- 1Department of Pharmacy, Children’s Hospital of Fudan University, Shanghai, China
- 2Department of Nephrology, Children’s Hospital of Fudan University, Shanghai, China
- 3Department of Neurology, Children’s Hospital of Fudan University, Shanghai, China
The purposes of this study were to explore the population pharmacokinetics and initial dose optimization of sirolimus improving drug blood level for seizure control in pediatric patients with tuberous sclerosis complex (TSC). Eighty pediatric patients diagnosed with TSC-related epilepsy were included for analysis. Sirolimus concentrations, physiological and biochemical indexes, and drug combination were collected to build a nonlinear mixed effect (NONMEM) model. Initial dose optimization was simulated by the Monte Carlo method. The weight and concomitant medication of oxcarbazepine affected sirolimus clearance. Without oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.07, 0.06, 0.05, 0.04, and 0.03 mg/kg/day were recommended for weights of 5–7.5, 7.5–11.5, 11.5–19, 19–40, and 40–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.05, 0.04, and 0.03 were recommended for weights of 5–8, 8–20, and 20–70 kg, respectively. With oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.09, 0.08, 0.07, 0.06, 0.05, and 0.04 mg/kg/day were recommended for weights of 5–7.5, 7.5–10, 10–13.5, 13.5–20, 20–35, and 35–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.06, 0.05, 0.04, and 0.03 were recommended for weights of 5–7, 7–14.5, 14.5–38, and 38–70 kg, respectively. The present study was the first to establish a population pharmacokinetic model of sirolimus improving drug blood level for seizure control in pediatric patients with TSC and recommend the initial dosage regimen.
Introduction
Tuberous sclerosis complex (TSC), is an autosomal dominant neurodermal syndrome, most of which are caused by abnormal organ development in the ectodermal tissue, involving multiple organs such as the brain, the skin, the peripheral nerves, and the kidney, and leading to the mammalian target of rapamycin (mTOR) pathway hyperactivation (Curatolo et al., 2008; van der Poest Clement et al., 2020). Its clinical features include epileptic seizures, facial angiofibroma, and decreased intelligence, among them, epilepsy is the most common neurologic complication and influences about 90% of patients, in which 2/3 are drug resistant (Chu-Shore et al., 2010; Kwan et al., 2010). If the seizure is not well controlled, it will seriously affect children’s intellectual development, reduce their quality of life, and increase the burden of the whole society (He et al., 2020). For the time being, the available treatments include a ketogenic diet, antiepileptic drugs, vagus nerve stimulation, and epilepsy surgery (He et al., 2020). However, the therapeutic outcomes of these treatments for individuals with TSC patients, in general, are not satisfactory (Curatolo et al., 2015; de Vries et al., 2015).
Sirolimus is an mTOR inhibitor and has been widely proved that it has good effects on treating many of the symptoms of TSC (Bissler et al., 2008; Davies et al., 2011; Nathan et al., 2015; Wataya-Kaneda et al., 2017). Moreover, He et al reported that sirolimus has a significant effect on seizures associated with TSC, and it could be used as the first-line medication for pediatric patients with TSC-associated epilepsy (He et al., 2020). However, sirolimus has a narrow therapeutic window with considerable inter- and intra-individual pharmacokinetic variabilities (Wang et al., 2020), making it difficult to design a sirolimus initial dosage regimen, especially for sirolimus improving drug blood level for seizure control in pediatric patients with TSC. Therefore, the purposes of this study were to explore the population pharmacokinetics and initial dose optimization of sirolimus improving drug blood level for seizure control in pediatric patients with TSC.
Methods
Patients
The study was approved by the Research Ethics Committee of Children’s Hospital of Fudan University (Ethical code: [2019] 019). In the present study, pediatric patients diagnosed with TSC-related epilepsy from May 2016 to October 2020 at the Children’s Hospital of Fudan University (Shanghai, China) were collected, retrospectively. The criteria for inclusion were as follows: 1) treated by sirolimus, 2) therapeutic drug monitoring for sirolimus. As for the study, it was a retrospective analysis and was approved by the ethics committee of our hospital without the need for written informed consent.
Therapeutic Drug Monitoring
In the present study, sirolimus was taken once a day and its concentrations were from therapeutic drug monitoring. Blood concentrations were collected prior to the subsequent administration, and the sirolimus concentrations used in the current research were trough concentrations. Sirolimus concentrations were detected using the Emit 2000 Sirolimus Assay (Siemens Healthcare Diagnostics Inc.) with a range of linear response, 3.5–30 ng/ml, whose values of inter-assay variability [coefficient of variation (CV%)] < 4.0%, and values of intra-assay CV (%) < 6.2% (Wang et al., 2020).
Population Pharmacokinetic Model
The population pharmacokinetic model was established with the nonlinear mixed-effects modeling software, NONMEM (edition 7, ICON Development Solutions, Ellicott City, MD, United States) and a first-order conditional estimation method with interaction (FOCE-I method). A one-compartment model with first-order elimination was used to describe the model because all the concentrations in this research were trough concentrations. The pharmacokinetic parameters, apparent oral clearance (CL/F), volume of distribution (V/F), and absorption rate constant [Ka, fixed at 0.485/h (Wang et al., 2020)], were included.
Random Effect Model
Inter-individual variabilities were shown in Eq. 1:
Ci, the individual parameter, included CL/F and V/F; TV(C), the typical individual parameter; ηi, symmetrical distribution, which was a random term with zero mean and variance omega^2 (ω2), when available, ηi would be added into CL/F and V/F, respectively.
Various random residual variabilities are shown in Eqs. 2–4:
Covariate Model
The relations of pharmacokinetic parameters with weight are shown in Eq. 5:
Pharmacokinetic parameters and the continuous covariates or categorical covariates are shown in Eqs. 6Eqs. 7, respectively:
The potential covariates included gender, age, weight, sirolimus dosage form, albumin, alanine transaminase, aspartate transaminase, creatinine, urea, total protein, total bile acid, direct bilirubin, total bilibrubin, hematocrit, hemoglobin, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and drug combination. The covariate inclusion criteria were calculated by objective function value (OFV) changes. The decrease of OFV greater than 3.84 (p < 0.05) was considered sufficient for inclusion in the base model. The increase of OFV greater than 6.63 (p < 0.01) was considered sufficient for significance in the final model.
Model Evaluation
The goodness-of-fit plots of model (observations vs. population predictions, observations vs. individual predictions, absolute value of weighted residuals of individual (│iWRES│) vs. individual predictions, and weighted residuals vs. time), distribution of weighted residuals for model (density vs. weighted residuals, and quantilies of weighted residuals vs. quantilies of normal), and visual predictive check (VPC) of model were used to estimate the final model. Additionally, the medians and 2.5th–97.5th percentiles of the results from bootstrap (repeated random sampling with replacement from the raw data base with 1,000 repetitions with different random sampling) were used for comparing with final model parameters.
Simulation
Initial dose optimizations were simulated by the Monte Carlo method, done using the NONMEM software (edition 7, ICON Development Solutions, Ellicott City, MD, United States). According to the report from He et al, the target concentration window of sirolimus improving seizure control in pediatric patients with TSC was 5–10 ng/ml (He et al., 2020). In the present study, we found weight, and concomitant medication of oxcarbazepine affected sirolimus clearance. Therefore, on the basis of whether oxcarbazepine was used in combination, and a once-daily sirolimus regimen or a twice-daily sirolimus regimen, we simulated different situations. In each situation, 1,000 virtual pediatric patients were simulated in ten dosages (0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, and 0.10 mg/kg/day) for eight weight groups (5, 10, 20, 30, 40, 50, 60, and 70 kg), respectively. The twice-daily sirolimus regimen was split evenly into two dosages a day. The probabilities of achieving the target concentration window were used as the evaluation criteria.
Results
Patients
A total of 80 children were included in the present study, 35 boys and 45 girls, aged from 0.61 to 16.61 years. Sirolimus dosage forms included tablet and solution, both dosage forms were used for 41 person-times, respectively (two children used two dosage forms). 188 sirolimus trough concentrations were collected, and the average number of concentrations was 2.35 per patient. The drug combination included carbamazepine, lamotrigine, levetiracetam, oxcarbazepine, topiramate, valproic acid, and vigabatrin. Table 1 shows the demographic data of patients.
Modeling
In the final model, weight was included as a covariant. In addition, concomitant medication of oxcarbazepine affected sirolimus clearance. Sirolimus dosage forms, or physiological and biochemical indexes were not included in the final model. Hence, the final models were as shown below in Eqs. 8, 9:
Evaluation
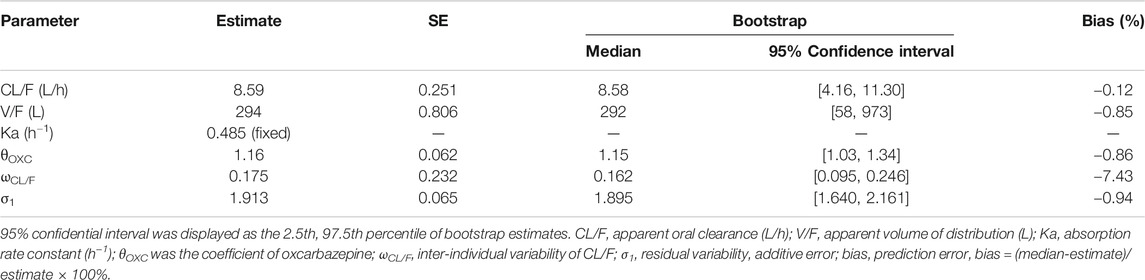
Figure 1 shows the final model evaluation. Figure 1A shows goodness-of-fit plots of model. Figures 1B,C show the distribution of weighted residuals. Figure 1D shows the visual predictive check of the model. We found that the distribution of the final model was normal, and most of the observed sirolimus concentrations were within the 95% prediction intervals from the simulation data, showing the prediction-corrected sirolimus concentrations were well predicted by the final model. The parameter estimates of the final model and bootstrap validation are shown in Table 2. The value of inter-individual variability of V/F (ωV/F) was very small, close to zero, so we dropped it. In addition, the additive error was selected as the best random residual variability. The median values of 1,000 bootstraps were close to the respective parameter values of the final model, indicating that the model was reliable and accurate.
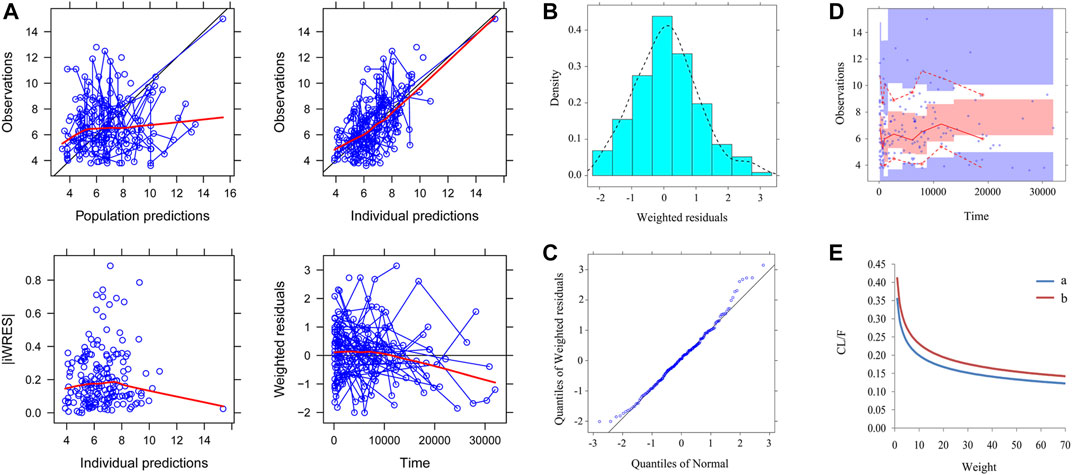
FIGURE 1. Model evaluation. (A) Goodness-of-fit plots of model (observations vs. population predictions, observations vs. individual predictions, absolute value of weighted residuals of individual (│iWRES│) vs. individual predictions, weighted residuals vs. time). (B) Density vs. weighted residuals. (C) Quantilies of weighted residuals vs. quantilies of normal. (D) Visual predictive check (VPC) of model. The middle solid line represents the median of the prediction-corrected concentrations. The lower and upper dashed lines are the 2.5th and 97.5th percentiles of the prediction-corrected concentrations. (E) Sirolimus apparent clearance rate (CL/F, L/h/kg) of pediatric patients with different weight, A: without oxcarbazepine, B: with oxcarbazepine.
Simulation
The sirolimus apparent clearance rate of pediatric patients with different weights are shown in Figure 1E, among which, line a was children without oxcarbazepine and line b was children with oxcarbazepine. With the same weight, the ratios of sirolimus clearance were 1:1.16 in children without oxcarbazepine and children with oxcarbazepine, respectively. Based on the final model, we simulated four different scenarios: 1) without oxcarbazepine, for once-daily sirolimus regimen; 2) without oxcarbazepine, for twice-daily sirolimus regimen; 3) with oxcarbazepine, for once-daily sirolimus regimen; 4) with oxcarbazepine, for twice-daily sirolimus regimen. The simulation of sirolimus concentrations at different initial dosages from the four different scenarios are shown in Figures 2–5, respectively. Figure 6 shows the probabilities of achieving the target concentrations of the four different scenarios; based on this, we recommended the optimal initial administration in each case, which are shown in Table 3. Without oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.07, 0.06, 0.05, 0.04, and 0.03 mg/kg/day were recommended for weights of 5–7.5, 7.5–11.5,11.5–19, 19–40, and 40–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.05, 0.04, and 0.03 were recommended for weights of 5–8, 8–20, and 20–70 kg, respectively. With oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.09, 0.08, 0.07, 0.06, 0.05, and 0.04 mg/kg/day were recommended for weights of 5–7.5, 7.5–10, 10–13.5, 13.5–20, 20–35, and 35–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.06, 0.05, 0.04, and 0.03 were recommended for weights of 5–7, 7–14.5, 14.5–38, and 38–70 kg, respectively.
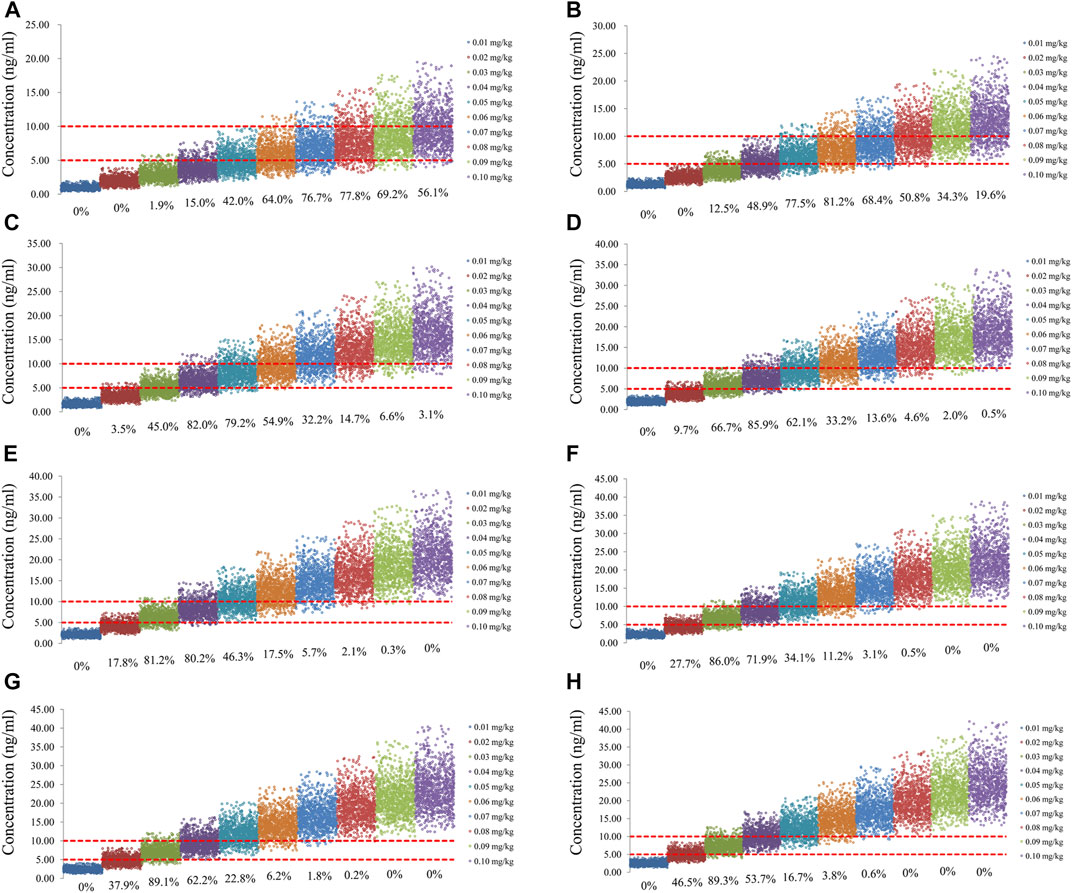
FIGURE 2. Simulation of sirolimus concentrations of once-daily sirolimus regimen without oxcarbazepine. (A) Pediatric patients weighing 5 kg. (B) Pediatric patients weighing 10 kg. (C) Pediatric patients weighing 20 kg. (D) Pediatric patients weighing 30 kg. (E) Pediatric patients weighing 40 kg. (F) Pediatric patients weighing 50 kg. (G) Pediatric patients weighing 60 kg. (H) Pediatric patients weighing 70 kg.
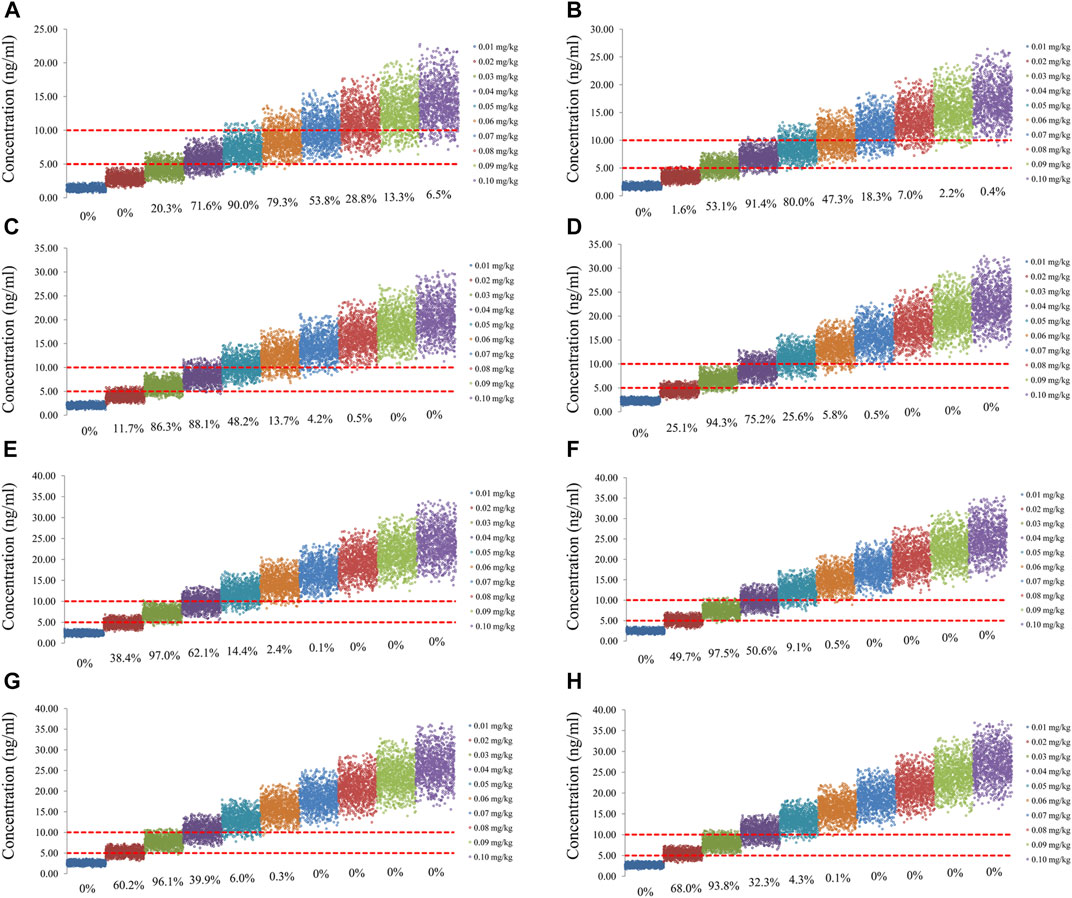
FIGURE 3. Simulation of sirolimus concentrations of twice-daily sirolimus regimen without oxcarbazepine. (A) Pediatric patients weighing 5 kg. (B) Pediatric patients weighing 10 kg. (C) Pediatric patients weighing 20 kg. (D) Pediatric patients weighing 30 kg. (E) Pediatric patients weighing 40 kg. (F) Pediatric patients weighing 50 kg. (G) Pediatric patients weighing 60 kg. (H) Pediatric patients weighing 70 kg.
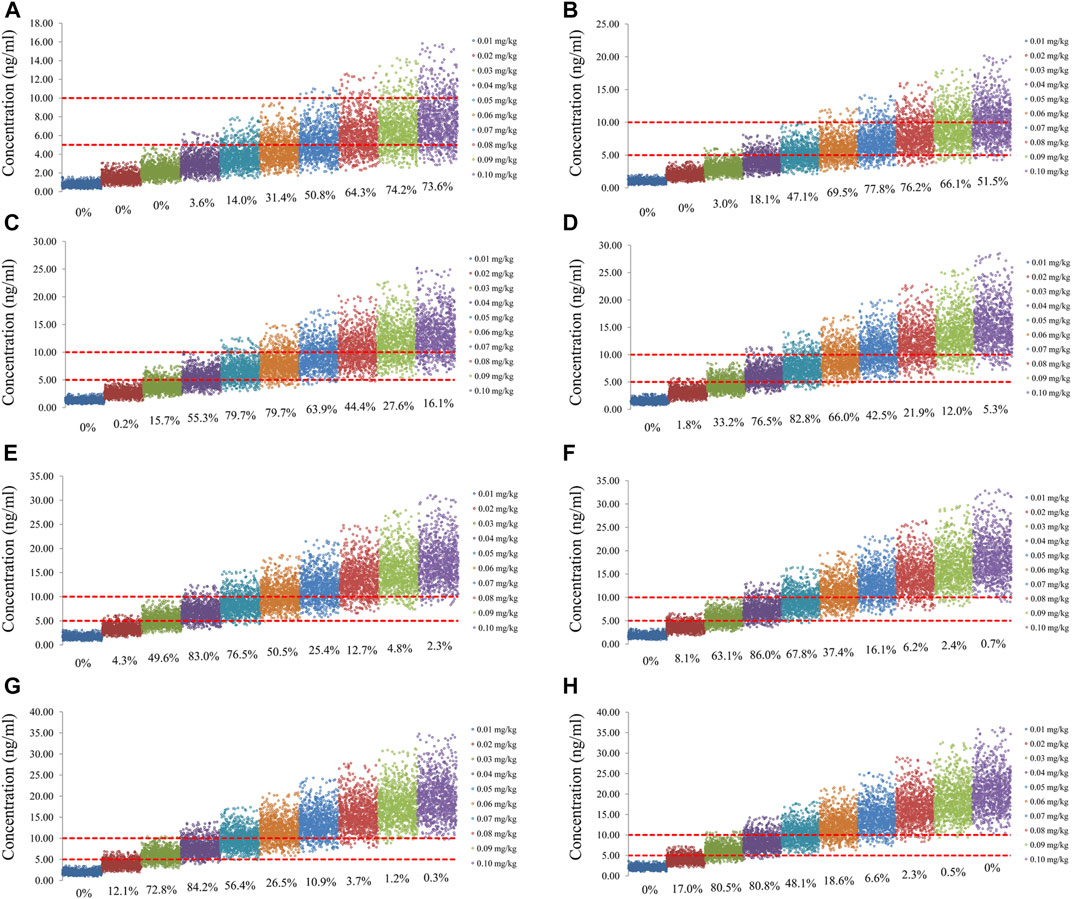
FIGURE 4. Simulation of sirolimus concentrations of once-daily sirolimus regimen with oxcarbazepine. (A) Pediatric patients weighing 5 kg. (B) Pediatric patients weighing 10 kg. (C) Pediatric patients weighing 20 kg. (D) Pediatric patients weighing 30 kg. (E) Pediatric patients weighing 40 kg. (F) Pediatric patients weighing 50 kg. (G) Pediatric patients weighing 60 kg. (H) Pediatric patients weighing 70 kg.
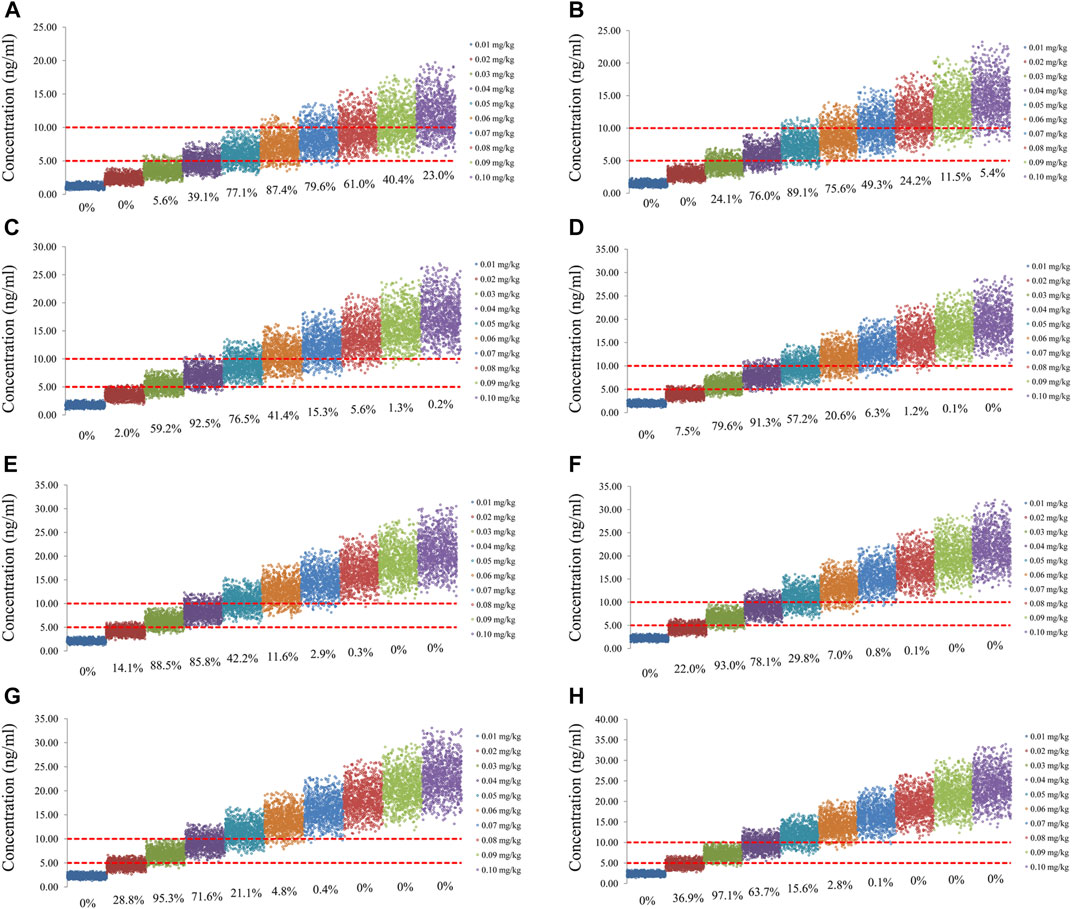
FIGURE 5. Simulation of sirolimus concentrations of twice-daily sirolimus regimen with oxcarbazepine. (A) Pediatric patients weighing 5 kg. (B) Pediatric patients weighing 10 kg. (C) Pediatric patients weighing 20 kg. (D) Pediatric patients weighing 30 kg. (E) Pediatric patients weighing 40 kg. (F) Pediatric patients weighing 50 kg. (G) Pediatric patients weighing 60 kg. (H) Pediatric patients weighing 70 kg.
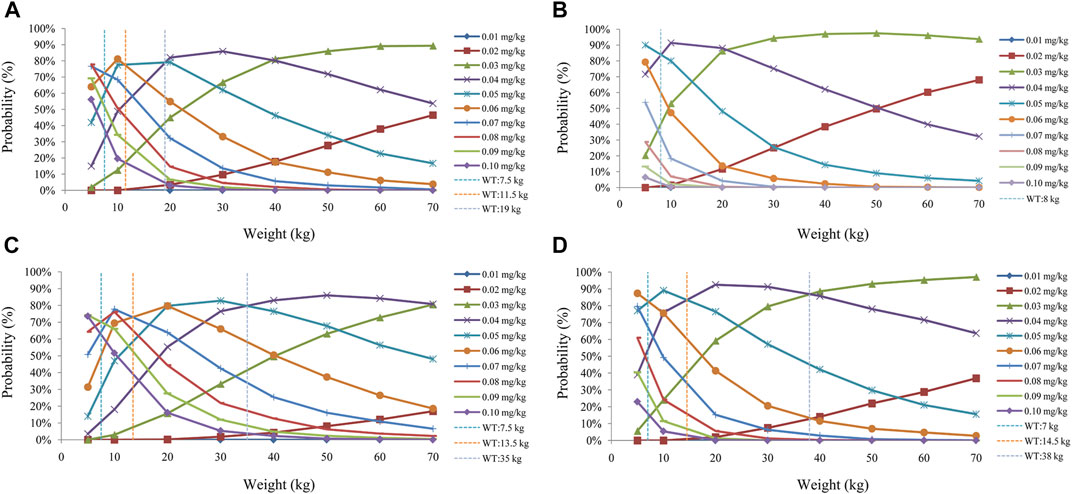
FIGURE 6. Probability to achieve the target concentrations. (A) Once-daily sirolimus regimen without concomitant medication of oxcarbazepine. (B) Twice-daily sirolimus regimen without concomitant medication of oxcarbazepine. (C) Once-daily sirolimus regimen with concomitant medication of oxcarbazepine. (D) Twice-daily sirolimus regimen with concomitant medication of oxcarbazepine.
Discussion
It is verified that mTOR inhibitors have good effects on treating TSC (Palavra et al., 2017; Franz and Krueger, 2018; Li et al., 2019). Krueger et al reported short-term safety of mTOR inhibitors in infants and very young children with TSC (Krueger et al., 2018). Saffari et al reported the safety and efficacy of mTOR inhibitor treatment in patients with TSC under 2 years of age (Saffari et al., 2019). Among them, everolimus is a registered mTOR inhibitor for epilepsy in TSC. French et al reported adjunctive everolimus treatment significantly reduced seizure frequency with a tolerable safety profile compared with placebo in patients with TSC and treatment-resistant seizures (French et al., 2016). In addition, He et al reported that sirolimus has a significant effect on seizures associated with TSC, and it could be used as the first-line medication for pediatric patients with TSC-associated epilepsy and the target concentration window of sirolimus was 5–10 ng/ml (He et al., 2020).
In the traditional clinical diagnosis and treatment process, sirolimus concentrations were monitored using therapeutic drug monitoring, subsequently adjusting the next sirolimus dosage based on concentrations. However, the initial dosage could not be recommended by the method. Luckily, the combination of population pharmacokinetics and Monte Carlo simulation could be used to recommend the optimum initial regimen, which has been successfully used in many drugs. For example, Zhao et al reported developmental population pharmacokinetics and dosing optimization of cefepime in neonates and young infants (Zhao et al., 2020). Therefore, the present study aimed to recommend the initial dosage regimen for sirolimus improving drug blood level for seizure control in pediatric patients with TSC based on the population pharmacokinetics and Monte Carlo simulation.
In our study, weight and concomitant medication of oxcarbazepine affected sirolimus clearance. It has been reported that there was a nonlinear relationship between drug clearance and weight in pediatric patients, which may be well described by allometric scaling with the coefficients of 0.75 for clearance and 1 for volume (Anderson and Holford, 2008; Hao et al., 2018). In addition, oxcarbazepine is a cytochrome P450 (CYP) 3A4 inducer (McGrane et al., 2018) and sirolimus is primarily metabolized by the cytochrome CYP3A4 and CYP3A5 (Zhang et al., 2017). Oxcarbazepine may accelerate sirolimus metabolism by inducing 3A4 activity. And at the same weight, the ratios of sirolimus clearance were 1:1.16 from children without oxcarbazepine and children with oxcarbazepine, respectively. These results suggested that the dose of sirolimus should be increased when oxcarbazepine was included in the epilepsy regimen. In addition, carbamazepine is also an inducer of CYP3A4. Theoretically, it may have potential interaction with sirolimus. However, carbamazepine could not be included as a statistically significant covariable in the present study. What is interesting about this is that it is not isolated, Chen et al reported evaluation of CYP3A4-based interactions of levomilnacipran with carbamazepine in healthy subjects, also no dose adjustment for levomilnacipran is suggested when levomilnacipran is co-administered with carbamazepine (Chen et al., 2015). We also found that sirolimus dosage forms, tablet or solution, had no significant effect on sirolimus clearance rate, suggesting that dosage forms suitable for specific age-groups of children can be selected according to the actual clinical needs.
Ultimately, based on the final model, we recommended the optimal initial administration in each case. Without oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.07, 0.06, 0.05, 0.04, and 0.03 mg/kg/day were recommended for weights of 5–7.5, 7.5–11.5,11.5–19, 19–40, and 40–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.05, 0.04, and 0.03 were recommended for weights of 5–8, 8–20, and 20–70 kg, respectively. With oxcarbazepine, for once-daily sirolimus regimen, the doses of 0.09, 0.08, 0.07, 0.06, 0.05, and 0.04 mg/kg/day were recommended for weights of 5–7.5, 7.5–10, 10–13.5, 13.5–20, 20–35, and 35–70 kg, respectively; for twice-daily sirolimus regimen, the doses of 0.06, 0.05, 0.04, and 0.03 were recommended for weights of 5–7, 7–14.5, 14.5–38, and 38–70 kg, respectively. In addition, we found that in the same situation, the twice-daily sirolimus regimen had higher probabilities of achieving the target concentrations, meanwhile reduced total use of sirolimus, which could reduce the medical cost to some extent. But, the downside was that it may increase the frequency of use and may decrease medication compliance in patients. Hence, the clinician or pharmacist should choose the specific selection of once-daily or twice-daily sirolimus regimen based on the actual clinical situation.
Of course, to some extent, CYP3A4 and CYP3A5 may influence sirolimus metabolism in adults (Tamashiro et al., 2017; Zhang et al., 2017). However, it was interesting in our previous study that the initial dosage recommendation for sirolimus in children with TSC, CYP3A4, and CYP3A5 polymorphism did not significantly affect sirolimus clearance rates (Wang et al., 2020). The main reason was that included children had a wide age span (Wang et al., 2020). And it was reported that gene expression in different age development processes had development-dependent characteristics; meanwhile, CYP3A expression isoforms levels were highly variable after birth (Lacroix et al., 1997). That is to say, the activity of drug-metabolizing enzymes of the same CYP3A genotype may be different in different age-groups and genotypes may not accurately explain the differences from sirolimus concentrations. Other studies had also confirmed that CYP3A genotypes were not included in population pharmacokinetics of sirolimus for children (Mizuno et al., 2017a; Mizuno et al., 2017b). Most important of all, the sirolimus pharmacogenomics was not tested routinely in current sirolimus therapeutics, and the weight-based dosage recommendations had more convenient and better clinical application advantages (Wang et al., 2020). Based on the above, the current study did not further examine the influence of CYP3A4 and CYP3A5 genotypes. However, further studies will be conducted on the newly identified polymorphism that may significantly affect sirolimus clearance in pediatric patients. In addition, it was an objective existence that sparse clinical sampling mostly contained insufficient information, which was also a limitation of our study. We will further verify the conclusion of our study through prospective intensive sampling in the future.
Conclusion
The present study was the first to establish a population pharmacokinetic model of sirolimus improving drug blood level for seizure control in pediatric patients with TSC and recommend the initial dosage regimen.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Children’s Hospital of Fudan University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZL, HX, and YW conceived and designed the study. XC, DW, LZ, JL,YH, GW, YZ, and QY collected the data. XC and DW built the model and evaluated the data. XC wrote the article. DW reviewed and edited the article. All authors read and approved the article.
Funding
This work was supported by the Shanghai Municipal Education Commission (No. HJW-R-2019-66-19), the Clinical Pharmacy Key Specialty Construction Project of Shanghai (No. YZ2017/5), and the scientific research project of Science and Technology Commission of Shanghai Municipality (No. 18DZ1910604, No.19XD1400900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Anderson, B. J., and Holford, N. H. (2008). Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48, 303–332. doi:10.1146/annurev.pharmtox.48.113006.094708
Bissler, J. J., McCormack, F. X., Young, L. R., Elwing, J. M., Chuck, G., Leonard, J. M., et al. (2008). Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358 (2), 140–151. doi:10.1056/NEJMoa063564
Chen, L., Boinpally, R., Gad, N., Greenberg, W. M., Wangsa, J., Periclou, A., et al. (2015). Evaluation of cytochrome P450 (CYP) 3A4-based interactions of levomilnacipran with ketoconazole, carbamazepine or alprazolam in healthy subjects. Clin. Drug Investig. 35 (10), 601–612. doi:10.1007/s40261-015-0318-2
Chu-Shore, C. J., Major, P., Camposano, S., Muzykewicz, D., and Thiele, E. A. (2010). The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 51 (7), 1236–1241. doi:10.1111/j.1528-1167.2009.02474.x
Curatolo, P., Bombardieri, R., and Jozwiak, S. (2008). Tuberous sclerosis. Lancet 372 (9639), 657–668. doi:10.1016/S0140-6736(08)61279-9
Curatolo, P., Moavero, R., and de Vries, P. J. (2015). Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 14 (7), 733–745. doi:10.1016/S1474-4422(15)00069-1
Davies, D. M., de Vries, P. J., Johnson, S. R., McCartney, D. L., Cox, J. A., Serra, A. L., et al. (2011). Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin. Cancer Res. 17 (12), 4071–4081. doi:10.1158/1078-0432.CCR-11-0445
de Vries, P. J., Whittemore, V. H., Leclezio, L., Byars, A. W., Dunn, D., Ess, K. C., et al. (2015). Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr. Neurol. 52 (1), 25–35. doi:10.1016/j.pediatrneurol.2014.10.004
Franz, D. N., and Krueger, D. A. (2018). mTOR inhibitor therapy as a disease modifying therapy for tuberous sclerosis complex. Am. J. Med. Genet. C Semin. Med. Genet. 178 (3), 365–373. doi:10.1002/ajmg.c.31655
French, J. A., Lawson, J. A., Yapici, Z., Ikeda, H., Polster, T., Nabbout, R., et al. (2016). Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388 (10056), 2153–2163. doi:10.1016/S0140-6736(16)31419-2
Hao, G. X., Huang, X., Zhang, D. F., Zheng, Y., Shi, H. Y., Li, Y., et al. (2018). Population pharmacokinetics of tacrolimus in children with nephrotic syndrome. Br. J. Clin. Pharmacol. 84 (8), 1748–1756. doi:10.1111/bcp.13605
He, W., Chen, J., Wang, Y. Y., Zhang, M. N., Lu, Q., Wang, Q. H., et al. (2020). Erratum to "Sirolimus improves seizure control in pediatric patients with tuberous sclerosis: a prospective cohort study" [Seizure: eur. J. Epilepsy, 79 (2020) 20-26]. Seizure 81, 342–426. doi:10.1016/j.seizure.2020.03.018
Krueger, D. A., Capal, J. K., Curatolo, P., Devinsky, O., Ess, K., Tzadok, M., et al. (2018). Short-term safety of mTOR inhibitors in infants and very young children with tuberous sclerosis complex (TSC): multicentre clinical experience. Eur. J. Paediatr. Neurol. 22 (6), 1066–1073. doi:10.1016/j.ejpn.2018.06.007
Kwan, P., Arzimanoglou, A., Berg, A. T., Brodie, M. J., Allen Hauser, W., Mathern, G., et al. (2010). Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 51 (6), 1069–1077. doi:10.1111/j.1528-1167.2009.02397.x
Lacroix, D., Sonnier, M., Moncion, A., Cheron, G., and Cresteil, T. (1997). Expression of CYP3A in the human liver--evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur. J. Biochem. 247 (2), 625–634. doi:10.1111/j.1432-1033.1997.00625.x
Li, M., Zhou, Y., Chen, C., Yang, T., Zhou, S., Chen, S., et al. (2019). Efficacy and safety of mTOR inhibitors (rapamycin and its analogues) for tuberous sclerosis complex: a meta-analysis. Orphanet J. Rare Dis. 14 (1), 39. doi:10.1186/s13023-019-1012-x
McGrane, I. R., Loveland, J. G., and de Leon, J. (2018). Possible oxcarbazepine inductive effects on aripiprazole metabolism: a case report. J. Pharm. Pract. 31 (3), 361–363. doi:10.1177/0897190017710523
Mizuno, T., Fukuda, T., Christians, U., Perentesis, J. P., Fouladi, M., and Vinks, A. A. (2017a). Population pharmacokinetics of temsirolimus and sirolimus in children with recurrent solid tumours: a report from the Children's Oncology Group. Br. J. Clin. Pharmacol. 83 (5), 1097–1107. doi:10.1111/bcp.13181
Mizuno, T., Fukuda, T., Emoto, C., Mobberley-Schuman, P. S., Hammill, A. M., Adams, D. M., et al. (2017b). Developmental pharmacokinetics of sirolimus: implications for precision dosing in neonates and infants with complicated vascular anomalies. Pediatr. Blood Cancer 64 (8). doi:10.1002/pbc.26470
Nathan, N., Wang, J. A., Li, S., Cowen, E. W., Haughey, M., Moss, J., et al. (2015). Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J. Am. Acad. Dermatol. 73 (5), 802–808. doi:10.1016/j.jaad.2015.07.018
Palavra, F., Robalo, C., and Reis, F. (2017). Recent advances and challenges of mTOR inhibitors use in the treatment of patients with tuberous sclerosis complex. Oxid Med. Cell Longev 2017, 9820181. doi:10.1155/2017/9820181
Saffari, A., Brösse, I., Wiemer-Kruel, A., Wilken, B., Kreuzaler, P., Hahn, A., et al. (2019). Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age - a multicenter retrospective study. Orphanet J. Rare Dis. 14 (1), 96. doi:10.1186/s13023-019-1077-6
Tamashiro, E. Y., Felipe, C. R., Genvigir, F. D. V., Rodrigues, A. C., Campos, A. B., Hirata, R. D. C., et al. (2017). Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metab. Pers Ther. 32 (2), 89–95. doi:10.1515/dmpt-2016-0036
van der Poest Clement, E., Jansen, F. E., Braun, K. P. J., and Peters, J. M. (2020). Update on drug management of refractory epilepsy in tuberous sclerosis complex. Paediatr. Drugs 22 (1), 73–84. doi:10.1007/s40272-019-00376-0
Wang, D. D., Chen, X., Xu, H., and Li, Z. P. (2020). Initial dosage recommendation for sirolimus in children with tuberous sclerosis complex. Front. Pharmacol. 11, 890. doi:10.3389/fphar.2020.00890
Wataya-Kaneda, M., Nakamura, A., Tanaka, M., Hayashi, M., Matsumoto, S., Yamamoto, K., et al. (2017). Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex : a randomized clinical trial. JAMA Dermatol. 153 (1), 39–48. doi:10.1001/jamadermatol.2016.3545
Zhang, J., Dai, Y., Liu, Z., Zhang, M., Li, C., Chen, D., et al. (2017). Effect of CYP3A4 and CYP3A5 genetic polymorphisms on the pharmacokinetics of sirolimus in healthy Chinese volunteers. Ther. Drug Monit. 39 (4), 406–411. doi:10.1097/FTD.0000000000000415
Keywords: population pharmacokinetics, initial dose optimization, sirolimus, seizure control, tuberous sclerosis complex
Citation: Chen X, Wang D, Zhu L, Lu J, Huang Y, Wang G, Zhu Y, Ye Q, Wang Y, Xu H and Li Z (2021) Population Pharmacokinetics and Initial Dose Optimization of Sirolimus Improving Drug Blood Level for Seizure Control in Pediatric Patients With Tuberous Sclerosis Complex. Front. Pharmacol. 12:647232. doi: 10.3389/fphar.2021.647232
Received: 29 December 2020; Accepted: 15 February 2021;
Published: 20 April 2021.
Edited by:
Venkata Kashyap Yellepeddi, The University of Utah, United StatesReviewed by:
Susanna Medellín, Autonomous University of San Luis Potosí, MexicoSergiusz Jozwiak, Medical University of Warsaw, Poland
Copyright © 2021 Chen, Wang, Zhu, Lu, Huang, Wang, Zhu, Ye, Wang, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Wang, eWl3YW5nQHNobXUuZWR1LmNu; Hong Xu, aHh1QHNobXUuZWR1LmNu; Zhiping Li, enBsaUBmdWRhbi5lZHUuY24=
†These authors have equally contributed to this work
 Xiao Chen
Xiao Chen Dongdong Wang
Dongdong Wang Lin Zhu1
Lin Zhu1 Jinmiao Lu
Jinmiao Lu Yi Wang
Yi Wang Zhiping Li
Zhiping Li