- 1Nutrition and Toxicology Division, Federal Institute of Industrial Research Oshodi, Lagos, Nigeria
- 2Faculty of Pharmacy, Barrett Hodgson University, Karachi, Pakistan
- 3Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan
- 4Department of Biochemistry, Adamawa State University, Mubi, Nigeria
- 5Department of Biochemistry, Ahmadu Bello University, Zaria, Nigeria
- 6Analytical Division, Federal Institute of Industrial Research Oshodi, Lagos, Nigeria
Glioblastoma multiforme (GBM) is a malignant primary type of brain cancer with high proliferation and metastasis rates due to involvement of the microglial cell. It is resistant against available chemotherapy. Many strategic protocols have been developed but prognosis and patient life has not improved substantially. In this study, the anti-metastatic and antioxidant effect of fatty acids from Clerodendrum volubile leaves were investigated in U87-MG (Human Glioblastoma Multiforme) cell lines. The extracted fatty acids were incubated with U87-MG cells for 48 h. The anti-proliferative effect was determined by MTT assay, while apoptosis and cell cycle were analyzed with BD FACSCalibur. The transwell assay protocol was utilized in the analysis of cell migration and invasion. The treated cell lines were also assessed for reduced glutathione (GSH) level, catalase, superoxide dismutase (SOD) and lipid peroxidation. The fatty acid extract showed significant inhibitory activity on cell proliferation and cell cycle progression, mitigated oxidative stress, and suppressed migration and invasion in U-87 MG cell lines. These results give credence to the therapeutic potential of this plant against cancer, especially GBM.
Introduction
Glioblastoma multiforme (GBM) is a malignant primary brain tumor common amongst the young kids with range of age < 13 years, characterized by high rates of proliferation, metastasis, and resistance to chemotherapy protocols (Brada et al., 2001; Puli et al., 2006; Markiewicz-Żukowska et al., 2013). These therapeutic failures represent a great difficulty in its treatment and management, leading to short survival rate, and loss of patient’s life quality (Puli et al., 2006). Its key epidemiologic risk includes age, sex, race, lifestyle behaviors, diet, and exposure to environmental factors including pollution from different sources (Efird, 2011).
Though epidemiology of glioblastoma has reported little or no occurrence in West Africa, changes in lifestyle particularly increased urbanization and adoption of western lifestyles and diet are however a major concern (Erukainure et al., 2016). An indigenous African diet often consists mainly of vegetable leaves, unrefined grains and spices, unlike the western counterpart consisting of sweetened desserts, saturated fats, and processed grains (Erukainure et al., 2016). Almost 30–40% of all cancers have been found to be preventive by healthy life style such as weight control, exercise, and physical activities (Potter and Potter, 1997; Jemal et al., 2011). Several studies have linked consumption of dietary fatty acids in diet with reduced brain cancer. Ketogenic diet characterized by high-saturated fats and low protein/carbohydrate, has proven to be effective during therapy and potent adjuvant management of malignancies in almost all types of cancers (Woolf and Scheck, 2015). Antal et al. (2014) reported increased sensitivity of glioblastoma cells to radiotherapy after treatment with arachidonic acid, docosahexaenoic acid, and γ-linolenic acid.
Clerodendrum volubile is among the common leafy vegetables in Southern part of Nigeria and it is well established for its medicinal use (Erukainure et al., 2010). It is indigenously known as Obenetete by the Itsekiris and Urhobos in the Delta of Niger. Commonly known as magic leaf, it is also used in the management and sometimes as adjunct for the treatment of diabetes mellitus, arthritis, rheumatism, ulcers, and many other diseases (Burkill, 1985). The phytochemicals of C. volubile and antioxidant activities have been reported (Adefegha and Oboh, 2010; Erukainure et al., 2011). Erukainure et al. (2014) isolated an iridoid glycoside from the leaves and reported its antioxidant activity in rats’ brain and hepatic tissues. In our previous study, we extracted dietary fatty acids from the leaves and investigated its effect on breast cancer cells (Erukainure et al., 2016). The fatty acids arrested cell cycle progression and down-regulated matrix metalloproteinase-9 in the breast cancer cells (Erukainure et al., 2016). Furthermore, molecular studies are required to prove the proclaimed medicinal uses of the extract of C. volubile leaves.
This present study aims to report the anti-proliferative, anti-oxidative, and anti-migratory and/or anti-metastatic activity of the fatty acid rich extracts from leaves of C. volubile on U87-MG cancer cells.
Materials and Methods
Plant Materials
Fresh C. volubile leaves, purchased from Ifon, Ondo State, Nigeria were identified and authenticated at the Department of Botany, University of Benin, Benin City, Nigeria (Voucher number: UBHC284).
The leaves were dried under shed, blended, and stored in deoxygenated container for further analysis (chemical, biochemical, and biological activities).
Extraction of Fatty Acids
The blended leaves were subjected to methanol extraction, followed by fractionation with solvents of increasing polarity, as described by Erukainure et al. (2016).
The concentrated hexane fraction was subjected to methanolysis using the method described by Nickavar et al. (2003).
Cell Cultures and Treatments
U87-MG cells were procured from American Type Culture Collection (ATCC). On arrival the ATCC instructions were followed and cells were submitted to the Bio-Bank of PCMD; ICCBS, University of Karachi, Karachi, Pakistan.
These cells were cultured in DMEM medium, 10% (v/v) fetal Bovine Serum (Sigma), L-glutamine 1% (v/v), penicillin 100 U/mL and streptomycin 100 μg/mL.
These newly seeded cells were kept in humidified incubator with 5% CO2.
Cellular Cytotoxicity Analysis Using MTT as a Dye
The anti-proliferative activity of the extracted fatty acids against U87-MG cancer cells was evaluated in a 96-well plate using standard MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay, as described by Mosmann (1983).
Cells (1 × 104 cells/mL) were seeded in 96-well plates.
After overnight incubation, the medium was replaced and 200 μL of fresh medium was added to each well, along with serial dilutions of the fatty acid extract (16, 32, 64, and 125 μg/mL, respectively).
Incubation at 48 h was done under same growth conditions, equal volume of the solution of dye MTT, 2 mg/mL, already prepared and preserved at -20°C was added to each well in triplicate manner.
After aspiration of nutrient media, 10% FBS and cell were then incubated for 4 h under same conditions as described for seeding the cells.
The nutrient media, 10% FBS 100 μL of DMSO were added to each well after aspiration of older media.
Absorbance was recorded at 570 nm wavelengths on a micro plate-reader (SoftMax PRO 4.3.1.LS, Molecular Devices, Sunnyvale, CA, United States).
The % inhibition was later calculated as follows:
Apoptotic Analysis by Propidium Iodide Flow Cytometry
Cells were seeded at 2 × 105/mL/well in 24-well plate.
These cells were then incubated (under same growth conditions as described above) at 37°C overnight.
These cells were incubated with the extracted fatty acids at 37°C under 5% CO2 for 48 h. Cells treated with DMSO served as a negative control.
After incubations, cells were typsinized and centrifuged at 2500 rpm for 3–5 min. They were washed twice using phosphate buffer saline (PBS) before re-suspending in propidium iodide (PI) buffer.
One milliliter PI (0.5 mg/mL) was added for 1 min to the cells and their viability was analyzed with BD FACSCalibur.
Fluorescent Activated Cells’ Cycle Analysis by Sorting (FACS)
U87-MG seeded in concentration of 1 million cells/mL/well using 6-well plate. The plate was incubated with the extracted fatty acid at 37°C for 48 h.
After incubation, the cells were then subjected to cell cycle analysis using flow cytometry on the BD Biosciences FACS machine, as described by Aliyu et al. (2013) and Erukainure et al. (2016).
The formula described by Pozarowski and Darzynkiewicz (2004) was used in calculating the duration of each phase.
Migration and Invasion Assay
This was carried out according to the method described by Yao et al. (2012) with slight modifications, as U87-MG is a sensitive cell line and requires gentle handling.
5 × 104 cells were seeded on top of the matrigel insert already prepared in top chamber with utmost care. Coating contained 150 μg matrigel on each membrane of top chamber.
The cells for both assays were trypsinized and resuspended in DMEM supplemented (700–900 μL) with 10% fetal bovine serum was gently pipetted down into the lower chambers. This was done in such a way that the surface of the media in lower chamber was just touching the lower side of the top matrigel-containing chamber.
The cells were incubated with the fatty acids extract of C. volubile in 120 μg/mL concentration at 37°C for 48 h for the migration and invasion assays, respectively. The cells at the top chambers were aspirated gently out.
Cells that adhered to the lower membrane of the inserts were fixed, and stained with solution of 20% methanol and 0.1% crystal violet.
They were subsequently counted and photographed with at 20–40× power-inverted microscope (Olympus Corp., Tokyo, Japan).
Determination of Oxidative Stress Parameters
For this analysis the U87-MG cells were specifically counted in concentration of 1 million cells/mL, and added to 24-well plate and were then treated with the fatty acid extract of C. volubile at a concentration of 120 μg/mL.
These cells were analyzed for total protein (Lowry et al., 1951), reduced glutathione (GSH) level (Ellman, 1959), catalase activity (Chance and Maehly, 1955), superoxide dismutase (SOD) (Kakkar et al., 1984) activity, and also malondialdehyde (MDA) level, (Chowdhury and Soulsby, 2002).
Inhibition of Chymotrypsin Activity
The α-chymotrypsin inhibitory activity of the extracted fatty acids was carried out as described by Cannell et al. (1988). Alpha-chymotrypsin (9 units/mL in 50 mM Tris-base buffer pH 7.6; Sigma Chemical Co., United States) was incubated with the fatty acid extract (2.5, 5.0, 10.0, and 20.0 μg/mL, respectively) for 20 min. A total of 100 pJ of substrate solution (N-Succinyl-phenyla1anine-pnitroanilide, 1 mg/mL of 50 mM Tris-Base buffer pH 7.6) was added to start the enzyme reaction. The absorbance of released p-nitroaniline was read at 410 nm.
Statistics
To validate the significance of results, each experiment was repeated at least three times. Results were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used in establishing statistical significance. While significant difference was established at P < 0.05 using LSD. Statistical analyses were carried out using SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL, United States).
Results
A dose-dependent cytotoxic activity of the fatty acid extract was observed in U87-MG cell lines with an IC50 of ∼120 μg/mL as shown in Figure 1, indicating a potent anti-proliferative effect.
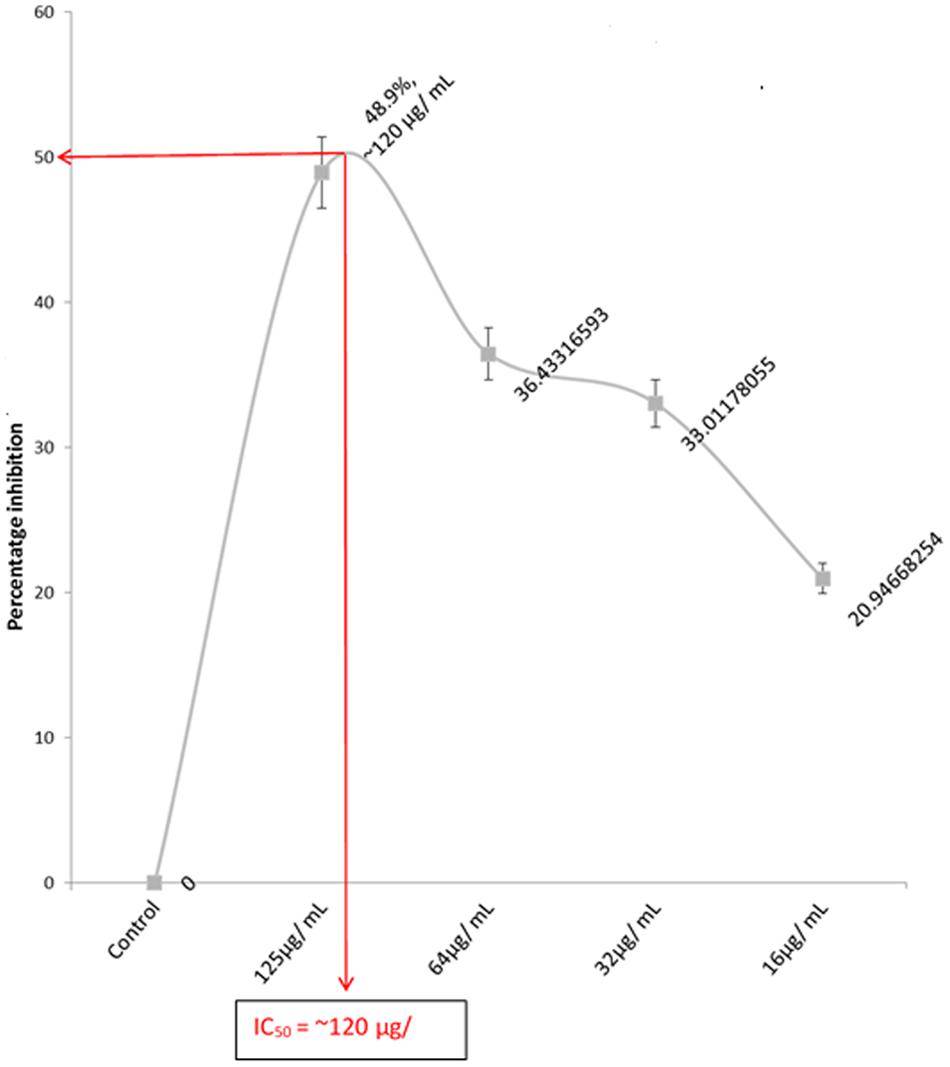
FIGURE 1. IC50 of the anti-proliferation effect of extracted fatty acids on U87 MG cell lines. Values = mean ± SD; n = 3.
Apoptosis analysis by PI flow cytometry (Figure 2) revealed high cell deaths as the concentration increased, which is an indication of a dose-dependent anti-tumor activity of the fatty acid extract in U87-MG cancer cells.
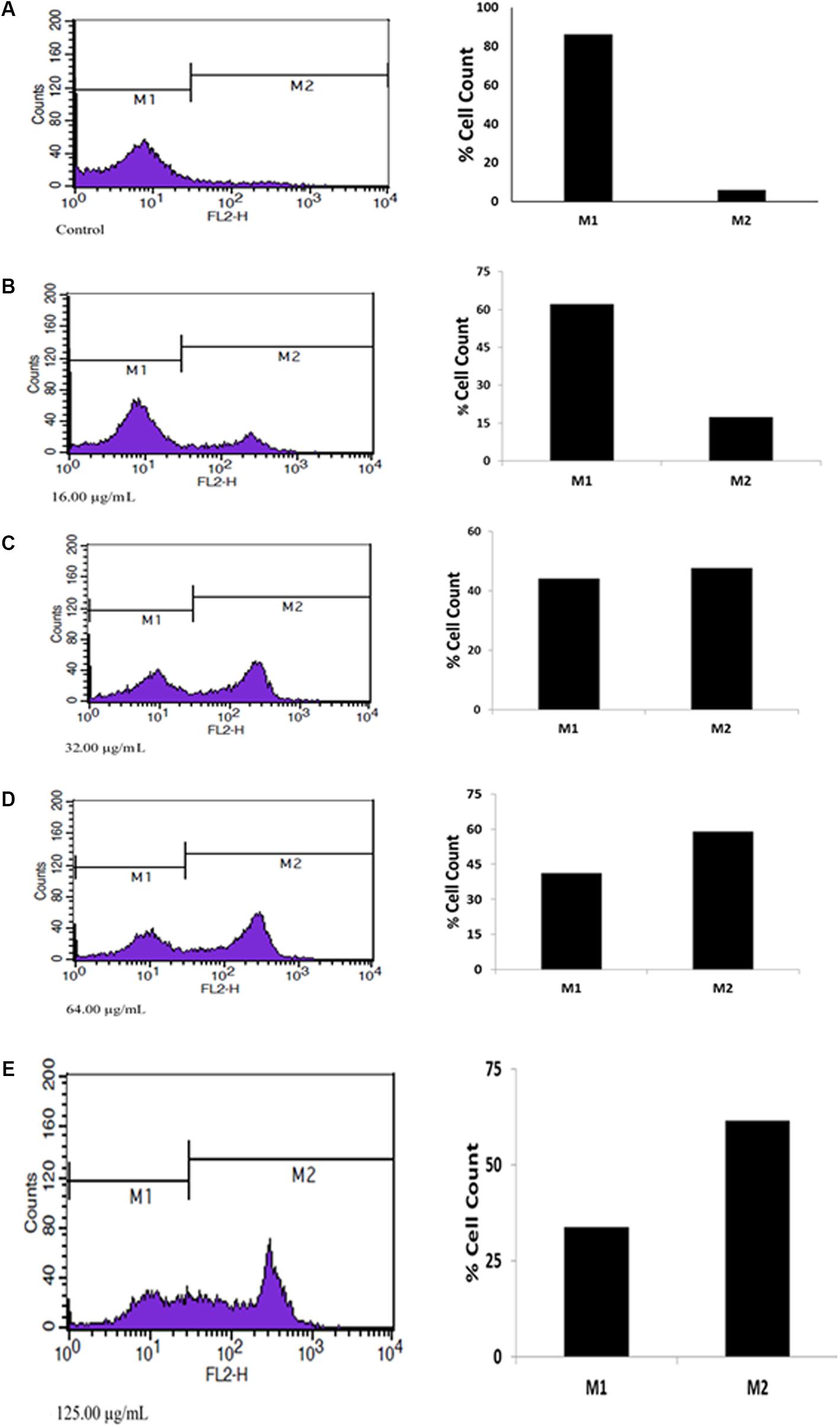
FIGURE 2. Apoptotic effect of extracted fatty acids on U87 MG cell lines. (A) Contol; (B) 16.00 μg/mL; (C) 32.00 μg/mL; (D) 64.00 μg/mL; (E) 125.00 μg/mL.
The quantity of DNA in each cancer cell population in G0/G1 and G2/M phases were significantly depleted in the fatty acids treated cells, as depicted in Figure 3. This was also observed with the simultaneous arrest of S phase of the cell cycle.
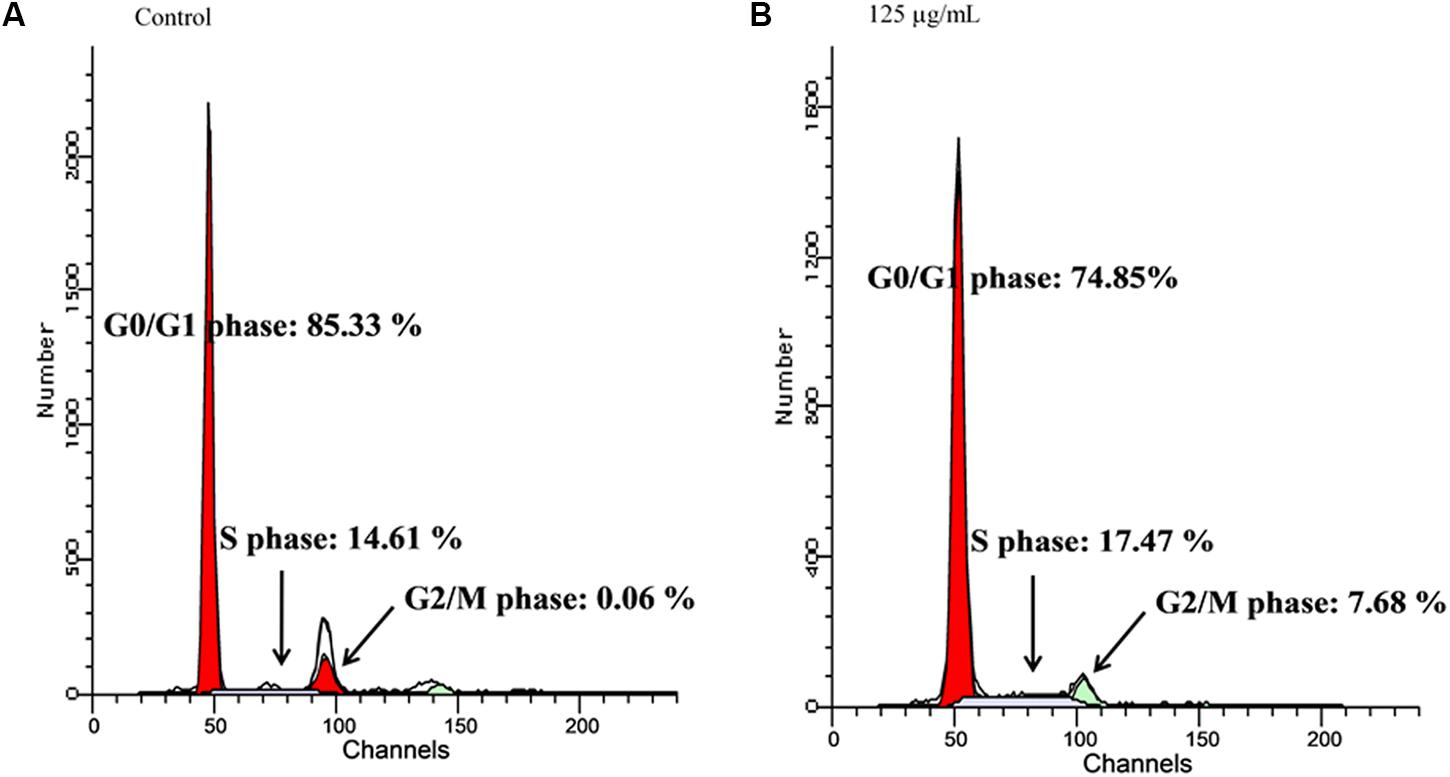
FIGURE 3. Effect of extracted fatty acids on cell cycle phase distribution of U-87 MG cell lines. (A) Control; (B) 125 μg/mL.
Transwell migration analysis revealed the migration of the untreated cells in all six parts of the chamber of 24-well transwell plate, as shown in Figures 4, 5A, indicating an occurrence of cell migration and/or invasion. However, the fatty acid treated cells showed little or no migration (Figure 5B), indicating a suppressive effect on cell migration and/or invasion.
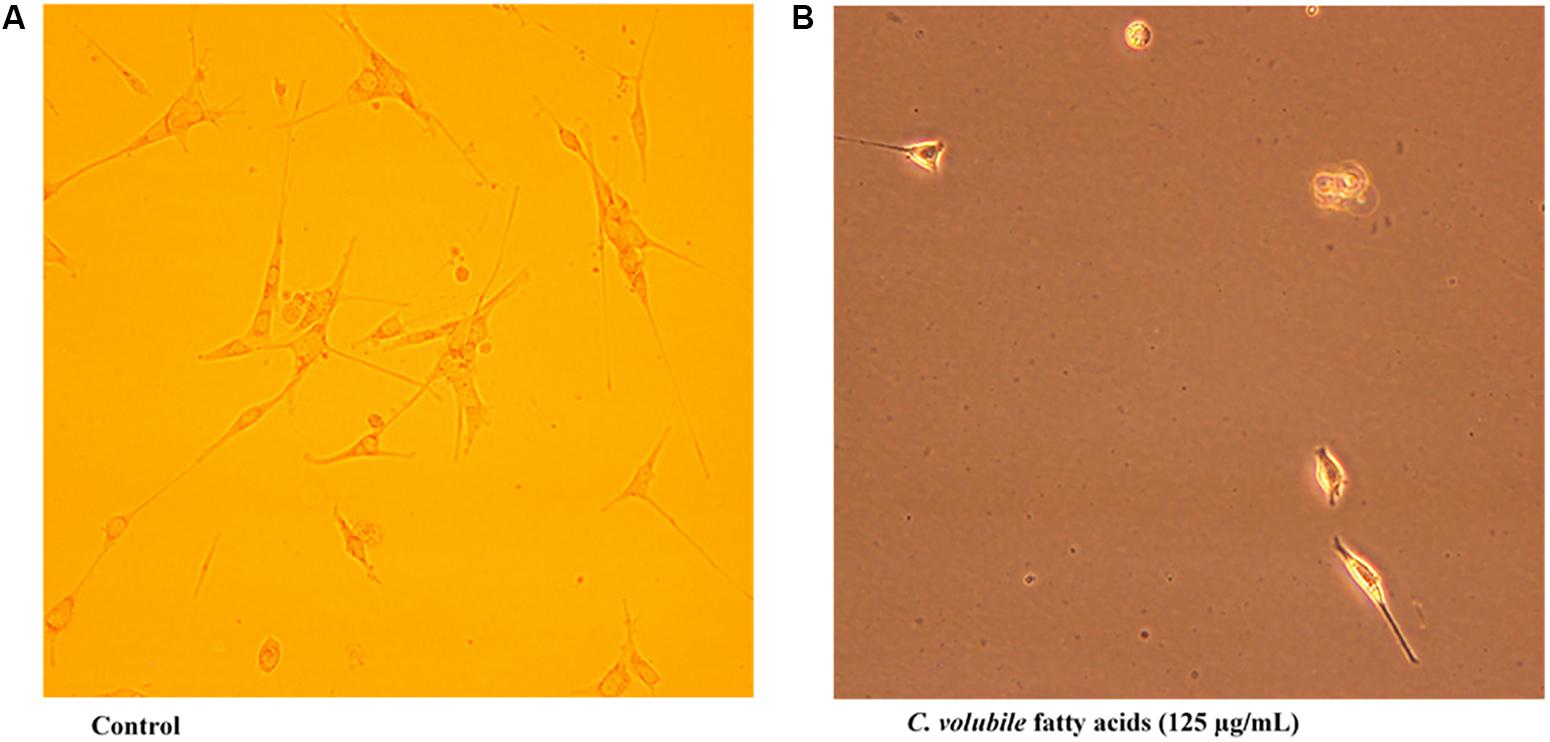
FIGURE 5. Effect of C. volubile fatty acids on cell migration and invasion of U87-MG (Human Neuronal Glioblastoma) cell lines (20× [2 mm]). (A) Control; (B) Fatty acids.
Incubation of cells and fatty acid extract led to significant (p < 0.05) increase in GSH level with a concomitant decrease in MDA level, indicating an anti-oxidative activity as shown in Figures 6A–D. The significant (p < 0.05) increase as well as slight increase in catalase and SOD activities, respectively, further indicates the antioxidant potency of the fatty acids.
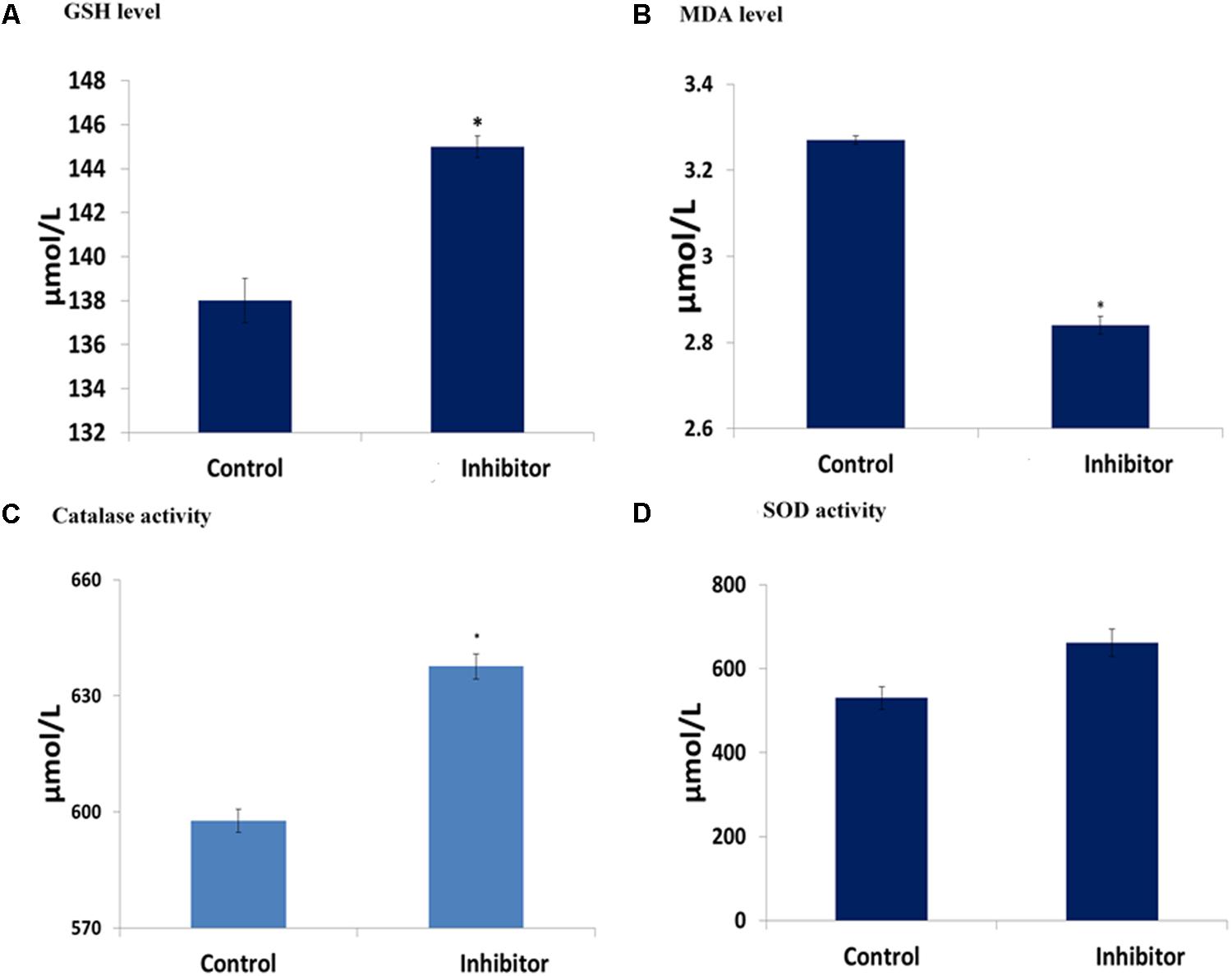
FIGURE 6. Antioxidative activities of C. volubile fatty acids in U87-MG (Human Neuronal Glioblastoma) cell lines. Values = mean ± SD; n = 3. (A) GSH level; (B) MDA level; (C) Catalase activity; (D) SOD activity.
The fatty acids displayed a dose-dependent inhibitory effect on α-chymotrypsin activity and its kinetics as presented in Figures 7A,B.
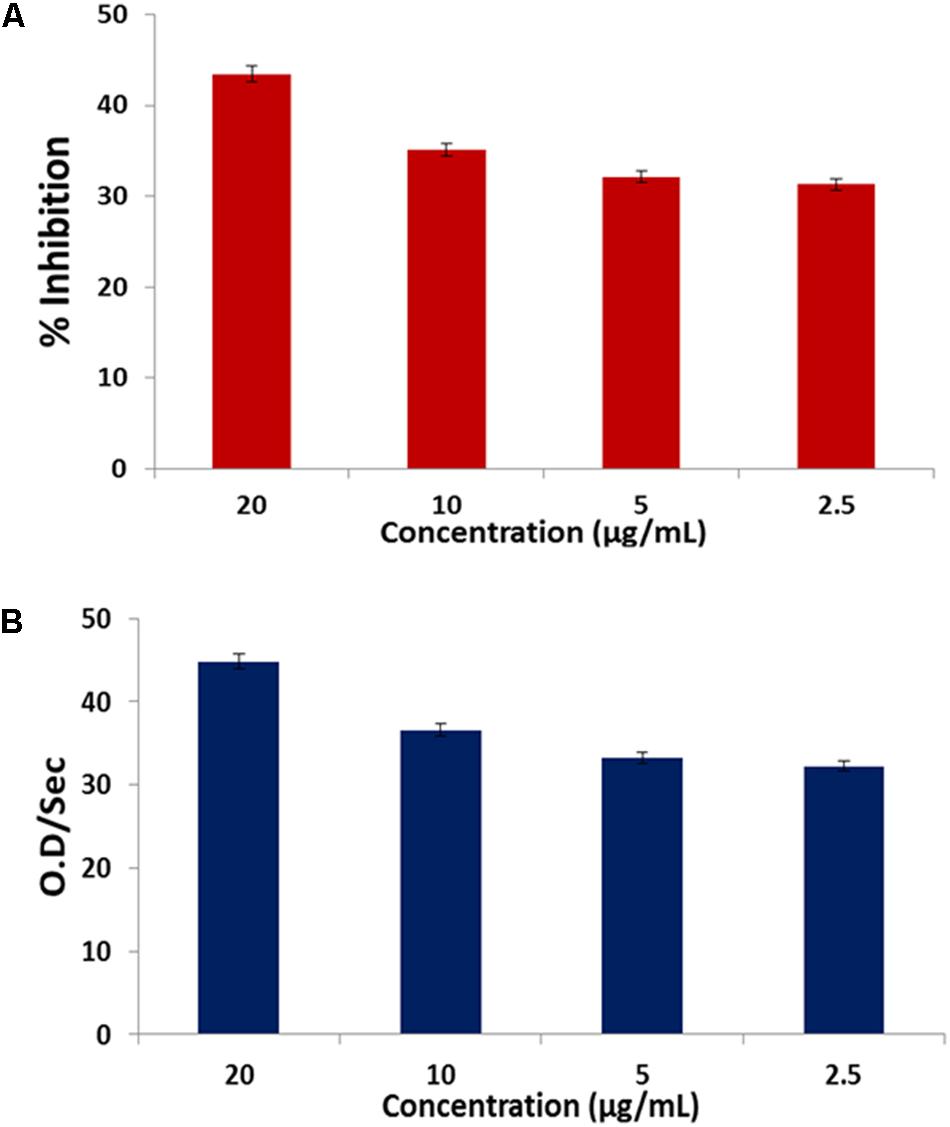
FIGURE 7. (A) Inhibitory effect of C. volubile fatty acids on α-chymotrypsin activity; (B) Effect of C. volubile fatty acids on kinetics (Vmax) of α-chymotrypsin. Values = mean ± SD; n = 3.
Discussion
Glioblastoma multiforme (GBM) has been recognized as a major brain tumor, and implicated in extensive invasion into neighboring brain tissue (Puli et al., 2006). This rapid invasion makes it difficult to treat especially while using surgical resection (Brada et al., 2001; Puli et al., 2006; Markiewicz-Żukowska et al., 2013). Hence, the need for an effective anti-proliferative agent that can suppress this invasion and/or migration with little or no side effects. In our previous study, we characterized the fatty acids extracted from C. volubile leaves using GC-MS to identify the fatty acid composition (Erukainure et al., 2016). Oleic acid was identified as the most abundant component, along with octadecanoic acid, n-hexadecanoic acid and 2-heptanone, 6-methyl in lesser quantities (Erukainure et al., 2016). In this present study, the suppressive effect of dietary fatty acids extracted from C. volubile was investigated on human glioblastoma multiforme cells with the aim of elucidating the possible molecular mechanism, along with its effect.
The cytotoxic effect of fatty acids on cancer cell lines has been reported in previous studies (Motaung et al., 1999). This corresponds with the observed cytotoxic effect of the extracted oil on U87-MG cells (Figure 1), thus indicating an anti-proliferative potential against GBM. This corresponds with our previous study of the anti-proliferative effect of C. volubile fatty against breast cancer, attributed to its high oleic acid concentration. The protective role of oleic acid against several cancers has been reported in previous epidemiological and animal studies (Carrillo et al., 2012). Thus, the observed anti-proliferative effect of the fatty acid extract, can be attributed to the high oleic acid content.
Novel natural therapies, targeted on induction of apoptosis in cancer cells, are gaining major interest. The role of apoptosis in cancer therapy is well emphasized in several studies. Most cancers manipulate and/or downregulates the antiapoptotic molecules, thereby evading this highly regulated program cell death (Koff et al., 2015). The observed apoptotic activity of the fatty acid extract as evident by the increased cell death (Figure 2), further indicates the anticancer potency of the fatty acids. This can be attributed to the arrest of S phase with concomitant depopulation of the DNA contents/cell population in G0/G1 as well as G2/M phases of the cell cycle (Figure 3). Cell cycle arrest has been implicated in the induction of apoptosis (Aliyu et al., 2013). Fauser et al. (2013) reported the induction of cell cycle by fatty acids.
Cell migration and invasion have been associated with the pathophysiology of cancer and in fact a major characteristic of malignant tumors and one of the key causes of cancer death (Bozzuto et al., 2010). In this study, the observed anti-migration, and invasion activity of the fatty acid extract on U87-MG cancer cells indicates a suppressive effect (Figure 5). This can be associated with the ability of the fatty acid enriched extract to inhibit α-chymotrypsin in vitro (Figure 7). Alpha-chymotrypsin has been implicated in the activation of metalloproteinases, and degradation of the extracellular matrix (ECM proteins), thus plays a major role in tumor growth and metastasis (Novak and Trnka, 2005; Mu et al., 2012). ECM acts as a physical barrier to migrating cells and must be degraded before the metastasis can occur. The in vitro inhibition of α-chymotrypsin (Figure 7) by the fatty acids therefore indicates a protective potential against ECM degradation. It can thus be suggested that the anti-metastatic action of the fatty acid extract could be facilitated by lowering the ability of glioblastoma cells to degrade the extracellular matrix components, by inhibiting the activities of α-chymotrypsin.
The implication of oxidative stress in the etiology of cancer has been reported (Erukainure et al., 2016). Oxidative stress arises due to an imbalance in the production of free radicals and the cell’s own antioxidant defenses (Erukainure et al., 2016). Often described as redox imbalance, it causes an impairment of normal cellular metabolism, which promotes malignancy, cancer initiation, and progression (Acharya et al., 2010). The observed reduced GSH level, SOD and catalase activities with concomitant increased MDA level in untreated U87-MG cells indicates an occurrence of oxidative stress (Figures 6A–D). Alteration of these enzymes has been reported in malignant cells and tumor tissues, thus suggesting an occurrence of redox imbalance in cancer cells (Barrera, 2012). The reversed levels and activities on following treatment with the fatty acids indicate an antioxidative potency against glioblastoma (Figure 6). Erukainure et al. (2016) reported a similar effect by same extract on MCF-7 cells, indicating the antioxidant protective effect of the leaf fatty acids against human invasive cancers. A remarkable association has been recognized between oxidative stress and increased uncontrollable drive of the G0/G1 and G2/M phases, leading to tumor metastasis and invasion (Pizarro et al., 2009; Chaudhary et al., 2013; Carrasco-Torres et al., 2017). This corresponds with the observed anti-oxidative activity, S phase arrest and concomitant depopulation of DNA contents/cell population in G0/G1 and G2/M phases of the cell cycle (Figure 3). This is also reflected by the decreased cell migration (Figure 5).
Fatty acids from C. volubile leaves could thus suppress tumor metastasis and/or invasion in human neuronal glioblastoma cells by the following mechanism: (1) attenuation of redox imbalance which inactivates the G0/G1 phase; (2) depopulation of DNA in the G0/G1 and G2/M phases with concurrent arrest of the S phase leading to inactivation of the cell cycle and apoptosis; and (3) Inhibition of α-chymotrypsin degradation of the extracellular matrix.
Conclusion
The results of the current study suggest a suppressive effect of the fatty acid extract of C. volubile leaves against tumor metastasis and/or invasion in human neuronal glioblastoma cells, thus demonstrating their preliminary therapeutic potential. This fatty acid rich extract may thus be proposed as dietary supplements for the treatment, and prevention of such ailments.
Author Contributions
OE, NA, AN, AM, GE, and MZ: conceived and designed the project. OE, AN, and NA: performed the MTT, apoptosis, transwell migration, and cell cycle assays. OE and AM: performed the oxidative stress assay. OE, AM, AO, and MZ: performed the statistical analysis. All authors were involved in the interpretation of results. OE, AM, GE, and MZ wrote the manuscript. All authors revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the consistent laboratory effort of Dr. Salman A. Khan from Molecular Oncology Laboratory, Dr. Panjwani Center for Molecular Medicine and Drug Research (PCMD), International Center for Chemical and Biological Sciences, University of Karachi, Karachi, Pakistan. OE acknowledges the World Academy of Sciences for the advancement of Science in Developing Countries (TWAS), Trieste, Italy, for ICCBS-TWAS Fellowship (2012) at the H.E.J. Research Institute of Chemistry, ICCBS, University of Karachi, Karachi, Pakistan.
References
Acharya, A., Das, I., Chandhok, D., and Saha, T. (2010). Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 3, 23–34. doi: 10.4161/oxim.3.1.10095
Adefegha, S., and Oboh, G. (2010). “Water Extractable Phytochemicals from some nigerian spices inhibit Fe2+-induced lipid peroxidation in Rat’s brain – in vitro. J. Food Process. Technol. 2, 1–6.
Aliyu, M., Odunola, O. A., Farooq, A. D., Rasheed, H., Mesaik, A. M., Choudhary, M. I., et al. (2013). Molecular mechanism of antiproliferation potential of Acacia honey on NCI-H460 cell line. Nutr. Cancer 65, 296–304. doi: 10.1080/01635581.2013.756920
Antal, O., Hackler, L., Shen, J., Mán, I., Hideghéty, K., Kitajka, K., et al. (2014). Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: cellular, biochemical and gene expression analysis. Lipid. Health Dis. 13, 142. doi: 10.1186/1476-511X-13-142
Barrera, G. (2012). Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012:137289. doi: 10.5402/2012/137289
Bozzuto, G., Ruggieri, P., and Molinari, A. (2010). Molecular aspects of tumor cell migration and invasion. Ann. Ist. Super. Sanita 46, 66–80. doi: 10.1590/S0021-25712010000100009
Brada, M., Hoang-Xuan, K., Rampling, R., Dietrich, P., Dirix, L., Macdonald, D., et al. (2001). Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann. Oncol. 12, 259–266.
Burkill, H. M. (1985). The Useful Plants of West Tropical Africa, Edn 2, Vol. 1. Richmond, VA: Royal Botanic Gardens, Kew.
Cannell, R. J., Kellam, S. J., Owsianka, A. M., and Walker, J. M. (1988). Results of a large scale screen of microalgae for the production of protease inhibitors. Planta Med. 54, 10–14. doi: 10.1055/s-2006-962319
Carrasco-Torres, G., Baltiérrez-Hoyos, R., Andrade-Jorge, E., Villa-Treviño, S., Trujillo-Ferrara, J. G., and Vásquez-Garzón, V. R. (2017). Cytotoxicity, oxidative stress, cell cycle arrest, and mitochondrial apoptosis after combined treatment of hepatocarcinoma cells with maleic anhydride derivatives and quercetin. Oxid. Med. Cell. Longev. 2017:2734976. doi: 10.1155/2017/2734976
Carrillo, C., Cavia Camarero, M. D. M., and Alonso de la Torre, S. (2012). Antitumor effect of oleic acid; mechanisms of action. A review. Nutr. Hosp. 27, 1860–1865. doi: 10.3305/nh.2012.27.6.6010
Chance, B., and Maehly, A. (1955). Assay of catalases and peroxidases. Method Enzymol. 2, 764–775. doi: 10.1016/S0076-6879(55)02300-8
Chaudhary, P., Sharma, R., Sahu, M., Vishwanatha, J. K., Awasthi, S., and Awasthi, Y. C. (2013). 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J. Biol. Chem. 288, 20532–20546. doi: 10.1074/jbc.M113.467662
Chowdhury, P., and Soulsby, M. (2002). Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Annal. Clin. Lab. Sci. 32, 188–192.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77. doi: 10.1016/0003-9861(59)90090-6
Erukainure, L., Oke, O., Owolabi, F., and Adenekan, S. (2010). Antioxidant nutrient properties and antioxidant activities of obenetete (Clerodendrum volubile), a nonconventional leafy vegetable consumed in Nigeria. Afric. J. Food Agric. Nutr. Dev. 10, 4156–4167. doi: 10.4314/ajfand.v10i10.62893
Erukainure, O., Oke, O., Ajiboye, A., and Okafor, O. (2011). Nutritional qualities and phytochemical constituents of Clerodendrum volubile, a tropical non-conventional vegetable. Int. Food Res. J. 18, 1393–1399.
Erukainure, O. L., Ebuehi, O. A., Choudhary, I. M., Adhikari, A., Hafizur, R. M., Perveen, S., et al. (2014). Iridoid glycoside from the leaves of Clerodendrum volubile beauv. Shows potent antioxidant activity against oxidative stress in rat brain and hepatic tissues. J. Diet. Suppl. 11, 19–29. doi: 10.3109/19390211.2013.859213
Erukainure, O. L., Zaruwa, M. Z., Choudhary, M. I., Naqvi, S. A., Ashraf, N., Hafizur, R. M., et al. (2016). Dietary fatty acids from leaves of clerodendrum volubile induce cell cycle arrest, downregulate matrix metalloproteinase-9 expression, and modulate redox status in human breast cancer. Nutr. Cancer 68, 634–645. doi: 10.1080/01635581.2016.1156714
Fauser, J., Matthews, G., Cummins, A., and Howarth, G. (2013). Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 59, 214–224. doi: 10.1159/000356067
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., and Forman, D. (2011). Global cancer statistics. CA Cancer J. Clin. 61, 69–90. doi: 10.3322/caac.20107
Kakkar, P., Das, B., and Viswanathan, P. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 21, 130–132.
Koff, J. L., Ramachandiran, S., and Bernal-Mizrachi, L. (2015). A time to kill: targeting apoptosis in cancer. Int. J. Mol. Sci. 16, 2942–2955. doi: 10.3390/ijms16022942
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Markiewicz-Żukowska, R., Borawska, M. H., Fiedorowicz, A., Naliwajko, S. K., Sawicka, D., and Car, H. (2013). Propolis changes the anticancer activity of temozolomide in U87MG human glioblastoma cell line. BMC Complement. Altern. Med. 13:50. doi: 10.1186/1472-6882-13-50
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immun. Methods 65, 55–63. doi: 10.1016/0022-1759(83)90303-4
Motaung, E., Prinsloo, S., Van Aswegen, C., Du Toit, P., Becker, P., and Du Plessis, D. (1999). Cytotoxicity of combined essential fatty acids on a human prostate cancer cell line. Prostagl. Leukot. Essent. Fatty Acid 61, 331–337. doi: 10.1054/plef.1999.0107
Mu, X.-M., Shi, W., Sun, L.-X., Li, H., Wang, Y.-R., Jiang, Z.-Z., et al. (2012). Pristimerin inhibits breast cancer cell migration by up-regulating regulator of G protein signaling 4 expression. Asian Pac. J. Cancer Prev. 13, 1097–1104. doi: 10.7314/APJCP.2012.13.4.1097
Nickavar, B., Mojab, F., Javidnia, K., and Amoli, M. A. R. (2003). Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Naturforsch. C 58, 629–631. doi: 10.1515/znc-2003-9-1004
Pizarro, J. G., Folch, J., Vazquez De la Torre, A., Verdaguer, E., Junyent, F., Jordán, J., et al. (2009). Oxidative stress-induced DNA damage and cell cycle regulation in B65 dopaminergic cell line. Free Radic. Res. 43, 985–994. doi: 10.1080/10715760903159188
Potter, J. D., and Potter, J. D. (1997). Food, Nutrition and the Prevention of Cancer: A Global Perspective: Summary. Washington, DC: American Institute of Cancer Research.
Pozarowski, P., and Darzynkiewicz, Z. (2004). Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 281, 301–311. doi: 10.1385/1-59259-811-0:301
Puli, S., Lai, J. C., and Bhushan, A. (2006). Inhibition of matrix degrading enzymes and invasion in human glioblastoma (U87MG) cells by isoflavones. J. Neuro Oncol. 79, 135–142. doi: 10.1007/s11060-006-9126-0
Woolf, E. C., and Scheck, A. C. (2015). The ketogenic diet for the treatment of malignant glioma. J. Lipid Res. 56, 5–10. doi: 10.1194/jlr.R046797
Keywords: cancer, Clerodendrum volubile, oxidative stress, tumor migration, unsaturated fatty acids
Citation: Erukainure OL, Ashraf N, Naqvi AS, Zaruwa MZ, Muhammad A, Odusote AD and Elemo GN (2018) Fatty Acids Rich Extract From Clerodendrum volubile Suppresses Cell Migration; Abates Oxidative Stress; and Regulates Cell Cycle Progression in Glioblastoma Multiforme (U87 MG) Cells. Front. Pharmacol. 9:251. doi: 10.3389/fphar.2018.00251
Received: 19 November 2017; Accepted: 06 March 2018;
Published: 20 March 2018.
Edited by:
Abdul Sadiq, University of Malakand, PakistanCopyright © 2018 Erukainure, Ashraf, Naqvi, Zaruwa, Muhammad, Odusote and Elemo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ochuko L. Erukainure, bG9yZWtzQHlhaG9vLmNvLnVr
 Ochuko L. Erukainure
Ochuko L. Erukainure Nadia Ashraf
Nadia Ashraf Asma S. Naqvi3
Asma S. Naqvi3 Aliyu Muhammad
Aliyu Muhammad