- 1Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
- 3Hainan Medical University, Hainan, China
Inflammatory disorders underlie varieties of human diseases. San-Huang-Xie-xin-Tang (SHXXT), composed with Rhizoma Rhei (Rheum palmatum L.), Rhizoma Coptidis (Coptis chinensis Franch), and Radix Scutellaria (Scutellaria baicalensis Georgi), is a famous formula which has been widely used in the fight against inflammatory abnormalities. Mutual reinforcement is one of the basic theories of traditional Chinese medicine. Here this article reviewed and analyzed the recent research on (1) How the main constituents of SHXXT impact on inflammation-associated signaling pathway molecules. (2) The interaction between the main constituents and efflux pumps or intestinal transporters. The goal of this work was to, (1) Provide evidence to support the theory of mutual reinforcement. (2) Clarify the key targets of SHXXT and suggest which targets need further investigation. (3) Give advice for the clinical use of SHXXT to elevated the absorption of main constituents and eventually promote oral bioavailability. We search literatures in scientific databases with key words of “each main SHXXT constituent,” in combination with “each main inflammatory pathway target molecule” or each main intestinal transporter, respectively. We report the effect of five main constituents on target molecules which lies in three main inflammatory signaling pathways, we as well investigate the interaction between constituents and intestinal transporter. We conclude, (1) The synergistic effect of constituents at both levels confirm the mutual reinforcement theory of TCM as it is proven in this work. (2) The effect of main constituents on downstream targets in nuclear need more further investigation. (3) Drug elevating the absorption of rhein, berberine and baicalein can be employed to promote oral bioavailability of SHXXT.
Introduction
Inflammation, a complex response triggered by pernicious stimuli like pathogens or irritants, verified to be involved in process of many diseases such as Alzheimer Disease, type 2 diabetes, rheumatoid arthritis, etc., (Chiapinotto Spiazzi et al., 2015; Garimella et al., 2015; Saito et al., 2015). Generally, inflammation is classified as acute and chronic type. Acute type only last a few days with neutrophil infiltration, while chronic type can last up to years with infiltrations of lymphocytes and macrophages (Ambrozova et al., 2016). Inflammatory pathways perform a crucial part for signal transduction and recent research provide genuine evidence showing NF-κB, MAPK and JAK/STAT are the three main pathways (Bertolini, 2012; Ottani et al., 2015).
As a famous traditional Chinese medicine (TCM) formula which has been used for centuries, San-Huang-Xie-Xin-Tang (SHXXT) displays good curative activation in the treatment of inflammatory disorders such as atherosclerosis (Wang Y. S. et al., 2011), upper respiratory tract infection (Ma et al., 2009; Kim et al., 2014), diabetic nephropathy (Wu et al., 2015), gastritis, gastric bleeding and peptic ulcers (Lo et al., 2005), and these protective effects are correlated with reactions of weakening inflammatory by suppressing cytokine/chemokine production. SHXXT has a quite simple composition with only three herbals, namely Radix et Rhizoma Rhei (Rheum palmatum L.) [RR, yields anthraquinones like emodin(Emo), rhein(Rhe) and aloe-emodin (Aem)], Rhizoma Coptidis (Coptis chinensis Franch) [RC, yields alkaloids like berberine(Ber) and coptisine(COP)], and Radix Scutellaria (Scutellaria baicalensis Georgi) [RS, yields flavonoids like baicalin(Bai) and baicalein (Bae)]. Previous studies show the basic effective constituents of SHXXT responsible for the anti-inflammatory effect may be Ber, Bai, Emo, Rhe, and Aem (Ma et al., 2009), plus, Bae is considered as a quality control indicator of RS (Zhang et al., 2013b). In regard of the bioavailability of SHXXT, A rapid and sensitive UPLC-ESI/MS method determined 17 active SHXXT constituents with good linearity in a relatively wide concentration ranges, among which, Bai is the most abundant. In bloodstream, the major forms of SHXXT include Bae, Emo, Aem and Rhe, while only the parent form of Rhe can be detected, and the conjugated effect may be accounted for their physicochemical property differences (Li et al., 2010; Shia et al., 2011).
Intestinal transporters (IT), such as P-gp, MRP, BCRP (Sampson et al., 2015), SGLT1 (Asano et al., 2004) and OCT (Bader et al., 2014), play a critical role in the process of intracellular and efflux transport. Numerous evidence illustrate the main constituents in SHXXT are the substrates of efflux transporters which leads to a very low oral bioavailability (Huang S. et al., 2011; He et al., 2014; Wei et al., 2014; Di et al., 2015). However, most studies only concentrate on solitary constituent, whether they have mutual effect on respective absorption remains to be elucidated.
There's growing evidence indicating that all those constituents above, while exclusively dosed, possess anti-inflammation effect by affecting a variety of target molecules in signaling pathways (Shih et al., 2007; Hamsa and Kuttan, 2012; Zhang et al., 2013a; Hu et al., 2014). We are all clear that, Chinese herbal combination should not only improve curative effects and reduce side effects, but also promote the mutual absorption of effective constituents. In this study, we review the recent studies and discuss how the three classic herbals of SHXXT, RS, RR, and RC, reach the goal of synergistic interaction at both pharmacodynamics and pharmacokinetic level.
Pharmacodynamic Level
Effect of the Active Constituents on Molecules in NF-κB Pathway
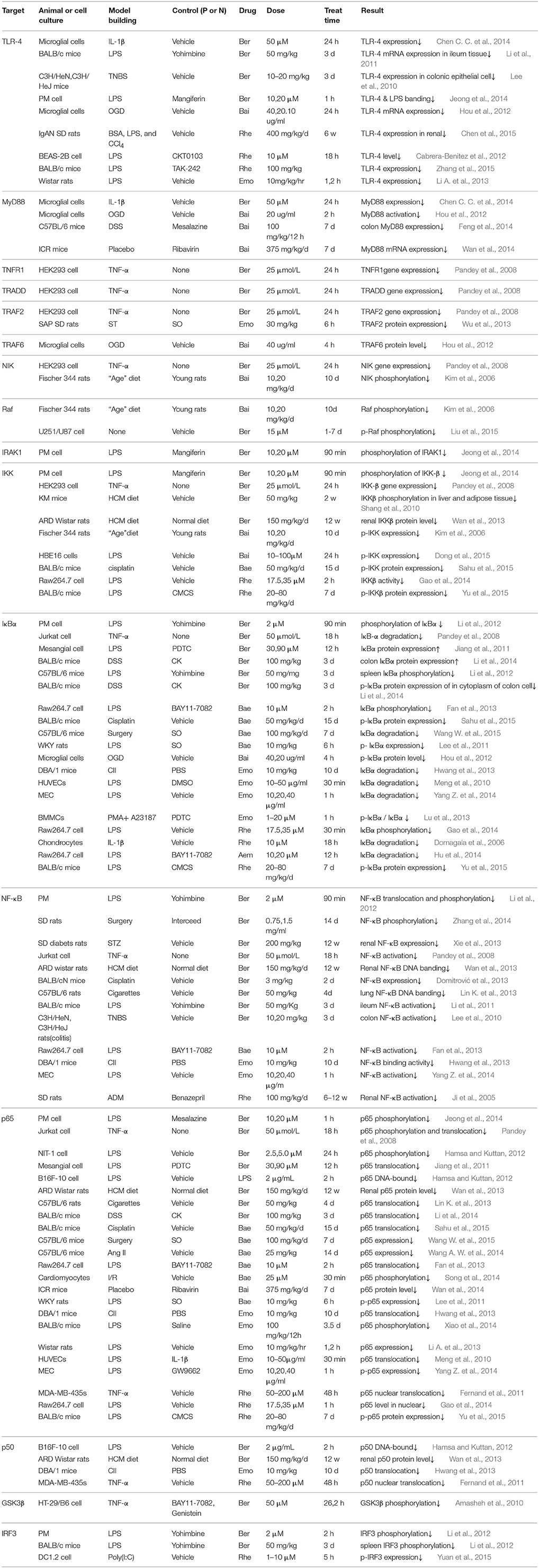
TLR-4 is the first described TLRs in mammals, it responds to LPS which can trigger NF-κB activation and pro-inflammatory cytokines secretion (Lee et al., 2010), constituents that can block the binding between TLR-4 and LPS are supposed to be valued in inflammation treatment (Wu et al., 2016). As summarized in Table 1, It is reported that Ber, Bai and Rhe exert inhibitory effect on TLR-4 expression in varies of models (Lee et al., 2010; Li et al., 2011; Cabrera-Benitez et al., 2012; Hou et al., 2012; Chen C. C. et al., 2014; Chen et al., 2015), and the combination of TLR-4 and LPS is observed to be blocked by Ber (Jeong et al., 2014). So, it seems that the anti-inflammatory mechanism of SHXXT begins at a really early stage, ever since LPS are interacting with upstream membrane protein.
It has been identified that, MyD88 is recruited by TLR4 at plasma membrane to stimulate the initial activation of IKK, and it may be responsible for the early peak in NF-κB activity (Cheng Z. et al., 2015). Apart from MyD88, there are many other adapter molecules (such as TRAF3, TRAM and TRADD) sharing similar activity. NIK will promote NF-κB activation once combined with TRAF2 (Lee et al., 2014). Among them, MyD88 has been most systemically studied both in vivo and in vitro. In respect of these adaptor molecules, Ber and Bai negatively regulate their protein expressions (Pandey et al., 2008; Hou et al., 2012; Lim et al., 2012; Chen C. C. et al., 2014; Feng et al., 2014; Wan et al., 2014), however the main constituents from RR are rarely mentioned.
Enzyme complex IKK (α-γ) have a crucial role in regulating NF-κB signaling pathway (Bagnéris et al., 2015). In general, IκBα forms a heterodimer with p65 (RELA) and p50 (NF-κb1), making NF-κB sequestered in cytoplasm. Once activated, IκBα goes phosphorylated meanwhile p65 is liberated and translocate into nuclear, which leads to gene transcription (Pandey et al., 2008). Depicted in Figure 1, the majority of current studies focus on upstream molecules from IKK to p65. Data in Table 1 show the main constituents of SHXXT can inhibit (1) the expression and phosphorylation of IKK, (2) the expression, phosphorylation and degradation of IκBα, (3) the expression, phosphorylation and translocation of p65 and (4) the expression, phosphorylation, DNA banding and activation of NF-κB in multiple in-vivo and in-vitro models, such as mesangial (Jiang et al., 2011), RAW264.7 (Fan et al., 2013), MEC (Yang Z. et al., 2014) etc., and ARD rats (Wan et al., 2013), C57BL/6 mice (Wang W. et al., 2015), DBA/1 mice (Hwang et al., 2013), etc.
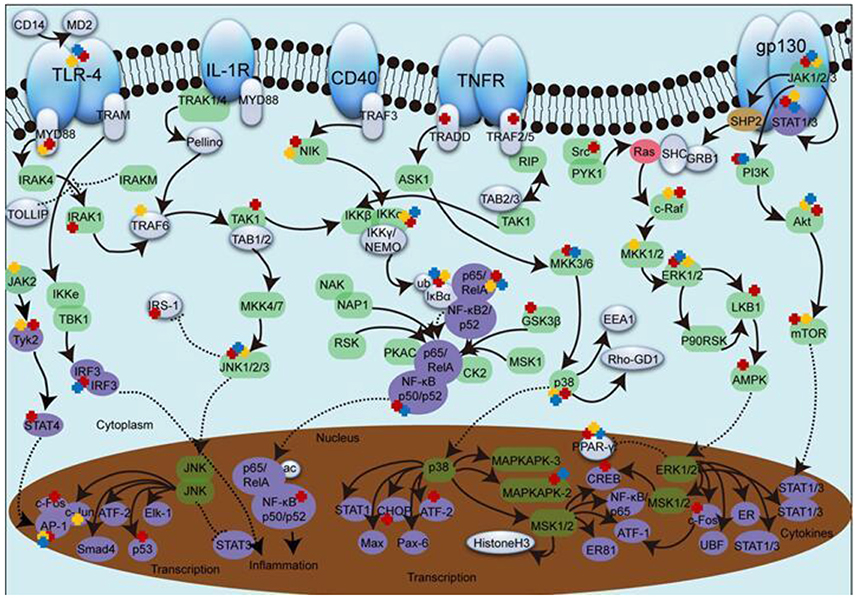
Figure 1. The effect of SHXXT alkaloids on inflammatory pathway molecules. 1. The green ellipse represents kinase. 2. The purple ellipse represents transcription factor. 3. The red ellipse represents GTpase. 4. The brown ellipse represents phosphatase. 5. The solid arrow represents direct stimulatory modification. 6. The dotted arrow represents translocation. 7. The dotted “T” represents direct inhibitory modification. 8. The red, yellow and blue cross represents the target influenced by RC, RS, and RR constituents, respectively.
We know that, GSK3β is not active until dephosphorylated, and the activation will promote inflammation process undergoes Alzheimer Disease and diabetes (Venna et al., 2015). IRF3 is a target of TLR-4 signaling pathway, acting as regulating and activating the transcription of interferon which results inflammatory responses (Cheng B. C. Y. et al., 2015). Briefly, phosphorylation of these two downstream molecules are both identified to be reversed by Ber or Rhe treatment in either animal or cell inflammatory model (Amasheh et al., 2010; Li et al., 2012; Yuan et al., 2015), which cover the effect shortage of RS constituents at this part.
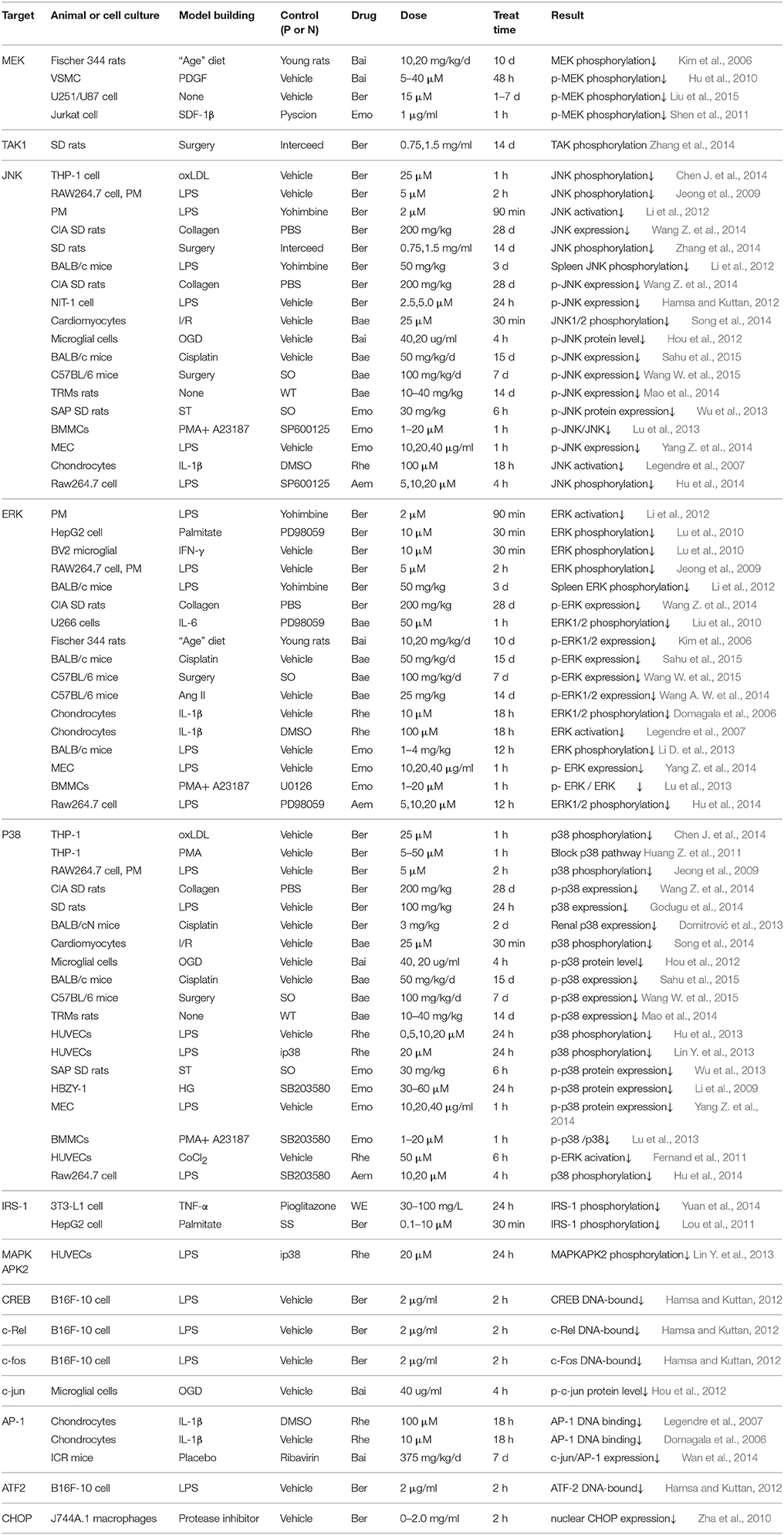
Effect of the Active Constituents on Molecules in MAPK Pathway
MAPK can be divided into several subfamilies including p38, ERK and JNK (Lou et al., 2011). Upstream TAK1 forms a complex consist of TAB1, TAB2, and TRAF6 and then sequentially activate MKK and JNK. The presence of Ras will activate c-raf, MEK and ERK, followed by c-fos regulation once transported into nucleus. Subsequently, the regulated c-fos recruits c-jun to form AP-1 complex (Figure 1).
Accumulative data shown in Table 2 leads to a conclusion that p38, ERK, and JNK attract the most focus of study in MAPK pathway. In-vitro study results reveal that the increased level of p38, ERK or JNK phosphorylation stimulated by cytokines/chemokines like LPS (Lin Y. et al., 2013), IL-1β (Legendre et al., 2007), oxLDL (Chen J. et al., 2014), PMA (Huang Z. et al., 2011), ischemia (Song et al., 2014), OGD (Hou et al., 2012), HG (Li et al., 2009) and CoCl2 (Fernand et al., 2011), or in-vivo elevated level induced by insulin (Lu et al., 2010), collagen (Wang Z. et al., 2014) and cisplatin (Sahu et al., 2015) can be significantly attenuated by either RR, RC, or RS constituent intervention. To further investigate whether p38, ERK and JNK are the only targets, molecules lied on the upstream and downstream are taken into consideration. Turns out, Ber, Bai as well as Rhe treatments all show inhibitory effect on MEK phosphorylation (Shen et al., 2011; Lim et al., 2012; Liu et al., 2015). Nevertheless, for the enhanced phosphorylation of TAK1, Ber is the only reported SHXXT constituent (Zhang et al., 2014). In addition, Ber, Bai, or Rhe also display markedly suppressing effect on endonuclear translocation factors like c-fos and CREB (Hamsa and Kuttan, 2012), c-jun (Hou et al., 2012), ATF-2(Legendre et al., 2007), CHOP (Zha et al., 2010), or AP-1 complex (Domagala et al., 2006).
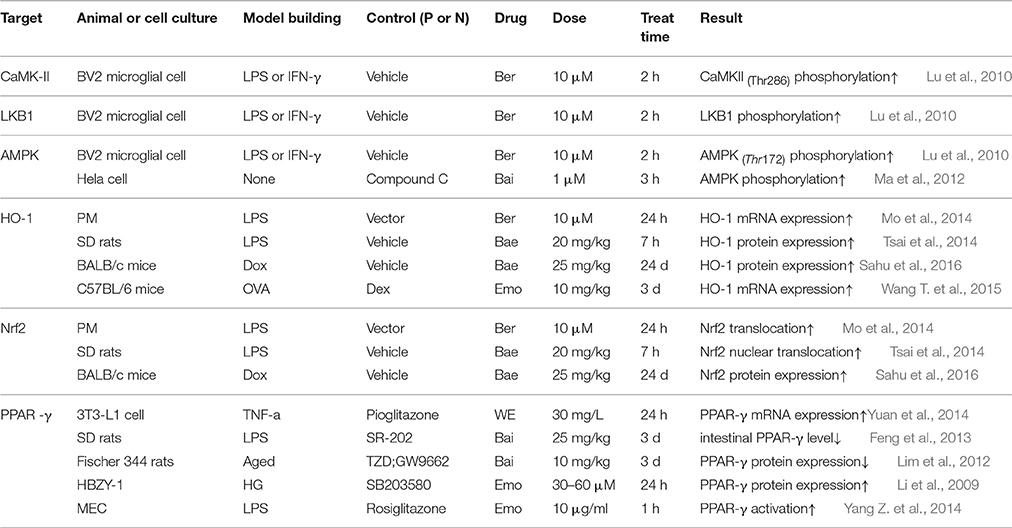
Effect of the Active Constituents on Molecules in AMPK Pathway
AMPK serves as a cellular energy sensor to modulate lipid metabolism, and it can be activated by upstream kinases like LKB1 and CaMKK (Yang Y. et al., 2014; Li N. S. et al., 2016). There is a mechanism underlined the relationship, thus once AMPK activated, the nuclear translocation of Nrf2 is promoted, which contribute to the diminution of pro-inflammatory cytokines production. Nrf2 can also drive downstream HO-1 expression in with the considerable beneficial protect effect against cell injury from inflammatory response like diabetes mellitus (Agca et al., 2014). PPAR-γ is identified as a primary regulator of gene expression for inflammation and a pharmacological receptor of insulin-sensitizing drugs (Choi et al., 2014).
As summed up in Table 3, the current study status demonstrate that Ber from RC exert the most comprehensive effect compared with other constituents form RR and RS, pathway molecules from upstream to downstream, including CaMKII, LKB1, PPAR-γ (Legendre et al., 2007), AMPK (Lu et al., 2010), Nrf2 and HO-1(Mo et al., 2014) are all verified to be the effective targets of Ber. In addition, Emo (Yang Z. et al., 2014; Wang T. et al., 2015), Bai and Bae (Lim et al., 2012; Ma et al., 2012; Feng et al., 2013; Tsai et al., 2014) as well affect some of those molecules. Given this investigation situation, it seems that constituents from either RR, RS, or RC can block AMPK pathway by cross-talk regulating pathway molecules.
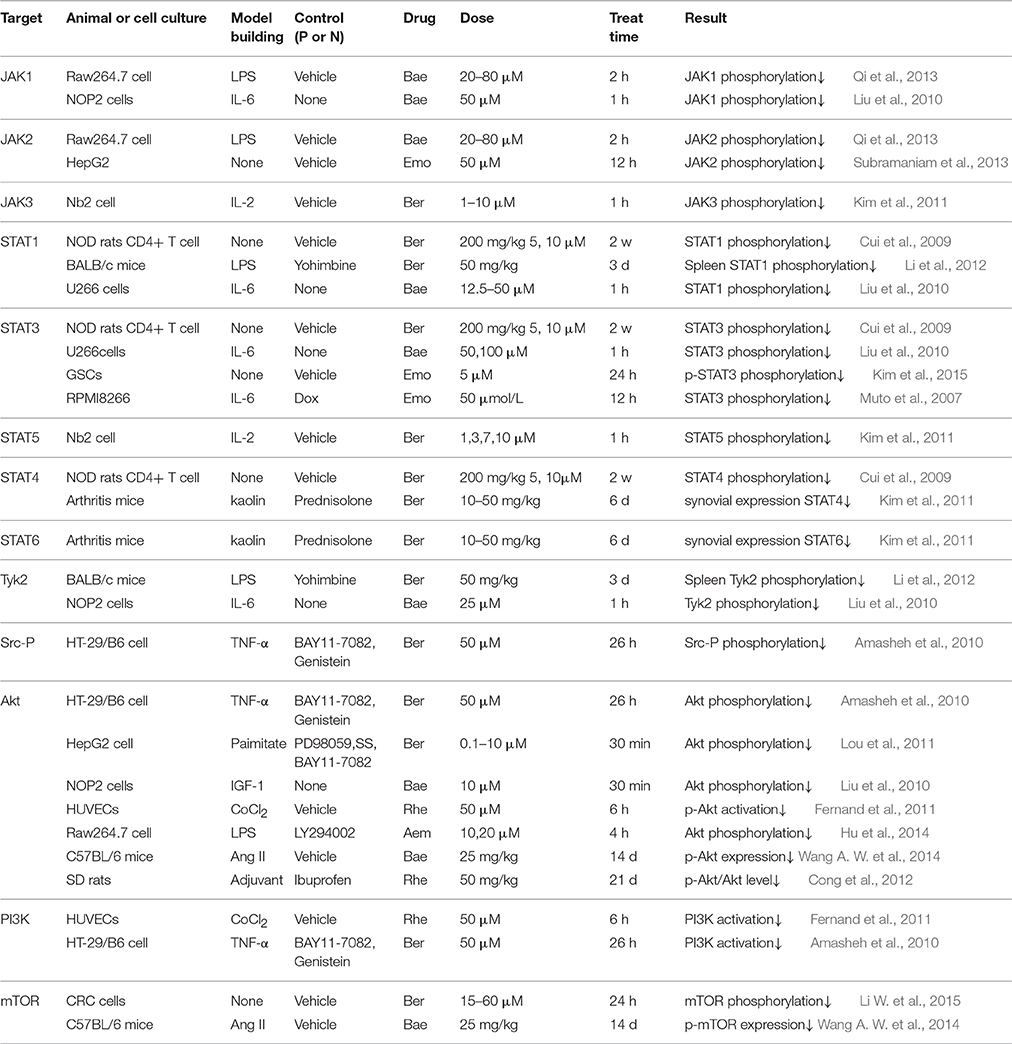
Effect of the Active Constituents on Molecules in JAK/STAT Pathway
The activation of JAK catalyze Tyr phosphorylation so that STAT can be combined with receptor protein, then transported into nucleus to regulate transcription. It has been reported that STAT1 and STAT5, the downstream molecules of IFN-γ, are also likely to be implicated in inflammation (Chmielewski et al., 2015; Li X. et al., 2015). Akt functions as emerging crucial regulator of multiple cellular processes, such as apoptosis, differentiation, survival, etc., (Piao et al., 2015). Moreover, recent studies indicate PI3K/Akt can lead to an elevated expression level of COX-2 and iNOs in inflammatory macrophages (Liou et al., 2014). Further activated mTOR can regulate cell growth, differentiation as well as transcription and it tends to perform abnormally in diabetes models (Hua and Hu, 2015).
For JAK/STAT pathway, constituents from RC, RS and RR are all showing inhibitory activity, typical targets include JAK (Kim et al., 2011; Qi et al., 2013; Subramaniam et al., 2013), STAT (Cui et al., 2009; Liu et al., 2010; Kim et al., 2015) and Akt (Lou et al., 2011; Hu et al., 2014; Wang A. W. et al., 2014), all of which are proved to be influenced by Ber, Bae, Bae, Emo or Aem in either in-vivo or in-vitro models (Table 4). On the other hand, results in the study concerning about downstream molecular present main RR constituent's effect-weakness on targets like Tyk2. Apparently, RS and RC cover the shortfalls of RR's poor activity in downstream pathway, which partly supports the synergistic theory of drug combination aiming at promoting curative effect.
Pharmacokinetic Level
Traditional Chinese medicines are frequently orally administrated and the absorption of active constituents are confirmed to be influenced by efflux pumps and intestinal transporters (ITs) (Park et al., 2012; Zumdick et al., 2012). In general, ITs widely distribute in intestinal membrane and can be divided into two categories. One accounts for external substance's intracellular transport, such as OCTs and SGLT1 (Moran et al., 2014; Couroussé and Gautron, 2015). The other one, like P-gp, MRP and BCRP, is functioning as efflux pump to make drug or toxin back to lumen (Yamagata et al., 2007; Juan et al., 2010; Zeng et al., 2015). There are many isolate reports showing SHXXT's main constituents have an unexpectedly low concentration in plasma with oral administration, making it challenged to explain its positive effects in inflammatory therapies.
In-vitro research on the efflux pump and ITs normally use Caco-2 cell or MDCK cell for they both have similar structure of differential intestinal epithelial cell with apical side and basolateral side (Chen et al., 2013; Schexnayder and Stratford, 2015; Obringer et al., 2016). Currently, it is verified that Bai from RS is the substrate of both MRP2 and BCRP (Kalapos-Kovács et al., 2015), and another RS constituent Bae is also pumped out by MRP (Zhang et al., 2007). Rhe, Emo and Aem from RR are substrate of BCRP, MRP and P-gp respectively (Wang J. et al., 2011; Liu et al., 2012; Ye et al., 2013), those ITs at least partly reduce the bioavailability of SHXXT constituents by diminishing their intracellular transport. Similarly, the absorption of Ber, Pal, Cop and Jat form RC is reported to be promoted by OCTs while inhibited by P-gp (Chen et al., 2008; Zhang et al., 2011; Sun et al., 2014). In addition to OCTs, SGLT1 also contributes to uptake (Zhang et al., 2012). Thus, any constituents in SHXXT which suppress the MRP2, BCRP, and P-gp activation or, on the other hand, up-regulate OCTs and SGLT1 activation may be considered to exert mutual reinforcement property by promoting bioavailability.
In return, constituents in SHXXT show retroaction on those efflux pump or ITs. Depicted in Figure 2, Firstly, P-gp, which reduces the absorption of Ber, Pal, Cop, Jar, and Aem, is proved to be inhibited by Bae treatment (Cho et al., 2011). Secondly, Rhe can suppress MRP's activation which may lead the increasing uptake of Aem, Bai, and Bae (Shia et al., 2013). Last but not least, Ber can as well decrease BCRP activation, which is capable of promoting the intracellular concentrations of Bai, Emo, and Rhe (Tan et al., 2013).
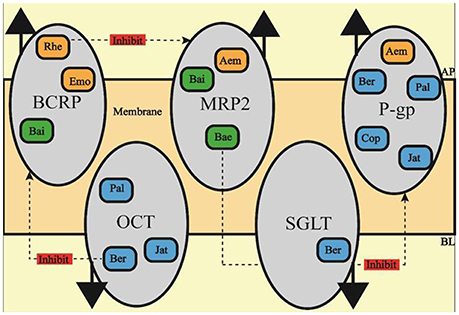
Figure 2. The effect of SHXXT constituents on ITs. Ber, berberine; Cop, coptisine; Pal, palmatine; Jat, jatrorrhizine; Bai, baicalin; Bae, baicalein; Emo, emodin; Aem, aloe-emodin; Rhe, rhein.
Discussion
TCM normally used as prescription so as to recruit active contents from different herbals. Modern mutual reinforcement theory believes pharmacodynamics effect after herbal combination is not simply equal to the summing up of each herbal, but to a certain extent, should be more than that. Under most circumstances, a prescription can bring out more advantages in regards of safety and efficacy aspects than a single herb does (Song et al., 2013). Apart from expanding effect on one specific part, the combination of several herbals can also give rise to respective effect on different parts, which in other words, supplement other herbals' disadvantages or helping other herbals to perform their property in a better way.
Inflammatory signal transduction is quite complex network, and suppression on any intersection can partly contribute to the prevention of inflammation process. SHXXT have been high-lighted based on their widely appearance in inflammation-associated treatment for centuries. Clinically, SHXXT is a preferred drug for “coexistence of cold and heat” (Zhang et al., 2013c). With the constantly deepen researches, it is widely used in the treatment for anti-pathogens, anti-inflammation, gastric mucosal protection, hemostasis, anti-diabetes and so on (Li and Guo, 2010). As depicted in Figure 1, target with three colored “cross” is to be influenced by constituents form all three compositions (TLR-4, ERK, JNK, p38, Akt, etc.), which lead a fold increase of the final effect. On the other hand, target with less than three “cross” suggest at least one composition was not valid at this part. For example,
(1) Ber from RC is reported to affect TAK1 and interaction between LPS and TLR-4, while RR and main RS constituents barely mentioned.
(2) Bae from RS and Ber from RC inhibit Tyk2 phosphorylation, while no main RR constituent has similar effect.
(3) Rhe from RR and Ber from RC reduce IRF3 phosphorylation level, while the effect of main constituent of RS isn't that clear, etc.
The connotative meaning of synergism at pharmacodynamics level is to enhance the effect on a certain target, as well as to expand target-affecting scope, just like what SHXXT constituents have performed. As for the pharmacokinetic level, shown in Figure 2, Ber form RS, Rhe form RR and Bae form RC is capable of improving the uptake or reducing the efflux of constituents from the other composition, which ultimately reaches the goal of synergistically influence inflammatory processes and eventually make this formula's anti-inflammatory action stronger and wider.
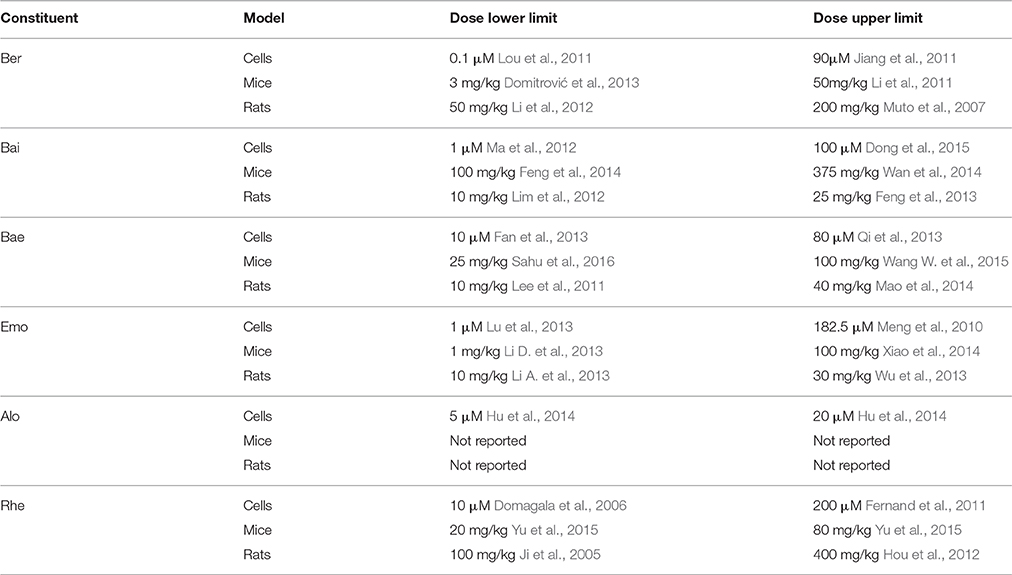
Nowadays, elevated attention has been paid to dose-effect relationship. There is a complicated process which can be expressed as “theory-methodology-formulation-medication-dosage” in TCM clinical therapeutics, showing how important for a formula prescription to have a specific herbal dosage (Zha et al., 2015). Basically for western medicine, these is a positive correlation between dose and toxicity. However, TCM at a large dosage tends to have good therapy efficacy with slight side effect (Wang et al., 1983). The dosage of Chinese herbals in clinical cases or experimental studies is usually at a relatively higher level than that documented in ancient TCM records (Peng, 2003; Sun, 2007). RR as an example, the dosage to treat cholestasis in clinical is more than four times the regular dose recommended in the Chinese pharmacopeia (Zhang et al., 2016). For now, the widespread explanation is that drug should be administrated to the patient with the correct disorder indications, otherwise it will produce dosage variety and individual detrimental effect (Zhao et al., 2015). As displayed in Table 5, dosage of constituents from SHXXT has a big range with no obvious rule to follow, it is possibly due to different tested animals or cells may have different drug sensitivities, but still need further clarification.
Conclusions
It is easy to find out not all the SHXXT constituents receive deep-enough investigation on their anti-inflammatory effect, the interaction between main SHXXT constituents and targets outside the nucleus get most focus. Besides, any drug elevating the absorption of Rhe, Ber, and Bae can be employed to promote oral bioavailability of SHXXT. Even though evidence shows P-gp, BCRP, and MRP really are inhibited while reports rarely cover the effect of SHXXT constituents on OCTs or SGLT. Hence, further investigation at these two levels is required to fully explain the mutual reinforcement relationship of RR, RC, and RS.
Author Contributions
JW: Prepare the manuscript; YH and LX: Search for the literatures; SL and YY: Draw the figures; XC and YZ: Do the summing work and accomplish the tables; WH: Polish language; XM and PW: Corresponding authors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81073118, 81274111 and 81473419).
Abbreviations
ADM, adriamycin amycin; Akt, Protein kinase B; extracellular signal-regulated kinase; AMPK, 5′ AMP-activated protein kinase; Ang II, angiotensin II; AP-1, activator protein 1; ATF, Activating transcription factor; BCRP, breast cancer resistance protein; CaMK, Calcium/calmodulin- dependent kinase; CHOP, C/EBP homologous protein 10; CK, ginsenoside metabolite compound K; CMC-Na, caboxy methyl cellulose; CREB, cAMP response element-binding protein; Dex, Dexamethasone; Dox, Doxorubicin; DSS, dextran sulfate sodium; ERK, extracellular signal-regulated kinase; GSK3β, Glycogen synthase kinase 3 beta; HCM, hypercholesterolemic; HG, high glucose; HO-1, HMOX1heme oxygenase (decycling) 1; I/R, ischemia/reperfusion; IKK, IκBα kinase; IκBα, inhibitor of nuclear factor κBα; iNOS, inducible nitric oxide synthase; IRAK, Interleukin-1 receptor-associated kinase; IRF3, Interferon regulatory factor 3; IRS-1, Insulin receptor substrate 1; JAK, junas kinase; JNK, c-Jun NH2-terminal kinase; LKB1, liver kinase B1; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MAPKAPK2, MAP kinase-activated protein kinase 2; MEK, Mitogen-activated protein kinase kinase; MRP, multidrug resistance associated protein; mTOR, mammalian target of rapamycin 2; MyD88, Myeloid differentiation primary response gene 88; NIK, NF-κB inducing kinase; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; OCT, organic cation transporter; OGD, oxygen–glucose deprivation; PDTC, Pyrrolidine dithiocarbamate; P-gp, P-glycoprotein; PI3K, phosphoinositide 3-kinase; PMA, Phorbol-12-myristate-13-acetate; Poly(I:C), Polyinosinic:polycytidylic acid; PPAR-γ, peroxisome proliferator-activated receptor γ; Raf, RAF proto-oncogene serine/threonine-protein kinase; SAP, severe acute pancreatitis; SGLT1, Na+-dependent glucose transporter; SO, sham operation; SS, sodium salicylate; ST, sodium taurocholate; STAT, signal transducer and activator of transcription; TAK1, transforming growth factor-b-activated kinase; TLR-4, toll-like receptor; TNBS, 2, 4, 6-trinitrobenzene sulfonic acid; TNFR1, tumor necrosis factor receptor 1; TRADD, Tumor necrosis factor receptor type 1-associated death domain protein; TRAF, TNF receptor associated factors; TRM, epilepsy-like tremor; Tyk2, Non-receptor tyrosine-protein kinase 2; VSMC, vascular smooth muscle cell; WKY, Wistar-Kyoto; WT, wild type.
References
Agca, C. A., Tuzcu, M., Hayirli, A., and Sahin, K. (2014). Taurine ameliorates neuropathy via regulating NF-κB and Nrf2/HO-1 signaling cascades in diabetic rats. Food Chem. Toxicol. 71, 116–121. doi: 10.1016/j.fct.2014.05.023
Amasheh, M., Fromm, A., Krug, S. M., Amasheh, S., Andres, S., Zeitz, M., et al. (2010). TNFα-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFκB signaling. J. Cell Sci. 123, 4145–4155. doi: 10.1242/jcs.070896
Ambrozova, G., Martiskova, H., Koudelka, A., Ravekes, T., Rudolph, T. K., Klinke, A., et al. (2016). Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses. Free Radic. Biol. Med. 90, 252–260. doi: 10.1016/j.freeradbiomed.2015.11.026
Asano, T., Ogihara, T., Katagiri, H., Sakoda, H., Ono, H., Fujishiro, M., et al. (2004). Glucose transporter and Na+/glucose cotransporter as molecular targets of anti-diabetic drugs. Curr. Med. Chem. 11, 2717–2724. doi: 10.2174/0929867043364360
Bader, S., Klein, J., and Diener, M. (2014). Choline acetyltransferase and organic cation transporters are responsible for synthesis and propionate-induced release of acetylcholine in colon epithelium. Eur. J. Pharmacol. 733, 23–33. doi: 10.1016/j.ejphar.2014.03.036
Bagnéris, C., Rogala, K. B., Baratchian, M., Zamfir, V., Kunze, M. B., Dagless, S., et al. (2015). Probing the solution structure of IκB Kinase (IKK) subunit γ and its interaction with kaposi sarcoma-associated herpes virus flice-interacting protein and IKK subunit β by EPR spectroscopy. J. Biol. Chem. 290, 16539–16549. doi: 10.1074/jbc.M114.622928
Bertolini, A. (2012). Drug-induced activation of the nervous control of inflammation: a novel possibility for the treatment of hypoxic damage. Eur. J. Pharmacol. 679, 1–8. doi: 10.1016/j.ejphar.2012.01.004
Cabrera-Benitez, N. E., Pérez-Roth, E., Casula, M., Ramos-Nuez, Á., Ríos-Luci, C., Rodríguez-Gallego, C., et al. (2012). Anti-inflammatory activity of a novel family of aryl ureas compounds in an endotoxin-induced airway epithelial cell injury model. PLoS ONE 7:e48468. doi: 10.1371/journal.pone.0048468
Chen, C. C., Hung, T. H., Lee, C. Y., Wang, L. F., Wu, C. H., Ke, C. H., et al. (2014). Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS ONE 9:e115694. doi: 10.1371/journal.pone.0115694
Chen, J., Cao, J., Fang, L., Liu, B., Zhou, Q., Sun, Y., et al. (2014). Berberine derivatives reduce atherosclerotic plaque size and vulnerability in apoE−/− mice. J. Transl. Med. 12, 326. doi: 10.1186/s12967-014-0326-7
Chen, X., Peng, S., Zeng, H., Fu, A., and Zhu, Q. (2015). Toll-like receptor 4 is involved in a protective effect of rhein on immunoglobulin A nephropathy. Indian J. Pharmacol. 47, 27. doi: 10.4103/0253-7613.150319
Chen, Y., Wang, X., Sun, H., Xing, D., Hu, J., Wai, Z., et al. (2008). Characterization of the transportation of berberine in Coptidis rhizoma extract through rat primary cultured cortical neurons. Biomed. Chromatogr. 22, 28–33. doi: 10.1002/bmc.889
Chen, Z. Z., Lu, Y., Du, S. Y., Shang, K. X., and Cai, C. B. (2013). Influence of borneol and muscone on geniposide transport through MDCK and MDCK-MDR1 cells as blood–brain barrier in vitro model. Int. J. Pharm. 456, 73–79. doi: 10.1016/j.ijpharm.2013.08.017
Cheng, B. C. Y., Yu, H., Su, T., Fu, X. Q., Guo, H., Li, T., et al. (2015). A herbal formula comprising Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits the production of inflammatory mediators and the IRAK-1/TAK1 and TBK1/IRF3 pathways in RAW 264.7 and THP-1 cells. J. Ethnopharmacol. 174, 195–199. doi: 10.1016/j.jep.2015.08.018
Cheng, Z., Taylor, B., Ourthiague, D. R., and Hoffmann, A. (2015). Distinct single-cell signaling characteristics are conferred by MyD88 and TRIF pathways during TLR4 activation. Sci. Signal. 8:ra69. doi: 10.1126/scisignal.aaa5208
Chiapinotto Spiazzi, C., Bucco, S. M., Pinto, I. A., Musacchio, V. L., Zanchi, M. M., Frasson, P. N., et al. (2015). Selenofuranoside ameliorates memory loss in Alzheimer-like sporadic dementia: aChE activity, oxidative stress, and inflammation involvement. Oxid. Med. Cell. Longev. 2015:976908. doi: 10.1155/2015/976908
Chmielewski, S., Piaszyk-Borychowska, A., Wesoly, J., and Bluyssen, H. A. (2015). STAT1 and IRF8 in vascular inflammation and cardiovascular disease: diagnostic and therapeutic potential. Int. Rev. Immunol. 25, 1–21. doi: 10.3109/08830185.2015.1087519
Cho, Y. A., Choi, J. S., and Burm, J. P. (2011). Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: a possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol. Rep. 63, 1066–1073. doi: 10.1016/S1734-1140(11)70624-7
Choi, S. S., Park, J., and Choi, J. H. (2014). Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 47, 599–608. doi: 10.5483/BMBRep.2014.47.11.174
Cong, X. D., Ding, M. J., Dai, D. Z., Wu, Y., Zhang, Y., and Dai, Y. (2012). ER stress, p66shc, and p-Akt/Akt mediate adjuvant-induced inflammation, which is blunted by argirein, a supermolecule and rhein in rats. Inflammation 35, 1031–1040. doi: 10.1007/s10753-011-9407-4
Couroussé, T., and Gautron, S. (2015). Role of organic cation transporters (OCTs) in the brain. Pharmacol. Ther. 146, 94–103. doi: 10.1016/j.pharmthera.2014.09.008
Cui, G., Qin, X., Zhang, Y., Gong, Z., Ge, B., and Zang, Y. Q. (2009). Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J. Biol. Chem. 284, 28420–28429. doi: 10.1074/jbc.M109.012674
Di, X., Wang, X., and Liu, Y. (2015). Effect of piperine on the bioavailability and pharmacokinetics of emodin rats. J. Pharm. Biomed. Anal. 115, 144–149. doi: 10.1016/j.jpba.2015.06.027
Domagala, F., Martin, G., Bogdanowicz, P., Ficheux, H., and Pujol, J. P. (2006). Inhibition of interleukin-1β-induced activation of MEK/ERK pathway and DNA binding of NF-κB and AP-1: Potential mechanism for Diacerein effects in osteoarthritis. Biorheology 43, 577–587.
Domitrović, R., Cvijanović, O., Pernjak-Pugel, E., Škoda, M., Mikelić, L., Crnčević-Orlić, Ž., et al. (2013). Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 62, 397–406. doi: 10.1016/j.fct.2013.09.003
Dong, S. J., Zhong, Y. Q., Lu, W. T., Li, G. H., Jiang, H. L., and Mao, B. (2015). Baicalin inhibits lipopolysaccharide-induced inflammation through signaling NF-κB pathway in HBE16 airway epithelial cells. Inflammation 38, 1493–1501. doi: 10.1007/s10753-015-0124-2
Fan, G. W., Zhang, Y., Jiang, X., Zhu, Y., Wang, B., Su, L., et al. (2013). Anti-inflammatory activity of baicalein in LPS-stimulated RAW264. 7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation 36, 1584–1591. doi: 10.1007/s10753-013-9703-2
Feng, A., Zhou, G., Yuan, X., Huang, X., Zhang, Z., and Zhang, T. (2013). Inhibitory effect of baicalin on iNOS and NO expression in intestinal mucosa of rats with acute endotoxemia. PLoS ONE 8:e80997. doi: 10.1371/journal.pone.0080997
Feng, J., Guo, C., Zhu, Y., Pang, L., Yang, Z., Zou, Y., et al. (2014). Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int. J. Clin. Exp. Med. 7, 4063–4072.
Fernand, V. E., Losso, J. N., Truax, R. E., Villar, E. E., Bwambok, D. K., Fakayode, S. O., et al. (2011). Rhein inhibits angiogenesis and the viability of hormone-dependent and-independent cancer cells under normoxic or hypoxic conditions in vitro. Chem. Biol. Interact. 192, 220–232. doi: 10.1016/j.cbi.2011.03.013
Gao, Y., Chen, X., Fang, L., Liu, F., Cai, R., Peng, C., et al. (2014). Rhein exerts pro-and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic. Biol. Med. 72, 104–112. doi: 10.1016/j.freeradbiomed.2014.04.001
Garimella, M. G., Kour, S., Piprode, V., Mittal, M., Kumar, A., Rani, L., et al. (2015). Adipose-derived mesenchymal stem cells prevent systemic bone loss in collagen-induced arthritis. J. Immunol. 195, 5136–5148. doi: 10.4049/jimmunol.1500332
Godugu, C., Patel, A. R., Doddapaneni, R., Somagoni, J., and Singh, M. (2014). Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 9:e89919. doi: 10.1371/journal.pone.0089919
Hamsa, T. P., and Kuttan, G. (2012). Antiangiogenic activity of Berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem. Toxicol. 35, 57–70. doi: 10.3109/01480545.2011.589437
He, W., Liu, G., Cai, H., Sun, X., Hou, W., Zhang, P., et al. (2014). Integrated pharmacokinetics of five protoBerberine-type alkaloids in normal and insomnic rats after single and multiple oral administration of Jiao-Tai-Wan. J. Ethnopharmacol. 154, 635–644. doi: 10.1016/j.jep.2014.04.040
Hou, J., Wang, J., Zhang, P., Li, D., Zhang, C., Zhao, H., et al. (2012). Baicalin attenuates proinflammatory cytokine production in oxygen–glucose deprived challenged rat microglial cells by inhibiting TLR4 signaling pathway. Int. Immunopharmacol. 14, 749–757. doi: 10.1016/j.intimp.2012.10.013
Hu, B., Zhang, H., Meng, X., Wang, F., and Wang, P. (2014). Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264. 7 macrophages. J. Ethnopharmacol. 153, 846–853. doi: 10.1016/j.jep.2014.03.059
Hu, G., Liu, J., Zhen, Y. Z., Wei, J., Qiao, Y., Lin, Y. J., et al. (2013). Rhein inhibits the expression of vascular cell adhesion molecule 1 in human umbilical vein endothelial cells with or without lipopolysaccharide stimulation. Am. J. Chin. Med. 41, 473–485. doi: 10.1142/S0192415X13500341
Hu, H. J., Han, M., Sun, R. H., Liu, B., and Wen, J. K. (2010). Baicalin inhibits VSMC proliferation and neointimal hyperplasia in rats. Basic Clin. Med. 30, 1252–1256.
Hua, F., and Hu, Z. (2015). TRIB3-P62 interaction, diabetes and autophagy. Oncotarget 6, 34061–34062. doi: 10.18632/oncotarget.6108
Huang, S., Chang, S. J., Yang, M., Chen, J. J., and Chang, W. H. (2011). Nanoscale hepatoprotective herbal decoction attenuates hepatic stellate cell activity and chloroform-induced liver damage in mice. Int. J. Nanomed. 6, 1365–1371. doi: 10.2147/IJN.S19503
Huang, Z., Wang, L., Meng, S., Wang, Y., Chen, T., and Wang, C. (2011). Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int. J. Cardiol. 146, 153–158. doi: 10.1016/j.ijcard.2009.06.023
Hwang, J. K., Noh, E. M., Moon, S. J., Kim, J. M., Kwon, K. B., Park, B. H., et al. (2013). Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology 52, 1583–1591. doi: 10.1093/rheumatology/ket178
Jeong, H. W., Hsu, K. C., Lee, J. W., Ham, M., Huh, J. Y., Shin, H. J., et al. (2009). Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 296, E955–E964. doi: 10.1152/ajpendo.90599.2008
Jeong, J. J., Jang, S. E., Hyam, S. R., Han, M. J., and Kim, D. H. (2014). The rhizome mixture of Anemarrhena asphodeloides and Coptidis chinensis ameliorates acute and chronic colitis in mice by inhibiting the binding of lipopolysaccharide to TLR4 and IRAK1 phosphorylation. Evid. Based Complement. Alternat. Med. 2014:809083. doi: 10.1155/2014/809083
Ji, Z. Q., Huang, C. W., Liang, C. J., Sun, W. W., Chen, B., and Tang, P. R. (2005). Effects of rhein on activity of caspase-3 in kidney and cell apoptosis on the progression of renal injury in glomerulosclerosis. Zhong Hua Yi Xue Za Zhi 85, 1836–1841.
Jiang, Q., Liu, P., Wu, X., Liu, W., Shen, X., Lan, T., et al. (2011). Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: involvement of NF-κB signaling pathway. Mol. Cell. Endocrinol. 331, 34–40. doi: 10.1016/j.mce.2010.07.023
Juan, M. E., González-Pons, E., and Planas, J. M. (2010). Multidrug resistance proteins restrain the intestinal absorption of trans-resveratrol in rats. J. Nutr. 140, 489–495. doi: 10.3945/jn.109.114959
Kalapos-Kovács, B., Magda, B., Jani, M., Fekete, Z., Szabó, P. T., Antal, I., et al. (2015). Multiple ABC transporters efflux baicalin. Phytother. Res. 29, 1987–1990. doi: 10.1002/ptr.5477
Kim, B. H., Kim, M., Yin, C. H., Jee, J. G., Sandoval, C., Lee, H., et al. (2011). Inhibition of the signalling kinase JAK3 alleviates inflammation in monoarthritic rats. Br. J. Pharmacol. 164, 106–118. doi: 10.1111/j.1476-5381.2011.01353.x
Kim, B. J., Kim, H., Lee, G. S., So, I., and Kim, S. J. (2014). Effects of San-Huang-Xie-Xin-tang, a traditional Chinese prescription for clearing away heat and toxin, on the pacemaker activities of interstitial cells of Cajal from the murine small intestine. J. Ethnopharmacol. 155, 744–752. doi: 10.1016/j.jep.2014.06.024
Kim, D. H., Kim, H. K., Park, S., Kim, J. Y., Zou, Y., Cho, K. H., et al. (2006). Short-term feeding of baicalin inhibits age-associated NF-κB activation. Mech. Ageing Dev. 127, 719–725. doi: 10.1016/j.mad.2006.05.002
Kim, J., Lee, J. S., Jung, J., Lim, I., Lee, J. Y., and Park, M. J. (2015). Emodin suppresses maintenance of stemness by augmenting proteosomal degradation of epidermal growth factor receptor/epidermal growth factor receptor variant III in glioma stem cells. Stem Cells Dev. 24, 284–295. doi: 10.1089/scd.2014.0210
Lee, I. A., Hyun, Y. J., and Kim, D. H. (2010). Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur. J. Pharmacol. 648, 162–170. doi: 10.1016/j.ejphar.2010.08.046
Lee, S., Challa-Malladi, M., Bratton, S. B., and Wright, C. W. (2014). Nuclear factor-κB-inducing kinase (NIK) contains an amino-terminal inhibitor of apoptosis (IAP)-binding motif (IBM) that potentiates NIK degradation by cellular IAP1 (c-IAP1). J. Biol. Chem. 289, 30680–30689. doi: 10.1074/jbc.M114.587808
Lee, Y. M., Cheng, P. Y., Chim, L. S., Kung, C. W., Ka, S. M., Chung, M. T., et al. (2011). Baicalein, an active component of Scutellaria baicalensis Georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J. Ethnopharmacol. 135, 179–185. doi: 10.1016/j.jep.2011.03.009
Legendre, F., Bogdanowicz, P., Martin, G., Domagala, F., Leclercq, S., Pujol, J., et al. (2007). Rhein, a diacerhein-derived metabolite, modulates the expression of matrix degrading enzymes and the cell proliferation of articular chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways. Clin. Exp. Rheumatol. 25, 546–555.
Li, A., Dong, L., Duan, M. L., Sun, K., Liu, Y. Y., Wang, M. X., et al. (2013). Emodin improves lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Microcirculation 20, 617–628. doi: 10.1111/micc.12061
Li, D., Zhang, N., Cao, Y., Zhang, W., Su, G., Sun, Y., et al. (2013). Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. Eur. J. Pharmacol. 705, 79–85. doi: 10.1016/j.ejphar.2013.02.021
Li, H. M., Wang, Y. Y., Wang, H. D., Cao, W. J., Yu, X. H., Lu, D. X., et al. (2011). Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol. Sin. 32, 1364–1372. doi: 10.1038/aps.2011.102
Li, H., Wang, Y., Zhang, H., Jia, B., Wang, D., Li, H., et al. (2012). Yohimbine enhances protection of berberine against LPS-induced mouse lethality through multiple mechanisms. PLoS ONE 7:e52863. doi: 10.1371/journal.pone.0052863
Li, J., Zhong, W., Wang, W., Hu, S., Yuan, J., Zhang, B., et al. (2014). Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS ONE 9:e87810. doi: 10.1371/journal.pone.0087810
Li, N. S., Zou, J. R., Lin, H., Ke, R., He, X. L., Xiao, L., et al. (2016). LKB1/AMPK inhibits TGF-β1 production and the TGF-β signaling pathway in breast cancer cells. Tumor Biol. 37, 8249–8258. doi: 10.1007/s13277-015-4639-9
Li, W., Hua, B., Saud, S. M., Lin, H., Hou, W., Matter, M. S., et al. (2015). Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinog. 54, 1096–1109. doi: 10.1002/mc.22179
Li, X., Liu, W., Wang, Q., Liu, P., Deng, Y., Lan, T., et al. (2009). Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol. Cell. Endocrinol. 307, 157–162. doi: 10.1016/j.mce.2009.03.006
Li, X., Wang, J., Cao, J., Ma, L., and Xu, J. (2015). Immunoregulation of bone marrow-derived mesenchymal stem cells on the chronic cigarette smoking-induced lung inflammation in rats. Biomed. Res. Int. 2015:932923. doi: 10.1155/2015/932923
Li, C. Y., Hu, Y. C., Lee Chao, P. D., Shia, C. S., Hsu, I. C., and Fang, S. H. (2010). Potential ex vivo immunomodulatory effects of San-Huang-Xie-Xin-Tang and its component herbs on mice and humans. J. Ethnopharmacol. 127, 292–298. doi: 10.1016/j.jep.2009.11.006
Li, Y., and Guo, C. (2010). The progress of modern pharmacological research of Sanhuang Xiexin decoction. Chin. Pharm. 21, 1048–1050.
Lim, H. A., Lee, E. K., Kim, J. M., Park, M. H., Kim, D. H., Choi, Y. J., et al. (2012). PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney. Biogerontology 13, 133–145. doi: 10.1007/s10522-011-9361-4
Lin, K., Liu, S., Shen, Y., and Li, Q. (2013). Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation 36, 1079–1086. doi: 10.1007/s10753-013-9640-0
Lin, Y., Zhen, Y., Liu, J., Wei, J., Tu, P., and Hu, G. (2013). Rhein lysinate inhibits monocyte adhesion to human umbilical vein endothelial cells by blocking p38 signaling pathway. Arch. Pharm. Res. 36, 1410–1418. doi: 10.1007/s12272-013-0156-9
Liou, C. J., Len, W. B., Wu, S. J., Lin, C. F., Wu, X. L., and Huang, W. C. (2014). Casticin inhibits COX-2 and iNOS expression via suppression of NF-κB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J. Ethnopharmacol. 158, 310–316. doi: 10.1016/j.jep.2014.10.046
Liu, Q., Xu, X., Zhao, M., Wei, Z., Li, X., Zhang, X., et al. (2015). Berberine Induces Senescence of Human Glioblastoma Cells by Downregulating the EGFR–MEK–ERK Signaling Pathway. Mol. Cancer Ther. 14, 355–363. doi: 10.1158/1535-7163.MCT-14-0634
Liu, S., Ma, Z., Cai, H., Li, Q., Rong, W., and Kawano, M. (2010). Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur. J. Haematol. 84, 137–144. doi: 10.1111/j.1600-0609.2009.01365.x
Liu, W., Feng, Q., Li, Y., Ye, L., Hu, M., and Liu, Z. (2012). Coupling of UDP-glucuronosyltransferases and multidrug resistance-associated proteins is responsible for the intestinal disposition and poor bioavailability of emodin. Toxicol. Appl. Pharmacol. 265, 316–324. doi: 10.1016/j.taap.2012.08.032
Lo, Y. C., Lin, Y. L., Yu, K. L., Lai, Y. H., Wu, Y. C., Ann, L. M., et al. (2005). San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J. Ethnopharmacol. 101, 68–74. doi: 10.1016/j.jep.2005.03.015
Lou, T., Zhang, Z., Xi, Z., Liu, K., Li, L., Liu, B., et al. (2011). Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 34, 659–667. doi: 10.1007/s10753-010-9276-2
Lu, D. Y., Tang, C. H., Chen, Y. H., and Wei, I. H. (2010). Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 110, 697–705. doi: 10.1002/jcb.22580
Lu, Y., Jeong, Y. T., Li, X., Kim, M. J., Park, P. H., Hwang, S. L., et al. (2013). Emodin isolated from Polygoni cuspidati Radix inhibits TNF-α and IL-6 release by blockading NF-κB and MAP kinase pathways in mast cells stimulated with PMA plus A23187. Biomol. Ther. (Seoul). 21, 435–441. doi: 10.4062/biomolther.2013.068
Ma, B. L., Ma, Y. M., Yan, D. M., Zhou, H., Shi, R., Wang, T. M., et al. (2009). Effective constituents in Xiexin Decoction for anti-inflammation. J. Ethnopharmacol. 125, 151–156. doi: 10.1016/j.jep.2009.05.035
Ma, Y., Yang, F., Wang, Y., Du, Z., Liu, D., Guo, H., et al. (2012). CaMKKβ is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS ONE 7:e47900. doi: 10.1371/journal.pone.0047900
Mao, X., Cao, Y., Li, X., Yin, J., Wang, Z., Zhang, Y., et al. (2014). Baicalein ameliorates cognitive deficits in epilepsy-like tremor rat. Neurol. Sci. 35, 1261–1268. doi: 10.1007/s10072-014-1695-7
Meng, G., Liu, Y., Lou, C., and Yang, H. (2010). Emodin suppresses lipopolysaccharide-induced pro-inflammatory responses and NF-κB activation by disrupting lipid rafts in CD14-negative endothelial cells. Br. J. Pharmacol. 161, 1628–1644. doi: 10.1111/j.1476-5381.2010.00993.x
Mo, C., Wang, L., Zhang, J., Numazawa, S., Tang, H., Tang, X., et al. (2014). The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-sked mice. Antioxid. Redox Signal. 20, 574–588. doi: 10.1089/ars.2012.5116
Moran, A. W., Al-Rammahi, M., Zhang, C., Bravo, D., Calsamiglia, S., and Shirazi-Beechey, S. P. (2014). Sweet taste receptor expression in ruminant intestine and its activation by artificial sweeteners to regulate glucose absorption. J. Dairy Sci. 97, 4955–4972. doi: 10.3168/jds.2014-8004
Muto, A., Hori, M., Sasaki, Y., Saitoh, A., Yasuda, I., Maekawa, T., et al. (2007). Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol. Cancer Ther. 6, 987–994. doi: 10.1158/1535-7163.MCT-06-0605
Obringer, C., Manwaring, J., Goebel, C., Hewitt, N. J., and Rothe, H. (2016). Suitability of the in vitro Caco-2 assay to predict the oral absorption of aromatic amine hair dyes. Toxicol. In vitro 32, 1–7. doi: 10.1016/j.tiv.2015.11.007
Ottani, A., Giuliani, D., Neri, L., Calevro, A., Canalini, F., Vandini, E., et al. (2015). NDP-α-MSH attenuates heart and liver responses to myocardial reperfusion via the vagus nerve and JAK/ERK/STAT signaling. Eur. J. Pharmacol. 769, 22–32. doi: 10.1016/j.ejphar.2015.10.022
Pandey, M. K., Sung, B., Kunnumakkara, A. B., Sethi, G., Chaturvedi, M. M., and Aggarwal, B. B. (2008). Berberine modifies cysteine 179 of IκBα kinase, suppresses nuclear factor-κB–regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 68, 5370–5379. doi: 10.1158/0008-5472.CAN-08-0511
Park, J. H., Park, J. H., Hur, H. J., Woo, J. S., and Lee, H. J. (2012). Effects of silymarin and formulation on the oral bioavailability of paclitaxel in rats. Eur. J. Pharm. Sci. 45, 296–301. doi: 10.1016/j.ejps.2011.11.021
Peng, Z. F. (2003). Medicine theory from Yi Xue Zhong Zhong Can Xi Lu of Zhang Xichun. Chin. J. Basic Med. Trad. Chin. Med. 9, 62–65.
Piao, Y., Li, Y., Xu, Q., Liu, J. W., Xing, C. Z., Xie, X. D., et al. (2015). Association of MTOR and AKT gene polymorphisms with susceptibility and survival of gastric cancer. PLoS ONE 10:e0136447. doi: 10.1371/journal.pone.0136447
Qi, Z., Yin, F., Lu, L., Shen, L., Qi, S., Lan, L., et al. (2013). Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 62, 845–855. doi: 10.1007/s00011-013-0639-7
Sahu, B. D., Kumar, J. M., Kuncha, M., Borkar, R. M., Srinivas, R., and Sistla, R. (2016). Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 144, 8–18. doi: 10.1016/j.lfs.2015.11.018
Sahu, B. D., Mahesh Kumar, J., and Sistla, R. (2015). Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS ONE 10:e0134139. doi: 10.1371/journal.pone.0134139
Saito, T., Hasegawa-Moriyama, M., Kurimoto, T., Yamada, T., Inada, E., and Kanmura, Y. (2015). Resolution of inflammation by resolvin D1 Is essential for peroxisome proliferator–activated receptor-γ–mediated analgesia during postincisional pain development in type 2 diabetes. J. Am. Soc. Anesthesiol. 123, 1420–1434. doi: 10.1097/aln.0000000000000892
Sampson, K. E., Brinker, A., Pratt, J., Venkatraman, N., Xiao, Y., Blasberg, J., et al. (2015). Zinc finger nuclease–mediated gene knockout results in loss of transport activity for P-glycoprotein, BCRP, and MRP2 in Caco-2 cells. Drug Metab. Dispos. 43, 199–207. doi: 10.1124/dmd.114.057216
Schexnayder, C., and Stratford, R. E. (2015). Genistein and Glyceollin Effects on ABCC2 (MRP2) and ABCG2 (BCRP) in Caco-2 Cells. Int. J. Environ. Res. Public Health 13:17. doi: 10.3390/ijerph13010017
Shang, W., Liu, J., Yu, X., and Zhao, J. (2010). Effects of berberine on serum levels of inflammatory factors and inflammatory signaling pathway in obese mice induced by high fat diet. Zhongguo Zhong Yao Za Zhi 35, 1474–1477.
Shen, M. Y., Liu, Y. J., Don, M. J., Liu, H. Y., Chen, Z. W., Mettling, C., et al. (2011). Combined phytochemistry and chemotaxis assays for identification and mechanistic analysis of anti-inflammatory phytochemicals in Fallopia japonica. PLoS ONE 6:e27480. doi: 10.1371/journal.pone.0027480
Shia, C. S., Hou, Y. C., Juang, S. H., Tsai, S. Y., Hsieh, P. H., Ho, L. C., et al. (2011). Metabolism and pharmacokinetics of San-Huang-Xie-Xin-Tang, a polyphenol-rich Chinese medicine formula, in rats and ex-vivo antioxidant activity. Evid. Based Complement. Alternat. Med. 2011:721293. doi: 10.1093/ecam/nep124
Shia, C. S., Juang, S. H., Tsai, S. Y., Lee Chao, P. D., and Hou, Y. C. (2013). Interaction of rhubarb and methotrexate in rats: in vivo and ex vivo approaches. Am. J. Chin. Med. 41, 1427–1438. doi: 10.1142/S0192415X1350095X
Shih, Y. T., Wu, D. C., Liu, C. M., Yang, Y. C., Chen, J., and Lo, Y. C. (2007). San-Huang-Xie-Xin-Tang inhibits Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells. J. Ethnopharmacol. 112, 537–544. doi: 10.1016/j.jep.2007.04.015
Song, L., Yang, H., Wang, H. X., Tian, C., Liu, Y., Zeng, X. J., et al. (2014). Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis 19, 567–580. doi: 10.1007/s10495-013-0946-z
Song, J., Li, J., Ji, Y., Wang, H., Zheng, S., and Gao, J. (2013). Pharmacokinetic–pharmacodynamic evaluation of the major component astragaloside IV on the immunomodulatory effects of Yu-ping-feng prescription. Eur. J. Drug Metab. Pharmacokinet. 39, 103–110. doi: 10.1007/s13318-013-0161-x
Subramaniam, A., Shanmugam, M. K., Ong, T. H., Li, F., Perumal, E., Chen, L., et al. (2013). Emodin inhibits growth and induces apoptosis in an orthotopic hepatocellular carcinoma model by blocking activation of STAT3. Br. J. Pharmacol. 170, 807–821. doi: 10.1111/bph.12302
Sun, Q. X. (2007). The dosage of radix aconiti lateralis and its decoction method. Trad. Chin. Med. J. 6, 11–16.
Sun, Y. H., He, X., Yang, X. L., Dong, C. L., Zhang, C. F., Song, Z. J., et al. (2014). Absorption characteristics of the total alkaloids from Mahonia bealei in an in situ single-pass intestinal perfusion assay. Chin. J. Nat. Med. 12, 554–560. doi: 10.,1016/s1875-5364(14)60085-6
Tan, K. W., Li, Y., Paxton, J. W., Birch, N. P., and Scheepens, A. (2013). Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Food Chem. 138, 2267–2274. doi: 10.1016/j.foodchem.2012.12.021
Tsai, C. L., Lin, Y. C., Wang, H. M., and Chou, T. C. (2014). Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J. Ethnopharmacol. 153, 197–206. doi: 10.1016/j.jep.2014.02.010
Venna, V. R., Benashski, S. E., Chauhan, A., and McCullough, L. D. (2015). Inhibition of glycogen synthase kinase-3β enhances cognitive recovery after stroke: the role of TAK1. Learn. Mem. 22, 336–343. doi: 10.1101/lm.038083.115
Wan, Q., Wang, H., Han, X., Lin, Y., Yang, Y., Gu, L., et al. (2014). Baicalin inhibits TLR7/MYD88 signaling pathway activation to suppress lung inflammation in mice infected with influenza A virus. Biomed. Rep. 2, 437–441. doi: 10.3892/br.2014.253
Wan, X., Chen, X., Liu, L., Zhao, Y., Huang, W. J., Zhang, Q., et al. (2013). Berberine ameliorates chronic kidney injury caused by atherosclerotic renovascular disease through the suppression of NFκB signaling pathway in rats. PLoS ONE 8:e59794. doi: 10.1371/journal.pone.0059794
Wang, C. B., He, J. P., Lei, Z. Y., and Chen, J. M. (1983). Treatment of paeoniae radix rubra by the method of cooling and activating blood with paeoniae radix rubra use in large dose. J. Tradit. Chin. Med. 6, 30–33. doi: 10.1186/1749-8546-6-30
Wang, J., Wang, P., Yang, Y., Meng, X., and Zhang, Y. (2011). Intestinal absorption of aloe-emodin using single-passintestinal perfusion method in rat. Zhongguo Zhong Yao Za Zhi 36, 2393–2398.
Wang, Y. S., Lin, R. T., Cheng, H. Y., Yang, S. F., Chou, W. W., and Hank Juo, S. H. (2011). Anti-atherogenic effect of San-Huang-Xie-Xin-Tang, a traditional Chinese medicine, in cultured human aortic smooth muscle cells. J. Ethnopharmacol. 133, 442–447. doi: 10.1016/j.jep.2010.10.018
Wang, T., Zhong, X. G., Li, Y. H., Jia, X., Zhang, S. J., Gao, Y. S., et al. (2015). Protective effect of emodin against airway inflammation in the ovalbumin-induced mouse model. Chin. J. Integr. Med. 21, 431–437. doi: 10.1007/s11655-014-1898-z
Wang, W., Zhou, P. H., Xu, C. G., Zhou, X. J., Hu, W., and Zhang, J. (2015). Baicalein attenuates renal fibrosis by inhibiting inflammation via down-regulating NF-κB and MAPK signal pathways. J. Mol. Histol. 46, 283–290. doi: 10.1007/s10735-015-9621-8
Wang, A. W., Song, L., Miao, J., Wang, H. X., Tian, C., Jiang, X., et al. (2014). Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERK1/2, NF-κB, and calcineurin signaling pathways in mice. Am. J. Hypertens. 28, 518–526. doi: 10.1093/ajh/hpu194
Wang, Z., Chen, Z., Yang, S., Wang, Y., Huang, Z., Gao, J., et al. (2014). Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation 37, 1789–1798. doi: 10.1007/s10753-014-9909-y
Wei, Y., Guo, J., Zheng, X., Wu, J., Zhou, Y., Yu, Y., et al. (2014). Preparation, pharmacokinetics and biodistribution of baicalin-loaded liposomes. Int. J. Nanomedicine 9, 3623. doi: 10.2147/IJN.S66312
Wu, J. S., Shi, R., Lu, X., Ma, Y. M., and Cheng, N. N. (2015). Combination of active components of xiexin decoction ameliorates renal fibrosis through the inhibition of NF-κB and TGF-β1/smad pathways in db/db diabetic mice. PLoS ONE 10:e0122661. doi: 10.1371/journal.pone.0122661
Wu, J., Zhang, H., Hu, B., Yang, L., Wang, P., Wang, F., et al. (2016). Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur. J. Pharmacol. 780, 106–114. doi: 10.1016/j.ejphar.2016.03.037
Wu, L., Cai, B., Zheng, S., Liu, X., Cai, H., and Li, H. (2013). Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation 36, 1020–1029. doi: 10.1007/s10753-013-9634-y
Xiao, M., Zhu, T., Zhang, W., Wang, T., Shen, Y. C., Wan, Q. F., et al. (2014). Emodin ameliorates LPS-induced acute lung injury, involving the inactivation of NF-κB in mice. Int. J. Mol. Sci. 15, 19355–19368. doi: 10.3390/ijms151119355
Xie, X., Chang, X., Chen, L., Huang, K., Huang, J., Wang, S., et al. (2013). Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol. Cell. Endocrinol. 381, 56–65. doi: 10.1016/j.mce.2013.07.019
Yamagata, T., Kusuhara, H., Morishita, M., Takayama, K., Benameur, H., and Sugiyama, Y. (2007). Improvement of the oral drug absorption of topotecan through the inhibition of intestinal xenobiotic efflux transporter, breast cancer resistance protein, by excipients. Drug Metab. Dispos. 35, 1142–1148. doi: 10.1124/dmd.106.014217
Yang, Y., Li, W., Liu, Y., Sun, Y., Li, Y., Yao, Q., et al. (2014). Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J. Nutr. Biochem. 25, 1207–1217. doi: 10.1016/j.jnutbio.2014.06.001
Yang, Z., Zhou, E., Wei, D., Li, D., Wei, Z., Zhang, W., et al. (2014). Emodin inhibits LPS-induced inflammatory response by activati ng PPAR-γ in mouse mammary epithelial cells. Int. Immunopharmacol. 21, 354–360. doi: 10.1016/j.intimp.2014.05.019
Ye, L., Lu, L., Li, Y., Zeng, S., Yang, X., Chen, W., et al. (2013). Potential role of ATP-binding cassette transporters in the intestinal transport of rhein. Food Chem. Toxicol. 58, 301–305. doi: 10.1016/j.fct.2013.04.044
Yu, C., Qi, D., Sun, J. F., Li, P., and Fan, H. Y. (2015). Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci. Rep. 5:11822. doi: 10.1038/srep11822
Yuan, Y., Li, X., Zaidi, S. A., Arnatt, C. K., Yu, X., Guo, C., et al. (2015). Small molecule inhibits activity of scavenger receptor A: lead identification and preliminary studies. Bioorg. Med. Chem. Lett. 25, 3179–3183. doi: 10.1016/j.bmcl.2015.05.090
Yuan, Y., Wang, X., Lu, X., Marunaka, Y., and Wang, X. (2014). Effect of Coptidis Rhizoma extracts in a water-based solution on insulin resistance in 3T3-L1 adipocytes. Biomed. Res. 35, 321–327. doi: 10.2220/biomedres.35.321
Zeng, X. Y., Dong, S., He, N. N., Jiang, C. J., Dai, Y., and Xia, Y. F. (2015). Comparative pharmacokinetics of arctigenin in normal and type 2 diabetic rats after oral and intravenous administration. Fitoterapia 105, 119–126. doi: 10.1016/j.fitote.2015.06.014
Zha, L. H., He, L. S., Lian, F. M., Zhen, Z., Ji, H. Y., Xu, L. P., et al. (2015). Clinical strategy for optimal traditional Chinese medicine (TCM) herbal dose selection in disease therapeutics: expert consensus on classic TCM herbal formula dose conversion. Am. J. Chin. Med. 43, 1515–1524. doi: 10.1142/S0192415X1550086X
Zhang, C. E., Niu, M., Li, R. Y., Feng, W. W., Ma, X., Dong, Q., et al. (2016). Untargeted metabolomics reveals dose-response characteristics for effect of rhubarb in a rat model of cholestasis. Front. Pharmacol. 7:85. doi: 10.3389/fphar.2016.00085
Zha, W., Liang, G., Xiao, J., Studer, E. J., Hylemon, P. B., Pandak, W. M. Jr., et al. (2010). Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS ONE 5:e9069. doi: 10.1371/journal.pone.0009069
Zhang, K., Jiao, X. F., Li, J. X., and Wang, X. W. (2015). Rhein inhibits lipopolysaccharide-induced intestinal injury during sepsis by blocking the toll-like receptor 4 nuclear factor-κB pathway. Mol. Med. Rep. 12, 4415–4421. doi: 10.3892/mmr.2015.3925
Zhang, L., Lin, G., Kovács, B., Jani, M., Krajcsi, P., and Zuo, Z. (2007). Mechanistic study on the intestinal absorption and disposition of baicalein. Eur. J. Pharm. Sci. 31, 221–231. doi: 10.1016/j.ejps.2007.04.001
Zhang, Q., Ma, Y. M., Wang, Z. T., and Wang, C. H. (2013a). Differences in pharmacokinetics and anti-inflammatory effects between decoction and maceration of Sanhuang Xiexin Tang in rats and mice. Planta Med. 79, 1666–1673. doi: 10.1055/s-0033-1350959
Zhang, Q., Wang, C. H., Ma, Y. M., Zhu, E. Y., and Wang, Z. T. (2013b). UPLC-ESI/MS determination of 17 active constituents in two categorized formulas of traditional Chinese medicine, Sanhuang Xiexin Tang and Fuzi Xiexin Tang: application in comparing the differences in decoctions and macerations. Biomed. Chromatogr. 27, 1079–1088. doi: 10.1002/bmc.2910
Zhang, Q., Wang, C. H., Ma, Y. M., and Wang, Z. T. (2013c). Preparative method of Sanhuang Xiexin Decoction and Fuzi Xiexin Decoction: research advances. J. Int. Pharm. Res. 40, 295–303.
Zhang, X., Qiu, F., Jiang, J., Gao, C., and Tan, Y. (2011). Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: involvement of P-glycoprotein. Xenobiotica 41, 290–296. doi: 10.3109/00498254.2010.529180
Zhang, Y., Li, X., Zhang, Q., Li, J., Ju, J., Du, N., et al. (2014). berberine hydrochloride prevents postsurgery intestinal adhesion and inflammation in rats. J. Pharmacol. Exp. Ther. 349, 417–426. doi: 10.1124/jpet.114.212795
Zhang, Y., Zhu, H. X., and Guo, L. W. (2012). Intestinal absorption of berberine alone and in combinations by rats single pass intestinal perfusion in situ. Yao Xue Xue Xao 47, 233–238.
Zhao, Y., Ma, X., Wang, J., Wen, R., Jia, L., Zhu, Y., et al. (2015). Large dose means significant effect–dose and effect relationship of Chi-Dan-Tui-Huang decoction on alpha-naphthylisothiocyanate-induced cholestatic hepatitis in rats. BMC Complement. Altern. Med. 15:104. doi: 10.1186/s12906-015-0637-0
Keywords: San-Huang-Xie-Xin-Tang, constituents, anti-inflammatory, NF-κB, MAPK, JAK/STAT, intestinal transporter
Citation: Wu J, Hu Y, Xiang L, Li S, Yuan Y, Chen X, Zhang Y, Huang W, Meng X and Wang P (2016) San-Huang-Xie-Xin-Tang Constituents Exert Drug-Drug Interaction of Mutual Reinforcement at Both Pharmacodynamics and Pharmacokinetic Level: A Review. Front. Pharmacol. 7:448. doi: 10.3389/fphar.2016.00448
Received: 07 September 2016; Accepted: 09 November 2016;
Published: 28 November 2016.
Edited by:
Pietro Minuz, University of Verona, ItalyReviewed by:
Satish Ramalingam, Chettinad Academy of Research and Education, IndiaEnnio Lubrano, University of Molise, Italy
Copyright © 2016 Wu, Hu, Xiang, Li, Yuan, Chen, Zhang, Huang, Meng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianli Meng, eGxtOTk5OUBob3RtYWlsLmNvbQ==
Ping Wang, dml2aWFuc2VjdG9yQGFsaXl1bi5jb20=
 Jiasi Wu
Jiasi Wu Yingfan Hu1
Yingfan Hu1 Ping Wang
Ping Wang