- 1Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2International Collaborative Center on Big Science Plan for Purine Signalling, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Rudolf Boehm Institute for Pharmacology and Toxicology, University of Leipzig, Leipzig, Germany
- 4Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom
- 5Achucarro Centre for Neuroscience, IKERBASQUE, Basque Foundation for Science, Bilbao, Spain
Introduction
The brain is the most complex organ of human body composed of several highly specialised and heterogeneous population of cells, represented by neurones, neuroglia (astrocytes, microglia, oligodendrocytes) and cells of brain vasculature. Neurones and neuroglia form neural circuits; different types of glial cells contribute to shaping and maintaining synaptic connections, plasticity, homeostasis, and network level activity through dynamic monitoring and alteration of central nervous system (CNS) functional architecture (Kettenmann et al., 2013; Allen and Lyons, 2018; Verkhratsky and Nedergaard, 2018; Augusto-Oliveira et al., 2020). Microglial cells are scions of foetal macrophages invading the neural tube early in embryonic development (Ginhoux et al., 2013); after settling in the nervous tissue these cells undergo the most remarkable metamorphoses acquiring specific morphology (small soma with long, ramified motile processes) and physiology. In particular, microglial cells gain receptors to neurotransmitters and neuromodulators, while retaining the pattern recognition receptors from their immune heritage; this extended complement of receptors makes microglia arguably the most “receptive” cells in the CNS (Kettenmann et al., 2011; Garaschuk and Verkhratsky, 2019). Among these many receptors, microglia possess several types of purinoceptors, which are linked to microglial housekeeping, neuroprotective and defensive capabilities (Verkhratsky et al., 2009; Tozaki-Saitoh et al., 2012). Purinergic signalling emerges as the key mechanism in the dynamic interactions between neurones and glial cells, with ATP being a classical neurotransmitter and a danger signal damage-associated molecular pattern (DAMP). This duality makes ATP and related purines versatile signalling molecules controlling microglial behaviours in both physiological and pathological context (Domercq et al., 2013; Illes et al., 2020).
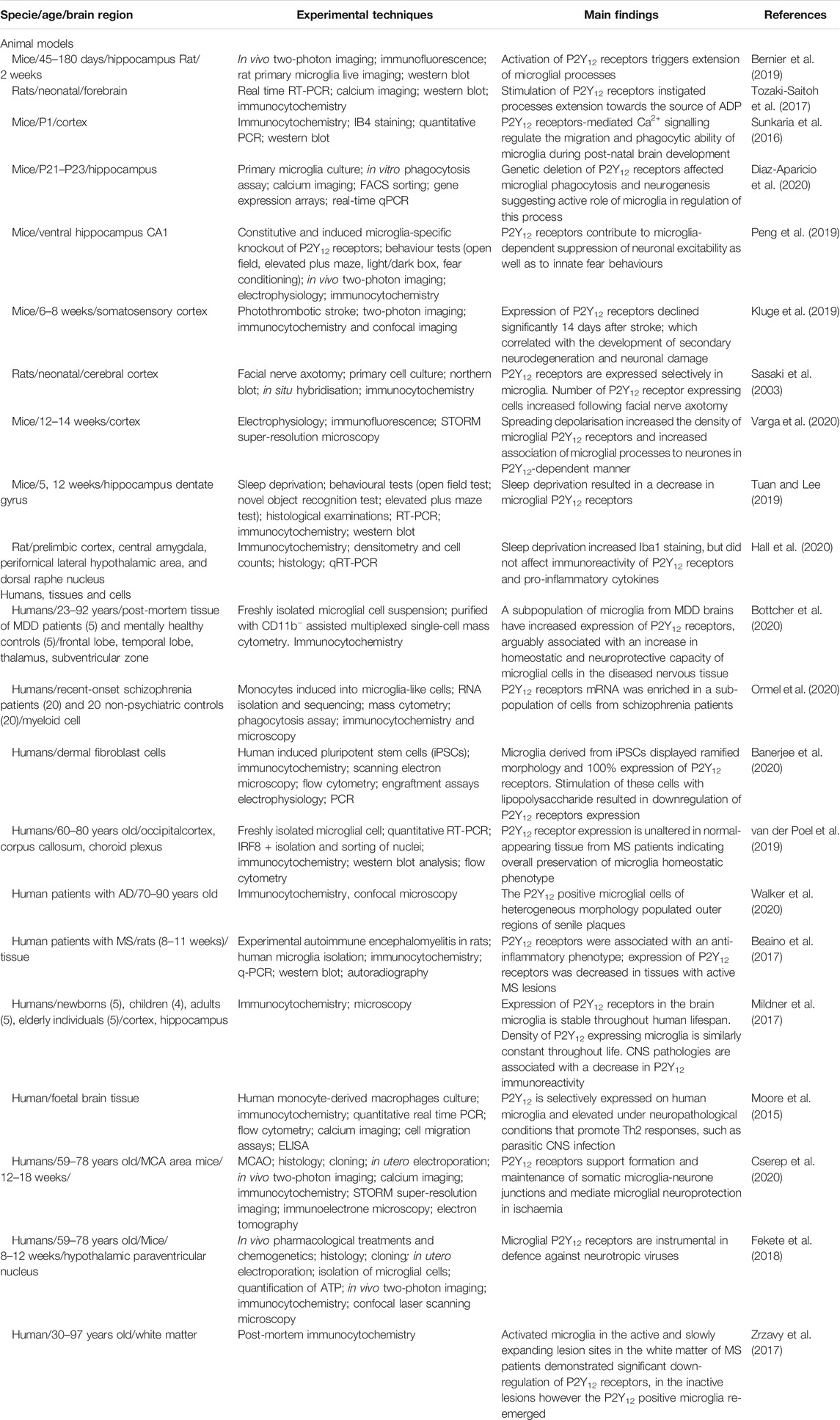
The metabotropic P2Y12 purinoceptor is of a particular relevance for microglia. First and foremost, the expression of this receptor distinguishes CNS resident microglia from peripheral macrophages (Sasaki et al., 2003; Haynes et al., 2006). Second, in the healthy brain P2Y12 receptors are universally and specifically expressed in microglia in all brain regions and across different species from rodents to humans (Sasaki et al., 2003; Mildner et al., 2017); the P2Y12 receptors are widely considered to be a signature of microglia in the healthy brain (Hickman et al., 2013; Bosco et al., 2018; Peng et al., 2019). Third, expression of P2Y12 receptors is stable from foetal state and throughout human lifespan (Crain et al., 2009; Mildner et al., 2017). The P2Y12 receptors share the seven-transmembrane topology characteristic for G-protein coupled receptors of P2Y family (Burnstock and Verkhratsky, 2012). The preferred agonist for P2Y12 receptors is adenosine diphosphate (ADP), which in the periphery acts as a major instigator of platelet aggregation and granule secretion thus supporting thrombogenesis (Liverani et al., 2014). In the CNS, microglial P2Y12 receptors are activated by ADP deriving from enzymatic degradation of ATP released from neurones, astrocytes and oligodendroglia during their physiological activity or following tissue damage (Abbracchio et al., 2009; Zimmermann et al., 2012). Metabotropic P2Y12 receptors are localised in the processes and in the somata of surveilling microglia, where they mediate various aspects of intercellular signalling targeting microglia (Table 1 and Posfai et al., 2019; Vainchtein and Molofsky, 2020).
Modes of Microglial Patrolling of the Healthy CNS: Role for P2Y12 Receptors
Microglial cells are indefatigable surveillants and overseers of the nervous tissue; their ramified processes are in constant move scanning CNS parenchyma (Davalos et al., 2005; Nimmerjahn et al., 2005) with a particular attention paid to neurones (Wake et al., 2009; Cserep et al., 2020). Microglial surveillance of the nervous tissue occurs in several distinct modes.
Microglia-Dendritic/Synaptic Patrolling
In the healthy brain microglial processes are constantly contacting synaptic contacts located on neuronal dendrites. These microglia-dendritic contacts are instrumental for synaptic pruning in early development, which removes silent, aberrant or redundant synapses by en passant phagocytosis (Sierra et al., 2010) thus contributing to shaping neuronal ensembles and supporting neuroplasticity (Kettenmann et al., 2013; Sakai, 2020). Synaptic pruning is controlled by neuronal complement system (Stevens et al., 2007; Schafer et al., 2012), which tags the synapses to be removed, and by neurone-derived chemokine CX3CL1 also known as fractalkine. Microglial cells specifically express fractalkine receptors, activation of which stimulates synaptic pruning by physiological phagocytosis (Paolicelli et al., 2011). At later developmental stages microglia can remove not only whole synapses but also synaptic fragments through the process known as trogocytosis (Weinhard et al., 2018).
Microglia-dendritic interactions are regulated by neuronal activity: an increase in neuronal firing increases the frequency and number of contacts between microglial processes and synapses (Li et al., 2013). Plastic remodelling of the nervous tissue involves substantial changes in microglial morphology, manifested in hyper-ramification of processes, decreased intrinsic motility of processes and increased number of contacts with synaptic sites. P2Y12 receptors play a primary role in these preocesses; pharmacological and genetic occlusion of these receptors suppressed both microglial changes and neuronal plasticity, thus revealing contribution of microglia to experience-induced reshaping of neuronal networks (Sipe et al., 2016).
Microglia-Somatic Patrolling
The second distinct type of microglial patrolling is aimed at neuronal somata. In the cortex microglial processes frequently contact neuronal cell bodies. The microglia process-neuronal somata contacts (defined as somatic microglial junctions) last for tens of minutes and even up to 1 h, which is much longer compared to microglia-dendritic or microglia-synaptic contacts which usually last for several minutes only (Cserep et al., 2020). Neuronal part of microglia-somatic junction contains mitochondria and secretory vesicles closely associated with plasmalemma; the microglial part of the junction was characterised by exceptionally high density of P2Y12 receptors. The P2Y12 receptors control formation of microglia-somatic junctions, as pharmacological blockade of these receptors halves the duration of microglia-somatic contacts. The microglia-somatic junctions seem to be particularly important for neuroprotection after ischemic attack: the stroke greatly increases microglial coverage of neuronal cell bodies; this increase requires operational P2Y12 receptors. Inhibition of P2Y12-mediated signalling negatively impacts on neurones, which experience greater calcium load and increased functional disconnection. Signalling between neurones and microglial processes at the somatic level is supported by neuronal mitochondria and ATP exocytosis from vesicular-nucleotide transporter (VNUT)-containing secretory vesicles: disruption of either impairs the microglia-somatic junction (Cserep et al., 2020). To summarise, microglial P2Y12 receptors provide for specialised interaction between neuronal cell bodies and microglial cells, interaction which appears to be critical for neuroprotection.
Microglia-Axonal Patrolling
Microglial processes establish intimate contacts with axon initial segments early in development and these contacts are maintained through adulthood probably supporting axonal structure (Baalman et al., 2015). Increased firing of the axon, reflective of neuronal hyperexcitability initiates further extension of microglial processes, which enwrap the axon and suppress axonal action potential generation, thus preventing excitotoxicity. Inhibition of microglial motility blocks this mechanism and facilitates neuronal death (Kato et al., 2016). Which microglial receptors are responsible for axonal patrolling remains unknown, although the involvement of fractalkine receptors has been excluded (Baalman et al., 2015).
Microglial Processes Converging Response—Counteracting Acute Lesions to the Nervous Tissue
Another type of microglial patrolling is associated with rapid convergence processes response, in which microglial processes swiftly move towards the site of potential injury. Thus response is regulated solely by P2Y12 receptors that detect the source of ATP/ADP as a potential damage signal (Davalos et al., 2005). The converging response of microglial processes represents a specific form of patrolling associated with primary defensive function of microglia. This response occurs at the initial stages of various neuropathologies. In particular, local cortical damage, associated with rapid increase in ATP/ADP instantly triggers microglial processes convergence towards the site of the lesion (Haynes et al., 2006). This directional extension of microglial processes involved activation of β1 integrin signalling cascade (Ohsawa et al., 2010). Microglial processes converge on axons after traumatic brain injury to reduce neuronal excitability (Benusa and Lafrenaye, 2020). Similarly, microglial processes move to and enwrap neurones and axons in experimental epilepsy, which again counteracts hyperexcitability and potentially limits the seizures (Eyo et al., 2014). Mechanistically, excessive neuronal activity results in activation of NMDA receptors, which trigger ATP release that translates, through activation of P2Y12 receptors, into converging microglial processes response (Dissing-Olesen et al., 2014) Genetic or pharmacological silencing of P2Y12 receptors obliterates microglial processes converging response in all these pathological contexts (Haynes et al., 2006; Eyo et al., 2014).
Microglial P2Y12 Receptors in Neurological Diseases
Pathological insults to the CNS invariably stimulate and recruit microglia (Kettenmann et al., 2011; Savage et al., 2019), triggering reactive microgliosis (the commonly used term “activation” is somewhat misleading; microglial cells are activated by numerous signals in physiological context, whereas microgliosis represent response to pathology and hence should be defined as reactivity). Purines and ATP are, as alluded earlier, classic damage-associated molecular patters (DAMP) conserved throughout the evolution (Verkhratsky and Burnstock, 2014). The P2Y12 receptor is intimately involved in the early stages of microglial response to the lesion, as discussed in previous chapter, and to the early stages of microgliotic response (Table 1). Stimulation of microglial P2Y12 receptors triggers microgliotic transformation into various reactive phenotypes that ultimately climaxes in amoeboid phagocyting microglia (Hanisch and Kettenmann, 2007; Savage et al., 2019). Genetic deletion of P2Y12 receptors results deficits in up-regulation of K+ outward rectifying channels and in membrane ruffling and chemotaxis of amoeboid microglia (Swiatkowski et al., 2016).
Reactive microgliosis however almost invariably results in down-regulation of expression of microglial P2Y12 receptors (Zrzavy et al., 2017). Injection of LPS into the striatum triggers massive activation of microglial cells associated with almost complete disappearance of P2Y12 receptors 4 days after the insult (Fukumoto et al., 2019); treatment of human induced pluripotent stem cells derived microglia with LPS likewise resulted in disappearance of P2Y12 receptors (Banerjee et al., 2020). Similarly, experimental stroke induced gradual and almost compete disappearance of microglial P2Y12 receptors (Kluge et al., 2019); down-regulation of P2Y12 receptors have been observed in microglia in several chronic neurological diseases (Mildner et al., 2017; Zrzavy et al., 2017). Recent investigations however have found P2Y12 receptors expression in microglia in several chronic neurological and neuropsychiatric conditions. The P2Y12-positive microglial cells were detected in the microglia freshly isolated from post-mortem brains of human patients suffering from major depressive disorder (Bottcher et al., 2020). Similarly microglia bearing P2Y12 receptors were found in the outer regions of senile plaques in post-mortem tissues from Alzheimer’s disease patients (Walker et al., 2020). These results indicate that P2Y12 microglia populate diseased brains, which might be associated with rise of defensive, safeguarding microglial phenotypes, distinct from reactive microglia.
Conclusion
The P2Y12 purinoceptors are signature receptors of microglia in the healthy brain. These receptors mediate patrolling behaviours of surveilling microglia and coordinate neuronal activity with operation of microglia. The P2Y12 receptors are instrumental for microglial response to neuropathological lesion, and are responsible for the initiation of reactive microgliosis. Reactive microglia as a rule do not express P2Y12 receptors, however in neurodegenerative and neuropsychiatric disease the population of P2Y12-bearing microglia (distinct form reactive microglia) remains; these cells arguably participate in defensive, safeguarding responses against neuropathology.
Author Contributions
Conceptualisation: S-SL and AV; writing—original draft preparation: S-SL and AV; literature review, writing—review and editing: S-SL, YT, PI, and AV.
Funding
Our work was supported by National Key R&D Program of China (2019YFC1709101), the Project First-Class Disciplines Development of Chengdu University of Traditional Chinese Medicine (CZYHW1901), the National Natural Science Foundation of China (81774437 and 81973969), and Science and Technology Program of Sichuan Province, China (2019YFH0108).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbracchio, M. P., Burnstock, G., Verkhratsky, A., and Zimmermann, H. (2009). Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32, 19–29. doi:10.1016/j.tins.2008.10.001
Allen, N. J., and Lyons, D. A. (2018). Glia as architects of central nervous system formation and function. Science. 362, 181–185. doi:10.1126/science.aat0473
Augusto-Oliveira, M., Arrifano, G. P., Takeda, P. Y., Lopes-Araujo, A., Santos-Sacramento, L., Anthony, D. C., et al. (2020). Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci. Biobehav. Rev. 118, 331–357. doi:10.1016/j.neubiorev.2020.07.039
Baalman, K., Marin, M. A., Ho, T. S., Godoy, M., Cherian, L., Robertson, C., et al. (2015). Axon initial segment-associated microglia. J. Neurosci. 35, 2283–2292. doi:10.1523/JNEUROSCI.3751-14.2015
Banerjee, P., Paza, E., Perkins, E. M., James, O. G., Kenkhuis, B., Lloyd, A. F., et al. (2020). Generation of pure monocultures of human microglia-like cells from induced pluripotent stem cells. Stem Cell Res. 49, 102046. doi:10.1016/j.scr.2020.102046
Beaino, W., Janssen, B., Kooij, G., van der Pol, S. M. A., van Het Hof, B., van Horssen, J., et al. (2017). Purinergic receptors P2Y12R and P2X7R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J. Neuroinflammation 14, 259. doi:10.1186/s12974-017-1034-z
Benusa, S. D., and Lafrenaye, A. D. (2020). Microglial process convergence on axonal segments in health and disease. Neuroimmunol. Neuroinflammation 7, 23–39. doi:10.20517/2347-8659.2019.28
Bernier, L. P., Bohlen, C. J., York, E. M., Choi, H. B., Kamyabi, A., Dissing-Olesen, L., et al. (2019). Nanoscale surveillance of the brain by microglia via cAMP-regulated filopodia. Cell Rep. 27, 2895–2908. doi:10.1016/j.celrep.2019.05.010
Bosco, D. B., Zheng, J., Xu, Z., Peng, J., Eyo, U. B., Tang, K., et al. (2018). RNAseq analysis of hippocampal microglia after kainic acid-induced seizures. Mol. Brain 11, 34. doi:10.1186/s13041-018-0376-5
Bottcher, C., Fernandez-Zapata, C., Snijders, G. J. L., Schlickeiser, S., Sneeboer, M. A. M., Kunkel, D., et al. (2020). Single-cell mass cytometry of microglia in major depressive disorder reveals a non-inflammatory phenotype with increased homeostatic marker expression. Transl. Psychiatry 10, 310. doi:10.1038/s41398-020-00992-2
Burnstock, G., and Verkhratsky, A. (2012). Purinergic signalling in the nervous system. Heidelberg, Germany: Springer-Verlag.
Crain, J. M., Nikodemova, M., and Watters, J. J. (2009). Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J. Neuroinflammation, 6, 24. doi:10.1186/1742-2094-6-24
Cserep, C., Posfai, B., Lenart, N., Fekete, R., Laszlo, Z. I., Lele, Z., et al. (2020). Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367, 528–537. doi:10.1126/science.aax6752
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. doi:10.1038/nn1472
Diaz-Aparicio, I., Paris, I., Sierra-Torre, V., Plaza-Zabala, A., Rodriguez-Iglesias, N., Marquez-Ropero, M., et al. (2020). Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J. Neurosci. 40, 1453–1482. doi:10.1523/JNEUROSCI.0993-19.2019
Dissing-Olesen, L., LeDue, J. M., Rungta, R. L., Hefendehl, J. K., Choi, H. B., and MacVicar, B. A. (2014). Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J. Neurosci. 34, 10511–10527. doi:10.1523/JNEUROSCI.0405-14.2014
Domercq, M., Vazquez-Villoldo, N., and Matute, C. (2013). Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 7, 49. doi:10.3389/fncel.2013.00049
Eyo, U. B., Peng, J., Swiatkowski, P., Mukherjee, A., Bispo, A., and Wu, L. J. (2014). Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J. Neurosci. 34, 10528–10540. doi:10.1523/JNEUROSCI.0416-14.2014
Fekete, R., Cserep, C., Lenart, N., Toth, K., Orsolits, B., Martinecz, B., et al. (2018). Microglia control the spread of neurotropic virus infection via P2Y12 signalling and recruit monocytes through P2Y12-independent mechanisms. Acta Neuropathol. 136, 461–482. doi:10.1007/s00401-018-1885-0
Fukumoto, Y., Tanaka, K. F., Parajuli, B., Shibata, K., Yoshioka, H., Kanemaru, K., et al. (2019). Neuroprotective effects of microglial P2Y1 receptors against ischemic neuronal injury. J. Cereb. Blood Flow Metab. 39, 2144–2156. doi:10.1177/0271678X18805317
Garaschuk, O., and Verkhratsky, A. (2019). Microglia: the neural cells of nonneural origin. Methods Mol. Biol. 2034, 3–11. doi:10.1007/978-1-4939-9658-2_1
Ginhoux, F., Lim, S., Hoeffel, G., Low, D., and Huber, T. (2013). Origin and differentiation of microglia. Front. Cell. Neurosci. 7, 45. doi:10.3389/fncel.2013.00045
Hall, S., Deurveilher, S., Robertson, G. S., and Semba, K. (2020). Homeostatic state of microglia in a rat model of chronic sleep restriction. Sleep 43 (11), zsaa108. doi:10.1093/sleep/zsaa108
Hanisch, U. K., and Kettenmann, H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394. doi:10.1038/nn1997
Haynes, S. E., Hollopeter, G., Yang, G., Kurpius, D., Dailey, M. E., Gan, W. B., et al. (2006). The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519. doi:10.1038/nn1805
Hickman, S. E., Kingery, N. D., Ohsumi, T. K., Borowsky, M. L., Wang, L. C., Means, T. K., et al. (2013). The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905. doi:10.1038/nn.3554
Illes, P., Rubini, P., Ulrich, H., Zhao, Y., and Tang, Y. (2020). Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells 9, 1108. doi:10.3390/cells9051108
Kato, G., Inada, H., Wake, H., Akiyoshi, R., Miyamoto, A., Eto, K., et al. (2016). Microglial contact prevents excess depolarization and rescues neurons from excitotoxicity. eNeuro 3, ENEURO.0004-16.2016. doi:10.1523/ENEURO.0004-16.2016
Kettenmann, H., Hanisch, U. K., Noda, M., and Verkhratsky, A. (2011). Physiology of microglia. Physiol. Rev. 91, 461–553. doi:10.1152/physrev.00011.2010
Kettenmann, H., Kirchhoff, F., and Verkhratsky, A. (2013). Microglia: new roles for the synaptic stripper. Neuron. 77, 10–18. doi:10.1016/j.neuron.2012.12.023
Kluge, M. G., Abdolhoseini, M., Zalewska, K., Ong, L. K., Johnson, S. J., Nilsson, M., et al. (2019). Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J. Cereb. Blood Flow Metab. 39, 2456–2470. doi:10.1177/0271678X18797346
Li, Y., Du, X. F., and Du, J. L. (2013). Resting microglia respond to and regulate neuronal activity in vivo. Commun. Integr. Biol. 6, e24493. doi:10.4161/cib.24493
Liverani, E., Kilpatrick, L. E., Tsygankov, A. Y., and Kunapuli, S. P. (2014). The role of P2Y12 receptor and activated platelets during inflammation. Curr. Drug Targets 15, 720–728. doi:10.2174/1389450115666140519162133
Mildner, A., Huang, H., Radke, J., Stenzel, W., and Priller, J. (2017). P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia. 65, 375–387. doi:10.1002/glia.23097
Moore, C. S., Ase, A. R., Kinsara, A., Rao, V. T., Michell-Robinson, M., Leong, S. Y., et al. (2015). P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2, e80. doi:10.1212/NXI.0000000000000080
Nimmerjahn, A., Kirchhoff, F., and Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. doi:10.1126/science.1110647
Ohsawa, K., Irino, Y., Sanagi, T., Nakamura, Y., Suzuki, E., Inoue, K., et al. (2010). P2Y12 receptor-mediated integrin-b1 activation regulates microglial process extension induced by ATP. Glia 58, 790–801. doi:10.1002/glia.20963
Ormel, P. R., Bottcher, C., Gigase, F. A. J., Missall, R. D., van Zuiden, W., Fernandez Zapata, M. C., et al. (2020). A characterization of the molecular phenotype and inflammatory response of schizophrenia patient-derived microglia-like cells. Brain Behav. Immun. 90, 196–207. doi:10.1016/j.bbi.2020.08.012
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. doi:10.1126/science.1202529
Peng, J., Liu, Y., Umpierre, A. D., Xie, M., Tian, D. S., Richardson, J. R., et al. (2019). Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol. Brain 12, 71. doi:10.1186/s13041-019-0492-x
Posfai, B., Cserep, C., Orsolits, B., and Denes, A. (2019). New insights into microglia-neuron interactions: a neuron’s perspective. Neuroscience 405, 103–117. doi:10.1016/j.neuroscience.2018.04.046
Sakai, J. (2020). Core concept: how synaptic pruning shapes neural wiring during development and, possibly, in disease. Proc. Natl. Acad. Sci. U.S.A. 117, 16096–16099. doi:10.1073/pnas.2010281117
Sasaki, Y., Hoshi, M., Akazawa, C., Nakamura, Y., Tsuzuki, H., Inoue, K., et al. (2003). Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44, 242–250. doi:10.1002/glia.10293
Savage, J. C., Carrier, M., and Tremblay, M. E. (2019). Morphology of microglia across contexts of health and disease. Methods Mol. Biol. 2034, 13–26. doi:10.1007/978-1-4939-9658-2_2
Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74, 691–705. doi:10.1016/j.neuron.2012.03.026
Sierra, A., Encinas, J. M., Deudero, J. J., Chancey, J. H., Enikolopov, G., Overstreet-Wadiche, L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495. doi:10.1016/j.stem.2010.08.014
Sipe, G. O., Lowery, R. L., Tremblay, M. E., Kelly, E. A., Lamantia, C. E., and Majewska, A. K. (2016). Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 7, 10905. doi:10.1038/ncomms10905
Stevens, B., Allen, N. J., Vazquez, L. E., Howell, G. R., Christopherson, K. S., Nouri, N., et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. doi:10.1016/j.cell.2007.10.036
Sunkaria, A., Bhardwaj, S., Halder, A., Yadav, A., and Sandhir, R. (2016). Migration and phagocytic ability of activated microglia during post-natal development is mediated by calcium-dependent purinergic signalling. Mol. Neurobiol. 53, 944–954. doi:10.1007/s12035-014-9064-3
Swiatkowski, P., Murugan, M., Eyo, U. B., Wang, Y., Rangaraju, S., Oh, S. B., et al. (2016). Activation of microglial P2Y12 receptor is required for outward potassium currents in response to neuronal injury. Neuroscience 318, 22–33. doi:10.1016/j.neuroscience.2016.01.008
Tozaki-Saitoh, H., Makoto, T., and Inoue, K. (2012). P2Y receptors in microglia and neuroinflammation. WIREs Membr. Transp. Signal 1, 493–501. doi:10.1002/wmts.46
Tozaki-Saitoh, H., Miyata, H., Yamashita, T., Matsushita, K., Tsuda, M., and Inoue, K. (2017). P2Y12 receptors in primary microglia activate nuclear factor of activated T-cell signaling to induce C-C chemokine 3 expression. J. Neurochem. 141, 100–110. doi:10.1111/jnc.13968
Tuan, L. H., and Lee, L. J. (2019). Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol. Dis. 130, 104517. doi:10.1016/j.nbd.2019.104517
Vainchtein, I. D., and Molofsky, A. V. (2020). Astrocytes and microglia: in sickness and in health. Trends Neurosci. 43, 144–154. doi:10.1016/j.tins.2020.01.003
van der Poel, M., Ulas, T., Mizee, M. R., Hsiao, C. C., Miedema, S. S. M., Adelia, , et al. (2019). Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10, 1139. doi:10.1038/s41467-019-08976-7
Varga, D. P., Menyhart, A., Posfai, B., Csaszar, E., Lenart, N., Cserep, C., et al. (2020). Microglia alter the threshold of spreading depolarization and related potassium uptake in the mouse brain. J. Cereb. Blood Flow Metab. 40, S67–S80. doi:10.1177/0271678X19900097
Verkhratsky, A., and Burnstock, G. (2014). Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays 36, 697–705. doi:10.1002/bies.201400024
Verkhratsky, A., Krishtal, O. A., and Burnstock, G. (2009). Purinoceptors on neuroglia. Mol. Neurobiol. 39, 190–208. doi:10.1007/s12035-009-8063-2
Verkhratsky, A., and Nedergaard, M. (2018). Physiology of astroglia. Physiol. Rev. 98, 239–389. doi:10.1152/physrev.00042.2016
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S., and Nabekura, J. (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980. doi:10.1523/JNEUROSCI.4363-08.2009
Walker, D. G., Tang, T. M., Mendsaikhan, A., Tooyama, I., Serrano, G. E., Sue, L. I., et al. (2020). Patterns of expression of purinergic receptor P2RY12, a putative marker for non-activated microglia, in aged and Alzheimer’s disease brains. Int. J. Mol. Sci. 21, 678. doi:10.3390/ijms21020678
Weinhard, L., di Bartolomei, G., Bolasco, G., Machado, P., Schieber, N. L., Neniskyte, U., et al. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 9, 1228. doi:10.1038/s41467-018-03566-5
Zimmermann, H., Zebisch, M., and Strater, N. (2012). Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8, 437–502. doi:10.1007/s11302-012-9309-4
Keywords: microglia, P2Y12 receptors, neurone-microglial crosstalk, purinergic signalling, neuroprotective
Citation: Lin S-S, Tang Y, Illes P and Verkhratsky A (2021) The Safeguarding Microglia: Central Role for P2Y12 Receptors. Front. Pharmacol. 11:627760. doi: 10.3389/fphar.2020.627760
Received: 10 November 2020; Accepted: 30 November 2020;
Published: 14 January 2021.
Edited by:
Elena Adinolfi, University of Ferrara, ItalyReviewed by:
Elisabetta Coppi, University of Florence, ItalyCopyright © 2021 Lin, Tang, Illes and Verkhratsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Si-Si Lin, bGluc2lzaUBzdHUuY2R1dGNtLmVkdS5jbg==; Alexei Verkhratsky, QWxleGVqLlZlcmtocmF0c2t5QG1hbmNoZXN0ZXIuYWMudWs=
 Si-Si Lin
Si-Si Lin Yong Tang
Yong Tang Peter Illes
Peter Illes Alexei Verkhratsky
Alexei Verkhratsky