- 1Division of Aging and Dementia, Department of Neurology, Columbia University Medical Center, New York, NY, United States
- 2Division of Cognitive Neuroscience, Department of Neurology, Columbia University Medical Center, New York, NY, United States
Alzheimer’s disease causes both cognitive and non-cognitive symptoms. There is increasing evidence that the presentation and course of Alzheimer’s disease is highly heterogenous. This heterogeneity presents challenges to patients, their families, and clinicians due to the difficulty in prognosticating future symptoms and functional impairment. Behavioral and psychiatric symptoms are emerging as a significant contributor to this clinical heterogeneity. These symptoms have been linked to multiple areas of neurodegeneration, which may suggest that they are representative of network-wide dysfunction in the brain. However, current diagnostic criteria for Alzheimer’s disease focus exclusively on the cognitive aspects of disease. Behavioral and psychiatric symptoms have been found in multiple studies to be related to disease severity and to contribute to disease progression over time. A better understanding of how behavioral and psychiatric symptoms relate to cognitive aspects of Alzheimer’s disease would help to refine the models of disease and hopefully lead to improved ability to develop therapeutic options for this devastating disease.
Introduction
Dementia is characterized by a decline in cognitive function when compared with others with similar age and education. It is an important cause of morbidity and mortality, especially in the elderly.
In 1906, Alois Alzheimer reported a case of a woman with prominent and progressive psychiatric symptoms and memory disturbance, who he followed for 5 years until her death (Maurer et al., 1997). Alzheimer’s disease is the most common cause of cognitive impairment, and its prevalence increases with age (Erkkinen et al., 2018). However, the rapidity of decline in this first patient has generally not been considered the usual course of the disease. In fact, there is significant heterogeneity in the rates and manners in which patients progress through the stages of Alzheimer’s disease (Mayeux et al., 1985).
Part of this heterogeneity is the presence of behavioral and psychiatric symptoms (BPSD). These symptoms affect over 80% of patients with AD over the course of disease, however their presentations are highly variable both between patients and over an individual’s disease course (Garre-Olmo et al., 2010a). Psychiatric symptoms can be present at all stages of disease, however specific symptoms are more common at different stages of disease. Although all symptoms worsen with disease severity, certain symptoms such as delusions, agitation, and apathy tend to become much more prevalent (Lyketsos et al., 2002).
The prevalence of psychiatric symptoms early in the course of dementia has become increasingly recognized. Mild behavioral impairment is a recently defined diagnostic construct that has been used to describe the presence of these symptoms, even in the absence clear cognitive change (Ismail et al., 2016).
Management of psychiatric symptoms is an important component of caring for these patients. Behavioral symptoms are a significant source of caregiver stress (Van Den Wijngaart et al., 2007) and contribute to the financial burden of caring for these patients (Murman et al., 2002; Schnaider Beeri et al., 2002; Herrmann N et al., 2006). These symptoms also contribute to earlier nursing home placement (Yaffe et al., 2002). A better understanding of BPSD and how they relate to neurodegenerative disease and its progression is important to patients, their families, and clinicians.
BPSD have been associated with overall clinical deterioration (Stella F et al., 2016), and there is evidence that patients with severe symptoms have identifiable neuroanatomical changes (Poulin et al., 2017). Delusions and hallucinations have been associated with atrophy within the neural networks that regulate complex behaviors (Rafii MS et al., 2014).
The ability to prognosticate clinical course is extremely important for clinicians as well as patients and their families. In addition, from a public health perspective, it is important to be able to predict costs and the health care resources needed to care for patients with AD as the population ages, as well as for identifying appropriate clinical targets for disease-modifying therapies. The trajectory of progression is not necessarily linear (Samtani et al., 2012) and is quite heterogeneous both between individuals and over the course of one case (Mayeux et al., 1985). To that end, several studies have tried to find ways of predicting clinical progression of disease. Although there are few FDA-approved treatments for BPSD at this time, the ability to predict disease course is highly valuable in its own right.
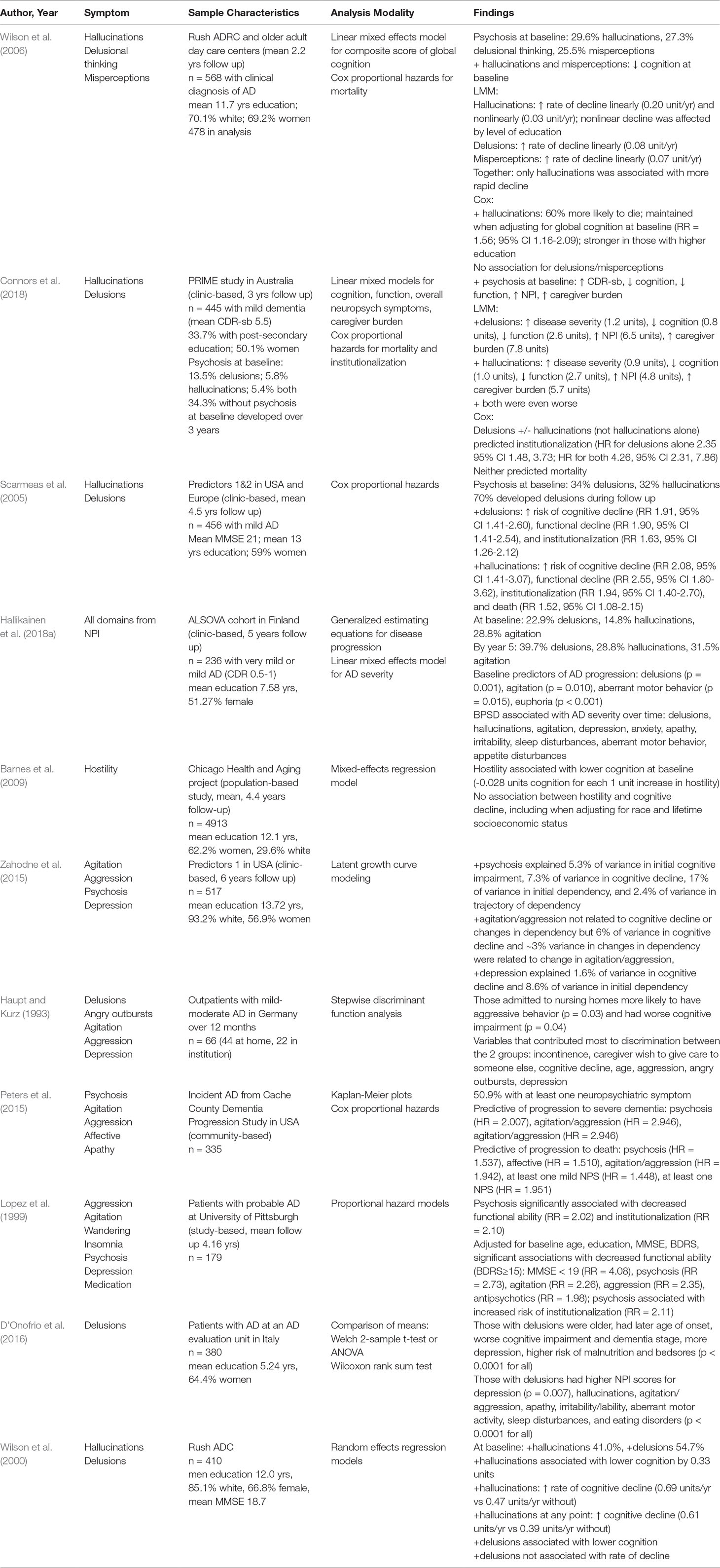
Although significant amounts of research have been devoted to a better understanding of the “negative” BPSD—namely, depression and anxiety—comparatively less has been devoted to “positive” symptoms, such as hallucinations and delusions. This review will discuss three major clusters of positive BPSD: hallucinations, delusions, and aggression/agitation. It will explore the underlying neural bases for these clusters of symptoms and discuss how these symptoms affect the rates of cognitive and functional decline in patients. A selection of the papers referenced are summarized in Table 1.
Hallucinations
Epidemiology/Neurobiology
The reported prevalence of hallucinations in Alzheimer’s disease is wide ranging, with some estimates from 12% to 33% (Leroi et al., 2003; Wilson et al., 2006; Scarmeas et al., 2005). Unlike dementia with Lewy bodies, where visual hallucinations are a core clinical feature (McKeith et al., 2017), the hallucinations of Alzheimer’s disease can be visual, auditory (Wilson et al., 2006), olfactory, or rarely, tactile (Devanand et al., 1992). Hallucinations in Alzheimer’s disease have been associated with lower education, non-Caucasian ethnicity, and worse severity of disease (Bassiony et al., 2000;Wilson et al., 2006).
Efforts to identify the anatomy underlying visual hallucinations have yielded variable results. Hallucinations have been associated with occipital atrophy (Holroyd et al., 2000) and hypoperfusion in left dorsolateral prefrontal, left medial temporal, and right parietal cortices (Lopez et al., 2001). One study found that atrophy in the right supramarginal gyrus predicted worsened hallucinations over 3 years (Donovan et al., 2014). It would be intuitive to propose that more global network dysfunction underlies the relationship between hallucinations and cognitive decline. However, there have been few formal studies assessing this network. The right anterior insula has been proposed as the “core region” for hallucinations, in part, due to its role in integrating external sensory input with the internal milieu (Blanc et al., 2014).
Deficiency in acetylcholine from the basal forebrain underlies attentional and arousal deficits in Alzheimer’s disease and other neurodegenerative diseases (Pepeu et al., 2013). Accordingly, acetylcholinerase inhibitors have long been a mainstay in the treatment of Alzheimer’s disease. It has been proposed that a form of cholinergic deficiency syndrome, which is characterized by restlessness, memory disturbances, and visual hallucinations, and was initially described as an iatrogenic syndrome from anticholinergic treatment, may exist in a more chronic form in neurodegenerative diseases (Lemstra et al., 2003). Although this proposal suggested that cholinergic deficiency syndrome is more specific to dementia with Lewy bodies, the symptoms of the syndrome are often present in patients with Alzheimer’s disease as well. An EEG study of patients with Alzheimer’s disease or dementia with Lewy bodies found that patients with hallucinations and Alzheimer’s disease had similar slowing of EEG activity as those with hallucinations and dementia with Lewy bodies, suggestive of cholinergic loss in both populations (Dauwan et al., 2018).
The effect of hallucinations on cognition has been associated with genetic predispositions. A large study using 900 autopsy-confirmed cases of AD from the National Alzheimer’s Coordinating Center (NACC) data to study the effect of psychosis and APO ε4 on cognition found that hallucinations were significantly associated with worse cognition, and that the presence of APO ε4 attenuated this relationship (Qian W et al., 2018). Interestingly, the presence of APO ε4 was also significantly associated with more Lewy body pathology in this study.
Additionally, hallucinations have been associated with sleep disturbances. It has been hypothesized that this is due to dysregulation of the neurotransmitter systems involved in sleep (Sinforiani et al., 2007). Hallucinations tend to be more likely to occur during sleep or during sleep–wake phase transitional phases (i.e. falling asleep and waking up) (Sinforiani et al., 2007). Intuitively, this supports an association between the sleep–wake cycle and the presence of hallucinations.
Contribution to Rates of Decline
Hallucinations are associated with more severe cognitive impairment (Wadsworth et al., 2012), are persistent (Holtzer et al., 2003), and may increase in incidence over time (Vilalta-Franch et al., 2013). There have been several studies both in community-based and clinic-based cohorts that assessed the relationship between hallucinations and the rate of decline. They have largely shown that the presence of hallucinations at baseline is associated with more rapid decline. Wilson et al. (2006) followed patients for an average of 2.2 years in the Rush Alzheimer’s Disease Center and community-dwelling adults and found that in addition to being related to poorer performance on cognitive screening (average MMSE of 10.7 for patients with hallucinations versus 14.1 for those without), patients demonstrated more rapid cognitive decline if they had hallucinations at baseline. Patients with hallucinations also had increased risk of mortality by the end of the study (RR, 1.55). Similarly, Connors et al. (2018) demonstrated that patients in memory clinics in Australia with hallucinations had worsened dementia severity, lower cognition and function, and greater caregiver burden over a 3-year period. Similar results have been found by several other studies (Forstl et al., 1993; Vilalta-Franch et al., 2013; Tchalla et al., 2018).
A limitation of any longitudinal study of hallucinations is that symptoms fluctuate over time (Devanand, 1999). Therefore, extended follow-up would be beneficial and contribute to improved robustness of the analysis. Hallikeinen et al. (2018a) analyzed data from the ALSOVA study, a cohort of patients with very mild and mild AD (CDR 0.5-1 at baseline) in Finland, using generalized estimated equations (GEE), and did not find evidence that hallucinations predicted disease severity over 5 years. However, using linear mixed models, they did find that hallucinations were significantly associated with Alzheimer’s disease severity over time.
In contrast, Scarmeas et al. (2005) analyzed data from participants in the Predictors 1 and Predictors 2 cohorts, which are longitudinal studies of patients diagnosed with probable Alzheimer’s disease in multiple centers in the United States and Europe (Stern et al., 1993; Scarmeas et al., 2004), for an average of 4.5 years, and up to 14 years, and using Cox analysis looked at the risk of reaching specified functional and cognitive endpoints. They found that the presence of hallucinations was associated with increased risk of cognitive (RR, 1.62) and functional (RR, 2.25) decline, institutionalization (RR, 1.60), and death (RR, 1.49). It is possible that part of the difference in results may be related to the use of different statistical measures.
An additional limitation of studying the role of hallucinations in Alzheimer’s disease is that it is often difficult to determine whether there is comorbid dementia with Lewy bodies (DLB). One large study found evidence of Lewy Body pathology in the brains of 60.7% of a cohort of clinically diagnosed Alzheimer’s disease. When NIA-RI criteria, which require AD pathology to make the diagnosis (The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease, 1997), were applied, Lewy bodies were found in 56.8% of the cohort (Hamilton, 2000). Furthermore, it can be clinically challenging to discriminate between the two conditions. Chung et al. found that pathology-confirmed AD did have distinct clinical phenotypes when co-occurring with Lewy body pathology (Chung et al., 2015). In contrast, Roudil et al. found that there were no significant clinical differences in many clinical and neuropsychological aspects between pathology-confirmed AD with Lewy bodies (either confined to the amygdala or more widespread in the cortex) and without, including the presence of hallucinations (Roudil et al., 2018), although this was a much smaller study and used a cohort that may have been worse cognitively.
Aggression/Agitation
Epidemiology/Neurobiology
Agitation and aggression are common in Alzheimer’s disease, with one large study of electronic health records estimating the prevalence of agitation as over 50% in mild cases (Halpern et al., 2019), as measured in a large study of electronic health records. It may be among the most significant contributors to longitudinal caregiver stress, possibly due to undermining a caregiver’s sense of security (Hallikainen et al., 2018b). However, there are fewer studies of agitation and aggression compared with other BPSD (Victoroff et al., 2018). One study of pathologically confirmed AD with intermediate or high pathology load found that patients with agitation and aggression tend to do worse on cognitive testing and are worse functionally (Sennik et al., 2017). It is a significant symptom from a public health perspective due to its association with higher healthcare costs through increased institutionalization (Costa et al., 2018) as well as “informal costs,” such as caregiver time (Rattinger et al., 2019).
Like hallucinations, the presence of agitation has been correlated with multiple anatomic locations. Agitation has been correlated with increased neurofibrillary tangle burden in the orbitofrontal and anterior cingulate cortices (Tekin et al., 2001). Supporting this is an anatomical study using ADNI data which found that the presence of worsening agitation and aggression was associated with greater atrophy in frontal, insular, amygdala, cingulate, and hippocampal regions of interest in patients with mild cognitive impairment and AD dementia over 2 years (Trzepacz et al., 2013). It has also been suggested that right frontal lobe dysfunction may be predominant (Lopez et al., 2001). Functional imaging studies have similarly suggested that agitation is associated with lower metabolism in frontal and temporal regions (Sultzer et al., 1995). Interestingly, Ehrenberg et al. (Ehrenberg et al., 2018) found that agitation was associated with neurofibrillary tangle pathology at Braak stages I to IV but not at levels V to VI, suggesting that subcortical pathology may be an important contributor as well.
Aggression has been linked to increased amyloid burden. Transgenic mice expressing a human APP mutation have been shown to be significantly more aggressive than non-transgenic littermates, even early on the course of disease (Alexander et al., 2011). In humans, a large study found that the presence of agitation/aggression was directly correlated to high burden of amyloid pathology in a cohort of pathologically confirmed AD (Sennik et al., 2017), and that other clinical diagnoses were attributed to patients with agitation/aggression, including dementia with Lewy Bodies and frontotemporal dementia. In this cohort, phosphorylated TDP-43 deposits were more common in male patients with agitation, indicating the difficulty in diagnosing patients appropriately when behavioral disturbances are present.
In a cohort of pathologically confirmed Alzheimer’s disease, the presence of a higher overall score on the Cohen-Mansfield Agitation Inventory (Cohen-Mansfield, 1986), a scale specifically for agitation and aggression in the elderly, was significantly associated with decreased levels of 5-HIAA in the hippocampus. In this cohort, the presence of physically nonaggressive behavior (which may still be distressing to the patient and caregiver) was significantly associated with increased dopamine catabolism in the cerebellum (Vermeiren et al., 2014). An autopsy-based study of individuals with a clinical diagnosis of Alzheimer’s disease showed that aggression was a significant predictor of decreased cholinergic innervation on autopsy, and overactivity was the best predictor of decreased serotonergic innervation (Garcia-Alloza et al., 2005).
Association With Rates of Decline
Agitation, alone and in combination with other BPSD, has been associated with disease severity over time, but inconsistently with disease progression. There are few studies that assess agitation as an isolated factor. Haupt and Kurz (Haupt and Kurz, 1993), in a small clinic-based study, found that aggression was one factor that predicted institutionalization over 1 year. Similarly, Peters et al. (2015) in the Cache County Dementia Progression Study, found that agitation/aggression was a significant predictor of the risks of both of severe dementia (defined as CDR ≥ 2 or MMSE ≤ 10) and death (HR 2.946, 1.942 respectively) in a Cox proportional hazards model, although the baseline status of participants in that cohort is unclear. Further, Lopez et al. (1999) in their study of the effects of psychiatric symptoms and psychiatric medications on disease progression found that the presence of either aggression or agitation was independently associated with a significantly increased risk of shorter time to significant functional impairment (RR, 2.35, 2.26, respectively) while controlling for age, education, sex, and baseline cognitive and functional status.
In contrast, Barnes and colleagues, using data from the Chicago Health and Aging Project, a longitudinal population-based study, found that hostility was both associated with worse cognition at baseline in both non-Hispanic whites and African Americans but not with cognitive decline over 4.4 years, in a mixed-effects regression model (Barnes et al., 2009). Zahodne et al. studied data from the Predictors 1 cohort and found that agitation/aggression at baseline were not correlated with cognition at baseline, functional decline, or cognitive decline in a latent growth curve model over 6 years. However, change over time in agitation/aggression did account for a small amount of the variability in cognitive decline over time (Zahodne et al., 2015). Similarly, in the ALSOVA study, agitation at the time of diagnosis did not predict disease progression but was significantly associated with severity of disease over 5 years (Hallikainen et al., 2018a).
Of note, Hallikainen et al. found that, along with agitation and aggression, aberrant motor behavior tended to track with disease severity and was a predictor of disease progression (Hallikainen et al., 2018a). Aberrant motor behaviors are sometimes, (Aalten et al., 2003; van der Linde et al., 2014) but not always, (Garre-Olmo et al., 2010b) clustered together with agitation in studies that have looked for grouping of neuropsychiatric symptoms out of the neuropsychiatric inventory (NPI) by methods such as factor analysis and latent class analysis.
Delusions
Epidemiology/Neurobiology
Delusions are a well-recognized symptom of Alzheimer’s disease although Holtzer et al. found that they may worsen initially and then become less prevalent over time (Holtzer R et al., 2003), which can make estimating its prevalence difficult. Delusions tend to be considered either “persecutory” or related to misidentification phenomena, such as Capgras syndrome or phantom boarder syndrome. The presence of delusions tends to be combined with the presence of hallucinations to create the construct of psychosis. Although psychosis certainly encompasses both of these symptoms, delusions may be representative of different neural circuits than hallucinations. Clinically as well, it is worthwhile considering the two symptoms separately. There are many circumstances where visual and auditory hallucinations may occur in the absence of fixed beliefs on the part of the patient believing that they are true, and delusions can occur in the absence of hallucinations. Interestingly, delusions may have a different impact on patients’ functioning than hallucinations or agitation. Bertrand and colleagues (Bertrand et al., 2017) found that patients with mild to moderate AD who had delusions had a decreased ability to express treatment choice preference, potentially impacting their ability to consent to medical care. In contrast, patients with hallucinations, agitation/aggression, and several other BPSD did not have weaknesses in decision-making abilities.
Structurally, the presence of delusions has been associated with decreased gray matter density in the right inferior frontal gyrus and inferior parietal lobule, as well as the left inferior and medial frontal gyri and claustrum in patients with mild AD (Bruen et al., 2008). Interestingly, Fischer and colleagues compared MRI scans in patients with MCI before and after the onset of delusions (generally within the span of 6 months), and found significant differences in gray matter morphology in 14 locations, including the bilateral insulae, the cerebellum, the right thalamus and posterior cingulate gyrus, and the left precuneus, left superior temporal gyrus, and parahippocampal gyrus (Fischer et al., 2016). During that time, some patients had converted from MCI to mild AD, and the mean cognition had worsened as well. The presence of delusions has also been associated with abnormalities in the integrity of white matter in the left parietooccipital region and the corpus callosum (Nakaaki et al., 2013), as well as advanced neurofibrillary tangle pathology (Ehrenberg et al., 2018).
Association With Rates of Decline
Delusions are often studied in conjunction with hallucinations. Their presence is often associated with poorer performance on cognitive testing cross-sectionally (Jeste et al., 1992) as well as faster decline longitudinally. Connors et al, in the PRIME study in Australia, found that the presence of delusions alone was associated with worse cognition, function, and dementia severity, as well as increased caregiver burden, over 3 years using a linear mixed model. Delusions also predicted institutionalization, but not mortality (Connors et al., 2018). Similarly, Scarmeas et al. found in the Predictors cohort that delusions were associated with increased risk for cognitive (RR, 1.50) and functional (RR, 1.41) decline as well as institutionalization (RR, 1.60) and mortality (RR, 1.49) (Scarmeas et al., 2005).
Interestingly, D’Onofrio and colleagues, in a single-center study, found that delusions were associated with a trend toward significantly longer disease duration in patients with mild to moderate AD (D’Onofrio et al., 2016). Further, Wilson et al. studied the effects of hallucinations and delusions over 4 years and found that delusions were not associated with the rate of cognitive decline (Wilson et al., 2000). There are few studies that separate delusions from hallucinations in longitudinal studies; however, the evidence regarding the effect of delusions on the rate of cognitive decline seems to be less conclusive. Additional studies would be useful in better understanding the effect of the presence of delusions.
Using BPSD to Inform Models of Disease
The clinical diagnosis of probable Alzheimer’s disease requires the presence of worsening cognition in either an amnestic or nonamnestic pattern (McKhann et al., 2011). Noncognitive symptoms are not considered in these diagnostic criteria; however, BPSD are a prevalent aspect of disease. Although the time point of disease during which these symptoms initially manifest varies, they persist over time and increase with worsening of disease. These symptoms may be more challenging for families to address than cognitive symptoms and represent a public health concern due to the increased morbidity, mortality, and associated healthcare costs. The areas of the brain which correlate to these symptoms are likely affected due to the spread of pathology across neural networks, and it remains unclear why these behavioral areas become affected early in some patients. The genetic and environmental contributors to the presence and timing of these symptoms have yet to be elucidated. The variable results in attempting to identify specific brain regions associated with specific symptoms suggests that the presence of BPSD may be reflective of network-wide dysfunctions. Indeed, the presence of hyperactivity, which includes agitation, has been linked to changes in the anterior salience network, which is important in generating appropriate responses to the external environment, in resting-state functional MRI (Balthazar et al., 2014). It is worth considering whether the presence of these symptoms should be considered as important in diagnosing Alzheimer’s disease as are cognitive changes. Due to the extensive heterogeneity in Alzheimer’s disease, different models of disease prediction may need to be developed. In fact, Razlighi et al, in developing and validating a longitudinal Grade of Membership (L-GoM) model to predict time to institutionalization, full-time care, and death in the Predictors 1 and 2 cohorts, included the presence of BPSD, such as psychosis and wandering (Razlighi et al., 2014). Further studies are needed to better understand these issues, so that ultimately effective symptomatic treatments can be developed.
Additionally, the relationship between neuropathological changes and BPSD is likely affected by outside factors. Casanova et al, in their review of clinicopathological correlates of BPSD, note that neuroleptics may increase the risk for cerebrovascular events in patients with dementia which may itself increase the risk for certain BPSD (namely, depression and apathy) and also worsen cognitive decline (Casanova et al., 2011). Neuroleptics and other antipsychotics may also have an impact on disease presentation and progression; these medications may reduce symptoms of BPSD, although a consensus of geriatric mental health experts noted that the evidence is limited and inconsistent with regard to agitation and aggression (Salzman et al., 2008). However, Lopez et al. (1999) found that antipsychotics were associated with increased functional decline, and hypothesized that this effect could be related to sedation or extrapyramidal effects. Nonpharmacologic therapies such as music, bright light, and pet therapy have also been noted to be possibly effective in reducing symptoms of BPSD, although such therapies are often part of a broader care program (Forlenza et al., 2017; Doody et al., 2001) and can be difficult to isolate for purposes of a trial. Many, but not all, studies looking at the effect of BPSD on disease progression take pharmacologic therapy into account; however, we were unable to find studies that accounted for nonpharmacologic approaches. If the use of nonpharmacologic therapies can be strengthened with additional clinical trials, this will need to be taken into account in future studies of BPSD as well.
Furthermore, the use of cholinesterase inhibitors and memantine may confound these effects as well; in the Predictors 2 cohort, cholinesterase inhibitors were associated with delayed functional decline, and memantine was associated with delayed time to death when controlled for several patient characteristics, including the presence of psychiatric symptoms (Zhu et al., 2013). Although this does not confer causality, it implies that current standard of care treatment for Alzheimer’s disease may affect the influence of BPSD on dementia severity.
An additional factor not usually accounted for is the role of social support or family involvement in care, whose relative absence has been associated with increased depression in the elderly (Sonnenberg et al., 2013). In contrast, Chan et al. found that those with a child or child-in-law as the caregiver were more likely to be reported as having psychopathy, although it is not clear whether this was due to a difference in prevalence of the symptoms or a difference in likelihood of reporting them (Chan et al., 2003). It is plausible that the presence of a more extensive social support network and family presence could help to alleviate distress caused by symptoms, such as hallucinations and perhaps mitigate the effect of these symptoms on functioning and cognitive decline. Further studies would be useful in clarifying this effect.
An important limitation in many studies has been the lack of pathologic confirmation of diagnosis. BPSD are present in many neurodegenerative diseases, such as Lewy body disease and frontotemporal dementia. Clinically, these entities can be difficult to distinguish from each other, particularly early in the course of disease. As an example, the frontal variant of Alzheimer’s disease may be misdiagnosed as frontotemporal dementia—one clinic-based study found that nearly 40% of cases diagnosed clinically as frontotemporal dementia were changed to a diagnosis of Alzheimer’s disease based on PET scan results (Ossenkoppele et al., 2013). Similarly, an autopsy series of Lewy body disease, Alzheimer’s disease, and AD with amygdala-predominant Lewy bodies found that the presence of visual hallucinations did not distinguish between the groups, although they tended to occur earlier in Lewy body disease. The underlying pathologies of Alzheimer’s disease and Lewy body disease frequently coexist (Hamilton, 2000), which can make it difficult to ascertain clinically which pathology is causing the symptoms.
Additionally, there is no standard method for estimating baseline effects on downstream events. Statistical methods that are commonly used are Cox proportional hazards, latent growth curve modeling, and generalized estimating equations. Each model has its strengths; however, it can be difficult to directly compare results obtained from different methods, leading to additional difficulty in using baseline characteristics to predict effects over time.
In the clinical setting, the presence of early hallucinations and agitation may direct the clinician’s diagnostic approach away from the possibility of underlying Alzheimer’s pathology. Current diagnostic criteria for other neurodegenerative disorders encourage this approach; however, this may be an incomplete understanding. It may be more useful to view the presence of BPSD in the setting of other cognitive changes as a relatively nonspecific symptom of underlying neural network dysfunction. Biomarker-based diagnosis in the form of imaging, as well as CSF and serum diagnostics, is likely to be increasingly important in refining clinical diagnostic criteria.
Conclusion
The ability to prognosticate is critically important for patients and their families. The balance of the evidence suggests that early presence of positive BPSD may predict faster progression of disease. This does not account for the effect of negative BPSD (namely, depression and apathy, which likely also contribute to progression of disease). Although the etiology underlying this effect is not well understood, it is likely a complex combination of genetic predisposition and comorbid neuropathology. Cohort studies that followed patients with early dementia have been vitally important in elucidating this effect, although few studies have followed patients for long enough to capture the variability over a long disease course. Additional long-term studies are needed to ensure the generalizability of these effects.
Author Contributions
RG and YS contributed to manuscript preparation and editing.
Funding
This work was supported by R01 AG007370 from the NIA and by 5T32 NS007153 from the NINDS (Elkind, PI).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References
Aalten, P., de Vugt, M. E., Lousberg, R., Korten, E., Jaspers, N., Senden, B., et al. (2003). Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement. Geriatr. Cogn. Disord. 15 (2), 99–105. doi: 10.1159/000067972
Alexander, G., Hanna, A., Serna, V., Younkin, L., Younkin, S., Janus, C. (2011). Increased aggression in males in transgenic Tg2576 mouse model of Alzheimer’s disease. Behav. Brain Res. 216 (1), 77–83. doi: 10.1016/j.bbr.2010.07.016
Balthazar, M. L., Pereira, F. R., Lopes, T. M., da Silva, E. L., Coan, A. C., Campos, B. M., et al. (2014). Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum. Brain Mapp. 35 (4), 1237–1246. doi: 10.1002/hbm.22248
Barnes, L. L., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S., Everson-Rose, S. A., Evans, D. A. (2009). Hostility and change in cognitive function over time in older blacks and whites. Psychosom. Med. 71, 6, 652–658. doi: 10.1097/PSY.0b013e3181a651b3
Bassiony, M. M., Steinberg, M. S., Warren, A., Rosenblatt, A., Baker, A. S., Lyketsos, C. G. (2000). Delusions and hallucinations in Alzheimer’s disease: prevalence and clinical correlates. Int. J. Geriatr. Psychiatry 15 (2), 99–107. doi: 10.1002/(SICI)1099-1166(200002)15:2<99::AID-GPS82>3.0.CO;2-5
Bertrand, E., van Duinkerken, E., Landeira-Fernandez, J., Dourado, M. C. N., Santos, R. L., Laks, J., et al. (2017). Behavioral and psychological symptoms impact clinical competence in Alzheimer’s disease. Front. Aging Neurosci 9 (182), 1–8. doi: 10.3389/fnagi.2017.00182
Blanc, F., Noblet, V., Philippi, N., Cretin, B., Foucher, J., Armspach, J.-P., et al. (2014). Right anterior insula: core region of hallucinations in cognitive neurodegenerative diseases. PLoS One 9 (12), e114774. doi: 10.1371/journal.pone.0114774
Bruen, P. D., McGeown, W. J., Venneri, A., Shanks, M. F. (2008). Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain 131 (9), 2455–2463. doi: 10.1093/brain/awn151
Casanova, M. F., Starkstein, S. E., Jellinger, KAJAN. (2011). Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta. Neuropathol. 122 (2), 117–135. doi: 10.1007/s00401-011-0821-3
Chan, D. C., Kasper, J. D., Black, B. S., Rabins, P. V. (2003). Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: the Memory and Medical Care Study. Int. J. Geriatr. Psychiatry 18 (2), 174–182. doi: 10.1002/gps.781
Chung, E. J., Babulal, G. M., Monsell, S. E., Cairns, N. J., Roe, C. M., Morris, J. C. (2015). Clinical features of Alzheimer disease with and without Lewy bodies. JAMA Neurol. 72 (7), 789–796. doi: 10.1001/jamaneurol.2015.0606
Cohen-Mansfield, J. (1986). Agitated behaviors in the elderly. J. Am. Geriatr. Soc. 34 (10), 722–727. doi: 10.1111/j.1532-5415.1986.tb04303.x
Connors, M. H., Ames, D., Woodward, M., Brodaty, H. (2018). Psychosis and clinical outcomes in Alzheimer disease: a longitudinal study. Am. J. Geriatr. Psychiatry 26 (3), 304–313. doi: 10.1016/j.jagp.2017.10.011
Costa, N., Wübker, A., De Mauléon, A., Zwakhalen, S. M. G., Challis, D., Leino-Kilpi, H., et al. (2018). Costs of care of agitation associated with dementia in 8 European countries: results from the right time place care study. JAMDA 19 (1), 95.e1–9.e10. doi 10.1016/j.jamda.2017.10.013
D’Onofrio, G., Panza, F., Sancarlo, D., Paris, F. F., Cascavilla, L., Mangiacotti, A., et al. (2016). Delusions in patients with Alzheimer’s disease: a multidimensional approach. J. Alzheimers Dis. 51 (2), 427–437. doi: 10.3233/JAD-150944
Dauwan, M., Linszen, M. M. J., Lemstra, A. W., Scheltens, P., Stam, C. J., Sommer, I. E. (2018). EEG-based neurophysiological indicators of hallucinations in Alzheimer’s disease: comparison with dementia with Lewy bodies. Neurobiol. Aging 67, 75–83. doi: 10.1016/j.neurobiolaging.2018.03.013
Devanand, D. (1999). The interrelations between psychosis, behavioral disturbances, and depression in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 13 (Suppl 2), S3–S8. doi: 10.1097/00002093-199911002-00002
Devanand, D. P., Miller, L., Richards, M., Marder, K., Bell, K., Mayeux, R., et al. (1992). The columbia university scale for psychopathology in Alzheimer’s disease. Arch Neurol. 49 (4), 371–376. doi: 10.1001/archneur.1992.00530280051022
Donovan, N. J., Wadsworth, L. P., Lorius, N., Locascio, J. J., Rentz, D. M., Johnson, K. A., et al. (2014). Regional cortical thinning predicts worsening apathy and halluciantions across the Alzheimer Disease Spectrum. Am. J. Geriatr. Psychiatry. 22 (11), 1168–1179. doi: 10.1016/j.jagp.2013.03.006
Doody, R. S., Stevens, J. C., Beck, C., Dubinsky, R. M., Kaye, J. A., Gwyther, L., et al. (2001). Practice parameter: management of dementia (an evidence-based review). Neurology. 56 (9), 1154–1166. doi: 10.1212/WNL.56.9.1154
Ehrenberg, A. J., Suemoto, C. K., Franca Resende, E. P., Petersen, C., Leite, R. E. P., Rodriguez, R. D., et al. (2018). Neuropathologic correlates of psychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 66 (1), 115–126. doi: 10.3233/JAD-180688
Erkkinen, M. G., Kim, M.-O., Geschwind, M. D. (2018). Clinical neurology and epidemiology of major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 10 (4), 1–44. doi: 10.1101/cshperspect.a033118
Fischer, C. E., Ting, W. K.-C., Millikin, C. P., Ismail, Z., Schweizer, T. A., Initiative, T. A. D. N. (2016). Gray matter atrophy in patients with mild cognitive impairment/Alzheimer’s disease over the course of developing delusions. Int. J. Geriatr. Psychiatry 31 (1), 76–82. doi: 10.1002/gps.4291
Forlenza, O. V., Loureiro, J. C., Pais, M. V., Stella, F. (2017). Recent advances in the management of neuropsychiatric symptoms in dementia. Curr. Opin. Psychiatry 30 (2), 151–158. doi: 10.1097/YCO.0000000000000309
Forstl, H. B. C., Geiger-Kabisch, C., Sattel, H., Schreiter-Gasser, U. (1993). Psychotic features and the course of Alzheimer’s disease: relationship to cognitive, electroencephalographic and computerized tomography findings. Acta Psychiatr. Scan. 87, 395–399. doi: 10.1111/j.1600-0447.1993.tb03394.x
Garcia-Alloza, M., Gil-Bea, F. J., Diez-Ariza, M., Chen, C. P. L. H., Francis, P. T., Lasheras, B., et al. (2005). Cholinergic–serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 43 (3), 442–449. doi: 10.1016/j.neuropsychologia.2004.06.007
Garre-Olmo, J., Lopez-Pousa, S., Vilalta-French, J., de Gracia Blanco, M., Bulbena Vilarrasa, A. (2010a). Grouping and trajectories of neuropsychiatric symptoms in patients with Alzheimer’s disease. J. Alzheimers Dis. 22, 1169–1180. doi: 10.3233/JAD-2010-101215
Garre-Olmo, J., Lopez-Pousa, S., Vilalta-French, J., de Gracia Blanco, M., Bulbena Vilarrasa, A. (2010b). Grouping and trajectories of the neuropsychiatric symptoms in patients with Alzheimer’s disease, Part I: symptom clusters. J. Alzheimers Dis. 22, 1157–1167. doi: 10.3233/JAD-2010-101212
Hallikainen, I., Hongisto, K., Valimaki, T., Hanninen, T., Martikainen, J., Koivisto, A. M. (2018a). The progression of neuropsychiatric symptoms in Alzheimer’s disease during a five-year follow-up: Kuopio ALSOVA Study. J. Alzheimers Dis. 61 (4), 1367–1376. doi: 10.3233/JAD-170697
Hallikainen, I., Koivisto, A. M., Välimäki, T. (2018b). The influence of the individual neuropsychiatric symptoms of people with Alzheimer disease on family caregiver distress—a longitudinal ALSOVA study. Int. J. Geriatr. Psychiatry 33 (9), 1207–1212. doi: 10.1002/gps.4911
Halpern, R., Seare, J., Tong, J., Hartry, A., Olaoye, A., Aigbogun, M. S. (2019). Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/dementia. Int. J. Geriatr. Psychiatry 34 (3), 420–431. doi: 10.1002/gps.5030
Hamilton, R. L. (2000). Lewy Bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 10 (3), 378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x
Haupt, M., Kurz, A. (1993). Predictors of nursing home placement in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 8 (9), 741–746. doi: 10.1002/gps.930080906
Herrmann N, L. K., Sambrook, R., Lesnikova, N., Hebert, R., McCracken, P., Robillard, A., et al. (2006). The contribution of neuropsychiatric symptoms to the cost of dementia care. Int. J. Geriatr. Psychiatry 21, 972–976. doi: 10.1002/gps.1594
Holroyd, S., Shepherd, ML J., Hunter Downs, I. (2000). Occipital atrophy is associated with visual hallucinations in Alzheimer’s disease. J. Neuropsychiatr Clin. Neurosci. 12 (1), 25–28. doi: 10.1176/jnp.12.1.25
Holtzer, R., Tang, M.-X., Devanand, D. P., Albert, S. M., Wegesin, D. J., Marder, K., et al. (2003). Psychopathological features in Alzheimer’s disease: course and relationship with cognitive status. J. Am. Geriatr. Soc. 51, 953–960. doi: 10.1046/j.1365-2389.2003.51308.x
Ismail, Z., Smith, E. E., Geda, Y., Sultzer, D., Brodaty, H., Smith, G., et al. (2016). Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 12 (2), 195–202. doi: 10.1016/j.jalz.2015.05.017
Jeste, D. V., Wragg, R. E., Salmon, D. P., Harris, M. J., Thal, L. J. (1992). Cognitive deficits of patients with Alzheimer’s disease with and without delusions. Am. J. Psychiatry. 149 (2), 184–189. doi: 10.1176/ajp.149.2.184
Lemstra, A. W., Eikelenboom, P., van Gool, W. A. (2003). The cholinergic deficiency syndrome and its therapeutic implications. Gerontology. 49 (1), 55–60. doi: 10.1159/000066508
Leroi, I., Voulgari, A., Breitner, J. C. S., Lyketsos, C. G. (2003). The epidemiology of psychosis in dementia. Am. J. Geriatr. Psychiatry 11 (1), 83–91. doi: 10.1097/00019442-200301000-00011
Lopez, O. L., Smith, G., Becker, J. T., Meltzer, C. C., DeKosky, S. T. (2001). The psychotic phenomenon in probable Alzheimer’s disease. J. Neuropsychiatr Clin. Neurosci. 13 (1), 50–55. doi: 10.1176/jnp.13.1.50
Lopez, O. L., Wisniewski, S. R., Becker, J. T., Boller, F., DeKosky, S. T. (1999). Psychiatric medication and abnormal behavior as predictors of progression in probable alzheimer disease. Arch Neurol. 56 (10), 1266–1272. doi: 10.1001/archneur.56.10.1266
Lyketsos, C. G., Lopez, O., Jones, B., Fitzpatrick, A. L., Breitner, J., DeKosky, S. (2002). Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 288 (12), 1475–1483. doi: 10.1001/jama.288.12.1475
Maurer, K., Volk, S., Gerbaldo, H. (1997). Auguste D and Alzheimer’s disease. Lancet. 349 (9064), 1546–1549. doi: 10.1016/S0140-6736(96)10203-8
Mayeux, R., Stern, Y., Spanton, S. (1985). Heterogeneity in dementia of the Alzheimer Type; evidence of subgroups. Neurology. 35, 453–461. doi: 10.1212/WNL.35.4.453
McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J.-P., Weintraub, D., et al. (2017). Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 89 (1), 88–100. doi: 10.1212/WNL.0000000000004058
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. J. Alzheimers Assoc. 7 (3), 263–269. doi: 10.1016/j.jalz.2011.03.005
Murman, D. L., Chen, Q., Powell, M. C., Kuo, S. B., Bradley, C. J., Colenda, C. C. (2002). The incremental direct costs associated with behavioral symptoms in AD. Neurology. 59 (11), 1721–1729. doi: 10.1212/01.WNL.0000036904.73393.E4
Nakaaki, S., Sato, J., Torii, K., Oka, M., Negi, A., Nakamae, T., et al. (2013). Decreased white matter integrity before the onset of delusions in patients with Alzheimer’s disease: diffusion tensor imaging. Neuropsychiatr. Dis. Treat 9, 25–29. doi: 10.2147/NDT.S38942
Ossenkoppele, R., Prins, N. D., Pijnenburg, Y. A., Lemstra, A. W., van der Flier, W. M., Adriaanse, S. F., et al. (2013). Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 9 (4), 414–421. doi: 10.1016/j.jalz.2012.07.003
Pepeu, G., Giovannini, M. G., Bracco, L. (2013). Effect of cholinesterase inhibitors on attention. Chem. Biol. Interact. 203 (1), 361–364. doi: 10.1016/j.cbi.2012.09.016
Peters, M. E., Schwartz, S., Han, D., Rabins, P. V., Steinberg, M., Tschanz, J. T., et al. (2015). Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death. Cache County Demen. Progression Stud. 172 (5), 460–465. doi: 10.1176/appi.ajp.2014.14040480
Poulin, S. P., Bergeron, D., Dickerson, B. C. (2017). Risk factors, neuroanatomical correlates, and outcome of neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 60, 483–493. doi: 10.3233/JAD-160767
Qian, W, F. C., Schweizer, T. A., Munoz, D. G. (2018). Association between psychosis phenotype and APOE genotype on the clinical profiles of Alzheimer’s disease. Curr. Alzheimer Res. 15 (2), 187–194. doi: 10.2174/1567205014666170829114346
Rafii, MS, T. C., Kin, H. T., Desikan, R. S., Fleisher, A. S., Katibian, D., Brewer, J. B., et al . (2014). Neuropsychiatric symptoms and regional neocortical atrophy in mild cognitive impairment and Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 29 (2), 159–165. doi: 10.1177/1533317513507373
Rattinger, G. B., Sanders, C. L., Vernon, E., Schwartz, S., Behrens, S., Lyketsos, C. G., et al. (2019). Neuropsychiatric symptoms in patients with dementia and the longitudinal costs of informal care in the Cache County population. Alzheimers Dement. 5, 81–88. doi: 10.1016/j.trci.2019.01.002
Razlighi, Q. R., Stallard, E., Brandt, J., Blacker, D., Albert, M., Scarmeas, N., et al. (2014). A new algorithm for predicting time to disease endpoints in Alzheimer’s disease patients. J. Alzheimers Dis. 38 (3), 661–668. doi: 10.3233/JAD-131142
Roudil, J., Deramecourt, V., Dufournet, B., Dubois, B., Ceccaldi, M., Duyckaerts, C., et al. (2018). Influence of Lewy pathology on Alzheimer’s disease phenotype: a retrospective clinico-pathological study. J. Alzheimers Dis. 63 (4), 1317–1323. doi: 10.3233/JAD-170914
Salzman, C., Jeste, D. V., Meyer, R. E., Cohen-Mansfield, J., Cummings, J., Grossberg, G. T., et al. (2008). Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J. Clin. Psychiatry 69 (6), 889–898. doi: 10.4088/JCP.v69n0602
Samtani, M. N., Farnum, M., Lobanov, V., Yang, E., Raghavan, N., Dibernardo, A., et al. (2012). An improved model for disease progression in patients from the Alzheimer’s disease neuroimaging initiative. J. Clin. Pharmacol. 52 (5), 629–644. doi: 10.1177/0091270011405497
Scarmeas, N., Hadjigeorgiou, G. M., Papadimitriou, A., Dubois, B., Sarazin, M., Brandt, J., et al. (2004). Motor signs during the course of Alzheimer disease. Neurology. 63 (6), 975. doi: 10.1212/01.WNL.0000138440.39918.0C
Scarmeas, N., Brandt, J., Albert, M., Hadjigeorgiou, G., Papadimitriou, A., Dubois, D. (2005). Delusions and hallucinations are associated with worse outcome in alzheimer disease. Arch Neurol. 62 (10), 1601–1608. doi: 10.1001/archneur.62.10.1601
Schnaider Beeri, M., Werner, P., Davidson, M., Noy, S. (2002). The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int. J. Geriatr. Psychiatry 17 (5), 403–408. doi: 10.1002/gps.490
Sennik, S., Schwizer, T. A., Fischer, C. E., Munoz, D. G. (2017). Risk factors and pathological substrates associated with agitation/aggression in Alzheimer’s disease: a preliminary study using NACC data. J. Alzheimers Dis. 55, 1519–1528. doi: 10.3233/JAD-160780
Sinforiani, E., Terzaghi, M., Pasotti, C., Zucchella, C., Zambrelli, E., Manni, R. (2007). Hallucinations and sleep–wake cycle in Alzheimer’s disease: a questionnaire-based study in 218 patients. Neurol. Sci. 28, 96–99. doi: 10.1007/s10072-007-0794-0
Sonnenberg, C. M., Deeg, D. J., van Tilburg, T. G., Vink, D., Stek, M. L., Beekman, A. T. (2013). Gender differences in the relation between depression and social support in later life. Int. Psychogeriatr. 25 (1), 61–70. doi: 10.1017/S1041610212001202
Stella, F, L. J., Govone, J. S., de Medeiros, K., Forlenza, O. V. (2016). Association of neuropsychiatric syndromes with global clinical deterioration in Alzheimer’s disease patients. Int. Psychogeriatr. 28 (5), 779–786. doi: 10.1017/S1041610215002069
Stern, Y., Folstein, M., Albert, M., Richards, M., Miller, L., Bylsma, F., et al. (1993). Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). Alzheimer Dis. Assoc. Disord. 7 (1), 3–21. doi: 10.1097/00002093-199307010-00002
Sultzer, D. L., Mahler, M. E., Mandelkern, M. A., Cummings, J. L., Van Gorp, W. G., Hinkin, C. H., et al. (1995). The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J. Neuropsychiatr Clin. Neurosci. 7 (4), 476–484. doi: 10.1176/jnp.7.4.476
Tchalla, A. E., Clement, J. P., Saulnier, I., Beaumatin, B., Lachal, F., Gayot, C., et al. (2018). Predictors of rapid cognitive decline in patients with mild-to-moderate Alzheimer disease: a prospective cohort study with 12-month follow up performed in memory clinics. Dement. Geriatr. Cogn. Disord. 45, 56–65. doi: 10.1159/000487938
Tekin, S., Mega, M., Masterman, D., Chow, T., Garakian, J., Vinters, H., et al. (2001). Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden in associated with agitation in Alzheimer disease. Ann. Neurol. 49, 355–361. doi: 10.1002/ana.72
The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. (1997). Consensus Recommendations for the Postmortem Diagnosis of Alzheimer’s disease. Neurobiol. Aging 18 (4, Supplement 1), S1–S2. doi: 10.1016/S0197-4580(97)00057-2
Trzepacz, P. T., Yu, P., Bhamidipati, P. K., Willis, B., Forrester, T., Tabas, L., et al. (2013). Frontolimbic atrophy is associated with agitation and aggression in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 9 (5, Supplement), S95–S104.e1. doi: 10.1016/j.jalz.2012.10.005
Van Den Wijngaart, M. A. G., Vernooij-Dassen, M. J. F. J., Felling, A. J. A. (2007). The influence of stressors, appraisal and personal conditions on the burden of spousal caregivers of persons with dementia. Aging Ment. Health 11 (6), 626–636. doi: 10.1080/13607860701368463
van der Linde, R. M., Dening, T., Matthews, F. E., Brayne, C. (2014). Grouping of behavioural and psychological symptoms of dementia. Int. J. Geriatr. Psychiatry 29 (6), 562–568. doi: 10.1002/gps.4037
Vermeiren, Y., Van Dam, D., Aerts, T., Engelborghs, S., De Deyn, P. P. (2014). Brain region-specific monoaminergic correlates of neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimers Dis. 41 (3), 819–833. doi: 10.3233/JAD-140309
Victoroff, J., Lin, F. V., Coburn, K. L., Shillcutt, S. D., Voon, V., Ducharme, S. (2018). Noncognitive behavioral changes associated with Alzheimer’s disease: implications of neuroimaging findings. J. Neuropsychiatry Clin. Neurosci. 30, 14–21. doi: 10.1176/appi.neuropsych.16080155
Vilalta-Franch, J L-PS, Calvo-Perxas, L., Garre-Olmo, J. (2013). Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am. J. Geriatr. Psychiatry 21, 1135–1143. doi: 10.1016/j.jagp.2013.01.051
Wadsworth, L. P., Lorius, N., Donovan, N. J., Locascio, J. J., Rentz, D. M., Johnson, K. A., et al. (2012). Neuropsychiatric Symptoms and Global Functional Impairment along the Alzheimer’s continuum. Dement. Geriatr. Cogn. Disord. 34, 96–111. doi: 10.1159/000342119
Wilson, R. S., Tang, Y., Aggarwal, N. T., Gilley, D. W., McCann, J. J., Bienias, J. L., et al. (2006). Hallucinations, cognitive decline, and death in Alzheimer’s disease. Neuroepidemiology 26, 68–75. doi: 10.1159/000090251
Wilson, R. S., Gilley, D. W., Bennett, D. A., Beckett, L. A., Evans, D. A. (2000). Hallucinations, delusions, and cognitive decline in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 69 (2), 172. doi: 10.1136/jnnp.69.2.172
Yaffe, K., Fox, P., Newcomer, R., Sands, L., Lindquist, K., Dane, K., et al. (2002). Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 287 (16), 2090–2097. doi: 10.1001/jama.287.16.2090
Zahodne, L. B., Ornstein, K., Cosentino, S., Devanand, D. P., Stern, Y. (2015). Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am. J. Geriatr. Psychiatry 23 (2), 130–140. doi: 10.1016/j.jagp.2013.03.014
Keywords: Alzheimer’s disease, behavioral and psychiatric symptoms, cognitive decline, functional decline, predictors of decline
Citation: Gottesman RT and Stern Y (2019) Behavioral and Psychiatric Symptoms of Dementia and Rate of Decline in Alzheimer’s Disease. Front. Pharmacol. 10:1062. doi: 10.3389/fphar.2019.01062
Received: 31 May 2019; Accepted: 20 August 2019;
Published: 24 September 2019.
Edited by:
Lydia Gimenez-Llort, Autonomous University of Barcelona, SpainReviewed by:
Kurt A. Jellinger, University of Vienna, AustriaLucio Tremolizzo, University of Milano-Bicocca, Italy
Copyright © 2019 Gottesman and Stern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaakov Stern, eXMxMUBjdW1jLmNvbHVtYmlhLmVkdQ==
 Reena T. Gottesman
Reena T. Gottesman Yaakov Stern
Yaakov Stern