Abstract
Cataract is the leading reason of blindness worldwide and is defined by the presence of any lens opacities or loss of transparency. The most common symptoms of cataract are impaired vision, decreased contrast sensitivity, color disturbance, and glare. Oxidative stress is among the main mechanisms involved in the development of age-related cataract. Surgery through phacoemulsification and intraocular lens implantation is the most effective method for cataract treatment, however, there are chances of serious complications and irreversible loss of vision associated with the surgery. Natural compounds consisting of antioxidant or anti-inflammatory secondary metabolites can serve as potential leads for anticataract agents. In this review, we tried to document medicinal plants and plant-based natural products used for cataract treatment worldwide, which are gathered from available ethnopharmacological/ethnobotanical data. We have extensively explored a number of recognized databases like Scifinder, PubMed, Science Direct, Google Scholar, and Scopus by using keywords and phrases such as “cataract”, “blindness”, “traditional medicine”, “ethnopharmacology”, “ethnobotany”, “herbs”, “medicinal plants”, or other relevant terms, and summarized the plants/phytoconstituents that are evaluated in different models of cataract and also tabulated 44 plants that are traditionally used in cataract in various folklore medical practices. Moreover, we also categorized the plants according to scientific studies carried out in different cataract models with their mechanisms of action.
Cataract: An Overview
The crystalline lens lies behind the iris and represents the dynamic part of the eye’s optical system, responsible for focusing the image onto the retina. Cataract is defined by the presence of any lens opacities or loss of transparency. The most common symptoms of cataract are impaired vision, decreased contrast sensitivity, color disturbance, and glare. Changes in the lens may also serve as markers for systemic health and aging in the over-all population (Song et al., 2014). According to the type of lens opacities, cataract is classified into three classical types: nuclear, posterior subcapsular, and cortical. These types can also be associated with each other and if untreated, they progress to total lens opacification. Some of the most common causes for cataract in adults are age, diabetes, steroid use, family history, or trauma. Congenital cataract has a significant prevalence, also.
Cataract is the foremost reason of blindness worldwide in spite of the technological advancements in eye surgery in the last two decades. In 2010, there were around 32 million blind people and 191 million were with poor vision. One in three blind people suffered from cataract (Khairallah et al., 2015). The World Health Organization (WHO) suggests that by 2020 the number of blind people will reach 90 million globally (Khairallah et al., 2015; Taylor, 2016). The strategy to fight this challenge is costly, aiming human resource, infrastructure development, and effective disease control. The latter is dependent on the characteristics of the specific disease. Prevalence of cataract increases with age, from 5% for patients of age 52–62 to 64% for patients over 70 years, in Europe (Prokofyeva et al., 2013). Age is a non-modifiable risk factor involved in the pathogenesis of cataract, hence the progressive aging of the population is an alarming issue. Identifying modifiable risk factors for cataract is imperative and may help to establish the preventive measures.
The surgical treatment for cataract consists of cataractous lens extraction and intraocular lens implant. It is the only current treatment available in order for patients to recover their visual function. This implies a significant cost and there is a significant lack of access to surgery, especially in the developing world. Despite good postoperative outcomes, complications are possible following cataract surgery. Studies have suggested that pseudophakia patients have a higher risk of retinal detachment. Endophthalmitis has also been reported in 0.12% of the operated cases (Toh et al., 2007). After the surgery, the mobility of the lens is lost and correcting glasses are usually necessary. This will only increase the expense and the discomfort for the patient and society. Medical treatment would be a desired alternative.
The most primitive written reference to cataract surgery was discovered in Sanskrit manuscripts dating back from the 5th century BCE. It was attributed to Sushruta, a well-known ancient plastic surgeon who described a procedure known as couching, in which the cataractous lens was displaced with a sharp tool to fall it into the vitreous cavity, clearing the visual axis, though the vision was significantly blurred as there were no corrective lenses or glasses (Uhr, 2003; Sachdev, 2017). Even at the time of Mesopotamia (ca. 3,000–4,000 BCE) records reveal that mysticism along with different animal products, vegetables, and minerals were utilized for the treatment of devil and spirits causing eye diseases. Hundreds of remedies were also described during the Greek era (ca. 460–375 BCE) for disorders of the eyes. Moreover, eye diseases are also described anatomically by Sushruta (as mentioned above), Galen and various medicinal and surgical procedures were described for the treatment of eye diseases (Duke-Elder, 1962; Albert and Edwards, 1996; Goodman, 1996). In 1748, the introduction of modern cataract surgery was done by Jacques Daviel in Paris, in which the cataractous lens is removed from the eye. Later on in 1753, Samuel Sharp of London presented the intracapsular procedure, wherein the whole lens was removed by an incision by put on thumb pressure. In 1867 silk sutures for cataract surgery was originally described by Henry Willard Williams of Boston (Uhr, 2003).
Cataract – Pathogenesis
Various mechanisms have been associated with age-related cataract pathogenesis. Lens opacities may appear due to changes in the microarchitecture, caused by mutations, biomechanical, or physical changes.
Mutations
Despite cataract being a multifactorial disease, sometimes mutations alone can cause lens opacities and this usually leads to congenital or pediatric cataract. Studies have presented more and more evidence that genetic factors are also part of age related cataract pathogenesis, raising the probability of molecular genetic relations between lens development and aging (Hejtmancik and Kantorow, 2004). Out of around 42 genes and loci that have been found to underlie congenital forms of cataract, a few of them have been linked with age associated cataract: EPHA2 (encodes a member of ephrin receptor of protein-tyrosine-kinases), CRYAA, CRYGS (both encode lens proteins), FYCO1 (encodes a scaffolding protein which is active in microtubule transport of autophagic vesicle), or TDRD7 (encodes an RNA-binding protein). The mutation p.Gly18Val in CRYGS results in a protein with normal structure in physiological conditions. The alterations in its structure occur after thermal or chemical injury. A similar mutation is Phe71Leu in CRYAA. The discovery of mutations in genes coding for TDRD7, EPHA2, and FYCO1 has provided the initial evidence for the functional importance of posttranscriptional mRNA regulation, ephrin signaling, and the autophagy pathway, respectively, in human lens transparency (Shiels and Hejtmancik, 2015).
Gene mutations underlying secondary forms of cataract could also play part in age related cataract formation. A mutation in gene on 17q of galactokinase 1 (GALK1) which is responsible for encoding of the first enzyme in galactose metabolism, trigger autosomal recessive GALK1 1-deficiency with hypergalactosemia and cataract as a result of galactitol accumulation and osmotic stress. A coding variation in GALK1 (p.A198V) generates enzyme instability associated with amplified risk of age-related cataract in the Japanese population (Okano et al., 2001).
Oxidative Stress
Oxidative stress is among the main mechanisms involved in the development of age-related cataract. Oxidative stress occurs when reactive compounds like the superoxide anion, hydroxyl radicals, and hydrogen peroxide are not neutralized by antioxidant enzymes and defense systems. Enzymes like catalase, SOD, and GPX are crucial for the homeostasis of the antioxidant system and ROS. When levels of ROS increase, this denatures the lens nucleic acids, proteins, and lipids, leading to mutations and cell apoptosis. Metabolic activities mostly take place in the lens epithelium. The lens epithelium uses the antioxidative enzymes in order to prevent damages caused by oxidative stress. Studies suggest that the highest concentration of SOD is in the lens epithelium (Rajkumar et al., 2013). These enzymes are also present in other parts of the lens and play a very important part in maintaining the lens clarity (Chang et al., 2013). SOD is responsible for converting superoxide anion into hydrogen peroxide, and then hydrogen peroxide is transformed into water by catalase or GPX. SOD enzyme activity is associated with cofactors like zinc, manganese, and copper. However, a decreased level of cofactors in cataractous lenses was not found. Experimental animal models show a decreased level of glutathione in the nucleus, therefore there is a higher susceptibility for oxidative damage and opacity formation (Giblin, 2000). Studies have shown that serum and aqueous humor levels of antioxidative enzymes are decreased in patients with cataract. However, there was no significant difference among different types of cataract and enzymes serum levels (Ohia et al., 2005; Wang et al., 2015).
Crystallins Problems
Crystallins, the major structural lens proteins have an imperative role in the lens transparency and acquire post-translational alterations during cataract formation, which lead to protein insolubility, aggregation and loss of lens transparency. Out of the three major crystallins, α-, β-, and γ-, α crystallins exhibit chaperone like activity, preventing them to aggregate. The chaperone activity is reduced in cataractous lenses. Prolonged hyperglycemic conditions increase the chances of crystallins deterioration (Reddy et al., 2014). Calcium activates calcium-binding proteins triggering changes in the shape and charge of the proteins. Elevated levels of calcium appear to induce proteolysis of crystallins by calpain, an intracellular cysteine protease. Activation of calpain, an intracellular cysteine protease, leads to proteolysis of the lens proteins. In order for calpains to activate, a high level of calcium is required (Obrosova et al., 2010). Studies demonstrate that the privation of an endogenous inhibitor of calpain, named calpastatin, could be linked to the initial changes that cause cataract (Nakajima et al., 2014). Some antioxidants have been reported to regulate calcium influx in selenite induced cataracts, for instance the flavonoid fraction of Brassica oleracea (Vibin et al., 2010).
Protein Structures
Alterations in the protein structure are also determined by UV exposure. Studies have shown that UVB generates more damage than UVA and that damages are prevented by the lens filters. After UV radiations, proteins suffer chemical reactions resulting in aggregations, decreasing the transparency of the lens (Cetinel et al., 2017). The crystalline lens is particularly exposed to phototoxic damage, because it absorbs most of UV radiation, together with cornea. The main association is with cortical cataract, most of the absorption occurring at the posterior surface of the lens. UV radiation can generate free radicals including oxygen-derived species, that cause lipid peroxydation of cellular membranes or can damage DNA directly (Youn et al., 2011). In vivo, induced cataract has no absolute threshold for UV exposure. UV induced cataract for in vivo exposure at UV-300 nm has a continuous dose-response function (Söderberg et al., 2016). UV radiation data from Eurosun library implied that rates of cataract were higher in regions with higher ambient UV-B radiation levels (Delcourt et al., 2014).
Medicinal Plants and Natural Products Used Against Cataract
Opacity of the lens is triggered by free radicals in most of the cases (Varma et al., 1984; Thiagarajan and Manikandan, 2013). Severe oxidative stress also leads to the protein modifications by free radicals, and several natural products from plants are helpful in the prevention of proteins insolubilization, which may delay the opacity of lenses (Bhadada et al., 2016b). Natural compounds constituting of antioxidant or anti-inflammatory secondary metabolites could be viewed as potentially optimal anticataract agents as antioxidant effect is among the major mechanisms for prevention of cataract in most of the cases, however, not all the plants possessing antioxidant potential could have anticataract properties. The role of plant polyphenols in anti–cataractogenic activities is also studied in the comprehensive manner either in vitro or in vivo (Rooban et al., 2009, 2011; Kim et al., 2011c; Wang et al., 2011; Sasikala et al., 2013; Sunkireddy et al., 2013; Ferlemi et al., 2016).
Although there is substantial basic and applied research in the field of cataract management by natural products, mostly ethno-pharmacological/ethnobotanical research, there are not many review papers available about the activity analysis of natural products against different cataract models. One paper focused on antioxidant containing plants against cataract was found with 41 plants investigating anti-cataract activity (Thiagarajan and Manikandan, 2013). Although there are few ethnopharmacological surveys and their reviews available (Maregesi et al., 2017), there is no detailed review available on the activities of different plants extracts and natural products in cataract models.
Methodology and Hypothesis
In this work, we attempted to gather and document the widely scattered information from various preclinical investigations and ethnopharmacological reports. We searched several web databases namely, Scifinder, ScienceDirect, Pubmed, Scopus, and Google Scholar. Boolean information retrieval method (Pohl et al., 2010) was applied using plant name with “AND” operator as also done in some other systemic reviews (Tewari et al., 2017, 2018) followed by “cataract” and using other different keywords such as “cataract”, “traditional medicine”, “ethnobotany”, “sodium selenite”, and “ethnopharmacology”.
The main research question we try to address in this paper is: “are medicinal plants/natural products used in various folk and traditional medicine of importance in the management of cataract?” and “what are the major preclinical in vitro/in vivo models that are used globally for the evaluation of cataract?”. We hypothesize that plants used in ethnomedicine are not only of potential importance but also preclinical studies conducted on various models of cataract could result in the development of potential drug candidates in future. This could be very rewarding for the scientists and scholars working in this area and also very beneficial for the patients to take forward the preclinically effective plants for clinical studies.
Results
Oxidative stress is involved in activation of MAPKs. Compounds resulted from the activation of MAPKs have been studied and were associated with cell apoptosis. The p38 MPAK was studied in vitro and it was shown that it is activated by hydrogen peroxide, induce cell apoptosis in lens epithelial cells and the antioxidant agents could reduce its effects. Inhibitors of p38 MAPK reduced ROS levels and apoptosis (Bai et al., 2015).
Lipids peroxidation is also a reason of age related cataract. This process has a negative impact on lipid–lipid and lipid–protein interactions. Research has shown high levels of hydroperoxides, oxy derivatives, and diene conjugates of phospholipid fatty acids in the aqueous humor of cataract patients. Also, studies have reported high levels of oxidation products of linoleic acid in patients with early cataract (Bai et al., 2015). A schematic representation of oxidative stress mechanisms involved in cataract etiology and action mechanisms of several medicinal plants with conducted pharmacological studies for the treatment of cataract are presented in Figure 1.
FIGURE 1
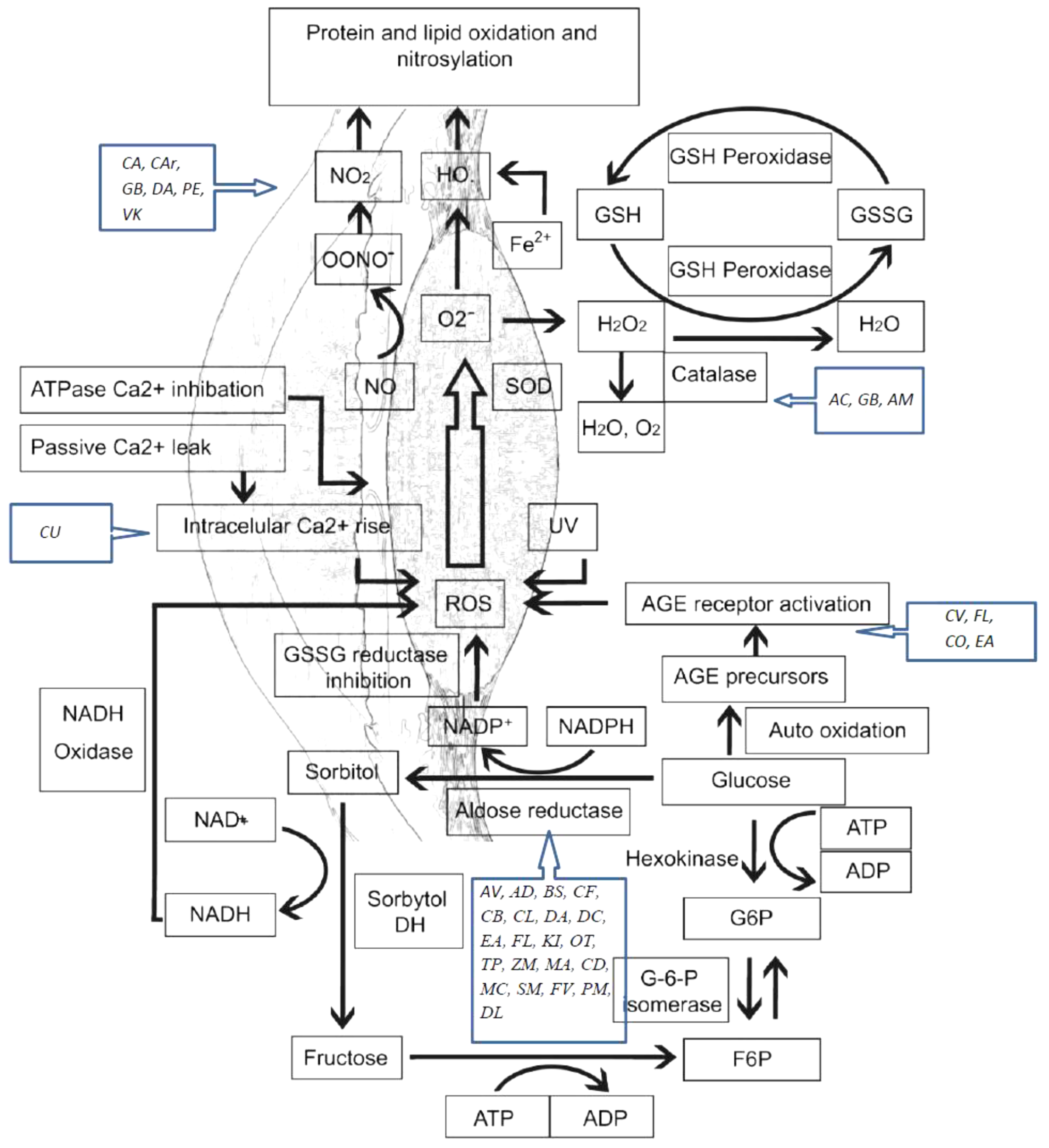
Oxidative stress mechanisms involved in cataract etiology and action mechanisms of several medicinal plants with conducted pharmacological studies for the treatment of cataract. AGE, advanced glycation end-products; ROS, reactive oxygen species; SOD, superoxide dismutase; GSH, glutathione; GSSG, glutathione disulfide; NO, nitric oxide. AC, Allium cepa; CA, Coffea arabica; CU, Curcumin; GB, Ginkgo biloba; AV, Adhatoda vasica; AM, Aegle marmelos; AD, Angelica dahurica; BS, Biophytum sensitivum; CB, Caesalpinia bonduc; CF, Cassia fistula; CV, Cinnamomum verum; CL, Curcuma longa; DA, Dendrobium aurantiacum var. denneanumis; DC, Dendrobium chrysotoxum; EA, Erigeron annuus; FL, Flavonoids; KI, KIOM-79; OT, Ocimum tenuiflorum; VK, Vitamin K; TP, Tephrosia purpurea; ZM, Zea mays; MA, Matteuorienate A; CD, Caesalpinia digyna; CO, Cornus officinalis; MC, Morinda citrifolia; SM, Salvia miltiorrhiza; FV, Foeniculum vulgare; PM, Pueraria montana; DL, Danshenol; C Ar, Citrus aurantium; PE, Phyllanthus emblica.
Polyol pathway is associated with diabetic cataract. Enzymes implicated in the polyol pathway, sorbitol dehydrogenase and AR are responsible for the conversion of glucose to fructose. Sorbitol, an intermediate compound, was found to produce cell lesions by modifying the membrane permeability. Therefore, accumulation of sorbitol leads to osmotic stress, collapse, and liquefaction of lens fibers resulting in loss of lens transparency (Pollreisz and Schmidt-Erfurth, 2010; Hashim and Zarina, 2012). AR converts glucose to sorbitol, dependent to NADPH. As a consequence, the level of NADPH decreases, also having a negative impact on the glutathione activity and the antioxidant system. In vivo and in vitro studies have shown that by inhibiting the activity of AR, the progression to cataract in patients with diabetes is reduced (Kim et al., 2011a; Ramana, 2011). The glycosylation pathway has also been linked to diabetic cataract. Excessive glucose level induces the glycation of proteins, generating superoxide radicals and AGEs in the process. Recent studies suggest that there is interdependence between the oxidative stress and polyol pathway, through AR and iNOS, responsible for the nitric oxide production during oxidation (Snow et al., 2015; Li et al., 2017) (Figure 1).
Here, we present details of plants evaluated against cataract with discussion of their possible mechanism of action (Figure 1 and Tables 1–4). Some important chemical structures of the natural products that are used against cataract or found in plants used in the management of cataract are also presented at Figure 2 (not all chemical structures are presented). We categorized the plants based upon the models evaluated. Table 1 describes the natural products used against cataract evaluated on selenite/sodium selenite induced cataracts, in Table 2 natural products used against cataract on preventive photooxidative damage is described, Table 3 deals with the natural products used against cataract on sugar-induced lens opacity/Streptozotocin induced diabetic cataract/galactose or glucose induced/ZDF models, in Table 4 AGEs-BSA crosslinking inhibition assay and lens AR activity models are described, and Table 5 describes the natural products used against cataract on hydrogen peroxide and naphthalene induced cataract and other miscellaneous models.
TABLE 1
| Plant (with part used)/natural product | Doses, concentrations and characteristics of extracts | Suggested/possible mechanism of action | References |
| Alangium salviifolium (L.f.) Wangerin (Syn. Alangium lamarckii Thwaites) (Leaves) | Alcoholic extract at an increasing concentration between 0 and 300 μg/mL (IC 106 ± 5.11 μg/mL) | Exhibit significant inhibitory effects on aldose reductase (AR) in the rat lens in vitro. | Kumar et al.,2011 |
| Allium cepa L. (Tuber/bulb) | 50% diluted juice | Prevention of selenite-induced cataract formation by increase in superoxide dismutase (SOD) and total antioxidant level and activities of glutathione peroxidase (GPX) in lens through instillation of juice in rat eyes. | Javadzadeh et al.,2009b |
| Allium sativum L. (Tuber/bulb) | Aqueous extract, 1 mL/kg body weight | Free radical scavenging activity (FRSA), antioxidant properties and associated with increased TA level, SOD and GPX activities in the lens. | Javadzadeh et al.,2009a |
| Aralia elata (Miq.) Seem. (Cortex) | Aqueous extract at 1 and 10 mg/mL (IC 11.3 μg/mL) | Inhibit AR and antioxidant activity. | Chung et al.,2005 |
| Brassica oleracea L. var. italica Plenck (Edible part) | Flavonoid fraction | Maintains antioxidant status, ionic balance via Ca2+ ATPase pump, inhibits calpain activation, lipid peroxidation, and protein insolubilization. | Vibin et al.,2010 |
| Caesalpinia digyna Rottler (Roots) | Alcoholic extract at an increasing concentration between 0 and 200 μg/mL (IC 46.29 ± 11.17 μg/mL) | AR inhibition. | Kumar et al.,2011 |
| Caffeic acid phenethyl ester | Caffeic acid phenethyl ester, subcutaneous | Suppressed cataract formation in rats by antioxidant property. | Doganay et al.,2002 |
| Camellia sinensis (L.) Kuntze (Leaves) | Extract (1 L of the solution contains 100 g of green tea, 400 mL of purified water and 535 mL of strong alcohol), intraperitoneally | Antioxidant activity. | Gupta et al.,2002 |
| Cassia fistula L. (Fruit pulp) | Sequential water, ethanol and chloroform extracts | AR inhibition. | Gacche and Dhole,2011a |
| Cochlospermum religiosum (L.) Alston (Leaves) | Isolated isorhamnetin-3-glucoside, 50 μg/mL | Retardation of selenite cataract in vitro via preventing oxidative stress, calcium accumulation and preclusion of lipid peroxidation. | Gayathri Devi et al.,2010 |
| Coffea arabica L. | 1 mL of Instant black coffee | Decreased level of total nitric oxide, tumor necrosis factor-α (TNF- α), Ca-ATPase, superoxide dismutase, interleukin (IL)-1β, preserved enzyme antioxidants and lens proteins. | El Okda et al.,2016 |
| Crataegus pinnatifida Bunge (Leaves) | Total flavonoids fraction | Inhibition of AR, FRSA. | Wang et al.,2011 |
| Crocus sativus L. (Stigmas) | Hydroalcoholic extract, intraperitoneal injections of saffron extract (60 mg/kg body weight) | Reinforcement of antioxidant condition, inhibits lipid peroxidation intensity, and inhibition of aqueous-soluble fraction of lens proteolysis. | Makri et al.,2013 |
| Curcuma longa L. (Rhizomes) | Curcumin (200 μM) | Oxidative stress inhibition and attenuation to cataract formation, ameliorated calcium-induced proteolysis. | Manikandan et al., 2009; Liao et al.,2016 |
| Cyanthillium cinereum (L.) H. Rob. (Syn. Vernonia cinerea Less.) (Leaves) | Isolated lupeol from flavonoid fraction that showed an IC 30 μg/mL against DPPH | Protection against formation of nuclear opacity in selenite-treated Sprague Dawley rat pups. | Asha et al.,2016 |
| Dregea volubilis (L.f.) Benth. ex Hook.f. (Leaves) | Isolated drevogenin D, 50 μg/mL | Antioxidant activity (affecting glutathione peroxidase, superoxide dismutase, catalase, and glutathione reductase), raises reduced glutathione and protein sulfhydryl levels, and decreases the lipid peroxidation levels. | Biju et al.,2007 |
| Ellagic acid | Ellagic acid 200 mg/kg body weight, i.p. | Inhibition of lipid peroxidation and maintains antioxidant defense system. | Sakthivel et al.,2008 |
| Emilia sonchifolia (L.) DC. ex DC. (Whole plant) | Flavonoid fraction 1.0 mg/kg body weight, i.p. | Antioxidant activity. | Lija et al.,2006 |
| Enicostemma hyssopifolium (Willd.) Verd. (Aerial parts) | C-glycosidic flavonoid including extract (IC 1.62; bitter fraction 2.40, Swertiamarin 7.59 and Swertisin 0.71 μg/mL) | AR inhibition. | Patel and Mishra,2009 |
| Eucalyptus deglupta Blume (Not given) | Ethanolic extract | AR inhibition. | Guzman and Guerrero,2005 |
| Ginkgo biloba L. (Egb761) | Extract 761 (0.35% 100 mg/kg body weight) | Prevents depletion of antioxidant enzymes, reduces oxidative stress, inhibition of lipid peroxidation and suppression of the TGF-β2/Smad pathway activation. | Lu et al., 2014; Cao et al.,2015 |
| Jacobaea maritima (L.) Pelser & Meijden [Syn. Cineraria maritima (L.) L.] (Aerial parts) | Ethanolic extract (300 μg/mL) | Increase in the activity of antioxidant enzymes and increase in the level of reduced glutathione in lens, reduces free radical generation. | Anitha et al.,2011, 2013 |
| Vitex negundo L. (Leaves) | Flavonoids | Maintenance of antioxidant status, by inhibition of ROS generation/lipid peroxidation in lens. | Rooban et al.,2012 |
| Moringa oleifera Lam. (Leaves) | Flavonoid fraction 2.5 μg/g body weight | Improvement of total antioxidant capability in lens, prevention of protein oxidation and lipid peroxidation. | Sasikala et al.,2010 |
| Origanum vulgare L. (Upper crust of beans) | Hydroethanolic extract (70%), 2 g/kg | Averts selenite-induced cataract through its antioxidant property. | Dailami et al.,2010 |
| Phyllanthus emblica L. (Syn. Emblica officinalis Gaertn.) (Fruits) | Aqueous extract, 26.19 mg/kg | Inhibition of sodium selenite induced cataract in rats though antioxidant property. | Nair et al.,2010 |
| Pleurotus ostreatus (Jacq. ex Fr.) P.Kumm. (Mushroom) | Ethanolic extract 250 μg/mL | Reduction of lipid peroxidation and increase in antioxidant enzymes. | Isai et al.,2009 |
| Rutin | Rutin | Alteration in protein profile and insolubilization of soluble protein. | Sasikala et al.,2013 |
| Senna tora (L.) Roxb. (Syn. Cassia tora L.) (Leaves) | Ethyl acetate fraction having anthraquinones and flavonoids, 5 μg/g body weight | Prevention of cytoskeletal protein denaturation in the lens, improvement of antioxidant capacity, and reduction in free radical generation. | Sreelakshmi and Abraham,2016 |
| Spathodea campanulata P.Beauv. (Flowers) | Exudate, 0.1 and 0.2 mg/mL | Counteracts cataract by antioxidant activity. | Gbemisola et al.,2014 |
| Syzygium malaccense (L.) Merr. & L.M.Perry (Not mentioned) | Ethanolic extract | AR inhibition. | Guzman and Guerrero,2005 |
| Tagetes erecta L. (Flowers) | Lutein and its ester at doses of 4, 40, and 400 mg/kg body weight | Antioxidant activity. | Harikumar et al.,2008 |
| Tephrosia purpurea (L.) Pers. (Whole plant) | Flavonoid rich fraction (40 mg/kg) or alcohol extract (300 mg/kg) | Maintenance of the antioxidant status and prevention of protein oxidation and lipid peroxidation in lens. | Bhadada et al.,2016a |
| Trigonella foenum-graecum L. (Seeds) | Lyophilized aqueous extract, (25, 50, and 100 μg/mL) | Antioxidant. | Gupta et al.,2010b |
| Triphala [An Ayurvedic formulation consisting of Emblica officinalis Gaertn., Terminalia chebula Retz., and Terminalia bellirica (Gaertn.) Roxb.] | Aqueous extract at 25, 50, and 75 mg/kg body weight i.p. | Restoration of GSH and reduced malondialdehyde levels. Substantial restoration in antioxidant enzymes activities like glutathione peroxidase, superoxide dismutase, catalase, and glutathione-s-transferase. | Gupta et al.,2010a |
| Vaccinium corymbosum L. (Leaves) | Decoctions (centrifuged, filtered, lyophilized), and dry extract, dissolved in sterilized normal saline, 100 mg/kg | Direct and indirect inhibition of lens calpains, anti-oxidant and chelating properties. | Ferlemi et al.,2016 |
| Vitex negundo L. | Flavonoids | Enhancement of antioxidant enzymes, maintains ionic balance and reduces the lens oxidative stress, prevention of changes in lens protein, loss of chaperone property, changes in lens structure, protective effect against oxidative damage. | Rooban et al.,2009, 2010, 2011 |
| Vitex negundo L. (Leaves) | Luteolin | Maintenance of antioxidant status via reducing ROS generation/lipid peroxidation in lens. | Rooban et al.,2012 |
| Vitis vinifera L. (Seed extract) | Proanthocyanidin/procyanidin-rich extract | Oxidative stress inhibition, suppression of lipid peroxidation, and free radicals and activation of inducible nitric oxide synthase (iNOS), and calpain II in lenses. Improvement of the antioxidant defense mechanisms of the lens. | Yamakoshi et al., 2002; Durukan et al., 2006; Zhang and Hu, 2012; Mani Satyam et al.,2014 |
| Withania somnifera (L.) Dunal (Procured extract) | Aqueous extract, 25–300 μg/mL | Inhibits lens AR activity. | Halder et al.,2003 |
Medicinal plants/natural products used against cataract on Selenite/sodium selenite induced cataract models, and suggested/possible mechanisms of action.
TABLE 2
| Plant (with part used)/ natural product | Doses, concentrations and characteristics of extracts | Suggested/possible mechanism of action | References |
| Astaxanthin | Astaxanthin (0–1 mM) | Prevention of cataract through protection of lens from oxidative insults and degradation by calcium-induced calpain. | Wu et al.,2006 |
| Citrus × aurantium L. (Peel) | Methanol-water extract, 100 and 200 mg/kg body weight | Delay in onset and maturation of naphthalene induced cataract vis prevention of the photo-oxidative damage produced by naphthalene. | Umamaheswari et al.,2011 |
| Ginkgo biloba L. (Leaves) | Standardized EGb761 extract (24% flavonol glycoside and 6% terpene lactones) | Protection from radiation induced cataracts in rat lens via antioxidant property. | Ertekin et al.,2004 |
| Lutein and Zeaxanthin | Lutein and Zeaxanthin | Protection of eye from oxidative stress and high-energy photons of blue light. | Moeller et al.,2000 |
Medicinal plants/natural products used against cataract on preventing photo-oxidative damage.
TABLE 3
| Plant (with part used)/natural product | Doses, concentrations and characteristics of extracts | Suggested/possible mechanism of action | References |
| Aegle marmelos (L.) Corrêa (Leaves) | Chloroform extract 150 mg and 300 mg/kg body weight, p.o. | Increases glutathione, catalase and superoxide dismutase, inhibits lens AR and decreases osmotic stress. | Panaskar et al., 2013; Sankeshi et al.,2013 |
| Allium sativum L. (Bulb) | Methanolic extract, 0.25 and 0.5 g/kg body weight, by forcible gut feeding | Antioxidant activity. | Raju et al.,2008 |
| Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch & Sav. (Roots) | Ether extract (100 μg/mL) (Byakangelicin) | Suppression of galactose induced cataract formation in diabetic rats via AR inhibiting property. | Shin et al.,1994, 1998 |
| Buddleja officinalis Maxim. (Flowers) | Apigenin | Inhibiting rat lens AR activity. | Matsuda et al.,1995 |
| Azadirachta indica A. Juss. (Not mentioned) | Aqueous extract, 25–300 μg/mL | Inhibits lens AR activity. | Halder et al.,2003 |
| Biophytum sensitivum (L.) DC. (Leaves) | Sequential water, ethanol and chloroform extracts | AR inhibition and antioxidant action. | Gacche and Dhole,2011a |
| Brassica juncea (L.) Czern. (Leaves) | Aqueous extract, 250 and 500 mg/kg | Effective activity against hyperglycemia induced oxidative and osmotic stress. | Valavala et al.,2011 |
| Brickellia arguta B. L. Rob (Not mentioned) | Ethanolic extract | AR inhibition. | Guzman and Guerrero,2005 |
| Caesalpinia bonduc (L.) Roxb. (Seeds) | Sequential water, ethanol and chloroform extracts | AR inhibition and antioxidant. | Gacche and Dhole,2011a |
| Cassia fistula (L.) (Fruit pulp) | Sequential water, ethanol and chloroform extracts | AR inhibition and antioxidant. | Gacche and Dhole,2011a |
| Catharanthus roseus (L.) G.Don (Leaves) | Sequential water, ethanol and chloroform extracts | Inhibiting AR activity and antioxidant action. | Gacche and Dhole,2011b |
| Chlorogenic acid | Chlorogenic acid (0.7–2.8 mM) | Inhibiting AR activity in galactose fed rats. | Kim et al.,2011a |
| Chromolaena odorata (L.) R.M.King & H.Rob. (Leaves) | Ethanol extract (200 and 400 mg/kg) | Decrease of oxidative stress. | Onkaramurthy et al.,2013 |
| Corydalis turtschaninovii Besser (Tuber) | Methanolic extract of the alkaloidal component (10–200 μg/mL) containing dehydrocorydaline | Inhibiting AR activity. | Kubo et al.,1994 |
| Curcuma longa L. (Rhizome) | Aqueous extract, 25–300 μg/mL | Prevents in vitro cataract via AR inhibitory activity. | Halder et al.,2003 |
| Dendrobium chrysotoxum Lindl. (Stems) | Gigantol | Inhibition of AR and AR gene expression. | Wu et al.,2017 |
| Dendrobium aurantiacum (F. Muell.) F. Muell. var. denneanumis (Kerr) Z.H.Tsi (Stems) | Gigantol | Attenuation in increase of AR, inducible nitric oxide synthase (iNOS) expression and opacification of rat lenses. | Fang et al.,2015 |
| Eclipta prostrata (L.) L. [Syn. Eclipta alba (L.) Hassk.] (Whole plant) | Ethanolic extract (flavonoids) | Inhibition of AR | Jaiswal et al.,2012 |
| Erigeron annuus (L.) Pers. (Flowers) | Isolated phenolic compounds | Inhibition of cataract via inhibiting protein glycation and AR in rat lens. | Jang et al.,2008 |
| Eugenia cordata (Sw.) DC.var. sintenisii (Kiaersk.) Krug & Urb. (Not mentioned) | Ethanolic extract | Inhibition of AR. | Guzman and Guerrero,2005 |
| Ficus glomerata L. (Fruits) | Sequential water, ethanol, and chloroform extracts | AR inhibition and maintaining of lens opacity. | Gacche and Dhole,2011b |
| Marsdenia sylvestris (Retz.) P.I.Forst. [Syn. Gymnema sylvestre (Retz.) R. Br.] (Leaves) | The polyol Conduritol A | Inhibition of AR. | Miyatake et al.,1994 |
| Thymus vulgaris L. (Leaves) | Methanolic extract and isolated compounds (Eriodictyol) | Suppression of the advanced glycation end products levels and fructosamines of albumin. | Morimitsu et al.,1995 |
| Genistein | Genistein | Increase connexin (Cx) 43 expression. | Huang et al.,2007 |
| Zingiber officinale Roscoe (Rhizomes) | Powder | Suppressing lens galactitol accumulation. | Saraswat et al.,2010 |
| Hydrocotyle bonariensis Comm. ex Lam. (Leaves) | Aqueous extract, 500 and 1,000 mg/kg | Reduction in lens protein insolubilization, lens peroxidation and increase in the antioxidant status of the lens. | Ajani et al.,2009 |
| Justicia adhatoda L. (Syn. Adhatoda vasica Nees.) (Procured extract) | Sequential water, ethanol and chloroform extracts | AR inhibition and antioxidant action. | Gacche and Dhole,2011a |
| KIOM-79 | (80% ethanol extract of parched Puerariae Radix, gingered). (Magnoliae cortex, Glycyrrhizae Radix and Euphorbiae Radix) (Magnolia officinalis, Pueraria lobata, Glycyrrhiza uralensis, Euphorbia pekinensis) (0–1,000 μg/mL) | AR inhibition. KIOM-79, an Inhibitor of AGEs–Protein Cross-linking, Prevents Progression of Nephropathy in Zucker Diabetic Fatty Rats. | Kim et al.,2011b |
| Magnolia fargesii (Finet & Gagnep.) W. C. Cheng (Flower buds) | Isolated scopoletin and tiliroside | Inhibition of rat lens aldose reductase (RLAR) activity; ex vivo cataractogenesis of rat lenses induced by xylose was inhibited by scopoletin. | Lee et al.,2010 |
| Mangifera indica L. | Ethanolic extract | AR inhibition and antioxidant activity. | Guzmán and Guerrero,2005 |
| Miyamayomena koraiensis (Nakai) Kitam. (Syn. Aster koraiensis Nakai) (Korean starwort) (Aerial part) | Extract of 100 and 200 mg/kg | Delay in the progression of lens opacification during the early diabetic cataractogenesis. | Kim et al.,2009 |
| Momordica charantia L. (Fruits) | Aqueous and ethanolic extracts, 200 and 400 mg/kg | Prevention of experimental diabetic cataract through reduction of plasma glucose levels. | Rathi et al.,2002 |
| Ocimum tenuiflorum L. (Syn. Ocimum sanctum L.) (Leaves) | Aqueous extract, 25–300 μg/mL | Prevents in vitro cataract by virtue of its aldose reductase inhibitory activity. | Halder et al.,2003 |
| Peonidin-3-glucoside | Peonidin-3-glucoside | Inhibits lens AR. | Morimitsu et al.,2002 |
| Phyllanthus emblica L. (Syn. Emblica officinalis Gaertn.) (Fruits) | Isolated β-glucogallin (0–40 μM) | Inhibition of AKR1B1. | Puppala et al.,2012 |
| Pterocarpus marsupium Roxb. (Bark) | Aqueous extract, 2 g/kg | Decreased opacity index. | Vats et al.,2004 |
| Pueraria montana (Lour.) Merr. var. lobata (Willd.) Sanjappa & Pradeep. (Roots) | Puerariafuran isolated from methanoilc extract | Inhibition of rat lens AR. | Kim et al.,2010 |
| Rutin | Rutin (10–100 μM) | Inhibits advanced glycation end products formation by prevention of dicarbonyls formation. | Muthenna et al.,2012 |
| Silybin | Silybin, 231 mg/day for 4 weeks | Reductions in the erythrocytic sorbitol level which lead to formation of glycation end products. | Zhang et al.,1995 |
| Silybum marianum (L.) Gaertn. (Seeds) | Silymarin 200 mg/kg/d, from extract | Antioxidative activity and increase in lens GSH and decrease in lipid peroxides (LPO) levels. | Fallah Huseini et al.,2009 |
| Syzygium cumini (L.) Skeels (Syn. Eugenia jambolana Lam.) (Kernels) | Aqueous and ethanolic extracts, 200 and 400 mg/kg | Significant reduction of plasma glucose. | Rathi et al.,2002 |
| Syzygium nervosum A.Cunn. ex DC. [Cleistocalyx operculatus (Roxb.) Merr. & L.M.Perry] (Dried flower buds) | Aqueous extract, 500 mg/kg bw/day | Indirect antihyperglycemic effect, decreases the levels of glucose, sorbitol, and fructose in diabetic rat lenses. | Mai et al.,2010 |
| Tephrosia purpurea (L.) Pers. (Whole plant) | Flavonoid rich fraction, 40 mg/kg/day, p.o, whole plant | AR enzyme inhibition and anti-oxidant activity. | Bhadada et al.,2016b |
| Theobroma cacao L. (Cacao liquor) | Crude polyphenol fraction (0.5% with diet) (Cyanidin) | Inhibits lens AR. | Osakabe et al.,2004 |
| Tinospora sinensis (Lour.) Merr. [Syn. Tinospora cordifolia (Willd.) Miers] (Procured stem extract) | Aqueous and ethanolic extracts, 200 and 400 mg/kg | Prevention of retinal oxidative stress, restoration of antioxidant enzyme levels and reduction in the angiogenic markers, vascular endothelial growth factor (VEGF) and protein kinase C (PKC) that are increased in diabetic retina. | Rathi et al., 2002; Rajalakshmi et al., 2009; Agrawal et al.,2012 |
| Triphala Ghrita | It’s an Ayurvedic formulation containing gallic acid | Delay in the onset and progression of galactose induced cataract through antioxidant activity. | Mahajan et al.,2011 |
| Vitamin K | Vitamin K | Lens Ca2+ homeostasis modulation and inhibition of osmotic and oxidative stress. | Sai Varsha et al.,2014 |
| Zea mays L. (Seed) | Hydroalcoholic extract, 2, 10, and 50 mg/mL | Decline in oxidative stress and inhibition of aldose reductase. | Thiraphatthanavong et al.,2014 |
| Zingiber officinale Roscoe (Rhizomes) | Powder | Reduction in the carbonyl stress, inhibition of osmotic stress by reduction in the activity of the polyol pathway, oxidative stress prevention. | Saraswat et al.,2010 |
Medicinal plants/natural products used against cataract on sugar-induced lens opacity/streptozotocin induced diabetic cataract/galactose, glucose and xylose induced/Zucker diabetic fatty (ZDF) aldose reductase rat models and possible mechanisms of action.
TABLE 4
| Plant (with part used)/natural product | Doses, concentrations and characteristics of extracts | Suggested/possible mechanism of action | References |
| Caesalpinia digyna Rottler (Roots) | Alcoholic extract at an increasing concentration between 0 and 200 μg/mL (IC 46.29 ± 11.17 μg/mL) | AR inhibition and antioxidant action. | Kumar et al.,2011 |
| Cinnamomum verum J.Presl (Bark) | Ethanolic extract fractions containing Procyanidin-B2, 1–3 mg | AGE inhibition of eye lens proteins under in vitro conditions and inhibition of the formation of glycosylated hemoglobin in human blood in ex vivo conditions. | Muthenna et al.,2013 |
| Cornus officinalis Siebold & Zucc. (Seeds) | EtOAc-soluble fraction (Galloyl glucoses) | Inhibition of formation of AGE, AGE- BSA cross-linking, and RLAR. | Lee et al.,2011 |
| Erigeron annuus (L.) Pers. (Leaves and stems) | 3,5-Di-O-caffeoyl-epi-quinic acid isolated from methanolic fraction, 5 μM | Inhibition of AGEs, AGEs-BSA cross-linking to collagen, RLAR formation, and prevention of lenses opacification. | Jang et al.,2010b |
| Flavonoids | Chrysin, apigenin, and baicalein | Inhibition of glycation, glycation induced lens opacity, AGEs, AR and lens protein aggregation. | Patil et al.,2016 |
| Hybanthus enneaspermus (L.) F.Muell. (Whole plant) | Different fractions from ethanolic extract, 0–300 μg/mL | Not clearly described. | Patel et al.,2012 |
| Magnolia biondii Pamp. [Syn. Magnolia fargesii (Finet & Gagnep.) W.C.Cheng] (Flower buds) | Isolated scopoletin and tiliroside | RLAR inhibition. | Lee et al.,2010 |
| Onoclea orientalis (Hook.) Hook. (Syn. Matteuccia orientalis Trev.) (Rhizomes) | Isolated compound Matteuorienate A, Matteuorienate B from the methanolic extract | AR inhibition. | Kadota et al.,1994 |
| Morinda citrifolia L. (Fruits) | Sequential water, ethanol, and chloroform extracts | AR inhibition and free radical scavenging activity. | Gacche and Dhole,2011b |
| Onoclea orientalis (Hook.) Hook. (Syn. Matteuccia orientalis Trev.) (Rhizomes) | Isolated compound from the methanolic extract Matteuorienate C | AR inhibition. | Basnet et al.,1995 |
| Platycodon grandiflorus (Jacq.) A.DC. (Flowers) | Isolated compounds from ethyl acetate soluble fractions [apigenin, luteolin, luteolin-7-O-β-D-glucopyranoside, luteolin-7-O-(6″-O-acetyl)-β-D-glucopyranoside, apigenin-7-O-β-D-glucopyranoside, apigenin-7-O-(6″-O-acetyl)-β-D-glucopyranoside, isorhamnetin-3-Oneohesperidoside, 4-O-caffeoylquinic acid, chlorogenic acid methyl ester, 4-O-β-D-glucopyranosyl caffeic acid] | Substantial inhibition of AGEs formation and RLAR. | Jang et al.,2010a |
| Salvia miltiorrhiza Bunge (Roots) | Constituents of methanolic extract Danshenol A Danshenol B, (-)-Danshexinkun A, Dihydrotanshinone I, Tanshinone IIA | AR inhibition. | Tezuka et al.,1997 |
Medicinal plants/natural products used against cataract on advanced glycation end products (AGE)- BSA cross-linking inhibition assay and lens aldose reductase activity models and possible mechanisms of action.
FIGURE 2
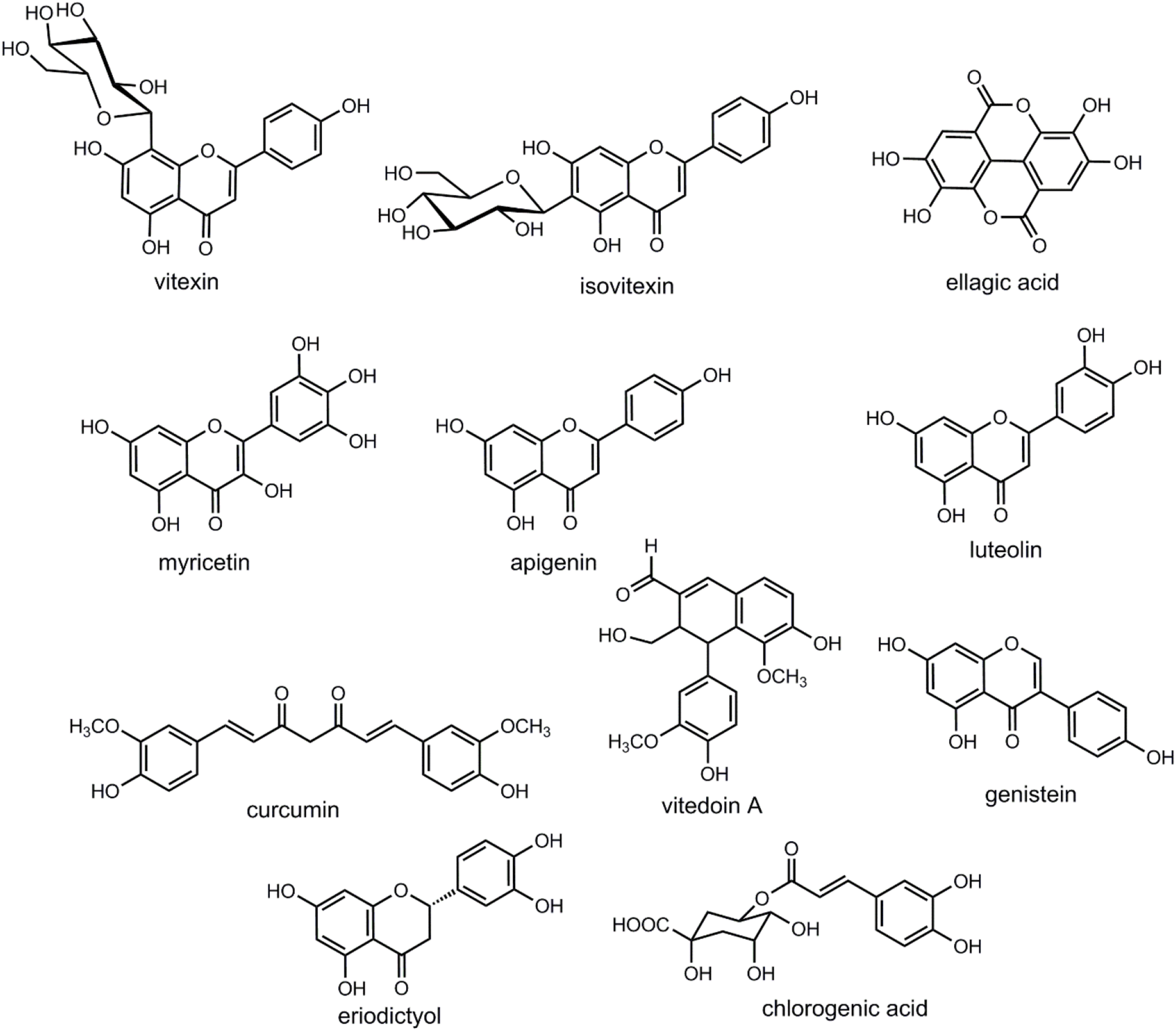
Chemical structures of some of the relevant natural products discussed in the context of cataract treatment.
TABLE 5
| Plant (with part used)/ natural product | Doses, concentrations and characteristics of extracts | Suggested/possible mechanism of action | References |
| Abies pindrow (Royle ex D.Don) Royle (Leaves) | Aqueous extract (5–20 mg/mL) | Inhibition of free radical generation. | Dubey et al.,2015b |
| Acorus calamus L. (Roots) | Methanolic extract and β-asarone | Significantly retarded experimental hydrogen peroxide induced cataractogenesis. | Kumar and Singh,2011 |
| Cistanche deserticola Y.C.Ma and SkQ1 (Stolons) | Fraction | Increase of the lens protein solubility and destroying of large protein aggregates, antioxidant action leads to elevation of tryptophan and kynurenine levels in the lens. | Snytnikova et al.,2012 |
| Elaeagnus rhamnoides (L.) A.Nelson (Syn. Hippophae rhamnoides L.) (Leaves) | Aqueous extract, 100–1,000 μg/mL | Regulation of oxidative stress and promotion of antioxidant systems. | Dubey et al.,2016 |
| Erythrina stricta Roxb. (Leaves) | Hydromethanolic extract (fractions), 200 mg/kg | Antioxidant activity, prevented the peroxidative damage caused by naphthalene. | Umamaheswari et al.,2010 |
| Foeniculum vulgare Mill. (Fruits) | Petroleum ether fraction, 10 mg/kg, twice daily | AR reduction and antioxidant action. | Dongare et al.,2012 |
| L-arginine | L-arginine | Blocking of carbonyl stress in the lens. | Fan et al.,2011 |
| Luffa cylindrica (L.) M.Roem. (Fruits) | Standardized extract, 5–30 μg/mL | Protection of lens proteins from oxidative damage. | Dubey et al.,2015a |
| Nigella sativa L. (Seeds) | Oil | Inhibiting of RNS generation, antioxidant action, and FRSA. | Taysi et al., 2015; Demir et al.,2016 |
| Ocimum tenuiflorum L. (Leaves) | Aqueous extract, 150 μg/mL | FRSA. | Halder et al.,2009 |
| Pueraria montana var. lobata (Will) Sanjappa & Pradeep [Syn. Pueraria lobata (Willd.) Ohwi] (Roots) | Puerariafuran isolated from methanolic extract | Inhibition of AR, xylose-induced lens opacity, and the oxidation in lenses. | Kim et al.,2010 |
| Vitis vinifera L. (Seed) | Extract constituting of 95% proanthocyanidins | Attenuates cell signaling, cell migration and inflammation, prevention of oxidative stress, inhibition of H2O2-induced phosphorylation of the p38 and c-Jun N-terminal kinase. | Jia et al.,2011 |
Medicinal plants/natural products used against cataract on hydrogen peroxide- and naphthalene induced cataract and other miscellaneous models and possible mechanisms of action.
Like in case of any other disease conditions, medicinal plants are being used in management of various eye ailments from ancient times. Medicinal plants are used in case of cataract, eye infections, conjunctivitis, eye dryness, and other eye disorders in many countries including India (Sandhu et al., 2011; Das et al., 2013; Rothe and Maheshwari, 2016), Bangladesh (Yusuf et al., 2006; Das et al., 2007, 2013), Nepal (Manandhar, 2002; Acharya and Pokhrel, 2006; Gewali, 2011; Watanabe et al., 2013), Sudan (Khalid et al., 2012), Tanzania (Maregesi et al., 2016), South Africa (Pendota et al., 2008), and many other regions of the world.
The literature analysis revealed that the sugar-induced or diabetic cataract models were the highest used models which were applied for the evaluation of around 39.84% of the plants/natural products. It was followed by selenite/sodium selenite induced cataract which is another common model of evaluation of cataract, and it accounts for around 36.71% of the plants/natural products. AGE-BSA crosslinking inhibition assay was used for the evaluation of 10.93%, and hydrogen peroxide and naphthalene induced cataract was account for evaluation of around 9.38% of the plants (Figure 3). In most of the cases especially for the diabetic cataract models, it was found that different antioxidant parameters like soluble protein, reduced glutathione, superoxide dismutase, lipid peroxidation were used (Bhadada et al., 2016b). Inhibition of AR was found as the most common hypothesis in these models (Bhadada et al., 2016b). Uses of in silico studies were also found common in some studies to explore the binding mode of the phytochemicals with the aldose reductase enzyme (Bhadada et al., 2016b; Patil et al., 2016).
FIGURE 3
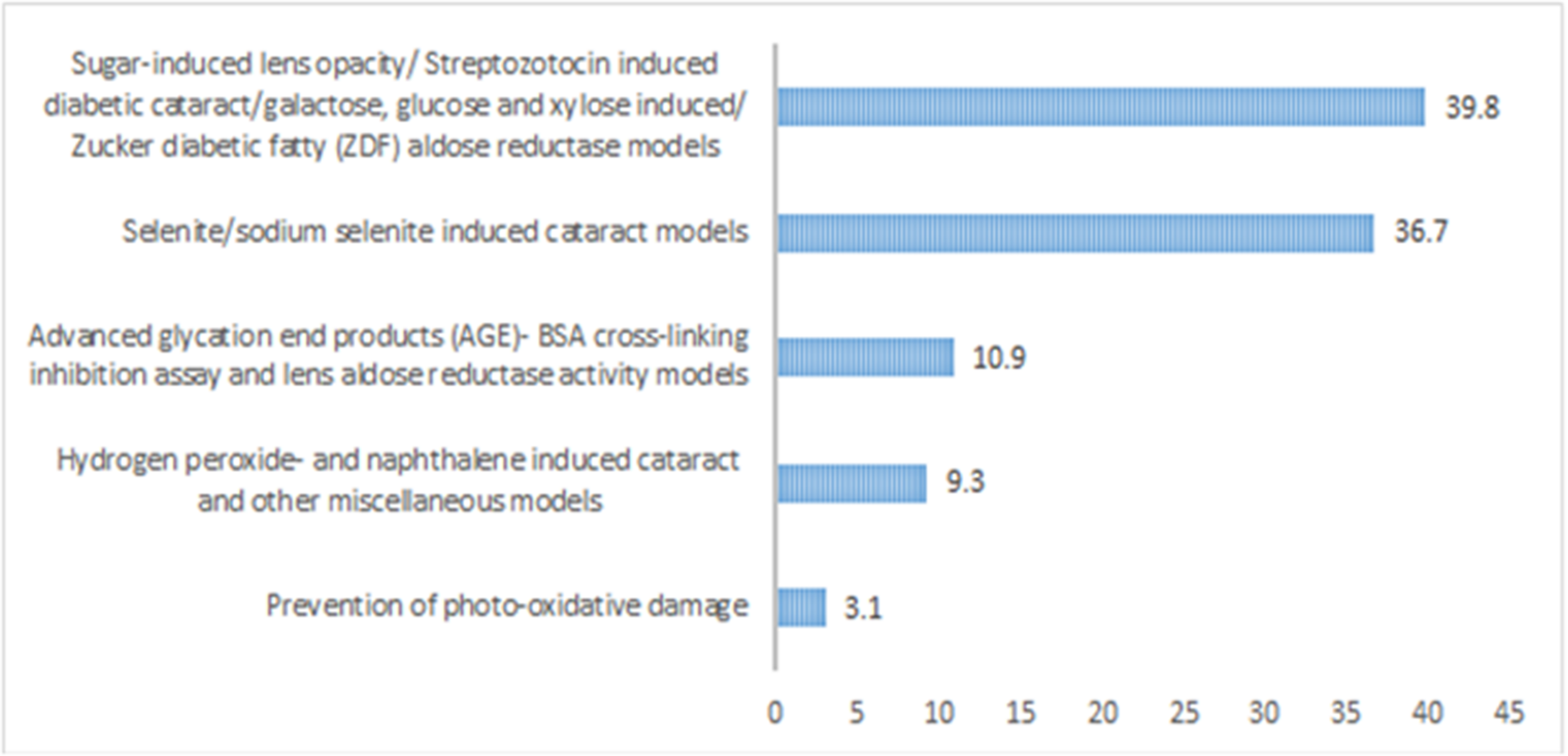
Percentage of different models used for evaluation of anticataract activity of plants/natural products.
In most of the studies, rats or rat pups lens were utilized as the model (Bhadada et al., 2016b; El Okda et al., 2016; Ferlemi et al., 2016; Sreelakshmi and Abraham, 2016) and in some cases fresh goat eyeballs were also used (Patil et al., 2016). In vitro studies were also utilized in large number of experiments. In some studies lens crystalline turbidity assay was used by estimation of lens protein turbidity using homogenized decapsulated porcine lenses which were procured from the local slaughterhouses in some cases (Ferlemi et al., 2016; Liao et al., 2016). Some other important factors in cataractogenesis like UV radiation was also used by researchers, and it was also proposed that some compounds can protect γ-crystallin from UV radiation damage and can act as potential anticataract agents (Liao et al., 2016).
Many of the mentioned plants showed potent anticataract activity in in vitro and in vivo models. Vitex negundo and Vitis vinifera were the plants in which sufficient preclinical studies were conducted and they may be of potential clinical use. It is also interesting that Vitex negundo was also used in the folk medicine in India (Kulkarni et al., 2008). The genus Ocimum was also one such genus which is utilized in folk medicine and was scientifically validated for its anticataract potential. Some other interesting findings were the use of Pleurotus ostreatus extract that prevented cataract in 75% of the tested rats (Isai et al., 2009). In a clinical study, although not directly against cataract, silybin improved the peripheral nerve conduction velocity and was reported as an effective aldose reductase inhibitor that can improve the disorder of polyol pathway in non-insulin dependent diabetes patients and prevent chronic complications of diabetes (Zhang et al., 1995) like cataract.
The detailed list of medicinal plants used in the management of cataract as reported in many ethnopharmacological surveys is given in Table 6. Singh et al. (2012) had also listed the medicinal plants used in management of cataract, however, the mechanistic insight was not performed and plants used in the management of cataract available till 2011 were covered (Singh et al., 2012).
TABLE 6
| Plant | Formulations and mode of administration | Major chemical constituents | Country | References |
| Abrus precatorius L. (Fabaceae) | Fresh leaves are squeezed and juice is used as eye drops | Abrine; trigonelline; abruslactone A; hemiphloin and abrusin | India/Tanzania | Ragasa et al., 2013; Garaniya and Bapodra, 2014; Maregesi et al.,2016 |
| Aloe vera (L.) Burm. f. (Asphodelaceae) | One drop of leaf juice twice a day is used as eye drop | Anthraquinones; aloe emodin and chrysophanol | Tanzania | Lee et al., 2013; Maregesi et al.,2017 |
| Barleria prionitis L. (Acanthaceae) | Leaf juice is used | Phenylethanoid glycoside; barlerinoside along with six known iridoid glycosides | Sri Lanka | Jaiswal et al., 2014; Rajamanoharan,2014 |
| Bidens pilosa L. (Asteraceae) | Juice of fresh leaves is used as eye drops. | Phenylheptatriyne; linoleic acid; linolenic acid; friedelin and friedelan-3 beta-ol | Tanzania | Geissberger and Sequin, 1991; Maregesi et al.,2016 |
| Boquila trifoliolata (DC.) Decne. (Lardizabalaceae) | Fresh leaves are squeezed and the juice is utilized as eye drops. | Oleanolic acid | Chile | Gusinde, 1936a, 1936b; Silva and Stück,1962 |
| Breynia vitis-idaea (Burm.f.) C.E.C.Fisch. (Syn. Breynia rhamnoides M. Arg.) (Euphorbiaceae) | Stem exudate is put in the eyes for 2–3 days in the morning | Breynin and breyniaionoside E | India | Parinitha et al., 2004; Meng et al.,2010 |
| Byttneria herbacea Roxb. (Malvaceae) | Root paste is used | Not described | India | Biswas et al.,2016 |
| Chusquea quila Kunth (Poaceae) | New stems are heated and the juice of the stem is received in a vessel and mixed with breast milk and Oxalis rosea | Holocellulose; lignin and α-Cellulose | Chile | Gusinde, 1936a, 1936b; Oliveira et al.,2016 |
| Citrus limon (L.) Osbeck (Rutaceae) | Salted lemon juice is used as eye drops. | Linalool; α-humulene; α-pinene and limonene | Tanzania | Golmakani and Moayyedi, 2015; Maregesi et al.,2017 |
| Coccinia grandis (L.) Voigt (Cucurbitaceae) | Juice of the stems is dripped into the eyes to treat cataract. | Cephalandrine A and cephalandrine B | Nepal | Gewali, 2011; Manandhar,2002 |
| Colocasia sp. (Araceae) | Leaves are cooked and eaten for 2–4 weeks or till curing. | Flavonoids; β-sitosterol and steroids | Bangladesh | Prajapati et al., 2011; Linky et al.,2015 |
| Commiphora edulis (Klotzsch) Engl. (Burseraceae) | Fresh latex which was produced on detachment of leaf is applied daily until recovery. | Not described | Tanzania | Maregesi et al.,2016 |
| Croton caudatus Geisel. (Euphorbiaceae) | Gum/sap is mixed with mustard oil and applied to eyes. | Bis(2,3-dihydroxypropyl) nonanedioate; 12-O- (α-methyl)butyrylphorbol-13-decanoate; 12-O- tiglylphorbol-13-decanoate; (9S,10R,11E,13R)- 9,10,13-trihydroxyoctadec-11-enoic acid; methyl (9S,10R,11E,13R)-9,10,13- trihydroxyoctadec-11-enoate; 4(1H)- quinolinone and 5-hydroxy-2-pyridinemethanol | Bangladesh | Linky et al., 2015; Su et al.,2016 |
| Croton bonplandianus Baill. (Euphorbiaceae) | Young stem juice is used as eye drops | Phorbol esters | India | Phillipson, 1995; Islam et al., 2010; Vashistha and Kaur,2013 |
| Diplolepis geminiflora (Decne.) Liede & Rapini (Apocynaceae) | The latex secreted when cutting a branch is applied over the eyes | Not described | Chile | Gusinde,1936a, b |
| Dolichos trilobus L. (Leguminosae) | Leaf juice is boiled, cooled and applied. | Doliroside A; phenols and tannins | Tanzania | Hedberg et al., 1983; Bisby,1994 |
| Duchesnea indica (Jacks.) Focke (Rosaceae) | Leaf juice is applied | Ellagitannins; ellagic acid glycosides; hydroxybenzoic acid; ellagic acid; hydroxycinnamic acid derivatives, and flavonols | India | Kapkoti et al., 2011; Zhu et al.,2015 |
| Eryngium paniculatum Cav. & Dombey ex F. Delaroche (Apiaceae) | Decoction of the root is put in the eyes | (E)-anethole; α-pinene; (-)-2,4,4-Trimethyl-3- formyl-2,5-cyclohexadienyl angelate | Chile | Gusinde, 1936a, 1936b; Cobos et al.,2002 |
| Erythrina indica Lam. (Papilionaceae) | Juice is put drop-wise in the affected eye | Lectin | India | Konozy et al., 2002; Parinitha et al., 2004; Biswas et al.,2016 |
| Euphorbia hirta L. (Euphorbiaceae) | Fresh latex obtained from detached leaves, three drops used for three times a day | Afzelin; quercitrin; myricitrin; rutin; quercetin; euphorbin-A; euphorbin-B; euphorbin-C and euphorbin-D | Tanzania | Kumar et al., 2010; Maregesi et al.,2016, 2017 |
| Fascicularia bicolor (Ruiz & Pav.) Mez (Bromeliaceae) | The juice of the young plant parts | Not described | Chile | Gusinde,1936a, b |
| Ficus benghalensis L. (Moraceae) | Milky juice is used | Alkaloids; glycosides, terpenoids; flavonoids; and tannins | Nepal | Acharya and Pokhrel, 2006; Ogunlowo et al.,2013 |
| Geranium core-core Steud. (Geraniaceae) | Powdered roots are placed on the eyes | Hexadecanoic acid; hexahydrofarnesyl acetone and tetracosane | Chile | Gusinde, 1936a, 1936b; Radulovic et al.,2011 |
| Ludwigia hyssopifolia (G.Don) Exell (Onagraceae) | Juice of this plant along with Ocimum americanum L.- Camphor type (2 drops thrice daily for 7–8 days) is given in the eye as a drop. | Piperine | Bangladesh | Yusuf et al., 2006; Das et al.,2007 |
| Marchantia polymorpha L. (Marchantiaceae) | Ointment of the crushed plant is prepared, applying it to the eyes | Polymorphatin A; Isorricardin D; 11,1′,13′- trihydroxyisorricardin; 2-[3-(hydroxymethyl)phenoxy]-3-[2-(4-hydroxyphenyl)ethyl]phenol; marchantin J and perrottetin E; 22-hydroxyhopane; 17(21)-hopene; 6α,22-dihydroxyhopane; 20α,22-dihydroxyhopane; 21,22-dihydroxyhopane; 6α, 11α, 22-trihydroxyhopane; 22,28-didroxyhopane; β-sitosterol and daucosterol | Chile | Gusinde, 1936a, 1936b; Fang et al.,2007, 2008 |
| Microglossa pyrifolia (Lam.) Kuntze (Asteraceae) | Root juice is used as eye drops | α-Humulene and α-pinene, Δ3-carene, (E)-β-ocimene and germacrene D | Tanzania | Hedberg et al., 1983; Boti et al.,2007 |
| Nepenthes khasiana Hook.f. (Nepenthaceae) | Not described | Droserone; 5-O-methyldroserone and naphthoquinones | India | Eilenberg et al., 2010; Dhal et al.,2011 |
| Nephrolepis biserrata (Sw.) Schott (Nephrolepidaceae) | Rhizome is scrubbed in the eyes | 1β,11α-Diacetoxy-11,12-epoxydrim-7-ene; 1β,6α,11α-triacetoxy-11,12-epoxydrim-7-ene; 1β,3β,11α-triacetoxy-11,12-epoxydrim-7-ene; 9(11)-fernene | Chile | Gusinde, 1936a, 1936b; Bottari et al., 1972; Siems et al.,1996 |
| Ocimum americanum L. (Lamiaceae) | Juice of O. americanum with Ludwigia hyssopifolia (two drops thrice daily for 7–8 days) is given in the eye as a drop | 1,8-Cineol; camphor; α-pinene and trans-α-bergamotene | Bangladesh | Yusuf et al., 2006; Bayala et al.,2014 |
| Oenothera acaulis Cav. (Onagraceae) | Stem juice is given in the eye as a drop. | Not described | Chile | Gusinde,1936a, b |
| Oxalis corniculata L. (Oxalidaceae) | Leaf juice is used. | Flavonoids; iso-vitexin; vitexin-2”-O-β–D-glucopyranoside; oleic acid; palmitic acid; linoleic acid; linolenic acid and stearic acid | India | Badwaik et al., 2011; Vashistha and Kaur,2013 |
| Oxalis rosea Jacq. (Oxalidaceae) | Plant material scrubbed in the eye. | Ascorbic acid; oxalic acid; dehydroascorbic acid; pyruvic acid and glyoxalic acid | Chile | Gusinde, 1936a, 1936b; Montes and Wilkomirsky, 1985; Das,1990 |
| Phyllanthus amarus Schum. &Thonn. (Phyllanthaceae) | Fresh leaves are squeezed and juice is utilized as eyes drops, 2–3 drops thrice daily for 7 days. | Amariin | Tanzania | Foo, 1993; Maregesi et al.,2016 |
| Ribes punctatum Ruiz & Pav. (Grossulariaceae) | Not described | Cyanidin-3-glucoside; cyanidin-3-rutinoside; delphinidin-3-rutinoside; delphinidin-3-glucoside; 3-caffeoylquinic acid; (epi)-gallocatechin and (epi)-catechin tetramers | Chile | Gusinde, 1936a, 1936b; Jiménez-Aspee et al.,2016 |
| Rumex usambarensis (Dammer) (Polygonaceae) | Aerial parts are squeezed and the juice is used as eye drops 2 times daily till recovery. | Chrysophanol, physcion, and emodin | Tanzania | Midiwo and Rukunga, 1985; Maregesi et al.,2016 |
| Stellaria media (L.) Vill. (Caryophyllaceae) | Aerial parts are scrubbed in the eyes | 2,4,5,7-tetramethyloctane; 6-methylheptyl-3′-hydroxy-2′-methylpropanoate; 2, 2,4-trimethyloctan-3-one; apigenin 6-C-β-D- galactopyranosyl-8-C-α-L-arabinopyranoside; apigenin 6-C-α-L- arabinopyranosyl-8-C-α-D-galactopyranoside | Chile | Gusinde, 1936a, 1936b; Hodisan and Sancraian, 1989; Kitanov, 1992; Pande et al., 1995; Hu et al., 2006; Vanhaecke et al., 2008; Sharma and Arora, 2012; Arora and Sharma,2014 |
| Solanum lycopersicum L. (Solanaceae) | Fresh leaves are squeezed and the juice is used as eye drops. | Adenosine | Tanzania | Fuentes et al., 2012; Maregesi et al.,2016 |
| Solanum virginianum L. (Solanaceae) | Seed is used | Arabinogalactan, glycosides | India | Pattanayak et al., 2012; Raja et al.,2014 |
| Swietenia macrophylla King (Meliaceae) | One drop of fresh latex produced from bark is used once daily | Swietemacrophyllanin; catechin and epicatechin | Tanzania | Falah et al., 2008; Maregesi et al.,2016 |
| Thunbergia grandiflora (Roxb. ex Rottl.) Roxb. (Acanthaceae) | Bubbles of 1–2 drops of the watery latex from the stem are blown gently into the affected eyes, 3 times a day for 4–5 days | Isounedoside and grandifloric acid | India | Ismail et al., 1996; Dipankar,2012 |
| Typha angustifolia L. (Typhaceae) | New stems are applied on the eye | Pentacosanoic acid; β-sitosterol; nonadecanol; naringenin; daucosterol; uracil typhaneoside; nicotinic acid; vanillic acid; succinic acid; thymine; stearic acid propanetriol ester | Chile | Gusinde, 1936a, 1936b; Jia et al., 1986; Liao et al., 1990; Chen et al., 2008; Varghese et al., 2009; Shukla et al.,2013 |
| Tridax procumbens (L.) L. (Asteraceae) | Leaf juice is dripped into the eyes to treat cataract | Procumbentin | Nepal | Manandhar,2002 |
| Vernonia amygdalina Delile (Asteraceae) | Fresh leaves are squeezed and the juice is used as eyes drops, 2–3 drops are used thrice daily for 7 days | Steroidal saponins; tannins; alkaloids; and flavonoids | Tanzania | Omoregie and Pal, 2016; Maregesi et al.,2017 |
| Vitex negundo L. (Lamiaceae) | NA | Vitedoin A; vitedoamine A; vitexdoin A; flavonoids; lignans; and terpenoids | India | Tewar et al., 2013; Shu et al.,2016 |
Medicinal plants reported globally by different ethnopharmacology/ethnobotanical surveys to be used in the treatment of cataract.
It was found that most of the surveys were conducted in different developing countries like Bangladesh, Chile, India, Nepal, and Tanzania (see Figure 4). Apart from the ethnobotanical surveys, several plants used in traditional medicine systems like Ayurveda were also found beneficial for cataract. One such good example of use of Ayurvedic formulation against cataract is the use of Triphala which showed good effect against cataract in in vitro and in vivo (Gupta et al., 2010a; Mahajan et al., 2011) studies and also was evaluated clinically and showed promising results (Bhati and Manjusha, 2015), however, more clinical studies are required involving larger patients for better scientific evidences. Plants used in Ayurveda like Momordica charantia, Eugenia jambolana, Pterocarpus marsupium, and Trigonella foenum-graecum prevented cataract development when observed in alloxan diabetic cataract model (Rathi et al., 2002; Vats et al., 2004).
FIGURE 4
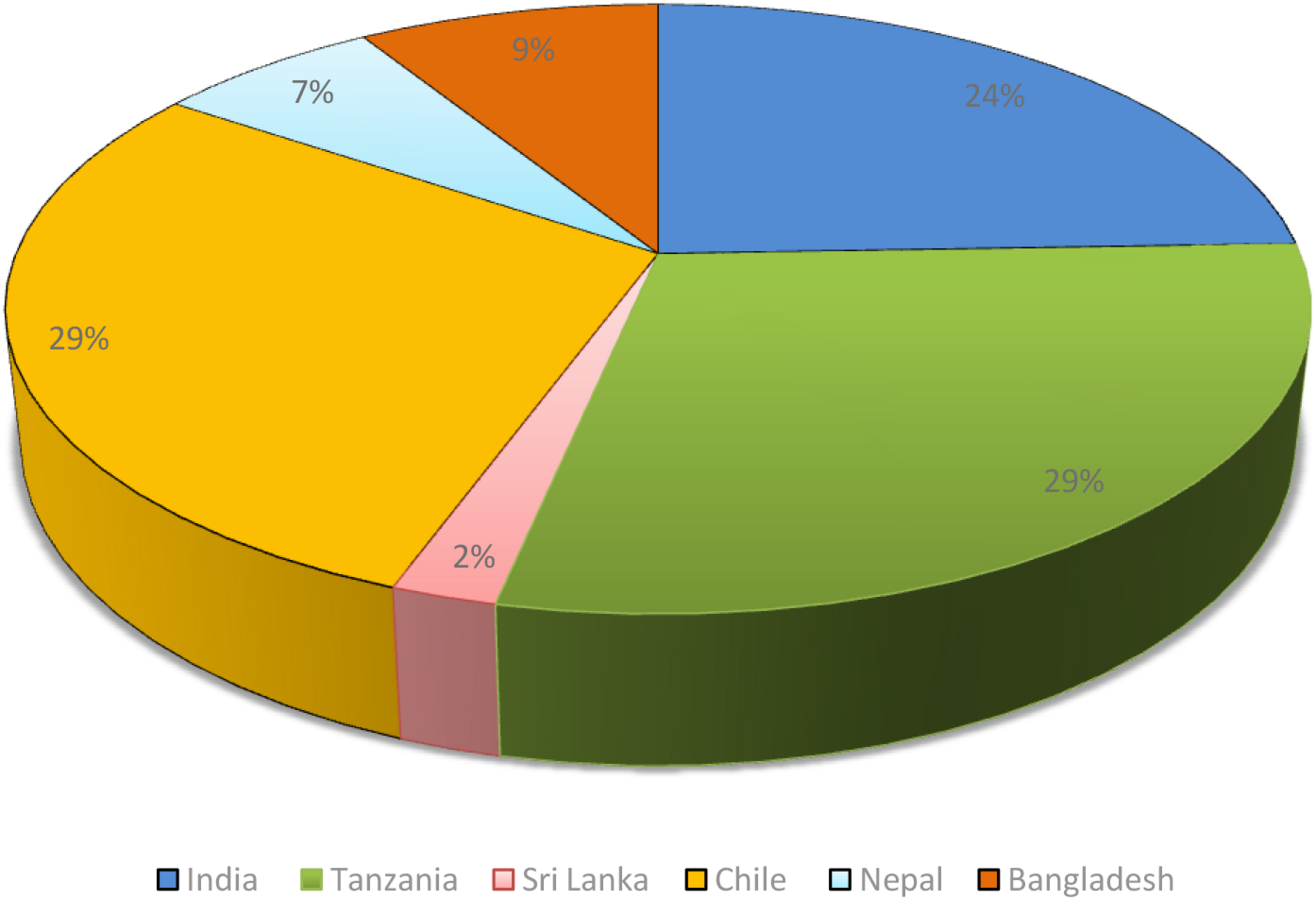
Distribution of plants used in folklore medicine.
Although, many plants have been utilized in various folklore medical practices, most of them are not scientifically validated. Moreover, some of these traditional practices may be harmful for the eyes as well. For instance, use of latex/sap of some Euphorbiaceae plants like Euphorbia hirta and Croton caudatus can be dangerous for eyes rather being beneficial. Moreover, sufficient care is obligatory while using the herbal medication for any of the eye diseases, as there is a case study that showed that cataract or development of cataract was aggravated after treatment with some unrevealed herbal medication in a 11 years old patient with atopic dermatitis (Kang et al., 2008).
This survey reveals that selenite/sodium selenite induced cataracts was the preferred model in studies with natural products used against cataract, followed by sugar-induced lens opacity/diabetes induced cataract models, AGE- BSA cross-linking inhibition assay, lens aldose reductase activity models, and hydrogen peroxide-induced cataract, and other miscellaneous models.
Diverse and sometimes complex phytochemicals present in numerous plants possess a broad spectrum of mechanisms for cataract treatment. One of the main mechanisms for the anticataract effect is the antioxidant effect (inhibitory effect on ROS formation). Several plants increase the activity of antioxidant enzymes as well. Some other important mechanisms involved are calpain inhibition, inhibition of lipid peroxidation, amelioration of calcium induced proteolysis, alteration in the protein profiles and the insolubilization of soluble proteins, attenuation in the inducible nitric oxide synthase expression, and AR inhibition.
For development of ocular drug delivery, various factors should be considered which affect the bioavailability of the ocular drugs. These factors include pH, structural forms of the drug, osmolarity, viscosity, tonicity, and the salt form of the drug (Goodman, 1996). Increasing scientific evidence clearly reveals that natural products have the potential to combat cataract at different levels but at the same time critical evaluation for the safety and toxicity profiles are required. Despite of potential evidence for the significance of natural products against cataract, the possible clinical trials are still lacking.
Conclusion
Ethnopharmacological evaluation of the medicinal plants used for cataract treatment could be a beneficial approach for the development of potential natural product-based therapies against cataract. In this work we have analyzed over 120 papers and found that around 44 medicinal plants/natural products are used for cataract management in different traditional and folk medicine systems. Possible mechanisms of around 118 plants/natural products (including repetition in different models) are also studied. It is interesting that most of the ethnobotanical survey studies are reported from many developing countries like Bangladesh, Chile, India, Nepal, and Tanzania. The medicinal plants are not only utilized in the folk medicine but many of the plants are also mentioned for cataract management in the traditional systems of medicine like in Ayurveda, traditional Chinese medicine and in Korean tradition medicine. However, it is still of utmost importance to document more comprehensively the plants utilized in the traditional medicine and rigorous preclinical and clinical studies are required to validate the use of such plants.
It was also notable that some combinations of plants were also used such as KIOM-79 which is a mixture of ethanol extract (80%) of parched Puerariae Radix, gingered Magnoliae Cortex, Glycyrrhizae Radix, and Euphorbiae Radix is used in Korean tradition medicine. Another such important combination formulation is ‘Triphala’ which is widely used Ayurvedic formulation in India containing fruits of Emblica officinalis Gaertn., Terminalia chebula Retz., and Terminalia belerica (Gaertn.) Roxb. However, many of the plants used in traditional medicines are not evaluated for their efficacy using rigorous scientific studies. Detailed mechanism-based in vitro and in vivo studies should be performed for the characterization of their possible pharmacological effects. On the other hand, evaluation of possible toxicity of these medicinal plants/natural products is also important as these medicines are directly applied in eyes and can have not just potential benefits but also harmful effects. Although there are numerous studies going on at preclinical level, clinical evidence for efficacy is still the need of the hour.
Statements
Author contributions
DT, OS, AM, DG, HD, JE, and AA drafted the initial manuscript. All authors improved, contributed, and agreed on the final version of the manuscript.
Acknowledgments
AM acknowledges the support by a grant of the Romanian Ministry of Research and Innovation, CNCS - UEFISCDI, project number PN-III-P1-1.1-PD-2016-1900, within PNCDI III. AA acknowledges the support by the Polish KNOW (Leading National Research Center) Scientific Consortium Healthy Animal—Safe Food, decision of Ministry of Science and Higher Education No. 05-1/KNOW2/2015. The authors would like to express their gratitude to Ms. Izabela Zene for her excellent technical help in designing Figure 1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- AGE
advanced glycation end products
- AKR1B1, aldo-keto reductase family 1
member B1
- AR
aldose reductase
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- Cx
connexin
- EPHA2: FRSA
free radical scavenging activity
- GPX
glutathione peroxidase
- GSH
glutathione
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LPO
lipid peroxides
- MAPKs
mitogen-activated protein kinase
- NADPH
nicotinamide adenine dinucleotide phosphate
- PKC
protein kinase C
- RLAR
rat lens aldose reductase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TGF-β2
transforming growth factor
- TNF-α
tumor necrosis factor-α
- UV
ultraviolet
- VEGF
vascular endothelial growth factor
- ZDF
zucker diabetic fatty.
References
1
AcharyaE.PokhrelB. (2006). Ethno-medicinal plants used by bantar of bhaudaha, Morang, Nepal.Our Nat.496–103.
2
AgrawalS. S.NaqviS.GuptaS. K.SrivastavaS. (2012). Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms.Food Chem. Toxicol.503126–3132. 10.1016/j.fct.2012.05.057
3
AjaniE. O.SalakoA. A.SharlieP. D.AkinleyeW. A.AdeoyeA. O.SalauB. A.et al (2009). Chemopreventive and remediation effect of Hydrocotyl bonariensis Comm. Ex Lam (Apiaceae) leave extract in galactose-induced cataract.J. Ethnopharmacol.123134–142. 10.1016/j.jep.2009.02.006
4
AlbertD. M.EdwardsD. D. (1996). The History of Ophthalmology.Cambridge: Blackwell Science.
5
AnithaT. S.AnnaduraiT.ThomasP. A.GeraldineP. (2011). Prevention of selenite-induced cataractogenesis by an ethanolic extract of Cineraria maritima: an experimental evaluation of the traditional eye medication.Biol. Trace Elem. Res.143425–436. 10.1007/s12011-010-8876-x
6
AnithaT. S.MuralidharanA. R.AnnaduraiT.JesudasanC. A. N.ThomasP. A.GeraldineP. (2013). Putative free radical-scavenging activity of an extract of Cineraria maritima in preventing selenite-induced cataractogenesis in Wistar rat pups.Mol. Vis.192551–2560.
7
AroraD.SharmaA. (2014). Isolation and characterization of the chemical constituents of Stellaria media Linn.Int. J. Pharm. Sci. Res.5:3669.
8
AshaR.Gayathri DeviV.AbrahamA. (2016). Lupeol, a pentacyclic triterpenoid isolated from Vernonia cinerea attenuate selenite induced cataract formation in sprague dawley rat pups.Chem. Biol. Interact.24520–29. 10.1016/j.cbi.2015.12.002
9
BadwaikH.SinghM. K.ThakurD.GiriT. K.TripathiD. K. (2011). The botany, chemistry, pharmacological and therapeutic application of Oxalis corniculata Linn-a review.Int. J. Phytomed.3:1.
10
BaiJ.ZhengY.DongL.CaiX.WangG.LiuP. (2015). Inhibition of p38 mitogen-activated protein kinase phosphorylation decreases H(2)O(2)-induced apoptosis in human lens epithelial cells.Graefes Arch. Clin. Exp. Ophthalmol.2531933–1940. 10.1007/s00417-015-3090-3093
11
BasnetP.KadotaS.HaseK.NambaT. (1995). Five new C-methyl flavonoids, the potent aldose reductase inhibitors from Matteuccia orientalis TREV.Chem. Pharm. Bull.431558–1564. 10.1248/cpb.43.1558
12
BayalaB.BassoleI. H. N.GnoulaC.NebieR.YonliA.MorelL.et al (2014). Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso.PLoS One9:e92122. 10.1371/journal.pone.0092122
13
BhadadaS. V.BhadadaV. J.GoyalR. K. (2016a). Preventive effect of Tephrosia purpurea on selenite-induced experimental cataract.Curr. Eye Res.41222–231. 10.3109/02713683.2015.1011281
14
BhadadaS. V.VyasV. K.GoyalR. K. (2016b). Protective effect of Tephrosia purpurea in diabetic cataract through aldose reductase inhibitory activity.Biomed. Pharmacother.83221–228. 10.1016/j.biopha.2016.05.018
15
BhatiH.ManjushaR. (2015). Clinical study on evaluation of anti-cataract effect of triphaladi ghana vati and elaneer kuzhambu anjana in timira (immature cataract).Ayu36283–289. 10.4103/0974-8520.182762
16
BijuP. G.DeviV. G.LijaY.AbrahamA. (2007). Protection against selenite cataract in rat lens by drevogenin D, a triterpenoid aglycone from Dregea volubilis.J. Med. Food10308–315. 10.1089/jmf.2006.054
17
BisbyF. (1994). Phytochemical Dictionary of the Leguminosae.Boca Raton, FL: CRC Press.
18
BiswasS.ShawR.BalaS.MazumdarA. (2016). Inventorization of some ayurvedic plants and their ethnomedicinal use in Kakrajhore forest area of West Bengal.J. Ethnopharmacol.197231–241. 10.1016/j.jep.2016.08.014
19
BotiJ. B.KoukouaG.N’GuessanT. Y.CasanovaJ. (2007). Chemical variability of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire.Flavour Fragr. J.2227–31. 10.1002/ffj.1743
20
BottariF.MarsiliA.MorelliI.PacchianiM. (1972). Aliphatic and triterpenoid hydrocarbons from ferns.Phytochemistry112519–2523.
21
CaoS.GaoM.WangN.LiuN.DuG.LuJ. (2015). Prevention of selenite-induced cataratogenesis by Ginkgo biloba extract (Egb761) in wistar rats.Curr. Eye Res.401028–1033. 10.3109/02713683.2014.980005
22
CetinelS.SemenchenkoV.ChoJ.-Y.SharafM. G.DamjiK. F.UnsworthL. D.et al (2017). UV-B induced fibrillization of crystallin protein mixtures.PLoS One12:e0177991. 10.1371/journal.pone.0177991
23
ChangD.ZhangX.RongS.ShaQ.LiuP.HanT.et al (2013). Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients.Oxid. Med. Cell. Longev.2013:587826. 10.1155/2013/587826
24
ChenP.YanH.ZhangL.DingA. (2008). Study on the constituents of Typha angustifolia L.Strait Pharm. J.12:33.
25
ChungY.-S.ChoiY.-H.LeeS.-J. A.ChoiS.LeeJ.KimH.et al (2005). Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo.J. Ethnopharmacol.10149–54.
26
CobosM. I.RodriguezJ. L.De PetreA.SpahnE.CasermeiroJ.LopezA. G.et al (2002). Composition of the essential oil of Eryngium paniculatum cav.J. Essent. Oil Res.1482–83.
27
DailamiK. N.AzadbakhtM.PharmZ. R.LashgariM. (2010). Prevention of selenite-induced cataractogenesis by Origanum vulgare extract.Pak. J. Biol. Sci.13743–747. 10.3923/pjbs.2010.743.747
28
DasB.KunduJ.BacharS. C.UddinM. A.KunduJ. K. (2007). Antitumor and antibacterial activity of ethylacetate extract of Ludwigia hyssopifolia Linn and its active principle piperine.Pak. J. Pharm. Sci.20128–131.
29
DasP. R.IslamM. T.MostafaM. N.RahmatullahM. (2013). Ethnomedicinal plants of the bauri tribal community of Moulvibazar district, Bangladesh.Anc. Sci. Life32:144. 10.4103/0257-7941.122997
30
DasS. (1990). Biochemical Studies of Different Species of Oxalis in Relation to their Medicinal Values.Ph.D. thesis, Department of Botany, Utkal University, Bhubaneswar.
31
DelcourtC.Cougnard-GrégoireA.BoniolM.CarrièreI.DoréJ.-F.DelyferM.-N.et al (2014). Lifetime exposure to ambient ultraviolet radiation and the risk for cataract extraction and age-related macular degeneration: the alienor study.Investig. Opthalmol. Vis. Sci.55:7619. 10.1167/iovs.14-14471
32
DemirE.TaysiS.AlB.DemirT.OkumusS.SaygiliO.et al (2016). The effects of Nigella sativa oil, thymoquinone, propolis, and caffeic acid phenethyl ester on radiation-induced cataract.Wien. Klin. Wochenschr.128587–595. 10.1007/s00508-015-0736-734
33
DhalY.SahuR. K.DeoB. (2011). Ethno medicinal survey of Koraput District, Odisha: an update.J. Pharm. Res.44142–4145.
34
DipankarD. (2012). Traditional ethno-medicinal plants use by the Darlong tribes in Tripura, Northeast India.Int. J. Ayurvedic Herb. Med.2954–966.
35
DoganayS.TurkozY.EverekliogluC.ErH.BozaranM.OzerolE. (2002). Use of caffeic acid phenethyl ester to prevent sodium-selenite-induced cataract in rat eyes.J. Cataract Refract. Surg.281457–1462.
36
DongareV.KulkarniC.KondawarM.MagdumC.HaldavnekarV.ArvindekarA. (2012). Inhibition of aldose reductase and anti-cataract action of trans-anethole isolated from Foeniculum vulgare Mill. fruits.Food Chem.132385–390. 10.1016/j.foodchem.2011.11.005
37
DubeyS.DeepP.SinghA. K. (2016). Phytochemical characterization and evaluation of anticataract potential of seabuckthorn leaf extract.Vet. Ophthalmol.19144–148. 10.1111/vop.12271
38
DubeyS.SahaS.KaithwasG.SarafS. A. (2015a). Effect of standardized fruit extract of Luffa cylindrica on oxidative stress markers in hydrogen peroxide induced cataract.Indian J. Pharmacol.47644–648. 10.4103/0253-7613.169586
39
DubeyS.SahaS.SarafS. A. (2015b). In vitro anti-cataract evaluation of standardised Abies pindrow leaf extract using isolated goat lenses.Nat. Prod. Res.291145–1148. 10.1080/14786419.2014.980250
40
Duke-ElderS. (1962). System of Ophthalmology Vol VII: The Foundations of Ophthalmology-Heredity, Pathology, Diagnosis and Therapeutics.Maryland Heights, MO: Mosby.
41
DurukanA. H.EverekliogluC.HurmericV.KerimogluH.ErdurmanC.BayraktarM. Z.et al (2006). Ingestion of IH636 grape seed proanthocyanidin extract to prevent selenite-induced oxidative stress in experimental cataract.J. Cataract Refract. Surg.321041–1045. 10.1016/j.jcrs.2006.02.041
42
EilenbergH.Pnini-CohenS.RahamimY.SionovE.SegalE.CarmeliS.et al (2010). Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana.J. Exp. Bot.61911–922. 10.1093/jxb/erp359
43
El OkdaE. A.MohamedM. M.ShaheedE. B.Abdel-MoeminA. R. (2016). Switching to instant black coffee modulates sodium selenite-induced cataract in rats.Ger. Med. Sci.14:Doc05. 10.3205/000232
44
ErtekinM. V.Koçerİ.Karslıoğluİ.TaysiS.GepdiremenA.SezenO.et al (2004). Effects of oral ginkgo biloba supplementation on cataract formation and oxidative stress occurring in lenses of rats exposed to total cranium radiotherapy.Jpn. J. Ophthalmol.48499–502. 10.1007/s10384-004-0101-z
45
FalahS.SuzukiT.KatayamaT. (2008). Chemical constituents from Swietenia macrophylla bark and their antioxidant activity.Pak. J. Biol. Sci.112007–2012.
46
Fallah HuseiniH.Zaree MahmodabadyA.HeshmatR.RazaM. (2009). The effect of Silybum marianum (L.) Gaertn. Seed extract (Silymarin) on galactose induced cataract formation in rats.J. Med. Plants17–12.
47
FanX.XiaoqinL.PottsB.StrauchC. M.NemetI.MonnierV. M. (2011). Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice.Mol. Vis.172221–2227.
48
FangH.HuX.WangM.WanW.YangQ.SunX.et al (2015). Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats.J. Ethnopharmacol.172238–246. 10.1016/j.jep.2015.06.034
49
FangL.GuoH.LouH. (2007). Three new bibenzyl derivatives from the chinese liverwort Marchantia polymorpha L.Helv. Chim. Acta90748–752.
50
FangL.NiuC.LouH. (2008). Studies on chemical constituents of Marchantia polymorpha L.Chin. Pharm. J.43:18.
51
FerlemiA.-V.MakriO. E.MermigkiP. G.LamariF. N.GeorgakopoulosC. D. (2016). Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: investigation of the effect on calpains, antioxidant and metal chelating properties.Exp. Eye Res.145258–268. 10.1016/j.exer.2016.01.012
52
FooL. Y. (1993). Amariin, a di-dehydrohexahydroxydiphenoyl hydrolysable tannin from Phyllanthus amarus.Phytochemistry33487–491.
53
FuentesE.CastroR.AstudilloL.CarrascoG.AlarconM.GutierrezM.et al (2012). Bioassay-guided isolation and HPLC determination of bioactive compound that relate to the antiplatelet activity (Adhesion, Secretion, and Aggregation) from Solanum lycopersicum.Evid. Based Compl. Alternat. Med.2012:147031. 10.1155/2012/147031
54
GaccheR. N.DholeN. A. (2011a). Aldose reductase inhibitory, anti-cataract and antioxidant potential of selected medicinal plants from the Marathwada region.India Nat. Prod. Res.25760–763. 10.1080/14786419.2010.536951
55
GaccheR. N.DholeN. A. (2011b). Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: an attempt to standardize the botanicals for amelioration of diabetes complications.Food Chem. Toxicol.491806–1813. 10.1016/j.fct.2011.04.032
56
GaraniyaN.BapodraA. (2014). Ethno botanical and Phytophrmacological potential of Abrus precatorius L.: a review.Asian Pac. J. Trop. Biomed.4S27–S34. 10.12980/APJTB.4.2014C1069
57
Gayathri DeviV.RoobanB. N.SasikalaV.SahasranamamV.AbrahamA. (2010). Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro.Toxicol. Vitr.241662–1669. 10.1016/j.tiv.2010.05.021
58
GbemisolaI. A.FaluyiJ. O.OsoniyiO. (2014). Evaluation of the effect of Spathodea campanulata flower bud exudate on cataractogenesis in rat lenses.Afr. J. Tradit. Compl. Altern. Med.1183–91.
59
GeissbergerP.SequinU. (1991). Constituents of Bidens pilosa L.: do the components found so far explain the use of this plant in traditional medicine?Acta Trop.48251–261.
60
GewaliM. B. (2011). Aspects of Traditional Medicine in Nepal.Oyama: University of Toyama.
61
GiblinF. J. (2000). Glutathione: a vital lens antioxidant.J. Ocul. Pharmacol. Ther.16121–135. 10.1089/jop.2000.16.121
62
GolmakaniM.MoayyediM. (2015). Comparison of heat and mass transfer of different microwave-assisted extraction methods of essential oil from Citrus limon (Lisbon variety) peel.Food Sci. Nutr.3506–518. 10.1002/fsn3.240
63
GoodmanL. S. (1996). Goodman and Gilman’s the Pharmacological Basis of Therapeutics.New York, NY: McGraw-Hill.
64
GuptaS. K.HalderN.SrivastavaS.TrivediD.JoshiS.VarmaS. D. (2002). Green tea (Camellia sinensis) protects against selenite-induced oxidative stress in experimental cataractogenesis.Ophthalmic Res.34258–263.
65
GuptaS. K.KalaiselvanV.SrivastavaS.AgrawalS. S.SaxenaR. (2010a). Evaluation of anticataract potential of Triphala in selenite-induced cataract: in vitro and in vivo studies.J. Ayurveda Integr. Med.1280–286. 10.4103/0975-9476.74425
66
GuptaS. K.KalaiselvanV.SrivastavaS.SaxenaR.AgrawalS. S. (2010b). Trigonella foenum-graecum (Fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis.Biol. Trace Elem. Res.136258–268. 10.1007/s12011-009-8540-8545
67
GusindeM. (1936a). Plantas medicinales que los indios Araucanos recomiendan.Anthropos31, 850–873.
68
GusindeM. (1936b). Plantas medicinales que los indios Araucanos recomiendan.Anthropos31, 555–571.
69
GuzmanA.GuerreroR. O. (2005). Inhibition of aldose reductase by herbs extracts and natural substances and their role in prevention of cataracts.Rev. Cuba. Plant Med.101–7.
70
GuzmánÁGuerreroR. O. (2005). Inhibition of aldose reductase by herbs extracts and natural substances and their role in prevention of cataracts.Rev. Cuba. Plantas Med.103–4.
71
HalderN.JoshiS.GuptaS. K. (2003). Lens aldose reductase inhibiting potential of some indigenous plants.J. Ethnopharmacol.86113–116. 10.1016/S0378-8741(03)00052-57
72
HalderN.JoshiS.NagT. C.TandonR.GuptaS. K. (2009). Ocimum sanctum extracts attenuate hydrogen peroxide induced cytotoxic ultrastructural changes in human lens epithelial cells.Phytother. Res.231734–1737. 10.1002/ptr.2831
73
HarikumarK. B.NimitaC. V.PreethiK. C.KuttanR.ShankaranarayanaM. L.DeshpandeJ. (2008). Toxicity profile of lutein and lutein ester isolated from marigold flowers (Tagetes erecta).Int. J. Toxicol.271–9. 10.1080/10915810701876265
74
HashimZ.ZarinaS. (2012). Osmotic stress induced oxidative damage: possible mechanism of cataract formation in diabetes.J. Diabetes Complications26275–279. 10.1016/j.jdiacomp.2012.04.005
75
HedbergI.HedbergO.MadatiP. J.MshigeniK. E.MshiuE. N.SamuelssonG. (1983). Inventory of plants used in traditional medicine in Tanzania. Part III. Plants of the families Papilionaceae-Vitaceae.J. Ethnopharmacol.9237–260.
76
HejtmancikJ. F.KantorowM. (2004). Molecular genetics of age-related cataract.Exp. Eye Res.793–9. 10.1016/j.exer.2004.03.014
77
HodisanV.SancraianA. (1989). Triterpenoid saponins from Stellaria media (L.) Cyr.Farmacia37:105.
78
HuY.-M.YeW.-C.LiQ.TianH.-Y.WangH.DuH.-Y. (2006). C-glycosylflavones from Stellaria media.Zhongguo Tianran Yaowu4420–424.
79
HuangR.ShiF.LeiT.SongY.HughesC. L.LiuG. (2007). Effect of the isoflavone genistein against galactose-induced cataracts in rats.Exp. Biol. Med.232118–125.
80
IsaiM.ElanchezhianR.SakthivelM.ChinnakkaruppanA.RajamohanM.JesudasanC. N.et al (2009). Anticataractogenic effect of an extract of the oyster mushroom, Pleurotus ostreatus, in an experimental animal model.Curr. Eye Res.34264–273. 10.1080/02713680902774069
81
IslamM. S.RahmanM. M.RahmanM. A.QayumM. A.AlamM. F. (2010). In vitro evaluation of Croton bonplandianum Baill. as potential antitumor properties using Agrobacterium tumefaciens.J. Agric. Technol.679–86.
82
IsmailL. D.el-AziziM. M.KhalifaT. I.StermitzF. R. (1996). Iridoid glycosides from Thunbergia grandiflora.Phytochemistry421223–1225.
83
JaiswalN.BhatiaV.SrivastavaS. P.SrivastavaA. K.TamrakarA. K. (2012). Antidiabetic effect of Eclipta alba associated with the inhibition of alpha-glucosidase and aldose reductase.Nat. Prod. Res.262363–2367. 10.1080/14786419.2012.662648
84
JaiswalS. K.DubeyM. K.DasS.RaoC. V. (2014). Gastroprotective effect of the iridoid fraction from Barleria prionitis leaves on experimentally-induced gastric ulceration.Chin. J. Nat. Med.12738–744. 10.1016/S1875-5364(14)60113-8
85
JangD. S.LeeY. M.JeongI. H.KimJ. S. (2010a). Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro.Arch. Pharm. Res.33875–880. 10.1007/s12272-010-0610-x
86
JangD. S.YooN. H.KimN. H.LeeY. M.KimC.-S.KimJ.et al (2010b). 3,5-Di-O-caffeoyl-epi-quinic acid from the leaves and stems of Erigeron annuus inhibits protein glycation, aldose reductase, and cataractogenesis.Biol. Pharm. Bull.33329–333.
87
JangD. S.YooN. H.LeeY. M.YooJ. L.KimY. S.KimJ. S. (2008). Constituents of the flowers of Erigeron annuus with inhibitory activity on the formation of advanced glycation end products (AGEs) and aldose reductase.Arch. Pharm. Res.31:900. 10.1007/s12272-001-1244-z
88
JavadzadehA.GhorbanihaghjoA.AramiS.RashtchizadehN.MesgariM.RafeeyM.et al (2009a). Prevention of selenite-induced cataractogenesis in wistar albino rats by aqueous extract of garlic.J. Ocul. Pharmacol. Ther.25395–400. 10.1089/jop.2009.0038
89
JavadzadehA.GhorbanihaghjoA.BonyadiS.RashidiM.MesgariM.RashtchizadehN.et al (2009b). Preventive effect of onion juice on selenite-induced experimental cataract.Indian J. Ophthalmol.57:185. 10.4103/0301-4738.49391
90
JiaS.-S.LiuY.-L.MaC.-M.YangS.-L.ZhouH.-M.ZhaoD.-C.et al (1986). Studies on the constituents of the flavonoids from the pollen of Typha angustfolia L.(Puhuang).Yao Xue Xue Bao21441–446.
91
JiaZ.SongZ.ZhaoY.WangX.LiuP. (2011). Grape seed proanthocyanidin extract protects human lens epithelial cells from oxidative stress via reducing NF-small ka, CyrillicB and MAPK protein expression.Mol. Vis.17210–217.
92
Jiménez-AspeeF.TheodulozC.VieiraM. N.Rodríguez-WernerM. A.SchmalfussE.WinterhalterP.et al (2016). Phenolics from the Patagonian currants Ribes spp: isolation, characterization and cytoprotective effect in human AGS cells.J. Funct. Foods2611–26.
93
KadotaS.BasnetP.HaseK.NambaT. (1994). Matteuorienate A and B, two new and potent aldose reductase inhibitors from Matteuccia orientalis (Hook.) Trev.Chem. Pharm. Bull.421712–1714.
94
KangK. D.KangS. M.YimH. B. (2008). Herbal medication aggravates cataract formation: a case report.J. Korean Med. Sci.23537–539. 10.3346/jkms.2008.23.3.537
95
KapkotiB.LodhiyalN.LodhiyalL. S. (2011). Ethno-medicinal plants and their uses by van panchayat people in nainital of kumaun region, Uttarakhand.Biolife2526–532.
96
KhairallahM.KahlounR.BourneR.LimburgH.FlaxmanS. R.JonasJ. B.et al (2015). Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010.Invest. Ophthalmol. Vis. Sci.566762–6769. 10.1167/iovs.15-17201
97
KhalidH.AbdallaW. E.AbdelgadirH.OpatzT.EfferthT. (2012). Gems from traditional north-African medicine: medicinal and aromatic plants from Sudan.Nat. Prod. Bioprospect.292–103. 10.1007/s13659-012-0015-2
98
KimC.-S.KimJ.LeeY. M.SohnE.JoK.KimJ. S. (2011a). Inhibitory effects of chlorogenic acid on aldose reductase activity in vitro and cataractogenesis in galactose-fed rats.Arch. Pharm. Res.34847–852. 10.1007/s12272-011-0519-z
99
KimJ.KimC.-S.SohnE.KimH.JeongI.-H.KimJ. S. (2011b). KIOM-79 prevents lens epithelial cell apoptosis and lens opacification in zucker diabetic fatty rats.Evid. Based Compl. Alternat. Med.2011:717921. 10.1155/2011/717921
100
KimJ.KimC.-S.SohnE.LeeY. M.KimJ. S. (2011c). KIOM-79 inhibits aldose reductase activity and cataractogenesis in Zucker diabetic fatty rats.J. Pharm. Pharmacol.631301–1308. 10.1111/j.2042-7158.2011.01341.x
101
KimC.-S.KimJ.-H.JeongI.-H.KimY.-S.LeeJ.JangD.-S.et al (2009). Slow development of diabetic cataract in streptozotocin-induced diabetic rats via inhibition of aldose reductase activity and sorbitol accumulation by use of Aster koraiensis extract.Korean J. Pharmacogn.40339–344.
102
KimN. H.KimY. S.LeeY. M.JangD. S.KimJ. S. (2010). Inhibition of aldose reductase and xylose-induced lens opacity by puerariafuran from the roots of Pueraria lobata.Biol. Pharm. Bull.331605–1609.
103
KitanovG. M. (1992). Phenolic acids and flavanoids from Stellaria media (L.) Vill.(caryophyllaceae).Pharmazie47470–471.
104
KonozyE. H. E.MulayR.FacaV.WardR. J.GreeneL. J.Roque-BarrieraM. C.et al (2002). Purification, some properties of a D-galactose-binding leaf lectin from Erythrina indica and further characterization of seed lectin.Biochimie841035–1043. 10.1016/S0300-9084(02)00003-2
105
KuboM.MatsudaH.TokuokaK.KobayashiY.TanakaT. (1994). Studies of anti-cataract drugs from natural sources. I. Effects of a methanolic extract and the alkaloidal components from Corydalis tuber on in vitro aldose reductase activity.Biol. Pharm. Bull.17458–459.
106
KulkarniR. R.VirkarA. D.D’melloP. (2008). Antioxidant and antiinflammatory activity of Vitex negundo.Indian J. Pharm. Sci.70838–840. 10.4103/0250-474X.49140
107
KumarD.SinghR. (2011). Anti cataract activity of Acorus calamus Linn. against hydrogen peroxide induced cataractogenesis in Goat eyes.Int. J. Pharm. Sci. Rev. Res.11112–115.
108
KumarR.PatelD. K.LalooD.SairamK.HemalathaS. (2011). Inhibitory effect of two Indian medicinal plants on aldose reductase of rat lens in vitro.Asian Pac. J. Trop. Med.4694–697. 10.1016/S1995-7645(11)60176-4
109
KumarS.MalhotraR.KumarD. (2010). Euphorbia hirta: its chemistry, traditional and medicinal uses, and pharmacological activities.Pharmacogn. Rev.458–61. 10.4103/0973-7847.65327
110
LeeJ.JangD. S.KimN. H.LeeY. M.KimJ.KimJ. S. (2011). Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo.Biol. Pharm. Bull.34443–446.
111
LeeJ.KimN. H.NamJ. W.LeeY. M.JangD. S.KimY. S.et al (2010). Scopoletin from the flower buds of Magnolia fargesii inhibits protein glycation, aldose reductase, and cataractogenesis ex vivo.Arch. Pharm. Res.331317–1323. 10.1007/s12272-010-0904-z
112
LeeY. S.JuH. K.KimY. J.LimT.-G.UddinM. R.KimY. B.et al (2013). Enhancement of anti-inflammatory activity of Aloe vera adventitious root extracts through the alteration of primary and secondary metabolites via salicylic acid elicitation.PLoS One8:e82479. 10.1371/journal.pone.0082479
113
LiX.LiuW.HuangX.XiongJ.WeiX. (2017). Interaction of AR and iNOS in lens epithelial cell: a new pathogenesis and potential therapeutic targets of diabetic cataract.Arch. Biochem. Biophys.61544–52. 10.1016/j.abb.2017.01.007
114
LiaoJ.-H.HuangY.-S.LinY.-C.HuangF.-Y.WuS.-H.WuT.-H. (2016). Anticataractogenesis mechanisms of curcumin and a comparison of its degradation products: an in vitro study.J. Agric. Food Chem.642080–2086. 10.1021/acs.jafc.6b00430
115
LiaoM.LiuY.XiaoP. (1990). Studies on the constituents of the flavonoids from the leaves of Typha angustifolia L.Acta Bot. Sin.32137–140.
116
LijaY.BijuP. G.ReeniA.CibinT. R.SahasranamamV.AbrahamA. (2006). Modulation of selenite cataract by the flavonoid fraction of Emilia sonchifolia in experimental animal models.Phytother. Res.201091–1095. 10.1002/ptr.2005
117
LinkyS. A.TonnyF. T.ZamanN.RahmanA.MimS. J.IslamE.et al (2015). Phytotherapy in Kishoreganj district, Bangladesh–Part I.World J. Pharm. Pharm. Sci.4274–283.
118
LuQ.YangT.ZhangM.DuL.LiuL.ZhangN.et al (2014). Preventative effects of Ginkgo biloba extract (EGb761) on high glucose-cultured opacity of rat lens.Phytother. Res.28767–773. 10.1002/ptr.5060
119
MahajanK. N.SinghaiA. K.VadnereG. P. (2011). Investigation on anticataract activity of Triphala ghrita.J. Chem.81438–1443.
120
MaiT. T.YamaguchiK.YamanakaM.LamN. T.OtsukaY.ChuyenN. V. (2010). Protective and anticataract effects of the aqueous extract of Cleistocalyx operculatus flower buds on beta-cells of streptozotocin-diabetic rats.J. Agric. Food Chem.584162–4168. 10.1021/jf904304w
121
MakriO. E.FerlemiA.-V.LamariF. N.GeorgakopoulosC. D. (2013). Saffron administration prevents selenite-induced cataractogenesis.Mol. Vis.191188–1197.
122
ManandharN. P. (2002). Plants and people of Nepal timber press.Oregon192526–527.
123
Mani SatyamS.Kurady BairyL.PirasanthanR.Lalit VaishnavR. (2014). Grape seed extract and zinc containing nutritional food supplement prevents onset and progression of age-related cataract in wistar rats.J. Nutr. Health Aging18524–530. 10.1007/s12603-014-0020-28
124
ManikandanR.ThiagarajanR.BeulajaS.ChindhuS.MariammalK.SudhandiranG.et al (2009). Anti-cataractogenic effect of curcumin and aminoguanidine against selenium-induced oxidative stress in the eye lens of wistar rat pups: an in vitro study using isolated lens.Chem. Biol. Interact.181202–209. 10.1016/j.cbi.2009.05.011
125
MaregesiS.KaukeB.KagasheG.KaaliR. (2016). Traditional eye medicines in tanzania: products, health risk awareness and safety evaluation.Herb. Med. Open Access2:1.
126
MaregesiS. M.MessoC. W.MathiasJ. (2017). Ethnomedical survey and safety evaluation of traditional eye medicines used in Misungwi district, Tanzania.J. Intercult. Ethnopharmacol.675–83. 10.5455/jice.20161116071244
127
MatsudaH.CaiH.KuboM.TosAH.IinumaM. (1995). Study on anti-cataract drugs from natural sources. II. Effects of buddlejae flos on in vitro aldose reductase activity.Biol. Pharm. Bull.18463–466.
128
MengD.-H.WuJ.WangL.-Y.ZhaoW.-M. (2010). Two new glycosides from Breynia vitis-idaea.J. Asian Nat. Prod. Res.12535–541. 10.1080/10286021003745452
129
MidiwoJ. O.RukungaG. M. (1985). Distribution of anthraquinone pigments in Rumex species of Kenya.Phytochemistry241390–1391.
130
MiyatakeK.KenshoG.FujimotoT.NoguchiE.ShinoharaM.TakenakaS.et al (1994). Effect of conduritol A, a polyol from gymnema sylvestre, on the development of diabetic cataracts in streptozotocin-treated rats and on aldose reductase.Biosci. Biotechnol. Biochem.58756–757.
131
MoellerS. M.JacquesP. F.BlumbergJ. B. (2000). The potential role of dietary xanthophylls in cataract and age-related macular degeneration.J. Am. Coll. Nutr.19522S–527S.
132
MontesM.WilkomirskyT. (1985). Medicina Popular Chilena.Chile: Universidad de Concepción, Concepción.
133
MorimitsuY.KubotaK.TashiroT.HashizumeE.KamiyaT.OsawaT. (2002). Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses.Int. Congr. Ser.1245503–508.
134
MorimitsuY.YoshidaK.EsakiS.HirotaA. (1995). Protein glycation inhibitors from thyme (Thymus vulgaris).Biosci. Biotechnol. Biochem.592018–2021.
135
MuthennaP.AkileshwariC.SaraswatM.ReddyG. B. (2012). Inhibition of advanced glycation end-product formation on eye lens protein by rutin.Br. J. Nutr.107941–949. 10.1017/S0007114511004077
136
MuthennaP.RaghuG.AkileshwariC.SinhaS. N.SuryanarayanaP.ReddyG. B. (2013). Inhibition of protein glycation by procyanidin-B2 enriched fraction of cinnamon: delay of diabetic cataract in rats.IUBMB Life65941–950. 10.1002/iub.1214
137
NairN. K.PatelK.GandhiT. (2010). Effect of aqueous extract of Embelica offcinalis on selenite induced cataract in rats.Iran. J. Pharm. Res.9147–152.
138
NakajimaT.ShearerT. R.AzumaM. (2014). Loss of calpastatin leads to activation of calpain in human lens epithelial cells.Invest. Ophthalmol. Vis. Sci.555278–5283. 10.1167/iovs.14-14778
139
ObrosovaI. G.ChungS. S. M.KadorP. F. (2010). Diabetic cataracts: mechanisms and management.Diabetes Metab. Res. Rev.26172–180. 10.1002/dmrr.1075
140
OgunlowoO. P.ArimahB. D.AdebayoM. A. (2013). Phytochemical analysis and comparison of in-vitro antimicrobial activities of the leaf, stem bark and root bark of Ficus benghalensis.IOSR J. Pharm.333–38.
141
OhiaS. E.OpereC. A.LedayA. M. (2005). Pharmacological consequences of oxidative stress in ocular tissues.Mutat. Res.57922–36. 10.1016/j.mrfmmm.2005.03.025
142
OkanoY.AsadaM.FujimotoA.OhtakeA.MurayamaK.HsiaoK.-J.et al (2001). A genetic factor for age-related cataract: identification and characterization of a novel galactokinase variant, “Osaka,” in asians.Am. J. Hum. Genet.681036–1042.
143
OliveiraP. E.CunhaA. G.ReyesG.GacitúaW.Petit-BreuilhX. (2016). Chusquea quila, a natural resource from Chile: its chemical, physical, and nanomechanical properties.BioResources1110057–10069.
144
OmoregieE. S.PalA. (2016). Antiplasmodial, antioxidant and immunomodulatory activities of ethanol extract of Vernonia amygdalina del. Leaf in Swiss mice.Avicenna J. Phytomed.6236–247.
145
OnkaramurthyM.VeerapurV. P.ThippeswamyB. S.ReddyT. N. M.RayappaH.BadamiS. (2013). Anti-diabetic and anti-cataract effects of Chromolaena odorata Linn., in streptozotocin-induced diabetic rats.J. Ethnopharmacol.145363–372. 10.1016/j.jep.2012.11.023
146
OsakabeN.YamagishiM.NatsumeM.YasudaA.OsawaT. (2004). Ingestion of proanthocyanidins derived from cacao inhibits diabetes-induced cataract formation in rats.Exp. Biol. Med.22933–39.
147
PanaskarS. N.JoglekarM. M.TaklikarS. S.HaldavnekarV. S.ArvindekarA. U. (2013). Aegle marmelos correa leaf extract prevents secondary complications in streptozotocin-induced diabetic rats and demonstration of limonene as a potent antiglycating agent.J. Pharm. Pharmacol.65884–894. 10.1111/jphp.12044
148
PandeA.ShuklaY. N.TripathiA. K. (1995). Lipid constituents from Stellaria media.Phytochemistry39709–711.
149
ParinithaM.HarishG. U.VivekN. C.MaheshT.ShivannaM. B. (2004). Ethno-botanical wealth of Bhadra wild life sanctuary in Karnataka.Indian J. Traditi. Knowl.337–50.
150
PatelD.KumarR.KumarM.SairamK.HemalathaS. (2012). Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn F. Muell.Asian Pac. J. Trop. Biomed.2134–139. 10.1016/S2221-1691(11)60207-4
151
PatelM. B.MishraS. M. (2009). Aldose reductase inhibitory activity of a C-glycosidic flavonoid derived from Enicostemma hyssopifolium.J. Compl. Integr. Med.6:5.
152
PatilK. K.MeshramR. J.DholeN. A.GaccheR. N. (2016). Role of dietary flavonoids in amelioration of sugar induced cataractogenesis.Arch. Biochem. Biophys.5931–11. 10.1016/j.abb.2016.01.015
153
PattanayakS.DuttaM. K.DebnathP. K.BandyopadhyayS. K.SahaB.MaityD. (2012). A study on ethno-medicinal use of some commonly available plants for wound healing and related activities in three southern districts of West Bengal, India.Explor. Anim. Med. Res.297–110.
154
PendotaS. C.GriersonD. S.AfolayanA. J. (2008). An ethnobotanical study of plants used for the treatment of eye infections in the Eastern Cape Province, South Africa.Pak. J. Biol. Sci.112051–2053.
155
PhillipsonJ. D. (1995). A matter of some sensitivity.Phytochemistry381319–1343.
156
PohlS.ZobelJ.MoffatA. (2010). “Extended boolean retrieval for systematic biomedical reviews,” in Proceedings of the Thirty-Third Australasian Conferenc on Computer Science - Volume 102 ACSC’10, (Darlinghurst, NSW: Australian Computer Society, Inc), 117–126.
157
PollreiszA.Schmidt-ErfurthU. (2010). Diabetic cataract—pathogenesis, epidemiology and treatment.J. Ophthalmol.2010:608751. 10.1155/2010/608751
158
PrajapatiR.KalariyaM.UmbarkarR.ParmarS.ShethN. (2011). Colocasia esculenta: a potent indigenous plant.Int. J. Nutr. Pharmacol. Neurol. Dis.1:90.
159
ProkofyevaE.WegenerA.ZrennerE. (2013). Cataract prevalence and prevention in Europe: a literature review.Acta Ophthalmol.91395–405. 10.1111/j.1755-3768.2012.02444.x
160
PuppalaM.PonderJ.SuryanarayanaP.ReddyG. B.PetrashJ. M.LaBarberaD. V. (2012). The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis.PLoS One7:e31399. 10.1371/journal.pone.0031399
161
RadulovicN.DekicM.Stojanovic-RadicZ.PalicR. (2011). Chemical composition and antimicrobial activity of the essential oils of Geranium columbinum L. and G. lucidum L.(Geraniaceae).Turk. J. Chem.35499–512.
162
RagasaC. Y.LorenaG. S.MandiaE. H.RagaD. D.ShenC.-C. (2013). Chemical constituents of Abrus precatorius.Am. J. Essent. Oils Nat. Prod.17–10.
163
RajaW.NosalovaG.GhoshK.SivovaV.NosalS.RayB. (2014). In vivo antitussive activity of a pectic arabinogalactan isolated from Solanum virginianum L. in guinea pigs.J. Ethnopharmacol.15641–46. 10.1016/j.jep.2014.08.012
164
RajalakshmiM.ElizaJ.PriyaC. E.NirmalaA.DaisyP. (2009). Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats.Afr. J. Pharm. Pharmacol.3171–180.
165
RajamanoharanP. R. (2014). An ethno botanical survey of medicinal plants in Sillalai, Jaffna, Northern Province, Sri Lanka.Int. J. Herb. Med.122–30.
166
RajkumarS.VasavadaA. R.PraveenM. R.AnanthanR.ReddyG. B.TripathiH.et al (2013). Exploration of molecular factors impairing superoxide dismutase isoforms activity in human senile cataractous lenses.Invest. Ophthalmol. Vis. Sci.546224–6233. 10.1167/iovs.13-11935
167
RajuT. N.KanthV. R.LavanyaK. (2008). Effect of methanolic extract of Allium sativum (AS) in delaying cataract in STZ-induced diabetic rats.J. Ocul. Biol. Dis. Infor.146–54. 10.1007/s12177-008-9003-5
168
RamanaK. V. (2011). ALDOSE REDUCTASE: new insights for an old enzyme.Biomol. Concepts2103–114. 10.1515/BMC.2011.002
169
RathiS. S.GroverJ. K.VikrantV.BiswasN. R. (2002). Prevention of experimental diabetic cataract by indian ayurvedic plant extracts.Phytother. Res.16774–777. 10.1002/ptr.1064
170
ReddyV. S.KumarC. U.ReddyG. B. (2014). Effect of chronic hyperglycemia on crystallin levels in rat lens.Biochem. Biophys. Res. Commun.446602–607. 10.1016/j.bbrc.2014.03.012
171
RoobanB. N.LijaY.BijuP. G.SasikalaV.SahasranamamV.AbrahamA. (2009). Vitex negundo attenuates calpain activation and cataractogenesis in selenite models.Exp. Eye Res.88575–582. 10.1016/j.exer.2008.11.020
172
RoobanB. N.SasikalaV.DeviV. G.SahasranamamV.AbrahamA. (2012). Prevention of selenite induced oxidative stress and cataractogenesis by luteolin isolated from Vitex negundo.Chem. Biol. Interact.19630–38. 10.1016/j.cbi.2012.01.005
173
RoobanB. N.SasikalaV.SahasranamamV.AbrahamA. (2010). Vitex negundo modulates selenite-induced opacification and cataractogensis in rat pups.Biol. Trace Elem. Res.138282–292. 10.1007/s12011-010-8633-8631
174
RoobanB. N.SasikalaV.SahasranamamV.AbrahamA. (2011). Analysis on the alterations of lens proteins by Vitex negundo in selenite cataract models.Mol. Vis.171239–1248.
175
RotheS. P.MaheshwariA. A. (2016). Traditional herbal medicine for ophthalmic diseases used by tribals in melghat region.Sciences675–78.
176
SachdevM. (2017). Cataract surgery: the journey thus far.Indian J. Ophthalmol.651273–1274. 10.4103/ijo.IJO-1098-17
177
Sai VarshaM. K. N.RamanT.ManikandanR. (2014). Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis.Exp. Eye Res.12873–82. 10.1016/j.exer.2014.09.007
178
SakthivelM.ElanchezhianR.RameshE.IsaiM.JesudasanC. N.ThomasP. A.et al (2008). Prevention of selenite-induced cataractogenesis in wistar rats by the polyphenol, ellagic acid.Exp. Eye Res.86251–259.
179
SandhuP. S.SinghB.GuptaV.BansalP.KumarD. (2011). Potential herbs used in ocular diseases.J. Pharm. Sci. Res.31127–1140.
180
SankeshiV.KumarP. A.NaikR. R.SridharG.KumarM. P.GopalV. V. H.et al (2013). Inhibition of aldose reductase by Aegle marmelos and its protective role in diabetic cataract.J. Ethnopharmacol.149215–221. 10.1016/j.jep.2013.06.025
181
SaraswatM.SuryanarayanaP.ReddyP. Y.PatilM. A.BalakrishnaN.ReddyG. B. (2010). Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats.Mol. Vis.161525–1537.
182
SasikalaV.RoobanB. N.PriyaS. G. S.SahasranamamV.AbrahamA. (2010). Moringa oleifera prevents selenite-induced cataractogenesis in rat pups.J. Ocul. Pharmacol. Ther.26441–447. 10.1089/jop.2010.0049
183
SasikalaV.RoobanB. N.SahasranamamV.AbrahamA. (2013). Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of alpha-crystallin.Graefes Arch. Clin. Exp. Ophthalmol.2511747–1755. 10.1007/s00417-013-2281-z
184
SharmaA.AroraD. (2012). Phytochemical and pharmacological potential of genus Stellaria: a review.J Pharm. Res.53591–3596.
185
ShielsA.HejtmancikJ. F. (2015). Molecular genetics of cataract.Prog. Mol. Biol. Transl. Sci.134203–218. 10.1016/bs.pmbts.2015.05.004
186
ShinK. H.ChungM. S.ChoT. S. (1994). Effects of Furanocoumarins from Angelica dahurica on aldose reductase and galactosemic cataract formation in rats.Arch. Pharm. Res.17331–336.
187
ShinK. H.LimS. S.KimD. K. (1998). Effect of byakangelicin, an aldose reductase inhibitor, on galactosemic cataracts, the polyol contents and Na+, K+ATPase activity in sciatic nerves of streptozotocin-induced diabetic rats.Phytomedicine5121–127. 10.1016/S0944-7113(98)80008-80001
188
ShuZ.LiX.RahmanK.QinL.ZhengC. (2016). Chemical fingerprint and quantitative analysis for the quality evaluation of Vitex negundo seeds by reversed-phase high-performance liquid chromatography coupled with hierarchical clustering analysis.J. Sep. Sci.39279–286. 10.1002/jssc.201500796
189
ShuklaR.SrivastavaS.DwivediP. K.SarkarS.GuptaS.MishraA. (2013). Preliminary pharmacognostical and phytochemical investigations on the various part of Typha angustifolia.Int. J. Biol. Phamr. Res.419–22.
190
SiemsK.WeigtF.WollenweberE. (1996). Drimanes from the epicuticular wax of the fern Nephrolepis biserrata.Phytochemistry411119–1121.
191
SilvaM.StückR. (1962). On the occurrence of oleanolic acid in Boquilla trifoliolata (DC) Dcne.Arch. Pharm. Ber. Dtsch. Pharm. Ges.29558–59.
192
SinghB.KumarD.SinghR. (2012). Phytotherapeutics for management and prevention of cataract generation.Phytopharmacology393–110.
193
SnowA.ShiehB.ChangK.-C.PalA.LenhartP.AmmarD.et al (2015). Aldose reductase expression as a risk factor for cataract.Chem. Biol. Interact.234247–253. 10.1016/j.cbi.2014.12.017
194
SnytnikovaO. A.TsentalovichY. P.StefanovaN. A.FursovaA. Z.KapteinR.SagdeevR. Z.et al (2012). The therapeutic effect of mitochondria-targeted antioxidant SkQ1 and Cistanche deserticola is associated with increased levels of tryptophan and kynurenine in the rat lens.Dokl. Biochem. Biophys.447300–303. 10.1134/S1607672912060087
195
SöderbergP. G.TalebizadehN.YuZ.GalichaninK. (2016). Does infrared or ultraviolet light damage the lens?Eye30241–246. 10.1038/eye.2015.266
196
SongE.SunH.XuY.MaY.ZhuH.PanC.-W. (2014). Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis.PLoS One9:e112054. 10.1371/journal.pone.0112054
197
SreelakshmiV.AbrahamA. (2016). Cassia tora leaves modulates selenite cataract by enhancing antioxidant status and preventing cytoskeletal protein loss in lenses of sprague dawley rat pups.J. Ethnopharmacol.178137–143. 10.1016/j.jep.2015.12.012
198
SuH.-G.YangH.MengC.-W.PengC.GuoL.DaiO. (2016). Study on chemical constituents of seeds of Croton tiglium and their cytotoxicities.Zhongguo Zhong Yao Za Zhi413620–3623. 10.4268/cjcmm20161920
199
SunkireddyP.JhaS. N.KanwarJ. R.YadavS. C. (2013). Natural antioxidant biomolecules promises future nanomedicine based therapy for cataract.Coll. Surfaces B Biointerfaces112554–562. 10.1016/j.colsurfb.2013.07.068
200
TaylorH. R. (2016). The global issue of vision loss and what we can do about it: José Rizal Medal 2015.Asia Pac. J. Ophthalmol.595–96. 10.1097/APO.0000000000000188
201
TaysiS.AbdulrahmanZ. K.OkumusS.DemirE.DemirT.AkanM.et al (2015). The radioprotective effect of Nigella sativa on nitrosative stress in lens tissue in radiation-induced cataract in rat.Cutan. Ocul. Toxicol.34101–106. 10.3109/15569527.2014.910802
202
TewarR. C.KotechaM.SharmaA. K.SharmaP. (2013). Ethno-medicinal heritage of chandi devi hill’s of Haridwar, Uttarakhand.Int. J. Innov. Res. Dev.2233–241.
203
TewariD.MocanA.ParvanovE. D.SahA. N.NabaviS. M.HuminieckiL.et al (2017). Ethnopharmacological approaches for therapy of jaundice: Part I.Front. Pharmacol.8:518. 10.3389/fphar.2017.00518
204
TewariD.StankiewiczA. M.MocanA.SahA. N.TzvetkovN. T.HuminieckiL.et al (2018). Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs.Front. Aging Neurosci.10:3. 10.3389/fnagi.2018.00003
205
TezukaY.KasimuR.BasnetP.NambaT.KadotaS. (1997). Aldose reductase inhibitory constituents of the root of Salvia miltiorhiza Bunge.Chem. Pharm. Bull.451306–1311.
206
ThiagarajanR.ManikandanR. (2013). Antioxidants and cataract.Free Radic. Res.47337–345. 10.3109/10715762.2013.777155
207
ThiraphatthanavongP.WattanathornJ.MuchimapuraS.WipaweeT.WannanonP.TerdthaiT.et al (2014). Preventive effect of Zea mays L. (purple waxy corn) on experimental diabetic cataract.Biomed. Res. Int.2014:507435. 10.1155/2014/507435
208
TohT.MortonJ.CoxonJ.ElderM. J. (2007). Medical treatment of cataract.Clin. Exp. Ophthalmol.35664–671. 10.1111/j.1442-9071.2007.01559.x
209
UhrB. W. (2003). History of ophthalmology at baylor university medical center.Proc. Bayl. Univ. Med. Cent.16435–438.
210
UmamaheswariM.AsokkumarK.LalithaV.SivashanmugamT.SubhadradeviV. (2011). Anticataract and antioxidant activities of Citrus aurantium L. Peel extract against naphthalene induced cataractogenesis in rats.J. Pharm. Res.4680–682.
211
UmamaheswariM.AsokkumarK.SivashanmugamT.SubhadradeviV.NeethuM. (2010). Anticataract activity of Erythrina stricta against naphthalene-induced cataractogenesis in rats.Bangladesh J. Pharmacol.577–81.
212
ValavalaV. K.VangipurapuR. K.BanamV. R.PulukurthiU. M. A.ReddyM.TurlapatiN. R. (2011). Effect of mustard (Brassica juncea) leaf extract on streptozotocin-induced diabetic cataract in wistar rats.J. Food Biochem.35109–124.
213
VanhaeckeM.Van den EndeW.LescrinierE.DyubankovaN. (2008). Isolation and characterization of a pentasaccharide from Stellaria media.J. Nat. Prod.711833–1836. 10.1021/np800274k
214
VargheseA.GavaniU.AbrahamS.BgtP.AshaJ. (2009). Phytochemical screening and antimicrobial investigation of Typha angustifolia Linn.Int. J. Chem. Sci.71905–1910.
215
VarmaS. D.ChandD.SharmaY. R.KuckJ. F. J.RichardsR. D. (1984). Oxidative stress on lens and cataract formation: role of light and oxygen.Curr. Eye Res.335–57.
216
VashisthaB. D.KaurM. (2013). Floristic and ethno botanical survey of Ambala District, Haryana.Int. J. Pharm. Biol. Sci.4353–360.
217
VatsV.YadavS. P.BiswasN. R.GroverJ. K. (2004). Anti-cataract activity of Pterocarpus marsupium bark and Trigonella foenum-graecum seeds extract in alloxan diabetic rats.J. Ethnopharmacol.93289–294. 10.1016/j.jep.2004.03.032
218
VibinM.Siva PriyaS. G.N RoobanB.SasikalaV.SahasranamamV.AbrahamA. (2010). Broccoli regulates protein alterations and cataractogenesis in selenite models.Curr. Eye Res.3599–107. 10.3109/02713680903428991
219
WangT.ZhangP.ZhaoC.ZhangY.LiuH.HuL.et al (2011). Prevention effect in selenite-induced cataract in vivo and antioxidative effects in vitro of Crataegus pinnatifida leaves.Biol. Trace Elem. Res.142106–116. 10.1007/s12011-010-8752-8758
220
WangX.SunJ.DangG. F.GaoY.DuanL.WuX. Y. (2015). Antioxidant content and cytological examination of aqueous fluid from patients with age-related cataracts at different stages.Genet. Mol. Res.146251–6255. 10.4238/2015.June.9.11
221
WatanabeT.RajbhandariK. R.MallaK. J.DevkotaH. P.YaharaS. (2013). A Handbook of Medicinal Plants of Nepal Supplement I.Kanagawa: Ayurseed Life Environmental Institute, 50–51.
222
WuJ.LiX.WanW.YangQ.MaW.ChenD.et al (2017). Gigantol from Dendrobium chrysotoxum Lindl. Binds and inhibits aldose reductase gene to exert its anti-cataract activity: an in vitro mechanistic study.J. Ethnopharmacol.198255–261. 10.1016/j.jep.2017.01.026
223
WuT.-H.LiaoJ.-H.HouW.-C.HuangF.-Y.MaherT. J.HuC.-C. (2006). Astaxanthin protects against oxidative stress and calcium-induced porcine lens protein degradation.J. Agric. Food Chem.542418–2423.
224
YamakoshiJ.SaitoM.KataokaS.TokutakeS. (2002). Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats.J. Agric. Food Chem.504983–4988. 10.1021/jf0201632
225
YounH.-Y.McCannaD. J.SivakJ. G.JonesL. W. (2011). In vitro ultraviolet-induced damage in human corneal, lens, and retinal pigment epithelial cells.Mol. Vis.17237–246.
226
YusufM.WahabM. A.ChowdhuryJ. U.BegumJ. (2006). Ethno-medico-botanical knowledge from Kaukhali proper and Betbunia of Rangamati District.Bangladesh J. Plant Taxon.1355–61.
227
ZhangJ.MaoX.ZhouY. (1995). Silybin decreases erythrocytic sorbitol level and improves peripheral nerve conduction velocity in patients with non-insulin dependent diabetes mellitus.Chin. J. Integr. Tradit. West. Med.184–86.
228
ZhangX.HuY. (2012). Inhibitory effects of grape seed proanthocyanidin extract on selenite-induced cataract formation and possible mechanism.J. Huazhong Univ. Sci. Technol. Med. Sci.32613–619. 10.1007/s11596-012-1006-6
229
ZhuM.DongX.GuoM. (2015). Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS.Molecules2022463–22475. 10.3390/molecules201219859
Summary
Keywords
medicinal plants, natural products, cataract, antioxidant, aldose reductase, lens opacity, MAPK
Citation
Tewari D, Samoilă O, Gocan D, Mocan A, Moldovan C, Devkota HP, Atanasov AG, Zengin G, Echeverría J, Vodnar D, Szabo B and Crişan G (2019) Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 10:466. doi: 10.3389/fphar.2019.00466
Received
16 August 2018
Accepted
12 April 2019
Published
13 June 2019
Volume
10 - 2019
Edited by
Marco Leonti, University of Cagliari, Italy
Reviewed by
Dezső Csupor, University of Szeged, Hungary; Guillermo Benítez, University of Granada, Spain
Updates
Copyright
© 2019 Tewari, Samoilă, Gocan, Mocan, Moldovan, Devkota, Atanasov, Zengin, Echeverría, Vodnar, Szabo and Crişan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrei Mocan, mocan.andrei@umfcluj.ro Atanas G. Atanasov, a.atanasov.mailbox@gmail.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Ethnopharmacology, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.