- 1Department of Neurology, Liaocheng People’s Hospital, Liaocheng, China
- 2Department of Intensive Care Unit, Yanggu People’s Hospital, Yanggu, China
Objective: Ginkgo leaf extract and dipyridamole injection (GDI), a kind of Chinese medicine preparation, has been considered as a promising supplementary treatment for ischemic stroke. The aim of this study was to systematically evaluate the clinical efficacy and safety of GDI mediated therapy for ischemic stroke.
Methods: PubMed, Cochrane Library, Medline, Embase, Web of Science, Wanfang database, Chinese Scientific Journal Database (VIP), China National Knowledge Infrastructure (CNKI) and Chinese Biological Medicine Database (CBM), were searched systematically for clinical trials of conventional treatments combined with GDI for ischemic stroke. The reported outcomes including overall response, hemorrheology and blood lipid indexes, and adverse events were systematically investigated.
Results: Data from thirty-nine trials including 3,182 ischemic stroke patients were involved. The results indicated that, compared with conventional treatments alone, the combination of conventional treatments with GDI obviously improved the overall response (odds ratio [OR] = 4.14, 95% confidence interval [CI] = 3.26–5.25, P < 0.00001), neurological status (National Institutes of Health Stroke Scale, OR = −3.13, 95% CI = −3.98 to −2.28, P < 0.00001) and activity of daily living (Barthel Index score, OR = 14.10, 95% CI = 9.51–18.68, P < 0.00001) of patients. Moreover, the hemorheology and blood lipids indexes of ischemic stroke patients were also significantly ameliorated after the combined therapy (P < 0.01). The frequency of adverse events did not differ significantly between the two groups (P > 0.05).
Conclusion: Evidence from the meta-analysis suggested that the combination of conventional treatments and GDI is safe and more effective in treating ischemic stroke than conventional treatments alone. Therefore, GDI mediated therapy could be recommended as an adjuvant treatment for ischemic stroke.
Introduction
Ischemia stroke is one of the common cerebrovascular diseases, and is a major cause of death and disability (Chen et al., 2019). It is characterized by the partial or complete loss of blood supply in part of the brain tissues, which account for about 80% of all stroke events (Chen et al., 2019). Ischemia results in reduced neuron number and interrupted neural axon network, and eventually resulting in the permanent loss of nerve tissue or disabled brain function (Xue et al., 2018). Over fifty million peoples are suffering ischemic stroke in the world, and nearly 50% of stroke survivors are left with disabling sequelae (Xue et al., 2018). Currently, the conventional therapy, including thrombolysis, controlling cerebral edema, neuroprotective agents, restoring blood supply to ischemic area, reducing blood viscosity, preventing and treating complications, controlling hypertension, etc. is the main clinical therapy for ischemia stroke (Ma et al., 2017; Chen et al., 2019). However, it functions mainly at the early stage of ischemia with a short time window, and therefore its clinical application is severely limited (Xue et al., 2018). Thus, the more effective agents for ischemia stroke patients are desirable.
Ginkgo extract is a Chinese traditional herb which made from the dried leaves of the Ginkgo biloba L (Ginkgoaceae) (Zeng et al., 2005), and has been widely applied as an effective complementary drug for brain disorder treatment in numerous hospitals of China. Ginkgo extract can protect the neurons against reactive oxygen species, regulates vasomotor, improves hemorheology and can also reduces infarction size by improving neurological function (Kampkotter et al., 2007; Nash and Shah, 2015; Tulsulkar et al., 2016; Liu et al., 2019). Zhang et al. (2018) demonstrated that ginkgo extract can inhibit astrocytic lipocalin-2 expression and alleviates neuro-inflammatory injury via the JAK2/STAT3 pathway after ischemic stroke. Ginkgo leaf extract and dipyridamole injection (GDI) is a compound preparation, which mainly consists of ginkgo flavone glycosides (24–25%), terpene lactones [ginkgolides (3.1%) and bilobalide (2.9%)] and dipyridamole (10%) (Zeng et al., 2005; Tan et al., 2018). Currently, several clinical trials indicated that conventional treatments combined with GDI exhibits more prominent therapeutic effects for ischemic stroke than conventional treatments alone (Wang et al., 2015; Li et al., 2017). However, the scientific evidence has not been systematically reviewed. Therefore, in this study, we conducted a meta-analysis to investigate the clinical efficacy and safety of GDI for ischemic stroke, in order to provide the best available evidence for clinical practice and further research planning on ischemia stroke treatment.
Materials and Methods
Search Strategy and Selection Criteria
This systematic review and meta-analysis was performed following the PRISMA guidelines and Cochrane Handbook. Literatures were searched across nine electronic databases, including PubMed, Cochrane Library, Medline, Embase, Web of Science, Wanfang database, Chinese Scientific Journal Database (VIP), China National Knowledge Infrastructure (CNKI) and Chinese Biological Medicine Database (CBM), before December 2018, with key terms “ginkgo biloba” or “ginkgo leaf extract” or “ginkgo dipyidamolum” or “ginkgo leaf extract and dipyridamole injection” or “yinxingdamo injection” and “ischemic stroke” or “cerebral infarction” or “brain infarction” or “cerebral ischemia” or “brain ischemia,” without language restriction (Supplementary Table 1). No language limits were applied.
Inclusion criteria: (1) Randomized controlled trials (RCTs) concerning ischemic stroke patients were included; (2) Research subjects (ischemic stroke patients) must meet WHO diagnostic criteria of ischemic stroke and exclude cerebral hemorrhage by brain computerized tomography (CT) or magnetic resonance imaging (MRI). (3) Articles involving more than 30 ischemic stroke patients; (4) There were no other medicines in combination with the conventional treatments in the experimental group, except for GDI, compared with the conventional treatments as a control; (5) Literatures comparing the clinical outcomes of conventional treatments plus GDI adjuvant therapy (experimental group) with conventional treatments alone (control group); (6) One or more outcome measures, including the overall response rate, neurological deficit score, serum level of CRP, hemorheology and blood lipid indexes, adverse events must be included in each study.
Exclusion criteria: (1) Studies not focus on GDI were excluded; (2) Inappropriate criteria in experimental or control group were excluded; (3) articles without sufficient available data were excluded; (4) Non-contrast articles, non-clinical studies, literature reviews, meta-analysis, meeting abstracts, case reports, repeated studies and experimental model researches were excluded.
Data Extraction and Quality Assessment
Data were extracted by two reviewers (PX and ZM) independently according to the same inclusion and exclusion criteria; disagreements were adjudicated by the third investigator (SL). The extracted characteristics comprised the following items: (a) first author’s names; (b) years of publication; (c) number of cases; (d) patient ages; (e) intervening measure; (f) dosage of GDI; (g) duration of treatment and (h) study parameter types. The quality of included trials was evaluated according to Cochrane Handbook (Zeng et al., 2015).
Outcome Definition
Clinical responses include treatment efficacy and adverse events. Treatment efficacy was evaluated in terms of the overall response rate (ORR), National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI) score, hemorrheology and blood lipid indexes. The hemorrheology indexes covered the following indicators: whole blood viscosity (WBV), plasma viscosity (PV), whole blood high-shear viscosity (WBHSV), whole blood low-shear viscosity (WBLSV), and content of fibrinogen (FIB). The blood lipid indicators [Plasma total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol (HDL-C); low density lipoprotein-cholesterol (LDL-C)] and plasma C reactive protein (CRP) of ischemic stroke patients were determined and compared between the GDI and non-GDI groups. Adverse events including fever, fullness in head, allergy, hemorrhage, palpitation, nausea and vomiting were taken into assessment.
Statistical Analysis
Statistical analysis was performed using the Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) and Stata 13.0 (Stata Corp., College Station, TX, USA). All data were expressed as odds ratio (OR) and 95% confidence intervals (CI), and P < 0.05 indicates difference with statistical significance. Heterogeneity among studies was estimated using the Cochran’s Q statistic and I2 tests, I2 > 50% or P < 0.1 indicated a high statistical heterogeneity (Jackson et al., 2012). A fixed-effects model was used to pool the estimates when heterogeneity was absent (I2 < 50%). Otherwise, a random effects model was selected.
Publication bias was numerically examined by Begg’s and Egger’s tests and presented by funnel plots. If publication bias existed, we used the trim-and-fill method to adjust the pooled estimates of the potential unpublished studies in the meta-analysis, which were compared with the original pooled OR (Liang et al., 2017; Lin et al., 2018). Sensitivity analysis was conducted to investigate the influence of different GDI dosages, sample sizes and research types on clinical efficacy.
Results
Search Results
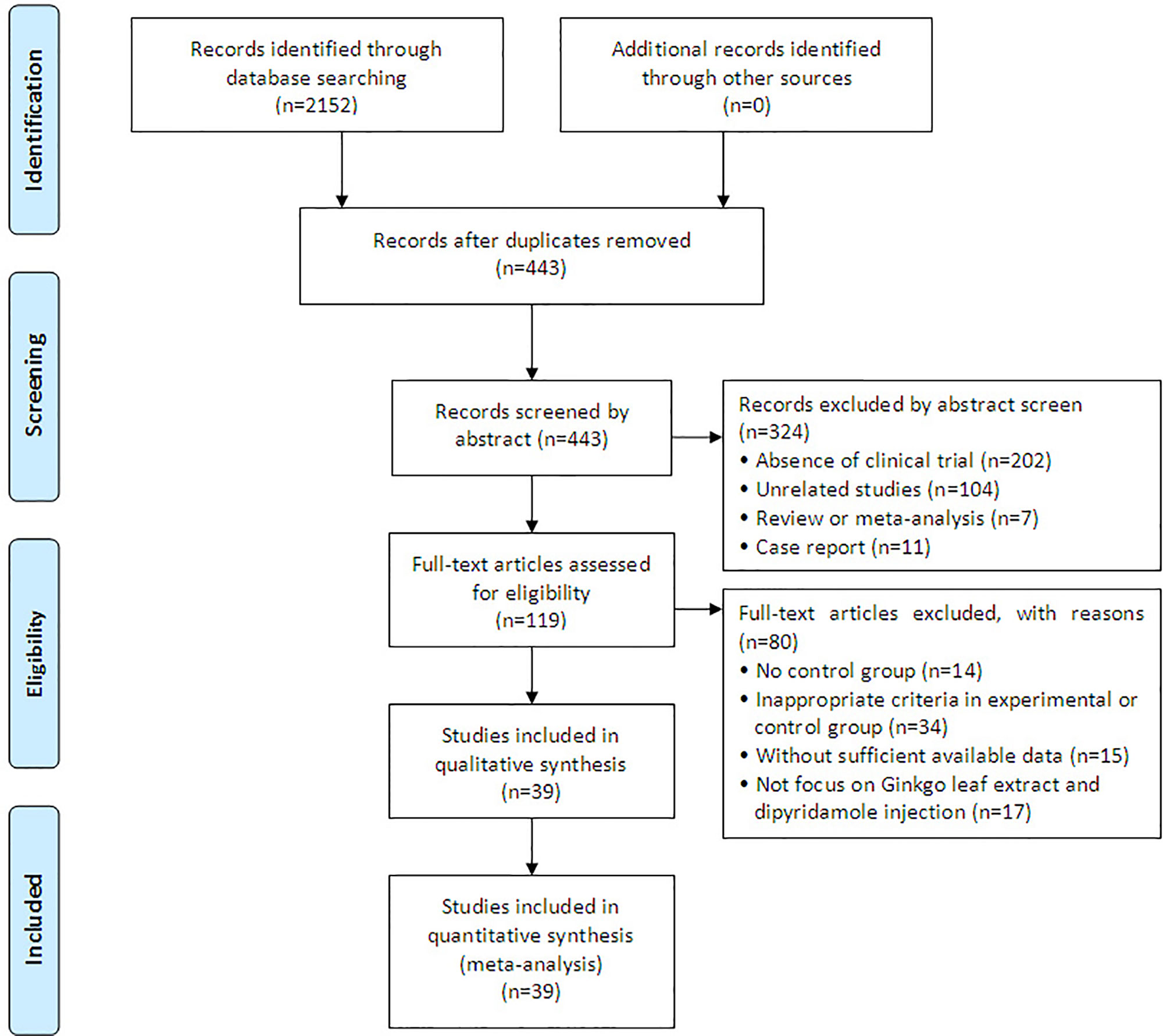
A total of 2,152 articles were identified with initial retrieve. 1,709 papers were excluded due to duplication. After title and abstract review, 324 articles were further excluded because they were not clinical trials (n = 202) or were unrelated studies (n = 104) or were reviews and meta-analysis (n = 7) or were case report (n = 11), leaving 119 studies as potentially relevant. After detailed assessment of full texts, articles without control group (n = 14), publications with inappropriate criteria of experimental or control group (n = 34), trials with insufficient data (n = 15) and studies not focus on GDI (n = 17) were excluded. Finally, 39 trials (Tang et al., 2009; Wang et al., 2009; Li, 2010; Long et al., 2010; Huang and Yuan, 2010; Tian et al., 2010; Zhou et al., 2010; Chen et al., 2011; Yang, 2012; Wang, 2012; Lan, 2013; Ding, 2013; Lin and Lin, 2013; Tang, 2013; Fang, 2014; Huang et al., 2014; Jiang, 2014; Yang, 2014; Wang, 2014a; Wang, 2014b; Chu, 2015; Liu, 2015; Sun, 2015; Zeng, 2015; Chen, 2016; Cui, 2016; Fu et al., 2016; Zhou et al., 2016; Li, 2016; Li, 2017; Dai, 2017; Guo, 2017; Wei, 2017; Ai, 2017; Zhang, 2017; Wang, 2018; Yi et al., 2018; Zhang, 2018; Zheng et al., 2018) involving 3,182 ischemic stroke patients were included in this analysis (Figure 1).
Patient Characteristics
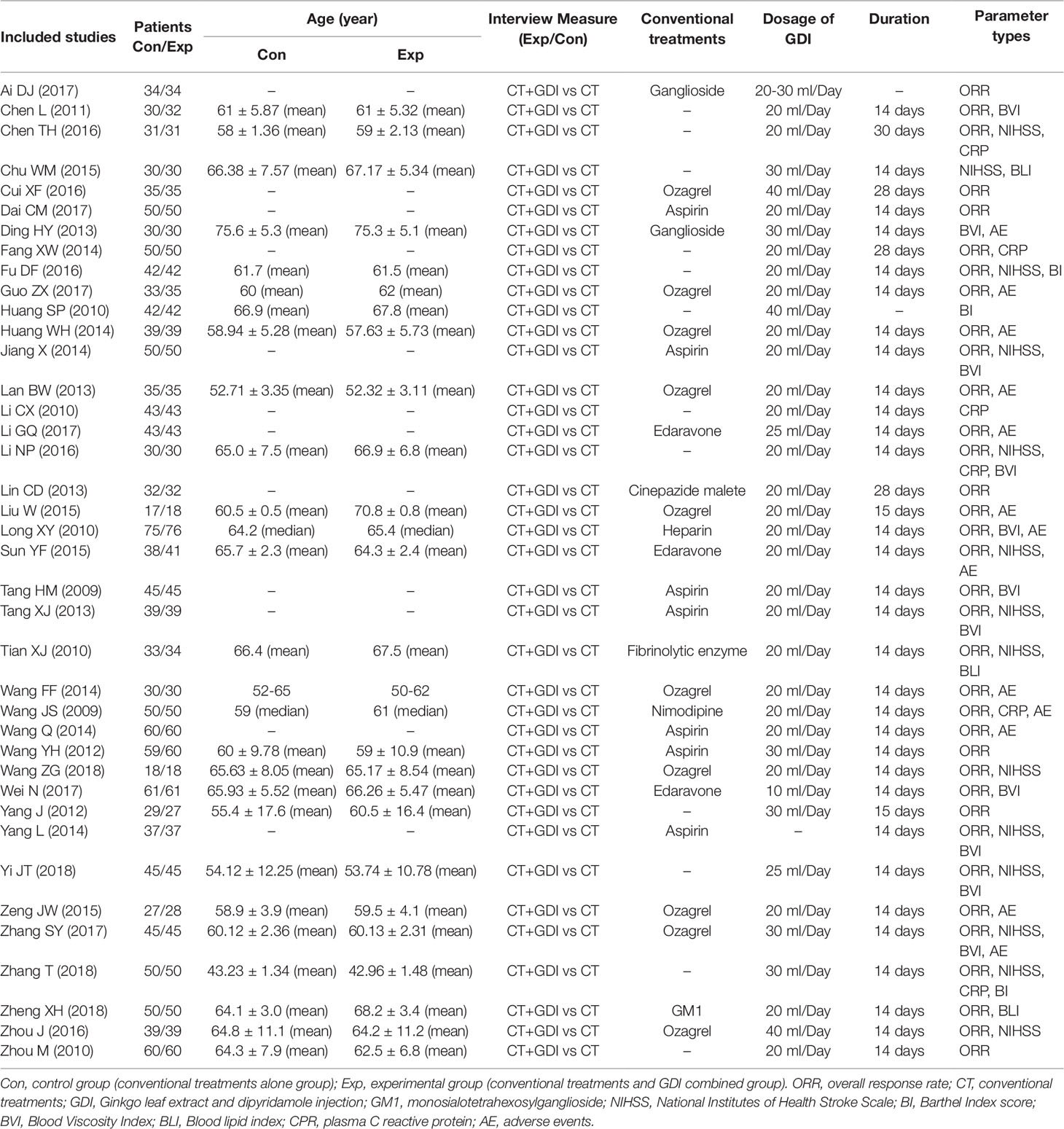
After selection, all included trials were performed in different medical centers of China. In total, 1,596 ischemic stroke patients were treated by conventional treatments in combination with GDI adjuvant therapy, while 1,589 patients were treated by conventional treatments alone. Detailed information of the involved studies and ischemic stroke patients is shown in Table 1. All included trials except one (Yang, 2014) clearly introduce the dosage of GDI. Twenty-two studies specifically describe the manufacturer of GDI and the remaining seventeen studies lacked clear description of production information (Supplementary Table 2). The compositions and concentrations of GDI in all included trials are the same (every 10ml GDI contained 9.0–11.0 mg total flavonoids and 3.6–4.4 mg dipyridamole). The Quality Standards of GDI in this study have been approved by Chinese State Food and Drug Administration (SFDA), and granted the Manufacturing Approve Number issued by Chinese SFDA. All pharmaceutical companies involved followed the quality processing procedure outlined in pharmacopeia.
Quality Assessment
The assessment of bias risk is shown in Figure 2. Thirty-four studies (Wang et al., 2009; Tang et al., 2009; Li, 2010; Huang and Yuan, 2010; Tian et al., 2010; Long et al., 2010; Zhou et al., 2010; Chen et al., 2011; Wang, 2012; Yang, 2012; Ding, 2013; Lin and Lin, 2013; Tang, 2013; Lan, 2013; Yang, 2014; Huang et al., 2014; Fang, 2014; Wang, 2014a; Wang, 2014b; Chu, 2015; Liu, 2015; Zeng, 2015; Cui, 2016; Fu et al., 2016; Li, 2016; Zhou et al., 2016; Chen, 2016; Zhang, 2017; Wei, 2017; Ai, 2017; Yi et al., 2018; Wang, 2018; Zhang, 2018; Zheng et al., 2018) were determined as low risk and the remaining five studies (Jiang, 2014; Sun, 2015; Dai, 2017; Guo, 2017; Li, 2017) were not true RCTs. All included trials did not provide clear description of performance and detection risks. The attrition risks of involved trials were low. Four trials (Huang and Yuan, 2010; Li, 2010; Ding, 2013; Chu, 2015) were considered as high reporting risk owing to lack of primary outcomes (ORR) and twenty-three studies (Tang et al., 2009; Zhou et al., 2010; Tian et al., 2010; Chen et al., 2011; Wang, 2012; Yang, 2012; Tang, 2013; Lin and Lin, 2013; Yang, 2014; Jiang, 2014; Fang, 2014; Fu et al., 2016; Li, 2016; Chen, 2016; Cui, 2016; Zhou et al., 2016; Ai, 2017; Dai, 2017; Wei, 2017; Zhang, 2018; Zheng et al., 2018; Wang, 2018; Yi et al., 2018) were considered as unclear reporting risk due to lack of safety assessment.
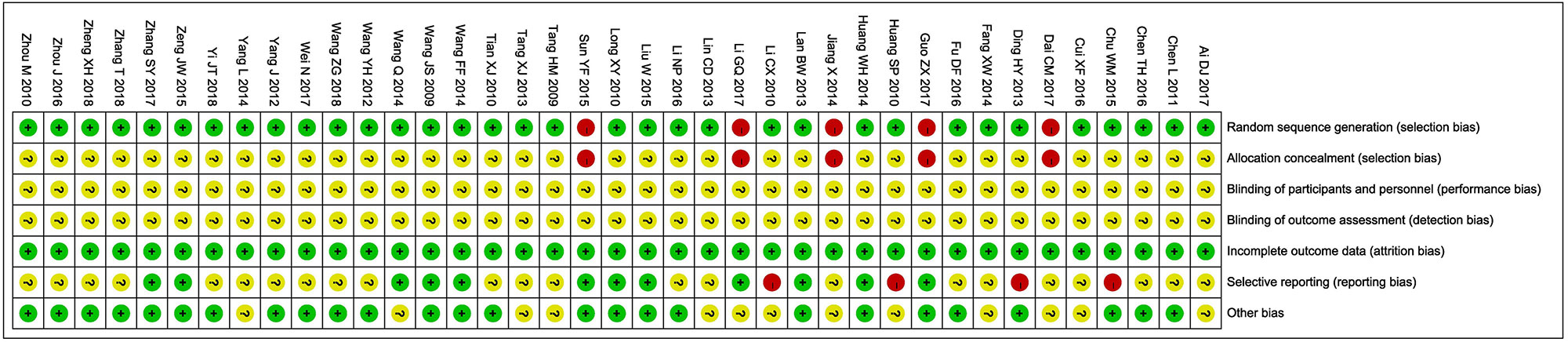
Figure 2 Risk of bias summary: review of authors’ judgments about each risk of bias item for included studies. Each color represents a different level of bias: red for high-risk, green for low-risk, and yellow for unclear-risk of bias.
ORR Assessments
Thirty-five clinical trials (Wang et al., 2009; Tang et al., 2009; Tian et al., 2010; Long et al., 2010; Zhou et al., 2010; Chen et al., 2011; Yang, 2012; Wang, 2012; Lan, 2013; Tang, 2013; Lin and Lin, 2013; Huang et al., 2014; Jiang, 2014; Wang, 2014a; Wang, 2014b; Fang, 2014; Yang, 2014; Liu, 2015; Sun, 2015; Zeng, 2015; Chen, 2016; Cui, 2016; Li, 2016; Zhou et al., 2016; Fu et al., 2016; Dai, 2017; Guo, 2017; Li, 2017; Wei, 2017; Zhang, 2017; Ai, 2017; Wang, 2018; Yi et al., 2018; Zhang, 2018; Zheng et al., 2018) involving 2,792 cases compared the ORR between the two groups (Figure 3). Our pooled results showed that patients underwent combined therapy had significantly improved ORR (OR = 4.14, 95% CI = 3.26-5.25, P < 0.00001) compared with conventional treatments alone. There was no heterogeneity, and a fixed-effect model was used to carry out the meta-analysis.
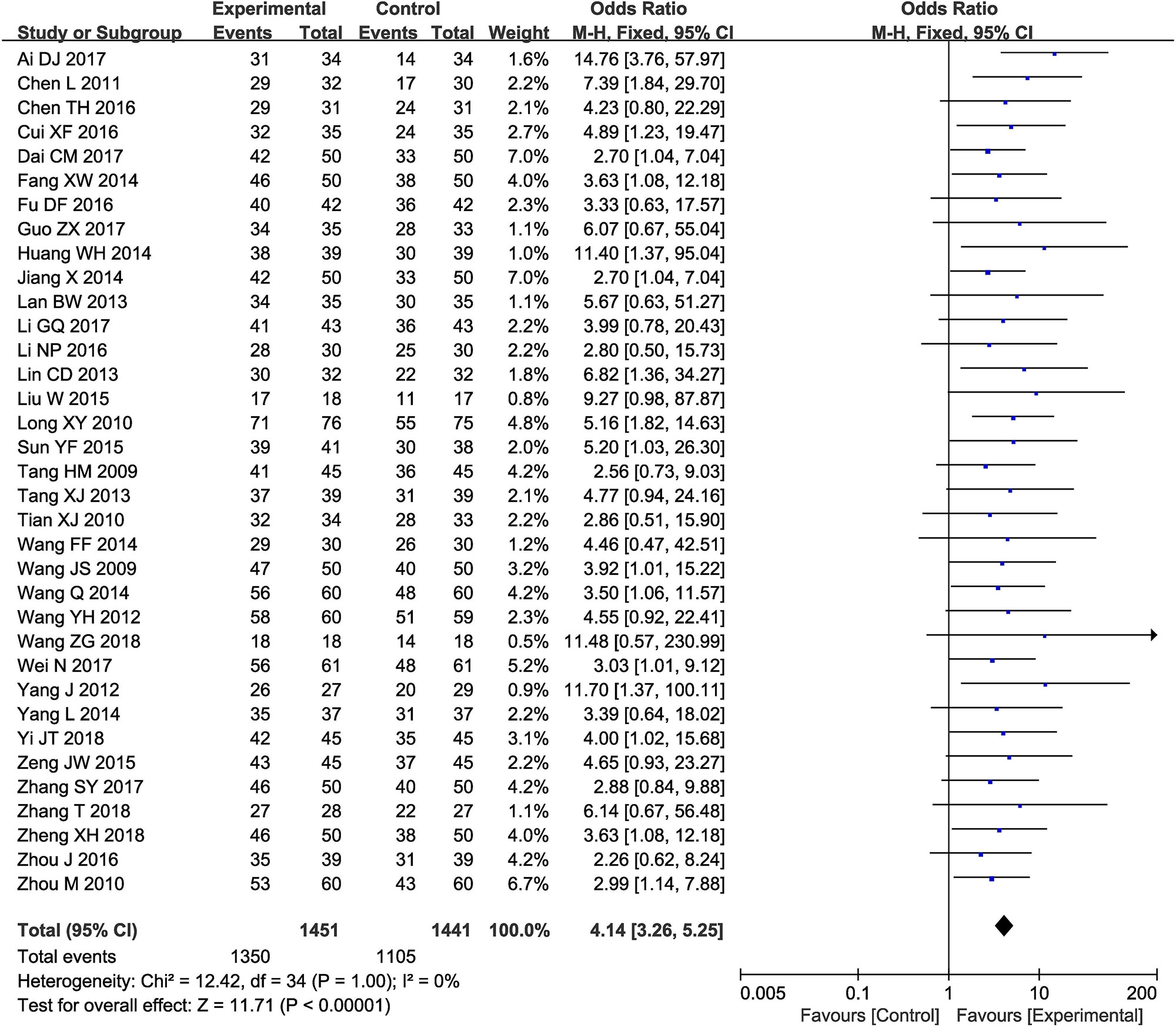
Figure 3 Forest plot of the comparison of overall response rate (ORR) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. GDI, Ginkgo leaf extract and dipyridamole injection. The fixed-effects meta-analysis model (Mantel-Haenszel method) was used.
NIHSS
Fourteen trials (Tian et al., 2010; Tang, 2013; Jiang, 2014; Yang, 2014; Chu, 2015; Sun, 2015; Chen, 2016; Fu et al., 2016; Li, 2016; Zhou et al., 2016; Zhang, 2017; Zhang, 2018; Yi et al., 2018; Wang, 2018) with 1,058 participants measured the neurological status according to the NIHSS (Figure 4). Results showed that the neurological status of ischemic oke patients received combined therapy was obviously improved compare to those treated by conventional treatments alone (OR = −3.13, 95% CI = −3.98 to −2.28, P < 0.00001). There was significant heterogeneity among the studies (I2 = 97%, P < 0.00001); therefore, a random-effects model was conducted to pool data and so any conclusions need to be made with caution.
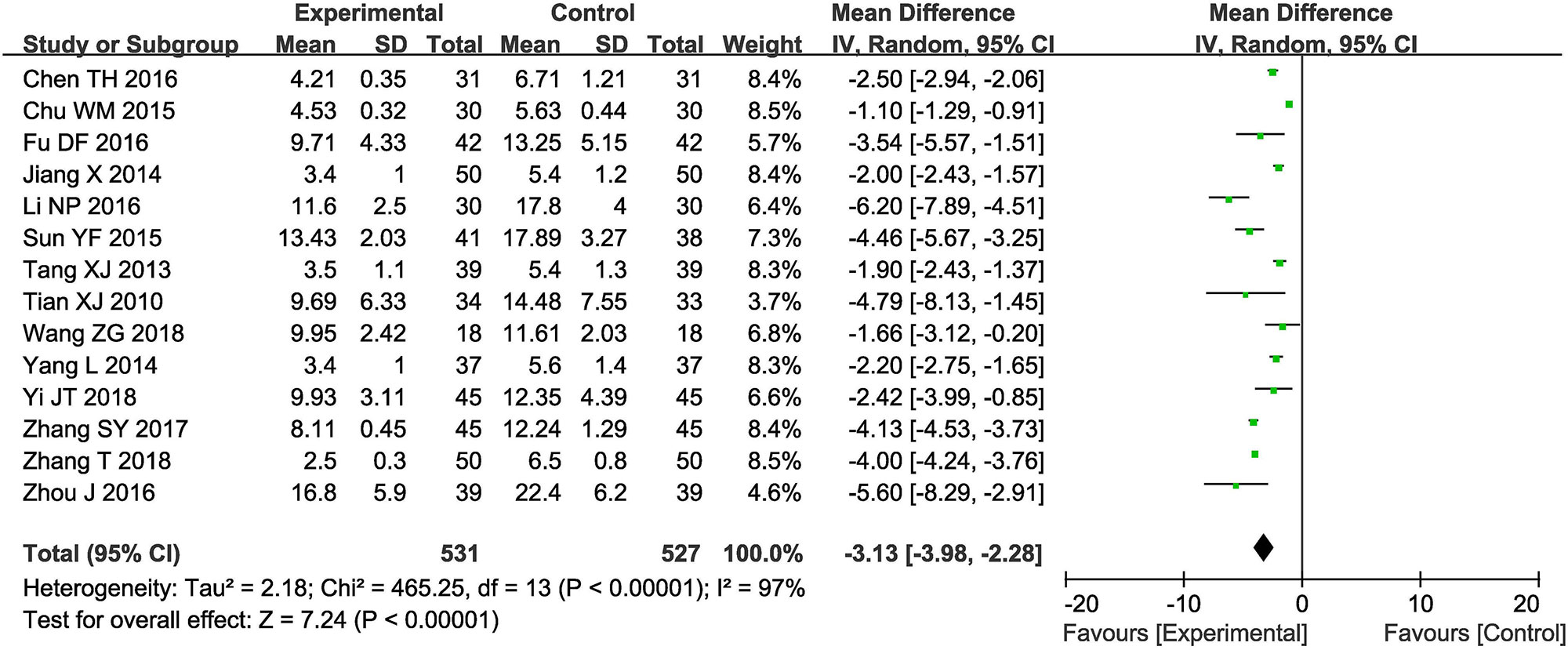
Figure 4 Forest plot of the comparison of National Institutes of Health Stroke Scale (NIHSS) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. GDI, Ginkgo leaf extract and dipyridamole injection. The random effects meta-analysis model (Inverse Variance method) was used.
Sensitivity Analysis Was Performed to Explore an Individual Study’s Influence on the Pooled Results by Deleting One Single Study Each Time From Pooled Analysis. as Supplementary Table 3 and Supplementary Figure 1 Signified, the Results Revealed That No Individual Studies Significantly Affected the NIHSS, Which Indicated Statistically Robust Results.
BI Score
Three trials (Huang and Yuan, 2010; Fu et al., 2016; Zhang, 2018) involving 368 ischemic stroke patients evaluated the activity of daily living according to the BI Score. As shown in Figure 5, the BI Score of ischemic stroke patients in the combined group were significantly higher than that of the control group (OR = 14.10, 95% CI = 9.51–18.68, P < 0.00001). A P-value = 0.04 and I2 = 69% indicated that there was significant heterogeneity among the studies; thus a random effect model was employed.
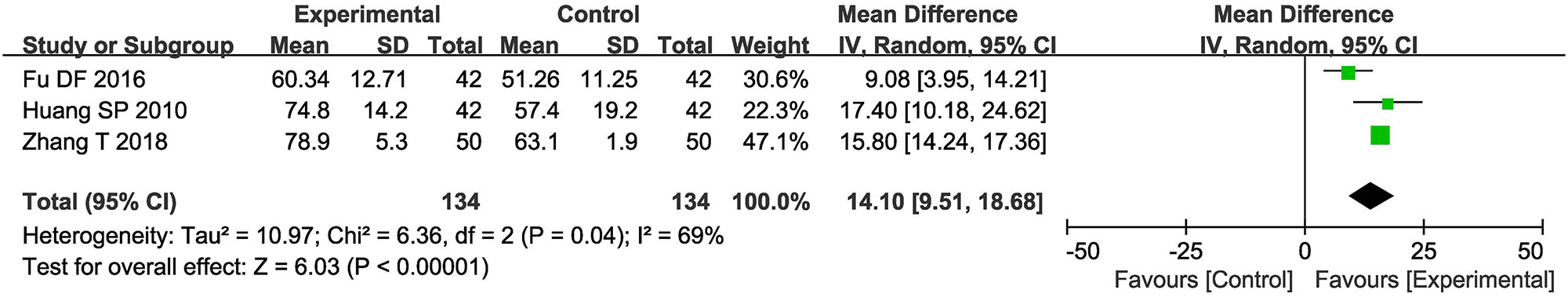
Figure 5 Forest plot of the comparison of Barthel Index (BI) score between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. GDI, Ginkgo leaf extract and dipyridamole injection. The random effects meta-analysis model (Inverse Variance method) was used.
CRP Level
There were six studies (Wang et al., 2009; Li, 2010; Fang, 2014; Li, 2016; Chen, 2016; Zhang, 2018) involving 508 patients measured the level of CRP (Supplementary Figure 2). The pooled analysis showed that compared with the conventional treatments alone, combined with GDI could significantly reduce the serum CRP levels of ischemic stroke patients (OR = −2.36, 95% CI = −3.25 to −1.48, P < 0.00001). There was statistical heterogeneity in CRP (P < 0.00001, I2 = 92%) according to the heterogeneity test. Therefore, the random effects model was used to pool this meta-analysis.
Hemorrheology Assessment
The hemorrheology of ischemic stroke patients was measured between GDI and non-GDI groups in eleven controlled studies (Tang et al., 2009; Long, et al., 2010; Chen, et al., 2011; Ding, 2013; Tang, 2013; Jiang, 2014; Yang, 2014; Li, 2016; Wei, 2017; Zhang, 2017; Yi et al., 2018) (Supplementary Figure 3). In this analysis, our results showed that the hemorrheology of ischemic stroke patients received combined therapy was significantly improved compare to those treated by conventional treatments alone, indicated by significantly reduced WBV (OR = −1.32, 95% CI = −1.40 to −1.24, P < 0.00001), PV (OR = −0.34, 95% CI = −0.51 to −0.17, P < 0.0001), WBHV (OR = −1.64, 95% CI = −2.39 to −0.89, P < 0.0001), WBLV (OR = −1.56, 95% CI = −2.33 to −0.79, P < 0.0001) and FIB (OR = −0.55, 95% CI = −1.43 to −0.34, P = 0.002). WBV (P = 0.99, I2 = 0%) was not heterogeneous among the studies, so fixed-effect model was used to analyzing its OR. Otherwise, random-effect model was used.
Blood Lipid Assessment
There were three studies (Tian et al., 2010; Chu, 2015; Zheng et al., 2018) reported the amelioration of blood lipid after the treatment of GDI and conventional therapy (Supplementary Figure 4). The meta-analysis revealed that the blood lipid level of patients was significantly improved compared with the conventional treatments alone, indicated by obviously decreased levels of TC (OR = −0.65, 95% CI = −0.82 to −0.48, P < 0.00001), TG (OR = −0.86, 95% CI = −1.24 to −0.49, P < 0.00001) and LDL-C (OR = −0.97, 95% CI = −1.24 to −0.70, P < 0.00001), and significantly increased HDL-C levels (OR = 0.23, 95% CI = 0.12–0.33, P < 0.0001) in blood. HDL-C (P = 0.68, I2 = 0%) was not heterogeneous among the studies, so fixed-effect model was used to analyzing its OR. Otherwise, random-effect model was used.
Adverse Events Assessment
Thirteen trials (Wang et al., 2009; Long et al., 2010; Ding, 2013; Lan, 2013; Wang, 2014a; Wang, 2014b; Huang et al., 2014; Liu, 2015; Sun, 2015; Zeng, 2015; Li, 2017; Guo, 2017; Zhang, 2017) involving 1,052 ischemic stroke patients evaluated the safety of GDI mediated therapy. The most common side effects of GDI treatment were fever, fullness in head, allergy, hemorrhage, palpitation, nausea and vomiting which usually subsided after symptomatic treatment. No severe adverse event occurred during GDI treatment, and the occurrence of these adverse reactions in the two groups was similar (Figure 6, Total side effects: OR = 0.74, 95% CI = 0.51–1.09, P = 0.13; Fever: OR = 0.85, 95% CI = 0.30–2.39, P = 0.75; Fullness in head: OR = 0.84, 95% CI = 0.29–2.45, P = 0.75; Allergy: OR = 0.49, 95% CI = 0.17–1.42, P = 0.19; Hemorrhage: OR = 1.00, 95% CI = 0.25–4.08, P = 1.00; Palpitation: OR = 2.08, 95% CI = 0.50–8.61, P = 0.31; Nausea and vomiting: OR = 1.00, 95% CI = 0.24–4.13, P = 1.00). Fixed-effect models were used to analyze OR rate because of low heterogeneity.
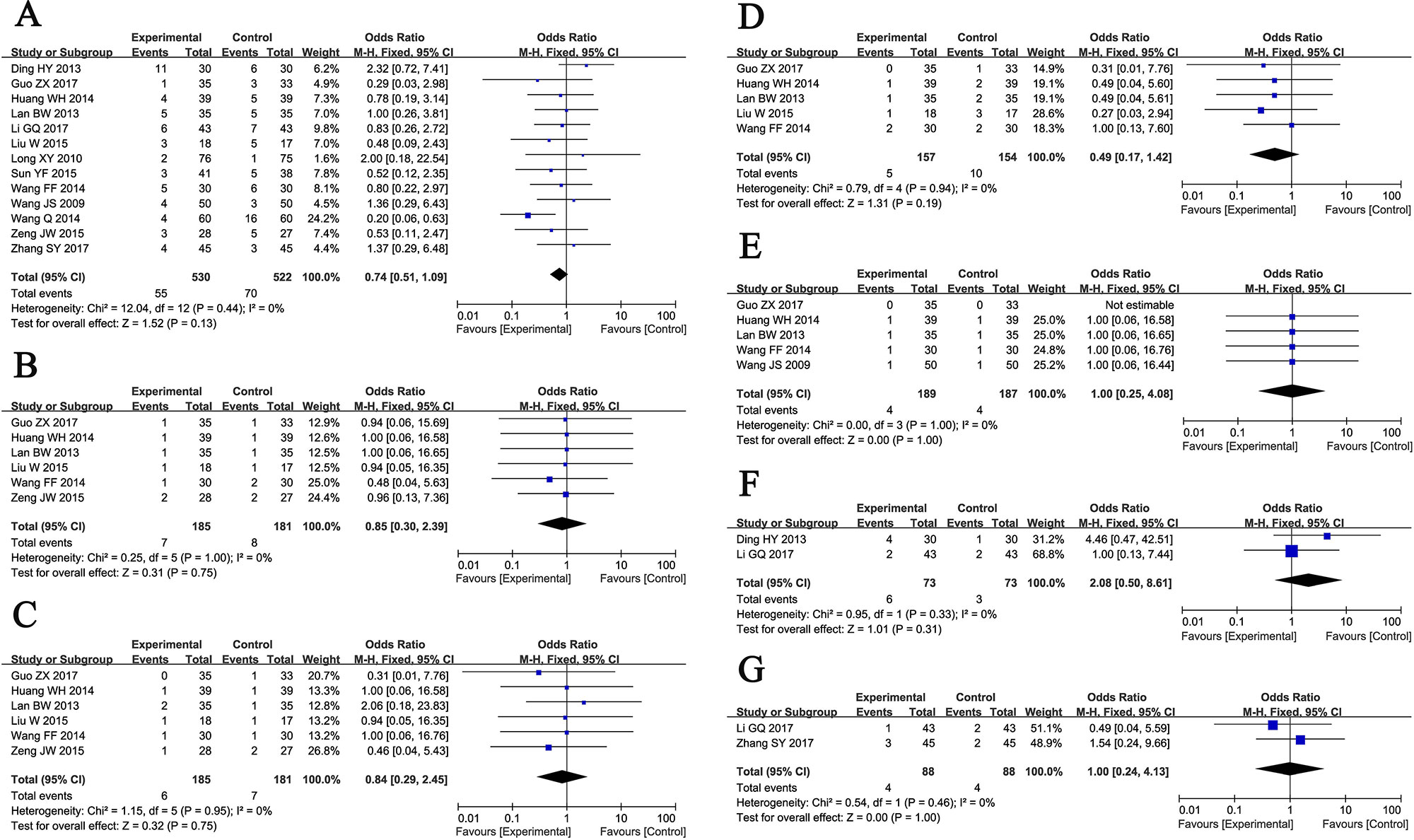
Figure 6 Forest plot of the comparison of adverse effects including total adverse effects (A), fever (B), fullness in head (C), allergy (D), hemorrhage (E), palpitation (F), nausea and vomiting (G) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group; GDI, Ginkgo leaf extract and dipyridamole injection. The fixed-effects meta-analysis model (Mantel-Haenszel method) was used.
Publication Bias
Publication bias was assessed visually by funnel plots. As shown in Figure 7, the funnel plots were symmetrical in NIHSS and total adverse events (TAE), but were asymmetrical in ORR.
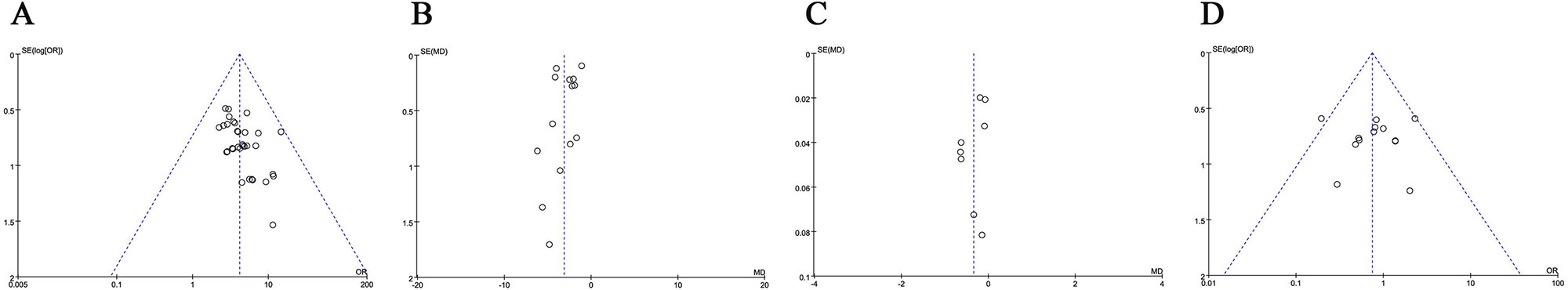
Figure 7 Funnel plot of overall response rate (ORR, A), National Institutes of Health Stroke Scale (NIHSS, B), plasma viscosity (PV, C) and total adverse effects (TAE, D).
We further assessed publication bias by Begg’s and Egger’s regression tests, and ORR was found with bias (Begg = 0.001; Egger = 0.001). No significant publication bias for NIHSS (Begg = 0.584; Egger = 0.638) and total side effects (Begg = 0.855; Egger = 0.986) was observed in these analyses. To determine if the bias affect the pooled risk of ORR, we conducted trim and filled analysis. The adjusted OR indicated same trend with the result of the primary analysis (before: P < 0.0001, after: P < 0.0001), reflecting the reliability of our primary conclusions.
Sensitivity Analysis
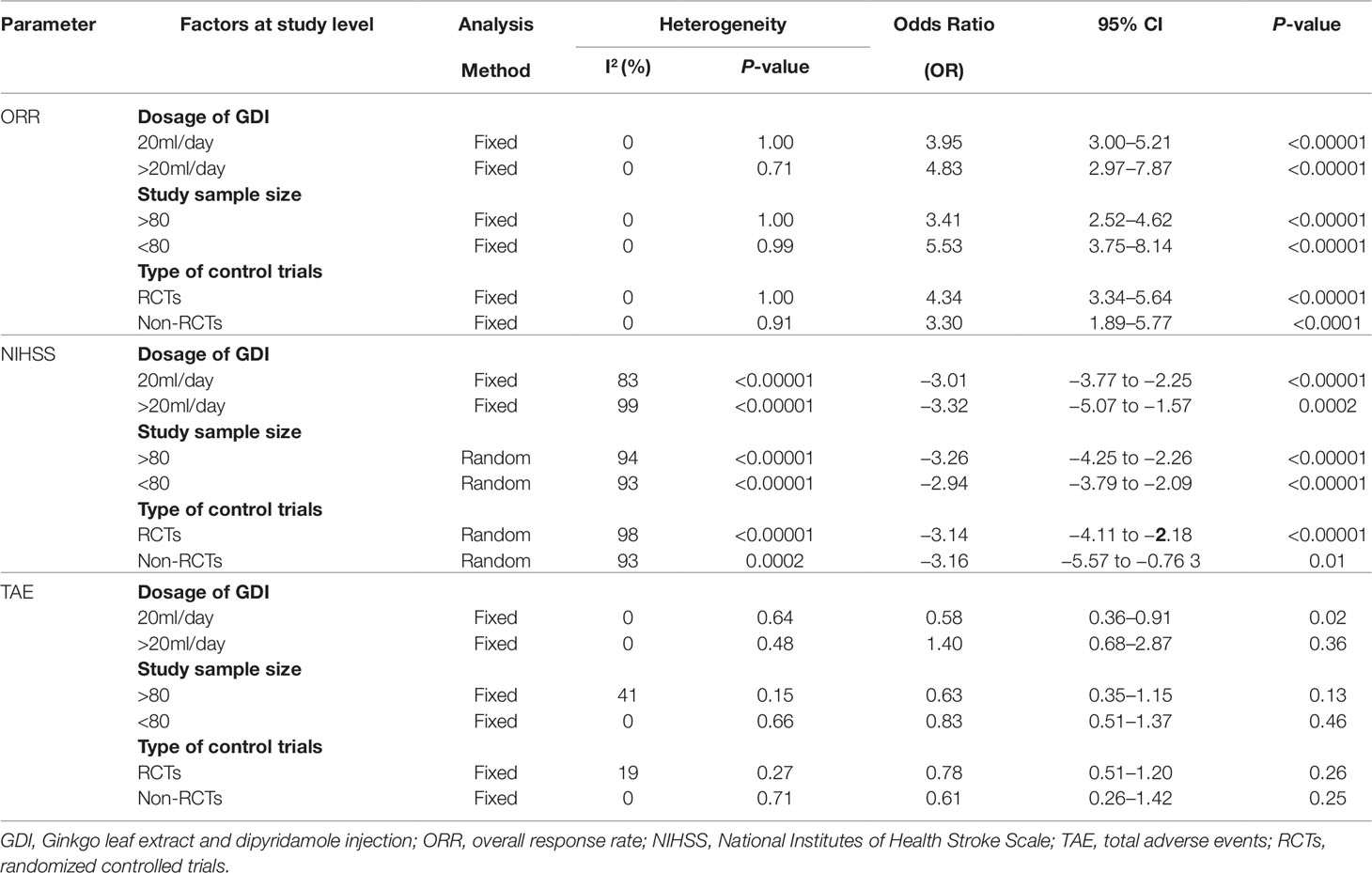
We also conducted subgroup analysis to explore the source of heterogeneity in ORR, NIHSS and TAE with respect to GDI dosages, sample sizes and types of involved studies. As shown in Table 2, our analysis results showed that no significant difference was found between different dosages of GDI, sample sizes and study types in ORR and NIHSS. Moreover, our results showed that GDI may alleviate the TAE caused by routine treatments when its dosage not more than 20 ml/day.
Discussion
Traditional Chinese medicine has been used to treat ischemic stroke in China during the past two thousand years. A survey in China showed that about 70% of doctors surveyed indicated that Chinese herb were effective complementary therapies for ischemic stroke (Zeng et al., 2005). GDI, a kind of Chinese medicine preparation, has been clinically applied as an effective adjuvant agent for reducing brain injuries, and enhancing functional recovery (Nash and Shah, 2015; Wang et al., 2015; Li et al., 2017). Even though there was statistical analysis of published literatures, a comprehensive and systematic evaluation of GDI for the treatment of ischemia stroke is still rare. In this analysis, we conducted a wide range of online search according strict inclusion and exclusion criteria, by which to provide an internationally accessible systematic review of the clinical efficacy and safety of GDI for the ischemia stroke.
The meta-analysis was carried out in thirty-five articles (Tang et al., 2009; Wang et al., 2009; Tian et al., 2010; Long et al., 2010; Zhou et al., 2010; Chen et al., 2011; Wang, 2012; Yang, 2012; Tang, 2013; Lan, 2013; Lin and Lin, 2013; Huang et al., 2014; Fang, 2014; Jiang, 2014; Wang, 2014a; Wang, 2014b; Yang, 2014; Liu, 2015; Zeng, 2015; Sun, 2015; Chen, 2016; Li, 2016; Zhou et al., 2016; Cui, 2016; Fu et al., 2016; Wei, 2017; Guo, 2017; Ai, 2017; Zhang, 2017; Dai, 2017; Li, 2017; Wang, 2018; Yi et al., 2018; Zhang, 2018; Zheng et al., 2018) to evaluate the ORR. Compared with conventional treatments alone, GDI combined with conventional treatments was associated with obviously higher ORR. Moreover, the combination therapy also significantly improved the neurological status and activity of daily living of ischemic stroke patients. CRP has important value in the prediction, prevention and prognosis of ischemic stroke (Matsuo et al., 2016; Yu et al., 2019). Our analysis results showed that the CRP level of patients was obviously decreased after conventional treatments and GDI combined therapy. Moreover, hemorheology and blood lipids indexes of patients were also significantly ameliorated. All these results indicated that GDI can protect ischemic stroke from injury, which may be related with its action on regulating the blood viscosity and level of blood lipid.
Safety is the top priority of a therapeutic strategy, and also a key factor for their clinical application and further development. This analysis confirmed the safety of GDI in ischemic stroke treatment. The most common side effects during GDI therapy were fever, fullness in head, allergy, hemorrhage, palpitation, nausea, and vomiting, and all of them did not differ significantly between the two groups. Therefore, GDI is a safe auxiliary medicine for ischemic stroke.
The analysis on therapeutic effects may be influenced by several factors. We used three clinical variables (GDI dosages, sample sizes, and research types) to interact with three outcome indicators (ORR, NIHSS, and TAE) and found that the TAE might be associated with GDI dosages. However, recent studies on the impact of these factors on the curative effect of GDI adjuvant therapy remain insufficient and further investigations such as biobliographic references that support this statement still should be performed.
There are some limitations in our analysis. As an important Chinese herb preparation, GDI was mainly applied in China, which may bring the unavoidable regional bias and subsequently influence the clinical application of GDI worldwide. In other countries such as America, India and Iran, G. biloba extract has also been used for acute ischemic stroke treatment and the prevention of cognitive decline (Garg et al., 1995; Dodge et al., 2008; Oskouei et al., 2013), but these studies were eventually excluded because they did not meet the inclusion criteria. We will keep following the updated researches on GDI mediated therapy for ischemic stroke in the world, and perform further systematical research on it. Moreover, several results showed significantly heterogeneity among the included trials, which may be due to the different ages of ischemic stroke patients and routine drug types. Therefore, the findings from our study should be dealt with some caution. Finally, a possible interaction between other drugs and ginkgo should be considered. However, our data were extracted from published papers and they did not provide sufficient information on this aspect. Therefore, based on currently available literatures, there are insufficient data to perform a statistical analysis to evaluate the correlation. We will keep paying close attention to this concern in our later studies
Conclusion
In summary, this meta-analysis indicated that GDI combined with conventional treatments was effective in treating ischemic stroke. Clinical application of GDI not only obviously enhanced the ORR of conventional treatments, but also effectively improved the blood viscosity and blood lipid level of ischemic stroke patients. However, because the low quality of some included trials increases risks and bias, the clinical efficacy and safety of GDI-mediate therapy for ischemic stroke still needs methodologically rigorous trials to verify.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
SL and ZM put forward this topic and designed this study. PX and ZM performed article screening, data collection and extraction, and manuscript writing. PX, ZM, and SL conducted the data analysis. SL polished the written English.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01403/full#supplementary-material
Supplementary Figure 1 | Sensitivity Analysis for National Institutes of Health Stroke Scale (NIHSS).
Supplementary Figure 2 | Forest plot of the comparison of plasma C reactive protein (CRP) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. GDI, Ginkgo leaf extract and dipyridamole injection. The random effects meta-analysis model (Inverse Variance method) was used.
Supplementary Figure 3 | Forest plot of the comparison of the hemorrheology indexes including WBV (A), PV (B), WBHSV (C), WBLSV (D) and FIB (E) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. WBV, whole blood viscosity; PV, plasma viscosity; WBHSV, whole blood high-shear viscosity; WBLSV, whole blood low-shear viscosity; FIB, content of fibrinogen; GDI, Ginkgo leaf extract and dipyridamole injection.
Supplementary Figure 4 | Forest plot of the comparison of the blood lipid indexes including TC (A), TG (B), HDL-C (C) and LDL-C (D) between the experimental and control group. Control group, conventional treatments alone group; Experimental group, conventional treatments and GDI combined group. TC, plasma total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; GDI, Ginkgo leaf extract and dipyridamole injection.
References
Ai, D. J. (2017). Effective method and effect analysis of drug treatment for cerebral infarction sequela. Med. Inform. 30, 116–117. doi: 10.3969/j.issn.1006-1959.2017.05.074
Chen, L., Zhang, T. Z., Li, M. L. (2011). Therapeutic effect observation of gingko dipyridamole for acute cerebral infarction. Healthmust-Readmagazine 9, 49.
Chen, Z., Xue, T., Huang, H., Xu, J., Shankar, S., Yu, H., et al. (2019). Efficacy and safety of sonothombolysis versus non-sonothombolysis in patients with acute ischemic stroke: a meta-analysis of randomized controlled trials. PloS One 14, e0210516. doi: 10.1371/journal.pone.0210516
Chen, T. H. (2016). Clinical observation of ginkgo leaf extract and dipyridamole injection in the treatment of type 2 diabetes patients with cerebral infarction. Shenzhen J. Integr. Trad. Chin. West. Med. 26, 27–28. doi: 10.16458/j.cnki.1007-0893.2016.18.013
Chu, W. M. (2015). Observation on efficacy of ginkgo leaf extract and dipyridamole injection in treatment of ischemic stroke patients. China Trop. Med. 15, 115–117. doi: 10.13604/j.cnki.46-1064/r.2015.01.041
Cui, X. F. (2016). Application effect analysis of gingko dipyridamole and ozagrel for the treatment of cerebral infarction. Clin. Res. 24, 103–104.
Dai, C. M. (2017). Effective observation of aspirin combined with gingko dipyridamole injection in the treatment of acute cerebral infarction. China Health Care Nutrit. 27, 104–105.
Ding, H. Y. (2013). Gingko dipyridamole injection combined with ganglioside for the treatment of 30 cases patients with acute cerebral infarction Shaanxi J. Trad. Chin. Med. 34, 288–289.
Dodge, H. H., Zitzelberger, T., Oken, B. S., Howieson, D., Kaye, J. (2008). A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology 70, 1809–1817. doi: 10.1212/01.wnl.0000303814.13509.db
Fang, X. W. (2014). Effect of ginkgo-dipyidamolum injection on CRP, IL-6 and sICAM-1 in the patients with type 2 diabetic with cerebral infraction. J. Hunan Normal Univ. (Med. Sci.) 11, 81–84.
Fu, D. F., Qiu, J., Yu, S. R., Li, W. H. (2016). Clinical experience of gingko dipyridamole in treating 42 cases patients with acute cerebral infarction. Stud. Trace Elem. Health 33, 13–14.
Garg, R. K., Nag, D., Agrawal, A. (1995). A double blind placebo controlled trial of ginkgo biloba extract in acute cerebral ischaemia. J. Assoc. Physicians. India. 43, 760–763.
Guo, Z. X. (2017). Clinical efficacy observation of gingko dipyridamole combined with Ozagrel injection in the treatment of cerebral infarction. Med. Inform. 30, 84–85. doi: 10.3969/j.issn.1006-1959.2017.12.052
Huang, S. P., Yuan, D. Y. (2010). Curative effect observation of gingko dipyridamole injection and early rehabilitation therapy in treating 42 cases of patients with cerebral infarction. Chin. J. Trad. Med. Sci. Tech. 17, 63–64. doi: 10.3969/j.issn.1005-7072.2010.01.029
Huang, W. H., Qiu, Z. X., Zeng, X. (2014). Clinical observation of gingko dipyridamole combined with Ozagrel injection in the treatment of cerebral infarction. Shenzhen J. Integr. Trad. Chin. West. Med. 24, 26–27.
Jackson, D., White, I. R., Riley, R. D. (2012). Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat. Med. 31, 3805–3820. doi: 10.1002/sim.5453
Jiang, X. (2014). Discussion on clinical efficacy of gingko dipyridamole combined with aspirin in the treatment of acute cerebral infarction. China Foreign Med. Treat. 29, 144–146. doi: 10.3969/j.issn.1674-0742.2014.29.074
Kampkotter, A., Pielarski, T., Rohrig, R., Timpel, C., Chovolou, Y., Watjen, W. (2007). The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol. Res. 55, 139–147. doi: 10.1016/j.phrs.2006.11.006
Lan, B. W. (2013). Effective observation of gingko dipyridamole combined with ozagrel injection in the treatment of cerebral infarction. Chin. J. Med. Guide 15, 1477–1479. doi: 10.3969/j.issn.1009-0959.2013.09.061
Li, S., Zhang, X., Fang, Q., Zhou, J., Zhang, M., Wang, H., et al. (2017). Ginkgo biloba extract improved cognitive and neurological functions of acute ischaemic stroke: a randomised controlled trial. Stroke Vasc. Neurol. 2, 189–197. doi: 10.1136/svn-2017-000104
Li, C. X. (2010). Clinical analysis of gingko dipyridamole in the treatment of patients with massive cerebral infarction. China Foreign Med. Treat. 29, 123. doi: 10.3969/j.issn.1674-0742.2010.09.095
Li, N. P. (2016). Clinical observation on treating ACI with the Yinxing Damo decoction. Clin. J. Chin. Med. 8, 65–66. doi: 10.3969/j.issn.1674-7860.2016.26.035
Li, G. Q. (2017). Clinical efficacy investigation of edaravone combined with gingko dipyridamole in the treatment of acute cerebral infarction. For All Health 11, 178. doi: 10.3969/j.issn.1009-6019.2017.01.238
Liang, M., Chen, Q., Zhang, Y., He, L., Wang, J., Cai, Y., et al. (2017). Impact of diabetes on the risk of bedsore in patients undergoing surgery: an updated quantitative analysis of cohort studies. Oncotarget 8, 14516–14524. doi: 10.18632/oncotarget.14312
Lin, C. D., Lin, C. S. (2013). Randomized parallel controlled study of Yinxing Damo injection combined with Kelinao in the treatment of acute cerebral infarction. J. Pract. Trad. Chin. Inter. Med. 27, 50–51. doi: 10.3969/j.issn.1671-7813.2013.06(s).26
Lin, L., Chu, H., Murad, M. H., Hong, C., Qu, Z., Cole, S. R., et al. (2018). Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 33 (8), 1260–1267. doi: 10.1007/s11606-018-4425-7
Liu, Q., Jin, Z., Xu, Z., Yang, H., Li, L., Li, G., et al. (2019). Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 24, 441–452. doi: 10.1007/s12192-019-00977-1
Liu, W. (2015). Effect analysis of Ozagrel and gingko dipyridamole in the treatment of acute cerebral infarction. Contemp. Med. Forum. 13, 270–271.
Long, X. Y., Jiang, M. C., Yin, L. P., Lin, Y. H. (2010). Clinical analysis of low molecular weight heparin combined with gingko dipyridamole in the treatment of acute cerebral infarction. Med. Inform. 5, 487–489. doi: 10.3969/j.issn.1006-1959-c.2010.03.025
Ma, X., Yang, Y. X., Chen, N., Xie, Q., Wang, T., He, X., et al. (2017). Meta-Analysis for Clinical Evaluation of Xingnaojing Injection for the Treatment of Cerebral Infarction. Front. Pharmacol. 8, 485. doi: 10.3389/fphar.2017.00485
Matsuo, R., Ago, T., Hata, J., Wakisaka, Y., Kuroda, J., Kuwashiro, T., et al. (2016). Plasma C-Reactive Protein and Clinical Outcomes after Acute Ischemic Stroke: A Prospective Observational Study. PloS One 11, e0156790. doi: 10.1371/journal.pone.0156790
Nash, K. M., Shah, Z. A. (2015). Current Perspectives on the Beneficial Role of Ginkgo biloba in Neurological and Cerebrovascular Disorders. Integr. Med. Insights 10, 1–9. doi: 10.4137/IMI.S25054
Oskouei, D. S., Rikhtegar, R., Hashemilar, M., Sadeghi-Bazargani, H., Sharifi-Bonab, M., Sadeghi-Hokmabadi, E., et al. (2013). The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 22, e557–e563. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.010
Sun, Y. F. (2015). Study on the clinical efficacy and safety of Yinxingdamo injection combined with edaravone in treatment of acute cerebral infarction. China Foreign Med. Treat. 34, 158–160. doi: 10.3969/j.issn.1674-0742.2015.28.065
Tan, D., Wu, J. R., Cui, Y. Y., Zhao, Y., Zhang, D., Liu, S., et al. (2018). Ginkgo Leaf Extract and Dipyridamole Injection as Adjuvant Treatment for Angina Pectoris: A Meta-Analysis of 41 Randomized Controlled Trials. Chin. J. Integr. Med. 24, 930–937. doi: 10.1007/s11655-018-2557-6
Tang, H. M., Tang, H. N., Chen, X. (2009). Effective observation of aspirin combined with gingko dipyridamole injection in the treatment of acute cerebral infarction. Chin. J. Misdiagnostics 9, 8844–8845.
Tang, X. J. (2013). Curative effect observation of aspirin combined with gingko dipyridamole for patients with acute cerebral infarction. Hebei Med. J. 35, 1188–1189. doi: 10.3969/j.issn.1002-7386.2013.08.033
Tian, X. J., Hao, J., Ji, S. B., Wang, Y. M., Li, H. H., Xing, H. X., et al. (2010). Clinical analysis of gingko dipyridamole combined with fibrinolytic enzyme in the treatment of early cerebral infarction. Lishizhen Med. Mater. Med. Res. 21, 20183–20184. doi: 10.3969/j.issn.1008-0805.2010.08.112
Tulsulkar, J., Glueck, B., Hinds, T. D., Jr., Shah, Z. A. (2016). Ginkgo biloba Extract Prevents Female Mice from Ischemic Brain Damage and the Mechanism Is Independent of the HO1/Wnt Pathway. Transl. Stroke Res. 7, 120–131. doi: 10.1007/s12975-015-0433-7
Wang, J. S., Xia, S. L., Li, Z. Z., Li, X. C. (2009). Role of Ginkgo bilobate extract combined with nimodipine in the treatment of early cerebral infarction. Chin. J. Ethnomed. Ethnopharm. 18, 101–102. doi: 10.3969/j.issn.1007-8517.2009.15.070
Wang, L., Zhang, T., Bai, K. (2015). [System evaluation on Ginkgo Biloba extract in the treatment of acute cerebral infarction]. J. Cent. South Univ. (Med. Sci.) 40, 1096–1102. doi: 10.11817/j.issn.1672-7347.2015.10.007
Wang, Y. H. (2012). Clinical effect observation of gingko dipyridamole combined with aspirin in the treatment of acute cerebral infarction. China Health Care Nutrit. 22, 2126. doi: 10.3969/j.issn.1004-7484(x).2012.07.455
Wang, F. F. (2014a). Clinical observation of gingko dipyridamole combined with Ozagrel injection in the treatment of advanced cerebral infarction. For All Health 8, 171–172. doi: 10.3969/j.issn.1009-6019.2014.12.227
Wang, Q. (2014b). Clinical efficacy observation of gingko dipyridamole combined with aspirin in the treatment of acute cerebral infarction. China Prac. Med. 9, 134–135.
Wang, Z. G. (2018). Clinical Study of gingko dipyridamole combined with Ozagrel intravenous drop in the treatment of cerebral infarction. Health Guide 31, 72. doi: 10.3969/j.issn.1006-6845.2018.31.065
Wei, N. (2017). Effect of Yinxingdamo injection and edaravone on the hemorheology and curative effect of patients with stroke. Syst. Med. 2, 33–36. doi: 10.19368/j.cnki.2096-1782.2017.06.033
Xue, P., Wang, M., Yan, G. (2018). Mesenchymal stem cell transplantation as an effective treatment strategy for ischemic stroke in Asia: a meta-analysis of controlled trials. Ther. Clin. Risk. Manage. 14, 909–928. doi: 10.2147/TCRM.S161326
Yang, J. (2012). Clinical observation of gingko dipyridamole in the treatment of 56 cases of cerebral infarction. J. Front. Med. 2, 157–158. doi: 10.3969/j.issn.2095-1752.2012.12.148
Yang, L. (2014). Clinical efficacy of gingko dipyridamole combined with aspirin in the treatment of acute cerebral infarction. For All Health 8, 491.
Yi, J. T., Wang, C. P., Jia, M. (2018). Effect of Ginkgo Biloba on fibrinolytic function and platelet parameters in patients with acute cerebral infarction. Chin. J. Thromb. Hemostasis 24, 1004–1006. doi: 10.3969/j.issn.1009-6213.2018.06.035
Yu, B., Yang, P., Xu, X., Shao, L. (2019). C-reactive protein for predicting all-cause mortality in patients with acute ischemic stroke: a meta-analysis. Biosci. Rep. 39. doi: 10.1042/BSR20181135
Zeng, X., Liu, M., Yang, Y., Li, Y., Asplund, K. (2005). Ginkgo biloba for acute ischaemic stroke. Cochrane Database Syst. Rev. 4, CD003691.
Zeng, X., Zhang, Y., Kwong, J. S., Zhang, C., Li, S., Sun, F., et al. (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 8, 2–10. doi: 10.1111/jebm.12141
Zeng, J. W. (2015). Efficacy and safety analysis of gingko dipyridamole adjuvant therapy for the treatment of cerebral infarction. Mod. Diag. Treat. 26, 94–95.
Zhang, Y., Liu, J., Yang, B., Zheng, Y., Yao, M., Sun, M., et al. (2018). Ginkgo biloba Extract Inhibits Astrocytic Lipocalin-2 Expression and Alleviates Neuroinflammatory Injury via the JAK2/STAT3 Pathway After Ischemic Brain Stroke. Front. Pharmacol. 9, 518. doi: 10.3389/fphar.2018.00518
Zhang, S. Y. (2017). Therapeutic effect observation and evaluation of gingko dipyridamole injection combined with Ozagrel on cerebral infarction. Pract. Clin. J. Integr. Trad. Chin. West. Med. 17, 42–43. doi: 10.13638/j.issn.1671-4040.2017.05.025
Zhang, T. (2018). Clinical study of Ginkgo Leaf Extract and Dipyridamole injection in the treatment of young and middle-aged patients with acute cerebral infarction. China Mod. Med. 25, 132–134. doi: 10.3969/j.issn.1674-4721.2018.01.041
Zheng, X. H., Zheng, X. D., Xiao, L., Peng, K. J. (2018). Effect of gingko dipyridamole combined with monosialotetrahexosylganglioside on blood lipid level in patients with cerebral infarction. Jiangxi Med. J. 53, 144–146. doi: 10.3969/j.issn.1006-2238.2018.2.020
Zhou, M., Tong, L. G., Feng, J. (2010). Effect observation of gingko dipyridamole injection on mild cognitive impairment after ischemic stroke. J. Emerg. Trad. Chin. Med. 19, 21–22. doi: 10.3969/j.issn.1004-745x.2010.01.013
Keywords: ginkgo leaf extract and dipyridamole injection, traditional Chinese medicine, conventional treatments, ischemic stroke, meta-analysis
Citation: Xue P, Ma Z and Liu S (2019) Efficacy and Safety of Ginkgo Leaf Extract and Dipyridamole Injection for Ischemic Stroke: A Systematic Review and Meta Analysis. Front. Pharmacol. 10:1403. doi: 10.3389/fphar.2019.01403
Received: 26 March 2019; Accepted: 04 November 2019;
Published: 04 December 2019.
Edited by:
Gerald A. Meininger, University of Missouri, United StatesReviewed by:
Ligia Salgueiro, University of Coimbra, PortugalMichael Heinrich, UCL School of Pharmacy, United Kingdom
Copyright © 2019 Xue, Ma and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuoya Ma, emh1b3lhbWFuZUAxNjMuY29t; Shuguang Liu, c2h1Z3VhbmdsZW1AMTYzLmNvbQ==
 Ping Xue1
Ping Xue1 Shuguang Liu
Shuguang Liu