- 1Department of Behavioral Neuroscience and Methamphetamine Abuse Research Center, Oregon Health & Science University, Portland, OR, United States
- 2Department of Genetics, Genomics and Informatics, University of Tennessee Health Sciences Center, Memphis, TN, United States
- 3VA Portland Health Care System, Portland, OR, United States
Major gene effects on traits associated with substance use disorders are rare. Previous findings in methamphetamine drinking (MADR) lines of mice, bred for high or low voluntary MA intake, and in null mutants demonstrate a major impact of the trace amine-associated receptor 1 (Taar1) gene on a triad of MA-related traits: MA consumption, MA-induced conditioned taste aversion and MA-induced hypothermia. While inbred strains are fundamentally genetically stable, rare spontaneous mutations can become fixed and result in new or aberrant phenotypes. A single nucleotide polymorphism in Taar1 that encodes a missense proline to threonine mutation in the second transmembrane domain (Taar1m1J) has been identified in the DBA/2J strain. MA is an agonist at this receptor, but the receptor produced by Taar1m1J does not respond to MA or endogenous ligands. In the present study, we used progeny of the C57BL/6J × DBA/2J F2 cross, the MADR lines, C57BL/6J × DBA/2J recombinant inbred strains, and DBA/2 mice sourced from four vendors to further examine Taar1-MA phenotype relations and to define the chronology of the fixation of the Taar1m1J mutation. Mice homozygous for Taar1m1J were found at high frequency early in selection for high MA intake in multiple replicates of the high MADR line, whereas Taar1m1J homozygotes were absent in the low MADR line. The homozygous Taar1m1J genotype is causally linked to increased MA intake, reduced MA-induced conditioned taste aversion, and reduced MA-induced hypothermia across models. Genotype-phenotype correlations range from 0.68 to 0.96. This Taar1 polymorphism exists in DBA/2J mice sourced directly from The Jackson Laboratory, but not DBA/2 mice sourced from Charles River (DBA/2NCrl), Envigo (formerly Harlan Sprague Dawley; DBA/2NHsd) or Taconic (DBA/2NTac). By genotyping archived samples from The Jackson Laboratory, we have determined that this mutation arose in 2001–2003. Our data strengthen the conclusion that the mutant Taar1m1J allele, which codes for a non-functional receptor protein, increases risk for multiple MA-related traits, including MA intake, in homozygous Taar1m1J individuals.
Introduction
Substance use disorders and related traits have been characterized as complex, based on their influence by a combination of multiple genetic and environmental factors. Quantitative trait locus (QTL) mapping and genome-wide association studies have identified locations of influential gene variants. Understandably, because of the polygenic nature of these traits, the amount of genetic variance accounted for by each independent QTL has often been relatively small (Buck et al., 1997; Vadasz et al., 2007; Hall et al., 2013; Yazdani et al., 2015). Furthermore, though some genes have repeatedly been identified (Hall et al., 2013), candidate gene polymorphism associations for various addiction-related traits have not always been reproducible (Hart et al., 2013; Forero et al., 2015). A welcome exception is a recently mapped reproducible QTL that accounts for more than 50% of the genetic variance in methamphetamine (MA) intake (Belknap et al., 2013) in multiple sets of mouse lines selectively bred for high vs. low two-bottle choice MA consumption, collectively referred to as the MA drinking (MADR) lines. Among the genes within the confidence interval of this QTL on mouse chromosome 10 is the trace amine-associated receptor 1 (Taar1) gene. The direct agonist activity of MA at the receptor (TAAR1) expressed by this gene (Bunzow et al., 2001) led us to examine sequence databases for potential Taar1 polymorphisms between the C57BL/6J and DBA/2J progenitor strains of the MADR lines. We found that the DBA/2J strain possesses a single nucleotide polymorphism (SNP) in Taar1 at position 229 that encodes a missense proline (CCC) to threonine (ACC) mutation in the second transmembrane domain of the TAAR1 protein, compared to the B6 strain and 27 other inbred strains, including four wild-derived strains (Shi et al., 2016). This mutant allele has been named Taar1m1J. The impact of this amino acid change is to eliminate TAAR1 function (Harkness et al., 2015; Shi et al., 2016).
Although there could be more than one gene within the chromosome 10 QTL that influences level of MA intake, several lines of evidence support the hypothesis that this Taar1 mutation is a quantitative trait nucleotide (QTN) with a major impact. First, selective breeding for MA intake is associated with a change in the frequency of the two Taar1 allele types so that after five generations of selective breeding, the MA high drinking (MAHDR) mice are homozygously fixed for the Taar1m1J allele, whereas the MA low drinking (MALDR) mice remain homozygous for the alternate, Taar1+, allele or are heterozygous (Harkness et al., 2015). MA intake is several-fold higher in MAHDR than MALDR lines (Wheeler et al., 2009; Shabani et al., 2011), and also in Taar1 knockout mice, compared to their wildtype littermates (Harkness et al., 2015). Therefore, homozgosity for both the naturally occurring mutation and an engineered Taar1 null mutation are associated with increased MA intake. Oral MA intake differences are not associated with differences in taste sensitivity (Wheeler et al., 2009; Shabani et al., 2011). In addition, MAHDR and Taar1 knockout mice both exhibit reduced sensitivity to aversive and physiological effects of MA that could limit voluntary MA consumption (Wheeler et al., 2009; Shabani et al., 2011, 2012, 2016; Harkness et al., 2015), supporting a role for Taar1 in these traits as well.
That the DBA/2J strain is the only one of the 27 strains we have examined that harbors the Taar1m1J allele, raised the question of when this mutation arose. We were able to partially answer the question by sequencing recombinant inbred (RI) strains that were derived at different time periods from the F2 cross of the C57BL/6J and DBA/2J strains (collectively, the BXD RI strains). None of the strains created beginning in 1969, 1991, or 1998 possess the threonine substitution, whereas those produced beginning in 2008 do (Shi et al., 2016). These data confirm that the mutation is relatively recent in origin. However, we sought to narrow down when the mutation appeared during this 10-year span, because this information is important for the interpretation of data collected in DBA/2J mice before and after the mutation arose for which TAAR1 function is relevant. In addition, because DBA/2 mice can be purchased from various vendors, we sought to determine if the mutation is present in stocks at other vendors or only in those supplied by The Jackson Laboratory (Bar Harbor, ME and Sacramento, CA), the source of all genetic material so far examined for this mutation. In addition to answering these questions, we used several mouse models to further examine the association of Taar1 genotype with the triad of genetically correlated MA-related traits: MA intake, MA-induced conditioned taste aversion (CTA) and MA-induced body temperature change (or thermal response). The models used were the C57BL/6J × DBA/2J F2 cross (F2 from this point forward), BXD RI strains, and DBA/2 stock from multiple vendors. Finally, we examined Taar1 allele frequencies at earlier stages of selective breeding in the MADR lines to test the prediction that fixation of the Taar1m1J allele occurs at an early stage of selection, consistent with a major single gene effect.
Materials and Methods
Subjects
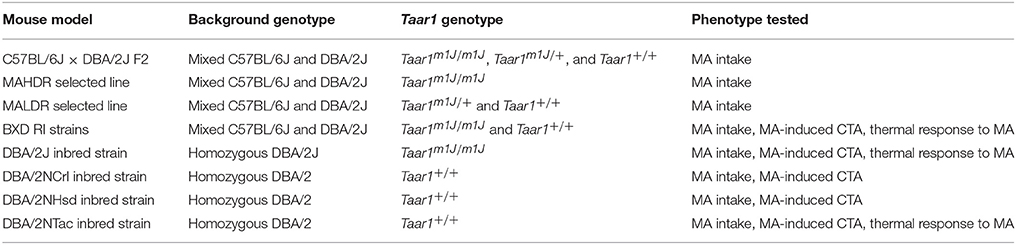
All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the VA Portland Health Care System (VAPORHCS). Mice were initially group-housed in polycarbonate shoebox cages (28.5 × 17.5 × 12 cm) with Bed-o'Cobs bedding (The Andersons, Inc., Maumee, OH) and maintained on a 12:12 h light:dark schedule, with lights on at 0600 h. Those that were not bred in-house were acclimated to the VAPORHCS for at least 2 weeks after shipping, prior to initiation of behavioral testing. Testing for MA-induced CTA and body temperature change was performed during the light phase, beginning no earlier than 2 h following lights on and no later than 2 h before lights off. The timing for assessment of MA intake is summarized in Figure 1. Mice had free access to laboratory rodent block food (Purina 5001or 5LOD PicoLab Rodent Diet; Animal Specialties, Woodburn, OR) and tap water at all times, except as described for specific procedures. See Table 1 for a summary of the genetic mouse models utilized.
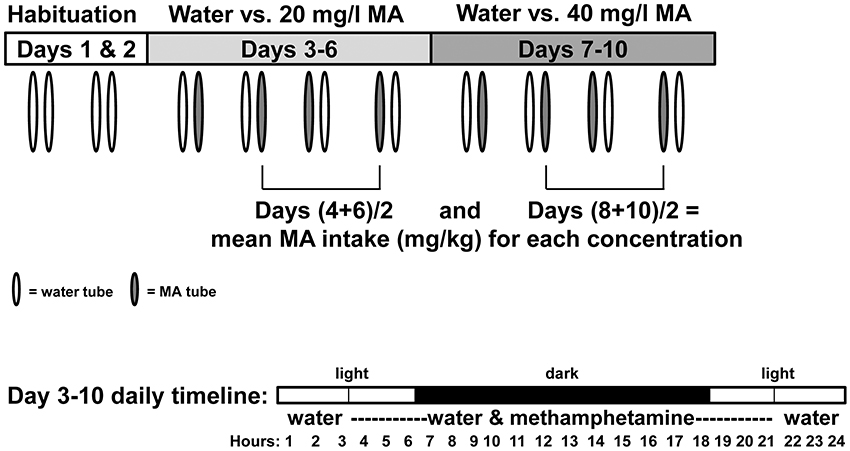
Figure 1. Diagram illustrating procedure for measuring two-bottle choice MA drinking. Mice were isolate-housed and given access to two, 25-ml graduated cylinders fitted with stoppers and sipper tubes for a 10-d period. On days 1 and 2, the tubes were filled with water. Mice were then given a choice between water and a solution of 20 mg MA/l tap water on days 3–6, and then 40 mg MA/l water on days 7–10. The MA tube was available for 18 h per day, beginning 3 h before the dark phase and ending 3 h into the light phase, as shown in the day 3–10 daily timeline. During the remaining 6 h, one tube containing water was available. The relative positions of the water and MA tubes on each day are indicated by tube coloration. Day 4 and 6 consumption values were averaged for each mouse and used to derive a mean MA intake value for the 20 mg/l concentration; Day 8 and 10 consumption values were averaged for each mouse and used to derive a mean MA intake value for the 40 mg/l concentration.
F2 Mice
For the creation of multiple serial replicate pairs of MADR selected lines that have been produced at 2-year intervals, the F2 cross of the C57BL/6J and DBA/2J strains were produced in the VAPORHCS animal facility and tested for two-bottle choice MA intake (Wheeler et al., 2009; Shabani et al., 2011). Mice of each strain and sex were purchased from The Jackson Laboratory and reciprocally crossed to create the F1 generation, which were then reciprocally crossed to create the F2. Multiple replicate MADR lines have been serially created. For the current analysis, the 156 F2 mice that were selected as breeders for the first selection (S1) generations of the replicate 3, 4, and 5 MADR lines (half of each sex), based on the amount of MA consumed using the two-bottle choice MA drinking procedure described later, were genotyped for the Taar1 polymorphism at position 229. Possible genotypes were homozygous Taar1m1J, homozygous Taar1+, and heterozygous for the two Taar1 allele types. Mice were 53–68 days of age at the start of testing for MA intake (mean ± SEM = 59 ± 0.5 days).
MADR Mice
Five replicate sets of MAHDR and MALDR lines have been produced. Taar1 position 229 SNP genotype and MA intake data for 208 MADR mice that served as breeders for various selection generations were available to examine genotype-phenotype association. Data were for mice selected as breeders from replicate 3 selection generation 3 (S3), replicate 4 S1, and replicate 5 S1 and S2. There were 26 mice (half of each sex) per selected line per replicate/generation. MA intake studies were initiated in these mice at 50–66 days of age (mean ± SEM = 59 ± 0.4 days).
BXD RI Mice
Breeding pairs for several BXD RI strains were obtained from RWW (University of Tennessee Health Science Center, Memphis, TN). Specific strains were chosen on the basis of their Taar1 position 229 genotype (Shi et al., 2016) to allow tests of the associations between Taar1 genotype and phenotypes. Breeders were established within the VAPORHCS and their offspring were tested for two-bottle choice MA intake, as described in Figure 1 and in our previous publications (Wheeler et al., 2009; Shabani et al., 2011). Mice from the following strains were tested and were homozygous for either the reference Taar1+ allele or the mutant Taar1m1J allele: Taar1+ genotype: BXD154, BXD161, BXD184, BXD196, BXD199, BXD205, BXD212, and BXD215; mutant Taar1m1J genotype: BXD113, BXD160, BXD171, BXD178, BXD186, BXD194, BXD204, and BXD210. The total number of mice tested for MA intake across all strains was 206 (120 female and 86 male), with a range of 3-28 per strain; mice were 53–114 days old (mean ± SEM = 79.2 ± 0.9). Some of the same BXD RI strains were also tested for MA-induced CTA and effects on core body temperature (independent sets of mice). For CTA, the strains were Taar1+ genotype: BXD154, BXD161, BXD196, BXD199, BXD205, and mutant Taar1m1J genotype: BXD113, BXD178, and BXD216; for the temperature study, the strains were Taar1+ genotype: BXD154, BXD161, BXD184, BXD196, BXD199, BXD205 and BXD212, and mutant Taar1m1J genotype: BXD113, BXD160, BXD171, BXD178, BXD194 and BXD210. The total number of mice tested across all strains for MA-induced CTA was 47 (24 female and 23 male) with a range of 1–7 per MA dose per strain, and mice were 72–98 days old (mean ± SEM = 83.2 ± 0.7 days). There were 223 BXD RI mice (117 female and 106 male) tested for MA effects on body temperature, with a range of 1–12 per MA dose per strain; mice were 49–124 days of age at the time of testing (mean ± SEM = 75 ± 0.9 days). The number of mice tested for some strains was small; however, to examine the genotype-phenotype associations, strain was not included as a factor; rather the BXD RI were considered as a single population. Thus, number tested per strain is informative, but not relevant to the current analysis, as all strains share a common C57BL/6J × DBA/2J background and the goal was to examine relationships with this particular genetic polymorphism.
Standard Inbred Strains
DBA/2 mice were purchased from several vendors, including DBA/2J from The Jackson Laboratory, DBA/2NCrl from Charles River (Wilmington, MA, USA), DBA/2NHsd from Envigo (Indianapolis, IN, USA; formerly Harlan Sprague Dawley), and DBA/2NTac from Taconic (Albany, NY, USA). Six mice were obtained per sex per vendor and were genotyped for Taar1 sequence at position 229 and tested for two-bottle choice MA consumption (Wheeler et al., 2009; Shabani et al., 2011). Mice were 56–70 days old at the initiation of testing (mean ± SEM = 56.7 ± 2.8 days). A separate cohort of mice from these four vendors (7–8 per sex per strain per dose) was tested for MA-induced CTA at age 73–93 days (mean ± SEM = 83.1 ± 1.1 days), and another cohort of DBA/2J and DBA/2NTac mice (11–12 per sex per strain per dose) was tested for MA-induced core temperature change at age 83–86 days (mean ± SEM = 84.5 ± 0.1 days); at the time of testing for thermal effects, DBA/2 mice were not available from some vendors.
Drugs, Reagents, and Biological Samples
(+)MA hydrochloride was purchased from Sigma (St. Louis, MO USA) and dissolved in tap water for drinking or in sterile 0.9% saline (Baxter Healthcare Corp., Deerfield, IL, USA) for injection. For CTA studies, sodium chloride (NaCl) was obtained from Sigma (St Louis, MO, USA) and 11.7 g of NaCl was dissolved in 1 liter of tap water to achieve a 0.2M solution. DNA samples used to narrow down the time when the spontaneous mutation arose were extracted from frozen spleen tissue archived at The Jackson Laboratory for DBA/2J mice from generations 208, 218, 221, and 225. These samples were sequenced locally for the Taar1 SNP at position 229. Additional samples from generations 215 and 216 were sequenced by the technical division at The Jackson Laboratory, using our procedures (described below).
Taar1 Sequencing
Genomic DNA was extracted from ear punch, tail, or spleen samples using QuickExtract DNA Extraction Solution (Epicenter, Madison, WI). Taar1 was amplified using a Hotstart DNA polymerase kit (Qiagen, Valecia, CA), with sequence-specific primers surrounding the region of interest (see Harkness et al., 2015 for primer details). After amplification, PCR products were run on an agarose gel and then purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Purified DNA samples were sequenced at the Oregon Health & Science University Sequencing Core using the forward and reverse primers to amplify the Taar1 gene. Sequences of the PCR products were aligned with the mouse Taar1 sequence (NM_053205.1). Some samples were genotyped using a rtPCR procedure that was more recently developed in our laboratory, simple to conduct and produces accurate sequence data, based on direct comparison of samples genotyped using both methods. Development of this rtPCR assay was based on standard Taqman methods (Shen et al., 2009) in which fluorescent probes were used to detect the SNP in the Taar1 gene.
Two-Bottle Choice MA Drinking
Procedures were as described for selective breeding for two-bottle choice MA drinking (Wheeler et al., 2009; Shabani et al., 2011) and are summarized in Figure 1. Two initial days when mice had access to 2 tubes filled with tap water served to familiarize them with the novel drinking apparatus and provide total volume intake data prior to MA access. The 18-h per day MA access period has been used in all of our selection studies; pilot studies indicate that MA intake is higher with 6 h withdrawal between MA access periods, compared to 24-h access. The MA and water tube positions were switched every other day to account for potential drinking side bias. Consumption values for the second and fourth days of access for each MA concentration are averaged for each mouse, because these days represent the second day after a tube position switch, when mice should be fully familiar with the location of the MA tube. MA consumption was measured in ml (accuracy = 0.2 ml) and then converted to mg/kg, based on body weight measured every 2 days. Total volume consumed (ml) prior to MA access and during the 18-h MA access periods were also analyzed.
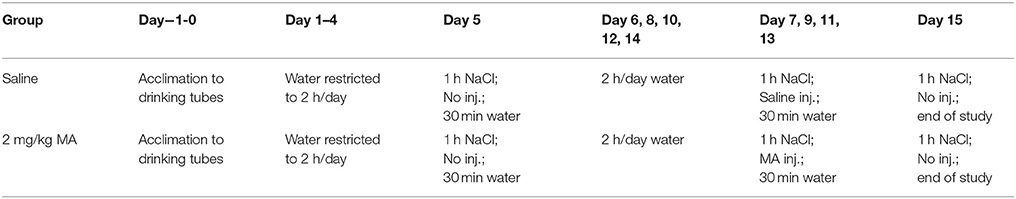
MA-Induced CTA
Mice were tested for sensitivity to the conditioned aversive effect of 2 mg/kg MA, using established procedures that utilize a 0.2M NaCl solution as a conditioned cue for the interoceptive effects of MA (Table 2). Mice with a low level of MA intake exhibit profound CTA to this dose of MA, indicated by a reduction in NaCl consumption (ml) across MA treatment days, whereas mice with a high level of MA intake exhibit no CTA to this dose (Wheeler et al., 2009; Shabani et al., 2011; Harkness et al., 2015). Mice were singly-housed, acclimated for 2 days to drinking from 10-ml graduated cylinders fitted with stoppers and sipper tubes (study days −1 and 0), and then their water access was limited to 2 h per day for a 4-day acclimation period to induce motivation to drink the novel NaCl solution (study days 1–4). Beginning on day 5, the NaCl solution was offered for 1 h every other day for a total of 6 presentations (days 5, 7, 9, 11, 13, and 15), and consumption was measured in ml by measuring tube volumes before and after each drinking session. Except on day 5 (when mice were introduced to the novel taste to reduce neophobia) and 15 (the last test day), mice were injected with saline or 2 mg/kg MA immediately after the 1-h NaCl drinking period. Day 5 data were not included in the analysis for CTA, consistent with our previous studies (Wheeler et al., 2009; Shabani et al., 2011; Harkness et al., 2015). About 3 h post-injection, mice were given access to water for 30 min each day to avoid dehydration. On intervening days, mice had 2 h of access to water and there were no experimental manipulations.
MA Effects on Core Body Temperature
Mice were tested for the effect of 2 mg/kg MA on body temperature at an ambient temperature of 21 ± 2°C. This dose of MA was chosen based on previous results indicating that mice with a low level of MA intake exhibit a profound hypothermic response to 2 mg/kg MA at this ambient temperature that is absent in mice with a higher level of MA intake (Harkness et al., 2015). Mice were weighed and then placed into individual perforated acrylic plastic cubicles (8 × 19 × 8 cm in W × H × L), that served to prevent huddling-associated alterations in body temperature (Crabbe et al., 1987, 1989). After placement, they remained undisturbed for 1 h and then a baseline rectal temperature was obtained (T0). Mice then received an injection of saline or MA, were returned to their respective cubicles, and rectal temperature was again measured at 30, 60, 90, 120, and 180 min post-injection (T30-180). This time-course was determined from our former research results (Harkness et al., 2015). To obtain these temperatures, each mouse was removed from its cubicle, gently restrained by the scruff of the neck, and a glycerin-coated, 5 mm probe attached to a Thermalert model TH-8 digital thermometer (Sensortek, Clifton, NJ) was inserted 2.5 cm into the rectum for 5 s. The mouse was then immediately returned to its cubicle.
Data Analysis
Statistica 64 Academic Software (Dell, Inc., Tulsa, OK, USA) was used for all analyses. Animals of both sexes were used in all experiments. MA intake (mg/kg), total volume consumed (ml), and NaCl volume intake (ml) data (for CTA studies) were analyzed by repeated measures analysis of variance (ANOVA) with MA concentration or test day as the repeated measure, and sex, genotype or vendor, replicate/generation, and dose as possible independent variables. MA-induced changes in body temperature were analyzed by repeated measures ANOVA with time as the repeated measure, and sex, genotype or vendor, and dose as possible independent variables. Effects were considered statistically significant at p < 0.05. Two-way interactions were interpreted using simple main effects analysis and post hoc Newman-Keuls mean comparisons were performed when appropriate. Pearson's r was used to calculate correlations between phenotypes and Taar1 genotype. The chi-squared test was used to compare expected vs. observed Taar1 genotype frequencies.
Results
Frequency of Taar1 Genotypes in F2 and MADR Mice
The key findings from this analysis are that there was a higher frequency of the homozygous Taar1m1J genotype in populations of mice chosen to serve as breeders for the high MA intake lines, compared to the low MA intake lines, and that this difference arose as early as in the F2 mice that served as breeders for the first selection generation.
F2 Mice
Taar1 genotype results for the 156 F2 mice that were tested for MA intake and selected to produce the S1 generations of the replicate 3, 4, and 5 MADR lines are illustrated in Figure 2. In Figure 2A (left panel) are the expected genotype frequencies for the null hypothesis that Taar1 genotype is not relevant to MA intake, alongside the actual frequencies of Taar1 genotypes. Also in Figure 2A (right panel) are genotype frequencies for the F2 mice selected as breeders specifically for the MAHDR vs. MALDR lines (replicates combined). The expected 1:2:1 ratio of Taar1m1J/m1J: Taar1m1J/+: Taar1+/+ genotypes for the null hypothesis (which is 39:78:39 for 156 mice) significantly diverged from the observed ratio of 31:56:69 (chi-squared = 30.9, p < 0.001; Figure 2A, left panel). The larger number of Taar1m1J homozygotes among the F2 chosen as breeders of the MAHDR lines, compared to MALDR lines, is apparent (68:1; Figure 2A, right panel). In Figure 2B are the expected genotype frequencies for the null hypothesis (which is 1:2:1 for each line or 6.5:13:6.5) and the observed genotype frequencies for each replicate, to illustrate consistency of the results. Again, the genotype frequencies for each population of F2 mice chosen to serve as breeders, based on their extreme high or low MA drinking phenotypes, significantly diverged from the expected frequencies. Chi-squared values were 161.3 (p < 0.001) and 24.8 (p < 0.001) for MAHDR and MALDR collapsed on replicate, respectively. The larger frequency of the Taar1m1J/m1J genotype in each MAHDR line, compared to each MALDR line is apparent; 20:1, 23:0 and 25:0 for replicate 3, 4, and 5, respectively.
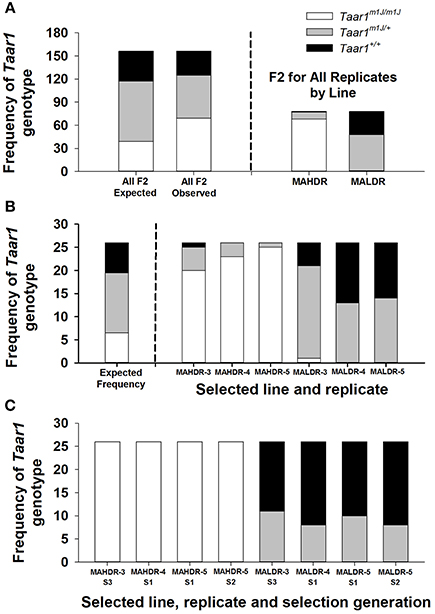
Figure 2. Taar1 genotype frequencies are altered by selective breeding for high and low levels of MA intake, beginning with a founding population of C57BL/6J × DBA/2J F2 mice. Shown in A,B are the frequencies of Taar1 genotypes for F2 mice that were tested for MA drinking and selected to produce the first selection generation (S1) offspring of several replicates of the MADR lines of mice. (A) Expected frequency (based on the null hypothesis of no role for Taar1 in MA intake) and observed frequency of homozygous Taar1m1J (in white), heterozygous Taar1m1J/+ (in gray), and homozygous Taar1+ (in black) mice among all selected F2 breeders (left) and observed frequencies for the F2 breeders separated by selected line (right). (B) Expected Taar1 genotype frequency (left) and observed frequencies for each line and replicate (right). (C) Taar1 genotype frequencies for the breeders of several replicates and selection generations (Sx) of the MAHDR and MALDR lines.
MADR Mice
Data from the F2 populations indicate rapid fixation of the Taar1m1J allele in the MAHDR lines. However, whether fixation of the Taar1+ allele was achieved or heterozygosity remained in the MALDR lines by the end of selection was unknown. To examine this, we searched our archives for animals from the various replicate sets of lines and generations for which we had both Taar1 genotype and MA intake data. The overall outcome of this analysis is that heterozygosity, rather than fixation of the Taar1+ allele, was retained in the MALDR lines at the end of selection. Taar1 genotype frequencies are shown in Figure 2C for four sets of animals. Genotyping results identified all MAHDR mice (n = 104) as homozygous Taar1m1J. This is consistent with the high frequency of Taar1m1J homozygotes that were selected from the F2 population to generate MAHDR lines of multiple replicates (Figure 2B). The MALDR mice were all heterozygous or homozygous Taar1+, again consistent with findings for the F2 mice that served as breeders of the MALDR lines (Figure 2B). When considering all 104 MALDR mice, the observed ratio of Taar1m1J/+: Taar1+/+ was 37:67. Assuming an equal probability of either genotype serving as a member of the breeding pairs for the MALDR lines, the expected ratio would be 1:4:7 Taar1m1J/m1J: Taar1m1J/+: Taar1+/+, since possible breeding combinations would be Taar1+/+ x Taar1+/+; Taar1m1J/+ x Taar1+/+, and Taar1m1J/+ x Taar1m1J/+. Thus, one animal in twelve would be expected to be a Taar1m1J homozygote, to exhibit higher MA intake, and be unlikely to be subsequently selected as a breeder. If all breeders are binned into the Taar1m1J/+ and Taar1+/+ classes, and the Taar1m1J/m1J animals are “lost,” the predicted ratio of Taar1m1J/+: Taar1+/+ would be 4:7, which is 38:66 for the current 104 MALDR breeders. The observed ratio of these genotypes was 34:67, which did not significantly differ from expectation, based on the chi-squared test. Frequencies by replicate/generation (Figure 2C) did not significantly differ from the expected 4:7 ratio (9.5:16.5 for the 26 animals per generation). Furthermore, there was little change in the ratio with increasing selection generation. The remaining heterozygosity is consistent with dominance of the Taar1+ allele for level of MA intake.
MA Drinking in F2 and MADR Mice
The key finding from this analysis in both the F2 and MADR mice is that Taar1 genotype is strongly associated with MA intake.
F2 Mice
MA consumption data for the F2 mice are presented in Figure 3A. A repeated measures ANOVA was performed on MA intake data, with sex, Taar1 genotype, replicate, and MA concentration as independent variables. There were no significant main or interaction effects involving sex, so data were further analyzed for the sexes combined. There was a significant Taar1 genotype × MA concentration interaction [F(2, 147) = 64.4, p < 0.0001]; homozygous Taar1m1J mice consumed significantly more MA than Taar1m1J/+ or homozygous Taar1+ mice for both MA concentrations, and consumed more MA when the concentration was 40 mg/l. Heterozygous Taar1m1J/+ mice and homozygous Taar1+ mice did not differ from each other or exhibit MA concentration-dependent effects. There was a significant Taar1 genotype × replicate interaction [F(4, 147) = 3.9, p < 0.01] that was associated with higher MA intake in homozygous Taar1m1J replicate 5 F2 mice, compared to homozygous Taar1m1J F2 mice from replicates 3 and 4.
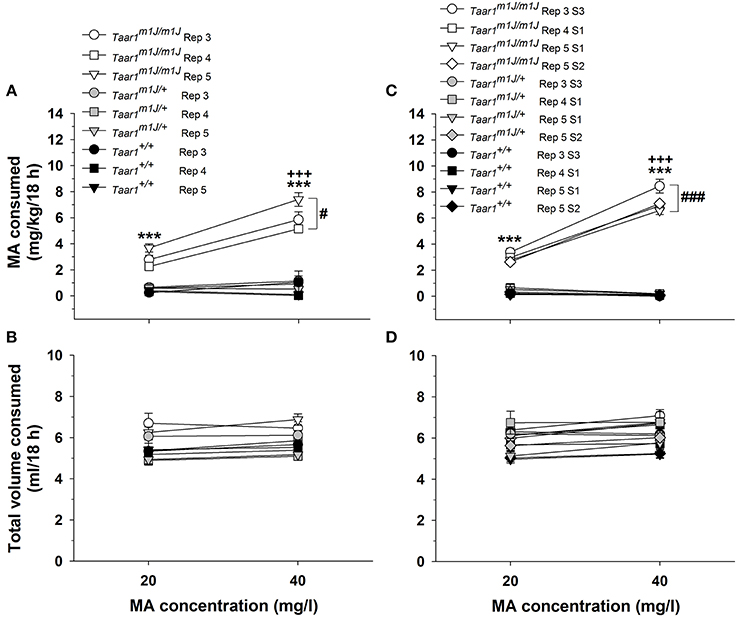
Figure 3. The homozygous Taar1m1J genotype is associated with higher MA intake. (A) Mean ± SEM MA consumption when MA was offered vs. water in 20 and 40 mg/l concentrations for F2 mice selected to produce the S1 generation MADR line offspring of replicate (Rep) 3–5, separated by Taar1 genotype; Taar1 genotype frequencies for these mice are those in Figures 2A,B. (B) Mean ± SEM total amount of fluid consumed by the same F2 mice from both the water and MA tubes, during the 18-h period when MA was available. (C) Mean ± SEM MA consumption when MA was offered vs. water in 20 and 40 mg/l concentrations to MADR mice of several replicates and selection generations, for which Taar1 genotype frequencies are shown in Figure 2C. (D) Mean ± SEM total amount of fluid consumed by the same MADR mice from both the water and MA tubes, during the 18-h period when MA was available. ***p < 0.001 for the comparison of homozygous Taar1m1J to heterozygous and homozygous Taar1+ mice; +++p < 0.001 for the comparison of MA consumption from the 20 and 40 mg/l MA concentrations within the homozygous Taar1m1J mice; #p < 0.05 for overall more MA consumed in Rep 5 compared to Rep 3 and Rep 4 homozygous Taar1m1J mice; ###p < 0.001 for overall more MA consumed in Rep 3 S3 Taar1m1J mice, compared to Rep 4 S1 and Rep 5 S1 and S2 Taar1m1J mice.
Total amount of fluid consumed during the time that MA was available was also impacted by Taar1 genotype in the F2 mice (Figure 3B). There were no significant effects involving sex in the initial analysis, so data were considered for the sexes combined. There were significant main effects of Taar1 genotype [F(2, 147) = 11.0, p < 0.0001], MA concentration [F(1, 147) = 8.3, p < 0.01] and replicate [F(2, 147) = 4.9, p < 0.01], but no significant interaction effects. Homozygous Taar1m1J mice consumed more total volume than Taar1m1J/+ or homozygous Taar1+ mice (mean ± SEM = 6.2 ± 0.1 vs. 5.4 ± 0.2 and 5.4 ± 0.2 ml, respectively; ps < 0.01), total volume consumed was greater when the 40 mg/l MA concentration was available (mean ± SEM = 5.6 ± 0.1 vs. 5.8 ± 0.1 ml for 20 and 40 mg/l, respectively; p < 0.01), and replicate 4 mice consumed less total volume than replicates 3 and 5 (mean ± SEM = 6.1 ± 0.2, 5.3 ± 0.2 and 5.7 ± 0.2 ml for replicate 3, 4, and 5, respectively; ps < 0.05). When water intake data for the same 18-h period averaged for the 2 days prior to MA access were similarly analyzed, there was no significant sex, Taar1 genotype, or replicate effect.
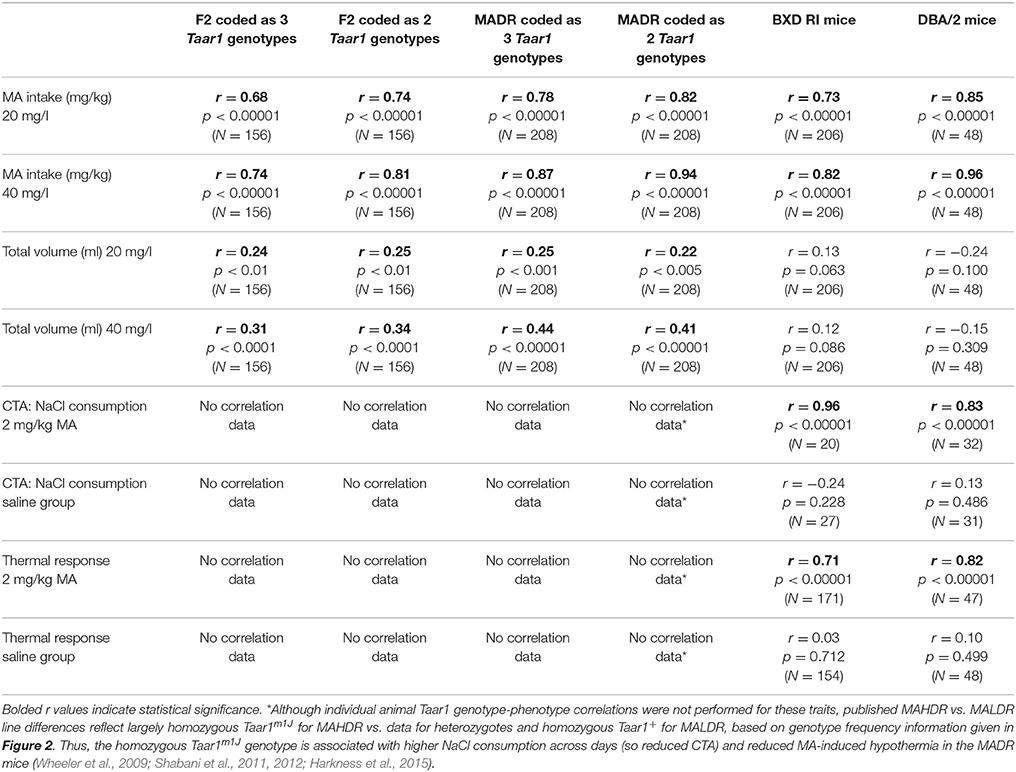
Finally, correlations were calculated between Taar1 genotype and intake measures. Results are presented in Table 3. First, correlations were considered for mice of the 3 possible Taar1 genotypes: Taar1m1J/m1J, Taar1m1J/+, and Taar1+/+. For MA intake, the correlations with MA intake were highly significant and Taar1 genotype accounted for 46 and 55% of the variance in MA intake for the 20 and 40 mg/l MA concentrations, respectively. For total volume, the correlations were smaller, but also significant, with Taar1 genotype accounting for 6 and 10% of the variance, during the periods when the 2 MA concentrations were available. Data were also examined with mice that were Taar1m1J/+, and Taar1+/+ coded as a single class, based on evidence for dominance of the Taar1+ allele on MA intake (Harkness et al., 2015). The amount of variance for which Taar1 genotype accounted increased to 55 and 66% for MA intake for the 20 and 40 mg/l concentrations, respectively (Table 3). Recoding the data in this way had a smaller impact on the amount of variance in total volume consumed accounted for by Taar1 genotype, with values of 6 and 12%.
MADR Mice
A repeated measures ANOVA was performed on MA intake data (Figure 3C), with sex, Taar1 genotype, replicate/generation and MA concentration as independent variables. There was a significant Taar1 genotype x MA concentration interaction [F(2, 183) = 272.9, p < 0.0001], but genotype-dependent effects were significant for both the 20 and 40 mg/l MA concentrations (ps < 0.001). Homozygous Taar1m1J mice consumed significantly more MA than heterozygotes and homozygous Taar1+ mice for both MA concentrations. MA intake was concentration-dependent only in homozygous Taar1m1J mice; they consumed more MA when the 40 mg/l concentration was available. There was also a significant Taar1 genotype × replicate/generation interaction [F(6, 183) = 4.0, p < 0.001]. The S3 homozygous Taar1m1J mice from replicate 3 consumed more MA compared to the S1 and S2 Taar1m1J homozygotes from the other replicates (mean ± SEM = 5.9 ± 0.15 vs. 5.0 ±.15, 4.7 ± 0.15, and 4.9 ± 0.15 mg/kg for replicate 3 S3 vs. replicate 4 S1, replicate 5 S1 and replicate 5 S2, respectively; ps < 0.001), supporting an effect of continued selection for high MA intake. There were no significant differences between the heterozygote and Taar1+ homozygote mice, within or across replicate/generation, indicating rapid fixation of the low MA intake trait in breeders chosen for selection. Finally, there was a significant sex × Taar1 genotype interaction [F(2, 183) = 7.7, p < 0.001]. Female, homozygous Taar1m1J mice consumed significantly more MA than male, homozygous Taar1m1J mice (mean ± SEM = 5.5 ± 0.1 and 4.7 ± 0.1 mg/kg, respectively; p < 0.001), whereas there were no sex differences within the heterozygote or Taar1+ homozygote genotypes.
There were no significant effects involving sex for total amount of fluid consumed during the time that MA was available, so data were considered for the sexes combined (Figure 3D). There was a significant main effect of replicate/generation [F(3, 195) = 5.1, p < 0.01]; total volume for S1 and S2 replicate 5 animals was less than for replicate 3 S3 mice (mean ± SEM = 5.7 ±.16 and 5.8 ±.16 vs. 6.4 ±.16 ml, respectively; ps < 0.01). There was also a significant Taar1 genotype x MA concentration interaction [F(2, 195) = 11.7, p < 0.0001]. Homozygous Taar1m1J mice consumed more total volume than homozygous Taar1+ mice, for both MA concentrations (mean ± SEM = 6.2 ±.11 vs. 5.5 ±.14 and 6.8 ±.11 vs. 5.6 ±.14 ml, respectively; ps < 0.01). Homozygous Taar1m1J mice consumed more total volume than mice heterozygous for Taar1 allele type only when the 40 mg/l MA concentration was offered (mean ± SEM = 6.8 ±.11 vs. 6.2 ±.19 ml, respectively; p < 0.01). Also, during the time that the 40 mg/l MA concentration was offered, mice heterozygous for the Taar1 allele consumed significantly more total volume than Taar1+ mice (mean ± SEM = 6.2 ±.19 vs. 5.6 ±.14 ml, respectively; p < 0.05). When water intake data for the same 18-h period averaged for the 2 days prior to MA access were similarly analyzed, there were no significant sex or Taar1 genotype effects.
Finally, correlations were calculated between Taar1 genotype and intake measures during the MA access period. Results are presented in Table 3. When data were coded as the 3 possible genotypes, Taar1 genotype accounted for 61 and 76% of the variance in MA intake for the 20 and 40 mg/l MA concentrations, respectively. For total volume, the correlations were smaller, but also significant, with Taar1 genotype accounting for 6 and 19% of the variance during the periods when the 2 MA concentrations were available. When data for the heterozygotes and Taar1+ homozygotes were coded as a single group, the amount of variance for which Taar1 genotype accounted increased to 67 and 88% for MA intake for the 20 and 40 mg/l MA concentrations, respectively. Recoding the data in this way had little impact for total volume consumed, with Taar1 genotype accounting for 5 and 17% of the variance.
Taar1 Genotype and MA Intake in BXD RI Mice
Data plotted in Figures 4A,B illustrate the frequency of the 2 possible Taar1 genotypes with regard to amount of MA consumed for the 206 BXD RI mice, and confirm the association of homozygosity for Taar1m1J with heightened MA intake. Because these mice are inbred, all individuals were either homozygous Taar1m1J (n = 95; 53 male and 42 female) or homozygous Taar1+ (n = 111; 67 male and 44 female). MA intake was impacted by Taar1 genotype. A repeated measures ANOVA with MA consumption data grouped on sex, Taar1 genotype, and MA concentration identified a significant Taar1 genotype x MA concentration interaction [F(1, 202) = 212.0, p < 0.0001], with homozygous Taar1m1J mice consuming significantly more MA from both the 20 (Figure 4A) and 40 (Figure 4B) mg/l concentration solutions, compared to homozygous Taar1+ mice (ps < 0.0001). Only the Taar1m1J homozygotes exhibited concentration-dependent MA intake and consumed significantly more MA when the MA concentration was 40 mg/l, compared to 20 mg/l (p < 0.0001). There was also a significant sex × Taar1 genotype interaction [F(2, 202) = 4.0, p < 0.05]. Female, homozygous Taar1m1J mice consumed about 0.5 mg/kg more MA compared to males (mean ± SEM = 3.4 ± 0.13 vs. 2.9 ± 0.15 mg/kg, respectively; p < 0.05); there was no sex difference in homozygous Taar1+ mice.
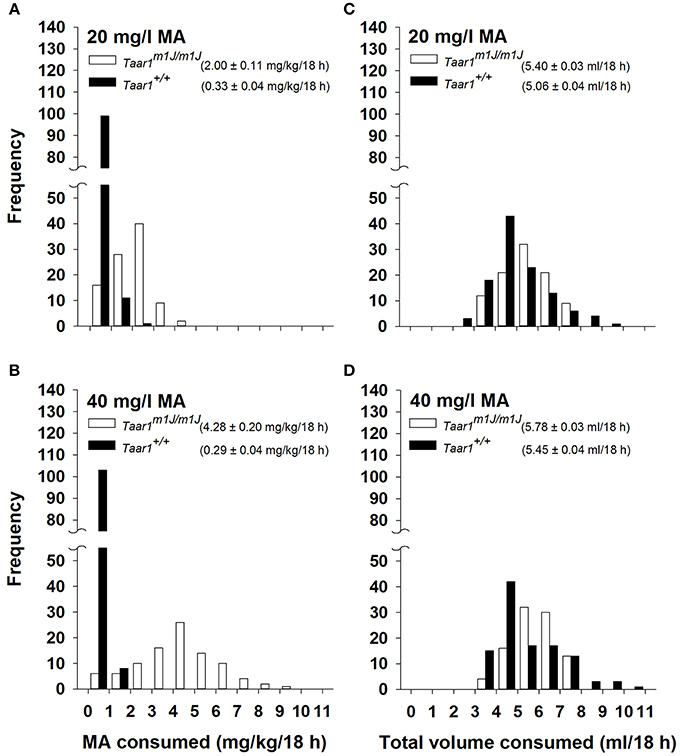
Figure 4. Frequency distributions for (A) 20 mg/l MA consumed, (B) 40 mg/l MA consumed, (C) total volume consumed during 20 mg/l MA access, and (D) total volume consumed during 40 mg/l MA access in 206 BXD RI mice, according to Taar1 genotype. Mean ± SEM MA or total volume consumed is given in the legend for each Taar1 genotype.
Taar1 genotype frequency data are plotted for total volume consumed in Figures 4C,D. There were no effects of sex for total volume and Taar1 genotype did not have a significant impact on total volume consumed. However, there was a significant effect of MA concentration, with about 0.39 ml more total volume consumed during the period when the 40 mg/l MA concentration was available [F(1, 202) = 65.7, p < 0.0001]. When water intake data for the same 18-h period averaged for the 2 days prior to MA access were similarly analyzed, there were no significant sex or Taar1 genotype effects. Taar1 genotype and total fluid intake were not significantly correlated (Table 3), whereas Taar1 genotype explained 53 and 67% of the variance in MA intake for the 2 MA concentrations, respectively, in the BXD RI mice.
Taar1 Genotype and MA Intake in DBA/2 Mice
The DBA/2 mice sourced from different vendors provide a more isogenic background on which to examine the relation between Taar1 genotype and MA intake. The key findings from this study are that Taar1 genotype differed in DBA/2 mice sourced from different vendors, all DBA/2 mice from The Jackson Laboratory were homozygous for the Taar1m1J allele, all DBA/2 mice from other vendors were homozygous for the Taar1+ allele, and homozygosity for the Taar1m1J allele was associated with greater MA intake. A repeated measures ANOVA was performed on MA intake (Figure 5A) with sex, vendor and MA concentration as independent variables. There were no significant main or interaction effects involving sex, so data were further analyzed for the sexes combined. There was a significant vendor x MA concentration interaction [F(3, 44) = 67.4; p < 0.0001]. Only the DBA/2J mice exhibited concentration-dependent MA intake, and there was a significant vendor effect for both MA concentrations (ps < 0.001). The DBA/2J mice had higher MA intake than DBA/2 mice from all other vendors at both the 20 and 40 mg/l concentrations, whereas there were no significant differences in MA intake among the DBA/2NCrl, DBA/2NTac, and DBA/2NHsd mice.
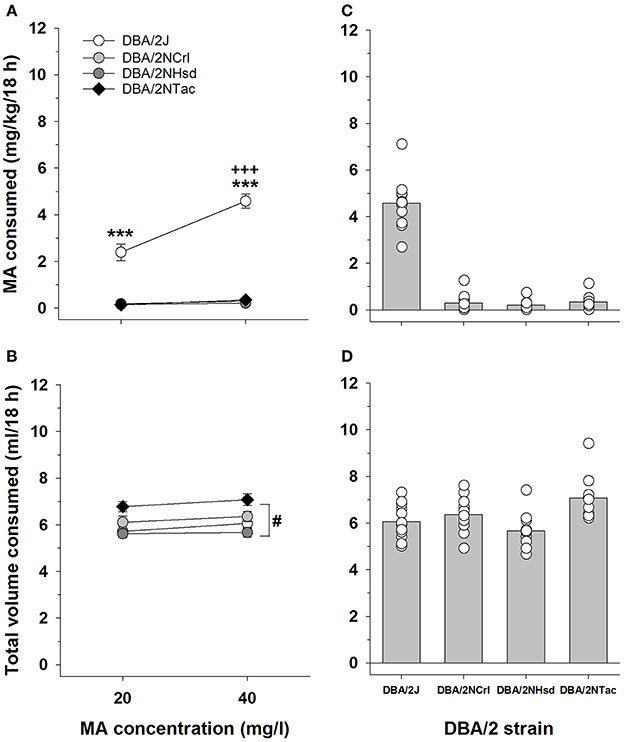
Figure 5. MA and total volume consumed differ across DBA/2 mice sourced from four vendors. (A) Mean ± SEM MA consumed when MA was offered vs. water in 20 and 40 mg/l concentrations to DBA/2 mice from The Jackson Laboratory (DBA/2J), Charles River (DBA/2NCrl), Envigo (DBA/2NHsd), and Taconic (DBA/2NTac). (B) Mean ± SEM total volume of fluid consumed from both the water and MA tubes, during the 18-h period when MA was available. (C) Means and individual MA intake amounts for the 40 mg/l MA concentration. (D) Means and individual total volumes consumed during the 18-h period when the 40 mg/l MA concentration was offered. ***p < 0.001 for the comparison of DBA/2J to mice from all other vendors; +++p < 0.001 for comparison of MA consumed from the 20 and 40 mg/l MA concentrations within the DBA/2J mice; #p < 0.05 for overall more total volume consumed in DBA/2NTac mice, compared to mice from all other vendors.
For total volume of fluid consumed (Figure 5B), there were no significant effects of sex in the initial analysis so data were considered for the sexes combined. There was a significant main effect of vendor [F(3, 44) = 8.0, p < 0.001], with the DBA/2NTac mice consuming significantly more fluid than the DBA/2 mice from other vendors (ps < 0.05), and comparable total volumes for the DBA/2J, DBA/2NCrl, and DBA/2NHsd mice. There was also a main effect of concentration [F(1, 44) = 9.8, p < 0.01], with about 0.25 ml more total volume consumed during the period when the 40 mg/l MA concentration was available. When water intake data for the same 18-h period averaged for the 2 days prior to MA access were similarly analyzed, there was a significant effect of vendor [F(3, 40) = 6.0, p < 0.01]. DBA/2NCrl mice consumed more water than DBA/2NhSD and DBA/2J mice, but not DBA/2NTac, which consumed more than DBA/2J.
Individual MA and total fluid intake values for the 40 mg/l MA concentration are plotted in Figures 5C,D to better illustrate the range and distribution of individual differences. There was a wider range of MA intake values for DBA/2J mice, compared to mice from the other vendors; however, there was no overlap of individual values for the DBA/2J animals with values for the other DBA/2 mice. In contrast, there was considerable overlap of individual values across all vendors for total volume. Taar1 genotype explained 72 and 92% of the variance in MA intake for the 20 and 40 mg/l MA concentrations, respectively, in the DBA/2 mice. The correlations between Taar1 genotype and total fluid intake were not significant (Table 3).
Taar1 Genotype and MA-Induced CTA
The main finding for the CTA studies is that Taar1 genotype was associated with sensitivity to MA-induced CTA, with homozygous Taar1m1J mice exhibiting resistance.
BXD RI mice
There were no significant effects of sex detected in the initial repeated measures ANOVA for NaCl consumption, with data grouped on sex, Taar1 genotype, MA dose and test day. Data for the sexes were combined and the analysis revealed a significant Taar1 genotype x MA dose × test day interaction [F(4, 172) = 30.2, p < 0.00001]. For the saline-treated mice (Figure 6A), there was no significant effect of Taar1 genotype, but there was a significant effect of test day for the amount consumed from the NaCl tube [F(4, 100) = 3.9, p < 0.01]; however, rather than a reduction in intake, there was a significant increase in intake on the final test day, compared to intake on the initial day (p < 0.05). For the 2 mg/kg MA-treated mice (Figure 6B), there was a significant Taar1 genotype × test day interaction [F(4, 72) = 47.9, p < 0.00001] that was associated with resistance of homozygous Taar1m1J mice to MA-induced CTA. Thus, there was no significant change in NaCl consumption across days in these mice, whereas intake significantly decreased across days in Taar1+ homozygotes (p < 0.0001). The Taar1+ homozygotes consumed significantly less from the NaCl tube, compared to Taar1m1J homozygotes on all days except test day 7 (the initial test day), at which time NaCl consumption was measured prior to pairing with MA. Correlations were examined between Taar1 genotype and NaCl intake during the final conditioning day (test day 15) as a measure of CTA. Taar1 genotype explained 92% of the variance in NaCl consumption in the MA-treated mice. This correlation was not significant for saline-treated mice (Table 3).
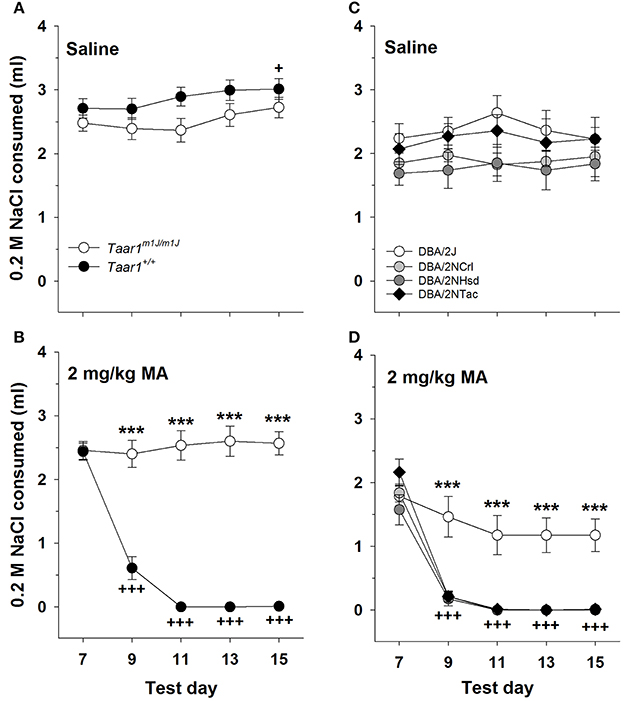
Figure 6. The Taar1m1J/m1J genotype is associated with insensitivity to MA-induced CTA. Mean ± SEM NaCl consumed initially (Test day 7) and after pairing with saline (A) or 2 mg/kg MA (B) injections (Test days 9–15) in homozygous Taar1m1J and Taar1+ BXD RI mice. For panels (A,B) ***p < 0.001 for the comparison of the two genotypes on the indicated test day; +p < 0.05 for overall NaCl intake on test day 15, compared to test day 9; +++p < 0.001 for NaCl intake in Taar1+/+ mice on each indicated day, compared to test day 7. Mean ± SEM NaCl consumed initially (Test day 7) and after pairing with saline (C) or 2 mg/kg MA (D) injections (Test days 9–15) in DBA/2 mice. For panels (C,D) ***p < 0.001 for the comparison of DBA/2J mice to mice of the other 3 vendors on the indicated test day; +++p < 0.001 for NaCl intake in DBA/2NTac, DBA/2NCrl and DBA/2NHsd mice on each indicated day, compared to test day 7.
DBA/2 Mice
In the initial repeated measures ANOVA for NaCl consumption, with data grouped on sex, vendor, MA dose and test day, there was a significant sex x MA dose interaction [F(1, 47) = 4.5, p < 0.05], but no other significant main or interaction effects involving sex. The interaction was due to greater overall intake of NaCl in male than female mice, only in the saline-treated animals (p < 0.01; mean ± SEM = 2.3 ± 0.1 and 1.9 ± 0.1 ml for males and females, respectively). We next examined the significant 3-way vendor × MA dose × test day interaction [F(12, 220) = 2.2, p < 0.05]. For the saline-treated mice (Figure 6C), there were no significant effects of vendor or day, indicating similar and stable NaCl intake. For the mice treated with 2 mg/kg MA (Figure 6D), there was a significant vendor x test day interaction [F(12, 112) = 5.1, p < 0.00001]. There were vendor-specific effects for all days (ps < 0.001) except the first day (day 7), when NaCl consumption was measured prior to any MA conditioning. On subsequent days, DBA/2J mice consumed significantly more NaCl than DBA/2 mice from the other three vendors. Furthermore, there were significant reductions in NaCl consumption across days in DBA/2NCrl, DBA/2NTac, and DBA/2NHsd mice, but not DBA/2J mice. When the correlation was examined between Taar1 genotype and NaCl intake for the final conditioning day (test day 15), Taar1 genotype explained 69% of the variance in NaCl consumption in the MA-treated mice. This correlation was not significant for saline-treated mice (Table 3).
Taar1 Genotype and MA Effects on Core Body Temperature
The main finding for these studies is that Taar1 genotype was associated with sensitivity to MA-induced hypothermia, with homozygous Taar1m1J mice exhibiting resistance.
BXD RI Mice
A repeated measures ANOVA on body temperature, with sex, Taar1 genotype, MA dose and time as factors, did not detect any significant main or interaction effects involving sex, so data were further analyzed for the sexes combined. There was a significant Taar1 genotype × dose × time interaction [F(5, 1605) = 50.7, p < 0.0001]. For the saline group (Figure 7A), there were significant main effects of Taar1 genotype [F(1, 760) = 12.6, p < 0.001] and time [F(5, 760) = 90.2, p < 0.0001]. Homozygous Taar1+ mice had a 0.5°C higher body temperature than homozygous Taar1m1J mice overall, and temperature declined by 0.8°C over time. For the 2 mg/kg MA group (Figure 7B), there was a significant Taar1 genotype x time interaction [F(5, 845) = 81.0; p < 0.0001]. Taar1+ homozygotes exhibited a hypothermic response to MA and had significantly lower body temperatures at 30–120 min post MA treatment, compared to their body temperature just prior to treatment (T0). Taar1m1J homozygotes were resistant to the hypothermic effects of MA and exhibited significant hyperthermia after MA treatment at all post-MA administration time points except T180, when their temperature was reduced compared to T0. This reduction was not likely related to MA treatment, as maximal hypothermia occurs at T30 and the mean temperature reduction was only 0.3°C. The disparate responses of the genotypes to MA were supported by significant differences between the Taar1 homozygote groups at 30–120 min. There was also a smaller, but significant difference at baseline, with the homozygous Taar1+ group having about a 0.2°C higher body temperature, compared to the homozygous Taar1m1J group.
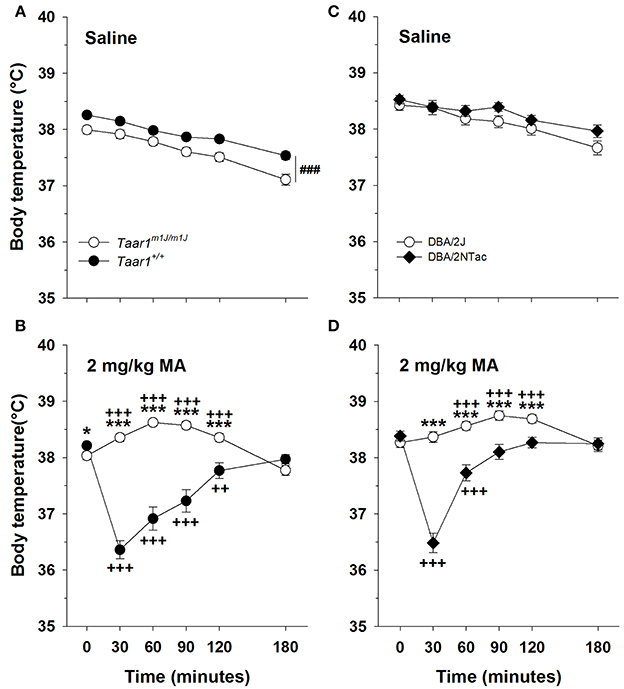
Figure 7. The homozygous Taar1m1J genotype is associated with resistance to MA-induced hypothermia. Mean ± SEM core temperature immediately before and for 3 h after (A) saline or (B) 2 mg/kg MA administration in BXD RI mice. Mean ± SEM core temperature immediately before and for 3 h after (C) saline or (D) 2 mg/kg MA administration in DBA/2J and DBA/2NTac mice. ###p < 0.001 for the main effect of genotype; *p < 0.05 and ***p < 0.001 for the genotype comparison at the indicated time point; ++p < 0.01 and +++p < 0.001 for temperature difference between the indicated time point and T0, within each genotype.
Because the most significant change in body temperature was at 30 min post MA treatment here and in previous studies (Harkness et al., 2015), the correspondence between Taar1 genotype and change in body temperature from baseline at 30 min (T30 minus T0) was examined for the saline and the MA groups (Table 3). For the MA group, the correlation was significant and Taar1 explained 50% of the variation in thermal response to MA. For the saline group, the correlation was not significant.
DBA/2J and DBA/2NTac
To examine the relationship between Taar1 and hypothermic response to MA on a more isogenic background, the DBA/2J and DBA/2NTac strains were compared. Consistent with all other findings, the DBA/2J strain, carrying the mutant Taar1m1J allele, was resistant to MA-induced hypothermia. A repeated measures ANOVA on body temperature, with sex, vendor, MA dose and time as factors, detected a significant sex × MA dose × time interaction [F(5, 435) = 3.6, p < 0.01], but no effects of sex involving vendor. Further examination within each dose group revealed a significant time × sex interaction only in the saline group [F(5, 215) = 5.3, p < 0.0001]. Males had a lower average body temperature than females of about 0.4°C, only at T180. Because this effect was not associated with vendor or MA treatment, a repeated measures ANOVA was performed on data for the sexes combined. There was a significant vendor × MA dose × time interaction [F(5, 455) = 20.6, p < 0.0001]. For the saline group (Figure 7C), the only significant result was an effect of time [F(5, 225) = 25.4, p < 0.0001]. For the 2 mg/kg MA group (Figure 7D), there was a significant vendor × time interaction [F(5, 230) = 41.6, p < 0.0001]. Body temperature was dependent on time in both the DBA/2NTac and DBA/2J mice (ps < 0.0001). DBA/2NTac mice displayed significant hypothermia at T30 and T60 post MA treatment, whereas the DBA/2J mice displayed a significant hyperthermic response at T60-120. Compared to DBA/2J mice, DBA/2NTac mice had significantly lower temperatures at all time points except T0 and T180.
The correlation between Taar1 genotype and change in body temperature from baseline at 30 min (T30-T0) was examined for the saline and the MA groups (Table 3). For the MA group, the correlation was significant and Taar1 explained 67% of the variation in thermal response to MA. For the saline group, the correlation was not significant.
Chronology for the Taar1 Mutation
Archived DNA was assayed for the Taar1 SNP in tissue samples obtained from The Jackson Laboratory for several DBA/2J mice from generations produced between 2001 and 2007. The Taar1 SNP that is associated with a non-functional receptor was not present in samples from animals prior to May of 2001, but was present in archived samples from DBA/2J mice beginning in 2003. Further investigation by personnel at The Jackson Laboratory provided evidence that mice from breeder pair 03-06347 (used for breeding in 2003) were homozygous for the mutation, which indicates that the Taar1 SNP arose after May of 2001, and by the time that new breeding stock for the DBA/2J mice was established at The Jackson Laboratory in 2003. The chronology is illustrated in Figure 8.
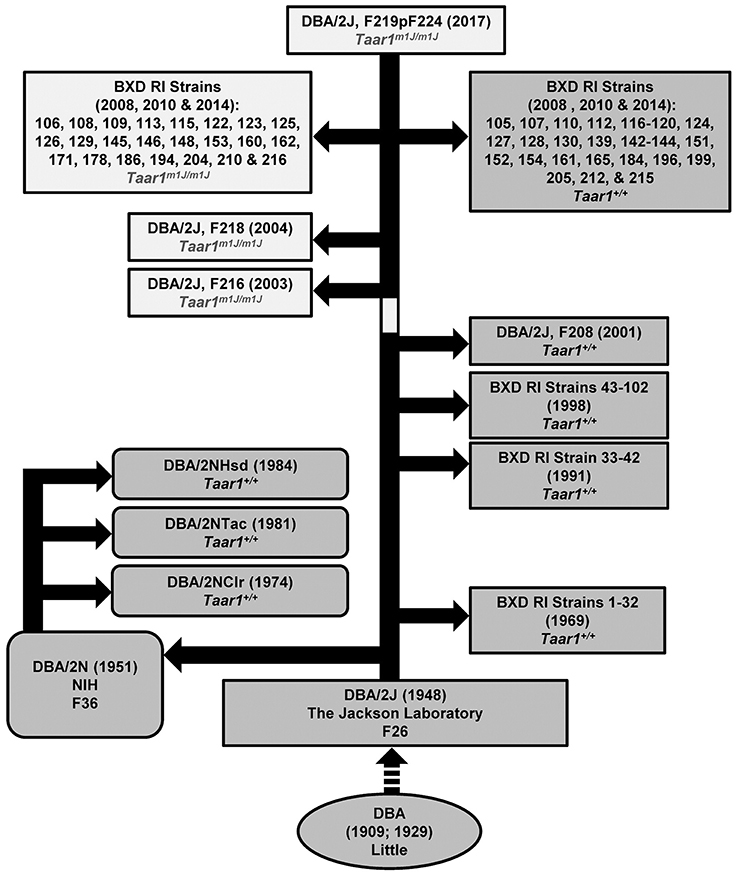
Figure 8. Chronology for the Taar1m1J allele. The historical sequence for DBA/2 mice is indicated, to the best of our knowledge. Dr. Clarence Cook Little began inbreeding the first DBA strain in 1909. In 1929, The Jackson Laboratory was established by Dr. Little, and strain crosses were initiated from which DBA sublines emerged, including the DBA/2. By 1948, the F26 generation had been produced at The Jackson Laboratory. F36 DBA/2 mice were distributed to the National Institutes of Health (NIH) in 1951, and named DBA/2N. Mice from NIH were distributed to Charles River, Taconic, and Harlan Sprague Dawley (now Envigo) in 1974, 1981, and 1984, respectively, where the mice were renamed to indicate source (DBA/2NClr, DBA/2NTac, and DBA/2NHsd, respectively). Current genotyping of mice sourced from those suppliers indicates that all are homozygous Taar1+. Multiple waves of BXD RI strains have been initiated beginning ca 1969, ca 1991, ca 1998, ca 2008, ca 2010, and ca 2014 (for details see Taylor et al., 1973, 1975, 1999; Peirce et al., 2004; http://www.genenetwork.org/mouseCross.html). DNA samples were available from many strains and Taar1 genotyping results are indicated. Some sequencing results for BXD RI strains not used in the current manuscript have been published (Shi et al., 2016). Only the more recently derived BXD RI strains possess the mutant Taar1m1J allele. Genotyping of DNA from generation F208, F216, and F218 DBA/2J mice indicates that the SNP in Taar1 at position 229 arose in 2001-2003 (indicated by the light coloration on the main bar of the figure). The dashed arrow indicates that some intermediary events have been omitted. Sequencing results for some additional strains can be found in Shi et al. (2016). Note that among strains BXD1-102, the following were directly sequenced for Taar1 position 229 genotype: 1, 2, 5, 6, 8, 9, 11, 12, 14–16, 18–25, 27–34, 36, 38–40, 42–45, 48, 48a, 51, 55, 56, 60, 63–66, 68–71, 73–75, 77, 79, 83, 84, 87, 89, 90, and 98–102.
Discussion
Results from the current studies strongly implicate an impact of TAAR1 function on MA intake and sensitivity to an aversive and a physiological response to MA. The triad of traits examined here has consistently been identified as genetically correlated in selectively bred MADR mice (Phillips and Shabani, 2015; Phillips et al., 2016), and previous data implicated Taar1 in the genetic associations (Harkness et al., 2015). The current data confirm the correspondence of higher MA intake, lack of sensitivity to MA-induced CTA and lack of sensitivity to MA-induced hypothermia with a Taar1 allele that codes for a non-functional receptor. Furthermore, Taar1 genotype frequency data support rapid fixation of the Taar1m1J allele in multiple replicates of the MAHDR line and retention of heterozygosity, indicating dominance of the Taar1+ allele for low MA intake in multiple MALDR lines, and that the Taar1m1J is not a dominant negative mutation.
The correlation between Taar1 genotype and each of the MA traits examined here was highly significant on a mixed C57BL/6J × DBA/2J background and an inbred DBA/2 strain background. For the MA intake selection phenotype, the amount of variance for which Taar1 genotype accounted tended to be greater on the isogenic background. Furthermore, the range of MA intake values for homozygous Taar1m1J mice tended to be wider for the populations with heterogeneous backgrounds. Thus, the lowest MA intake amount for an inbred DBA/2J mouse was 2.68 mg/kg and there was no overlap in MA intake values between individuals sourced from The Jackson Laboratory and the DBA/2 mice from other vendors that do not possess the Taar1m1J allele (Figure 5C). However, in the BXD RI mice, several homozygous Taar1m1J individuals consumed no MA and the intake values for the homozygous Taar1m1J and Taar1+ populations overlapped (Figures 4A,B). Overall, the data suggest that polymorphisms associated with the C57BL/6J strain impact MA consumption, either independently or epistatically. These individual differences are of interest, because they suggest that there are individuals with genotypes that can counteract the high risk associated with the Taar1 mutation, which could be studied to identify novel mechanisms for treatment.
The Taar1 SNP at position 229 in the DBA/2J strain was traced to a spontaneous event occurring at The Jackson Laboratory in 2001–2003. DBA/2 mice sourced from other common vendors do not possess the SNP and have MA traits characteristic of mice that possess the alternative allele. The current findings predict that for data collected prior to 2001, the C57BL/6 and DBA/2 mice, regardless of vendor, would not have differed for the traits examined here (or perhaps traits measured similarly). There are no published MA drinking data in C57BL/6 or DBA/2 mice prior to ours in 2014; in that study DBA/2J mice consumed more MA than C57BL/6J mice (Eastwood and Phillips, 2014). However, Meliska et al. (1995) published data in C57BL/6NHsd and DBA/2NHsd mice for amphetamine consumption and found significantly greater intake in C57BL/6NHsd females, compared to DBA/2NHsd, the opposite strain difference to ours. This difference was not found in males. Although we predicted that mice of these strains would not differ in meth(amphetamine) intake prior to the appearance of the Taar1 SNP, the reversal of the strain difference could reflect other alleles in the C57BL/6 strain background that impact this trait in the absence of the Taar1 polymorphism. In a recent study by Fultz et al. (2017), C57BL/6J mice were offered four bottles of MA of differing concentrations during 2 h of the dark cycle, with no water choice. Total consumption ranged from about 0.3–0.7 mg/kg. Low MA intake levels (up to ~0.18 mg/kg/1 h session) were also found in C57BL/6J mice in an operant oral MA self-administration study (Szumlinski et al., 2017). DBA/2J mice were not included in these studies; however, Sharpe et al. (2014) measured intravenous self-administration of MA in DBA/2J mice during 1-h operant sessions and obtained levels of about 4 mg/kg at the highest infusion dose (0.15 mg/kg/infusion). Also relevant are results for TAAR1 partial agonists. The partial agonist, RO5203648 reduced MA self-administration in rats, while not affecting sucrose self-administration (Cotter et al., 2015). Another partial TAAR1 agonist, RO5263397 reduced the breakpoint for MA self-administration and reduced MA-primed reinstatement of responding after extinction. However, when substituted for MA, the partial agonist did not sustain responding (Pei et al., 2017). These recently published studies support a role for TAAR1 in MA intake and reflect lower voluntary intake in C57BL/6 mice that possess a functional TAAR1, compared to DBA/2 mice that lack TAAR1 function, consistent with our findings.
With regard to meth(amphetamine)-induced body temperature change and CTA, there have been several relevant strain comparison studies, all performed prior to when the Taar1 SNP arose or in strains from vendors other than The Jackson Laboratory. The earliest of these studies measured temperature after 2, 10, and 20 mg/kg d-amphetamine in DBA/2J and C57BL/6J mice. Both strains exhibited a hypothermic response to the 2 mg/kg dose, consistent with the expectation for animals with functional TAAR1. DBA/2J mice exhibited hyperthermic responses to the higher amphetamine doses that were largely absent in C57BL/6J mice (Seale et al., 1985). Another study examined temperature responses to 4, 8, and 16 mg/kg MA in DBA/2J and C57BL/6J mice, 48 min after treatment. The strains did not exhibit significant thermal responses to any of the MA doses, although there was significant hypothermia or hyperthermia that varied among the BXD RI strains that were also tested (Grisel et al., 1997). Kita et al. (1998) examined thermal responses in DBA/2N and C57BL/6N mice, 1 h after each of four treatments with 2 or 4 mg/kg MA given every 2 h. Hyperthermia was observed in both strains, beginning with the second injection. Responses appeared to be largely comparable, although statistics were not given to allow this to be fully assessed. Thus, results vary considerably across these studies, as did drugs, doses and number of treatments, but agree in general with the prediction that the DBA/2 and C57BL/6 mice did not differ in hypothermic response to amphetamines prior to when the Taar1 SNP arose. Finally, C57BL/6JICo and DBA/2JICo mice (Charles River, Italy) exhibited comparable 1 and 2 mg/kg MA-induced CTA (Orsini et al., 2004).
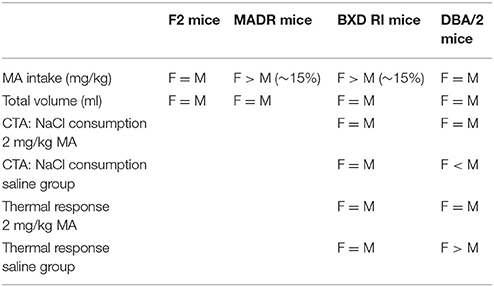
Table 4 summarizes the results for the effects of sex in the current studies. For most studies, sex did not have a significant impact. However, for MA intake, a sex difference was found in two of four studies. For the mice chosen as breeders of the MADR lines, females of the higher MA-intake homozygous Taar1m1J genotype consumed about 15% more MA than males. A similar result was obtained in the BXD RI mice, but not in the F2 or DBA/2 inbred mice. We have observed a significant sex difference in MA intake in some (Wheeler et al., 2009; Shabani et al., 2011, 2016), but not all (Eastwood and Phillips, 2014; Harkness et al., 2015; Shabani et al., 2016) of our prior studies. When a difference has been found, females have consumed more MA than males and the difference has been of about the same magnitude as reported here. A recent study in humans, including 413 males and 369 females who use MA, reported that females had heavier, more frequent and greater lifetime episodes of MA use and were more likely to be MA-dependent and to experience withdrawal than males (Rungnirundorn et al., 2017). Although there is some evidence for more MA intake in females in the rodent studies, the sex effect has been modest. For other traits, sex effects were inconsistently found.
Total volume consumed was examined in the MA intake studies to determine whether fluid intake was associated with level of MA intake. In addition, the association of Taar1 genotype with total volume was examined. In the F2 and MADR mice, Taar1 genotype was positively associated with total volume consumed; thus the homozygous Taar1m1J genotype was associated with greater total volume. Taar1 genotype explained 5–19% of the variance in total volume consumed during the time when MA was offered. However, there was no impact of Taar1 genotype on water intake during the 2 days prior to MA access. Since greater total volume was associated with the higher MA intake, homozygous Taar1m1J genotype, it is possible that stimulant effects of MA increase overall activity and generate thirst. However, this effect was not found in BXD RI or DBA/2 mice from the various vendors, and MAHDR mice that consumed an average of about 13 mg/kg MA in a binge drinking model did not consume more total volume than MALDR mice that consumed an average of 1 mg/kg MA (Shabani et al., 2016). In our selection studies, we have sometimes found the MAHDR line to consume about 0.5 ml more total volume than the MALDR line (Wheeler et al., 2009; Shabani et al., 2011). In general, Taar1 associations with total volume are not consistent and are modest compared to associations with MA intake.
A high resolution gene map was generated in 2006 for the BXD RI strains (Shifman et al., 2006) and 52 SNPs were identified as new mutations for which variation corresponded with the phases during which the strains were derived. There were three phases of BXD RI development prior to 2006 (derived beginning in 1969, 1991, and 1998); the Taar1 SNP was not among the 52 identified SNPs, since it arose in 2001–2003. However, the former analysis confirms that spontaneous mutations are not rare. For example, a spontaneous mutation in Gpnmb, a gene that contributes to pigmentary-related eye diseases, is known to have been fixed in the DBA/2J stock (Libby et al., 2005; Lu et al., 2011) and an in-frame deletion in centrosomal/ciliary protein Cep290 that produces retinal degeneration was fixed in BXD24 (Chang et al., 2006). In the case of the Taar1 SNP, we have determined that the mutation is a non-synonymous substitution that alters the amino acid sequence and function of the resulting receptor, as well as having a significant impact on several MA-related traits. Taar1 is located on mouse chromosome 10 in a cluster of Taar genes (from 23,920,387 to 24,109,564 base pairs) and the SNP in Taar1 at position 229 is the only sequence variant between the reference C57BL/6J strain and the DBA/2J strain for this entire cluster of genes (Shi et al., 2016).
Unlike the receptor expressed by Taar1+, the receptor expressed by Taar1m1J does not respond in situ to MA, or to the trace amines, β-phenethylamine (β-PEA) or tyramine (Harkness et al., 2015; Shi et al., 2016). Thus, the spontaneously occurring SNP created a functional TAAR1 knockout for which a receptor is expressed, but not stimulated by agonists to elicit a cAMP response. We and others have generated data in classical knockout mice in which the Taar1 gene was genetically altered via homologous recombination and does not produce a receptor protein. Results in knockout mice for MA-related traits have been similar to those for the MAHDR mice (Achat-Mendes et al., 2012; Harkness et al., 2015), suggesting that the trait alterations are not dependent upon the expression of the non-functional protein. However, it remains possible that the receptor expressed by the Taar1m1J allele has some function other than that regulated by agonist binding. Also remaining to be studied are the potential functional and behavioral consequences of the full slate of almost 50 non-synonymous SNPs reported for the human-TAAR1 (dbSNP database, NCBI). Shi et al. (2016) examined agonist-stimulated cAMP production for eight human receptor variants by constructing them into the TAAR1 reference human sequence and transfecting them into CHO-K1 cells. All variants expressed a receptor, and a cAMP response to β-PEA was observed in cells transfected with the reference sequence, whereas a reduced cAMP response or no response was observed for other variants. There is the possibility that these genetic variants could impact response to MA, risk for a MA use disorder, or response to treatment. In theory, individuals carrying non-functional variants should be at greatest risk, but would not be subject to treatment with TAAR1 agonists or partial agonists. However, it is possible that those with greatly reduced TAAR1 function could benefit from TAAR1-targeted treatments (Sotnikova et al., 2009; Cotter et al., 2015; Jing and Li, 2015; Edelmann et al., 2016; Pei et al., 2016; Phillips et al., 2016).
Author Contributions
CR: Development of experimental protocols, supervision of technical support, statistical analysis, interpretation of data, and wrote the manuscript interactively with TP. HB, ZZ, JE, JM, and NV: Data acquisition and entry, and checking of entered data for accuracy. RW: Provided some mouse breeding stock and assisted in data interpretation and manuscript editing. TP: Experimental design, analysis and interpretation of all data, and wrote the manuscript interactively with CR.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding provided by NIH NIDA P50DA018165, U01DA041579, P30DA04423, and T32DA007262; NIH NIAAA R24AA020245; the Department of Veterans Affairs and the VA Research Career Scientist Program. The authors would like to thank Dr. Jim Hagarman, Ms. Melissa Berry, Ms. MaryEllen Joseph, and Ms. Jane Ober for working with us to obtain genetic samples and for discussions pertaining to the genetic stability program and mouse colony practices at The Jackson Laboratory.
References
Achat-Mendes, C., Lynch, L. J., Sullivan, K. A., Vallender, E. J., and Miller, G. M. (2012). Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol. Biochem. Behav. 101, 201–207. doi: 10.1016/j.pbb.2011.10.025
Belknap, J. K., McWeeney, S., Reed, C., Burkhart-Kasch, S., McKinnon, C. S., Li, N., et al. (2013). Genetic factors involved in risk for methamphetamine intake and sensitization. Mamm. Genome 24, 446–458. doi: 10.1007/s00335-013-9484-9
Buck, K. J., Metten, P., Belknap, J. K., and Crabbe, J. C. (1997). Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J. Neurosci. 17, 3946–3955.
Bunzow, J. R., Sonders, M. S., Arttamangkul, S., Harrison, L. M., Zhang, G., Quigley, D. I., et al. (2001). Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 60, 1181–1188. doi: 10.1124/mol.60.6.1181
Chang, B., Khanna, H., Hawes, N., Jimeno, D., He, S., Lillo, C., et al. (2006). In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 15, 1847–1857. doi: 10.1093/hmg/ddl107
Cotter, R., Pei, Y., Mus, L., Harmeier, A., Gainetdinov, R. R., Hoener, M. C., et al. (2015). The trace amine-associated receptor 1 modulates methamphetamine's neurochemical and behavioral effects. Front. Neurosci. 9:39. doi: 10.3389/fnins.2015.00039
Crabbe, J. C., Feller, D. J., and Dorow, J. S. (1989). Sensitivity and tolerance to ethanol-induced hypothermia in genetically selected mice. J. Pharmacol. Exp. Ther. 249, 456–461.
Crabbe, J. C., Kosobud, A., Tam, B. R., Young, E. R., and Deutsch, C. M. (1987). Genetic selection of mouse lines sensitive (cold) and resistant (hot) to acute ethanol hypothermia. Alcohol Drug Res. 7, 163–174.
Eastwood, E. C., and Phillips, T. J. (2014). Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict. Biol. 19, 370–379. doi: 10.1111/adb.12003
Edelmann, M. R., Hartung, T., Trussardi, R., Iding, H., Galley, G., Pflieger, P., et al. (2016). Synthesis of enantiomerically pure [14 C]-labelled morpholine derivatives for a class of trace amine-associate receptor 1 agonists. J. Label. Comp. Radiopharm. 59, 635–639. doi: 10.1002/jlcr.3403
Forero, D. A., López-León, S., Shin, H. D., Park, B. L., and Kim, D.-J. (2015). Meta-analysis of six genes (BDNF, DRD1, DRD3, DRD4, GRIN2B and MAOA) involved in neuroplasticity and the risk for alcohol dependence. Drug Alcohol Depend. 149, 259–263. doi: 10.1016/j.drugalcdep.2015.01.017
Fultz, E. K., Martin, D. L., Hudson, C. N., Kippin, T. E., and Szumlinski, K. K. (2017). Methamphetamine-alcohol interactions in murine models of sequential and simultaneous oral drug-taking. Drug Alcohol Depend. 177, 178–186. doi: 10.1016/j.drugalcdep.2017.03.026
Grisel, J. E., Belknap, J. K., O'Toole, L. A., Helms, M. L., Wenger, C. D., and Crabbe, J. C. (1997). Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J. Neurosci. 17, 745–754.
Hall, F. S., Drgonova, J., Jain, S., and Uhl, G. R. (2013). Implications of genome wide association studies for addiction: are our a priori assumptions all wrong? Pharmacol. Ther. 140, 267–279. doi: 10.1016/j.pharmthera.2013.07.006
Harkness, J. H., Shi, X., Janowsky, A., and Phillips, T. J. (2015). Trace amine-associated receptor 1 regulation of methamphetamine intake and related traits. Neuropsychopharmacology 40, 2175–2184. doi: 10.1038/npp.2015.61
Hart, A. B., de Wit, H., and Palmer, A. A. (2013). Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology 38, 802–816. doi: 10.1038/npp.2012.245
Jing, L., and Li, J. X. (2015). Trace amine-associated receptor 1: a promising target for the treatment of psychostimulant addiction. Eur. J. Pharmacol. 761, 345–352. doi: 10.1016/j.ejphar.2015.06.019
Kita, T., Paku, S., Takahashi, M., Kubo, K., Wagner, G. C., and Nakashima, T. (1998). Methamphetamine-induced neurotoxicity in BALB/c, DBA/2N and C57BL/6N mice. Neuropharmacology 37, 1177–1184. doi: 10.1016/S0028-3908(98)00106-3
Libby, R. T., Anderson, M. G., Pang, I. H., Robinson, Z. H., Savinova, O. V., Cosma, I. M., et al. (2005). Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 22, 637–648. doi: 10.1017/S0952523805225130
Lu, H., Wang, X., Pullen, M., Guan, H., Chen, H., Sahu, S., et al. (2011). Genetic dissection of the Gpnmb network in the eye. Invest. Ophthalmol. Vis. Sci. 52, 4132–4142. doi: 10.1167/iovs.10-6493
Meliska, C. J., Bartke, A., McGlacken, G., and Jensen, R. A. (1995). Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol. Biochem. Behav. 50, 619–626. doi: 10.1016/0091-3057(94)00354-8
Orsini, C., Buchini, F., Piazza, P. V., Puglisi-Allegra, S., and Cabib, S. (2004). Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology 172, 264–270. doi: 10.1007/s00213-003-1647-z
Pei, Y., Asif-Malik, A., and Canales, J. J. (2016). Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front. Neurosci. 10:148. doi: 10.3389/fnins.2016.00148
Pei, Y., Asif-Malik, A., Hoener, M., and Canales, J. J. (2017). A partial trace amine-associated receptor 1 agonist exhibits properties consistent with a methamphetamine substitution treatment. Addict. Biol. 22, 1246–1256. doi: 10.1111/adb.12410
Peirce, J. L., Lu, L., Gu, J., Silver, L. M., and Williams, R. W. (2004). A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 5:7. doi: 10.1186/1471-2156-5-7
Phillips, T. J., Mootz, J. R., and Reed, C. (2016). Identification of treatment targets in a genetic mouse model of voluntary methamphetamine drinking. Int. Rev. Neurobiol. 126, 39–85. doi: 10.1016/bs.irn.2016.02.001
Phillips, T. J., and Shabani, S. (2015). An animal model of differential genetic risk for methamphetamine intake. Front. Neurosci. 9:327. doi: 10.3389/fnins.2015.00327
Rungnirundorn, T., Verachai, V., Gelernter, J., Malison, R. T., and Kalayasiri, R. (2017). Sex differences in methamphetamine use and dependence in a Thai treatment center. J. Addict. Med. 11, 19–27. doi: 10.1097/ADM.0000000000000262
Seale, T. W., Carney, J. M., Johnson, P., and Rennert, O. M. (1985). Inheritance of amphetamine-induced thermoregulatory responses in inbred mice. Pharmacol. Biochem. Behav. 23, 373–377. doi: 10.1016/0091-3057(85)90008-5
Shabani, S., Houlton, S. K., Hellmuth, L., Mojica, E., Mootz, J. R., Zhu, Z., et al. (2016). A mouse model for binge-level methamphetamine use. Front. Neurosci. 10:493. doi: 10.3389/fnins.2016.00493
Shabani, S., McKinnon, C. S., Cunningham, C. L., and Phillips, T. J. (2012). Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology 62, 1134–1141. doi: 10.1016/j.neuropharm.2011.11.005
Shabani, S., McKinnon, C. S., Reed, C., Cunningham, C. L., and Phillips, T. J. (2011). Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 10, 625–636. doi: 10.1111/j.1601-183X.2011.00700.x
Sharpe, A. L., Varela, E., Bettinger, L., and Beckstead, M. J. (2014). Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int. J. Neuropsychopharmacol. 18:pyu073. doi: 10.1093/ijnp/pyu073
Shen, G. Q., Abdullah, K. G., and Wang, Q. K. (2009). The TaqMan method for SNP genotyping. Methods Mol. Biol. 578, 293–306. doi: 10.1007/978-1-60327-411-1_19
Shi, X., Walter, N. A., Harkness, J. H., Neve, K. A., Williams, R. W., Lu, L., et al. (2016). Genetic polymorphisms affect mouse and human trace amine-associated receptor 1 function. PLoS ONE 11:e0152581. doi: 10.1371/journal.pone.0152581
Shifman, S., Bell, J. T., Copley, R. R., Taylor, M. S., Williams, R. W., Mott, R., et al. (2006). A high-resolution single nucleotide polymorphism genetic map of the mouse genome. PLoS Biol. 4:e395. doi: 10.1371/journal.pbio.0040395
Sotnikova, T. D., Caron, M. G., and Gainetdinov, R. R. (2009). Trace amine-associated receptors as emerging therapeutic targets. Mol. Pharmacol. 76, 229–235. doi: 10.1124/mol.109.055970
Szumlinski, K. K., Lominac, K. D., Campbell, R. R., Cohen, M., Fultz, E. K., Brown, C. N., et al. (2017). Methamphetamine addiction vulnerability: the glutamate, the bad, and the ugly. Biol. Psychiatry 81, 959–970. doi: 10.1016/j.biopsych.2016.10.005
Taylor, B. A., Bailey, D. W., Cherry, M., Riblet, R., and Weigert, M. (1975). Genes for immunoglobulin heavy chain and serum prealbumin protein are linked in mouse. Nature 256, 644–646.
Taylor, B. A., Heiniger, H. J., and Meier, H. (1973). Genetic analysis of resistance to cadmium-induced testicular damage in mice. Proc. Soc. Exp. Biol. Med. 143, 629–633.
Taylor, B. S., Wnek, C., Kotlus, B. S., Roemer, N., MacTaggart, T., and Phillips, S. J. (1999). Genotyping new BXD recombinant inbred mouse strains and comparison of BCD and consensus maps. Mamm. Genome 10, 335–348.
Vadasz, C., Saito, M., Gyetvai, B. M., Oros, M., Szakall, I., Kovacs, K. M., et al. (2007). Mapping of QTLs for oral alcohol self-administration in B6.C and B6.I quasi-congenic RQI strains. Neurochem. Res. 32, 1099–1112. doi: 10.1007/s11064-006-9234-4
Wheeler, J. M., Reed, C., Burkhart-Kasch, S., Li, N., Cunningham, C. L., Janowsky, A., et al. (2009). Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 8, 758–771. doi: 10.1111/j.1601-183X.2009.00522.x
Keywords: addiction, BXD RI strains, conditioned taste aversion, drinking, hypothermia, selected lines, substance use disorder, trace amine-associated receptor 1
Citation: Reed C, Baba H, Zhu Z, Erk J, Mootz JR, Varra NM, Williams RW and Phillips TJ (2018) A Spontaneous Mutation in Taar1 Impacts Methamphetamine-Related Traits Exclusively in DBA/2 Mice from a Single Vendor. Front. Pharmacol. 8:993. doi: 10.3389/fphar.2017.00993
Received: 14 November 2017; Accepted: 26 December 2017;
Published: 22 January 2018.
Edited by:
Damiana Leo, University of Mons, BelgiumReviewed by:
Francesco Papaleo, Fondazione Istituto Italiano di Technologia, ItalyDietmar Krautwurst, Leibniz Institute for Food Systems Biology, Technical University of Munich (LSB), Germany
Copyright © 2018 Reed, Baba, Zhu, Erk, Mootz, Varra, Williams and Phillips. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara J. Phillips, cGhpbGxpcHRAb2hzdS5lZHU=
 Cheryl Reed
Cheryl Reed Harue Baba1
Harue Baba1 John R. Mootz
John R. Mootz Nicholas M. Varra
Nicholas M. Varra Robert W. Williams
Robert W. Williams Tamara J. Phillips
Tamara J. Phillips