- Department of Clinical Veterinary Medicine, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
Acute lung injury (ALI) is a complex syndrome with sepsis occurring in critical patients, who usually lack effective therapy. Nuciferine is a primary bioactive component extracted from the lotus leaf, and it displays extensive pharmacological functions, including anti-cancer, anti-inflammatory, and antioxidant properties. Nevertheless, the effects of nuciferine on lipopolysaccharide (LPS)-stimulated ALI in mice has not been investigated. ALI of mice stimulated by LPS was used to determine the anti-inflammatory function of nuciferine. The molecular mechanism of nuciferine was performed on RAW264.7 macrophage cells. The results of pathological section, myeloperoxidase activity and lung wet/dry ratio showed that nuciferine alleviated LPS-induced lung injury (p < 0.05). qRT-PCR and ELISA experiments suggested that nuciferine inhibited TNF-α, IL-6, and IL-1β secretion in tissues and RAW264.7 cells but increased IL-10 secretion (p < 0.05). Molecular studies showed that TLR4 expression and nuclear factor (NF)-κB activation were both inhibited by nuciferine treatment (p < 0.05). To further investigate the anti-inflammatory mechanism of nuciferine, TLR4 was knocked down. When TLR4 was silenced, LPS induced the production of IL-1β, and TNF-α was markedly decreased by TLR4-siRNA and nuciferine treatment in LPS-induced RAW264.7 cells (p < 0.05). These results suggested that nuciferine had the ability to protect against LPS-stimulated ALI. Thus, nuciferine may be a potential drug for treating LPS-induced pulmonary inflammation.
Introduction
Acute lung injury (ALI) is a complex syndrome of sepsis in critical patients, which is closely related to high morbidity and mortality (Abdelmageed et al., 2015). ALI’s characteristics are hypoxemia, alveolar capillary membrane disruption, and pulmonary edema (Wu et al., 2016a). In addition, the acute respiratory distress syndrome is a more severe form of ALI, and the mortality rate remains as high as approximately 40% (Huang et al., 2016).
Lipopolysaccharide (LPS), an ingredient of endotoxin in the outer membrane of Gram-negative bacteria, has been widely used to induce ALI in mice (Sun et al., 2010; Zhao et al., 2014; Chao et al., 2017). LPS has great biological activity and enters the bloodstream, leading to serious inflammatory responses (Wu et al., 2016b). It is well-known that the pro-inflammatory mediator secretions, such as IL-1β, IL-6, and TNF-α, will increase sharply (Wang et al., 2011). These excessive expressions of pro-inflammatory cytokines contribute to lung edema and alveolar hemorrhage (Zhao et al., 2014). Moreover, these cytokines are activated by the NF-κB signaling pathway (Cho et al., 2014). TLR4, an important member of the toll like receptor (TLR) family, is needed for the response of LPS and participates in host resistance against Gram-negative bacteria (Wu et al., 2012). An increasing body of evidence has suggested that TLR4 is stimulated by LPS and can activate the NF-κB signaling pathway (Cho et al., 2014; Li et al., 2016; Wang et al., 2017). Thus, this approach may represent a useful strategy for treatment of ALI by interfering with the TLR4 activated signaling pathway.
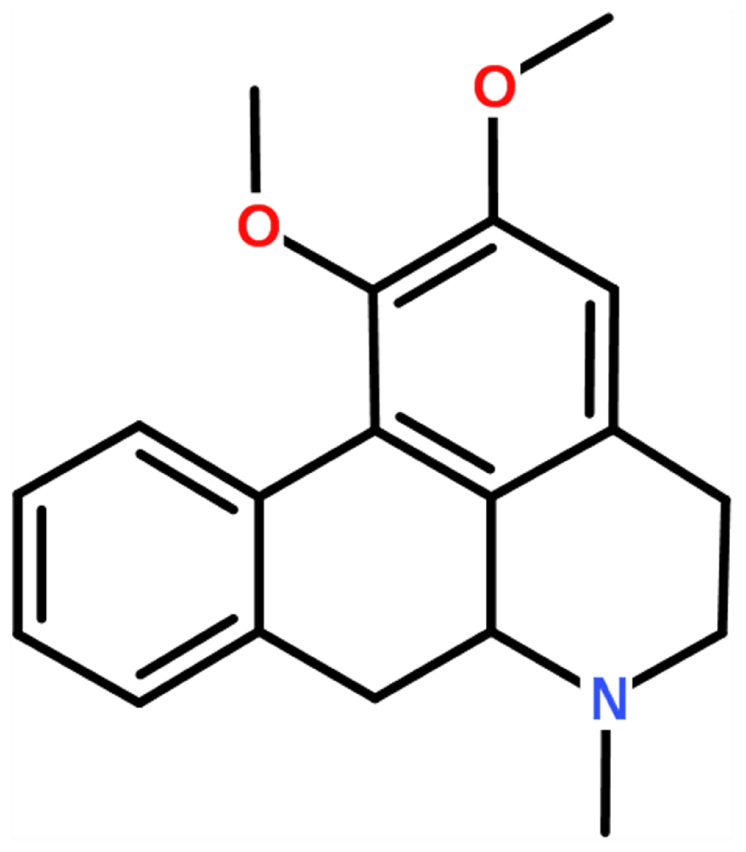
Recently, researchers have increasingly focused on natural products for their protective roles in the management and prevention of oxidative stress or inflammatory-related diseases and because they have fewer side effects (Menghini et al., 2016; Ferrante et al., 2017; Locatelli et al., 2017). Nuciferine (C19H21NO2, the chemical structure is shown in Figure 1) is a main bioactive component obtained from the lotus leaf and has extensive pharmacological functions, such as anti-cancer, anti-inflammatory, reduced body weight and antioxidant actions (Liu et al., 2014, 2015; Zhang et al., 2015; Qi et al., 2016; Wang et al., 2016). Macrophages are critical components in the connection between innate immunity and metabolism and tissue balance (A-González and Castrillo, 2011). Although the anti-inflammatory activity of nuciferine has been reported, there is no evidence that has elucidated the effects of nuciferine on LPS-induced ALI in mice and RAW264.7 cells.
In the present study, we aimed to explore the anti-inflammatory effects of nuciferine on pro-inflammatory mediator production in LPS-induced ALI in mice and RAW264.7 cells. Additionally, the molecular mechanisms involved were also explored.
Materials and Methods
Reagents
Nuciferine (purity > 98%) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (China). RAW264.7 cells were obtained from the American Type Culture Collection (United States). LPS was obtained from Sigma (United States). IL-1β, IL-6, IL-10, and TNF-α ELISA kits were obtained from BioLegend (United States). Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb, NF-κB p65 (D14E12) XP® Rabbit mAb, Phospho-IκBα (Ser32) (14D4) Rabbit mAb, IκBα (L35A5) Mouse mAb, and TLR4, β-actin were obtained from Cell Signaling Technology (Beverly, United States).
Experimental Processing
Fifty BALB/c mice (8 weeks old) were purchased from the Experimental Animal Center of Wuhan University (China). Mice were exposed to 12-h normal illumination and provided free food intake at room temperature and 65% humidity for 1 week before the experiments. This study was conducted in accordance with the prescribed experimental animal guidelines. All of the agreements were responsible for the Hubei Province experimental animal research center and Huazhong Agricultural University ethics committee (No. HZAUMO-2015-12).
Lipopolysaccharide was dissolved in phosphate buffered saline (PBS). Mice were randomly grouped as follows (each group of 10 mice): control group (PBS), LPS group (0.5 mg/kg), nuciferine (10 and 20 mg/kg)+LPS groups, and nuciferine (10 mg/kg) group. Establishment of the LPS stimulated ALI mouse model was based on a previous literature description (Lv et al., 2015). In brief, intranasal perfusion of LPS to induce ALI in mice. After 24 h of LPS stimulation, an intraperitoneal injection with nuciferine (10 and 20 mg/kg) was performed three times at 6, 12, and 18 h in the nuciferine groups. The same treatment in the control group was performed with PBS. Next, the mice were anesthetized with pentobarbital sodium and executed. Finally, the lung tissues were harvested and preserved in -80°C.
Histopathological Examination
Ten percent formalin was used to fix lung tissue for 24 h. Next, tissues were embedded in paraffin, sliced, and hematoxylin-eosin stained. Finally, light microscopy was performed to evaluate the pathological changes in lung morphology.
Lung Wet/Dry (W/D) Ratio
The wet lung tissue from mice was separated and its weight was recorded using a precision electronic balance (SHIMADZU AUY120, Japan). Next, the lung tissue was placed in a constant temperature blower dryer at 60°C to dry for 72 h. The dry weight of the lung tissue was measured again. To estimate pulmonary edema, the lung W/D ratio was calculated as previously described (Wu et al., 2016a).
Preparation of Bronchoalveolar Lavage Fluid (BALF)
Bronchoalveolar lavage fluid was used to prepare endotracheal intubation and rinsed with sterile physiological saline three times according to the literature description (Abdelmageed et al., 2015). BALF solution was centrifuged at 3000 rpm for 15 min, and the supernatants were later collected and preserved in -80°C until analysis.
Myeloperoxidase (MPO) Assay
The lung tissues from mice were collected, 100 mg tissues were homogenated with PBS (w/v = 1:19), and the tissue homogenate was subsequently centrifuged at 12000 rpm for 10 min. The supernatant was later collected. Detection of MPO activity in the supernatant was performed with a kit from the Nanjing Biological Co., Ltd., in accordance with the manufacturer’s instructions. The absorbance was measured with spectrophotometry at 460 nm.
Cell Cultivation and Processing
RAW264.7 macrophage cells were cultured in high glucose DMEM medium supplemented with 10% FBS at 37°C in five percent CO2 incubator. Cells were pretreated with nuciferine (10 and 20 μg/mL) for 1 h before being challenged with LPS (0.5 μg/mL) for 6 h. Cells without any treatment acted as the control group.
Cell Viability Assay
To determine the cytotoxicity stimulated by nuciferine, MTT experiments were carried out in accordance with the manufacturer’s instructions. The cells (1 × 105/mL) were plated in 96-well plates and cultured for 3 h in a 37°C incubator. Subsequently, the cells were treated with nuciferine (10 and 20 μg/mL) for 24 h. MTT (5 mg/mL) was added to the 96-well plate for 4 h, the supernatant was removed, and then one hundred microliters of DMSO was added. The absorbance value was measured at 570 nm with an ELISA Reader.
Analysis of Cytokines
The effects of nuciferine on LPS induced cytokine secretion were detected in cells and tissues. Tissues were homogenized with pre-cooled PBS, then centrifuged for 15 min at 12,000 rpm. Next, the supernatants were harvested. The supernatants of cells were also collected. The levels of TNF-α, IL-6, IL-1β, and IL-10 in all supernatants were determined with ELISA kits in accordance with the producer’s instructions. The absorbance value was measured at 450 nm with an ELISA Reader.
qRT-PCR Assay
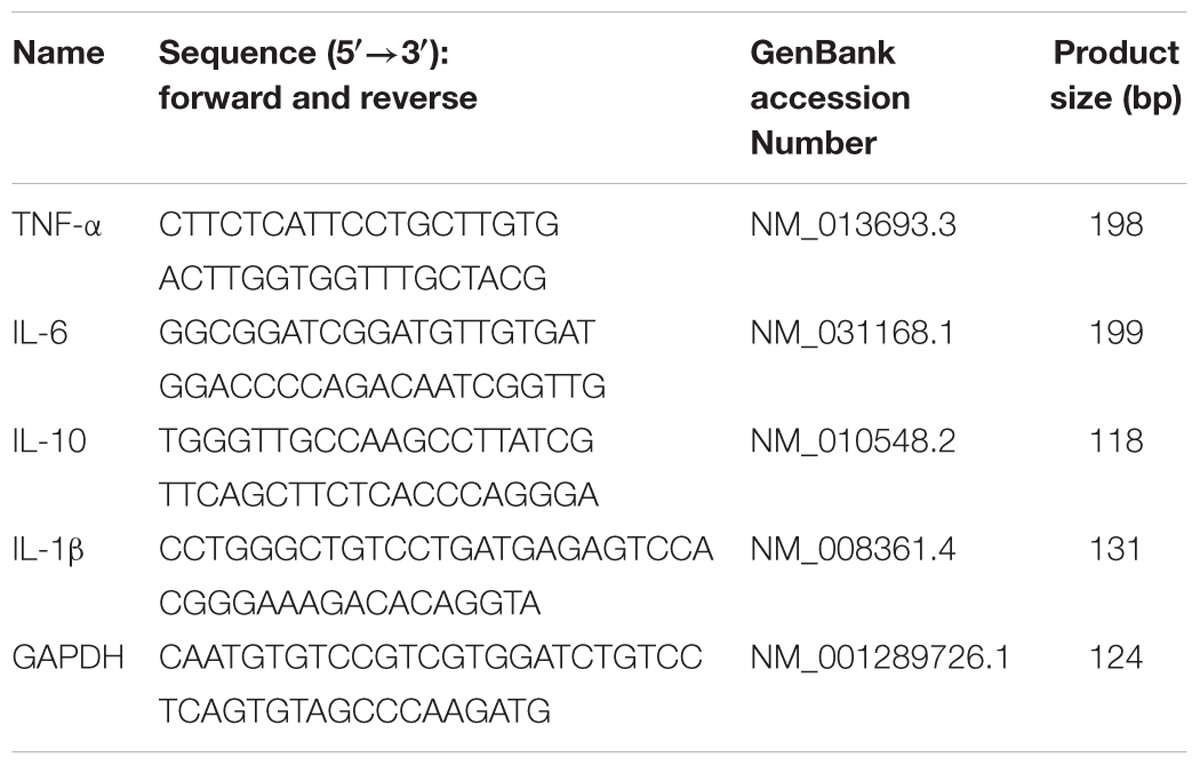
The total RNA of lung tissues and cells was extracted by Trizol agent. Determination of the purity of RNA was performed at 260/280 nm, and then reverse transcribed into cDNA. Primers used for detecting the expression of mRNA are displayed in Table 1. PCR reaction conditions were as follows: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 60 s, and 72°C for 60 s. The relative expression levels of target genes were standardized with the GAPDH gene using the 2-ΔΔCt method.
Immunoblotting Detection
Tissues and RAW264.7 cells were lysed with lysate of RIPA adding phosphatase inhibitor and centrifuged at 12,000 rpm for 10 min. The protein concentration was detected using a BCA kit. Next, protein was separated by 10% SDS-PAGE and transported to PVDF membranes. It was sealed with 5% skimmed milk powder for 2 h and the protein was hybridized with the primary antibody at 4°C for 12 h. Subsequently, membranes were incubated with secondary antibodies containing horseradish peroxidase at 25°C for 1 h. The chemiluminescence signal was imaged with the ECL Plus Western Blotting Detection System.
Small Interfering RNA (siRNA) Transfection
TLR4-siRNA, and negative control siRNA were obtained from GenePharma Co., Ltd. (Shanghai, China). For TLR4-siRNAtransfection, RAW264.7 cells (1 × 105 cells mL-1) were grown in six well culture-plates and allowed to reach approximately 60% confluence. Transfection was carried out in Opti-MEM medium with negative control siRNA (NC-siRNA), TLR4-siRNA through LipofectamineTM 2000 in accordance with the producer’s protocol. After 6 h, pretreatment with nuciferine (20 μg/mL) was performed for 1 h, LPS (1 μg/mL) was added for 6 h in the treatment group. Finally, cells were cleaved for further analysis.
Immunofluorescence Staining
RAW264.7 macrophage cells (1 × 104 mL-1) were passaged into 12-well culture plates. An immune fluorescence experiment was carried out after RAW264.7 cells were processed as indicated. RAW264.7 cell slices were incubated with TLR4 antibodies at 4°C for 12 h, and incubated with secondary antibodies in the dark for 2 h at room temperature. Subsequently, the nucleus was counterstained with DAPI and visualized under a fluorescence microscope (magnification 400×). The fluorescence values were processed by Image-Pro software to quantified the IOD and then averaged IOD of each section.
Statistics Analysis
The SPSS 16.0 software was used to analyze the data. The results were presented as the mean ± SEM. The comparisons between the groups were conducted by an ANOVA followed by Student’s t-test. The p-values less than 0.05 were considered to be significant.
Results
Effects of Nuciferine on LPS-Stimulated Lung Injury
The severity of LPS-induced ALI was determined by histological analysis and the BALF method. Lung morphology was observed in each group (Figure 2A). The results displayed that the structure of the alveolar wall was normal in the control group (Figure 2B). However, thickening of the alveolar wall, alveolar hyperemia, and infiltration of inflammatory cells were observed in the LPS group (Figure 2C). After treatment with nuciferine, alveolar hyperemia, inflammatory cell infiltration and thickening became greatly improvement (Figures 2D–F). Additionally, the inflammatory mediators in the BALF group were measured via an ELISA assay. The results indicated that IL-1β and TNF-α secretions were markedly increased after the LPS challenge, which was decreased under nuciferine treatment, especially at a high concentration (Figure 2G).
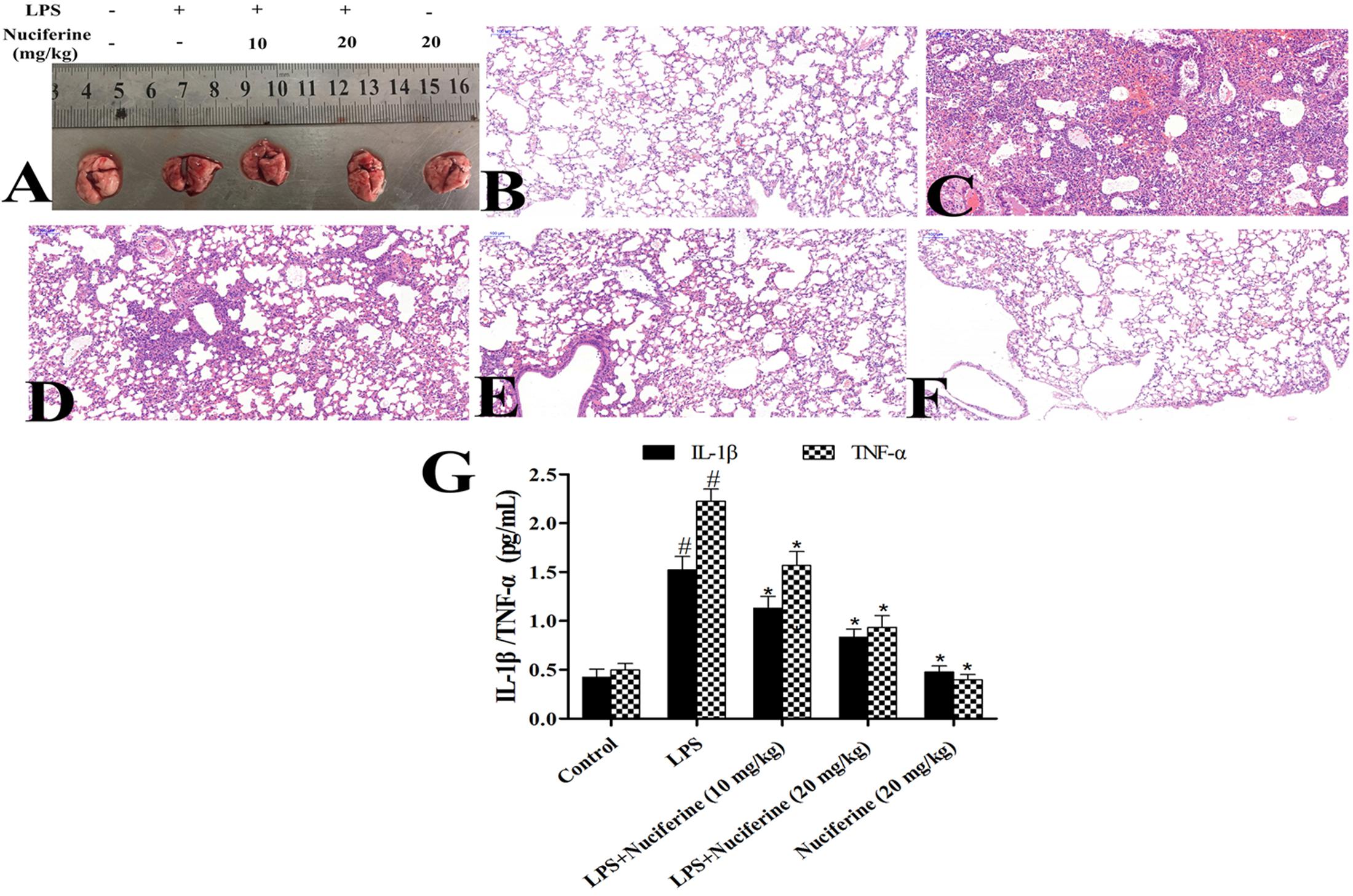
FIGURE 2. Effects of nuciferine on lipopolysaccharide (LPS)-induced lung injury. (A) Morphology of the lung. (B) Control group. (C) LPS group. (D,E) LPS + nuciferine (10 and 20 mg/kg) groups. (F) Nuciferine (20 mg/kg) group. (G) Bronchoalveolar lavage fluid was determined with an ELISA kit. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Effects of Nuciferine on MPO Activity, Lung W/D Ratio and Cell Vitality
Myeloperoxidase activity, a marker of neutrophil influx into tissue, is another method to evaluate the phagocytosis of parenchyma infiltration (Krawisz et al., 1984; Wu et al., 2016a). Additionally, it has been reported that pulmonary edema is one of the main characteristics in LPS induced ALI (Qiushi et al., 2015). Thus, the effects of nuciferine on LPS stimulated pulmonary damage were also determined by MPO activity and the lung W/D ratio. As shown in Figure 3A, compared with the control group, the lung W/D ratio increased dramatically under LPS stimulation. However, nuciferine treatment could greatly reduce the lung W/D ratio. To further estimate the effects of nuciferine on LPS-stimulated ALI, MPO activity was also assessed. As shown in Figure 3B, LPS greatly increased MPO activity, which was significantly decreased with nuciferine treatment. The potential toxicity of nuciferine to RAW264.7 cells was evaluated with an MTT experiment. As displayed in Figure 3C, the cell vitality was not susceptible to nuciferine treatment.
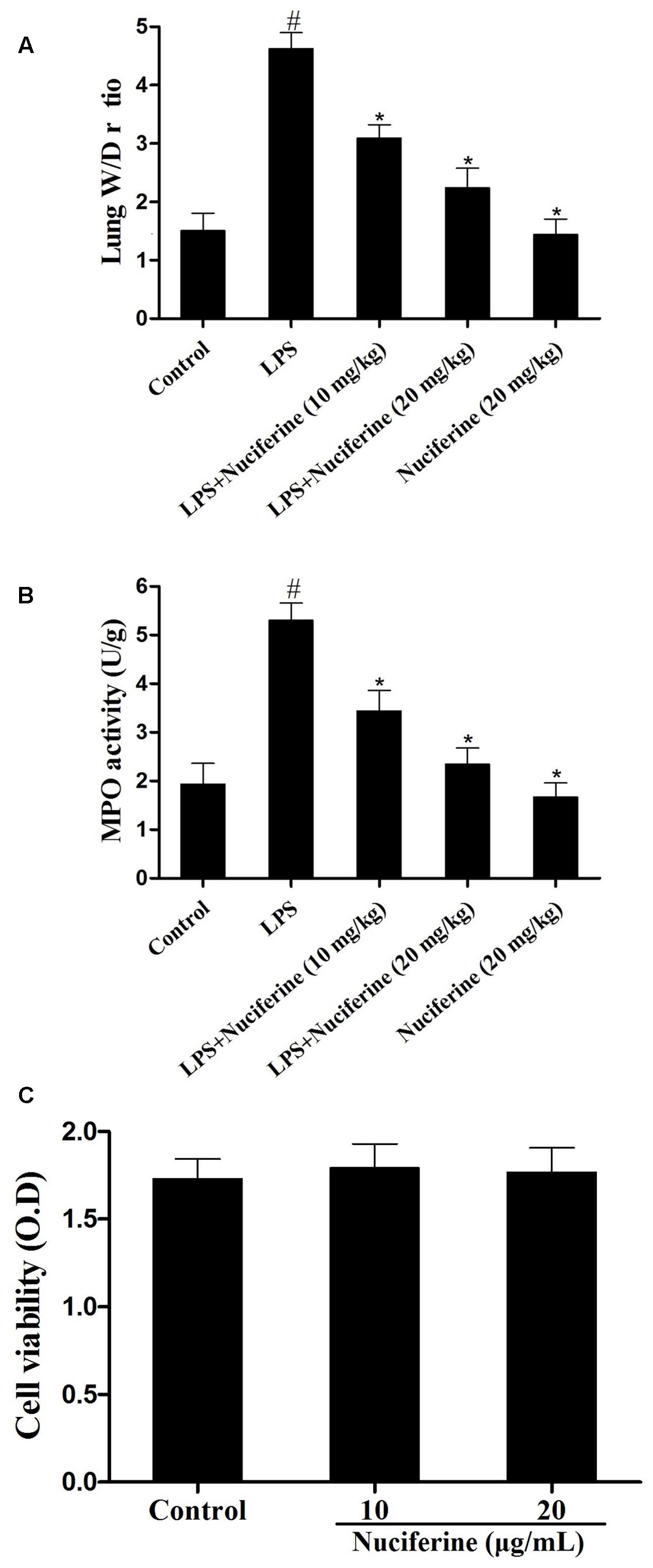
FIGURE 3. Effects of nuciferine on the lung W/D ratio, MPO activity and cell viability. The lung W/D ratio and MPO activity were determined as described in section “Materials and Methods.” (A) Lung W/D ratio. (B) MPO activity. (C) RAW264.7 cells were cultured with various concentrations of nuciferine (0–20 μg/mL) for 24 h. Cell viability was measured using an MTT assay. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Effects of Nuciferine on Pro-inflammatory Mediator Production
Pro-inflammatory mediator levels in cells and tissues were detected by qRT-PCR and ELISA assays. qRT-PCR results are displayed in Figure 4A. LPS markedly increased the IL-1β, IL-6, and TNF-α mRNA levels. Nuciferine treatment greatly inhibited the TNF-α, IL-6, and IL-1β mRNA levels in tissues and cells, but the expression of IL-10 was increased in tissues and cells. ELISA results suggested that nuciferine treatment inhibited the TNF-α, IL-6, and IL-1β protein expression levels compared to LPS stimulation but increased the IL-10 secretion in tissues and cells (Figure 4B).
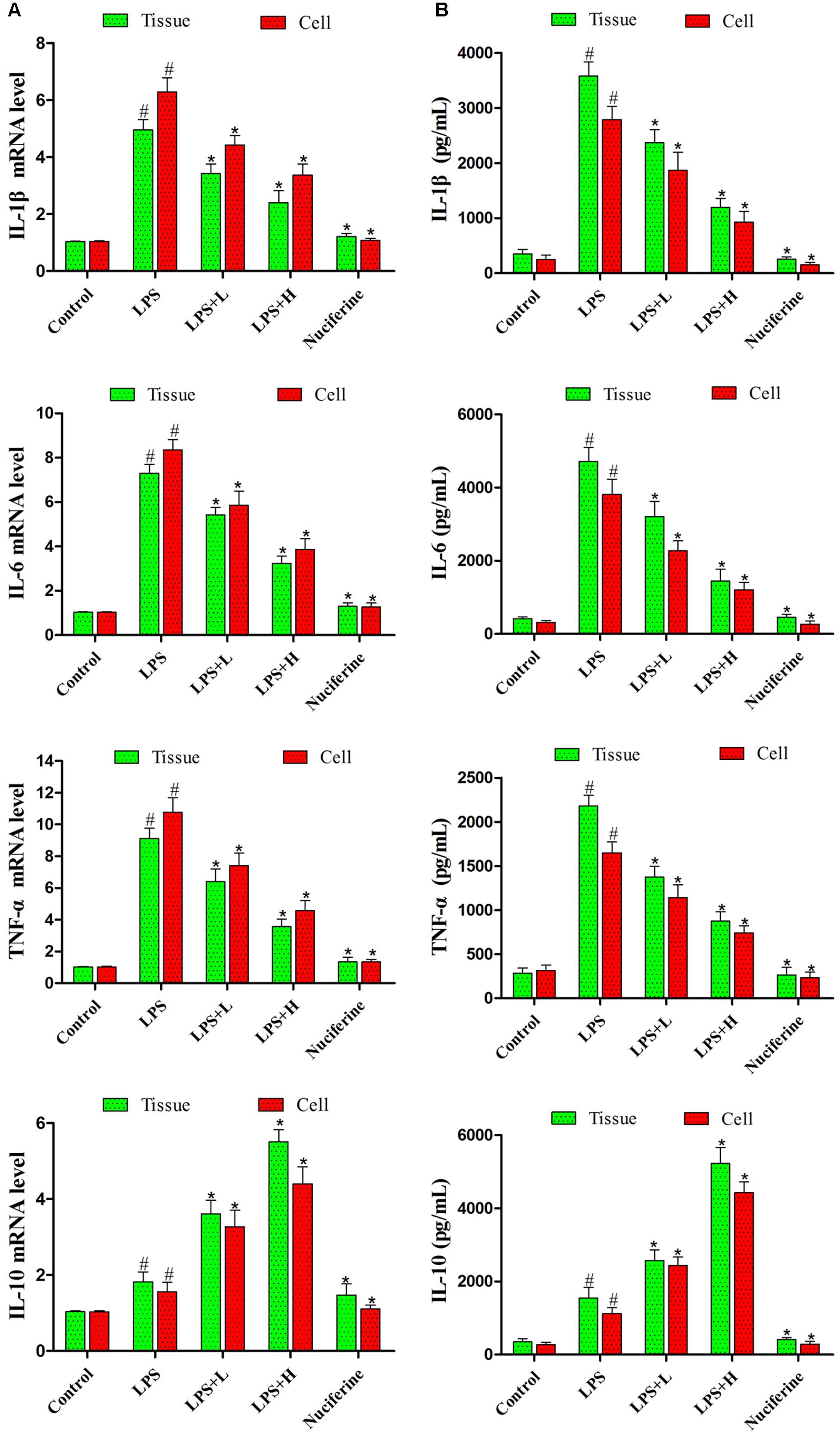
FIGURE 4. Effects of nuciferine on the secretion of cytokines. (A) The level of cytokines TNF-α, IL-6, IL-1β, and IL-10 mRNAs induced by LPS were detected qRT-PCR in tissues and cells. GAPDH served as the control. (B) The expressions of TNF-α, IL-6, IL-1β, and IL-10 were detected using an ELISA kit in tissues and cells. L and H indicate the tissues treated with 10 and 20 mg/kg nuciferine, respectively, or the cells treated with 10 and 20 μg/mL nuciferine, respectively. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Effects of Nuciferineon the Expression of TLR4
TLR4 is identified as a signaling receptor for LPS and is thought to have a role in innate recognition of bacteria (Akashi et al., 2000). The effect of nuciferine on TLR4 expression was detected with an immunoblot assay in tissues and cells. As displayed in Figure 5, TLR4 expression was increased by the LPS challenge. Nuciferine treatment obviously suppressed LPS-stimulated TLR4 expression in lung tissues and cells.
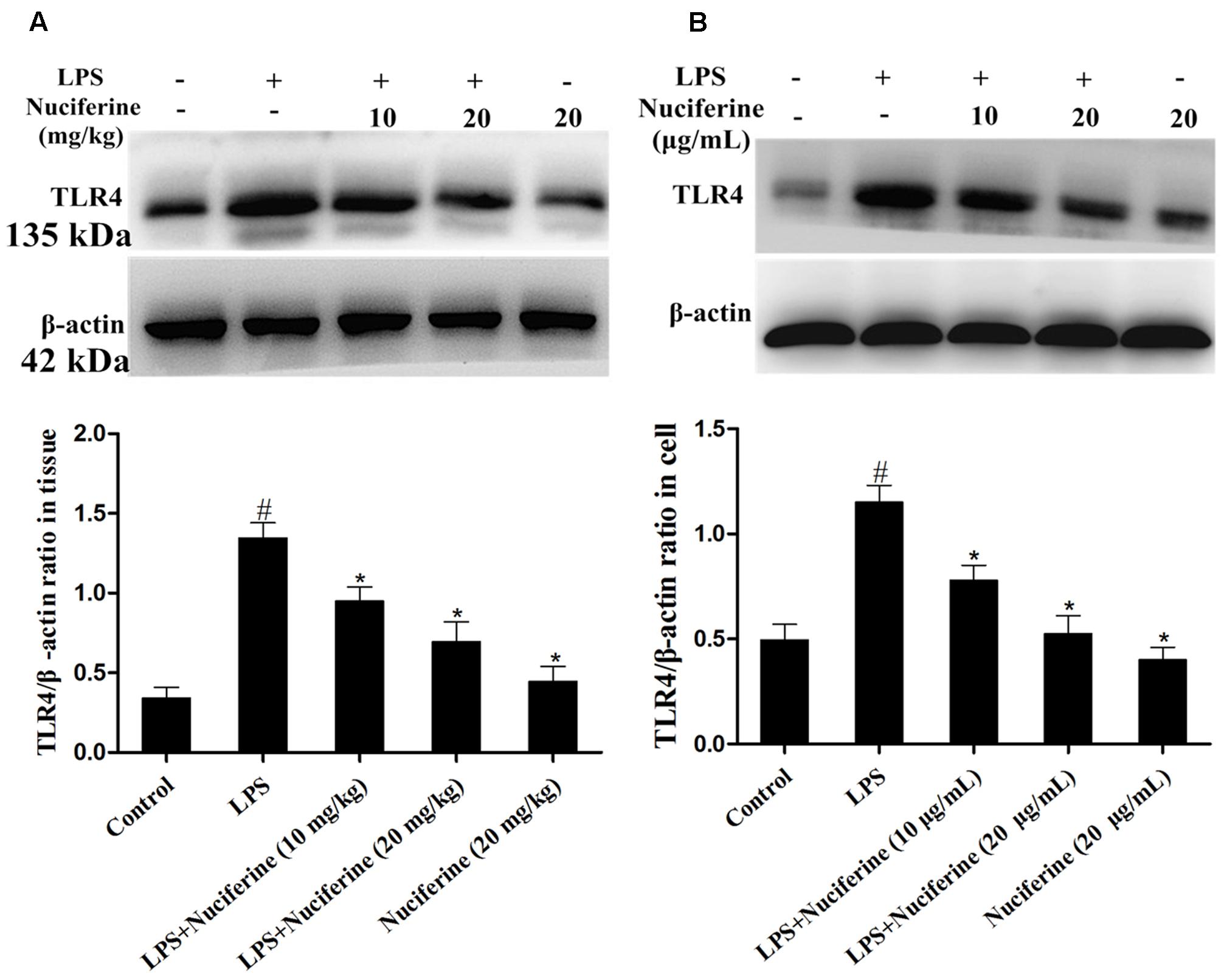
FIGURE 5. Effects of nuciferine on the expression of TLR4. (A) The effects of nuciferine on TLR4 expression was determined by Western blotting in lung tissues. (B) The effects of nuciferine on TLR4 expression was determined by Western blotting in RAW264.7 cells. β-actin served as the control. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Effects of Nuciferine on Activation of the NF-κB Pathway
The NF-κB pathway exerts a vital role in the inflammatory processes of LPS stimulation (Yang et al., 2016). We measured the effect of nuciferine on the LPS-stimulated NF-κB pathway activation in lung tissues and cells using a Western blot method. As shown in Figure 6A, the phosphorylation of p65 and IκBα proteins was clearly increased by the LPS challenge. In contrast, the phosphorylation of p65 and IκBα proteins were markedly decreased by the nuciferine treatment. The phosphorylation of p65 and IκBα proteins were greatly increased by the LPS challenge, which was reduced by nuciferine treatment in RAW264.7 cells (Figure 6B).
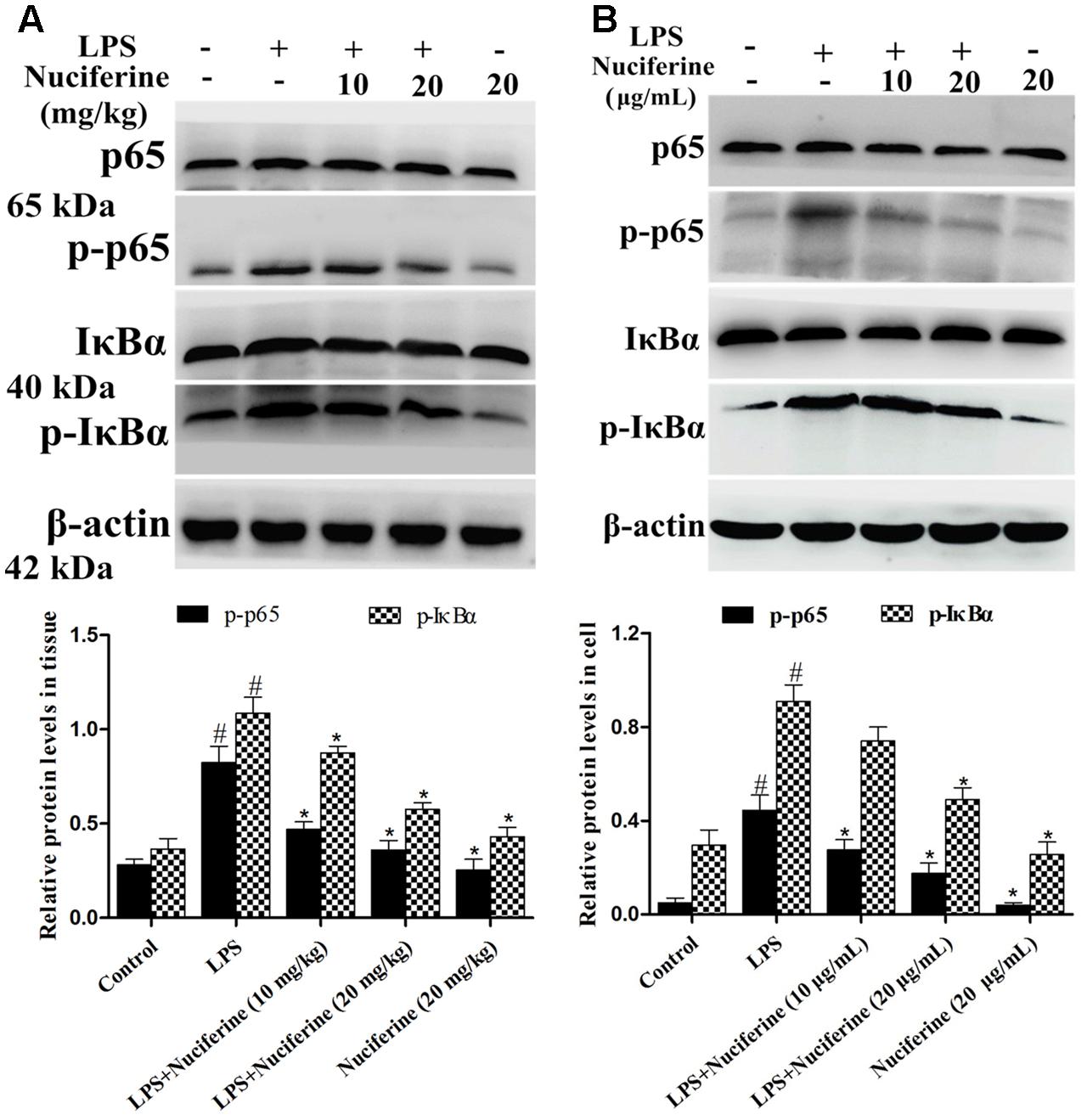
FIGURE 6. Effects of nuciferine on NF-κB pathway activation. (A) Expression of IκBα and p65 proteins in LPS-induced acute lung injury. (B) Expression of IκBα and p65 proteins in LPS-stimulated RAW264.7 cells. β-actin served as the control. These antibodies were both obtained from the Cell Signaling Technology [Phospho-NF-κB p65 (Ser536) (93H1) Rabbit mAb, NF-κB p65 (D14E12) XP® Rabbit mAb, Phospho-IκBα (Ser32) (14D4) Rabbit mAb, IκBα (L35A5) Mouse mAb]. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Effects of TLR4-siRNA Transfection on LPS-Induced Inflammatory Responses
To further confirm the anti-inflammatory mechanism of nuciferine is through the TLR4-mediated pathway, specific interference RNA (TLR4-siRNA) transfection was performed in RAW264.7 cells. RAW264.7 cells were transfected with control siRNA or TLR4 siRNA. The immunofluorescence technique was employed to detect TLR4 expression, and the results indicated that TLR4 expression was markedly decreased by TLR4-siRNA and nuciferine treatment (Figure 7A). After TLR4 interference, LPS induced the secretion of pro-inflammatory cytokines, which were measured using an ELISA kit. The results suggested the IL-1β and TNF-α secretions were clearly reduced by TLR4-siRNA and nuciferine treatment (Figure 7B). These results suggested that nuciferine inhibited the inflammatory response via the TLR4-mediated NF-κB signaling pathway.
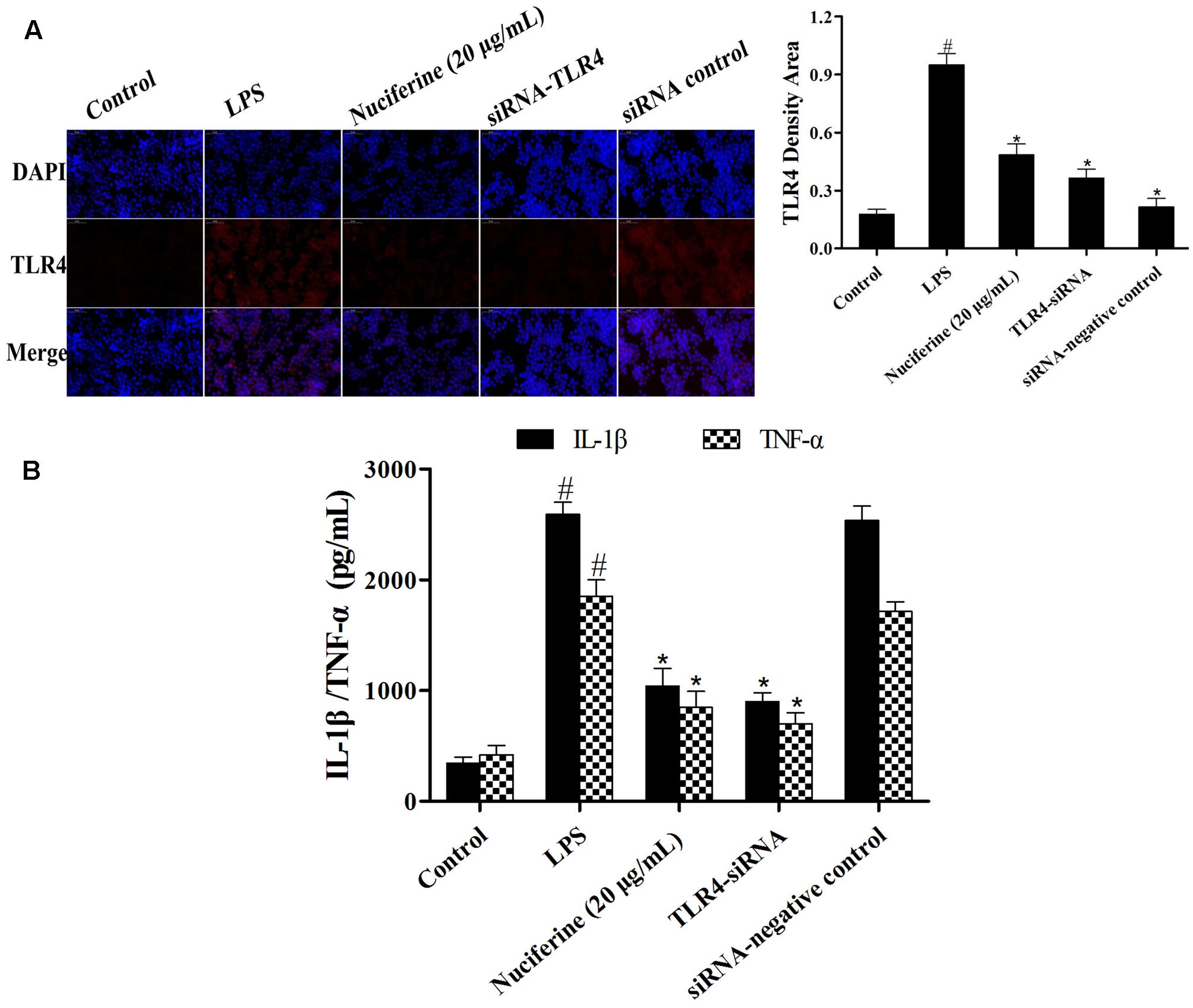
FIGURE 7. Effects of TLR4-siRNA transfection on LPS-induced inflammatory responses. (A) RAW264.7 cells were subjected to transfection with either control siRNA or TLR4 siRNA. The immunofluorescence technique was performed to determine TLR4 expression. Quantification of TLR4 positive density in each group. (B) After TLR4 interference, the LPS induced the production of pro-inflammatory cytokines IL-1β and TNF-α, which were measured using an ELISA kit. Data are represented as the mean ± SEM of three independent experiments. #p < 0.05 vs. Control group, ∗p < 0.05 vs. LPS group.
Discussion
Acute lung injury is characterized by activated neutrophils entering the pulmonary vasculature and alveoli, causing local lung or systemic inflammatory responses (Aulakh et al., 2014). LPS, a part of the cell wall of a Gram-negative bacterium, is often used to induce ALI (Agorreta et al., 2003; Alba-Loureiro et al., 2004; Wu et al., 2016a). It has been shown that nuciferine has several pharmacological characteristics, such as the anti-inflammatory activity (Wang et al., 2016). In this study, we observed that LPS induced inflammatory cell infiltration and alveolar congestion, which was significantly relieved by nuciferine treatment. The results of an in vivo study demonstrated that nuciferine relieves inflammatory injury in LPS-induced ALI.
Although inflammation is beneficial to the host’s immune defense, excessive inflammatory reactions cause damage (Kuriakose et al., 2014). Following the innate immune system activation, strong pro-inflammatory signals are generated, which not only plays important role in the stable balance of the immune system but is also important for protecting the host from the harmful effects of most inflammation and subsequent tissue repair (Cohen, 2002; Netea et al., 2004). RAW264.7 cell is not only a good anti-inflammatory screening model in vitro but is also frequently used for investigating the mechanism involved (Duan et al., 2010; Hu et al., 2017). It has been reported that inflammatory mediators increase dramatically during the LPS-induced inflammatory process (Agrawal et al., 2016). We observed that the secretions of these pro-inflammatory cytokines were reduced by nuciferine. Moreover, NF-κB served as a major regulator of immunological and inflammatory reactions (Ando et al., 2010). This protein regulates the transcription of various inflammatory cytokines with products that are involved in LPS-induced inflammation (An et al., 2002; Ma et al., 2012). The results showed that nuciferine administration suppressed phosphorylated p65 and IκBα in LPS stimulated ALI and cells.
Toll like receptor signaling is essential for immunity to various intracellular pathogens (Sasindran and Torrelles, 2011). Most of the studies indicated that LPS-regulated activation of TLR4 culminates in NF-κB transcriptional activity and pro-inflammatory mediator secretion (Abreu et al., 2001; Ando et al., 2002; Wu et al., 2016b). Thus, we hypothesized that the anti-inflammatory effect of nuciferine was achieved by suppressing activation of the TLR4-mediated NF-κB pathway. The present data showed that TLR4 expression was increased in the LPS challenge, but it was decreased in nuciferine administration. To further confirm the anti-inflammatory mechanism of nuciferine was via TLR4-mediated pathway, TLR4 was knocked down. When TLR4 was silenced, we observed that LPS induced pro-inflammatory cytokine secretion was significantly decreased by TLR4-siRNA or nuciferine treatment in LPS-induced RAW264.7 cells. Subsequently, the expression of pro-inflammatory mediators activated by NF-κB was determined in TLR4-siRNA interfering cells. The above results indicated nuciferine suppressed NF-κB activation through regulation of the TLR4 pathway.
Taken together, the results of this study demonstrated the pro-inflammatory mediators IL-1β, IL-6, and TNF-α expressions were inhibited by nuciferine administration. Moreover, the therapeutic effects of nuciferine on LPS-stimulated ALI and cells may act by suppressing the expression of TLR4 and its downstream mediated NF-κB signaling pathway. Accordingly, nuciferine might be an effective drug in preventing and treating LPS induced ALI.
Ethics Statement
All experiments involving live mice were performed according to the stipulated rules for the experimental usage of laboratory animals. All protocols were followed by the Laboratory Animal Research Center of Hubei Province and the ethics committee of Huazhong Agricultural University (Permit number: HZAUMO-2015-12).
Author Contributions
HW, and GD contributed to the conception and design of the study. HW, KJ, SG, and JY performed assays and furnished the lab study. YY and GZ performed data collection. HW, KJ, and GD conducted data analysis. HW and CQ drafted the manuscript. HW, KJ, and GD revised the paper. The authors have read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 31472254 and 31772816).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers LB, MP and handling Editor declared their shared affiliation.
References
Abdelmageed, M. E., El-Awady, M. S., Abdelrahim, M., and Suddek, G. M. (2015). LPS-RS attenuation of lipopolysaccharide-induced acute lung injury involves NF-kappaB inhibition. Can. J. Physiol. Pharmacol. doi: 10.1139/cjpp-2015-0219 [Epub ahead of print].
Abreu, M. T., Vora, P., Faure, E., Thomas, L. S., Arnold, E. T., and Arditi, M. (2001). Decreased expression of toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167, 1609–1616.
A-González, N., and Castrillo, A. (2011). Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim. Biophys. Acta 1812, 982–994. doi: 10.1016/j.bbadis.2010.12.015
Agorreta, J., Garayoa, M., Montuenga, L. M., and Zulueta, J. J. (2003). Effects of acute hypoxia and lipopolysaccharide on nitric oxide synthase-2 expression in acute lung injury. Am. J. Respir. Crit. Care Med. 168, 287–296. doi: 10.1164/rccm.200209-1027OC
Agrawal, V., Sawhney, N., Hickey, E., and McCarthy, J. V. (2016). Loss of presenilin 2 function is associated with defective LPS-mediated innate immune responsiveness. Mol. Neurobiol. 53, 3428–3438. doi: 10.1007/s12035-015-9285-0
Akashi, S., Shimazu, R., Ogata, H., Nagai, Y., Takeda, K., Kimoto, M., et al. (2000). Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164, 3471–3475.
Alba-Loureiro, T. C., Martins, E. F., Miyasaka, C. K., Lopes, L. R., Landgraf, R. G., Jancar, S., et al. (2004). Evidence that arachidonic acid derived from neutrophils and prostaglandin E2 are associated with the induction of acute lung inflammation by lipopolysaccharide of Escherichia coli. Inflamm. Res. 53, 658–663. doi: 10.1007/s00011-004-1308-7
An, H., Yu, Y., Zhang, M., Xu, H., Qi, R., Yan, X., et al. (2002). Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 106, 38–45.
Ando, I., Tsukumo, Y., Wakabayashi, T., Akashi, S., Miyake, K., Kataoka, T., et al. (2002). Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. Int. Immunopharmacol. 2, 1155–1162.
Ando, K., Hasegawa, K., Shindo, K., Furusawa, T., Fujino, T., Kikugawa, K., et al. (2010). Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 277, 2051–2066. doi: 10.1111/j.1742-4658.2010.07620.x
Aulakh, G. K., Suri, S. S., and Singh, B. (2014). Angiostatin inhibits acute lung injury in a mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L58–L68. doi: 10.1152/ajplung.00368.2012
Cho, B. O., So, Y., Jin, C. H., Nam, B. M., Yee, S. T., and Jeong, I. Y. (2014). 3-deoxysilybin exerts anti-inflammatory effects by suppressing NF-kappaB activation in lipopolysaccharide-stimulated RAW264.7 macrophages. Biosci. Biotechnol. Biochem. 78, 2051–2058. doi: 10.1080/09168451.2014.948377
Chao, W., Deng, J. S., Huang, S. S., Li, P. Y., Liang, Y. C., and Huang, G. J. (2017). 3, 4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 50, 77–86. doi: 10.1016/j.intimp.2017.06.014
Duan, G. J., Zhu, J., Wan, J. Y., Li, X., Ge, X. D., Liu, L. M., et al. (2010). A synthetic MD-2 mimetic peptide attenuates lipopolysaccharide-induced inflammatory responses in vivo and in vitro. Int. Immunopharmacol. 10, 1091–1100. doi: 10.1016/j.intimp.2010.06.010
Ferrante, C., Recinella, L., Locatelli, M., Guglielmi, P., Secci, D., Leporini, L., et al. (2017). Protective effects induced by microwave-assisted aqueous Harpagophytum extract on rat cortex synaptosomes challenged with amyloid beta-peptide. Phytother. Res. 31, 1257–1264. doi: 10.1002/ptr.5850
Hu, Y., Tao, L., Tan, H., Zhang, M., Shimizu, K., Zhang, F., et al. (2017). An active drimane-type lactone from Polygonum jucundum attenuates lipopolysaccharide-induced acute lung injury in mice through TLR4-MAPKs signaling pathway. Inflammation 40, 1204–1213. doi: 10.1007/s10753-017-0563-z
Huang, J., Li, L., Yuan, W., Zheng, L., and Guo, Z. (2016). NEMO-binding domain peptide attenuates lipopolysaccharide-induced acute lung injury by inhibiting the NF-kappaB signaling pathway. Mediators Inflamm. 2016:7349603.
Krawisz, J. E., Sharon, P., and Stenson, W. F. (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87, 1344–1350.
Kuriakose, S., Muleme, H., Onyilagha, C., Okeke, E., and Uzonna, J. E. (2014). Diminazene aceturate (Berenil) modulates LPS induced pro-inflammatory cytokine production by inhibiting phosphorylation of MAPKs and STAT proteins. Innate Immun. 20, 760–773. doi: 10.1177/1753425913507488
Li, Z., Xiao, X., and Yang, M. (2016). Asiatic acid inhibits lipopolysaccharide-induced acute lung injury in mice. Inflammation 39, 1642–1648. doi: 10.1007/s10753-016-0398-z
Liu, C. M., Kao, C. L., Wu, H. M., Li, W. J., Huang, C. T., Li, H. T., et al. (2014). Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 19, 17829–17838. doi: 10.3390/molecules191117829
Liu, W., Yi, D. D., Guo, J. L., Xiang, Z. X., Deng, L. F., and He, L. (2015). Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/beta-catenin signaling in non-small cell lung cancer. J. Ethnopharmacol. 165, 83–93. doi: 10.1016/j.jep.2015.02.015
Locatelli, M., Ferrante, C., Carradori, S., Secci, D., Leporini, L., Chiavaroli, A., et al. (2017). Optimization of aqueous extraction and biological activity of harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 31, 937–944. doi: 10.1002/ptr.5821
Lv, H., Zhu, C., Liao, Y., Gao, Y., Lu, G., Zhong, W., et al. (2015). Tenuigenin ameliorates acute lung injury by inhibiting NF-kappaB and MAPK signalling pathways. Respir. Physiol. Neurobiol. 216, 43–51. doi: 10.1016/j.resp.2015.04.010
Ma, J., Liang, H., Jin, H. R., Dat, N. T., Zhang, S. Y., Jiang, Y. Z., et al. (2012). Yangonin blocks tumor necrosis factor-alpha-induced nuclear factor-kappaB-dependent transcription by inhibiting the transactivation potential of the RelA/p65 subunit. J. Pharmacol. Sci. 118, 447–454.
Menghini, L., Ferrante, C., Leporini, L., Recinella, L., Chiavaroli, A., Leone, S., et al. (2016). An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 30, 1513–1518. doi: 10.1002/ptr.5655
Netea, M. G., Van der Meer, J. W., and Kullberg, B. J. (2004). Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 12, 484–488. doi: 10.1016/j.tim.2004.09.004
Qi, Q., Li, R., Li, H. Y., Cao, Y. B., Bai, M., Fan, X. J., et al. (2016). Identification of the anti-tumor activity and mechanisms of nuciferine through a network pharmacology approach. Acta Pharmacol. Sin. 37, 963–972. doi: 10.1038/aps.2016.53
Qiushi, W., Guanghua, L., and Guangquan, X. (2015). Acanthoic acid ameliorates lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 750, 32–38. doi: 10.1016/j.ejphar.2015.01.023
Sasindran, S. J., and Torrelles, J. B. (2011). Mycobacterium tuberculosis infection and inflammation: what is beneficial for the host and for the bacterium? Front. Microbiol. 2:2. doi: 10.3389/fmicb.2011.00002
Sun, C. Y., Pei, C. Q., Zang, B. X., Wang, L., and Jin, M. (2010). The ability of hydroxysafflor yellow a to attenuate lipopolysaccharide-induced pulmonary inflammatory injury in mice. Phytother. Res. 24, 1788–1795. doi: 10.1002/ptr.3166
Wang, D., Liu, M., Wang, Y., Luo, M., Wang, J., Dai, C., et al. (2011). Synthetic LXR agonist T0901317 attenuates lipopolysaccharide-induced acute lung injury in rats. Int. Immunopharmacol. 11, 2098–2103. doi: 10.1016/j.intimp.2011.09.002
Wang, M. X., Zhao, X. J., Chen, T. Y., Liu, Y. L., Jiao, R. Q., Zhang, J. H., et al. (2016). Nuciferine alleviates renal injury by inhibiting inflammatory responses in fructose-fed rats. J. Agric. Food Chem. doi: 10.1021/acs.jafc.6b03031 [Epub ahead of print].
Wang, W., Hu, X., Shen, P., Zhang, N., and Fu, Y. (2017). Sodium houttuyfonate inhibits LPS-induced inflammatory response via suppressing TLR4/NF-kB signaling pathway in bovine mammary epithelial cells. Microb. Pathog. 107, 12–16. doi: 10.1016/j.micpath.2017.03.011
Wu, H., Zhao, G., Jiang, K., Chen, X., Zhu, Z., Qiu, C., et al. (2016a). Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kappaB and MAPK activation. Int. Immunopharmacol. 35, 315–322. doi: 10.1016/j.intimp.2016.04.013
Wu, H., Zhao, G., Jiang, K., Li, C., Qiu, C., and Deng, G. (2016b). Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-kappaB activation. J. Agric. Food Chem. 64, 6171–6178. doi: 10.1021/acs.jafc.6b02304
Wu, Y., Li, W., Zhou, C., Lu, F., Gao, T., Liu, Y., et al. (2012). Ketamine inhibits lipopolysaccharide-induced astrocytes activation by suppressing TLR4/NF-kB pathway. Cell Physiol. Biochem. 30, 609–617. doi: 10.1159/000341442
Yang, Y. I., Woo, J. H., Seo, Y. J., Lee, K. T., Lim, Y., and Choi, J. H. (2016). Protective effect of brown alga phlorotannins against hyper-inflammatory responses in lipopolysaccharide-induced sepsis models. J. Agric. Food Chem. 64, 570–578. doi: 10.1021/acs.jafc.5b04482
Zhang, D. D., Zhang, J. G., Wu, X., Liu, Y., Gu, S. Y., Zhu, G. H., et al. (2015). Nuciferine downregulates Per-Arnt-Sim kinase expression during its alleviation of lipogenesis and inflammation on oleic acid-induced hepatic steatosis in HepG2 cells. Front. Pharmacol. 6:238. doi: 10.3389/fphar.2015.00238
Keywords: nuciferine, anti-inflammation, acute lung injury, toll-like receptor 4, nuclear factor-κB
Citation: Wu H, Yang Y, Guo S, Yang J, Jiang K, Zhao G, Qiu C and Deng G (2017) Nuciferine Ameliorates Inflammatory Responses by Inhibiting the TLR4-Mediated Pathway in Lipopolysaccharide-Induced Acute Lung Injury. Front. Pharmacol. 8:939. doi: 10.3389/fphar.2017.00939
Received: 27 September 2017; Accepted: 11 December 2017;
Published: 21 December 2017.
Edited by:
Patrizia Ballerini, Università degli Studi “G. d’Annunzio” Chieti – Pescara, ItalyReviewed by:
Jessica Roos, Goethe University Frankfurt, GermanyLuigi Brunetti, Università degli Studi “G. d’Annunzio” Chieti – Pescara, Italy
Mirko Pesce, Università degli Studi “G. d’Annunzio” Chieti – Pescara, Italy
Copyright © 2017 Wu, Yang, Guo, Yang, Jiang, Zhao, Qiu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ganzhen Deng, Z2FuemhlbmRlbmdAc29odS5jb20=
 Haichong Wu
Haichong Wu Kangfeng Jiang
Kangfeng Jiang Gan Zhao
Gan Zhao Ganzhen Deng
Ganzhen Deng