- 1School of Biological and Chemical Engineering, Zhejiang University of Science and Technology, Hangzhou, China
- 2The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, China
- 4The First Affiliated Hospital, Zhejiang Chinese Medical University, Hangzhou, China
- 5Department of Physicochemistry and Toxicology, Center for Disease Control and Prevention of Zhejiang Province, Hangzhou, China
- 6Experimental and Training Center, Zhejiang Pharmaceutical College, Ningbo, China
- 7Department of Orthopaedics, Changzheng Hospital, Second Military Medical University, Shanghai, China
- 8Department of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, Johannes Gutenberg University, Mainz, Germany
Background: As a degenerative joint disease with severe cartilage destruction and pain, osteoarthritis (OA) has no satisfactory therapy to date. In traditional Chinese medicine (TCM), Aconitum carmichaeli Debeaux derived Hei-shun-pian (Hsp) has been developed for joint pain treatment. However, it causes adverse events in OA patients. Long-time decoction has been traditionally applied to reduce the aconite toxicity of Hsp and other aconite herbs, but its detoxifying effect is uncertain.
Methods: Hsp was extracted with dilute decoction times (30, 60, and 120 min) and evaluated by toxicological, chemical, pharmacological assays. Acute toxicity assay and chemical analysis were employed to determine the toxicity and chemoprofile of Hsp extracts, respectively. Since the detoxified Hsp (dHsp) was defined, its therapeutic effect was evaluated by using an OA rat model induced by monosodium iodoacetate. dHsp at 14 g/kg was orally administered for 28 days, and the pain assessments (mechanical withdrawal threshold and thermal withdrawal latency) and histopathological analyses (HE and safranin-O staining) were performed. Real-time PCR (qPCR) was applied to determine the molecular actions of dHsp on cartilage tissue and on chondrocytes. MTT assay was conducted to evaluate the effect of dHsp on the cell viability of chondrocytes. The cellular and molecular assays were also conducted to analyze the functions of chemical components in dHsp.
Results: The chemoprofile result showed that the contents of toxic alkaloids (aconitine, mesaconitine, and hypaconitine) were decreased but that of non-toxic alkaloids (benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine) were increased with increasing decoction time. Acute toxicity assay showed that only Hsp extract with 120 min decoction was non-toxic within the therapeutic dose range. Thus, it was defined as dHsp for further experiment. In OA experiment, dHsp significantly attenuated joint pain and prevented articular degeneration from MIA attack. qPCR data showed that dHsp restored the abnormal expressions of Col10, Mmp2, Sox5, Adamts4/5/9, and up-regulated Col2 expression in rat cartilage. In vitro, dHsp-containing serum significantly proliferated rat chondrocytes and regulated the gene expressions of Col2, Mmp1, Adamts9, and Aggrecan in a similar way as the in vivo data. Moreover, aconitine, mesaconitine, and hypaconitine exerted cytotoxic effects on chondrocytes, while benzoylaconitine and benzoylhypaconitine except benzoylmesaconitine exhibited similar molecular actions to dHsp, indicating contributions of benzoylaconitine and benzoylhypaconitine to dHsp.
Conclusions: This study defined dHsp and demonstrated dHsp as a potential analgesic and disease modifying agent against OA with molecular actions on the suppression of chondrocyte hypertrophy and extracellular matrix degradation, providing a promising TCM candidate for OA therapy.
Introduction
Osteoarthritis (OA) is a progressive joint disease characterized by cartilage degradation, sclerosis of subchondral bone and osteophyte formation, resulting in chronic joint pain, joint stiffness, and disability. OA is the main cause of lower extremity disability around the globe, with hip and knee OA accounting for 17 million years lived with disability or 2.2% of all-cause years lived with disability (O’Neill et al., 2018). The incidence and severity of OA in women are higher than that in men. It is estimated that the lifetime risk of knee OA is about 40% in men and 47% in women (Johnson and Hunter, 2014). Even worse, due to the aging of the global population and the aggravation of obesity, the incidence of OA is getting higher and is expected to double by the year 2020 (Johnson and Hunter, 2014; Mandl, 2019). Current OA treatments are mostly targeting the symptomatic relief of pain and inflammation for joint function improvement. However, none of them can modify the OA progression, and their therapeutic outcomes are often associated with incomplete relief and side effects (Bijlsma et al., 2011). Therefore, development of novel anti-OA therapeutics is still sorely needed.
The lateral root of Aconitum carmichaeli Debx (family Ranunculaceae), named Fu-zi in China, is a widely used traditional Chinese medicine (TCM) with cardiotonic, analgesic, and anti-inflammatory activities. It was originally described by the earliest Pharmacopeia of China, “Shennong Materia Medica” (24−220 AD). However, Fu-zi is highly toxic, and diester-diterpenoid alkaloids (DDAs) such as aconitine, mesaconitine and hypaconitine are its main toxic components (He et al., 2017). These components can cause toxic side effects on the cardiovascular, nervous, respiratory and digestive systems, which can be mainly manifested as arrhythmia, hypotension, hypothermia, respiratory depression, muscle paralysis and central nervous dysfunction, and may even lead to death in severe cases (Chan, 2009). These side effects limited the clinical application of Fu-zi. Traditionally, processing methods have been developed for reduction of Fu-zi′s toxicity prior to prescription (China Pharmacopeia Committee, 2015). Hei-shun-pian (Hsp) is such a processed product that has been widely used as a principal herb in TCM formulas for treatment of joint pain, including Gan-cao Fu-zi Tang and Fu-zi Tang (Huang, 2013). Modern clinical studies have reported the therapeutic effects of those formulas on knee OA (Deng, 2008). Recently, we also reported in a clinical trial that Fu-zi Tang effectively alleviated knee pain and improved life quality of patients with mild to moderate knee OA (Liu et al., 2016). Nevertheless, adverse events still occurred with Fu-zi Tang treatment in those patients, indicating that Hsp remained toxic. It is well known that long-time decoction is a traditional and useful processing method for detoxifying aconite toxicity, resulting in derivatization of non-toxic alkaloids (benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine) from toxic alkaloids (Tong et al., 2013). However, there is no standard procedure to optimize detoxification, resulting in potential risks for patients. To evaluate the traditional detoxifying effect on Hsp and to explore, whether or not the long-time decocted Hsp remains therapeutically effective, we conducted acute toxicity assay and chemical analysis to determine the detoxicated Hsp (dHsp) and then employed an OA rat model to evaluate the therapeutic effect of dHsp. Afterwards, the molecular actions of dHsp on cartilage tissue and chondrocytes were clarified by real time PCR. This is the first report regarding the development and evaluation of dHsp.
Materials and Methods
Preparation of Hsp Extracts
Hei-shun-pian, a processed product of the lateral root of Aconitum carmichaeli Debx (Ranunculaceae) was harvested from Jiangyou (Sichuan, China) and authenticated by the authors (voucher specimen No. 081102). Detoxifying processing was applied in accordance with the procedure described in our previous report (Tong et al., 2013). The specific steps are as follows: The materials were powdered and evenly divided into three samples. Each sample was soaked in 10-fold water for 30 min, followed by boiling and decocting for 30 min, 60 min, and 120 min, respectively. Then, the water extract of each sample was collected after filtration, and 8-fold the amount of water was added to each residue, which is the same as the above procedure to decoct again, and to combine the first-stage extracts with the second-stage extracts of each sample. Finally, each supernatant was concentrated by rotary evaporation and freeze-dried to powder for storage and diluted into 1.0 g/ml for use. The extracts with 30 min decoction, 60 min decoction, 120 min decoction were labeled as Hsp-30, Hsp-60, and Hsp-120, respectively.
Chemicals and Reagents
Standard substances (HPLC grade) of aconitine (98.01% of purity, batch number: MUST-19110905), mesaconitine (99.14% of purity, batch number: MUST-19111311) and hypaconitine (99.09% of purity, batch number: MUST-19080210), benzoylaconitine (99.44% of purity, batch number: MUST-19103010), benzoylmesaconitine (99.66% of purity, batch number: MUST-19032807), and benzoylhypaconitine (99.46% of purity, batch number: MUST-20022710) were purchased from Chengdu Must Bioscience and Technology CO., LTD (Chengdu, China). Methanol and acetonitrile were of HPLC grade (Tedia, Fairfield, USA). Ammonium acetate and tetrahydrofuran were of analytical grade. Mono-iodoacetate (MIA) was purchased from Sigma (St. Louis, MO, United States). Iscove’s modified Dulbecco’s medium (IMDM), fetal bovine serum (FBS) and 0.25% trypsin were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, United States). 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (Taufkirchen, Germany). TRIzol reagent was purchased from Thermo Fisher Scientific Inc. The real time polymerase chain reaction (PCR) kit was purchased from Takara Biotechnology Co., Ltd. (Dalian, China). All antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, United States).
Animals and Cell Line
Male SD rats (180–220 g) and Kunming mice with both sexes (18–22 g) were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Sciences (Grade SPF II Certificate No. SCXK2017-0005) and housed in ventilated cages at 22 ± 1°C under a 12/12 h light/dark cycle with water and food ad libitum. Primary chondrocyte was isolated from the rat articular cartilage and cultured as previously described (Wang et al., 2013).
Chemical Analysis
HPLC analysis was performed on an Agilent 1260 Infinity HPLC system (Agilent Technologies, CA, United States). Chromatographic separation was carried out on a Hypersil BDS C18 column (250×4.6 mm, 5 μm) (Dalian Elite Analytical Instrument Co., Ltd) at 30°C. The mobile phase consisted of 0.1 mol/l ammonium acetate solution and acetonitrile-tetrahydrofuran (100:50, v/v) with a flow rate of 1.0 ml/min. The elution gradient was started at 85% ammonium acetate solution, followed by decreasing the ammonium acetate solution to 74% within 40 min, subsequently, the mobile phase was switched to 85% ammonium acetate again for 45 min. The sample injection volume was 10 μl, and the detection wavelength was 235 nm. The standard substances of aconitine, mesaconitine and hypaconitine were dissolved in methanol to obtain working standard solutions. The data was analyzed to identify and quantify the aconitine, mesaconitine, and hypaconitine in Hsp-30, Hsp-60, and Hsp-120 extracts by two-point external standard method.
The UPLC-MS analysis was performed on an Acquity UPLC system (Waters, MA, USA) equipped with a Xevo TQ-S triple quadrupoleelectrospray ionization (ESI) MS (Waters, MA, USA) operated in positive ESI-mode. Chromatographic separation was carried out on an Acquity BEH C18 column (100 mm × 2.1 mm, particle size 1.7 μm) maintained at 40°C. The mobile phase consisted of 5 mM ammonium formate solution (0.1% formic acid) and methyl alcohol (55:45, v/v) with a flow rate of 0.4 ml/min. The sample injection volume was 5 μl. The standard substances of benzoylaconitine, benzoylmesaconitine and benzoylhypaconitine were dissolved in methyl alcohol to obtain working standard solutions. The data was analyzed to identify and quantify the benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine in Hsp-30, Hsp-60, and Hsp-120 extracts.
Acute Toxicity Assay
Male and female Kunming mice were randomly divided into 16 groups with 8 animals each (4 male and 4 female). Before oral administration, all mice were fasted for 12 h with water ad libitum. Each five groups were orally given one type of detoxicated Hsp (Hsp-30, Hsp-60, or Hsp-120) in doses of 40, 60, 80, 100, 120 g/kg, respectively. The control group received an equal volume of water. After a single administration, the animals were observed closely during first 24 h and were kept under observation up to 14 days. The mortality of each group was observed. As an index for acute toxicity, LD50 with associated 95% confidence limits (CL) was determined by the Bliss’s method.
Therapeutic Evaluation
A total of 30 rats were used for therapeutic evaluation of dHsp. All rats were randomly and equally divided into three groups: NC as normal control group, OA as model group, and OA+dHsp as dHsp treated OA group. The OA and OA+dHsp groups were intra-articularly injected with 50 μl of 20 mg/ml MIA through the patella ligament of rat knees using a 100 μl microliter syringe to establish the OA model, while NC group was treated with 50 μl of saline in a same way. Besides the MIA injection, rats in OA+dHsp group were simultaneously treated with oral administration of 14 g/kg dHsp, while OA and NC group were given the equal volume of saline, respectively. The treatment lasted for 28 days, and the pain-related mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were tested after the last treatment. In addition, the serum of rats from NC group and OA+dHsp group were collected to obtain blank serum and dHsp-containing serum for in vitro experiment. Finally, all the animals were sacrificed under anesthesia, and the knee joints were taken immediately for histopathological and real time PCR assay.
Pain-Related Behavioral Observation
The TWL and MWT were measured by a Plantar Test apparatus (UgoBasile, Italy) and the von Frey test, respectively. The rats were placed in a transparent plastic box at room temperature (25 ± 2) °C. After the rats were quiet (stop combing hair and exploring activities), the cross mark on the tester was placed in the center of the left rear sole of the rats, and the instrument was opened away from the foot pad. Each rat was measured three times. In order to prevent the rats from being scalded by thermal radiation, the upper limit of time and temperature was set as 20 s and 35°C, respectively. For testing the mechanical pain threshold, the von Frey needle of the instrument was used to press the plantar surface of the left and right hind paws of each rat about three times. The rats showed rapid withdrawal of claws and licking of claws as the positive reaction of each test.
Histopathological Observation
The rat joints on one side were dissected immediately after sacrifice and fixed in 4% paraformaldehyde for 48 h, followed by decalcification with 5% hydrochloric acid for 96 h. After paraffin embedding, samples were sectioned (4~5 μm) and stained with HE (hematoxylin and eosin) and SO (safranin-O) using routine process. The HE stained slides were photographed under the microscope. According to Mankin’s scoring system, statistical grading was carried out in the range of 0–13 points through double-blind observation.
Cell Viability Assay
The chondrocyte viability was determined by MTT assay. The 2nd generation of chondrocytes were seeded on 96-well plates at a density of 5×103 cells/well in 200 ml medium for 24 h, and treated with dHsp-containing serum at 2.5, 5, 10, 15% for 24, 48, and 72 h and then treated with aconitine, mesaconitine, hypaconitine, benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine at dose ranges according to their concentrations in Hsp-120. A total of 20 ml MTT solution (5.0 mg/ml) was added to each well and incubated at 37°C for 4 h. 150 ml DMSO was subsequently added to each well to dissolve the formazan crystals and the optical density (OD) value was measured at 490 nm with a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, United States). Proliferative rate (%) = (dHsp-treated OD/untreated OD) ×100.
Real-Time PCR (qPCR)
qPCR assay was performed to test the relative mRNA expression of OA-related genes of rat joints obtained from the animal experiment and chondrocytes by using an ABI QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). An aliquot of chondrocytes were pre-treated with TNF-α (10 ng/ml) for 6 h, and then treated with dHsp-containing serum (15%) for 24 h. Another aliquot of chondrocytes were treated with benzoylaconitine, benzoylmesaconitine and benzoylhypaconitine for 24 h at doses corresponding to their concentrations in Hsp-120. Total RNA was extracted from cartilage tissue or chondrocytes using TRIzol reagent and reverse transcription was performed to produce cDNA. The final PCR reaction system was 20.0 μl, comprising 10.0 μl SYBR® Premix Ex Taq II (Tli RnaseH Plus), 0.8 μl PCR Forward Primer, 0.8 μl PCR Reverse Primer, 2.0 μl template cDNA, 0.4 μl ROX Reference Dye, and 6.0 μl ddH2O. The qPCR reaction conditions were as follows: 95°C for 30 s for initial denaturation, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 34 s, and extension at 72°C for 40 s. At the end of each reaction, melting curve analysis was performed. β-actin was used as the reference gene and the 2-ΔΔCT method was used to analyze the relative mRNA expressions (Table 1).
Statistical Analysis
Data were expressed as mean ± SD and subjected to one-way ANOVA, followed by Fisher’s least significant difference (LSD) comparison. All analyses were performed using an updated version of DPS software (Tang and Feng, 2007).
Results
Chemoprofiles of Hsp Extracts
The HPLC chromatogram comparison of toxic components in Hsp-30, Hsp-60, and Hsp-120 extracts were shown in Figures 1A–C. The retention time of aconitine, mesaconitine, and hypaconitine in all samples were 21.091 – 22.092 min, 11.162 – 11.816 min, and 44.705 – 47.280 min, respectively. There were no remarkable chromatographic differences in the peak time of Hsp samples with different decocting times. Nevertheless, upon decoction with water, the peak height of each compound decreased gradually in a time-dependent manner, and the peak of aconitine was even found disappeared after 120 min decoction. The concentrations of three toxic components in Hsp-30, Hsp-60, and Hsp-120 are shown in Figure 1D. The highest concentrations were found in Hsp-30, which attained 1.04 ± 0.03 μg/g for aconitine, 3.57 ± 0.07 μg/g for mesaconitine, 10.44 ± 0.20 μg/g for hypaconitine. Upon decoction with water, a stepwise decrease of each concentration was observed in different Hsp extracts with increasing decoction time. Especially when the decoction time attained 120 min, the concentrations of the three compounds reached the lowest level with undetected aconitine, 0.37 ± 0.01 μg/g of mesaconitine, and 5.47 ± 0.16 μg/g of hypaconitine. Thus, Hsp-120 was selected as dHsp for the following animal experiment.
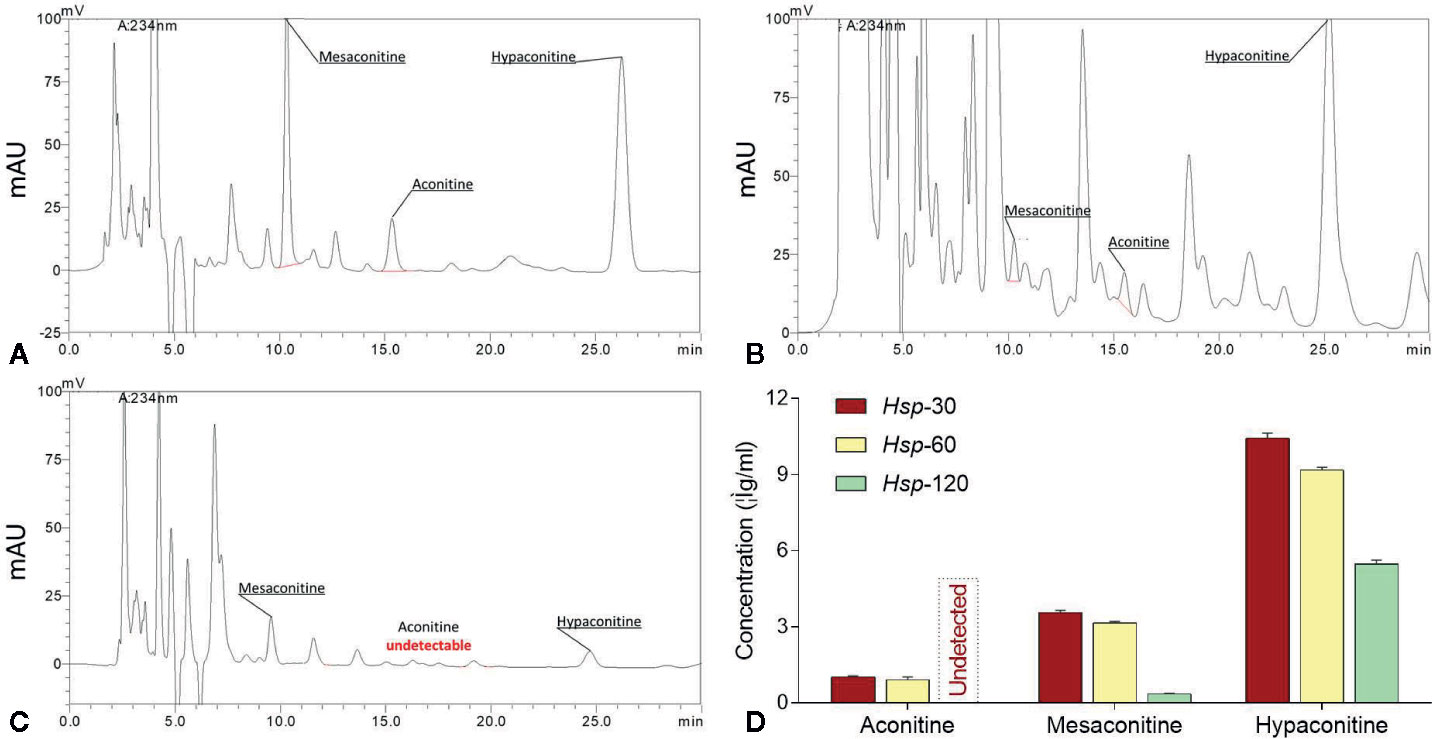
Figure 1 HPLC chromatograms of Hsp-30 (A), Hsp-60 (B), and Hsp-120 (C), and concentrations of aconitine, mesaconitine, and hypaconitine in Hsp-30, Hsp-60, and Hsp-120 (D).
UPLC-MS was applied to identify and quantify the non-toxic derivatives of aconitines in Hsp-30, Hsp-60, and Hsp-120. As shown in Figure 2, benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine were all present in Hsp-30, Hsp-60, and Hsp-120, while their concentrations were increased with increasing decoction time. For example, benzoylaconitine was increased from 38.33 ± 0.44 μg/g in Hsp-30 to 58.08 ± 0.25 μg/g in Hsp-120, benzoylmesaconitine was increased from 197.20 ± 0.25 μg/g in Hsp-30 to 279.56 ± 0.36 μg/g in Hsp-120, and benzoylhypaconitine was increased from 44.90 ± 0.05 μg/g in Hsp-30 to 87.86 ± 0.09 μg/g in Hsp-120.
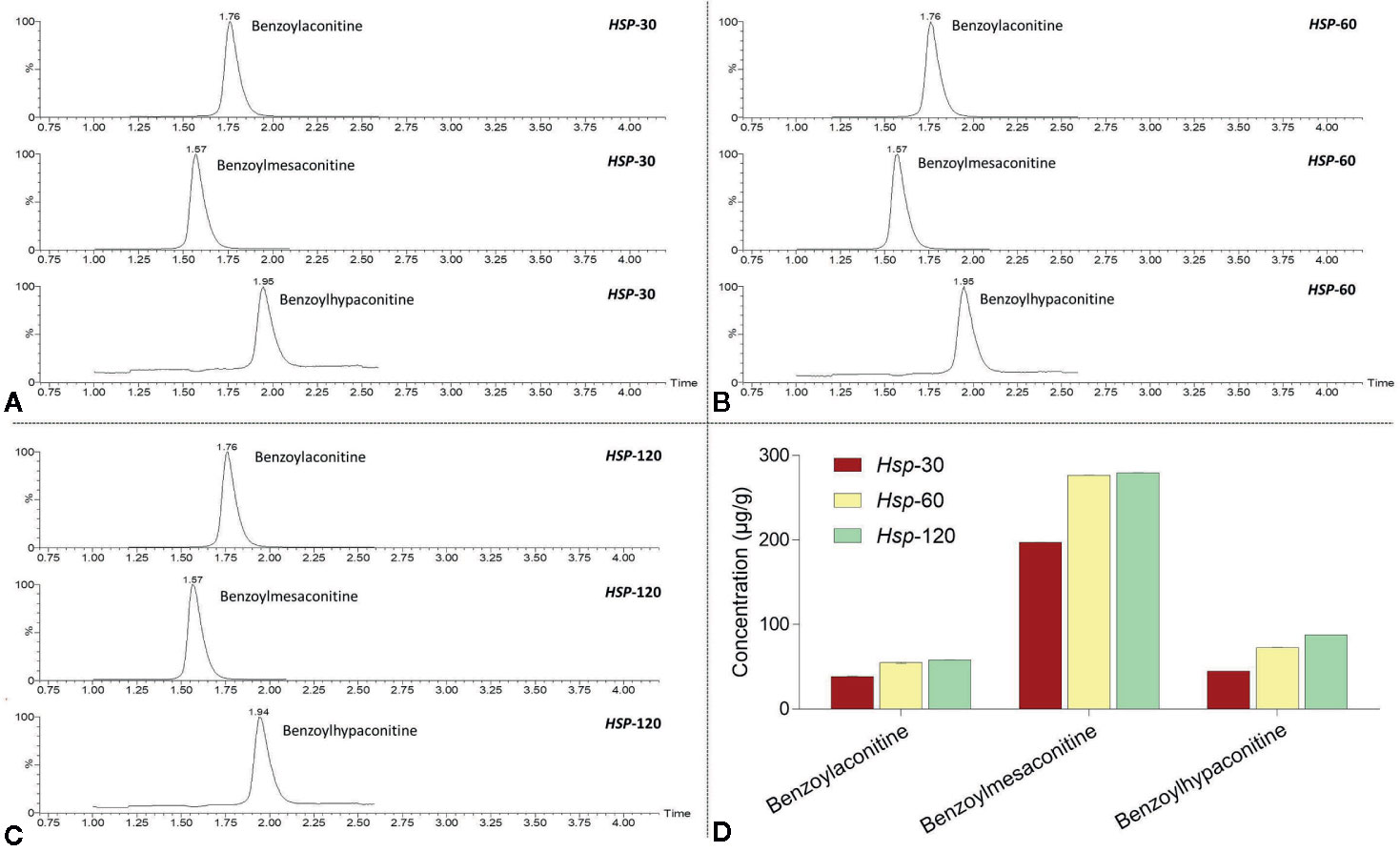
Figure 2 UPLC-MS chromatograms of Hsp-30 (A), Hsp-60 (B), and Hsp-120 (C), and concentrations of benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine in Hsp-30, Hsp-60, and Hsp-120 (D).
Acute Toxicity of Hsp Extracts
Our preliminary experiment found a rational dose range of dHsp being 40 to 120 g/kg for the acute toxicity test, since 120 g/kg almost reached the maximum saturated solubility. As shown in Figure 3, within 24 h, the death was initiated by Hsp-30 at 80 g/kg and Hsp-60 at 100 g/kg with mortalities of 37.5% and 12.5%, respectively. At the end, the mice exposed to Hsp-30 at 120 g/kg were all dead with LD50 of 81.1 (68.0 to 96.8) k/kg, indicating a concentration-dependent and time-dependent manner of toxicity. Hsp-60 showed lesser acute toxicity than Hsp-30, which caused final mortality of 37.5% at 120 g/kg. However, no death occurred under the treatment of Hsp-120 within the dose and time ranges, indicating a certain safety of Hsp-120.
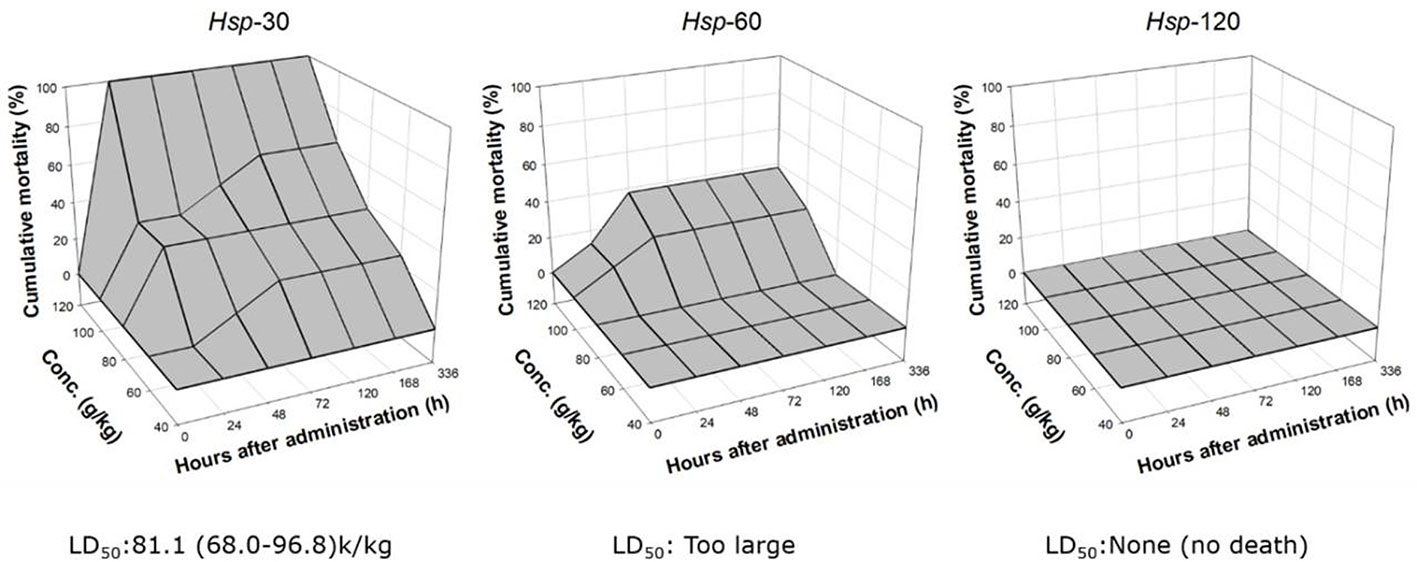
Figure 3 Cumulative mortalities of mice after oral administration of Hsp-30, Hsp-60, and Hsp-120 (g/kg) with LD50 value and associated 95% confidence limits.
In Vivo Anti-OA Effect of dHsp
MWT and TWL, respectively, reflect two behavioral responses: mechanical hyperalgesia and thermal hyperalgesia. As shown in Figure 4A, MWT and TWL were significantly decreased with OA modeling (both P < 0.05 versus NC group), while the abnormal levels were significantly restored by dHsp after 28 days treatment (both P < 0.01 versus OA group). Histopathological results with HE and SO staining are shown in Figure 4B. Severe cartilage damage, characterized by loss of chondrocytes and disorganization of extracelluar matrix, was observed in the OA group with significant increase of Mankin’s score (Figure 4C, P < 0.01 versus NC group). As expected, the damaged phenotype was obviously relieved by dHsp treatment with significant decrease of Mankin’s score (Figure 4C, P < 0.01 versus OA group), in which the chondrocytes and extracelluar matrix were protected.
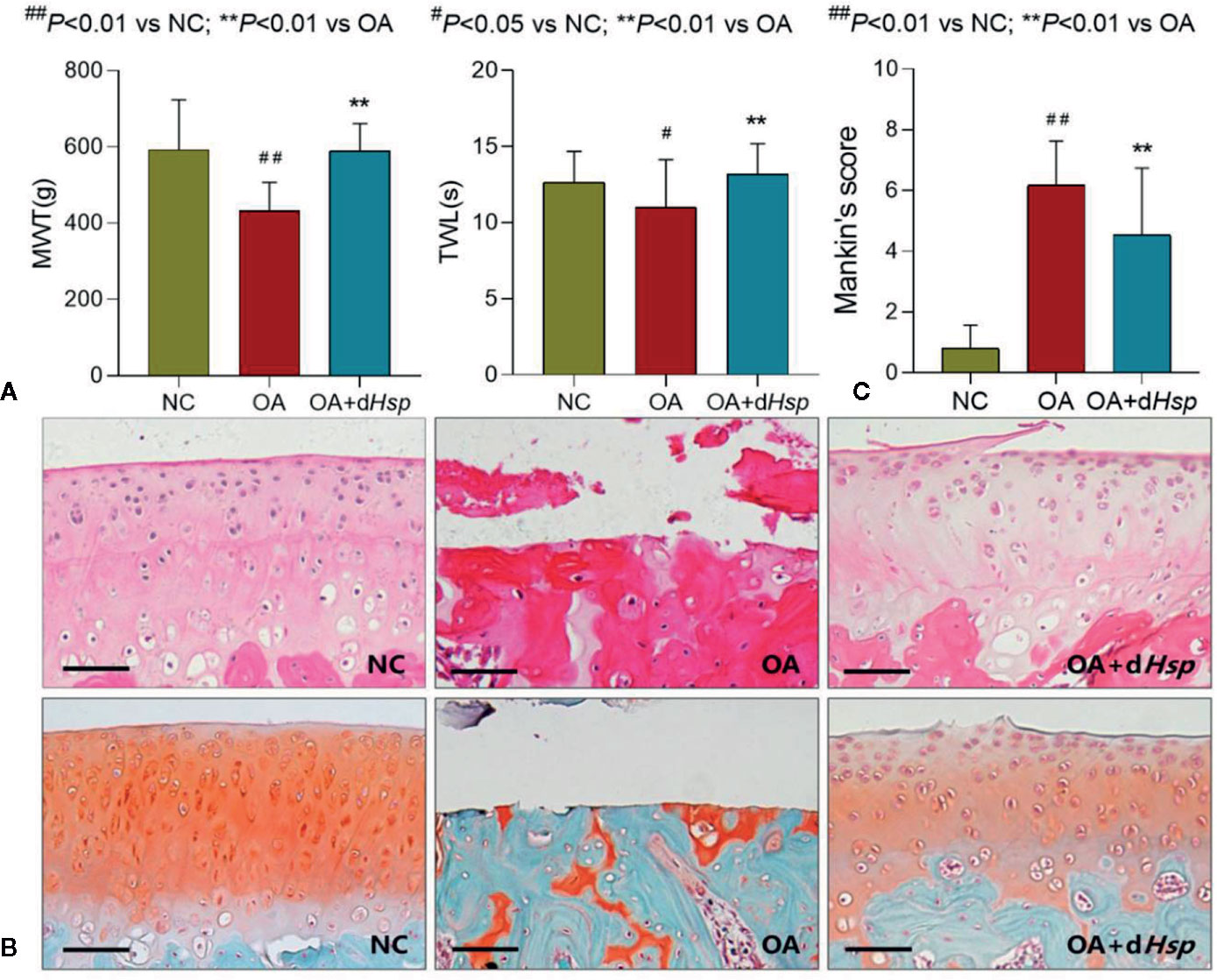
Figure 4 (A) MWT (g), TWL (s) results of the left and right hind paws of rats tested after final dHsp treatment (28 d); (B) Histopathological observation (HE and Safranin-O staining); (C) Mankin’s scoring of rat knee joints at day 28 after dHsp treatment. Values are presented as mean ± SD. #P < 0.05 vs. NC group; ##P<0.01 vs. NC group; **P<0.01 vs. OA group. Scale bar = 100 μm.
Molecular Actions of dHsp on Cartilage
The molecular actions of dHsp on the expression of OA-related genes in cartilage tissue were determined using qPCR assay. As shown in Figure 5A, MIA significantly up-regulated the expressions of Col10, Mmp2, Sox5, and Adamts4/5/9, as compared with that of NC group (all P < 0.01). After treatment of dHsp, the abnormal expressions of those genes were significantly restored as compared with that of OA group (all P < 0.05 or 0.01). There was no obvious difference of Col2 expression among all groups (P > 0.05).
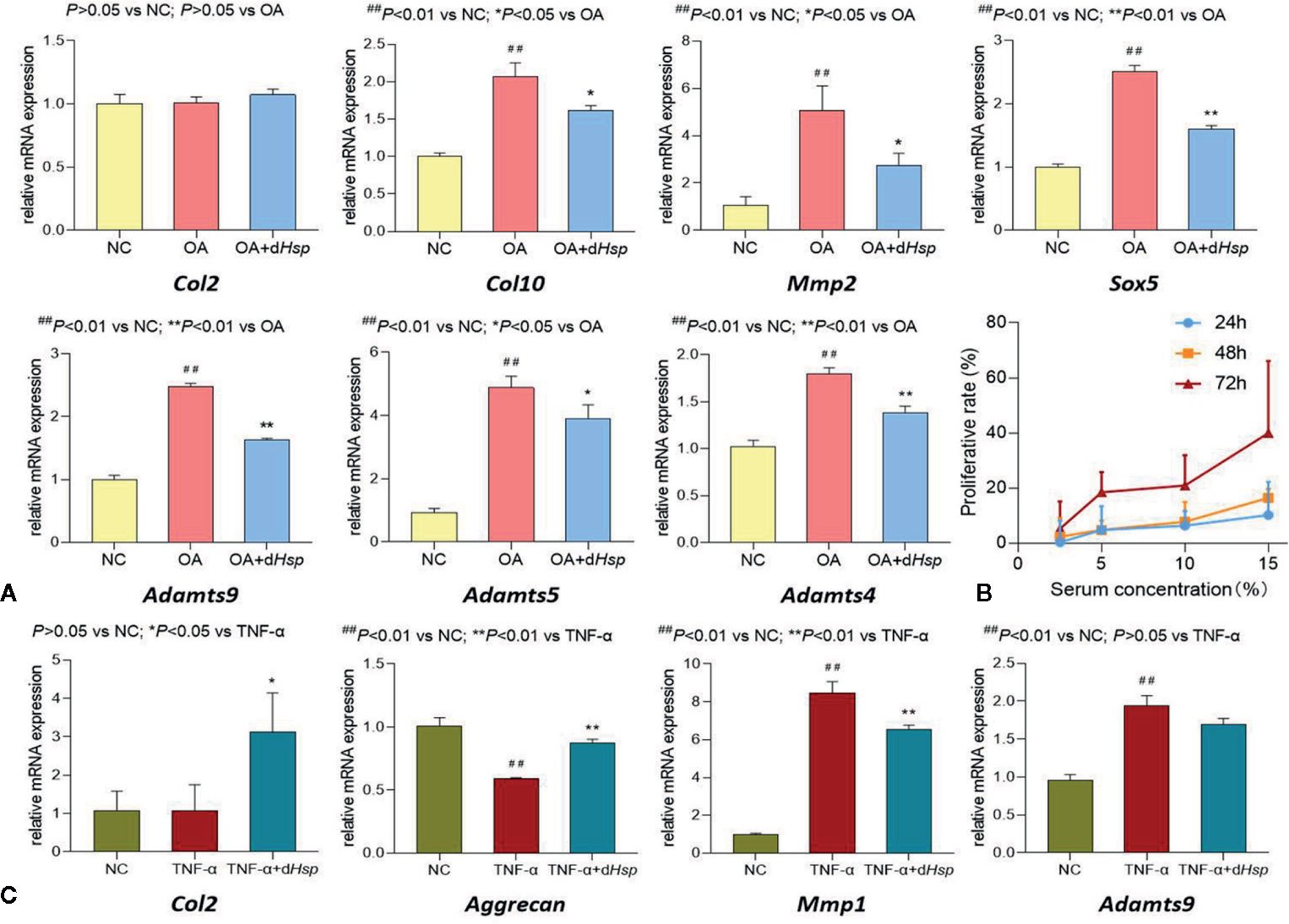
Figure 5 (A) Relative mRNA expressions of OA-related genes in cartilage tissue after oral administration of saline (NC group), saline (OA group), and 14 g/kg dHsp (OA+ dHsp group). (B) Cell viability of chondrocytes treated with dHsp-containing serum for 24, 48, and 72 h. (C) Relative mRNA expressions of target genes in chondrocytes treated with TNF-α or TNF-α plus dHsp-containing serum for 24 h. Values are presented as mean ± SD. ##P<0.01 vs. NC group; *P<0.05 or **P<0.01 vs. TNF-α group.
Cellular and Molecular Actions of dHsp-Containing Serum on Chondrocytes
To mimic the in vivo behavior of dHsp, dHsp-containing serum was applied for in vitro experiments. The proliferative effect of dHsp-containing serum on chondrocytes was assessed by MTT assay. As shown in Figure 5B, dHsp-containing serum at concentration range from 2.5% to 15% significantly increased cell viability of chondrocytes. The proliferative rate was increased from 0.3% to 10.2% after 24 h treatment, from 2.3% to 16.4% after 48 h treatment, and from 5.2% to 40.0% after 72 h treatment. It indicated that dHsp-containing serum induced proliferation of chondrocytes in concentration-dependent and time-dependent manner.
The molecular actions of dHsp-containing serum on the expression of OA related genes in chondrocytes were determined by qPCR assay. The rat primary chondrocytes were pretreated with TNF-α to induce inflammatory response for mimicking the pathological condition of OA. As shown in Figure 5C, TNF-α significantly down-regulated the mRNA expression of Aggrecan and up-regulated the mRNA expressions of Mmp1 and Adamts9, as compared with that of NC group (all P < 0.01). After 24 h treatment of dHsp-containing serum, the abnormal expressions of those genes were all restored toward the normal levels, as compared with that of TNF-α group (all P < 0.05 except for Adamts9). Similar to the in vivo result, there was no obvious difference of Col2 expression between NC and TNF-α groups (P > 0.05). Nevertheless, after 24 h treatment of dHsp-containing serum, the Col2 expression was significantly up-regulated as compared with that of TNF-α group (P < 0.05).
Cellular and Molecular Actions of dHsp-Contained Compounds on Chondrocytes
To further explore the potential mechanism of dHsp on OA, cellular and molecular effects of dHsp-contained alkaloids on chondrocytes were studied. MTT assay was conducted to evaluate the effect of aconitine, mesaconitine, hypaconitine, benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine on chondrocytes. For evaluating the contribution of each compound to dHsp, the dose range for each compound was selected in accordance with their concentrations in dHsp. For example, 5, 10, and 20 μg/ml were used as low, middle and high doses for aconitine, mesaconitine, hypaconitine, since the highest concentration of toxic aconitine in dHsp was 5.47 ± 0.16 μg/ml (hypaconitine). Moreover, 30, 60, and 120 μg/ml were used as low, middle and high doses for benzoylaconitine, 140, 280, and 560 μg/ml were used as low, middle and high doses for benzoylmesaconitine, and 45, 90, and 180 μg/ml were used as low, middle and high doses for benzoylhypaconitine, in which their middle doses were similar to their concentrations in dHsp.
As shown in Figure 6A, after 24 h treatment, aconitine, mesaconitine, and hypaconitine exerted significant inhibitory effects on chondrocytes at their dose ranges (all P < 0.01), indicating cytotoxicity of these compounds against chondrocytes. Besides, benzoylaconitine and benzoylhypaconitine at their dose ranges had non-toxic effect on chondrocytes, while benzoylmesaconitine at high dose exerted significant inhibitory effect (P < 0.01), suggesting that these compounds at their dose ranges, except benzoylmesaconitine at high dose, may contribute to the therapeutic effect of dHsp. Thus, these three compounds were selected for the following qPCR assay. As shown in Figure 6B, as compared with control group, benzoylaconitine at middle dose significantly down-regulated the expressions of Adamts4 and Adamts5 with slight down-regulatory effect on Col10 and Adamts9, while benzoylhypaconitine at middle dose significantly down-regulated the expressions of Col10, Adamts4, Adamts5, and Adamts9, suggesting these compounds as therapeutic components of dHsp. However, benzoylmesaconitine at middle dose significantly up-regulated the expressions of Adamts4, Adamts5, and Adamts9 with slight up-regulatory effect on Col10, indicating a negative contribution of this compound to dHsp.
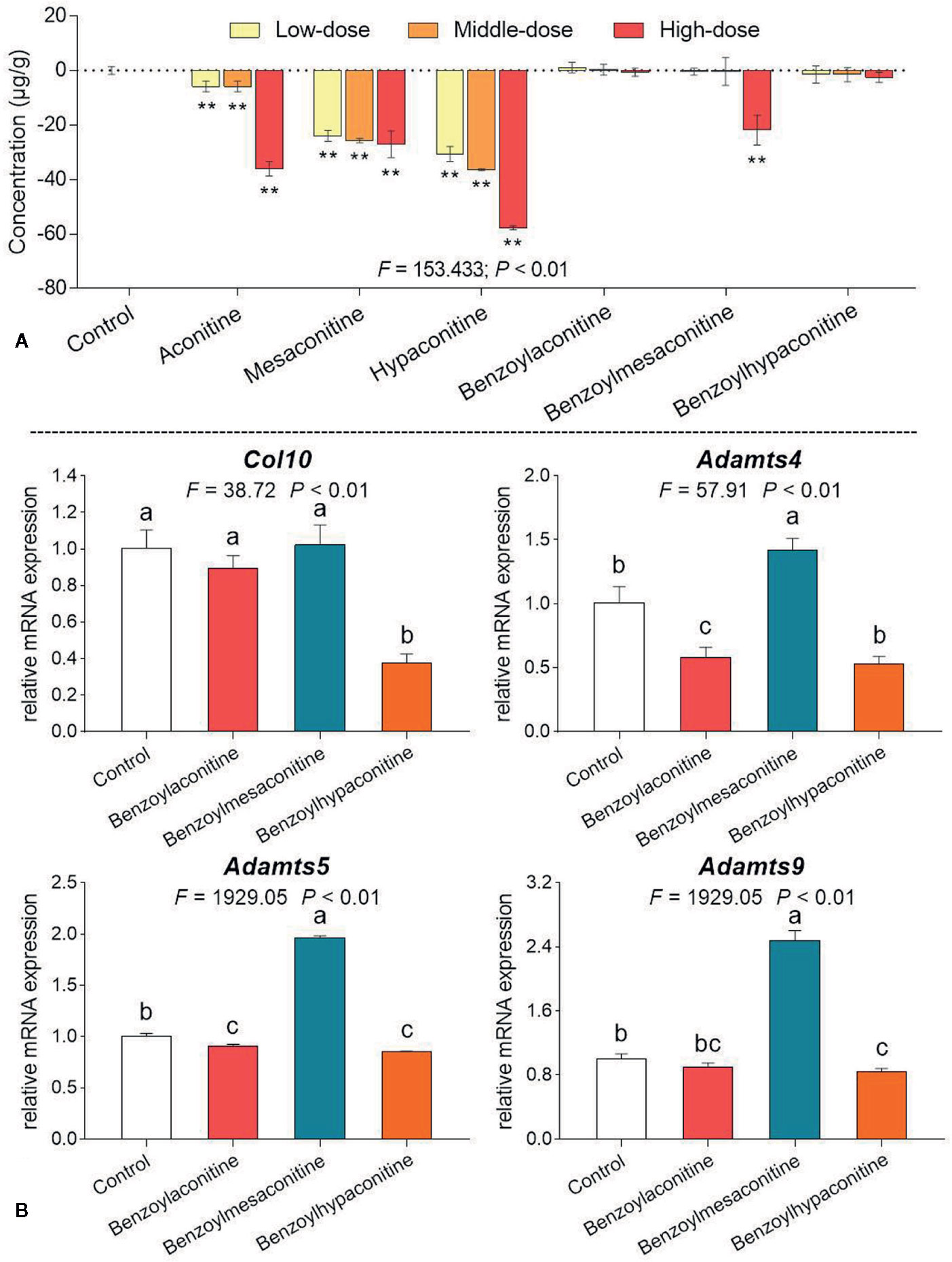
Figure 6 Cell viability of chondrocytes with treatments of aconitine, mesaconitine, hypaconitine, benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine (A) and relative mRNA expressions of OA-related genes in chondrocytes with treatments of benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine (B). Values are presented as mean ± SD. By means of LSD multiple comparisons, data with same lowercase letter indicate no significant difference between each other, while data with different letters indicate significant difference with each other. **P<0.01 vs. control group.
Discussion
Joint impairment-associated pain is the key clinical feature of OA, mainly caused by synovial neurogenic inflammation and subchondral nerve damage (Neogi et al., 2016). With the onset of joint impairment, pain occurs and progressively disables the joint capacity and movement in OA patients. Thus, it is very important to reach pain relief during OA therapy and to employ specific preclinical model of OA that can be used to evaluate not only disease-modifying effects but also analgesic activity. MIA-induced rat model is a minimally invasive, rapidly developed, and reproducible OA model, which causes histomorphological and functional joint impairment as well as pain behavior similar to human OA (Pitcher et al., 2016). MIA injected in articular cavity disrupts cartilage glycolysis and chondrocyte metabolism by inhibition of glyceraldehye-3-phosphate dehydrogenase, thereby inducing chondrocyte death, cartilage destruction and subchondral bone exposure (Lampropoulou-Adamidou et al., 2014). Subchondral bone is abundantly innervated and could potentially be a source of OA pain (Philpott et al., 2017). In addition, the severity of OA progression in this model can be controlled by MIA in a dose-dependent manner, therefore, the model of OA induced by MIA has become one of the most popular models to research the pain course and intervention effect of OA (Ogbonna et al., 2013; Chin et al., 2019).
As a commonly used TCM product, Hsp has attracted much attention for its therapeutic efficacy. However, it remains potentially toxic to patients (Liu et al., 2016). Long-time decoction is a traditional and rational method for detoxifying Hsp, the principle of which is that acetyl group at C8 and benzoyl group at C14 of DDAs are hydrolyzed under the action of water and heat, thereby reducing its content to obtain less toxic monoester-diterpenoid alkaloids and non-toxic non-esterified diterpenoid alkaloids (Yang et al., 2018). Up to now, it remains uncertain, how long time should be used and what effect it has. Previously, by means of long-time (120 min) decoction, we successfully removed the acute toxicity of Bai-fu-pian (Bfp), another kind of Fu-zi, and demonstrated unaltered therapeutic efficacy of detoxified Bfp (dBfp) on rheumatoid arthritis (Tong et al., 2013). It suggests that 120 min decoction might be effective in detoxifying Hsp. Considering the difference between Bfp (with no peel) and Hsp (with peel) as well as the more common use of Hsp in clinic, it is necessary to demonstrate whether long-time (120 min) decoction is also effective in detoxifying Hsp without alteration of therapeutic efficacy. Therefore, this study extracted Hsp with gradient decoction times (30, 60, and 120 min) and studied their acute toxicity and chemoprofiles. The results showed that, with increasing decocting time, the toxicity and concentrations of toxic alkaloids of Hsp were time-dependently decreased and the concentration of non-toxic alkaloid derivatives increased (Figures 1–3). Hsp with 120 min decoction was found to contain minimal levels of toxic components (Figure 1) and maximal levels of non-toxic derivatives (Figure 2). Also, it was non-toxic within the maximal dose range (Figure 3). In fact, 120 min is the upper time limit for the decoction, since more than 120 min would be too long to be practical. Thus, we defined the 120 min decocted Hsp as detoxicated Hsp (dHsp). Afterwards, an animal model of OA was employed to evaluate the therapeutic effect of dHsp. After 28 day treatment, the analgesic effect of dHsp was determined by assessments of mechanical and thermal sensitivity (Figure 4A). Meanwhile, the histopathological evidence combined with Mankin’s grading analysis exhibited the chondroprotective effect of dHsp. To further clarify the molecular actions of dHsp, qPCR assay was conducted on both cartilage and chondrocytes. dHsp significantly restored the abnormal expressions of Col10, Mmp2, Sox5, and Adamts4/5/9 and up-regulated the Col2 expression in damaged cartilage tissue. It also significantly restored the abnormal expressions of Aggrecan, Mmp1 and Adamts9 and up-regulated the Col2 expression in TNF-α treated chondrocytes (Figures 5A, C). Moreover, the results of MTT assay showed that dHsp significantly increased the cell viability of chondrocytes (Figure 5B). Further cellular and molecular assays showed that, among the six alkaloid components of Hsp, toxic alkaloids (aconitine, mesaconitine, and hypaconitine) were cytotoxic to chondrocytes, while benzoylaconitine and benzoylhypaconitine exhibited molecular actions similar to dHsp (Figure 6). The result suggests that benzoylaconitine and benzoylhypaconitine are therapeutic components of dHsp, which give positive contribution to the anti-OA effect of dHsp.
The destruction of cartilage in the process of OA is closely related to the degradation of cartilage extracellular matrix (ECM) (Guilak et al., 2018). The ECM of cartilage is mainly composed of collagens and proteoglycan. Col2 is the main collagen in the ECM and plays an important role in the metabolism and stability of cartilage. Col10 is another collagen existing rarely in the healthy cartilage but mainly in the degenerative cartilage. It is highly expressed by hypertrophic chondrocytes as a marker of hypertrophic regeneration. In sections of human osteoarthritic cartilage, Col10 was found around hypertrophic chondrocyte clusters in the deep zone close to tidemark (He et al., 2019). Besides the collagens, aggrecan, known as a cartilage-specific proteoglycan core protein, is another crucial component of cartilage ECM (Hu et al., 2019). In the OA state, aggrecan was significantly degraded in the cartilage, and the expression of aggrecan was inversely related to the OA progression (Eid et al., 2006). In contrary, MMPs and ADAMTSs are enzymes positively related to the OA progression, which can destroy the integrity and function of cartilage by hydrolyzing the ECM (Malemud, 2019). Of these, MMP1 exerts the strongest degradation effect on Col2 as a collagenase produced by synovial cells (Mehana et al., 2019). MMP2, known as gelatinase A, is also an important factor in the pathogenesis of OA (Zeng et al., 2015). Among the members of ADAMTSs, ADAMTS9, ADAMTS 5 and ADAMTS 4 have been shown to greatly induce degradation of cartilage aggrecan and fibrosis of collagen, resulting in the loss of cartilage compression strength and eventual occurrence of OA (J. Bondeson et al., 2008; Ohtsuki et al., 2019). Sox5 acts as a transcription factor associated with chondrogenesis. It is a highly expressed in synovium with inflammation, and the degree of cartilage destruction can be significantly reversed when the expression of Sox5 is silenced (Feng et al., 2016). In this study, we found that the molecular actions of dHsp were mediated by the regulation of gene expressions of Col2, Aggrecan, Col10, Mmp1, Mmp2, Adamts4/5/9, and Sox5 in cartilage tissue and chondrocytes, suggesting a mechanism of dHsp in association with the inhibition of ECM degradation and chondrocyte hypertrophy.
Conclusions
This is the first study reported that dHsp has anti-OA effect independent of its toxicity, which provides a safe candidate for TCM formulation in OA therapy. So far, there are two issues warranting further investigation. Firstly, dHsp has not completely reversed the OA histopathological changes in this study, since many chondrocytes remained lost on the cartilage. It might be due to the excessive severity of our MIA model. More OA models with moderate severity are needed for further evaluation the dHsp′s effect. Secondly, although the representative alkaloids in dHsp have been analyzed by this study, the roles of other components in dHsp remain unclear and need to be explored in future.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request..
Ethics Statement
The animal study was reviewed and approved by the Medical Norms and Ethics Committee of Zhejiang Chinese Medical University.
Author Contributions
LZha performed and funded the main work for revision; TL contributed to the main work of this study. RW performed the chemical analysis and wrote the paper. JX performed the MTT and qPCR assays for revision. LZho and LY contributed to the molecular experiment. ZH performed the UPLC-MS assay. HL participated in cellular experiments. FL contributed to the design of this study. WD and HW designed and funded this study. LS designed this study and finalized the data analysis. PT provided research ideas. SZ participated in the data analysis. TE improved the experimental design and the writing of this paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81774331, 81873049, 81804125), the Zhejiang Provincial Natural Science Foundation of China (Grant No. LY18H270004), the Zhejiang Provincial Science and Technology Project of Traditional Chinese Medicine of China (Grant No. 2016ZZ011), the Zhejiang Provincial Key Construction University Superiority Characteristic Discipline (Traditional Chinese Pharmacology) Opening Foundation of China (Grant No. ZYX2018006), and the Ningbo Natural Science Foundation (2014A610277).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
DDAs, diester-diterpenoid alkaloids; dHsp, detoxicated Hsp; DMSO, dimethyl sulfoxide; ECM, extracellular matrix; FBS, fetal bovine serum; HE, hematoxylin and eosin; Hsp, Hei-shun-pian; IMDM, Iscove’s modified Dulbecco’s medium; LSD, least significant difference; MIA, mono-iodoacetate; MWT, mechanical withdrawal threshold; OA, osteoarthritis; PCR, real time polymerase chain reaction; SO, safranin-O; TCM, traditional Chinese medicine; TWL, thermal withdrawal latency.
References
Bijlsma, J. W. J., Berenbaum, F., Lafeber, F. P. J. G. (2011). Osteoarthritis: an update with relevance for clinical practice. Lancet 377 (9783), 2115–2126. doi: 10.1016/s0140-6736(11)60243-2
Bondeson, J., Wainwright, S., Hughes, C., Caterson, B. (2008). The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin. Exp. Rheumatol. 26, 139–145.
Chan, T. Y. (2009). Aconite poisoning. Clin. Toxicol. (Phila) 47 (4), 279–285. doi: 10.1080/15563650902904407
Chin, K. Y., Wong, S. K., Japar Sidik, F. Z., Abdul Hamid, J., Abas, N. H., Mohd Ramli, E. S., et al. (2019). The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate. Int. J. Environ. Res. Public Health 16 (16), 2897. doi: 10.3390/ijerph16162897
China Pharmacopeia Committee (2015). Pharmacopeia of the People’s Republic of China (The First Division) (Beijing: China Chemical Industry Press), 191.
Deng, W. (2008). Clinical research on Gancao Fuzi decoction in treating osteoarthritis of knee joint. J. Chin. Med. Mater. 31 (07), 1107–1110. doi: 10.13863/j.issn1001-4454.2008.07.007
Eid, K., Thornhill, T. S., Glowacki, J. (2006). Chondrocyte gene expression in osteoarthritis: Correlation with disease severity. J. Orthop. Res. 24 (5), 1062–1068. doi: 10.1002/jor.20137
Feng, X., Shi, Y., Xu, L., Peng, Q., Wang, F., Wang, X., et al. (2016). Modulation of IL-6 induced RANKL expression in arthritic synovium by a transcription factor SOX5. Sci. Rep. 6, 32001. doi: 10.1038/srep32001
Guilak, F., Nims, R. J., Dicks, A., Wu, C. L., Meulenbelt, I. (2018). Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 71–72, 40–50. doi: 10.1016/j.matbio.2018.05.008
He, F., Wang, C. J., Xie, Y., Cheng, C. S., Liu, Z. Q., Liu, L., et al. (2017). Simultaneous quantification of nine aconitum alkaloids in Aconiti Lateralis Radix Praeparata and related products using UHPLC-QQQ-MS/MS. Sci. Rep. 7 (1), 13023. doi: 10.1038/s41598-017-13499-6
He, Y., Manon-Jensen, T., Arendt-Nielsen, L., Petersen, K. K., Christiansen, T., Samuels, J., et al. (2019). Potential diagnostic value of a type X collagen neo-epitope biomarker for knee osteoarthritis. Osteoarthritis Cartilage 27 (4), 611–620. doi: 10.1016/j.joca.2019.01.001
Hu, N., Gong, X., Yin, S., Li, Q., Chen, H., Li, Y., et al. (2019). Saxagliptin suppresses degradation of type II collagen and aggrecan in primary human chondrocytes: a therapeutic implication in osteoarthritis. Artif. Cells Nanomed. Biotechnol. 47 (1), 3239–3245. doi: 10.1080/21691401.2019.1647223
Johnson, V. L., Hunter, D. J. (2014). The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 28 (1), 5–15. doi: 10.1016/j.berh.2014.01.004
Lampropoulou-Adamidou, K., Lelovas, P., Karadimas, E. V., Liakou, C., Triantafillopoulos, I. K., Dontas, I., et al. (2014). Useful animal models for the research of osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 24 (3), 263–271. doi: 10.1007/s00590-013-1205-2
Liu, F., Shan, L., Tong, P., Zou, J., Xiao, L. (2016). Clinical study on Fuzi Tang for treatment of mild-to-moderate knee osteoarthritis with Cold-dampness stagnation syndrome. J. Tradit. Chin. Orthoped. Traumatol. 28 (01), 10–13.
Malemud, C. J. (2019). Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 165, 33–40. doi: 10.1016/j.bcp.2019.02.033
Mandl, L. A. (2019). Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage 27 (3), 359–364. doi: 10.1016/j.joca.2018.11.001
Mehana, E. E., Khafaga, A. F., El-Blehi, S. S. (2019). The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 234, 116786. doi: 10.1016/j.lfs.2019.116786
Neogi, T., Guermazi, A., Roemer, F., Nevitt, M. C., Scholz, J., Arendt-Nielsen, L., et al. (2016). Association of Joint Inflammation With Pain Sensitization in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 68 (3), 654–661. doi: 10.1002/art.39488
O’Neill, T. W., McCabe, P. S., McBeth, J. (2018). Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 32 (2), 312–326. doi: 10.1016/j.berh.2018.10.007
Ogbonna, A. C., Clark, A. K., Gentry, C., Hobbs, C., Malcangio, M. (2013). Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur. J. Pain 17 (4), 514–526. doi: 10.1002/j.1532-2149.2012.00223.x
Ohtsuki, T., Shinaoka, A., Kumagishi-Shinaoka, K., Asano, K., Hatipoglu, O. F., Inagaki, J., et al. (2019). Mechanical strain attenuates cytokine-induced ADAMTS9 expression via transient receptor potential vanilloid type 1. Exp. Cell Res. 383 (2), 111556. doi: 10.1016/j.yexcr.2019.111556
Philpott, H. T., O’Brien, M., McDougall, J. J. (2017). Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 158 (12), 2442–2451. doi: 10.1097/j.pain.0000000000001052
Pitcher, T., Sousa-Valente, J., Malcangio, M. (2016). The Monoiodoacetate Model of Osteoarthritis Pain in the Mouse. J. Vis. Exp. (111), 53746. doi: 10.3791/53746
Tang, Q. Y., Feng, M. G. (2007). DPS data processing system experimental design, statistical analysis and data mining. Beijing Sci. Press. pp. 43–80.
Tong, P., Wu, C., Wang, X., Hu, H., Jin, H., Li, C., et al. (2013). Development and assessment of a complete-detoxication strategy for Fuzi (lateral root of Aconitum carmichaeli) and its application in rheumatoid arthritis therapy. J. Ethnopharmacol. 146 (2), 562–571. doi: 10.1016/j.jep.2013.01.025
Wang, J., Zhu, X., Liu, L., Shi, X., Yin, L., Zhang, Y., et al. (2013). Effects of strontium on collagen content and expression of related genes in rat chondrocytes cultured in vitro. Biol. Trace Elem. Res. 153 (1-3), 212–219. doi: 10.1007/s12011-013-9640-9
Yang, M., Ji, X., Zuo, Z. (2018). Relationships between the Toxicities of Radix Aconiti Lateralis Preparata (Fuzi) and the Toxicokinetics of Its Main Diester-Diterpenoid Alkaloids. Toxins (Basel) 10 (10), 391. doi: 10.3390/toxins10100391
Keywords: Aconitum carmichaeli, traditional Chinese medicine, osteoarthritis, monosodium iodoacetate, pain behaviour, analgesia
Citation: Zhang L, Li T, Wang R, Xu J, Zhou L, Yan L, Hu Z, Li H, Liu F, Du W, Tong P, Wu H, Zhang S, Shan L and Efferth T (2020) Evaluation of Long-Time Decoction-Detoxicated Hei-Shun-Pian (Processed Aconitum carmichaeli Debeaux Lateral Root With Peel) for Its Acute Toxicity and Therapeutic Effect on Mono-Iodoacetate Induced Osteoarthritis. Front. Pharmacol. 11:1053. doi: 10.3389/fphar.2020.01053
Received: 08 January 2020; Accepted: 29 June 2020;
Published: 24 July 2020.
Edited by:
Jing-Yan Han, Peking University, ChinaReviewed by:
Jinming Zhang, Chengdu University of Traditional Chinese Medicine, ChinaSubhalakshmi Ghosh, Independent Researcher, Kolkata, India
Copyright © 2020 Zhang, Li, Wang, Xu, Zhou, Yan, Hu, Li, Liu, Du, Tong, Wu, Zhang, Shan and Efferth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxi Du, cHVycGxlcmFpbmVlckAxNjMuY29t; Huiling Wu, enl3aGxAemp1LmVkdS5jbg==; Letian Shan, bGV0aWFuLnNoYW5AZm94bWFpbC5jb20=
†These authors have contributed equally to this work
 Lei Zhang1†
Lei Zhang1† Rongrong Wang
Rongrong Wang Li Zhou
Li Zhou Li Yan
Li Yan Peijian Tong
Peijian Tong Shanxing Zhang
Shanxing Zhang Letian Shan
Letian Shan Thomas Efferth
Thomas Efferth