- 1Department of Biological and Medical Physics, Moscow Institute of Physics and Technology, Dolgoprudny, Russia
- 2Institute of Biology, Komi Scientific Center of Ural Branch of RAS, Syktyvkar, Russia
- 3Department of Ecology, Syktyvkar State University, Syktyvkar, Russia
- 4Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
- 5Insilico Medicine, Inc., Johns Hopkins University, Baltimore, MD, United States
Flavonoids is an intensively studied group of natural compounds with antioxidant, antineoplastic, antihyperglycemic, cardioprotective, and neuroprotective properties. The present study intends to investigate the geroprotective action of three selected flavonoids (naringin, luteolin, chrysin) in two model organisms, Caenorhabditis elegans and Drosophila melanogaster. Luteolin and chrysin were shown to improve lifespan parameters when administered to both model organisms. The observed positive effects of these flavonoids in D. melanogaster were limited to females and were not associated with reduced fecundity or locomotor impairment. The life-extending effects of flavonoids were observed in N2 wild-type worms but absent in aak-2(gt33) mutants implying that these effects can be associated with AMP-activated protein kinase activity. Naringin improved lifespan parameters of C. elegans, but had no effect on D. melanogaster females; in some cases, naringin was found to decrease the lifespan of males. Compared to chrysin and luteolin, however, naringin more effectively activates Nrf2 target genes (particularly, GstD1) under oxidative stress. Then we compared molecular mechanisms of studied compounds and a well-known geroprotector rapamycin, using software tool GeroScope. There are no transcriptomic data on luteolin or chrysin provided by LINCS Project database. The bioinformatics comparison of transcriptomics data for A549 and MCF7 human cell lines treated with rapamycin or naringin revealed that these compounds share just a few common signaling pathways and quite distinct in their geroprotective action. Thus, based on C. elegans effects of naringin, luteolin, chrysin on lifespan we have revealed new potential geroprotectors.
Introduction
Experiments conducted on various model organisms have shown that it is possible to pharmacologically modify the activity of longevity-associated signaling pathways. Flavonoids are a group of naturally occurring biologically active compounds (Zern and Fernandez, 2005; Vauzour et al., 2008; Kumar and Pandey, 2013; Brodowska, 2017). The geroprotective effects of different flavonoids on model organisms have been explored in numerous studies, the majority of which use Caenorhabditis elegans as a model organism to investigate the effects of flavanols, flavonols, and plant extracts (Pallauf et al., 2017). However, this area of research has not been without contention. Experiments involving the use of one compound have produced inconsistent or controversial results when performed on multiple model organisms. For example, quercetin was found to increase the lifespan of Saccharomyces cerevisiae (Belinha et al., 2007) and C. elegans (Kampkotter et al., 2008; Saul et al., 2008; Pietsch et al., 2009), but had no effect on mice (Jones and Hughes, 1982; Spindler et al., 2013). Those studies highlights the importance of the reproducibility of a compound’s geroprotective effects among different model organisms, particularly given that longevity-associated signaling pathways are highly evolutionarily conservative (Moskalev et al., 2016). For example, the increased lifespan of multiple organisms was observed for the anti-inflammatory drug ibuprofen (He et al., 2014) and the immunosuppressant rapamycin (Harrison et al., 2009; Bjedov et al., 2010; Robida-Stubbs et al., 2012).
Recently, it was reported that the flavone baicalein increases the lifespan and stress resistance of C. elegans (Havermann et al., 2013, 2016). Flavones are a subgroup of flavonoids with antineoplastic (Seelinger et al., 2008), anti-inflammatory (Ahad et al., 2014), and antihyperglycemic properties (Vinayagam and Xu, 2015). To further investigate the geroprotective activity of this subgroup, we first verified their effects on C. elegans lifespan via two other flavones, luteolin (3′,4′,5,7-tetrahydroxyflavone), and chrysin (5,7-dihydroxyflavone). Luteolin is abundant in the human diet, contained in foods like broccoli, carrots, parsley, and bird’s eye chilies, among others (Miean and Mohamed, 2001). Chrysin is less prevalent in the human diet but can be found in honey and propolis (Gambelunghe et al., 2003). The impact of those flavones on resistance of two model organisms to different stress conditions was also investigated. Subsequently, we comprehensibly examined Drosophila melanogaster as a model organism, studying various physiological parameters (fecundity, spontaneous activity) in addition to lifespan assays. To explain the observed effects, we also investigated the molecular mechanisms of flavonoids’ action using RT-PCR method as well as C. elegans mutant and D. melanogaster transgenic strains. It is known that flavonoids can influence evolutionary conserved signaling pathways. For example, they can induce (AMP)-activated protein kinase (AMPK) (Hwang et al., 2011; Pu et al., 2012; Shao et al., 2012). Thus we performed experiments with TG38 aak-2(gt33) C. elegans strain, which has a deletion in the aak2 gene. This gene codes one out of two homologs of alpha catalytic subunits of mammalian AMPK (Lee et al., 2008). In D. melanogaster the influence of compounds on AMPK gene expression was measured. Using D. melanogaster model we also investigated the effects of selected flavonoids on Keap1/Nrf2 signaling pathway by measuring the expression values of certain genes coding proteins of this pathway and expression level of GstD1-GFP reporter that is Nrf2 target under both stress and non-stress conditions. We have extended our study to include naringin, a flavanone-7-O-glycoside found in citrus fruits and juices, since naringenin has recently been shown to have hormetic effects on D. melanogaster longevity (Chattopadhyay et al., 2016) and glycosidic forms of flavonols are also known to be bioactive (Lee et al., 2015). As the hormetic effects are usually considered as the ability to induce cell stress defense program in the absence of stress, the effects of selected flavonoids on expression of some stress response genes was studied.
Materials and Methods
C. elegans Lifespan Assay
In this experiment, two C. elegans strains were used; the Bristol strain N2, kindly provided by Yelena Budovskaya (University of Amsterdam), and the TG38 aak-2(gt33) strain, kindly provided by Alexander Mironov (Engelhardt Institute of Molecular Biology, Moscow). The experiments were performed in liquid culture at 20°C according to a slightly modified version of the protocol set forth by Solis and Petrascheck (2011). In brief, worms were synchronized with a bleach solution. After hatching, they were placed in 96-well plates with an S-complete solution and Escherichia coli OP50 as a food source. To prevent the production of progeny, a 0.12 mM solution of 5-fluoro-2′-deoxyuridine (FUDR) (Sigma) was added to C. elegans approximately 45 h after adding the bacteria to L1 worms. Amphotericin B was used at a final concentration of 0.1 μg/mL. To prevent starvation, 5 μl of OP50 (2 × 109 CFU/ml) was added to each well on the 5th, 12th, and 19th day of the worms’ adulthood. The DMSO stock solutions of luteolin (Sigma), chrysin (Acros Organics) and naringin (Acros Organics) were added to N2 nematodes at final concentrations 0.5, 1, 5, 10, and 100 μM. The experiments on the aak-2(gt33) mutant strain were performed using the above concentrations of flavonoids, demonstrating the effect of flavonoids on wild-type worms. This mutant strain has a deletion in the aak2 gene coding, leaving one out of two alpha catalytic subunits of AMPK (Lee et al., 2008). For each experimental group in each experiment 45–110 nematodes were used with the total amount of worms in all performed experiments being no less than 170 for N2 Bristol strain and 110 for TG38 aak-2(gt33) strain. The number of dead worms was counted three times per week. Wells with less than five or more than 15 worms were eliminated to control for the effects of varied food availability. Vibratory perturbation and light exposure were used to induce movement and improve the accuracy of the count. It should be mentioned that in some cases, sediment was observed. The experiment was replicated three times. Some flavonoid concentrations were tested only twice. The statistical analysis was performed for each experiment and for the combined data of all experiments. The survival functions were counted using the Kaplan–Meier method and plotted as survival curves (Kaplan and Meier, 1992). The mean, median, minimum and maximum lifespans as well as the age of 90% mortality were calculated. The statistical significance of differences in survival data was estimated using the non-parametric Kolmogorov–Smirnov (Fleming et al., 1980), Cox–Mantel (Mantel, 1966) and Gehan-Breslow-Wilcoxon (Breslow, 1970) tests. The statistical significance of differences in maximum lifespan (the age of 90% mortality) was calculated using Wang–Allison test (Wang et al., 2004). The aging process was also assessed by calculating age-dependent (α) and initial (R0) mortality rates in the Gompertz equation (μ(x) = R0 eαx) and mortality rate doubling time (MRDT) using the formula MRDT = ln2/α (Finch, 1990). All calculations were performed using Statistica 6.1 (StatSoft, United States) and software environment for statistical computing and graphics R 2.15.1. The effects were considered to be significant if they were observed in at least two out of three experiments in addition to the data from the three experiments combined.
C. elegans Stress Resistance Assay
The experiments to assess stress resistance were performed on the 5th day of flavonoid treatment. In experiments regarding oxidative stress, 100 mM paraquat (Methyl Viologen, Acros Organics) was added to nematodes. In experiments testing heat shock, the worms were heated to 33°C. The number of dead worms was counted after 24 h. For each type of stress, the experiment was performed in triplicates. For each experimental group in each experiment 35–117 nematodes were used with the total amount in all performed experiments being no less than 170 for each variant. The data of three experiments was combined. The statistical comparison of the percentage of dead animals for combined data was made using the Fisher ϕ-test (Fisher, 1954).
D. melanogaster Lifespan Assay
The wild-type Canton-S D. melanogaster (Bloomington Stock Center, United States) were separated by sex and housed in 25 mm × 95 mm vials (30 flies per vial) with sugar-yeast medium of the following composition per 1 L: dry yeast—8 g, agar—7 g, sugar—30 g, semolina—30 g, propionic acid—8 drops (Ashburner, 1989). For each experimental group 100–150 flies were used. The flies were kept at 25°C with a 12-h light/dark cycle. The DMSO stock solutions of the studied compounds were dissolved in the yeast paste at final concentrations or 0.3, 0.5, and 1 μM. These concentrations were chosen as they are considered physiological (Kanazawa, 2011). This mixture was applied to the surface of the nutrient medium starting on the first day of adulthood. The flies were transferred into new bottles twice per week. The number of dead flies was counted no less than three times per week. The experiment was replicated three times. The same statistical analyses as for worms was performed.
D. melanogaster Stress Resistance Assay
The experiments on stress resistance were performed on the 10th day of flavonoid treatment. For each experimental group 100–150 flies were used. In experiments testing oxidative stress, flies were moved into vials with filter paper soaked in a 20 mM paraquat solution (Methyl Viologen, Sigma) in 5% sucrose (0.2 mL per vial) after 2 h of starvation. The flies were starved by transfer into vials with a filter paper saturated with water instead of the nutrient medium. In experiments regarding heat shock stress, the vials containing the flies and the nutrient medium were warmed to 35°C. The number of dead flies was counted twice a day. For each type of stress, the experiment was performed in triplicates. The data of three experiments was combined. The significance of differences between groups for combined data was evaluated with the Fisher ϕ-test (Fisher, 1954).
D. melanogaster Fecundity Assay
The fecundity of females was assessed once per week by placing three females and three males of the same age into vials with fresh nutrient colored with activated carbon and allowing 24 h for egg laying. After 24 h had passed, the number of eggs per female was counted. For each experimental variant, 50 fertile females were used. The males were replaced with young flies once per month. The statistical significance between the cumulative curves was evaluated with the χ2 test using the program Statistica 6.1 (StatSoft, United States) (Fisher, 1954).
D. melanogaster Spontaneous Activity Assay
For each experimental variant, 30 flies were selected (10 flies per vial). Males and females were analyzed separately. The flies were kept in standard conditions and transferred to new media twice per week. The spontaneous activity was tested for 24 h each week with the hardware-software complex “Drosophila Population Monitor” (TriKinetics, Inc., United States). The statistical analyses were performed with the χ2 test using the program Statistica 6.1 (StatSoft, United States) (Fisher, 1954).
RT-PCR
In this test, only 0.3 and 1.0 μM concentrations of the selected compounds were studied. On the 10th day of flavonoid consumption, the flies were homogenized. RNA was extracted using QIAzol Lysis Reagent (Qiagen, Netherlands) with subsequent isopropanol precipitation. RNA was purified with the DNase I, Amplification Grade kit (Life Technologies, Breda, the Netherlands) in accordance with the manufacturer’s protocol. The synthesis of cDNA was performed using Revert Aid reverse transcriptase as recommended by Fermentas. Real-time PCR was carried out on the 7500 Real-Time PCR System (Applied Biosystems, United States) using the following procedure: (1) denaturation for 10 min at 95°C, (2) denaturation for 15 s at 95°C, (3) annealing for 30 s at 60°C, (4) elongation for 30 s at 60°C. Steps 2–4 were repeated 50 times.
EF1α and β-Tubulin were used for expression normalization as they were the most stable among the four tested genes (Actin, RpL32, EF1α, β-Tubulin). The relative expression of the studied genes was calculated as described earlier (Zhikrevetskaya et al., 2015). The primer sequences for studied genes are presented in Supplementary Table 1.
The differences between relative expression values were counted as significant only if they were statistically significant according to the Student’s t-test and at least a twofold change in expression of the studied gene was considered due to biological variations in expression levels of reference genes (Log2FC > 1).
Quantification of GFP Reporter Gene Expression
In this experiment, the D. melanogaster transgenic line with GstD1-GFP reporter was used. The line was kindly provided by Dr. Tower (University of Southern California, Los Angeles, CA, United States).
To measure GstD1-GFP reporter expression, flies were anesthetized and photographed using a fluorescent microscope “MICMED-2 var.11” (LOMO, Russia) and video systems based on the Olympus C7070 digital camera (Olympus, Japan) on the 10th and 30th days of flavonoid consumption. The corrected total cell fluorescence (CTCF) was calculated using ImageJ software (National Institutes of Health, United States) as described elsewhere (Cali et al., 2015). The same assay was performed on flies treated for 12 h with 20 mM paraquat (Methyl Viologen, Sigma) in 5% sucrose (0.2 mL per vial). For each experimental group 10 flies were used.
Pathway-Level Similarity Analysis
Signaling pathway analysis is a common approach to gain insight from large-scale transcriptomic and proteomic data. To obtain signaling pathway activation scores (PASs), we utilized the in silico Pathway Activation Network Decomposition Analysis (iPANDA), which we applied to large-scale transcriptomic datasets as a method for biomarker development (Ozerov et al., 2016). In contrast to other methods, iPANDA generates PASs by using precalculated gene coexpression data in combination with gene importance factors quantified according to the degree of differential gene expression and pathway topology decomposition.
To obtain signaling PASs, we utilized transcriptional response data provided by LINCS Project1. We chose gene expression samples of drug-induced transcriptional response from A549 and MCF7 cell lines. The collection of signaling pathways is obtained from the SABiosciences collection2, in which there are 378 signaling pathways. A total of 15,489 compounds were considered. The similarity between two compounds was measured as a percent of commonly up- or down-regulated pathways. The pathway-level similarity analysis was performed only for naringin as there is no transcriptional response data for chrysin and luteolin.
Results
The Effects of Studied Flavonoids on C. elegans Lifespan
The studied flavonoids had positive effects on median lifespan of nematodes in certain concentrations (Supplementary Table 2). The most pronounced effects of naringin were observed for concentrations of 5 μM (Figure 1A). The addition of naringin in this concentration increased the median lifespan of worms by 7.7–15.4% (p < 0.05) in all performed experiments. The median and maximum lifespan were increased by 6.3–6.7 and 16.6–25% (p < 0.05), respectively, after the addition of luteolin at a concentration of 100 μM (Figure 1B). Chrysin, in a concentration of 10 μM, significantly increased the median lifespan by 6.7–30.7% (p < 0.05) in two experiments out of three (Figure 1C). However, the pro-longevity action of flavonoids was absent among nematodes with the aak2 mutation (Figure 1D). These data suggest that the effects were at least partly associated with AMPK (Supplementary Table 3).
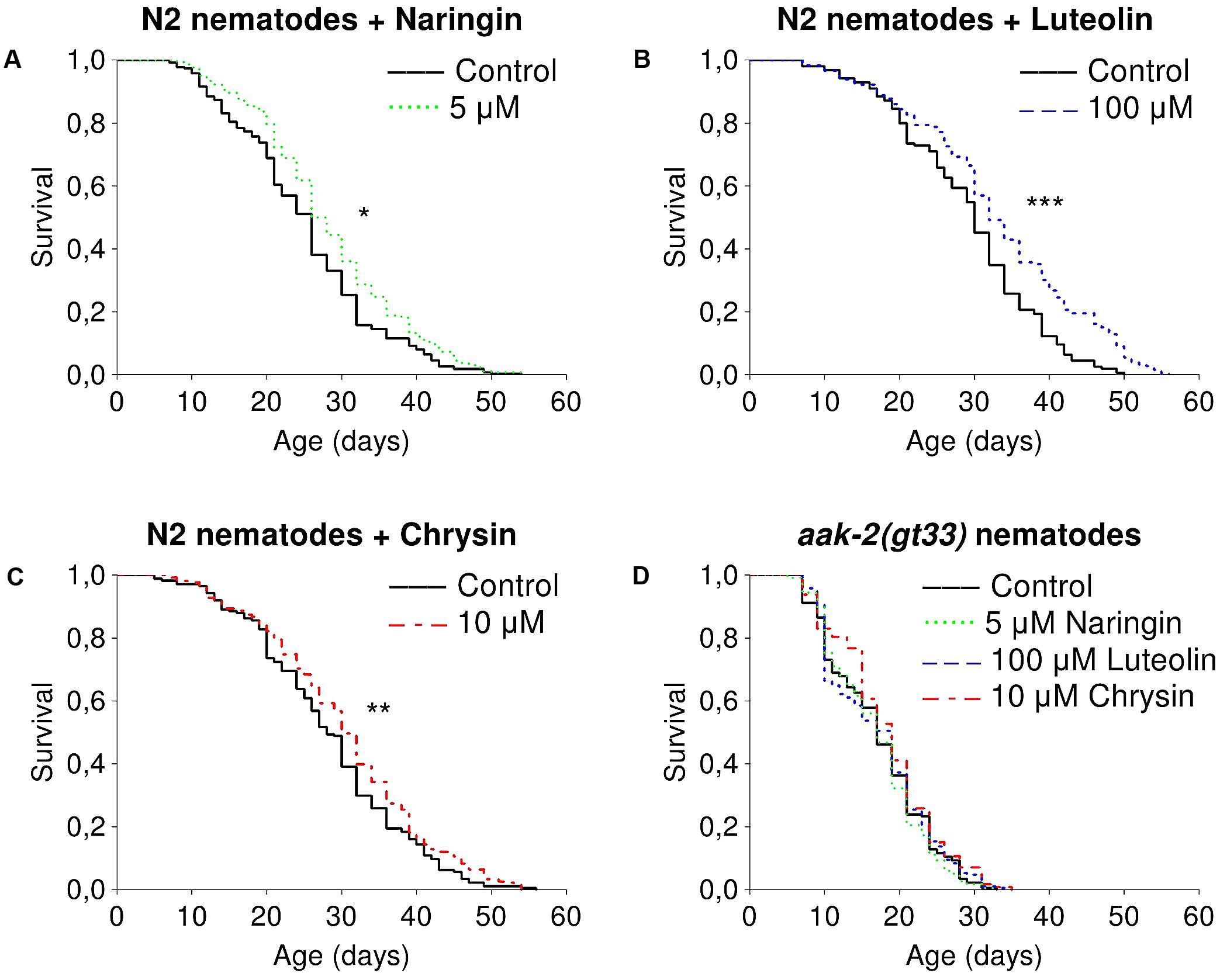
FIGURE 1. Survivorship curves of Caenorhabditis elegans N2 Bristol (A–C) and TG38 aak-2(gt33) (D) strains treated with flavonoids (combined data of two-three independent replicates); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Kolmogorov–Smirnov test.
The Effects of Studied Flavonoids on C. elegans Stress Resistance
Naringin did not affect heat shock resistance in C. elegans (Figure 2B) but increased the number of dead nematodes by 54–111% (p < 0.01) in experiments on oxidative stress (Figure 2A). In most cases, chrysin and luteolin did not significantly affect, or in some cases decreased, the survival rate of nematodes under stress conditions (p < 0.05) (Figures 2C–F).
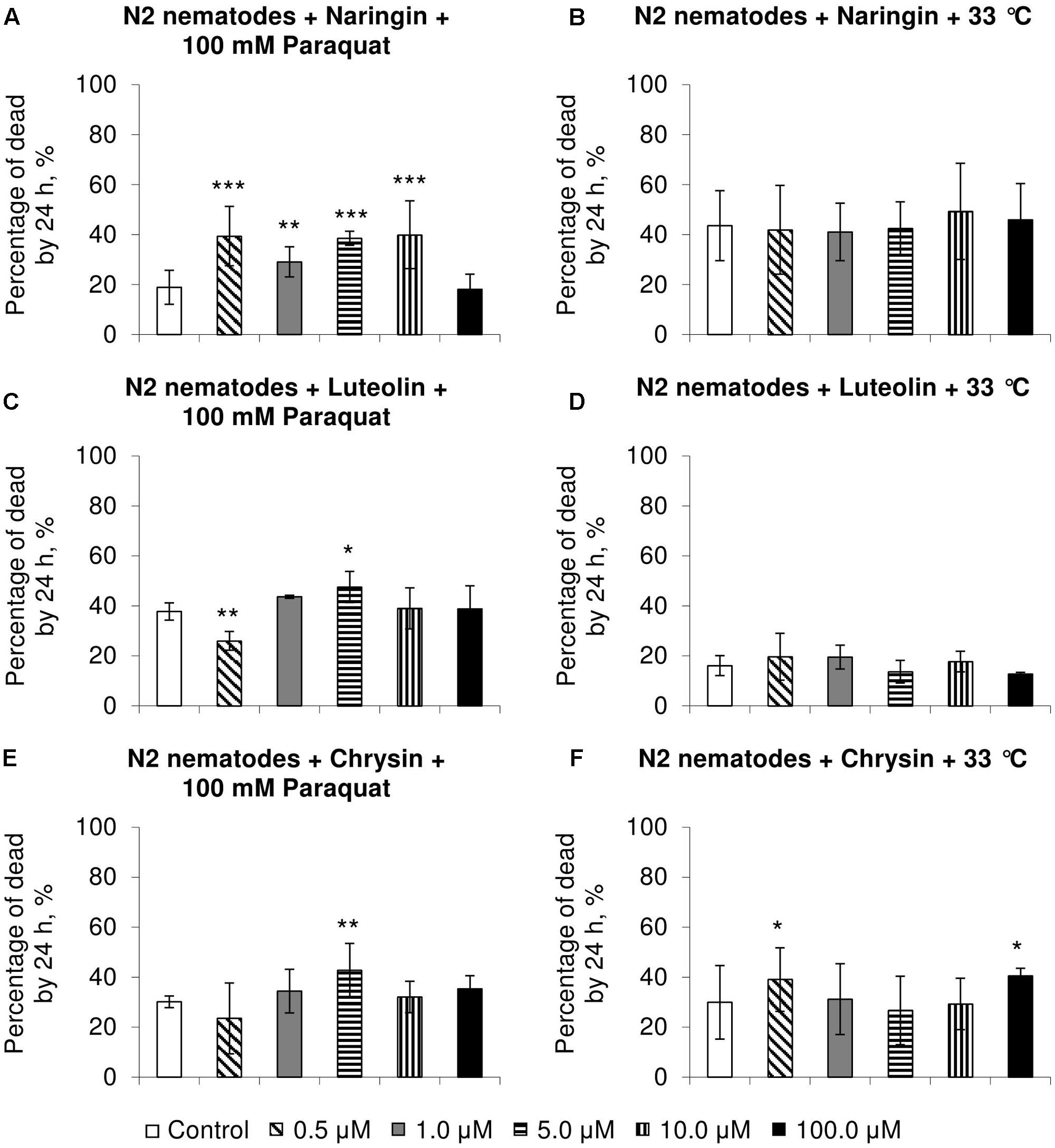
FIGURE 2. Resistance of Caenorhabditis elegans to oxidative stress (100 mM paraquat) (A,C,E) and heat shock (33°C) (B,D,F) after treatment with naringin (A,B), luteolin (C,D), and chrysin (E,F) (combined data of three independent replicates); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Fisher’s exact test.
The Effects of Studied Flavonoids on D. melanogaster Lifespan
Naringin had no significant effect on lifespan parameters of females, but in high concentration (1 μM) decreased the median lifespan of males by 6.5–20% (p < 0.05) in most cases (Supplementary Table 4 and Figure 3A). The addition of luteolin and chrysin, in all studied concentrations, increased the median lifespan of females by 1.8–12.1% (p < 0.01) and the maximum lifespan by 6.2–21.9% (p < 0.05) in most cases (Supplementary Tables 5, 6). However, no significant and stable effects were observed for males (Figures 3B,C).
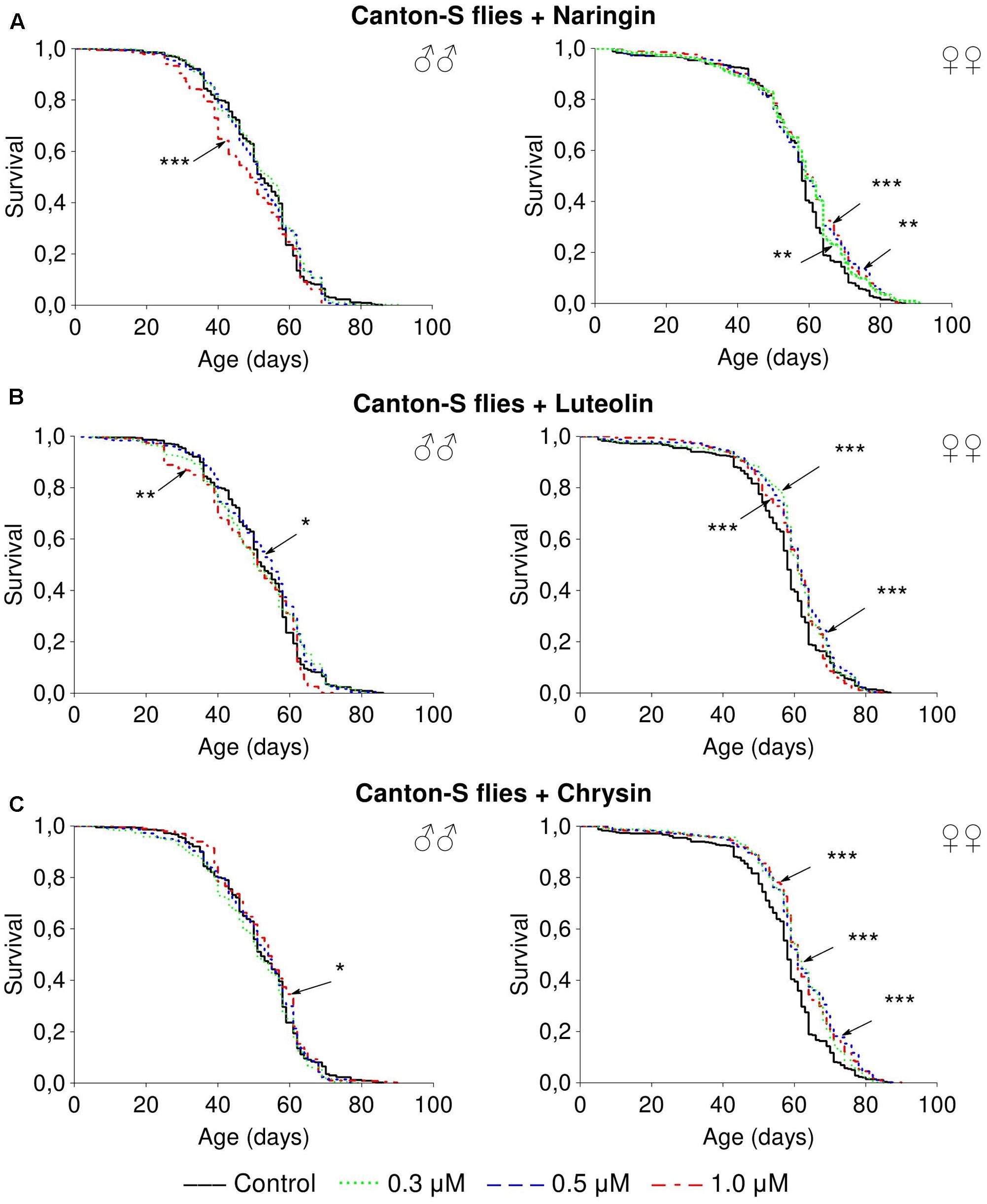
FIGURE 3. Survivorship curves of Drosophila melanogaster males (♂♂) and females (♀♀) treated with naringin (A), luteolin (B), and chrisin (C) (combined data of three independent replications); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, Kolmogorov–Smirnov test.
The Effects of Studied Flavonoids on D. melanogaster Stress Resistance
In most cases, naringin either did not or negatively affected the stress resistance of females (p < 0.05) (Figures 4A, 5A, 6A). In the lowest concentration (0.3 μM), luteolin improved starvation resistance in females (Figure 6B). Other studied concentrations, however, were found to diminish their resistance to both paraquat and heat shock (p < 0.05) (Figures 4B, 5B). Chrysin had no effects on stress resistance of females (Figures 4C, 5C, 6C).
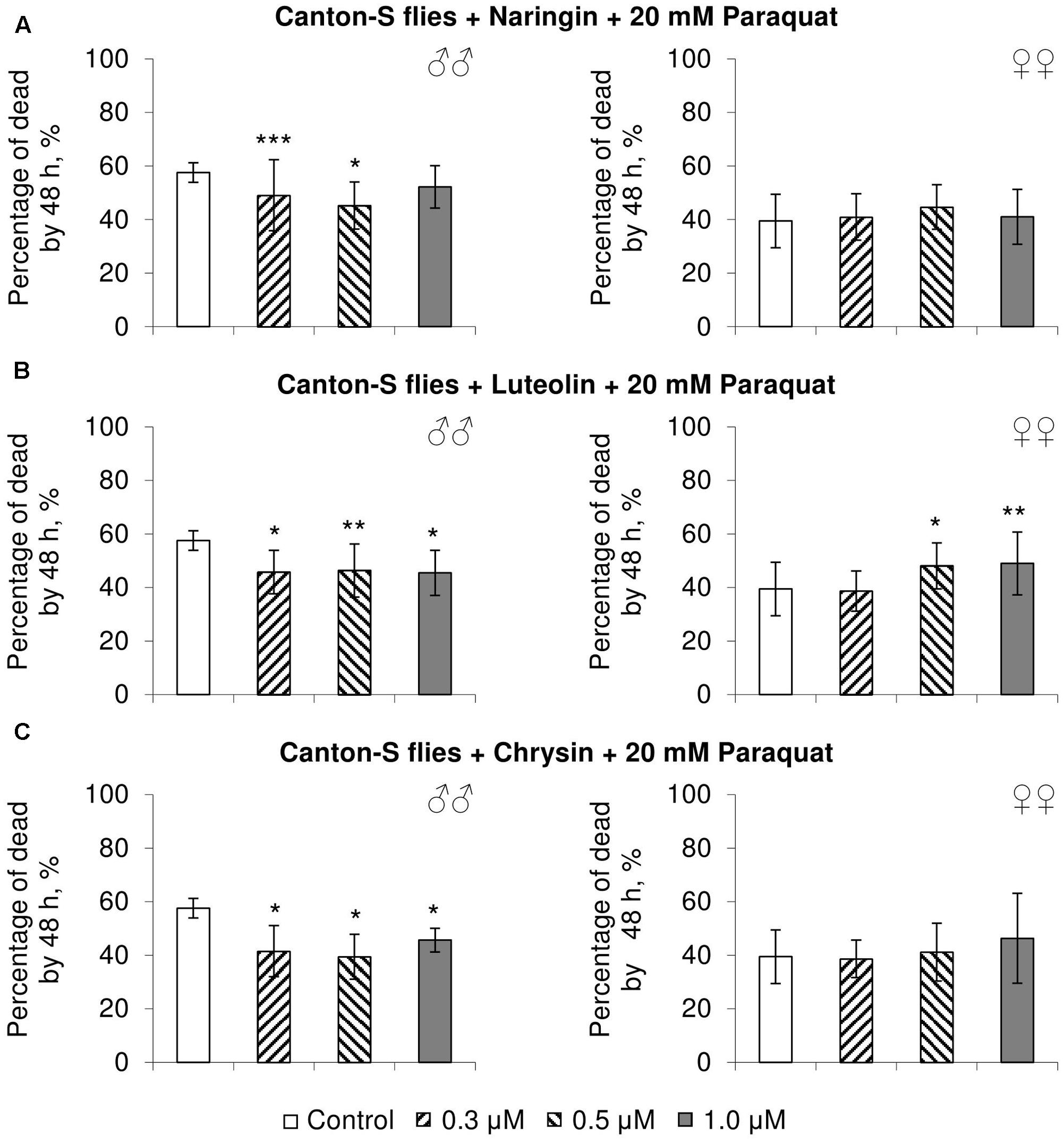
FIGURE 4. Resistance of Drosophila melanogaster males (♂♂) and females (♀♀) to oxidative stress (20 mM paraquat) after treatment with naringin (A), luteolin (B), and chrysin (C) (combined data of three independent replicates); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, Fisher’s exact test.
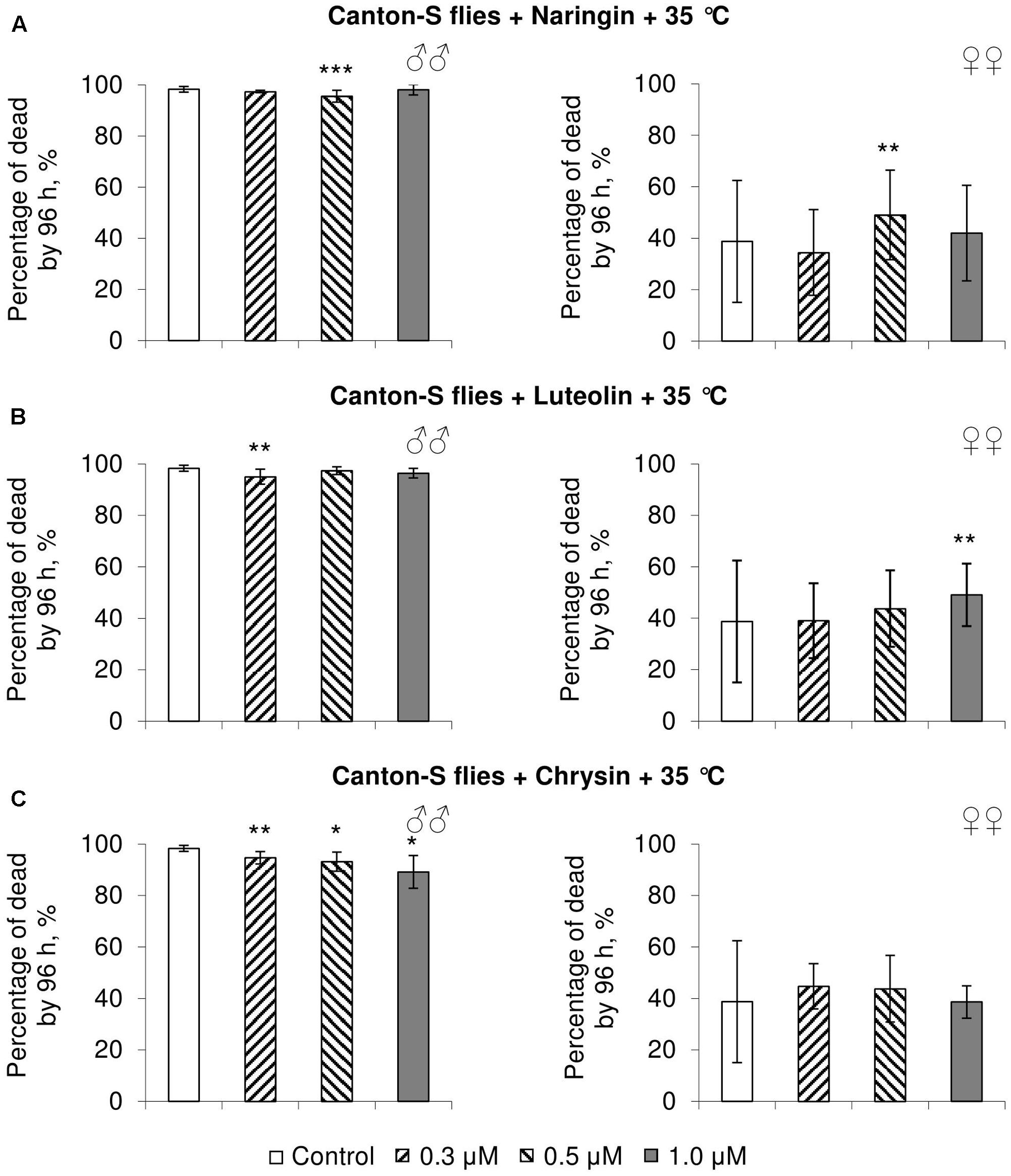
FIGURE 5. Resistance of Drosophila melanogaster males (♂♂) and females (♀♀) to heat shock (35°C) after treatment with naringin (A), luteolin (B), and chrysin (C) (combined data of three independent replicates); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, Fisher’s exact test.
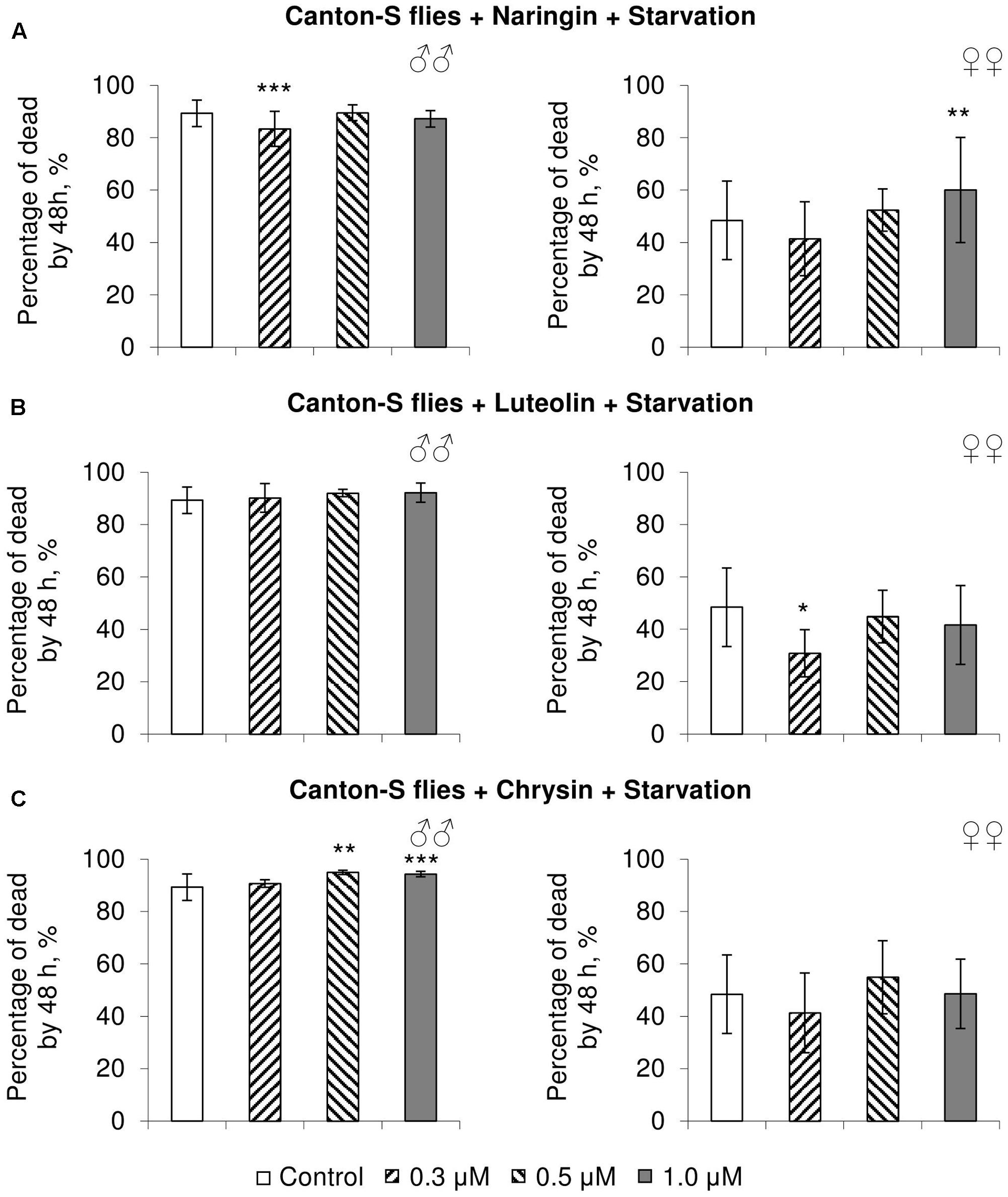
FIGURE 6. Resistance of Drosophila melanogaster males (♂♂) and females (♀♀) to starvation after treatment with naringin (A), luteolin (B), and chrysin (C) (combined data of three independent replicates); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, Fisher’s exact test.
In most concentrations, all three studied compounds were found to reduce the number of dead males by 14.8–31.4% after incubating for 48 h with paraquat (p < 0.05) (Figure 4). The addition of chrysin improved male heat shock resistance by 3.7–9.3% to thermal shock but abated their starvation resistance by 5.6–6.4% (p < 0.05) (Figures 5C, 6C). The effects of both naringin and luteolin on heat shock and starvation resistance in males had in most cases no significance (Figures 5A,B, 6A,B).
The Effects of Studied Flavonoids on Flies’ Fecundity and Locomotor Activity
The addition of the studied compounds, in all tested concentrations, was found to increase the number of eggs laid per female (Figure 7). However, the most remarkable changes as compared to the control flies were observed in older flies. At that time, the number of eggs laid was found to be increased by up to 3.6 times for naringin, 2.9 times for luteolin, and 2.1 times for chrysin (p < 0.05).
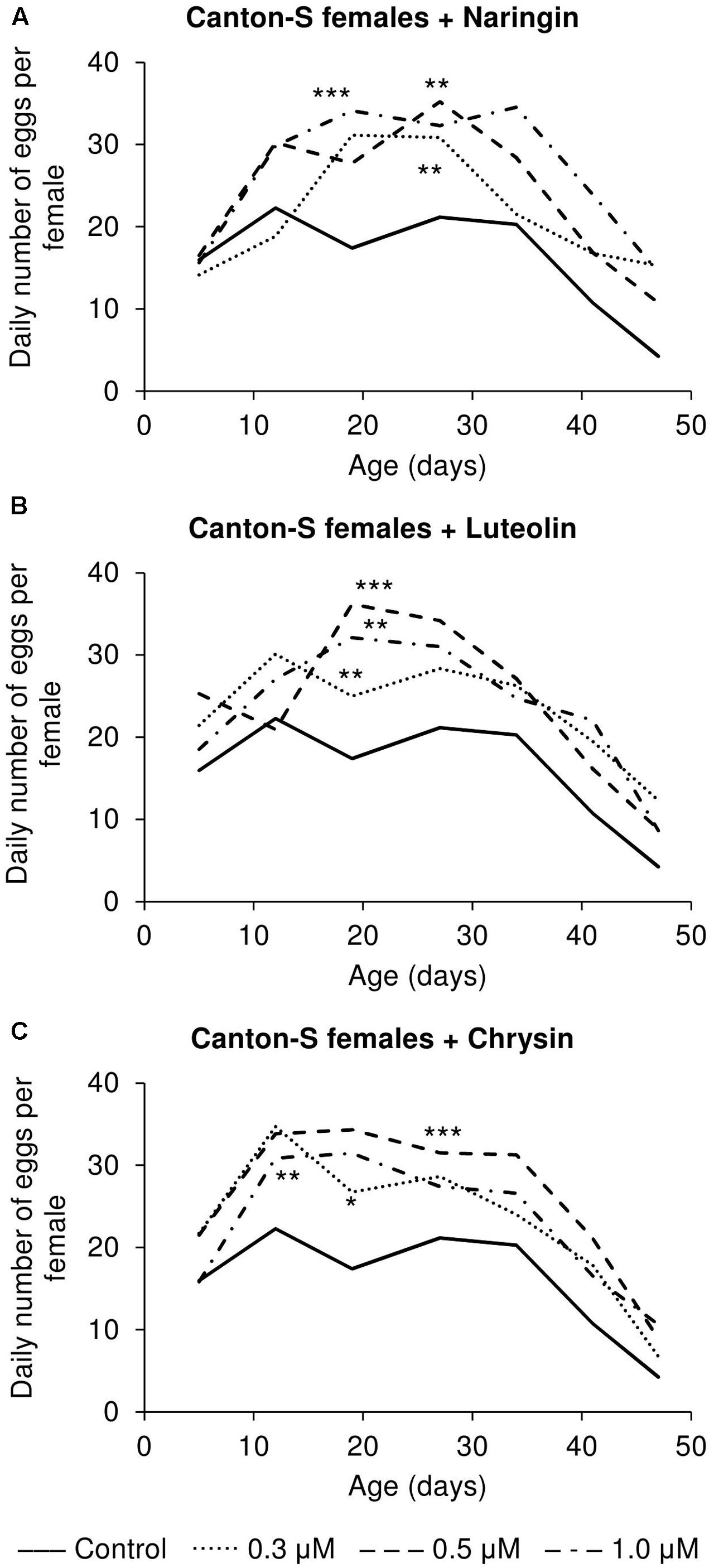
FIGURE 7. Fecundity activity of Drosophila melanogaster females treated with naringin (A), luteolin (B), and chrysin (C); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, χ2 test.
The effects that the tested flavonoids had on spontaneous activity were not so univocal. Even though the statistical analysis revealed changes compared to control flies, it can be concluded that the effects were mainly neutral. While those flavonoids increased the spontaneous activity of both males and females for some time-intervals, they appear to have had deleterious effects over other time periods (Figure 8).
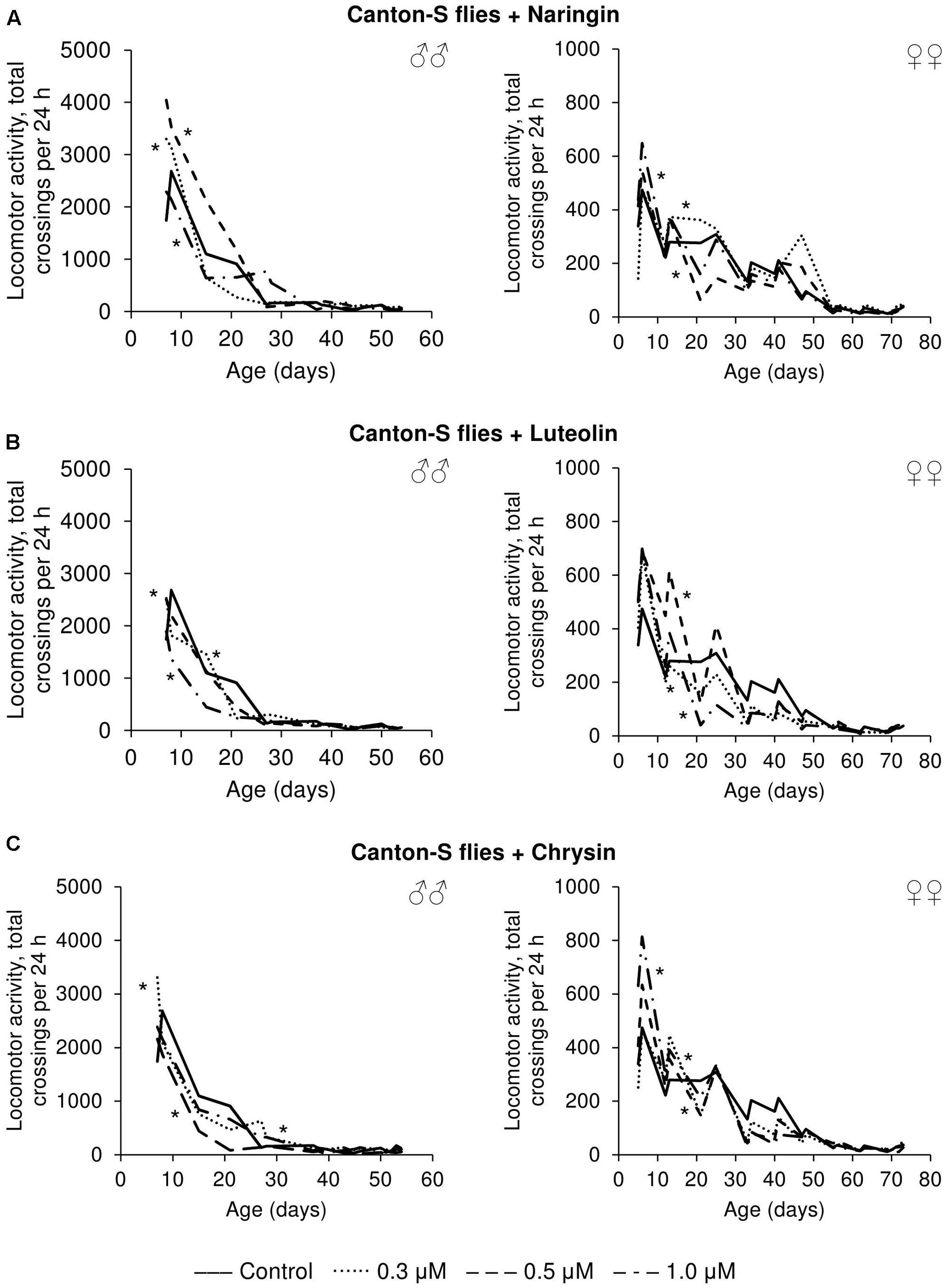
FIGURE 8. Spontaneous locomotor activity of Drosophila melanogaster males (♂♂) and females (♀♀) treated with naringin (A), luteolin (B), and chrysin (C); ∗p < 0.001, χ2 test.
The Effects of Studied Flavonoids on Flies’ Stress Response Genes Expression
The effects of the studied flavonoids on the expression of stress-response genes were investigated. Among 15 studied genes, only the changes in the Hsp70 gene can be considered to be significant (Figure 9). In females, Hsp70 expression levels were decreased by 2.1–5.7 times (p < 0.05) after the addition of all studied concentrations of chrysin and luteolin. The effects were dose-dependent, with higher flavonoid concentration corresponding to a pronounced decrease in the activity of the Hsp70 gene. The same effect was observed in females for high concentrations of naringin. In males, 1 μM naringin was found to reduce the expression of the Hsp70 gene by 6.9 times (p < 0.05). Luteolin and chrysin, however, did not significantly affect this gene in males. The results for all other genes were not considered to be significant.
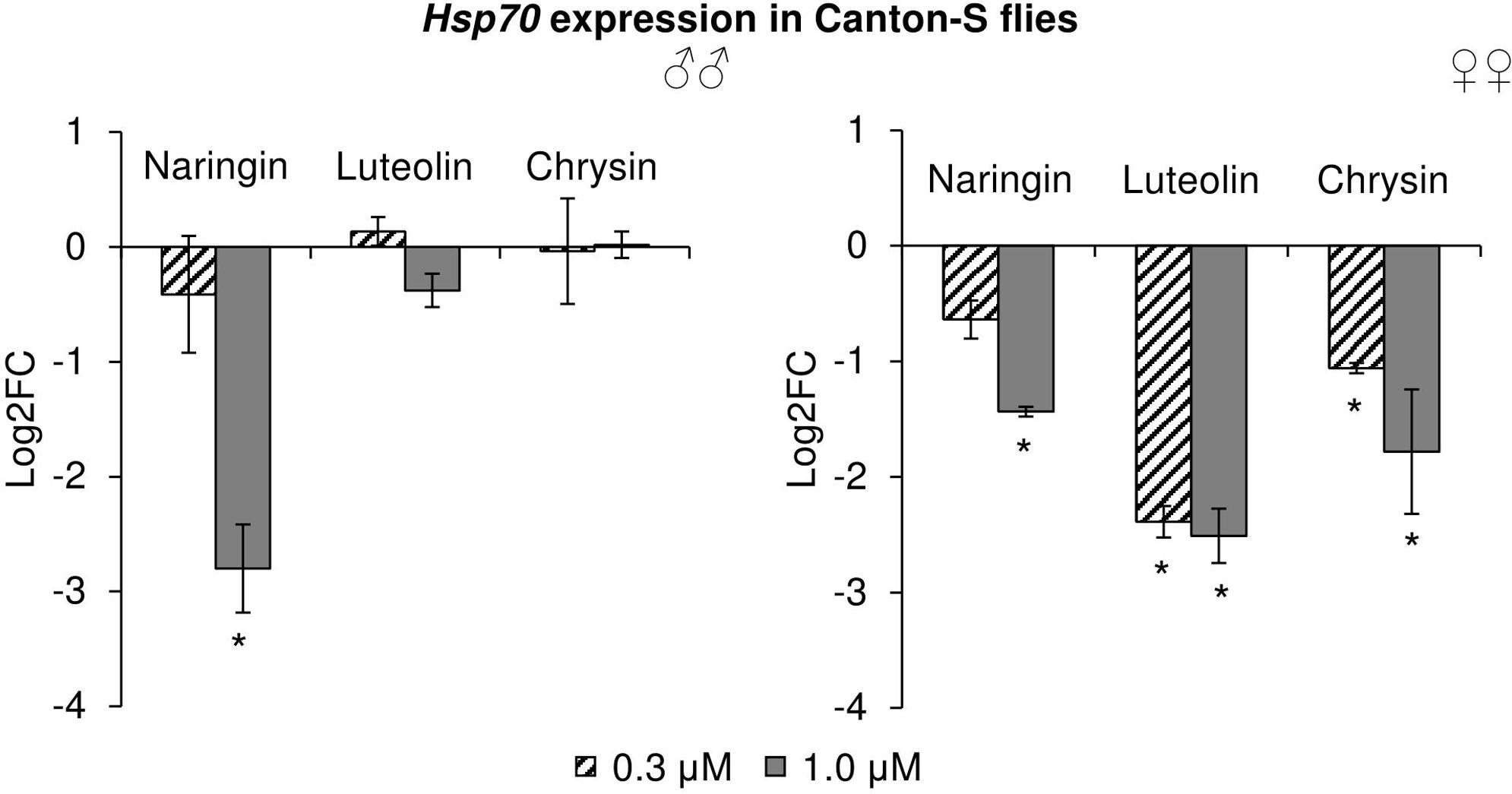
FIGURE 9. Expression of Hsp70 gene in Drosophila melanogaster males (♂♂) and females (♀♀) treated with flavonoids (combined data of three independent replicates); ∗p < 0.001, χ2 test.
The Effects of Studied Flavonoids on Keap1/Nrf2 Signaling Pathway in Flies
The effects of the studied compounds on the Keap1/Nrf2 signaling pathway in flies was tested. No significant effects were observed on the mRNA levels of genes CncC (coding the Nrf2 homolog in flies), Keap1 (coding the CncC inhibitor), or GclC (coding the CncC target gene). GstD1 is another target gene of CncC (Sykiotis and Bohmann, 2008). The flies with the GstD1-GFP reporter gene were used to study the effects of flavonoids on the organism’s ability to induce the expression of the Keap1/Nrf2 signaling pathway target gene under both normal and stress conditions. In most cases, chrysin did not affect GstD1-GFP reporter expression in either males or females under both normal and stress conditions as compared to the GstD1-GFP expression level in either the control group or after 12 h of paraquat treatment (Figures 10C, 11C). In contrast, the expression of this gene was observed to decrease after the addition of luteolin in most cases for both males and females after 10 days of treatment under non-stress conditions (Figures 10B, 11B). No effects were observed for males after the addition of naringin (Figure 10A). However, naringin was found to increase GstD1-GFP reporter level in females under stress conditions after both 10 and 30 days of treatment and in normal conditions after 30 days (Figure 11A). At the same time, it should be noted that 12 h of paraquat treatment on the 10th day of adulthood was not sufficient to significantly increase GstD1-GFP reporter level in control flies. In the case of females treated with those flavonoids, the increase was dramatic. Thus, it can be concluded that these flavonoids improve the speed of Nrf2 target genes activation under stress conditions.
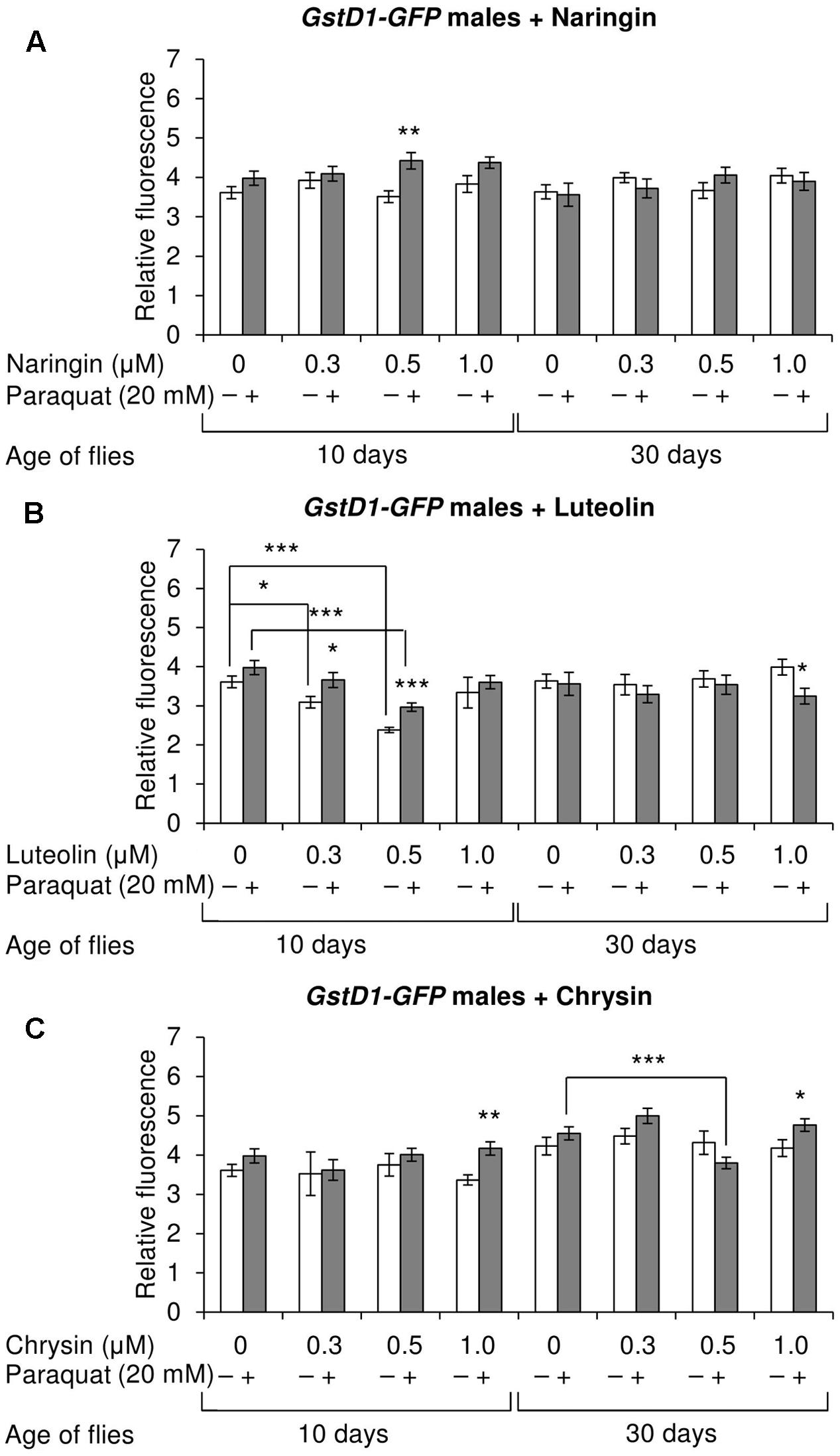
FIGURE 10. Relative fluorescence of GstD1-GFP reporter in Drosophila melanogaster males after treatment with naringin (A), luteolin (B), and chrysin (C); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, χ2 test.
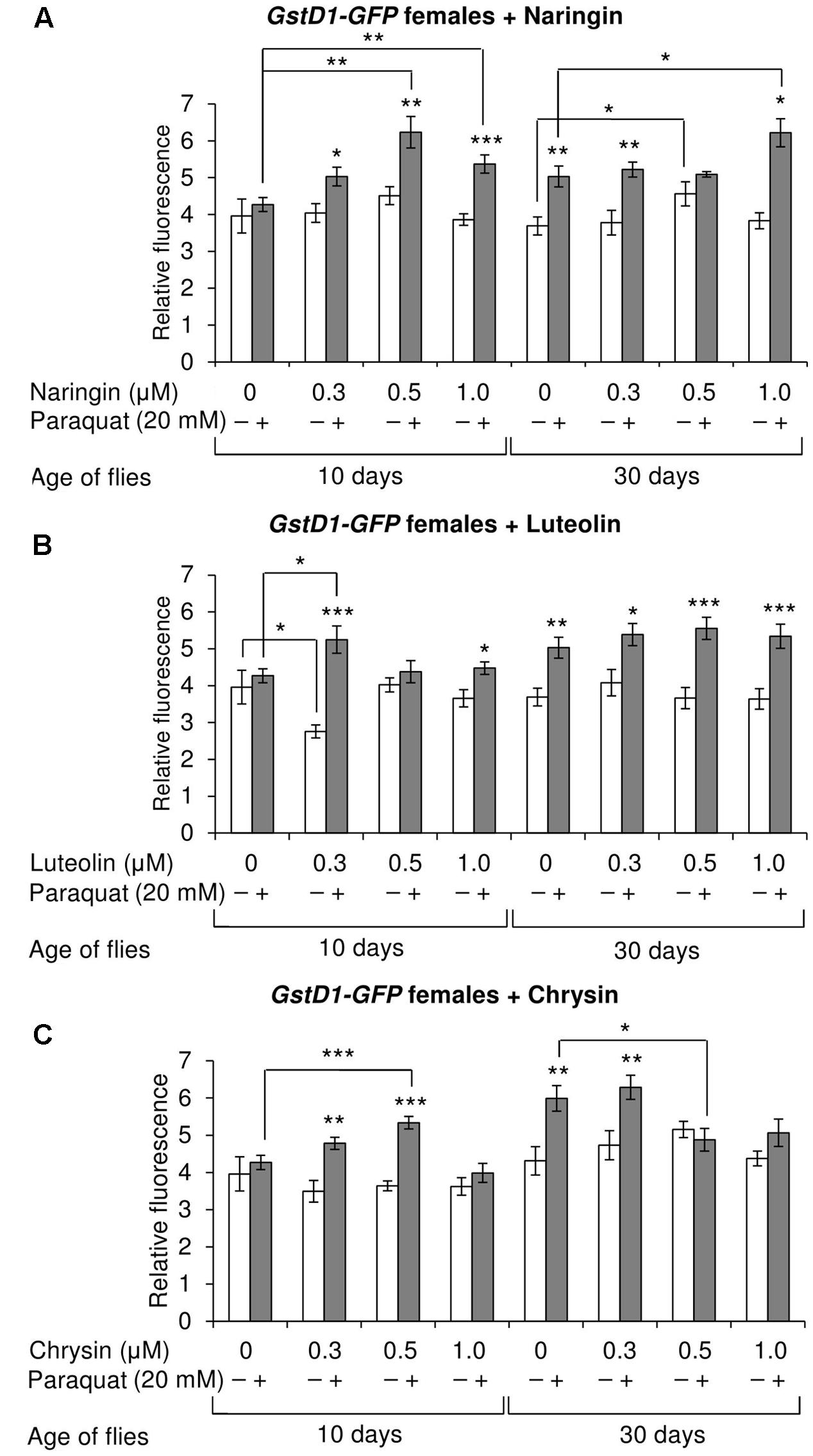
FIGURE 11. Relative fluorescence of GstD1-GFP reporter in Drosophila melanogaster females after treatment with naringin (A), luteolin (B), and chrysin (C); ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.01, χ2 test.
Similarity at Pathway Level
To determine pathway-level similarity, the iPANDA algorithm was applied. For each compound, perturbation PASs were calculated for 378 pathways. The similarity between the pathway activation signatures of natural compounds and rapamycin was evaluated by the number of commonly up- and down-regulated pathways between 15,489 compounds. Results of the pathway-level analysis are depicted in Supplementary Tables 7, 8.
The best similarity (rank 348 out of 15,489) was observed between naringin (time of exposure 24 h, concentration 10 μM) and rapamycin (time of exposure 24 h, concentration 0.4 μM) in A549 cell line, but in this case, the amount of commonly regulated pathways was only 88 out of 378 analyzed. The highest amount of commonly regulated pathways between naringin and rapamycin was 229 and was observed in MCF7 cells. However, when comparing to other compounds the rank of naringin is quite low (rank 1306 out of 15,489). Thus, it can be concluded that naringin and rapamycin have quite distinct mechanisms of action at least in A549 and MCF7 cells.
Discussion
In certain concentrations, the studied compounds improved the lifespan parameters of the C. elegans model organism. Luteolin and chrysin also improved the lifespan parameters of D. melanogaster females without reducing aging-dependent physiological parameters (fecundity and locomotor activity) but did not affect males. Naringin did not affect the lifespan of females and, at the highest concentration (1 μM), decreased the lifespan parameters of males. Additionally, flavonoids had either no effect or, in high concentrations, decreased the stress resistance of nematodes and flies.
For females, all studied compounds were associated with decreased Hsp70 gene expression. For males, only naringin was associated with decreased Hsp70 gene expression. The down-regulation of the Hsp70 gene is a known effect of flavonoids like quercetin (Tatsuta et al., 2014), fisetin (Kim et al., 2015), and epigallocatechin-3-gallate (Tran et al., 2010). Hsp70 proteins protect cells from various kinds of stress by participating in proteostasis and playing a role in cellular processes such as apoptosis or proliferation via interaction with regulatory proteins (Mayer and Bukau, 2005; Gong and Golic, 2006). Hsp70 overexpression is associated with increased lifespan in flies (Tatar et al., 1997). On the other hand, it was observed that flies with higher levels of Hsp70-GFP reporter die sooner than flies with lower levels (Yang and Tower, 2009). Thus, low levels of Hsp70 can be a biomarker of younger biological age.
The results of our experiments on the C. elegans mutant strain also suggest that the observed positive effects on nematode lifespan were at least partly associated with (AMP)-activated protein kinase (AMPK). Interestingly, according to the literature, the activation of AMPK leads to a decline of Hsp70 mRNA stability (Wang et al., 2010). For example, the flavonoid quercetin down-regulated Hsp70 via AMPK activation in HeLa cells (Jung et al., 2010). The ability to activate AMPK was also shown for luteolin (Hwang et al., 2011), chrysin (Shao et al., 2012), and naringin (Pu et al., 2012). AMPK is a protein kinase that controls cellular metabolism by activating some signaling pathways like Nrf2/SKN-1 and FOXO/DAF-16 and inhibiting others like TOR (Salminen and Kaarniranta, 2012). The overexpression of the AMPK gene in nematodes has been found to increase the lifespan of nematodes and flies (Apfeld et al., 2004; Stenesen et al., 2013; Ulgherait et al., 2014). The overexpression of this gene in the Drosophila nervous system has been demonstrated to result in greater effects on lifespan in females and was not associated with the decreased fecundity or spontaneous physical activity (Ulgherait et al., 2014). In our experiments, the effects were also more pronounced among D. melanogaster females and did not adversely affect fecundity or spontaneous physical activity. However, it is also possible that the more dramatic expression of these effects in females is a result of their higher food consumption (Wong et al., 2009).
According to the literature, another flavone, baicalein, has been found to improve the lifespan and stress resistance of C. elegans by activating the Keap1/Nrf2 signaling pathway, which regulates the cell-protection mechanisms (Havermann et al., 2013, 2016). However, chrysin and luteolin can either inhibit or activate this pathway depending on the biological model used in the experiments (Keum and Choi, 2014). For example, luteolin has been shown to inhibit the Keap1/Nrf2 signaling pathway in human hepatoma (HepG2), rat liver epithelial (RL-34), and mouse hepatoma (Hepa1c1c7) cells treated with dioxin (TCDD) (Zhang et al., 2014). The same effect was observed in another study on human lung carcinoma A549 (NSCLC), human breast carcinoma (MCF7), and human colon cancer (Caco2) cell lines (Tang et al., 2011). The effects were associated with a decrease of Nrf2 stability (Tang et al., 2011; Zhang et al., 2014). Similarly, chrysin was found to inhibit this signaling pathway in parental human hepatocellular carcinoma cells (Bel-7402/ADM) (Gao et al., 2013). At the same time, both chrysin and luteolin have been demonstrated to induce Nrf2 activity in hepatocytes isolated from male Sprague–Dawley rats, thus increasing their resistance to oxidative stress (Huang et al., 2013). In our experiments, chrysin and luteolin had no effects on the level of Nrf2 target activation under both stress and non-stress conditions. In some variants, luteolin even decreased the expression of the GstD1-GFP reporter. In contrast, naringin increased the level of GstD1-GFP reporter under normal and non-stress conditions. Naringin’s ability to activate the Keap1/Nrf2 signaling pathway is consistent with other studies (Gopinath and Sudhandiran, 2012; Chen et al., 2015). However, it is known that the level of Nrf2 target genes does not decrease with age, but the ability to activate under stress conditions does (Rahman et al., 2013). Our data imply that all three studied compounds increase the speed of Keap1/Nrf2 signaling pathway activation under paraquat treatment in flies.
Luteolin and chrysin have two and zero hydroxyl groups, respectively, on the B-ring. According to literature, the ability of flavonols, another subclass of flavonoids, to increase the lifespan of model organisms depends on the number of hydroxyl groups on their B-ring, with more hydroxyl groups being associated with more pronounced effects (Grunz et al., 2012). In our experiments, we observed no drastic differences between the effects of luteolin and chrysin on lifespan parameters of C. elegans and D. melanogaster. However, the addition of luteolin resulted in a more pronounced decrease in Hsp70 mRNA levels after 10 days of treatment. Furthermore, in contrast to chrysin, luteolin also decreased the level of the GstD1-GFP reporter gene in some cases. Thus, luteolin is more biologically active than chrysin.
The results of our experiments demonstrate the ability of the flavones chrysin and luteolin to improve the lifespan of both C. elegans and D. melanogaster biological models. The possible mechanism of their action is through AMPK activation. Even though naringin demonstrated no positive effects on the lifespan of D. melanogaster, it produced the most pronounced effects on Nrf2 target activation. The transcriptional response data analysis of the A549 and MCF7 cell lines (resulted from the LINCS Project) revealed that rapamycin and naringin activate and inhibit some common signaling pathways. However, mostly their mechanisms of action are different.
Author Contributions
EL, EP, MV, AZ, and AM wrote the manuscript text. EL, NZ, EP, AK, MV, EM, and SL carried out the experiments and processed the statistical analysis. AM supervised the research and the text of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Russian Science Foundation (Grant/Award Number: 14-50-00060).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2017.00884/full#supplementary-material
Footnotes
References
Ahad, A., Ganai, A. A., Mujeeb, M., and Siddiqui, W. A. (2014). Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 279, 1–7. doi: 10.1016/j.taap.2014.05.007
Apfeld, J., O’Connor, G., McDonagh, T., DiStefano, P. S., and Curtis, R. (2004). The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 18, 3004–3009. doi: 10.1101/gad.1255404
Ashburner, M. (1989). Drosophila: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Belinha, I., Amorim, M. A., Rodrigues, P., de Freitas, V., Moradas-Ferreira, P., Mateus, N., et al. (2007). Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J. Agric. Food Chem. 55, 2446–2451. doi: 10.1021/jf063302e
Bjedov, I., Toivonen, J. M., Kerr, F., Slack, C., Jacobson, J., Foley, A., et al. (2010). Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46. doi: 10.1016/j.cmet.2009.11.010
Breslow, N. (1970). A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 57, 579–594.
Brodowska, K. M. (2017). Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 7, 108–123. doi: 10.5281/zenodo.545778
Cali, T., Ottolini, D., Soriano, M. E., and Brini, M. (2015). A new split-GFP-based probe reveals DJ-1 translocation into the mitochondrial matrix to sustain ATP synthesis upon nutrient deprivation. Hum. Mol. Genet. 24, 1045–1060. doi: 10.1093/hmg/ddu519
Chattopadhyay, D., Sen, S., Chatterjee, R., Roy, D., James, J., and Thirumurugan, K. (2016). Context- and dose-dependent modulatory effects of naringenin on survival and development of Drosophila melanogaster. Biogerontology 17, 383–393. doi: 10.1007/s10522-015-9624-6
Chen, R. C., Sun, G. B., Wang, J., Zhang, H. J., and Sun, X. B. (2015). Naringin protects against anoxia/reoxygenation-induced apoptosis in H9c2 cells via the Nrf2 signaling pathway. Food Funct. 6, 1331–1344. doi: 10.1039/c4fo01164c
Finch, C. E. (1990). Longevity, Senescence, and the Genome. The John D and Catherine T MacArthur Foundation Series on Mental Health and Development. Chicago, IL: University of Chicago Press.
Fleming, T. R., Ofallon, J. R., and Obrien, P. C. (1980). Modified Kolmogorov-Smirnov test procedures with application to arbitrarily right-censored data. Biometrics 36, 607–625. doi: 10.2307/2556114
Gambelunghe, C., Rossi, R., Sommavilla, M., Ferranti, C., Rossi, R., Ciculi, C., et al. (2003). Effects of chrysin on urinary testosterone levels in human males. J. Med. Food 6, 387–390. doi: 10.1089/109662003772519967
Gao, A. M., Ke, Z. P., Shi, F., Sun, G. C., and Chen, H. (2013). Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem. Biol. Interact. 206, 100–108. doi: 10.1016/j.cbi.2013.08.008
Gong, W. J., and Golic, K. G. (2006). Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics 172, 275–286. doi: 10.1534/genetics.105.048793
Gopinath, K., and Sudhandiran, G. (2012). Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 227, 134–143. doi: 10.1016/j.neuroscience.2012.07.060
Grunz, G., Haas, K., Soukup, S., Klingenspor, M., Kulling, S. E., Daniel, H., et al. (2012). Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 133, 1–10. doi: 10.1016/j.mad.2011.11.005
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. doi: 10.1038/nature08221
Havermann, S., Humpf, H. U., and Watjen, W. (2016). Baicalein modulates stress-resistance and life span in C. elegans via SKN-1 but not DAF-16. Fitoterapia 113, 123–127. doi: 10.1016/j.fitote.2016.06.018
Havermann, S., Rohrig, R., Chovolou, Y., Humpf, H. U., and Watjen, W. (2013). Molecular effects of baicalein in Hct116 cells and Caenorhabditis elegans: activation of the Nrf2 signaling pathway and prolongation of lifespan. J. Agric. Food Chem. 61, 2158–2164. doi: 10.1021/jf304553g
He, C., Tsuchiyama, S. K., Nguyen, Q. T., Plyusnina, E. N., Terrill, S. R., Sahibzada, S., et al. (2014). Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLOS Genet. 10:e1004860. doi: 10.1371/journal.pgen.1004860
Huang, C. S., Lii, C. K., Lin, A. H., Yeh, Y. W., Yao, H. T., Li, C. C., et al. (2013). Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 87, 167–178. doi: 10.1007/s00204-012-0913-4
Hwang, J. T., Park, O. J., Lee, Y. K., Sung, M. J., Hur, H. J., Kim, M. S., et al. (2011). Anti-tumor effect of luteolin is accompanied by AMP-activated protein kinase and nuclear factor-kappaB modulation in HepG2 hepatocarcinoma cells. Int. J. Mol. Med. 28, 25–31. doi: 10.3892/ijmm.2011.667
Jones, E., and Hughes, R. E. (1982). Quercetin, flavonoids and the life-span of mice. Exp. Gerontol. 17, 213–217.
Jung, J. H., Lee, J. O., Kim, J. H., Lee, S. K., You, G. Y., Park, S. H., et al. (2010). Quercetin suppresses HeLa cell viability via AMPK-induced HSP70 and EGFR down-regulation. J. Cell. Physiol. 223, 408–414. doi: 10.1002/jcp.22049
Kampkotter, A., Timpel, C., Zurawski, R. F., Ruhl, S., Chovolou, Y., Proksch, P., et al. (2008). Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 149, 314–323. doi: 10.1016/j.cbpb.2007.10.004
Kanazawa, K. (2011). Bioavailability of non-nutrients for preventing lifestyle-related diseases. Trends Food Sci. Technol. 22, 655–659. doi: 10.1016/j.tifs.2011.06.005
Kaplan, E. L., and Meier, P. (1992). “Nonparametric estimation from incomplete observations,” in Breakthroughs in Statistics, eds S. Kotz and N. Johnson (New York, NY: Springer), 319–337.
Keum, Y. S., and Choi, B. Y. (2014). Molecular and chemical regulation of the Keap1-Nrf2 signaling pathway. Molecules 19, 10074–10089. doi: 10.3390/molecules190710074
Kim, J. A., Lee, S., Kim, D. E., Kim, M., Kwon, B. M., and Han, D. C. (2015). Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis 36, 696–706. doi: 10.1093/carcin/bgv045
Kumar, S., and Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013:162750. doi: 10.1155/2013/162750
Lee, E. B., Kim, J. H., Cha, Y. S., Kim, M., Song, S. B., Cha, D. S., et al. (2015). Lifespan extending and stress resistant properties of vitexin from Vigna angularis in Caenorhabditis elegans. Biomol. Ther. 23, 582–589. doi: 10.4062/biomolther.2015.128
Lee, H., Cho, J. S., Lambacher, N., Lee, J., Lee, S. J., Lee, T. H., et al. (2008). The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 283, 14988–14993. doi: 10.1074/jbc.M709115200
Mantel, N. (1966). Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 50, 163–170.
Mayer, M. P., and Bukau, B. (2005). Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 62, 670–684. doi: 10.1007/s00018-004-4464-6
Miean, K. H., and Mohamed, S. (2001). Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 49, 3106–3112. doi: 10.1021/jf000892m
Moskalev, A., Chernyagina, E., Tsvetkov, V., Fedintsev, A., Shaposhnikov, M., Krut’ko, V., et al. (2016). Developing criteria for evaluation of geroprotectors as a key stage toward translation to the clinic. Aging Cell 15, 407–415. doi: 10.1111/acel.12463
Ozerov, I. V., Lezhnina, K. V., Izumchenko, E., Artemov, A. V., Medintsev, S., Vanhaelen, Q., et al. (2016). In silico pathway activation network decomposition analysis (iPANDA) as a method for biomarker development. Nat. Commun. 7:13427. doi: 10.1038/ncomms13427
Pallauf, K., Duckstein, N., and Rimbach, G. (2017). A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 76, 145–162. doi: 10.1017/S0029665116000720
Pietsch, K., Saul, N., Menzel, R., Sturzenbaum, S. R., and Steinberg, C. E. (2009). Quercetin mediated lifespan extension in Caenorhabditis elegans is modulated by age-1, daf-2, sek-1 and unc-43. Biogerontology 10, 565–578. doi: 10.1007/s10522-008-9199-6
Pu, P., Gao, D. M., Mohamed, S., Chen, J., Zhang, J., Zhou, X. Y., et al. (2012). Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biochem. Biophys. 518, 61–70. doi: 10.1016/j.abb.2011.11.026
Rahman, M. M., Sykiotis, G. P., Nishimura, M., Bodmer, R., and Bohmann, D. (2013). Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell 12, 554–562. doi: 10.1111/acel.12078
Robida-Stubbs, S., Glover-Cutter, K., Lamming, D. W., Mizunuma, M., Narasimhan, S. D., Neumann-Haefelin, E., et al. (2012). TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724. doi: 10.1016/j.cmet.2012.04.007
Salminen, A., and Kaarniranta, K. (2012). AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 11, 230–241. doi: 10.1016/j.arr.2011.12.005
Saul, N., Pietsch, K., Menzel, R., and Steinberg, C. E. (2008). Quercetin-mediated longevity in Caenorhabditis elegans: Is DAF-16 involved? Mech. Ageing Dev. 129, 611–613. doi: 10.1016/j.mad.2008.07.001
Seelinger, G., Merfort, I., Wolfle, U., and Schempp, C. M. (2008). Anti-carcinogenic effects of the flavonoid luteolin. Molecules 13, 2628–2651. doi: 10.3390/molecules13102628
Shao, J. J., Zhang, A. P., Qin, W., Zheng, L., Zhu, Y. F., and Chen, X. (2012). AMP-activated protein kinase (AMPK) activation is involved in chrysin-induced growth inhibition and apoptosis in cultured A549 lung cancer cells. Biochem. Biophys. Res. Commun. 423, 448–453. doi: 10.1016/j.bbrc.2012.05.123
Solis, G. M., and Petrascheck, M. (2011). Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J. Vis. Exp. 49:e2496. doi: 10.3791/2496
Spindler, S. R., Mote, P. L., Flegal, J. M., and Teter, B. (2013). Influence on longevity of blueberry, cinnamon, green and black tea, pomegranate, sesame, curcumin, morin, pycnogenol, quercetin, and taxifolin fed iso-calorically to long-lived, F1 hybrid mice. Rejuvenation Res. 16, 143–151. doi: 10.1089/rej.2012.1386
Stenesen, D., Suh, J. M., Seo, J., Yu, K., Lee, K. S., Kim, J. S., et al. (2013). Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 17, 101–112. doi: 10.1016/j.cmet.2012.12.006
Sykiotis, G. P., and Bohmann, D. (2008). Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76–85. doi: 10.1016/j.devcel.2007.12.002
Tang, X., Wang, H., Fan, L., Wu, X., Xin, A., Ren, H., et al. (2011). Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic. Biol. Med. 50, 1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008
Tatar, M., Khazaeli, A. A., and Curtsinger, J. W. (1997). Chaperoning extended life. Nature 390, 30. doi: 10.1038/36237
Tatsuta, T., Hosono, M., Ogawa, Y., Inage, K., Sugawara, S., and Nitta, K. (2014). Downregulation of Hsp70 inhibits apoptosis induced by sialic acid-binding lectin (leczyme). Oncol. Rep. 31, 13–18. doi: 10.3892/or.2013.2814
Tran, P. L., Kim, S. A., Choi, H. S., Yoon, J. H., and Ahn, S. G. (2010). Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer 10:276. doi: 10.1186/1471-2407-10-276
Ulgherait, M., Rana, A., Rera, M., Graniel, J., and Walker, D. W. (2014). AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 8, 1767–1780. doi: 10.1016/j.celrep.2014.08.006
Vauzour, D., Vafeiadou, K., Rodriguez-Mateos, A., Rendeiro, C., and Spencer, J. P. (2008). The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 3, 115–126. doi: 10.1007/s12263-008-0091-4
Vinayagam, R., and Xu, B. (2015). Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr. Metab. 12, 60. doi: 10.1186/s12986-015-0057-7
Wang, C., Li, Q., Redden, D. T., Weindruch, R., and Allison, D. B. (2004). Statistical methods for testing effects on “maximum lifespan”. Mech. Ageing Dev. 125, 629–632. doi: 10.1016/j.mad.2004.07.003
Wang, T., Yu, Q., Chen, J., Deng, B., Qian, L., and Le, Y. (2010). PP2A mediated AMPK inhibition promotes HSP70 expression in heat shock response. PLOS ONE 5:e13096. doi: 10.1371/journal.pone.0013096
Wong, R., Piper, M. D., Wertheim, B., and Partridge, L. (2009). Quantification of food intake in Drosophila. PLOS ONE 4:e6063. doi: 10.1371/journal.pone.0006063
Yang, J., and Tower, J. (2009). Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. J. Gerontol. A Biol. Sci. Med. Sci. 64, 828–838. doi: 10.1093/gerona/glp054
Zern, T. L., and Fernandez, M. L. (2005). Cardioprotective effects of dietary polyphenols. J. Nutr. 135, 2291–2294.
Zhang, T., Kimura, Y., Jiang, S., Harada, K., Yamashita, Y., and Ashida, H. (2014). Luteolin modulates expression of drug-metabolizing enzymes through the AhR and Nrf2 pathways in hepatic cells. Arch. Biochem. Biophys. 557, 36–46. doi: 10.1016/j.abb.2014.05.023
Keywords: lifespan, D. melanogaster, C. elegans, chrysin, luteolin, naringin
Citation: Lashmanova E, Zemskaya N, Proshkina E, Kudryavtseva A, Volosnikova M, Marusich E, Leonov S, Zhavoronkov A and Moskalev A (2017) The Evaluation of Geroprotective Effects of Selected Flavonoids in Drosophila melanogaster and Caenorhabditis elegans. Front. Pharmacol. 8:884. doi: 10.3389/fphar.2017.00884
Received: 11 October 2017; Accepted: 16 November 2017;
Published: 07 December 2017.
Edited by:
Paul Roos Ernsberger, Case Western Reserve University School of Medicine, United StatesReviewed by:
Hong Zhan, University of Wisconsin-Madison, United StatesMedardo Hernández, Complutense University of Madrid, Spain
Copyright © 2017 Lashmanova, Zemskaya, Proshkina, Kudryavtseva, Volosnikova, Marusich, Leonov, Zhavoronkov and Moskalev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Moskalev, YW1vc2thbGV2QGxpc3QucnU=
 Ekaterina Lashmanova
Ekaterina Lashmanova Nadezhda Zemskaya
Nadezhda Zemskaya Ekaterina Proshkina
Ekaterina Proshkina Anna Kudryavtseva
Anna Kudryavtseva Marina Volosnikova
Marina Volosnikova Elena Marusich
Elena Marusich Sergey Leonov
Sergey Leonov Alex Zhavoronkov
Alex Zhavoronkov Alexey Moskalev
Alexey Moskalev