- 1Research Division of Clinical Pharmacology, First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 2Department of Gastroenterology, First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 3Department of Pharmacy, Jiangsu Shengze Hospital, Suzhou, China
Background: Not all patients with acid-related disorders receiving proton pump inhibitor (PP) treatment get adequate gastric pH control. The genetic variation of receptors, metabolic enzymes, and transporters are known to cause failures of therapies. We have conducted a study to evaluate the influence of gastric H+/K+-ATPase, CYP2C19, and ABCB1 polymorphisms on the pharmacokinetic and pharmacodynamic profiles of dexlansoprazole injection in healthy Chinese subjects.
Methods: A total of 51 subjects were enrolled for pharmacokinetic and pharmacodynamic study after a single intravenous administration of 20 or 30 mg dexlansoprazole. Plasma concentrations were determined using a chiral liquid chromatography-mass spectrometry method. The intragastric pH and baseline-adjusted intragastric pH parameters were introduced to evaluate the pharmacodynamic characters. Genotyping was performed by polymerase chain reaction.
Results: The pharmacokinetic parameters were significantly influenced by CYP2C19 phenotypes, and gastric acid secretion inhibition were affected by both gastric H+/K+-ATPase and CYP2C19 polymorphisms. Gastric H+/K+-ATPase genotypes had greater effects than CYP2C19 genotypes on the suppression of gastric acid secretion.
Conclusion: Gastric H+/K+-ATPase polymorphism may be one of the main reasons that cause insufficient gastric acid inhibition.
Introduction
Gastric hydrogen-potassium adenosine triphosphatase (H+/K+-ATPase, proton pump), an α,β-heterodimeric enzyme, is responsible for the final step of gastric acid production (Abe et al., 2011). It serves as the target of proton pump inhibitors (PPIs) for the treatment of gastric acid-related diseases including peptic ulcer disease, gastro-esophageal reflux disease (GERD), erosive esophagitis, and acute upper gastrointestinal hemorrhage (Mullin et al., 2009; Gralnek et al., 2015). Approximately 20% of people in the western population experience symptoms of GERD regularly (Hershcovici et al., 2011), and the prevalence of peptic ulcer disease is over 16% in Chinese populat!ion (Lau et al., 2013). However, the acid-suppressive effect of PPIs varies between individuals, for instance, about 19% of GERD patients experience persistent symptoms after standard doses of PPIs therapy due to inadequate acid inhibition (Katz, 2006).
The inhibition of gastric acid secretion caused by PPIs depends on their covalent binding to gastric H+/K+-ATPase (Sachs et al., 2007). The α-subunit of this enzyme, constituted of 1,034 amino acids, has 10 transmembrane segments (TMs) (Shin and Sachs, 2004, 2006). Besides one common site (cysteine 813 at exoplasmic vestibule entry into TM6) that binds with all PPIs, omeprazole binds to cysteine 892, lansoprazole binds to cysteines 892 and 321, pantoprazole binds to cysteine 822 and rabeprazole binds to cysteines 892, 822, and 321(Besancon et al., 1997; Shin and Sachs, 2004, 2006). The affinity of inhibitors is decreased when amino acids mutate at the extra cellular end of TM6, the loop between TM5 and TM6, or the luminal end of TM8 (Sachs et al., 2007), which would lead to lower acid secretion activity. Single nucleotide polymorphisms (SNPs) of the α-subunit may have a correlation with the configuration of gastric H+/K+-ATPase, which may lead to differences in individual gastric acid inhibition by PPIs (Li et al., 2013). However, the relation between SNPs of H+/K+-ATPase and the inhibition of acid secretion by PPIs in humans has not been investigated.
Besides the binding between PPIs and proton pump, the pharmacological response of acid inhibition is also related to the systemic exposure of drugs (Vakily et al., 2009). PPIs are extensively metabolized by cytochrome P450 (CYP) 2C19 (Mullin et al., 2009). Our previous studies showed that there were significant relationships between CYP2C19 polymorphisms and pharmacokinetics or pharmacodynamics after healthy volunteers were administered omeprazole (Wang et al., 2010; Feng et al., 2015), rabeprazole (Wang et al., 2011), lansoprazole (Wang et al., 2012), or pantoprazole (Gawrońska-Szklarz et al., 2012). Lansoprazole is a substrate of ABCB1 (ATP-binding cassette, sub-family B, member 1) protein, which pumps xenobiotics (such as drugs) out of cells (Aller et al., 2009). The pharmacokinetic (PK) and pharmacodynamic (PD) difference between wild-type and mutant types of ABCB1 C3435T after an oral administration of lansoprazole is inconsistent (Kodaira et al., 2009; Li et al., 2014). To date, the influence of CYP2C19 and ABCB1 genetic polymorphisms on PK and PD of dexlansoprazole have not been reported.
Being the R-enantiomer of lansoprazole, dexlansoprazole has a lower clearance and a higher systemic exposure than the S-enantiomer, which could provide improved PK profiles in humans (Katsuki et al., 1996; Metz et al., 2009; Sun et al., 2015). The currently marketed formulation of dexlansoprazole is a dual delayed-release (DDR) capsule indicated for erosive esophagitis and GERD, which was first approved by the FDA in 2009. The novel formulation for injection was developed for the treatment of acute upper gastrointestinal hemorrhage by providing consistently high gastric pH (Gisbert et al., 2001).
The aim of this study is to evaluate the influence of gastric H+/K+-ATPase, CYP2C19, and ABCB1 polymorphisms on the gastric acid inhibition and pharmacokinetics profiles of dexlansoprazole injection in healthy Chinese subjects.
Materials and Methods
Study Design
This study was an open-label and single-center clinical trial (China Food and Drug Administration registration: 2013L01977). The protocol was approved by the Ethics Committee of First Affiliated Hospital with Nanjing Medical University and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guideline. All participants gave written informed consent prior to the enrollment.
Subjects
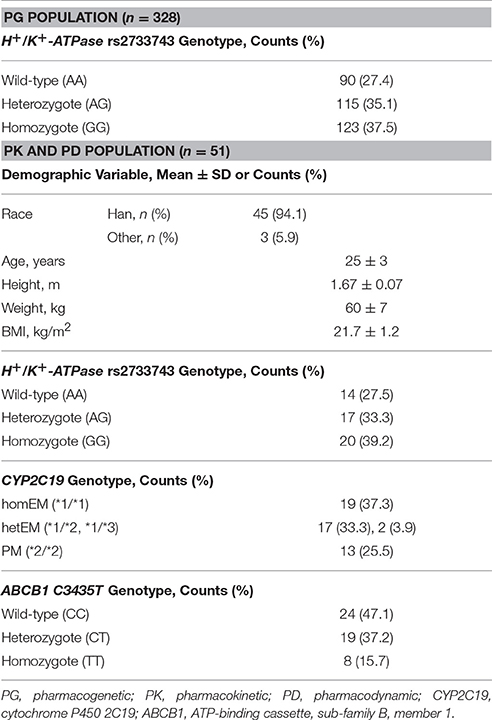
A total of 328 subjects were enrolled for the genetic analysis of gastric H+/K+-ATPase polymorphisms. Among this pharmacogenetic population, 51 subjects participating in the PK and PD study were sampled for genotyping of CYP2C19 and ABCB1 additionally.
Eligible subjects for PK and PD study were selected from healthy Chinese subjects aged 18–40 years, with a body mass index of 19–24 kg/m2. Subjects were examined to be healthy on the basis of medical history, physical examination, laboratory examination, and 12-lead electrocardiogram. The following exclusion criteria were applied to subjects in PK and PD study: a history of clinically significant cardiovascular, hepatic, renal or gastrointestinal diseases; a history of nervous system or muscle disease; seizure or other psychiatric disorders; a history of known allergy or intolerance to any drugs; a history of tobacco, alcohol, or drug abuse; a positive outcome of Helicobacter pylori infection test; those with abnormalities in clinical laboratory parameters; reception of an experimental drug or donation of blood 3 months prior to the first dose; pregnant or nursing female.
All subjects were confined to the Phase I unit beginning with the evening before baseline intubation and kept to a regular schedule. An overnight fasting (12 h) was required before administration, while standard meals were provided 4 h post-dose.
Study Drug and Administration
Dexlansoprazole injection (30 mg per vial) used in the PK and PD study was manufactured by Nanjing Yoko Pharma Co., Ltd. (Nanjing, China). Subjects received an intravenous infusion of 20 or 30 mg dexlansoprazole injection in sterile saline solution (100 mL/h, 60 min). The study drug was administrated with an infusing pump (SA213, Lifepum, Beijing, China) and given through an intravenous catheter in the antecubital vein opposite to the one from which blood samples were taken.
Pharmacokinetic Measurement
To determine the pharmacokinetic properties of dexlansoprazole injection in human, a series of venous blood samples (5 mL) were collected in heparinized tubes at 0 (pre-dose), 10, 20, 40, 60, and 75 min, 1.5, 2, 2.5, 3, 4, 6, 9, 12, 16, 24, and 36 h post-dose. Samples were centrifuged immediately at 4°C for 10 min at 3,500 revolutions per minute, then separated and stored at −80°C for PK analysis.
A chiral liquid chromatography-mass spectrometry method was developed and validated for the simultaneous determination of (R)- and (S)-lansoprazole in human plasma, with the calibration curve of 500–3,000 ng/mL for both (R)- and (S)-lansoprazole (Sun et al., 2015). Baseline chiral separation was achieved on a Chiralpak IC column. The mass spectrometric analysis was performed using a QTrap 5500 mass spectrometer (AB Sciex, Concord, Ontario, Canada).
The PK parameter of Cmax (maximum concentration) and Tmax (the time to Cmax) were obtained directly from the observed data. Other PK parameters including t1/2 (elimination half-life), AUCPK (area under the plasma concentration-time curve), Vd (volume of distribution), and CL (clearance) were calculated using non-compartmental methods with Phoenix WinNonlin version 6.4 (Pharsight Inc., CA, USA).
Pharmacodynamic Measurement
An ambulatory pH recorder (Orion II, MMS, USA) with a disp osable pH catheter (VersaFlex, MMS, USA) was used to monitor intragastric pH. The electrode was placed in water for at least 10 min and then calibrated using standard buffer solutions (pH 1.07 and 7.01) before intubation. Both calibration and intubation processes were conducted under the guidance of MMS Virtual Instructor Program. The catheter, trans nasally placed 10 cm under the lower esophageal sphincter, was identified by a sharp decrease in pH (Wang et al., 2010, 2011). Subjects were intubated on baseline and the day of dosing at about 7:30 a.m., and the intragastric pH data were recorded continuously for 24 h from 8:00 a.m. Intragastric pH was sampled every second, and the data were stored in a MMS Database program (MMS, USA). The PD parameters including Median (pH), Mean (pH), TpH≥3 (%), TpH≥4 (%), TpH≥6 (%), TpH≥6, and AUCpH−t were analyzed by the MMS Database program.
Median (pH) and mean (pH) were well-investigated in most studies, and the percentage time of pH ≥ 4 (or pH ≥ 4 holding time ratio) was the benchmark for predicting clinical efficacy of PPIs in treating acid-related diseases (Metz et al., 2009). Novel parameter TpH≥6 (time to first gastric pH ≥ 6, min) and (area under pH-time curve from τ1 to τ2, pH·s) were defined to evaluate the velocity and extent of pharmacodynamics with more accuracy. All parameters were calculated and compared according to time periods of 0–24, 0–4, 4–10, 10–14, and 14–24 h post dosing.
The parameter was calculated as follows:
Where t represented the time duration measured by seconds, was the area between the pH line = 10 and the pH-time curve, which was generated by the MMS software.
Baseline-adjusted PD parameters were calculated as follows: for example, ΔAUCpH−t = AUCpH−t − AUCpH−tbaseline, ΔMean (pH) = Mean (pH) − Mean (pH)baseline, and ΔMedian (pH) = Median (pH) − Median (pH)baseline.
Gastric H+/K+-ATPase, CYP2C19, and ABCB1 Genotyping
Genomic DNA was extracted from leucocytes of blood samples using a commercially available kit (RelaxGene Blood DNA System, Tiangen, Beijing, China) according to the manufacturer's protocol. A total of 14 exons of ATP4A gene were sequenced using primers as Oksanen (Oksanen et al., 2006) published. Genotyping for H+/K+-ATPase (rs2733743), CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), CYP2C19*17 (rs12248560), and ABCB1 C3435T (rs1045642) variant alleles was performed by polymerase chain reaction followed by Sanger sequencing. DNA sequencing was processed using BigDye® Terminator Cycle Sequencing v3.1 kit and DNA Analyzer (3730xl, Applied Biosystems, USA), which was provided by Realgene Biotech (Nanjing, China) and Sangon Biotech (Shanghai, China). Subjects participating in PK and PD studies were genotyped into three groups according to CYP2C19 wild-type gene, *2 and *3, namely homEM (*1/*1), hetEM (*1/*2 and *1/*3), and PM (*2/*2, *2/*3, and *3/*3).
Statistical Analysis
Descriptive statistics was utilized to summarize demographic and biologic characteristics of subjects. Continuous variables (PK and PD parameters) were expressed as Mean ± SD, and categorical variables as frequencies and percentages. Genotype frequencies were tested for Hardy-Weinberg equilibrium. Statistical comparison of PK and PD parameters was performed by an independent sample test and one-way analysis of variance (ANOVA) with two-sided P < 0.05 considered statistically significant. Statistical analysis was performed using SPSS software version 22.0 (IBM Corp., USA).
Results
Study Population
Demographic and biologic variables are summarized in Table 1. Genotype and allele frequencies of gastric H+/K+-ATPase (rs2733743), CYP2C19*2 (rs4244285), and ABCB1 C3435T (rs1045642) were distributed according to the Hardy-Weinberg equilibrium in the study population. No CYP2C19*17 (rs12248560) mutant was observed in the PK and PD population. In the pharmacogenetic analysis of the gene encoding the gastric H+/K+-ATPase α-subunit, one non-synonymous variation (rs2733743) was found in most subjects with 115 heterozygotes (35.1%) and 123 homozygotes (37.5%). The recorded SNP rs2733743, a homozygous A → G transition (c.823T>C) in ATP4A, alters amino acid 265 of gastric H+/K+-ATPase α subunit from valine to alanine (Yang et al., 2012). Subjects were then classified into three types as gastric H+/K+-ATPase wildtype, heterozygote and homozygote (AA, AG, and GG, respectively) accordingly.
Pharmacokinetic Data
Mean plasma concentration-time profile of dexlansoprazole after single intravenous administration is shown in Figure 1A. The PK parameters for dexlansoprazole injection grouped by dose are summarized in Table 2. The Cmax and AUCPK exhibit dose proportionality with no statistical difference in ln (Cmax/dose) and ln (AUCPK/dose) between the 20 and 30 mg groups. The PK parameters of t1/2, CL, and Vd were independent of dosage, and no significant gender-related difference was observed in PK parameters except that the Vd of male subjects was slightly higher than that of female subjects.
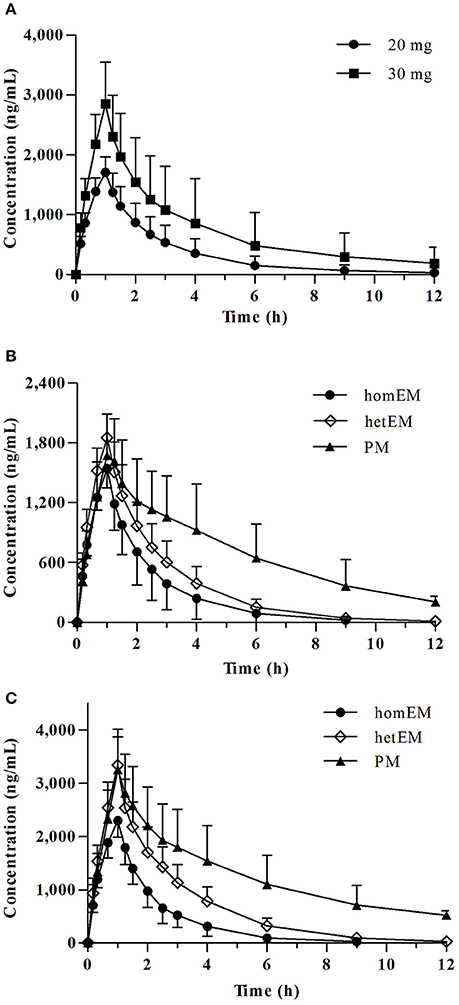
Figure 1. Plasma concentration-time profiles for dexlansoprazole 20 and 30 mg. (A) Mean ± SD concentration-time curve for dexlansoprazole after a single intravenous dosing of 20 vs. 30 mg. Comparison of plasma concentrations following intravenous administration of dexlansoprazole (B) 20 mg (n = 8, 9, 6 for homEM, hetEM, and PM) and (C) 30 mg (n = 11, 10, 7 for homEM, hetEM, and PM) by CYP2C19 phenotype.
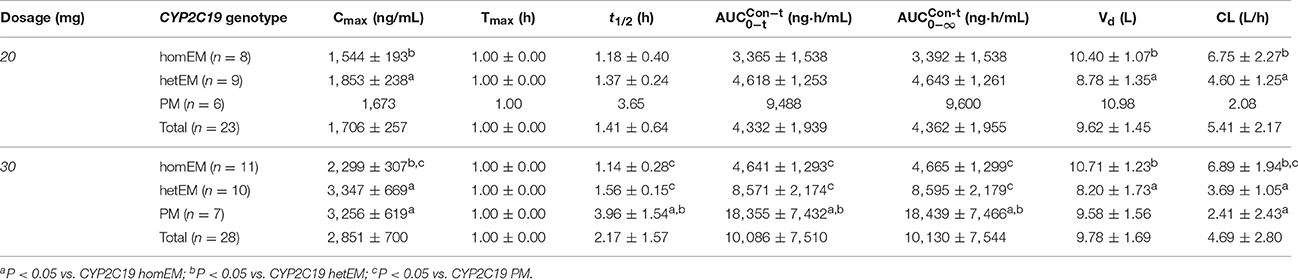
Table 2. PK parameters for dexlansoprazole following a single intravenous administration of 20 or 30 mg by CYP2C19 phenotype (Mean ± SD).
CYP2C19 PMs presented a slower elimination and higher exposure (Cmax and AUCPK) compared to CYP2C19 homEMs, while the elimination and exposure of CYP2C19 hetEMs were intermediate between that of PMs and homEMs (Figures 1B,C). The PK parameters grouped by CYP2C19 phenotype are summarized in Table 2. In the 30 mg group, CYP2C19 PMs exhibit significantly higher Cmax, AUCPK, and t1/2 than homEMs and presented remarkably higher AUCPK and t1/2 than those of CYP2C19 hetEMs. CYP2C19 hetEMs shows a significantly higher Cmax and AUCPK compared to CYP2C19 homEMs. As the previous study (Kodaira et al., 2009) revealed, no statistical significant differences were observed among ABCB1 genotypes.
Pharmacodynamic Data
The 24-h intragastric pH-time curves before and after intravenous dosing are presented in Figure 2A. The rapidity of onset of increase in intragastric pH was dose-dependent with the time to first gastric pH ≥ 6 (TpH≥6) of 76 (± 29) min for the 20 mg group and 58 (± 25) min for the 30 mg group. The mean intragastric pH, pH ≥ 4 and pH ≥ 6 holding times (TpH≥4 (%), TpH≥6 (%), the percentage of time that intragastric pH was above 4 or 6) for the 30 mg group was obviously higher than the 20 mg group. The baseline-adjusted intragastric pH-time curves (post-dose pH minus the baseline value) are shown in Figure 2B, and the baseline-adjusted intragastric pH was dose-dependent.
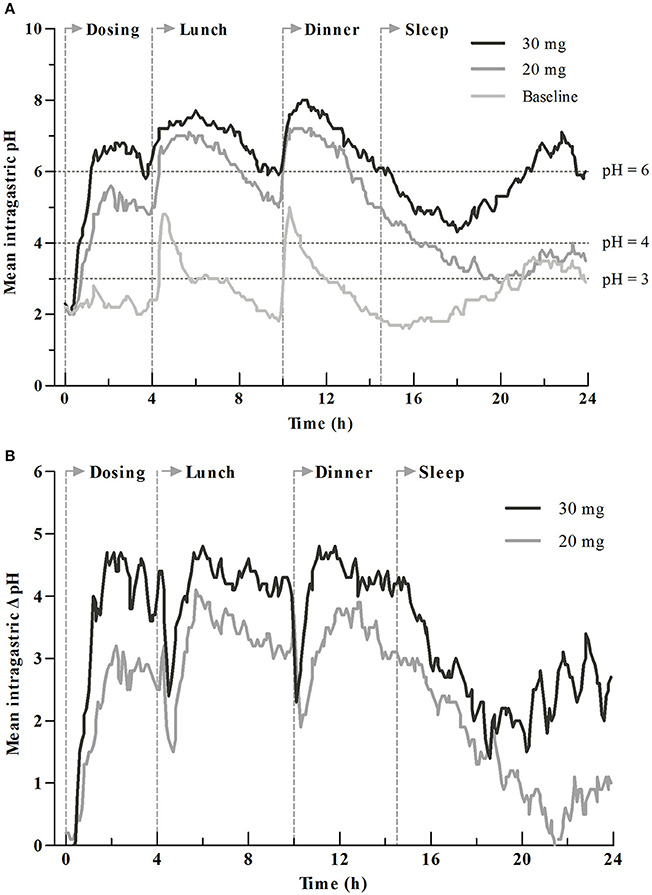
Figure 2. Mean intragastric pH profiles for dexlansoprazole 20 and 30 mg. (A) Comparison of intragastric pH before and after drug administration. (B) Comparison of baseline-adjusted intragastric pH by dosage. Time to first gastric pH ≥ 6 (TpH≥6) was 76 ± 29 min for 20 mg group and 58 ± 25 min for 30 mg group. Vertical lines at 0, 4, 10, and 14.5 h represent the time for intravenous dosing, lunch, dinner, and sleep, respectively.
The PD parameters are summarized in Table 3. The PD parameters after intravenous administration of 20 or 30 mg dexlansoprazole were significant increased (P < 0.05) compared to baseline. There was a significant elevation in PD parameters in the 30 mg group than the 20 mg group during initial time (0–4 h), overnight hours (14–24 h), and the whole time (0–24 h) (P < 0.05) after intravenous dosing at about 8:00 a.m. Mean and median intragastric pH for the 30 mg group was significantly higher than those of the 20 mg group during 0–4, 14–24, and 0–24 h after dosing. A similar increment was observed in delta(Δ)-values (values after dosing minus the corresponding values at baseline) of mean and median intragastric pH from 20 to 30 mg. No significant differences in mean and median pH were noticed between 20 and 30 mg during the 4–10 h interval, except that Δ-mean pH for the 30 mg group was higher than that of the 20 mg group (4.2 ± 1.2 vs. 3.3 ± 1.4, P = 0.043). Statistically significant differences in TpH≥3 (%) and TpH≥4 (%) were observed during time intervals of 0–24, 0–4, and 14–24 h. Pairwise comparisons of baseline-adjusted TpH≥3 (%) and TpH≥4 (%) were significantly greater for 30 mg compared with 20 mg during 0–24, 0–4, and 14–24 h intervals. Significant differences were observed in both TpH≥6 (%) and its Δ-values between dosage groups during 0–24, 0–4, 4–10, and 14–24 h intervals. Statistically significant higher values of novel PD parameter (area under pH-time curve from τ1 to τ2) were obtained with the 30 mg group compared with the 20 mg group during 0–24, 0–4, and 14–24 h intervals. Corresponding Δ-values for , , , and were significantly higher with the 30 mg group vs. the 20 mg group. No statistically significant difference of above-mentioned PD parameters and Δ-values was observed between the 20 and 30 mg groups during 10–14 h post-dosing.
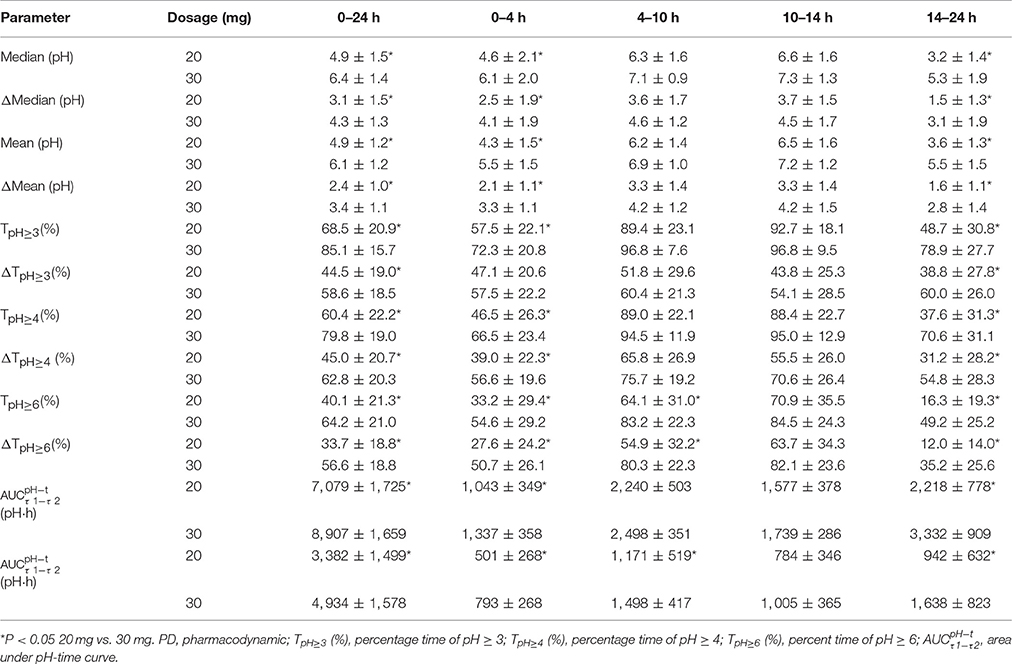
Table 3. PD parameters after single intravenous administration of dexlansoprazole 20 or 30 mg (Mean ± SD).
PD parameters divided by CYP2C19 phenotype in the 30 mg group are shown in Figures 3A1–A4, and mean values are summarized in Table 4. No significant differences in PD parameters were noticed between CYP2C19 homEMs, hetEMs, and PMs across all time periods, except that TpH≥6 (%) was higher with PMs than homEMs during the 14–24 h interval. However, analysis in Δ-values for mean (pH), median (pH), TpH≥3 (%), TpH≥4 (%), TpH≥6 (%), and showed statistically significant variations between homEMs and PMs during the 0–24 and 14–24 h intervals. Statistically significant difference between hetEMs and homEMs were found in Δ-TpH≥6 (%) during the 14–24 h interval additionally.
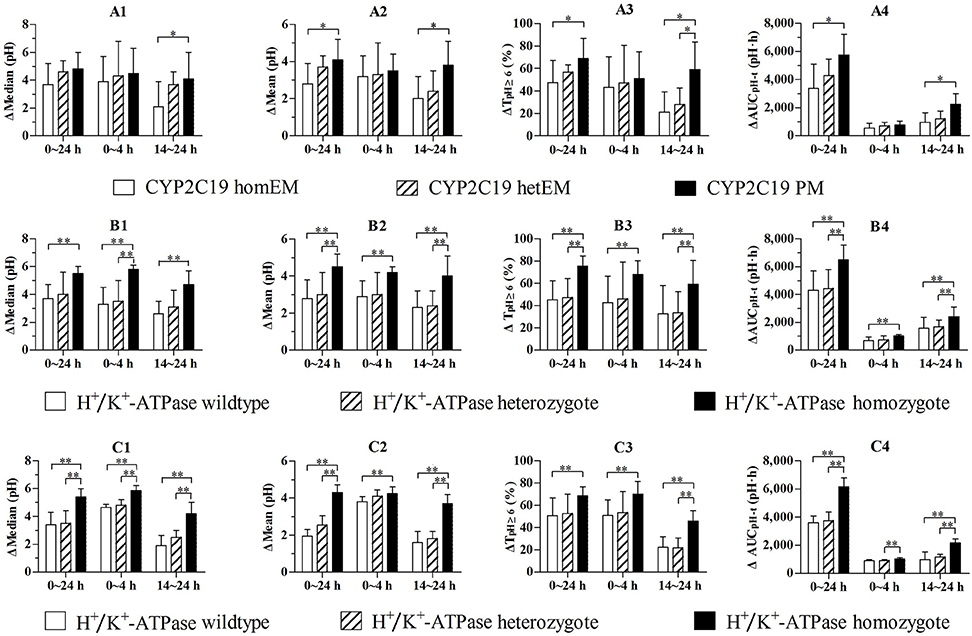
Figure 3. (A1–A4) Comparison of baseline-adjusted PD parameters by CYP2C19 phenotype following single intravenous administration of 30 mg (n = 7, 10, 11 for CYP2C19 wildtype, heterozygote, and homozygote). (B1–B4) Comparison of baseline-adjusted PD parameters of subjects in the 30 mg group by H+/K+-ATPase genotype (n = 8, 10, 10 for H+/K+-ATPase wildtype, heterozygote, and homozygote). (C1–C4) Comparison of baseline-adjusted PD parameters of CYP2C19 EMs by H+/K+-ATPase genotype (n = 6, 8, 7 for H+, K+-ATPase wildtype, heterozygote, and homozygote). *P < 0.05 vs. CYP2C19 PM, **P < 0.05 vs. H+/K+-ATPase homozygote.
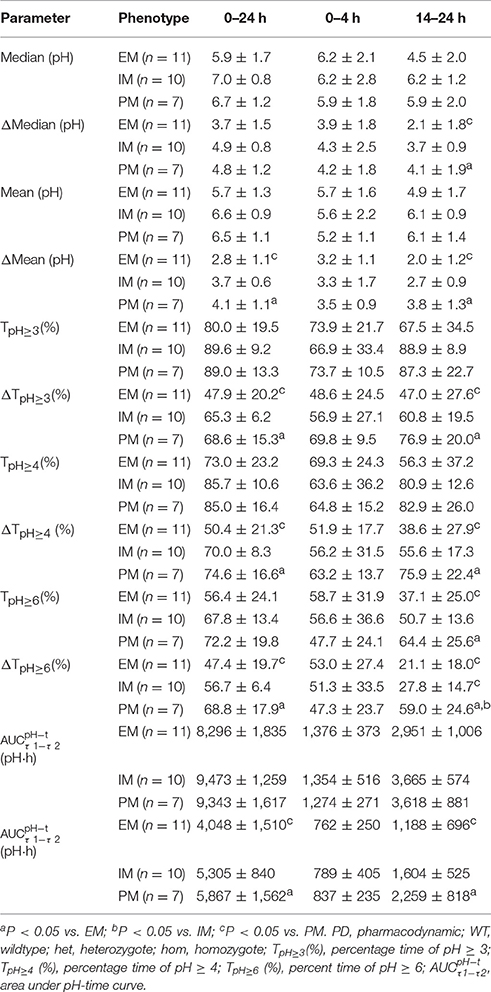
Table 4. PD parameters by CYP2C19 phenotype after single intravenous administration of dexlansoprazole 30 mg (Mean ± SD).
PD parameters divided by gastric H+/K+-ATPase rs2733743 wildtypes, heterozygotes and homozygotes in the 30 mg group are shown in Figures 3B1–B4, and mean values are summarized in Table 5. No significant differences in PD parameters were noticed between gastric H+/K+-ATPase homEMs, hetEMs, and PMs across all time periods. However, analysis in Δ-values for mean (pH), median (pH), TpH≥3 (%), TpH≥4 (%), TpH≥6 (%), and showed statistically significant variation between homEMs and PMs during 0–24, 0–4, and 14–24 h intervals. Statistically significant differences between hetEMs and homEMs were found in Δ-median pH, Δ-mean pH, Δ-TpH≥6 (%), and Δ- during 0–24, 14–24, or 0–4 h intervals, respectively.
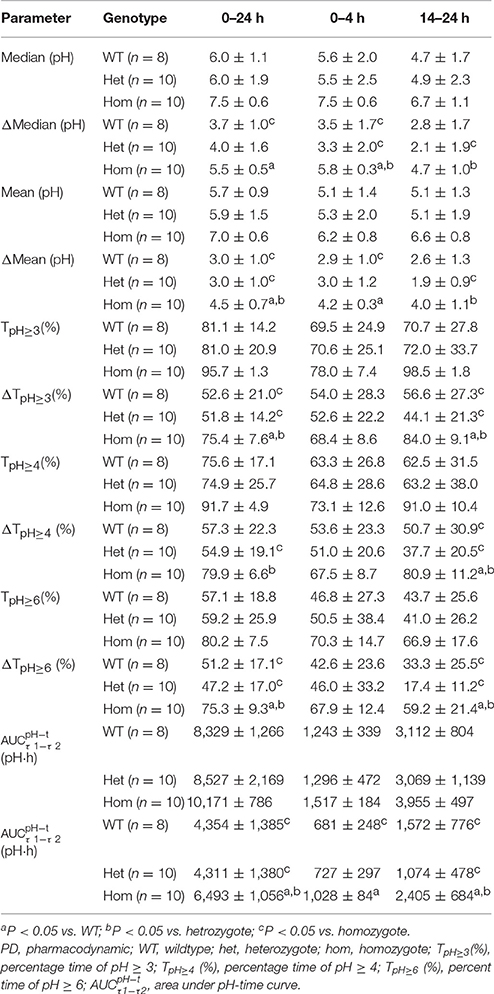
Table 5. PD parameters by H+/K+-ATPase genotype after single intravenous administration of dexlansoprazole 30 mg (Mean ± SD).
PD parameters divided by gastric H+/K+-ATPase rs2733743 wildtypes, heterozygotes, and homozygotes based on CYP2C19 EMs in the 30 mg group are shown in Figures 3C1–C4, and mean values are summarized in Table 6. No significant differences in PD parameters were noticed between gastric H+/K+-ATPase homEMs, hetEMs, and PMs across all time periods, except that TpH≥4 (%) was higher with homEMs than hetEMs during 0–4 h interval. However, analysis in Δ-values for mean (pH), median (pH), TpH≥3 (%), TpH≥4 (%), TpH≥6 (%), and showed statistically significant higher values in gastric H+/K+-ATPase homEMs than those in PMs during 0–24, 0–4, and 14–24 h intervals. Statistically significant differences between hetEMs and homEMs were found in Δ-median pH, Δ-mean pH, Δ-TpH≥6 (%), and Δ- during 0–24, 14–24, or 0–4 h intervals, respectively.
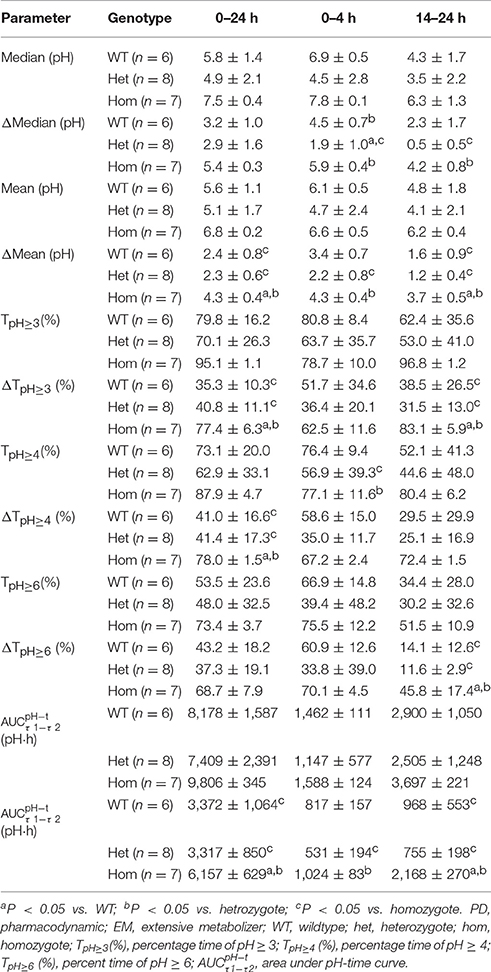
Table 6. PD parameters of CYP2C19 EMs by H+/K+-ATPase genotype after single intravenous administration of dexlansoprazole 30 mg (Mean ± SD).
Discussion
PPIs are acid-liable, so they are usually prepared as enteric-coated formulation to protect from degradation in the stomach when given orally (Horn and Howden, 2005). Oral administrated PPIs in humans are absorbed in the proximal small intestine and suffered from many factors, including preparation disintegrate and release, gastrointestinal motility rate, and the first-pass metabolism (Vanderhoff and Tahboub, 2002; Horn and Howden, 2005; Zhang et al., 2011; Chai et al., 2017). The pharmacokinetics of PPIs after oral administration, compared with IV administration, are affected by the dosage form, food and drink, gastrointestinal physiological conditions, and other various factors (Vanderhoff and Tahboub, 2002; Horn and Howden, 2005; Zhang et al., 2011; Chai et al., 2017). Interindividual variation of PK parameters after oral administration were larger than that after IV route (Amer et al., 2004; Freston et al., 2004; Thota et al., 2013). In order to decrease the influence of drug absorption and the first-pass metabolism on the inhibition of acid secretion by PPIs, the intravenous infusion was superior to others. Dexlansoprazole was selected to conduct the study to reduce the influence of the stereoselective PK of enantiomers (Katsuki et al., 1996; Metz et al., 2009; Sun et al., 2015).
The interindividual variation of PK parameters of lansoprazole and dexlansoprazole by CYP2C19 phenotype were smaller than that of the un-phenotype group (Qiao et al., 2006; Xu et al., 2010; Li et al., 2014). Moreover, the interindividual variation of PK parameters by CYP2C19 phenotype after IV administration were smaller than that after oral administration. To minimize the impact of metabolic phenotypes of CYP2C19, intravenous infusion of dexlansoprazole injection in CYP2C19 EMs population were optimized to elucidate the relation between SNPs of H+/K+-ATPase and the inhibition of acid secretion.
The gastric acid inhibition of PPIs depends on their irreversible covalent binding to gastric H+/K+-ATPase, leading to a much longer duration of suppression than its plasma half-life (Sachs et al., 2007). Our results showed remarkably higher intragastric pH after dosing in all subjects when compared to their own baseline, particularly during the nocturnal time. The elevation in intragastric pH was dose-dependent and the higher the dose the greater the increments of gastric pH inhibition were observed. So far, whether the efficacy of PPIs is affected by gastric H+/K+-ATPase polymorphism in humans has not been clearly established. Our results reveal that gastric H+/K+-ATPase rs2733743 homozygotes have significantly stronger gastric acid inhibition than wildtypes after administration of dexlansoprazole injection.
Main PK parameters (such as Cmax, AUCPK, and half-life) of dexlansoprazole were higher than the data of lansoprazole injection at the same dosage (Yang et al., 2012). Results of the current study show that t1/2 and AUCPK for CYP2C19 PMs were about 3- to 4-fold of those for CYP2C19 homEMs in the same dose group, which was consistent with previous studies of PK performance affected by CYP2C19 polymorphism after oral and intravenous administration of lansoprazole (Hu et al., 2004; Wang et al., 2012). There were statistically significant differences of baseline-adjusted mean pH (Δ ) and TpH≥6 (%) between PMs and homEMs during the 0–24 or 14–24 h intervals. The stronger and longer acid inhibition in PMs could be explained by much higher Cmax and AUCPK caused by a slower clearance and a higher system exposure. Lansoprazole is also a substrate of ABCB1, but the significant effects of ABCB1 polymorphism on pharmacokinetics and pharmacodynamics of dexlansoprazole have not been found in this study.
Further analysis comparing the pharmacodynamic parameters between gastric H+/K+-ATPase and CYP2C19 genotypes in the dexlansoprazole 30 mg group was aiming to reveal which genotype would suppress gastric acid secretion more strongly. The results showed that there was no significant difference between CYP2C19 homEMs, hetEMs, and PMs during the first 4 h after intravenous administration of dexlansoprazole, while gastric H+/K+-ATPase homozygotes had significantly higher gastric acid inhibition effects of Δ , TpH≥3(%), and TpH≥4 (%) than gastric H+/K+-ATPase heterozygotes or wildtypes. And in the CYP2C19 EMs, we also get the same results in that the subjects classified as gastric H+/K+-ATPase rs2733743 homozygotes had significantly higher gastric acid inhibition effects than those classified as H+/K+-ATPase heterozygotes or wildtypes.
In the summary, we propose that gastric H+/K+-ATPase rs2733743 genotypes have greater effects than CYP2C19 genotypes on the suppression of gastric acid secretion. In the meanwhile, we could explain why some patients could not get satisfactory gastric acid inhibition after a conventional dose of PPIs.
Conclusion
Gastric H+/K+-ATPase polymorphism affects intragastric pH-values after PPIs dosing, which may cause insufficient gastric acid inhibition. Gastric H+/K+-ATPase genotypes had greater effects than CYP2C19 genotypes on the suppression of gastric acid secretion. A single dose of 30 mg dexlansoprazole injection provides more effective acid inhibition compared to 20 mg.
Author Contributions
Each of the authors participated in this research by contributing to the conception and design of the study (YW and YC), study management (YW, LS, YL, HZ, MW, LX, JC, MB, HL, PPZ, XZ, and PZ), performance of laboratory experiments (LS, YF, and ZY), and statistical analysis and interpretation (YW, LS, YL, JW, and LM).
Funding
This project was supported by the grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions, National Natural Sciences Foundation of China (81273593, 81673515, 81503160), Natural Science Foundation of Jiangsu Province (BK20161591), Six Talent Peaks Project in Jiangsu Province (2014-YY-001), Jiangsu Provincial Medical Youth Talent (QNRC2016215), Suzhou science and education Youth Project (KJXW2016067), and Suzhou industrial technology innovation (SYSD2016046).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the contributions from the study sponsor Nanjing Yoko Pharma Co., Ltd.
References
Abe, K., Tani, K., and Fujiyoshi, Y. (2011). Conformational rearrangement of gastric H(+),K(+)-ATPase induced by an acid suppressant. Nat. Commun. 2, 155. doi: 10.1038/ncomms1154
Aller, S. G., Yu, J., Ward, A., Weng, Y., Chittaboina, S., Zhuo, R., et al. (2009). Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718–1722. doi: 10.1126/science.1168750
Amer, F., Karol, M. D., Pan, W. J., Griffin, J. S., Lukasik, N. L., Locke, C. S., et al. (2004). Comparison of the pharmacokinetics of lansoprazole 15- and 30-mg sachets for suspension vs. intact capsules. Clin. Ther. 26, 2076–2083. doi: 10.1016/j.clinthera.2004.12.008
Besancon, M., Simon, A., Sachs, G., and Shin, J. M. (1997). Sites of reaction of the gastric H,K-ATPase with extracytoplasmic thiol reagents. J. Biol. Chem. 272, 22438–22446. doi: 10.1074/jbc.272.36.22438
Chai, R. R., Meng, G. F., Ding, J. N., and Zhu, Q. Q. (2017). Study progress in pharmacokinetics of proton pump inhibitors. China Pharmacist. 20, 331–333. doi: 10.3969/j.issn.1008-049X.2017.02.040
Feng, S., Cleary, Y., Parrott, N., Hu, P., Weber, C., Wang, Y., et al. (2015). Evaluating a physiologically based pharmacokinetic model for prediction of omeprazole clearance and assessing ethnic sensitivity in CYP2C19 metabolic pathway. Eur. J. Clin. Pharmacol. 71, 617–624. doi: 10.1007/s00228-015-1834-y
Freston, J. W., Pilmer, B. L., Chiu, Y. L., Wang, Q., Stolle, J. C., Griffin, J. S., et al. (2004). Evaluation of the pharmacokinetics and pharmacodynamics of intravenous lansoprazole. Aliment. Pharmacol. Ther. 19, 1111–1122. doi: 10.1111/j.1365-2036.2004.01942.x
Gawrońska-Szklarz, B., Adamiak-Giera, U., Wyska, E., Kurzawski, M., Gornik, W., Kaldonska, M., et al. (2012). CYP2C19 polymorphism affects single-dose pharmacokinetics of oral pantoprazole in healthy volunteers. Eur. J. Clin. Pharmacol. 68, 1267–1274. doi: 10.1007/s00228-012-1252-3
Gisbert, J. P., González, L., Calvet, X., Roqu,é, M., Gabriel, R., and Pajares, J. M. (2001). Proton pump inhibitors vs. H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment. Pharmacol. Ther. 15, 917–926. doi: 10.1046/j.1365-2036.2001.01012.x
Gralnek, I. M., Dumonceau, J. M., Kuipers, E. J., Lanas, A., Sanders, D. S., Kurien, M., et al. (2015). Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 47, a1–a46. doi: 10.1055/s-0034-1393172
Hershcovici, T., Jha, L. K., and Fass, R. (2011). Dexlansoprazole MR: a review. Ann. Med. 43, 366–374. doi: 10.3109/07853890.2011.554429
Horn, J. R., and Howden, C. W. (2005). Review article: similarities and differences among delayed-release proton-pump inhibitor formulations. Aliment. Pharmacol. Ther. 3, 20–24. doi: 10.1111/j.1365-2036.2005.02714.x
Hu, Y. R., Qiao, H. L., and Kan, Q. C. (2004). Pharmacokinetics of lansoprazole in Chinese healthy subjects in relation to CYP2C19 genotypes. Acta Pharmacol. Sin. 25, 986–990.
Katsuki, H., Yagi, H., Arimori, K., Nakamura, C., Nakano, M., Katafuchi, S., et al. (1996). Determination of R(+)- and S(-)-lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humans. Pharm. Res. 13, 611–615. doi: 10.1023/A:1016062508580
Katz, P. O. (2006). Review article: intragastric and oesophageal pH monitoring in patients with gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 23(Suppl. 1), 3–11. doi: 10.1111/j.1365-2036.2006.02801.x
Kodaira, C., Sugimoto, M., Nishino, M., Yamade, M., Shirai, N., Uchida, S., et al. (2009). Effect of MDR1 C3435T polymorphism on lansoprazole in healthy Japanese subjects. Eur. J. Clin. Pharmacol. 65, 593–600. doi: 10.1007/s00228-009-0625-8
Lau, J. Y., Barkun, A., Fan, D. M., Kuipers, E. J., Yang, Y. S., and Chan, F. K. (2013). Challenges in the management of acute peptic ulcer bleeding. Lancet 381, 2033–2043. doi: 10.1016/S0140-6736(13)60596-6
Li, C. Y., Zhang, J., Chu, J. H., Xu, M. J., Ju, W. Z., Liu, F., et al. (2014). A correlative study of polymorphisms of CYP2C19 and MDR1 C3435T with the pharmacokinetic profiles of lansoprazole and its main metabolites following single oral administration in healthy adult Chinese subjects. Eur. J. Drug Metab. Pharmacokinet. 39, 121–128. doi: 10.1007/s13318-013-0148-7
Li, H., Meng, L., Liu, F., Wei, J. F., and Wang, Y. Q. (2013). H+/K+-ATPase inhibitors: a patent review. Expert Opin. Ther. Pat. 23, 99–111. doi: 10.1517/13543776.2013.741121
Metz, D. C., Vakily, M., Dixit, T., and Mulford, D. (2009). Review article: dual delayed release formulation of dexlansoprazole MR, a novel approach to overcome the limitations of conventional single release proton pump inhibitor therapy. Aliment. Pharmacol. Ther. 29, 928–937. doi: 10.1111/j.1365-2036.2009.03984.x
Mullin, J. M., Gabello, M., Murray, L. J., Farrel, C. P., Bellows, J., Wolov, K. R., et al. (2009). Proton pump inhibitors: actions and reactions. Drug Discov. Today 14, 647–660. doi: 10.1016/j.drudis.2009.03.014
Oksanen, A. M., Lemmelä, S. M., Järvelä, I. E., and Rautelin, H. I. (2006). Sequence analysis of the genes encoding for H+/K+-ATPase in autoimmune gastritis. Ann. Med. 38, 287–293. doi: 10.1080/07853890600673260
Qiao, H. L., Hu, Y. R., Tian, X., Jia, L. J., Gao, N., Zhang, L. R., et al. (2006). Pharmacokinetics of three proton pump inhibitors in Chinese subjects in relation to the CYP2C19 genotype. Eur. J. Clin. Pharmacol. 62, 107–112. doi: 10.1007/s00228-005-0063-1
Sachs, G., Shin, J. M., Vagin, O., Lambrecht, N., Yakubov, I., and Munson, K. (2007). The gastric H,K ATPase as a drug target: past, present, and future. J. Clin. Gastroenterol. 41(Suppl. 2), S226–S242. doi: 10.1097/MCG.0b013e31803233b7
Shin, J. M., and Sachs, G. (2004). Differences in binding properties of two proton pump inhibitors on the gastric H+,K+-ATPase in vivo. Biochem. Pharmacol. 68, 2117–2127. doi: 10.1016/j.bcp.2004.07.035
Shin, J. M., and Sachs, G. (2006). Gastric H,K-ATPase as a drug target. Dig. Dis. Sci. 51, 823–833. doi: 10.1007/s10620-005-9042-8
Sun, L., Cao, Y., Jiao, H., Fang, Y., Yang, Z., Bian, M., et al. (2015). Enantioselective determination of (R)- and (S)-lansoprazole in human plasma by chiral liquid chromatography with mass spectrometry and its application to a stereoselective pharmacokinetic study. J. Sep. Sci. 38, 3696–3703. doi: 10.1002/jssc.201500653
Thota, S., Khan, S. M., Tippabhotla, S. K., Battula, R., Gadiko, C., and Vobalaboina, V. (2013). Bioequivalence of two lansoprazole delayed release capsules 30 mg in healthy male volunteers under fasting, fed and fasting-applesauce conditions: a partial replicate crossover study design to estimate the pharmacokinetics of highly variable drugs. Drug Res. (Stuttg). 63, 551–557. doi: 10.1055/s-0033-1347236
Vakily, M., Zhang, W., Wu, J., Atkinson, S. N., and Mulford, D. (2009). Pharmacokinetics and pharmacodynamics of a known active PPI with a novel Dual Delayed Release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trials. Curr. Med. Res. Opin. 25, 627–638. doi: 10.1185/03007990802693883
Vanderhoff, B. T., and Tahboub, R. M. (2002). Proton pump inhibitors: an update. Am. Fam. Physician. 66, 273–281.
Wang, Y., Yuan, Y., Meng, L., Fan, H., Xu, J., Zhang, H., et al. (2011). Study of the pharmacokinetics and intragastric pH of rabeprazole given as successive intravenous infusion to healthy Chinese subjects. Eur. J. Clin. Pharmacol. 67, 25–31. doi: 10.1007/s00228-010-0949-4
Wang, Y., Zhang, P., Jiang, N., Gong, X., Wang, D., Tang, N., et al. (2010). Influence of CYP2C19 on the relationship between pharmacokinetics and intragastric pH of omeprazole administered by successive intravenous infusions in Chinese healthy subjects. Eur. J. Clin. Pharmacol. 66, 563–569. doi: 10.1007/s00228-010-0821-6
Wang, Y., Zhang, P., Jiang, N., Gong, X., Wang, D., Tang, N., et al. (2012). Influence of CYP2C19 on the pharmacokinetics of lansoprazole administrated by single and successive intravenous infusion in healthy Chinese subjects. Drugs Ther. Stud. 2, 9–14. doi: 10.4081/dts.2012.e3
Xu, H. R., Chen, W. L., Li, X. N., and Chu, N. N. (2010). The effect of CYP2C19 activity on pharmacokinetics of lansoprazole and its active metabolites in healthy subjects. Pharm. Biol. 48, 947–952. doi: 10.3109/13880200903300220
Yang, M., Liu, M., Zhang, Y., Wang, X., Xiao, X., and Liu, H. (2012). Pharmacokinetics of lansoprazole and its main metabolites after single intravenous doses in healthy Chinese subjects. Xenobiotica 42, 1156–1162. doi: 10.3109/00498254.2012.687119
Keywords: gastric H+/K+-ATPase, gastric acid, pharmacokinetics, pharmacogenomics, CYP2C19
Citation: Sun L-N, Cao Y, Li Y-Q, Fang Y-Q, Zhang H-W, Wang M-F, Xie L-J, Chen J, Yang Z-C, Bian M-L, Li H, Zhang P-P, Wei J-F, Meng L, Zhang X-H, Zhao P and Wang Y-Q (2017) Impact of Gastric H+/K+-ATPase rs2733743 on the Intragastric pH-Values of Dexlansoprazole Injection in Chinese Subjects. Front. Pharmacol. 8:670. doi: 10.3389/fphar.2017.00670
Received: 06 July 2017; Accepted: 07 September 2017;
Published: 22 September 2017.
Edited by:
Ruixin Zhu, Tongji University, ChinaReviewed by:
Su-Jun Lee, Inje University, South KoreaRohit Gundamaraju, University of Tasmania, Australia
Copyright © 2017 Sun, Cao, Li, Fang, Zhang, Wang, Xie, Chen, Yang, Bian, Li, Zhang, Wei, Meng, Zhang, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Qing Wang, d3lxanNwaEAxNjMuY29t
†These authors have contributed equally to this work as co-first authors.
 Lu-Ning Sun
Lu-Ning Sun Yang Cao2†
Yang Cao2† Yue-Qi Li
Yue-Qi Li Yong-Qing Wang
Yong-Qing Wang