- 1Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education, College of Life Sciences, Northwest University, Xi’an, China
- 2Department of Pharmacy, Xi ‘an Mental Health Center, Xi’an, China
- 3College of Chemistry & Chemical Engineering, Xi ‘an Shiyou University, Xi’an, China
As the first and key step of traditional Chinese medicine (TCM)-guided drug development, lead discovery necessitates continuous exploration of new methodology for screening bioactive compounds from TCM. This work intends to establish a strategy for rapidly recognizing β2-adrenergic receptor (β2-AR) target compounds from the fruit of Siraitia grosvenorii (LHG). The method involved immobilization of β2-AR onto amino-microsphere to synthesize the receptor column, the combination of the column to high-performance liquid chromatography (HPLC) to screen bioactive compounds of LHG, the identification of the compounds by HPLC coupled with mass spectrometry (MS), and the evaluation of druggability through pharmacokinetic examination by HPLC–MS/MS. Mogroside V was screened and identified as the β2-AR-targeted bioactive compounds in LHG. This compound exhibited desired pharmacokinetic behavior including the time to reach peak plasma concentrations of 45 min, the relatively low elimination of 138.5 min, and the high bioavailability. These parameters indicated that mogroside V has a good druggability for the development of new drugs fighting β2-AR-mediated respiratory ailments like asthma. The combination of the methods in this work is probably becoming a powerful strategy for screening and early evaluating the bioactive compounds specifically binding to G-protein-coupled receptor target from complex matrices including TCM.
Introduction
Traditional medicine like traditional Chinese medicine (TCM) has made a great contribution to fight ailments and to protect the health of oriental people. In practice, many herbs have been prescribed as food additives in single form besides the utilization of complex prescription. Such practice necessitates the recognition of bioactive compounds not only the complex prescription but also the single herb in medicine, chemistry, and food industry (Sun et al., 2013; Bernardini et al., 2017; Zhang, 2017). This has been partly hindered for several reasons, including the complex composition, dialectical flexibility, and diverse therapeutic mechanism of TCM (Zhao et al., 2015). To address this issue, ongoing works are urgently needed to pursue the new assays that enable rapid recognition of the bioactive compounds in a single herb of complex prescription.
A survey of the literature has resulted in several modern techniques that have potential in screening bioactive compounds from TCM. These methodologies include bioactivity-guided fragmentation and separation (Sun et al., 2018; Li et al., 2019; Sari and Kececi, 2019), serum pharmacochemistry (He et al., 2015; Han et al., 2016), metabolomics (Liu et al., 2018; Wu et al., 2019), and computer virtualization technology (Mofidifar et al., 2018; Van Hilten et al., 2019). Among these assays, the bioactivity-guided fragmentation and separation are the most classic and broadly accepted method for screening compounds of interest for TCM. Despite the successful application during a long period, this method has been limited due to the loss of bioactive compounds during the gradual separating procedure. Serum pharmacochemistry has focused on identification of the compounds in serum after administration of certain TCM. Owing to such improvement, this method has the capacity to address the issue of bioactive compound loss generated by bioactivity-guided fragmentation and separation method. As a powerful method, metabolomics display an outstanding capacity to discover the biomarker of TCM and elucidate the associated mechanism. The application of this method is partly limitedly ascribed to the requirement of relatively expensive instrumental platform. Computer virtualization technology is a theoretical method that is powerful for exploration of the binding mechanism of target–compound interaction. The main issue of this method is the lack of capability in complex sample analysis. Taken together, the development of new assays for screening bioactive compounds from complex sample is still on the way to address these abovementioned issues.
Biomedical chromatography (Li et al., 2015; Wang et al., 2017a; Han et al., 2018) has the property of incorporating the specificity of target–drug recognition into the separating procedure of chromatographic analysis. It is believed to be a powerful tool for analyzing complex samples regarding the purpose of screening bioactive compounds specifically binding to the immobilized target. Although their application is successful in many cases, such methods need further improvement because most of the current methods concentrate on the transporting protein like serum albumin but much less on the largest drug targets such as G-protein-coupled receptors. Taking inspiration from the pioneer work by Wainer et al. (Wainer et al., 1999; Moaddel and Wainer, 2006; Krzysztof et al., 2010), we have developed a series of immobilized G-protein-coupled receptor-based chromatographic methods (Zhao et al., 2010; Zhao et al., 2012; Zeng et al., 2018). Broad application and validation of these methods in real sample are required to make them a powerful tool for screening bioactive compounds of TCM.
Siraitia grosvenorii (Swingle) C. Jeffrey ex A. M. Lu & Z. Y. Zhang is primarily cultivated in Guangxi, Guangdong, Hunan, and Jiangxi provinces of South China. The fruits of this plant, known as Luo Han Guo (LHG) in China, as a traditional medicine, have been widely used in treating coughs, sore throats, and constipation (Li et al., 2007; Qi et al., 2008; Lu et al., 2012). The mechanism of these diseases is confirmed to be associated with the signaling pathway of β2-adrenergic receptor (β2-AR) (Tamaoki et al., 2010; Cortez et al., 2016; Balabolkin, et al., 2017). Inspired by these reports, we hypothesized that S. grosvenorii has bioactive compounds that specifically bind to β2-AR. This work intends to analyze the aqueous extract of the herb by immobilized β2-AR-based chromatographic methods. Druggability of the screened compound was also evaluated through the analysis of pharmacokinetic behavior by high-performance liquid chromatography (HPLC) coupled with electrospray (ESI) and tandem mass spectrometry (MS/MS).
Materials and Methods
Materials and Instruments
Reference standards of salbutamol (batch no. 100328-200703) and terbutaline (batch no.100273-201202) were obtained from the National Institutes for Food and Drug Control (Beijing, China). Mogroside V (batch no. 181201A) was from Nanjing Daosifu Biotechnology (Nanjing, China). Notoginsenoside R1 (batch no. 110745-200415) (Figure 1A) was utilized as internal standard (IS) and was obtained from the National Institutes for Food and Drug Control (Beijing, China). ICI 118551 hydrochloride (batch no. 045M4622V) was bought from Sigma (St. Louis, MO, USA) and was a selective β2-AR antagonist. Ampicillin (Amp) was purchased from Regal Biology Technology Co (Shanghai, China). All other reagents were analytically pure unless especially stated.
The fruits of S. grosvenorii (LHG) were purchased from Beijing Tongrentang Herbal (Xi’an, China) and authenticated by professor Fang Minfeng, Department of Traditional Chinese Medicine of Northwest University. Voucher specimens were deposited at College of Life Sciences, Northwest University (Xi’an China), No. NWU-CLS-20181023-72. The packing machine was supplied by Dalian Elite Analytical Instruments Company (Dalian, China). The chromatographic system consisted of an Elite 3100 series apparatus (Dalian Elite Analytical Instruments Company, Dalian, China), which was equipped with an isocratic pump, a column oven, and an ultraviolet–visible detector. Separation and identification of the bioactive compounds were carried out on an Agilent 1100 high-performance liquid chromatography (HPLC) including a G1379A vacuum degasser, a G1311A quaternary pump, a G1316A thermostatted column oven, and a G1313A autosampler (Agilent, Germany) coupled with an SL Agilent 1100 tandem mass spectrometer, which was equipped with an electrospray ionization (ESI) source. In vitro pharmacological activity of the screened bioactive compounds was assessed by BL-420s biological function experiment system (Chengdu Techman Science and Technology Ltd, Chengdu, China). In vitro tissues were maintained in a physiological environment by SV-8 isothermal perfusion device (Chengdu Techman Science and Technology Ltd, Chengdu, China) and HSS-1(b) thermostatic bath (Chengdu Instrument Factory, Chengdu, China).
Methods
Preparativon of LHG Extract
The aqueous extract of LHG was prepared by hot refluxing. Briefly, 100-g dried LHG was immersed in 10-folds of water and remained for 30 min. Subsequent treatment of the herb was performed by hot refluxing method with duration of 60, 50, and 40 min for each time. The suspension of each time was collected together and filtered by vacuum filtration. The filtrate was concentrated to 50 ml by rotary evaporation at 65°C. The result solution was stored at 4°C for further use.
Synthesis of Immobilized β2-AR Stationary Phase
According to the method in our previous work, we constructed the plasmid of pReceiver-β2-AR-Halo and transferred the clones into Escherichia coli BL21 (DE3) (Zeng et al., 2018). We incubated the clones in 50-ml Luria–Bertani (LB) medium containing 100 μg/mL of ampicillin overnight at 37°C and propagated the culture into self-induction medium for an additional 12.0-h incubation. We collected the cells by centrifugation and suspended the cell pellets in lysis buffer followed by incubation for 30 min at 37°C. This suspension was disrupted by ultrasonication and was centrifuged to collect the supernatant for immobilization.
The halo-tagged β2-AR protein was immobilized on the surface of amino-microspheres using the reported method (Zeng et al., 2018). Briefly, 6-chloroacetic acid-modified amino-microspheres were suspended in DMF containing N,N-diisopropylethylamine and HATU under room temperature. The suspension was stirred 4.0 h to activate the microspheres. The activated microspheres were collected by filtering the suspension and were totally rinsed by phosphate buffer (20 mM, pH = 7.4). The immobilization of β2-AR was achieved by mixing the activated spheres with cell lysates using phosphate buffer (20 mM, pH = 7.4) as the solvent. This suspension was stirred 2.0 h and was filtered to collect the immobilized receptor by centrifugation. The unbound protein was removed by rinsing the microspheres using phosphate buffer (20 mM, pH = 7.4). The result microspheres were packed into a stainless-steel column (50 mm × 4.6 mm) using phosphate buffer (20 mM, pH = 7.4) as slurry and propulsive agent under a pressure of 400 bar.
Characterization of the β2-AR Column
Sodium nitrite was used as a negative control to determine the void time of the chromatographic system. In this case, the detection wavelength was set at 254 nm. The specificity of the immobilized β2-AR column was characterized by retention behaviors of salbutamol and terbutaline (agonists of β2-AR) using ammonium acetate (5 mM, pH = 7.4) as the mobile phase. The detection wavelength was 276 nm. The stability of the column was examined by continuous analyses of the retention times and peak profiles for 3 weeks.
Screening of the Bioactive Compounds in LHG
Prior to screening the bioactive compounds of LHG, we analyzed the extract of the herb using reversed-phase HPLC coupled with trap mass spectrometry. Here, the separation was performed on an Inertsil ODS-3-C18 column (5 μm, 4.6 mm × 150 mm). The mobile phase was 0.2% (v/v) formic acid water (A) and acetonitrile (B). Gradient elution was set at 10% B to 26% B (0–5 min), 26% B (5–15 min), 26% B to 33% B (15–25 min), 33% B to 80% B (25–30 min), and 80% B to 10% B (30–35 min). The flow rate was 0.6 ml/min, and the column temperature was 30°C. MS detection was performed in a negative mode with the scan range of 100–2,000 amu. The nebulizing gas pressure was 40 psi. The flow rate and temperature of the dry gas were 8.0 L/min and 350°C, respectively.
We utilized the column containing immobilized β2-AR to analyze the aqueous extract of LHG. The mobile phase was ammonium acetate buffer (5 mM, pH = 7.4), and the flow rate was 0.2 ml/min with a sampling volume of 20 μl under a detection wavelength of 203 nm. The retention peak that has a retention time longer than 0.7 min was collected as the bioactive compounds specifically binding to β2-AR. Such collection was further separated and identified by HPLC–MS. The separating and identifying conditions for this case were identical to those in the analysis of the extract by reversed-phase HPLC–MS.
Pharmacokinetic Study
Animals
Male Sprague–Dawley (SD) rats (240 ± 20 g) were purchased from the Experimental Animal Center of Xi’an Jiaotong University (Xi’an, China). The animals were maintained in a room under controlled temperature (24 ± 1°C) and humidity (50–70%) with 12-h light/dark cycle. The rats were fed with standard laboratory food and acclimatized to the environment for a week before the experiment. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals under the approval of the Animal Experimentation Ethics Committee at Northwest University.
HPLC–MS/MS Conditions for Pharmacokinetic Study
All the plasma samples were analyzed on an Inertsil ODS-3-C18 column (5 μm, 4.6 mm × 150 mm). The mobile phase for quantitative determination of mogroside V in rat plasma consisted of 29% acetonitrile/0.2% (v/v) formic acid at a flow rate of 0.6 ml/min. The injection volume was 20 μl under a detection wavelength of 203 nm.
The detection of mogroside V in plasma sample was achieved in negative ion mode. The optimized mass conditions include the following: capillary voltage of 3,500 V, drying gas of 8 L/min at 350°C, nebulizer pressure of 40 psi at 350°C, and ion scan range of 100–2,000 amu. Under these conditions, multi-reaction monitoring (MRM) mode was carried out to specifically and sensitively detect the mogroside V. The mass transitions were m/z 1,285.6 → 1,123.6 for mogroside V and m/z 931.3 → 637.5 for IS (notoginsenoside R1).
Preparation of Standards and Quality Control Samples
The standard stock solutions of mogroside V and IS were prepared by methanol to reach the concentration of 1.0 mg/ml. Diverse concentrations (244.1, 488.3, 976.6, 1,953.1, 7,812.5, 15,625, 31,250, and 62,500 ng/ml) of working solutions for mogroside V were prepared by diluting the stock solutions with blank plasma. A similar method was utilized to prepare the working solution of IS (50 μg/ml). All these solutions were stored at 4°C.
Calibration standards were prepared by spiking the working solutions (20 μl) into blank rat plasma (180 μl) to acquire the concentrations of 24.4, 48.8, 97.7, 195.3, 781.3, 1,562.5, 3,125, and 6,250 ng/ml. Quality control (QC) samples were prepared using the same method at 30 ng/ml (LQC), 500 ng/ml (MQC), and 5,000 ng/ml (HQC).
Pretreatment of Plasma Sample
An aliquot of 10 μl of IS working solution was added into an aliquot of 90 μl of plasma samples. Vortexing the mixture for 2.0 min, we centrifuged the plasma for 10 min with a speed of 12,000 rpm at a temperature of 4°C. The supernatant was collected and transferred to another 0.5-ml centrifuge tube and then blow dried with nitrogen. We applied 200 μl of methanol to redissolve it, which was used for analysis.
Validation of the Method for Pharmacokinetic Study
Selectivity and Specificity
The selectivity and specificity of the method were evaluated by analyzing the chromatograms of six different batches of blank plasma, blank plasma spiked with mogroside V, and IS, and the samples after administration of mogroside V.
Linearity of Calibration Curves and Lower Limits of Quantification
The calibration curve was obtained by the peak area ratios (y) of mogroside V to IS against the theoretical concentration (x) of mogroside V. Linearity of calibration curves was assessed by a linear regression using 1/x2 as weighting factor. The lower limit of quantification (LLOQ) having signal/noise (S/N) ratio ≥ 10 with acceptable accuracy [relative error (RE)] and precision [relative standard deviation (RSD)] of less than 20% was defined as the lowest drug concentration of the calibration curve.
Accuracy and Precision
Accuracy and precision were evaluated at three QC levels in six replicates on the same day and three analytical batches on six continuous days. Intra-day and inter-day precision [relative standard deviation (RSD)] and the accuracy [relative error (RE)] of such cases were required to be less than ±15%.
Extraction Recovery
The extraction recoveries of the analytes were determined at three levels of QC samples. They were calculated by comparing the mean peak area of QC samples with those of the blank plasma spiked with neat solutions after extraction (n = 6).
Stability
The stability of mogroside V in plasma was evaluated at three QC levels in six replicates under varieties of storing and processing conditions. The results of the peak area of QC samples were compared with those from freshly prepared standard samples. The QC samples with recoveries of 85–115% were defined as having good stability under the desired conditions. Short-term stability was assessed after QC samples were saved at room temperature for 14 h. Subsequently, freeze–thaw stability was assessed after three freeze (−20°C) and thaw (room temperature) cycles of QC samples. Long-term stability was examined after QC samples were saved at −20°C for 1 month.
Pharmacokinetic Study of Mogroside V
Eighteen rats were randomly divided into three groups of six for each group, including blank control, mogroside V, and LHG groups. The blank group was orally administered with normal saline. The other two groups were administered with mogroside V at a dose of 200 mg/kg and LHG aqueous extraction at a dose of 12.5 ml/kg (equivalent to 200 mg/kg mogroside V). Blood samples were collected in heparin sodium vacutainers at post-dose 5, 15, 30, 45, 60, 90, 120, 240, 360, 480, and 720 min. The plasma was pre-treated by centrifugation and frozen at −80°C until further analysis. The pharmacokinetic parameters including the area under curve (AUC0–720, and AUC0–∞), the maximum plasma concentration (Cmax), the time to achieve maximum plasma concentration (Tmax), and the elimination half-life (t1/2z) were calculated by Drug and Statistics 3.0.0 software (DAS, T. C. M., Shanghai, China). All data were recorded as the mean ± standard deviation (SD), and the 95% confidence interval was also estimated when necessary.
Results and Discussion
Feasibility of Immobilized β2-AR in Screening Bioactive Compounds of LHG
It is reported that the aqueous extract of LHG has significant inhibitory effect on cough of mice induced by concentrated ammonium hydroxide or sulfur dioxide. The extract is also attractive for increasing the secretion function of the respiratory tract and has a laxative effect on normal or constipated mice and anti-spasmolytic effect on isolated small intestine (Wang et al., 1998). Other publications have showed that the aqueous extract of LHG and Momordica glycosides could significantly reduce the cough numbers and increase the amount of tracheal secretions in cavy and mice, respectively (Chen et al., 2006; Li et al., 2008). As one of the effective components of LHG, mogroside V (Figure 1B) is extensively discussed for reducing the cough times and prolonged the incubation period for cough of mice (Liu et al., 2007). It is also effective for extending the phenol red excretion of mice trachea, which indicates that mogroside V has a certain expectorant effect. In addition, mogroside V has obvious capability to antagonize spasmodic contraction of the ileum and trachea caused by histamine. These results have indicated that mogroside V has certain contribution to the antitussive, expectorant, and spasmolytic effects of LHG.
In a pharmacological view, these ailments are closely associated with the signaling pathway of β2-AR. For instance, the work by Freund-Michel et al. (2010) has declared that β2-AR agonists exhibit antitussive properties in guinea pigs through direct inhibition of sensory nerve activity, independent of bronchodilation. These aforementioned reports have demonstrated that there are bioactive compounds targeting β2-AR in LHG extract; mogroside V is possible to become a ligand of β2-AR. Regarding these demonstrations, we proposed a good feasibility of immobilized β2-AR in screening bioactive compounds binding to the receptor from LHG extract.
Analysis of LHG Extract by RP–HPLC–MS/MS
S. grosvenorii is a medicinal and edible plant that belongs to Cucurbitaceae vine family. It contains diverse bioactive ingredients, such as triterpenoids, flavonoids, lignans, furans, sugars, fatty acids, and steroid ingredients. Previous reports have isolated and identified nearly 50 kinds of triterpenoids, seven kinds of flavonoids, and 59 kinds of fatty acids from the herb (Chaturvedula and Prakash, 2011; Chaturvedula and Meneni, 2015; Takemoto et al., 1983a, b, c; Qing et al., 2017). Takemoto et al. (1983a), Takemoto et al. (1983b) and Takemoto et al. (1983c) have confirmed mogroside IV, mogroside V, and mogroside VI isolated from S. grosvenorii as tetracyclic triterpene compound that exhibits a wide range of notable biological activities. As a member of the triterpene glycosides, mogrosides are regarded as the major effective constituents of S. grosvenorii. Afterward, a large group of triterpene glycosides were identified from dried and fresh fruits of the herb. These compounds include mogroside IV, mogroside V, mogroside III, 11-oxidized-mogrol V, mogroside IE1, mogroside IIIE, siamenoside I, mogroside IVa, mogroside IIA1, mogroside IIA2, glycoside VI, mogroside A, isomogroside V, and mogroside IIE (Chaturvedula and Prakash, 2011; Prakash and Chaturvedula, 2014; Qing et al., 2017).
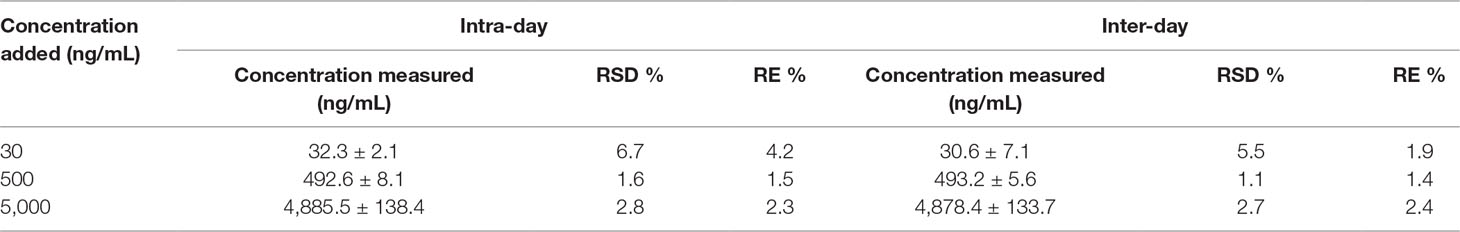
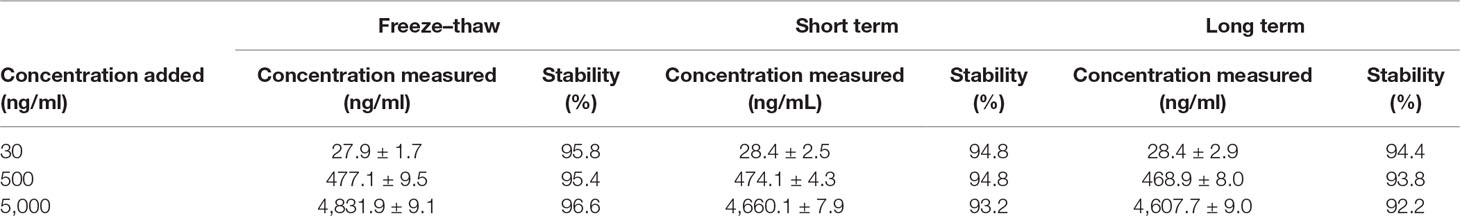
Figure 2 illustrates the total ion current chromatogram of the aqueous extract. With reference to those reports, we identified 17 compounds in the herb. Among them, 12 were triterpene saponins, and 5 were not yet identified to our scope of knowledge (Table 1). Additionally, the content of mogroside V was determined as 0.8% of S. grosvenorii, which is much higher than the value of 0.5% required by Chinese Pharmacopoeia 2015 Edition. This result confirmed the quality of S. grosvenorii collected in this work.
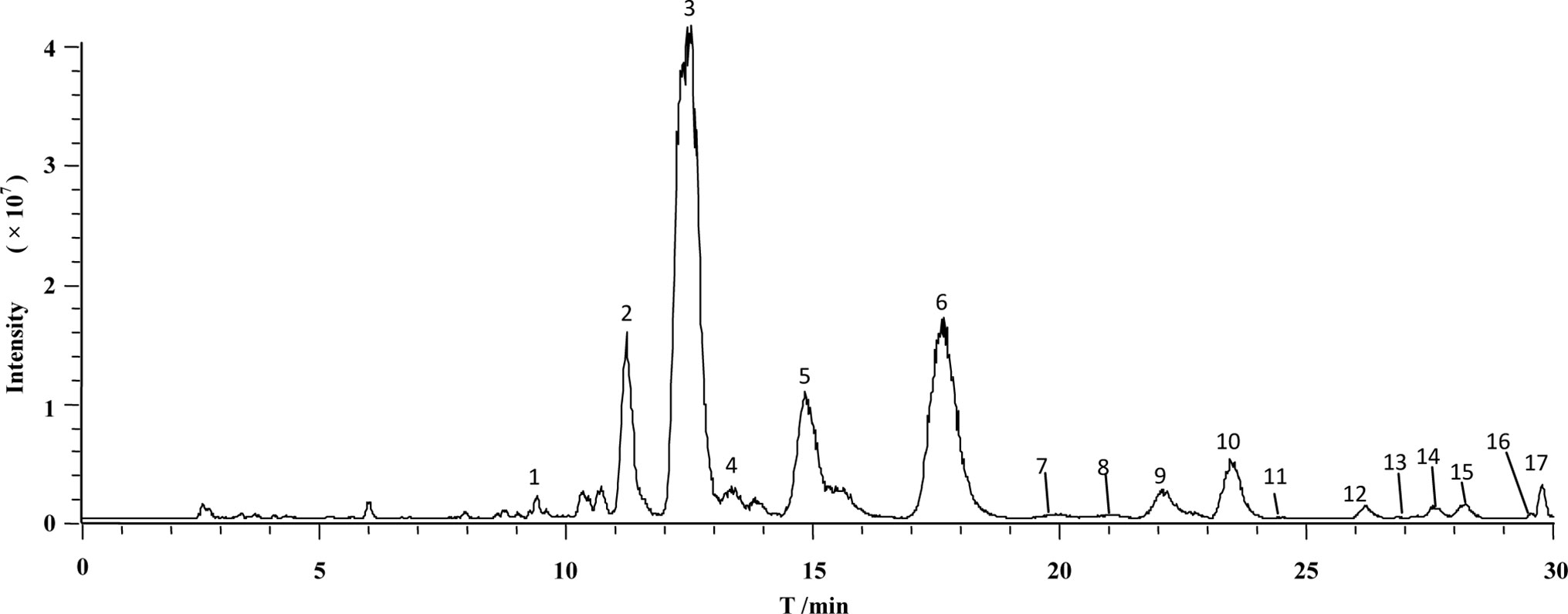
Figure 2 Representative chromatogram of total ion current of LHG extract by reversed-phase high-performance liquid chromatography coupled with mass spectrometry.
Characterization of β2-AR Column
Specificity of the immobilized β2-AR column was tested by comparing the retention behaviors of salbutamol and terbutaline on the column with those of sodium nitrite. Each of the drugs was injected three times, and the retention time was determined to be 3.2 min for salbutamol, 3.9 min for terbutaline, and 0.7 min for sodium nitrite (Figure 3). Under the same conditions, prazosin and terazosin (specific ligands of α1A-adrenergic receptor) displayed retention times of 0.8 and 1.0 min. These values were far lower than the retention times of salbutamol and terbutaline and were approximately equal to the retention time of sodium nitrite. This provided a proof of high specificity of the immobilized β2-AR and indicated that the immobilized β2-AR has the capacity to recognize its specific ligands.
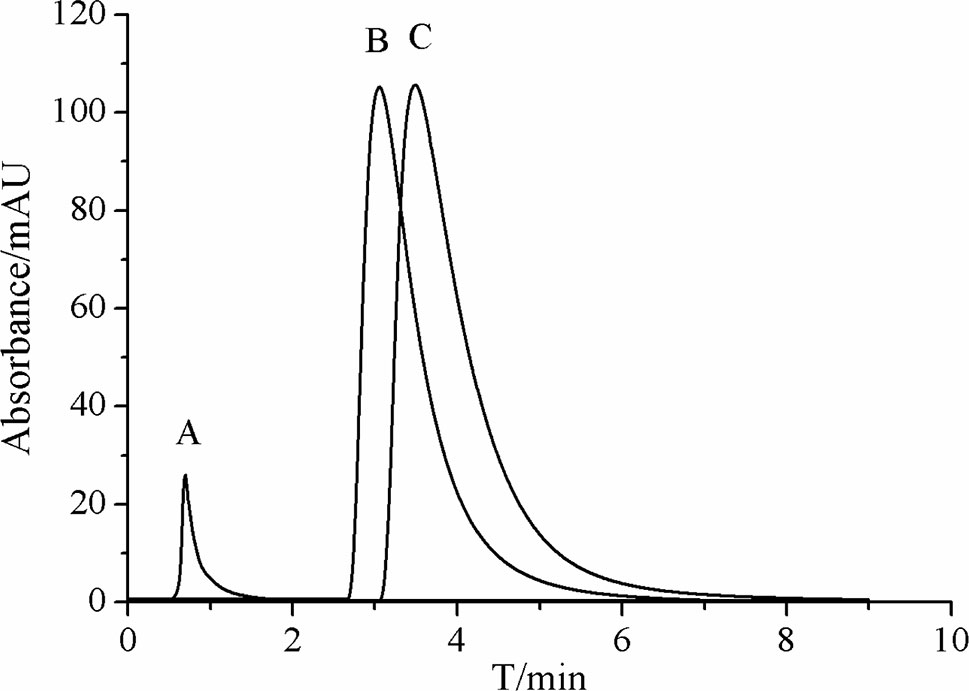
Figure 3 Representative chromatograms of (A) sodium nitrite, (B) salbutamol, and (C) terbutaline on immobilized β2-AR column by high-performance liquid chromatography.
The other characterization of the immobilized β2-AR column was the stability investigation. We performed such examination by continuously analyzing the retention times of salbutamol and terbutaline for 3 weeks. The relative standard deviations (RSDs) of their retention times were calculated to be 3.8% and 2.9%, respectively. The chromatogram profiles of the two drugs showed no significant variations in 3 weeks. This result demonstrated that the immobilized β2-AR was stable at least in 3 weeks. In that duration, the column remains the bioactivity of recognizing and capturing the unknown ligands of the receptor from complex matrices. It is possible to be utilized for screening β2-AR-targeted compounds from S. grosvenorii.
Screening the Bioactive Compounds of LHG
LHG, the fruit of S. grosvenorii, is a widely prescribed TCM for the treatment of lung injury, sore throat, and constipation for thousands of years. The chemical constitutions of LHG are cucurbitane triterpene glycosides, glucose, and lipids. A cocktail of references has revealed that the extracted bioactive compounds from S. grosvenorii possess good anticancer, antidiabetic, antioxidant, and anti-inflammatory activities (Li et al., 2007; Qi et al., 2008; Lu et al., 2012). The anti-inflammatory effect of the extract was achieved by down-regulating the expression of pro-inflammatory cytokines, iNOS, COX-2, and IL-6 plus up-regulating the inflammation protective genes expression including PARP1 and MAPK9 (Shi et al., 2014). This mechanism has been reported as one of the signaling pathways of β2-AR. As a member of superfamily of G-protein-coupled receptors, β2-AR has exhibited unambiguous action in curing diseases of respiratory system like asthma. Such curative effect has mainly deepened the initiation of β2-AR signaling pathway by forming a receptor–ligand complex. Taken together, we hypothesize that there are β2-AR-targeted bioactive compounds in LHG.
In the current work, we extracted LHG through a hot refluxing method. Such method has been broadly accepted as a powerful assay for achieving most of the components in LHG. Figure 4A displays the chromatogram of LHG aqueous extract on immobilized β2-AR column. We found two intensive peaks with retention times of 0.9 and 4.3 min. Regarding the void time of the chromatographic system, we collected the peak at 4.3 min as the compound of interest. We further separated this collection by an Inertsil ODS-3-C18 column (5 μm, 4.6 mm × 150 mm) and identified the elution by trap mass spectrometry (Figure 4B). The full-scan mass spectrum of this compound showed a deprotonated ion [M−H]− at m/z 1,285.6 in negative mode. MS/MS result revealed that the father ion yields two daughter ions at m/z 1,123.6 and m/z 961.6 by loss of a monomolecular and a bimolecular of C6H10O5. Such mass pattern was in line with MS/MS information of the reference standard of mogroside V. On the basis of these results, we reasoned that the peak at 4.3 min on β2-AR column as the bioactive compound binding to the receptor in LHG. Such identification was reasonable since mogroside V has clear action on curing ailments mediated by β2-AR.
Figure 4 Representative chromatograms of (A) LHG extract on the β2-AR column. (B) Total ion current of eluted compounds by reversed-phase high-performance liquid chromatography coupled with mass spectrometry.
Comparison With Other Screening Methods
The screening of bioactive compounds from TCM is in the central part of TCM-guided drug development. Owing to this important role, such research has attracted great attention of researchers in pharmaceutical, chemistry, and biology. Wang et al. (2012) constructed a comprehensive two-dimensional (2D) HPLC system where liposome chromatographic column was utilized as the first dimensional column and the silica monolithic column was regarded as the second dimensional chromatographic column. They applied the system in screening bioactive compounds from Schisandra chinensis and identified 14 compounds of the retained peaks. In another report, Wang et al. (2017b) established a comprehensive 2D PC-3 cell membrane chromatography (CMC) system to screen the bioactive compounds of anti-prostate cancer from Sophorae flavescentis Ait. and confirmed the structures of five compounds from the herb extract. Li et al. (2013) created an HPLC method taking human albumin as stationary phase to explore the leading compounds by in vitro PPB. Wang et al. (2000) utilized immobilized human serum albumin to screen the bioactive compounds from Artemisia capillaris and confirmed two strong retention peaks of scoparone and capillarisin. Tong et al. (2014) extracted lipid rafts from brain glioma U251 cells by synthesizing immobilized lipid to screen for anti-tumor bioactive compounds from Galla chinensis. Despite their great contribution to promote the development of TCM-guided drugs, the aforementioned reports appear to have certain limitations, like lower separating capacity and specificity. This often makes false-positive results during the utilization of these assays. The exploration of new methodologies still remains open to rapidly recognize, separate, and identify compounds of interest from TCM.
Our group proposed a concept-guided methodology named as receptor chromatography (Li et al., 2015; Wang et al., 2017a; Zeng et al., 2018) to address the limitations of the above assays. In most cases, this method has immobilized the main target like G-protein-coupled receptor of approved drugs onto solid support to achieve column using the immobilized target as stationary phases. Successful applications of the stationary phases have demonstrated high sensitivity, specificity, and stability when it comes to screening the target-binding bioactive compounds. Zhao et al. (2010) screened and identified four bioactive compounds in the aqueous extract of Coptis chinensis Franch. by β2-AR chromatography. Wang et al. (2017a) analyzed Shuang-Huang-Lian prescription and identified chlorogenic acid as the bioactive compound. This work utilized β2-AR chromatographic method for the pursuit of bioactive compounds targeting the receptor from the aqueous extract of LHG. Owing to the high separating capacity, we rapidly recognized mogroside V as β2-AR binding bioactive compound. Comparing the methods including immobilized liposome chromatography, we reasoned that the proposed method is possible to enhance the role of chromatographic methods in TCM-guided drug development.
Mogroside V Exhibited β2-AR-Associated Bronchodilator Activities
To prove the bronchodilatory activity of mogroside V, we tested the effects of the compound on the isolated tracheal strips in vitro. The potential β2-AR-mediating relaxant mechanisms of the compound were also evaluated in the absence and presence of a specific inhibitor. This was performed with the scarification of rats weighing 250–300 g by exsanguination from the abdominal aorta. We collected the tracheal tissues between the larynx and the lungs and immediately immersed them in cold oxygenated Krebs solution (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, and 9.8 mM glucose; pH 7.4). After the removal of adhesive tissues, we spirally isolated the tracheal segment with three cartilage rings to assay the tracheal strip reactivity. Such strips were mounted onto two L-shaped stainless-steel holders connected with a force–displacement transducer. The isometric tension was determined in a tissue bath by a PowerLab data acquisition system.
We aerated the pre-warmed Krebs solution (37°C) with a gas mixture of 95% O2 and 5% CO2. At initial tests, the tracheal strips with tensions of 1.0-g mass were equilibrated for 60 min. The resulting strips were stimulated with high-potassium (K+) solution (63 mM NaCl, 60 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, and 9.8 mM glucose; pH 7.4) to evoke a contractile response in the presence or absence of ICI 118551 (10 μM). When the tensions achieved a plateau value, we cumulatively included mogroside V to induce a relaxant response. Figure 5 illustrates the influences of mogroside V from 0.1 μM to 1.0 mM on the pre-contracted strip by high-potassium (K+) solution. We observed a concentration-dependent manner of mogroside V on the relaxation of tracheal strips in the absence of ICI 118551. Such response disappeared when ICI 118551 was simultaneously applied to the pre-contracted strips. Since ICI 118551 is a specific inhibitor of β2-AR, these results suggested that mogroside V is an agonist of the receptor, and the bronchodilator activities of the compound were mediated by β2-AR. By these results, we concluded that receptor chromatography is powerful for screening specific ligands of a receptor from traditional Chinese medicine.
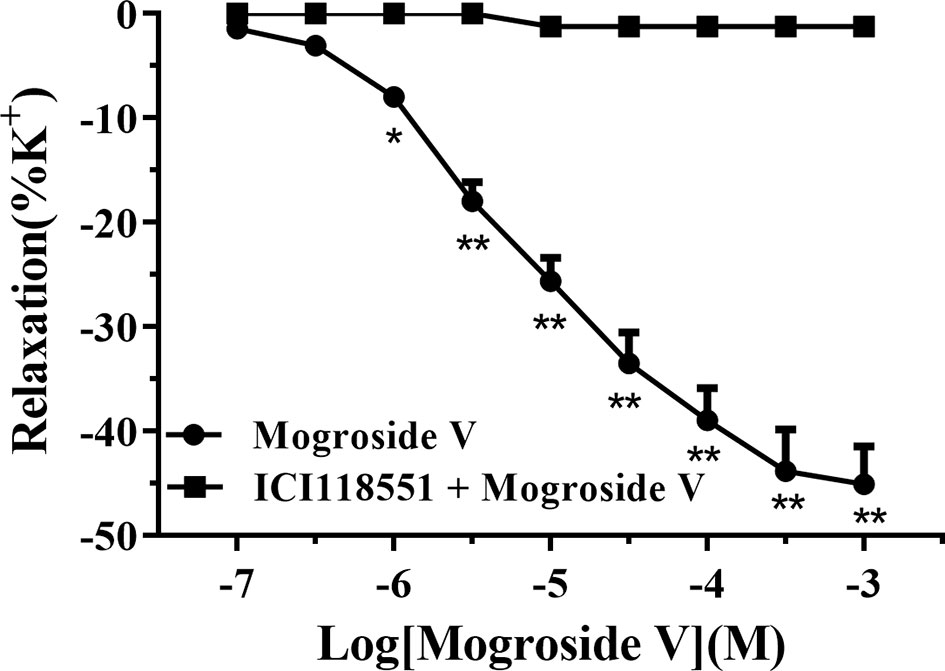
Figure 5 The dose–response curves of mogroside V for the relaxation effect on rat tracheal smooth muscle pre-contracted by high K+ solution (60 mM) in vitro. Each point represents the means ± SEM of each group (n = 6) from two separate experiments. *p < 0.05, **p < 0.01 versus the ICI 118551 treated group.
Method Validation for Pharmacokinetics
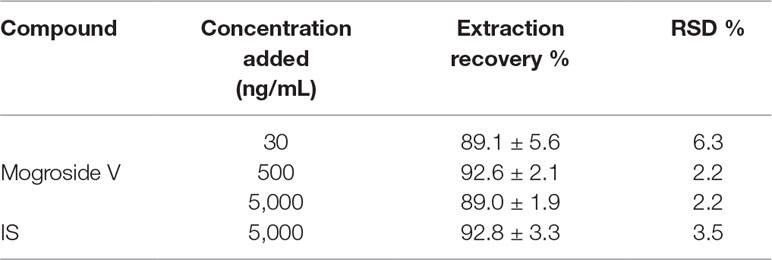
The MRM chromatograms of blank plasma, QC samples [30 ng/ml (LQC), 500 ng/ml (MQC), and 5,000 ng/ml (HQC)], and plasma collected after 200 mg/kg of oral administration of mogroside V at 45 min are shown in Figures 6A–E, respectively. It is obvious that there is no endogenous interference at the retention times of mogroside V (3.6 min) and IS (4.7 min). The calibration curves showed good linearity at the range of 24.4–6,250 ng/ml. The equation of the regression was y = 0.0037x − 0.0067 (R2 = 0.9987), where y is the peak area ratio of mogroside V to the IS and x is the concentration of mogroside V. Under the proposed conditions, the LLOQ of mogroside V in plasma was determined as 5 ng/mL. The data of intra-day and inter-day accuracy and precision are summarized in Table 2. Both the intra-day and inter-day accuracy (RE) and the precision (RSD) were less than 15%. This result was in good accordance with the requirement of bio-assay for pharmacokinetic study by the guideline of Guidance for Industry Bioanalytical Method Validation (U.S. Food and Drug Administration, 2018). The extraction recovery data are summarized in Table 3 where the recoveries of mogroside V in plasma were all above 85%. The stability data are listed in Table 4. This demonstrated that the process of sample preparation generates little variation. Under diverse storing and processing conditions, mogroside V showed a good stability.
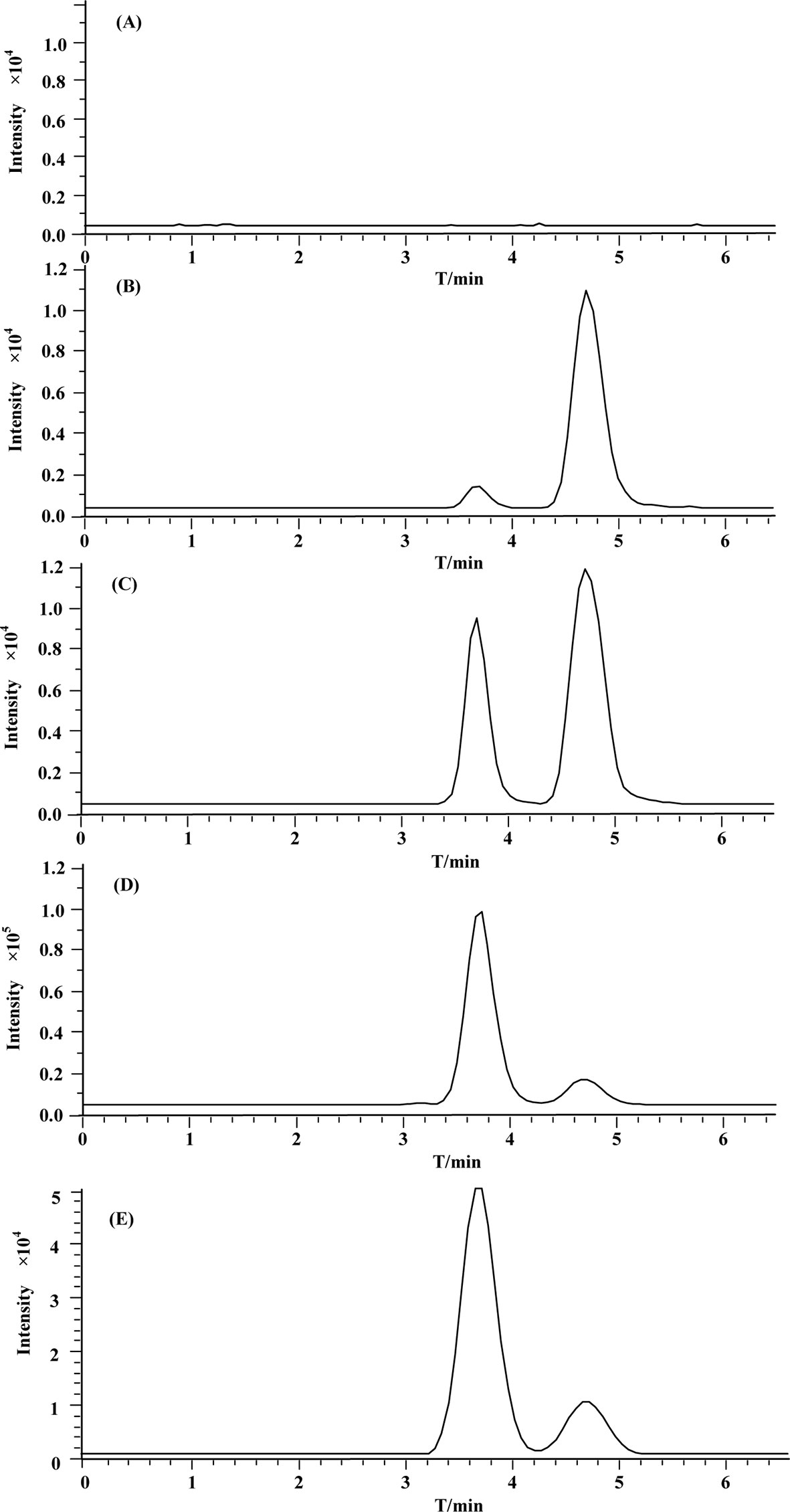
Figure 6 Representative MRM chromatograms of (A) blank rat plasma; (B–D) (QC samples (30 ng/mL (LQC), 500 ng/mL (MQC), and 5,000 ng/mL (HQC), respectively); (E) rat plasma sample at 45 min after 200 mg/kg oral administration of mogroside V.
Regarding the validated test, we summarized that the proposed HPLC–MS/MS method is sensitive and reliable for the determination of mogroside V in rat plasma. The method has the capacity to analyze the pharmacokinetic behavior of mogroside V in rat plasma after administration of LHG extract.
Pharmacokinetic Study
The validated assay was applied in the pharmacokinetic study of mogroside V and LHG extract in rat plasma after oral administration. The concentration–time curves are described in Figure 7. The corresponding pharmacokinetic parameters are summarized in Table 5.
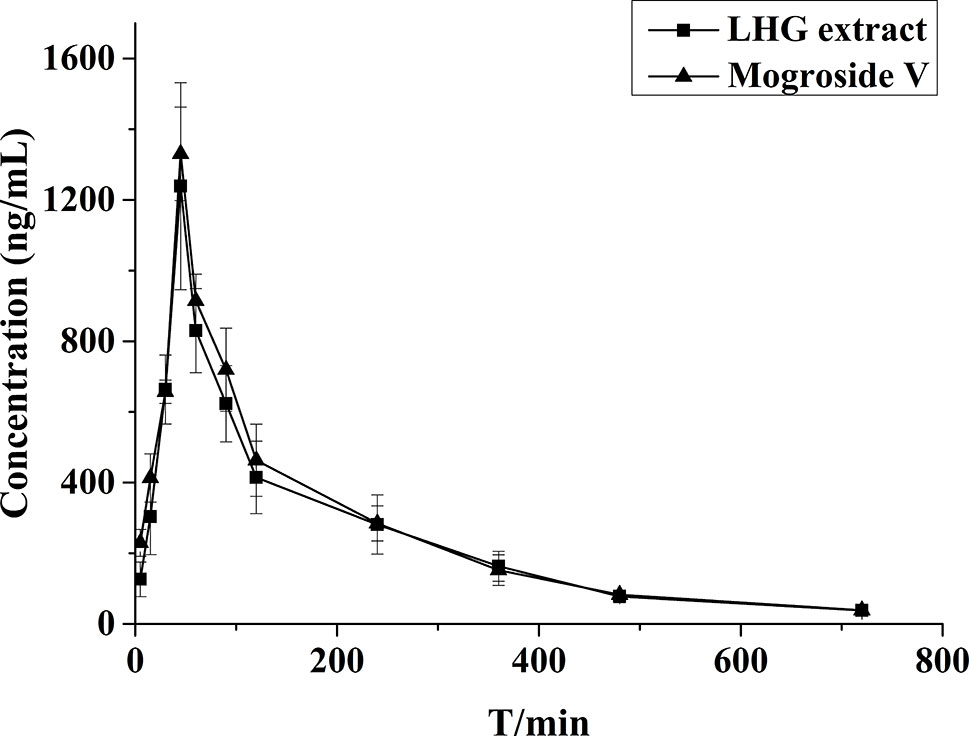
Figure 7 Mean plasma concentration–time profiles of rats after oral administration of mogroside V (200 mg/kg) and LHG extract (12.5 ml/kg).
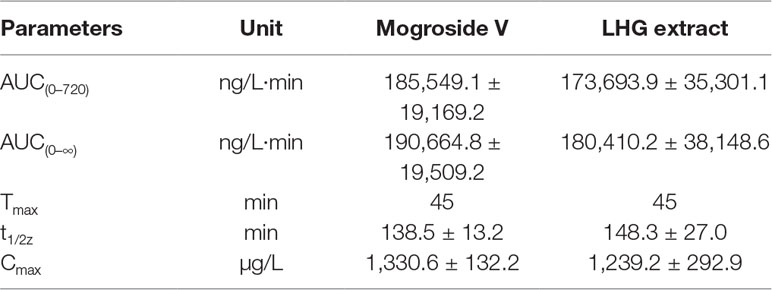
Table 5 Pharmacokinetic parameters of mogroside V (200 mg/kg) and LHG extract (12.5 ml/kg) orally administered to rats (n = 6 per time point).
By contrastive analysis, we obtained an identical pharmacokinetic behavior between mogroside V and LHG extract after oral administration of desired dose. Both mogroside V and LHG extract showed the time to reach peak plasma concentrations (Cmax) at 45 min. The corresponding plasma concentrations were 1,330.6 ± 132.2 and 1,239.2 ± 292.9 ng/ml, respectively. The AUC(0–720) and AUC(0–∞) of mogroside V were 185,549.1 ± 19,169.2 and 190,664.8 ± 19,509.2 ng/L·min, respectively, which changed to 173,693.9 ± 35,301.1 and 180,410.2 ± 38,148.6 ng/L·min when LHG extract was applied. No clear difference was found between the two groups of data, which confirmed the equal dosage of mogroside V during the administration LHG and the compound alone. The ratio of the AUC value was below 120%, providing a proof of rationale time points for blood collection to pursue the pharmacokinetic investigation (Shi et al., 2019). Taking the identical pharmacokinetic behavior into account, we inferred that mogroside V is the representative compounds of LHG for treatment of β2-AR-mediating ailments.
A few reports have declared that mogroside V is detectable in rat plasma after tail intravenous injection (1.12 or 2.0 mg/kg) and intraperitoneal administration (1.12 mg/kg) (Zhou et al., 2018), while it disappears after oral administration even though the dose was increased to 5 mg/kg (Luo, 2017). We reasoned that the undetectable results in the case of oral administration are mainly ascribed to the relatively low dose of the compound. Such dose was far lower than the requirement of the compound according to the instruction of LHG in Pharmacopoeia of the People’s Republic of China. On this account, we increased the dose to 200 mg/kg for the pharmacokinetic study. Such dose is in line with the pharmacological study of the compound and has proved to be nontoxic according to the toxicological examination of mogroside V after oral administration by Qin et al. (2006). The study in this work displayed desired pharmacokinetic parameters like slow elimination of mogroside V after oral administration of LHG or the compound alone. This provided a proof of good druggability for mogroside V and indicated that the compound has potential to become a lead compound for further investigation.
Conclusions
The immobilized β2-AR chromatography coupled with HPLC–MS was utilized to screen and identify the bioactive compounds of LHG. Mogroside V was identified as the bioactive compound binding to the β2-AR. The druggability of mogroside V was evaluated through the analysis of pharmacokinetic behavior by HPLC–ESI–MS/MS, which illustrated that mogroside V is the main representative of LHG. It demonstrates good druggability for the treatment of β2-AR-mediated ailments including asthma. The method of this work is possible to become a powerful strategy for screening bioactive compounds from TCM and pre-evaluating its druggability.
Data Availability
All datasets generated for this study are included in the manuscript and the supplementary files.
Ethics Statement
This study was carried out in accordance with the recommendations of Guidelines in Care and Use of Animal under the approval of the Animal Experimentation Ethics Committee at Northwest University.
Author Contributions
XJ, JL, BS and QiL carried out experiments and wrote the manuscript, XJ, JG and GF analyzed experimental results, ZC, QiaL, XiaZ and JC revised the manuscript, XinZ conceived and designed the experiments.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 21705126, 21775119, and 81702832).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Balabolkin, I., Bulgakova, V., Bryantseva, O., Pinelis, V., Tyumentseva, E., Zhurkova, N. (2017). The relationship between genetic polymorphisms of beta2-adrenergic receptors and efficiency of the broncholytic therapy among Russian children with bronchial asthma. Allergy 72, 332–332.
Bernardini, S., Tiezzi, A., Laghezza, M. V., Ovidi, E. (2017). Natural products for human health: an historical overview of the drug discovery approaches. Nat. Prod. Res. 32, 1–25. doi: 10.1080/14786419.2017.1356838
Chaturvedula, V. S. P., Meneni, S. R. (2015). A new cucurbitane glycoside from Siraitia grosvenorii. Nat. Prod. Commun. 10, 1521–1523. doi: 10.1177/1934578X1501000908
Chaturvedula, V. S. P., Prakash, I. (2011). Cucurbitane glycosides from Siraitia grosvenorii. J. Carbohyd. Chem. 30, 16–26. doi: 10.1080/07328303.2011.583511
Chen, Y., Fan, X. B., Wang, Y. X., Hang, X. M. (2006). Functional study of natural food sweetener mogrosides. China Food Addit. 1, 41–43.
Cortez, E., Castro, M., Matos, A., Lourenco, M., Ferreira, J., Bicho, M. (2016). The role of beta2-adrenergic receptor polymorphism in bronchial asthma. Allergy 71, 279–279.
Freund-Michel, V. C., Birrell, M. A., Giembycz, M. A., Hele, D. J., Haj-Yahia, S., Belvisi, M. G. (2010). Beta(2)-agonists block tussive responses in guinea pigs via an atypical cAMP-dependent pathway. Eur. Resp. J. 35, 647–654. doi: 10.1183/09031936.00034009
Han, F., Liu, T. F., Yin, R., Zhang, X. S., Ma, L., Xu, R., et al. (2016). UHPLC–FT–ICR–MS combined with serum pharmacochemistry for bioactive compounds discovery of Zhi-Zi-Da-Huang-decoction against alcohol-induced hepatotoxicity in rats. RSC Adv. 6, 108917–108927. doi: 10.1039/C6RA19422B
Han, S., Lv, Y. N., Wei, F., Fu, J., Hu, Q., Wang, S. C. (2018). Screening of bioactive components from traditional Chinese medicines using cell membrane chromatography coupled with mass spectrometry. Phytochem. Anal. 29, 341–350. doi: 10.1002/pca.2756
He, J. L., Zhao, J. W., Ma, Z. C., Wang, Y. G., Liang, Q. D., Tan, H. L., et al. (2015). Serum pharmacochemistry analysis using UPLC–Q-TOF/MS after oral administration to rats of Shenfu decoction. Evid. Based Compl. Alt. 1–12. doi: 10.1155/2015/973930
Krzysztof, J., Lawrence, T., Lucita, J., Anthony Yiu-Ho, W., Rui-Ping, X., Wainer, I. W. (2010). The effect of stereochemistry on the thermodynamic characteristics of the binding of fenoterol stereoisomers to the beta(2)-adrenoceptor. Biochem. Pharmacol. 79, 1610–1615. doi: 10.1016/j.bcp.2010.01.035
Li, D., Ikeda, T., Huang, Y., Liu, J., Nohara, T., Sakamoto, T., et al. (2007). Seasonal variation of mogrosides in Lo Han Kuo (Siraitia grosvenori) fruits. J. Nat. Med. Tokyo 61, 307–312. doi: 10.1007/s11418-006-0130-7
Li, J., Li, P. B., Yuan, G. J. (2008). The antitussive effect of water extract of grosvenor momordica fruit. J. Hainan Med. Coll. 14, 16–18. doi: 10.13210/j.cnki.jhnu.2008.01.005
Li, Q., Wang, J., Zheng, Y. Q. Y., Yang, L. J., Zhang, Y. J., Bian, L. J., et al. (2015). Comparison of zonal elution and nonlinear chromatography in determination of the interaction between seven drugs and immobilised beta(2)-adrenoceptor. J. Chromatogr. A 1401, 75–83. doi: 10.1016/j.chroma.2015.05.012
Li, Y. C., Kuo, P. C., Yang, M. L., Chen, T. Y., Hwang, T. L., Chiang, C. C., et al. (2019). Chemical constituents of the leaves of Peltophorum pterocarpum and their bioactivity. Molecules 24. doi: 10.3390/molecules24020240
Li, Y. F., Zhang, X. Q., Hu, W. Y., Li, Z., Liu, P. X., Zhang, Z. Q. (2013). Rapid screening of drug-protein binding using high-performance affinity chromatography with columns containing immobilized human serum albumin. J. Anal. Methods Chem. 1–7. doi: 10.1155/2013/439039
Liu, M., Gong, X., Quan, Y., Zhou, Y., Li, Y., Peng, C. (2018). A Cell-based metabonomics approach to investigate the varied influences of chrysophanol-8-O-beta-d-glucoside with different concentrations on L-02 cells. Front. Pharmacol. 9, 1530. doi: 10.3389/fphar.2018.01530
Liu, T., Wang, X. H., LI, C., Zhang, Y., WU, G. L., Li, D. C., et al. (2007). Study on the antitussive, expectorant and antispasmodic effect of saponin V from momordica grosvenori. Chin. Pharm. J. 42, 1534–1536. doi: 10.3321/j.issn:1001-2494.2007.20.005
Lu, F. L., Li, D. P., Fu, C. M., Liu, J. L., Huang, Y. L., Chen, Y. Y., et al. (2012). Studies on chemical fingerprints of Siraitia grosvenorii fruits (Luo Han Guo) by HPLC. J. Nat. Med. Tokyo 66, 70–76. doi: 10.1007/s11418-011-0555-5
Luo, Z. L. (2017). The biosynthesis of cucubitadienol and the biological activities of cucurbitane-type. dissertation/doctor’s thesis. Chinese Academy of Medical Science & Peking Union Medical College. Pulishier location: Beijing.
Moaddel, R., Wainer, I. W. (2006). Development of immobilized membrane-based affinity columns for use in the online characterization of membrane bound proteins and for targeted affinity isolations. Anal. Chim. Acta 564, 97–105. doi: 10.1016/j.aca.2005.09.020
Mofidifar, S., Sohraby, F., Bagheri, M., Aryapour, H. (2018). Repurposing existing drugs for new AMPK activators as a strategy to extend lifespan: a computer-aided drug discovery study. Biogerontology 19, 133–143. doi: 10.1007/s10522-018-9744-x
Prakash, I., Chaturvedula, V. S. P. (2014). Additional new minor cucurbitane glycosides from Siraitia grosvenorii. Molecules 19, 3669–3680. doi: 10.3390/molecules19033669
Qi, X. Y., Chen, W. J., Zhang, L. Q., Xie, B. J. (2008). Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr. Res. 28, 278–284. doi: 10.1016/j.nutres.2008.02.008
Qin, X., Xiaojian, S., Ronggan, L., Yuxian, W., Zhunian, T., Shouji, G., et al. (2006). Subchronic 90-day oral (Gavage) toxicity study of a Luo Han Guo mogroside extract in dogs. Food Chem. Toxicol. 44, 2106–2109. doi: 10.1016/j.fct.2006.07.023
Qing, Z. X., Zhao, H., Tang, Q., Mo, C. M., Huang, P., Cheng, P., et al. (2017). Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 138, 240–248. doi: 10.1016/j.jpba.2017.01.059
Sari, A., Kececi, Z. (2019). Phytochemical investigations on chemical constituents of Taraxacum bessarabicum (Hornem.) Hand.-Mazz. subsp. bessarabicum (Hornem.) Hand.-Mazz. Iran. J. Pharm. Res. 18, 400–405.
Shi, B. M., Yang, L. J., Gao, T., Ma, C. C., Li, Q. N., Nan, Y. F., et al. (2019). Pharmacokinetic profile and metabolite identification of bornyl caffeate and caffeic acid in rats by high performance liquid chromatography coupled with mass spectrometry. RSC Adv. 9, 4015–4027. doi: 10.1039/C8RA07972B
Shi, D., Zheng, M., Wang, Y., Liu, C., Chen, S. (2014). Protective effects and mechanisms of mogroside V on LPS-induced acute lung injury in mice. Pharm. Biol. 52, 729–734. doi: 10.3109/13880209.2013.867451
Sun, R., Sun, L., Han, C. (2018). Partial-least-squares and canonical-correlation analysis of chemical constituents and active ingredients of new types of Chinese mulberries. Food Sci. Nutr. 6, 1950–1959. doi: 10.1002/fsn3.753
Sun, Y. G., Du, Y. F., Yang, K., Chang, L., Cao, L., Ren, Y. P., et al. (2013). A comparative study on the pharmacokinetics of a traditional Chinese herbal preparation with the single herb extracts in rats by LC-MS/MS method. J. Pharm. Biomed. Anal. 81-82, 34–43. doi: 10.1016/j.jpba.2013.03.022
Takemoto, T., Arihara, S., Nakajima, T., Okuhira, M. (1983a). Studies on the constituents of fructus Momordicae. I. On the sweet principle. Yakugaku Zasshi. 103, 1151–1154. doi: 10.1248/yakushi1947.103.11_1151
Takemoto, T., Arihara, S., Nakajima, T., Okuhira, M. (1983b). Studies on the constituents of fructus Momordicae. II. Structure of sapogenin. Yakugaku Zasshi. 103, 1155–1166. doi: 10.1248/yakushi1947.103.11_1155
Takemoto, T., Arihara, S., Nakajima, T., Okuhira, M. (1983c). Studies on the constituents of fructus Momordicae. III. Structure of mogrosides. Yakugaku Zasshi. 103, 1167–1173. doi: 10.1248/yakushi1947.103.11_1167
Tamaoki, J., Yokohori, N., Tagaya, E., Kirishi, S., Miyamoto, Y., Ochiai, K., et al. (2010). Comparable effect of a leukotriene receptor antagonist and long-acting beta2-adrenergic agonist in cough variant asthma. Allergy Asthma Proc. Official J. Reg. State Allergy Soc. 31, 78. doi: 10.2500/aap.2010.31.3366
Tong, S., Sun, C., Cao, X., Zheng, Q., Zhang, H., Firempong, C. K., et al. (2014). Development and thermodynamic evaluation of novel lipid raft stationary phase chromatography for screening potential antitumor agents. Biomed Chromatogr. 28, 1615–1623. doi: 10.1002/bmc.3189
U.S. Food and Drug Administration (2018), Bioanalytical method validation available guidance for industry, Available at: https://www.fda.gov/media/70858/download.
Van Hilten, N., Chevillard, F., Kolb, P. (2019). Virtual compound libraries in computer-assisted drug discovery. J. Chem. Inf. Model 59, 644–651. doi: 10.1021/acs.jcim.8b00737
Wainer, I. W., Zhang, Y., Xiao, Y., Kellar, K. J. (1999). Liquid chromatographic studies with immobilized neuronal nicotinic acetylcholine receptor stationary phases: effects of receptor subtypes, pH and ionic strength on drug-receptor interactions. J. Chromatogr. B Biomed. Sci. Appl. 724, 65–72. doi: 10.1016/S0378-4347(98)00579-9
Wang, H. L., Zou, H. F., Ni, J. Y., Kong, L., Gao, S., Guo, B. C. (2000). Fractionation and analysis of Artemisia capillaris Thunb. by affinity chromatography with human serum albumin as stationary phase. J. Chromatogr. A 870, 501–510. doi: 10.1016/S0021-9673(99)01062-6
Wang, J., Li, F., Zeng, K., Li, Q., Zhao, X., Zheng, X. (2017a). Bioactive compounds of Shuang-Huang-Lian prescription and an insight into its binding mechanism by beta2-adrenoceptor chromatography coupled with site-directed molecular docking. J. Sep. Sci. 40, 4357–4365. doi: 10.1002/jssc.201700522
Wang, Q., Li, A. Y., Huang, R. Q., Lin, L. (1998). The expectorant, cough and laxative effect of Luo-Han-Guo. J. Guangxi Coll. TCM 15, 62–64.
Wang, Q., Xu, J., Li, X., Zhang, D., Han, Y., Zhang, X. (2017b). Comprehensive two-dimensional PC-3 prostate cancer cell membrane chromatography for screening anti-tumor components from Radix Sophorae flavescentis. J. Sep. Sci. 40, 2688–2693. doi: 10.1002/jssc.201700208
Wang, S., Wang, C., Zhao, X., Mao, S., Wu, Y., Fan, G. (2012). Comprehensive two-dimensional high performance liquid chromatography system with immobilized liposome chromatography column and monolithic column for separation of the traditional Chinese medicine Schisandra chinensis. Anal. Chim. Acta 713, 121–129. doi: 10.1016/j.aca.2011.10.062
Wu, B., Xiao, X., Li, S., Zuo, G. (2019). Transcriptomics and metabonomics of the anti-aging properties of total flavones of Epimedium in relation to lipid metabolism. J. Ethnopharmacol. 229, 73–80. doi: 10.1016/j.jep.2018.09.039
Zeng, K., Li, Q., Wang, J., Yin, G., Zhang, Y., Xiao, C. (2018). One-step methodology for the direct covalent capture of GPCRs from complex matrices onto solid surfaces based on the bioorthogonal reaction between haloalkane dehalogenase and chloroalkanes. Chem. Sci. 9, 446–456. doi: 10.1039/C7SC03887A
Zhang, Y. X. (2017). Pharmacological study on traditional Chinese medicine and natural product in China. Chin. J. Pharmacol. Toxicol. 31, 941. doi: 10.3867/j.issn.1000-3002.2017.10.001
Zhao, X., Nan, Y., Xiao, C., Zheng, J., Zheng, X., Wei, Y., et al. (2010). Screening the bioactive compounds in aqueous extract of Coptidis rhizoma which specifically bind to rabbit lung tissues beta2-adrenoceptor using an affinity chromatographic selection method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878, 2029–2034. doi: 10.1016/j.jchromb.2010.05.040
Zhao, X., Zheng, X., Fan, T., Li, Z., Zhang, Y., Zheng, J. (2015). A novel drug discovery strategy inspired by traditional medicine philosophies. Science 347, S38–S40.
Zhao, X. F., Lu, H. Y., Huang, J., Zheng, J. B., Zheng, X. H., Zhang, Y. Y. (2012). Binding interaction between prazosin and immobilized receptor by frontal analysis. Chromatographia 75, 411-415. doi: 10.1007/s10337-012-2198-4
Keywords: high-throughput screening, pharmacokinetics, receptor chromatography, β2-adrenergic receptor, Siraitia grosvenorii
Citation: Jia X, Liu J, Shi B, Liang Q, Gao J, Feng G, Chang Z, Li Q, Zhang X, Chen J and Zhao X (2019) Screening Bioactive Compounds of Siraitia grosvenorii by Immobilized β2-Adrenergic Receptor Chromatography and Druggability Evaluation. Front. Pharmacol. 10:915. doi: 10.3389/fphar.2019.00915
Received: 21 March 2019; Accepted: 19 July 2019;
Published: 16 August 2019.
Edited by:
Xijun Wang, Heilongjiang University of Chinese Medicine, ChinaReviewed by:
Victoria Samanidou, Aristotle University of Thessaloniki, GreeceKai Xiao, Second Military Medical University, China
Copyright © 2019 Jia, Liu, Shi, Liang, Gao, Feng, Chang, Li, Zhang, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinfeng Zhao, emhhb3hmQG53dS5lZHUuY24=
†These authors contributed equally to this work.
 Xiaoni Jia1,2†
Xiaoni Jia1,2† Baimei Shi
Baimei Shi Xinfeng Zhao
Xinfeng Zhao