- Department of Neurology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Objective: Migraine is a complex, prevalent and disabling neurological disorder characterized by recurrent episodes of headache without ideal treatment. We aim to assess the current available evidence of herbal Chuanxiong (Ligusticum chuanxiong Hort. root) formulae for the treatment of migraine according to the high-quality randomized controlled trials (RCTs).
Methods: English and Chinese electronic databases were searched from their inceptions until March 2017. The methodological quality of included study was assessed by the Cochrane Collaboration risk of bias tool. RCTs with Cochrane risk of bias (RoB) score ≥4 were included in the analyses. Meta-analysis was conducted using RevMan 5.3 software. Publication bias was assessed by funnel plot analysis and Egger's test.
Results: Nineteen RCTs with 1832 participants were identified. The studies investigated the Chuanxiong formulae vs. placebo (n = 5), Chuanxiong formulae vs. conventional pharmacotherapy (CP) (n = 13 with 15 comparisons), and Chuanxiong formulae plus CP vs. CP (n = 1). Meta-analysis indicated that Chuanxiong formulae could reduce frequency, duration, days and pain severity of migraine and improve the total clinical efficacy rate (P < 0.05). Adverse event monitoring was reported in 16 out of 19 studies and occurrence rate of adverse event was low.
Conclusion: The findings of present study indicated that Chuanxiong formulae exerted the symptom reliefs of for migraine.
Introduction
Migraine is characterized as the recurrent episodes of headaches and related symptoms, occurring in 14.70% proportion of population worldwide (Vos et al., 2012). The Global Burden of Disease (GBD) Survey listed migraine as the third most prevalent disorder in 2010 (Vos et al., 2012) and seventh position among the leading causes of disability on a global basis in 2015 (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016). According to a population-based door-to-door survey of primary headaches in China, the estimated 1-year prevalence of migraine was 9.3% (Yu et al., 2012). The disorder represents a huge socioeconomic burden with a population of over 1.3 billion in China. The total estimated annual cost of primary headache disorders was CNY 672.7 billion, accounting for 2.24% of gross domestic product (GDP) (Yu et al., 2012). Therapeutic agents, including non-steroidal anti-inflammatory drugs (NSAIDs) (aspirin, diclofenac, ibuprofen, naproxen), opioids (butorphanol nasal spray) and triptans (almotriptan; eletriptan; frovatriptan; naratriptan; rizatriptan) are common used in clinic (Carville et al., 2012). In particular, triptans are the first-line acute treatments (Worthington et al., 2013). However, triptans are contraindicated in patients with a history of symptomatic peripheral, coronary, and cerebrovascular disease and severe hypertension (Dodick, 2018). NSAIDs may induce gastrointestinal (Kirthi et al., 2013) and cardiovascular disorders (Moore et al., 2014). Opioids are associated with the incidence of habituation, addiction, tolerance and withdrawal syndromes (Levin, 2014), Furthermore, frequent use of these medications may be contributed to medication-overuse headache (MOH) (Scher et al., 2017). In a word, their applications are still greatly limited by their tolerability and adverse effects. The effective management of headache disorders remains a moving field and a potential challenge to the neurologist (Sinclair et al., 2015). Thus, many migraine patients resort to complementary and alternative medicine (CAM).
Traditional Chinese medicine (TCM), a main form of CAM, has been used for medical treatment of headache in China for the thousands of years and now is still used worldwide. The rhizome of Ligusticum chuanxiong Hort. (Chuanxiong) originated from Divine Husbandman's Classic of the Materia Medica (Shen Nong Ben Cao Jing), is a well-known TCM herb (China Pharmacopoeia Committee, 2005). Based on the literature review, Chuanxiong formulae are the most common used Chinese classical and/or patent prescription for treating headache both in ancient and modern time (Zheng Q. et al., 2013; Li et al., 2015). In spite of thousands of years' application history, the efficacy and safety evaluation of Chuanxiong formulae also should be scientifically performed. Previous systematic reviews (Zhou et al., 2013; Li et al., 2015) of TCM for migraine prevented to make firm conclusions because of poor methodological quality of the primary studies. Therefore, the aim of this study is to assess the available evidence of Chuanxiong formulae for migraine according to high-quality randomized controlled trials (RCTs).
Methods
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Moher et al., 2010) and our previous study (Yang et al., 2017).
Search Strategy
PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP) and Wanfang Database were retrieved in English or in Chinese by using the following search terms: “(migraine OR headache) AND (traditional Chinese medicine OR herbal medicine OR TCM OR integrative medicine OR Integrated Traditional and Western Medicine).” The search time ranged from the inception of each database until March 2017. Moreover, we also manually searched the additional relevant studies, using the references of the systematic reviews that published previously. Specific herb name “Chuanxiong” was not specifically searched to ensure that eligible herbal formulae were included as much as possible.
Eligibility Criteria
Type of participants: The adult participants with migraine of any gender or ethnicity were eligible for inclusion. The widely used diagnosis criteria of headache were Classification and Diagnostic criteria for headache disorders, cranial neuralgias and facial pain (ICHD-1) (Headache Classification Committee of the International Headache Society (IHS), 1988), The international classification of headache disorder, 2nd edition (ICHD-2) (Headache Classification Committee of the International Headache Society (IHS), 2004) and The international classification of headache disorder, 3rd edition (ICHD-3) (Headache Classification Committee of the International Headache Society (IHS), 2013).
Type of study: Only RCTs evaluating the efficacy and safety of Chuanxiong formulae for migraine were eligible. Trials that only mentioned the word “randomization” without any description of the random allocation process were excluded. Quasi-RCTs studies, which allocated participants according to the date of birth, hospital record number, date of admission or identity (ID) number, were also excluded.
Type of intervention: Herbal formulae that must include the herb Chuanxiong was used in the experiment group. There was no limitation on the form of the drug (e.g., liquid, direction, pill, and capsule), dosage, frequency or duration of the treatment. The intervention of control groups included placebo or conventional pharmacotherapy (CP).
Type of outcome measures: The primary outcomes were evaluated by headache frequency, headache duration, headache days and pain intensity. The secondary outcomes measurements were the total clinical effective rate and adverse events.
Exclusion Criteria
Studies were excluded if they did not meet the above eligibility criteria. Additionally, trials with any one of the following conditions were excluded: (1) case series, reviews, observation study, animal researches and pharmacological experiments; (2) duplicated publications; (3) TCM that were used in both treatment group and control group. (4) combined with other CAM therapy, e.g., yoga, massage, Tai Chi, Qigong, acupuncture and moxibustion.
Study Selection
Two reviewers independently screened the titles and abstracts to select eligible RCTs. Full text of the studies that potentially met the predefined criteria were obtained and read. When datasets overlapped or were duplicated, only the most recent information was included. Disagreements about the study selection were resolved by discussing with the corresponding author.
Data Extraction
Two reviewers independently extracted data from the eligible trials using a pre-designed standard data extract form. The following details were extracted: (1) publication year and the first authors' names, publication language, type of headache disorders,diagnosis standard; (2) the characteristics of participants, including number, sex, mean age, course of disease; (3) treatment information, including details of interventions management, course of treatment, follow-up period. (4) outcome measurement and adverse effect. In studies with multiple comparison groups, the most relevant comparison group was chosen for analysis. If outcomes were presented from the studies at different time points, we extracted data from the last time point of treatment. When there were inconsistencies, the corresponding author participated in the extraction. And the original authors of trials were contacted for missing data and additional information.
Quality Assessment
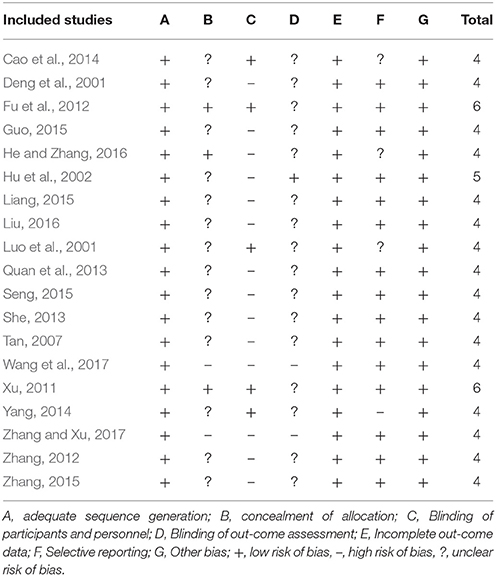
Methodological quality of included studies was assessed by using the risk of bias (RoB) tools in accordance with Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2011). Seven components were as follows: A. adequate sequence generation; B. concealment of allocation; C. blinding (participants and personnel); D. blinding (outcome assessor); E. incomplete outcome data addressed (ITT analysis); F. selective reporting; G. other potential threat to validity. Each of these indicators was categorized as low risk of bias, high risk of bias and unclear. In the scale of zero to seven, we included the studies to enter the final analysis only when they met at least four items. Disagreements between two reviewers about the assessment of quality of included literatures were solved through consultation with corresponding authors.
Chuanxiong Formulae Composition
The constituent of Chuanxiong formulae in each included study was recorded. The frequency of use for specific herb was calculated and those with cumulative frequencies over 50% are described in detail.
Data Analysis
Information from eligible studies was aggregated to produce a quantitative summary using the software Cochrane Collaboration Review Manage (RevMan 5.3). Continuous data (headache frequency, headache duration, headache days, pain intensity scales) were expressed as mean difference (MD) or standardized mean difference (SMD) whereas dichotomous data (clinical effective rate) were reported as relative risk (RR) with 95% confidence intervals (CI). Statistical heterogeneity among trials was assessed using the chi-squared test and I2 statistic. If no heterogeneity exists (P > 0.1, I2 < 50%), a fixed effect model (FEM) was applied; otherwise the random effect model (REM) was generally a more plausible match. Sensitivity analysis was performed by changing analysis combination to explore the impact of confounding factors. Meanwhile, in consideration of the differences in participants, interventions and treatment, the subgroup analysis was planned to conduct using the Z-test. The differences between the treatment groups and control groups were considered to be statistically significant when P < 0.05. If more than10 studies were included in each outcome, funnel plots and Egger's test were used to examine publication bias.
Results
Description of Studies
A total of 7238 studies were retrieved through searching five electronic databases and other sources. After duplication removed, 5365 records remained. By screening the titles and abstracts, 3467 records were excluded; among which 3096 studies were not related to headache, 31 papers were animal experiments, 15 were mechanism studies and 325 were reviews, protocols, experiences, or case reports. By reading the full text, 1879 studies were removed, including 131 that had improper control interventions, 234 that were lack of control group, 54 that have no full text available, 757 that were not real RCTs, 40 that did not use Chuanxiong formulae, 121 that were other types of headaches, 472 that contained other CAM therapy, such as acupuncture, massage or scraping, and 70 that had low methodological quality. Ultimately, 19 eligible studies with Cochrane RoB score ≥4 were included for this study (Deng et al., 2001; Luo et al., 2001; Hu et al., 2002; Tan, 2007; Xu, 2011; Fu et al., 2012; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Cao et al., 2014; Yang, 2014; Guo, 2015; Liang, 2015; Seng, 2015; He and Zhang, 2016; Liu, 2016; Wang et al., 2017; Zhang and Xu, 2017). A PRISMA flow chart depicted the search process and study selection (Figure 1).
Study Characteristics
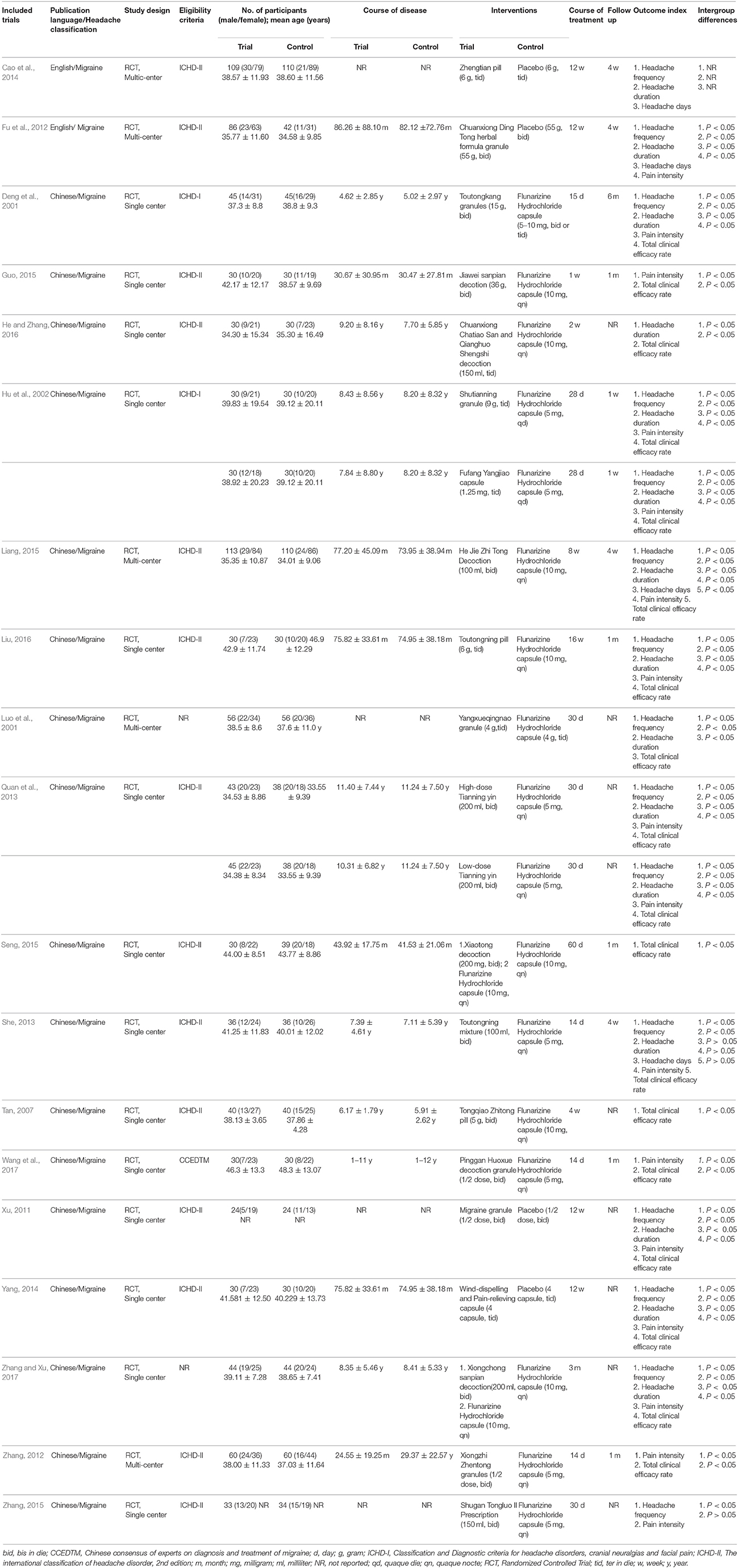
The characteristics of the 19 included trials with 21 comparisons were summarized in Table 1. All eligible studies were conducted in China. Two articles published in English (Fu et al., 2012; Cao et al., 2014), while the rest of articles published in Chinese (Deng et al., 2001; Luo et al., 2001; Hu et al., 2002; Tan, 2007; Xu, 2011; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Yang, 2014; Guo, 2015; Liang, 2015; Seng, 2015; He and Zhang, 2016; Liu, 2016; Wang et al., 2017). There were 17 RCTs with two arms (Deng et al., 2001; Luo et al., 2001; Tan, 2007; Xu, 2011; Fu et al., 2012; Zhang, 2012, 2015; She, 2013; Cao et al., 2014; Yang, 2014; Guo, 2015; Liang, 2015; Seng, 2015; He and Zhang, 2016; Liu, 2016; Wang et al., 2017; Zhang and Xu, 2017), 2 RCTs with three arms (Hu et al., 2002; Quan et al., 2013). Two main diagnostic criteria for migraine were ICHD-I and ICHD-II.The sample size of the included studies ranged from 48 to 223, enrolling a total of 1832 participants, 974 patients in treatment groups and 858 patients serving as controls. Five studies compared Chuanxiong formulae alone with placebo (Luo et al., 2001; Xu, 2011; Fu et al., 2012; Cao et al., 2014; Yang, 2014) and 12 studies compared Chuanxiong formulae with CP (Deng et al., 2001; Hu et al., 2002; Tan, 2007; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Guo, 2015; Liang, 2015; He and Zhang, 2016; Liu, 2016; Wang et al., 2017). Two studies combined Chuanxiong formulae with CP vs. CP (Seng, 2015; Zhang and Xu, 2017). The CP all was Flunarizine Hydrochloride. The preparations used in 19 RCTs with 21 comparisons were administered orally in decoctions (9 comparisons), granules (7 comparisons), capsules (2 comparisons) and pills (3 comparisons). The treatment duration ranged from 1 to 16 weeks. Eleven studies mentioned the duration of follow-up, which lasted from 1 week to 6 months (Deng et al., 2001; Hu et al., 2002; Fu et al., 2012; Zhang, 2012; She, 2013; Cao et al., 2014; Guo, 2015; Liang, 2015; Seng, 2015; Liu, 2016; Wang et al., 2017).
Description of the Chuanxiong Formulae
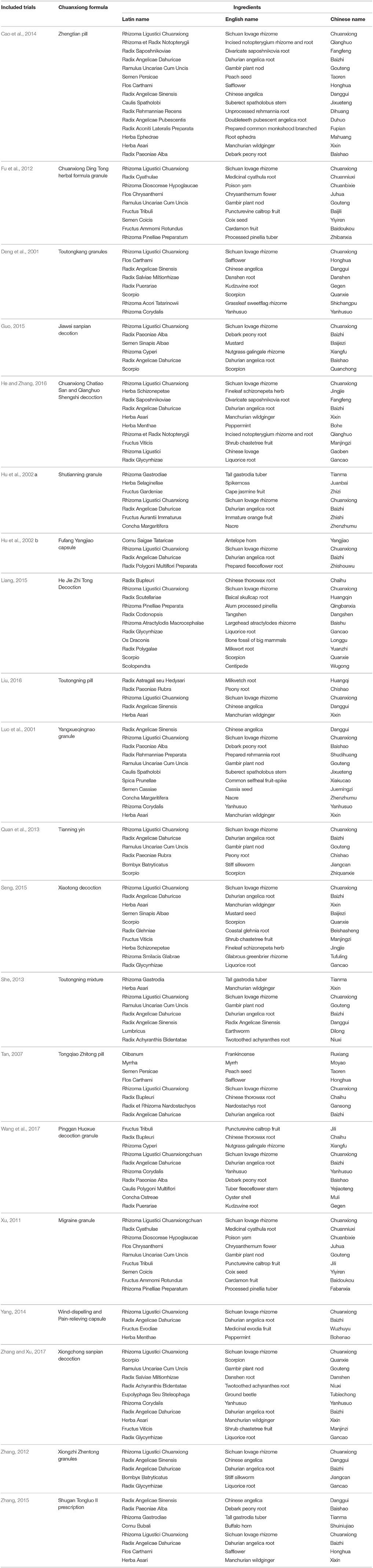
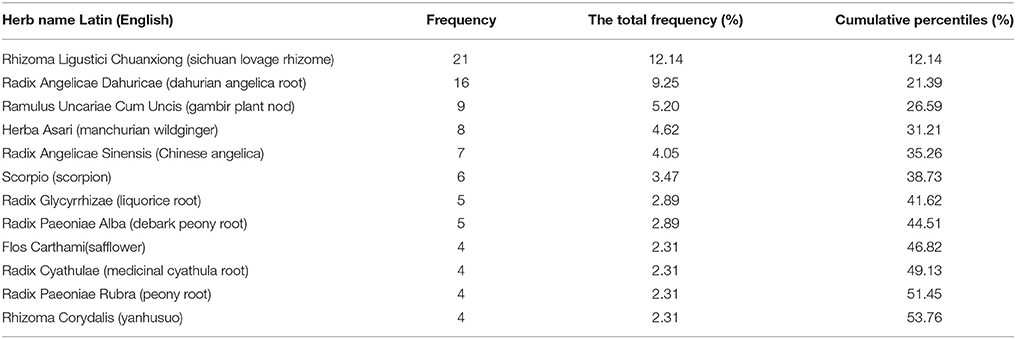
The constituent of Chuanxiong formulae in each included study was detailed in Table 2. Sixty-four herbs were used in the 19 different Chuanxiong formulae. The top 12 most frequently used herbs were ordinally Rhizoma Ligustici Chuanxiong (sichuan lovage rhizome), Radix Angelicae Dahuricae (dahurian angelica root), Ramulus Uncariae Cum Uncis (gambir plant nod), Herba Asari (manchurian wildginger), Radix Angelicae Sinensis (Chinese angelica), Scorpio (scorpion), Radix Glycyrrhizae (liquorice root), Radix Paeoniae Alba (debark peony root), Flos Carthami (safflower), Radix Cyathulae (medicinal cyathula root), Radix Paeoniae Rubra (peony root), Rhizoma Corydalis (yanhusuo), which were used more than 4 times (Table 3).
RoB
RoB assessment is shown in Table 4. All included studies were described as “randomized” with appropriate methods of sequence generation. Twelve studies used a random number table in the allocation of participants (Deng et al., 2001; Luo et al., 2001; Hu et al., 2002; Tan, 2007; Quan et al., 2013; She, 2013; Guo, 2015; Seng, 2015; Zhang, 2015; Liu, 2016; Wang et al., 2017; Zhang and Xu, 2017); three studies applied Statistical Analysis System (SAS) software (Zhang, 2012; Liang, 2015; He and Zhang, 2016); two studies were central assignment (Xu, 2011; Fu et al., 2012); one study employed Statistical Product and Service Solutions (SPSS) software to generate the random numbers (Yang, 2014) and another one mentioned randomization by computer-generated stochastic system (Cao et al., 2014). These 19 studies were assessed to be low RoB in the domain of sequence generation. One study applied “sealed envelopes” (He and Zhang, 2016) and two studies applied central allocation concealment in the trial design (Xu, 2011; Fu et al., 2012). Five studies were double blindness (Luo et al., 2001; Xu, 2011; Fu et al., 2012; Cao et al., 2014; Yang, 2014). All studies either had dropouts with adequate explanations and appropriate methods to treat missing data or had no dropouts. Finally, 16 out of 19 studies were at low RoB from other sources including funding, protocols, conflicts of interest, and baseline balance (Deng et al., 2001; Hu et al., 2002; Tan, 2007; Xu, 2011; Fu et al., 2012; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Yang, 2014; Guo, 2015; Liang, 2015; Seng, 2015; Liu, 2016; Wang et al., 2017; Zhang and Xu, 2017), except for 3 studies that did not reported available funding or protocols was therefore at unclear RoB (Luo et al., 2001; Cao et al., 2014; He and Zhang, 2016).
Effectiveness
Migraine Frequency
Thirteen studies evaluated the frequency of migraine attack in a month, and data showed a significant reduction both in studies that compared with placebo (SMD = −0.65, 95% CI −0.93 to −0.38, P < 0.00001, heterogeneity χ2 = 8.67, P = 0.07, I2 = 54%, Figure 2; Luo et al., 2001; Xu, 2011; Fu et al., 2012; Cao et al., 2014; Yang, 2014) and compared with CP (SMD = −1.05, 95% CI −1.28 to −0.82, P < 0.00001, heterogeneity χ2 = 17.95, P = 0.02, I2 = 55%, Figure 2; Deng et al., 2001; Hu et al., 2002; Quan et al., 2013; She, 2013; Liang, 2015; Zhang, 2015; Liu, 2016). Only one study (Zhang and Xu, 2017) compared Chuanxiong formulae plus CP with CP alone. The result of the study favored the combined treatment with P < 0.05.
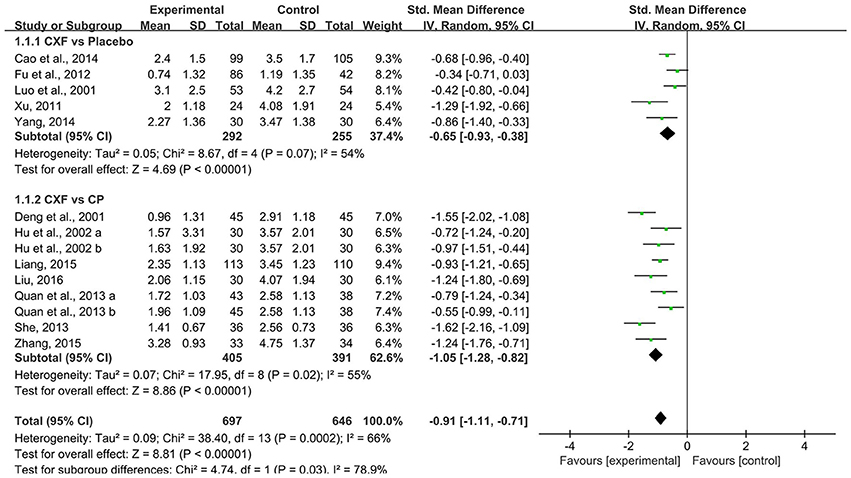
Figure 2. The forest plot of the efficacy of Chuanxiong formulae on the migraine frequency. CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
Migraine Duration
There were 12 trials with 14 comparisons reported headache duration as outcome measure. Meta-analysis demonstrated that Chuanxiong formulae were significantly better at reducing the duration of migraine than placebo (SMD = −0.50, 95% CI −0.68 to −0.32, P < 0.00001, heterogeneity χ2 = 4.34, P = 0.36, I2 = 8%, Figure 3; Xu, 2011; Fu et al., 2012; Cao et al., 2014; Yang, 2014) and CP (SMD = −0.76, 95% CI −0.99 to −0.52, P < 0.00001, heterogeneity χ2 = 19.50, P = 0.01, I2 = 59%, Figure 3; Deng et al., 2001; Hu et al., 2002; Quan et al., 2013; She, 2013; Liang, 2015; He and Zhang, 2016; Liu, 2016). There was homogeneity for this outcome in the placebo comparison but not in the Chuanxiong formulae vs. CP comparison. After excluding one study (Deng et al., 2001) which had relatively short course of disease, the result still indicated a benefit in the Chuanxiong formulae groups (SMD −0.62, 95% CI −0.78 to −0.47, P < 0.00001, heterogeneity χ2 = 1.47, P = 0.98, I2 = 0%). For the comparison of Chuanxiong formulae plus CP vs. CP, one study (Zhang and Xu, 2017) demonstrated that combined treatment had better effect than conventional medicine alone (P < 0.05).
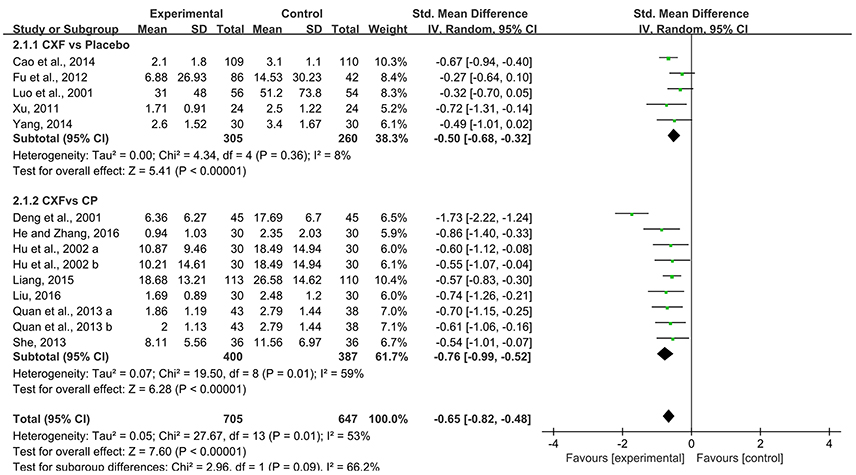
Figure 3. The forest plot of the efficacy of Chuanxiong formulae on the migraine duration. CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
Migraine Days
Four studies analyzed showed a statistically significant difference in the outcome of migraine days. For two multi-center RCTs (Fu et al., 2012; Cao et al., 2014) that compared Chuanxiong formulae with placebo, the data of migraine days in Chuanxiong formulae was significantly lower (MD = −0.74, 95% CI −1.30 to −0.18, P = 0.01, heterogeneity χ2 = 0.08, P = 0.78, I2 = 0%, Figure 4). For comparisons with CP, there was a benefit for the Chinese herbal medicine (CHM) group as well (MD = −0.50, 95% CI −0.80 to −0.20, P = 0.001, heterogeneity χ2 = 0.00, P = 1.00, I2 = 0%, Figure 4; She, 2013; Liang, 2015).
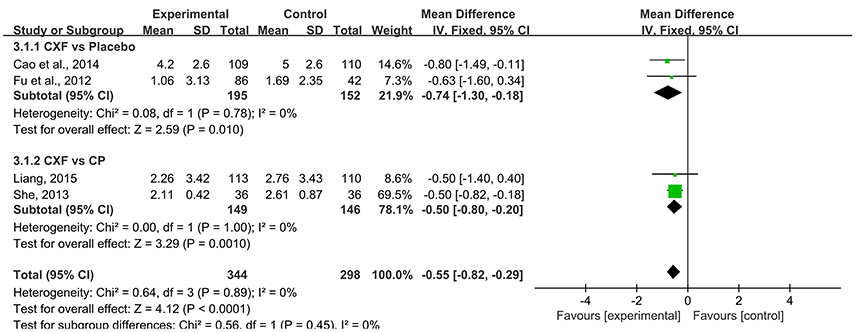
Figure 4. The forest plot of the efficacy of Chuanxiong formulae on the migraine days. CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
Pain Intensity
Pain intensity of migraine was observed in 14 studies. Pooled data showed that Chuanxiong formulae were significantly better at relieving the pain compared with placebo in 3 studies (SMD = −0.71, 95% CI −0.98 to −0.43, P < 0.00001, heterogeneity χ2 = 1.45, P = 0.48, I2 = 0%, Figure 5; Xu, 2011; Fu et al., 2012; Yang, 2014) and with CP in 10 studies (SMD = −0.67, 95% CI −0.84 to −0.47, P < 0.00001, heterogeneity χ2 = 22.59, P = 0.02, I2 = 51%, Figure 5; Deng et al., 2001; Hu et al., 2002; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Guo, 2015; Liang, 2015; Liu, 2016; Wang et al., 2017). One study (Zhang and Xu, 2017) indicated that the pain score of CHM plus CP groups was significantly lower than that of the CP group (P < 0.05).
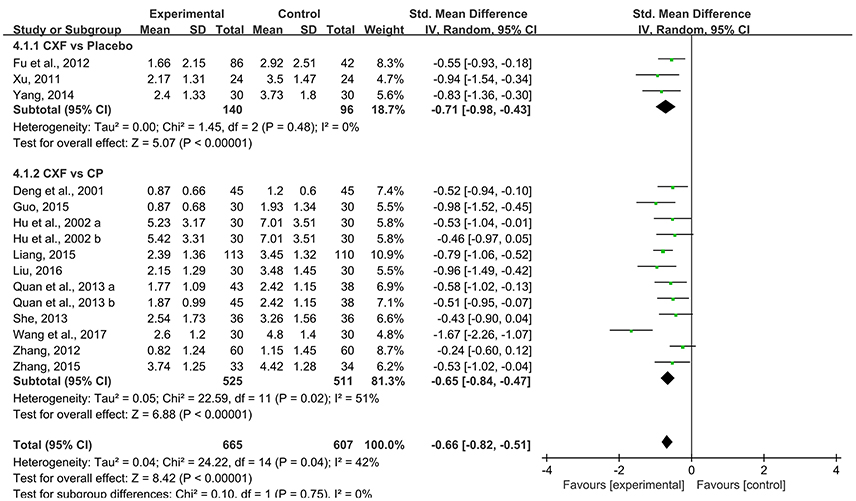
Figure 5. The forest plot of the efficacy of Chuanxiong formulae on pain intensity. CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
The Total Clinical Efficacy Rate
The total clinical efficacy rate was reported in 16 studies with 18 comparisons. There were significant improvement comparing Chuanxiong formulae with placebo (RR = 3.55, 95% CI 2.44–5.17, P < 0.00001, heterogeneity χ2 = 0.13, P = 0.94, I2 = 0%, Figure 6; Luo et al., 2001; Xu, 2011; Yang, 2014). Compared with CP, the pooled data showed that Chuanxiong formulae was superior to CP (RR = 1.25, 95% CI 1.18–1.33, P < 0.00001, heterogeneity χ2 = 20.27, P = 0.06, I2 = 41%, Figure 6; Deng et al., 2001; Hu et al., 2002; Tan, 2007; Zhang, 2012; Quan et al., 2013; She, 2013; Guo, 2015; Liang, 2015; He and Zhang, 2016; Liu, 2016; Wang et al., 2017). Two studies (Seng, 2015; Zhang and Xu, 2017) showed that there was a benefit for the Chuanxiong formulae plus CP group when compared with CP (RR = 1.24, 95% CI 1.06–1.45, P = 0.007, heterogeneity χ2 = 0.01, P = 0.91, I2 = 0%, Figure 6).
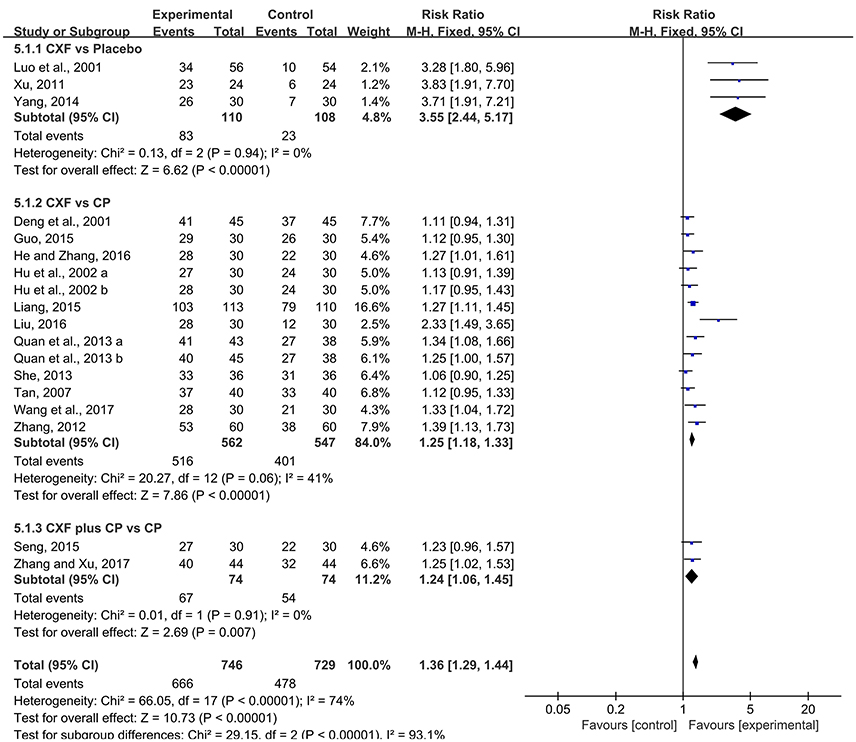
Figure 6. The forest plot of the efficacy of Chuanxiong formulae on the clinical efficacy rate. CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
Adverse Events
Sixteen out of 19 studies (Luo et al., 2001; Hu et al., 2002; Tan, 2007; Xu, 2011; Fu et al., 2012; Zhang, 2012, 2015; Quan et al., 2013; She, 2013; Cao et al., 2014; Yang, 2014; Guo, 2015; Seng, 2015; Liu, 2016; Wang et al., 2017; Zhang and Xu, 2017) reported the adverse events occurring during the treatment, in which a total of 61/742 (8.22%) patients suffered adverse events in the trial groups and 56/623 (8.99%) patients did so in control groups, and the rest three studies (Deng et al., 2001; Liang, 2015; He and Zhang, 2016) did not mention any information about adverse events. Ten studies (Tan, 2007; Xu, 2011; Zhang, 2012, 2015; Quan et al., 2013; Yang, 2014; Guo, 2015; Seng, 2015; Liu, 2016; Wang et al., 2017) stated that no adverse event happened during the treatment. In the 3 studies (Luo et al., 2001; She, 2013; Cao et al., 2014) with adequate information of adverse events, 40 cases reported that there were adverse reactions of the gastrointestinal reactions including indigestion, bloating and flatulence, epigastric pain, abdominal pain, constipation, vomiting and nausea in the experimental group, whereas it was occurred in 38 cases in the control group. Adverse reactions of nervous system such as somnolence, insomnia, dizziness is the second most frequent, 13 cases in trial groups and 15 cases in control groups. Adverse events of all studies were generally mild both in the Chuanxiong formulae and control groups. One study (Luo et al., 2001) reported that a patient suffered severe chest congestion and nausea, but the investigator did not consider the event to be related to study medication.
Publication Bias
Funnel plots were reviewed for four outcomes (Figure 7). The results showed symmetrical distribution for the outcomes of migraine frequency (Egger's test t = −1.17, 95% CI −6.58 to 1.95, p = 0.263), migraine duration (Egger's test t = −1.27, 95% CI −5.44 to 1. 42, p = 0.227), and pain intensity (Egger's test t = −0.96, 95% CI −4.79 to 1.82, P = 0.352), which did not suggest an obvious publication bias. However, there was a significant bias in the total clinical efficacy rate with Egger's test (t = 6.37, 95% CI 2.58 to 5.16, p < 0.001). Because the number of studies in the outcome of migraine days was limited (n = 4), funnel plot and Egger's test were not appropriate.
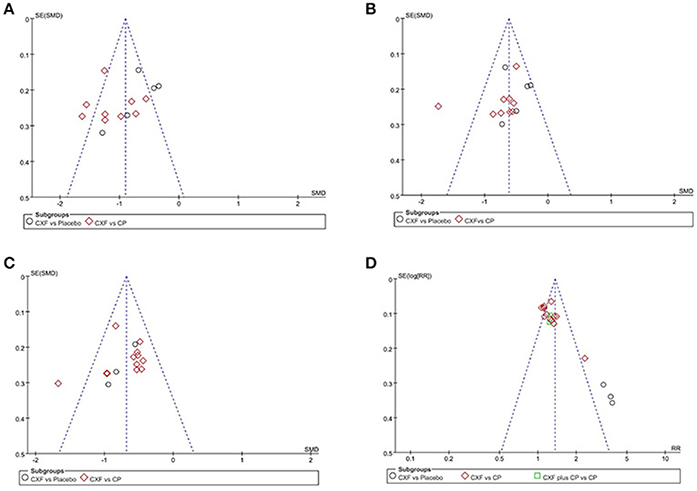
Figure 7. The funnel plots of the efficacy of Chuanxiong formulae on the migraine frequency (A), migraine duration (B), pain intensity (C), and the clinical efficacy rate (D). CXF, Chuanxiong formulae; CP, conventional pharmacotherapy.
Discussion
Summary of Evidence
A former review (Zhou et al., 2013) published in 2013 found some evidence of supporting the use of TCM for migraine; however the poor methodological quality and significant publication bias prevented the author making firm conclusions. Our previous review (Li et al., 2015) in 2015 also demonstrated that Chuanxiong Chadiao powder may be effective and safe for the treatment of headache. This is a systematic review of 19 high-quality RCTs with 1832 participants to determine the efficacy and safety of Chuanxiong formulae for migraine. The present study indicated that Chuanxiong formulae provided statistically significant benefits in terms of reducing frequency, duration, days, pain severity of migraine and improving the total clinical efficacy rate. In addition, Chuanxiong formulae appeared to be generally safe and well tolerated. Current evidence supported that Chuanxiong formulae could be an alternative drugs for the symptom treatment of migraine.
Limitations
There are several limitations in the primary studies. Firstly, although we included the high-quality RCTs according to a cumulative score of at least 4 out of 7 for the Cochrane RoB tool domains, the methodological details was still not adequate in some studies. Only 3 studies (Xu, 2011; Fu et al., 2012; He and Zhang, 2016) described a proper method of allocation concealment and 5 studies (Luo et al., 2001; Xu, 2011; Fu et al., 2012; Cao et al., 2014; Yang, 2014) employed the blinding procedure. Some studies were unable to be blinded, due to the fact that TCM is special in color, smell and taste, in contrast to the standard capsule of Flunarizine Hydrochloride. However, no study used a double-dummy technique to reduce the difference of drugs between the experiment and control groups. Blinding makes it difficult to bias results intentionally or unintentionally and helps ensure the credibility of study conclusions (Day and Altman, 2000). In addition, the intervention of trials with inadequate allocation concealment is 18% more “beneficial” than in trials with adequate concealment (Higgins and Green, 2011). Secondly, migraine affects approximately 18% of women and 6% of men (Lipton et al., 2007). The ratio of gender is amplified in the included RCTs. This gender selection bias should be avoided by recruiting males to an extent. Thirdly, relatively long treatment periods could increase the power of the trial by providing more stable estimates for the efficacy of Chuanxiong formulae. However, the treatment duration ranged from 1 to 16 weeks. The long-term safety of Chuanxiong formulae for headache could not be determined because follow-up period in the studies ranged from 1 week to 6 months. Guidelines for controlled trials of drugs in migraine recommends that treatment periods is no less than 3 months in phase II RCTs and up to 6 months in phase III trials, and every 4 weeks visits is necessary (Tfelt-Hansen et al., 2012). Fourthly, due to the context in terms of traditional culture and the barrier of language, all RCTs were in English or in Chinese and have been conducted in Chinese population, which restricts the generalizability of the findings. Fifthly, migraine treatment can be divided into acute treatment and preventive treatment (Antonaci et al., 2016). It is difficult to differentiate the effectiveness of Chuanxiong formulae in two kinds of treatments because the weakness rooted in primary studies. In fact, acute treatment is focused on single episodes of headache and no RCTs were designed specifically for acute treatment of Chuanxiong. Thus, further particular trial design of acute treatment of Chuanxiong is needed.
Implications for Practice
The use of TCM in treating many common neurological ailments has been paid more attention over the years (Ma et al., 2009). Chuanxiong is widely used in TCM for headache. The main active ingredients of Chuanxiong for migrain include tetramethylpyrazine (TMP), senkyunolide A, ferulic acid (FA) and ligustilide (Ran et al., 2011). The significant pharmacological activities of Chuanxiong and its main compounds are as follows: (1) Antioxidant effects: TMP, FA and ligustilide could reduce the production of intracellular reactive oxygen species (ROS) and nitric oxide (NO), and the expression of inducible nitric oxide synthase (iNOS) (Wong et al., 2007; Chung et al., 2012; Zheng Z. et al., 2013; Cao et al., 2015; Ren et al., 2017). TMP and FA inhibit the activity of NADPH oxidase via ERK signaling pathway and NF-κB pathway respectively (Wong et al., 2007; Cao et al., 2015). (2) Antiinflammatory effects: TMP, senkyunolide A and ligustilide could down regulate the activation and proliferation of astrocytic, the production and bioactivity of tumor necrosis factor α (TNF-α), and the expression of cyclooxygenase-2 (COX-2) protein (Liu et al., 2005; Chung et al., 2012; Feng et al., 2012; Jiang et al., 2017). (3) Antiapoptotic effects: Ligustilide prevented neuronal apoptosis in both parietal cortex and hippocampus through regulation of mitochondrion metabolism (Feng et al., 2012) TMP could decrease the levels of miR-214-3p and increase the expression level of Bcl2l2 (Fan and Wu, 2017). FA was mainly through TLR4/MyD88 signaling pathway and NF-kB pathway (Cao et al., 2015; Ren et al., 2017). (4) Antinociceptive effects: TMP could inhibit the expression of P2X3 receptor in the trigeminal ganglia (TG), exhibiting potential effect on pain relief (Xiong et al., 2017). Ligustilide could activate the transient receptor potential cationic channel ankyrin 1 (TRPA1) (Zhong et al., 2011) and display high affinities with 5-hydroxytryptamine (5-HT) 1D receptors (Du et al., 2015) and 5-HT 7 receptors (Deng et al., 2006), regulating the release of calcitonin gene-related protein (CGRP) which can cause vasodilatation. Thus, Chuanxiong formulae are likely to be multi-targeting therapy for the multi-hit driven migraine pathogenesis. However, it remains to clarify the nature of the ingredients of the mixture and the mechanisms of action of Chuanxiong. This should be the object of further studies.
Implications for Further Studies
Firstly, we suggested that the protocol of clinical trials must register in clinical trials registry platform and CONSORT 2010 statement should be applied in trial reporting and publication. Secondly, in order to facilitate more reliable comparison of study results, more consistency in the use of the international standard on migraine clinical trials, such as guidelines for controlled trials of drugs in migraine: 3rd edition, which consist of the following parts: selection of patients, trial design, evaluation of results and statistics (Tfelt-Hansen et al., 2012). The type of migraine should be illustrated definitely in trials, which could give precise evidence for clinic. Meanwhile, we also recommend the appropriate sample size that calculated before enrollment, ideal length of treatment and follow-up, adequate randomization methods, sufficient blinding, and intent-to-treat (ITT) analyses in future RCTs. Thirdly, Radix Angelicae Dahuricae, Ramulus Uncariae Cum Uncis, Herba Asari, Radix Angelicae Sinensis, and Scorpio were the most frequently used herbs, which should be considered firstly when formulating optimal combination of Chuanxiong with other herbal ingredients. Finally, the exact pathomechanism of migraine and the pharmacological mechanism of Chuanxiong remain largely unknown, which should be further investigated.
Conclusion
The present findings indicated that Chuanxiong formulae provided statistically significant benefits for migraine and were generally safe. Thus, the available evidence of present study supported the alternative use of Chuanxiong formulae for migraine.
Author Contributions
Study conception and design: GZ and CS; Acquisition, analysis and/or interpretation of data: CS, QX, YS, YW, ZH and GZ; Final approval and overall responsibility for this published work: GZ.
Funding
This work was financially supported by the grant of National Natural Science Foundation of China (81573750/81473491/81173395/H2902); the Young and Middle-Aged University Discipline Leaders of Zhejiang Province, China (2013277); Zhejiang Provincial Program for the Cultivation of High-level Health talents (2015).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EP and handling Editor declared their shared affiliation.
Abbreviations
5-HT, 5-hydroxytryptamine; CAM, complementary and alternative medicine; CGRP, calcitonin gene-related protein; CHM, Chinese herbal medicine; CI, confidence intervals; CNKI, China National Knowledge Infrastructure; COX-2, cyclooxygenase-2; CP, conventional pharmacotherapy; FA, ferulic acid; FEM, fixed effect model; GBD, global burden of disease. GDP, gross domestic product; ICHD-1, Classification and Diagnostic criteria for headache disorders, cranial neuralgias and facial pain; ICHD-2, The international classification of headache disorder, 2nd edition; ICHD-3, The international classification of headache disorder, 3rd edition; ID, identity; iNOS, reactive oxygen species; ITT, intent-to-treat; MD, mean difference; miR-214-3p, microRNA-214-3p; MOH, medication-overuse headache; NO, nitric oxide; NSAIDs, non-steroidal anti-inflammatory drugs; RCTs, randomized controlled trials; REM, random effect model; RoB, risk of bias; ROS, reactive oxygen species; RR, relative risk; SAS, Statistical Analysis System; SMD, standardized mean difference; SPSS, Statistical Product and Service Solutions; TCM, traditional Chinese medicine; TG, trigeminal ganglia; TMP, tetramethylpyrazine; TNF-α, tumor necrosis factor α; TRPA1, transient receptor potential cationic channel ankyrin 1. VIP, Chinese Science and Technology Periodical Database.
References
Antonaci, F., Ghiotto, N., Wu, S., Pucci, E., and Costa, A. (2016). Recent advances in migraine therapy. Springerplus. 5:637. doi: 10.1186/s40064-016-2211-8
Cao, K. G., Yu, L. H., Gao, Y., Fan, Y. P., Zhao, J. J., Zhang, X. Z., et al. (2014). Efficacy of Zhengtian pill for migraine prophylaxis: a randomized, multicenter, double-blind, placebo-controlled, parallel-group study. Eur. J. Integr. Med. 6, 259–267. doi: 10.1016/j.eujim.2014.01.005
Cao, Y. J., Zhang, Y. M., Qi, J. P., Liu, R., Zhang, H., and He, L. C. (2015). Ferulic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NADPH oxidase and NF-κB pathway. Int. Immunopharmacol. 28, 1018–1025. doi: 10.1016/j.intimp.2015.07.037
Carville, S., Padhi, S., Reason, T., and Underwood, M., and Guideline Development Group (2012). Diagnosis and management of headaches in young people and adults: summary of NICE guidance. BMJ. 345:e5765. doi: 10.1136/bmj.e5765
China Pharmacopoeia Committee (2005). Pharmacopoeia of the People's Republic of China, 1st Division. Beijing: Chemical Industry Press.
Chung, J. W., Choi, R. J., Seo, E. K., Nam, J. W., Dong, M. S., Shin, E. M., et al. (2012). Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch. Pharm. Res. 35, 723–732. doi: 10.1007/s12272-012-0417-z
Day, S. J., and Altman, D. G. (2000). Statistics notes: blinding in clinical trials and other studies. BMJ 321:504. doi: 10.1136/bmj.321.7259.504
Deng, S., Chen, S. N., Yao, P., Nikolic, D., van Breemen, R. B., Bolton, J. L., et al. (2006). Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat. Prod. 69, 536–541. doi: 10.1021/np050301s
Deng, Y. J., Wang, J. J., He, F. Y., Liu, W. Y., Gu, X., and Huang, X. P. (2001). Clinical observation of Toutongkang granules in treatment of migraine. Hunan Guid. J. Tradit. Chin. Med. Pharm. 7, 48–50. doi: 10.3969/j.issn.1672-951X.2001.02.002 (in Chinese).
Du, H., Zhou, N., Li, J. J., and Fan, F. (2015). A cell membrane chromatography method for investigation of 5-hydroxytryptamine receptor-ligustilide interaction. Chin. J. Chromatogr. 33, 530–534. (in Chinese) doi: 10.3724/SP.J.1123.2015.01003
Fan, Y., and Wu, Y. (2017). Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed. Pharmacother. 94, 827–833. doi: 10.1016/j.biopha.2017.07.162
Feng, Z., Lu, Y., Wu, X., Zhao, P., Li, J., Peng, B., et al. (2012). Ligustilide alleviates brain damage and improves cognitive function in rats of chronic cerebral hypoperfusion. J. Ethnopharmacol. 144, 313–321. doi: 10.1016/j.jep.2012.09.014
Fu, C., Yu, L., Zou, Y., Cao, K. G., Zhao, J. J., Gong, H. Y., et al. (2012). Efficacy of chuanxiong ding tong herbal formula granule in the treatment and prophylactic of migraine patients: a randomized, double-blind, multicenter, placebo-controlled trial. Evid. Based Compl. Alternat. Med. 2012:967968. doi: 10.1155/2012/967968
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602. doi: 10.1016/S0140-6736(16)31678-6
Guo, Y. K. (2015). Clinical Observation of Jiawei Sanpian Decoction in Treatment of Migraine(Wind-Phlem Stasis Type). Dissertation, Henan University of Chinese Medicine (in Chinese).
He, J. B., and Zhang, X. Y. (2016). The randomized parallel controlled study of Chuanxiong Chatiao San and Qianghuo Shengshi decoction treating migraine. J. Pract. Tradit. Chin. Intern. Med. 30, 96–98. doi: 10.13729/j.issn.1671-7813.2016.05.40 (in Chinese).
Headache Classification Committee of the International Headache Society (IHS) (1988). Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 8(Suppl. 7), 1–96. doi: 10.1111/j.1468-2982.1991.tb00022.x
Headache Classification Committee of the International Headache Society (IHS) (2004). The international classification of headache disorders: 2nd edition. Cephalalgia 24(Suppl. 1), 9–160. doi: 10.1111/j.1468-2982.2003.00824.x
Headache Classification Committee of the International Headache Society (IHS) (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 33, 629–808. doi: 10.1177/0333102413485658
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 343:d5928. doi: 10.1136/bmj.d5928
Higgins, J., and Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1, Updated March 2011. Oxford: The Cochrane Collaboration.
Hu, Z. Q., Song, L. G., and Mei, T. (2002). Clinical and experimental study on treatment of migraine with shutianning granule. Chin. J. Integr. Tradit. West Med. 22, 581–583. doi: 10.3321/j.issn:1003-5370.2002.08.006 (in Chinese).
Jiang, L., Pan, C. L., Wang, C. Y., Liu, B. Q., Han, Y., Hu, L., et al. (2017). Selective suppression of the JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates neuropathic pain in rats. J. Neuroinflam. 14:174. doi: 10.1186/s12974-017-0947-x
Kirthi, V., Derry, S., and Moore, R. A. (2013). Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst. Rev. 30:CD008041. doi: 10.1002/14651858.CD008041.pub3
Li, J. H., Cao, X. P., Wei, J. J., Song, L., Liao, F. J., Zheng, G. Q., et al. (2015). Chuanxiong chadiao powder, a famous Chinese herbal prescription, for headache: a systematic review and meta-analysis. Complement Ther. Med. 23, 577–590. doi: 10.1016/j.ctim.2015.06.012
Liang, B. (2015). The Clinical Research of He jie zhi Tong Decoction in Treating Migraine of Stagnation of Liver and Deficiency Spleen. Dissertation, Changchun University of Chinese Medicine, Changchun. (in Chinese)
Lipton, R. B., Bigal, M. E., Diamond, M., Freitag, F., Reed, M. L., Stewart, W. F., et al. (2007). Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68, 343–349. doi: 10.1212/01.wnl.0000252808.97649.21
Liu, H. Y. (2016). Clinical Observation of Toutongning Pill in the Treatment of Migraine With Qi Deficiency and Blood Stasis Type. Dissertation, Shanxi University of Chinese Medicine. (in Chinese)
Liu, L., Ning, Z. Q., Shan, S., Zhang, K., Deng, T., Lu, X. P., et al. (2005). Phthalide Lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappaB activation. Planta Med. 71, 808–813. doi: 10.1055/s-2005-871231
Luo, S., Wang, X. D., Kuang, P. G., Jia, J. P., Yang, Z. J., Zhou, B. Y., et al. (2001). A clinical study of Yangxueqingnaokeli in preventive treament of migraine. Chin. J. Neurol. 34, 291–294. doi: 10.3760/j.issn:1006-7876.2001.05.012 (in Chinese).
Ma, X. H., Zheng, C. J., Han, L. Y., Xie, B., Jia, J., Cao, Z. W., et al. (2009). Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov. Today 14, 579–588. doi: 10.1016/j.drudis.2009.03.012
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G., and PRISMA Group (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Moore, N., Salvo, F., Duong, M., Blin, P., and Pariente, A. (2014). Cardiovascular risks associated with low-dose ibuprofen and diclofenac as used OTC. Expert Opin. Drug Saf. 13, 167–179. doi: 10.1517/14740338.2014.846324
Quan, Y. P., Li, F., Wang, W., Wang, N., Chang, H. J., Wu, N. B., et al. (2013). Clinical effect observation of different doses of Tian Ning Yin on migraine. Global Tradit. Chin. Med. 6, 351–353. doi: 10.3969/j.issn.1674-1749.2013.05.011 (in Chinese).
Ran, X., Ma, L., Peng, C., Zhang, H., and Qin, L. P. (2011). Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm. Biol. 49, 1180–1189. doi: 10.3109/13880209.2011.576346
Ren, Z., Zhang, R., Li, Y., Li, Y., Yang, Z., and Yang, H. (2017). Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 40, 1444–1456. doi: 10.3892/ijmm.2017.3127
Scher, A. I., Rizzoli, P. B., and Loder, E. W. (2017). Medication overuse headache: an entrenched idea in need of scrutiny. Neurology 89, 1296–1304. doi: 10.1212/WNL.0000000000004371
Seng, Z. F. (2015). Clinical Study of Xiaotong Decoction on Migraine (The Type Of Coagulated Cold and Blood Stasis). Dissertation, Henan University of Chinese Medicine. (in Chinese)
She, Y. M. (2013). Clinical Observation of the Treatment of Tou Tong Ning Mixture on Migrain (Liver Wind Agitation, Blood Stasis Obstructing the Collaterals). Dissertation, Hubei University of Chinese Medicine. (in Chinese)
Sinclair, A. J., Sturrock, A., Davies, B., and Matharu, M. (2015). Headache management: pharmacological approaches. Pract. Neurol. 15, 411–423. doi: 10.1136/practneurol-2015-001167
Tan, J. (2007). Clinical Study About the Effect of Tongqiao Zhitong Pill on Blood-stasis Type Migraine. Dissertation, Hunan University of Chinese Medicine. (in Chinese)
Tfelt-Hansen, P., Pascual, J., Ramadan, N., Dahlöf, C., D'Amico, D., Diener, H. C., et al. (2012). International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 32, 6–38. doi: 10.1177/0333102411417901
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. doi: 10.1016/S0140-6736(12)61729-2
Wang, L., Wang, K., Wang, X. Y., Li, Y., Dou, J. J., and Zhang, L. P. (2017). Clinical analysis on pinggan huoxue decoction granules in treating migraine of liver stagnation and blood stasis. Chin. Med. Mod. Dist. Educ. Chin. 15, 83–85. doi: 10.3969/j.issn.1672-2779.2017.10.037 (in Chinese).
Wong, K. L., Wu, K. C., Wu, R. S., Chou, Y. H., Cheng, T. H., and Hong, H. J. (2007). Tetramethylpyrazine inhibits angiotensin II-increased NAD(P)H oxidase activity and subsequent proliferation in rat aortic smooth muscle cells. Am. J. Chin. Med. 35, 1021–1035. doi: 10.1142/S0192415X0700548X
Worthington, I., Pringsheim, T., Gawel, M. J., Gladstone, J., Cooper, P., Dilli, E., et al. (2013). Canadian Headache Society Guideline: acute drug therapy for migraine headache. Can. J. Neurol. Sci. 5(Suppl. 3), S1–S80. doi: 10.1017/S0317167100017819
Xiong, W., Tan, M., He, L., Ou, X., Jin, Y., Yang, G., et al. (2017). Inhibitory effects of tetramethylpyrazine on pain transmission of trigeminal neuralgia in CCI-ION rats. Brain Res Bull. 134, 72–78. doi: 10.1016/j.brainresbull.2017.07.005
Xu, Y. L. (2011). The Clinical Research of Pinggan Xifeng Huayu Tongluo Treatment Migraine (Liver Wind Carry Blood Stasis Syndrome). Dissertation, Changchun University of Chinese Medicine. (in Chinese)
Yang, D. D. (2014). Wind-Dispelling and Pain-Relieving Capsule in the Treatment of Migraine (Which is Also Called Slowed Blood Flow in Traditional Chinese medicine) Clinical Research. Dissertation, Changchun University of Chinese Medicine, Changchun. (in Chinese)
Yang, W. T., Zheng, X. W., Chen, S., Shan, C. S., Xu, Q. Q., Zhu, J. Z., et al. (2017). Chinese herbal medicine for Alzheimer's disease: clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. doi: 10.1016/j.bcp.2017.07.002
Yu, S., Liu, R., Zhao, G., Yang, X., Qiao, X., Feng, J., et al. (2012). The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 52, 582–591. doi: 10.1111/j.1526-4610.2011.02061.x
Zhang, G. N., and Xu, Y. L. (2017). Study on Xiongchongsanpian Decoction in treatment of migraine with turbid phlegm disturbing mind syndrome. Acta Chin. Med. 32, 285–289. doi: 10.16368/j.issn.1674-8999.2017.02.073 (in Chinese).
Zhang, L. L. (2012). Clinical Study on Migraine Treated With Removing Obstruction in Collaterals for Relieving Pain Basic on Collateral Theory of TCM, Dissertation, Nanjing University of Chinese Medicine, Nanjing. (in Chinese)
Zhang, R. (2015). The Clinical Research of Treatment Migraine (Liver Stagnation and Blood Stasis) of Hospital Preparation Shugan Tongluo Prescription. Dissertation, Changchun University of Chinese Medicine, Changchun. (in Chinese)
Zheng, Q., Wei, S. F., Xiong, W. H., Xue, X., Yu, J., Wu, Z. F., et al. (2013). Analysis on application of Chuanxiong Rhizoma in Chinese patent medicine formula for treating headache. Chin. Tradit. Herbal Drugs 44, 2777–2781. doi: 10.7501/j.issn.0253-2670.2013.19.027
Zheng, Z., Li, Z., Chen, S., Pan, J., and Ma, X. (2013). Tetramethylpyrazine attenuates TNF-α-induced iNOS expression in human endothelial cells: involvement of Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Exp. Cell Res. 319, 2145–2151. doi: 10.1016/j.yexcr.2013.05.018
Zhong, J., Pollastro, F., Prenen, J., Zhu, Z., Appendino, G., and Nilius, B. (2011). Ligustilide: a novel TRPA1 modulator. Pflug. Arch. 462, 841–849. doi: 10.1007/s00424-011-1021-7
Keywords: headache, pain, Ligusticum chuanxiong Hort. Root, Traditional Chinese medicine, Chinese herbal medicine
Citation: Shan C-S, Xu Q-Q, Shi Y-H, Wang Y, He Z-X and Zheng G-Q (2018) Chuanxiong Formulae for Migraine: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. Front. Pharmacol. 9:589. doi: 10.3389/fphar.2018.00589
Received: 08 March 2018; Accepted: 16 May 2018;
Published: 27 June 2018.
Edited by:
Francisco R. Nieto, University of Granada, SpainReviewed by:
Cristina Sampaio, CHDI Foundation, United StatesNasiara Karim, University of Malakand, Pakistan
Esperanza Del Pozo, University of Granada, Spain
Copyright © 2018 Shan, Xu, Shi, Wang, He and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Qing Zheng, Z3FfemhlbmdAc29odS5jb20=
 Chun-Shuo Shan
Chun-Shuo Shan Guo-Qing Zheng
Guo-Qing Zheng