- 1College of Veterinary Medicine, Northeast Agricultural University, Harbin, China
- 2Heilongjiang Key Laboratory for Animal Disease Control and Pharmaceutical Development, Harbin, China
- 3Heilongjiang Academy of Agricultural Sciences, Harbin, China
Streptococcus suis is difficult to treat and responsible for various infections in humans and pigs. It can also form biofilms and induce persistent infections. Rhizoma Coptidis is a medicinal plant widely used in Traditional Chinese Medicine. Although the inhibitory effects of Rhizoma Coptidis on biofilm formation have been investigated in several studies, the ability of Rhizoma Coptidis to inhibit S. suis biofilm formation and the underlying mechanisms have not yet been reported. In this study, we showed that sub-minimal inhibitory concentrations (25 and 50 μg mL-1) of water extracts of Rhizoma Coptidis (Coptis deltoidea C.Y.Cheng & P.K.Hsiao, obtained from Sichuan Province) were sufficient to inhibit biofilm formation, as shown in the tissue culture plate (TCP) method and scanning electron microscopy. Real-time PCR and iTRAQ were used to measure gene and protein expression in S. suis. Sub-minimum inhibitory concentrations (25 and 50 μg mL-1) of Rhizoma Coptidis water extracts inhibited S. suis adhesion significantly in an anti-adherence assay. Some genes, such as gapdh, sly, and mrp, and proteins, such as antigen-like protein, CPS16V, and methyltransferase H, involved in adhesion were significantly modulated in cells treated with 50 μg mL-1 of Rhizoma Coptidis water extracts compared to untreated cells. The results from this study suggest that compounds in Rhizoma Coptidis water extracts play an important role in inhibiting adhesion of S. suis cells and, therefore, biofilm formation.
Introduction
Streptococcus suis is a pathogen causing huge economic and financial losses in the pork industry and an emerging threat to human health (Staats et al., 1997; Hill et al., 2005; Lun et al., 2007). S. suis can form biofilms, trapping nutrients, and shielding the pathogen from antagonistic effects (Brady et al., 2008; Wang et al., 2011). Biofilms are consortia of microorganisms attached to biotic or abiotic surfaces. Generally, the initial step in biofilm formation is a non-specific, reversible attachment of bacteria to substrate surfaces. Once permanently attached, the bacteria start to synthesize insoluble exopolysaccharides that encase the adherent bacteria in a three-dimensional matrix (Costerton et al., 1987). Therefore, reducing S. suis adhesion to surfaces may be an effective way to mitigate biofilm formation.
Studies have suggested that some genes and proteins play crucial roles in a series of complex molecular processes leading to biofilm formation (Sauer, 2003; Latasa et al., 2006; Beloin et al., 2008; Gaddy and Actis, 2009). A previous study reported deletion of the atl gene from S. suis type 2 strain HA9801, which encodes an autolysin, reduced adhesion to HEp-2 cells by 50% compared with wild-type S. suis, suggesting a role for Alt in biofilm formation and cell adhesion (Ju et al., 2012). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an S. suis protein, has been identified as an adhesin. GAPDH mediates cell adhesion, encouraging biofilm production (Brassard et al., 2004; Wang and Lu, 2007). Muramidase-released protein (MRP) is a cell wall protein allowing bacteria to resist phagocytosis by macrophages and aids adhesion to epithelial cells (Liang et al., 2011). MRP induces expression of the cell surface protein BapA1 in Streptococcus pneumonia. Deletion of mrp reduces the bacterium’s ability to aggregate and form biofilms (Liang et al., 2011).
Rhizoma Coptidis (RC), used for over 2000 years in Traditional Chinese Medicine, has been studied for its antibacterial, antiviral, anti-inflammatory, anti-hyperglycemic, and hypolipidemic effects (Ye et al., 2009; Wu et al., 2014). In recent years, there has been a surge in the study of plants rich in bioactive components. These components have been shown to possess various beneficial properties including anti-adhesive effects (Dixon, 2001). It is reported that Rhizoma Coptidis can inhibit biofilm formation by Staphylococcus epidermidis (Wang et al., 2009). Previous studies on the anti-pathogenic effects of Rhizoma Coptidis have focused on its anti-biofilm activity (Zhu and Li, 2006; Hayashi et al., 2007; Yu et al., 2007). Our previous study indicated that a water extract from Rhizoma Coptidis (C. deltoidea C.Y.Cheng & P.K.Hsiao, obtained from Sichuan Province), berberine hydrochloride and coptisine can all inhibit S. suis biofilm formation in a tissue culture plate (TCP) assay (Liu et al., 2015), though the mechanisms involved are poorly understood.
Since in the previous study we only found that water extract from Rhizoma Coptidis could interfere with the formation of S. suis biofilms, but we did not know the mechanism involved. So the aim of this study was therefore to investigate the mechanisms by which Rhizoma Coptidis (C. deltoidea C.Y.Cheng & P.K.Hsiao, obtained from Sichuan Province) extracts disrupt S. suis biofilm formation and bacterial adherence, and to guide strategies to prevent S. suis biofilm infection.
Materials and Methods
Preparation of Extract
Rhizoma Coptidis (C. deltoidea C.Y.Cheng & P.K.Hsiao, obtained from Sichuan Province) was purchased as a crude drug from the Beijing Tong Ren Tang Pharmacy. Its identity was authenticated by Professor Mingxia Bai at the Horticulture Branch of the Heilongjiang Academy of Agricultural Sciences. A berberine standard (877-200001) was purchased from the Ministry of Health of Drug Products. To generate water extracts of Rhizoma Coptidis, 50 g Rhizoma Coptidis powder were boiled in 500 mL distilled water for 60 min at 100°C before decanting and filtration. The filtrate was collected and added to 300 mL of distilled water and boiled for 60 min at 100°C. The final filtrate mass was lyophilized and concentrated into a dried powder with a yield of 0.25 g mL-1 and stored at 4°C. The amount of berberine, the major active ingredient, in Rhizoma Coptidis water extracts was measured by high-performance liquid chromatography (HPLC) on a Waters Alliance HPLC system (Waters e2695, United States) consisting of a binary pump and a UV/Vis detector. Separation was carried out using a 5 μm DL-Cl8 column (4.6 mm × 150 mm, Japan) at 37°C. Acetonitrile (solvent A) and 0.05 M potassium dihydrogen phosphate (solvent B) were used as the mobile phase at a ratio of 40:60 (solvent A:solvent B), supplemented with 0.015 M sodium dodecyl sulfate. The flow rate was set at 1.2 mL min-1. A detection wavelength of 345 nm and an injection volume of 5 μL were used in the study. The amount of berberine in the Rhizoma Coptidis aqueous extract was determined by comparing the HPLC retention time to the authentic standard. Quantification of berberine in the aqueous extract was done using a linear calibration plot of the peak area in HPLC at 345 nm against concentration using the external standard method. The calibration curve was calculated by plotting peak areas against six different concentrations of the standard solutions (0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mg mL-1).
Minimum Inhibitory Concentrations
The MIC (minimal inhibitory concentration) was determined by the microtiter broth dilution method, as recommended by the Clinical and Laboratory Standards Institute [CLSI] (2016). Dilutions were performed in Todd-Hewitt Broth (THB) medium using 1 × 106 colony-forming units (CFU) of bacteria per milliliter. Cell suspensions (100 μL) were inoculated into 96-well microtiter plates in the presence of Rhizoma Coptidis water extracts with different final concentrations (0, 1.625, 3.125, 6.25, 12.5, 25, 50, 100, or 200 μg mL-1). Azithromycin was used as a positive control, with the susceptibility (MIC) of S. suis ATCC 700794 to azithromycin found to be 32 μg mL-1 (Yang et al., 2016). Inoculated microplates were incubated at 37°C for 24 h before examination. Susceptibility (MIC) of S. suis ATCC700794 to Rhizoma Coptidis water extracts was 100 μg mL-1 (Berberine, the active ingredient, in Rhizoma Coptidis water extracts was 36.3 μg mL-1).
Growth Conditions of S. suis Biofilms
Streptococcus suis ATCC700794 was grown overnight in THB (Sigma-Aldrich) at 37°C with constant shaking. Biofilm culture production was described previously (Wang et al., 2011). Briefly, S. suis grown in THB medium at 37°C was added to a 1% fibrinogen solution in 100 mm polystyrene dishes and grown for 24 h. After decanting the growth medium, plates were thoroughly rinsed twice with 50 mM Tris–HC1 (pH 7.5). Biofilms were then harvested by scraping. Cells were sonicated for 5 min and centrifuged at 12,000 × g for 10 min at 4°C. Supernatants were then removed and cell pellets were washed twice with 50 mM Tris–HC1 (pH 7.5).
Determination of the Effect of Rhizoma Coptidis Water Extracts on Biofilm Formation by TCP Assay
Streptococcus suis cultures in mid-exponential growth phase with an optical density of 0.2 at 600 nm (OD600) were used for TCP assays. In each well of a 96-well plate, 100 μL of S. suis culture and 100 μL of Rhizoma Coptidis water extract were combined. The final tested concentrations were 6.25, 12.5, 25, or 50 μg mL-1. Wells filled with sterile growth medium were included as blank controls, azithromycin (1/2MIC) as a positive control. Wells containing 200 μL culture without extract served as negative controls. After incubation at 37°C for 24 h, all wells were washed with sterile phosphate-buffered saline (PBS) and stained with crystal violet.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was performed as described previously (Zhao et al., 2015). Briefly, cultures were diluted to an OD600 of 0.1 before adding 2 mL to wells of a six-well microplate containing a 10 mm × 10 mm sterilized rough organic membrane (Mosutech Co., Ltd., Shanghai, China). After incubation without shaking for 24 h at 37°C, medium and planktonic bacteria were removed by washing with sterile PBS. Biofilms were then prepared for analysis (Zhao et al., 2015).
Anti-adherence Activity of Extract Against S. suis
Anti-adherence to organic membranes. Assays were prepared as previously described (Hamada et al., 1981). Briefly, S. suis ATCC700794 cultures at mid-exponential growth phase were diluted to an OD600 of 0.1 before combining with 2 mL of THB or sub-MICs of Rhizoma Coptidis water extract in a six-well microplate containing a 10 mm × 10 mm sterilized rough organic membrane (Mosutech Co., Ltd., Shanghai, China). After incubation without shaking for 24 h at 37°C, planktonic cells were decanted. Attached cells were removed by addition of 0.5 M sodium hydroxide. Adherence was quantified by OD600. Percentage adherence = [OD600 of adhered cells/(OD600 of adhered cells + OD600 of planktonic cells)].
Anti-adherence to cells. Assays were prepared as described previously (Lalonde et al., 2000) with slight modifications. Briefly, PK-15 cells were cultured in DMEM (Hyclone) and grown in 75 cm × 75 cm flasks at 37°C with 5% CO2. Confluent monolayers of PK-15 cells (1.0 × 105 cells per well) were cultured in 96-well plates (Corning, NY, United States). S. suis cells, either supplemented with sub-MICs of Rhizoma Coptidis water extracts or untreated, were added to each well at an MOI of 100:1 and incubated at 37°C to allow cells to attach. After 4 h, plates were washed twice with PBS and cells were lysed with sterile distilled water on ice. Both adherent and intracellular bacteria were counted on THB agar. Both assays were repeated three times.
RNA Isolation and Real-Time PCR
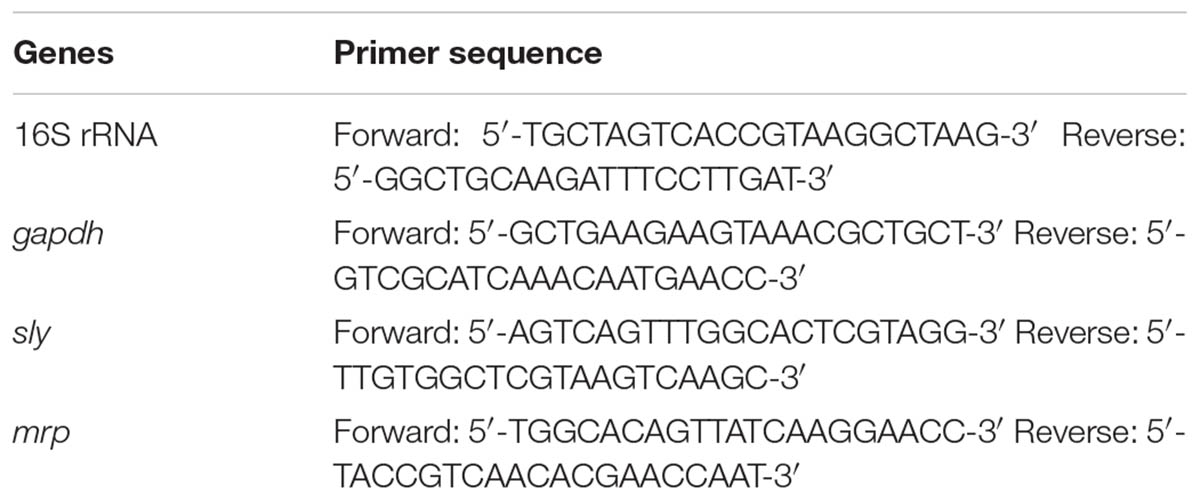
Real-time PCR was performed as described previously (Yang et al., 2015). The primer sequences used in the experiment were shown in Table 1. To investigate the effect of Rhizoma Coptidis water extracts on gene expression, mid-log growth phase cultures of S. suis were supplemented with 50 μg mL-1 extract and incubated at 37°C for 24 h. Cells without extract served as control. Cultures were centrifuged at 10,000 × g for 5 min before treatment with an RNase Remover I (Huayueyang Ltd., Beijing, China). Total RNA levels were determined using the E.Z.N.ATM. Bacterial RNA isolation kit. Real-time PCR for each sample was performed as previously described (Yang et al., 2015).
iTRAQ Analysis
Protein was extracted from S. suis cells either treated with 50 μg mL-1 Rhizoma Coptidis water extract or left untreated (Wang et al., 2011). iTRAQ analysis was performed at Shanghai Applied Protein Technology Co., Ltd. (APT, Shanghai, China). Three biological replicates were evaluated to minimize the influence of less reliable quantitative information. iTRAQ analysis was performed as previously described (Zhao et al., 2015).
Statistical Analysis
Values were calculated as the mean of individual experiments in triplicate and compared to those of the control groups. Differences between two mean values were calculated by Student’s t-test using SPSS 11.0.0 statistical software, with p-values below 5% designated as statistically significant.
Results
Amounts of the Active Ingredient Berberine in Rhizoma Coptidis Water Extracts
High-performance liquid chromatography chromatograms of a Rhizoma Coptidis water extract and a standard solution of berberine are shown in Figure 1. The retention time of berberine agreed well with the authentic compound (15.43 min). The calibration curve equation was y = 7E + 06x + 28,724, R2 = 0.999. Using the calibration curve, the portion of berberine, the active ingredient, in Rhizoma Coptidis water extracts was calculated to be 36.30%.
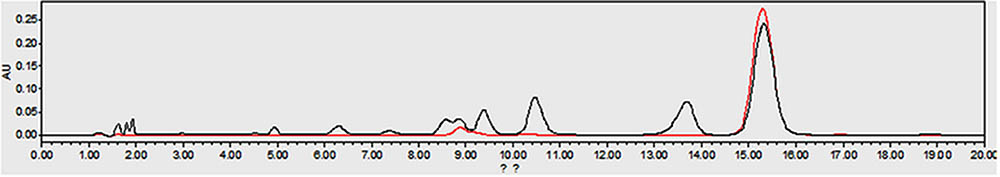
FIGURE 1. The HPLC chromatograms of the Rhizoma Coptidis aqueous extract (black, retention time 15.43 min) and the berberine standard (red, retention time 15.43 min).
Effect of Rhizoma Coptidis Water Extracts Against Biofilm Formation in Vitro
The TCP method allows quantitative detection of S. suis biofilm formation at 24 h. Four different doses of Rhizoma Coptidis water extract were tested against S. suis biofilms (Figure 2). At 12.5 and 6.25 μg mL-1, the OD600 of S. suis ATCC700794 were lower than the negative control. At 25 and 50 μg mL-1, there was significant inhibition (p < 0.05) of S. suis biofilm formation, suggesting that these concentrations were more effective than 6.25 or 12.5 μg mL-1 at inhibiting biofilm formation.
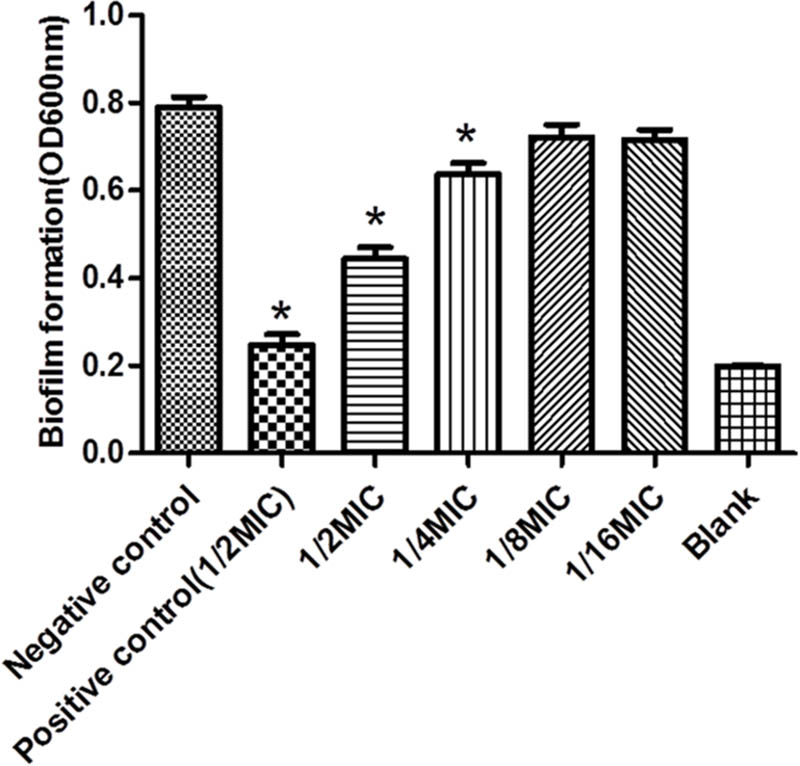
FIGURE 2. Effect of Rhizoma Coptidis at different concentrations on S. suis ATCC700794 biofilm formation. Data are expressed as means ± standard. Significant decrease (∗p<0.05) compared to control biofilm formation of S. suis in vitro.
Scanning Electron Microscopy
Scanning electron microscopy was performed to examine the effects of 50 μg mL-1 Rhizoma Coptidis water extract on S. suis biofilm formation. In the absence of extract, the surface of the rough organic membrane was observed to be almost entirely covered by aggregates and micro-colonies of S. suis (Figure 3A). However, when 50 μg mL-1 extract was added, most of the cell aggregates were dispersed (Figure 3B), suggesting that S. suis biofilm formation was inhibited by the extract in vitro.
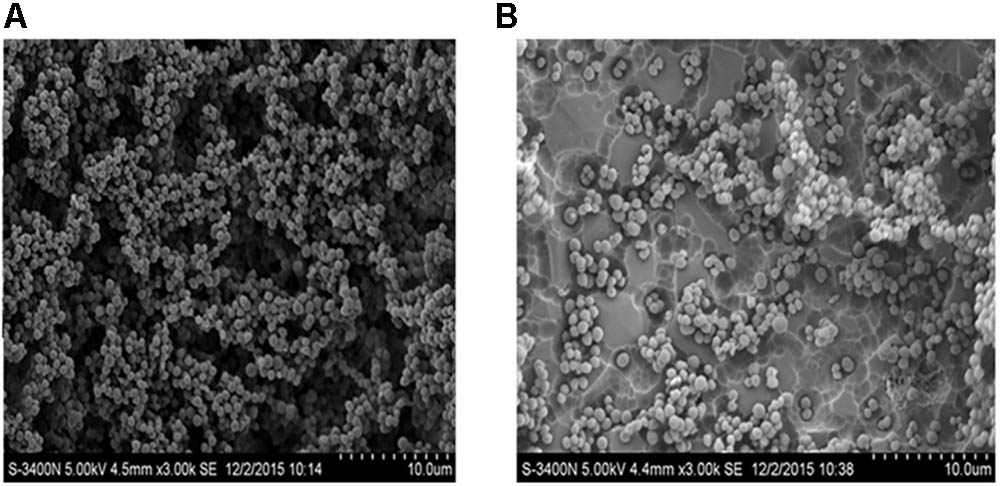
FIGURE 3. Scanning electron microscopy of biofilm of S. suis grown in THB broth. (A) With 0 MIC (0 μg mL-1) concentration of aqueous extracts of Rhizoma Coptidis; (B) with 1/2 × MIC (50 μg mL-1) concentration of aqueous extracts of Rhizoma Coptidis.
Anti-adherence Activity of Extract Against S. suis
The inhibitory effects of Rhizoma Coptidis water extract on adherence of S. suis to glass were tested at several concentrations (Figure 4). The extract inhibited adherence to organic membranes (Figure 4A) and PK-15 cells (Figure 4B).
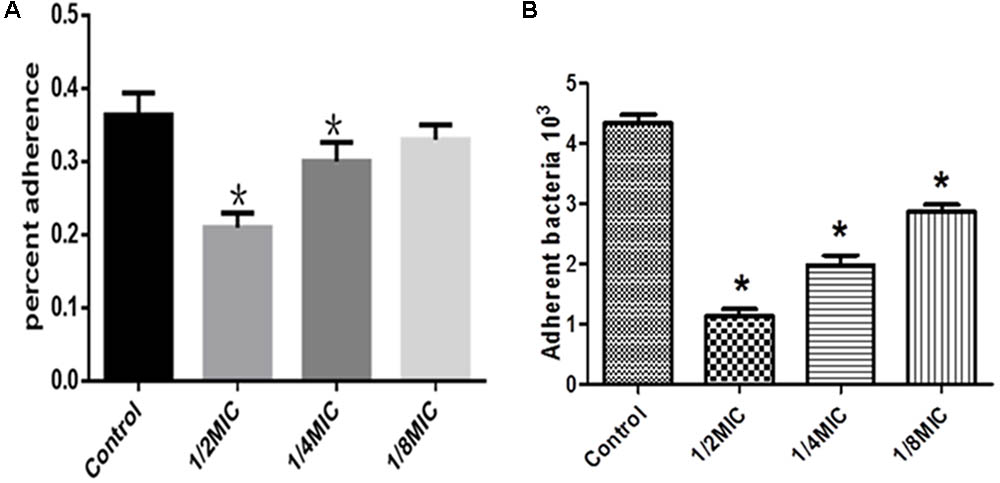
FIGURE 4. Effect of Rhizoma Coptidis at different concentrations on S. suis ATCC700794 adhesion to glass (A), or PK-15 cells (B). Data are expressed as means ± standard. Significant decrease (∗p < 0.05) compared to control in vitro.
The Effect of Rhizoma Coptidis Water Extracts on Gene Expression
The expression profiles of gapdh, sly, and mrp in S. suis were determined 24 h post-treatment with 50 μg mL-1 Rhizoma Coptidis water extract. In treated cultures, gapdh, sly, and mrp gene expression levels were suppressed compared to untreated samples (Figure 5).
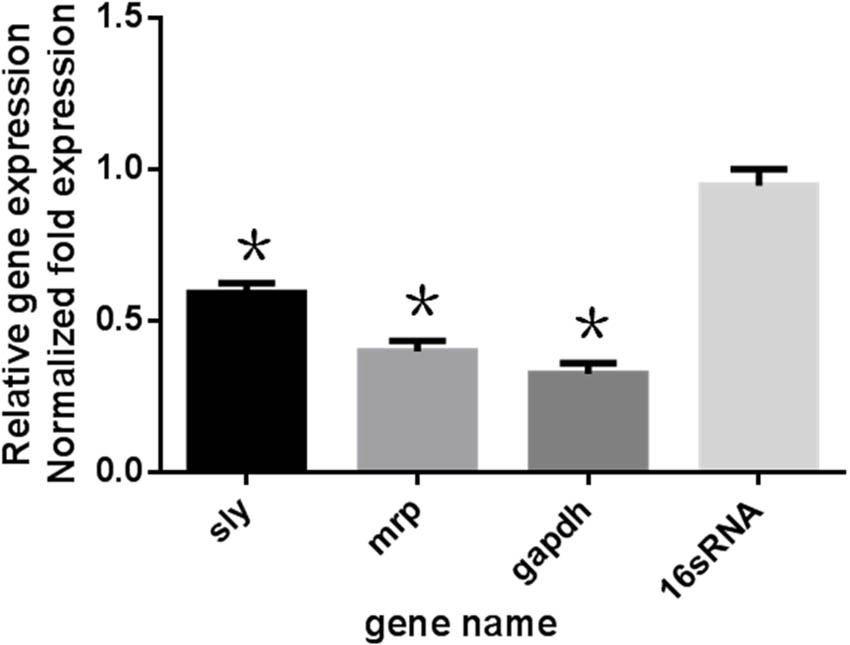
FIGURE 5. Effect of 1/2MIC of aqueous extracts of Rhizoma Coptidis on mRNA decreased expression of genes in S. suis ATCC700794. Data are expressed as means ± standard deviations. The expression was normalized to 16S rRNA. Controls refer to the absence of aqueous extracts of Rhizoma Coptidis. Significantly different (∗p < 0.05) compared to untreated control bacteria.
Rhizoma Coptidis Water Extracts Inhibit Biofilm Formation and Modulate Protein Expression by iTRAQ
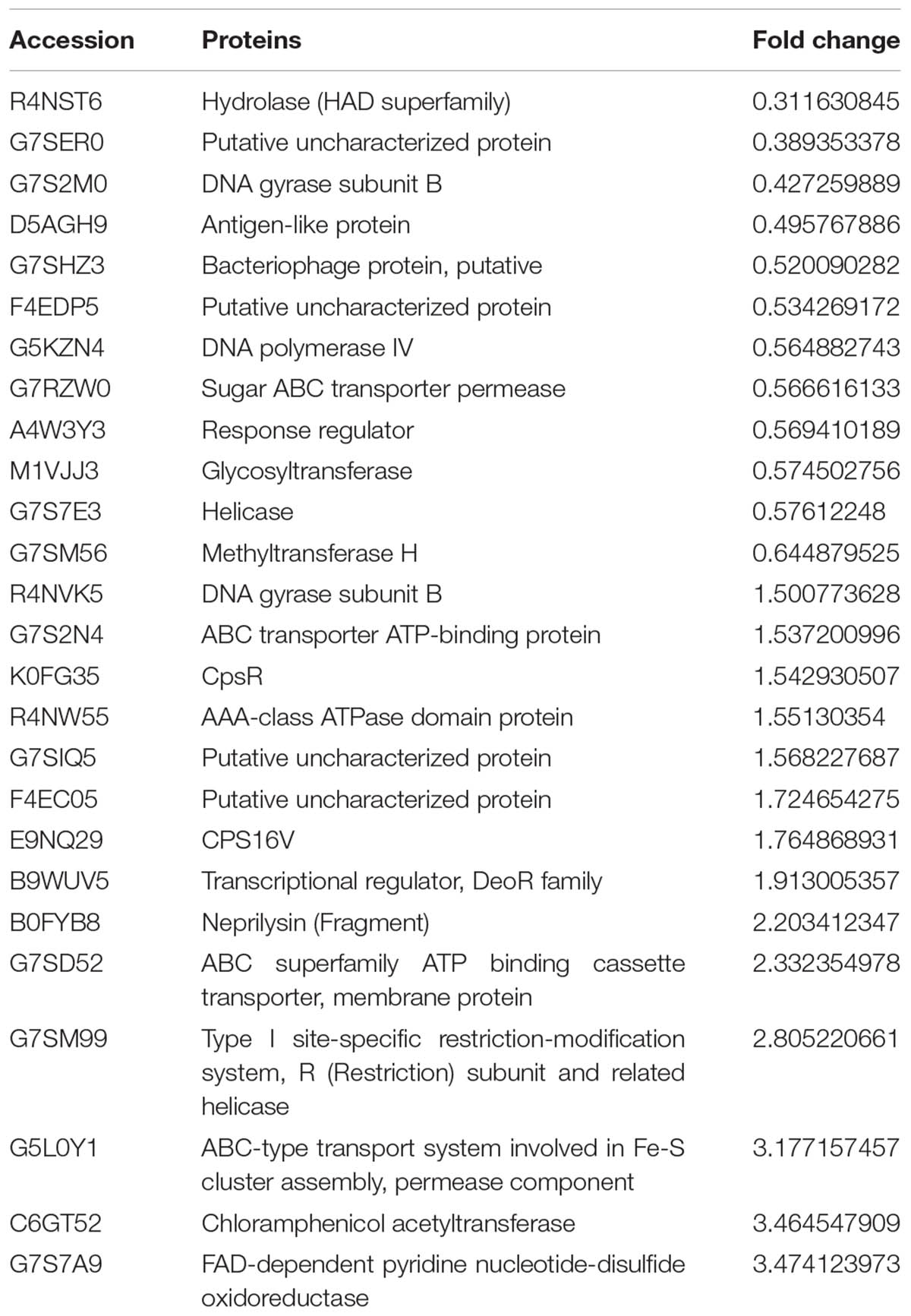
Streptococcus suis cultures were incubated with extract for 24 h before measurement using iTRAQ. Changes in protein expression levels were observed, with some proteins changing by more than 1.5-fold and others by less than 0.67-fold (p < 0.05). Of the 26 proteins tested using iTRAQ after treatment with 50 μg mL-1 extract, expression of 15 proteins increased and 11 were suppressed (Table 2). Among the suppressed proteins were antigen-like protein (D5AGH9), hydrolase (R4NST6), methyltransferase H (G7SM56), glycosyltransferase (M1VJJ3), and helicase (G7S7E3). These proteins had fold-change values of 0.495767886, 0.311630845, 0.644879525, 0.574502756, and 0.57612248, respectively.
Discussion
We investigated the relationship between Rhizoma Coptidis water extracts and S. suis biofilm formation. Previous studies have suggested that there is a relationship between some antimicrobial agents and biofilm formation (Majtan et al., 2008; Nucleo et al., 2009; Mishra et al., 2014; Zhao et al., 2015; Wang et al., 2016). In our study, sub-MICs of Rhizoma Coptidis water extracts could inhibit biofilm formation of S. suis in vitro, as observed in a TCP assay. Most studies on the anti-infective activities of Rhizoma Coptidis have focused on its anti-biofilm effects, with little or no attention paid to the specific mechanisms this effect. We detected anti-adherence activity of Rhizoma Coptidis water extracts against S. suis, suggesting that extracts of this plant inhibit adherence to organic membranes and PK-15 cells. Our results suggest that the anti-adherence activity of Rhizoma Coptidis water extracts is an important factor in inhibiting S. suis biofilm formation.
In this study, we identified S. suis genes gapdh, sly, and mrp as potential targets of Rhizoma Coptidis water extracts. These genes are thought to play key roles in infection and invasion (Liang et al., 2011; Ju et al., 2012) and have been shown to be important in biofilm formation and adhesion. Treatment with 50 μg mL-1 of extract suppressed gapdh, sly, and mrp gene expression. We speculate that this downregulation may be the cause of a reduction in S. suis adhesion and therefore biofilm formation. However, the detailed molecular mechanisms behind this reduction are still unknown and should be addressed in further studies.
Using iTRAQ, we found that 26 proteins were differentially expressed upon treatment of S. suis with 50 μg mL-1 Rhizoma Coptidis water extract compared to untreated cells. Of these proteins, 11 proteins, implicated in surface adhesion and biofilm formation, were significantly suppressed. These include an antigen-like protein (D5AGH9), hydrolase (R4NST6), methyltransferase H (G7SM56), glycosyltransferase (M1VJJ3), and helicase (G7S7E3) (Table 2). Antigen-like protein (D5AGH9) has been identified as a novel matricellular protein that promotes cell adhesion and spreading (Tajiri et al., 2010). Hydrolase, from the haloacid dehydrogenase superfamily (R4NST6), plays an important role in Paracoccidioides brasiliensis adherence to host cells (Hernandez et al., 2010). A previous study showed that deletion of an orphan C5-cytosine methyltransferase, similar to methyltransferase H (G7SM56), has a significant effect on the expression of genes responsible for pathogenic growth (Kumar et al., 2012). Over-expression of the putative Brucella glycosyltransferase can lead to development of clumping and increased adhesion to polystyrene plates (Dabral et al., 2015). Furthermore, a recent study showed that mutation of the hrpB gene, which encodes RNA helicase, can reduce surface adhesion and inhibit disease spread in citrus leaves (Granato et al., 2016).
Biofilm formation and adhesion were reduced by treatment with 50 μg mL-1 of Rhizoma Coptidis water extract, likely by downregulation of expression of the proteins discussed above. In contrast, loss of capsular polysaccharides has previously been described to facilitate and speed up biofilm formation (Qin et al., 2013). Our results suggest that treatment of S. suis cells by the extract might cause upregulation of CpsR (K0FG35) and CPS16V (E9NQ29), proteins involved capsular polysaccharide formation, and reduced biofilm formation and adhesion.
Our results show that sub-MICs of Rhizoma Coptidis water extracts could inhibit biofilm formation, though the mechanism of action is unclear. We observed anti-adherence activity of the extract on S. suis. We also found that expression levels of genes and proteins involved in adhesion were significantly altered in cells treated with sub-MICs of Rhizoma Coptidis water extracts compared to untreated cells. Our results indicate that Rhizoma Coptidis water extracts inhibit S. suis biofilm formation by limiting adhesion.
Author Contributions
Y-HL designed the whole experiment. The other authors are responsible for completing the experiment.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 31472231), the earmarked fund for China Agriculture Research System-35, scientific research project of Heilongjiang Province Education Department (No. 12531037).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Shanghai Applied Protein Technology Co., Ltd. for the help with iTRAQ.
References
Beloin, C., Roux, A., and Ghigo, J. M. (2008). Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 322, 249–289. doi: 10.1007/978-3-540-75418-3_12
Brady, R. A., Leid, J. G., Calhoun, J. H., Costerton, J. W., and Shirtliff, M. E. (2008). Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52, 13–22. doi: 10.1111/j.1574-695X.2007.00357.x
Brassard, J., Gottschalk, M., and Quessy, S. (2004). Cloning and purification of the Streptococcus suis serotype 2 glyceraldehyde-3-phosphate dehydrogenase and its involvement as an adhesin. Vet. Microbiol. 102, 87–94. doi: 10.1016/j.vetmic.2004.05.008
Costerton, J. W., Cheng, K. J., Geesey, G. G., Ladd, T. I., Nickel, J. C., Dasgupta, M., et al. (1987). Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41, 435–464. doi: 10.1146/annurev.mi.41.100187.002251
Clinical and Laboratory Standards Institute [CLSI] (2016). M100S Performance Standards for Antimicrobial Susceptibility Testing, 26th Edn. Wayne, PA: CLSI.
Dabral, N., Jain-Gupta, N., Seleem, M. N., Sriranganathan, N., and Vemulapalli, R. (2015). Overexpression of Brucella putative glycosyltransferase WbkA in B. abortus RB51 leads to production of exopolysaccharide. Front. Cell. Infect. Microbiol. 5:54. doi: 10.3389/fcimb.2015.00054
Dixon, R. A. (2001). Natural products and plant disease resistance. Nature 411, 843–847. doi: 10.1038/35081178
Gaddy, J. A., and Actis, L. A. (2009). Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 4, 273–278. doi: 10.2217/fmb.09.5
Granato, L. M., Picchi, S. C., Andrade, M. D., Takita, M. A., De Souza, A. A., Wang, N., et al. (2016). The ATP-dependent RNA helicase HrpB plays an important role in motility and biofilm formation in Xanthomonas citri subsp citri. BMC Microbiol. 16:55. doi: 10.1186/s12866-016-0655-1
Hamada, S., Torii, M., Kotani, S., and Tsuchitani, Y. (1981). Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect. Immun. 32, 364–372.
Hayashi, K., Minoda, K., Nagaoka, Y., Hayashi, T., and Uesato, S. (2007). Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg. Med. Chem. Lett. 17, 1562–1564. doi: 10.1016/j.bmcl.2006.12.085
Hernandez, O., Almeida, A. J., Gonzalez, A., Garcia, A. M., Tamayo, D., Cano, L. E., et al. (2010). A 32-kilodalton hydrolase plays an important role in Paracoccidioides brasiliensis adherence to host cells and influences pathogenicity. Infect. Immun. 78, 5280–5286. doi: 10.1128/IAI.00692-10
Hill, J. E., Gottschalk, M., Brousseau, R., Harel, J., Hemmingsen, S. M., and Goh, S. H. (2005). Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 107, 63–69. doi: 10.1016/j.vetmic.2005.01.003
Ju, C. X., Gu, H. W., and Lu, C. P. (2012). Characterization and functional analysis of atl, a novel gene encoding autolysin in Streptococcus suis. J. Bacteriol. 194, 1464–1473. doi: 10.1128/JB.06231-11
Kumar, R., Mukhopadhyay, A. K., Ghosh, P., and Rao, D. N. (2012). Comparative transcriptomics of H. pylori strains AM5, SS1 and their hpyAVIBM deletion mutants: possible roles of cytosine methylation. PLoS One 7:e42303. doi: 10.1371/journal.pone.0042303
Lalonde, M., Segura, M., Lacouture, S., and Gottschalk, M. (2000). Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology 146(Pt 8), 1913–1921. doi: 10.1099/00221287-146-8-1913
Latasa, C., Solano, C., Penades, J. R., and Lasa, I. (2006). Biofilm-associated proteins. C. R. Biol. 329, 849–857. doi: 10.1016/j.crvi.2006.07.008
Liang, X., Chen, Y. Y., Ruiz, T., and Wu, H. (2011). New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect. Immun. 79, 3239–3248. doi: 10.1128/IAI.00029-11
Liu, B., Han, Q., Sheng, Z. L., Chen, J. Q., Chen, X. Y., Wei, S. G., et al. (2015). Intervention effects of water extract, berberine hydrochloride and coptisine from Rhizoma Coptidis against Streptococcus suis biofilm in vitro. Chin. J. Vet. Med. 51, 16–19.
Lun, Z. R., Wang, Q. P., Chen, X. G., Li, A. X., and Zhu, X. Q. (2007). Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect. Dis. 7, 201–209. doi: 10.1016/S1473-3099(07)70001-4
Majtan, J., Majtanova, L., Xu, M., and Majtan, V. (2008). In vitro effect of subinhibitory concentrations of antibiotics on biofilm formation by clinical strains of Salmonella enterica serovar Typhimurium isolated in Slovakia. J. Appl. Microbiol. 104, 1294–1301. doi: 10.1111/j.1365-2672.2007.03653.x
Mishra, N. N., Ali, S., and Shukla, P. K. (2014). Arachidonic acid affects biofilm formation and PGE2 level in Candida albicans and non-albicans species in presence of subinhibitory concentration of fluconazole and terbinafine. Braz. J. Infect. Dis. 18, 287–293. doi: 10.1016/j.bjid.2013.09.006
Nucleo, E., Steffanoni, L., Fugazza, G., Migliavacca, R., Giacobone, E., Navarra, A., et al. (2009). Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol. 9:270. doi: 10.1186/1471-2180-9-270
Qin, L., Kida, Y., Imamura, Y., Kuwano, K., and Watanabe, H. (2013). Impaired capsular polysaccharide is relevant to enhanced biofilm formation and lower virulence in Streptococcus pneumoniae. J. Infect. Chemother. 19, 261–271. doi: 10.1007/s10156-012-0495-3
Sauer, K. (2003). The genomics and proteomics of biofilm formation. Genome Biol. 4:219. doi: 10.1186/gb-2003-4-6-219
Staats, J. J., Feder, I., Okwumabua, O., and Chengappa, M. M. (1997). Streptococcus suis: past and present. Vet. Res. Commun. 21, 381–407. doi: 10.1023/A:1005870317757
Tajiri, Y., Igarashi, T., Li, D., Mukai, K., Suematsu, M., Fukui, E., et al. (2010). Tubulointerstitial nephritis antigen-like 1 is expressed in the uterus and binds with integrins in decidualized endometrium during postimplantation in mice. Biol. Reprod. 82, 263–270. doi: 10.1095/biolreprod.109.080028
Wang, K. C., and Lu, C. P. (2007). Adhesion activity of glyceraldehyde-3-phosphate dehydrogenase in a Chinese Streptococcus suis type 2 strain. Berl. Munch. Tierarztl. Wochenschr. 120, 207–209.
Wang, S., Yang, Y. B., Zhao, Y. L., Zhao, H. H., Bai, J. W., Chen, J. Q., et al. (2016). Sub-MIC tylosin inhibits Streptococcus suis biofilm formation and results in differential protein expression. Front. Microbiol. 7:384. doi: 10.3389/fmicb.2016.00384
Wang, X. Q., Qiu, S. J., Yao, X., Tang, T. T., Dai, K. R., and Zhu, Z. A. (2009). Berberine inhibits Staphylococcus epidermidis adhesion and biofilm formation on the surface of titanium alloy. J. Orthop. Res. 27, 1487–1492. doi: 10.1002/jor.20917
Wang, Y., Zhang, W., Wu, Z. F., and Lu, C. P. (2011). Reduced virulence is an important characteristic of biofilm infection of Streptococcus suis. FEMS Microbiol. Lett. 316, 36–43. doi: 10.1111/j.1574-6968.2010.02189.x
Wu, H., He, K., Wang, Y. Z., Xue, D. F., Ning, N., Zou, Z. Y., et al. (2014). The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma coptidis. Phytomedicine 21, 1373–1381. doi: 10.1016/j.phymed.2014.05.002
Yang, Y. B., Wang, S., Wang, C., Huang, Q. Y., Bai, J. W., Chen, J. Q., et al. (2015). Emodin affects biofilm formation and expression of virulence factors in Streptococcus suis ATCC700794. Arch. Microbiol. 197, 1173–1180. doi: 10.1007/s00203-015-1158-4
Yang, Y. B., Chen, J. Q., Zhao, Y. L., Bai, J. W., Ding, W. Y., Zhou, Y. H., et al. (2016). Sub-MICs of azithromycin decrease biofilm formation of Streptococcus suis and increase capsular polysaccharide content of S. suis. Front. Microbiol. 7:1659. doi: 10.3389/fmicb.2016.01659
Ye, X., Feng, Y., Tong, Y., Ng, K. M., Tsao, S., Lau, G. K., et al. (2009). Hepatoprotective effects of coptidis rhizoma aqueous extract on carbon tetrachloride-induced acute liver hepatotoxicity in rats. J. Ethnopharmacol. 124, 130–136. doi: 10.1016/j.jep.2009.04.003
Yu, Y., Yi, Z. B., and Liang, Y. Z. (2007). Validate antibacterial mode and find main bioactive components of traditional Chinese medicine Aquilegia oxysepala. Bioorg. Med. Chem. Lett. 17, 1855–1859. doi: 10.1016/j.bmcl.2007.01.032
Zhao, Y. L., Zhou, Y. H., Chen, J. Q., Huang, Q. Y., Han, Q., Liu, B., et al. (2015). Quantitative proteomic analysis of sub-MIC erythromycin inhibiting biofilm formation of S. suis in vitro. J. Proteomics 116, 1–14. doi: 10.1016/j.jprot.2014.12.019
Keywords: S. suis, biofilm, Rhizoma Coptidis, adhesion, real-time PCR, iTRAQ technology
Citation: Li Y-H, Zhou Y-H, Ren Y-Z, Xu C-G, Liu X, Liu B, Chen J-Q, Ding W-Y, Zhao Y-L, Yang Y-B, Wang S and Liu D (2018) Inhibition of Streptococcus suis Adhesion and Biofilm Formation in Vitro by Water Extracts of Rhizoma Coptidis. Front. Pharmacol. 9:371. doi: 10.3389/fphar.2018.00371
Received: 19 October 2017; Accepted: 29 March 2018;
Published: 16 April 2018.
Edited by:
Victor Kuete, University of Dschang, CameroonReviewed by:
Letizia Angiolella, Sapienza Università di Roma, ItalySusan Semple, University of South Australia, Australia
Copyright © 2018 Li, Zhou, Ren, Xu, Liu, Liu, Chen, Ding, Zhao, Yang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-Hua Li, bGl5YW5odWExOTcwQDE2My5jb20=
 Yan-Hua Li
Yan-Hua Li Yong-Hui Zhou
Yong-Hui Zhou Yong-Zhi Ren1,2
Yong-Zhi Ren1,2 Jian-Qing Chen
Jian-Qing Chen Yu-Lin Zhao
Yu-Lin Zhao Yan-Bei Yang
Yan-Bei Yang