- 1Neurology Imaging Unit, Imperial College London, London, United Kingdom
- 2Geriatrics Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
According to the World Alzheimer’s report, dementia was estimated to affect 50 million worldwide in 2018, number expected to increase to more than 150 million within 30 years. Alzheimer’s disease is the most common type of dementia, accounting on its own for 2/3 of all dementia cases. The initial signs and symptoms of Alzheimer’s disease relate to progressive cognitive decline, inexorably progressing until the loss of independence. Neuropsychiatric and behavioral symptoms may occur during the progression of the disease; around 20% of patients without any behavioral symptoms at the diagnosis will experience some of them within 2 years. Consequences are early institutionalization, lower quality of life, of both patients and carers, and more severe cognitive impairment. Treatment options for behavioral symptoms include pharmacological and non-pharmacological approaches. The latter are usually preferred, since antipsychotic therapy is not free from several, and often serious, adverse events. However, behavioral symptoms are not always controllable with non-pharmacological intervention. The psychotropic class of medication more frequently prescribed for behavioral symptoms are atypical antipsychotics; among them, risperidone is the only one licensed for the treatment of aggression, in Europe but not in the USA. On that regard, the use of antipsychotic drugs should be limited, due to the increased risk of mortality, stroke, hallucination, and higher risk of relapse after discontinuation. Some new agents are under evaluation, such as pimavanserin and lumateperone. In this review, we are evaluating the current available pharmacological options to treat behavioral symptoms as well as the forthcoming new agents.
Introduction
According to the World Alzheimer’s Report 2018, 50 million people are living with dementia worldwide (A.s.D. International, 2018), and Alzheimer’s disease (AD) accounts on its own for almost 2/3 of all dementia cases. AD is a devastating neurodegenerative disease characterised by progressive cognitive impairment, usually affecting, at the beginning, episodic memory. As the disease progresses, other cognitive domains become affected, leading to the loss of independence. Behavioral and psychological symptoms in dementia (BPSD) are a group of behavior, mood, perception, or thought disturbances manifesting with anxiety, agitation, delusions and hallucination (Abraha et al., 2017). The incidence of perceptual abnormalities in patient with dementia is high; around 20% of subjects with no behavioral symptoms at the diagnosis will develop some of them within 2 years (Abraha et al., 2017). Experiencing BPSD leads to institutionalization, greater cognitive impairment, worse quality of life and carers’ distress, increased mortality (Creese et al., 2018), prolonged in-hospital stay, and more difficult discharge (Davies et al., 2018). The features of the psychiatric symptoms in AD usually differ from the ones in other psychiatric disorders. Compared to schizophrenia, delusions in AD are simpler, mostly characterized by misbelief, distrust about family members, theft, and suspiciousness of abandon (De Deyn et al., 2013). Hallucinations in AD are usually visual (Lanctot et al., 2017), more rarely somatic and olfactory (El Haj et al., 2017). Agitation has been related to structural and functional impairment of emotional circuits, leading, for example, to increased perception of threat (Lanctot et al., 2017).
Unfortunately, the management of BPSD is complicated and challenging; the available licensed treatment is limited, the success of the therapy varies, and the spectrum of possible adverse events limits the choices (Lane et al., 2018). The preferred approach is the non-pharmacological one; however, despite multiple studies, there is not enough homogeneity in sample size, intervention adopted and follow-up period (Abraha et al., 2017). An evidence-based algorithm has recently been designed and proposed by multidisciplinary teams, to better manage behavioral symptoms and lead clinicians on the therapeutic choice (Davies et al., 2018).
In this review we evaluate the use of antipsychotic drugs in AD. The search was conducted in PubMed and Scopus, with keywords and search criteria as detailed in the Supplementary Material.
Antipsychotic Drugs
Antipsychotic drugs can be divided in two groups: typical and atypical, depending on their strength as antagonists to dopamine D2 receptors, higher for the typical ones, and to the 5-hydroxytryptamine-A (5-HT2A) receptors, characteristic of the atypical ones (Ballard and Howard, 2006). Among the typical antipsychotic category, haloperidol remains the most prescribed; several concerns have been raised because of their low safety profile. Sedation, extrapyramidal symptoms (EPS), orthostatic hypotension, and anticholinergic effects were concerning safety issues, mainly due to the strong and long-lasting binding of D2 receptors across the whole brain regions, together with several other receptors (De Deyn et al., 2013). Risperidone, olanzapine, and quetiapine are atypical neuroleptics. The binding to D2 receptors is more targeted to selected brain regions, related to the psychotic symptoms, sparing the ones linked to the motor symptoms; these are due to an antagonist action on 5-HT2A receptors as well, or to a shorter blockage of D2 receptors (De Deyn et al., 2013). The use of antipsychotic drugs to treat agitation tracks back to the 60s (Ballard et al., 2011); since then, due to increasing evidences of safety concerns, their prescription has been widely reduced (Ballard et al., 2011).
Atypical Antipsychotic Drugs
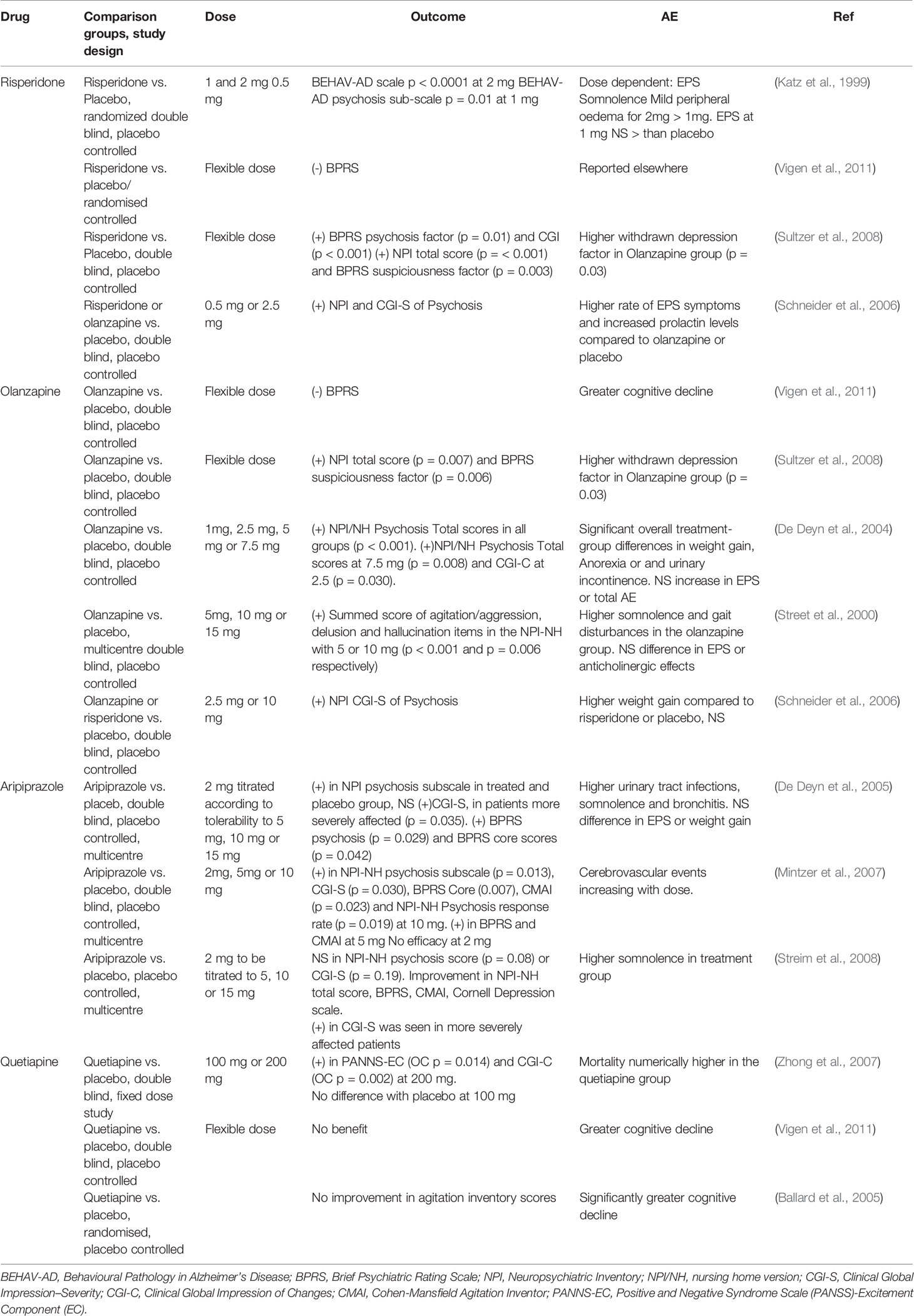
Risperidone is a dopamine, serotonin, and noradrenalin receptor antagonist (Davies et al., 2018), licensed for the treatment of maniac and mixed bipolar disorder episodes and is approved for short-term treatment of behavioral symptom in dementia in UK, but not in the US (Davies et al., 2018). Several clinical trials evaluated the efficacy of risperidone in the treatment for BPSD. A significant improvement in aggression, measured with the BEHAV-AD (Behavioural Pathology in Alzheimer’s Disease) scale, was seen with risperidone at 1 and 2 mg, with better outcome on the higher dose (–1.50 [–2.05, –0.95] p < 0.0001), compared to placebo (Katz et al., 1999; Ballard and Howard, 2006). Some difference was also seen at the dose of 1 mg, compared to placebo, in treating psychosis (–0.14 [–0.25, –0.03] p = 0.01) (Katz et al., 1999; Ballard and Howard, 2006). The largest randomized controlled study (RCT) “Cognitive Effects of A typical Antipsychotic Medications in Patients with Alzheimer’s Disease” (CATIE-AD) gave interesting results. A cohort of 421 patients with AD, followed up in an outpatient setting for psychosis or agitation/aggression, was randomized to receive placebo, risperidone, quetiapine, or olanzapine for 36 weeks, with cognitive assessment at baseline, 12, 24, and 36 weeks (Vigen et al., 2011). The risperidone or olanzapine groups showed greater cognitive decline in the cognitive summary score (sum of 18 cognitive tests), while the olanzapine one had a greater decline in the mini mental state examination (MMSE); the BPRS cognitive factor was worse in quetiapine group, compared to the placebo group (Vigen et al., 2011). The phase 1 outcomes of CATIE-AD showed that, compared to placebo, the group receiving risperidone had greater improvement in the Brief Psychiatric Rating Scale (BPRS) psychosis factor and in the Clinical Global Impression of Changes (CGI-C) (Sultzer et al., 2008). The groups receiving either risperidone or olanzapine had greater improvement in the Neuropsychiatric Inventory scale (NPI) and BPRS hostile suspiciousness factor (Sultzer et al., 2008). However, the final results of the study did not show any significant advantages in the improvement in the CGI-C scale; adverse event and discontinuation due to poor tolerability favoured placebo (Schneider et al., 2006). The multicentre Antipsychotic Discontinuation in Alzheimer’s disease (ADAD) trial evaluated a cohort of 180 patients with AD and agitation or psychosis. The whole cohort received risperidone for 16 weeks, and then was randomized to proceed with risperidone for 32 weeks, receive risperidone for 16 weeks and placebo for further 16 weeks, or receive placebo for 32 weeks (Patel et al., 2017). The discontinuation of the drug led to increased risk of relapse during the randomization window, particularly for severe hallucinations at baseline (Patel et al., 2017). The dementia antipsychotic withdrawal trial (DART-AD) randomized patients with AD in care facilities to continue the current medication (thioridazine, chlorpromazine, haloperidol, trifluoperazine, or risperidone) or switch to placebo for 12 months (Ballard et al., 2009), with mortality rate as a primary outcome. Among the whole cohort, the mortality was higher in the subject continuing the medication, compared to the placebo branch (Ballard et al., 2009).
Olanzapine is an atypical antipsychotic, licensed for the treatment of schizophrenia and bipolar disorder in adults. Studies have been conducted to evaluate the efficacy in controlling behavioral symptoms in dementia. De Deyn et al. conducted a large study with > 600 AD patients with delusions or hallucinations, randomized to receive either placebo or a fixed dose of olanzapine (1.0, 2.5, 5.0, or 7.5 mg/day) for 10 weeks. An improvement in the sum of delusion and hallucination scores of the NPI was seen in the group treated, from the 2.5 mg dose, with higher efficacy for the 7.5 mg dose (De Deyn et al., 2004). Another study compared the efficacy and tolerability of a flexible dose of olanzapine and risperidone vs. placebo in the NPI and Clinical Global Impression–Severity (CGI-S) of Psychosis scale. Improvement has been noticed in all the three groups, with a higher rate of adverse events and withdrawal in the risperidone and olanzapine group (Deberdt et al., 2005). In a multicentre, double-blinded and placebo-controlled trial, 206 older patients AD patients in nursing home, showing psychotic or behavioral symptoms, were randomized to receive olanzapine (5, 10, or 15 mg/die) or placebo. Significant improvement in the summed scores of agitation/aggression, delusion and hallucination items in the NPI-nursing home version (NPI-NH) was seen in patient treated with lower doses of olanzapine, while the higher dose did not differ from the placebo. Six weeks treatment with either placebo or three different doses of olanzapine favoured the use of lower doses in controlling behavioral symptoms, compared to placebo or 15mg/day. The treatment was well tolerated. Apart from somnolence and gait disturbances, commoner in the treatment group, no other potential side effects were seen compared to placebo (Street et al., 2000).
Aripiprazole is an atypical antipsychotic drug licensed for the treatment of schizophrenia in adults and adolescents, mania in bipolar disease in children, adolescents and adults, autism, major depression in adults. Due to the safer profile, a broader use has been done, including psychosis in older patients with dementia (De Deyn et al., 2013). A 10 weeks double blind, placebo-controlled and multicentre study randomized 208 AD patients with delusions or hallucination, to receive aripiprazole 2 mg/day, possibly titrated to higher dosages, or placebo. Significant improvement was seen in the CGI-S of illness, and in the mean CGI-improvements, in patients more severely affected. Significant improvement has also been demonstrated in the BPRS psychosis and BPRS core scores, but the overall conclusion was that the improvement was only modest over placebo (De Deyn et al., 2005). A double-blind, multicentre and placebo-controlled study assigned institutionalized AD patients with psychotic symptoms to aripiprazole (2, 5 or 10 mg/day) or placebo, for 10 weeks. Significant improvement in the NPI-NH psychosis, delusion, agitation/aggression, anxiety, and irritability subscales was seen in the 10 mg treatment group. Some improvement was also seen in the Cohen-Mansfield Agitation Inventory (CMAI) scores in the 5 and 10 mg group, and the mean CGI-S was better in the 10 mg group (Mintzer et al., 2007). The higher efficacy of 10 mg of aripiprazole in controlling behavioral symptoms has been confirmed in another multicentre, placebo-controlled trial on institutionalized AD patients (Streim et al., 2008).
Quetiapine is licensed for the treatment of schizophrenia and bipolar disorder in adults, prescribed off-label to treat post-traumatic stress disorder, anxiety, insomnia, and behavioral symptoms in dementia. Two different doses of quetiapine (100 or 200 mg/daily) vs. placebo tested in a cohort of AD patients showed greater improvement, at a dose of 200 mg, in the Positive and Negative Syndrome Scale (PANSS)-Excitement Component (EC) score, as well as in the CGI-C (observation analysed as Last Observation Carried Forward (LOCF): p = 0.014 and Observed cases (OC): p = 0.002). No advantages over placebo at lower dose (Zhong et al., 2007). Quetiapine did not show any benefit compared to placebo or other antipsychotic drugs in the CATIE-AD study (Vigen et al., 2011). Another RCT compared quetiapine, rivastigmine, and placebo over 26 weeks in institutionalized AD patients; the endpoint was the improvement in agitation, measured with the CMAI scale, and cognition (Ballard et al., 2005). Neither rivastigmine nor quetiapine showed significant benefit compared to placebo, and quetiapine was associated with worse cognitive decline (Ballard et al., 2005). Table 1 summarizes the above-mentioned studies. Overall, a meta-analysis by Ma et al. pooling RCTs with different antipsychotics, demonstrated their higher efficacy as assessed with NPI, CGI-C, CGI-S, BPRS, and CMAI, compared to placebo (Ma et al., 2014).
Antipsychotics and Safety
Neuroleptic drugs have been related to several adverse event and safety issues. The increasing concerns about adverse events and tolerability led the FDA to include a warning, in the form of a “black box” in the atypical and typical antipsychotic labels, to limit the prescription (Dorsey et al., 2010). The warning has also been issued by the European Medicines Agency (EMA); the committee for Medicinal Products for Human Use recommended including a warning of increased risk of death for all conventional antipsychotics in the older patients with dementia. Among the most frequent and widely reported side effect of neuroleptic, drowsiness, tardive dyskinesia and parkinsonism have been reported (Ballard and Howard, 2006). Moreover, increased risk of venous thromboembolic events (VTE) has been demonstrated in patients treated with antipsychotics, both in general and older population (Jonsson et al., 2018). In a meta-analysis in 2006, Ballard et al. reported several side effects of neuroleptics; haloperidol, among the first generation ones, was associated with EPS and drowsiness (Ballard and Howard, 2006). A systematic review aiming to compare the rates of EPS with second generation antipsychotic drugs with the first-generation ones, confirmed a reduced incidence with the former, across different pathologies and age groups (Correll et al., 2004). Among atypical neuroleptics, risperidone showed increased EPS at the dose of 1 mg and 2 mg; dose-dependent increase in somnolence and peripheral oedema was also seen in institutionalized AD patients (Katz et al., 1999). The higher incidence of EPS, somnolence, urinary tract infection (UTI), has been confirmed in two more recent studies, among nursing home residents with AD, vascular dementia or mixed (Mintzer et al., 2006). Gait disturbances and somnolence were also more common in patients treated with olanzapine compared to placebo (Street et al., 2000). Moreover, the impact on cardiovascular system is also a concern. In particular, elongation of the QT interval, torsade de pointes (TdP), and sudden cardiac death have a known relationship with antipsychotic drugs (Sicouri and Antzelevitch, 2018). A case control study on a population aged 65 years or above, undertaking antipsychotics at possible or conditional risk of TdP, demonstrated higher TdP risk for drugs classified as “known TdP risk, ” with risk ranking of haloperidol > risperidone > olanzapine > quetiapine (Danielsson et al., 2016). The risk of VTE is estimated to be double, compared to the general population, especially within the first three months of therapy, and several mechanisms are potentially responsible for this association. The reduced physical activity of older patients seems to play a role and, risk increases when associating more than one neuroleptic (Oglodek et al., 2018). Patients receiving typical antipsychotics have been reported to have elevated levels of antiphospholipid antibodies (including anticoagulants and anticardiolipin antibodies), which are associated with an increased VTE risk. Moreover, atypical antipsychotics may cause metabolic syndrome, which is per se a VTE risk factor (Oglodek et al., 2018).
Increased incidence of cerebrovascular disease has been demonstrated in randomized controlled studies in older patients (Herrmann and Lanctot, 2005), although the matter is debated. A recent meta-analysis demonstrated a more than doubled risk of stroke in general population using antipsychotics, which although appears to be lower in population with dementia. No association was seen with myocardial infarction, although the heterogeneity of the available studies limits the strength of the conclusion (Zivkovic et al., 2019). The meta-analysis by Ma et al., together with the efficacy of the antipsychotic therapy, showed higher risk for adverse events, such EPS (OR 1.74), cerebrovascular events (OR 2.5), somnolence (OR 2.95), gait disturbances (OR 3.35), oedema (OR 1.8), UTI (OR 1.35), and death (OR 1.52) (Ma et al., 2014). A large meta-analysis was published in 2018, including more than 380,000 patients with dementia, including more than 80,000 using antipsychotics, and 359,235 patients without dementia (Ralph and Espinet, 2018). The results showed the HR (hazard ratio) for all-cause mortality in patients treated with antipsychotic drugs 1.9–2.19, with higher risk within the first 180 days, and dose related. The risk was similar in subjects with and without dementia (Ralph and Espinet, 2018).
Treating patients with dementia often means treating older patients, presenting with polypathology, polypharmacy, and different sensitivity to the effect of psychotropic drugs. For this reason, potential drug drug-drug interaction needs to be considered (Pasqualetti et al., 2015). Taken together, the need of new therapeutic options, with a safer profile, is urgently needed, to better address such a delicate problem like the management of older patients and often frail patients.
Different Approaches and New Options
Different pharmacological approaches for the treatment of behavioral symptoms have been evaluated.
Acetylcholinesterase inhibitors (AChEI) demonstrated a mild effect on BPSD, particularly on agitation, delusion, aggression, or hallucination (Masopust et al., 2018); more benefits have been seen in Lewy Body dementia-related hallucinations (Creese et al., 2018). A recent meta-analysis demonstrated the efficacy of memantine in controlling “positive” symptoms such as aggression, agitation, delusions, disinhibition, compared to control; it was also effective on hallucinations (Kishi et al., 2017). The combined use of AChEI+memantine has been evaluated in a meta-analysis by Matsunaga et al; the results showed a better outcome of the combined therapy compared to AChEI alone in both behavioral and functional scores, with also a positive trend in cognitive performance (Matsunaga et al., 2014). A further meta-analysis confirmed the better outcome in terms of both cognitive and behavioral symptoms of the combined therapy (Chen et al., 2017).
Among selective serotonin uptake inhibitors, sertraline showed some efficacy in a cohort of patients with mild to moderate agitation; citalopram gave good results on agitation in the face, however, of possible QTc prolongation. (Davies et al., 2018). When compared to atypical antipsychotic, such as perphenazine, risperidone, or quetiapine, improvement in agitation has been seen, with less adverse event for the citalopram-treated group (Ahmed et al., 2019). Trazodone has been tested as well; reduction in agitation could be a consequence of sedation due to its histaminergic effect (Davies et al., 2018). Anticonvulsants, such as carbamazepine and gabapentin, showed some potential effects, however in case reports or small and not RCT trials (Creese et al., 2018; Davies et al., 2018).
An algorithm for the therapeutic approach to agitation and aggression in patients with AD or mixed dementia, revised by a multidisciplinary team, was recently proposed (Davies et al., 2018). The algorithm hypothesizes a sequential use of medication, basing on evidences, efficacy, safety and tolerability. Risperidone is the first step of treatment (Davies et al., 2018), followed by quetiapine or aripiprazole; titration and switching-drug time-point are also suggested. (Davies et al., 2018).
Atypical antipsychotic drugs, such as primavanserin, lumateperone, and brexpiprazole, are currently under evaluation. Primavanserin, approved for the treatment of hallucinations and delusions in Parkinson’s disease, works reducing the baseline activity of 5HT2A receptors, which are upregulated in psychotic symptoms, mediating the effects of 5HT2C without antagonizing D2 receptors. A phase II, double blind, placebo-controlled study in UK showed significant improvement in the NPI-NH psychosis score in the treatment group compared to placebo, at 6 weeks of treatment (Ahmed et al., 2019). However, concerns were raised about the reliability of the results, due to the small effect, only observed at 6 weeks, and the potential safety issues (Schneider, 2018). Nonetheless, another double blind, placebo-controlled study is undergoing to evaluate its efficacy in preventing relapse of psychotic symptoms (Ahmed et al., 2019). A phase III double blind, placebo-controlled study in AD patients with clinically significant agitation, is currently undergoing with lumateperone, a first-in-class agent in development for schizophrenia that acts synergistically through serotonergic, dopaminergic and glutamatergic systems (Porsteinsson and Antonsdottir, 2017). Brexpiprazole, a novel serotonin-dopamine receptor modulator with partial agonist activity at serotonin1A and dopamine2/3 receptors, has been approved for the treatment of schizophrenia and major depressive disorders (Porsteinsson and Antonsdottir, 2017). Two Phase III, randomized, double blind, placebo-controlled studies were conducted in AD patients with agitation; only one showed improvement in the CMAI scores. However, a further Phase III study is ongoing (Ahmed et al., 2019).
Conclusions
AD is a growing healthcare, social, and economic problem. The increased life expectancy with the consequent progressive population ageing lead to higher incidence and prevalence of age-related, chronic diseases, mirrored by the complexity of older patients. Despite the improved diagnostic approach to AD, no disease-modifying medications exist. Behavioral symptoms represent part of the complexity of dementia, and are related to worse cognitive outcome; their treatment is particularly challenging, especially when facing complex and frail patients. Nonetheless, non-pharmacological treatments have shown promising effects in reducing behavioral and psychological symptoms of dementia. Among the available treatments licensed for psychiatric diseases, only risperidone is approved in Europe for behavioral disorders in dementia; other antipsychotics are prescribed “off label.” However, some trials are currently undergoing with new potential medications with safer profile.
Author Contributions
VC, RA, and CO equally contributed to scientific literature research and analysis. VC wrote the manuscript. FM designed the study, analyzed the scientific literature, and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.01465/full#supplementary-material
References
Abraha, I., Rimland, J. M., Trotta, F. M., Dell’Aquila, G., Cruz-Jentoft, A., Petrovic, M., et al. (2017). Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open 7e012759. doi: 10.1136/bmjopen-2016-012759
Ahmed, M., Malik, M., Teselink, J., Lanctot, K. L., Herrmann, N. (2019). Current agents in development for treating behavioral and psychological symptoms associated with dementia. Drugs Aging. 36 (7), 589–605. doi: 10.1007/s40266-019-00668-7
Ballard, C., Howard, R. (2006). Neuroleptic drugs in dementia: benefits and harm. Nat. Rev. Neurosci. 7, 492–500. doi: 10.1038/nrn1926
Ballard, C., Margallo-Lana, M., Juszczak, E., Douglas, S., Swann, A., Thomas, A., et al. (2005). Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. Bmj-Brit Med. J. 330, 874–877. doi: 10.1136/bmj.38369.459988.8F
Ballard, C., Hanney, M. L., Theodoulou, M., Douglas, S., McShane, R., Kossakowski, K., et al. (2009). The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 8, 151–157. doi: 10.1016/S1474-4422(08)70295-3
Ballard, C., Creese, B., Corbett, A., Aarsland, D. (2011). Atypical antipsychotics for the treatment of behavioral and psychological symptoms in dementia, with a particular focus on longer term outcomes and mortality. Expert Opin. Drug Saf. 10, 35–43. doi: 10.1517/14740338.2010.506711
Chen, R., Chan, P. T., Chu, H., Lin, Y. C., Chang, P. C., Chen, C. Y., et al. (2017). Treatment effects between monotherapy of donepezil versus combination with memantine for Alzheimer disease: a metaanalysis. PloS One 12. doi: 10.1371/journal.pone.0183586
Correll, C. U., Leucht, S., Kane, J. M. (2004). Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am. J. Psychiatry 161, 414–425. doi: 10.1176/appi.ajp.161.3.414
Creese, B., Da Silva, M. V., Johar, I., Ballard, C. (2018). The modern role of antipsychotics for the treatment of agitation and psychosis in Alzheimer’s disease. Expert Rev. Neurother. 18, 461–467. doi: 10.1080/14737175.2018.1476140
Danielsson, B., Collin, J., Jonasdottir Bergman, G., Borg, N., Salmi, P., Fastbom, J. (2016). Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults - a Swedish nationwide study. Br. J. Clin. Pharmacol. 81, 773–783. doi: 10.1111/bcp.12829
Davies, S. J., Burhan, A. M., Kim, D., Gerretsen, P., Graff-Guerrero, A., Woo, V. L., et al. (2018). Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J. Psychopharmacol. 32, 509–523. doi: 10.1177/0269881117744996
De Deyn, P. P., Carrasco, M. M., Deberdt, W., Jeandel, C., Hay, D. P., Feldman, P. D., et al. (2004). Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. Int. J. Geriatr. Psych. 19, 115–126. doi: 10.1002/gps.1032
De Deyn, P., Jeste, D. V., Swanink, R., Kostic, D., Breder, C., Carson, W. H., et al. (2005). Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. J. Clin. Psychopharmacol. 25, 463–467. doi: 10.1097/01.jcp.0000178415.22309.8f
De Deyn, P. P., Drenth, A. F., Kremer, B. P., Oude Voshaar, R. C., Van Dam, D. (2013). Aripiprazole in the treatment of Alzheimer’s disease. Expert Opin. Pharmacother. 14, 459–474. doi: 10.1517/14656566.2013.764989
Deberdt, W. G., Dysken, M. W., Rappaport, S. A., Feldman, P. D., Young, C. A., Hay, D. P., et al. (2005). Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am. J. Geriat. Psychiat. 13, 722–730. doi: 10.1097/00019442-200508000-00012
Dorsey, E. R., Rabbani, A., Gallagher, S. A., Conti, R. M., Alexander, G. C. (2010). Impact of FDA black box advisory on antipsychotic medication use. Arch. Intern. Med. 170, 96–103. doi: 10.1001/archinternmed.2009.456
El Haj, M., Roche, J., Jardri, R., Kapogiannis, D., Gallouj, K., Antoine, P. (2017). Clinical and neurocognitive aspects of hallucinations in Alzheimer’s disease. Neurosci. Biobehav. R. 83, 713–720. doi: 10.1016/j.neubiorev.2017.02.021
Herrmann, N., Lanctot, K. L. (2005). Do atypical antipsychotics cause stroke? CNS Drugs 19, 91–103. doi: 10.2165/00023210-200519020-00001
Jonsson, A. K., Schill, J., Olsson, H., Spigset, O., Hagg, S. (2018). Venous thromboembolism during treatment with Antipsychotics: a review of current evidence. CNS Drugs 32, 47–64. doi: 10.1007/s40263-018-0495-7
Katz, I. R., Jeste, D. V., Mintzer, J. E., Clyde, C., Napolitano, J., Brecher, M. (1999). Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J. Clin. Psychiatry 60, 107–115. doi: 10.4088/JCP.v60n0207
Kishi, T., Matsunaga, S., Iwata, N. (2017). The effects of memantine on behavioral disturbances in patients with Alzheimer’s disease: a meta-analysis. Neuropsych. Dis. Treat 13, 1909–1928. doi: 10.2147/NDT.S142839
Lanctot, K. L., Amatniek, J., Ancoli-Israel, S., Arnold, S. E., Ballard, C., Cohen-Mansfield, J., et al. (2017). Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimers Dement. (N. Y.) 3, 440–449. doi: 10.1016/j.trci.2017.07.001
Lane, C. A., Hardy, J., Schott, J. M. (2018). Alzheimer’s disease. Eur. J. Neurol. 25, 59–70. doi: 10.1111/ene.13439
Ma, H., Huang, Y. L., Cong, Z. T., Wang, Y., Jiang, W. H., Gao, S. H., et al. (2014). The efficacy and safety of atypical antipsychotics for the treatment of Dementia: A meta-analysis of randomized placebo-controlled trials. J. Alzheimers Dis. 42, 915–937. doi: 10.3233/JAD-140579
Masopust, J., Protopopova, D., Valis, M., Pavelek, Z., Klimova, B. (2018). Treatment of behavioral and psychological symptoms of dementias with psychopharmaceuticals: a review. Neuropsychiatr. Dis. Treat 14, 1211–1220. doi: 10.2147/NDT.S163842
Matsunaga, S., Kishi, T., Iwata, N. (2014). Combination therapy with cholinesterase inhibitors and memantine for Alzheimer’s disease: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 18. doi: 10.1016/j.jalz.2014.05.1710
Mintzer, J., Greenspan, A., Caers, I., Van Hove, I., Kushner, S., Weiner, M., et al. (2006). Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. Am. J. Geriatr. Psychiatry 14, 280–291. doi: 10.1097/01.JGP.0000194643.63245.8c
Mintzer, J. E., Tune, L. E., Breder, C. D., Swanink, R., Marcus, R. N., McQuade, R. D., et al. (2007). Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am. J. Geriatr. Psychiatry 15, 918–931. doi: 10.1097/JGP.0b013e3181557b47
Oglodek, E. A., Just, M. J., Grzesinska, A. D., Araszkiewicz, A., Szromek, A. R. (2018). The impact of antipsychotics as a risk factor for thromboembolism. Pharmacol. Rep. 70, 533–539. doi: 10.1016/j.pharep.2017.12.003
Pasqualetti, G., Tognini, S., Calsolaro, V., Polini, A., Monzani, F. (2015). Potential drug-drug interactions in Alzheimer patients with behavioral symptoms. Clin. Interv. Aging 10, 1457–1466. doi: 10.2147/CIA.S87466
Patel, A. N., Lee, S., Andrews, H. F., Pelton, G. H., Schultz, S. K., Sultzer, D. L., et al. (2017). Prediction of relapse after discontinuation of antipsychotic treatment in alzheimer’s disease: the role of hallucinations. Am. J. Psychiat. 174, 362–369. doi: 10.1176/appi.ajp.2016.16020226
Porsteinsson, A. P., Antonsdottir, I. M. (2017). An update on the advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin. Pharmacother. 18, 611–620. doi: 10.1080/14656566.2017.1307340
Ralph, S. J., Espinet, A. J. (2018). Increased all-cause mortality by antipsychotic drugs: updated review and meta-analysis in dementia and general mental health care. J. Alzheimers Dis. Rep. 2, 1–26. doi: 10.3233/ADR-170042
Schneider, L. S., Tariot, P. N., Dagerman, K. S., Davis, S. M., Hsiao, J. K., Ismail, M. S., et al. (2006). Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N. Engl. J. Med. 355, 1525–1538. doi: 10.1056/NEJMoa061240
Schneider, L. S. (2018). Pimavanserin for patients with Alzheimer’s disease psychosis. Lancet Neurol. 17, 194–195. doi: 10.1016/S1474-4422(18)30052-8
Sicouri, S., Antzelevitch, C. (2018). Mechanisms underlying the actions of antidepressant and antipsychotic drugs that cause sudden Cardiac Arrest. Arrhyth. Electrophysi. 7, 199–209. doi: 10.15420/aer.2018.29.2
Street, J. S., Clark, W. S., Gannon, K. S., Cummings, J. L., Bymaster, F. P., Tamura, R. N., et al. (2000). Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch. Gen. Psychiatry 57, 968–976. doi: 10.1001/archpsyc.57.10.968
Streim, J. E., Porsteinsson, A. P., Breder, C. D., Swanink, R., Marcus, R., McQuade, R., et al. (2008). A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. Am. J. Geriatr. Psychiatry 16, 537–550. doi: 10.1097/JGP.0b013e318165db77
Sultzer, D. L., Davis, S. M., Tariot, P. N., Dagerman, K. S., Lebowitz, B. D., Lyketsos, C. G., et al. (2008). Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am. J. Psychiatry 165, 844–854. doi: 10.1176/appi.ajp.2008.07111779
Vigen, C. L. P., Mack, W. J., Keefe, R. S. E., Sano, M., Sultzer, D. L., Stroup, T. S., et al. (2011). cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s Disease: outcomes From CATIE-AD. Am. J. Psychiat. 168, 831–839. doi: 10.1176/appi.ajp.2011.08121844
Zhong, K. X., Tariot, P. N., Mintzer, J., Minkwitz, M. C., Devine, N. A. (2007). Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Curr. Alzheimer Res. 4, 81–93. doi: 10.2174/156720507779939805
Keywords: Alzheimer’s disease, antipsychotic drugs, D2 receptors, 5-HT2A receptors, agitation, hallucinations
Citation: Calsolaro V, Antognoli R, Okoye C and Monzani F (2019) The Use of Antipsychotic Drugs for Treating Behavioral Symptoms in Alzheimer’s Disease. Front. Pharmacol. 10:1465. doi: 10.3389/fphar.2019.01465
Received: 22 June 2019; Accepted: 13 November 2019;
Published: 06 December 2019.
Edited by:
Bjorn Johansson, Karolinska Institutet (KI), SwedenReviewed by:
Fabricio Ferreira de Oliveira, Elysian Clinic, BrazilRyota Kobayashi, Yamagata University, Japan
Christiane Gasse, Aarhus University Hospital, Denmark
Copyright © 2019 Calsolaro, Antognoli, Okoye and Monzani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Monzani, ZmFiaW8ubW9uemFuaUBtZWQudW5pcGkuaXQ=
 Valeria Calsolaro
Valeria Calsolaro Rachele Antognoli
Rachele Antognoli Chukwuma Okoye
Chukwuma Okoye Fabio Monzani
Fabio Monzani