- 1Department of Rehabilitation Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
- 2Department of Physical Medicine and Rehabilitation, National Taiwan University Hospital Bei-Hu Branch, Taipei, Taiwan
- 3Department of Rehabilitation Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
- 4Rehabilitation Clinic, Bangkok Hospital Chiang Mai, Chiang Mai, Thailand
- 5Division of Pharmacy Practice, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 6Center of Health Outcomes Research and Therapeutic Safety (Cohorts), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 7Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN), School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand
- 8Division of Pharmaceutical Care, Department of Pharmacy, Phrae Hospital, Phrae, Thailand
Background: Peripheral nerve entrapment syndromes commonly result in pain, discomfort, and ensuing sensory and motor impairment. Many conservative measures have been proposed as treatment, local injection being one of those measures. Now with high-resolution ultrasound, anatomical details can be visualized allowing diagnosis and more accurate injection treatment. Ultrasound-guided injection technique using a range of injectates to mechanically release and decompress the entrapped nerves has therefore developed called hydrodissection or perineural injection therapy. Several different injectates from normal saline, local anesthetics, corticosteroids, 5% dextrose in water (D5W), and platelet-rich plasma (PRP) are available and present clinical challenges when selecting agents regarding effectiveness and safety.
Aims: To systematically search and summarize the clinical evidence and mechanism of different commonly used injectates for ultrasound-guided hydrodissection entrapment neuropathy treatment.
Methods: Four databases, including PubMed, EMBASE, Scopus, and Cochrane were systematically searched from the inception of the database up to August 22, 2020. Studies evaluating the effectiveness and safety of different commonly used injectates for ultrasound-guided hydrodissection entrapment neuropathy treatment were included. Injectate efficacy presents clinical effects on pain intensity, clinical symptoms/function, and physical performance, electrodiagnostic findings, and nerve cross-sectional areas. Safety outcomes and mechanism of action of each injectate were also described.
Results: From ten ultrasound-guided hydrodissection studies, nine studies were conducted in carpal tunnel syndrome and one study was performed in ulnar neuropathy at the elbow. All studies compared different interventions with different comparisons. Injectates included normal saline, D5W, corticosteroids, local anesthetics, hyaluronidase, and PRP. Five studies investigated PRP or PRP plus splinting comparisons. Both D5W and PRP showed a consistently favorable outcome than those in the control group or corticosteroids. The improved outcomes were also observed in comparison groups using injections with normal saline, local anesthetics, or corticosteroids, or splinting. No serious adverse events were reported. Local steroid injection side effects were reported in only one study.
Conclusion: Ultrasound-guided hydrodissection is a safe and effective treatment for peripheral nerve entrapment. Injectate selection should be considered based on the injectate mechanism, effectiveness, and safety profile.
Introduction
Peripheral nerves are susceptible to pressure-induced injury as they travel along different anatomical structures resulting in entrapment neuropathy (Trescot and Brown, 2015). Pressure-induced injury can result from mechanical compression, constriction, overstretching, or edema. The cause of compression can be exogeneous; caused by instruments or other non-bodily structures, or endogeneous; caused from the patient’s body (Toussaint et al., 2010). In cases of endogenous causes, the compression can be external to the nerve or internal, as the compressive structure originates from one of the nerve’s components itself. Entrapment may occur at various sites in the body whether between muscles or bones, around blood vessels, across joints, and through tunnels or fascial penetration sites (Toussaint et al., 2010). The most common site of entrapment is the median nerve at the wrist or carpal tunnel syndrome (CTS) and the second most common is the ulnar nerve at the elbow or cubital tunnel syndrome (CuTS) (Doughty and Bowley, 2019). Other reported less common sites include lateral femoral cutaneous nerve, lateral antebrachial cutaneous nerve, and medial superior cluneal nerves (Tagliafico et al., 2011; Chang et al., 2017; Wu and Boudier-Revéret, 2019). Entrapment can disturb sensory and/or motor function resulting in neuropathic pain, discomfort, and weakness (Toussaint et al., 2010; Schmid et al., 2013). Nerve compression leads to segmental intraneural ischemia disrupting the blood-nerve barrier and dysfunction of the intraneural circulation, intraneural edema formation, and ectopic impulse generation of both mechanosensitive and nociceptive neurons resulting in neuropathic pain with varying severity (Schmid et al., 2013; Trescot and Brown, 2015). Activated C-fibers may produce and release pain-producing and degenerative neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) resulting in chronic neurogenic inflammation (Ji et al., 2018). With prolonged compression, demyelination and axonal loss follow, as well as nerve fascicles swelling leading to epineural fibrosis. Many treatment options are available to counter the effect of entrapment, conservative measures include splinting, tendon and nerve gliding exercise, physical modalities, and corticosteroids injection (Huisstede et al., 2010; Kooner et al., 2019). Patients who respond poorly to those measures become candidates for surgical decompression or reconstruction (Lauder et al., 2019). At present, high-resolution ultrasound plays important role in the diagnosis of entrapment neuropathy and guided injection delivering a range of injectates, for example, normal saline, corticosteroids, local anesthetics, dextrose, and platelet-rich plasma (PRP). This procedure, known as hydrodissection or perineural injection, provides not only a mechanical effect to release and decompress the entrapped nerves but also a pharmacological effect relieving pain and promoting recovery through numerous mechanisms (Chang et al., 2020; Lam et al., 2020; Reeves and Rabago, 2020). There has been a considerable increase in interest and publications of this procedure regarding the benefits and disadvantages or adverse effects of each different agent (Catapano et al., 2020; Lam et al., 2020; Lin et al., 2020). As clinicians planning to perform such a procedure, agent selection is usually based on effectiveness and safety. Therefore, the present systematic review aims to present the effectiveness and safety of different commonly used injectates for ultrasound-guided hydrodissection entrapment neuropathy treatment, explain relevant mechanism of action and discuss practical issues with agent selection as well as highlight knowledge gaps found.
Methods
This systematic review was carried out and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009).
Data Sources and Search Strategy
EMBASE, Scopus, Cochrane, and PubMed were systematically searched from their establishment to August 22, 2020. The Medical Subject Headings (MeSH) were utilized as applicable. The bibliography lists of associated articles were explored. Strategic search terms included “nerve hydrodissection”, “injectates”, “steroid”, “saline”, “platelet-rich plasma”, and “5% dextrose” with slight modifications based on the database. There was no language restriction.
Study Selection
From these articles, the included studies were selected according to the following criteria: 1) carried out in patients age over 18 years with peripheral nerve entrapment syndrome; 2) patients received guided ultrasound; and 3) clinical effects of intervention were evaluated comparing perineural injections with non-surgical treatments for peripheral nerve entrapment syndrome. Animal studies and studies are not displayed as original research such as comments, expert opinions, case reports, case series, conference meeting abstracts, surveys, reviews, editorials, systematic reviews, meta-analyses, observational study, and letters were excluded. Two investigators (M.B. and S.K.) separately assessed each title, abstract, and full-text article for possibly eligible studies. Disagreements were resolved by consensus.
Data Extraction and Outcome Measures
Data extractions from all possibly appropriate articles were performed independently by the two reviewers (M.B. and S.K.). When discrepancies occurred, they were resolved by consensus discussions with a third reviewer (S.S.). The data extracted and described included the following: region, study design, diagnosis, treatment allocation, characteristics of participants (such as age, sex, and the number of participants), follow-up interval, efficacy outcome, and safety outcome. The outcomes of interest were pain, measured by visual analog scale (VAS), clinical symptoms and function measured by the Boston Carpal Tunnel Questionnaire (BCTQ) separately as a symptom severity scale (BCTQs) and a functional status scale (BCTQf) or as a single combined scale (BCTQ combined), and lastly, by the Quick-Disability of Arm Shoulder and Hand (Q-DASH) questionnaire. Also used were participant-rated clinical outcome assessments by subjective symptom changes and global assessment of treatment results, other physical performances were measured by finger pinch strength (kg), monofilament testing score, static and dynamic two-point discrimination scores. Electrodiagnostic findings (EDS) were measured by sensory nerve conduction velocity (SNCV, m/s), distal motor latency (DML, ms), motor nerve conduction study (MNCS, m/s), distal compound motor action potential amplitude (CMAP, mV), sensory latency (ms), and sensory nerve action potential amplitude (SNAP, mV), and ultrasound measurement of nerve cross-sectional area (CSA).
Quality Assessment
The quality of the individual study was appraised independently by two investigators (S.K. and S.S.) using the Cochrane Risk-of-bias tool 2.0 (RoB 2.0) for randomized controlled trials. This tool includes six domains for methodological evaluation: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, and 6) selective reporting. Each study was classified as having a low risk, high risk, or unclear risk. Disagreements were resolved by discussion.
Statistical Analysis
Overall effects were analyzed and stratified according to clinical effect and intervention for treating peripheral nerve entrapment syndrome. If data was available, a pairwise or network meta-analysis with a DerSimonian-Laird random-effects model was used to estimate treatment effects, pooled risk ratios (RR), or weighted mean differences (WMD) along with 95% confidence intervals (CI) for dichotomous and continuous outcomes, respectively. Statistical heterogeneity between studies was assessed using the I2 values. I2 values lower around 25%, 25%–75%, and greater than 75% indicate low, moderate, and high heterogeneity, respectively (DerSimonian and Laird, 1986; Higgins et al., 2003). The software used for data analysis was STATA version 14 (STATA Corp, College Station, TX, USA).
Results
Study Selection
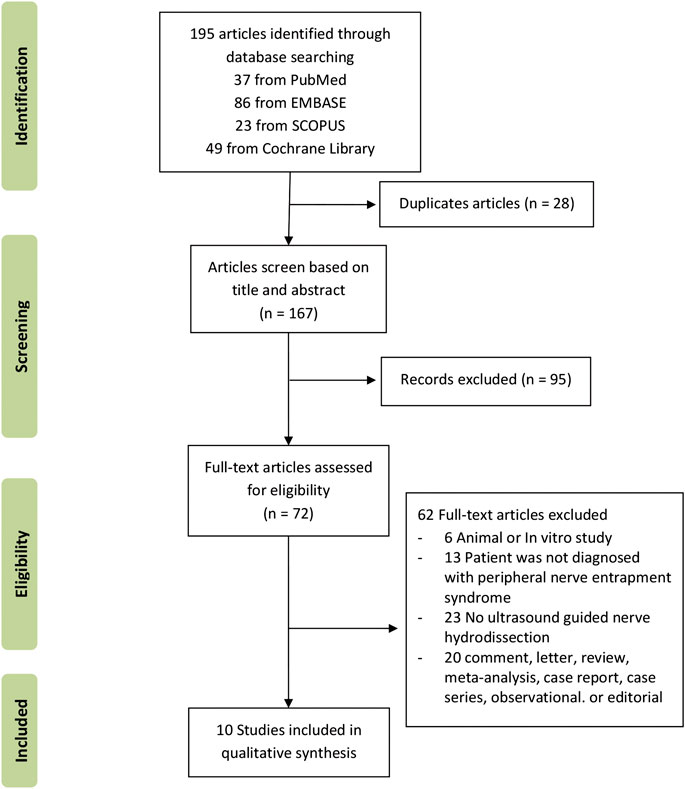
A total of 195 records were identified through database searching (n = 195). A total of 167 records remained after duplicates were removed. Of the remaining 167 records, ninety-five were deemed ineligible based on title and abstract. Of the 72 articles qualified for a full-text review, sixty-two full-text articles were excluded because they did not meet the study eligibility criteria. The flow chart in Figure 1 presents the results describing the exclusions at different stages during the review process. Ten studies were included in this systematic review (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019).
Characteristics of Included Studies
The general characteristics of the included studies are presented in Table 1. Of the ten included studies, seven studies were conducted in patients with mild to moderate carpal tunnel syndrome (CTS), two in patients with moderate carpal tunnel syndrome (CTS), and one in patients with cubital tunnel syndrome (CuTS). Four studies were from Taiwan, two were from Egypt, one was from Turkey, one was from Greece, one was from the United States and one was from the Netherlands. The study design of the ten studies included five randomized double-blind controlled trials, three randomized single-blind controlled trials, one triple-blind randomized controlled trial, and one prospective quasi-experimental trial. This systematic review included 569 patients with 570 affected wrists. The majority (>75%) of the overall participants were women in eight out of ten studies. The average age in the patient group in the included studies ranged from 38.3 to 66.1 years.
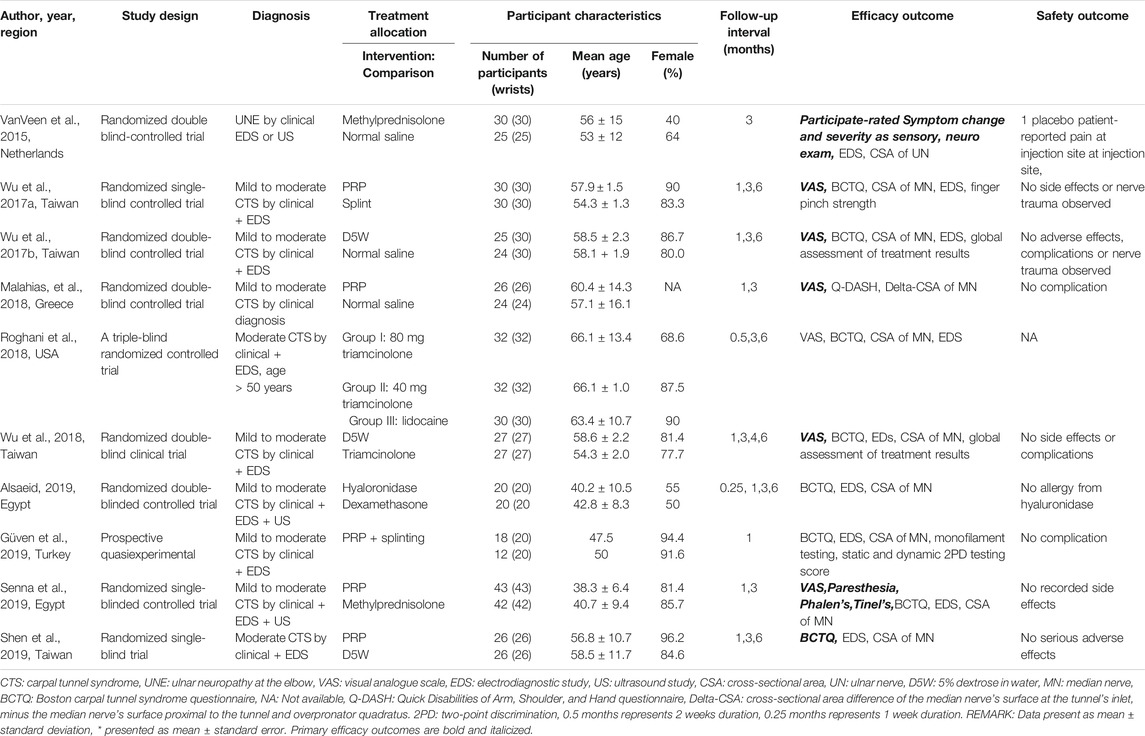
TABLE 1. Characteristics of the included randomized controlled studies using different injectates (corticosteroids, dextrose, PRP) for ultrasound-guided injection treatment of peripheral nerve entrapment.
All ten studies compared the different ultrasound-guided interventions to different comparison injectate or other conservative treatment methods, none compared a matched intervention and comparison group. Intervention injectate ranges from corticosteroids, 5% dextrose (D5W), platelet-rich plasma alone, or platelet-rich plasma (PRP) combined with splinting as an intervention and hyaluronidase. Three studies used normal saline (NSS) as a control injectate, each study compared corticosteroids, D5W, and PRP, respectively, as an intervention to NSS control (van Veen et al., 2015; Wu et al., 2017b; Malahias et al., 2018). Two studies used splinting as a control conservative treatment method, each study compared PRP and PRP combined with splinting, respectively, as an intervention to splinting control (Wu et al., 2017a; Güven et al., 2019). The remaining five studies compared two different injectates or different doses of an injectate as the following details, one study compared D5W with corticosteroids, one study compared different doses of corticosteroids with local anesthetics, one study compared hyaluronidase with corticosteroids as an adjuvant to local anesthetics (LA), one study compared PRP with steroid and one study compared PRP with D5W (Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Senna et al., 2019; Shen et al., 2019). Regarding efficacy outcome measurement used, the visual analog scale for pain (VAS) was used in six studies (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Senna et al., 2019). Clinical symptoms and function measured by the Boston carpal tunnel questionnaire (BCTQ) separately as a symptom severity scale (BCTQs) and functional status scale (BCTQf) were used in seven studies (Wu et al., 2017a; Wu et al., 2017b; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). BCTQ was used as a combined single scale in one study (Roghani et al., 2018). Participant-rated clinical outcome assessment by subjective symptom change was used in one study (van Veen et al., 2015) and two studies by the same investigator used a global assessment of treatment results as a participant-rated tool (Wu et al., 2017b; Wu et al., 2018). For physical performance, one study measured finger pinch strength (Wu et al., 2017a), one study measured monofilament testing scores, static and dynamic two-point discrimination scores (Güven et al., 2019). Nine studies measured electrodiagnostic parameters (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). All ten studies measured the cross-sectional area of the investigated nerve (CSA) (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). VAS was the primary outcome in six studies (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Senna et al., 2019), while BTCQ was the primary outcome in one study (Shen et al., 2019). All ten studies used an in-plane ultrasound-guided injection technique (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). The shortest duration for post-injection follow up was at one week (0.25 months) interval in one study (Alsaeid, 2019), the maximum follow-up duration was six months in six studies (Wu et al., 2017a; Wu et al., 2017b; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Shen et al., 2019). Nine studies reported side effects or adverse events outcomes (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). Only one of those nine studies reported adverse events after injection while the other eight studies reported no post-injection side effects or adverse events (van Veen et al., 2015). One study, however, did not mention these side effects or adverse events outcomes (Roghani et al., 2018).
Assessment of Risk of Bias
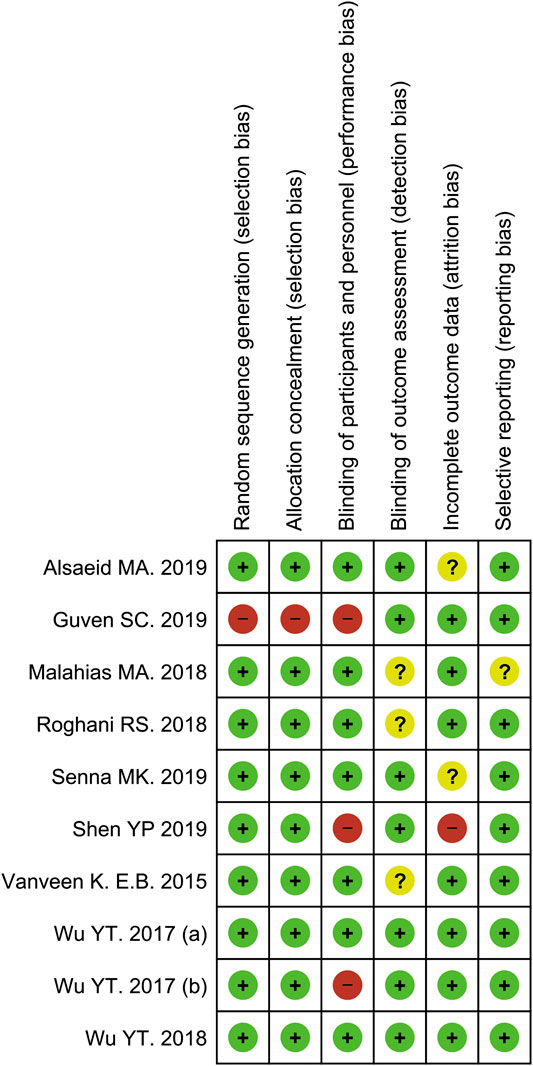
The methodological quality assessments of the included studies were revealed with the Cochrane risk of bias 2.0 tool. In this analysis, two studies were classified as low risk of bias (Wu et al., 2017b; Wu et al., 2018), three studies yielded a high risk of bias (Wu et al., 2017a; Güven et al., 2019; Shen et al., 2019), with the remaining five studies had an unclear risk of bias (van Veen et al., 2015; Malahias et al., 2018; Roghani et al., 2018; Alsaeid, 2019; Senna et al., 2019). Details of the quality assessment by the Cochrane risk of bias 2.0 tool is presented in Figures 2, 3.
Effect on Pain Intensity (VAS)
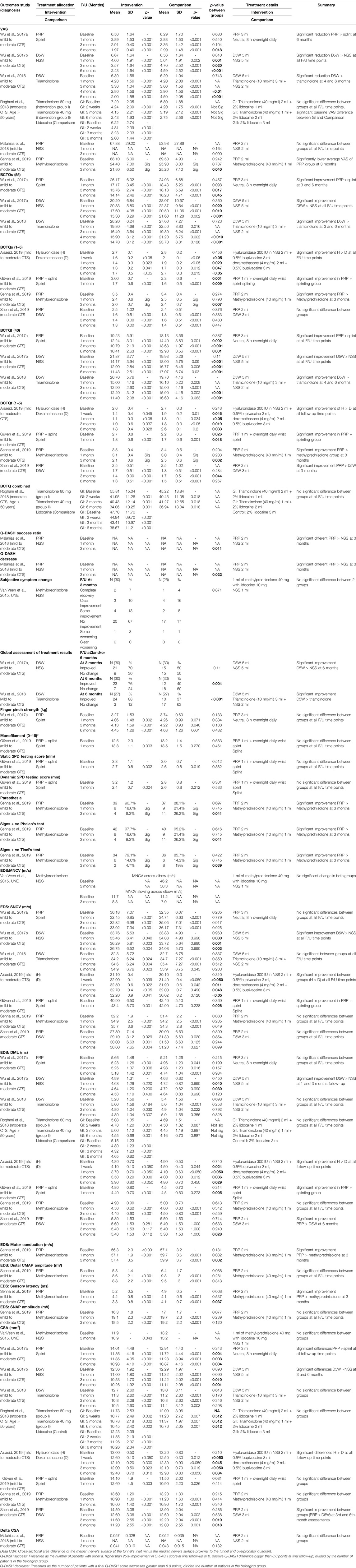
To measure pain intensity, a visual analog scale (VAS) was used in six studies Wu et al., 2017a; Wu et al., 2017b; Wu et al., 2018; Roghani et al.,2018; Malahias et al.,2018, and Senna et al.,2019) (Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2017a; Wu et al., 2017b; Wu et al., 2018). Two studies by Wu et al., 2017b and Malahias et al., 2018 used hydrodissection with normal saline as a control group (Malahias et al., 2018; Wu et al., 2017b). A study by Wu et al., 2017b compared D5W with normal saline as a control group (Wu et al., 2017b). A study by Malahias et al. compared PRP with normal saline as a control group (Malahias et al., 2018). Both studies showed greater VAS reduction in the intervention group, however, the difference between groups was not significant in a Malahias et al. study (p = 0.09) (Malahias et al., 2018). In a study by Wu et al., 2017b, there was a significant VAS reduction between both groups (D5W vs NSS) at all follow up time points at 1, 3,6 months (mean differences: −2.07, 95% CI = −1.15 to −2.99, p < 0.001 at one month; −3.1, 95% CI = −2.25 to −3.95, p < 0.001 at 3 months; −4.24, 95% CI = −3.39 to −5.09, p < 0.001 at 6 months) (Wu et al., 2017b). A study by Wu et al., 2017a that compared PRP vs splinting as a control group, showed significantly greater VAS reduction in PRP group at 6 months (mean difference: −4.53, 95% CI = −3.91 to −5.15, p < 0.001). Both groups showed significant VAS reduction at all follow-up time points at 1, 3, 6 months (Wu et al., 2017a). A study by Wu et al., 2018 compared D5W with triamcinolone, showed significantly greater VAS reduction in the D5W group at four months (mean difference: −3.6, 95% CI = −2.6 to −4.5, p < 0.001) and six months (mean difference: −4.3, 95% CI = −3.2 to −5.4, p < 0.001) with the greatest difference between group observed at six months (Wu et al., 2018). A study by Senna et al. compared PRP with methylprednisolone showed significantly lower average VAS in the PRP group at 3 months follow-up (mean difference: −46.3, 95% CI = −43.62 to −48.98, p < 0.001) see Table 2 for p between groups (Senna et al., 2019). Both groups showed significant VAS reduction at all follow-up time points at 1, 3, 4, 6 months (Wu et al., 2018). A study by Roghani et al. compared two different doses of steroids (80 mg vs 40 mg triamcinolone) vs local anesthetics (2% lidocaine) as a control group, showed no significant VAS differences between groups at all follow-up time points at 2 weeks, 3, 6 months (Roghani et al., 2018). Nevertheless, each of the three groups showed a significant VAS reduction within-group at all follow-up time points (Roghani et al., 2018).
Effect on Clinical Symptoms, Function, and Physical Performance
Standardized outcome measures specific for carpal tunnel syndrome and upper extremity disorders were used in nine carpal tunnel syndrome studies (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). The Boston Carpal Tunnel Syndrome questionnaire (BCTQ) was used in eight studies, except Malahias et al. which used the Quick Disability of Arms Shoulders and Hands (Q-DASH) questionnaire. For the ulnar nerve entrapment study by vanVeen et al. the authors developed a 6-point subjective symptom change scoring system for patients to rate their symptoms (van Veen et al., 2015). Two studies, Wu et al., 2017b and Wu et al., 2018 added global assessment of treatment results as another patient-rated outcome measure (Wu et al., 2017b; Wu et al., 2018). Physical performance including finger pinch strength, monofilament test scores, static 2-point discrimination test (static 2PD) and dynamic 2-point discrimination test (dynamic 2PD), paresthesia symptoms, positive Tinel’s sign, and Phalen’s test. Finger pinch strength were measured by Wu et al. (Wu et al., 2017a). Monofilament test scores, static 2-point discrimination test (static 2PD), and dynamic two point discrimination test (dynamic 2PD) were measured by Güven et al. (Güven et al., 2019). Paresthesia symptoms, positive Tinel’s sign, and Phalen’s test were measured by Senna et al. (Senna et al., 2019).
Boston Carpal Tunnel Syndrome Questionnaire (BCTQ)
For BCTQs (55) outcomes, Two studies Wu et al., 2017b and Wu et al., 2018 used D5W as an intervention group (Wu et al., 2017b; Wu et al., 2018). A study by Wu et al., 2018 compared D5W with triamcinolone. (Wu et al., 2018). Another study (Wu et al., 2017b in 2017 compared D5W with normal saline (Wu et al., 2017b). Both studies showed greater results on BCTQs (55) in the intervention group. In a study by Wu et al., 2017b showed significant improvement BCTQs (55) in the D5W group at all follow up time points; at 1 month (mean difference: −9.37, 95% CI = −6.09 to −12.65, p < 0.001), 3 months (mean difference: −12.6, 95% CI = −9.63 to −15.57, p < 0.001), and 6 months (mean difference: −14.9, 95% CI = −12.13 to −17.67, p < 0.001) with difference between group observed after 3 months (Wu et al., 2017b). In the same way of another research by Wu et al., 2017a compared PRP with splinting as a control group, there was a significant improvement BCTQs (55) at 3 and 6 months (mean differences: −10.41, 95% CI = −7.99 to −12.83, p < 0.001 and −12.03, 95% CI = −9.65 to −14.41, p < 0.001) with the observed difference between groups after three months as well (Wu et al., 2018).
For BCTQs (1–5) outcomes, two studies (Alsaeid and Senna et al.) used corticosteroid medications (dexamethasone and methylprednisolone) as a comparison group (Alsaeid, 2019; Senna et al., 2019). A study by Alsaeid compared dexamethasone with hyaluronidase as an intervention group. This study showed significant BCTQs (1–5) improvement in hyaluronidase group at all follow up time points (mean differences were: −1.1, 95% CI = −0.99 to −1.20, p < 0.05 at 1 week; −1.3, 95% CI = −1.16 to −1.44, p = 0.023 at 1 month; −1.4, 95% CI = −1.29 to −1.50, p = 0.041 at 3 months; −1, 95% CI = −0.77 to −1.23, p < 0.05 at 6 months) (Alsaeid, 2019). Similarly in a Senna et al. study, which compared methylprednisolone with PRP, the result showed significant BCTQs (1–5) improvement in PRP group at 3 months (mean difference: −1.5, 95% CI = −1.26 to −1.74, p < 0.001) (Senna et al., 2019). Furthermore, in a study by Shen et al., using PRP compared with D5W, the results did not show significant difference of BCTQs (1–5) between groups at all follow up time points (Shen et al., 2019). This might imply that both PRP and D5W gave positive clinical symptom effect for moderate CTS.
For BCTQf (40) measurement, two studies by Wu et al., 2017b and Wu et al., 2018 used D5W as an intervention group (Wu et al., 2017b; Wu et al., 2018). A study by Wu et al., 2017b compared D5W with normal saline (Wu et al., 2017b). Another study by Wu et al., 2018 compared D5W with triamcinolone (Wu et al., 2018). Both studies showed positive result on D5W group in BCTQf (40), which presented significant BCTQf (40) improvement at 1, 3, 6 months (mean differences were: −6.99, 95% CI = −4.57 to −9.41, p < 0.001 at 1 month; −8.44, 95% CI = −6.14 to −10.74, p < 0.001 at 3 months; −8.82, 95% CI = -6.46 to −11.18, p < 0.001 at 6 months) and 4, 6 months (mean differences: −8.5, 95% CI −5.97 to −11.03, p < 0.001 at 4 months and −9.3, 95% CI = −6.93 to −11.67, p < 0.001 at 6 months) respectively (Wu et al., 2017b; Wu et al., 2018).
For BCTQf (1–5) measurement, Two studies (Alsaeid and Senna et al.) used corticosteroid medication (dexamethasone and methylprednisolone) as a comparison group (Alsaeid, 2019; Senna et al., 2019). A study by Alsaeid compared dexamethasone with hyaluronidase as an intervention group. This study showed significant BCTQf (1–5) improvement in hyaluronidase group at all follow up time points (mean differences: −1.2, 95% CI = −0.94 to −1.46, p = 0.045 at 1 week; −1.5, 95% CI = −1.27 to −1.73, p < 0.05 at 1 month; −1.6, 95% CI = −1.27 to −1.92, p = 0.037 at 3 months; −0.8, 95% CI = −0.54 to −1.06, p = 0.028 at 6 months) (Alsaeid, 2019). Similarly with Senna et al. which compared Methylprednisolone with PRP as an intervention group. The result showed significant positive effect on PRP in BCTQf (1–5) at 3 months (mean difference: −1.4, 95% CI = −1.18 to −1.62, p < 0.001) (Senna et al., 2019). Another PRP study, Güven et al. studied mild to moderate CTS, compared PRP plus splinting with splinting alone, delta analysis showed significantly greater improvement in PRP plus splinting group (p = 0.018) (Güven et al., 2019).
For BCTQ combined score (BCTQ combined), used in a study by Roghani et al. compared triamcinolone 80 mg, triamcinolone 40 mg, and lidocaine as a comparison group. The results did not show a significant difference between all three groups at all follow-up time points (Roghani et al., 2018).
For Q-DASH success ratio and Q-DASH decrease, the study by Malahias et al. used PRP as an intervention group, which compared with NSS as a comparison group. This results showed significantly greater improvement in PRP comparing to the NSS group at 3 months (Malahias et al., 2018).
Subjective Symptom Changes and Global Assessment of Treatment Results
In the subjective symptom change, the study by vanVeen et al. compared methylprednisolone with NSS as a comparison group, which did not present a significant difference between methylprednisolone and NSS groups at all follow-up time points (van Veen et al., 2015). On the other hand, for global assessment of treatment results. two studies by Wu et al., 2017b and Wu et al., 2018 used D5W as an intervention group. Each study compared D5W with NSS and triamcinolone, respectively. Both studies showed significantly greater improvement on a global assessment of treatment results in the D5W group at 6 months (Wu et al., 2017b; Wu et al., 2018).
Physical Performance
Two studies by Wu et al., 2017a and Güven et al. used splinting as a comparison group. The study by Wu et al., 2017a compared splinting with PRP as an intervention group, whose results did not show a significant difference between groups on finger pinch strength at all follow-up time points (Wu et al., 2017a). Similarly, a study by Güven et al., compared splinting with PRP as an intervention group. The result does not present a significant difference between groups on monofilament, static 2PD test, and dynamic 2PD test at all follow-up time points (Güven et al., 2019). Another study by Senna et al. used PRP as an intervention group, comparing with methylprednisolone. The results showed significant improvement on paresthesia (p-value between-group = 0.041), positive Phalen’s test (p-value between groups = 0.041), and positive Tinel’s sign (p-value between groups = 0.039) in PRP group at three months (Senna et al., 2019).
Effect on an Electrodiagnostic Study (EDS)
Of the ten studies, nine studies in carpal tunnel syndrome patients had EDS performed on median nerves (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). Only one study in cubital tunnel syndrome patients had EDS performed on the ulnar nerve (van Veen et al., 2015). Sensory nerve conduction velocity (SNCV) and distal motor latency (DML) are the most commonly evaluated parameters as they were evaluated in all nine carpal tunnel syndrome studies.
SNCV was measured in median nerve studies. Three of the studies, studied in mild to moderate CTS, showed significant improvement between groups at all follow-up time points. Wu et al., 2017b compared injectate with NSS as a control, showed significantly greater improvement in D5W than NSS group at 1, 3 and 6 months (mean differences: 1.70, 95% CI = 1.39 to 4.79, p = 0.040 at 1 month; 2.53, 95% CI = 0.40 to 5.46, p = 0.003 at 3 months; 2.99, 95% CI = 0.13 to 6.11, p = 0.004 at 6 months) (Wu et al., 2017b). In another study, Alsaeid compared injectate with injectate, showed significantly greater improvement in hyaluronidase group than dexamethasone group at 1 week, first, third, and sixth month (mean differences were: 1.80, 95% CI = 1.61 to 1.99, p = 0.039 at 1 week; 1.40, 95% CI = 1.07 to 1.73, p = 0.022 at 1 month; 1.60, 95% CI = 1.34 to 1.86, p < 0.050 at 3 months; 1.10, 95% CI = 0.65 to 1.55, p = 0.041 at 6 months) (Alsaeid, 2019). Güven et al. studied in mild to moderate CTS, compared PRP plus splinting with splinting alone, delta analysis showed significantly greater improvement in PRP plus splinting group (p = 0.026) (Güven et al., 2019).
DML was also measured in the median nerve study. Four of the studies showed significant improvement between groups. Wu et al., 2017b studied mild to moderate CTS, compared D5W with NSS as a control, showed significantly greater improvement in dextrose group than NSS group at 1 and 3 months (mean differences were: −0.21, 95% CI = −0.45 to −0.87, p = 0.220 at 1 month; −0.25, 95% CI = −0.40 to −0.90, p = 0.200 at 3 months) (Wu et al., 2017b). Alsaeid study of mild to moderate CTS, compared hyaluronidase with dexamethasone, showing significantly greater improvement in the hyaluronidase group at all follow-up time points (mean differences were: 0.70, 95%CI = -0.38 to -1.02, p < 0.050 at 1 week, −1.10, 95% CI = −0.65 to −1.55, p < 0.050 at 1 month; −1.30, 95%CI = −0.97 to −1.63, p = 0.030 at 3 months; −0.90, 95% CI = −0.42 to −1.38, p < 0.050 at 6 months) (Alsaeid, 2019). Güven et al. studied in mild to moderate CTS, compared PRP plus splinting with splinting alone, delta analysis showed significantly greater improvement in the PRP plus splinting group (p = 0.005) (Güven et al., 2019). Shen et al. studied moderate CTS, compared PRP with D5W, the results showed significantly greater improvement in the PRP group at six months (mean differences: −0.4, 95% CI = −0.45 to −1.25, p = 0.112) (Shen et al., 2019).
Sensory latency was measured in a study by Senna et al. There was significantly greater improvement in the PRP group than methylprednisolone group at three months (mean difference: −1.40, 95% CI = −1.11 to −1.69, p < 0.001) (Senna et al., 2019). Distal CMAP amplitude and SNAP amplitude was measured also in a study by Senna et al. However, there were no significant differences between the PRP and methylprednisolone groups (Senna et al., 2019).
A study by vanVeen et al. measured MNCV of ulnar nerve across the elbow and MNCV slowing across the elbow. However, there were no significant differences between the methylprednisolone and NSS groups (van Veen et al., 2015).
Effect on Nerve Cross-Sectional Area (CSA)
All ten studies measured the CSA of the studied nerve (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). Two of the studies showed significant different improvements between groups in longer follow-up assessments (at 3 and 6 months), one was a study by Wu et al., 2017b and another was by Shen et al. (Wu et al., 2017b; Shen et al., 2019). A study by Wu et al., 2017b, comparing D5W with NSS as a control, showed significantly greater improvement in dextrose group than NSS group (mean difference: −1.83, 95% CI = −0.89 to −2.77, p < 0.001 at 3 months; −2.11, 95% CI = −1.11 to −3.09, p < 0.001 at 6 months) (Wu et al., 2017b). In a study by Shen et al., comparing PRP with D5W, showed significantly greater improvement in PRP group than dextrose group (mean difference: −2.9, 95% CI = −1.33 to −4.47, p < 0.001 at 3 months; −3.3, 95% CI = −1.73 to −4.87, p < 0.001, at 6 months) (Shen et al., 2019). A study by Wu et al. 2017(a), comparing PRP with splinting, showed significant improvement between groups at all follow up time points at 1, 3 and 6 months (mean difference: −2.15, 95% CI = −0.09 to −4.39, p < 0.001 at 1 month; −2.66, 95%CI = −0.45 to −4.87, p < 0.001 at 3 months; −3.08, 95%CI = −0.86 to −5.30, p < 0.001 at 6 months) (Wu et al., 2017a).
Safety Outcomes
Adverse effects were reported in only one study on ulnar nerve entrapment at the elbow by vanVeen et al.; comparing methylprednisolone and NSS. Five patients reported a complication. One of the five patients received a placebo and reported pain at the site of injection (n = 25 in the placebo group, 4%). Four patients were treated with methylprednisolone, one reported swelling at the injection site, one had pain at the injection site, one had a swollen hand, and one had depigmentation at the injection site (n = 30 in methylprednisolone group, 13.3%) (van Veen et al., 2015). One study did not report adverse effects (Roghani et al., 2018). Seven studies reported no complications, nerve trauma, or serious adverse effects observed during the study (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Wu et al., 2018; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). One study reported no allergy to hyaluronidase (Alsaeid, 2019).
Discussion
To the author’s knowledge, this study is the only systematic review selecting only ultrasound-guided hydrodissection articles. This systematic review retrieved ten eligible studies on ultrasound-guided hydrodissection for treatment of entrapment neuropathy with different injectates (van Veen et al., 2015; Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). The majority of studies were conducted in patients with mild to moderate carpal tunnel syndrome (CTS), the most common entrapment neuropathy (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Wu et al., 2018; Alsaeid, 2019; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019). All studies compared different interventions with different factors, none of the studies could be matched, therefore, a pairwise or network meta-analysis was infeasible. The authors selected studies using ultrasound-guided hydrodissection so that any clinical effect differences would unlikely result from needle misplacement, minimizing interference with result evaluation. Injectates used in the selected studies were normal saline, local anesthetics, corticosteroids, dextrose, platelet-rich plasma, and hyaluronidase. Each injectate offered different clinical effects of interest including pain, clinical symptoms, and function, physical performance, electrodiagnostic findings, and nerve cross-sectional area because of various mechanisms, both mechanical decompression effect and pharmacologic effects of the injectates. Each injectate mechanism was described in the following paragraphs.
Normal saline (NSS) or 0.9% sodium chloride (NaCl) is a crystalloid fluid with an osmolarity of 30.8 mOsmol/L and a pH range of 4.5–7. Within every 100 ml of 0.9% sodium chloride injection, there is an equal amount (154 mEq) of sodium and chloride ions (Baxter Corporation, 2016; Tonog and Lakhkar, 2020). For hydrodissection purposes, it can be used on its own or as a diluent for other injectates, for example, corticosteroids or local anesthetics, acting mainly as perineural space expander without intrinsic inflammatory reducing or nerve repairing effects (Chang et al., 2020). Of the 10 studies, three studies used NSS as a control injectate compared with methylprednisolone (van Veen et al., 2015), D5W (Wu et al., 2017b), and PRP (Malahias et al., 2018).
Local anesthetic (LAs) is the primary pain-reducing agent for the procedure, often serving as a combination agent with steroids (Chang et al., 2020). Local anesthetics share the same chemical composition (pharmacophore) of three structural domains: an aromatic group, a terminal amine group, and a hydrocarbon chain being ester or amide linkage connecting these two groups. Therefore, they are classified structurally as ester-linked LAs or amine-linked LAs (Tetzlaff, 2000; Page et al., 2006). From the included studies in this systematic review, the most commonly used agent for hydrodissection was lidocaine, ranging from 1–2% concentration with injected volume of 1–2 ml (van Veen et al., 2015; Wu et al., 2017b; Malahias et al., 2018; Roghani et al., 2018; Güven et al., 2019; Shen et al., 2019). Only one study used 3 ml of 0.5% bupivacaine (Alsaeid, 2019). Both agents belong to amide-linked LAs and the preparation was without vasopressors. LAs reduce pain directly by reversibly blocking voltage-gated sodium channels within an axon, especially the axons of afferent nociceptors, which are Aδ-fibers and C-fibers, these fibers play a major role in pain perception. Lidocaine has pKa lower than bupivacaine, 7.9 vs 8.1, respectively, this allows more rapid onset, moderate hydrophilicity allowing moderate potency and adequate duration of action of around 1–2 h. Because of higher pKa, bupivacaine provides slower onset and much longer duration of action and higher potency (Becker and Reed, 2006; Becker and Reed, 2012; Schulman and Strichartz., 2012). In addition to anesthetic properties, LAs may play an anti-inflammatory role as reported in a systematic review and may be considered as a single agent for hydrodissection when steroid is less preferred, for example, in elderly patients with diabetes mellitus (Caracas et al., 2009; Roghani et al., 2018).
Corticosteroids are a strong anti-inflammatory agent and provide pain relief mainly through anti-inflammatory mechanisms including inhibitory effects on cytokines, reducing inflammatory mediators such as leukotrienes, prostaglandins, and platelet-activating factors, preventing the recruitment and activation of several inflammatory cells including lymphocytes, eosinophils, basophils, and macrophages (Guyre et al., 1988; Barnes et al., 1993). Corticosteroids also reduce edema by reducing capillary permeability and blood flow, and also reduce granulation tissue formation (Schwiebert et al., 1996). Synthetic steroid preparations for local injection are available with varying anti-inflammatory potencies, glucocorticoid effect, mineralocorticoid activities, solubility, and duration of actions. Commonly used injectable steroids, such as triamcinolone, methylprednisolone, and dexamethasone are derivatives of prednisolone. They are compounds with an-OH (hydroxyl) group, having intrinsic glucocorticoid property, and are ready to act without prior conversion in the liver (Garg and Adler, 2012). The first two preparations are in microcrystalline suspension form with extensive particle aggregation while dexamethasone preparation is in clear solution form. The particulate form potentially gives a longer duration of action than the non-particulate form as the particles were slowly released (MacMahon et al., 2009). Of the ten studies, five used corticosteroids; one used dexamethasone (Alsaeid, 2019), two used triamcinolone (Roghani et al., 2018; Wu et al., 2018), and two used methylprednisolone (van Veen et al., 2015; Senna et al., 2019) with injected volume ranging from 1–2 ml. From the described mechanism, corticosteroids provide a clinical effect of pain reduction, improving symptoms, decreased CSA due to edema reduction, allowing more space around the nerve, enable the electrophysiologic findings to improve.
Five percent dextrose in water or D5W is an isotonic solution of dextrose in a form of d-glucose, containing 278 mmol/L of dextrose. How D5W relieves neuropathic pain in the perineural injection is still rather unclear. A hypothesis has been proposed that D5W relieves pain through a sensorineural mechanism by downregulating the transient receptor potential vanilloid receptor 1 (TRPV-1) which is usually upregulated in cases of chronic neuropathic pain (Malek et al., 2015; Reeves and Rabago, 2020). This hypothesis on the mechanism of pain reduction has been made from a pilot study using mannitol to reduce capsaicin-induced pain (Bertrand et al., 2015; Reeves et al., 2016). Another mechanism is by decreasing C-fibers activation by reversing hypoglycemic status which induces excessive C-fibers activation (MacIver and Tanelian, 1992). Even though there are studies that consistently report clinical benefits compared with injection control, evidence of nervous tissue proliferation remains unclear (Reeves and Rabago, 2020). Dextrose predominately provides pain reduction, and also improving symptoms, function, electrophysiologic findings, and CSA reduction. Of the ten studies, three studies used D5W for injectates, D5W is the intervention injectate of interest in two studies, one comparing with NSS control and one comparing with triamcinolone (Wu et al., 2017b; Wu et al., 2018), another study D5W was used as a comparative injectate against PRP (Shen et al., 2019) with injected volume range from 3 to 5 ml.
Platelet-rich plasma (PRP) is a portion of the plasma fraction of autologous blood with a platelet concentration above the baseline (before centrifugation). Once activated, secretory granules release many mediators important in homeostatic, growth factors, and cytokines affecting inflammation, angiogenesis, facilitating the natural healing process and promote regeneration in many tissue types (Andia and Abate, 2013; Alves and Grimalt, 2018). Growth factors important in promoting axonal regrowth and angiogenesis include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and transforming growth factor (TGF-β), vascular endothelial growth factor (VEGF), and insulin-like growth factor-1 (IGF-1) (Borselli et al., 2010; Kim et al., 2014). The PRP fraction may contain a supraphysiologic concentration of platelets ranging from two to five times the baseline concentration (Le et al., 2018). Due to different preparation protocols, yielded PRP component; platelet concentration, presence or absence of leukocytes and erythrocytes, and also the timing of activation, tends to vary from study to study (Lansdown and Fortier, 2017). By promoting axonal regrowth, PRP not only reduces pain but also restores the nerve’s function and preserves the properties of the target muscles (Frostick et al., 1998; Kuffler, 2013). Because of PRP’s regenerating mechanism, PRP provides broad clinical effect from pain reduction, improving symptoms, function, electrophysiologic findings as well as CSA reduction. Of the ten studies, two studies compared PRP with conservative measure, one compared PRP alone with splinting and another compared PRP plus splinting with splinting alone (Wu et al., 2017a; Güven et al., 2019). One study compared PRP with normal saline (Malahias et al., 2018), two studies compared PRP with another injectate being methylprednisolone and D5W (Senna et al., 2019; Shen et al., 2019). Injected PRP volume range from 2 to 3 ml. Only one study gave specific details of the PRP component describing 3 ml of injected PRP with a platelet concentration of 2.7 ± 0.4 times, leukocytes count 1.2 ± 0.4 (Wu et al., 2017a).
Hyaluronidase is a mucolytic enzyme derived from mammalian tissue or synthesized in vitro in pure form (rHuPH20) using recombinant technology. Hyaluronidase lowers the viscosity of hyaluronan, a constituent of the extracellular matrix, thereby increasing tissue permeability (Dunn et al., 2010). For hydrodissection purposes, it is used as an adhesiolysis agent to release the entrapped nerve. One study compared hyaluronidase (300 IU) with dexamethasone as an adjuvant to 0.5% bupivacaine, the clinical effect it provided included symptoms, electrophysiologic findings, and CSA improvement (Alsaeid, 2019).
From the selected studies, pain (VAS) reduction was significantly achieved greater than NSS control or splitting into studies using D5W and PRP (Wu et al., 2017a; Wu et al., 2017b). When comparing one injectate to another, one study showed greater VAS reduction in intervention injectate (D5W) comparing to triamcinolone (Wu et al., 2018), another study comparing PRP to methylprednisolone showed lower average VAS in the PRP group than methylprednisolone group at the three-month follow up (Senna et al., 2019). For clinical symptoms, function, and physical performance, the improvement was significantly greater than NSS control or splitting into studies using D5W and PRP (Wu et al., 2017a; Wu et al., 2017b; Malahias et al., 2018; Güven et al., 2019). When comparing one injectate to another, D5W, PRP, and hyaluronidase gave greater improvement than their steroids counterparts (Wu et al., 2018; Alsaeid, 2019; Senna et al., 2019). Regarding main electrodiagnostic parameters (SNCV and DML) findings, D5W and hyaluronidase resulted in superior outcomes comparing to NSS and dexamethasone, respectively (Wu et al., 2017b; Alsaeid, 2019). PRP plus splinting also significantly improved main electrodiagnostic parameters (Güven et al., 2019). Another PRP study evaluated sensory latency and PRP showed superior outcomes compared to dextrose (Shen et al., 2019). All studies measured studied nerve cross-sectional area, the greater reduction was observed using D5W with NSS control, and PRP with splinting control (Wu et al., 2017a; Wu et al., 2017b). One study showed that PRP also achieved greater CSA reduction than D5W (Shen et al., 2019). Different doses of corticosteroids did not result in significant differences between doses in any outcomes (Roghani et al., 2018). From the main findings, D5W gave consistently superior effects comparing to NSS control or triamcinolone across all outcomes measured with the greatest magnitude of difference in later follow-up months (3,4 or 6 months) (Wu et al., 2017b; Wu et al., 2018). PRP demonstrated superior pain, clinical symptoms, and CSA reduction when comparing to NSS or splinting (Wu et al., 2017a; Malahias et al., 2018). PRP plus splinting resulted in greater electrodiagnostic parameters improvement than splinting alone (Güven et al., 2019). Therefore, D5W and PRP could be considered the preferred injectates for mild to moderate CTS. This finding also corresponds to the recent meta-analysis investigating regenerative injections for CTS (Lin et al., 2020). It is noticeable that, in a study comparing the two (D5W vs PRP), both gave significant improvement after hydrodissection, significantly greater improvement parameters in the PRP group consisted of BCTQf, DML, and CSA (Shen et al., 2019). This is quite expected as both were effective, showing many significant outcome improvements comparing to NSS or splint control. Of note, is the recent injectate, hyaluronidase, giving superior effects comparing to dexamethasone in clinical symptoms and electrodiagnostic findings. Considering adverse events, the only study reported adverse event was ulnar nerve study using corticosteorids, the events were common side effects from local steroids injection including pain, swelling and depigmentation at the injection site (vanVeen et al., 2015). The other eight CTS studies reported no adverse events. Different anatomy of injected sites might explain the situation, as the tissue covering ulnar nerve at the elbow region is very thin and without structurally containing boundaries, the injectate may infiltrate after injection up to the subcutaneous layer, even with ultrasound guidance, unlike the median nerve which is located inside the carpal tunnel. Even though no studies report severe allergic reaction or systemic toxicity of injectates, there is still a potential for severe allergic reaction when injecting with local anesthetics, corticosteroids and hyaluronidase as the drug vehicles or preservatives in the preparation may provoke severe allergic reactions in some patients (MacMahon et al., 2009; Becker and Reed, 2012).
The most investigated injectate among nine CTS studies was PRP, being the intervention injectate in five studies (Wu et al., 2017a; Malahias et al., 2018; Güven et al., 2019; Senna et al., 2019; Shen et al., 2019), followed by dextrose, in two studies (Wu et al., 2017b; Wu et al., 2018). This has shown the trend toward the need for injectates with regenerative effects, expecting longer and more permanent recovery. As it is well-established now that corticosteroid injections in CTS provide good but short-term clinical symptoms relief. Even surgical treatment may not always restore the nerve function (Huisstede et al., 2010). One injectate that has just recently been seen in entrapment hydrodissection publications is hyaluronidase, primarily used in the ophthalmology field or for lysis of epidural adhesion, this was included in one of the selected studies (Dunn et al., 2010; Alsaeid, 2019). The only corticosteroid study in CTS was by Roghani et al., studying different doses of steroids compared with local anesthetics, still another pharmacologic agent, as a control group. This study particularly aimed at finding the optimal corticosteroid dose for use in elderly patients, different from other corticosteroid studies (Roghani et al., 2018). Interesting findings from the study was the control group (local anesthetics alone) experienced significant pain reduction, improved symptoms, and reduced CSA like the steroids group. The authors proposed that this may result from the potential anti-inflammatory effect of local anesthetics (Roghani et al., 2018). The only study investigating the effect of corticosteroids compared with a normal saline control was by vanVeen et al. As ulnar nerve entrapment is less common than CTS, less publications with much less controlled-trials publications exist. Corticosteroids remain the primary investigated or reported agent for ulnar nerve entrapment, therefore, possibilities exist for investigating other types of injectates. The challenges when evaluating PRP studies remained the undetermined dosage of platelets in PRP as many studies did not provide a full description. For studies using NSS as the control group, there was also a noticeable improvement in the group, implying the effectiveness of hydrodissection partly did come from a purely mechanical decompression. This effect was demonstrated in a randomized controlled trial study comparing ultrasound-guided hydrodissection with NSS and subcutaneous injection with NSS (Wu et al., 2019). Considering the potential local and transient blood sugar elevation side effects of steroid injections, especially in the elderly or patients with elevated blood sugar, D5W or PRP might be a more preferable option for these groups.
There are several limitations in this systematic review, first, all ten studies compared different interventions and comparisons, none could be combined for further analysis. Second, of ten studies, three were from the same investigator's group, this might limit the generalization of results as the study population was limited. Third, the follow-up interval was rather diverse with a maximum follow-up time at six months, which might be insufficient for evaluating regenerative effects. Fourth, the varying injected volume among the studies might also vary the clinical outcome as larger volume tends to provide greater mechanical decompression.
To further enhance knowledge of ultrasound-guided hydrodissection procedure, more studies on different nerves and locations are encouraged as well as in varied population groups to promote generalizability. Also, for PRP and D5W studies of longer duration than six months should be pursued. For future PRP studies, a full PRP preparation protocol together with detailed PRP components should be explained thoroughly as the information will be very helpful when comparing studies.
Conclusion
In summary, this systematic review shown the effectiveness and safety of ultrasound-guided hydrodissection injectates ranging from NSS, D5W, local anesthetics, corticosteroids, PRP, and hyaluronidase. All injectates can provide a clinical effect on their own. In comparative cases, D5W and PRP demonstrated a consistent superior clinical effect against the comparative agent or other conservative measures. With ultrasound-guidance, no serious adverse events occurred, except local side effects after corticosteroid injections.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MB, KC, SS, and SK conceived and designed the study, SK and MB searched and selected studies, MB, SP, KK, TV, and SK extracted essentials information. SK and SS assessed the risk of bias. MB, SP, KK, SK, and TV discussed the results and drafted the manuscript. All authors approved the final manuscript.
Funding
Open-access publication fees are supported by the faculty of medicine, Chaing Mai University. This work was supported by a grant from the Unit of Excellence on Clinical Outcomes Research and IntegratioN (UNICORN) Grant number: FF64-UoE003, School of Pharmaceutical Sciences, University of Phayao. The funding source had no role in the study design, collection, analysis and interpretation of data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer W-TW declared a shared affiliation, with no collaboration, with one of the authors, K-VC, to the handling editor at the time of review.
Acknowledgments
The authors wish to thank the Research Administration section and the Library of Faculty of Medicine, Chiang Mai University for administrative and technical support.
Abbreviations
2PD, 2-point discrimination; BCTQ, boston carpal tunnel questionnaire; BCTQs, boston carpal tunnel questionnaire symptom severity scale; BCTQf, boston carpal tunnel questionnaire functional status scale; BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene related peptide; CMAP, compound muscle action potential; CSA, cross-sectional area; CTS, carpal tunnel syndrome; CuTS, cubital tunnel syndrome; D5W, 5% dextrose in water; DML, distal motor latency; EDS, electrodiagnostic study; IGF-1, insulin-like growth factor-1; IL, interleukin; LAs, local anesthetics; MeSH, medical subject headings; MNCS, motor nerve conduction study; NaCl, sodium chloride; NGF, nerve growth factor; NSS, normal saline; OH, hydroxyl; PRISMA, preferred reporting items for systematic reviews and meta-analyses; PRP, platelet-rich plasma; RoB, risk-of-bias; Q-DASH, quick disabilities of arm shoulder and hand questionnaire; SNAP, Sensory nerve action potential.
References
Alsaeid, M. A. (2019). Dexamethasone versus hyaluronidase as an adjuvant to local anesthetics in the ultrasound-guided hydrodissection of the median nerve for the treatment of carpal tunnel syndrome patients. Anesth. Essays Res. 13, 417–422. doi:10.4103/aer.AER_104_19
Alves, R., and Grimalt, R. (2018). A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin. Appendage. Disord. 4, 18–24. doi:10.1159/000477353
Andia, I., and Abate, M. (2013). Platelet-rich plasma: underlying biology and clinical correlates. Regen. Med. 8, 645–658. doi:10.2217/rme.13.59
Barnes, P. J., Adcock, I., Spedding, M., and Vanhoutte, P. M. (1993). Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci. 14, 436–441. doi:10.1016/0165-6147(93)90184-l
Baxter Corporation (2016). Prescribing information:0.9% sodium chloride injection.USP in VIAFLEX plastic container. Florida, FL: Baxter International IncAvailable at: https://www.baxter.ca/sites/g/files/ebysai1431/files/2018-11/0.9pct_NaCl_Inj_Viaflex_EN.pdf (Accessed August 18, 2020).
Becker, D. E., and Reed, K. L. (2012). Local anesthetics: review of pharmacological considerations. Anesth. Prog. 59, 90–93. doi:10.2344/0003-3006-59.2.90
Becker, D. E., and Reed, K. L. (2006). Essentials of local anesthetic pharmacology. Anesth. Prog. 53, 98–110. doi:10.2344/0003-3006(2006)53[98:EOLAP]2.0.CO;2
Bertrand, H., Kyriazis, M., Reeves, K. D., Lyftogt, J., and Rabago, D. (2015). Topical mannitol reduces capsaicin-induced pain: results of a pilot-level, double-blind, randomized controlled trial. Pharm. Manag. PM R 7, 1111–1117. doi:10.1016/j.pmrj.2015.05.002
Borselli, C., Storrie, H., Benesch-Lee, F., Shvartsman, D., Cezar, C., Lichtman, J. W., et al. (2010). Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. U.S.A. 107, 3287. doi:10.1073/pnas.0903875106
Catapano, M., Catapano, J., Borschel, G., Alavinia, S. M., Robinson, L. R., and Mittal, N. (2020). Effectiveness of platelet-rich plasma injections for nonsurgical management of carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 101, 897–906. doi:10.1016/j.apmr.2019.10.193
Chang, K. V., Hsu, S. H., Wu, W. T., and Özçakar, L. (2017). Ultrasonographic technique for imaging and injecting the superior cluneal nerve. Am. J. Phys. Med. Rehabil. 96, e117–e118. doi:10.1097/PHM.0000000000000642
Chang, K. V., Wu, W. T., and Özçakar, L. (2020). Ultrasound imaging and guidance in peripheral nerve entrapment: hydrodissection highlighted. Pain Manag. 10, 97–106. doi:10.2217/pmt-2019-0056
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Contr. Clin. Trials 7, 177–188. doi:10.1016/0197-2456(86)90046-2
Doughty, C. T., and Bowley, M. P. (2019). Entrapment neuropathies of the upper extremity. Med. Clin. 103, 357–370. doi:10.1016/j.mcna.2018.10.012
Dunn, A. L., Heavner, J. E., Racz, G., and Day, M. (2010). Hyaluronidase: a review of approved formulations, indications and off-label use in chronic pain management. Expet Opin. Biol. Ther. 10, 127–131. doi:10.1517/14712590903490382
Frostick, S. P., Yin, Q., and Kemp, G. J. (1998). Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18, 397–405. doi:10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f
Garg, R., and Adler, G. K. (2012). “Pharmacology of the adrenal cortex,” in Principles of pharmacology: the pathophysiologic basis of drug therapy. Editors D. E. Golan, A. H. Tashjian, E. Armstrong, and A. W. Armstrong 3rd.Edn, (Pennsylvania, PA: Lippincott Williams & Wilkins), 489–504.
Güven, S. C., Özçakar, L., Kaymak, B., Kara, M., and Akıncı, A. (2019). Short‐term effectiveness of platelet‐rich plasma in carpal tunnel syndrome: a controlled study. J. Tissue. Eng. Regen. Med. 13, 709–714. doi:10.1002/term.2815
Guyre, P. M., Girard, M. T., Morganelli, P. M., and Manganiello, P. D. (1988). Glucocorticoid effects on the production and actions of immune cytokines. J. Steroid Biochem. 30, 89–93. doi:10.1016/0022-4731(88)90080-5
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327, 557–560. doi:10.1136/bmj.327.7414.557
Huisstede, B. M., Hoogvliet, P., Randsdorp, M. S., Glerum, S., van Middelkoop, M., and Koes, B. W. (2010). Carpal tunnel syndrome. Part I: effectiveness of nonsurgical treatments--a systematic review. Arch. Phys. Med. Rehabil. 91, 981–1004. doi:10.1016/j.apmr.2010.03.022
Ji, R. R., Nackley, A., Huh, Y., Terrando, N., and Maixner, W. (2018). Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129, 343–366. doi:10.1097/ALN.0000000000002130
Kim, J., Jeon, W., Kim, D., Rhyu, I., Kim, Y., Youn, I., et al. (2014). An inside-out vein graft filled with platelet-rich plasma for repair of a short sciatic nerve defect in rats. Neural Regen. Res. 9, 1351–1357. doi:10.4103/1673-5374.137587
Kooner, S., Cinats, D., Kwong, C., Matthewson, G., and Dhaliwal, G. (2019). Conservative treatment of cubital tunnel syndrome: a systematic review. Orthop. Rev. 11, 7955. doi:10.4081/or.2019.7955
Kuffler, D. P. (2013). Platelet-rich plasma and the elimination of neuropathic pain. Mol. Neurobiol. 48, 315–332. doi:10.1007/s12035-013-8494-7
Lam, K. H. S., Hung, C. Y., Chiang, Y. P., Onishi, K., Su, D. C. J., Clark, T. B., et al. (2020). Ultrasound-guided nerve hydrodissection for pain management: rationale, methods, current literature, and theoretical mechanisms. J. Pain Res. 13, 1957–1968. doi:10.2147/JPR.S247208
Lansdown, D. A., and Fortier, L. A. (2017). Platelet-rich plasma: formulations, preparations, constituents, and their effects. Operat. Tech. Sports Med. 25, 7–12. doi:10.1053/j.otsm.2016.12.002
Lauder, A., Mithani, S., and Leversedge, F. J. (2019). Management of recalcitrant carpal tunnel syndrome. J. Am. Acad. Orthop. Surg. 27, 551–562. doi:10.5435/JAAOS-D-18-00004
Le, A. D. K., Enweze, L., DeBaun, M. R., and Dragoo, J. L. (2018). Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med 11, 624–634. doi:10.1007/s12178-018-9527-7
Lin, C.-P., Chang, K.-V., Huang, Y.-K., Wu, W.-T., and Özçakar, L. (2020). Regenerative injections including 5% dextrose and platelet-rich plasma for the treatment of carpal tunnel syndrome: a systematic review and network meta-analysis. Pharmaceuticals 13, 49. doi:10.3390/ph13030049
MacIver, M. B., and Tanelian, D. L. (1992). Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology 76, 617–623. doi:10.1097/00000542-199204000-00020
MacMahon, P. J., Eustace, S. J., and Kavanagh, E. C. (2009). Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology 252, 647–661. doi:10.1148/radiol.2523081929
Malahias, M. A., Nikolaou, V. S., Johnson, E. O., Kaseta, M. K., Kazas, S. T., and Babis, G. C. (2018). Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: a placebo-controlled clinical study. J. Tissue. Eng. Regen. Med. 12, e1480–e1488. doi:10.1002/term.2566
Malek, N., Pajak, A., Kolosowska, N., Kucharczyk, M., and Starowicz, K. (2015). The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell. Neurosci. 65, 1–10. doi:10.1016/j.mcn.2015.02.001
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012. doi:10.1016/j.jclinepi.2009.06.005
Mooney, H. C., Maciel, J. V., Martins, P. M., de Souza, M. M., and Maia, L. C. (2009). The use of lidocaine as an anti-inflammatory substance: a systematic review. J. Dent. 37, 93–97. doi:10.1016/j.jdent.2008.10.005
Page, C., Curtis, M., Walker, M., and Hoffman, B. (2006). “Drugs used in anesthesia and critical care,” in Integrated pharmacology. Editors M. C. Clive Page, Michael. Walker, and Bria. Hoffman 3rd Edn (Missouri, MO: Elsevier Mosby), 571–587.
Reeves, D., and Rabago, D. (2020). PM&R Knowledge Now. Therapeutic injection of dextrose: prolotherapy, perineural injection therapy and hydrodissection. Available at:https://now.aapmr.org/therapeutic-injection-of-dextrose-prolotherapy-perineural-injection-therapy-and-hydrodissection/ (Accessed May 04, 2020).
Reeves, K. D., Sit, R. W., and Rabago, D. P. (2016). Dextrose prolotherapy: a narrative review of basic science, clinical research, and best treatment recommendations. Phys. Med. Rehabil. Clin 27, 783–823. doi:10.1016/j.pmr.2016.06.001
Roghani, R. S., Holisaz, M. T., Tarkashvand, M., Delbari, A., Gohari, F., Boon, A. J., et al. (2018). Different doses of steroid injection in elderly patients with carpal tunnel syndrome: a triple-blind, randomized, controlled trial. Clin. Interv. Aging 13, 117–124. doi:10.2147/cia.S151290
Schmid, A. B., Nee, R. J., and Coppieters, M. W. (2013). Reappraising entrapment neuropathies--mechanisms, diagnosis and management. Man. Ther. 18, 449–457. doi:10.1016/j.math.2013.07.006
Schulman, J. M., and Strichartz, G. R. (2012). “Local anesthetic pharmacology,” in Principles of pharmacology: the pathophysiologic basis of drug therapy. Editors D. E. Golan, A. H. Tashjian, E. Armstrong, and A. W. Armstrong 3 rd. Edn (Pennsylvania, PA: Lippincott Williams & Wilkins), 147–161.
Schwiebert, L. M., Beck, L. A., Stellato, C., Bickel, C. A., Bochner, B. S., Schleimer, R. P., et al. (1996). Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions. J. Allergy Clin. Immunol. 97, 143–152. doi:10.1016/s0091-6749(96)80214-4
Senna, M. K., Shaat, R. M., and Ali, A. A. A. (2019). Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin. Rheumatol. 38, 3643–3654. doi:10.1007/s10067-019-04719-7
Shen, Y. P., Li, T. Y., Chou, Y. C., Ho, T. Y., Ke, M. J., Chen, L. C., et al. (2019). Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: a prospective randomized, single-blind, head-to-head comparative trial. J. Tissue. Eng. Regen. Med. 13, 2009–2017. doi:10.1002/term.2950
Tagliafico, A., Serafini, G., Lacelli, F., Perrone, N., Valsania, V., and Martinoli, C. (2011). Ultrasound-guided treatment of meralgia paresthetica (lateral femoral cutaneous neuropathy): technical description and results of treatment in 20 consecutive patients. J. Ultrasound Med. 30, 1341–1346. doi:10.7863/jum.2011.30.10.1341
Tetzlaff, J. E. (2000). The pharmacology of local anesthetics. Anesthesiol. Clin. 18, 217. doi:10.1016/s0889-8537(05)70161-9
Tonog, P., and Lakhkar, A. (2020). Normal saline. StatPearls Publishing. Available at:https://www.ncbi.nlm.nih.gov/books/NBK545210/ (Accessed July 15, 2020).
Toussaint, C. P., Perry, E. C., Pisansky, M. T., and Anderson, D. E. (2010). What’s new in the diagnosis and treatment of peripheral nerve entrapment neuropathies. Neurol. Clin. 28, 979–1004. doi:10.1016/j.ncl.2010.03.017
Trescot, A., and Brown, M. (2015). Peripheral nerve entrapment, hydrodissection, and neural regenerative strategies. Tech. Reg. Anesth. Pain Manag. 19, 85–93. doi:10.1053/j.trap.2016.09.015
van Veen, K. E., Alblas, K. C., Alons, I. M., Kerklaan, J. P., Siegersma, M. C., Wesstein, M., et al. (2015). Corticosteroid injection in patients with ulnar neuropathy at the elbow: a randomized, double-blind, placebo-controlled trial. Muscle Nerve 52, 380–385. doi:10.1002/mus.24551
Wu, C. H., and Boudier-Revéret, M. (2019). Ultrasound-guided steroid injections for lateral antebrachial cutaneous nerve entrapment within postsurgical scar. Am. J. Phys. Med. Rehabil. 98, e106. doi:10.1097/phm.0000000000001150
Wu, Y. T., Chen, S. R., Li, T. Y., Ho, T. Y., Shen, Y. P., Tsai, C. K., et al. (2019). Nerve hydrodissection for carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Muscle Nerve 59, 174–180. doi:10.1002/mus.26358
Wu, Y. T., Ho, T. Y., Chou, Y. C., Ke, M. J., Li, T. Y., Huang, G. S., et al. (2017a). Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Sci. Rep. 7, 94. doi:10.1038/s41598-017-00224-6
Wu, Y. T., Ho, T. Y., Chou, Y. C., Ke, M. J., Li, T. Y., Tsai, C. K., et al. (2017b). Six-month efficacy of perineural dextrose for carpal tunnel syndrome: a prospective, randomized, double-blind, controlled trial. Mayo Clin. Proc. 92, 1179–1189. doi:10.1016/j.mayocp.2017.05.025
Keywords: entrapment neuropathy, ultrasound-guided hydrodissection, peripheral nerve, perineural injection, injectate, carpal tunnel syndrome, cubital tunnel syndrome
Citation: Buntragulpoontawee M, Chang K-V, Vitoonpong T, Pornjaksawan S, Kitisak K, Saokaew S and Kanchanasurakit S (2021) The Effectiveness and Safety of Commonly Used Injectates for Ultrasound-Guided Hydrodissection Treatment of Peripheral Nerve Entrapment Syndromes: A Systematic Review. Front. Pharmacol. 11:621150. doi: 10.3389/fphar.2020.621150
Received: 25 October 2020; Accepted: 30 December 2020;
Published: 05 March 2021.
Edited by:
Ahmad Reza Dehpour, Tehran University of Medical Sciences, IranReviewed by:
Wei-Ting Wu, National Taiwan University Hospital Bei-Hu branch, TaiwanKamal Mezian, Charles University, Czechia
Copyright © 2021 Buntragulpoontawee, Chang, Vitoonpong, Pornjaksawan, Kitisak, Saokaew and Kanchanasurakit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sukrit Kanchanasurakit, c3Vrcml0LmthQHVwLmFjLnRo, c3Vrcml0X3J4QGhvdG1haWwuY29t
 Montana Buntragulpoontawee
Montana Buntragulpoontawee Ke-Vin Chang
Ke-Vin Chang Timporn Vitoonpong
Timporn Vitoonpong Sineenard Pornjaksawan
Sineenard Pornjaksawan Kittipong Kitisak
Kittipong Kitisak Surasak Saokaew
Surasak Saokaew Sukrit Kanchanasurakit
Sukrit Kanchanasurakit