- 1Institute of Clinical Pharmacology, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Pharmacology and Toxicology, University of Kansas, Lawrence, KS, United States
- 3School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
Hypoxia-inducible factor-1 (HIF-1)–induced angiogenesis has been involved in numerous pathological conditions, and it may be harmful or beneficial depending on the types of diseases. Exploration on angiogenesis has sparked hopes in providing novel therapeutic approaches on multiple diseases with high mortality rates, such as cancer and ischemic stroke. The HIF-1 pathway is considered to be a major regulator of angiogenesis. HIF-1 seems to be involved in the vascular formation process by synergistic correlations with other proangiogenic factors in cancer and cerebrovascular disease. The regulation of HIF-1–dependent angiogenesis is related to the modulation of HIF-1 bioactivity by regulating HIF-1α transcription or protein translation, HIF-1α DNA binding, HIF-1α and HIF-1α dimerization, and HIF-1 degradation. Traditional Chinese herbal medicines have a long history of clinical use in both cancer and stroke treatments in Asia. Growing evidence has demonstrated potential proangiogenic benefits of Chinese herbal medicines in ischemic stroke, whereas tumor angiogenesis could be inhibited by the active components in Chinese herbal medicines. The objective of this review is to provide comprehensive insight on the effects of Chinese herbal medicines on angiogenesis by regulating HIF-1 pathways in both cancer and ischemic stroke.
Introduction
Angiogenesis is the formation and remodeling of new blood vessels and capillaries from the existing vasculature through interaction among cellular matrix, cytokines, and proteases. It plays a pivotal role in diffusion exchange of metabolites and nutrients in all the tissues and organs of the human body (Shi, 2009; Kusumbe et al., 2014), occurring throughout our lives in both diseased and healthy states. Changes in metabolism result in proportional changes in angiogenesis and, therefore, proportional changes in capillarity. Oxygen is crucial for this process. Hypoxia occurs when there is reduced oxygen supply and/or increased oxygen demand. It is the principal physiological stimulus for inducing angiogenesis, which provides a stimulus-response pathway that tries to maintain adequate oxygenation in pathological status, such as tumor growth and ischemic stroke (Mengozzi et al., 2012; Brown, 2016). There has been great interest during the past decades in regulating angiogenesis as a therapeutic target for cancer and ischemic stroke. The current clinical application based on the principle of angiogenesis includes antiangiogenic therapy and proangiogenic therapy. Antiangiogenic therapy has been used for cancer treatment, which inhibits the delivery of oxygen and nutrients to cancer cells. On the other hand, proangiogenic therapies in ischemic stroke could be beneficial by increasing blood flow. Hypoxia-inducible factor-1 (HIF-1), a regulator of essential adaptive responses to hypoxia-induced angiogenesis, is highly expressed under hypoxic conditions, such as aggressive tumors and ischemic brains (Sendoel et al., 2010; Berlow et al., 2017). HIF-1 has been suggested to be an important target in treating cancer and ischemic stroke by regulating the transcriptional activity of its downstream genes. The activity and accumulation of HIF-1α protein were found to be regulated at different levels, such as regulating HIF-1α synthesis stability or transactivation throughout its life cycle inside the cells (Yeom et al., 2011; Soleymani Abyaneh et al., 2017).
Traditional Chinese herbal medicine has a long history of clinical use in both cancer and stroke treatments in Asia. Chinese herbal medicines often use a variety of herbs in different complex combinations to enhance their therapeutic effects or reduce their toxicity. Growing evidence has demonstrated potential proangiogenic benefits of Chinese herbal medicines in ischemic stroke whereas tumor angiogenesis could be inhibited by the active components in herbal medicines (Hong et al., 2015; Gandin et al., 2016; Hong et al., 2016; Guo et al., 2018). Thus, the objective of this review is to provide comprehensive insight on how Chinese herbal medicines impact angiogenesis by regulating HIF-1 pathways in both cancer and ischemic stroke. In this study, we tried to give a systematic and timely update about the effects and mechanisms of several Chinese herbal medicines targeting HIF-1 pathways in cancer or ischemic stroke, such as Xue-Fu-Zhu-Yu decoction, ginsenosides, Pien Tze Huang, Yi Ai Fang, baicalein, and curcumin. Their mechanisms of antiangiogenesis or proangiogenesis behaviors, potential toxicity, or side effects and future research directions were discussed.
Method
Both clinical trials and basic research on Chinese herbal medicines that target the HIF-1 pathway were included to assess their efficacy and underlying mechanisms. One Chinese database (China Journals Full-Text Database) and four English databases (AMED, MEDLINE, EMBASE, and The CENTRAL) were applied in our study to retrieve more recent publications on this topic. Chinese herbal medicines and their active compounds for ischemic stroke or cancer treatment will be included in this review paper if more than two research papers have described the in vitro and in vivo studies of the particular subject or of any paper describing clinical trials on the subject.
Hypoxia-Induced Angiogenesis
Hypoxia is the nonphysiological exposure to low oxygen tension of cells or tissues, which is associated with various pathological events, such as stroke, inflammation, and cancer. These pathological events induce the restoration of oxygen homeostasis by activating repair mechanisms such as angiogenesis. Hypoxia-induced angiogenesis includes several steps (Figure 1). 1) Exposure to low oxygen tension upregulates the expression of proangiogenic growth factors that activate their receptors (Sendoel et al., 2010; Berlow et al., 2017). 2) Vascular permeability increases in response to vascular endothelial growth factor (VEGF), thereby inducing the exudation of plasma proteins that form a primitive scaffold for migrating endothelial cells. Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) exhibit antagonistic properties during the development of the vessel. Ang-1 is critical for vessel maturation, adhesion, migration, and survival, whereas Ang-2 is involved in vessel destabilization and promoting cell death. Yet, when it is in conjunction with VEGFs, Ang-2 can promote neovascularization (Jain and Carmeliet, 2012). The matrix metalloproteinases (MMPs) such as MMP2 and MMP9 can further induce angiogenesis by degrading matrix components (Ota et al., 2009; Kang et al., 2012). 3) Proliferative endothelial cells assemble and form a lumen by migrating to a distant location (Nieuwenhuis et al., 2017). In this stage, several proteins can promote endothelial cell survival, adhesion, and migration, such as VE-cadherin and integrins αβ. After new vessels are formed, pericytes and smooth muscle cells will stabilize the walls and prevent leakage by surrounding the novel capillaries. Other factors including Ang-1 and platelet-derived growth factor receptor (PDGFR) also take part in the maturation of novel capillaries (Rivera and Bergers, 2014).
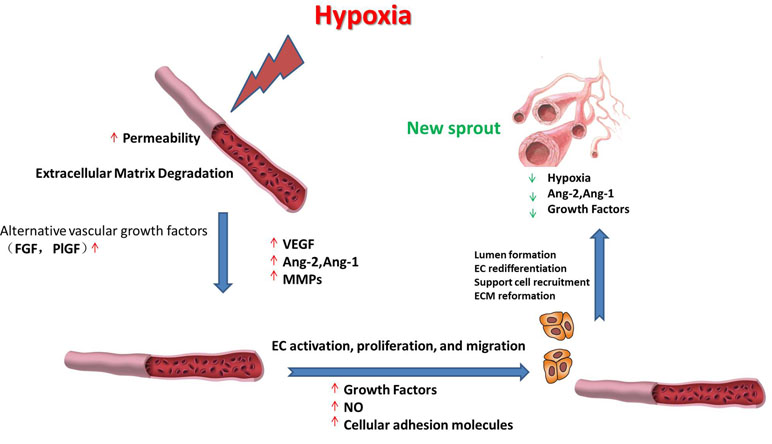
Figure 1 Schematic representation of the roles of vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), matrix metalloproteinases (MMPs), and various growth factors during hypoxia-induced angiogenesis. The processes include upregulating the expression of proangiogenic factors; the synergistic effects of VEGF, Ang-1, and Ang-2 on angiogenesis; degrading the matrix components; new vessel formation; and stabilization, as described in detail in the text.
Hypoxia-induced angiogenesis shows significant differences in signal pathways compared with physiological angiogenesis. For example, physiological angiogenesis in embryonic development requires activating the VEGF pathway, whereas hypoxia-induced angiogenesis such as tumor angiogenesis can also induce angiogenesis by recruiting myeloid cells and upregulate alternative vascular growth factors in addition to VEGF, such as fibroblast growth factor (FGF) and placental growth factor (PlGF). Although postischemic tissue revascularization is crucial for recovery in brain tissues after ischemic stroke (Li Q. et al., 2018) or in the heart after myocardial infarction (Chen R. et al., 2018), the activation of angiogenesis is harmful in disorders such as macular degeneration and cancer (Pio et al., 2013). Therefore, there is great interest in regulating angiogenesis as a possible therapeutic method for different kinds of diseases. Elucidating the molecular mechanism of hypoxia-induced angiogenesis will help in the identification of potential therapeutic targets and improve therapeutic effects.
Hypoxia-Inducible Factor-1
Changes in oxygen supply represent a pivotal physiological stimulus for all eukaryotic cells that require adequate oxygen consumption for intracellular metabolic reactions. In addition to its contribution to the maintenance of intracellular bioenergetics by producing mitochondrial ATP, O2 also serves as a universal electron acceptor in various biochemical pathways. Therefore, genes involved in responding to hypoxia are highly conserved during evolution. HIF-1 is an oxygen-dependent transcriptional activator, which is composed of HIF-1α, the alpha subunit, and the aryl hydrocarbon receptor nuclear translocator (Arnt), the beta subunit. Both subunits belong to the bHLH-PAS (Per/Arnt/Sim) family. HIF-1 is induced in hypoxic cells and binds to the cis-acting hypoxia response element (HRE) of the human EPO gene, which is required for erythropoietin synthesis (Aldo and Elisabetta, 2018; Zhu and Zhang, 2018). Intracellular oxygen concentration levels can affect the subcellular localization and protein activity of the HIF-1α subunit, whereas the expression of HIF-1β is not regulated by the oxygen level (Wang et al., 2018). The HIF-1α and HIF-1β subunits are similar in structure, and both contain two PAS domains. The bHLH and PAS domains are critical for the heterodimer formation of HIF-1α and HIF-1β and for DNA binding. The HIF-1α subunit contains N-terminal transactivation domains (TAD-N) and C-terminal transactivation domains (TAD-C) concatenated by an inhibitory domain (Figure 2). The TAD-N is continuous with protein stability that overlaps with the oxygen-dependent degradation (ODD) domain. The TAD-C is independent of protein stability that interacts with p300/CBP and is critical for transcription activity. The HIF-1α protein is unstable (half-life = 5 min) and is modified by various posttranscriptional regulations, including phosphorylation, hydroxylation, ubiquitination, acetylation, and nitrosation. Factor inhibiting HIF-1 (FIH-1) hydroxylates asparagine-803 of HIF-1α within the TAD-C under normoxic conditions, which inhibits the interaction of HIF-1α with transcriptional coactivators. The molecular mechanisms of the pivotal role of HIF-1 in the regulation of angiogenesis have been revealed in recent years. Recent studies have demonstrated that HIF-1 activity in human tissues can induce angiogenesis in the following ways: 1) by activating the transcription of various angiogenic genes or their receptors such as ANGPT1, ANGPT2, VEGF, PlGF, and PDGFB (Chen et al., 2017); 2) by modulating proangiogenic chemokines and receptors (SDF-1α, sphingosine-1-phosphate, stromal cell–derived factor 1α, receptor CXCR4, sphingosine-1-phosphate receptors, and C-X-C chemokine receptor type 4), thus promoting the recruitment of endothelial progenitor cells to the hypoxic site (Soni and Padwad, 2017); and 3) by facilitating cell cycle progression and DNA replication in endothelial cells (Toth and Warfel, 2017). Through the phosphoinositide 3-kinase (PI3K) or Ras/MAPK pathway, several growth factors and their cognate receptors can influence cellular responses to hypoxia and regulate the expression of HIF-1α. Previous studies have shown that inhibition of PI3K pathway downregulates both basal and mitogen-induced HIF-1α expression (Cheng et al., 2018). In general, the modifications of HIF-1 are rapidly and precisely regulated according to the cellular oxygen concentration by multiple signaling. The hypoxia-induced angiogenesis is a highly complex and orchestrated process in human disease. HIF-1 was found to be a major modulator of hypoxia-induced angiogenesis by synergistic correlations with various proangiogenic factors and regulates many genes that play important roles in angiogenesis (Table 1). Thus, HIF-1 modulation could offer therapeutic benefits for various hypoxia pathologies, including diseases with high mortality and morbidity rates, such as cancer and ischemic stroke.
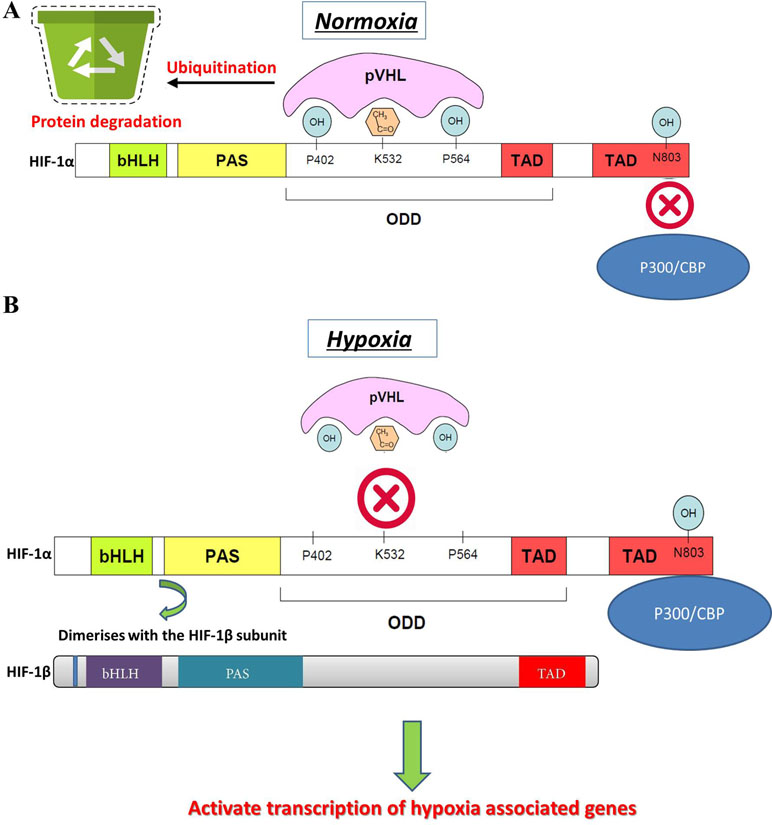
Figure 2 HIF-1α gene structure, stability, and activation. (A) Normal oxygen level induces the degradation of HIF-1α by hydroxylation or acetylation-mediated VHL binding and also transcriptional activity of HIF-1α. (B) Under hypoxic conditions, VHL is not prolyl-hydroxylated and cannot bind to HIF-1α protein, which leads to a decreased rate of HIF-1α degradation. Hypoxia promotes the interaction of HIF-1α within CBP/p300 and induces dimerization of HIF-1α with HIF-1β, which results in HIF-1 transcription factor formation. The active HIF-1 will further bind to HREs and activate the transcription of downstream genes.
Hypoxia-Induced Angiogenesis in Cancer and the Role of Hif-1
Because of the expansive growth activities within malignant tumor, cancer cells are highly metabolic. However, the poorly vascularized original tissue structure leads to inadequate oxygen supply for tumor progression. Hypoxia is commonly observed in the microenvironment of cancer, which arises in cancer via the uncontrolled proliferation driven by the oncogene of cancer cells in the absence of an efficient vascular bed. As a result of rapid cell proliferation, the cancer cell quickly exhausts the oxygen supply and nutrient from the normal vasculature, which leads to hypoxia. In previous studies, the relationship between hypoxia and tumor progression has been proven by O2-sensitive microsensors (Semenza, 2003; Bohonowych et al., 2011). Clinical studies have shown that patients with hypoxic cervical tumors, head and neck cancer, and sarcoma of soft tissue may have worse disease-free survival than that of patients with normally aerated tumors. The inadequate oxygen supply at the tumor tissue may induce tumor progression through selective pressure by the mutation of cancer suppressor genes, which may reduce tumor cells’ apoptotic capacity and promote tumor growth. Another key characteristic of the hypoxic response in tumor is the modulation of multiple genes that promote angiogenesis to fortify oxygen supply (Zagzag et al., 2000).
Cancer growth and metastasis depend on lymphangiogenesis and neovascularization triggered by hypoxia signals from cancer cells. Cancer cells under hypoxic conditions will upregulate the expression of PDGF, Ang-2, stromal-derived factor 1 (SDF-1), and VEGF, which are crucial in endothelial cell activation and promoting neoangiogenesis. Activated HIF-1 plays a crucial role in hypoxia-adaptive responses of the tumor cells through transcriptional activation of these proangiogenesis genes. As shown in previous studies, HIF-1 can mediate acute hypoxia-induced VEGF expression in neuroblastoma, whereas HIF-2 modulates VEGF expression during prolonged hypoxia (Maxwell et al., 1999). Furthermore, VEGF expression under hypoxia may increase the activity of other proangiogenic factors and their receptors; thus, vessel outgrowth was stimulated through multiple factors. This so-called “angiogenic switching” induces tumor angiogenesis and stimulates tumor growth by supplying nutrients and oxygen by newly formed vessels (Singh et al., 2017). During the cellular adaptation to hypoxic stress, PI3K/AKT/mTOR and MAPK signaling pathways are involved in hypoxia-induced tumor angiogenesis by various growth factors that bind to toll-like receptors (TLRs), alarmin receptors, receptor tyrosine kinases, and G protein–coupled receptors on cell surface, which may also activate HIF-1 (De Francesco et al., 2018). The mitogen-activated protein kinase (MAPK) and PI3K pathways are activated by the combination of growth factor with its cognate receptor tyrosine kinase. PI3K promotes the activation of the downstream mammalian target of rapamycin (mTOR) and serine/threonine kinase AKT. mTOR further induces p70 S6 kinase (S6K) and its substrate phosphorylation then induces HIF-1α protein synthesis. In the MAPK pathway, the extracellular signal-regulated kinase (ERK) is activated by the upstream signal cascade (RAS/RAF/MEK). Activated ERK promotes the phosphorylation of eukaryotic translation initiation factor 4E (eIF-4E) binding protein (4E-BP1) and MAP kinase interacting kinase (MNK). MNK can also phosphorylate eukaryotic translation initiation factor 4E (eIF-4E) directly. Then, the HIF-1α mRNA translation is activated (Rius et al., 2008; Ban et al., 2017; Aldo and Elisabetta, 2018). Key cellular responses to the hypoxic tumor microenvironment triggered by HIF-1 and its downstream targets increase the vascular formation, cancer invasiveness, and resistance to treatment (Liu H. et al., 2018).
Hypoxia-induced tumor angiogenesis is stimulated and regulated by both activator and inhibitor molecules. However, simple upregulation of the activity of proangiogenesis factors is not sufficient for neovascularization of the tumor. Negative regulators or endogenous inhibitors of vessel growth also need to be downregulated, such as the thrombospondin-1 and thrombospondin-2. In recent years, various anticancer agents have been developed by targeting these angiogenic activator or inhibitor molecules in malignant tumor. A number of antiangiogenesis drugs have been approved by the U.S. Food and Drug Administration (FDA) for treating progressive cancer. So far, most of these drugs are molecular targeted agents that were developed specifically to target VEGF or its receptors, such as bevacizumab (Avastin) and vandetanib (Caprelsa) (Li et al., 2018a). During the last two decades, interest in the role of HIF-1 in tumor angiogenesis has grown exponentially since its identification and molecular characterization in human cancer. Much progress has been made recently about the cellular and molecular mechanism of HIF-1 and its involvement in cancer growth and metastasis based on the analysis of experimental animal models and human cancer biopsies.
In brief, activation of HIF-1 in cancer cells is one of the key masters orchestrating their adaptation mechanism to the hypoxic conditions. Considering the pivotal roles of HIF-1 in tumor angiogenesis, there has been great interest in developing novel anticancer agents inhibiting the related pathway. As we know, HIF-1 modulation in cancer cells is a complex network including various signal cascades and overlapping mechanisms, each of which might act as a potential target to selectively intervene cancer.
Chinese Herbal Medicines Mediate Antiangiogenic Factors by Regulating Hif-1 Pathways in Cancer Treatment
The use of Chinese herbal medicines to treat cancer dates back centuries in ancient traditional folklore in China and Asian countries (Qin et al., 2018; Oyenihi and Smith, 2019). Many herbal extracts and herbal soups have been reported that could relieve clinical symptoms, improve quality of life, and reduce side effects in cancer therapy (Dong et al., 2010; Xu et al., 2014; Tian et al., 2010). In view of the importance of HIF-1 in tumor angiogenesis, the development of herbal medicine inhibitors for this pathway has attracted wide interest. It is clear that the regulation of HIF-1 is a highly complex network cascade and overlapping mechanisms involving multiple targets and signaling pathways, such as HIF-1α mRNA expression, HIF-1α protein expression, and HIF-1 transcriptional activity. As shown in Figures 2 and 3, we have concluded that Chinese herbal medicines can regulate HIF-1 by targeting different targets that exert antiangiogenic effects in cancer therapy.
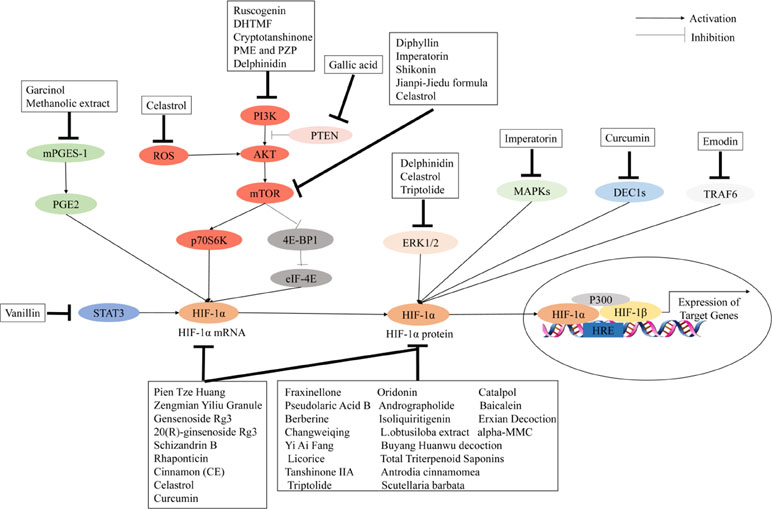
Figure 3 Chinese herbal medicines inhibit the activation of the HIF-1 pathway in cancer treatment through different targets.
Inhibitors of HIF-1α mRNA and/or Protein Expression
Numerous herbal medicines that inhibit HIF-1α mRNA and/or protein expression have significant antiangiogenic effects. Berberine, the main active ingredient isolated from Coptis chinensis, has been shown to decrease the expression of HIF-1α and VEGF in esophageal cancer, hepatocellular carcinoma, prostate cancer, nasopharyngeal carcinoma, and lung cancer (Fu et al., 2013; Yang et al., 2013; Tsang et al., 2015; Zhang et al., 2014a; Zhang C. et al., 2014). Isoliquiritigenin, a natural product derived from liquorice, could significantly decrease VEGF expression by promoting HIF-1α degradation in breast cancer cells (Wang et al., 2013). Ginsenoside Rg3 is one of the active ingredients in ginseng. Chen et al. (2010) reported that ginsenoside Rg3 could inhibit VEGF expression through downregulation of HIF-1α protein in various human cancers. Wang et al. (2009) reported that ginsenoside Rg3 could inhibit HIF-1α and VEGF expression during hypoxia and inhibit hep-2 cell growth by affecting cell cycle progression. Another report has shown that 20(R)-ginsenoside Rg3 could inhibit tumor angiogenesis by suppressing the expression of VEGF, MMP9, and HIF-1α in a mouse model of Lewis lung cancer (Geng et al., 2016). Schisandrin B (Sch B) is the most abundant dibenzocyclooctadiene lignan in Schisandra chinensis. Lv et al. (2015) found that Sch B could inhibit the migration and invasion of A549 cells by decreasing the expressions of HIF-1, VEGF, MMP-2, and MMP-9 in vitro. Scutellaria barbata is widely used in the treatment of cancer in traditional Chinese medicine. Shiau et al. (2014) found that S. barbata could play an antiangiogenic role by targeting the HIF-1α signaling pathway and reducing the expression of VEGF. Hu et al. (2012) used a mouse model of ovarian carcinoma xenograft to study the underlying anticancer mechanisms of Zengmian Yiliu granule (ZMYLG), a traditional Chinese formula. ZMYLG could downregulate the protein expression and mRNA of HIF-1α and VEGF and exert antiangiogenic effects on ovarian carcinoma xenografts. Triptolide (TPL) is an active ingredient extracted from triptolide and widely used in cancer treatment. Li et al. (2018b) found that TPL could inhibit angiogenesis by reducing the expression of HIF-1α and VEGF in a dose-dependent manner. Protein alpha-momorcharin (alpha-MMC) is isolated from seeds of the bitter gourd Momordica charantia. Pan et al. (2014) showed that alpha-MMC has significant inhibitory effects on normal and hypoxic nasal-pharyngeal cancer cells by blocking HIF-1α signaling such as the expression of VEGF and UPR. Baicalein, a type of flavonoid isolated from the roots of Scutellaria baicalensis, could suppress tumor growth, which is associated with a reduction of HIF-1α and VEGF in an orthotopic glioma mouse model (Wang and Jiang, 2015) (Figure 4). Table 2 lists important Chinese herbal medicines that act on HIF-1 mRNA and/or protein expression.
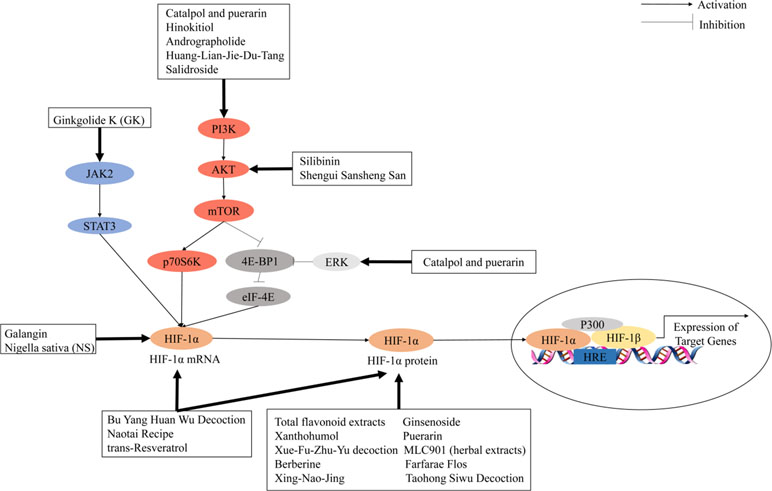
Figure 4 Herbal medicines promote the activation of the HIF-1 pathway through different targets in ischemic stroke.

Table 2 Chinese Herbal Medicines and Their Molecular Targets That Are Regulated by the HIF-1 Pathway in Cancer.
Inhibitors of HIF-1 Transcriptional Activity
So far, several herbal medicines have been shown to inhibit tumor angiogenesis through downregulating HIF-1 activation by inhibiting its transcriptional activity. Shiau et al. explored the underlying mechanisms of S. barbata on regulating HIF-1–dependent expression of VEGF. Hypoxia induces angiogenesis by upregulating VEGF expression. However, after treatment with S. barbata, the expression of VEGF was downregulated in lung cancer cells. In addition, S. barbata inhibited the proliferation and migration of endothelial cells under a hypoxic environment. S. barbata suppressed the transcriptional activity of HIF-1α and promoted the phosphorylation of the upstream signal molecule AKT (Shiau et al., 2014). Triptolide is the major active compound in traditional Chinese medicine (TCM) herb Tripterygium wilfordii Hook F. Triptolide exhibits significant chemotherapeutic effects against cancer based on its antiangiogenesis and drug resistance circumvention activities. Various biological molecules suppressed by triptolide have been identified as its potential targets. Triptolide could downregulate the transcriptional activity of HIF-1α and further decrease the transcriptional activity of its target genes including VEGF (Zhou et al., 2010).
Inhibitors of Signal Transduction Pathways
Several Chinese herbal medicines have been reported to act on different signaling pathways to indirectly regulate HIF-1 activation and exert antiangiogenic effects in cancer treatment. Imperatorin is an active natural furocoumarin ingredient from Angelica dahurica. Mi et al. (2017) reported that imperatorin administration could inhibit tumor growth and tumor angiogenesis in vivo and in vitro and downregulate HIF-1α activation by targeting the mTOR/p70S6K/4E-BP1 and MAPK pathways. 5,3’-Dihydroxy-6,7,4’-trimethoxyflavanone (DHTMF) is one of the main ingredients of Vitex rotundifolia. Kim et al. (2015) showed that DHTMF could inhibit angiogenesis and induce apoptosis by decreasing the expression levels of HIF-1α and VEGF via the Akt/mTOR pathway in cancer cells. Kim et al. (2017) reported that diphyllin, a natural component of traditional Chinese medicine, could regulate the mTORC1/HIF-1α/VEGF pathway in the treatment of esophageal cancer (Kim et al., 2017). Curcumin is an active molecule isolated from the dried rhizome of Curcuma longa. Wang D. et al. (2017) found that curcumin could downregulate the HIF-1α, VEGF, DEC1, and STAT3 signal transduction pathways in the treatment of gastric cancer. Garcinol (camboginol) is a natural polyisoprenylated benzophenone isolated from dried rind of the Garcinia indica. Ranjbarnejad et al. (2017a) found that garcinol could inhibit VEGF, MMP2/9, and CXCR4 expression by targeting the mPGES-1/PGE2/HIF-1a pathway. Herbal medicines targeting signal transduction pathways are reported in Table 2.
Activation of Hif-1–Dependent Angiogenesis in Ischemic Stroke
Stroke is one of the major causes of death and long-term disability worldwide. About 50% of patients who have suffered from a stroke live less than 1 year (Zhang and Chopp, 2009). There are two main types of stroke: ischemic and hemorrhagic. Ischemic strokes account for about 85% of all strokes, which is caused by a sudden halt of blood supply to the brain tissue because of ischemia and can result in permanent brain injury (Senior, 2001). The thrombotic or embolic occlusion of a cerebral artery will lead to irreversible neuronal cell death and further induce serious brain injury at the core of the infarct immediately. In addition, the secondary injury will result in the expansion of the area of brain injury, which can continue for an extended period after the first ischemic attack (Chopp and Li, 2002). Thus, reestablishment of the functional cerebral microvasculature network will improve regional blood supply and promote stroke recovery. Angiogenesis is a fundamental pathological process in malignant tumor growth and development. However, it may also occur as an advantageous defense response against hypoxia in ischemic stroke by improving blood supply to the brain tissue. Previous research has shown that angiogenesis is positively correlated to the survival rate of ischemic stroke patients, indicating that regulation of the neovascular growth in the ischemic brain could be a pivotal target for ischemic stroke treatment. Numerous studies have shown that the HIF-1 signaling pathway is likely involved in promoting angiogenesis after ischemic stroke in the brain (Zhang et al., 2011). As a transcription factor in response to hypoxia, HIF-1 activity is increased in brains after ischemic attacks. In 1996, it was first reported that both subunit mRNAs of HIF-1 were upregulated in the brains of mice or rats when they were exposed to a hypoxic environment for 30 to 60 min (Jiang et al., 1996). Another study showed that HIF-1α expression was dramatically increased in the cerebral cortex of a rat after 60 min of recovery from cardiac arrest and remained boosted for more than 10 h. In addition, HIF-1α mRNA expression was fortified after focal ischemia in rat brain tissue. The increase was detected 8 h after the onset of ischemia and further elevated at 20 and 25 h (Zaman et al., 1999). These results demonstrate that the activity of HIF-1 is increased in ischemic brains and that the level of HIF-1α expression is heterogeneous. It has been reported that the HIF-1–mediated VEGF/Notch1 signaling pathway plays a crucial role in the development of angiogenesis in the ischemic brain. Apart from VEGF signaling, other complex mechanisms may also take part in HIF-1–mediated angiogenesis regulation after ischemic stroke. The expressions of angiogenesis–related genes such as the endothelin-1 (ET1), adrenomedullin (ADM), α1B-adrenergic receptor, nitric oxide synthase, Ang-2, stromal-derived growth factor-1 (SDF-1), PDGF-B, PlGF, and heme oxygenease-1 (HO-1) are also modulated by HIF-1 (Weih et al., 1999; Zhang et al., 2007; Yeh et al., 2008). In addition, HIF-1 mediated the regulation of collagen prolyl hydroxylase, MMPs, and plasminogen activator receptor and inhibitor (PAI) expression, which further modulates matrix metabolism and vascular maturation in the ischemic brain (Zou et al., 2018).
Because of the potentially pivotal roles in promoting angiogenesis by HIF-1 after ischemic stroke, it has been recommended that upregulation of HIF-1 activity is a highly promising therapeutic strategy for ischemic brain injury. Thus, the mechanism of HIF-1–induced angiogenesis in ischemic cerebral tissue has drawn much attention and is under extensive exploration. Currently, the only FDA-approved therapy for focal occlusive ischemia in the brain is the administration of the thrombolytic agent tissue plasminogen activator (tPA), which may have the risk of bleeding complications (Ohsawa et al., 2005). Thus, it is imperative to develop additional approaches to enhance therapeutic safety in ischemic stroke treatment. In recent years, several studies have raised great interest on the role of HIF-1 activation in the prognosis of ischemic stroke and whether upregulation of HIF-1 could benefit this disease. Therapeutic activation of HIF-1 applied before the ischemic stress or in the peri-ischemic period may theoretically enhance the natural response of angiogenesis in ischemic stroke patients. Some strategies have been used successfully on experimental activation of HIF-1 in ischemic disease animal models. For example, knocking out the central ODD domain will promote the activity of HIF-1α. The expression of such an HIF-1α transgenic protein in mouse models leads to significant activation of HIF-1 transcriptional targets and angiogenesis (Lin-Holderer et al., 2016). In addition, those neovessels are not leaky, and the intensive vascularity will not induce edema. This result contrasts with that of another study of VEGF therapy wherein edema is frequently detected and demonstrates that HIF-1 activation might keep away from this potential side effect in ischemic disease treatment (Ryou et al., 2015). Other studies have tried to use genetic therapy targeting to activate HIF-1 in the rabbit hind limb ischemia model and rat myocardial infarction model; these therapies improved angiogenesis and increased blood flow to the ischemic area (Li et al., 2016). Another focus on improving HIF-1–induced angiogenesis is suppressing the degradation of HIF-1α. For example, a macrophage-derived peptide called PR39 can interact with the proteasome and inhibit HIF degradation. Animal experiments have confirmed that PR39 treatment can improve peri-infarct angiogenesis in ischemic cardiac tissue (Hao et al., 2009). Besides using proteasome inhibitors, overexpression of peptides corresponding to the VHL-binding prolyl hydroxylation sites in HIF-1 also inhibits the degradation process of HIF-1α and further enhances angiogenesis in ischemic tissue. Another combined treatment with transgenic stem cells was applied in ischemic stroke rats. Rat bone marrow–derived mesenchymal stem cells were transfected with adenovirus containing HIF-1α genes with mutations at Asn 803 and Pro 564 sites, which prevent HIF-1 degradation. The cells with transgenic genes were injected into the cerebral artery occlusion of rats. After a week, improved angiogenesis and reduced infarction in brain tissue were observed; the rats’ ischemic stroke symptoms were also relieved (Li C. et al., 2017). HIF prolyl 4-hydroxylase domain proteins (PHD) are among the most pivotal inhibitors of the HIF-1 pathway. Suppression of the HIF-1 PHD by small molecular agents or genetic therapy may also inhibit HIF-1 degradation and activate the downstream gene’s transcriptional activity (Liu Y. et al., 2018). One study verified that PHD ablation in neurons improved ischemic stroke recovery in mice through endogenous adaptive angiogenesis by activation of the HIF-VEGF signaling (Mi et al., 2018). In general, the activation of HIF-1–dependent angiogenesis may provide therapeutic potential in ischemic and hypoxic cerebrovascular diseases. The central role of HIF-1 in the modulation of the hypoxia-correlated pathway has provided a promising approach for the development of novel therapeutic agents for ischemic stroke.
Chinese Medicines Mediate Angiogenic Factors to Promote Angiogenesis by Regulating the Hif-1 Pathway After Ischemic Stroke
Herbal medicines, including herbal formulas, herbal extract, and chemical ingredients, have been widely used in the treatment of cardiovascular and cerebrovascular diseases for centuries because of reduced side effects (Fan et al., 2017). Previous studies indicated that herbal medicines are often used as an alternative therapy for prevention, treatment, and rehabilitation interventions of ischemic stroke (Table 3). As an important component of cerebral angiogenesis in patients with ischemic stroke, there has been great attention in developing activators targeting the HIF-1 pathway. HIF-1 activation can be induced by regulation of one of the following pathways: HIF-1 mRNA expression, HIF-1 protein expression, or signal transduction pathways. Figure 3 summarizes the treatment of ischemic stroke by herbal medicines that regulate HIF-1α to promote angiogenesis through the different mechanisms.

Table 3 Herbal Medicines and Their Molecular Targets Regulated by the HIF-1 Pathway in Ischemic Stroke.
Activators of HIF-1 mRNA and/or Protein Expression
Several Chinese herbal medicines that target upregulating HIF-1 mRNA and/or protein expression have proangiogenic effects in ischemic stroke treatment. Ginsenoside, a major active ingredient of ginseng, has been demonstrated to be effective in the treatment of acute ischemic stroke. Gao et al. (2018) found that ginsenoside has therapeutic effects on cerebral ischemia and hypoxic injury through the HIF-1α–VEGF pathway in an oxygen-glucose deprivation/reperfusion (OGD/R) model of neural stem cells (NSCs). Xanthohumol, an ingredient of beer, is the principal prenylated flavonoid in hops (Humulus lupulus L). Yen et al. reported that xanthohumol-induced neuroprotection is associated with many factors such as HIF-1α, iNOS, and TNF-α. He et al. established a rat model of transient middle cerebral artery occlusion (tMCAO), followed by 24 h of reperfusion (Yen et al., 2012). Administration of total flavonoid extracts (TFC) could improve neurological deficits, reduce infarct volume, and promote angiogenesis by increasing the expression of HIF-1α, VEGF, Ang-1, Dll4, Notch1, and CD31 (He et al., 2018). MLC901, an herbal extract preparation modified from the TCM herbal formula, has been proven to have neuroprotective and neurorestorative properties in preclinical models of stroke, traumatic brain injury, and global cerebral ischemia. Gandin et al. (2016) found that 5-week pretreatment with MLC901 in MCAO-induced cerebral ischemia mouse models could regulate the expression of HIF-1α and its downstream targets, such as VEGF, Ang-1, and Ang-2. Chen et al. showed that administration of TCM formula Bu Yang Huan Wu (BYHW) decoction decreased cerebral edema, the neurological deficient score, and brain infarct volume in a rat model of cerebral ischemia/reperfusion (I/R) injury. Furthermore, BYHW treatment markedly decreased the mRNA and protein levels of HIF-1α and VEGF compared with those of the model treatment (Chen Z. et al., 2018). Nigella sativa (NS) is one of the widely used herbs from the family ranunculaceae. Soleimannejad et al. (2017) found that the N. sativa extract was associated with increased expression of VEGF and HIF-1α, markers of brain angiogenesis after total cerebral ischemia in rats. Puerarin, a major isoflavonoid isolated from the Chinese medicinal herb Radix puerariae (kudzu root), is widely used for treating cardiovascular disease in clinics. Chang et al. (2009) used a tMCAO rat model to study the effects of puerarin. Administration of puerarin inhibited the expression of HIF-1α, TNF-α, iNOS, caspase-3, and many factors, and it may be an ideal therapeutic measure after ischemia-reperfusion brain injury. Xue-Fu-Zhu-Yu decoction (XFZYD) is a traditional Chinese medicine formula widely used in cardiovascular diseases. Lee et al. found that XFZYD administration slightly reduced infarct volume compared with that of solvent-treated rats. However, the combination of XFZYD and recombinant tissue plasminogen activator (rt-PA) significantly reduced the infarct volume in cerebral ischemic areas. In addition, rt-PA administration significantly reduced the expression of TNF-α and iNOS but did not decrease the expression of HIF-1α or caspase-3, whereas XFZYD administration significantly reduced the expression of all these proteins in the ischemic region. In addition, XFZYD administration significantly enhanced the reduction of rt-pa–mediated TNF-α, iNOS, HIF-1α, and active caspase-3 expression (Lee et al., 2011). Galangin, a commonly used antioxidant, is a natural flavonoid derived from the rhizome of Alpina officinarum Hance. Wu et al. (2015) showed that galangin could promote angiogenesis and vascular remodeling to improve neurological function scores and the cerebral infarct area by upregulating the Wnt/β-catenin and HIF-1α/VEGF signaling pathway in a MCAO rat model. Herbal medicines targeting HIF-1 mRNA and/or protein expression are provided in Table 2.
Activators of Signal Transduction Pathways
Several studies have shown that Chinese herbal medicines can also target different signal transduction pathways to upregulate HIF-1-induced angiogenesis. Huang-Lian-Jie-Du-Tang (HLJDT) is a classical heat-clearing and detoxicating formula of traditional Chinese medicine. Zhang et al. found that HLJDT preconditioning in the MCAO rat model could decrease the cerebral infarction volume, neurological deficient score, and cerebral water content. In addition, HLJDT preconditioning in cerebral cortical neurons in vitro under oxygen and glucose deprivation (OGD) could increase HIF-1α, VEGF, and erythropoietin (EPO) expression levels and activation of the PI3K/AKT signaling pathway (Zhang et al., 2014b). Shengui Sansheng San (SSS), a traditional Chinese herbal formula, has been used for stroke for more than 300 years. B. Liu et al. (2018) showed that SSS could activate AKT/mTOR/HIF-1α and ERK1/2 signals to facilitate VEGF production, resulting in angiogenesis after stroke in the rat MCAO model. Ginkgolide K (GK) is an extract isolated from the leaves of Ginkgo biloba. Chen et al. have used a tMCAO mouse model to verify the pharmacological properties of GK. GK treatment could significantly increase the expressions of HIF-1α and VEGF in the tMCAO model. In the OGD/R model of bEnd.3 cells, GK-induced upregulation of HIF-1α and VEGF could be eliminated by JAK2/STAT3 inhibitor AG490 (Chen et al., 2018). Andrographolide is a bicyclic diterpenoid lactone from the leaves of Andrographis paniculata (Acanthaceae). Chen et al. suggested that andrographolide could ameliorate brain injury in ischemic stroke by PI3K/AKT–dependent activation of the NF-κB and further activation of HIF-1α pathways in vivo and in vitro (Chern et al., 2011). As a conclusion, the herbal medicines targeting HIF-1 signal transduction pathways are provided in Table 2.
Conclusions and Future Directions
HIF-1–induced angiogenesis has been involved in numerous pathological conditions, and it may be harmful or beneficial depending on the types of specific disease. Since the 1970s, the exploration on angiogenesis has sparked hopes in providing novel therapeutic approaches in multiple diseases with high mortality rates, such as cancers and ischemic stroke. Depending on different types of diseases and the expected treatment effects, angiogenesis-targeted therapies have different approaches. Generally, the clinical application of angiogenesis can be classified into two different strategies: antiangiogenesis (cancer) and proangiogenesis (ischemic stroke). The induction of angiogenesis for therapeutic purposes in ischemic stroke can be directly stimulated by various angiogenic factors, such as PlGF, VEGF, PDGF, and FGF, some of which have been applied in preclinical and clinical studies. However, treatments only using proangiogenic factors to induce angiogenesis were proven to be insufficient in ischemic disease; thus, novel treatments that can stabilize neovascularization with high-efficiency are required for better therapeutic effects. Therefore, HIF-1–induced angiogenesis may be a promising strategy for ischemic cerebrovascular disease. HIF-1 activation in ischemic cerebrovascular disease leads to a more mature and stable vascular formation compared with that of traditional proangiogenic factor therapy, wherein neovascularization tends to be leaky. Instead of proangiogenesis in ischemic stroke therapy, cancer treatments are based on suppression of angiogenesis for inhibiting tumor growth and metastasis. Current therapies are focused on suppressing VEGF activity, such as sunitinib (VEGFR2 inhibitor) and bevacizumab (VEGF inhibitor) target therapy. Because of the pivotal role of the HIF-1 pathway in modulating the activation of various proangiogenic factors in cancers, HIF-1 has been considered as a promising target for developing novel anticancer agents. Suppression of HIF-1–dependent angiogenesis involves the modulation of HIF-1 activity by regulating HIF-1α transcription and protein translation, HIF-1α DNA binding, HIF-1α and HIF-1β dimerization, and HIF-1 degradation. Considering the profound impact of HIF-1 on cancer progression and the unsatisfactory efficacy of current treatment protocols, several clinical trials are being conducted with potential antiangiogenesis agents that involve protein degradation, downregulation, or inactivation of HIF-1. It is noteworthy that, within a single herb concoction, sometimes we can find both inhibitors and activators of HIF-1, which will complicate the use of herbal medicines under clinical conditions. For example, ginsenoside Rg3 and ginsenoside Rg1 are both natural triterpenoid saponins extracted from red ginseng. Previous studies showed that ginsenoside Rg3 could inhibit tumor angiogenesis by decreasing the expression of HIF-1 in various cancers, whereas ginsenoside Rg1 might inhibit myocardial ischemia and reperfusion injury by activating HIF-1 (Chen et al., 2010; Yuan et al., 2019).
With a long history of more than 2,000 years of clinical use, Chinese herbal medicine is emerging as a complementary and alternative choice for its multitargeted, multileveled, and coordinated intervention effects against complex disorders, such as cancer and ischemic stroke. Research results from many in vitro and in vivo studies have demonstrated that several Chinese herbal formulations, herbs, or herbal compounds can induce or inhibit angiogenesis through multiple cellular mechanisms. Numerous preclinical studies have provided supportive evidence for using Chinese herbal medicines as a novel antiangiogenesis therapy for cancer or proangiogenesis therapy for ischemic stroke by targeting the HIF-1 pathway. However, the overall scientific evidence to back the application of Chinese herbal medicines for the management of cancer and ischemic stroke remains limited, and the results of these researches are sometimes contradictory and inconclusive. The underlying reasons for these inconsistencies include the complex chemical and pharmacological properties of Chinese herbal medicines and the interactions between the multiple bioactive ingredients of Chinese herbal medicines. More researches are needed to gain a better understanding of the dual effects of Chinese herbal medicines on angiogenesis in cancer and ischemic stroke treatment. In addition, despite the long history of Chinese herbal medicines in the treatment of cancer and ischemic stroke, well-controlled clinical studies with herbal medicinal products used for treating these diseases are still limited. More rigorously designed, controlled, randomized, international, multicenter clinical trials are urgently required for further validating Chinese herbal medicine efficacy in cancer and ischemic stroke treatment. Finally, as a double-edged sword, the important role of HIF-1 in angiogenesis should be considered as a promising target for treating cancer or ischemic stroke. The possible side effects and potential risk of angiogenesis-related complications by Chinese herbal medicines should also be considered when applying the HIF-1 target strategy for management of ischemic stroke and cancer.
Author Contributions
MH and HS wrote the manuscript. NW, H-YT, QW, and YF revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81673627), Guangzhou Science Technology and Innovation Commission Research Projects (201805010005), Research Grant Council, HKSAR (Project code: RGC GRF 17152116), and Commissioner for Innovation Technology, HKSAR (Project code: ITS/091/16FX).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agrawal, M., Kumar, V., Singh, A. K., Kashyap, M. P., Khanna, V. K., Siddiqui, M. A., et al. (2013). trans-Resveratrol protects ischemic PC12 Cells by inhibiting the hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem. Neurosci. 4 (2), 285–294. doi: 10.1021/cn300143m
Al-Anazi, A., Parhar, R., Saleh, S., Al-Hijailan, R., Inglis, A., Al-Jufan, M., et al. (2018). Intracellular calcium and NF-kB regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Sci. 212, 275–284. doi: 10.1016/j.lfs.2018.10.014
Aldo, P., Elisabetta, C. (2018). Role of HIF-1 in cancer progression: novel insights. Curr. Mol. Med. 18 (6), 343–351. doi: 10.2174/1566524018666181109121849.
Ambrosini, S., Sarchielli, E., Comeglio, P., Porfirio, B., Gallina, P., Morelli, A., et al. (2015). Fibroblast growth factor and endothelin-1 receptors mediate the response of human striatal precursor cells to hypoxia. Neuroscience 289, 123–133. doi: 10.1016/j.neuroscience.2014.12.073
Bae, M. K., Kim, S. H., Jeong, J. W., Lee, Y. M., Kim, H. S., Kim, S. R., et al. (2006). Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 15 (6), 1557–62. doi: 10.3892/or.15.6.1557
Ban, H. S., Kim, B. K., Lee, H., Kim, H. M., Harmalkar, D., Nam, M., et al. (2017). The novel hypoxia-inducible factor-1alpha inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis. 8 (6), e2843. doi: 10.1038/cddis.2017.235
Belaidi, E., Morand, J., Gras, E., Pepin, J. L., Godin-Ribuot, D. (2016). Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol. Ther. 168, 1–11. doi: 10.1016/j.pharmthera.2016.07.010
Beppu, K., Nakamura, K., Linehan, W. M., Rapisarda, A., Thiele, C. J. (2005). Topotecan blocks hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression induced by insulin-like growth factor-I in neuroblastoma cells. Cancer Res. 65 (11), 4775–4781. doi: 10.1158/0008-5472.CAN-04-3332
Berlow, R. B., Dyson, H. J., Wright, P. E. (2017). Hypersensitive termination of the hypoxic response by a disordered protein switch. Nature 543 (7645), 447–451. doi: 10.1038/nature21705
Bluff, J. E., Menakuru, S. R., Cross, S. S., Higham, S. E., Balasubramanian, S. P., Brown, N. J., et al. (2009). Angiogenesis is associated with the onset of hyperplasia in human ductal breast disease. Br. J. Cancer 101 (4), 666–672. doi: 10.1038/sj.bjc.6605196
Bohonowych, J. E., Peng, S., Gopal, U., Hance, M. W., Wing, S. B., Argraves, K. M., et al. (2011). Comparative analysis of novel and conventional Hsp90 inhibitors on HIF activity and angiogenic potential in clear cell renal cell carcinoma: implications for clinical evaluation. BMC Cancer 11, 520. doi: 10.1186/1471-2407-11-520
Brown, C. (2016). Targeted therapy: an elusive cancer target. Nature 537 (7620), S106–108. doi: 10.1038/537S106a
Carroll, V. A., Ashcroft, M. (2006). Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 66 (12), 6264–6270. doi: 10.1158/0008-5472.CAN-05-2519
Chang, Y., Hsieh, C. Y., Peng, Z. A., Yen, T. L., Hsiao, G., Chou, D. S., et al. (2009). Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. J. Biomed. Sci. 16, 9. doi: 10.1186/1423-0127-16-9
Chen, Q. J., Zhang, M. Z., Wang, L. X. (2010). Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell Physiol. Biochem. 26 (6), 849–858. doi: 10.1159/000323994
Chen, Y., Zhu, H. B., Liao, J., Yi, Y. Q., Wang, G. Z., Tong, L., et al. (2014). [Regulation of naotai recipe on the expression of HIF-lalpha/VEGF signaling pathway in cerebral ischemia/reperfusion rats]. Zhongguo. Zhong. Xi. Yi. Jie. He. Za. Zhi. 34 (10), 1225–1230.
Chen, H., Feng, J., Zhang, Y., Shen, A., Chen, Y., Lin, J., et al. (2015). Pien Tze Huang inhibits hypoxia-induced angiogenesis via HIF-1 alpha/VEGF-A pathway in colorectal cancer. Evid. Based Complement. Alternat. Med. 2015, 454279. doi: 10.1155/2015/454279
Chen, C., Tang, Q., Zhang, Y., Dai, M., Jiang, Y., Wang, H., et al. (2017). Metabolic reprogramming by HIF-1 activation enhances survivability of human adipose-derived stem cells in ischaemic microenvironments. Cell Prolif. 50 (5). doi: 10.1111/cpr.12363
Chen, H., Liu, P., Zhang, T., Gao, Y., Zhang, Y., Shen, X., et al. (2018). Effects of diphyllin as a novel V-ATPase inhibitor on TE-1 and ECA-109 cells. Oncol. Rep. 39 (3), 921–928. doi: 10.3892/or.2018.6191
Chen, M., Zou, W., Chen, M., Cao, L., Ding, J., Xiao, W., et al. (2018). Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur. J. Pharmacol. 833, 221–229. doi: 10.1016/j.ejphar.2018.06.012
Chen, R., Cai, X., Liu, J., Bai, B., Li, X. (2018). Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 215, 31–42. doi: 10.1016/j.lfs.2018.10.047
Chen, Y., Sun, Y., Li, W., Wei, H., Long, T., Li, H., et al. (2018). Systems pharmacology dissection of the anti-stroke mechanism for the Chinese traditional medicine Xing-Nao-Jing. J. Pharmacol. Sci. 136 (1), 16–25. doi: 10.1016/j.jphs.2017.11.005
Chen, Z. Z., Gong, X., Guo, Q., Zhao, H., Wang, L. (2018). Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 alpha, VEGF and promotion beta-ENaC expression. J. Ethnopharmacol. 228, 70–81. doi: 10.1016/j.jep.2018.09.017
Cheng, Z., Fu, J., Liu, G., Zhang, L., Xu, Q., Wang, S. Y. (2018). Angiogenesis in JAK2 V617F positive myeloproliferative neoplasms and ruxolitinib decrease VEGF, HIF-1 enesis in JAK2 V617F positive cells. Leuk. Lymphoma. 59 (1), 196–203. doi: 10.1080/10428194.2017.1324155
Chern, C. M., Liou, K. T., Wang, Y. H., Liao, J. F., Yen, J. C., Shen, Y. C. (2011). Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta. Med. 77 (15), 1669–1679. doi: 10.1055/s-0030-1271019
Chopp, M., Li, Y. (2002). Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1 (2), 92–100. doi: 10.1016/S1474-4422(02)00040-6
Copple, B. L. (2010). Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-beta-dependent mechanisms. Liver Int. 30 (5), 669–682. doi: 10.1111/j.1478-3231.2010.02205.x
Das, L., Vinayak, M. (2014). Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One 9 (6), e99583. doi: 10.1371/journal.pone.0099583
De Francesco, E. M., Maggiolini, M., Musti, A. M. (2018). Crosstalk between Notch, HIF-1alpha and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 19 (7), 2011. doi: 10.3390/ijms19072011
Dong, H., Lin, W., Wu, J., Chen, T. (2010). Flavonoids activate pregnane x receptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC Biochem. 11, 23. doi: 10.1186/1471-2091-11-23
Fan, X. X., Li, F., Lv, Y. N., Zhang, Y., Kou, J. P., Yu, B. Y. (2017). An integrated shotgun proteomics and bioinformatics approach for analysis of brain proteins from MCAO model using serial affinity chromatograph with four active ingredients from Shengmai preparations as ligands. Neurochem. Int. 103, 45–56. doi: 10.1016/j.neuint.2016.12.017
Forbes, A., Anoopkumar-Dukie, S., Chess-Williams, R., McDermott, C. (2016). Relative cytotoxic potencies and cell death mechanisms of alpha1 -adrenoceptor antagonists in prostate cancer cell lines. Prostate 76 (8), 757–766. doi: 10.1002/pros.23167
Fortenbery, G. W., Sarathy, B., Carraway, K. R., Mansfield, K. D. (2018). Hypoxic stabilization of mRNA is HIF-independent but requires mtROS. Cell Mol. Biol. Lett. 23, 48. doi: 10.1186/s11658-018-0112-2
Freise, C., Ruehl, M., Erben, U., Neumann, U., Seehofer, D., Kim, K. Y., et al. (2011). A hepatoprotective Lindera obtusiloba extract suppresses growth and attenuates insulin like growth factor-1 receptor signaling and NF-kappaB activity in human liver cancer cell lines. BMC Complement. Altern. Med. 11, 39. doi: 10.1186/1472-6882-11-39
Fu, L., Chen, W., Guo, W., Wang, J., Tian, Y., Shi, D., et al. (2013). Berberine targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS One 8 (7), e69240. doi: 10.1371/journal.pone.0069240
Fu, P., Du, F., Chen, W., Yao, M., Lv, K., Liu, Y. (2014). Tanshinone IIA blocks epithelial-mesenchymal transition through HIF-1alpha downregulation, reversing hypoxia-induced chemotherapy resistance in breast cancer cell lines. Oncol. Rep. 31 (6), 2561–2568. doi: 10.3892/or.2014.3140
Gandin, C., Widmann, C., Lazdunski, M., Heurteaux, C. (2016). MLC901 favors angiogenesis and associated recovery after ischemic stroke in mice. Cerebrovasc. Dis. 42 (1-2), 139–154. doi: 10.1159/000444810
Gao, J., Bai, H., Li, Q., Li, J., Wan, F., Tian, M., et al. (2018). In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion. Int. J. Mol. Med. 41 (1), 353–363. doi: 10.3892/ijmm.2017.3253
Geng, L., Fan, J., Gao, Q. L., Yu, J., Hua, B. J. (2016). Preliminary study for the roles and mechanisms of 20(R)-ginsenoside Rg3 and PEG-PLGA-Rg3 nanoparticles in the Lewis lung cancer mice. Beijing Da. Xue. Xue. Bao. Yi. Xue. Ban. 48 (3), 496–501.
Gramley, F., Lorenzen, J., Jedamzik, B., Gatter, K., Koellensperger, E., Munzel, T., et al. (2010). Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc. Pathol. 19 (2), 102–111. doi: 10.1016/j.carpath.2008.11.001
Guo, D., Murdoch, C. E., Liu, T., Qu, J., Jiao, S., Wang, Y., et al. (2018). Therapeutic angiogenesis of Chinese herbal medicines in ischemic heart disease: a review. Front. Pharmacol. 9, 428. doi: 10.3389/fphar.2018.00428
Han, X., Sun, S., Zhao, M., Cheng, X., Chen, G., Lin, S., et al. (2014). Celastrol stimulates hypoxia-inducible factor-1 activity in tumor cells by initiating the ROS/Akt/p70S6K signaling pathway and enhancing hypoxia-inducible factor-1alpha protein synthesis. PLoS One 9 (11), e112470. doi: 10.1371/journal.pone.0112470
Hao, Y. W., Sun, L. J., Liu, Y., Wang, Q. Y., Yang, G. X. (2009). [Secretory expression of PR39 following adeno-associated viral-encoding fusion gene transfer induces angiogenesis in hypoxia chick embryo]. Zhonghua. Xin. Xue. Guan. Bing. Za. Zhi. 37 (8), 746–749.
He, Z., Chen, A. Y., Rojanasakul, Y., Rankin, G. O., Chen, Y. C. (2016). Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 35 (1), 291–297. doi: 10.3892/or.2015.4354
He, Q., Li, S., Li, L., Hu, F., Weng, N., Fan, X., et al. (2018). Total Flavonoids in Caragana (TFC) promotes angiogenesis and enhances cerebral perfusion in a rat model of ischemic stroke. Front. Neurosci. 12, 635. doi: 10.3389/fnins.2018.00635
Hong, M., Wang, N., Tan, H. Y., Tsao, S. W., Feng, Y. (2015). MicroRNAs and Chinese medicinal herbs: new possibilities in cancer therapy. Cancers (Basel) 7 (3), 1643–1657. doi: 10.3390/cancers7030855
Hong, M., Tan, H. Y., Li, S., Cheung, F., Wang, N., Nagamatsu, T., et al. (2016). Cancer stem cells: the potential targets of Chinese medicines and their active compounds. Int. J. Mol. Sci. 17 (6), 893. doi: 10.3390/ijms17060893
Hou, F., Li, W., Shi, Q., Li, H., Liu, S., Zong, S., et al. (2016). Yi Ai Fang, a traditional Chinese herbal formula, impacts the vasculogenic mimicry formation of human colorectal cancer through HIF-1alpha and epithelial mesenchymal transition. BMC Complement. Altern. Med. 16 (1), 428. doi: 10.1186/s12906-016-1419-z
Hsu, H. W., Wall, N. R., Hsueh, C. T., Kim, S., Ferris, R. L., Chen, C. S., et al. (2014). Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 50 (1), 19–26. doi: 10.1016/j.oraloncology.2013.10.003
Hu, X. X., Zhang, Q. H., Qi, C. (2012). Anti-angiogenic effects of zengmian YiIiu granule on ovarian carcinoma xenograft. Zhongguo. Zhong. Xi. Yi. Jie. He. Za. Zhi. 32 (7), 970–974.
Hua, H., Zhu, Y., Song, Y. H. (2018). Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 101, 115–122. doi: 10.1016/j.biopha.2018.02.031
Huang, L., Zhang, Z., Zhang, S., Ren, J., Zhang, R., Zeng, H., et al. (2011). Inhibitory action of Celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1alpha pathway. Int. J. Mol. Med. 27 (3), 407–415. doi: 10.3892/ijmm.2011.600
Hwang, J. H., Kumar, V. R., Kang, S. Y., Jung, H. W., Park, Y. K. (2018). Effects of flower buds extract of Tussilago farfara on focal cerebral ischemia in rats and inflammatory response in BV2 microglia. Chin. J. Integr. Med. 24 (11), 844–852. doi: 10.1007/s11655-018-2936-4
Jain, R. K., Carmeliet, P. (2012). SnapShot: tumor angiogenesis. Cell 149 (6), 1408–1408 e1. doi: 10.1016/j.cell.2012.05.025
Jayakumar, T., Hsu, W. H., Yen, T. L., Luo, J. Y., Kuo, Y. C., Fong, T. H., et al. (2013). Hinokitiol, a natural tropolone derivative, offers neuroprotection from thromboembolic stroke in vivo. Evid. Based Complement. Alternat. Med. 2013, 840487. doi: 10.1155/2013/840487
Jia, L. Y., Wu, X. J., Gao, Y., Rankin, G. O., Pigliacampi, A., Bucur, H., et al. (2017). Inhibitory effects of total triterpenoid saponins isolated from the seeds of the tea plant (Camellia sinensis) on human ovarian cancer cells. Molecules 22 (10), 1649. doi: 10.3390/molecules22101649
Jiang, B. H., Semenza, G. L., Bauer, C., Marti, H. H. (1996). Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 271 (4 Pt 1), C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172
Kabei, K., Tateishi, Y., Nozaki, M., Tanaka, M., Shiota, M., Osada-Oka, M., et al. (2018). Role of hypoxia-inducible factor-1 in the development of renal fibrosis in mouse obstructed kidney: special references to HIF-1 dependent gene expression of profibrogenic molecules. J. Pharmacol. Sci. 136 (1), 31–38. doi: 10.1016/j.jphs.2017.12.004
Kang, S. G., Zhou, G., Yang, P., Liu, Y., Sun, B., Huynh, T., et al. (2012). Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc. Natl. Acad. Sci. U.S.A. 109 (38), 15431–15436. doi: 10.1073/pnas.1204600109
Kaul, D. K., Fabry, M. E., Suzuka, S. M., Zhang, X. (2013). Antisickling fetal hemoglobin reduces hypoxia-inducible factor-1alpha expression in normoxic sickle mice: microvascular implications. Am. J. Physiol. Heart Circ. Physiol. 304 (1), H42–50. doi: 10.1152/ajpheart.00296.2012
Kim, A., Ma, J. Y. (2018). Rhaponticin decreases the metastatic and angiogenic abilities of cancer cells via suppression of the HIF1alpha pathway. Int. J. Oncol. 53 (3), 1160–1170. doi: 10.3892/ijo.2018.4479
Kim, K. M., Heo, D. R., Lee, J., Park, J. S., Baek, M. G., Yi, J. M., et al. (2015). 5,3’-Dihydroxy-6,7,4’-trimethoxyflavanone exerts its anticancer and antiangiogenesis effects through regulation of the Akt/mTOR signaling pathway in human lung cancer cells. Chem. Biol. Interact. 225, 32–9. doi: 10.1016/j.cbi.2014.10.033
Kim, M. H., Jeong, Y. J., Cho, H. J., Hoe, H. S., Park, K. K., Park, Y. Y., et al. (2017). Delphinidin inhibits angiogenesis through the suppression of HIF-1alpha and VEGF expression in A549 lung cancer cells. Oncol. Rep. 37 (2), 777–784. doi: 10.3892/or.2016.5296
Kondisetty, S., Menon, K. N., Pooleri, G. K. (2018). Fibronectin protein expression in renal cell carcinoma in correlation with clinical stage of tumour. Biomark Res. 6, 23. doi: 10.1186/s40364-018-0137-8
Kusumbe, A. P., Ramasamy, S. K., Adams, R. H. (2014). Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507 (7492), 323–328. doi: 10.1038/nature13145
Laurenzana, A., Chilla, A., Luciani, C., Peppicelli, S., Biagioni, A., Bianchini, F., et al. (2017). uPA/uPAR system activation drives a glycolytic phenotype in melanoma cells. Int. J. Cancer 141 (6), 1190–1200. doi: 10.1002/ijc.30817
Lee, J. J., Hsu, W. H., Yen, T. L., Chang, N. C., Luo, Y. J., Hsiao, G., et al. (2011). Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J. Ethnopharmacol. 134 (3), 824–830. doi: 10.1016/j.jep.2011.01.033
Li, J., Fan, Z. Z., Sun, J., Xu, J. H. (2011). In vitro antimetastatic effect of Changweiqing through antiinvasion of hypoxic colorectal carcinoma LoVo cells. Chin. J. Integr. Med. 17 (7), 517–24. doi: 10.1007/s11655-011-0785-0
Li, Y., Liu, X., Zhou, T., Kelley, M. R., Edwards, P., Gao, H., et al. (2014). Inhibition of APE1/Ref-1 redox activity rescues human retinal pigment epithelial cells from oxidative stress and reduces choroidal neovascularization. Redox. Biol. 2, 485–494. doi: 10.1016/j.redox.2014.01.023
Li, G., Shan, C., Liu, L., Zhou, T., Zhou, J., Hu, X., et al. (2015). Tanshinone IIA inhibits HIF-1alpha and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PLoS One 10 (2), e0117440. doi: 10.1371/journal.pone.0117440
Li, L., Saliba, P., Reischl, S., Marti, H. H., Kunze, R. (2016). Neuronal deficiency of HIF prolyl 4-hydroxylase 2 in mice improves ischemic stroke recovery in an HIF dependent manner. Neurobiol. Dis. 91, 221–235. doi: 10.1016/j.nbd.2016.03.018
Li, C., Zhang, B., Zhu, Y., Li, Y., Liu, P., Gao, B., et al. (2017). Post-stroke constraint-induced movement therapy increases functional recovery, angiogenesis, and neurogenesis with enhanced expression of HIF-1alpha and VEGF. Curr. Neurovasc. Res. 14 (4), 368–377. doi: 10.2174/1567202614666171128120558
Li, M. Y., Mi, C., Wang, K. S., Wang, Z., Zuo, H. X., Piao, L. X., et al. (2017). Shikonin suppresses proliferation and induces cell cycle arrest through the inhibition of hypoxia-inducible factor-1alpha signaling. Chem. Biol. Interact. 274, 58–67. doi: 10.1016/j.cbi.2017.06.029
Li, C., Wang, Q., Shen, S., Wei, X., Li, G. (2018). Oridonin inhibits VEGF-A-associated angiogenesis and epithelial-mesenchymal transition of breast cancer in vitro and in vivo. Oncol. Lett. 16 (2), 2289–2298. doi: 10.3892/ol.2018.8943
Li, Q. F., Decker-Rockefeller, B., Bajaj, A., Pumiglia, K. (2018). Activation of ras in the vascular endothelium induces brain vascular malformations and hemorrhagic stroke. Cell Rep. 24 (11), 2869–2882. doi: 10.1016/j.celrep.2018.08.025
Li, X., Ma, S., Yang, P., Sun, B., Zhang, Y., Sun, Y., et al. (2018). Anticancer effects of curcumin on nude mice bearing lung cancer A549 cell subsets SP and NSP cells. Oncol. Lett. 16 (5), 6756–6762. doi: 10.3892/ol.2018.9488
Li, X., Lu, Q., Xie, W., Wang, Y., Wang, G. (2018). Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/beta-Catenin signaling. Biochem. Biophys. Res. Commun. 496 (2), 443–449. doi: 10.1016/j.bbrc.2018.01.052
Lin-Holderer, J., Li, L., Gruneberg, D., Marti, H. H., Kunze, R. (2016). Fumaric acid esters promote neuronal survival upon ischemic stress through activation of the Nrf2 but not HIF-1 signaling pathway. Neuropharmacology 105, 228–240. doi: 10.1016/j.neuropharm.2016.01.023
Liu, Y. M., Liu, Y. K., Lan, K. L., Lee, Y. W., Tsai, T. H., Chen, Y. J. (2013). Medicinal fungus antrodia cinnamomea inhibits growth and cancer stem cell characteristics of hepatocellular carcinoma. Evid. Based Complement. Alternat. Med. 2013, 569737. doi: 10.1155/2013/569737
Liu, Y., Tang, Q., Shao, S., Chen, Y., Chen, W., Xu, X. (2017). Lyophilized powder of catalpol and puerarin protected cerebral vessels from ischemia by its anti-apoptosis on endothelial cells. Int. J. Biol. Sci. 13 (3), 327–338. doi: 10.7150/ijbs.17751
Liu, B., Luo, C., Zheng, Z., Xia, Z., Zhang, Q., Ke, C., et al. (2018). Shengui Sansheng San extraction is an angiogenic switch via regulations of AKT/mTOR, ERK1/2 and Notch1 signal pathways after ischemic stroke. Phytomedicine 44, 20–31. doi: 10.1016/j.phymed.2018.04.025
Liu, H., Tang, L., Li, X., Li, H. (2018). Triptolide inhibits vascular endothelial growth factor-mediated angiogenesis in human breast cancer cells. Exp. Ther. Med. 16 (2), 830–836. doi: 10.3892/etm.2018.6200
Liu, Y., Ran, H., Xiao, Y., Wang, H., Chen, Y., Chen, W., et al. (2018). Knockdown of HIF-1alpha impairs post-ischemic vascular reconstruction in the brain via deficient homing and sprouting bmEPCs. Brain Pathol. 28 (6). doi: 10.1111/bpa.12628
Llurba, E., Sanchez, O., Ferrer, Q., Nicolaides, K. H., Ruiz, A., Dominguez, C., et al. (2014). Maternal and foetal angiogenic imbalance in congenital heart defects. Eur. Heart. J. 35 (11), 701–707. doi: 10.1093/eurheartj/eht389
Lv, X. J., Zhao, L. J., Hao, Y. Q., Su, Z. Z., Li, J. Y., Du, Y. W., et al. (2015). Schisandrin B inhibits the proliferation of human lung adenocarcinoma A549 cells by inducing cycle arrest and apoptosis. Int. J. Clin. Exp. Med. 8 (5), 6926–6936.
Ma, J., Han, L. Z., Liang, H., Mi, C., Shi, H., Lee, J. J., et al. (2014). Celastrol inhibits the HIF-1alpha pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol. Rep. 32 (1), 235–42. doi: 10.3892/or.2014.3211
Magierowski, M., Magierowska, K., Hubalewska-Mazgaj, M., Surmiak, M., Sliwowski, Z., Wierdak, M., et al. (2018). Cross-talk between hydrogen sulfide and carbon monoxide in the mechanism of experimental gastric ulcers healing, regulation of gastric blood flow and accompanying inflammation. Biochem. Pharmacol. 149, 131–142. doi: 10.1016/j.bcp.2017.11.020
Manresa, M. C., Taylor, C. T. (2017). Hypoxia inducible factor (HIF) hydroxylases as regulators of intestinal epithelial barrier function. Cell Mol. Gastroenterol. Hepatol. 3 (3), 303–315. doi: 10.1016/j.jcmgh.2017.02.004
Mao, D., Lei, S., Ma, J., Shi, L., Zhang, S., Huang, J., et al. (2016). [Effect of jianpi-jiedu formula on tumor angiogenesis-relevant genes expression in colorectal cancer]. Zhong. Nan. Da. Xue. Xue. Bao. Yi. Xue. Ban. 41 (12), 1297–1304.
Mathew, T., Sarada, S. K. S. (2018). Intonation of Nrf2 and Hif1-alpha pathway by curcumin prophylaxis: a potential strategy to augment survival signaling under hypoxia. Respir. Physiol. Neurobiol. 258, 12–24. doi: 10.1016/j.resp.2018.09.008
Matsumoto, L., Hirota, Y., Saito-Fujita, T., Takeda, N., Tanaka, T., Hiraoka, T., et al. (2018). HIF2alpha in the uterine stroma permits embryo invasion and luminal epithelium detachment. J. Clin. Invest. 128 (7), 3186–3197. doi: 10.1172/JCI98931
Matteucci, E., Modora, S., Simone, M., Desiderio, M. A. (2003). Hepatocyte growth factor induces apoptosis through the extrinsic pathway in hepatoma cells: favouring role of hypoxia-inducible factor-1 deficiency. Oncogene 22 (26), 4062–4073. doi: 10.1038/sj.onc.1206519
Maxwell, P. H., Wiesener, M. S., Chang, G. W., Clifford, S. C., Vaux, E. C., Cockman, M. E., et al. (1999). The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399 (6733), 271–275. doi: 10.1038/20459
Mengozzi, M., Cervellini, I., Villa, P., Erbayraktar, Z., Gokmen, N., Yilmaz, O., et al. (2012). Erythropoietin-induced changes in brain gene expression reveal induction of synaptic plasticity genes in experimental stroke. Proc. Natl. Acad. Sci. U.S.A. 109 (24), 9617–9622. doi: 10.1073/pnas.1200554109
Mi, C., Ma, J., Wang, K. S., Zuo, H. X., Wang, Z., Li, M. Y., et al. (2017). Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1alpha via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J. Ethnopharmacol. 203, 27–38. doi: 10.1016/j.jep.2017.03.033
Mi, D. H., Fang, H. J., Zheng, G. H., Liang, X. H., Ding, Y. R., Liu, X., et al. (2018). DPP-4 inhibitors promote proliferation and migration of rat brain microvascular endothelial cells under hypoxic/high-glucose conditions, potentially through the SIRT1/HIF-1/VEGF pathway. CNS Neurosci. Ther. 25 (3). doi: 10.1111/cns.13042
Miki, K., Unno, N., Nagata, T., Uchijima, M., Konno, H., Koide, Y., et al. (2004). Butyrate suppresses hypoxia-inducible factor-1 activity in intestinal epithelial cells under hypoxic conditions. Shock 22 (5), 446–452. doi: 10.1097/01.shk.0000140664.80530.bd
Min, L., Ling, W., Hua, R., Qi, H., Chen, S., Wang, H., et al. (2016). Antiangiogenic therapy for normalization of tumor vasculature: a potential effect of Buyang Huanwu decoction on nude mice bearing human hepatocellular carcinoma xenografts with high metastatic potential. Mol. Med. Rep. 13 (3), 2518–2526. doi: 10.3892/mmr.2016.4854
Muller, S., Djudjaj, S., Lange, J., Iacovescu, M., Goppelt-Struebe, M., Boor, P. (2018). HIF stabilization inhibits renal epithelial cell migration and is associated with cytoskeletal alterations. Sci. Rep. 8 (1), 9497. doi: 10.1038/s41598-018-27918-9
Nieuwenhuis, J., Adamopoulos, A., Bleijerveld, O. B., Mazouzi, A., Stickel, E., Celie, P., et al. (2017). Vasohibins encode tubulin detyrosinating activity. Science 358 (6369), 1453–1456. doi: 10.1126/science.aao5676
Ohradanova, A., Gradin, K., Barathova, M., Zatovicova, M., Holotnakova, T., Kopacek, J., et al. (2008). Hypoxia upregulates expression of human endosialin gene via hypoxia-inducible factor 2. Br. J. Cancer 99 (8), 1348–1356. doi: 10.1038/sj.bjc.6604685
Ohsawa, S., Hamada, S., Kakinuma, Y., Yagi, T., Miura, M. (2005). Novel function of neuronal PAS domain protein 1 in erythropoietin expression in neuronal cells. J. Neurosci. Res. 79 (4), 451–458. doi: 10.1002/jnr.20365
Ota, I., Li, X. Y., Hu, Y., Weiss, S. J. (2009). Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl. Acad. Sci. U.S.A. 106 (48), 20318–20323. doi: 10.1073/pnas.0910962106
Oyenihi, A. B., Smith, C. (2019). Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 229, 54–72. doi: 10.1016/j.jep.2018.09.037
Pahlman, S., Lund, L. R., Jogi, A. (2015). Differential HIF-1alpha and HIF-2alpha expression in mammary epithelial cells during fat pad invasion, lactation, and involution. PLoS One 10 (5), e0125771. doi: 10.1371/journal.pone.0125771
Pan, W. L., Wong, J. H., Fang, E. F., Chan, Y. S., Ng, T. B., Cheung, R. C. (2014). Preferential cytotoxicity of the type I ribosome inactivating protein alpha-momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochem. Pharmacol. 89 (3), 329–339. doi: 10.1016/j.bcp.2014.03.004
Park, S. Y., Kang, J. H., Jeong, K. J., Lee, J., Han, J. W., Choi, W. S., et al. (2011). Norepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanism. Int. J. Cancer 128 (10), 2306–2316. doi: 10.1002/ijc.25589
Park, S. Y., Kwon, S. J., Lim, S. S., Kim, J. K., Lee, K. W., Park, J. H. (2016). Licoricidin, an active compound in the hexane/ethanol extract of Glycyrrhiza uralensis, inhibits lung metastasis of 4T1 murine mammary carcinoma cells. Int. J. Mol. Sci. 17 (6), 934. doi: 10.3390/ijms17060934
Park, E. J., Lee, Y. M., Oh, T. I., Kim, B. M., Lim, B. O., Lim, J. H. (2017). Vanillin suppresses cell motility by inhibiting STAT3-Mediated HIF-1alpha mRNA expression in malignant melanoma cells. Int. J. Mol. Sci. 18 (3), 532. doi: 10.3390/ijms18030532
Pena-Mercado, E., Garcia-Lorenzana, M., Arechaga-Ocampo, E., Gonzalez-De la Rosa, C. H., Beltran, N. E. (2018). Evaluation of HIF-1alpha and iNOS in ischemia/reperfusion gastric model: bioimpedance, histological and immunohistochemical analyses. Histol. Histopathol. 33 (8), 815–823.
Peterle, G. T., Maia, L. L., Trivilin, L. O., de Oliveira, M. M., Dos Santos, J. G., Mendes, S. O., et al. (2018). PAI-1, CAIX, and VEGFA expressions as prognosis markers in oral squamous cell carcinoma. J. Oral Pathol. Med. 47 (6), 566–574. doi: 10.1111/jop.12721
Pio, R., Ajona, D., Lambris, J. D. (2013). Complement inhibition in cancer therapy. Semin. Immunol. 25 (1), 54–64. doi: 10.1016/j.smim.2013.04.001
Prangsaengtong, O., Jantaree, P., Lirdprapamongkol, K., Svasti, J., Koizumi, K. (2018). Shikonin suppresses lymphangiogenesis via NF-kappaB/HIF-1alpha axis inhibition. Biol. Pharm. Bull. 41 (11), 1659–1666. doi: 10.1248/bpb.b18-00329
Qin, J. J., Li, X., Hunt, C., Wang, W., Wang, H., Zhang, R. (2018). Natural products targeting the p53-MDM2 pathway and mutant p53: recent advances and implications in cancer medicine. Genes. Dis. 5 (3), 204–219. doi: 10.1016/j.gendis.2018.07.002
Ranjbarnejad, T., Saidijam, M., Moradkhani, S., Najafi, R. (2017a). Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Prostaglandins Other Lipid Mediat. 131, 1–8. doi: 10.1016/j.prostaglandins.2017.05.003
Ranjbarnejad, T., Saidijam, M., Tafakh, M. S., Pourjafar, M., Talebzadeh, F., Najafi, R. (2017b). Garcinol exhibits anti-proliferative activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells. Hum. Exp. Toxicol. 36 (7), 692–700. doi: 10.1177/0960327116660865
Rausch, L. K., Hofer, M., Pramsohler, S., Kaser, S., Ebenbichler, C., Haacke, S., et al. (2018). Adiponectin, leptin and visfatin in hypoxia and its effect for weight loss in obesity. Front. Endocrinol. (Lausanne) 9, 615. doi: 10.3389/fendo.2018.00615
Rius, J., Guma, M., Schachtrup, C., Akassoglou, K., Zinkernagel, A. S., Nizet, V., et al. (2008). NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453 (7196), 807–811. doi: 10.1038/nature06905
Rivera, L. B., Bergers, G. (2014). Angiogenesis. Science 344 (6191), 1449–1450. doi: 10.1126/science.1257071
Ryou, M. G., Choudhury, G. R., Li, W., Winters, A., Yuan, F., Liu, R., et al. (2015). Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience 301, 193–203. doi: 10.1016/j.neuroscience.2015.05.064
Sathya, S., Sudhagar, S., Vidhya Priya, M., Bharathi Raja, R., Muthusamy, V. S., Niranjali Devaraj, S., et al. (2010). 3beta-hydroxylup-20(29)-ene-27,28-dioic acid dimethyl ester, a novel natural product from Plumbago zeylanica inhibits the proliferation and migration of MDA-MB-231 cells. Chem. Biol. Interact. 188 (3), 412–420. doi: 10.1016/j.cbi.2010.07.019
Sato, K., Morimoto, N., Kurata, T., Mimoto, T., Miyazaki, K., Ikeda, Y., et al. (2012). Impaired response of hypoxic sensor protein HIF-1alpha and its downstream proteins in the spinal motor neurons of ALS model mice. Brain Res. 1473, 55–62. doi: 10.1016/j.brainres.2012.07.040
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3 (10), 721–732. doi: 10.1038/nrc1187
Sena, J. A., Wang, L., Pawlus, M. R., Hu, C. J. (2014). HIFs enhance the transcriptional activation and splicing of adrenomedullin. Mol. Cancer Res. 12 (5), 728–741. doi: 10.1158/1541-7786.MCR-13-0607
Sendoel, A., Kohler, I., Fellmann, C., Lowe, S. W., Hengartner, M. O. (2010). HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature 465 (7298), 577–583. doi: 10.1038/nature09141
Senior, K. (2001). Angiogenesis and functional recovery demonstrated after minor stroke. Lancet 358 (9284), 817. doi: 10.1016/S0140-6736(01)06014-7
Sharma, D., Singh, P., Singh, S. S. (2018). beta-N-oxalyl-L-alpha,beta-diaminopropionic acid induces wound healing by stabilizing HIF-1alpha and modulating associated protein expression. Phytomedicine 44, 9–19. doi: 10.1016/j.phymed.2018.04.024
Shi, H. (2009). Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 16 (34), 4593–600. doi: 10.2174/092986709789760779
Shi, G. H., Zhou, L. (2018). Emodin suppresses angiogenesis and metastasis in anaplastic thyroid cancer by affecting TRAF6mediated pathways in vivo and in vitro. Mol. Med. Rep. 5191–5197. doi: 10.3892/mmr.2018.9510
Shi, L., Zhang, G., Zheng, Z., Lu, B., Ji, L. (2017). Andrographolide reduced VEGFA expression in hepatoma cancer cells by inactivating HIF-1alpha: the involvement of JNK and MTA1/HDCA. Chem. Biol. Interact. 273, 228–236. doi: 10.1016/j.cbi.2017.06.024
Shiau, A. L., Shen, Y. T., Hsieh, J. L., Wu, C. L., Lee, C. H. (2014). Scutellaria barbata inhibits angiogenesis through downregulation of HIF-1 alpha in lung tumor. Environ. Toxicol. 29 (4), 363–70. doi: 10.1002/tox.21763
Singh, D., Arora, R., Kaur, P., Singh, B., Mannan, R., Arora, S. (2017). Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci. 7, 62. doi: 10.1186/s13578-017-0190-2
Soleimannejad, K., Rahmani, A., Hatefi, M., Khataminia, M., Hafezi Ahmadi, M. R., Asadollahi, K. (2017). Effects of nigella sativa extract on markers of cerebral angiogenesis after global ischemia of brain in rats. J. Stroke Cerebrovasc. Dis. 26 (7), 1514–1520. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.040
Soleymani Abyaneh, H., Gupta, N., Radziwon-Balicka, A., Jurasz, P., Seubert, J., Lai, R., et al. (2017). STAT3 but not HIF-1alpha is important in mediating hypoxia-induced chemoresistance in MDA-MB-231, a triple negative breast cancer cell line. Cancers (Basel) 9 (10), 137. doi: 10.3390/cancers9100137
Soni, S., Padwad, Y. S. (2017). HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta. Oncol. 56 (4), 503–515. doi: 10.1080/0284186X.2017.1301680
Su, M. T., Lin, S. H., Chen, Y. C., Kuo, P. L. (2014). Gene-gene interactions and gene polymorphisms of VEGFA and EG-VEGF gene systems in recurrent pregnancy loss. J. Assist. Reprod. Genet. 31 (6), 699–705. doi: 10.1007/s10815-014-0223-2
Sui, H., Zhao, J., Zhou, L., Wen, H., Deng, W., Li, C., et al. (2017). Tanshinone IIA inhibits beta-catenin/VEGF-mediated angiogenesis by targeting TGF-beta1 in normoxic and HIF-1alpha in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 403, 86–97. doi: 10.1016/j.canlet.2017.05.013
Suvanish Kumar, V. S., Pretorius, E., Rajanikant, G. K. (2018). The synergistic combination of everolimus and paroxetine exerts post-ischemic neuroprotection in vitro. Cell Mol. Neurobiol. 38 (7), 1383–1397. doi: 10.1007/s10571-018-0605-6
Taheem, D. K., Foyt, D. A., Loaiza, S., Ferreira, S. A., Ilic, D., Auner, H. W., et al. (2018). Differential regulation of human bone marrow mesenchymal stromal cell chondrogenesis by hypoxia inducible factor-1alpha hydroxylase inhibitors. Stem. Cells. 36 (9), 1380–1392. doi: 10.1002/stem.2844
Tal, R., Shaish, A., Barshack, I., Polak-Charcon, S., Afek, A., Volkov, A., et al. (2010). Effects of hypoxia-inducible factor-1alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 177 (6), 2950–2962. doi: 10.2353/ajpath.2010.090800
Tian, G., Guo, L., Gao, W. (2010). Use of compound Chinese medicine in the treatment of lung cancer. Curr. Drug Discov. Technol. 7 (1), 32–36. doi: 10.2174/157016310791162776
Toth, R. K., Warfel, N. A. (2017). Strange bedfellows: nuclear factor, erythroid 2-like 2 (Nrf2) and hypoxia-inducible factor 1 (HIF-1) in tumor hypoxia. Antioxidants (Basel) 6 (2), E27. doi: 10.3390/antiox6020027
Toullec, A., Buard, V., Rannou, E., Tarlet, G., Guipaud, O., Robine, S., (2018). HIF-1alpha deletion in the endothelium, but not in the epithelium, protects from radiation-induced enteritis. Cell Mol. Gastroenterol. Hepatol. 5 (1), 15–30. doi: 10.1016/j.jcmgh.2017.08.001
Trollmann, R., Muhlberger, T., Richter, M., Boie, G., Feigenspan, A., Brackmann, F., et al. (2018). Differential regulation of angiogenesis in the developing mouse brain in response to exogenous activation of the hypoxia-inducible transcription factor system. Brain Res. 1688, 91–102. doi: 10.1016/j.brainres.2018.03.012
Tsai, I. T., Kuo, C. C., Liou, J. P., Chang, J. Y. (2018). Novel microtubule inhibitor MPT0B098 inhibits hypoxia-induced epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. J. Biomed. Sci. 25 (1), 28. doi: 10.1186/s12929-018-0432-6
Tsang, C. M., Cheung, K. C., Cheung, Y. C., Man, K., Lui, V. W., Tsao, S. W., et al. (2015). Berberine suppresses Id-1 expression and inhibits the growth and development of lung metastases in hepatocellular carcinoma. Biochim. Biophys. Acta. 1852 (3), 541–551. doi: 10.1016/j.bbadis.2014.12.004
Tyszka-Czochara, M., Lasota, M., Majka, M. (2018). Caffeic acid and metformin inhibit invasive phenotype induced by TGF-beta1 in C-4I and HTB-35/SiHa human cervical squamous carcinoma cells by acting on different molecular targets. Int. J. Mol. Sci. 19 (1), E266. doi: 10.3390/ijms19010266
Wang, F. R., Jiang, Y. S. (2015). Effect of treatment with baicalein on the intracerebral tumor growth and survival of orthotopic glioma models. J. Neurooncol. 124 (1), 5–11. doi: 10.1007/s11060-015-1804-3
Wang, B. S., Zhang, L. S., Song, D. M., Zhang, J. H., Liu, Y. M. (2009). [Effect of gensenoside Rg3 on apoptosis of Hep-2 and expression of HIF-1alha in human laryngeal cancer cell line under anoxic conditions]. Zhong. Yao. Cai. 32 (1), 102–106.
Wang, C., Wang, Z., Zhang, X., Zhang, X., Dong, L., Xing, Y., et al. (2012). Protection by silibinin against experimental ischemic stroke: up-regulated pAkt, pmTOR, HIF-1alpha and Bcl-2, down-regulated Bax, NF-kappaB expression. Neurosci. Lett. 529 (1), 45–50. doi: 10.1016/j.neulet.2012.08.078
Wang, Z., Wang, N., Han, S., Wang, D., Mo, S., Yu, L., (2013). Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS One 8 (7), e68566. doi: 10.1371/journal.pone.0068566
Wang, D., Xin, Y., Tian, Y., Li, W., Sun, D., Yang, Y. (2017). Pseudolaric acid B inhibits gastric cancer cell metastasis in vitro and in haematogenous dissemination model through PI3K/AKT, ERK1/2 and mitochondria-mediated apoptosis pathways. Exp. Cell. Res. 352 (1), 34–44. doi: 10.1016/j.yexcr.2017.01.012
Wang, X. P., Wang, Q. X., Lin, H. P., Chang, N. (2017). Anti-tumor bioactivities of curcumin on mice loaded with gastric carcinoma. Food Funct. 8 (9), 3319–3326. doi: 10.1039/C7FO00555E
Wang, W., Xu, B., Xuan, H., Ge, Y., Wang, Y., Wang, L., et al. (2018). Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J. Vasc. Surg. 68 (5), 1538–1550 e2. doi: 10.1016/j.jvs.2017.09.030
Wei, Y., Hong, H., Zhang, X., Lai, W., Wang, Y., Chu, K., et al. (2017). Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation 40 (4), 1297–1309. doi: 10.1007/s10753-017-0573-x
Weih, M., Bergk, A., Isaev, N. K., Ruscher, K., Megow, D., Riepe, M., et al. (1999). Induction of ischemic tolerance in rat cortical neurons by 3-nitropropionic acid: chemical preconditioning. Neurosci. Lett. 272 (3), 207–210. doi: 10.1016/S0304-3940(99)00594-7
Woodley, D. T., Fan, J., Cheng, C. F., Li, Y., Chen, M., Bu, G., et al. (2009). Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90alpha autocrine signaling to promote keratinocyte migration. J. Cell. Sci. 122 (Pt 10), 1495–1498. doi: 10.1242/jcs.047894
Wu, C., Chen, J., Chen, C., Wang, W., Wen, L., Gao, K., et al. (2015). Wnt/beta-catenin coupled with HIF-1alpha/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 5, 16151. doi: 10.1038/srep16151
Xing, Y., Mi, C., Wang, Z., Zhang, Z. H., Li, M. Y., Zuo, H. X., et al. (2018). Fraxinellone has anticancer activity in vivo by inhibiting programmed cell death-ligand 1 expression by reducing hypoxia-inducible factor-1alpha and STAT3. Pharmacol. Res. 135, 166–180. doi: 10.1016/j.phrs.2018.08.004
Xu, W., Yang, G., Xu, Y., Zhang, Q., Fu, Q., Yu, J., et al. (2014). The possibility of traditional Chinese medicine as maintenance therapy for advanced nonsmall cell lung cancer. Evid. Based Complement. Alternat. Med. 2014, 278917. doi: 10.1155/2014/278917
Yamakawa, M., Liu, L. X., Belanger, A. J., Date, T., Kuriyama, T., Goldberg, M. A., et al. (2004). Expression of angiopoietins in renal epithelial and clear cell carcinoma cells: regulation by hypoxia and participation in angiogenesis. Am. J. Physiol. Renal. Physiol. 287 (4), F649–657. doi: 10.1152/ajprenal.00028.2004
Yang, X., Yang, B., Cai, J., Zhang, C., Zhang, Q., Xu, L., et al. (2013). Berberine enhances radiosensitivity of esophageal squamous cancer by targeting HIF-1alpha in vitro and in vivo. Cancer. Biol. Ther. 14 (11), 1068–1073. doi: 10.4161/cbt.26426
Yeh, S. H., Hung, J. J., Gean, P. W., Chang, W. C. (2008). Hypoxia-inducible factor-1alpha protects cultured cortical neurons from lipopolysaccharide-induced cell death via regulation of NR1 expression. J. Neurosci. 28 (52), 14259–14270. doi: 10.1523/JNEUROSCI.4258-08.2008
Yen, T. L., Hsu, C. K., Lu, W. J., Hsieh, C. Y., Hsiao, G., Chou, D. S., et al. (2012). Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J. Agric. Food Chem. 60 (8), 1937–1944. doi: 10.1021/jf204909p
Yen, T. L., Ong, E. T., Lin, K. H., Chang, C. C., Jayakumar, T., Lin, S. C., et al. (2014). Potential advantages of Chinese medicine Taohong Siwu Decoction () combined with tissue-plasminogen activator for alleviating middle cerebral artery occlusion-induced embolic stroke in rats. Chin. J. Integr. Med. doi: 10.1007/s11655-014-1847-x
Yeom, C. J., Zeng, L., Zhu, Y., Hiraoka, M., Harada, H. (2011). Strategies to assess hypoxic/hif-1-active cancer cells for the development of innovative radiation therapy. Cancers (Basel) 3 (3), 3610–3631. doi: 10.3390/cancers3033610
Yu, X., Tong, Y., Kwok, H. F., Sze, S. C., Zhong, L., Lau, C. B., et al. (2012). Anti-angiogenic activity of Erxian Decoction, a traditional Chinese herbal formula, in zebrafish. Biol. Pharm. Bull. 35 (12), 2119–2127. doi: 10.1248/bpb.b12-00130
Yuan, C., Wang, H., Yuan, Z. (2019). Ginsenoside Rg1 inhibits myocardial ischaemia and reperfusion injury via HIF-1 alpha-ERK signalling pathways in a diabetic rat model. Pharmazie 74 (3), 157–162.
Zagzag, D., Zhong, H., Scalzitti, J. M., Laughner, E., Simons, J. W., Semenza, G. L. (2000). Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer 88 (11), 2606–2618. doi: 10.1002/1097-0142(20000601)88:11<2606::AID-CNCR25>3.0.CO;2-W
Zaman, K., Ryu, H., Hall, D., O'Donovan, K., Lin, K. I., Miller, M. P., (1999). Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. 19 (22), 9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999
Zdralevic, M., Vucetic, M., Daher, B., Marchiq, I., Parks, S. K., Pouyssegur, J. (2018). Disrupting the ‘Warburg effect’ re-routes cancer cells to OXPHOS offering a vulnerability point via‘ferroptosis’-induced cell death. Adv. Biol. Regul. 68, 55–63. doi: 10.1016/j.jbior.2017.12.002
Zeinali, T., Mansoori, B., Mohammadi, A., Baradaran, B. (2018). Regulatory mechanisms of miR-145 expression and the importance of its function in cancer metastasis. Biomed. Pharmacother. 109, 195–207. doi: 10.1016/j.biopha.2018.10.037
Zhang, Z. G., Chopp, M. (2009). Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 8 (5), 491–500. doi: 10.1016/S1474-4422(09)70061-4
Zhang, S., Zhang, Z., Sandhu, G., Ma, X., Yang, X., Geiger, J. D., et al. (2007). Evidence of oxidative stress-induced BNIP3 expression in amyloid beta neurotoxicity. Brain Res. 1138, 221–230. doi: 10.1016/j.brainres.2006.12.086
Zhang, Z., Yan, J., Chang, Y., Du Yan, S., Shi, H. (2011). Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem. 18 (28), 4335–4343. doi: 10.2174/092986711797200426
Zhang, Q., Qian, Z., Pan, L., Li, H., Zhu, H. (2012). Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta. Physiol. Hung. 99 (3), 311–323. doi: 10.1556/APhysiol.99.2012.3.8
Zhang, C., Yang, X., Zhang, Q., Yang, B., Xu, L., Qin, Q., et al. (2014). Berberine radiosensitizes human nasopharyngeal carcinoma by suppressing hypoxia-inducible factor-1alpha expression. Acta. Otolaryngol. 134 (2), 185–192. doi: 10.3109/00016489.2013.850176
Zhang, Q., Zhang, C., Yang, X., Yang, B., Wang, J., Kang, Y., et al. (2014a). Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn. Pathol. 9, 98. doi: 10.1186/1746-1596-9-98
Zhang, Q., Bian, H., Li, Y., Guo, L., Tang, Y., Zhu, H. (2014b). Preconditioning with the traditional Chinese medicine Huang-Lian-Jie-Du-Tang initiates HIF-1alpha-dependent neuroprotection against cerebral ischemia in rats. J. Ethnopharmacol. 154 (2), 443–452. doi: 10.1016/j.jep.2014.04.022
Zhang, K., Han, E. S., Dellinger, T. H., Lu, J., Nam, S., Anderson, R. A., et al. (2017). Cinnamon extract reduces VEGF expression via suppressing HIF-1alpha gene expression and inhibits tumor growth in mice. Mol. Carcinog. 56 (2), 436–446. doi: 10.1002/mc.22506
Zhang, L., Chen, C., Duanmu, J., Wu, Y., Tao, J., Yang, A., et al. (2018). Cryptotanshinone inhibits the growth and invasion of colon cancer by suppressing inflammation and tumor angiogenesis through modulating MMP/TIMP system, PI3K/Akt/mTOR signaling and HIF-1alpha nuclear translocation. Int. Immunopharmacol. 65, 429–437. doi: 10.1016/j.intimp.2018.10.035
Zhou, Z. L., Luo, Z. G., Yu, B., Jiang, Y., Chen, Y., Feng, J. M., et al. (2010). Increased accumulation of hypoxia-inducible factor-1alpha with reduced transcriptional activity mediates the antitumor effect of triptolide. Mol. Cancer 9, 268. doi: 10.1186/1476-4598-9-268
Zhu, H., Zhang, S. (2018). Hypoxia inducible factor-1alpha/vascular endothelial growth factor signaling activation correlates with response to radiotherapy and its inhibition reduces hypoxia-induced angiogenesis in lung cancer. J. Cell. Biochem. 119 (9), 7707–7718. doi: 10.1002/jcb.27120
Zhu, P., Wu, Y., Yang, A., Fu, X., Mao, M., Liu, Z. (2017). Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 95, 68–76. doi: 10.1016/j.biopha.2017.08.049
Keywords: angiogenesis, herbal medicine, hypoxia-inducible factor-1, cancer, ischemic stroke
Citation: Hong M, Shi H, Wang N, Tan H-Y, Wang Q and Feng Y (2019) Dual Effects of Chinese Herbal Medicines on Angiogenesis in Cancer and Ischemic Stroke Treatments: Role of HIF-1 Network. Front. Pharmacol. 10:696. doi: 10.3389/fphar.2019.00696
Received: 12 March 2019; Accepted: 29 May 2019;
Published: 26 June 2019.
Edited by:
Issy Laher, University of British Columbia, CanadaReviewed by:
Wentzel Christoffel Gelderblom, Cape Peninsula University of Technology, South AfricaMaria Luisa Del Moral, Universidad de Jaén, Spain
Copyright © 2019 Hong, Shi, Wang, Tan, Wang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yibin Feng, eWZlbmdAaGt1Lmhr; Qi Wang, d2FuZ3FpQGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work.
 Ming Hong
Ming Hong Honglian Shi
Honglian Shi Ning Wang
Ning Wang Hor-Yue Tan
Hor-Yue Tan Qi Wang
Qi Wang Yibin Feng
Yibin Feng