi: 10.1002/hep.21665
Wai, C. T., Greenson, J. K., Fontana, R. J., Kalbfleisch, J. D., Marrero, J. A., Conjeevaram, H. S., et al. (2003). A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526. doi: 10.1053/jhep.2003.50346
Walsh, K. M., Timms, P., Campbell, S., MacSween, R. N., and Morris, A. J. (1999). Plasma levels of matrix metalloproteinase-2 (MMP-2) and tissue inhibitors of metalloproteinases -1 and -2 (TIMP-1 and TIMP-2) as noninvasive markers of liver disease in chronic hepatitis C: comparison using ROC analysis. Dig. Dis. Sci. 44, 624–630. doi: 10.1023/A:1026630129025
Wang, J.-H., Changchien, C.-S., Hung, C.-H., Eng, H.-L., Tung, W.-C., Kee, K.-M., et al. (2009). FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J. Gastroenterol. 44, 439–446. doi: 10.1007/s00535-009-0017-y
Wang, Q. B., Zhu, H., Liu, H. L., and Zhang, B. (2012). Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: a meta-analysis. Hepatology 56, 239–247. doi: 10.1002/hep.25610
Wang, Y., Ganger, D. R., Levitsky, J., Sternick, L. A., McCarthy, R. J., Chen, Z. E., et al. (2011). Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am. J. Roentgenol. 196, 553–561. doi: 10.2214/AJR.10.4580
William, M. C. R., Michael, V., Robert, T., Michael, B., Alastair, B., Detlef, S., et al. (2004). Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127, 1704–1713. doi: 10.1053/j.gastro.2004.08.052
Wong, G. L. H., Wong, V. W. S., Choi, P. C. L., Chan, A. W. H., and Chan, H. L. Y. (2010b). Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment. Pharmacol. Ther. 31, 1095–1103. doi: 10.1111/j.1365-2036.2010.04276.x
Wong, G. L., Wong, V. W., Chim, A. M., Yiu, K. K., Chu, S. H., Li, M. K., et al. (2011). Factors associated with unreliable liver stiffness measurement and its failure with transient elastography in the Chinese population. J. Gastroenterol. Hepatol. 26, 300–305. doi: 10.1111/j.1440-1746.2010.06510.x
Wong, V. W., Chu, W. C., Wong, G. L., Chan, R. S., Chim, A. M., Ong, A., et al. (2012). Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 61, 409–415. doi: 10.1136/gutjnl-2011-300342
Wong, V. W., Vergniol, J., Wong, G. L., Foucher, J., Chan, H. L., Le Bail, B., et al. (2010a). Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 51, 454–462. doi: 10.1002/hep.23312
Wu, Z., Matsui, O., Kitao, A., Kozaka, K., Koda, W., Kobayashi, S., et al. (2015). Hepatitis C related chronic liver cirrhosis: feasibility of texture analysis of MR images for classification of fibrosis stage and necroinflammatory activity grade. PLoS ONE 10:e0118297. doi: 10.1371/journal.pone.0118297
Xiao, G., Yang, J., and Yan, L. (2015). Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 61, 292–302. doi: 10.1002/hep.27382
Xie, Q., Zhou, X., Huang, P., Wei, J., Wang, W., and Zheng, S. (2014). The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS ONE 9:e92772. doi: 10.1371/journal.pone.0092772
Yabu, K., Kiyosawa, K., Mori, H., Matsumoto, A., Yoshizawa, K., Tanaka, E., et al. (1994). Serum collagen type IV for the assessment of fibrosis and resistance to interferon therapy in chronic hepatitis C. Scand. J. Gastroenterol. 29, 474–479. doi: 10.3109/00365529409096841
Yada, N., Kudo, M., Morikawa, H., Fujimoto, K., Kato, M., and Kawada, N. (2013). Assessment of liver fibrosis with real-time tissue elastography in chronic viral hepatitis. Oncology 84(Suppl. 1), 13–20. doi: 10.1159/000345884
Yoneda, M., Mawatari, H., Fujita, K., Endo, H., Iida, H., Nozaki, Y., et al. (2008). Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig. Liver Dis. 40, 371–378. doi: 10.1016/j.dld.2007.10.019
Yoneda, M., Suzuki, K., Kato, S., Fujita, K., Nozaki, Y., Hosono, K., et al. (2010). Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 256, 640–647. doi: 10.1148/radiol.10091662
Yoon, J. H., Lee, J. M., Han, J. K., and Choi, B. I. (2014). Shear wave elastography for liver stiffness measurement in clinical sonographic examinations: evaluation of intraobserver reproducibility, technical failure, and unreliable stiffness measurements. J. Ultrasound Med. 33, 437–447. doi: 10.7863/ultra.33.3.437
Zarski, J.-P., Sturm, N., Guechot, J., Paris, A., Zafrani, E.-S., Asselah, T., et al. (2012). Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J. Hepatol. 56, 55–62. doi: 10.1016/j.jhep.2011.05.024
Zeng, J., Liu, G.-J., Huang, Z.-P., Zheng, J., Wu, T., Zheng, R.-Q., et al. (2014). Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur. Radiol. 24, 2572–2581. doi: 10.1007/s00330-014-3292-9
Zeng, M. D., Lu, L. G., Mao, Y. M., Qiu, D. K., Li, J. Q., Wan, M. B., et al. (2005). Prediction of significant fibrosis in HBeAg-positive patients with chronic hepatitis B by a noninvasive model. Hepatology 42, 1437–1445. doi: 10.1002/hep.20960
Zhao, X., Huang, M., Zhu, Q., Wang, T., and Liu, Q. (2015). The relationship between liver function and liver parenchymal contrast enhancement on Gd-BOPTA-enhanced MR imaging in the hepatocyte phase. Magn. Reson. Imaging 33, 768–773. doi: 10.1016/j.mri.2015.03.006
Keywords: hepatology, fibrosis, biomarkers, elastography, MRI
Citation: Chin JL, Pavlides M, Moolla A and Ryan JD (2016) Non-invasive Markers of Liver Fibrosis: Adjuncts or Alternatives to Liver Biopsy? Front. Pharmacol. 7:159. doi: 10.3389/fphar.2016.00159
Received: 19 April 2016; Accepted: 31 May 2016;
Published: 20 June 2016.
Edited by:
Kotambail Venkatesha Udupa, St. Vincent's University Hospital, IrelandReviewed by:
Sergey V. Ryzhov, Maine Medical Center Research Institute, USAEmmanuel Tsochatzis, Royal Free Hospital and University College London, UK
Copyright © 2016 Chin, Pavlides, Moolla and Ryan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John D. Ryan, amRwcnlhbkB5YWhvby5jby51aw==
†Joint first authors.
 Jun L. Chin
Jun L. Chin Michael Pavlides
Michael Pavlides Ahmad Moolla3†
Ahmad Moolla3† John D. Ryan
John D. Ryan