- 1Department of Cancer Biology/Pathology, Wayne State University, Detroit, MI, United States
- 2Department of Internal Medicine, Conemaugh Memorial Medical Center, Johnstown, PA, United States
- 3Department of Hematology/Oncology, University of Iowa, Iowa City, IA, United States
Pancreatic cancer is an aggressive malignancy with poor survival. Research has indicated the association of few genetic aberrations with pancreatic cancer. The data regarding the prevalence of microsatellite instability in pancreatic cancer is diverse and controversial. However, it could be an actionable target in pancreatic cancer especially due to availability of immune checkpoint inhibitors which has demonstrated promising results in different types of cancers. We present a case of pancreatic cancer whose microsatellite instability status was identified on liquid biopsy (circulating tumor DNA testing). Our patient showed a dramatic ongoing durable response to immunotherapy. We were able to do serial monitoring with liquid biopsy that showed clinical utility and validity.
Background
Pancreatic cancer is a challenging disease with unfavorable outcomes. Pancreatic ductal adenocarcinoma (PDAC) constitutes around 90% of all malignant pancreatic cancers (Hackeng et al., 2016). As opposed to other common cancers, there has been a rise in incidence and mortality rates of PDAC (Ryerson et al., 2016). According to the American Cancer Society, it is the fourth leading cause of cancer-related mortality in both males and females with an estimated number of new cases and deaths in the United States in 2019 as 56,770 and 45,750, respectively (Siegel et al., 2019). PDAC is associated with poor prognosis, having a 5-year survival rate of 8%, owing to its presentation as an advanced disease, being resistant to different drug regimens and a distinct tumor microenvironment with condensed desmoplasia (Mahadevan and Von Hoff, 2007; Yachida and Iacobuzio-Donahue, 2009; Erkan et al., 2010; Erkan et al., 2012; Liu et al., 2017). Chemotherapy, both in adjuvant and neoadjuvant settings, has remained the treatment of choice for most patients with PDAC. However, it has not been helpful in a significant improvement of survival of these patients. This leaves surgery as the only curative option but fewer than 20% of patients present with a resectable disease at diagnosis (DeWitt et al., 2004). The unavailability of a useful diagnostic and prognostic biomarker has always been a concern for PDAC (Herreros-Villanueva and Bujanda, 2016).
PDAC could be associated with several targets including KRAS, TP53, TGF-β, WNT, NOTCH, SMAD4, CDKN2A, ARID1A, MLL3, and TGFBR2 (Bailey et al., 2016; Chou et al., 2018). Important are aberrations in BRCA and DNA repair for whom PARP-inhibitors are now an option. Small proportion (1%–2%) of PDAC is also associated with microsatellite instability (MSI) (Barrett et al., 2017; Humphris et al., 2017; Hu et al., 2018). MSI results from mismatch repair deficiency (dMMR) and consists of repetitive 1–6 base pairs of DNA (Umar and Kunkel, 1996; Humphris et al., 2017). dMMR or loss of functional ability of any of the mismatch repair proteins (MLH1, MSH2, MSH6, and PMS2) hinders the effective DNA replication process. Research has indicated that cancers with dMMR and MSI-H respond very well to immune checkpoint inhibitors. This implies that targeting the immune checkpoints including programmed cell death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4) encourages T cells to fight cancer cells. This has been well-elaborated for melanoma and lung cancer, but data is lacking for PDAC.
Herein, we report a case of PDAC whose MSI-H status was identified on liquid biopsy which is also known as circulating tumor DNA (ctDNA) testing. Our patient showed a dramatic response to immunotherapy which was again assessed on ctDNA assays.
Case Presentation
Patient is an 81-year old female who had unrelenting pain in her stomach in January 2018. She had an ultrasound done by her gastroenterologist that showed a mass in the pancreatic head versus body. A follow-up CT scan confirmed the ultrasound findings and also showed one possible enlarged lymph node. This was followed up by MRI at Mayo Clinic, Florida in March 2018, which showed locally advanced pancreatic mass centered at the junction of the body and tail measuring 5.1 × 6.4 × 5.2 cm. The results also showed encasement of the first jejunal arterial and venous branches with adjacent mass effect causing narrowing of the superior mesenteric vein confluence. Enlarged right common iliac artery chain lymph node measuring 1.4 × 1.1 cm and indeterminate lesions in the posterior aspect of the vertebral body at T9 were noted.
A baseline ctDNA test was obtained, which is at present part of our standard of care at Mayo Clinic, Florida for patients with GI malignancies. Testing is performed through commercially available platforms. In this particular patient, this was done through Guardant360 that showed SMAD4 R361H, TP53R213L, KRAS G12D, RET A640A, KIT K412K, NTRK3 R630G, ARID1A G1711fs and the highest variant allele fraction was noted to be 2.2% as shown in Table 1. She was started on neoadjuvant chemotherapy with gemcitabine and paclitaxel since surgery was not an option, but there was not much response in her repeat scans in May 2018, though there was a decrease in the highest variant allele fraction to 1.2%. She was then switched to 5-fluorouracil with nanoliposomal irinotecan (5-FU was held due to DPD heterozygosity on pharmacogenomics testing). Repeat CT scan in July 2018 showed decrease in the size of a mass in the pancreas which measures 3.4 × 3.1 cm compared with 6.5 × 5.1 cm. Moreover, highest variant allele fraction dropped down to 0.8% on ctDNA testing in July 2018. Chemoradiation with capecitabine was added to her treatment plan in August 2018.
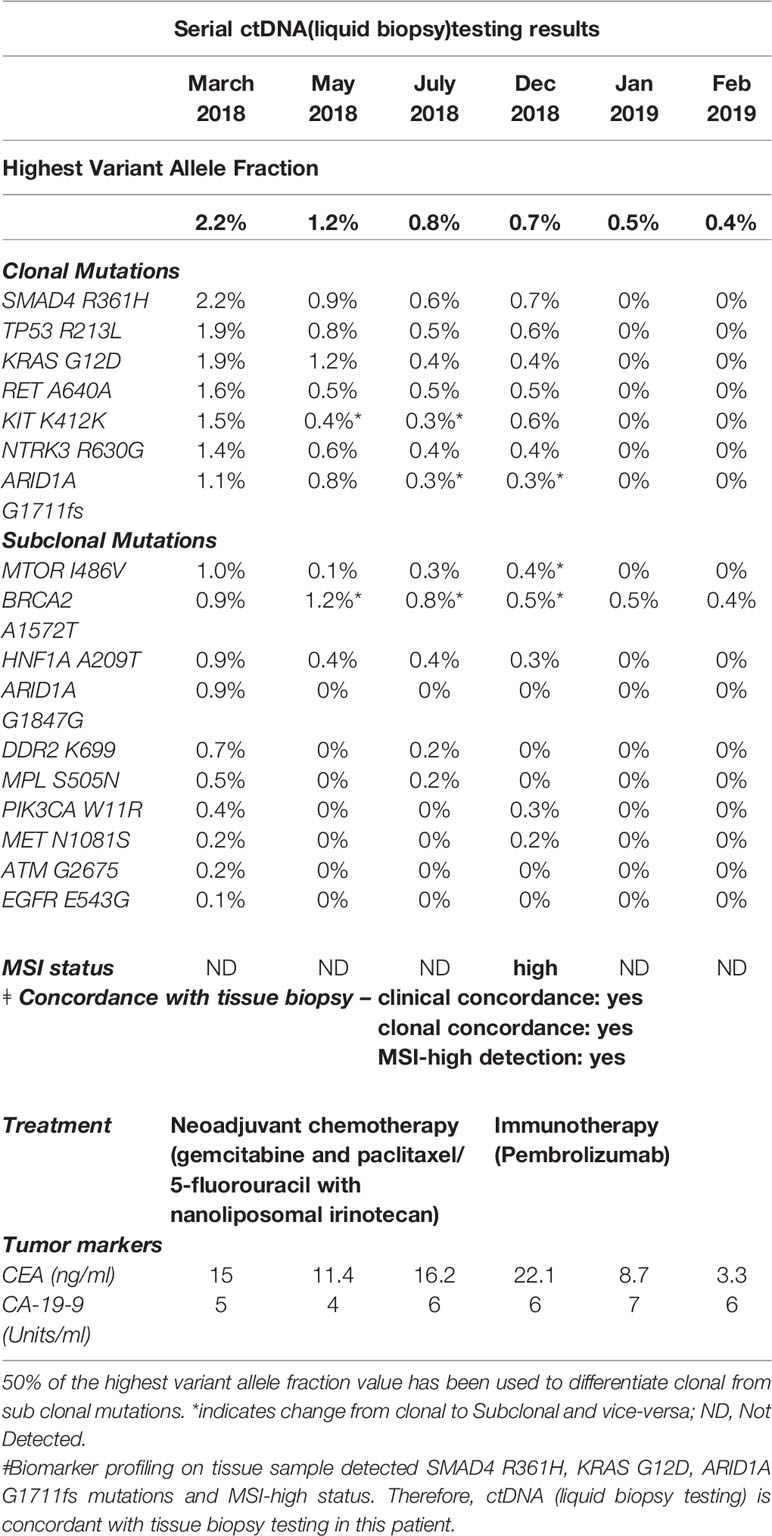
Table 1 Serial circulating tumor DNA evaluation in our patient with MSI-H/dMMR pancreatic ductal adenocarcinoma and excellent response to pembrolizumab. As noted, the circulating tumor DNA in the SMAD4 R361H mutation has gone down from 2.2% to 0%.
In December 2018, CT scan of the chest showed a new right lower lung lobe nodule suspicious for metastatic disease along with a persistent mass in the body of the pancreas. In the repeat ctDNA testing in December 2018, she was noted to be MSI-High/mismatch repair deficient (dMMR). Furthermore, mismatch repair immunohistochemistry on the tissue sample showed loss of MLH1 and PSM2 proteins. Therefore, she was considered a great candidate for immunotherapy pembrolizumab on-label and was started on it. Dramatic improvement was noticed within 4 weeks of treatment with pembrolizumab and repeat ctDNA testing in January 2019 showed loss of all the above noted mutations. Furthermore, the highest variant allele fraction dropped down to 0.5% and patient continues to be on this therapy as depicted in Figure 1.
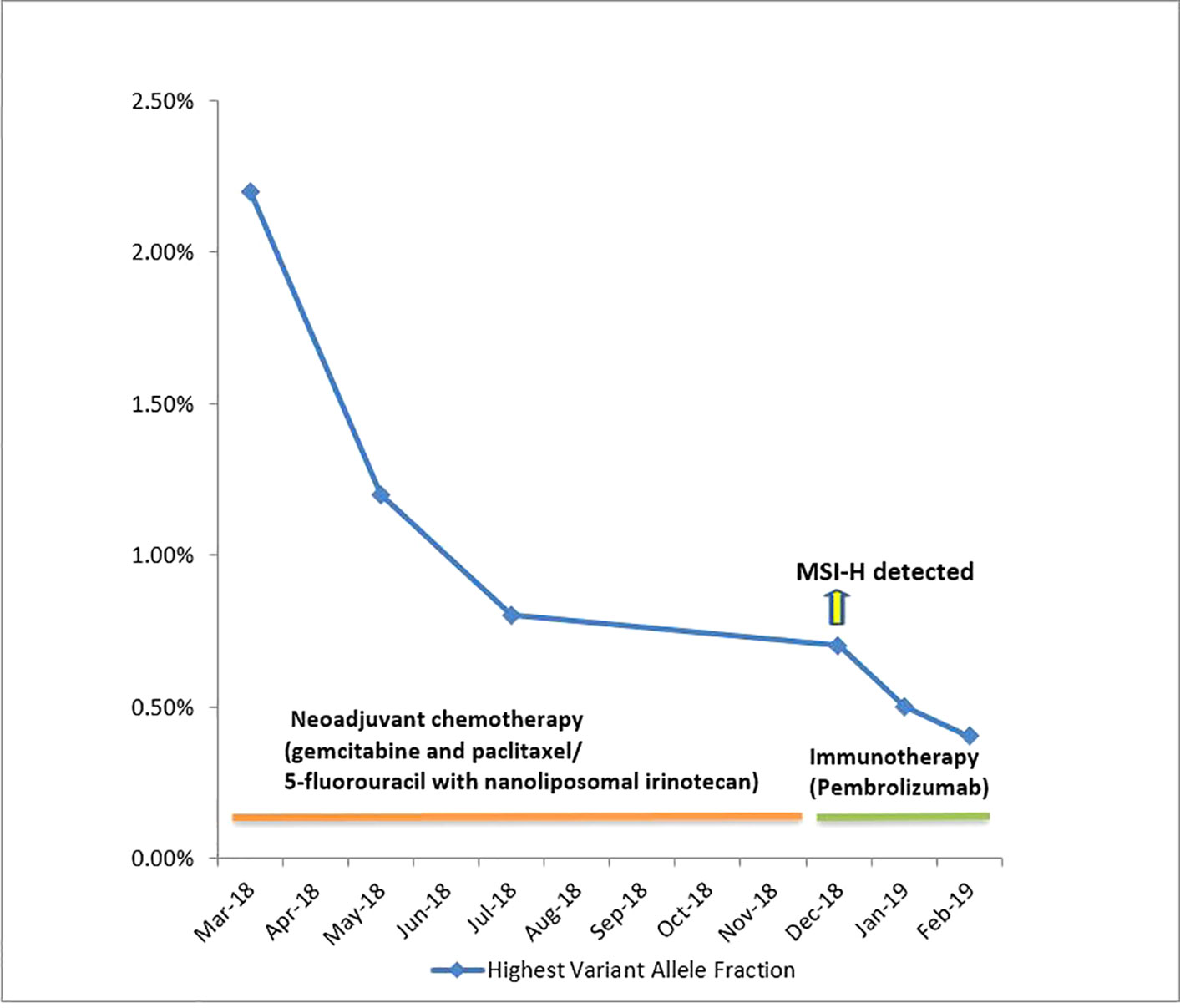
Figure 1 Serial liquid biopsies and changes in Highest Variant Allele Fractions of ctDNA over the course of therapy. MSI-H detected in ctDNA testing done in December 2018. MSI-H was not detected in ctDNA reports of January 2019 and February 2019.
Discussion
The management of PDAC is a complex task. To begin with, tissue biopsy has been the diagnostic test of choice historically which is a cumbersome technique. Moreover, it is troublesome for the patients to have repeated serial tissue biopsies in order to monitor the response to therapy. In the past decade, liquid biopsy has emerged as a noninvasive and patient-convenient technique that has demonstrated the clinical utility and validity for various cancers. However, it has not been widely incorporated into clinical practice for the management of all types of cancers especially PDAC because of sparsity of evidence. Our case showed the promising role of liquid biopsy not only in detection of the underlying genetic aberration but also in determination of the treatment response in PDAC.
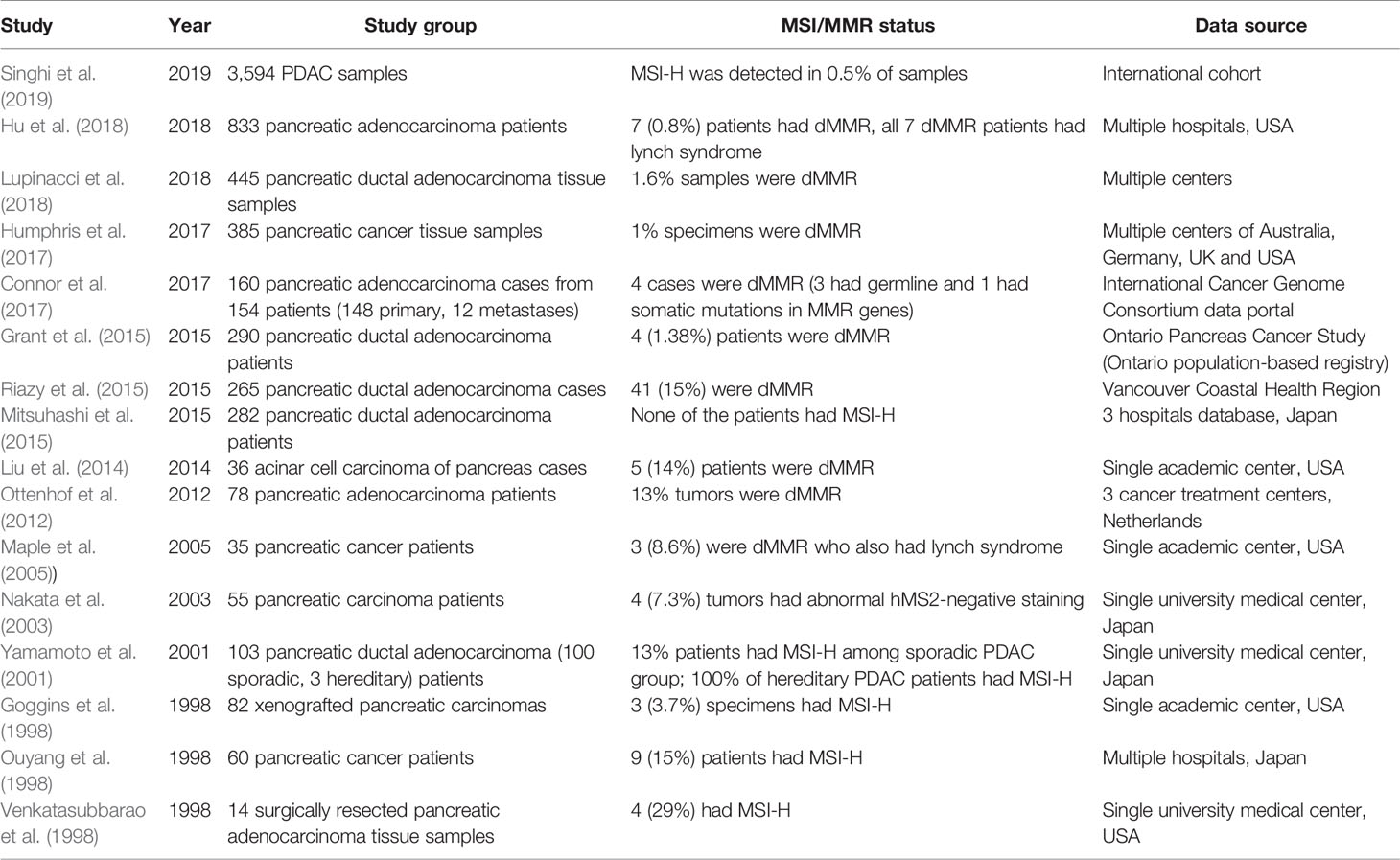
The available data on the prevalence of dMMR and MSI-H in PDAC is limited and heterogeneous. Previous studies have demonstrated the existence of MSI-H in PDAC, however, the findings were controversial. Yamamoto et al. investigated 103 PDAC patients and the reported incidence of MSI-H in their cohort was 16%. The researchers also reported the finding of MSI-H in 100% of their patients with hereditary PDAC (n = 3) (Yamamoto et al., 2001). Goggins et al. studied 82 xenografts of pancreatic carcinoma and reported that 3.7% of their specimens had MSI-H (Goggins et al., 1998). Ouyang et al. investigated 60 pancreatic cancer patients and found MSI-H in 9 (15%) patients of their cohort (Ouyang et al., 1998). Venkatasubbarao et al. reported the presence of MSI-H in 4 (29%) out of their 14 surgically resected samples of pancreatic adenocarcinoma (Venkatasubbarao et al., 1998). Recently, Hu et al. investigated 833 PDAC patients and found dMMR in 7 (0.8%) patients of their cohort (Hu et al., 2018). The summary of studies looking at pancreatic carcinoma and MSI-H/dMMR expression are demonstrated in Table 2. The disagreement in prevalence of MSI-H in PDAC might be due to confounding factors including differences in sample sizes, test techniques and diversity of tumor histology (Macherla et al., 2018).
Our patient demonstrated a phenomenal response to immunotherapy. The tumor mutation burden pre- and post-treatment was well-picked on ctDNA assays. Recent research has highlighted the clinical utility and validity of ctDNA testing in the management of various gastrointestinal cancers including PDAC (Shahjehan et al., 2019). Our findings also suggest that serial monitoring of tumor mutation burden can serve as a potential prognostic biomarker in pancreatic cancer. Previously, this was not feasible before the implication of ctDNA testing. The advent of immune checkpoint inhibitors has revolutionized the management of various MSI-H/dMMR tumors especially non-small cell lung carcinoma and melanoma. In May 2017, the United States Food and Drug Administration (FDA) approved pembrolizumab, a PD-1 inhibitor, for the treatment of MSI-H/dMMR solid tumors regardless of site of origin or histology that didn’t respond or have metastasized after the introduction of first line agents (Lemery et al., 2017). The role of immunotherapy in PDAC has been studied in various clinical trials, however, no objective/complete response was achieved (Brahmer et al., 2012; Patnaik et al., 2015). The potential factors responsible for resistance of PDAC to immunotherapy could be low immunogenicity, decreased tumor mutation burden and inherent quality of being unlikely to be detected by the immune system (Evans et al., 2016).
There are several ongoing clinical trials that are investigating the role of various anti-PD-1/PD-L1 agents including pembrolizumab, nivolumab, etc. as monotherapy as well as combination regimens in PDAC. A recent phase I study investigated the role of a combination regimen consisting of a tumor-associated macrophage-targeting agent cabiralizumab and the anti-PD-1 nivolumab in metastatic PDAC. The combination regimen demonstrated a confirmed objective response in 4 of 31 (13%) patients. The researchers also revealed that all of these 4 patients with confirmed objective response had MSI-H and didn’t show response to anti‒PD-1 or PD-L1 monotherapy (NCT02526017) (Wainberg ZA et al., 2017).
Le et al. investigated the efficacy of PD-1 blockade in the management of 12 types of solid tumors including pancreatic cancer in a multicenter phase 2 study. They enrolled all dMMR cancer patients (n = 86) who had at least one prior therapy and developed a progressive disease. The estimated objective and complete response rates were 53% and 21%, respectively. Of note, the subset analysis for pancreatic cancer (n = 8) demonstrated an objective response rate of 62%. The researchers indicated that the complete and partial responses were attained in 2 (25%) and 3 (37%) pancreatic cancer patients respectively. For the colorectal cancer (n = 40), their results showed the objective and complete response rates of 52% and 12%, respectively (NCT01876511). The study is still ongoing and manifests the value of immune checkpoint inhibitors in pancreatic cancer (Le et al., 2017).
Conclusion and Future Directions
Pancreatic cancer is a lethal cancer with poor outcomes in spite of the recent breakthroughs in combination chemotherapy regimens. Immune checkpoint inhibitors have exhibited strong responses in several MSI-H solid tumors but there is lack of evidence regarding pancreatic cancer. Liquid biopsy could be incorporated in the management of these patients to record serial assessments of tumor mutation burden and to detect microsatellite instability, where obtaining tissue is often very difficult.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
Written informed consent was obtained from the patient for the publication of this case report.
Author Contributions
SK and FS drafted the initial draft manuscript with guidance from PK. Author PK revised the manuscript and further edited by all the authors. All authors approved the final draft for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bailey, P., Chang, D. K., Nones, K., Johns, A. L., Patch, A.-M., Gingras, M.-C. (2016). Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531 (7592), 47–52. doi: 10.1038/nature16965
Barrett, M. T., Deiotte, R., Lenkiewicz, E., Malasi, S., Holley, T., Evers, L. (2017). Clinical study of genomic drivers in pancreatic ductal adenocarcinoma. Br. J. Cancer 117 (4), 572–582. doi: 10.1038/bjc.2017.209
Brahmer, J. R., Tykodi, S. S., Chow, L. Q., Hwu, W. J., Topalian, S. L., Hwu, P. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366 (26), 2455–2465. doi: 10.1056/NEJMoa1200694
Chou, A., Froio, D., Nagrial, A. M., Parkin, A., Murphy, K. J., Chin, V. T. (2018). Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut 67 (12), 2142–2155. doi: 10.1136/gutjnl-2017-315144
Connor, A. A., Denroche, R. E., Jang, G. H., Timms, L., Kalimuthu, S. N., Selander, I. (2017). Association of distinct mutational signatures with correlates of increased immune activity in pancreatic ductal adenocarcinoma. JAMA Oncol. 3 (6), 774–783. doi: 10.1001/jamaoncol.2016.3916
DeWitt, J., Devereaux, B., Chriswell, M., McGreevy, K., Howard, T., Imperiale, T. F. (2004). Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 141 (10), 753–763. doi: 10.7326/0003-4819-141-10-200411160-00006
Erkan, M., Reiser-Erkan, C., Michalski, C. W., Kleeff, J. (2010). Tumor microenvironment and progression of pancreatic cancer. Exp. Oncol. 32 (3), 128–131.
Erkan, M., Reiser-Erkan, C., Michalski, C. W., Kong, B., Esposito, I., Friess, H., et al. (2012). The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr. Mol. Med. 12 (3), 288–303. doi: 10.2174/156652412799218921
Evans, M. S., Diamond, M. S., Rech, A. J., Chao, T., Richardson, M. W., Lin, J. H. (2016). Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight 1 (14). doi: 10.1172/jci.insight.88328
Goggins, M., Offerhaus, G. J., Hilgers, W., Griffin, C. A., Shekher, M., Tang, D.. (1998). Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild-type K-ras and characteristic histopathology. poor differentiation, a syncytial growth pattern, and pushing borders suggest RER+. Am. J. Pathol. 152 (6), 1501–1507.
Grant, R. C., Selander, I., Connor, A. A., Selvarajah, S., Borgida, A., Briollais, L. (2015). Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology 148 (3), 556–564. doi: 10.1053/j.gastro.2014.11.042
Hackeng, W. M., Hruban, R. H., Offerhaus, G. J., Brosens, L. A. (2016). Surgical and molecular pathology of pancreatic neoplasms. Diagn. Pathol. 11 (1), 47. doi: 10.1186/s13000-016-0497-z
Herreros-Villanueva, M., Bujanda, L. (2016). Non-invasive biomarkers in pancreatic cancer diagnosis: what we need versus what we have. Ann. Transl. Med. 4 (7), 134. doi: 10.21037/atm.2016.03.44
Hu, Z. I., Shia, J., Stadler, Z. K., Varghese, A. M., Capanu, M., Salo-Mullen, E. (2018). Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin. Cancer Res. 24 (6), 1326–1336. doi: 10.1158/1078-0432.CCR-17-3099
Humphris, J. L., Patch, A. M., Nones, K., Bailey, P. J., Johns, A. L., McKay, S. (2017). Hypermutation in pancreatic cancer. Gastroenterology 152 (1), 68–74.e2. doi: 10.1053/j.gastro.2016.09.060
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 (6349), 409–413. doi: 10.1126/science.aan6733
Lemery, S., Keegan, P., Pazdur, R. (2017). First FDA approval agnostic of cancer site - when a biomarker defines the indication. N. Engl. J. Med. 377 (15), 1409–1412. doi: 10.1056/NEJMp1709968
Liu, W., Shia, J., Gönen, M., Lowery, M. A., O'Reilly, E. M., Klimstra, D. S. (2014). DNA mismatch repair abnormalities in acinar cell carcinoma of the pancreas: frequency and clinical significance. Pancreas 43 (8), 1264–1270. doi: 10.1097/MPA.0000000000000190
Liu, Q., Liao, Q., Zhao, Y. (2017). Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 17, p. 68. doi: 10.1186/s12935-017-0437-3
Lupinacci, R. M., Goloudina, A., Buhard, O., Bachet, J. B., Maréchal, R., Demetter, P. (2018). Prevalence of microsatellite instability in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology 154 (4), 1061–1065. doi: 10.1053/j.gastro.2017.11.009
Macherla, S., Laks, S., Naqash, A. R., Bulumulle, A., Zervos, E., Muzaffar, M. (2018). Emerging Role of Immune Checkpoint Blockade in Pancreatic Cancer. Int. J. Mol. Sci. 19 (11). doi: 10.3390/ijms19113505
Mahadevan, D., Von Hoff, D. D. (2007). Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 6 (4), 1186–1197. doi: 10.1158/1535-7163.MCT-06-0686
Maple, J. T., Smyrk, T. C., Boardman, L. A., Johnson, R. A., Thibodeau, S. N., Chari, S. T. (2005). Defective DNA mismatch repair in long-term (> or = 3 years) survivors with pancreatic cancer. Pancreatology 5 (2-3), 220–7; discussion 227-8. doi: 10.1159/000085275
Mitsuhashi, K., Nosho, K., Sukawa, Y., Matsunaga, Y., Ito, M., Kurihara, H. (2015). Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6 (9), 7209–7220. doi: 10.18632/oncotarget.3109
Nakata, B., Wang, Y. Q., Yashiro, M., Ohira, M., Ishikawa, T., Nishino, H., et al. (2003). Negative hMSH2 protein expression in pancreatic carcinoma may predict a better prognosis of patients. Oncol. Rep. 10 (4), 997–1000. doi: 10.3892/or.10.4.997
Ottenhof, N. A., Morsink, F. H., Ten Kate, F., van Noorden, C. J., Offerhaus, G. J. (2012). Multivariate analysis of immunohistochemical evaluation of protein expression in pancreatic ductal adenocarcinoma reveals prognostic significance for persistent Smad4 expression only. Cell Oncol. (Dordr) 35 (2), 119–126. doi: 10.1007/s13402-012-0072-x
Ouyang, H., Furukawa, T., Abe, T., Kato, Y., Horii, A. (1998). The BAX gene, the promoter of apoptosis, is mutated in genetically unstable cancers of the colorectum, stomach, and endometrium. Clin. Cancer Res. 4 (4), 1071–1074.
Patnaik, A., Kang, S. P., Rasco, D., Papadopoulos, K. P., Elassaiss-Schaap, J., Beeram, M. (2015). Phase I study of pembrolizumab (MK-3475; Anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin. Cancer Res. 21 (19), 4286–4293. doi: 10.1158/1078-0432.CCR-14-2607
Riazy, M., Kalloger, S. E., Sheffield, B. S., Peixoto, R. D., Li-Chang, H. H., Scudamore, C. H. (2015). Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod. Pathol. 28 (10), 1383–1389. doi: 10.1038/modpathol.2015.89
Ryerson, A. B., Eheman, C. R., Altekruse, S. F., Ward, J. W., Jemal, A., Sherman, R. L. (2016). Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122 (9), 1312–1337. doi: 10.1002/cncr.29936
Shahjehan, F., Kamatham, S., Kasi, P. M. (2019). Role of circulating tumor dna in gastrointestinal cancers: update from abstracts and sessions at ASCO 2018. Front. Oncol. 9, 358–358. doi: 10.3389/fonc.2019.00358
Siegel, R. L., Miller, K. D., Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69 (1), 7–34. doi: 10.3322/caac.21551
Singhi, A. D., George, B., Greenbowe, J. R., Chung, J., Suh, J., Maitra, A. (2019). Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomarkers. Gastroenterology 156 (8), 2242–2253.e4. doi: 10.1053/j.gastro.2019.02.037
Umar, A., Kunkel, T. A. (1996). DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur. J. Biochem. 238 (2), 297–307. doi: 10.1111/j.1432-1033.1996.0297z.x
Venkatasubbarao, K., Ahmed, M. M., Swiderski, C., Harp, C., Lee, E. Y., McGrath, P., et al. (1998). Novel mutations in the polyadenine tract of the transforming growth factor beta type II receptor gene are found in a subpopulation of human pancreatic adenocarcinomas. Genes Chromosomes Cancer 22 (2), 138–144. doi: 10.1002/(SICI)1098-2264(199806)22:2<138::AID-GCC8>3.0.CO;2-Y
Wainberg, Z. A., Piha-Paul, S. A., Luke, J., Kim, E. J., Thompson, J. A., Britten, C. D. (2017). First-in-human phase 1 dose-escalation and expansion of a novel combination, anti-CSF-1 receptor (cabiralizumab) plus anti-PD-1 (nivolumab), in patients with advanced solid tumors. In 2017 Annual Meeting of the Society for Immunotherapy of Cancer. National Harbor, MD.
Yachida, S., Iacobuzio-Donahue, C. A. (2009). The pathology and genetics of metastatic pancreatic cancer. Arch. Pathol. Lab. Med. 133 (3), 413–422. doi: 10.1043/1543-2165-133.3.413
Keywords: circulating tumor DNA, microsatellite instability, pancreatic cancer, immunotherapy, pembrolizumab
Citation: Kamatham S, Shahjehan F and Kasi PM (2020) Circulating Tumor DNA-Based Detection of Microsatellite Instability and Response to Immunotherapy in Pancreatic Cancer. Front. Pharmacol. 11:23. doi: 10.3389/fphar.2020.00023
Received: 10 October 2019; Accepted: 08 January 2020;
Published: 10 February 2020.
Edited by:
Gloria Ravegnini, University of Bologna, ItalyReviewed by:
Marina Pajic, Garvan Institute of Medical Research, AustraliaSamuel J. Klempner, Massachusetts General Hospital Cancer Center, United States
Copyright © 2020 Kamatham, Shahjehan and Kasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pashtoon Murtaza Kasi, UGFzaHRvb24ta2FzaUB1aW93YS5lZHU=
 Saivaishnavi Kamatham
Saivaishnavi Kamatham Faisal Shahjehan
Faisal Shahjehan Pashtoon Murtaza Kasi
Pashtoon Murtaza Kasi