- 1Department of Neurosurgery, University of Michigan Medical School, Ann Arbor, MI, United States
- 2Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor, MI, United States
- 3Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States
- 4Masonic Cancer Center, University of Minnesota, Minneapolis, MN, United States
- 5Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, MI, United States
- 6Biointerfaces Institute, University of Michigan, Ann Arbor, MI, United States
- 7Department of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
- 8Rogel Cancer Center, University of Michigan Medical School, Ann Arbor, MI, United States
- 9Biosciences Initiative in Brain Cancer, University of Michigan, Ann Arbor, MI, United States
Gliomas are one of the most lethal types of cancers accounting for ∼80% of all central nervous system (CNS) primary malignancies. Among gliomas, glioblastomas (GBM) are the most aggressive, characterized by a median patient survival of fewer than 15 months. Recent molecular characterization studies uncovered the genetic signatures and methylation status of gliomas and correlate these with clinical prognosis. The most relevant molecular characteristics for the new glioma classification are IDH mutation, chromosome 1p/19q deletion, histone mutations, and other genetic parameters such as ATRX loss, TP53, and TERT mutations, as well as DNA methylation levels. Similar to other solid tumors, glioma progression is impacted by the complex interactions between the tumor cells and immune cells within the tumor microenvironment. The immune system’s response to cancer can impact the glioma’s survival, proliferation, and invasiveness. Salient characteristics of gliomas include enhanced vascularization, stimulation of a hypoxic tumor microenvironment, increased oxidative stress, and an immune suppressive milieu. These processes promote the neuro-inflammatory tumor microenvironment which can lead to the loss of blood-brain barrier (BBB) integrity. The consequences of a compromised BBB are deleteriously exposing the brain to potentially harmful concentrations of substances from the peripheral circulation, adversely affecting neuronal signaling, and abnormal immune cell infiltration; all of which can lead to disruption of brain homeostasis. In this review, we first describe the unique features of inflammation in CNS tumors. We then discuss the mechanisms of tumor-initiating neuro-inflammatory microenvironment and its impact on tumor invasion and progression. Finally, we also discuss potential pharmacological interventions that can be used to target neuro-inflammation in gliomas.
Introduction
Gliomas are the most commonly diagnosed malignant primary tumors that arise in the brain (Louis et al., 2016; Ceccarelli et al., 2016; Schwartzbaum et al., 2006). The traditional classification of gliomas is based on the histological features according to the microscopic similarity with the putative cell of origin along glial precursor cell lineages (i.e., astrocytes, oligodendrocytes, etc) and malignancy grade (Grade I-IV). Gliomas that lack features of aggressiveness and grow slower are classified as Low-Grade Gliomas (LGG) and correspond with WHO grades I and II, while gliomas that have hallmarks of aggressiveness and are more proliferative are classified as High-Grade Gliomas (HGG) and correspond with WHO grades III and IV. Although histopathologic classification is more established, it has the disadvantage of potential observer bias, particularly between grade III and IV tumors (van den Bent et al., 2010). In the last decade, the availability of glioma datasets has facilitated the correlation of genetic, transcriptional, and epigenetic signatures with clinical features (Noushmehr et al., 2010; Sturm et al., 2012; Verhaak et al., 2010).
The current stratification of gliomas is established by the WHO Classification of Central Nervous System Tumors, which was updated in 2016 (Louis et al., 2016; Wesseling and Capper, 2018). According to the new classification, gliomas are divided into diffusely infiltrating gliomas, which include grade II and III astrocytic tumors, grade II and III oligodendrogliomas, grade IV glioblastoma, and diffuse midline gliomas (Louis et al., 2016). This recent update is the first to include molecular parameters into consideration, besides the commonly used histopathological parameters. The more relevant molecular markers incorporated in glioma classification are IDH mutations, 1p19q deletion, MGMT promoter methylation, TERT promoter mutations, ATRX loss of function mutations, and p53 loss of function mutations and mutations in isocitrate dehydrogenase 1 and 2 genes (IDH1/2 m) (Louis et al., 2016).
IDH1/2 m defines a distinct subgroup of glioma (GBM) and is clinically associated with favorable outcomes. IDHm have been identified in grade II and III astrocytomas, oligodendrogliomas, and oligoastrocytomas, and in secondary glioblastomas (which refers to gliomas with high-grade hallmarks such as nuclear atypia, and/or cellular pleomorphism as well as microvascular proliferation and/or necrosis), which result from tumor recurrence. IDH1m tumors are classified into two molecular subgroups: the first group carries a codeletion of the chromosomal bands 1p and 19q and TERT promoter mutation. Most of these gliomas are histologically defined as oligodendrogliomas. On the other hand, IDHm without 1p and 19q codeletion are mostly P53 and ATRX mutant, associated with hypermethylation phenotype (G-CIMP high) and astrocytic histology. The incorporation of IDHm status into diffuse glioma classification was in part prompted by its relevance in the clinical outcome of the tumors.
Diffuse gliomas with wild-type IDH (IDH-wt), even when they may be histologically defined as grade II or III, tend to behave like more aggressive glioblastomas. IDH-wt grade IV glioblastomas (GBM) are the most common malignant primary brain tumors. GBMs are densely cellular, pleomorphic tumors with mitotic activity and either microvascular proliferation or necrosis, or both. Histologic variants of this group include epithelioid glioblastoma (which normally occurs in children and young adults and carries BRAF V600 E mutations), along with giant cell GBM and gliosarcoma. The prognosis for all glioblastoma variants is poor, with survival commonly less than two years. Despite the clear differences between them, pediatric gliomas are still classified according to the histological resemblance to adult gliomas. As an exception, the WHO 2016 classification incorporated the entity of histone H3 K27 M diffuse midline glioma, which mainly occurs in pediatric patients and regardless of the histological grade, has a poor prognosis. Our molecular knowledge of pediatric gliomas has been refined over the last few years, and some molecular markers, such as H3 G34R/V, BRAF, NF1 mutations, and PDGFRα amplifications, are now becoming relevant for the decision of clinical interventions (Koschmann et al., 2016; Miklja et al., 2019; Haase et al., 2020).
Several aspects make it difficult to efficiently treat gliomas, i.e., the anatomical location, the presence of the blood-brain barrier (BBB), and the restricted immune reactivity within the CNS. The anatomical location, together with the infiltrative nature of high-grade glioma, makes the total resection of the tumor mass virtually impossible. The blood-brain barrier hampers the delivery of therapeutic compounds to the tumor site. Despite these limitations, intense research has opened up opportunities to explore tailored therapies for different glioma subtypes with particular molecular lesions that are currently under clinical trials (Haase et al., 2020).
Currently, the standard of care (SOC) for GBM is comprised of surgery to remove the tumor mass, followed by radiation therapy in combination with Temozolomide (TMZ) administration. The incorporation of TMZ in GBM treatment leads to a small progression-free survival (PFS) improvement (5 vs. 6.9 months), in addition to a significant benefit in 2-years overall survival (11 vs. 27%) (Brandes et al., 2008). The MGMT promoter methylation can predict the TMZ therapy efficacy, and in many glioma subtypes which do not have MGMT promoter methylation, TMZ does not improve patients’ outcome. The extent of tumor resection is the most prominent treatment factor associated with survival (Brown et al., 2016; Molinaro et al., 2019).
Blood-Brain Barrier
The healthy Blood-Brain Barrier (BBB) is a selectively permeable barrier secured by endothelial cells linked by tight junctions, pericytes embedded in a basement membrane. Astrocytic end-feet is also anchored to the basement membrane (Varatharaj and Galea, 2017; Ros et al., 2018). Under homeostasis, these components protect the brain from toxic materials, regulate the transport of essential nutrients, and maintain a stable brain environment (Bergers and Benjamin, 2003; Ros et al., 2018). When BBB integrity is compromised, for example by glioma-associated inflammation, these cell layers change in both anatomy and function, altering the permeability of blood vessels in the brain (Figure 1; Erickson et al., 2012; Varatharaj and Galea, 2017).
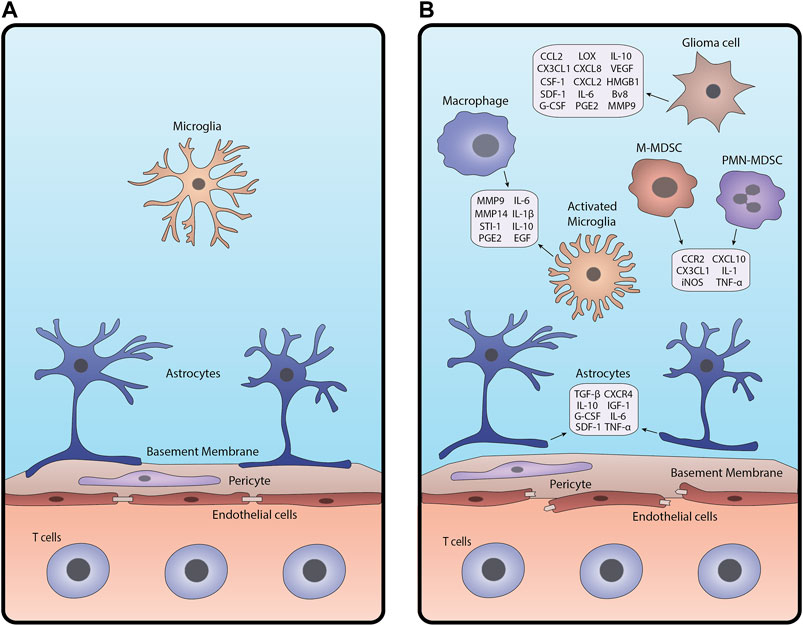
FIGURE 1. Schematic of the BBB under (A) homeostasis and (B) glioma-associated inflammation. In inflamed conditions (B), junctions between endothelial cells break apart, pericytes are disrupted, and astrocytic endfeet detach from the basement membrane. Glioma cells, macrophages and microglia, astrocytes, and MDSCs release cytokines and other factors that contribute to BBB breakdown and create an inflammatory tumor microenvironment.
Gliomas are characterized by the activation of numerous pathways that drive gliomagenesis. These include but are not limited to activation of hypoxia, abnormal angiogenesis, and tissue remodeling which disrupt the BBB and trigger an inflammatory response in the brain microenvironment (Bergers and Benjamin, 2003; Allavena et al., 2008; Ros et al., 2018). Response to hypoxic conditions is mediated by hypoxia-inducible factor-alpha (HIF-α), regulating the expression of angiogenic and inflammatory factors such as vascular endothelial growth factor (VEGF) (Murat et al., 2009; Belykh et al., 2020). VEGF disrupts the cellular barrier around existing blood vessels, pulling endothelial cells away to form new capillaries with fenestrations and fewer tight junctions (Dubois et al., 2014; Belykh et al., 2020). This results in an enhanced infiltration of cellular and plasma components into the brain, further exacerbating brain homeostasis.
While the intact BBB permits immune surveillance by effector immune cells, compromised BBB allows excessive immune cell infiltration magnifying inflammatory responses (Vries et al., 1998; Sonar and Lal, 2018). Tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) produced by inflamed immune cells such as tumor-associated macrophages (TAMs) promote the expression of inflammatory molecules in other cell types (Allavena et al., 2008; Sethi et al., 2008; Kore and Abraham, 2014). Moreover, reactive astrocytes, microglia, and endothelial cells can secrete mediators including transforming growth factor (TGF-β) and metalloproteinases (MMP2 and MMP9), augmenting the inflammatory tumor microenvironment (Sethi et al., 2008; Könnecke and Bechmann, 2013). Altered TGF-β signaling reduces the expression of cell adhesion molecules in pericytes and endothelial cells, weakening their attachments to the surrounding vessel (Joseph et al., 2013; Sonar and Lal, 2018). MMPs secreted by activated microglia cleave extracellular (ECM) components of the basal lamina, which alters the function of the basement membrane (Wolburg et al., 2012; Könnecke and Bechmann, 2013).
Glioma Tumor Microenvironment
Previously, the CNS was thought to be an ‘immune privileged’ site. This idea was favored following the rejection of transferred foreign tissue into the brain parenchyma (Murphy and Sturm, 1923; Medawar, 1948; Galea et al., 2007). Further investigation demonstrated that this immune privilege status of the CNS is relative to other tissues and organs and it depends on the existence of neuroinflammation. This has led to the implementation of clinical trials aiming to develop immunotherapies against glioma. However, most of these trials have failed to show benefits in glioma patients due to the immunosuppressive nature of the glioma tumor microenvironment (TME) that hampers effective anti-glioma immune response. In addition to the infiltrating immune cells, glioma cells express immune suppressive molecules such as interleukin-6, interleukin-10, TGF-β, LDH5, galectin-1 (gal-1), and prostaglandin-E which reprogram the infiltrating immune cells to a pro-tumor phenotype (Figure 2; Van Meir, 1995; Huettner et al., 1997; Crane et al., 2012; Perng and Lim, 2015). Interleukin-10 not only inhibits the functions of DCs and macrophages but also has a pro-inflammatory and inhibitory effect against T-cell activation and proliferation (Moore et al., 2001). Gal-1 enhances tumor cell migration and induces T-cell apoptosis and the skewing of tumor-infiltrating macrophages to the immunosuppressive M2 type (Verschuere et al., 2011).
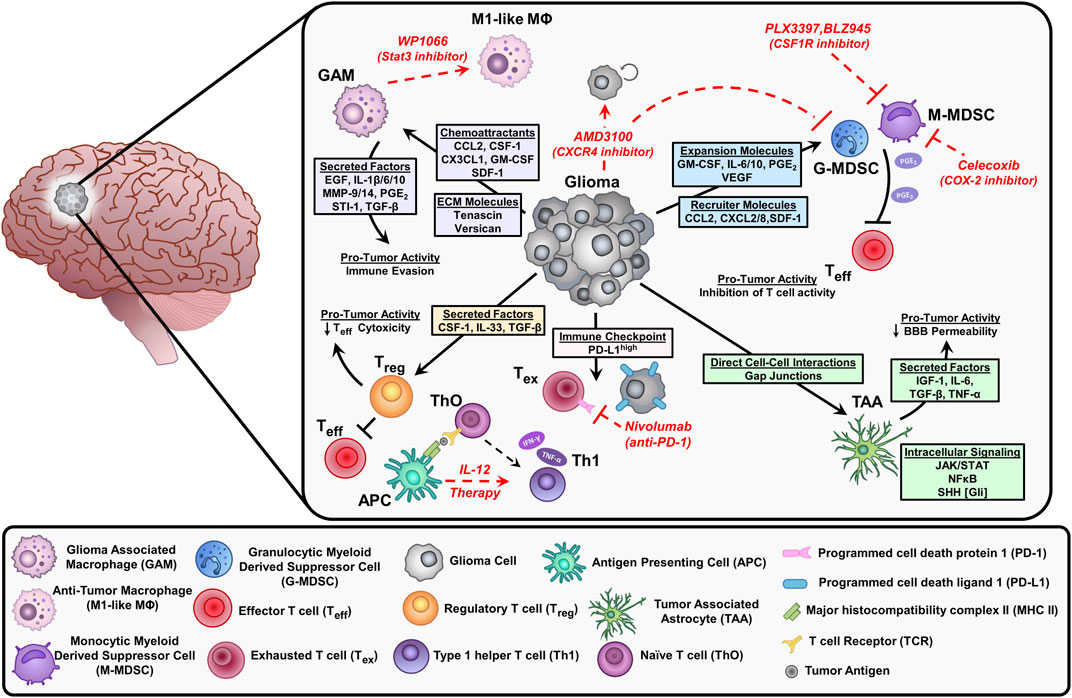
FIGURE 2. Depiction of the immunosuppressive environment in glioblastoma and current therapeutic approaches (red) to stimulate anti-tumor responses or inhibit suppressive immune cell populations. Glioma cells release chemoattractants and cytokines that recruit immune cells into the tumor microenvironment (TME). These factors elicit pro-tumor activities that inhibit effector T cells (Teff) and contribute to tumor progression. Glioma cells can also directly interact with immune cells and astrocytes impacting the effectiveness of chemotherapy. Inhibition of Stat3 by WP1066 reduces glioma-associated macrophages (GAM) by blocking intracellular signals induced by IL-10 to promote an anti-tumor macrophage (M1-like MΦ) phenotype. The CXCR4 antagonist AMD3100 prevents the binding of stromal cell-derived factor (SDF-1), resulting in decreased tumor proliferation and blocks the migration of myeloid-derived suppressor cells (MDSCs) in the TME. The CSF1R inhibitors (PLX3397, BLZ945) suppress the infiltration of myeloid cells (macrophages and MDSCs) within the TME. MDSCs synthesize prostaglandin E2 (PGE2) via COX-2 which inhibits effector T cells by reducing Interferon-gamma (IFN-γ) release. The COX-2 inhibitor, celecoxib reduces MDSC immunosuppressive activity. Interleukin-12 (IL-12) is secreted by antigen-presenting cells (APC) like dendritic cells and macrophages, exogenous IL-12 therapy triggers a pro-inflammatory transition of naïve T cells (ThO) to type 1 helper T cells (Th1) leading to an increase in IFN-y and tumor necrosis factor (TNF-α) secretion. Tumor cells express high levels of programmed cell death ligand (PD-L1) on their cell surface, which leads to exhausted T cells (Tex) by immune checkpoint signaling through their receptor programmed cell death protein 1 (PD-1). Inhibition of PD-1 with nivolumab reduces T cell exhaustion.
Myeloid cells are the most frequent immune cell type in the glioma TME, accounting for ∼60% of all glioma infiltrating immune cells. In GBM, myeloid-derived suppressor cells (MDSCs) are major immunosuppressive cells that hamper glioma immune response (Kamran et al., 2017; Alghamri et al., 2020). Clinically, the frequency of MDSCs is associated with an unfavorable prognosis in glioma (Rahbar et al., 2016). Besides MDSCs, glioma-associated macrophages/microglia (GAMs) represent a major population of immune cells infiltrating gliomas (Pyonteck et al., 2013; Brown et al., 2018; Chen and Hambardzumyan, 2018). Several chemokines and cytokines, including CCL2 and CX3CL1, are responsible for GAMs recruitments and expansion in glioma (Okada et al., 2009; Held-Feindt et al., 2010; da Fonseca and Badie, 2013). Within the lymphoid cells, T cells and NK cells represent the major effector cells in glioma (Kmiecik et al., 2013; Kmiecik et al., 2014), albeit they represent a small percentage of the total immune cells within the glioma TME (about 2% of immune-infiltrating cells). Tregs are also infiltrated in the glioma TME, they elicit a strong immunosuppressive response against anti-glioma T-cells (Chang et al., 2016). Multiple GBM-derived factors are responsible for Tregs recruitment including CCL22, CCL2, TGF-β, or indoleamine 2,3-dioxygenase 1 (IDO1) (Akasaki et al., 2004; Akhurst and Hata, 2012; Wainwright et al., 2012; Chang et al., 2016). Glioma-induced tissue hypoxia has also been shown to be responsible for the activation of regulatory T-cells (Saetta et al., 2011; Razavi et al., 2016). Collectively, recruitment and activation of immunosuppressive cells decrease the efficacy of anti-glioma immune response. Below, we will discuss in detail how some of these cells are recruited and activated to the glioma TME to support glioma invasion.
Role of Myeloid Cells (Microglia/Macrophages, MDSCs)
GAMs make up a high proportion of myeloid cells encountered in the glioblastoma microenvironment accounting for up to 30% of the tumor mass (Russo and Cappoli, 2018). Microglia are the brain’s resident myeloid cells, which are not replenished by blood-derived monocytes under normal physiological conditions. Instead, they function as a self-sustaining population with an extended capacity to proliferate (Schetters et al., 2018). In contrast, peripheral macrophages are derived from hematopoietic stem cells arising in the bone marrow and migrate to the glioma microenvironment partially due to the breakdown of the blood-brain barrier (Russo and Cappoli, 2018). GAMs are recruited to the glioma microenvironment via the glioma cells’ release of chemoattractant: CCL2, CX3CL1, colony-stimulating factor (CSF-1), stromal cell-derived factor 1 (SDF-1), granulocyte/macrophage-colony stimulating factor (GM-CSF), and lysine oxidase (LOX) (Figure 2; Zhang et al., 2012; Roesch et al., 2018; Gutmann and Kettenmann, 2019; Chen et al., 2019). Under pathological conditions, microglia and peripheral macrophages/monocytes activate, proliferate, and contribute to the disruption of immunological homeostasis (Gutmann and Kettenmann, 2019; Sankowski et al., 2019). In the glioma microenvironment, macrophages are impacted to elicit pro-tumoral effects through the activation of immunosuppressive pathways enhancing glioma progression (Hambardzumyan et al., 2016; Roesch et al., 2018). For example, glioma cells produce let7, tenascin-C (TNC), and versican resulting in increased production of cytokines and matrix metalloproteases (MMP9 and MMP14) by GAMs (Hambardzumyan et al., 2016; Gutmann and Kettenmann, 2019). In response to these signals, GAMs release multiple cytokines such as transforming growth factor ß (TGF-β), stress-inducible protein 1 (STI-1), prostaglandin E2 (PGE2), IL-6, IL-1β, IL-10, and epidermal growth factor (EGF), which promote glioma cell proliferation and inhibit T cells function (Hambardzumyan et al., 2016; Gutmann and Kettenmann, 2019). GAMs’ depletion and/or inhibition by chlodronate and microglial inhibitory factor (MIF/TKP) drastically reduced tumor growth, further suggesting GAMs as a potential therapeutic target (Markovic et al., 2009; Zhai et al., 2011).
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells that express high levels of immunosuppressive molecules and inhibit anti-tumor immunity (Grabowski et al., 2021). These cells can derive from monocytic (M-MDSCs) or granulocytic (PMN-MDSCs) origin (Grabowski et al., 2021). M-MDSCs have been shown to have greater immunosuppressive capability and are more common in the blood of GBM patients; whereas, PMN-MDSCs make up a greater portion of MDSCs in the glioma microenvironment (Mi et al., 2020). Tumor-derived cytokines are the major drivers of MDSCs expansion in the glioma microenvironment. These can be divided into two classes: MDSCs recruiters (such as CCL2, CXCL8, SDF-1, and CXCL2) and MDSCs expanders (such as IL-6, PGE2, IL-10, VEGF, and GM-CSF) (Mi et al., 2020; Miyazaki et al., 2020). These cytokines result in the recruitment and expansion of MDSCs infiltrating the glioma microenvironment. There, MDSCs suppress mainly T cell and NK cell functions (Gieryng et al., 2017). This inhibition is triggered by multiple mechanisms including induction of oxidative stress, inhibition of T cell migration, expression of T cell inhibitory ligands, and depletion of critical T cell metabolites (Mi et al., 2020; Grabowski et al., 2021). The establishment of MDSCs as a major immunosuppressive population identifies them as a target for anti-glioma therapy.
Several immunotherapies are being evaluated in clinical trials to target the immunosuppressive and pro-tumoral myeloid cells. The large majority of these immunotherapies target the pro-tumoral myeloid cell recruitment to the glioma. Representatively, the chemoattractant molecules responsible for the myeloid cell migration to the glioma, for example, CSF-1R, avβ3/5 integrins, and CXCR4 are being targeted in multiple trials (Russo and Cappoli, 2018; Roesch et al., 2018). PLX3397 (ClinicalTrials.gov NCT identifiers: CNCT01349036 and NCT01790503) and BLZ945 (ClinicalTrials.gov NCT identifier: NCT02829723) are CSF-1R inhibitors currently being evaluated for their efficacy as glioblastoma treatments (Butowski et al., 2015; Colman et al., 2018). Unfortunately, patients treated with PLX3397 alone showed no significant improvement, nor did PLX3397 improve the results when combined with the current standard of care (SOC), temozolomide chemoradiotherapy. The trial utilizing BLZ945, alone and in combination with the PD-1 checkpoint receptor inhibitor PDR001, as a treatment of glioblastoma, is still ongoing. An inhibitor of avβ3 and avβ5 integrins, cilengitide, in combination with SOC has been evaluated in clinical trials (ClinicalTrials.gov NCT identifier: NCT00689221) and shown no survival benefits compared to SOC alone. CXCR4 inhibitor, AMD3100, is in clinical trials for treating GBM, and one completed trial of AMD3100 in combination with SOC (ClinicalTrials.gov NCT identifier: NCT01977677) showed an improvement in tumor control (Thomas et al., 2019). In addition to inhibiting the recruitment of myeloid cells, some immunotherapy agents are also being evaluated in trials to target the functions of GAMs and MDSCs (Roesch et al., 2018; Mi et al., 2020). For stimulating GAM anti-tumoral activity, the small molecule inhibitor of STAT3, WP1066, is being evaluated in clinical trials (ClinicalTrials.gov NCT identifiers: NCT01904123 and NCT04334863). Research has shown that WP1066 causes GAM upregulation of costimulatory molecules CD80 and CD86, but no results have been released for the WP1066 clinical trials (Hussain et al., 2007). Studies have shown that COX-2 inhibition suppresses MDSC activation, however COX-2 inhibition, via celecoxib, failed to show any favorable outcome in clinical settings (ClinicalTrials.gov NCT identifier: NCT00112502) (Penas-Prado et al., 2015; Mi et al., 2020).
Role of T-Cells
T cells are the largest group of lymphocytes infiltrating the glioma microenvironment and are key factors for effective anti-glioma immunity (Yang et al., 2010; Mohme and Neidert, 2020). The number of CD8 T cells infiltrating the glioma microenvironment is correlated with positive outcomes in clinical settings (Yang et al., 2010; Gieryng et al., 2017). The first step for initiation and development of anti-glioma CD8+ T cells, i.e., T cell priming, takes place in lymphoid tissues (cervical draining lymph nodes). The sequence of events begins with antigen loading on DCs and culminates with CD8+ T cell‐mediated tumor cytolysis (Dranoff, 2004; Farhood et al., 2019). DCs cross-present the tumor-associated antigen via MHC class I molecules to CD8+ T cells to trigger the formation of cytotoxic anti-glioma CD8+ T cells (CTLs) (Joffre et al., 2012; Spranger and Gajewski, 2018). The activated CTLs use two main pathways to induce target cell apoptosis, i.e., granule exocytosis and Fas ligand (FasL)‐mediated apoptosis (Voskoboinik et al., 2006; Anel et al., 1994; de Saint Basile et al., 2010; Hassin et al., 2011). The former is mediated by releasing granular proteins such as granzymes (Gzs)and perforin from the CTLs. The second mechanism is triggered by the FasL pathway which induces apoptosis through activation of caspases. This prompts the release of cytochrome c and other essential enzymes in the target cells. Other effector molecules being releases by the CTLs, such as interferon‐γ (IFN‐γ) and tumor necrosis factor α (TNF‐α), augment their cytotoxicity toward cancer cells [(Schoenborn and Wilson, 2007; Farhood et al., 2019)].
However, studies found that the majority of CD8+ T cells infiltrating the glioma microenvironment are in an exhausted, hypo-responsive state (Woroniecka et al., 2018). Exhaustion is a hypo-responsive T-cell state resulting from chronic antigenic exposure under sub-optimal conditions. This state represents a specific transcriptional program that is often characterized by up-regulation of various co-inhibitory receptors including PD-1, LAG-3, and TIM-3, on the T cells (Koyama et al., 2016; Kadiyala et al., 2020; Mohme and Neidert, 2020). These exhausted CD8+ T cells (CD8+ Tex cells) have lower secretion of their effector cytokines, but they still provide some, albeit reduced antitumor immunity (Woroniecka et al., 2018).
On the other hand, CD4+ T cells primarily mediate anti-tumor immunity by providing help for CD8+ CTL and antibody responses, as well as via secretion of effector cytokines such as interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα). CD4+ T cells are highly versatile, polyfunctional cells that can differentiate into one of several diverse functional subtypes (Tay et al., 2021). The most extensively studies subtype in glioma immunology is Tregs. While exhausted CD8 T cells are merely non-functional, Tregs acquire immunosuppressive function and have pro-tumor functions within the glioma microenvironment (Andaloussi and Lesniak, 2006; Mu et al., 2017). Tregs suppress the anti-tumor immune response directly via expression of immunosuppression inducing ligands, such as PD-L1 and CTLA-4, or indirectly via the release of anti-inflammatory cytokines, including IL-10 and TGF-β (Grabowski et al., 2021). Therefore, Tregs represent an attractive target to enhance glioma immunotherapy.
A variety of therapeutic approaches targeting the T cell compartment in gliomas are being evaluated for clinical testing. Monoclonal antibody-based therapies targeting the immune checkpoint molecules mainly PD-1, PD-L1, and CTLA-4 are being used to block the direct cell-to-cell contact-mediated inhibition of CD8+ T cells (Domingues et al., 2016; Wang et al., 2019). So far, clinical trials using the immune checkpoint inhibitors separately have shown inconsistent results in improving patient survival (Wang et al., 2019). For example, CheckMate 143 (ClinicalTrials.gov NCT identifier: NCT02017717) investigated the use of nivolumab, an anti-PD-1 monoclonal antibody, on patients with recurrent GBM, in a phase III clinical trial, but the trial was ended due to failure to improve overall survival compared to bevacizumab, an anti-VEGF therapy (Mitchell and Dey, 2019). However, this study prompted further investigation by showing the efficacy of nivolumab on the overall survival of a subset of recurrent GBM patients (Mitchell and Dey, 2019). A later phase II clinical trial (ClinicalTrials.gov NCT identifier: NCT02550249) demonstrated the safety of presurgical (neoadjuvant) nivolumab in a set of primary and recurrent GBM patients. Although no obvious clinical benefit was observed in recurrent GBM patients, 2 of the 3 primary patients treated with neoadjuvant nivolumab showed long-term survival (Schalper et al., 2019). Potential reasons for this lack of efficacy are low infiltration of CD8+ T cells in the tumor and several immune checkpoint molecules being expressed simultaneously. Several combination therapies are being tested to counteract these reasons for low efficacy encountered when using single-treatment modalities. These combination therapies utilize multiple checkpoint inhibitors along with DC-based vaccines to stimulate more CD8+ T cell recruitment (Wang et al., 2019; Miyazaki et al., 2020). ClinicalTrials.gov NCT identifier: NCT02529072 is an example of a recently tested DC-based vaccine combined with the nivolumab on recurrent gliomas. This study was terminated early due to adverse clinical effects, inefficacy of the combination and nivolumab alone treatments, and the failure of nivolumab alone to improve GBM survival (Peters et al., 2019). The combination immune checkpoint inhibitor therapies haven’t shown consistent efficacy yet, but more research has the potential to optimize the immune checkpoint inhibitor combinations to enhance clinical outcomes (Wang et al., 2019).
Role of Astrocytes
The brain microenvironment consists of multiple cell types, including the most abundant glial cell, astrocytes. Astrocytes constitute the majority of brain cells ∼50% of all brain cells and, therefore, they are expected to play a major role in GBM [(O'Brien et al., 2013; Gieryng et al., 2017)]. GBM cells activate tumor-associated astrocytes (TAAs) to enhance GBM invasion within healthy brain tissue (Guan et al., 2018; Henrik Heiland et al., 2019). TAAs are also involved in tumor progression and resistance to radiation and chemotherapy (Yang et al., 2014). Astrocytes themselves can become tumorigenic, which can be triggered by aberrant signaling in essential genes related to astrocyte development. For example, the nuclear factor kappa‐B (NF‐κB)‐ pathway has been shown to be involved in the transformation of normal astrocytes into TAAs which enhance GBM evasion (Figure 2; Kim et al., 2014). Recently, STAT‐3 was found to play a major role in the induction of reactive astrocytes that contribute to the metastatic melanoma to the brain (Priego et al., 2018). Moreover, astrocytes secrete factors that maintain the tight junctions of the blood-brain barrier (BBB) (Abbott et al., 2006; Alvarez et al., 2011; Heithoff et al., 2021), which in turn regulates the success or failure of metastatic cells extravasating the brain. Tumor cells within the brain make direct contact with astrocytes through gap junctions (Soroceanu et al., 2001; Horng et al., 2017), leading to increased chemoresistance (Chen et al., 2015). Astrocytes also promote the release of degradative enzymes, cytokines, chemokines, and growth factors, thereby promoting tumor cell proliferation, survival, and invasion (Brandao et al., 2019).
Distinct astrocytic phenotype exists in the tumor environment, which leads to a large release of anti-inflammatory cytokines such as TGFβ, IL10, and G-CSF through JAK/STAT pathway activation (Henrik Heiland et al., 2019). Inhibition of the JAK/STAT pathway shifts the balance of pro- and anti-inflammatory cytokines toward a pro-inflammatory environment. The complex interaction of astrocytes and microglial cells can further contribute to the immunosuppressive glioma TME, suggesting that tumor-associated astrocytes contribute to anti-inflammatory responses (Henrik Heiland et al., 2019). Studies show that astrocytes with activated sonic hedgehog (SHH) signaling pathways are localized mainly in the glioma perivascular niche (Becher et al., 2008). Moreover, dysregulation of SHH signaling leads to enhanced proliferation of precursor cells likely to be involved in initiating the formation of gliomas (Clement et al., 2007; Komada, 2012). Moreover, the dysregulation of CXCL12/CXCR4 signaling induces aberrant astrocyte proliferation, which could also contribute to glioma progression (Bonavia et al., 2003). In response to CNS injury, reactive astrocytes can secrete IL‐6, tumor growth factor‐β (TGF‐β), insulin growth factor‐1 (IGF‐1), and tumor necrosis factor‐α (TNF‐α), (Seike et al., 2011; Placone et al., 2016). Furthermore, GDF-15 overexpressed in reactive astrocytes caused an enhanced proliferation of GBM cells in vitro while GDF-15 deficient cells exhibit decreased in vivo tumor growth (Roth et al., 2010; Zamanian et al., 2012). Astrocytes can also promote the invasion of CD133 + GBM stem Cells in the brain microenvironment (Rath et al., 2013). Overall, these processes directly or indirectly cause astrocytes transformation and promote gliomagenesis.
Role of Cytokines
Cytokines are multifunctional molecules that control neo-angiogenesis, proliferation, invasion, and immune cell infiltration within the TME. Dysregulation of tumor-associated cytokine results in failure by the immune system to recognize tumor cells and thereby suppresses effective cell-mediated immunity (Zhu et al., 2012). Brain cells, tumor cells, and immune cells cross-talk through the complex cytokine network in the tumor microenvironment which promotes GBM progression (Iwami et al., 2011).
Glioma cells express multiple immune-suppressive cytokines such as transforming growth factor-beta (TGF-β), IL-10, IL-4, IL-6, and IL-13, all of which interfere directly or indirectly with anti-glioma immune response (Debinski et al., 1999; Gomez and Kruse, 2006; Perng and Lim, 2015; Brown et al., 2016; Harshyne et al., 2016). IL-33 has been shown to drive tumor progression, reduce overall survival, and orchestrate the GBM microenvironment to overcome resistance to immunotherapy (De Boeck et al., 2020). Both pro-inflammatory cytokines interleukin-6 (IL-6) and colony-stimulating factor-1 (CSF-1) trigger an immunosuppressive environment in GBM by inhibiting T-cell functions (Hao et al., 2002; Bender et al., 2010).
Despite the fact that most glioma-derived cytokines promote an inflammatory TME and are associated with glioma progression, some cytokines have been shown to have antitumor activity and have a therapeutic value. For example, IL-12 is endogenously secreted by antigen-presenting cells (APCs) which elicit adaptive cell-mediated immune responses (Heufler et al., 1996). However, despite the encouraging results of glioma eradication in mouse models, clinical studies using recombinant IL-12 in patients with advanced cancer were discontinued due to poor tolerability (Atkins et al., 1997; Leonard et al., 1997).
Another novel strategy used to target recurrent glioma is based on the differential expression of the IL-13 receptor by GBMs (IL13Rα2) that differs from the physiological IL4R/IL13R receptor expressed by normal cells. Adenovirus vector encoding mutated IL-13 (binds specifically to IL13Rα2) fused to active portion Pseudomonas exotoxin (Ad.mhIL-4. TRE.mhIL-13-PE) showed effective anti-GBM cytotoxicity, with minimal neurotoxicity (Kunwar, 2003; Hsieh and Lesniak, 2005; Mineharu et al., 2012). A similar strategy using a recombinant form of IL-13 fused to Pseudomonas Exotoxin A (IL13-PE38QQR) was introduced into GBM cells by convection-enhanced delivery (CED) method (Kunwar, 2003; Hsieh and Lesniak, 2005). This strategy is in multiple phase I/II clinical trials for the treatment of patients with recurrent malignant gliomas. Results from these trials indicated that local delivery of IL13-PE38QQR is safe and associated with an increase in median survival (Kunwar, 2003; Hsieh and Lesniak, 2005). Weiss et al. have recently developed fusion molecules that directly deliver cytokines to the tumors after systemic administration to promote immune responses. They combined immunostimulatory cytokines, such as IL2, IL12, or TNF, with an L19 antibody against GBM-specific fibronectin (Weiss et al., 2020). The resulting fusion immune-cytokines can be administered intravenously and accumulate in tumors, showing encouraging results in mouse models and a pilot study in human patients [NCT03779230]. Uncovering the modulatory role of glioma-derived suppressor factors and understanding the complex crosstalk between glioma cells and their microenvironment will assist in developing therapeutic strategies for glioma eradication.
Role of Oxidative Stress
Chronic inflammation is the leading cause of oxidative stress in astrocytes and microglia.(Salazar-Ramiro et al., 2016). Excessive ROS production can induce damage at different cellular processes, such as metabolism, signaling molecules, and most importantly at the genomic level (including mitochondrial and chromosomal DNA).(Redza-Dutordoir and Averill-Bates, 2016). Changes in cellular ROS is one of the hallmarks that favor GBM cells’ survival and proliferation and provide further protection from apoptosis (Salazar-Ramiro et al., 2016). Genetic lesions commonly found in glioma are the leading cause of oxidative stress and genetic instability. GBMs exhibit epidermal growth factor receptor (EGFR) amplification, phosphatase and tensin homolog (PTEN) mutation, and loss of chromosome 10 all of which shift the balance toward higher ROS production within the tumor microenvironment (Reardon et al., 2015). For instance, constant activation of EGFR enhances the formation of intracellular ROS in neoplastic cells (Miller et al., 2007). This increase in ROS further enhances the tyrosine autophosphorylation by EGFR (Weng et al., 2018). Similarly, ROS can oxidize the PTEN protein and induce the disulfide bond formation between Cys71 and Cys124 within the N-terminal phosphatase domain resulting in the inactivation of the PTEN tumor suppressor activity (Lee et al., 2002; Kwon et al., 2004). Also, several studies suggested that TP53 mutation drives tumor progression by sustaining an oncogenic oxidant intracellular environment through altering the signaling pathways and of redox-related enzymes. For example, a mutation in TP53 deactivates mitochondrial-super oxide dismutase-2 (SOD2), and glutathione peroxidase (GPx), causing the accumulation of O2− and H2O2, respectively (Hussain et al., 2004; Macedo et al., 2012; Cordani et al., 2020). All these processes induce aberration in the interstitial microenvironment and can drive glioma progression through excessive ROS production by tumor-infiltrating immune cells (Li et al., 2013). ROS-induced DNA damage triggers the release of a classical DAMP i.e. high-mobility group 1 (HMGB1) molecule which is released after oxidation of cysteine residues promoting genomic instability in GBM cells (Kang et al., 2013). TLRs can bind to the released HMGB1 and signal via the NF-κB, pathway causing enhanced production of pro-inflammatory cytokines, exacerbating ROS production in GBM cells [(Galdiero et al., 2013; Gieryng et al., 2017)].
The presence of ROS within the glioma TME can increase the immunosuppressive phenotype of immune cells infiltrating the TME, which interferes with effective immunotherapy. This immunosuppressive effect can be direct or indirect. Examples of direct ROS-mediated immunosuppression include the accumulation of Tregs (Kachikwu et al., 2011), M2-macrophages (Tsai et al., 2007), as well as interfering with dendritic cell activation and maturation (Kim et al., 2007). The indirect effect of ROS-mediated immunosuppressive TME includes the upregulation of PD-L1 by the tumor cells (Bailly, 2020; Raninga et al., 2020) as well enhanced expression of cytokines and chemokines that will recruit immunosuppressive myeloid cells (Bianchi et al., 2014; Eckert et al., 2018).
Studies also revealed that lower amount of nitric oxide (NO) produced through inducible nitric oxide synthase (iNOS/NOS2) in various tumors, including gliomas, can promote angiogenesis, invasion, and cellular proliferation (Fahey and Girotti, 2019; Maccallini et al., 2020). iNOS is a key regulator of glioma transformation downstream of the EGFRvIII/STAT3 signaling pathway. STAT3 directly binds to the promoter of the iNOS and thereby stimulates its expression (Jahani-Asl and Bonni, 2013). Moreover, iNOS-derived NO in glioma cells elicits resistance to various therapies including 5-aminolevulinic acid (ALA)-based photodynamic therapy (PDT) and endows gliomas of greater proliferation and aggressiveness (Fahey and Girotti, 2019; Maccallini et al., 2020). Several lines of evidence have showed that decreased proliferation of glioma cells could be mediated by selective inhibition of iNOS such as 1,400 W (N-(3-(aminomethyl) benzyl) acetamidine) (Fedorov et al., 2003) or mercapto-ethyl guanidine (MEG) (Southan et al., 1996) which inhibit the iNOS activity.
Generally, the CNS is composed of high fatty acids content which contributes to its high metabolic activity, making it particularly sensitive to oxidative damages by ROS (Panov et al., 2014). Neurons and astrocytes are equipped with antioxidant systems such as glutathione (GSSG-GSH) that defend these cells from oxidative damage. However, due to the high expression of SOD and catalase enzymes in astrocytes, there is an accelerated conversion of superoxide to hydrogen peroxide in these cells (Dokic et al., 2012). This makes astrocytes particularly sensitive to damage induced by ROS, leading to genetic instability and exacerbating neuroinflammation. The negative regulation of SOD-1 results in increased ROS production and enhanced phosphorylation of the signaling molecules Akt (Ghosh et al., 2010). This triggers rearrangement of the actin cytoskeleton downstream PI3K/Akt pathways; favoring the invasion and migration of GBM cells (Qian et al., 2005; Ghosh et al., 2010). Tumor cells exploit this inflammatory environment to invade, proliferate and sustain angiogenesis, by producing vascular endothelial growth factor (VEGF), Bv8, and MMP9 (Cao et al., 2013).
Immunotherapy in Glioma
The concept of tumor immunotherapy involves activation of the immune system to eradicate the tumor by stimulating effector components or blocking immune suppressors. Glioblastoma is a “cold tumor” in that it harbors a low mutational load with low infiltrating cytotoxic T-cells, making it difficult to mount an effective immune response. Moreover, glioma is highly infiltrated by immunosuppressive immune cells such as regulatory T cells, and myeloid cells that actively promote inflammatory microenvironment and suppress the effector anti-tumor immune response. Despite all these challenges, Immunotherapy for glioma is a promising area of active investigation (Dunn-Pirio and Vlahovic, 2017; Kamran et al., 2018). Various preclinical studies have demonstrated the success of immunotherapy-based approaches in animal models and many phase I and II clinical trials have shown immunotherapy to be safe and in some cases lead to improved progression-free survival (PFS) (Phuphanich et al., 2013; Bloch et al., 2014; Mitchell et al., 2015). Below, we provide an overview of the immunological approaches which yielded promising results in the preclinical setting and are currently being tested in the clinic. A list of these trials are summarized in Table 1.
CAR-T Cells
Adoptive transfer of chimeric antigen receptor (CAR) T cells is a promising immunotherapy glioma treatment (Migliorini et al., 2018; Akhavan et al., 2019). CAR T cells are T cells that have been removed from the patients and modified to have tumor antigen-binding receptor specificity before adoptively transferring them back to the patient (Akhavan et al., 2019). This therapy increases the ability of T cells to recognize and target cancerous cells. The current CAR T cells in clinical trials target IL-13Rα2, epidermal growth factor receptor variant III (EGFRvIII), and human epidermal growth factor receptor 2 (HER2) (Migliorini et al., 2018). These targets were selected due to their expression by glioma cells and negligible expression by normal brain cells since CAR T cell targeting of normal brain cells could result in lethal levels of toxicity (Akhavan et al., 2019). The clinical trials, ClinicalTrials.gov NCT identifiers: NCT02208362, NCT01454596, NCT01109095, targeting IL-13Rα2, EGFRvIII, and HER2, respectively, provide useful insights on the immunotherapeutic ability of CAR T cells against gliomas (Migliorini et al., 2018). These trials showed the CAR T cells’: low toxicity, moderate ability to traffic to the glioma microenvironment, limited ability to target glioma cells, and inconsistent results in increasing overall survival (Migliorini et al., 2018; Akhavan et al., 2019; Wang et al., 2020). For example, HER2-specific CAR T cells, in the NCT01109095 trial, were used to treat 17 patients with progressive GBM (Ahmed et al., 2017). Of these, 8 patients had clinical benefits, and the overall survival of the cohort was 11.1 months post T cell infusion (Ahmed et al., 2017). Recently, several therapies have been designed to improve the efficacy of CAR T cells by combining them with checkpoint inhibitors, such as PD-1, CTLA4, and PD-L1, but the clinical trials are still ongoing and have not released results (Akhavan et al., 2019).
HSC Transplantation
Hematopoietic stem-cell transplantation (HSCT) refers to a procedure in which hematopoietic stem cells are infused to restore bone marrow function in cancer patients who receive harmful doses of cytotoxic drugs or radiation therapy. Studies showed HSCs infusion is associated with enhanced recruitment and migration of tumor-specific T-cells within the tumor bed (Flores et al., 2015). This effect is mediated by HSCs-derived cytokines such as CCL3 (MIP-1α) that facilitates the subsequent recruitment of activated tumor-specific T cells causing enhanced anti-glioma immune response (Flores et al., 2015; Bryukhovetskiy et al., 2016). HSCs may be obtained from the transplant recipient (autologous HSCT) or a donor (allogeneic HSCT) and harvested from bone marrow, peripheral blood, or umbilical cord blood shortly after delivery of neonates. An early study compared survival outcomes of 27 children with recurrent malignant astrocytomas who underwent myeloablative chemotherapy and autologous HSCT with a matched historical cohort (n = 56) that received standard chemotherapy following tumor recurrence (Finlay et al., 2008). The results of this study suggest myeloablative chemotherapy with autologous HSCT can produce long-term survival among children with recurrent malignant astrocytoma. Bouffet et al. reported on a series of 22 children and young adults with high-grade gliomas treated with autologous HSCT, the response rate was 29% with one complete and three partial responses (Bouffet et al., 1997). However, the authors concluded that overall survival using this procedure was no better than that reported with conventional treatments (Bouffet et al., 1997). . In conclusion, HSC therapy may extend glioma patients' survival particularly for those patients being treated with high dose chemotherapy regimens. However, the dose of chemotherapeutics used in this treatment regimen should be carefully balanced to avoid intolerance and toxic side effects (Brandes et al., 2001; Egan et al., 2016).
Gene Therapies and Virotherapies
Gene therapy is a therapeutic approach that involves the insertion of the genetic material into a tissue or organ to treat a variety of diseases, including solid cancers, such as brain tumors. These genetic elements include whole genes, oligonucleotides, or regulatory elements which are usually inserted into the target cells either by gene delivery vectors or liposomal-nanoparticle formulations. (Asad et al., 2017; Kamran et al., 2018). In glioma, gene therapy is an attractive approach due to the fact that its local administration can overcome the challenges needed to bypass the BBB, and therefore it may have lower systemic side effects (Okura et al., 2014). Albeit, its clinical application still presents many challenges (Lowenstein et al., 2009).
Among prevalent gene therapy approaches for cancer, suicide gene therapy is one of the most studied approaches for the treatment of glioma. Currently, there are around 20 Phase-I/II clinical trials testing the effectiveness of adenoviral vectors mediated suicide gene therapies in multiple subtypes of glioma. An ongoing Phase-I trial evaluates the safety and tolerability of Ad-TK in combination with systemic administration of the prodrug valacyclovir in combination with SOC and Nivolumab in patients with HGG (NCT03576612). The trial is still ongoing with no results posted.
Another example of a clinically applied conditional cytotoxic/suicidal approach is the delivery of the cytosine deaminase (CD) in cancer cells. CD is expressed mainly by yeast and bacteria but is absent in mammalian cells (Takahashi et al., 2014). GBM cells expressing CD will be susceptible to 5-fluorocytosine, due to it is the ability to convert it to its active form Fluorouracil (5-FU) (Okura et al., 2014; Takahashi et al., 2014). The initial Phase-I clinical trial of Toca 511, a replication-competent RV encoding CD, showed safety and good tolerability, with effective tumoricidal responses in patients with recurrent high-grade glioma (Cloughesy et al., 2018). However, a recent phase III clinical trial (NCT02414165) did not show improved overall survival compared to the SOC (Cloughesy et al., 2020).
Another attractive approach is immune stimulatory gene therapy which entails the expression of immune activators in glioma cells to enhance the anti-tumor immune response.
Two independent Phase-I trials (NCT02026271, NCT03330197) investigating the tolerability of Ad-RTS-hIL-12 in combination with veledimex (VDX) showed promising results (Chiocca et al., 2019). In a separate Phase-I trial, the safety of Ad-RTS-Hil-12/VDX is being tested in combination with Nivolumab (NCT03636477) (Garcia-Fabiani et al., 2020). In addition, a Phase-II trial is studying the inducible hIL-12 in combination with PD-1 antibody Libtayo (NCT04006119) (Garcia-Fabiani et al., 2020).
In an effort to overcome the shortcomings of monotherapies, combination therapies have been developed. We have pioneered the combination of Ad-Flt3L and Ad-TK (Castro et al., 2014; Kamran et al., 2018). In this therapeutic approach, adenoviral vectors encoding for herpes simplex type 1-thymidine kinase (TK) and FMS-like Tyrosine kinase 3 ligand (Flt3L) are delivered into the brain where glioma cell death is induced upon systemic ganciclovir treatment (Ali et al., 2005). This triggers the release of tumor-associated antigens and damage-associated molecular pattern molecules (DAMPs) into the tumor microenvironment, triggering an antitumor immune response (Kamran et al., 2018; Altshuler et al., 2020). Our results indicate that the release of DAMPs such as HMGB1 from Ad-TK infected tumor cells is required for the efficacy of Ad-TK + Ad-Flt3L mediated immunotherapy (Candolfi et al., 2009; Curtin et al., 2009). Flt3L increases the migration and infiltration of DCs into the TME. This glioma infiltrating DCs can phagocytose antigens that are released during TK-induced glioma cell death (Curtin et al., 2009; Candolfi et al., 2012). Moreover, HMGB1 released from dying tumor cells activates DCs through stimulation of TLR2 pathway. These activated DCs then transport the tumor antigens to the draining lymph nodes to generate T-cell mediated cytotoxic immune responses against tumor cells (Curtin et al., 2009). This strategy effectively eradicated the tumor in multiple glioma models and granted long-term survival in tumor-bearing mice (Mineharu et al., 2011). Results from Phase-I dose-escalating trial (NCT01811992) showed well-tolerability and safety when giving a combination of AdVs expressing TK and Flt3L. There was also higher infiltrating DCs and lymphoid cells within the TME patients receiving the gene therapy (Lowenstein et al., 2019).
Dendritic Cells Vaccines
Dendritic Cells (DCs) are professional antigen-presenting cells (APC) that are able to engulf antigenic proteins, process peptides, and traffic to the draining lymph nodes (dLNs) where they present antigens to naive T-cells (Palucka and Banchereau, 1999). This process leads to induction and activation of effector T-cell, the goal standard for anti-tumor immune response. Administration tumor antigen pulsed-DCs has been validated in multiple solid tumors such as melanoma, prostate cancer, and renal cell carcinoma (Schadendorf et al., 2006; Su et al., 2003; Anguille et al., 2014). In glioma, DCs therapy represents an attractive approach especially because of the paucity of professional DCs in GBM (Eagles et al., 2018; Srivastava et al., 2019). Preclinical models have shown promising results for DCs treatment in combination with PD-1 blockade (Antonios et al., 2016). In addition, phase I clinical trial revealed that the combination of TMZ with DCs therapy is safe and tolerable in GBM patients (Vik-Mo et al., 2013; Hunn et al., 2015; Akasaki et al., 2016). Moreover, autologous DCs pulsed with autologous tumor lysate (DCVax) are currently being investigated in phase III clinical trials for newly diagnosed GBM (NCT00045968). Beside the direct administration of autologous DCs to glioma patients, the activation and recruitment of endogenous DCs in vivo have been shown to be safe and effective in glioma patients. This is illustrated by infusion of TLR ligands, or by intratumoral injection of Flt3L which causes robust DCs expansion and activation (Hou et al., 2008; Belderbos et al., 2019; Jones et al., 2010). Another trial tests the efficacy of DCs loaded with glioma-associated protein (pp65) along with the lysosomal associated protein (LAMP) (CMV-pp65-LAMP mRNA-loaded DCs) in combination with the TMZ and GM-CSF. This study showed increased progression-free survival (PFS) and OS, and upregulation in IFNγ levels (Batich et al., 2017) (NCT00639639). These trials showed that DCs therapy is safe, and well-tolerated in glioma patients and elicited a minimal inflammatory response. Future trials will further reveal the effectiveness of this treatment on patients with recurrent GBM and its impact on overall survival.
Peptide Vaccines
Peptide vaccines are developed based on peptides derived from tumor-associated antigens (TAAgs) and used to induce effective anti-tumor T-cell response (Lee et al., 2020). Some of the TAAgs tested in glioma, survivin, WT-1 protein, EphrinA2, and IL13RA2, have been investigated as potential peptide vaccines (NCT04013672, NCT03149003, NCT02078648); however, therapeutic efficacy has been limited (Platten et al., 2018). The low efficacy of TAAgs vaccines could be attributed to the tolerance mechanisms to self-antigens, which also implies the risks for off-target toxicity (Hollingsworth and Jansen, 2019). Alternatively, neoantigens that are exclusively expressed in tumor cells can be employed in peptide vaccines. The major challenge for glioma lies in the epitope selection: it harbors only 30–50 non-synonymous mutations per megabase in glioblastomas, and roughly 1–3% of the mutations result in immunogenic neoantigens (Schumacher and Schreiber, 2015; Johanns and Dunn, 2017). Despite the low mutational burden, some shared neoepitopes, such as EGFRvIII and IDH1R132H, are identified, and corresponding vaccines have been extensively evaluated in clinical trials (Weller et al., 2017; Platten et al., 2018; Reardon et al., 2020). Recently, a peptide vaccine targetting the most common mutation in glioma IDH1m (R132H) has been developed and showed promising results in Phase-I clinical trial (NCT02454634) (Platten et al., 2021). Overall, neoantigen vaccines demonstrate good safety and immunogenicity, but the response rate and survival benefits are marginal. It has been recognized that neoantigens are usually unique, and the subclonal expression would lead to spontaneous antigen loss, opening avenues for immune escape and tumor progression (Lim et al., 2018).
High-Density Lipoprotein Nanoparticle Vaccines
The tantalizing results and heterogeneity of neoantigen expression emphasize the need for the development of personalized vaccination. Currently, neoantigen identification is based on the whole-exome sequencing (WES), followed by computational predications of the antigenicity through HLA binding (Kreiter et al., 2015), which can be further confirmed by the transcriptome analysis with mass spectrometry (Yadav et al., 2014). In particular, Kuai, et al. have developed a sHDL nanodisc composed of phospholipids and apolipoprotein A1-mimetic peptides for neoantigen vaccination (Kuai et al., 2017). After coupling with Cytosine-phosphorothioate-guanine CpG oligonucleotide, a TLR-9 agonist, nanodiscs promoted co-delivery of Ag/CpG to lymphoid organs, eliciting strong neoantigen-specific anti-tumor CD8+ T-cell responses in murine models of melanoma and colon carcinoma. Taking one step further, Scheetz et al. developed the personalized peptide vaccine against gliomas with the sHDL nanodisc platform (Scheetz et al., 2020). Three neoantigens of GL261 tumor cells were screened by in silico MHC-I affinity prediction algorithms and subsequent immunogenicity verification in tumor-bearing mice. Selected peptides were loaded to nanodiscs together with CpG. Immunizing mice with the vaccine plus anti-PD-L1 therapy improved the survival rate to approximately 90% in the subcutaneous GL261 tumor model, and it showed 33% complete tumor regression in the orthotopic model, which represented a significant survival advantage compared with soluble neoantigens plus anti-PD-L1 treatments. Nanodisc vaccination elicited robust IFN-γ+ T cell response against all three epitopes, and the addition of anti-PD-L1 further augmented the T cell responses. TME analyses on CNS tumors showed an increased frequency of CD8+ T cells with lower levels of PD-1 expression. Meanwhile, compared with the PBS group, lower Tregs frequency and higher M1:M2-like macrophage ratio was observed in tumor tissues, suggesting a reversed immunosuppressive milieu. In parallel, the therapeutic efficacy of nanodiscs with either mIDH1123–132 or mIDH1126–141 neoantigens was evaluated in a genetically engineered mIDH1 murine glioma model. The results showed that nanodiscs vaccination significantly suppressed tumor growth and extended the survival rate. All survived mice showed resistance to tumor recurrence upon rechallenge with mIDH1 neurospheres in the contralateral hemisphere.
Aside from TAAs and neoantigens, cancer stem cells (CSCs) and other non-neoplastic compartments may serve as potential targets for gliomas. Nanodiscs delivering CSC-derived ALDH1-A1 and -A3 peptides have been shown to induce potent anti-tumor activity and prolong animal survival in multiple mouse models (Hassani Najafabadi et al., 2020). Overall, sHDL nanodisc serves as a versatile platform with potent anti-tumor efficacy.
Other Immunotherapies
Immune checkpoint inhibitors targeting the program death receptors and its ligand (PD-1/PD-L1) and T lymphocyte-associated antigen-4 (CTLA-4) have significantly enhanced immunotherapy efficacy for selected solid tumors (Ferris et al., 2010; Ferris et al., 2016; Bouffet et al., 2016). However, CNS tumors at best have a moderate response to current immunotherapies (Kurtulus et al., 2015; Bouffet et al., 2016). Recently, there has been increasing interest in alternative immunotherapy strategies including the synergy between PD1, TIM3, and LAG3 blockade and CAR T cells therapy (NCT02661282) for the treatment of gliomas [(Woo et al., 2012; Ahmed et al., 2017; Kim et al., 2017; Chheda et al., 2018)]. Recently, there have been advances with an alternative immune checkpoint using a novel immune checkpoint inhibitor peptide ligand. The CD200 immune checkpoint is modulated by an inhibitory receptor (CD200R1) and multiple activation receptors (CD200AR) (Wright et al., 2000; Gorczynski et al., 2000; Wright et al., 2003; Gorczynski et al., 2004; Wong et al., 2012; Damiani et al., 2015). Rigorous studies by several groups have provided evidence that targeting the CD200 checkpoint enhances immunotherapy efficacy (Kretz-Rommel et al., 2007; Copland et al., 2007; Gorczynski et al., 2011; Rygiel et al., 2012; Gorczynski et al., 2013). To this end, a monoclonal antibody against the CD200 protein (Samalizumab) (Adler, 2010) was evaluated in a clinical trial in 2008 for relapsed refractory B-cell chronic lymphocytic leukemia (B-CLL) and multiple myeloma (NCT00648739) (Adler, 2010). Alexion reported that 95% of the patients with B-CLL had up to 98% reduction in CD200+CD4+ T cells. The loss of these immune cells created an immunocompromized state and may be the reason why only 36% of the patients had a 10% reduction in bulk disease. Investigators have taken a new approach for improving immunotherapies by targeting the CD200ARs on antigen-presenting cells with a peptide ligand. They reported that the peptide ligand activates the immune system enhancing cytokine/chemokine production, Co-stimulatory molecules CD80/CD86 and MHC-II enhancing an antigen response while downregulating the inhibitory CD200R1 and PD-1 (Moertel et al., 2014; Xiong et al., 2016; Xiong et al., 2019). They reported that treatment with autologous tumor lysate given concomitantly with the peptide ligand significantly enhanced survival in a spontaneous canine high-grade glioma trial with pet-owned dogs (Olin et al., 2019) and recently initiated a phase I adult glioblastoma recurrent trial (NCT04642937) (Table 1).
Prospects and Conclusion
In this review, we discuss multiple mechanisms by which gliomas induce the development of an immunosuppressive TME and evade immune surveillance. Various components within the tumor microenvironment such as secreted immune-modulating factors, hypoxia, and oxidative stress result in activation of the surrounding astrocytes and microglia to an inflammatory state. This suppressive microenvironment is further enhanced by infiltrating immunosuppressive myeloid cells, immunosuppressive macrophages, exhausted T cells, and Tregs which contribute to a tumor-supportive TME, promoting glioma cell growth and invasion. We also discuss recent advances in immunotherapeutic strategies that if used in combination could provide promising novel approaches that could elicit clinical benefit. These include adding immune checkpoint blockade to immune-mediated gene therapies, virotherapy, CAR-T cells, dendritic cells’ vaccines, or nanoparticle-mediated vaccination technologies.
Despite the advances in treatment regimen in multiple solid tumors in recent years, the SOC for glioma has not changed since 2005 with the inclusion of TMZ. The prognosis for GBM remains dismal with no major improvements in the median or overall survival. Major challenges need to be overcome in gliomas, such as tumor heterogeneity, the immunosuppressive TME (MDSCs, TAMS, Tregs), the low mutational burden, and the antigen heterogeneity in order to elicit effective immunotherapies. Further studies are crucial to delineate the complex mechanisms and interactions between tumor cells, immune cells, tumor stroma, resident brain cells, and the brain and tumor vasculature.
Over the past 2 decades, a pressing need to deeply profile the glioma TME has led investigators to integrate data obtained from traditional approaches with those obtained with new, single-cell technologies, including high parameter mass cytometry, single-cell sequencing, and high-resolution imaging. These advances have enabled us to investigate in detail the complexity of the TME and to interrogate in-depth previously unexplored tumor-infiltrating cell types. This expanded our understanding of the multifaceted tumor ecosystems and allowed us to profile cellular heterogeneity, dynamicity and plasticity, and complex cell-cell interactions.
Current open clinical trials testing immunotherapy in GBM could provide promising avenues for gene therapy, CAR-T cells as well as vaccine therapies in various combinatorial treatment strategies. These combinatorial approaches which have proven beneficial in the preclinical setting could prove particularly valuable in the clinical arena, when targeting multiple immune checkpoints or when adding cytokine therapy, likely to result in better outcomes. Furthermore, recent results from trials of neoadjuvant immune checkpoint blockade provide a new cause for optimism as we work to decode mechanisms for treatment resistance. On the other hand, an increased understanding of the possible indicators of side effects of immunotherapies will lead to improved clinical care and make the application of immunotherapies far safer and well-tolerated for glioma patients. Despite formidable challenges, with the advent of single-cell transcriptomic analysis (Wang et al., 2019; Gibellini et al., 2020; Mathewson et al., 2021), high parameter mass cytometry (Hartmann et al., 2019; Wang et al., 2019; Gohil et al., 2021), and high-resolution Hyperion imaging technologies (Carvajal-Hausdorf et al., 2019; Xie et al., 2020), the glioma immunotherapy field is undergoing unprecedented growth in the discovery of novel therapeutic targets and interventions, that we envisage will lead to effective novel treatments for this devastating brain cancer in the near future.
Author Contributions
MSA, BM, CSH, SH, SF, RT, AD, KB, MO, JM, AS, AM, PL, and MC wrote the manuscript, with overall guidance, revisions, and edits from PL and MC. CSH and SC prepared Figures. MSA, PL, MC reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the National Institutes of Health/National Institute of Neurological Disorders andamp; Stroke (NIH/NINDS) Grants R21-NS091555, R37NS094804, and R01NS074387 to MC; R01NS076991, R01NS082311, and R01NS096756 to PL; Rogel Cancer Center Scholar Award and Forbes Senior Research Scholar Award to MC; National institutes of Health/National Institute of Biomedical Imaging and Bioengineering (NIH/NIBIB) Grant R01-EB022563 to JM, PL, and MC; University of Michigan M-Cube; the Department of Neurosurgery; the University of Michigan Rogel Comprehensive Cancer Center; the Pediatric Brain Tumor Foundation (BTF), Leah’s Happy Hearts Foundation, The Chad Tough Foundation (Ian's Friends Foundation (IFF)), and the Biosciences Initiative in Brain Cancer to MC and PL. NIH/NCI T32- CA009676 Post-Doctoral Fellowship to MSA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Karin Muraszko for her academic leadership and Marta Edwards, Brandye Hill, and Katherine Wood for administrative and technical assistance.
References
Abbott, N. J., Rönnbäck, L., and Hansson, E. (2006). Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53. doi:10.1038/nrn1824
Adler, I. (2010). Alexion Reports Results from First Clinical Trial of Novel Anti-Cancer Antibody Samalizumab at ASH Annual Meeting. http://news.alexionpharma.com/press-release/product-news/alexion-reports-results-first-clinical-trial-novel-anti-cancer-antibody.
Ahmed, N., Brawley, V., Hegde, M., Bielamowicz, K., Kalra, M., Landi, D., et al. (2017). HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma. JAMA Oncol. 3, 1094–1101. doi:10.1001/jamaoncol.2017.0184
Akasaki, Y., Liu, G., Chung, N. H. C., Ehtesham, M., Black, K. L., and Yu, J. S. (2004). Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J. Immunol. 173, 4352–4359. doi:10.4049/jimmunol.173.7.4352
Akasaki, Y., Kikuchi, T., Homma, S., Koido, S., Ohkusa, T., Tasaki, T., et al. (2016). Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol. Immunother. 65, 1499–1509. doi:10.1007/s00262-016-1905-7
Akhavan, D., Alizadeh, D., Wang, D., Weist, M. R., Shepphird, J. K., and Brown, C. E. (2019). CAR T cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 290, 60–84. doi:10.1111/imr.12773
Akhurst, R., and Hata, A. (2012). Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 11, 791–811. doi:10.1038/nrd3810
Alghamri, A. R., Thalla, R., Kamran, N., Zhang, L., Mohd Faisal, S., Ventosa, M., et al. (2020). Castro MG G-CSF Secreted by Epigenetically Reprogrammed Mutant IDH1 Glioma Stem Cells Reverses the Myeloid Cells'-Mediated Immunosuppressive Tumor Microenvironment. BioRxiv 2020, 215954. doi:10.1101/2020.07.22.215954
Ali, S., King, G. D., Curtin, J. F., Candolfi, M., Xiong, W., Liu, C., et al. (2005). Combined Immunostimulation and Conditional Cytotoxic Gene Therapy Provide Long-Term Survival in a Large Glioma Model. Cancer Res. 65, 7194–7204. doi:10.1158/0008-5472.can-04-3434
Allavena, P., Sica, A., Solinas, G., Porta, C., and Mantovani, A. (2008). The Inflammatory Micro-Environment in Tumor Progression: The Role of Tumor-Associated Macrophages. Crit. Rev. Oncology/Hematology 66, 1–9. doi:10.1016/j.critrevonc.2007.07.004
Altshuler, D. B., Kadiyala, P., Nuñez, F. J., Nuñez, F. M., Carney, S., Alghamri, M. S., et al. (2020). Prospects of biological and synthetic pharmacotherapies for glioblastoma. Expert Opinion Biol. Ther. 20, 305–317. doi:10.1080/14712598.2020.1713085
Alvarez, J. I., Dodelet-Devillers, A., Kebir, H., Ifergan, I., Fabre, P. J., Terouz, S., et al. (2011). The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731. doi:10.1126/science.1206936
Andaloussi, A. E., and Lesniak, M. S. (2006). An Increase in CD4+CD25+FOXP3+ Regulatory T Cells in Tumor-Infiltrating Lymphocytes of Human Glioblastoma Multiforme1. Neuro-oncology 8, 234–243. doi:10.1215/15228517-2006-006
Anel, A., Buferne, M., Boyer, C., Schmitt-Verhulst, A.-M., and Golstein, P. (1994). T Cell Receptor-Induced Fas Ligand Expression in CYtotoxic T Lymphocyte Clones Is Blocked by Protein Tyrosine Kinase Inhibitors and Cyclosporin A. Eur. J. Immunol. 24, 2469–2476. doi:10.1002/eji.1830241032
Anguille, S., Smits, E. L., Lion, E., van Tendeloo, V. F., and Berneman, Z. N. (2014). Clinical use of Dendritic Cells for Cancer Therapy. Lancet Oncol. 15, e257–e267. doi:10.1016/s1470-2045(13)70585-0
Antonios, J. P., Soto, H., Everson, R. G., Orpilla, J., Moughon, D., Shin, N., et al. (2016). PD-1 Blockade Enhances the Vaccination-Induced Immune Response in Glioma. JCI Insight 1. doi:10.1172/jci.insight.87059
Asad, A. S., Moreno Ayala, M. A., Gottardo, M. F., Zuccato, C., Nicola Candia, A. J., Zanetti, F. A., et al. (2017). Viral Gene Therapy for Breast Cancer: Progress and Challenges. Expert Opinion Biol. Ther. 17, 945–959. doi:10.1080/14712598.2017.1338684
Atkins, M. B., Robertson, M. J., Gordon, M., Lotze, M. T., DeCoste, M., DuBois, J. S., et al. (1997). Phase I Evaluation of Intravenous Recombinant Human Interleukin 12 in Patients With Advanced Malignancies. Clin. Cancer Res. 3, 409–17.
Bailly, C. (2020). Regulation of PD-L1 Expression on Cancer Cells With ROS-Modulating Drugs. Life Sci. 246, 117403. doi:10.1016/j.lfs.2020.117403
Batich, K. A., Reap, E. A., Archer, G. E., Sanchez-Perez, L., Nair, S. K., Schmittling, R. J., et al. (2017). Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 23, 1898–1909. doi:10.1158/1078-0432.ccr-16-2057
Becher, O. J., Hambardzumyan, D., Fomchenko, E. I., Momota, H., Mainwaring, L., Bleau, A.-M., et al. (2008). Gli Activity Correlates With Tumor Grade in Platelet-Derived Growth Factor-Induced Gliomas. Cancer Res. 68, 2241–2249. doi:10.1158/0008-5472.can-07-6350
Belderbos, R. A., Aerts, J. G. J. V., and Vroman, H. (2019). Enhancing Dendritic Cell Therapy in Solid Tumors with Immunomodulating Conventional Treatment. Mol. Ther. - Oncolytics 13, 67–81. doi:10.1016/j.omto.2019.03.007
Belykh, E., Shaffer, K. V., Lin, C., Byvaltsev, V. A., Preul, M. C., and Chen, L. (2020). Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 10. doi:10.3389/fonc.2020.00739
Bender, A. M., Collier, L. S., Rodriguez, F. J., Tieu, C., Larson, J. D., Halder, C., et al. (2010). Sleeping beauty-mediated somatic mutagenesis implicates CSF1 in the formation of high-grade astrocytomas. Cancer Res. 70, 3557–3565. doi:10.1158/0008-5472.can-09-4674
Bergers, G., and Benjamin, L. E. (2003). Tumorigenesis and the angiogenic switch. Nature Rev. Cancer 3. doi:10.1038/nrc1093
Bianchi, G., Vuerich, M., Pellegatti, P., Marimpietri, D., Emionite, L., Marigo, I., et al. (2014). ATP/P2X7 Axis Modulates Myeloid-Derived Suppressor Cell Functions in Neuroblastoma Microenvironment. Cell Death Dis. 5, e1135. doi:10.1038/cddis.2014.109
Bloch, O., Crane, C. A., Fuks, Y., Kaur, R., Aghi, M. K., Berger, M. S., et al. (2014). Heat-Shock Protein Peptide Complex-96 Vaccination for Recurrent Glioblastoma: A Phase II, Single-Arm Trial. Neuro-Oncology 16, 274–279. doi:10.1093/neuonc/not203
Bonavia, R., Bajetto, A., Barbero, S., Pirani, P., Florio, T., and Schettini, G. (2003). Chemokines and Their Receptors in the CNS: Expression of CXCL12/SDF-1 and CXCR4 and Their Role in Astrocyte Proliferation. Toxicol. Lett. 139, 181–189. doi:10.1016/s0378-4274(02)00432-0
Bouffet, E., Mottolese, C., Jouvet, A., Philip, I., Frappaz, D., Carrie, C., et al. (1997). Etoposide and Thiotepa Followed by Abmt (Autologous Bone Marrow Transplantation) in Children and Young Adults With High-Grade Gliomas. Eur. J. Cancer 33, 91–95. doi:10.1016/s0959-8049(96)00369-3
Bouffet, E., Larouche, V., Campbell, B. B., Merico, D., de Borja, R., Aronson, M., et al. (2016). Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. Jco 34, 2206–2211. doi:10.1200/jco.2016.66.6552
Brandao, M., Simon, T., Critchley, G., and Giamas, G. (2019). Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 67, 779–790. doi:10.1002/glia.23520
Brandes, A. A., Palmisano, V., Pasetto, L. M., Basso, U., and Monfardini, S. (2001). High-dose chemotherapy with bone marrow rescue for high-grade gliomas in adults. Cancer Invest. 19, 41–48. doi:10.1081/cnv-100000084
Brandes, A. A., Franceschi, E., Tosoni, A., Blatt, V., Pession, A., Tallini, G., et al. (2008). MGMTPromoter Methylation Status Can Predict the Incidence and Outcome of Pseudoprogression After Concomitant Radiochemotherapy in Newly Diagnosed Glioblastoma Patients. Jco 26, 2192–2197. doi:10.1200/jco.2007.14.8163
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, J. R., Naranjo, A., et al. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 375, 2561–2569. doi:10.1056/nejmoa1610497
Brown, T. J., Brennan, M. C., Li, M., Church, E. W., Brandmeir, N. J., Rakszawski, K. L., et al. (2016). Association of the Extent of Resection With Survival in Glioblastoma. JAMA Oncol. 2, 1460–1469. doi:10.1001/jamaoncol.2016.1373
Brown, N. F., Carter, T. J., Ottaviani, D., and Mulholland, P. (2018). Harnessing the Immune System in Glioblastoma. Br. J. Cancer 119, 1171–1181. doi:10.1038/s41416-018-0258-8
Bryukhovetskiy, I. S., Dyuizen, I. V., Shevchenko, V. E., Bryukhovetskiy, A. S., Mischenko, P. V., Milkina, E. V., et al. (2016). Hematopoietic Stem Cells as a Tool for the Treatment of Glioblastoma Multiforme. Mol. Med. Rep. 14, 4511–4520. doi:10.3892/mmr.2016.5852
Butowski, N., Colman, H., De Groot, J. F., Omuro, A. M., Nayak, L., Wen, P. Y., et al. (2015). Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro. Oncol. 18, 557–564. doi:10.1093/neuonc/nov245
Candolfi, M., Yagiz, K., Foulad, D., Alzadeh, G. E., Tesarfreund, M., Muhammad, A. K. M. G., et al. (2009). Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin. Cancer Res. 15, 4401–4414. doi:10.1158/1078-0432.ccr-09-0155
Candolfi, M., King, G. D., Yagiz, K., Curtin, J. F., Mineharu, Y., Muhammad, A. G., et al. (2012). Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia 14, 757–IN26. doi:10.1593/neo.12794
Cao, Z., Shang, B., Zhang, G., Miele, L., Sarkar, F. H., Wang, Z., et al. (2013). Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1836, 273–286. doi:10.1016/j.bbcan.2013.08.001
Carvajal-Hausdorf, D. E., Patsenker, J., Stanton, K. P., Villarroel-Espindola, F., Esch, A., Montgomery, R. R., et al. (2019). Multiplexed (18-Plex) Measurement of Signaling Targets and Cytotoxic T Cells in Trastuzumab-Treated Patients using Imaging Mass Cytometry. Clin. Cancer Res. 25, 3054–3062. doi:10.1158/1078-0432.ccr-18-2599
Castro, M. G., Candolfi, M., Wilson, T. J., Calinescu, A., Paran, C., Kamran, N., et al. (2014). Adenoviral vector-mediated gene therapy for gliomas: coming of age. Expert Opinion Biol. Therapy 14, 1241–1257. doi:10.1517/14712598.2014.915307
Ceccarelli, M., Barthel, F. P., Malta, T. M., Sabedot, T. S., Salama, S. R., Murray, B. A., et al. (2016). Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 164, 550–563. doi:10.1016/j.cell.2015.12.028
Chang, A. L., Miska, J., Wainwright, D. A., Dey, M., Rivetta, C. V., Yu, D., et al. (2016). CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 76, 5671–5682. doi:10.1158/0008-5472.can-16-0144
Chen, Z., and Hambardzumyan, D. (2018). Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 9. doi:10.3389/fimmu.2018.01004
Chen, W., Wang, D., Du, X., He, Y., Chen, S., Shao, Q., et al. (2015). Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med. Oncol. 32, 43. doi:10.1007/s12032-015-0487-0
Chen, R., Keoni, C., Waker, C. A., Lober, R. M., Chen, Y.-H., and Gutmann, D. H. (2019). KIAA1549-BRAF Expression Establishes a Permissive Tumor Microenvironment Through NFκB-Mediated CCL2 Production. Neoplasia 21, 52–60. doi:10.1016/j.neo.2018.11.007
Chheda, Z. S., Kohanbash, G., Okada, K., Jahan, N., Sidney, J., Pecoraro, M., et al. (2018). Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J. Exp. Med. 215, 141–157. doi:10.1084/jem.20171046
Chiocca, E. A., Yu, J. S., Lukas, R. V., Solomon, I. H., Ligon, K. L., Nakashima, H., et al. (2019). Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: Results of a phase 1 trial. Sci. Transl. Med. 11. doi:10.1126/scitranslmed.aaw5680
Clement, V., Sanchez, P., de Tribolet, N., Radovanovic, I., and Ruiz i Altaba, A. (2007). HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 17, 165–172. doi:10.1016/j.cub.2006.11.033
Cloughesy, T. F., Landolfi, J., Vogelbaum, M. A., Ostertag, D., Elder, J. B., Bloomfield, S., et al. (2018). Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro. Oncol. 20, 1383–1392. doi:10.1093/neuonc/noy075
Cloughesy, T. F., Petrecca, K., Walbert, T., Butowski, N., Salacz, M., Perry, J., et al. (2020). Effect of Vocimagene Amiretrorepvec in Combination With Flucytosine vs Standard of Care on Survival Following Tumor Resection in Patients With Recurrent High-Grade Glioma. JAMA Oncol. 6, 1939–1946. doi:10.1001/jamaoncol.2020.3161
Colman, H., Raizer, J. J., Walbert, T., Plotkin, S. R., Chamberlain, M. C., Wong, E. T., et al. (2018). Phase 1b/2 study of pexidartinib (PEX) in combination with radiation therapy (XRT) and temozolomide (TMZ) in newly diagnosed glioblastoma. Jco 36, 2015. doi:10.1200/jco.2018.36.15_suppl.2015
Copland, D. A., Calder, C. J., Raveney, B. J. E., Nicholson, L. B., Phillips, J., Cherwinski, H., et al. (2007). Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am. J. Pathol. 171, 580–588. doi:10.2353/ajpath.2007.070272
Cordani, M., Butera, G., Pacchiana, R., Masetto, F., Mullappilly, N., Riganti, C., et al. (2020). Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 10. doi:10.3390/biom10030361
Crane, C. A., Ahn, B. J., Han, S. J., and Parsa, A. T. (2012). Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro-Oncology 14, 584–595. doi:10.1093/neuonc/nos014
Curtin, J. F., Liu, N., Candolfi, M., Xiong, W., Assi, H., Yagiz, K., et al. (2009). HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 6, e1000010. doi:10.1371/journal.pmed.1000010
da Fonseca, A. C., and Badie, B. (2013). Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin. Dev. Immunol. 2013, 264124. doi:10.1155/2013/264124
Damiani, D., Tiribelli, M., Raspadori, D., Sirianni, S., Meneghel, A., Cavalllin, M., et al. (2015). Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget 6, 30212–21. doi:10.18632/oncotarget.4901
De Boeck, A., Ahn, B. Y., D'Mello, C., Lun, X., Menon, S. V., Alshehri, M. M., et al. (2020). Glioma-derived IL-33 orchestrates an inflammatory brain tumor microenvironment that accelerates glioma progression. Nat. Commun. 11, 4997. doi:10.1038/s41467-020-18569-4
de Saint Basile, G., Ménasché, G., and Fischer, A. (2010). Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat. Rev. Immunol. 10, 568–579. doi:10.1038/nri2803
Debinski, W, Gibo, DM, Slagle, B, Powers, SK, and Gillespie, GY (1999). Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int. J. Oncol. 15, 481–6. doi:10.3892/ijo.15.3.481
Dokic, I., Hartmann, C., Herold-Mende, C., and Régnier-Vigouroux, A. (2012). Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress. Glia 60, 1785–1800. doi:10.1002/glia.22397
Domingues, P., González-Tablas, M., Otero, Á., Pascual, D., Miranda, D., Ruiz, L., et al. (2016). Tumor infiltrating immune cells in gliomas and meningiomas. Brain, Behavior, Immunity 53, 1–15. doi:10.1016/j.bbi.2015.07.019
Dranoff, G. (2004). Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 4, 11–22. doi:10.1038/nrc1252
Dubois, L. G., Campanati, L., Righy, C., D’Andrea-Meira, I., Spohr, T. C. L. d. S. e., Porto-Carreiro, I., et al. (2014). Gliomas and the vascular fragility of the blood brain barrier. Front. Cellular Neurosci. 8. doi:10.3389/fncel.2014.00418
Dunn-Pirio, A. M., and Vlahovic, G. (2017). Immunotherapy approaches in the treatment of malignant brain tumors. Cancer 123, 734–750. doi:10.1002/cncr.30371
Eagles, M., Nassiri, F., Badhiwala, J., Suppiah, S., Almenawer, S., Zadeh, G., et al. (2018). Dendritic cell vaccines for high-grade gliomas. Tcrm 14, 1299–1313. doi:10.2147/tcrm.s135865
Eckert, F., Schilbach, K., Klumpp, L., Bardoscia, L., Sezgin, E.C., Schwab, M., et al. (2018). Potential Role of CXCR4 Targeting in the Context of Radiotherapy and Immunotherapy of Cancer. Front. Immunol. 9, 3018. doi:10.3389/fimmu.2018.03018
Egan, G., Cervone, K. A., Philips, P. C., Belasco, J. B., Finlay, J. L., and Gardner, S. L. (2016). Phase I study of temozolomide in combination with thiotepa and carboplatin with autologous hematopoietic cell rescue in patients with malignant brain tumors with minimal residual disease. Bone Marrow Transpl. 51, 542–545. doi:10.1038/bmt.2015.313
Erickson, M. A., Dohi, K., and Banks, W. A. (2012). Neuroinflammation: A Common Pathway in CNS Diseases as Mediated at the Blood-Brain Barrier. Neuroimmunomodulation 19. doi:10.1159/000330247
Fahey, J. M., and Girotti, A. W. (2019). Nitric Oxide Antagonism to Anti-Glioblastoma Photodynamic Therapy: Mitigation by Inhibitors of Nitric Oxide Generation. Cancers 11. doi:10.3390/cancers11020231
Farhood, B., Najafi, M., and Mortezaee, K. (2019). CD8+cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 234, 8509–8521. doi:10.1002/jcp.27782
Fedorov, R., Hartmann, E., Ghosh, D. K., and Schlichting, I. (2003). Structural Basis for the Specificity of the Nitric-oxide Synthase Inhibitors W1400 and N ω - Propyl - l - Arg for the Inducible and Neuronal Isoforms *. J. Biol. Chem. 278, 45818–45825.
Ferris, R. L., Jaffee, E. M., and Ferrone, S. (2010). Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. Jco 28, 4390–4399. doi:10.1200/jco.2009.27.6360
Ferris, R. L., Blumenschein, G., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2016). Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 375, 1856–1867. doi:10.1056/nejmoa1602252
Finlay, J. L., Dhall, G., Boyett, J. M., Dunkel, I. J., Gardner, S. L., Goldman, S., et al. (2008). Myeloablative chemotherapy with autologous bone marrow rescue in children and adolescents with recurrent malignant astrocytoma: outcome compared with conventional chemotherapy: a report from the Children's Oncology Group. Pediatr. Blood Cancer 51, 806–811. doi:10.1002/pbc.21732
Flores, C., Pham, C., Snyder, D., Yang, S., Sanchez-Perez, L., Sayour, E., et al. (2015). Novel role of hematopoietic stem cells in immunologic rejection of malignant gliomas. Oncoimmunology 4, e994374. doi:10.4161/2162402x.2014.994374
Galdiero, M. R., Bonavita, E., Barajon, I., Garlanda, C., Mantovani, A., and Jaillon, S. (2013). Tumor associated macrophages and neutrophils in cancer. Immunobiology 218, 1402–1410. doi:10.1016/j.imbio.2013.06.003
Galea, I., Bechmann, I., and Perry, V. H. (2007). What is immune privilege (not)? Trends Immunol. 28, 12–18. doi:10.1016/j.it.2006.11.004
Garcia-Fabiani, M. B., Ventosa, M., Comba, A., Candolfi, M., Nicola Candia, A. J., Alghamri, M. S., et al. (2020). Immunotherapy for gliomas: shedding light on progress in preclinical and clinical development Expert Opin. Investig. Drugs 29, 659–684. doi:10.1080/13543784.2020.1768528
Ghosh, S., Tewari, R., Dixit, D., and Sen, E. (2010). TNFα induced oxidative stress dependent Akt signaling affects actin cytoskeletal organization in glioma cells. Neurochem. Int. 56, 194–201. doi:10.1016/j.neuint.2009.10.003
Gibellini, L., De Biasi, S., Porta, C., Lo Tartaro, D., Depenni, R., Pellacani, G., et al. (2020). Single-Cell Approaches to Profile the Response to Immune Checkpoint Inhibitors. Front. Immunol. 11. doi:10.3389/fimmu.2020.00490
Gieryng, A., Pszczolkowska, D., Walentynowicz, K. A., Rajan, W. D., and Kaminska, B. (2017). Immune microenvironment of gliomas. Lab Invest. 97, 498–518. doi:10.1038/labinvest.2017.19
Gohil, S. H., Iorgulescu, J. B., Braun, D. A., Keskin, D. B., and Livak, K. J. (2021). Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 244–256. doi:10.1038/s41571-020-00449-x
Gomez, G. G., and Kruse, C. A. (2006). Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther. Mol. Biol. 10, 133–146.
Gorczynski, R. M., Yu, K., and Clark, D. (2000). Receptor engagement on cells expressing a ligand for the tolerance-inducing molecule OX2 induces an immunoregulatory population that inhibits alloreactivity in vitro and in vivo. J. Immunol. 165, 4854–4860. doi:10.4049/jimmunol.165.9.4854
Gorczynski, R., Chen, Z., Kai, Y., Lee, L., Wong, S., and Marsden, P. A. (2004). CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 172, 7744–7749. doi:10.4049/jimmunol.172.12.7744
Gorczynski, R. M., Clark, D. A., Erin, N., and Khatri, I. (2011). Role of CD200 expression in regulation of metastasis of EMT6 tumor cells in mice. Breast Cancer Res. Treat. 130, 49–60. doi:10.1007/s10549-010-1259-3
Gorczynski, R. M., Chen, Z., Khatri, I., Podnos, A., and Yu, K. (2013). Cure of metastatic growth of EMT6 tumor cells in mice following manipulation of CD200:CD200R signaling. Breast Cancer Res Treat 142, 271–282. doi:10.1007/s10549-013-2735-3
Grabowski, M. M., Sankey, E. W., Ryan, K. J., Chongsathidkiet, P., Lorrey, S. J., Wilkinson, D. S., et al. (2021). Immune suppression in gliomas. J. Neurooncol. 151, 3–12. doi:10.1007/s11060-020-03483-y
Guan, X., Hasan, M. N., Maniar, S., Jia, W., and Sun, D. (2018). Reactive Astrocytes in Glioblastoma Multiforme. Mol. Neurobiol. 55, 6927–6938. doi:10.1007/s12035-018-0880-8
Gutmann, D. H., and Kettenmann, H. (2019). Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 104, 442–449. doi:10.1016/j.neuron.2019.08.028
Haase, S., Nuñez, F.M., Gauss, J.C., Thompson, S., Brumley, E., Lowenstein, P., et al. (2020). Hemispherical Pediatric High-Grade Glioma: Molecular Basis and Therapeutic Opportunities. Int. J. Mol. Sci. 21. doi:10.3390/ijms21249654
Hambardzumyan, D., Gutmann, D. H., and Kettenmann, H. (2016). The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27. doi:10.1038/nn.4185
Hao, C., Parney, I. F., Roa, W. H., Turner, J., Petruk, K. C., and Ramsay, D. A. (2002). Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 103, 171–8. doi:10.1007/s004010100448
Harshyne, L. A., Nasca, B. J., Kenyon, L. C., Andrews, D. W., and Hooper, D. C. (2016). Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro. Oncol. 18, 206–215. doi:10.1093/neuonc/nov107
Hartmann, F. J., Babdor, J., Gherardini, P. F., Amir, E.-A. D., Jones, K., Sahaf, B., et al. (2019). Comprehensive Immune Monitoring of Clinical Trials to Advance Human Immunotherapy. Cell Rep. 28, 819–831. doi:10.1016/j.celrep.2019.06.049
Hassani Najafabadi, A., Zhang, J., Aikins, M. E., Najaf Abadi, Z. I., Liao, F., Qin, Y., et al. (2020). Cancer Immunotherapy via Targeting Cancer Stem Cells Using Vaccine Nanodiscs. Nano Lett. 20, 7783–7792. doi:10.1021/acs.nanolett.0c03414
Hassin, D., Garber, O. G., Meiraz, A., Schiffenbauer, Y. S., and Berke, G. (2011). Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 133, 190–196. doi:10.1111/j.1365-2567.2011.03426.x
Heithoff, B. P., George, K. K., Phares, A. N., Zuidhoek, I. A., Munoz‐Ballester, C., and Robel, S. (2021). Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 69, 436–472. doi:10.1002/glia.23908
Held-Feindt, J., Hattermann, K., Müerköster, S. S., Wedderkopp, H., Knerlich-Lukoschus, F., Ungefroren, H., et al. (2010). CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exper. Cell Res. 316, 1553–1566. doi:10.1016/j.yexcr.2010.02.018
Henrik Heiland, D., Ravi, V. M., Behringer, S. P., Frenking, J. H., Wurm, J., Joseph, K., et al. (2019). Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat. Commun. 10, 2541. doi:10.1038/s41467-019-10493-6
Heufler, C., Koch, F., Stanzl, U., Topar, G., Wysocka, M., Trinchieri, G., et al. (1996). Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur. J. Immunol. 26, 659–668. doi:10.1002/eji.1830260323
Hollingsworth, R.E., and Jansen, K. (2019). Turning the corner on therapeutic cancer vaccines. npj Vaccines 4, 1–10. doi:10.1038/s41541-019-0103-y
Horng, S., Therattil, A., Moyon, S., Gordon, A., Kim, K., Argaw, A. T., et al. (2017). Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J. Clin. Invest. 127, 3136–3151. doi:10.1172/jci91301
Hou, B., Reizis, B., and DeFranco, A. L. (2008). Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282. doi:10.1016/j.immuni.2008.05.016
Hsieh, J. C., and Lesniak, M. S. (2005). Surgical management of high-grade gliomas. Exp. Rev. Neurother. 5, 33–39. doi:10.1586/14737175.5.6.s33
Huettner, C., Czub, S., Kerkau, S., Roggendorf, W., and Tonn, J. C. (1997). Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 17, 3217–24.
Hunn, M. K., Bauer, E., Wood, C. E., Gasser, O., Dzhelali, M., Ancelet, L. R., et al. (2015). Dendritic cell vaccination combined with temozolomide retreatment: results of a phase I trial in patients with recurrent glioblastoma multiforme. J. Neurooncol. 121, 319–329. doi:10.1007/s11060-014-1635-7
Hussain, S. P., Amstad, P., He, P., Robles, A., Lupold, S., Kaneko, I., et al. (2004). p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 64, 2350–2356. doi:10.1158/0008-5472.can-2287-2
Hussain, S. F., Kong, L.-Y., Jordan, J., Conrad, C., Madden, T., Fokt, I., et al. (2007). A Novel Small Molecule Inhibitor of Signal Transducers and Activators of Transcription 3 Reverses Immune Tolerance in Malignant Glioma Patients. Cancer Res. 67, 9630–9636. doi:10.1158/0008-5472.can-07-1243
Iwami, K., Natsume, A., and Wakabayashi, T. (2011). Cytokine networks in glioma. Neurosurg. Rev. 34, 253–264. doi:10.1007/s10143-011-0320-y
Jahani-Asl, A., and Bonni, A. (2013). iNOS: a potential therapeutic target for malignant glioma. Cmm 13, 1241–1249. doi:10.2174/1566524011313080002
Joffre, O. P., Segura, E., Savina, A., and Amigorena, S. (2012). Cross-presentation by dendritic cells. Nat. Rev. ImmunolImmunol. 12, 557–569. doi:10.1038/nri3254
Johanns, T. M., and Dunn, G. P. (2017). Applied Cancer Immunogenomics. Cancer J. (Sudbury, Mass.) 23, 125–130. doi:10.1097/ppo.0000000000000247
Jones, S. C., Brahmakshatriya, V., Huston, G., Dibble, J., and Swain, S. L. (2010). TLR-Activated Dendritic Cells Enhance the Response of Aged Naive CD4 T Cells via an IL-6-Dependent Mechanism. J. I. 185, 6783–6794. doi:10.4049/jimmunol.0901296
Joseph, J. V., Balasubramaniyan, V., Walenkamp, A., and Kruyt, F. A .E. (2013). TGF-β as a therapeutic target in high grade gliomas – Promises and challenges. Biochem. Pharmacol. 85. doi:10.1016/j.bcp.2012.11.005
Kachikwu, E. L., Iwamoto, K. S., Liao, Y.-P., DeMarco, J. J., Agazaryan, N., Economou, J. S., et al. (2011). Radiation enhances regulatory T cell representation. Int. J. Radiation Oncology*Biology*Physics 81, 1128–1135. doi:10.1016/j.ijrobp.2010.09.034
Kadiyala, P., Carney, S. V., Gauss, J.C ., Garcia-Fabiani, M. B., Haase, S., Alghamri, M. S., et al. (2020). Inhibition of 2-Hydroxyglutarate Elicits Metabolic-reprograming and Mutant IDH1 Glioma Immunity in Mice. J. Clin. Invest. 131, e139542. doi:10.1172/JCI139542
Kamran, N., Kadiyala, P., Saxena, M., Candolfi, M., Li, Y., Moreno-Ayala, M. A., et al. (2017). Immunosuppressive Myeloid Cells' Blockade in the Glioma Microenvironment Enhances the Efficacy of Immune-Stimulatory Gene Therapy. Mol. Ther. 25, 232–248. doi:10.1016/j.ymthe.2016.10.003
Kamran, N., Alghamri, M. S., Nunez, F. J., Shah, D., Asad, A. S., Candolfi, M., et al. (2018). Current state and future prospects of immunotherapy for glioma. Immunotherapy 10, 317–339. doi:10.2217/imt-2017-0122
Kang, R., Zhang, Q., Zeh, H. J., Lotze, M. T., and Tang, D. (2013). HMGB1 in cancer: good, bad, or both? Clin. Cancer Res. 19, 4046–4057. doi:10.1158/1078-0432.ccr-13-0495
Kim, H.-J., Barajas, B., Chan, R. C.-F., and Nel, A. E. (2007). Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence. J. Aller. Clin. Immunol. 119, 1225–1233. doi:10.1016/j.jaci.2007.01.016
Kim, J.-K., Jin, X., Sohn, Y.-W., Jin, X., Jeon, H.-Y., Kim, E.-J., et al. (2014). Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett. 353, 194–200. doi:10.1016/j.canlet.2014.07.034
Kim, J. E., Patel, M. A., Mangraviti, A., Kim, E. S., Theodros, D., Velarde, E., et al. (2017). Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 23, 124–136. doi:10.1158/1078-0432.ccr-15-1535
Kmiecik, J., Poli, A., Brons, N. H. C., Waha, A., Eide, G. E., Enger, P. Ø., et al. (2013). Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 264, 71–83. doi:10.1016/j.jneuroim.2013.08.013
Kmiecik, J., Zimmer, J., and Chekenya, M. (2014). Natural killer cells in intracranial neoplasms: presence and therapeutic efficacy against brain tumours. J. Neurooncol. 116, 1–9. doi:10.1007/s11060-013-1265-5
Komada, M. (2012). Sonic hedgehog signaling coordinates the proliferation and differentiation of neural stem/progenitor cells by regulating cell cycle kinetics during development of the neocortex. Congenit Anom (Kyoto) 52, 72–77. doi:10.1111/j.1741-4520.2012.00368.x
Könnecke, H., and Bechmann, I. (2013). The Role of Microglia and Matrix Metalloproteinases Involvement in Neuroinflammation and Gliomas. Clinical and Developmental Immunology 2013, 914104. doi:10.1155/2013/914104
Kore, R. A., and Abraham, E. C. (2014). Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochemical and Biophysical Research Communications 453. doi:10.1016/j.bbrc.2014.09.068
Koschmann, C., Calinescu, A.-A., Nunez, F. J., Mackay, A., Fazal-Salom, J., Thomas, D., et al. (2016). ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci. Transl. Med. 8, 328ra28. doi:10.1126/scitranslmed.aac8228
Koyama, S., Akbay, E.A., Li, Y.Y., Herter-Sprie, G.S., Buczkowski, K.A., Richards, W.G., et al. (2016). Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications 7, 10501. doi:10.1038/ncomms10501
Kreiter, S., Vormehr, M., Van de Roemer, N., Diken, M., Löwer, M., Diekmann, J., et al. (2015). Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696. doi:10.1038/nature14426
Kretz-Rommel, A., Qin, F., Dakappagari, N., Ravey, E. P., McWhirter, J., Oltean, D., et al. (2007). CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol 178, 5595–5605. doi:10.4049/jimmunol.178.9.5595
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A., and Moon, J. J. (2017). Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Mater 16, 489–496. doi:10.1038/nmat4822
Kunwar, S. (2003). Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88, 105–111. doi:10.1007/978-3-7091-6090-9_16
Kurtulus, S., Sakuishi, K., Ngiow, S.-F., Joller, N., Tan, D. J., Teng, M. W. L., et al. (2015). TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest 125, 4053–4062. doi:10.1172/jci81187
Kwon, J., Lee, S.-R., Yang, K.-S., Ahn, Y., Kim, Y. J., Stadtman, E. R., et al. (2004). Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proceedings of the National Academy of Sciences 101, 16419–16424. doi:10.1073/pnas.0407396101
Lee, S.-R., Yang, K.-S., Kwon, J., Lee, C., Jeong, W., and Rhee, S. G. (2002). Reversible inactivation of the tumor suppressor PTEN by H2O2. Journal of Biological Chemistry 277, 20336–20342. doi:10.1074/jbc.m111899200
Lee, KL, Schlom, J, and Hamilton, DH (2020). Combination therapies utilizing neoepitope-targeted vaccines. Cancer Immunol. Immunother. 70, 875–885. doi:10.1007/s00262-020-02729-y
Leonard, JP, Sherman, ML, Fisher, GL, Buchanan, LJ, Larsen, G, Atkins, MB, et al. (1997). Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90, 2541–8.
Li, G., Tang, D., and Lotze, M. T. (2013). Ménage à Trois in stress: DAMPs, redox and autophagy. Seminars in Cancer Biology 23, 380–390. doi:10.1016/j.semcancer.2013.08.002
Lim, M., Xia, Y., Bettegowda, C., and Weller, M. (2018). Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422–442. doi:10.1038/s41571-018-0003-5
Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., et al. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131, 803–820. doi:10.1007/s00401-016-1545-1
Lowenstein, PR, Lowenstein, ED, and Castro, MG (2009). Challenges in the evaluation, consent, ethics and history of early clinical trials - Implications of the Tuskegee ‘trial' for safer and more ethical clinical trials. Curr Opin Mol Ther 11, 481–4.
Lowenstein, P., A Orringer, D., Sagher, O., Heth, J., Hervey-Jumper, S., Gerald Mammoser, A., et al. (2019). Atim-44. a Phase I First-in-human Trial of Two Adenoviral Vectors Expressing Hsv1-Tk and Flt3L for Treating Newly Diagnosed Resectable Malignant Glioma: Therapeutic Reprogramming of the Brain Immune System. Neuro-Oncology 21, vi11. doi:10.1093/neuonc/noz175.042
Maccallini, C., Gallorini, M., Cataldi, A., and Amoroso, R. (2020). Targeting iNOS As a Valuable Strategy for the Therapy of Glioma. ChemMedChem 15, 339–344. doi:10.1002/cmdc.201900580
Macedo, G.S., Lisboa da Motta, L., Giacomazzi, J., Netto, C.B., Manfredini, V., Vanzin, C.S., et al. (2012). Increased oxidative damage in carriers of the germline TP53 p.R337H mutation. PLoS One 7, e47010. doi:10.1371/journal.pone.0047010
Markovic, D. S., Vinnakota, K., Chirasani, S., Synowitz, M., Raguet, H., Stock, K., et al. (2009). Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proceedings of the National Academy of Sciences 106, 12530–12535. doi:10.1073/pnas.0804273106
Mathewson, N. D., Ashenberg, O., Tirosh, I., Gritsch, S., Perez, E. M., Marx, S., et al. (2021). Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 184, 1281–1298. doi:10.1016/j.cell.2021.01.022
Medawar, PB (1948). Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol 29, 58–69.
Mi, Y., Guo, N., Luan, J., Cheng, J., Hu, Z., Jiang, P., et al. (2020). The Emerging Role of Myeloid-Derived Suppressor Cells in the Glioma Immune Suppressive Microenvironment. Frontiers in Immunology 11, 737. doi:10.3389/fimmu.2020.00737
Migliorini, D., Dietrich, P.-Y., Stupp, R., Linette, G. P., Posey, A. D., and June, C. H. (2018). CAR T-Cell Therapies in Glioblastoma: A First Look. Clin Cancer Res 24, 535–540. doi:10.1158/1078-0432.ccr-17-2871
Miklja, Z., Pasternak, A., Stallard, S., Nicolaides, T., Kline-Nunnally, C., Cole, B., et al. (2019). Molecular profiling and targeted therapy in pediatric gliomas: review and consensus recommendations. Neuro Oncol 21, 968–980. doi:10.1093/neuonc/noz022
Miller, E. W., Tulyathan, O., Isacoff, E. Y., and Chang, C. J. (2007). Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol 3, 263–267. doi:10.1038/nchembio871
Mineharu, Y., King, G. D., Muhammad, A. G., Bannykh, S., Kroeger, K. M., Liu, C., et al. (2011). Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res 17, 4705–4718. doi:10.1158/1078-0432.ccr-11-0915
Mineharu, Y., Muhammad, A. G., Yagiz, K., Candolfi, M., Kroeger, K. M., Xiong, W., et al. (2012). Gene Therapy-Mediated Reprogramming Tumor Infiltrating T Cells Using IL-2 and Inhibiting NF-κB Signaling Improves the Efficacy of Immunotherapy in a Brain Cancer Model. Neurotherapeutics 9, 827–843. doi:10.1007/s13311-012-0144-7
Mitchell, D., and Dey, M. (2019). Neoadjuvant anti-PD-1 immunotherapy for recurrent glioblastoma. Transl Cancer Res TCR 8, S577–S579. doi:10.21037/tcr.2019.05.24
Mitchell, D. A., Batich, K. A., Gunn, M. D., Huang, M.-N., Sanchez-Perez, L., Nair, S. K., et al. (2015). Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519, 366–369. doi:10.1038/nature14320
Miyazaki, T., Ishikawa, E., Sugii, N., and Matsuda, M. (2020). Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment. Cancers 12. doi:10.3390/cancers12071960
Moertel, C.L., Xia, J., LaRue, R., Waldron, N.N., Andersen, B.M., Prins, R.M., et al. (2014). CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J Immunother Cancer 2, 46. doi:10.1186/s40425-014-0046-9
Mohme, M., and Neidert, M.C. (2020). Tumor-Specific T Cell Activation in Malignant Brain Tumors. Frontiers in Immunology 11, 205. doi:10.3389/fimmu.2020.00205
Molinaro, A. M., Taylor, J. W., Wiencke, J. K., and Wrensch, M. R. (2019). Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 15, 405–417. doi:10.1038/s41582-019-0220-2
Moore, K. W., de Waal Malefyt, R., Coffman, R. L., and O'Garra, A. (2001). Interleukin-10and Theinterleukin-10 Receptor. Annu. Rev. Immunol. 19, 683–765. doi:10.1146/annurev.immunol.19.1.683
Mu, L., Yang, C., Gao, Q., Long, Y., Ge, H., DeLeon, G., et al. (2017). CD4+ and Perivascular Foxp3+ T Cells in Glioma Correlate with Angiogenesis and Tumor Progression. Frontiers in immunology 8, 1451. doi:10.3389/fimmu.2017.01451
Murat, A., Migliavacca, E., Hussain, S. F., Heimberger, A. B., Hamou, M.-F., et al. (2009). Modulation of Angiogenic and Inflammatory Response in Glioblastoma by Hypoxia. PLOS ONE 4, e5947. doi:10.1371/journal.pone.0005947
Murphy, J. B., and Sturm, E. (1923). CONDITIONS DETERMINING THE TRANSPLANTABILITY OF TISSUES IN THE BRAIN. The Journal of experimental medicine 38, 183–197. doi:10.1084/jem.38.2.183
Noushmehr, H., Weisenberger, D. J., Diefes, K., Phillips, H. S., Pujara, K., Berman, B. P., et al. (2010). Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer cell 17, 510–522. doi:10.1016/j.ccr.2010.03.017
O'Brien, E. R., Howarth, C., and Sibson, N. R. (2013). The role of astrocytes in CNS tumors: pre-clinical models and novel imaging approaches. Front Cell Neurosci 7, 40. doi:10.3389/fncel.2013.00040
Okada, M, Saio, M, Kito, Y, Ohe, N, Yano, H, Yoshimura, S, et al. (2009). Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol 34, 1621–7. doi:10.3892/ijo_00000292
Okura, H., Smith, C. A., and Rutka, J. T. (2014). Gene therapy for malignant glioma. Molecular and Cellular Therapies 2, 21. doi:10.1186/2052-8426-2-21
Olin, M. R., Ampudia-Mesias, E., Pennell, C.A., Sarver, A., Chen, C.C., Moertel, C.L., et al. (2019). Treatment Combining CD200 Immune Checkpoint Inhibitor and Tumor-Lysate Vaccination after Surgery for Pet Dogs with High-Grade Glioma. Cancers (Basel) 11. doi:10.3390/cancers11020137
Palucka, K., and Banchereau, J. (1999). Dendritic cells: a link between innate and adaptive immunity. Journal of clinical immunology 19, 12–25. doi:10.1023/a:1020558317162
Panov, A., Orynbayeva, Z., Vavilin, V., and Lyakhovich, V. (2014). Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014, 472459. doi:10.1155/2014/472459
Penas-Prado, M., Hess, K. R., Fisch, M. J., Lagrone, L. W., Groves, M. D., Levin, V. A., et al. (2015). Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro-oncology 17, 266–273. doi:10.1093/neuonc/nou155
Perng, P., and Lim, M. (2015). Immunosuppressive Mechanisms of Malignant Gliomas: Parallels at Non-CNS Sites. Front Oncol 5, 153. doi:10.3389/fonc.2015.00153
Peters, K. B., Archer, G. E., Norberg, P., Xie, W., Threatt, S., Lipp, E. S., et al. (2019). Safety of nivolumab in combination with dendritic cell vaccines in recurrent high-grade glioma. Jco 37, e13526. doi:10.1200/jco.2019.37.15_suppl.e13526
Phuphanich, S., Wheeler, C. J., Rudnick, J. D., Mazer, M., Wang, H., Nuño, M. A., et al. (2013). Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 62, 125–135. doi:10.1007/s00262-012-1319-0
Placone, A. L., Quiñones-Hinojosa, A., and Searson, P. C. (2016). The role of astrocytes in the progression of brain cancer: complicating the picture of the tumor microenvironment. Tumor Biol. 37, 61–69. doi:10.1007/s13277-015-4242-0
Platten, M., Schilling, D., Bunse, L., Wick, A., Bunse, T., Riehl, D., et al. (2018). ATIM-33. NOA-16: a first-in-man multicenter phase I clinical trial of the German Neurooncology working group evaluating a mutation-specific peptide vaccine targeting IDH1R132H in patients with newly diagnosed malignant ASTROCYTOMAS. Neuro-oncology 20, vi8–vi9. doi:10.1093/neuonc/noy148.028
Platten, M., Bunse, L., Riehl, D., Bunse, T., Ochs, K., and Wick, W. (2018). Vaccine strategies in gliomas. Current treatment options in neurology 20, 1–9. doi:10.1007/s11940-018-0498-1
Platten, M., Bunse, L., Wick, A., Bunse, T., Le Cornet, L., Harting, I., et al. (2021). A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 592, 463–468. doi:10.1038/s41586-021-03363-z
Priego, N., Zhu, L., Monteiro, C., Mulders, M., Wasilewski, D., Bindeman, W., et al. (2018). STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24, 1024–1035. doi:10.1038/s41591-018-0044-4
Pyonteck, S. M., Akkari, L., Schuhmacher, A. J., Bowman, R. L., Sevenich, L., Quail, D. F., et al. (2013). CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19, 1264–1272. doi:10.1038/nm.3337
Qian, Y., Zhong, X., Flynn, D. C., Zheng, J. Z., Qiao, M., Wu, C., et al. (2005). ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene 24, 3154–3165. doi:10.1038/sj.onc.1208525
Rahbar, A., Cederarv, M., Wolmer-Solberg, N., Tammik, C., Stragliotto, G., Peredo, I., et al. (2016). Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. OncoImmunology 5, e1075693. doi:10.1080/2162402x.2015.1075693
Raninga, P. V., Lee, A. C., Sinha, D., Shih, Y. Y., Mittal, D., Makhale, A., et al. (2020). Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti‐PD‐L1 antibody for treatment of triple‐negative breast cancer. Int. J. Cancer 146, 123–136. doi:10.1002/ijc.32410
Rath, B.H., Fair, J.M., Jamal, M., Camphausen, K., and Tofilon, P.J. (2013). Astrocytes enhance the invasion potential of glioblastoma stem-like cells. PLoS One 8, e54752. doi:10.1371/journal.pone.0054752
Razavi, S.-M., Lee, K.E., Jin, B.E., Aujla, P.S., Gholamin, S., and Li, G. (2016). Immune Evasion Strategies of Glioblastoma. Front Surg 3, 11. doi:10.3389/fsurg.2016.00011
Reardon, DA, Ligon, KL, Chiocca, EA, and Wen, PY (2015). One size should not fit all: advancing toward personalized glioblastoma therapy. Discov Med 19, 471–7.
Reardon, D. A., Desjardins, A., Vredenburgh, J. J., O'Rourke, D. M., Tran, D. D., Fink, K. L., et al. (2020). Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): results of a double-blind randomized phase II trial. Clin Cancer Res 26, 1586–1594. doi:10.1158/1078-0432.ccr-18-1140
Redza-Dutordoir, M., and Averill-Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1863, 2977–2992. doi:10.1016/j.bbamcr.2016.09.012
Roesch, S., Rapp, C., Dettling, S., and Herold-Mende, C. (2018). When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma. Ijms 19, 436. doi:10.3390/ijms19020436
Ros, M.D., Gregorio, V.D., Iorio, A., Giunti, L., Guidi, M., Martino, M.d., et al. (2018). Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. International Journal of Molecular Sciences 19.
Roth, P., Junker, M., Tritschler, I., Mittelbronn, M., Dombrowski, Y., Breit, S. N., et al. (2010). GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin Cancer Res 16, 3851–3859. doi:10.1158/1078-0432.ccr-10-0705
Russo, C. D., and Cappoli, N. (2018). Glioma associated microglia/macrophages, a potential pharmacological target to promote antitumor inflammatory immune response in the treatment of glioblastoma. Nn 5, 36. doi:10.20517/2347-8659.2018.42
Rygiel, T. P., Karnam, G., Goverse, G., van der Marel, A. P. J., Greuter, M. J., van Schaarenburg, R. A., et al. (2012). CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 31, 2979–2988. doi:10.1038/onc.2011.477
Saetta, A. A., Levidou, G., El-Habr, E. A., Panayotidis, I., Samaras, V., Thymara, I., et al. (2011). Expression of pERK and pAKT in human astrocytomas: correlation with IDH1-R132H presence, vascular endothelial growth factor, microvascular characteristics and clinical outcome. Virchows Arch 458, 749–759. doi:10.1007/s00428-011-1074-1
Salazar-Ramiro, A., Ramirez-Ortega, D., Perez de la Cruz, V., Hernandez-Pedro, N.Y., Gonzalez-Esquivel, D.F., Sotelo, J., et al. (2016). Role of Redox Status in Development of Glioblastoma. Front Immunol 7, 156. doi:10.3389/fimmu.2016.00156
Sankowski, R., Böttcher, C., Masuda, T., Geirsdottir, L., Sagar, , Sindram, E., et al. (2019). Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci 22, 2098–2110. doi:10.1038/s41593-019-0532-y
Schadendorf, D., Ugurel, S., Schuler-Thurner, B., Nestle, F. O., Enk, A., Bröcker, E.-B., et al. (2006). Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Annals of Oncology 17, 563–570. doi:10.1093/annonc/mdj138
Schalper, K. A., Rodriguez-Ruiz, M. E., Diez-Valle, R., López-Janeiro, A., Porciuncula, A., Idoate, M. A., et al. (2019). Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 25, 470–476. doi:10.1038/s41591-018-0339-5
Scheetz, L., Kadiyala, P., Sun, X., Son, S., Hassani Najafabadi, A., Aikins, M., et al. (2020). Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas. Clin Cancer Res 26, 4369–4380. doi:10.1158/1078-0432.ccr-20-0341
Schetters, S.T.T., Gomez-Nicola, D., Garcia-Vallejo, J.J., and Van Kooyk, Y. (2018). Neuroinflammation: Microglia and T Cells Get Ready to Tango. Frontiers in Immunology 8, 1905. doi:10.3389/fimmu.2017.01905
Schoenborn, J. R., and Wilson, C. B. (2007). Regulation of Interferon‐γ During Innate and Adaptive Immune Responses. Advances in immunology 96, 41–101. doi:10.1016/s0065-2776(07)96002-2
Schumacher, T. N., and Schreiber, R. D. (2015). Neoantigens in cancer immunotherapy. Science 348, 69–74. doi:10.1126/science.aaa4971
Schwartzbaum, J. A., Fisher, J. L., Aldape, K. D., and Wrensch, M. (2006). Epidemiology and molecular pathology of glioma. Nat Rev Neurol 2, 494–503. doi:10.1038/ncpneuro0289
Seike, T., Fujita, K., Yamakawa, Y., Kido, M. A., Takiguchi, S., Teramoto, N., et al. (2011). Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis 28, 13–25. doi:10.1007/s10585-010-9354-8
Sethi, G., Aggarwal, B.B., and Sung, B. (2008). TNF: A master switch for inflammation to cancer. Front Biosci Volume, 5094–5107. doi:10.2741/3066
Sonar, S.A., and Lal, G. (2018). Blood-brain barrier and its function during inflammation and autoimmunity. Journal of Leukocyte Biology 103. doi:10.1002/jlb.1ru1117-428r
Soroceanu, L., Manning, T. J., and Sontheimer, H. (2001). Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 33, 107–117. doi:10.1002/1098-1136(200102)33:2<107::aid-glia1010>3.0.co;2-4
Southan, G. J., Zingarelli, B., O'Connor, M., Salzman, A. L., and Szabó, C. (1996). Spontaneous rearrangement of aminoalkylisothioureas into mercaptoalkylguanidines, a novel class of nitric oxide synthase inhibitors with selectivity towards the inducible isoform. British journal of pharmacology 117, 619–632. doi:10.1111/j.1476-5381.1996.tb15236.x
Spranger, S., and Gajewski, T. F. (2018). Mechanisms of Tumor Cell-Intrinsic Immune Evasion. Annu. Rev. Cancer Biol. 2, 213–228. doi:10.1146/annurev-cancerbio-030617-050606
Srivastava, S., Jackson, C., Kim, T., Choi, J., and Lim, M. (2019). A Characterization of Dendritic Cells and Their Role in Immunotherapy in Glioblastoma: From Preclinical Studies to Clinical Trials. Cancers 11, 537. doi:10.3390/cancers11040537
Sturm, D., Witt, H., Hovestadt, V., Khuong-Quang, D.-A., Jones, D. T. W., Konermann, C., et al. (2012). Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer cell 22, 425–437. doi:10.1016/j.ccr.2012.08.024
Su, Z, Dannull, J, Heiser, A, Yancey, D, Pruitt, S, Madden, J, et al. (2003). Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res 63, 2127–33.
Takahashi, M., Valdes, G., Hiraoka, K., Inagaki, A., Kamijima, S., Micewicz, E., et al. (2014). Radiosensitization of gliomas by intracellular generation of 5-fluorouracil potentiates prodrug activator gene therapy with a retroviral replicating vector. Cancer Gene Ther 21, 405–410. doi:10.1038/cgt.2014.38
Tay, R. E., Richardson, E. K., and Toh, H. C. (2021). Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 28, 5–17. doi:10.1038/s41417-020-0183-x
Thomas, R. P., Nagpal, S., Iv, M., Soltys, S. G., Bertrand, S., Pelpola, J. S., et al. (2019). Macrophage Exclusion after Radiation Therapy (MERT): A First in Human Phase I/II Trial using a CXCR4 Inhibitor in Glioblastoma. Clin Cancer Res 25, 6948–6957. doi:10.1158/1078-0432.ccr-19-1421
Tsai, C.-S., Chen, F.-H., Wang, C.-C., Huang, H.-L., Jung, S.-M., Wu, C.-J., et al. (2007). Macrophages From Irradiated Tumors Express Higher Levels of iNOS, Arginase-I and COX-2, and Promote Tumor Growth. International Journal of Radiation Oncology*Biology*Physics 68, 499–507. doi:10.1016/j.ijrobp.2007.01.041
van den Bent, M. J., Dubbink, H. J., Marie, Y., Brandes, A. A., Taphoorn, M. J. B., Wesseling, P., et al. (2010). IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res 16, 1597–1604. doi:10.1158/1078-0432.ccr-09-2902
Van Meir, E. G. (1995). Cytokines and tumors of the central nervous system. Glia 15, 264–288. doi:10.1002/glia.440150308
Varatharaj, A., and Galea, I. (2017). The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity 60. doi:10.1016/j.bbi.2016.03.010
Verhaak, R. G. W., Hoadley, K. A., Purdom, E., Wang, V., Qi, Y., Wilkerson, M. D., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell 17, 98–110. doi:10.1016/j.ccr.2009.12.020
Verschuere, T., De Vleeschouwer, S., Lefranc, F., Kiss, R., and Van Gool, S. W. (2011). Galectin-1 and immunotherapy for brain cancer. Expert Review of Neurotherapeutics 11, 533–543. doi:10.1586/ern.11.40
Vik-Mo, E. O., Nyakas, M., Mikkelsen, B. V., Moe, M. C., Due-Tønnesen, P., Suso, E. M. I., et al. (2013). Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 62, 1499–1509. doi:10.1007/s00262-013-1453-3
Voskoboinik, I., Smyth, M. J., and Trapani, J. A. (2006). Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol 6, 940–952. doi:10.1038/nri1983
Vries, H.d., Kuiper, J., Boer, A.d., Berkel, T.V., and Breimer, D.D. (1998). The Blood-Brain Barrier in Neuroinflammatory Diseases. Pharmacological Reviews 2, 143–156.
Wainwright, D. A., Balyasnikova, I. V., Chang, A. L., Ahmed, A. U., Moon, K.-S., Auffinger, B., et al. (2012). IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18, 6110–6121. doi:10.1158/1078-0432.ccr-12-2130
Wang, H., Diaz, A.K., Shaw, T.I., Li, Y., Niu, M., Cho, J.-H., et al. (2019). Deep multiomics profiling of brain tumors identifies signaling networks downstream of cancer driver genes. Nature communications 10, 3718. doi:10.1038/s41467-019-11661-4
Wang, X., Guo, G., Guan, H., Yu, Y., Lu, J., and Yu, J. (2019). Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. Journal of experimental & clinical cancer research : CR 38, 87. doi:10.1186/s13046-019-1085-3
Wang, J., Shen, F., Yao, Y., Wang, L.-l., Zhu, Y., and Hu, J. (2020). Adoptive Cell Therapy: A Novel and Potential Immunotherapy for Glioblastoma. Frontiers in Oncology 10, 59. doi:10.3389/fonc.2020.00059
Weiss, T., Puca, E., Silginer, M., Hemmerle, T., Pazahr, S., Bink, A., et al. (2020). Immunocytokines are a promising immunotherapeutic approach against glioblastoma. Sci Transl Med 12. doi:10.1126/scitranslmed.abb2311
Weller, M., Butowski, N., Tran, D. D., Recht, L. D., Lim, M., Hirte, H., et al. (2017). Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. The Lancet Oncology 18, 1373–1385. doi:10.1016/s1470-2045(17)30517-x
Weng, M.S., Chang, J.H., Hung, W.Y., Yang, Y.C., and Chien, M.H. (2018). The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J Exp Clin Cancer Res 37, 61. doi:10.1186/s13046-018-0728-0
Wesseling, P., and Capper, D. (2018). WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol 44, 139–150. doi:10.1111/nan.12432
Wolburg, H., Noell, S., Fallier-Becker, P., Mack, A. F., and Wolburg-Buchholz, K. (2012). The disturbed blood-brain barrier in human glioblastoma. Molecular Aspects of Medicine 33, 579–589. doi:10.1016/j.mam.2012.02.003
Wong, K. K., Brenneman, F., Chesney, A., Spaner, D. E., and Gorczynski, R. M. (2012). Soluble CD200 is critical to engraft chronic lymphocytic leukemia cells in immunocompromised mice. Cancer Res 72, 4931–4943. doi:10.1158/0008-5472.can-12-1390
Woo, S.-R., Turnis, M. E., Goldberg, M. V., Bankoti, J., Selby, M., Nirschl, C. J., et al. (2012). Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 72, 917–927. doi:10.1158/0008-5472.can-11-1620
Woroniecka, K., Chongsathidkiet, P., Rhodin, K., Kemeny, H., Dechant, C., Farber, S. H., et al. (2018). T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res 24, 4175–4186. doi:10.1158/1078-0432.ccr-17-1846
Wright, G. J., Puklavec, M. J., Willis, A. C., Hoek, R. M., Sedgwick, J. D., Brown, M. H., et al. (2000). Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 13, 233–242. doi:10.1016/s1074-7613(00)00023-6
Wright, G. J., Cherwinski, H., Foster-Cuevas, M., Brooke, G., Puklavec, M. J., Bigler, M., et al. (2003). Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 171, 3034–3046. doi:10.4049/jimmunol.171.6.3034
Xie, S., Shan, X.-F., Yau, V., Zhang, J.-Y., Zhang, X.-Y., Yan, Y.-P., et al. (2020). Hyperion imaging system reveals heterogeneous tumor microenvironment of oral squamous cell carcinoma patients at T1N0M0 stage. Ann Transl Med 8, 1513. doi:10.21037/atm-20-7194
Xiong, Z., Ampudia-Mesias, E., Shaver, R., Horbinski, C. M., Moertel, C. L., and Olin, M. R. (2016). Tumor-derived vaccines containing CD200 inhibit immune activation: implications for immunotherapy. Immunotherapy 8, 1059–1071. doi:10.2217/imt-2016-0033
Xiong, Z., Ampudia Mesias, E., Pluhar, G. E., Rathe, S. K., Largaespada, D. A., Sham, Y. Y., et al. (2019). CD200 Checkpoint Reversal: A Novel Approach to Immunotherapy. Clin Cancer Res 26, 232–241. doi:10.1158/1078-0432.ccr-19-2234
Yadav, M., Jhunjhunwala, S., Phung, Q. T., Lupardus, P., Tanguay, J., Bumbaca, S., et al. (2014). Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576. doi:10.1038/nature14001
Yang, I., Tihan, T., Han, S. J., Wrensch, M. R., Wiencke, J., Sughrue, M. E., et al. (2010). CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. Journal of Clinical Neuroscience 17, 1381–1385. doi:10.1016/j.jocn.2010.03.031
Yang, N., Yan, T., Zhu, H., Liang, X., Leiss, L., Sakariassen, P.O., et al. (2014). A co-culture model with brain tumor-specific bioluminescence demonstrates astrocyte-induced drug resistance in glioblastoma. J Transl Med 12, 278. doi:10.1186/s12967-014-0278-y
Zamanian, J. L., Xu, L., Foo, L. C., Nouri, N., Zhou, L., Giffard, R. G., et al. (2012). Genomic analysis of reactive astrogliosis. Journal of Neuroscience 32, 6391–6410. doi:10.1523/jneurosci.6221-11.2012
Zhai, H., Heppner, F. L., and Tsirka, S. E. (2011). Microglia/macrophages promote glioma progression. Glia 59, 472–485. doi:10.1002/glia.21117
Zhang, J., Sarkar, S., Cua, R., Zhou, Y., Hader, W., and Yong, V. W. (2012). A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis 33, 312–319. doi:10.1093/carcin/bgr289
Keywords: immunosuppression, inflammation, tumor microenvironment, glioma, immunotherapy
Citation: Alghamri MS, McClellan BL, Hartlage CS, Haase S, Faisal SM, Thalla R, Dabaja A, Banerjee K, Carney SV, Mujeeb AA, Olin MR, Moon JJ, Schwendeman A, Lowenstein PR and Castro MG (2021) Targeting Neuroinflammation in Brain Cancer: Uncovering Mechanisms, Pharmacological Targets, and Neuropharmaceutical Developments. Front. Pharmacol. 12:680021. doi: 10.3389/fphar.2021.680021
Received: 12 March 2021; Accepted: 04 May 2021;
Published: 18 May 2021.
Edited by:
Maria Grazia Bottone, University of Pavia, ItalyReviewed by:
Marco Peviani, University of Pavia, ItalyMarta Llansola, Principe Felipe Research Center (CIPF), Spain
Copyright © 2021 Alghamri, McClellan, Hartlage, Haase, Faisal, Thalla, Dabaja, Banerjee, Carney, Mujeeb, Olin, Moon, Schwendeman, Lowenstein and Castro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria G. Castro, bWFyaWFjYXNAdW1pY2guZWR1
 Mahmoud S. Alghamri
Mahmoud S. Alghamri Brandon L. McClellan
Brandon L. McClellan Carson S. Hartlage1,2
Carson S. Hartlage1,2 Santiago Haase
Santiago Haase Syed Mohd Faisal
Syed Mohd Faisal Ali Dabaja
Ali Dabaja Kaushik Banerjee
Kaushik Banerjee Anzar A. Mujeeb
Anzar A. Mujeeb James J. Moon
James J. Moon Pedro R. Lowenstein
Pedro R. Lowenstein Maria G. Castro
Maria G. Castro