- 1Department of Medicine, Surgery and Neuroscience, Rheumatology Unit, Clinic for the Diagnosis and Management of Hand Osteoarthritis, Azienda Ospedaliera Universitaria Senese, Siena, Italy
- 2Department of Medicine, Surgery and Neuroscience, Orthopedics and Traumatology Unit, University of Siena, Siena, Italy
Thumb-base osteoarthritis (TBOA) is a common condition, mostly affecting post-menopausal women, often inducing a significant impact on quality of life and hand functionality. Despite its high prevalence and disability, the therapeutic options in TBOA are still limited and few have been investigated. Among the pharmacological strategies for TBOA management, it would be worthwhile to mention the injection-based therapy. Unfortunately, its efficacy is still the subject of debate. Indeed, the 2018 update of the European League Against Rheumatism (EULAR) recommendations for the management of hand osteoarthritis (OA) stated that intra-articular (IA) injections of glucocorticoids should not generally be used, but may be considered in patients with painful interphalangeal joints, without any specific mention to the TBOA localization and to other widely used injections agents, such as hyaluronic acid (HA) and platelet-rich plasma (PRP). Even American College of Rheumatology (ACR) experts conditionally recommended against IA HA injections in patients with TBOA, while they conditionally encouraged IA glucocorticoids. However, the recommendations from international scientific societies don’t often reflect the clinical practice of physicians who routinely take care of TBOA patients; indeed, corticosteroid injections are a mainstay of therapy in OA, especially for patients with pain refractory to oral treatments and HA is considered as a safe and effective treatment. The discrepancy with the literature data is due to the great heterogeneity of the clinical trials published in this field: indeed, the studies differ for methodology and protocol design, outcome measures, treatment (different formulations of HA, steroids, PRP, and schedules) and times of follow-up. For these reasons, the current review will provide deep insight into the injection-based therapy for TBOA, with particular attention to the different employed agents, the variety of the schedule treatments, the most common injection techniques, and the obtained results in terms of efficacy and safety. In depth, we will discuss the available literature on corticosteroids and HA injections for TBOA and the emerging role of PRP and other injection agents for this condition. We will consider in our analysis not only randomized controlled trials (RCTs) but also recent pilot or retrospective studies trying to step forward to identify satisfactory management strategies for TBOA.
Introduction
Thumb-base osteoarthritis (TBOA) is a highly prevalent condition affecting middle-aged and older people; the condition increases with age, is more common in women—particularly post-menopausal—and it is often bilateral (Dahaghin et al., 2005; Haugen et al., 2011; Kloppenburg et al., 2017).
The prevalence of symptomatic TBOA among people aged >50 years was estimated from 5 to 7%, while the prevalence of radiographic TBOA is higher, ranging from 45 to 60% (Sodha et al., 2005; Sonne-Holm and Jacobsen, 2006).
The main symptoms of TBOA are pain, localized to the base of the thumb, stiffness, tenderness and loss of range of motion. The impairment function reduces the ability to perform activities of daily living, such as writing, opening a jar, turning a car key, and turning a door or handling small objects. In the more advanced stages, thenar muscle wasting combined with subluxation and adduction of the thumb metacarpal can induce a characteristic “squaring” joint deformity. Furthermore, patients with concomitant osteoarthritis (OA) of the interphalangeal (IP) joints and TBOA complain of more pain, functional disability, and reduced quality of life (Bijsterbosch et al., 2010; Tenti et al., 2020).
Despite its high prevalence and disability, the therapeutic options for TBOA are still limited and few investigated; its management usually requires a combination of non-pharmacological, pharmacological, and surgical strategies with a multidisciplinary approach (Kloppenburg et al., 2017).
Among the pharmacological strategies, it would be worthwhile to mention the use of intra-articular (IA) injection-based therapy with corticosteroid or hyaluronic acid (HA). Unfortunately, its efficacy is still the subject of debate and not universally shared by the current guidelines for the management of hand OA.
The 2007 European League Against Rheumatism (EULAR) recommendations for hand OA support the use of IA long-acting corticosteroids for painful flares of OA, especially for TBOA (Zhang et al., 2007).
Conversely, the 2018 update of EULAR recommendations state that IA injections of steroids should not generally be used, but may be considered in patients with painful IP joints, without any specific mention to the TBOA localization and to other widely used IA agents, as HA and platelet-rich plasma (PRP) (Kloppenburg et al., 2019). Even American College of Rheumatology (ACR) experts conditionally recommend against IA HA injections in patients with TBOA, while they conditionally encourage IA glucocorticoids (Kolasinski et al., 2020).
However, the recommendations from international scientific societies do not often reflect the clinical practice of all physicians who routinely take care of TBOA patients; indeed, corticosteroid injections are a mainstay of therapy in OA, especially for patients with pain refractory to oral treatments, and HA is considered as a safe and effective therapeutic option.
Considering the high prevalence of a disabling disease, such as TBOA, we aimed to perform a narrative review analyzing the current evidence on the efficacy and safety of the intra-articular therapy. For this purpose, we grouped the literature evidence for different used IA drugs (corticosteroids, hyaluronate, PRP, or other medications), adding a discussion to find the gaps in this area and to identify where additional research is needed.
Methods
Data Sources and Searches
We created a comprehensive search strategy aimed to capture all relevant papers concerning injection-based therapy for TBOA. The search strategy was applied to the following bibliographic databases: Cochrane Library, PubMed, MEDLINE, EMBASE, Web of Science, and Scopus, using the terms “thumb-base joint osteoarthritis,” “trapezio-metacarpal joint osteoarthritis,” “first carpo-metacarpal joint osteoarthritis,” “rizoartrhosis” in combination with “intra-articular injections,” “injection-based therapy,” “steroid injections,” “hyaluronic acid injections,” “platelet-rich plasma injections,” and “prolotherapy.” Additional articles were identified by searching bibliographies of each paper. Furthermore, we searched www.clinicaltrials.gov for active and/or recently completed clinical trials testing agents for IA therapy of TBOA.
We conducted the search of the literature in October 2020.
Inclusion/Exclusion Criteria
In this narrative review, we included all studies analyzing an injection-based intervention for patients suffering from TBOA. In particular, articles were considered eligible if they met the following criteria: 1) diagnosis of TBOA of the study population, according to the ACR criteria for hand OA (Altman et al., 1990); 2) any study design, including not only randomized controlled trials (RCTs), but even prospective open label or retrospective studies; 3) any studies presenting at least an evaluation of the efficacy, in terms of both pain and function, and tolerability of injection-based therapy; 4) any type of pharmacological agents or medical devices injected; 5) any injection approach included (with any or no image guidance); 6) studies published from 2000 to October 2020, totally written in English language. Studies were excluded if they did not evaluate the effects of injection therapy on both pain and function; review articles, studies not published as a full article (conference abstracts) and papers not totally written in the English language were also not considered.
Selection of Studies
Initially, duplicates were removed and relevant trials were independently screened by checking titles, keywords, and abstracts by two authors (T. S., M. N.). The references of the selected articles and all significant reviews on the topic were also checked to identify other potential papers. Then, a full-text evaluation of the selected studies was performed by the same authors (T. S, M. N.) to determine whether the trials met the inclusion criteria regarding design, study population, outcomes, and interventions. Disagreement between the two reviewers was solved by involving a third author (F. A.).
Data Extraction
Data were independently extracted and aggregated into a Microsoft Excel®spreadsheet database by two authors (C. S. and G. S.). In particular, the data extraction sheet was designed to collect data about the study design, participants, details on the interventions undertaken, types of outcome measures evaluated, duration of follow-up, loss to follow-up, and results. Any inconsistencies between the two authors were solved by consensus discussion or by involving a third reviewer (F. A.) in case of persistent disagreement.
Outcomes and Data Analysis
Patient-reported pain and function were considered the main outcomes of interest; possible side effects related to the injection-based therapy were also recorded. A priori we defined as short-term follow-up, a follow-up period ranging from one week to 3 months, medium-term follow-up a period ranging from 3 to 6 months, and long-term follow-up above 6 months. Descriptive analysis was performed for all demographic data, interventions, and outcome parameters to facilitate narrative interpretation and comparison among the studies.
Results
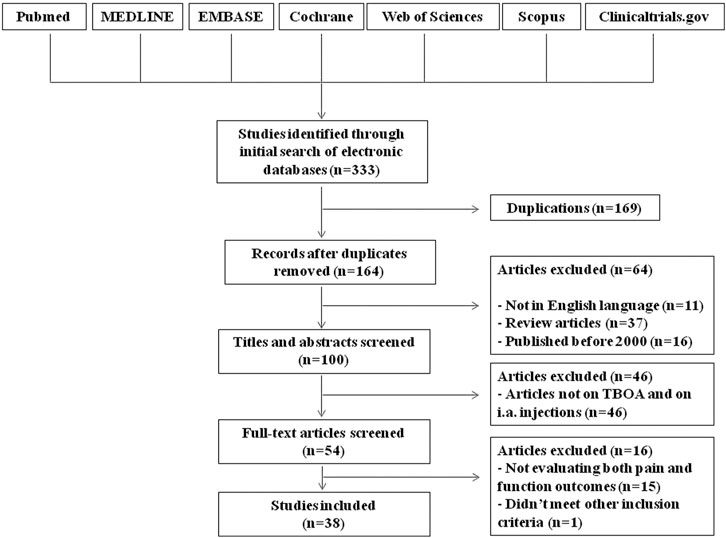
Literature Search Results and Trials Characteristics
In total, 164 potential eligible studies were found; no additional papers were obtained by hand searching of references. Of these, 11 studies were excluded because they were written in a language other than English, 37 because they were review articles and 16 because were published before 2000. Based on the title and the abstract content, 46 of these articles were not included in our review. The full texts of the remaining 54 studies were read, and a further 16 studies were excluded because they did not meet other inclusion criteria (Figure 1). We identified 38 assessable studies, six analyzing the effect of IA injections of corticosteroids, 20 evaluating the effects of IA hyaluronic acid, of whom seven in comparison to steroids, five dealing with IA injections of PRP and the remaining seven exploring new emerging IA therapy. Additionally, we identified two study protocols for trials planned for the coming years.
Corticosteroid Injections
Intra-articular corticosteroids have been used for decades in the management of symptomatic OA and remain a common practice given their potent anti-inflammatory properties and the favorable cost/effectiveness profile. Steroid injection is typically reserved to patients not responding to systemically delivered drugs or who do not tolerate pharmacological treatments (Jüni et al., 2015). The choice of the drug depends on the experience and preference of the physician, but generally includes triamcinolone, methylprednisolone, and betamethasone.
Intra-articular injection of steroid is mostly used and studied for inflammatory and degenerative disease of large joints, such as the knee, while the scientific evidence for TBOA is limited and conflicting. The characteristics of the few studies found by our literature research are summarized in Table 1.
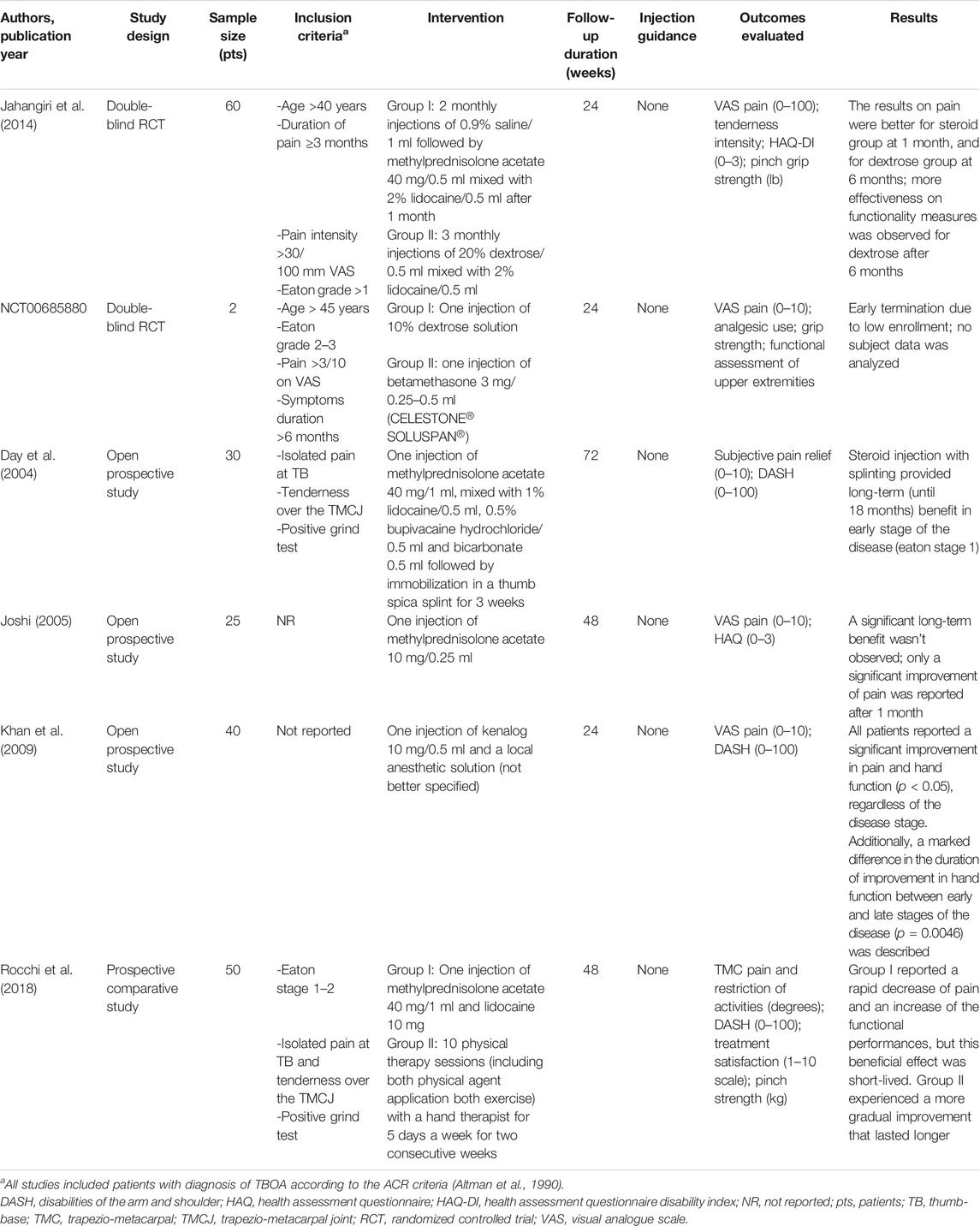
TABLE 1. Summary of studies investigating intra-articular injections of corticosteroids for the treatment of thumb-base osteoarthritis.
A double-blind RCT compared the efficacy of IA steroids (methylprednisolone acetate 40 mg) with a 20% dextrose solution (prolotherapy treatment), both mixed with 0.5 ml of 2% lidocaine (Jahangiri et al., 2014). In this study, sixty patients with TBOA beyond stage one of the Eaton classification (Eaton and Glickel, 1987) were selected and randomly assigned to corticosteroids or prolotherapy. One group received two monthly placebo injections with a 0.9% saline solution and in the third month the steroid, the other one was treated with three monthly IA dextrose solution. The efficacy of the treatment was evaluated at 1, 2, and 6 months after the third injection. Methylprednisolone appeared more effective in the short-term, but at the sixth month the results showed a remarkable difference in favor of dextrose. No severe side effects were reported for prolotherapy.
Another randomized double-blind trial comparing IA 10% dextrose solution to betamethasone injection for the treatment of symptomatic TBOA was performed by the Mayo Hand Clinic (Clinicaltrials.gov, NCT00685880). The study started in 2008, but it appears to have been discontinued because of the small number of patients recruited.
A number of non-RCTs investigated the effectiveness and tolerability of IA steroid for TBOA. Thirty patients with TBOA were included in a long-term prospective open study and treated with a single injection of methylprednisolone acetate (40 mg) and 0.5 ml of 1% lidocaine followed by the use of a thumb spica splint for three weeks (Day et al., 2004). The clinical evaluation provided long-term (until 18 months) benefit in early stage of the disease, while in the severe form of disease (Eaton stage 4) the treatment appeared ineffective. On the contrary, Joshi R (Joshi, 2005) in a prospective case series of 25 patients treated with a single injection of 10 mg of methylprednisolone acetate showed a significant improvement of pain after 1 month, but not in the following observations at 3, 6, and 12 months. The Author did not report any information about the stage of the disease or about the concomitant use of other pharmacological or non-pharmacological treatments during the study period.
Khan et al. (Khan et al., 2009) conducted a prospective open study in 40 patients with TBOA to evaluate the improvement in pain and function of the hand after a single IA corticosteroid injection (triamcinolone acetonide 10 mg) and a local anesthetic solution. The symptomatic effect was evident in all patients in the short-term evaluation (2–4 weeks), but the duration of this benefit was different according to the stage of the disease.
Rocchi et al. (Rocchi et al., 2018) compared, prospectively, the effect of 10 sessions of physiotherapy to a single IA injection of methylprednisolone acetate (40 mg) and lidocaine in 50 patients with TBOA at early stages. The patients receiving IA therapy reported a rapid decrease of pain and an increase of the functional performances, but this beneficial effect was not maintained in the long-term follow-up (12 months). The group treated with physiotherapy (heat application, passive and active mobilization, massage, and stretching) experienced a more gradual improvement that lasted longer.
We did not report in this analysis the trials by Meenagh et al. (Meenagh et al., 2004) and by Swindells et al. (Swindells et al., 2010), because they evaluated only the effects of IA steroids on pain and not on functionality, as determined by our inclusion criteria.
Hyaluronic Acid Injections
HA represents another well-known IA treatment for OA; its use is based on its ability to restore the rheological properties of the synovial fluid and thus to decrease pain and improve functionality. For these reasons, it can represent a valid and safe alternative to IA corticosteroids in OA patients not responding to non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics. The role of viscosupplementation with HA is nowadays worldwide recognized for the treatment of knee OA, but its usefulness has been recently suggested also for other joints, such as hip, ankle, shoulder, temporomandibular joint, and thumb (Henrotin et al., 2015). However, as demonstrated in recent systematic reviews and meta-analysis, the scientific evidence on the efficacy of the IA therapy with HA in TBOA is still subject of debate, and often limited by the great heterogeneity of the trials performed in this field (Trellu et al., 2015; Kroon et al., 2016; Riley et al., 2019). The main sources of heterogeneity are represented by different HA formulations employed with variable injection schedules and IA techniques, different periods of follow-up and a great variety of assessed outcomes.
We identified a total of 20 papers, including nine RCTs, two retrospective comparative studies and nine open label trials evaluating the effects of the IA therapy with HA in TBOA patients. In the controlled studies, the comparator treatment was represented by IA corticosteroids (7 papers), IA saline solution (one paper) and extracorporeal shock wave therapy (ESWT) (one paper). The remaining controlled trials evaluated different schedules of IA HA in one case, and assessed a combination therapy with IA HA and IA ketorolac vs. IA HA alone in another one.
Hyaluronic Acid Versus Corticosteroids Injections
The individual characteristics of each study (6 RCTs and one retrospective comparative study) are reported in Table 2. A direct comparison among these trials is not possible, considering the great heterogeneity of the studies for a variety of parameters. Four research papers evaluated, as HA formulation, sodium hyaluronate from different commercial brands, two studies analyzed hylan and another one considered a hybrid formulation of HA. As corticosteroid comparator, the Authors chose triamcinolone acetonide in four cases, although with different dosages, betamethasone disodium phosphate in two works and methylprednisolone in the remaining one. Injections courses ranged from a single injection to three weekly injections. The length of follow-up was of 6 months for all trials, except from one in which the follow-up lasted until 12 months. Image guidance was employed in only one study. The only outcome parameter evaluated in all studies was pain by a visual analogue scale (VAS). Functionality was assessed by a variety of different tests.
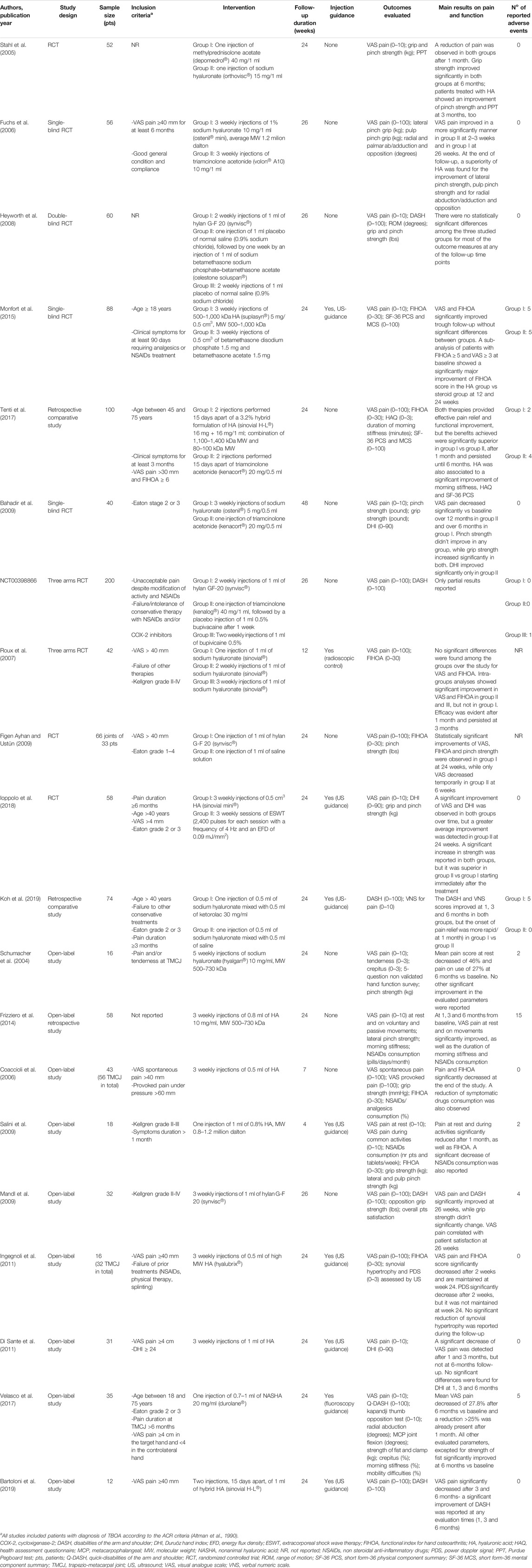
TABLE 2. Summary of studies investigating intra-articular injections of hyaluronic acid for the treatment of thumb-base osteoarthritis.
Concerning the efficacy of the results, the RCTs by Stahl et al. (Stahl et al., 2005) and Fuchs et al. (Fuchs et al., 2006) showed a significant effect of both IA steroid and IA HA on pain relief (VAS) and function improvement (assessed by grip strength in the former study and by pinch grip and pulp pinch grip in the latter). However, Stahl et al. (Stahl et al., 2005) observed a significant improvement of the functional Purdue Pegboard Test (PPT), which measures the fine hand function, only in the HA group. Consistent with these results, Fuchs et al. (Fuchs et al., 2006) found a superiority of HA over steroids in all assessed parameters (VAS pain, grip power, and range of motion) in the medium-term. The more recent 6-months, single-blind, RCT by Heyworth et al. (Heyworth et al., 2008) reported no statistically significant differences among the three studied groups, of whom one was treated with two IA injections of hylan, one with a single injection of normal saline (0.9% sodium chloride) followed, after a week, by IA betamethasone, and another one with two IA injections of normal saline; however, a positive trend in hand function, assessed by Disabilities of the Arm, Shoulder, and Hand (DASH) scores, was observed in patients treated with HA. A positive trend in hand function, measured by Functional Index for Hand Osteoarthritis (FIHOA) score, was observed in patients treated with IA HA [3 weekly injections of a formulation of HA with molecular weight (MW) 500–1,000 kDa] also by Monfort et al. (Monfort et al., 2015) in a 6-months single-blinded randomized trial vs. betamethasone. These findings became particularly evident and reached statistical significance when patients with more severe symptoms (FIHOA score of at least five and VAS score of 50 or more) were considered for analysis.
These encouraging data on the HA therapy in patients with TBOA were recently confirmed by a 6-months retrospective comparative study which assessed the efficacy of a new hybrid formulation of HA vs. triamcinolone acetonide in 100 patients (Tenti et al., 2017). The Authors found both IA therapies effective in controlling pain (by VAS) and improving joint functionality (by FIHOA), but the benefits achieved were significantly superior in the HA group than in the steroid group after 1 month and until the end of follow-up. Furthermore, the HA formulation studied also resulted in an association with a significant decrease in the duration of morning stiffness and with a significant improvement of Health Assessment Questionnaire (HAQ) and physical component summary (PCS)-SF-36.
Contrasting results were reported by Bahadir et al. (Bahadir et al., 2009) in an RCT evaluating in the long-term 20 patients treated with a single injection of 20 mg triamcinolone acetonide and 20 patients who received three weekly injections of 5 mg sodium hyaluronate. Pain levels were significantly decreased in both groups, but the beneficial effect persisted until 12 months only in the steroid group; similarly, the improvement in hand functionality, assessed by the Duruoz Hand Index (DHI), reached statistical significance only in patients treated with triamcinolone.
Interestingly, the protocol of a new randomized multicenter study, the RHIZ’ART trial, aimed to analyze, for the first time, the possible synergistic effect of corticosteroids associated with HA, compared to steroid alone, in TBOA patients, was published last year (Cormier et al., 2019). The Authors would like to compare VAS pain, Cochin score, grip strength and opposition force, 3 months after a single injection of 0.5 ml of corticosteroid and 0.5 ml of physiological saline or 0.5 ml of corticosteroid and 0.5 ml of HA and would like to continue the follow-up until 12 months.
A phase three triple-blind (participants, care provider, investigator) RCT comparing the safety and effectiveness of hyaluronan (Hylan G-F20 injected once a week for two consecutive weeks) to corticosteroids (triamcinolone, 40 mg injected the first week, followed by a placebo injection of 1 ml 0.5% bupivacaine the second week) and local anesthetic (Bupivicaine 0.5% 1 ml injected once a week for 2 weeks) in relieving symptoms of TBOA has recently been completed (Clinicaltrials.gov, NCT00398866). Unfortunately, only partial results have been reported.
Hyaluronic Acid Versus Other IA Treatment Comparators
Considering the lack of guidelines for the IA HA treatment schedule, in 2007 Roux et al. (Roux et al., 2007) compared the efficacy on pain and function of one, two, or three IA injections of 1 ml sodium hyaluronate, performed weekly under radioscopic control in the carpometacarpal joint of 44 patients. No significant differences were found among the three groups over the study period (3 months) for VAS pain and FIHOA, while intra-groups differences between baseline and the end of follow-up were significant only for patients treated with two or three injections.
In a 6-months Turkish RCT conducted in 2009, IA HA was compared to IA saline injection in 33 women with bilateral TBOA; in particular, hands of the same patient were divided to hylan G-F 20 injection and saline injection, randomly. The Authors found a significant improvement of VAS pain, FIHOA, and pinch strength at the 24th week only in the hylan group, while a short-term (at the sixth week) placebo analgesic effect was described for the control group (Figen Ayhan and Ustün, 2009).
In another RCT on 58 TBOA patients, three weekly IA injections of 0.5 cm3 HA were compared to ESWT performed once a week for three consecutive weeks. Although a significant improvement in VAS pain, DHI score and grip and pinch strength was observed in both groups at 3 and 6 months, a greater benefit was reported in the ESWT group for all the assessed parameters (Ioppolo et al., 2018).
Finally, very recently, in a retrospective comparative study, Koh et al. (Koh et al., 2019) treated 74 TBOA patients with ultrasound-guided IA injection of 0.5 ml of sodium hyaluronate and 0.5 ml of ketorolac or 0.5 ml of sodium hyaluronate and 0.5 ml of saline. The DASH and verbal numeric scale (VNS) pain scores improved at 1, 3, and 6 months post-injection in both groups, but the pain reduction was significantly more rapid (at 1 month) after the injection of HA plus ketorolac compared to HA alone, suggesting a possible role of this combined IA therapy for a fast onset of analgesia.
Hyaluronic Acid in Open Label Trials
In the last two decades, a variety of papers investigating the potential efficacy of different formulations of HA have been published (Table 2).
In 2004 the open-label study by Schumacher et al. (Schumacher et al., 2004) provided preliminary evidence that a cycle of five weekly injections of low MW (500–730 kDa) HA into the trapezio-metacarpal joint of 16 TBOA patients, was effective in reducing pain at 6 months follow-up, although a significant effect on pinch strength could not be observed. The beneficial effects of the same HA formulation have been subsequently confirmed by a retrospective open study conducted by Frizziero et al. (Frizziero et al., 2014). The Authors demonstrated that 58 patients treated with three weekly IA injections of low MW HA (500–730 kDa) presented a significant reduction of pain at rest and on voluntary or passive movements of flexion, extension, abduction, and rotation (on a 0–10 mm VAS scale), of morning stiffness duration and of NSAIDs consumption at any evaluation time (1, 3, and 6 months); furthermore, a significant improvement of radial and palmar ab-/adduction was registered at each follow-up visit.
The use of IA HA for TBOA was encouraged also in two different studies by Coaccioli et al. (Coaccioli et al., 2006) and Salini et al. (Salini et al., 2009); however, both trials were limited by a very short-term follow-up (1 month). In the former trial, 43 TBOA patients for a total of 56 trapezio-metacarpal joints were treated with three weekly injections of 0.5 ml HA and experienced a significant reduction of VAS pain, FIHOA score and NSAIDs/analgesic consumption, other than a significant improvement of grip strength after 1 month from the first injection (Coaccioli et al., 2006). In the latter study, a small group of TBOA patients (n = 18) received a single ultrasound-guided injection of a formulation of HA with a MW of 0.8–1.2 million Dalton; a significant decrease of pain at rest and during activities, as well as of FIHOA score were reported at the end of 1 month follow-up, together with a significant reduction of NSAIDs intake (Salini et al., 2009).
Other HA formulations also resulted to be beneficial for patients with TBOA in open label pilot trials. In an American study on 32 patients, a cycle of three weekly injections of hylan G-F 20 determined a significant improvement of VAS pain and DASH score (Mandl et al., 2009). In 2011, Ingegnoli et al. (Ingegnoli et al., 2011) evaluated the effects of three ultrasound-guided IA injections, performed 1 week apart, with high MW HA in 32 TB joints of 16 patients. The Authors reported a significant clinical improvement, characterized by VAS pain and FIHOA score decrease, 2 weeks after the injections and this effect persisted until 6 months. At the same time, a significant reduction of power doppler signal was observed at 2 weeks, suggesting a potential role of HA in reducing local inflammation, although this result was not maintained at week 24. In the same year, an Italian trial assessed the efficacy of an ultrasound-guided procedure for the treatment of TBOA with HA. Thirty-one patients received three weekly injections of 1 ml HA and experienced a statistically significant VAS reduction at 1 and 3 months after the end of the IA therapy, but not a 6-months follow-up; no significant differences were described for DHI at any evaluation times (Di Sante et al., 2011).
More recently, a 6-months, prospective, open-label study investigated the effects of a single IA injection of nonanimal hyaluronic acid (NASHA) into the trapezio-metacarpal joint of 35 TBOA patients. This HA formulation differs from the others above mentioned for the presence of synthetic cross-linking which creates a three-dimension gel network, responsible for an increased viscosity and half-life. The Authors reported a significant mean change from baseline in VAS pain score at any evaluation times (month 1, 3, and 6) with a reduction of 27.8% at 6 months. Further, a significant improvement of quickDASH, Kapandji thumb opposition test, radial abduction, metacarpal flexion, and strength of clamp scores were observed at the end of follow-up (Velasco et al., 2017). Finally, an open study on a small sample of patients (n = 12) confirmed the positive results of the above-mentioned study by Tenti et al. (Tenti et al., 2017) on the use of an hybrid formulation of HA. Indeed, the Authors reported a statistically significant reduction of VAS pain after 3 and 6 months and a significant improvement of DASH score at 1, 3, and 6 months (Bartoloni et al., 2019).
We did not report in this analysis the trial by Dauvissat et al. (Dauvissat et al., 2018) on a single injection of mannitol-modified cross-linked HA in patients with TBOA, because it evaluated only the effects on pain and not on functionality, as determined by our inclusion criteria.
Platelet-Rich Plasma Injections
PRP is an autologous blood product derived by centrifugation of the whole blood and characterized by a high concentration of platelets above the normal levels (Marx, 2001). Many protocols for preparing PRP exist; one possibility is to include the leukocyte-containing buffy coat obtaining the so-called leukocyte-rich PRP, while another one is to exclude leukocytes resulting in the so-called leukocyte-poor PRP which is the standard PRP preparation for OA (Evans et al., 2020). Its use for the treatment of OA of large joints, particularly knee and hip OA, has emerged since the first decade of twenty-first century. The rationale of efficacy of this IA treatment lies on its ability to reverse pro-inflammatory processes and to modify the microenvironment inside the joint, restoring the articular homeostasis (Ornetti et al., 2016). In depth, after PRP injection, a subset of cytokines and growth factors, as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), interleukin-1 receptor antagonist (IL-1RA), soluble receptor of tumor necrosis factor-alpha (TNF-alpha) transforming growth factor-beta (TGF-beta), and many others, are released into the joint, through the degranulation of the platelets α-granules. Globally, these mediators exert an anti-catabolic and anti-inflammatory action, modulate the metabolic functions of chondrocytes and subchondral bone and stimulate fibroblasts to synthesize HA (Moussa et al., 2017).
Actually, only a very limited number of papers, often with a very small sample size and a not controlled design, investigating the possible efficacy of PRP in TBOA are published (Table 3).
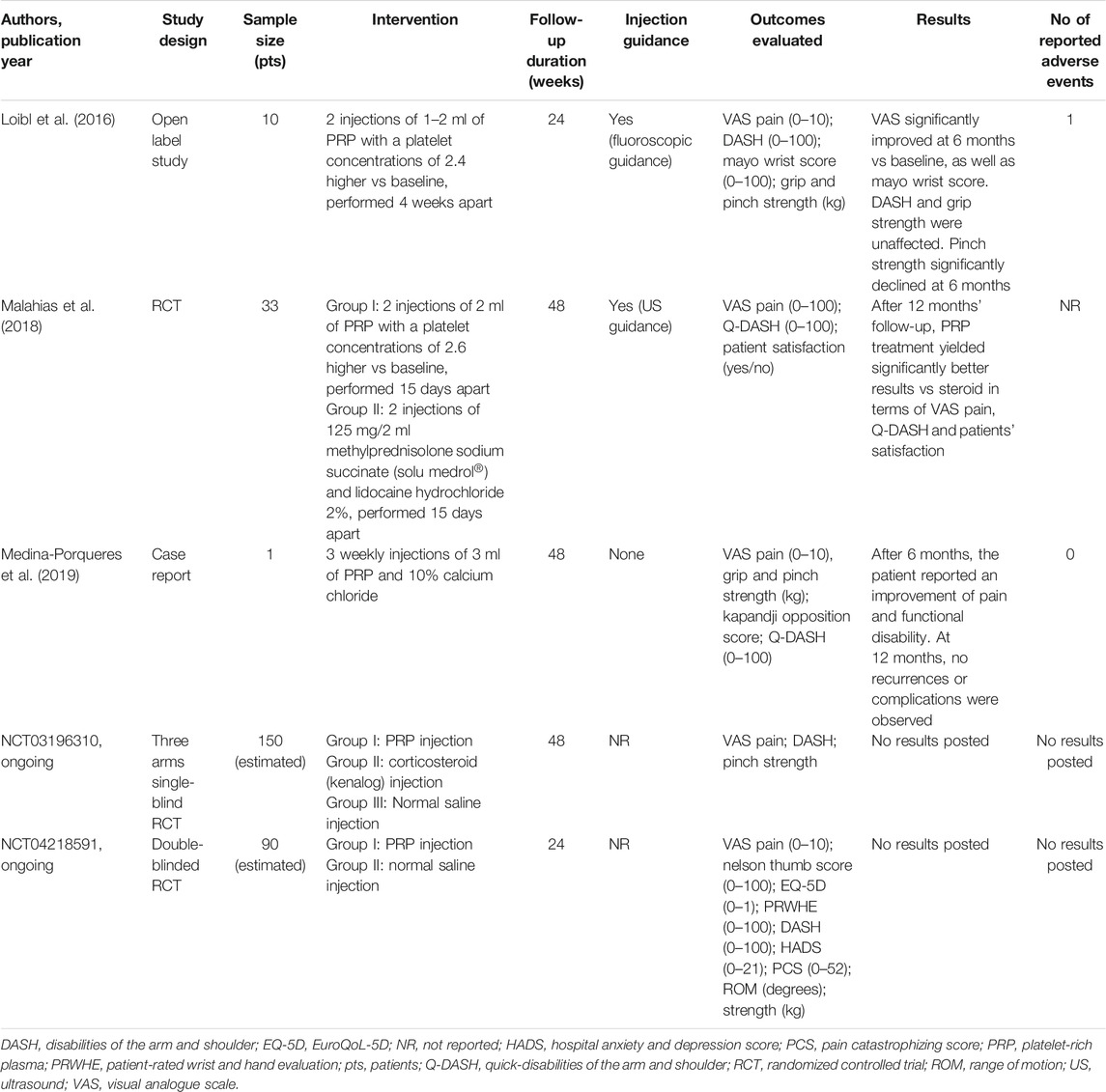
TABLE 3. Summary of studies investigating intra-articular injections of platelet-rich plasma for the treatment of thumb-base osteoarthritis.
The first one dates back to 2016 and analyzed the effect of two IA injections of 1–2 ml of PRP, administered 4 weeks apart to a small number of patients (n = 10). After 6 months of follow-up, the Authors reported a significant improvement of VAS pain and Mayo wrist score, while no differences vs. baseline were observed for DASH score and grip strength (Loibl et al., 2016). These results are supported by a RCT published in 2018 and assessed the efficacy of two ultrasound-guided IA PRP injections, performed 2 weeks apart, in 16 patients compared to two ultrasound-guided IA methylprednisolone and lidocaine injections at a 2-weeks interval in 17 patients. The Authors demonstrated a significant efficacy of PRP in improving pain (measured by VAS) and function (assessed by quick-DASH) both in the mid- (3 months) and long-term (12 months) with a superior effect of PRP compared to steroids at 12 months of follow-up (Malahias et al., 2018). The beneficial effect of PRP in TBOA was supported also by the case report by Medina-Porqueres et al. (Medina-Porqueres et al., 2019). The Authors reported the clinical history of a pianist affected by TBOA and treated with three weekly IA PRP injections who experienced a significant improvement of VAS pain, grip and pinch strength, and quick-DASH score after 6 months; at 12 months follow-up no recurrences or complications were identified.
There are two ongoing clinical trials with IA PRP for TBOA registered in ClinicalTrials.gov. A single-blind (patients) study with IA injections of leukocyte depleted PRP vs. triamcinolone acetonide and vs. placebo (normal saline) for TBOA started in United States in September 2018 (NCT03196310); no results have been reported yet.
Finally, a double-blind randomized trial is currently ongoing in Sweden in patients with radiological Eaton class 1–3 of TBOA comparing the efficacy of PRP vs. placebo (saline solution) (NCT04218591).
New Emerging Intra-articular Therapies
New data are emerging about the possible use of IA injections based on mesenchymal-derived stem cell populations for the treatment of OA, due to their properties of providing mechanical support into the joint and stimulating cartilage repair and regeneration (Bosetti et al., 2016); however, the evidence for TBOA is still very limited. In Table 4 is reported the summary of the studies investigating such kind of IA treatment in TBOA.
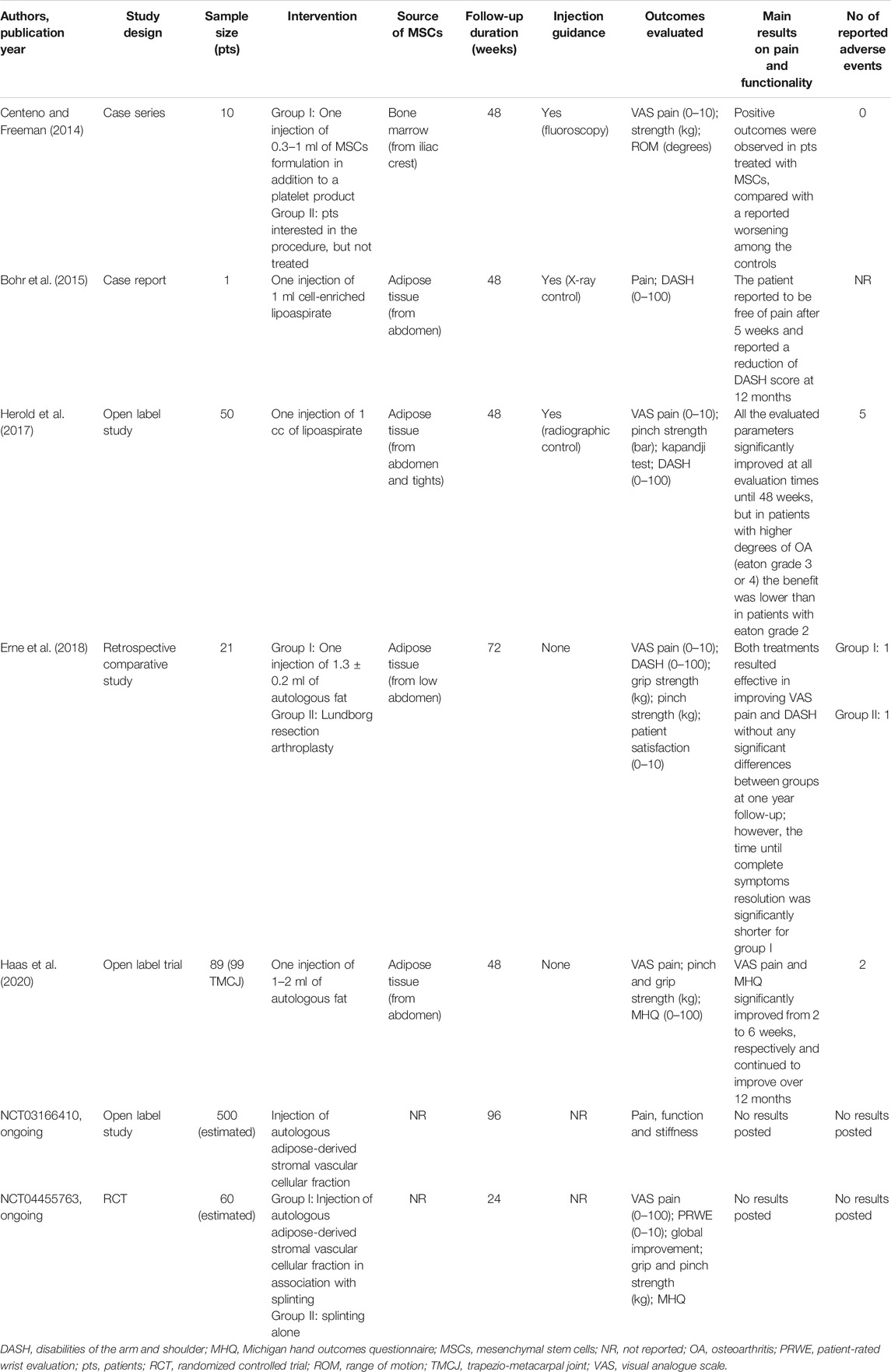
TABLE 4. Summary of studies investigating intra-articular injections of mesenchymal-derived stem cell populations for the treatment of thumb-base osteoarthritis.
A case series on a small study population investigated the efficacy of fluoroscopy-guided IA injections of autologous mesenchymal stem cells, derived from bone marrow aspirate of iliac crest, administered to six patients, and compared to four participants who remained untreated. The Authors reported positive encouraging results for both pain and function after one year of follow-up, although they claimed caution for the several limitations of the study (Centeno and Freeman, 2014). Subsequently, Bohr et al. (Bohr et al., 2015) described the case of a 62-year old man, affected by TBOA, treated with cell-enriched lipoaspirate arhroplasty, after abdominal liposuction, who experienced pain relief after five weeks and a significant improvement vs. baseline of DASH score after one year. Herold et al. (Herold et al., 2017) confirmed the positive results in their prospective open study, which included 50 TBOA patients treated with IA injection of processed autologous fat. This therapy resulted beneficial in terms of VAS score, DASH score, grip, and pinch strength at 12 months follow-up. However, a sub-groups analysis showed significantly better outcomes in patients at Eaton stage 2, while only partial or no improvement in stage 3 or 4.
More recently, Erne et al. (Erne et al., 2018) performed a retrospective study aimed to compare the results of a surgical technique of trapeziectomy with autologous fat injections. Twelve patients underwent the Lundborg resection arthroplasty, while nine patients received autologous fat injection, harvested from their own abdomen. Both treatments resulted effective in improving pain and function (measured by VAS and DASH questionnaires, respectively) without any significant differences between groups at one-year follow-up; however, autologous fat injections seemed to determine a shorter time until symptoms resolution and shorter operative time compared with Lundborg arthroplasty.
Data on a wider cohort of TBOA patients (n = 99) treated with autologous fat injection was derived from the most recent study by Haas et al. (Haas et al., 2020). They reported that pain during activities at 2 and 6 weeks as well as 3, 6, and 12 months was significantly lower than at baseline. Furthermore, Michigan Hand Outcome Questionnaire (MHQ) scores were significantly higher at 6 weeks, 3, 6, and 12 months.
Two open label studies are currently undergoing to evaluate the safety and efficacy of injection therapy with autologous stromal vascular fraction (SVF) derived from adipose tissue (Clinicaltrials.gov, NCT03166410; NCT04455763). The SVF exerts anti-inflammatory, immunosuppressive and chondroprotective effects; due to its potential properties being tried in treating patients with different OA localizations (Pak et al., 2018).
Interestingly, still ongoing at the Cochin Hospital of Paris is an RCT aimed to evaluate the possible efficacy of IA injections of botulinum toxin A, associated with splinting, and compared to IA injection of saline associated with splinting. The rationale for use of botulinum toxin A in OA lies on its potential role in suppressing the release of some mediators involved in nociception (Gil et al., 2018).
Safety of Intra-articular Therapy
In general, IA therapy represents a valid and safe alternative in OA patients with multiple comorbidities, for whom pharmacological treatments often present a not favorable risk/benefit ratio or are contraindicated. However, IA therapy is not free of several side effects.
In particular, corticosteroids are known to be associated with both local reactions, as skin atrophy or hypopigmentation, acute corticosteroid-microcrystalline joint flare and hemarthrosis and both systemic effects, including facial flush, hyperglycemia, blood pressure increase, Tachon’s syndrome, vagal reaction and hypersensitivity (Nguyen and Rannou, 2017). Furthermore, it is noteworthy to report the potential chondrotoxicity of IA steroids which still remains one of the more debated issues in this field. Indeed, some in vitro and animal studies demonstrated that corticosteroids can have an adverse effect on cartilage, especially at high doses, probably due to its ability to modulate cartilage proteins production and breakdown (Wernecke et al., 2015). From a clinical point of view, some trials showed a greater cartilage volume loss in patients treated with IA steroids compared to placebo (McAlindon et al., 2017; Zeng et al., 2019).
In the trials on TBOA patients summarized in this review, the adverse events related to IA steroids injections are not discussed in depth and rarely reported. The side effects occurred in a minority of patients and consisted mainly in temporary acute local pain starting 1–6 h after the injections and resolved spontaneously after one or two days. Only one patient reported mild skin atrophy and hypopigmentation (Day et al., 2004; Joshi, 2005; Khan et al., 2009; Jahangiri et al., 2014; Rocchi et al., 2018).
Intra-articular HA is usually recognized as a safe treatment for OA; the incidence of adverse events in RCTs, especially on knee OA, is rather low. The most frequent side effects consist of mild transient local reactions, such as pain, swelling, flares, and effusion at the site injection, while systemic events are seldom reported. Furthermore, rare cases of acute pseudoseptic reactions are observed in association with avian high MW cross-linked HA (Nguyen and Rannou, 2017). Actually, there is no evidence of a direct influence of the number of joint injections on the occurrence of side effects, while high MW and cross-linked formulations of HA were more frequently associated to local reactions and post-injection flares in comparison with intermediate or low MW (Reichenbach et al., 2007; Nguyen and Rannou, 2017).
The analysis of the literature papers on IA HA therapy for TBOA patients, confirmed what had already been demonstrated for HA treatment safety in OA in general. Indeed, several trials did not report any side effects after HA injections and others documented only minor local adverse reactions consisting of pain and/or swelling at the site injection, usually lasting a few hours and were spontaneously resolved (Schumacher et al., 2004; Stahl et al., 2005; Coaccioli et al., 2006; Fuchs et al., 2006; Roux et al., 2007; Heyworth et al., 2008; Bahadir et al., 2009; Figen Ayhan and Ustün, 2009; Salini et al., 2009; Di Sante et al., 2011; Ingegnoli et al., 2011; Frizziero et al., 2014; Monfort et al., 2015; Tenti et al., 2017; Ioppolo et al., 2018; Bartoloni et al., 2019; Koh et al., 2019). Only in two different studies evaluating high MW and cross-linked HA formulations, local adverse events of moderate intensity and needing ice, NSAIDs and/or selective cyclooxygenase-2 inhibitors (COXIBs) for resolution were recorded (Mandl et al., 2009; Velasco et al., 2017).
In 2007 Karalezli et al. (Karalezli et al., 2007) conducted a prospective study on 16 TBOA patients to analyze pain and tolerability of viscosupplementation therapy with HA. Patients underwent a cycle of three weekly injections of 0.3 cm3 sodium hyaluronate: eight patients under fluoroscopy control (group A) and the others without fluoroscopy control (group B). The results confirmed the tolerability of IA HA therapy, but pain and discomfort are frequent during the injection procedure with a major degree of pain experienced by subjects from group B.
Furthermore, the analysis of an American database containing data of patients with TBOA, the Truven MarketScan® Databases, revealed that both steroid both HA injections were associated with early post-operative complications after surgical treatment of TBOA. In particular, infectious complications were associated with corticosteroids injections, while wound-healing complications were found to be related mainly to IA HA therapy (Giladi et al., 2018).
The current evidence suggests a comparable safety profile of PRP to IA HA with self-limited post-injection pain and swelling representing the most frequent reported adverse events (Nguyen and Rannou, 2017). Unfortunately, there are no data available about the tolerability of PRP injections for TBOA.
The few studies on the IA therapy with mesenchymal-derived stem cell populations did not show severe complications and consisted mainly in persisting pain after the procedure injection. In particular, Herold et al. (Herold et al., 2017) observed a transient paraesthesia of branches of the superficial radial nerve that completely resolved after 2 months in two patients, while three patients underwent additional surgical treatment for insufficient pain relief induced by the injection therapy. Also, Haas et al. (Haas et al., 2020) reported that in 2% of the cases, further operation was needed for persisting pain. Similarly, Erne et al. (Erne et al., 2018) found one patient who needed revision surgery because of persisting pain.
Discussion
The present narrative review provides an updated and comprehensive overview of the efficacy and safety of different IA injection-based therapies currently employed for the management of TBOA. Concerning IA steroids and HA, it seems that IA HA may be useful in TBOA, especially in improving functional capacity and IA corticosteroids in reducing painful symptomatology (Trellu et al., 2015; Kroon et al., 2016; Riley et al., 2019), but the current evidence remains equivocal and inconclusive. Indeed, in agreement of what has already been reported by some systematic reviews and meta-analysis with a robust methodological quality, the great heterogeneity among the trials published until now does not deserve a definite conclusion about the efficacy of these treatments and whether an injection-based therapy is more effective than another one (Trellu et al., 2015; Kroon et al., 2016; Riley et al., 2019). First of all, the studies differed for the design, with only few RCTs or retrospective comparative studies; in almost all cases, they were small single-center studies with a very limited number of patients. The population analyzed was heterogeneous, particularly for the severity of the radiological grade, evaluated according to different criteria (Kellgren-Lawrence or Eaton grade). Only twelve studies included a specific symptom threshold for inclusion (e.g., VAS ≥ 30 mm, VAS ≥ 40 mm, and FIHOA ≥ 6) (Coaccioli et al., 2006; Fuchs et al., 2006; Roux et al., 2007; Figen Ayhan and Ustün, 2009; Di Sante et al., 2011; Ingegnoli et al., 2011; Jahangiri et al., 2014; Tenti et al., 2017; Velasco et al., 2017; Ioppolo et al., 2018; Bartoloni et al., 2019). This factor is a potential source of bias in interpreting the trials’ results, considering that including participants with relatively low levels of symptoms could make less likely that a clinical meaningful difference in outcomes could be obtained. For these reasons, both Osteoarthritis Research Society International (OARSI) and European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) recommendations for the conduct of pharmacological clinical trials in hand OA recommend a minimum cut-off for inclusion in terms of pain and function (Kloppenburg et al., 2015a; Reginster et al., 2018).
Another important source of heterogeneity is represented by different formulations of IA corticosteroids and HA tested with different injected volumes. Among steroids, triamcinolone acetonide, methylprednisolone and betamethasone are the most frequent used; there are no evidence supporting the superiority of a formulation over another one in TBOA, although in large joints OA, triamcinolone acetonide seems to have a greater effectiveness (Cushman et al., 2018). The HA preparations explored in the above discussed trials included HA of different MW (low, intermediate, and high), hylan, cross-linked HA and hybrid formulations. Unfortunately, no data are available about a possible difference in efficacy according to MW and viscosity in TBOA. The number of injections was variable ranging from one to three injections both for steroids and HA, as well as the technique of IA injections. In this sense, particularly debated was the accuracy of TBOA injections with and without imaging guidance, nowadays represented essentially by ultrasound. Indeed, the consensus statement on viscosupplementation (Henrotin et al., 2015) suggested to inject the trapezio-metacarpal joint under fluoroscopy or ultrasonography guidance and a recent United States cadaveric study showed a 25% higher accuracy when thumb-base joint was injected with ultrasound guidance compared to no imaging control (To et al., 2017). Conversely, other studies demonstrated success rates comparable with those obtained under ultrasound-control when the injections were performed by an experienced physician based on palpation of landmarks (Helm et al., 2003; Mandl et al., 2006).
Furthermore, another important element of heterogeneity is represented by a great variety of analyzed outcomes. Outcome Measures in Rheumatology (OMERACT) consensus recommended to evaluate in hand OA clinical trials pain, functional capacity, joint activity, and patient global assessment (Kloppenburg et al., 2015b). Few studies followed these suggestions, and for hand functionality different scores were often used, sometimes evaluating not only the hand, but the arm in its globality; few papers investigated FIHOA, validated in hand OA and considered a reliable measure of hand functionality (Kloppenburg et al., 2015b).
Also, the times of follow-up are extremely variable, ranging from 1 to 12 months, contributing to make difficult the comparison across the studies.
Another important point often poorly explored is represented by the description of the concomitant pharmacological and non-pharmacological therapy for TBOA. Indeed, in real-world application, TBOA is managed not only with injections, but with a multidisciplinary approach, so we think that more detailed information, particularly on the concomitant use of NSAIDs/analgesics and splint, can provide useful clinical implications.
Concerning PRP and mesenchymal-derived stem cell populations injections, the data are encouraging, but still too limited for any kind of conclusion. In particular, the small sample size of the analyzed studies makes it very difficult to extrapolate the results to a large scale population. Furthermore, a better understanding of the mechanism of actions of PRP and mesenchymal-derived stem cell populations and a standardized preparation method are needed to achieve a higher level of evidence in this field. It is possible that in the future both therapies can obtain a place in the management of TBOA, mainly thanks to their properties of promoting healing cartilage defects, stem cell proliferation and preventing chondrocytes and extra-cellular matrix degradation (Bonetti et al., 2020).
The tolerability of all the discussed IA therapies were found to be quite good. Local side effects are the most frequently reported and consisted mainly of painful, moderate, local inflammatory reactions at the injection site. Corticosteroids injections have the disadvantages to potentially determine skin and/or ligaments alterations, particularly in the case of repeated injections and in diabetic subjects. However, the most serious risk for IA injections remains septic arthritis which has not been described in any of the above-presented studies.
The current review of the scientific literature allowed us to find out some important points which, in our opinion, deserve further investigation. First of all, the discrepancy between the clinical experience of several physicians with expertize in this field, and the published recommendations from international scientific societies has become more evident throughout the last few years. This gap deriving from the literature evidence, which is methodologically very poor, is likely to determine negative implications, restricting patients’ access to this valuable treatment option and accelerating the referrals to the surgery, a more expensive strategy and without minor risks. In our opinion, the only way to solve this discrepancy is to realize well-designed and well-conducted controlled trials, preferably double-blind RCTs or real-life studies on a large sample size of patients. Further, there is a need for homogenous trials which can follow the OARSI and ESCEO criteria for the conduct of clinical studies in hand OA, not only in selecting patients, but also in defining the most reliable pain and function outcomes (Kloppenburg et al., 2015a; Reginster et al., 2018). The follow-up should be performed in the long-term with results at 1 year. The injection procedure should be standardized, as well as the schedule of the injected agent. At this regard, we think that studies of comparisons between the different IA therapies and placebo, between different agents within the same class and between different IA treatment belonging to various pharmaceutical categories should be encouraged. Also comparing the injection-based therapies with other conservative strategies including oral pharmacological drugs, exercise, splint, different kinds of physical therapies, as laser therapy or extracorporeal shockwave therapy, should be very interesting. Finally, to understand if some disease characteristics (e.g., radiological grade) could be useful in helping clinicians in the choice of the IA therapy, should be desirable.
The main limitation of this review lies in its narrative nature with all the limitations inherent to a non-rigorous systematic review. In particular, this paper did not identify the quality and the strength of the discussed trials, and has not been built on a robust methodology structure. Further limitations are those intrinsic to the included papers which presented several consistent methodological flaws, as the not randomized controlled design.
Conclusion
The intra-articular injection of therapeutic agents is an attractive strategy for the local treatment of TBOA, which takes a place within the multidisciplinary approach for the management of hand OA. However, the current evidence remains equivocal. The main reason behind this is related to the poor methodology of the available scientific studies, which makes the results quite inconclusive. Some data supported the clinical usefulness of IA HA, especially in improving functional capacity and of IA corticosteroids in reducing painful symptomatology; new emerging and encouraging results derived from PRP and mesenchymal-derived stem cell populations, but they are still preliminary. At this regard, we auspicate a growing development of the scientific evidence in the field of regenerative medicine until now poorly explored in TBOA. For an exhaustive understanding of all therapeutic possibilities related to the different intra-articular agents in TBOA patients there is a need for large, independent, methodologically robust RCTs with long-term follow-up.
Research Agenda
• To publish well-conducted double-blind RCTs on a large TBOA population and with a long-term follow-up
• To use standardized selection criteria and standardized efficacy outcomes to make the different studies uniform and comparable
• To uniform the injection technique and the therapeutic regimens (dosage, number of injections, kind of formulation of steroid and HA)
• To compare the IA agents with each other, with placebo, and with other conservative therapeutic options
To find out if a corticosteroid or HA formulation is superior to another one in TBOA
• To study the additional symptomatic effect of the different IA therapies, combined with other therapeutic options such as pharmacological management, physiotherapy and splinting
• To identify patients and disease characteristics useful to guide the choice of the IA agent
Author Contributions
AF and ST conceived the topic and the design of work. ST and NM contributed to the literature search, while SC and SG organized the database and contributed to the analysis and interpretation of the literature data. AF solved disagreement between the two Authors. AF and ST wrote the first draft of the manuscript. SC, NM, and SG revised the paper critically for important intellectual content and provided approval for publication of the content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Altman, R., Alarcón, G., Appelrouth, D., Bloch, D., Borenstein, D., Brandt, K., et al. (1990). The American college of rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis. Rheum. 33, 601–610. doi:10.1002/art.1780331101
Bahadir, C., Onal, B., Dayan, V. Y., and Gürer, N. (2009). Comparison of therapeutic effects of sodium hyaluronate and corticosteroid injections on trapeziometacarpal joint osteoarthritis. Clin. Rheumatol. 28, 529–533. doi:10.1007/s10067-008-1079-6
Bartoloni, E., Luccioli, F., La Paglia, G. M. C., Cafaro, G., Marcucci, E., and Gerli, R. (2019). Effect of Sinovial High-Low® injections in trapeziometacarpal osteoarthritis. Clin. Exp. Rheumatol. 37, 166.
Bijsterbosch, J., Visser, W., Kroon, H. M., Stamm, T., Meulenbelt, I., Huizinga, T. W., et al. (2010). Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann. Rheum. Dis. 69, 585–587. doi:10.1136/ard.2009.104562
Bohr, S., Rennekampff, H. O., and Pallua, N. (2015). Cell-enriched lipoaspirate arthroplasty: a novel approach to first carpometacarpal joint arthritis. Hand Surg. 20, 479–481. doi:10.1142/S0218810415720259
Bonetti, M. A., Rovere, G., Fulchignoni, C., De Santis, V., Ziranu, A., Maccauro, G., et al. (2020). Autologous fat transplantation for the treatment of trapeziometacarpal joint osteoarthritis. Orthop. Rev. (Pavia) 12, 8666. doi:10.4081/or.2020.8666
Bosetti, M., Borrone, A., Follenzi, A., Messaggio, F., Tremolada, C., and Cannas, M. (2016). Human lipoaspirate as autologous injectable Active scaffold for one-step repair of cartilage defects. Cel Transpl. 25, 1043–1056. doi:10.3727/096368915X689514
Centeno, C. J., and Freeman, M. D. (2014). Percutaneous injection of autologous, culture-expanded mesenchymal stem cells into carpometacarpal hand joints: a case series with an untreated comparison group. Wien Med. Wochenschr. 164, 83–87. doi:10.1007/s10354-013-0222-4
Coaccioli, S., Pinoca, F., and Puxeddu, A. (2006). Short term efficacy of intra-articular injection of hyaluronic acid in osteoarthritis of the first carpometacarpal joint in a preliminary open pilot study. Clin. Ter. 157, 321–325.
Cormier, G., Le Goff, B., Denis, A., Varin, S., Auzanneau, L., Dimet, J., et al. (2019). Corticosteroids injections versus corticosteroids with hyaluronic acid injections in rhizarthrosis: the randomised multicentre RHIZ’ART trial study protocol. BMJ Open 9, e022553. doi:10.1136/bmjopen-2018-022553
Cushman, D. M., Bruno, B., Christiansen, J., Schultz, A., and McCormick, Z. L. (2018). Efficacy of injected corticosteroid type, dose, and volume for pain in large joints: a narrative review. PM R. 10, 748–757. doi:10.1016/j.pmrj.2018.01.002
Dahaghin, S., Bierma-Zeinstra, S. M. A., Ginai, A. Z., Pols, H. A. P., Hazes, J. M. W., and Koes, B. W. (2005). Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann. Rheum. Dis. 64, 682–687. doi:10.1136/ard.2003.017087
Dauvissat, J., Rizzo, C., Lellouche, H., Porterie, J., Melac-Ducamp, S., Locquet, V., et al. (2018). Safety and predictive factors of short-term efficacy of a single injection of mannitol-modified cross-linked hyaluronic acid in patients with trapeziometacarpal osteoarthritis. Results of a multicentre prospective open-label pilot study (INSTINCT trial). Clin. Med. Insights Arthritis Musculoskelet. Disord. 11, 1179544118782901. doi:10.1177/1179544118782901
Day, C. S., Gelberman, R., Patel, A. A., Vogt, M. T., Ditsios, K., and Boyer, M. I. (2004). Basal joint osteoarthritis of the thumb: a prospective trial of steroid injection and splinting. J. Hand Surg. Am. 29, 247–251. doi:10.1016/j.jhsa.2003.12.002
Di Sante, L., Cacchio, A., Scettri, P., Paoloni, M., Ioppolo, F., and Santilli, V. (2011). Ultrasound-guided procedure for the treatment of trapeziometacarpal osteoarthritis. Clin. Rheumatol. 30, 1195–1200. doi:10.1007/s10067-011-1730-5
Eaton, R. G., and Glickel, S. Z. (1987). Trapeziometacarpal osteoarthritis, staging as a rationale for treatment. Hand Clin. 3, 455–471.
Erne, H. C., Cerny, M. K., Ehrl, D., Bauer, A. T., Schmauss, V., Moog, P., et al. (2018). Autologous fat injection versus Lundborg resection arthroplasty for the treatment of trapeziometacarpal joint osteoarthritis. Plast. Reconstr. Surg. 141, 119–124. doi:10.1097/PRS.0000000000003913
Evans, A., Ibrahim, M., Pope, R., Mwangi, J., Botros, M., Johnson, S. P., et al. (2020). Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J. Orthop. 18, 226–236. doi:10.1016/j.jor.2020.01.037
Figen Ayhan, F., and Ustün, N. (2009). The evaluation of efficacy and tolerability of Hylan G-F 20 in bilateral thumb base osteoarthritis: 6 months follow-up. Clin. Rheumatol. 28, 535–541. doi:10.1007/s10067-008-1080-0
Frizziero, A., Maffulli, N., Masiero, S., and Frizziero, L. (2014). Six-months pain relief and functional recovery after intra-articular injections with hyaluronic acid (mw 500–730 kDa) in trapeziometacarpal osteoarthritis. Muscles Ligaments Tendons J. 4, 256–261.
Fuchs, S., Mönikes, R., Wohlmeiner, A., and Heyse, T. (2006). Intra-articular hyaluronic acid compared with corticoid injections for the treatment of rhizarthrosis. Osteoarthritis Cartilage 14, 82–88. doi:10.1016/j.joca.2005.07.016
Gil, C., Abdoul, H., Campagna, R., Guerini, H., Ieong, E., Chagny, F., et al. (2018). Intra-articular botulinum toxin A for base-of-thumb osteoarthritis: protocol for a randomised trial (RHIBOT). BMJ Open 8, e022337. doi:10.1136/bmjopen-2018-022337
Giladi, A. M., Rahgozar, P., Zhong, L., and Chung, K. C. (2018). Corticosteroid or hyaluronic acid injections to the carpometacarpal joint of the thumb joint are associated with early complications after subsequent surgery. J. Hand Surg. Eur. 43, 1106–1110. doi:10.1177/1753193418805391
Haas, E. M., Eisele, A., Arnoldi, A., Paolini, M., Ehrl, D., Volkmer, E., et al. (2020). One-year outcomes of intraarticular fat transplantation for thumb carpometacarpal joint osteoarthritis: case review of 99 joints. Plast. Reconstr. Surg. 145, 151–159. doi:10.1097/PRS.0000000000006378
Haugen, I. K., Englund, M., Aliabadi, P., Niu, J., Clancy, M., Kvien, T. K., et al. (2011). Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann. Rheum. Dis. 70, 1581–1586. doi:10.1136/ard.2011.150078
Helm, A. T., Higgins, G., Rajkumar, P., and Redfern, D. R. (2003). Accuracy of intra-articular injections for osteoarthritis of the trapeziometacarpal joint. Int. J. Clin. Pract. 57, 265–266.
Henrotin, Y., Raman, R., Richette, P., Bard, H., Jerosch, J., Conrozier, T., et al. (2015). Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin. Arthritis Rheum. 45, 140–149. doi:10.1016/j.semarthrit.2015.04.011
Herold, C., Rennekampff, H. O., Groddeck, R., and Allert, S. (2017). Autologous fat transfer for thumb carpometacarpal joint osteoarthritis: a prospective study. Plast. Reconstr. Surg. 140, 327–335. doi:10.1097/PRS.0000000000003510
Heyworth, B. E., Lee, J. H., Kim, P. D., Lipton, C. B., Strauch, R. J., and Rosenwasser, M. P. (2008). Hylan versus corticosteroid versus placebo for treatment of basal joint arthritis: a prospective, randomized, double-blinded clinical trial. J. Hand Surg. Am. 33, 40–48. doi:10.1016/j.jhsa.2007.10.009
Ingegnoli, F., Soldi, A., and Meroni, P. L. (2011). Power Doppler sonography and clinical monitoring for hyaluronic Acid treatment of rhizarthrosis: a pilot study. J. Hand Microsurg. 3, 51–54. doi:10.1007/s12593-011-0037-8
Ioppolo, F., Saracino, F., Rizzo, R. S., Monacelli, G., Lanni, D., Di Sante, L., et al. (2018). Comparison between extracorporeal shock wave therapy and intra-articular hyaluronic acid injections in the treatment of first carpometacarpal joint osteoarthritis. Ann. Rehabil. Med. 42, 92–100. doi:10.5535/arm.2018.42.1.92
Jahangiri, A., Moghaddam, F. R., and Najafi, S. (2014). Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J. Orthop. Sci. 19, 737–743. doi:10.1007/s00776-014-0587-2
Joshi, R. (2005). Intraarticular corticosteroid injection for first carpometacarpal osteoarthritis. J. Rheumatol. 32, 1305–1306.
Jüni, P., Hari, R., Rutjes, A. W., Fischer, R., Silletta, M. G., Reichenbach, S., et al. (2015). Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst. Rev. Cd005328. doi:10.1002/14651858.CD005328.pub3
Karalezli, N., Ogun, T. C., Kartal, S., Saracgil, S. N., Yel, M., and Tuncay, I. (2007). The pain associated with intraarticular hyaluronic acid injections for trapeziometacarpal osteoarthritis. Clin. Rheumatol. 26, 569–571. doi:10.1007/s10067-006-0354-7
Khan, M., Waseem, M., Raza, A., and Derham, D. (2009). Quantitative assessment of improvement with single corticosteroid injection in thumb CMC joint osteoarthritis?. Open Orthop. J. 3, 48–51. doi:10.2174/1874325000903010048
Kloppenburg, M., Bøyesen, P., Visser, A. W., Haugen, I. K., Boers, M., Boonen, A., et al. (2015b). Report from the OMERACT hand osteoarthritis working group: set of core domains and preliminary set of instruments for use in clinical trials and observational studies. J. Rheumatol. 42, 2190–2197. doi:10.3899/jrheum.141017
Kloppenburg, M., Kroon, F. P., Blanco, F. J., Doherty, M., Dziedzic, K. S., Greibrokk, E., et al. (2019). 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann. Rheum. Dis. 78, 16–24. doi:10.1136/annrheumdis-2018-213826
Kloppenburg, M., Maheu, E., Kraus, V. B., Cicuttini, F., Doherty, M., Dreiser, R. L., et al. (2015a). OARSI hand clinical trial recommendations work GroupOARSI clinical trials recommendations: design and conduct of clinical trials for hand osteoarthritis. Osteoarthritis Cartilage 23, 772–786. doi:10.1016/j.joca.2015.03.007
Kloppenburg, M., van Beest, S., and Kroon, F. P. B. (2017). Thumb base osteoarthritis: a hand osteoarthritis subset requiring a distinct approach. Best Pract. Res. Clin. Rheumatol. 31, 649–660. doi:10.1016/j.berh.2018.08.007
Koh, S. H., Lee, S. C., Lee, W. Y., Kim, J., and Park, Y. (2019). Ultrasound-guided intra-articular injection of hyaluronic acid and ketorolac for osteoarthritis of the carpometacarpal joint of the thumb: a retrospective comparative study. Medicine (Baltimore) 98, e15506. doi:10.1097/MD.0000000000015506
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., and Block, J. (2020). 2019 American College of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72, 220–233. doi:10.1002/art.41142
Kroon, F. P., Rubio, R., Schoones, J. W., and Kloppenburg, M. (2016). Intra-articular therapies in the treatment of hand osteoarthritis: a systematic literature review. Drugs Aging 33, 119–133. doi:10.1007/s40266-015-0330-5
Loibl, M., Lang, S., Dendl, L. M., Nerlich, M., Angele, P., Gehmert, S., et al. (2016). Leukocyte-reduced platelet-rich plasma treatment of basal thumb arthritis: a pilot study. Biomed. Res. Int. 2016, 9262909. doi:10.1155/2016/9262909
Malahias, M. A., Roumeliotis, L., Nikolaou, V. S., Chronopoulos, E., Sourlas, I., and Babis, G. C. (2018). Platelet-rich plasma versus corticosteroid intra-articular injections for the treatment of trapeziometacarpal arthritis: a prospective randomized controlled clinical trial. CARTILAGE. 12(1):51-61. doi:10.1177/1947603518805230
Mandl, L. A., Hotchkiss, R. N., Adler, R. S., Ariola, L. A., and Katz, J. N. (2006). Can the carpometacarpal joint be injected accurately in the office setting? Implications for therapy. J. Rheumatol. 33, 1137–1139.
Mandl, L. A., Hotchkiss, R. N., Adler, R. S., Lyman, S., Daluiski, A., Wolfe, S. W., et al. (2009). Injectable hyaluronan for the treatment of carpometacarpal osteoarthritis: open label pilot trial. Curr. Med. Res. Opin. 25, 2103–2108. doi:10.1185/03007990903084016
Marx, R. E. (2001). Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant. Dent 10, 225–228. doi:10.1097/00008505-200110000-00002
McAlindon, T. E., LaValley, M. P., Harvey, W. F., Price, L. L., Driban, J. B., Zhang, M., et al. (2017). Ward rj. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 317, 1967–1975. doi:10.1001/jama.2017.5283
Medina-Porqueres, I., Martin-Garcia, P., Sanz-De Diego, S., Reyes-Eldblom, M., and Cantero-Tellez, R. (2019). Platelet-rich plasma for thumb carpometacarpal joint osteoarthritis in a professional pianist: case-based review. Rheumatol. Int. 39, 2167–2175. doi:10.1007/s00296-019-04454-x
Meenagh, G. K., Patton, J., Kynes, C., and Wright, G. D. (2004). A randomised controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann. Rheum. Dis. 63, 1260–1263. doi:10.1136/ard.2003.015438
Monfort, J., Rotés-Sala, D., Segalés, N., Montañes, F. J., Orellana, C., Llorente-Onaindia, J., et al. (2015). Comparative efficacy of intra-articular hyaluronic acid and corticoid injections in osteoarthritis of the first carpometacarpal joint: results of a 6-month single-masked randomized study. Jt. Bone Spine 82, 116–121. doi:10.1016/j.jbspin.2014.08.008
Moussa, M., Lajeunesse, D., Hilal, G., El Atat, O., Haykal, G., Serhal, R., et al. (2017). Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp. Cel Res. 352, 146–156. doi:10.1016/j.yexcr.2017.02.012
Nguyen, C., and Rannou, F. (2017). The safety of intra-articular injections for the treatment of knee osteoarthritis: a critical narrative review. Expert Opin. Drug Saf. 16, 897–902. doi:10.1080/14740338.2017.1344211
Ornetti, P., Nourissat, G., Berenbaum, F., Sellam, J., Richette, P., and Chevalier, X. (2016). Under the aegis of the Osteoarthritis Section of the French Society for Rheumatology (Société Française de Rhumatologie, SFR).Does platelet-rich plasma have a role in the treatment of osteoarthritis?. Jt. Bone Spine 83, 31–36. doi:10.1016/j.jbspin.2015.05.002
Pak, J., Lee, J. H., Pak, N., Pak, Y., Park, K. S., Jeon, J. H., et al. (2018). Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: updated status. Int. J. Mol. Sci. 19, 2146. doi:10.3390/ijms19072146
Reginster, J. L., Arden, N. K., Haugen, I. K., Rannou, F., Cavalier, E., Bruyère, O., et al. (2018). Guidelines for the conduct of pharmacological clinical trials in hand osteoarthritis: consensus of a working group of the European society on clinical and economic Aspects of Osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Semin. Arthritis Rheum. 48, 1–8. doi:10.1016/j.semarthrit.2017.12.003
Reichenbach, S., Blank, S., Rutjes, A. W., Shang, A., King, E. A., Dieppe, P. A., et al. (2007). Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 57, 1410–1418. doi:10.1002/art.23103
Riley, N., Vella-Baldacchino, M., Thurley, N., Hopewell, S., Carr, A. J., and Dean, B. J. F. (2019). Injection therapy for base of thumb osteoarthritis: a systematic review and meta-analysis. BMJ Open 9, e027507. doi:10.1136/bmjopen-2018-027507
Rocchi, L., Merolli, A., Giordani, L., Albensi, C., and Foti, C. (2018). Trapeziometacarpal joint osteoarthritis: a prospective trial on two widespread conservative therapies. Muscles Ligaments Tendons J. 7, 603–610. doi:10.11138/mltj/2017.7.4.603
Roux, C., Fontas, E., Breuil, V., Brocq, O., Albert, C., and Euller-Ziegler, L. (2007). Injection of intra-articular sodium hyaluronidate (Sinovial) into the carpometacarpal joint of the thumb (CMC1) in osteoarthritis. A prospective evaluation of efficacy. Jt. Bone Spine 74, 368–372. doi:10.1016/j.jbspin.2006.08.008
Salini, V., De Amicis, D., Abate, M., Natale, M. A., and Di Iorio, A. (2009). Ultrasound-guided hyaluronic acid injection in carpometacarpal osteoarthritis: short-term results. Int. J. Immunopathol. Pharmacol. 22, 455–460. doi:10.1177/039463200902200222
Schumacher, H. R., Meador, R., Sieck, M., and Mohammed, Y. (2004). Pilot investigation of hyaluronate injections for first metacarpal-carpal (MC-C) osteoarthritis. J. Clin. Rheumatol. 10, 59–62. doi:10.1097/01.rhu.0000120894.49180.99
Sodha, S., Ring, D., Zurakowski, D., and Jupiter, J. B. (2005). Prevalence of osteoarthrosis of the trapeziometacarpal joint. J. Bone Jt. Surg. Am. 87, 2614–2618. doi:10.2106/JBJS.E.00104
Sonne-Holm, S., and Jacobsen, S. (2006). Osteoarthritis of the first carpometacarpal joint: a study of radiology and clinical epidemiology. Results from the Copenhagen Osteoarthritis Study. Osteoarthritis Cartilage 14, 496–500. doi:10.1016/j.joca.2005.12.001
Stahl, S., Karsh-Zafrir, I., Ratzon, N., and Rosenberg, N. (2005). Comparison of intraarticular injection of depot corticosteroid and hyaluronic acid for treatment of degenerative trapeziometacarpal joints. J. Clin. Rheumatol. 11, 299–302. doi:10.1097/01.rhu.0000191194.39926.c9
Swindells, M. G., Logan, A. J., Armstrong, D. J., Chan, P., Burke, F. D., and Lindau, T. R. (2010). The benefit of radiologically-guided steroid injections for trapeziometacarpal osteoarthritis. Ann. R. Coll. Surg. Engl. 92, 680–684. doi:10.1308/003588410X12699663905078
Tenti, S., Ferretti, F., Gusinu, R., Gallo, I., Giannotti, S., Pozza, A., et al. (2020). Impact of thumb osteoarthritis on pain, function, and quality of life: a comparative study between erosive and non-erosive hand osteoarthritis. Clin. Rheumatol. 39, 2195–2206. doi:10.1007/s10067-020-04982-z
Tenti, S., Pascarelli, N. A., Giannotti, S., Galeazzi, M., Giordano, N., and Fioravanti, A. (2017). Can hybrid hyaluronic acid represent a valid approach to treat rizoarthrosis? A retrospective comparative study. BMC Musculoskelet. Disord. 18, 444. doi:10.1186/s12891-017-1809-5
To, P., McClary, K. N., Sinclair, M. K., Stout, B. A., Foad, M., Hiratzka, S., et al. (2017). The accuracy of common hand injections with and without ultrasound: an anatomical study. Hand (N Y) 12, 591–596. doi:10.1177/1558944717692086
Trellu, S., Dadoun, S., Berenbaum, F., Fautrel, B., and Gossec, L. (2015). Intra-articular injections in thumb osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Jt. Bone Spine 82, 315–319. doi:10.1016/j.jbspin.2015.02.002
Velasco, E., Ribera, M. V., and Pi, J. (2017). Single-arm open-label study of Durolane (NASHA nonanimal hyaluronic acid) for the treatment of osteoarthritis of the thumb. Open Access Rheumatol. 9, 61–66. doi:10.2147/OARRR.S128675
Wernecke, C., Braun, H. J., and Dragoo, J. L. (2015). The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop. J. Sports Med. 3, 2325967115581163. doi:10.1177/2325967115581163
Zeng, C., Lane, N. E., Hunter, D. J., Wei, J., Choi, H. K., McAlindon, T. E., et al. (2019). Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage 27, 855–862. doi:10.1016/j.joca.2019.01.007
Zhang, W., Doherty, M., Leeb, B. F., Alekseeva, L., Arden, N. K., Bijlsma, J. W., et al. (2007). EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann. Rheum. Dis. 66, 377–388. doi:10.1136/ard.2006.062091
Keywords: thumb-base osteoarthritis, trapezio-metacarpal osteoarthritis, first carpo-metacarpal osteoarthritis, rizoartrhosis, intra-articular injection, hyaluronic acid, corticosteroids, platelet-rich plasma
Citation: Tenti S, Cheleschi S, Mondanelli N, Giannotti S and Fioravanti A (2021) New Trends in Injection-Based Therapy for Thumb-Base Osteoarthritis: Where Are We and where Are We Going?. Front. Pharmacol. 12:637904. doi: 10.3389/fphar.2021.637904
Received: 04 December 2020; Accepted: 23 February 2021;
Published: 13 April 2021.
Edited by:
Annalisa Bruno, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Piotr Bełdowski, University of Technology and Life Sciences in Bydgoszcz, PolandSeyed M. Rayegani, Shahid Beheshti University, Iran
Copyright © 2021 Tenti, Cheleschi, Mondanelli, Giannotti and Fioravanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Cheleschi, c2FyYWNoZWxlc2NoaUBob3RtYWlsLmNvbQ==
 Sara Tenti
Sara Tenti Sara Cheleschi
Sara Cheleschi Nicola Mondanelli
Nicola Mondanelli Stefano Giannotti2
Stefano Giannotti2 Antonella Fioravanti
Antonella Fioravanti