- 1Department of Bioengineering, Zhuhai Campus Zunyi Medical University, Zhuhai, China
- 2The College of Life Sciences, Northwest University, Xi’an, China
- 3Honghui Hospital, Xi’an Jiaotong University, Xi’an, China
Actinidia chinensis Planch. (A. chinensis), commonly known as Chinese kiwifruit, is a China native fruit, which becomes increasingly popular due to attractive economic, nutritional, and health benefits properties. The whole plant including fruits, leaves, vines, and roots of A. chinensis are used mainly as food or additive in food products and as folk medicine in China. It is a good source of triterpenoids, polyphenols, vitamin C, carbohydrate, amino acid, and minerals. These constituents render the A. chinensis with a wide range of pharmacological properties including antitumor, antioxidant, anti-inflammatory, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective activities, suggesting that it may possibly be value in the prevention and treatment of pathologies associated to cancer, oxidative stress, and aging. This minireview provides a brief knowledge about the recent advances in chemistry, biological activities, utilization, and storage of Chinese kiwifruit. Future research directions on how to better use of this crop are suggested.
Introduction
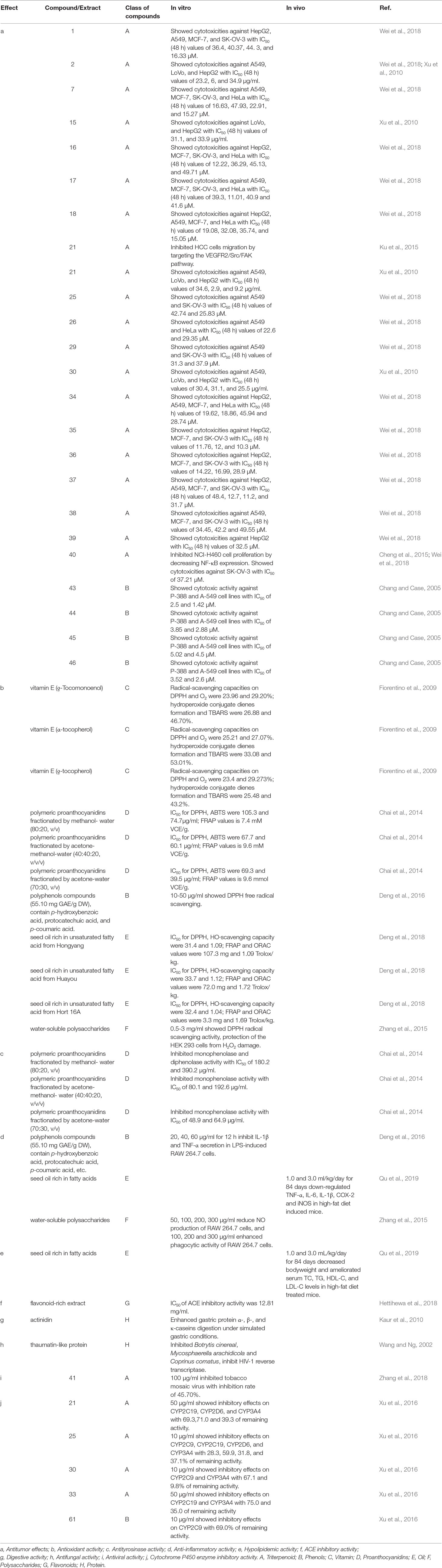
Actinidia chinensis Planch. (A. chinensis), commonly known as “Chinese kiwifruit” (English), “中华猕猴桃” (Chinese), and characterized by excessive vegetative vigor, is a woody perennial, deciduous, and functionally dioecious medicinal plant in the family Actinidiaceae (Flora of China, 2007; The Plant List, 2013). It is native to China and has been cultivated in New Zealand, United States, Greece, Italy, Chile, France, Japan, and Korea (Li and Zhu, 2017; Ma et al., 2017). In China, they are mainly distributed in temperate to warm-temperate zones such as Shaanxi, Gansu, Henan, Guangdong, Guangxi, Fujian, Guizhou, Yunnan, Sichuan, as well as the middle and lower reaches of the Yangtze River basin, especially in Yiling district in Yichang city, Hubei province (Figure 1) (Flora of China, 2007). There are 13 A. chinensis cultivars, especially “Hongyang,” “Jintao,” and “Huayou,” are developed for commercial production in China (Sharon, 2016), and more than three ones such as “Sungold,” “Charm,” and “Hort16A” developed in New Zealand (Henare, 2016) (Table 1).
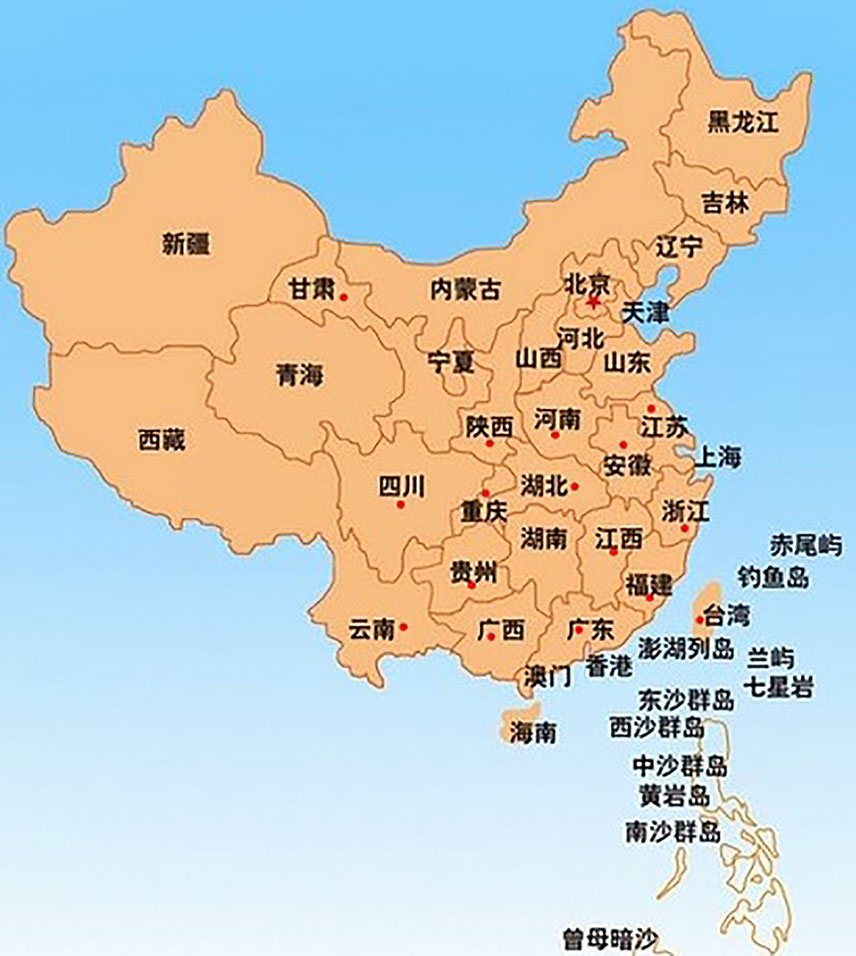
Figure 1 The red spots in the map depicted the main region of A. chinensis distribution in China (Flora of China, 2007; https://www.newasp.net/soft/105257.html).
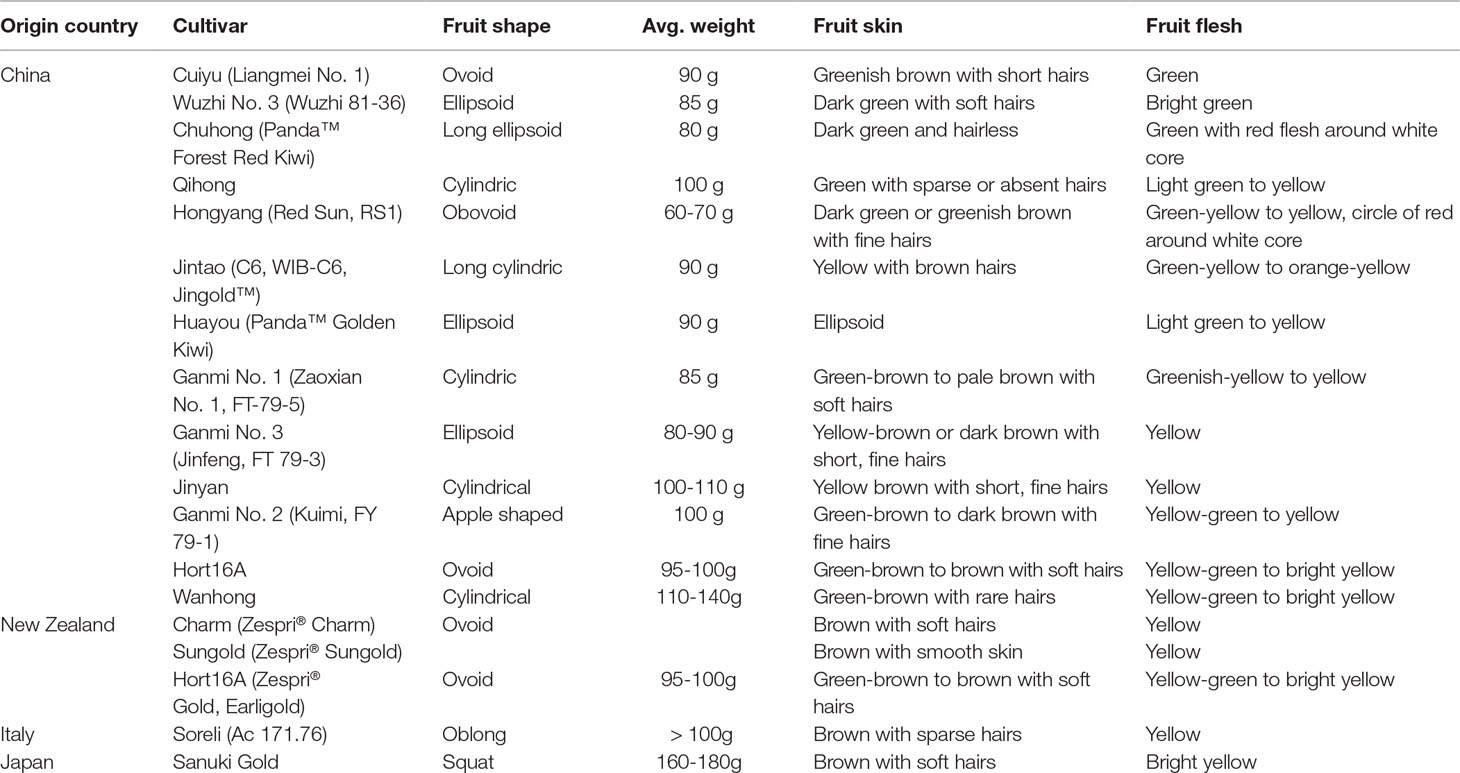
Table 1 A. chinensis cultivars developed for commercial production (Henare, 2016).
There are two varieties accepted by The Plant List that include A. chinensis and A. chinensis var. setosa H.L.Li (The Plant List, 2013). The fruit of A. chinensis is the largest one in Actinidia genus, and it has the greatest economic, medicinal, and edible significance in terms of production and utilization. Its relevant pictures are showed in Figure 2. Generally, Chinese kiwifruit with a cross-sectional radius of about 3 cm is oval-shaped densely covered with yellowish-brown hairs. The flesh color of fruit skin is green to yellow, and the average fruit weight is 20–120 g. The fruit is a tasty, nutritious food that can be eaten fresh directly. Today, a range of kiwifruit processed products with the attractive eating quality and nutritional benefits has been developed including juice, preserved fruit, yogurt, wine, canned fruit, dried kiwi slices, fruit vegetable juice drinks, milk beverage, and vinegar. Apart from being a food and natural health product, the whole plant (fruits, branches and leaves, vines and roots) of A. chinensis has been used as traditional folk medicine in China (He et al., 2017; Wei et al., 2018; Fang et al., 2019). The ripe kiwifruit, tastes sweet and sour, acts on the spleen, stomach, and kidney meridians, has improving properties on dyspepsia, loss of appetite, and vomiting. The branches and leaves have been used to treat arthronalgia, bleeding, empyrosis, and ulcer. The vine has appetizing, heat clearing, and wind-dampness dispelling effects and is used to treat indigestion, aundice, and urolithiasis. The root and bark of A. chinensis taste bitter and astringent, and they have various medical effects such as wind and heat dispelling, blood circulation improving, and detumescence properties, and are used for the treatment of rheumatoid arthritis, bruises, furuncle, swelling, filariasis, hepatitis, and dysentery (Xie, 1975). However, people with weak spleen and stomach should be cautious in taking A. chinensis (Xie, 1975). To date, only very few modern studies have been done on potential toxic and side effects of A. chinensis, which should be highlighted in future research.
The principal chemical composition of the whole plant of A. chinensis include polyphenol, triterpenoids and derivatives, carotenoids, polysaccharides, amino acids, vitamins, essential oils, and microelements. (Papunidze et al., 2001; Chang and Case, 2005; Ma et al., 2017; Wang et al., 2017; Twidle et al., 2018). Among these ingredients, the main bioactive constituents are phenolic compounds, triterpenes, and the major nutritional composition are vitamin C, vitamin E, dietary fiber, and microelements, which make up a relatively significant share of the daily value (Table 2). Pharmacological results have revealed various promising bioactivities to A. chinensis including antitumor, antioxidant, anti-inflammatory, antimicrobial, immunoregulatory, hypolipemic and antidiabetic, cardiovascular protective, hypnotic effects, and ACE inhibitory activities (Deng et al., 2013; Niu et al., 2016; Sun et al., 2017; Xia et al., 2017; Deng et al., 2018; Hou et al., 2018; Fang et al., 2019). Much of these bioactivities of A. chinensis are consistent with those observed in traditional folk medicine. More importantly, A. chinensis showed significantly antitumor and antioxidant properties, and these effects could be depended on the presence of a range of triterpenoids, polysaccharide, and phenolic compounds (Chang and Case, 2005; Wei et al., 2018; Fang et al., 2019). However, the information on the chemical and biological activities of A. chinensis is scattered. In this review, we intend to systematically summarize the recent advances in nutritional composition, chemistry, and biological activities of A. chinensis and also provide future research directions for better utilize and develop it as a sustainable crop.
Chemical Composition
Nutritional Composition
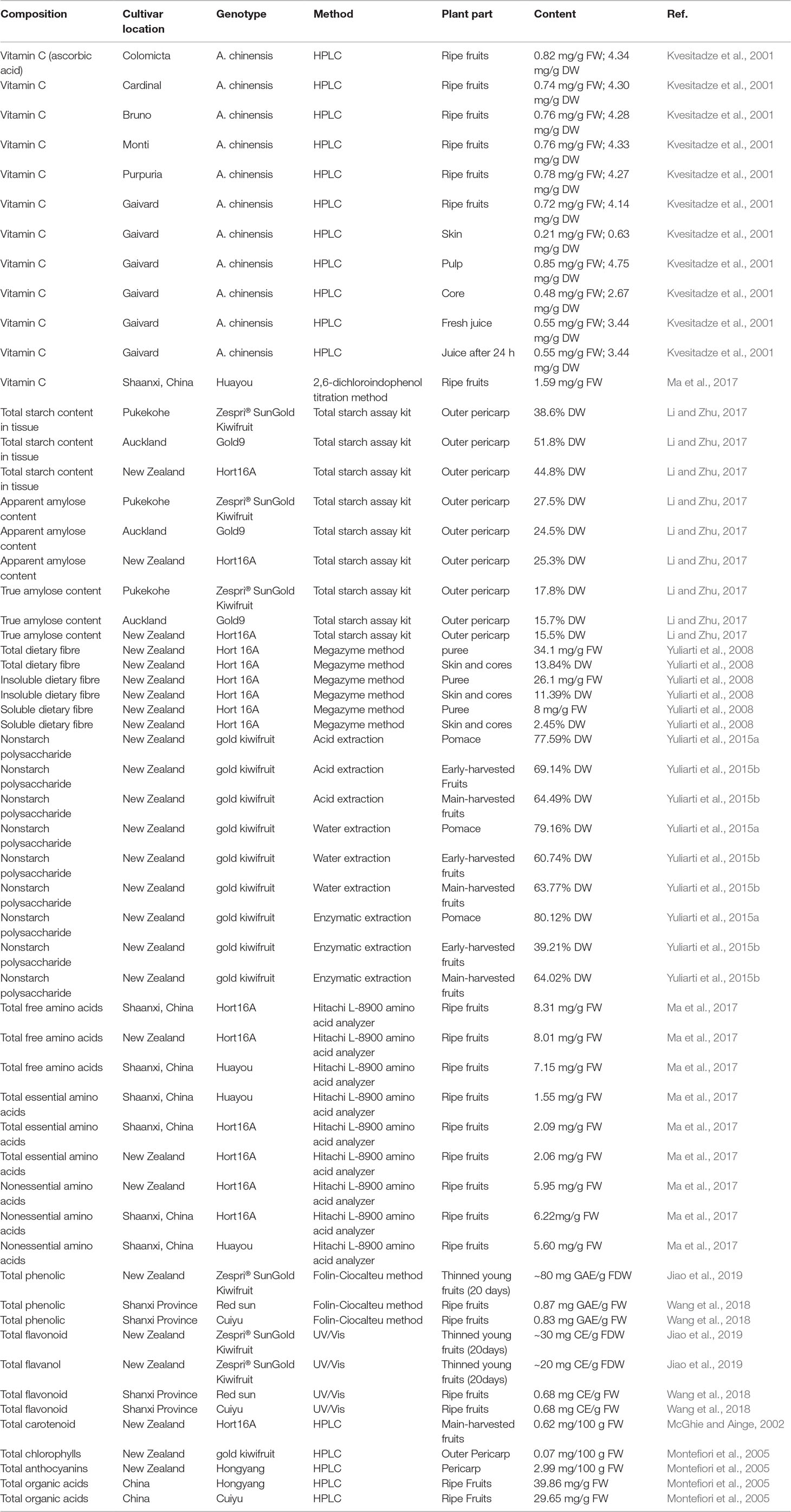
Chinese kiwifruit, known as the “king of fruits,” is a fruit with high-pulp juices, thick flesh, delicious taste, and rich nutrition and has a higher commercial and economic value. It is a rich source of various nutrients including vitamins, carbohydrate, sugar, minerals, amino acids, protein, fatty acids (e.g., linoleic acid), and carotenoids. Table 2 lists the nutritional composition of sun-gold kiwifruit reported from the USDA Food Composition Database (United States Department of Agriculture, USDA Food Composition Databases, 2018). Table 3 shows the chemical content of A. chinensis fruit (Chang and Case, 2005; Cui et al., 2007; Zhou et al., 2009; Xu et al., 2010; He et al., 2014; He et al., 2015a; He et al., 2015b; Xu et al., 2016; Twidle et al., 2018; Sivakumaran et al., 2018; Wei et al., 2018; Zhang et al., 2018). Of particular note, nutritional composition in kiwifruit is vitamin C (1.61 mg/g) and minerals K (3.15 mg/g). The average vitamin C content of Huayou, Jintao, Ganmi-1, Ganmi-2, Ganmi-3, Wuzhi-3, and Cuiyu cultivated in China are 1.59, 1.49, 0.86, 1.34, 0.97, 2.88, and 1.18 mg/g, respectively. Meanwhile, the vitamin C in SunGold was 1.61 mg/g edible flesh, followed by other varieties Sweet Green (1.5 mg/g) and green “Hayward” (0.85 mg/g) (Sivakumaran et al., 2018). Especially, the vitamin C content in kiwifruit is higher than that determined in lemon, orange, strawberry, and grapefruits (Ma et al., 2017).
The data show evidence that the sun-gold kiwifruit is high in carbohydrate (15.79%) and sugars (12.3%). The total starches contents were found for outer pericarp and core tissues ranged from 38.6% to 51.8% and 34.6% to 40.7% DW in three harvesting A. chinensis varieties, and the starches in core have higher amylose content (20.7%–23.3%) and enzyme susceptibility. However, the crystallinity degree, granule size, and gelatinization parameters of starches in core are somewhat lower (Li and Zhu, 2017). The kiwifruit peel contains a higher total pectin content (3.7%–4.2%) than that of pulp (1.6%–2.1%) (Meng et al., 2017). Xia et al. (2017) analyzed the polysaccharide from Hongyang using water extraction, followed by column chromatography, high performance gel permeation chromatography, HPLC, and Fourier transform infrared spectroscopy. The results indicated that the polysaccharide of Hogyang fruit consisted of the following monosaccharides: D-galactose (25.45%), D-galacturonic acid (25.25%), L-arabinose (20.51%), L-rhamnose (17.78%), D-glucose (6.14%), D-mannose (2.13%), D-xylose (1.03%), D-glucuronic acid (0.97%), and D-fucose (0.74%). These studies confirmed the utilization potential of Chinese kiwifruit as an incredibly healthy food and loaded with important nutrients and health benefits for human consumption.
Kiwifruit contained 18 free amino acids. Briefly, the total essential amino acid contents in Jintao, Hongyang, Huayou, and Hort16A cultivated in China are 2.59, 1.55, 2.0, and 2.09 mg/g FW, whereas the total essential amino acid in Hort16A cultivated in New Zealand was 2.06 mg/g FW. Meanwhile, Jintao, Hort16A and Hongyang also had a high amount of nonessential amino acid and total free amino acid. The most abundant amino acids detected in the kiwifruit were arginine, glutamic acid, lysine, phenylalanine, aspartic acid, and tyrosine (Ma et al., 2017). There are a number of total saturated lipids including C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0 with the content of 1.49, 0.05, 0.14, 0.14, 0.09, 0.9, and 0.14 mg/g in edible flesh portion of A. chinensis. Meanwhile, the content of monounsaturated fatty acids C16:1 and C18:1 are 0.09 and 0.27 mg/g, and the polyunsaturated fatty acids C18:2 and C18:3 are 1.13 and 0.77 mg/g (Drummond, 2013). In fact, the kiwifruit seed oil is rich in unsaturated fatty acids (89.92%), notably linolenic acid, which accounts for 60.59% of total seed oil (Luan et al., 2017). Υ-tocopherol, Υ-tocotrienol, and ƍ-tocotrienol are identified in kiwifruit seed oil (Fiorentino et al., 2009). Besides, minerals like calcium, iron, potassium, magnesium, sodium, phosphorus, copper, manganese, zinc, iodine, selenium, and vitamins including vitamin A, β-carotene, lutein, zeaxanthin, riboflavin, niacin, pantothenic acid, vitamin B6, folate, tocopherol, vitamin E, vitamin K, and choline are identified in kiwifruit (Sivakumaran et al., 2018). Thus, these data suggest that kiwifruit is an interesting fruit for daily nutrition and energy suppliers.
Phytochemicals
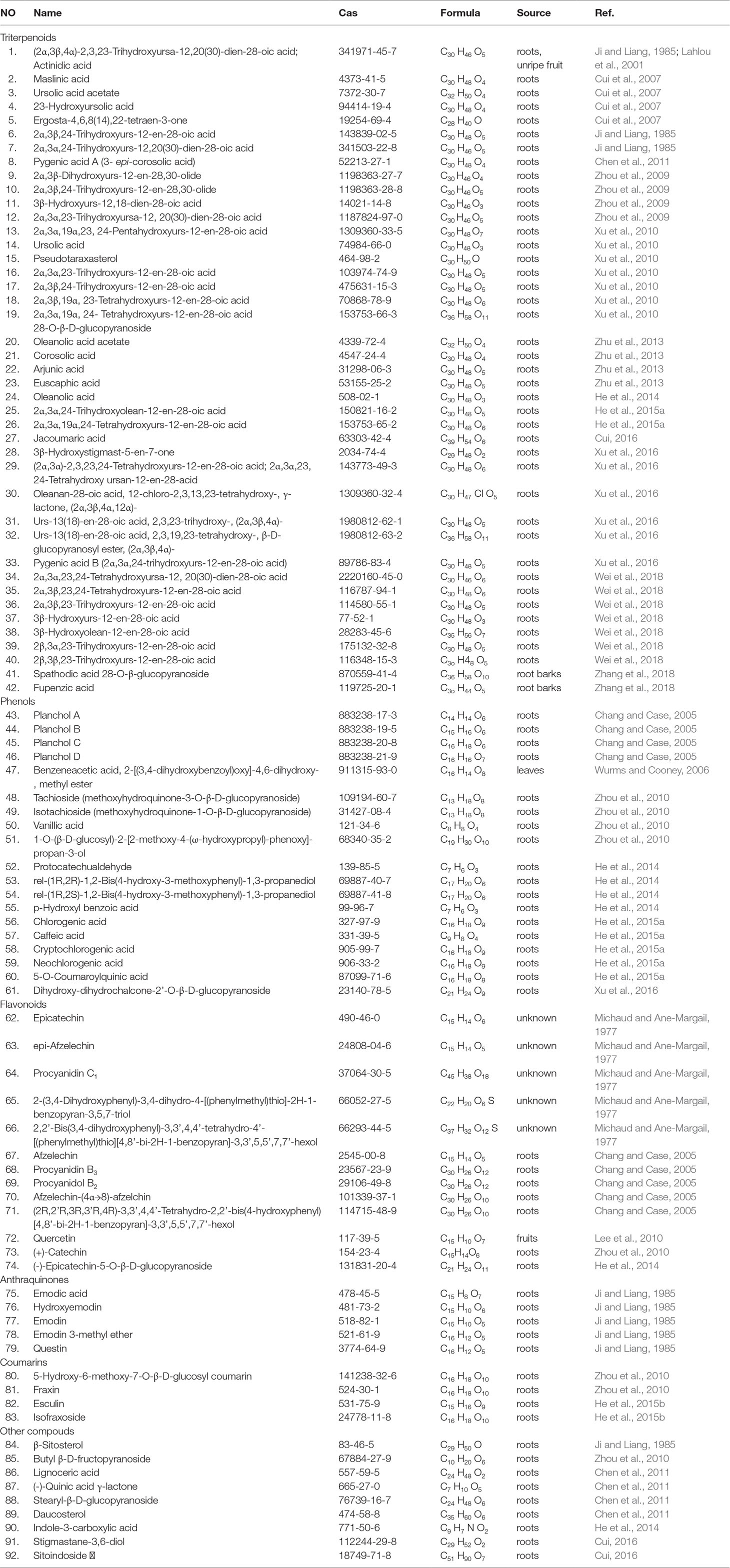
A range of phytochemicals, including triterpenoids, saponins, and phenolic compounds (flavonoids, polyphenols, anthraquinones, and coumarins) varying in structures, were found and identified in A. chinensis. The major constituents isolated and identified in leaves and roots of A. chinensis are listed in Table 4.
Triterpenoids
Currently, triterpenoids have been the major research focus of A. chinensis components due to their promising antitumor properties. To date, 42 triterpenoids have been isolated and identified mainly from roots of A. chinensis. The commonly triterpenoids found in roots of A. chinensis are 12-en-28-oic acids of oleanane and ursane type. It is noteworthy that some of these triterpenoids (1-2, 7, 15-18, 21, 25-26, 29-30, and 34-40) have significant antitumor activity and deserve further research and development.
Phenolic Compounds
The phenolic compounds abundantly presented in different botanical parts of A. chinensis, and they have drawn increasing attention. These compounds include phenols, flavonoids, and flavanols are characterized by antitumor, antioxidant, and free radicals scavenging properties. HPLC-PAD and UPLC-QqQ-MS/MS-based methods have been used generally for the identification and quantification of these phenolic compounds (Ma et al., 2017; Jiao et al., 2019). The total phenolic, flavonoid, and flavanol contents from young A. chinensis kiwifruits “Zespri® SunGold Kiwifruit” growing in 20 days are 82.84 mg GAE/g FDW, 30.08 catechin/g equivalents FDW, and 20.20 catechin/g equivalents FDW. Meanwhile, the total phenolic, flavonoid, and flavanol contents presented in young A. chinensis kiwifruits growing in 60 days and mature kiwifruits are gradually decreasing, indicating polyphenol content possesses a decreasing pattern during fruit ripening (Jiao et al., 2019). The major chemical composition of phenolics detected in young “Zespri® SunGold Kiwifruit” are epicatechin, quercitrin, rutin, catechin, chlorogenic acid, ferulic acid, and vanillic acid. Based on UPLC-TOF/MS and UPLC-QqQ/MS method, Zhao et al., 2014 showed that the radix A. chinensis contained catechin derivatives, quinic acid derivatives, coumarin derivatives, caffeic acid, and p-coumaric acid (Zhao et al., 2014), showing that A. chinensis appears to be a good source of phenolics.
Volatile Compound and Essential Oil
The volatile components of A. chinensis var. chinensis fruit and flowers have been profiled by GC-MS. The dominant volatile components of eating-ripe firmness fruit are straight-chain aldehydes, alcohols, and esters, such as hexanal, decanal, octanal, nonanal, benzaldehyde, acetaldehyde, hex-E2-enal, 1,8-cineole, ethanol, hexanol, methyl butanoate, and ethyl octanoate (Wang et al., 2011). The volatile components of flowers included (3E,6E)-α-farnesene (38.8%), pentadecane (12.49%), (+)-germacrene D (8.55%), heptadecane (8.01%), (8Z)-heptadecene (7.72%), 2-phenylethano (4.69%), (3Z,6Z,9Z)-heptadecatriene (2.54%), and nonadecane (1.98%) (Twidle et al., 2018). It can be found that terpenes and straight chain alkenes were dominant in flowers of A. chinensis var. chinensis, which contained nearly >92% of the total ion counts. Importantly, many of these compounds possess strong and interesting aroma. However, the volatile components gradually changed during maturation. The essential oil of roots of A. chinensis have been profiled by GC-MS, and the major essential oil in roots are dodecane (29.39%), octane (5.16%), decane (2.94%), paeonal (2.81%), camphor (2.77%), n-decanoic acid (2.64%), 4-Methyldodecane (2.45%), undecane (2.16%), and linalool oxide (2.1%) (Yu et al., 2009).
Carotenoid and Chlorophyll
Carotenoids and chlorophyll are responsible for the color and attractiveness of kiwifruit fruits, as well as provide nutritional values. The carotenoids detected in the red-fleshed genotypes of A. chinensis fruit (Hort16A) are 9′-cis-neoxanthin, violaxanthin, antheraxanthin, lutein, zeaxanthin, β-cryptoxanthin, and β-carotene (McGhie and Ainge, 2002; Montefiori et al., 2005). Chlorophylls a and b are the dominant chlorophylls in Hort16A (McGhie and Ainge, 2002).
Quality Determination
Ripe kiwifruit is susceptible to environmental and itself. Usually, human sensory evaluation method can directly identify the fruit shape, color, surface, pulp, and flavor, but there is little information about swelling, ripening, and other agents present in fruit. Physical and chemical method including firmness and microbial are used effectively to determine the quality condition of kiwifruit. Some new instrument detection methods with accurate analysis ability such as GC, GC-MS, HPLC, UPLC-QqQ-MS/MS, and electronic nose combined with surface acoustic wave resonator are developed for the fruit and its products quality rapid analysis (Kvesitadze et al., 2001; Montefiori et al., 2005; Liu and Hui, 2015; Jiao et al., 2019). As to radix A. chinensis, the systematical method like UPLC-TOF/MS and UPLC-QqQ/MS is commonly applied to quality evaluation and active components analysis for A. chinensis (Zhao et al., 2014). Therefore, there are many high accurate analysis methods for rapid quality evaluation, but there is a lack of effective and standardized quality and safety standard for kiwifruit in China. Thus, there is an urgent demand for developing specific functional components and quality evaluation indicators for standardization and quality control of the fruit and its products.
Biological Activities
A. chinensis contains a range of bioactive compounds accounting for natural pharmacological properties including antitumor, antioxidant, anti-inflammatory, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective activities, and most of these biological activities support its traditional use. Table 5 shows the major biological activities of compound or extract from A. chinensis.
Antitumor Activity
Crude extracts, fractions, and isolated compounds from A. chinensis exhibited strong inhibition against tumor growth in various forms of human cancer cells. These cancer cells were hepatocellular carcinoma cells HepG2 (Xu et al., 2010; Zuo et al., 2012), Hep3B, SMMC7721, MHCC97L, MHCC97H, HCCLM3 (Fang et al., 2019), HL-7702 (He et al., 2017), Huh7 (Hou et al., 2018), lung cancer cells NCI-H460 and NCI-H1299 (Lv et al., 2018), colon cancer cells HT-29, LoVo, and SW480, pharyngeal carcinoma cell lines Fadu and HEP-2, gastric cancer cells SGC-7901, BGC-823, MKN-49P, and MFC, as well as other cancer cells like A549, P-388, MCF-7, SK-OV-3, and HeLa (Chang and Case, 2005; Xu et al., 2010; Xu et al., 2010; Zuo et al., 2012; Shen et al., 2014; Xia et al., 2017; Gu et al., 2017; Wang et al., 2017; Wei et al., 2018). These reported antitumor activities are consistent with the traditional usage such as liver cancer, lung cancer, colon cancer, esophagus cancer, and gastric cancer.
A large number of triterpenoids in roots of A. chinensis especially those with carboxyl group showed marked cytotoxicity against various types of cancer cells in vitro. Especially, compounds 1-2, 7, 15-18, 21, 25-26, 29-30, 34-40, and 43-46 exhibited remarkable antitumor activity against on A549, HepG2, LVOV, MCF-7, HeLa, and/or HepG2 in vitro (Table 5). Additionally, the polysaccharide of Hogyang fruit showed notable inhibitory against tumor cells lines SGC7901, MCF-7, HT29, HepG2, and NCI-H460 with IC50 of were 0.28, 0.31, 0.58, 0.64, and 0.65 µM, respectively (Xia et al., 2017). In vivo, a polysaccharide isolated from the roots of A. chinensis showed antitumor activity by prolonging the life of EAC or P388 cells-induced tumor mice and inhibiting the DNA synthesis in EAC cells (Lin, 1988). Early treatment and long-term treatment with water extracts of roots from A. chinensis with 2 g/kg/day strongly attenuated the malignant behavior of HCC in mice by decreasing DLX2 expression (Fang et al., 2019).
The molecular mechanism of the inhibition against tumor growth and the apoptosis promoting of the fractions and isolated compounds were due to downregulate DLX2 gene expression and VEGFR2/Src/FAK pathway, inhibit cholesterol metabolism by upregulating PCSK9 signaling pathway, regulate gene encoding laminin subunit beta-3 pathways, and decreased NF-κB and EP3 expression. Meanwhile, the antioxidation and anti-inflammation are also important and possible mechanisms. The triterpenoids, polysaccharides, and phenolic compounds were identified as the major bioactive compounds in the extract from A. chinensis roots with antitumor properties (Chang and Case, 2005; Wei et al., 2018), which provides new way to search for treating cancers with natural therapeutic compounds. Overall, A. chinensis has prominent antitumor potential and has a good health benefit for people, however, the further in vivo and clinical studies on antitumor properties of A. chinensis are needed for confirmation.
Antioxidant Activity
Antioxidant activity of bioactive compounds of A. chinensis have been the mostly studied by various in vitro and in vivo assays. These in vitro assays consisted of both chemical and biological assays like DPPH, ABTS, FRAP, HO·, ORAC, oxidative stress by H2O2, and lipid oxidation (Chai et al., 2014; Lee et al., 2015; Hwang et al., 2017; Deng et al., 2018). The in vivo assays were based on SOD, GSH, ALT, AST, oxidative DNA damage, and lipid oxidation (Iwasawa et al., 2011; Sun et al., 2017; Deng et al., 2018; Wang et al., 2018). The above results showed that A. chinensis is a good source of bioactive compounds with antioxidant properties to various extents. The antioxidant capacities of kiwifruit are greatly attributed to polyphenols, flavonoid, unsaturated fatty acid, and vitamin C. In addition, the different extraction methods, different plant parts, and genetic diversity of kiwifruit demonstrated different antioxidant activities. The peel showed the strongest antioxidant activity, followed by the pulp and the core. The antioxidant activity of kiwifruit peel was mainly depended on plenty of phenolic substances, and the antioxidant activity of the pulp was mainly attributed to the existence of a large amount of vitamin C (Zhang et al., 2016). The seed oil of Hort 16A and Hongyang are attractive materials rich in unsaturated fatty acid demonstrated radical scavenging capacities for FRAP, DPPH, HO·, and ORAC with IC50 of 3.3 mgTrolox/kg, 32.4 mg/ml, 1.04 mg/ml, 1.69 mgTrolox/kg, and 107.3 mgTrolox/kg, 31.4 mg/ml, 1.09 mg/ml, 1.99 mgTrolox/kg, respectively (Deng et al., 2018). The radical scavenging capacities of fresh and freeze-dried Hort 16A rich in phenolics and flavonoids for ABTS, DPPH, and ORAC were 8.8, 8.8, 98.3, and 6.0, 5.0, and 40.3 mg VCE/g, respectively (Hwang et al., 2017). The radical scavenging capacities of Red sun and Cuiyu rich in phenolics and flavonoids for ABTS, DPPH, ORAC, and FRAP were 1.35, 1.01, 10.78, 1.50 and 1.32, 0.9, 8.87, 1.28 mg VCE/g, respectively (Wang et al., 2018). Oral administration of kiwifruit protected lymphocytes against oxidative DNA damage, inhibit lipid oxidation in mice, increased SOD and GSH, and lowered ALT and AST levels in the patients (Sun et al., 2017). Therefore, A. chinensis possess confirmed antioxidant capacity and it seems that appropriate extraction methods, appropriate genotypes, and plant parts can be screened to maximize the antioxidant properties of A. chinensis.
Anti-Inflammatory Activity
Anti-inflammatory activity of A. chinensis has been proved in vivo and in vitro models. On high-fat diet-induced obese C57BL/6 mice models, consecutive consumption the seeds oil of A. chinensis with 1.0 and 3.0 ml/kg·bw ameliorated obesity-induced inflammation by down-regulating the mRNA expression of related to inflammation adipokines, such as TNF-α, IL-6, IL-1β, COX-2, and iNOS (Qu et al., 2019). The aqueous and ethyl acetate extracts demonstrated anti-inflammatory activity in inflammatory bowel disease models of the IL-10 gene-deficient mice (Edmunds et al., 2012). In patients with type-2 diabetes mellitus, the fruit juice of A. chinensis showed preventative activity on inflammation by activating Keap1 and Nrf2 via upregulating miR-424 (Sun et al., 2017). On the cellular level, polyphenols mainly composed of protocatechuic acid, p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, and ferulic acid from seeds of A. chinensis at concentration of 40 and 60 μg/ml for 12 h decreased the secretion of pro-inflammatory cytokines IL-1β and TNF-α in LPS-induced RAW 264.7 cells (Deng et al., 2016). Therefore, the anti-inflammatory potential A. chinensis seeds mainly depend on the synergetic effect of these polyphenols, and it may be used to prevent a variety of inflammation related diseases.
Antibacterial Activity
All the extracts including skin, pulp, seeds, and stems showed bactericidal against Staphylococcus aureus, Streptococcus pyogenes, S. faecalis, Salmonella typhi, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumonia. The skin and pulp extracts showed inhibition activity against S. aureus and S. pyogenes with MIC values of 8 and 4 μg/ml, but they showed moderate inhibition activity against S. faecalis, S. typhi, P. mirabilis, P. aeruginosa, E. coli, and K. pneumonia with MIC values ranging from 16 to 128 μg/ml. The leaves and stems extract just inhibited S. pyogenes and P. aeruginosa with MIC values of both 64 and 32 μg/ml. The seeds extracts showed an exclusively bacteriostatic activity against these selected strains of bacteria with MIC values of between 1 and 8 μg/ml (Basile et al., 1997). Polyphenol from seeds of A. chinensis showed significant bactericidal against Bacillus cereus, B. subtilis, Shigella flexneri, and Salmonella Typhi, and bacteriostatic against B. thuringiensis. We can find that the antimicrobial activity of the polyphenol extract on gram-positive bacteria is higher than that of gram-negative bacteria (Deng et al., 2013). Therefore, kiwifruit seeds are potential food processing material for their antimicrobial activity.
Immunoregulatory Activity
Consumption of the aqueous extracts of whole fresh fruit of Hort16Aat 375 mg/kg for 12 days enhanced both innate and acquired immunity in cholera vaccine and tetanus/diphtheria vaccine models in Balb/c mice, showing a beneficial effect on healthy (Shu et al., 2008). The homopolysaccharide derivatived by O-sulfation from the roots of A. chinensis at concentration of 10 and 50 μg/ml activated phagocytic activity and increased NO production of RAW 264.7 macrophages, and the activity of sulfated polysaccharides is strongly related to the degree of the sulfation (Niu et al., 2016), and treatment with 50-300 μg/ml water-soluble polysaccharides dose-dependently stimulated NO production and phagocytic activity of RAW 264.7macrophages (Zhang et al., 2015). It remains to clarify the detailed mechanism of immunoregulatory activity and the responsible compositions for this valid action.
Hypolipemic and Antidiabetic Activities
Administered the seed oil of A. chinensis rich in fatty acids at 1.0 and 3.0 ml/kg·bw daily over 12 consecutive weeks significantly lowered bodyweight gain, inguinal fat tissue weight, and the accumulation of TC, TG, HDL-C, and LDL-C in liver of the high-fat diet-induced obese C57BL/6 mice. Meanwhile, long-term consumption of the seed oil of A. chinensis up-regulated the expression of thermogenesis-related genes like PPAR-γ, UCP1, PGC1-α, and PRDM16, down-regulated FAS expression, and altered the gut microbiota by decreasing the Firmicutes-to-Bacteroidetes ratio (Qu et al., 2019). In addition, the seed oil from A. chinensis supplementation improved insulin resistance and alleviated hyperglycemia by reducing HOMA-IR index and blood glucose in high fat diet-induced obese mice (Qu et al., 2019). Thus, the lipid lowering potential of A. chinensis seed provide a basis theory for food industries.
Cardiovascular Protective Effects
In H9c2 rat cardiac myocytes cells induced by hypoxia in cardiomyocytes treated with angiotensin II, treatment with 1.25 and 2.5 mg/ml polysaccharide of A. chinensis alleviated cardiac hypertrophy, decreased mitochondrial dysfunction and reduced cardiomyocytes apoptosis by decreasing the apoptosis-associated genes expression like mitochondria associated-1 and caspases3/8/9, and cleaving caspases-3/8/9. Additionally, the protective effects of polysaccharide against hypoxia-induced apoptosis may be attributable to inactivate the ERK1/2 and PI3K/AKT signaling pathways (Wang et al., 2018). The polysaccharide of A. chinensis can be potentially used in the treatment of heart disease. However, it is noteworthy that polysaccharide at high dose (10 mg/ml) suppressed the cardiomyocytes viability.
Hypnotic Effects
Oral administration of ethanol extracts from A. chinensis peel at dose of 250, 500, and 1,000 mg/kg dose-dependently decreased sleep latency and increased sleep duration in pentobarbital-treated mice. Especially, the sequentially partitioned with ethyl acetate fraction rich in flavonoids (1.63 mg QE/g) at 250 mg/kg exert significantly hypnotic effects and this sedative-hypnotic activity could be inhibited by GABAA-BZD receptor antagonist flumazenil. The flavonoids may be attributable to hypnotic activity via allosteric GABAA-BZD receptor modulation, but the precise mechanisms and the existing individual flavonoids are needed to be evaluated in the future (Yang et al., 2013).
Ace Inhibitory Activity
The 70% aqueous acetone extracts partitioning with hexane rich in flavonoid from Hort 16A dose-dependently inhibited ACE activity with IC50 of 12.81 mg/ml using a fluorescence-based biochemical assay. LC-MS/MS showed that the higher total phenolic and total flavonoid contents are identified in this extract. UPLC-MS/MS showed that polyphenols (231.32 µg/g DW) in the extract are mainly flavonols, flavanols, and phenolic acids. Specifically, quercetin-3-O-galactoside (205.19 µg/g DW), quercetin-3-O-glucoside (0.45 µg/g DW), quercetin-3-O-rhamnoside (0.61 µg/g DW), quercetin-3-O-rutinoside (0.29 µg/g DW), epicatechin (5.15 µg/g DW), catechin (0.75 µg/g DW), epigallocatechin (0.61 µg/g DW), phloridzin (2.03 µg/g DW), and isoferulic acid (15.12 µg/g DW) are major compounds in the extract (Hettihewa et al., 2018). These compounds could be responsible for the observed in vitro ACE inhibitory activity of Hort 16A fruit, though the active compounds identifying and in vivo animal studies remain to be investigated and conducted.
Dermatological Activity
The raw polysaccharides with >90% carbohydrate and 5.2% residual protein from the fresh fruit of A. chinensis at 10 μg/ml showed a significantly proliferation-promoting on cell proliferation rates of HaCaT cell line and primary keratinocytes (NHK), and it also significantly promoted proliferation of human dermal fibroblasts at 132 and 198 μg/ml. Meanwhile, treatment of the polysaccharides at 200 μg/ml significantly stimulated ATP-synthesis, promoted mitochondrial activity and energy metabolism of HaCaT keratinocytes, and significantly increased collagen synthesis in dermal skin equivalents (Deters et al., 2005). Kiwifruit pericarp proanthocyanidins mainly contained B-type propelargonidins, procyanidins, procyanidins gallate, and prodelphinidins showed strongly inhibition activity on tyrosinase, indicating that it can be used as whitening agents (Chai et al., 2014).
Cytochrome P450 Enzyme Inhibitory Activities
Cytochrome P450 system in liver plays an important role in drug metabolism. It transforms drug from hydrophobic to hydrophilic, which is easier to excrete. The 90% EtOH extract of A. chinensis root at 50 μg/ml exhibited inhibition activities on CYP2C9, CYP2D6, and CYP3A4 in human liver tissue with the 69.0, 76.3, and 53.3% of remaining activity, respectively. The inhibitory effect of the crude extract could be largely attributed to the presence of triterpenoids (Xu et al., 2016). It is worth noting that the combination of crude extracts or these triterpenoids with other medical herbs or drugs may lead to drug interaction with cytochrome CYPs at pharmacokinetic and pharmacodynamic levels, which indicates that people should cautiously consume A. chinensis fruit when taken medicine.
Processing and Utilization
Chinese kiwifruit is a very high nutritional value of nourishing and consumers’ favorite fruit, which has shown application potential in food, medicine, and health products industry. China is the largest kiwifruit producer in the world. In 2016, kiwifruit production in China reached 2.41 million tons per year, accounting for 56.0% of the world’s total kiwifruit production (United Nations Food and Agriculture Organization, 2016). To date, a series of commercially available products has been processed due to abundant nutrient substance and claimed health benefits. These Chinese kiwifruit related products include sliced fruit, juice, preserved fruit, yogurt, wine, canned fruit, dried kiwi slices, fruit vegetable juice drinks, biscuits, milk beverage, whipped cream, baked goods, vinegar, and oil capsule. Furthermore, various different parts of A. chinensis showed different uses. Briefly, the leaves contain protein, starch, and polyphenols, which may be developed as an excellent source of natural products. The beautiful and fragrant of Chinese kiwifruit flowers rich in honey juice and volatiles can be used as high-quality honey source. Kiwifruit peel residue as sources of high-quality pectin can be used as functional ingredient for food products. Chinese kiwifruit seeds rich in essential fatty acids, protein, and dietary fiber can be used in food and health products industry (Xie, 1975; Garcia et al., 2012). The roots and barks contain ursolic acid, oleanolic acid, and quercetin, which have antitumor effect against liver cancer, lung cancer, gastric cancer, esophageal cancer, colorectal cancer, and cervical cancer (Chang and Case, 2005; Xu et al., 2010; Wei et al., 2018). The different parts of A. chinensis are widely used as pharmaceutical raw materials in medicine for prevention and treatment of tumors. In addition, the various claimed nutritional and pharmacological properties including strong antitumor, antioxidation, and anti-inflammatory potential of various extracts or active compounds of A. chinensis indicated that they could be further developed for functional food with added-commercial value or effective and safe drug formulations.
Storage Methods
Chinese kiwifruit has a short postharvest life because of fast softening and serious decay. Preservation of Chinese kiwifruit for prolonged periods is particularly important. Freezing and frozen storage is currently the most common method, which can effectively inhibit the softening of kiwifruit and prolong its postharvest life. However, kiwifruit is cold-sensitive and very susceptible to chilling injury when storage at the temperature between −2°C and 2.5°C for a long time (Gerasopoulos et al., 2006; Ma et al., 2014). Interestingly, dipped by water for 10 min at 45°C to low temperature storage can prevent chilling injury development to kiwifruit. Meanwhile, the kiwifruit pretreated at 45°C and then stored at 0°C for 90 days showed higher firmness and soluble solids content, and MDA content and lipoxygenase activity in kiwifruit are reduced. However, pretreated at 20 and 55°C were ineffective at alleviating chilling tolerance (Ma et al., 2014). Various other treatments including preharvest calcium chloride sprays (Gerasopoulos and Drogoudi, 2005), putrescine (Yang et al., 2016), preharvest chilling (Sfakiotakis et al., 2005), and gradual cooling (Yang et al., 2013) have also been used to alleviate chilling injury in kiwifruit.
After harvest, kiwifruit is highly perishable, and its nutritional ingredients and quality decline rapidly due to the influence of internal biochemical reactions and external environment. The modified atmosphere packaging, chitosan, 1-methylcyclopropene, ClO2, ozone, tea polyphenols, protein, lipid composite film, oxalate, salicylic acid, and citric acid have been used individually or combined to alleviate physicochemical quality changes for postharvest of kiwifruit (Huang et al., 2017). The ozone treatment induced the ripening process, delayed the microbial growth, and influenced the content of vitamin C, polyphenols, flavonoids, and carotenoids (Goffi et al., 2019). The chitosan combined with salicylic acid treatment during storage at room temperature for 14 days provides a significantly effective preservative effect by delayed vitamin C and soluble solids decomposition, inhibiting moisture loss and acidity change, and maintaining texture and surface color of Chinese kiwifruit in 14 days of storage at room temperature (Huang et al., 2017).
Conclusions
Chinese kiwifruit and related products are increasingly popular throughout the world due to the remarkably economic, nutritional, and health benefits values. It is a good source of phenolic compounds, vitamin C, carbohydrates, sugars, amino acids, and minerals. Of particular note in kiwifruit is vitamin C and minerals K. The phenolic compounds present in Chinese kiwifruit are organic acids and flavonoids, and fruit peel and flesh, leaf, vine, and roots also contain a variety of these phenolic components. The major components of the roots are triterpenoids characterized by 12-en-28-oic acids of oleanane and ursane type. Terpenes, straight chain alkenes, alcohols, and esters were dominant volatile components in flowers and roots of A. chinensis. These chemical compounds render the A. chinensis with a range of sensory quality, nutritional, and pharmacological properties as proved by in vitro and in vivo studies. The claimed biological activity of isolated compounds, fractions, or crude extracts include antitumor, antioxidant, anti-inflammatory, antibacterial, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective effects. Of particular note is that these claimed biological activities such as antitumor, antioxidant, and immunoregulatory may be greatly attributed to the existence of triterpenoids, polyphenols, flavonoid, polysaccharide, unsaturated fatty acid, and vitamin C. These findings suggest that Chinese kiwifruit can be useful in the prevention and treatment of pathologies associated to cancer, oxidative stress, and aging.
There are also research opportunities to better development, utilization, and protection kiwifruit for human consumption. Cytochrome P450 inhibitory activities, toxicity analysis, qualitative and quantitative metabolite research, effective and standardized quality standard building, and clinical studies should be encouraged to conducted for safe daily consumption. Meanwhile, the synergism and attenuation effects, metabolic behavior of various ingredients, as well as the in vivo and molecular mechanisms studies responsible for the observed biological properties should be conducted. It is also found that some of the A. chinensis cultivars were only supported by a few studies, and confirmative studies should be conducted to verify their health effects. Apart from the fruit, other plant parts of kiwifruit including leaves and roots should also be explored for effective utilization. The effective method and technology for the storage and preservation of kiwifruit during preharvest and postharvest remain to be explored to avoid the frequent chilling damage, soft rot, and mildew, and also decrease and improve the change of the chemical profile and bioactivity properties during storage.
Author Contributions
XC and YM obtained the literatures. JF, ZZ, and XH wrote the manuscript. XH, LH, and YL gave ideas and edited the manuscript. All authors approved the paper for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
A549, Human alveolar basal epithelial cells; ABTS, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; ACE, Angiotensin converting enzyme; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CE, Catechin equivalents; COX-2, Cyclooxygenase-2; CYP2C9, Cytochrome P450 2C9; CYP2D6, Cytochrome P450 2D6; CYP3A4, Cytochrome P450 3A4; CYPs, Cytochrome P450; DLX2, Distal-less-like homeobox protein; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DW, Dry weight; EP3, Prostaglandin E2 receptor subtype 3; ERK1/2, Extracellular regulated protein kinases; FAS, Fatty acid synthase; FDW, Freeze-dried weight; FRAP, Ferric ion reducing antioxidant power; FW, flesh weight; GABA-BZD, γ-aminobutyric acid-benzodiazepine; GAE, Gallic acid equivalents; GC-MS, Gas chromatography-mass spectrometer; GSH, Glutathione; HaCaT, Human immortalized keratinocytes; HCC, Hepatocellular carcinoma; HDL-C, High density lipoprotein cholesterol; HEK 293, Human embryonic kidney 293 cells; HepG2, Liver hepatocellular cells; HO·, Hydroxyl radical; HOMA-IR, Homeostasis model assessment for insulin resistance; HPLC, High-performance liquid chromatography; IC50, Half maximal inhibitory concentration; IL-1β, Interleukin-1β; IL-6, Interleukin-6; iNOS, Inducible nitric oxide synthase; Keap1, Kelch-like ECH-associated protein 1; LC-MS/MS, Liquid chromatography-tandem mass spectrometry; LDL-C, Low density lipoprotein cholesterol; LoVo, Colorectal cancer cell line; MCF-7, Human breast adenocarcinoma cell line; MDA, Malondialdehyde; MIC, Minimum inhibitory concentration; NCI-H460, Large cell lung cancer cells; NF-κB, Nuclear factor-kappa B; NO, Nitric oxide; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; ORAC, Oxygen radical absorbance capacity; P-388, Mouse leukemia cells; PGC1-α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K/AKT, Phosphatidylinositol 3-kinase/protein kinase B; PPAR-γ, Peroxisome proliferators-activated receptors; PRDM16, PR domain containing 16; QE, Quercetin equivalents; RAW 264.7, Mouse leukaemic monocyte macrophage cell line; SK-OV-3, Human ovarian epithelial cancer cells; SOD, Superoxide dismutase; TC, Total cholesterol; TG, Triglyceride; TNF-α, Tumor necrosis factor alpha; UCP1, Uncoupling protein 1; UPLC-MS/MS, Ultra-high-performance liquid chromatography-tandem mass spectrometry; UPLC-QqQ-MS/MS, Ultra-high-performance hydrophilic interaction liquid chromatography-triple quadrupole tandem mass; UPLC-TOF/MS, Ultra-performance liquid chromatography quadrupole-time of flight mass spectrometry; USDA, United States Department of Agriculture; UV/Vis, Ultraviolet-visible spectroscopy; VCE, Vitamin C equivalents; VEGFR2/Src/FAK, Vascular endothelial growth factor receptor 2/Src/focal adhesion kinase.
References
Basile, A., Vuotto, M. L., Violante, U., Sorbo, S., Martone, G., Castaldo-Cobianchi, R. (1997). Antibacterial activity in Actinidia chinensis, Feijoa sellowiana and Aberia caffra. Inter. J. Antimicrob. Ag. 8, 199–203. doi: 10.1016/S0924-8579(97)00376-2
Chai, W. M., Shi, Y., Feng, H. L., Xu, L., Xiang, Z. H., Gao, Y. S., et al. (2014). Structure characterization and antityrosinase mechanism of polymeric proanthocyanidins fractionated from kiwifruit pericarp. J. Agric. Food Chem. 62, 6382–6389. doi: 10.1021/jf501009v
Chang, J., Case, R. (2005). Cytotoxic phenolic constituents from the root of Actinidia chinensis. Planta Med. 71, 955–959. doi: 10.1055/s-2005-871225
Chen, X., Yang, S., Bai, S. (2011). Chemical constituents in root of Actinidia chinensis. Zhongcaoyao. 42, 841–843. doi: 10.7666/d.y1883489
Cheng, Q. L., Li, H. L., Huang, Z. Q., Chen, Y. J., Liu, T. S. (2015). 2β, 3β, 23-trihydroxy-urs-12-ene-28-olic acid (TUA) isolated from Actinidia chinensis radix inhibits NCI-H460 cell proliferation by decreasing NF-κB expression. Chem.Biol. Interact. 240, 1–11. doi: 10.1016/j.cbi.2015.06.038
Cui, Y. (2016). Study on chemical constituents in ethyl acetate extracts from the root of Actinidia chinensis.Hubei Agri. Sci. 55, 5359–5360. doi: 10.14088/j.cnki.issn0439-8114.2016.20.048.
Cui, Y., Zhang, X., Chen, J., Zhang, Y., Lin, X., Zhou, L. (2007). Chemical constituents from root of Actinidia chinensis. Zhongguo Zhongyao Zazhi. 32, 1663–1665. doi: 10.3321/j.issn:1001-5302.2007.16.016
Deng, J., Liu, Q., Zhang, C., Cao, W., Fan, D., Yang, H. (2016). Extraction optimization of polyphenols from waste kiwi fruit seeds (Actinidia chinensis Planch.) and evaluation of its antioxidant and anti-inflammatory properties. Molecules 21, pii: E832. doi: 10.3390/molecules21070832
Deng, J. J., Liu, Q. Q., Zhang, Q., Zhang, C., Liu, D., Fan, D. D., et al. (2018). Comparative study on composition, physicochemical and antioxidant characteristics of different varieties of kiwifruit seed oil in China. Food Chem. 264, 411–418. doi: 10.1016/j.foodchem.2018.05.063
Deng, J. J., Yang, H. X., Fan, D. D., Cao, W., Luo, Y. E. (2013). Antibacterial activities of polyphenolic extract from kiwi fruit (Actinidia chinensis Planch.) seeds. J. Pure Appl. Microbio. 7, 491–494.
Deters, A. M., Schröder, K. R., Hensel, A. (2005). Kiwi fruit (Actinidia chinensis) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell. Physiol. 202, 717–722. doi: 10.1002/jcp.20161
Drummond, L.. (2013). The composition and nutritional value of kiwifruit. Adv. Food Nutr. Res. Chapter 3 . 68, 33–54. doi: 10.1016/B978-0-12-394294-4.00003-1
Edmunds, S. J., Roy, N. C., Davy, M., Cooney, J. M., Barnett, M. P., Zhu, S., et al. (2012). Effects of kiwifruit extracts on colonic gene and protein expression levels in IL-10 gene-deficient mice. Br. J. Nutr. 108, 113–129. doi: 10.1017/S0007114511005241
Fang, T. T., Fang, Y., Xu, X. J., He, M. Y., Zhao, Z. Y., Huang, P. X., et al. (2019). Actinidia chinensis Planch. root extract attenuates proliferation and metastasis of hepatocellular carcinoma by inhibiting epithelial-mesenchymal transition. J. Ethnopharm. 231, 474–485. doi: 10.1016/j.jep.2018.11.014
Fiorentino, A., Mastellone, C., D’Abrosca, B., Pacifico, S., Scognamiglio, M., Cefarelli, G., et al. (2009). ƍ-Tocomonoenol: a new vitamin E from kiwi (Actinidia chinensis) fruits. Food Chem. 115, 187–192. doi: 10.1016/j.foodchem.2008.11.094
Garcia, C. V., Quek, S. Y., Stevenson, R. J., Winz, R. A. (2012). Kiwifruit flavour: a review. Trends Food Sci. & Tech. 24, 82–91. doi: 10.1016/j.tifs.2011.08.012
Gerasopoulos, D., Drogoudi, P. D. (2005). Summer-pruning and preharvest calcium chloride sprays affect storability and low temperature breakdown incidence in kiwifruit. Postharvest Biol. Technol. 36, 303–308. doi: 10.1016/j.postharvbio.2005.01.005
Gerasopoulos, D., Chlioumis, G., Sfakiotakis, E. (2006). Non-freezing points below zero induce low-temperature breakdown of kiwifruit at harvest. J. Sci. Food Agric. 86, 886–890. doi: 10.1002/jsfa.2429
Goffi, V., Zampella, L., Forniti, R., Petriccione, M., Botondi, R. (2019). Effects of ozone postharvest treatment on physicochemical and qualitative traits of Actinidia chinensis‘Soreli’ during cold storage. J. Sci. Food Agric. 99, 5654–5661. doi: 10.1002/jsfa.9823
Gu, J., Li, J., Yang, X. D., Shang, Z. W., Wei, H. Q., Jiang, X. J., et al. (2017). Study on inhibition effect of Actinidia chinensis Planch. extracted by chloroform proliferation of HEP-2 Cells. Chin. Arch. Tradi. Chin. Med. 35, 2835–2838. doi: 10.13193/j.issn.1673-7717.2017.11.028
He, G. N., Hu, X. M., Wang, H., Fan, L., Wang, B. C. (2015a). Chemical constituents in root of Actinidia chinensis Planch. Zhonghua Zhongyiyao Zazhi. 30, 498–500.
He, J., Ma, B. Z., Wang, X. X., Liu, F., Qin, W. J., Zhang, X. L., et al. (2015b). Chemical constituents from the root of Actinidia chinensis (II). Zhongguo Yaoxue Zazhi. 50, 1960–1963. doi: 10.11669/cpj.2014.03.005
He, J., Ma, B. Z., Zhao, T., Wang, W., Wei, F. L., Lu, J., et al. (2014). Chemical constituents from the roots of Actinidia chinensis. Zhongguo Yaoxue Zazhi. 49, 184–186. doi: 10.11669/cpj.2014.03.005
He, M. Y., Hou, J. J., Wang, L. Y., Zheng, M. H., Fang, T. T., Wang, X. D., et al. (2017). Actinidia chinensis Planch. root extract inhibits cholesterol metabolism in hepatocellular carcinoma through upregulation of PCSK9. Oncotarget 8, 42136–42148. doi: 10.18632/oncotarget.15010
Hettihewa, S. K., Hemar, Y., Rupasinghe, H. P. V. (2018). Flavonoid-rich extract of Actinidia macrosperma (a wild kiwifruit) inhibits angiotensin-converting enzyme in vitro. Food 7, 146. doi: 10.3390/foods7090146
Henare, S. J. (2016). The nutritional composition of kiwifruit (Actinidia spp.). Nutr. Compos. Fruit Cultivars, Chapter 15, 337–368. doi: 10.1016/B978-0-12-408117-8.00015-5
Hou, J. J., Wang, L. Y., Wu, D. J. (2018). The root of Actinidia chinensis inhibits hepatocellular carcinomas cells through LAMB3. Cell Biol. Toxicol. 34, 321–332. doi: 10.1007/s10565-017-9416-7
Huang, Z. Y., Li, J., Zhang, J. F., Gao, Y. Y., Hui, G. H. (2017). Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch.) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 11, 184–191. doi: 10.1007/s11694-016-9385-1
Hwang, J. S., Cho, C. H., Baik, M. Y., Park, S. K., Heo, H. J., Cho, Y. S., et al. (2017). Effects of freeze-drying on antioxidant and anticholinesterase activities in various cultivars of kiwifruit (Actinidia spp.). Food Sci. Biotechnol. 26, 221–228. doi: 10.1007/s10068-017-0030-5
Iwasawa, H., Morita, E., Yui, S., Yamazaki, M.. (2011). Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol. Pharm. Bull. 34, 128–134. doi: 10.1248/bpb.34.128
Ji, Z., Liang, X. (1985). Chemical constituents of the root of Actinidia chinensis Planch. Yaoxue Xuebao 20, 778–781.
Jiao, Y., Chen, D. L., Fan, M. T., Young, Qu, S. (2019). UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 281, 97–105. doi: 10.1016/j.foodchem.2018.12.062
Kaur, L., Rutherfurd, S. M., Moughan, P. J., Drummond, L., Boland, M. J. (2010). Actinidin enhances gastric protein digestion as assessed using an in vitro gastric digestion model. J. Agri. Food Chem. 58, 5068–5073. doi: 10.1021/jf903332a
Ku, C. Y., Wang, Y. R., Lin, H. Y., Lu, S. C., Lin, J. Y. (2015). Corosolic acid inhibits hepatocellular carcinoma cell migration by targeting the VEGFR2/Src/FAK pathway. PLoS One. 10, e0126725. doi: 10.1371/journal.pone.0126725
Kvesitadze, G. I., Kalandiya, A. G., Papunidze, S. G., Vanidze, M. R. (2001). Identification and quantification of ascorbic acid in kiwi fruit by high-performance liquid chromatography. Appl. Biochem. Micro. 37, 215–218. doi: 10.1023/A:1002848302873
Lahlou, E. H., Hirai, N., Kamo, T., Tsuda, M., Ohigashi, H. (2001). Actinidic acid, a new triterpene phytoalexin from unripe kiwi fruit. Biosci. Biotech. Bioch. 65, 480–483. doi: 10.1271/bbb.65.480
Lee, D. E., Shin, B. J., Hur, H. J., Kim, J. H., Kim, J., Kang, N. J., et al. (2010). Quercetin, the active phenolic component in kiwifruit, prevents hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Brit. J. Nutr. 104, 164–170. doi: 10.1017/S0007114510000346
Lee, I., Lee, B. H., Eom, S. H., Oh, C. S., Kang, H., Cho, Y. S., et al. (2015). Antioxidant capacity and protective effects on neuronal PC-12 cells of domestic bred kiwifruit. Korean J. Hortic. Sci. Technol. 33, 259–267. doi: 10.7235/hort.2015.14123
Li, D. X., Zhu, F. (2017). Physicochemical properties of kiwifruit starch. Food Chem. 220, 129–136. doi: 10.1016/j.foodchem.2016.09.192
Lin, P. F. (1988). Antitumor effect of Actinidia chinensis polysaccharide on murine tumor. Zhonghua Zhong Liu Za Zhi. 10, 441–444.
Liu, W., Hui, G. H. (2015). Kiwi fruit (Actinidia chinensis) quality determination based on surface acoustic wave resonator combined with electronic nose. Bioengineered 6, 1–9. doi: 10.1080/21655979.2014.996430
Luan, X., Li, X. J., Guo, M. M. (2017). Xiangxi kiwifruit seed component and characteristics of kiwifruit seed oil. Chin. Oils Fats. 42, 136–139. doi: 10.3969/j.issn.1003-7969.2017.08.030
Lv, J. P., Wang, L. Y., Shen, H., Wang, X. D. (2018). Regulatory roles of OASL in lung cancer cell sensitivity to Actinidia chinensis Planch. root extract (acRoots). Cell Biol. Toxicol. 34, 207–218. doi: 10.1007/s10565-018-9422-4
Ma, Q. S., Suo, J. T., Huber, D. J., Dong, X. Q., Han, Y., Zhang, Z. K., et al. (2014). Effect of hot water treatments on chilling injury and expression of a new C-repeat binding factor (CBF) in ‘Hongyang’ kiwifruit during low temperature storage. Postharvest Biol. Technol. 97, 102–110. doi: 10.1016/j.postharvbio.2014.05.018
Ma, T. T., Sun, X. Y., Zhao, J. M., You, Y. L., Lei, Y. S., Gao, G. T., et al. (2017). Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 218, 294–304. doi: 10.1016/j.foodchem.2016.09.081
McGhie, T. K., Ainge, G. D. (2002). Color in ruit of the genus Actinidia: carotenoid and chlorophyll compositions. J. Agri. Food Chem. 50, 117–121. doi: 10.1021/jf010677l
Meng, F. C., Cao, J. F., Zhang, Y. T., Chang, M. Y. (2017). Optimization of extraction technology of pectin from kiwifruit peel and pulp by ultrasound-assisted extraction. J. Green Sci. Technol. 14, 283–285. doi: 10.16663/j.cnki.lskj.2017.14.101
Michaud, J., Ane-Margail, M. (1977). Analytical study of catechuic tannins. I. Flavanolic oligomers of Actinidia chinensis Planch. Babin, Bull. Soc. Parma Bord. 116, 52–64.
Montefiori, M., McGhie, T. K., Costa, G., Ferguson, A. R. (2005). Pigments in the fruit of red-fleshed kiwifruit (Actinidia chinensis and Actinidia deliciosa). J. Agric. Food Chem. 53, 9526–9530. doi: 10.1021/jf051629u
Niu, H., Song, D., Sun, Y. L., Zhang, W. X., Mu, H. B., Duan, J. Y. (2016). Preparation and sulfation of an α-glucan from Actinidia chinensis roots and their potential activities. Int. J. Biol. Macromol. 92, 981–987. doi: 10.1016/j.ijbiomac.2016.07.091
Papunidze, G., Kalandiya, A., Papunidze, S., Vanidze, M. (2001). Kiwifruit chemical composition. Bull. Ge. Acad. Sci. 164, 544–546.
Qu, L., Liu, Q., Zhang, Q., Tuo, X., Fan, D., Deng, J., et al. (2019). Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 125, 85–94. doi: 10.1016/j.fct.2018.12.046
Sfakiotakis, E., Chlioumis, G., Gerasopoulos, D. (2005). Preharvest chilling reduces low temperature breakdown incidence of kiwifruit. Postharvest Biol. Technol. 38, 169–174. doi: 10.1016/j.postharvbio.2005.06.010
Sharon, J. H. (2016). The nutritional composition of kiwifruit (Actinidia spp.). Nutr. Compos. Fruit Cultivars. 15, 343–345. doi: 10.1016/B978-0-12-408117-8.00015-5
Shen, L., Zhang, G. J., Zhang, G. S., Song, W. Y., Xu, G. H. (2014). Effect of Actinidia chinensis polysaccharide on apoptosis of MFC and their orthotopic transplanted tumor of gastric cancer. Chin. Trad. Herb. Drugs. 45, 673–678. doi: 10.7501/j.issn.0253-2670.2014.05.015
Shu, Q., De Silva, U. M., Chen, S., Peng, W. D., Ahmed, M., Lu, G. J., et al. (2008). Kiwifruit extract enhances markers of innate and acquired immunity in a murine model. Food Agri. Immunol. 19, 149–161. doi: 10.1080/09540100802117198
Sivakumaran, S., Huffman, L., Sivakumaran, S., Drummond, L. (2018). The nutritional composition of Zespri SunGold kiwifruit and ZespriSweet green kiwifruit. Food Chem. 238, 195–202. doi: 10.1016/j.foodchem.2016.08.118
Sun, L. F., Li, X. F., Li, G., Dai, B., Tan, W. (2017). Actinidia chinensis Planch. improves the indices of antioxidant and anti-inflammation status of type 2 diabetes mellitus by activating keap1 and nrf2 via the upregulation of microRNA-424. Oxid. Med. Cell. Longev. 2017, 7038789. doi: 10.1155/2017/7038789
The Plant List (2013). Version 1.1. Published on the internet; http://www.theplantlist.org/ (accessed 1st January).
Twidle, A. M., Barker, D., Seal, A. G., Fedrizzi, B., Suckling, D. M. (2018). Identification of floral volatiles and pollinator responses in kiwifruit cultivars, Actinidia chinensis var. chinensis. J. Chem. Ecol. 44, 406–415. doi: 10.1007/s10886-018-0936-2
United Nations Food and Agriculture Organization. (2016). Kiwifruit production in 2016. “Crops/World Regions/Production Quantity from pick lists”. Retrieved 2018-02-20.
United States Department of Agriculture USDA Food Composition Databases. (2018). https://ndb.nal.usda.gov/ndb/. Accessed on April, 2018.
Wang, H. X., Ng, T. B. (2002). Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochem. 61, 1–6. doi: 10.1016/S0031-9422(02)00144-9
Wang, M. Y., MacRae, E., Wohlers, M., Marsh, K. (2011). Changes in volatile production and sensory quality of kiwifruit during fruit maturation in Actinidia deliciosa‘Hayward’ and A. chinensis‘Hort16A’. Postharvest Biol. Technol. 59, 16–24. doi: 10.1016/j.postharvbio.2010.08.010
Wang, Q., Lei, X. X., Yang, X. D., Xin, T., Li, Y. Q. (2017). Purification of total triterpenes from the root of Actinidia chinensis Planch. and its antitumor activity. Guangdong Med. J. 38, 514–518. doi: 10.3969/j.issn.1001-9448.2017.04.005
Wang, Q., Xu, Y. F., Gao, Y., Wang, Q. (2018). Actinidia chinensis Planch. polysaccharide protects against hypoxia-induced apoptosis of cardiomyocytes in vitro. Mol. Med. Rep. 18, 193–201. doi: 10.3892/mmr.2018.8953
Wang, Y., Zhao, C. L., Li, J. Y., Liang, Y. J., Yang, R. Q., Liu, J. Y., et al. (2018). Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotec. Biotechnol. Equip. 32, 1–8. doi: 10.1080/13102818.2018.1443400
Wei, L. B., Ma, S. Y., Liu, H. X., Huang, C. S., Liao, N. (2018). Cytotoxic triterpenoids from roots of Actinidia chinensis. Chem. Biodivers. 15, e1700454. doi: 10.1002/cbdv.201700454
Wurms, K. V., Cooney, J. M. (2006). Isolation of a new phenolic compound, 3, 5-dihydroxy-2-(methoxycarbonylmethyl) phenyl 3, 4-dihydroxybenzoate, from leaves of Actinidia chinensis (kiwifruit). Asian J. Biochem. 1, 325–332. doi: 10.3923/ajb.2006.325.332
Xia, C., Chen, J., Zhang, Y. J., Zhu, Y. Q., Li, H. J., Deng, J. L. (2017). Isolation, purification, monosaccharide composition and anticancer proliferation activity of polysaccharide fraction from Hongyang kiwifruit (Actinidia chinensis). Food Sci. 38, 126–131. doi: 10.7506/spkx1002-6630-201721020
Xie, Z. W. (1975). National compilation of Chinese herbal medicine Vol. I. Beijing: People’s Health Publishing House, 820–821.
Xu, Y. X., Xiang, Z. B., Jin, Y. S., Shen, Y., Chen, H. S. (2010). Two new triterpenoids from the roots of Actinidia chinensis.Fitoterapia. 81, 920–924. doi: 10.1016/j.fitote.2010.06.007
Xu, Y. X., Xiang, Z. B., Jin, Y. S., Xu, W., Sun, L. N., Chen, W. S., et al. (2016). Constituents from the roots of Actinidia chinensis and their cytochrome P450 enzyme inhibitory activities. Chem. Biodivers. 13, 1454–1459. doi: 10.1002/cbdv.201500518
Yang, H. L., Lee, Y. C., Han, K. S., Singh, H. S., Yoon, M., Park, J. H., et al. (2013). Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 136, 160–163. doi: 10.1016/j.foodchem.2012.07.111
Yang, Q., Rao, J., Yi, S., Meng, K., Wu, J., Hou, Y. (2013). Antioxidant enzyme activity and chilling injury during low-temperature storage of kiwifruit cv Hongyang exposed to gradual postharvest cooling. Horticul. Environ. Biotechnol. 53, 505–512. doi: 10.1007/s13580-012-0101-8
Yang, Q., Wang, F., Rao, J. (2016). Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in kiwifruit. PLoS One. 11, e0162159. doi: 10.1371/journal.pone.0162159
Yu, A. N., Tian, D. T., Qu, W. Y., Tan, Z. W., Song, X. J. (2009). Chemical composition of the essential oil of the roots of Actinidia chinensis from China. Chem. Nat. Com. 45, 108–109. doi: 10.1007/s10600-009-9235-z
Yuliarti, O., Goh, K. K., Matia-Merino, L., Mawson, J., Brennan, C. (2015a). Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 187, 290–296. doi: 10.1016/j.foodchem.2015.03.148
Yuliarti, O., Goh, K. K., Matia-Merino, L., Mawson, J., Drummond, L., Brennan, C. S. (2008). Effect of extraction techniques and conditions on the physicochemical properties of the water soluble polysaccharides from gold kiwifruit (Actinidia chinensis). Inter. J. Food Sci. Tec. 43, 2268–2277. doi: 10.1111/j.1365-2621.2008.01866.x
Yuliarti, O., Matia-Merino, L., Goh, K. K., Mawson, J., Williams, M. A., Brennan, C. (2015b). Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 166, 479–485. doi: 10.1016/j.foodchem.2014.06.055
Zhang, L., Zhang, W. X., Wang, Q. J., Wang, D. D., Dong, D. Q., Mu, H. B., et al. (2015). Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol. 72, 975–983. doi: 10.1016/j.ijbiomac.2014.09.056
Zhang, T., Li, C., Luo, A. W., Tang, M. L., Chen, H., Li, X. L., et al. (2016). Antioxidant activities in vitro of different fruit parts of eight kiwifruit varieties. Food Sci. 37, 88–93. doi: 10.7506/spkx1002-6630-201619015
Zhang, X. Y., Zhou, Y., Wei, Z. P., Shen, J., Wang, L. K., Ma, Z. Q., et al. (2018). Antiphytoviral toxins of Actinidia chinensis root bark (ACRB) extract: laboratory and semi-field trials. Pest Manag. Sci. 74, 1630–1636. doi: 10.1002/ps.4854
Zhao, T., He, J., Wang, X., Ma, B., Wang, X., Zhang, L., et al. (2014). Rapid detection and characterization of major phenolic compounds in radix Actinidia chinensis Planch. by ultra-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 98, 311–320. doi: 10.1016/j.jpba.2014.05.019
Zhou, X. F., Liu, Y. H., Tang, L., Zhang, P., Wu, J. Z. (2010). Chemical constituents from the roots of Actinidia chinensis. Chem. Nat. Compd. 46, 308–309. doi: 10.1007/s10600-010-9599-0
Zhou, X. F., Zhang, P., Pi, H. F., Zhang, Y. H., Ruan, H. L., Wang, H., et al. (2009). Triterpenoids from the roots of Actinidia chinensis. Chem. Biodivers. 6, 1202–1207. doi: 10.1002/cbdv.200800214
Zhu, W. J., Yu, D. H., Zhao, M., Lin, M. G., Lu, Q., Wang, Q. W., et al. (2013). Antiangiogenic triterpenes isolated from Chinese herbal medicine Actinidia chinensis Planch. Anticancer Ag. Med. Chem. 13, 195–198. doi: 10.2174/1871520611313020002
Keywords: Actinidia chinensis, nutritional composition, chemistry, pharmacological properties, antitumor, antioxidant
Citation: He X, Fang J, Chen X, Zhao Z, Li Y, Meng Y and Huang L (2019) Actinidia chinensis Planch.: A Review of Chemistry and Pharmacology. Front. Pharmacol. 10:1236. doi: 10.3389/fphar.2019.01236
Received: 10 April 2019; Accepted: 27 September 2019;
Published: 30 October 2019.
Edited by:
Monique S.J. Simmonds, Royal Botanic Gardens, Kew, United KingdomReviewed by:
Marinella De Leo, University of Pisa, ItalyJianping Chen, Shenzhen Traditional Chinese Medicine Hospital, China
Copyright © 2019 He, Fang, Chen, Zhao, Li, Meng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xirui He, eGlydWloZTYxMDUxOTRAMTYzLmNvbQ==
 Xirui He
Xirui He Jiacheng Fang2
Jiacheng Fang2