- 1Department of Natural Products and Alternative Medicine, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
- 3Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
- 4Department of Pharmaceutics, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt
- 6Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Assiut Branch, Assiut, Egypt
- 7Department of Pharmaceutical Chemistry, College of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
Vasodilators are an important class of antihypertensive agents. However, they have limited clinical use due to the reflex tachycardia associated with their use which masks most of its antihypertensive effect and raises cardiac risk. Chemical investigation of Psiadia punctulata afforded five major methoxylated flavonoids (1–5) three of which (1, 4, and 5) showed vasodilator activity. Linoleic acid-based self-nanoemulsifying drug delivery system (SNEDDS) was utilized to develop intravenous (IV) formulations that contain compounds 1, 4, or 5. The antihypertensive effect of the prepared SNEDDS formulations, loaded with each of the vasodilator compounds, was tested in the angiotensin-induced rat model of hypertension. Rats were subjected to real-time recording of blood hemodynamics and surface Electrocardiogram (ECG) while the pharmaceutical formulations were individually slowly injected in cumulative doses. Among the tested formulations, only that contains umuhengerin (1) and 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavone (5) showed potent antihypertensive effects. Low IV doses, from the prepared SNEDDS, containing either compound 1 or 5 showed a marked reduction in the elevated systolic blood pressure by 10 mmHg at 12 μg/kg and by more than 20 mmHg at 36 μg/kg. The developed SNEDDS formulation containing either compound 1 or 5 significantly reduced the elevated diastolic, pulse pressure, dicrotic notch pressure, and the systolic–dicrotic notch pressure difference. Moreover, both formulations decreased the ejection duration and increased the non-ejection duration while they did not affect the time to peak. Both formulations did not affect the AV conduction as appear from the lack of effect on p duration and PR intervals. Similarly, they did not affect the ventricular repolarization as no effect on QTc or JT interval. Both formulations decreased the R wave amplitude but increased the T wave amplitude. In conclusion, the careful selection of linoleic acid for the development of SNEDDS formulation rescues the vasodilating effect of P. punctulata compounds from being masked by the reflex tachycardia that is commonly associated with the decrease in peripheral resistance by most vasodilators. The prepared SNEDDS formulation could be suggested as an effective medication in the treatment of hypertensive emergencies, after clinical evaluation.
Introduction
Hypertension is one of the most common chronic medical problems affecting more than one billion people all over the world (Chockalingam et al., 2006). The “silent” nature of hypertension can lead a majority of hypertensive patients to the emergency room with a hypertensive emergency, an important clinical entity, which if not recognized and acted upon promptly, can result in life-threatening adverse outcomes (Mallidi et al., 2015). Vasodilators form the mainstay of treatment in hypertensive emergencies (Mallidi et al., 2015) and a valuable agent in the treatment of systemic hypertension (Hariri and Patel, 2021). However, they have a limited clinical role due to the reflex tachycardia associated with their use, as a consequence of the medication-induced baroreflex response compensating for the sudden medication decrease in vascular resistance (Kester et al., 2012; Rascher and Paech, 2020), furthermore, reflex tachycardia might propagate aortic dissection (Estrera et al., 2006).
Angiotensin II, the major biologically active component of the renin-angiotensin system, contributes to the regulation of vascular tone, salt and water balance, and blood pressure (Kurdi and Booz, 2011; Cuthbert et al., 2020). Angiotensin II is well known for its strong vasoconstricting effect that leads to an increase in blood pressure (Rascher and Paech, 2020). Infusion experiments using angiotensin II establish a qualitative and quantitative correlation between elevated concentrations of plasma angiotensin II and elevated arterial pressure (White et al., 1989).
In recent years, there has been a growing interest in the use of bioactive compounds from plant sources to treat hypertension, owing to their efficacy, safety, relative availability, and low cost (Verma et al., 2020). Different phytoconstituents, including flavonoids, alkaloids, and terpenoids have been identified as vasodilators from plants (Micucci et al., 2020).
Psiadia punctulata (DC.) Vatke (Asteraceae) is a small shrub mostly found in some east African countries including Eritrea, Saudi Arabia, and North East India (Kidane et al., 2018). Methanol extract of the whole plant is reported to show a blood pressure-lowering effect, phrenic neuromuscular nerve blocking effect and relaxant properties on the smooth muscle of mice trachea (Achola et al., 1998). In our previous work, we have isolated five methoxylated flavonoids from P. punctulata, out of which three compounds namely; umuhengerin (1), luteolin-3′,4` -dimethyl ether (4), and 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavone (5) have produced significant vasodilation which is mediated through endothelial nitric oxide pathway (Abdallah et al., 2020). However, the utilization of the previously isolated vasodilators in treating hypertension invivo has not been examined yet.
Linoleic acid is an essential n-6 polyunsaturated fatty acid required for normal growth and development at 1–2% of daily energy (Taha, 2020). It has been used in the development of many drug delivery systems such as liposomes, in the treatment of melasma (Irby et al., 2017); nanoemulsion, to enhance oral bioavailability of simvastatin (Singh et al., 2016); and the development of self-nanoemulsifying drug delivery system (SNEDDS), to improve quercetin hepatoprotective activity (Van Staden et al., 2020). Previous reports suggest that linolenic acid may protect against coronary artery disease and related death (Marangoni et al., 2020). The linoleic acid protection was attributed to its effect on heart rate suggesting possible antiarrhythmic activity (Christensen et al., 2005).
The present study aimed to evaluate the antihypertensive activity for the isolated methoxylated flavonoids from P. punctulata after administration of an IV formulation based on SNEDDS containing linoleic acid to unmask the effect of reflex tachycardia.
Materials and Methods
Material
Polyethylene glycol (PEG) 400, oleic acid, and Tween® 80 were purchased from Sigma–Aldrich (St. Louis, MO). Linoleic acid was obtained from Acros organics (Fair Lawn, New Jersey). Angiotensin II and dimethyl sulfoxide (DMSO) from (Sigma-Aldrich, Munich, Germany) were used in the biological study.
Plant Material and Isolation of Active Compounds
The methods of collection, extraction, and isolation of the methoxy flavonoids from the flowering aerial parts of P. punctulata were mentioned in detail in our previous publication (Abdallah et al., 2019).
Development of IV Formulation
Preparation of Ternary Phase Mixtures
In this study, self-nanoemulsifying drug delivery system (SNEDDS) was utilized to develop the IV formulation. PEG 400 was selected as a cosurfactant, tween 80 (polysorbate 80) was selected as a surfactant. Three oils namely, olive oil, linoleic acid, and oleic acid were screened to select the oil that produces SNEDDS with the lowest globule size. The selection of the surfactant and cosurfactant was based on our previously published work (Ahmed et al., 2014; El-Say et al., 2015; Ahmed et al., 2018). The selection of the oils was based on their biocompatibility that renders the formulation suitable for IV administration. Seven different formulations containing different quantities of oil, surfactant, and cosurfactant were proposed and their composition is illustrated in Table 1. Briefly, 1 g of SNEDDS was prepared by accurately weighing the calculated amount of oil, surfactant, and cosurfactant in an Eppendorf tube. Each mixture was vortex for 30 s until a homogenous dispersion was obtained. The total weight of the oil, surfactant and cosurfactant in any SNEDDS mixture was always added to 100%.
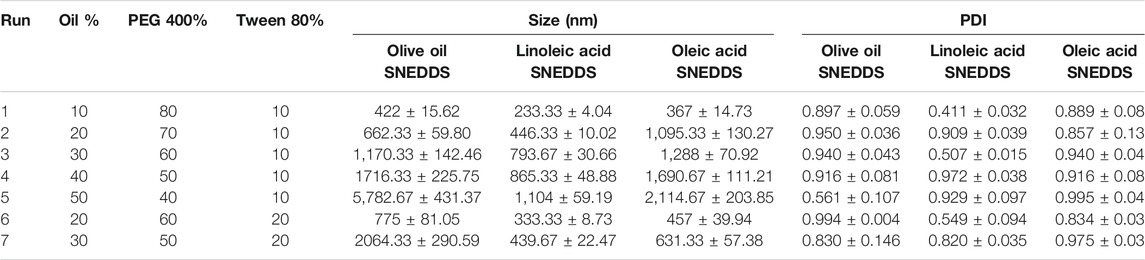
TABLE 1. Composition of the prepared self-nanoemulsifying drug delivery system (SNEDDS) and the obtained results for the size and polydispersity index.
Characterization of the Prepared SNEDDS
Known weight (1 g) of the prepared SNEDDS formulation was added to 20 ml of distilled water on a magnetic stirrer. Stirring was continued until the formation of a homogenous dispersion (nano-emulsion). The globule size and polydispersity index (PDI) of the obtained emulsions were determined using Malvern Zetasizer Nano ZSP, Malvern Panalytical Ltd. (Malvern, United Kingdom). Dynamic light scattering with non-invasive backscatter optics was the technique used to measure the size. An average of three readings was recorded.
Preparation of the Medicated IV Formulation
Known weight (2.1 mg) of the freeze-dried isolated compounds was separately added to 1 g of the selected SNEDDS formulation which contains 10% linoleic acid, 80% PEG 400, and 10% tween 80. The mixture was vortex until the complete dissolving of the compound in the SNEDDS formulation. The medicated SNEDDS (1 g) was added to 20 ml double distilled water on a magnetic stirrer. Stirring was continued until the formation of a homogenous mixture that was sterile filtered using Ministar® single-use 0.45 mm, non-pyrogenic syringe filter of Sterile-ED, Sartorius Stedim Biotech GmbH (Goettingen, Germany) to prepare medicated IV formulations. Plain (non-medicated) IV formulation was also prepared for comparative study.
Biological Study
Animals
Six-week-old male Wistar rats with a weight of (250–275 g) were obtained from Zagazig University. They were kept in clear cages made of polypropylene and with good ventilation (3–4 rats in each cage), under constant environmental conditions of 22 ± 2°C temperature, 50–60% relative humidity, and 12-h day and night cycle. Unlimited rodent pellet food and purified water were provided to the rats. The experimental design and animal handling procedures were as indicated by the guidelines of the Ethical Committee for Animal Handling at Zagazig University (approval number ZU-IACUC/3/F/33/2021).
Blood Pressure Recording
The blood pressure was recorded invasively in real-time following the procedure outlined in our previous publications (Azhar and El-Bassossy, 2014; El-Bassossy and Watson, 2015; El-Bassossy et al., 2017b; El-Bassossy et al., 2017c). The rats were subjected to anesthesia with a single intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Animals’ body temperature was held at 37°C through a rectal probe and automated heating pads. A micro-tip pressure-volume catheter (PV catheter, SPR-901, Millar Instruments, Houston, TX, United States of America) was inserted via a small opening into the right carotid artery. This instrument can continuously monitor arterial pressure. The micro-tip catheter was linked via a Power Lab Data Interface to a computer running the Lab Chart professional software (v8.0, AD Instruments, Bella Vista, Australia) incorporating a blood pressure (BP) module. Following a stabilization time of 5 min, readings were continuously recorded. The BP module was employed to real-time monitor all hemodynamic parameters including; systolic BP, diastolic BP, heart rate, pulse pressure, dicrotic notch pressure, ejection duration, non-ejection duration and time to peak.
Electrocardiogram (ECG) Recording
A Powerlab® system (AD Instruments, Bella Vista, Australia) linked to a computer running the LabChart professional software with the ECG module was employed to record the standard surface ECG according to the methodology outlined in a previous report by our group (El-Bassossy et al., 2016; El-Bassossy et al., 2017a; El-Bassossy et al., 2018). The ECG module quantitatively assesses the various elements of the ECG including; p duration, PR interval, QTc interval, JT interval, R amplitude and T amplitude.
Acute Induction of Hypertension
After 10 min (stabilization period) of basal recording of invasive blood pressure and ECG, the standard dose of angiotensin II (120 ng/min/kg) that were commonly used in osmotic mini-pumps (Gonzalez et al., 2014; El-Bassossy et al., 2018) was slowly infused through the femoral vein using a syringe pump and continued throughout the experiment duration.
Animals Treatment
Animals were divided into 4 groups each of 6 rats: saline group, vehicle (the plain IV formulation), IV formulation containing compound 1 group, and IV formulation containing compound 5 group. After 10 min (stabilization period) of starting angiotensin infusion; the saline and the plain and the medicated IV formulations containing either compound 1 or 5 were injected into the femoral vein in doses of 12, 24, 36 μg/kg every 10 min each in 0.1 ml injection volume. Invasive blood hemodynamics and ECG were continuously recorded throughout the experiment. Saline (0.3 ml) was injected in time control experiments.
Effects on Serum Sodium and Potassium Levels
At the end of the experiment, blood samples were obtained from the femoral vein. Levels of sodium and potassium in blood were analyzed by colorimetric methods using a spectrophotometer using the commercially available kits. The method is based on the reaction of sodium or potassium with a selective chromogen producing a chromophore whose absorbance varies directly as the concentration of sodium or potassium in the test specimen.
Statistical Analysis
Values of the present study presented in form of mean ± SEM. Statistical analysis was carried out using the Prism 5 computer program (Graph Pad, United States). Statistical comparison of blood hemodynamics and electrocardiogram parameters was done using repeated measures Two-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test of baseline-corrected data, while the statistical comparison of electrolyte levels was done by using one way ANOVA, followed by Newman–Keuls’ post hoc test. p < 0.05 was considered significant.
Results and Discussion
To the best of our knowledge, the current study is the first to report a potent antihypertensive activity for a linoleic acid-based SNEDDS IV formulation containing methoxy flavonoid (umuhengerin or 5,3′-dihydroxy- 6,7,4′,5′-tetramethoxyflavone), isolated from P. punctulata. The formulation caused a significant antihypertensive effect through vasodilatation and a decrease in peripheral resistance without reflex tachycardia. This behavior could be detected through the ability of the developed formulation to reduce the elevated diastolic, pulse pressure, dicrotic notch pressure, and the systolic-dicrotic notch pressure difference. The developed IV formulation could be considered as an effective medication in the treatment of hypertensive emergencies, after clinical evaluation.
Characterization of Isolated Compounds
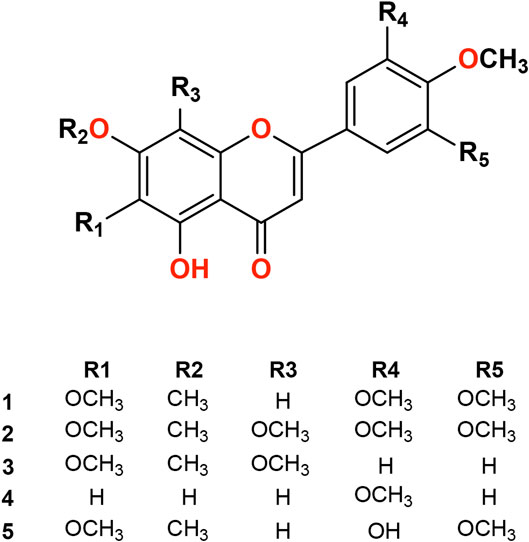
Isolated compounds from P. punctulata were identified based on their NMR data and comparison with previously published data. The compounds (Figure 1) were identified as umuhengerin (1), gardenin A (2), gardenin B (3), luteolin-3′,4′ -dimethyl ether (4), and 5,3′-dihydroxy-6,7,4′,5′-tetramethoxyflavone (5).
SNEEDS Formulation
Three different SNEDDS groups, of seven runs each, containing PEG 400 as a cosurfactant, tween 80 as a surfactant and one of the studied oils (olive oil, linoleic acid, and oleic acid) were prepared and characterized for the globule size and PDI, and the obtained results are represented in Table 1.
The aim was to develop a nano-emulsion with the lowest globule size. The united states pharmacopeia (USP) <729> stated that the mean globule size of an injectable emulsion should be <500 nm (Pharmacopeia., 2019). Injectable emulsions that reach a diameter of 5 μm or more have a high tendency to become entrapped in small capillaries, especially those found in the brain or the lungs, which may cause embolism (Nilsson et al., 2019). In this study, as the concentration of oil was decreased the globule size was decreased, the effect that could be attributed to the availability of more surfactant molecules, at low oil concentration, that adsorbs and form a closely pack surfactant film at the oil/water interface. This effect leads to the formation of a stable system of low interfacial tension and hence small globule size. Based on the obtained results for particle size, SNEDDS formulation which contains 10% linoleic acid, 80% PEG 400, and 10% tween 80 was used to develop a medicated IV formulation containing the isolated compounds.
Biological Study
The Biological study of the isolated compounds revealed significant vasodilation activity of compounds 1, 4, and 5 however, the anti-hypertensive effect was observed only with compounds 1 and 5.
Effects of IV Formulations Contain Compound 1 or 5 on Systolic and Diastolic Blood Pressure and Heart Rate
As shown in Figures 2,3A, intravenous injection of formulations containing compound 1 or 5 in doses of 12, 24, and 36 μg/kg resulted in a gradual dose-dependent reduction in the elevated systolic blood pressure induced by angiotensin after 10 min of each dose injection (p < 0.05). The reduction in diastolic blood pressure was also gradual and reached a plateau with statistical significance at doses 24 and 36 μg/kg (p < 0.05, Figure 3B) compared to the group administered the plain IV formulation. Intravenous injection of the plain IV formulation resulted in a significant reduction in heart rate compared to the saline group, while the IV formulations containing compounds 1 or 5 did not produce any further effect on heart rate compared with the plain IV formulation. (Figure 3C). The vasodilating effects of the P. punctulata isolated compounds; 1 and 5 were previously reported by our teamwork (Abdallah et al., 2020), but this behavior does not guarantee the antihypertensive efficacy as evidence by the lack of antihypertensive efficacy of compound 4. Compounds usually have multiple effects and mechanisms, and the net result is a summation of all these effects. The carefully selected vehicle ingredients helped in having a vehicle with a significant reduction of heart rate per se. Among many oils that can be used in SNEDDS preparation, linoleic acid with the reported (Christensen et al., 2005) effect on heart rate was selected. This effect on heart rate rescues the vasodilating effect of compounds isolated from P. punctulata from being masked by the reflex tachycardia that commonly associated with the decrease in peripheral resistance by most vasodilators.
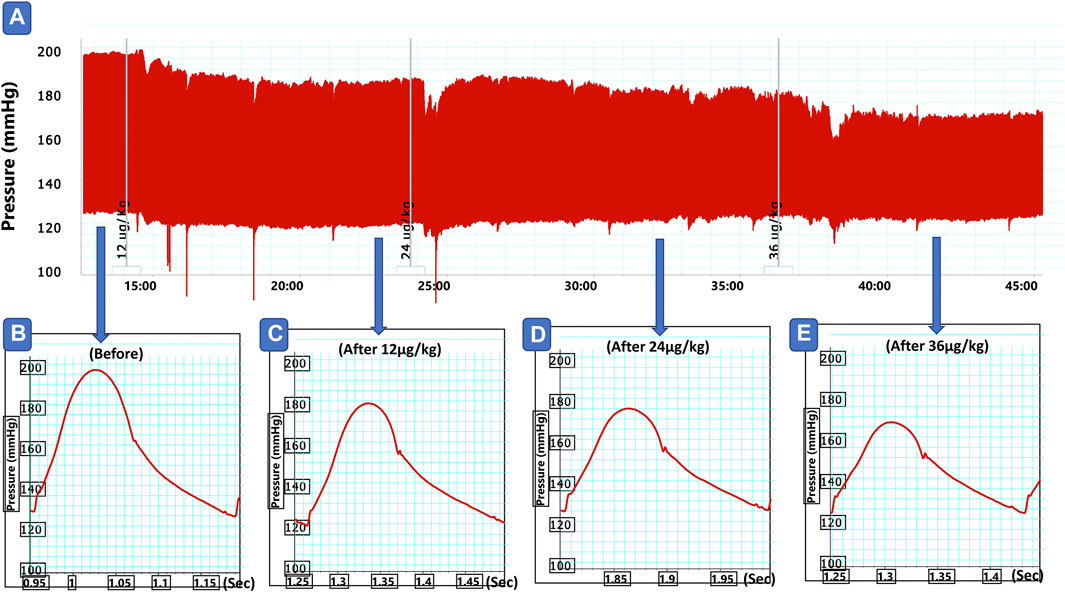
FIGURE 2. Representative traces of blood hemodynamic invasive recording (A), before (B), and after slow intravenous injection of formulation 1 in doses of 12 (C), 24 (D) and (E) 36 μg/kg in angiotensin model of hypertensive rats.
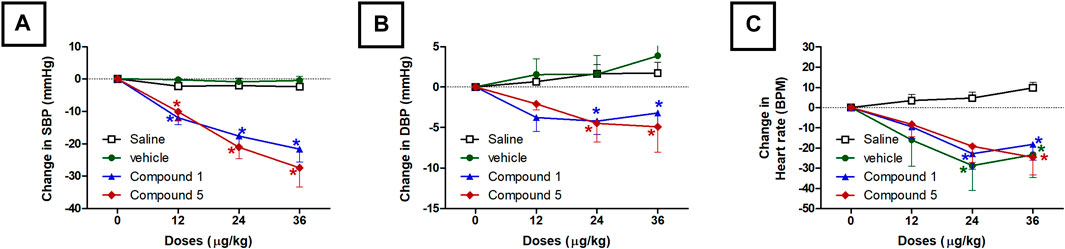
FIGURE 3. Effect of slow intravenous injection of formulations 1 and 5 on the systolic blood pressure (SBP, A), diastolic blood pressure (DBP, B), and the heart rate (C). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
Effects of IV Formulations Contain Compound 1 or 5 on Pulse Pressure, Dicrotic Notch Pressure, and SDP Difference
Pulse pressure is a marker for increased large arterial stiffness (Safar, 2018) and is a strong predictor of cardiovascular risk and hypertensive organ damage (Litwin et al., 2019). Both formulations contain compounds 1 and 5 resulted in a significant reduction in pulse pressure started after 10 min of intravenous injection of doses 24 and 36 μg/kg compared to plain IV formulation (p < 0.05, Figure 4A). The ability of both IV formulations to reduce pulse pressure is related to their ability to improve arterial compliance through reducing systolic blood pressure. Intravenous injection of doses 24 and 36 μg/kg of formulations containing compounds 1 and 5 resulted in a significant reduction in dicrotic notch pressure started after 10 min of injection compared to plain IV formulation (p < 0.05, Figure 4B). The dicrotic notch is a ubiquitous feature of the pressure waveform in the aorta. It is universally considered to be a marker for the end of the ventricular ejection period and is used routinely to calculate ejection duration in clinical practice (Nirmalan and Dark, 2014; Gamrah et al., 2020). Moreover, formulations contain compound 1 or 5 resulted in a significant reduction in the difference between systolic and dicrotic pressure (SDP difference) compared to plain IV formulation at doses 12, 24, and 36 μg/kg (p < 0.05, Figure 4C). The SDP difference is important in reflecting the coupling between myocardial contractility and a given afterload (Morelli et al., 2020). Previously Senzaki et al suggested that increased afterload rather than reduced contractility impairs ventricular-arterial coupling (Senzaki et al., 2006).
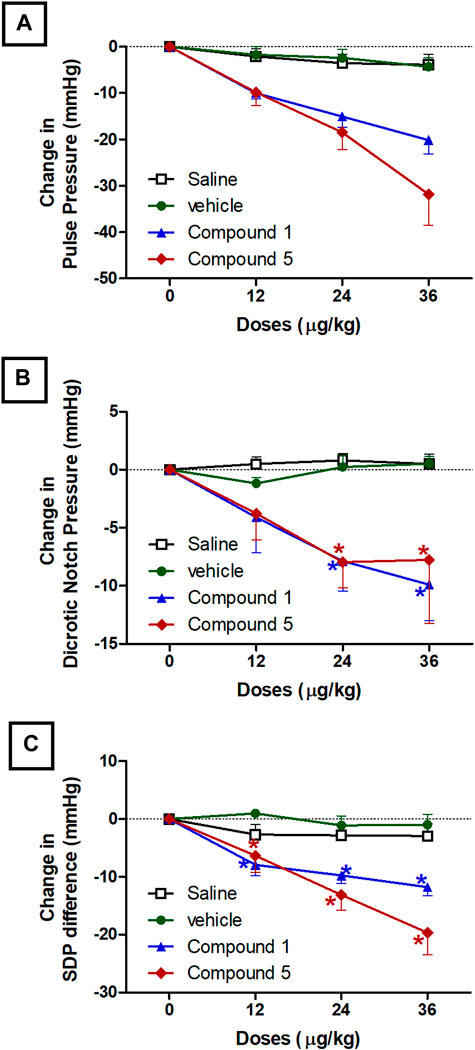
FIGURE 4. Effect of slow intravenous injection of formulations 1 and 5 on the pulse pressure (A), dicrotic notch pressure (B), and the systolic blood pressure-dicrotic notch pressure difference (SDP-difference, (C). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
Effects of IV Formulations Contain Compound 1 or 5 on Ejection Duration, Non-ejection Duration, and Time to Peak
Left ventricular systolic ejection duration is the duration of systolic ejection in milliseconds and is a reproducible measure of systolic performance (Avadhani et al., 2018) and its prolongation is a useful sign of aortic stenosis (Kligfield et al., 1984). However, the non-ejection duration reflects the periods when all heart valves are closed. Figure 5 shows that intravenous injection of formulation contains compound 1 resulted in a significant decrease in ejection duration at doses 24 and 36 μg/kg and a significant increase in non-ejection duration at dose 36 μg/kg but no change in time to peak compared to plain IV formulation. While formulation contains compound 5 resulted in a significant decrease in ejection duration and a significant increase in non-ejection duration at dose 36 μg/kg and didn’t change in time to peak compared to plain IV formulation (p < 0.05). The ejection duration from the left ventricle has been significantly prolonged in the patients with central systolic blood pressure higher than brachial systolic blood pressure (Bulas et al., 2017) and its prolongation concurrently widens the pulse pressure, which leads to ventricular hypertrophy (Toprak et al., 2009).
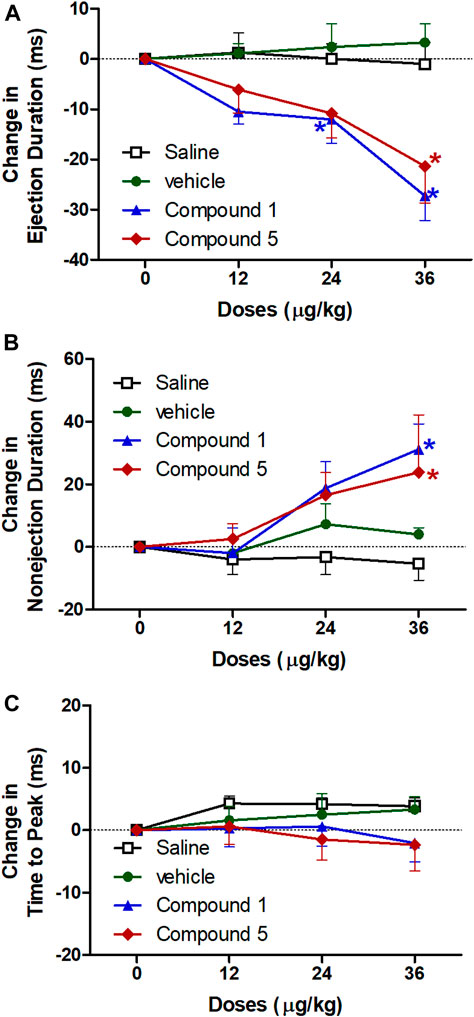
FIGURE 5. Effect of slow intravenous injection of formulations 1 and 5 on the ejection duration (A), nonejection duration (B), and the time to peak (C). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
Effects of IV Formulations Contain Compound 1 or 5 on Cardiac Electrophysiology
ECG signals and the information obtained through the analysis of these signals constitute the main source of diagnosis for many cardiovascular system diseases including hypertension (Yanık et al., 2020). In the current study injection of formulations containing compound 1 or 5 did not significantly affect atrial conductivity or the propagation of the impulse through the AV node and the conduction system to the ventricles as they did not affect the P-wave duration or PR interval compared to plain IV formulation (Figure 6). Similarly, the entire ventricular activity from the beginning of ventricular depolarization through the plateau phase to the ventricular repolarization was not affected by intravenous injection of formulations containing compound 1 or 5 as it had no significant effect on either QTc or JT intervals compared to plain IV formulation in hypertensive rats infused with angiotensin II (Figure 7).
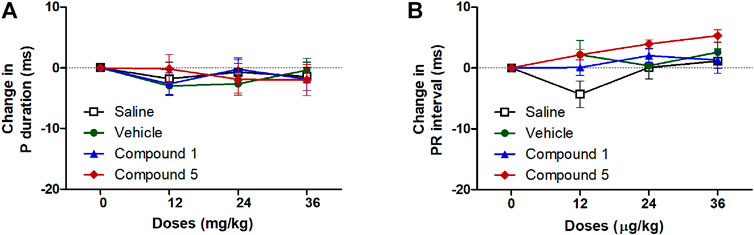
FIGURE 6. Effect of slow intravenous injection of formulations 1 and 5 on the p wave duration (A) and PR interval (B). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
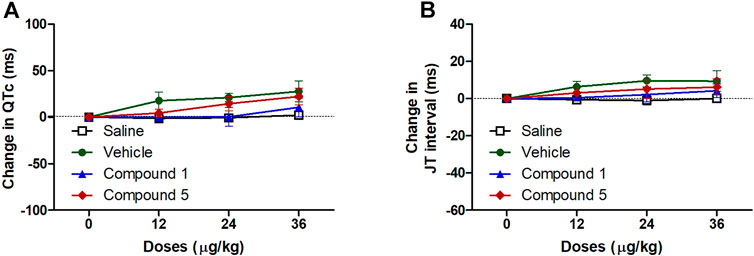
FIGURE 7. Effect of slow intravenous injection of formulations 1 and 5 on the QTc interval (A) and the JT interval (B). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
On the other hand, vehicle intravenous injection resulted in a reduction in T wave amplitude, this reduction is significantly different from the saline group at dose 36 μg/kg (p < 0.05, Figure 8B). Both formulations contain compound 1 or 5 resulted in a significant decrease in R wave amplitude which represents early ventricular depolarization and an increase in T wave amplitude which reflects ventricular repolarization compared to vehicle group only at dose 36 μg/kg of formulation 5 (p < 0.05, Figure 8). Its obvious from these results that the effect of formulations containing compound 1 or 5 is on the amplitudes not the duration of the electrical vectors reflecting ventricular activity.
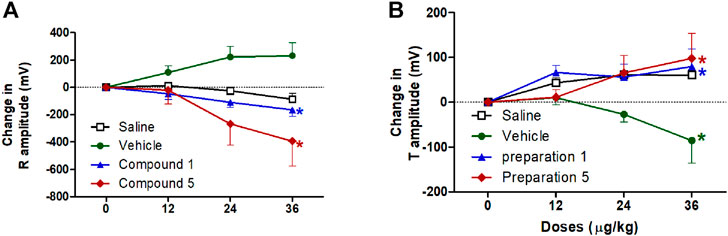
FIGURE 8. Effect of slow intravenous injection of formulations 1 and 5 on the R amplitude (A) and the T amplitude (B). Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values; by two Way ANOVA and Bonferroni post hoc test.
Effects of IV Formulations Contain Compound 1 or 5 on Serum Levels of Sodium and Potassium
Sodium and potassium are two important electrolytes that are closely associated with changes in cardiovascular structures and functions (Zhao et al., 2018). High serum sodium can be a marker of risk for increased pulse pressure, a surrogate index of arterial stiffness, in individuals at high risk for cardiovascular events (Nowak et al., 2019). As shown in Figure 9A, intravenous injection of the preparations vehicle resulted in a significant reduction in serum sodium level (p < 0.05) compared to the saline group. While compound 1 formulation significantly increased serum sodium compared with vehicle (p < 0.05) returning it to the initial level of saline control, compound 5 preparation produced a significant further reduction in serum sodium compared with the vehicle (p < 0.05).
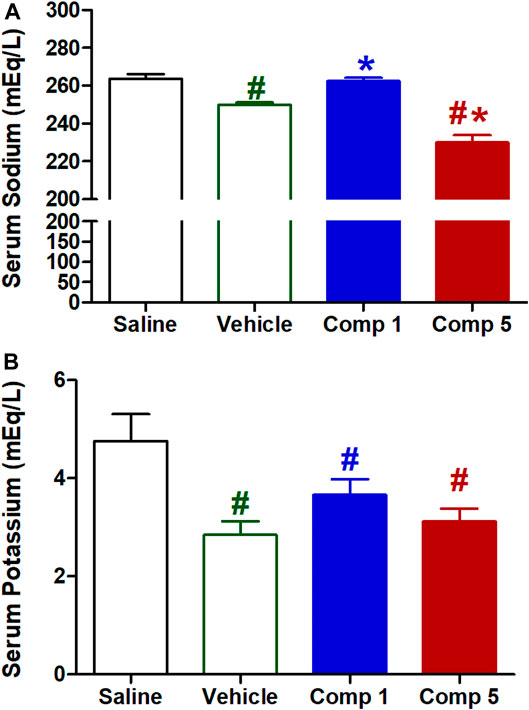
FIGURE 9. Effect of slow intravenous injection of formulations 1 and 5 on serum sodium (A) and potassium (B) levels. Data presented as mean ± standard error of 6 animals. *p < 0.05, compared with the corresponding vehicle control values, #p < 0.05, compared with the corresponding saline control values; by One Way ANOVA and Newman Keuls post hoc test.
On the other hand, intravenous injection of the preparations vehicle resulted in a significant reduction in serum potassium level (p < 0.05, Figure 9B) compared to the saline group. While neither compound 1 nor 5 preparations produced significant further reductions in serum potassium levels, they still significantly reduced from the saline control (p < 0.05). The ability of formulation contains compound 5 to reduce serum sodium level could arise from its direct vasodilation which increases renal blood flow and then increasing glomerular filtration. However, further studies are required to determine the underlying mechanisms involved with sodium and potassium excretion and the precise location of action in the nephron.
In conclusion, vasodilators produced reflex tachycardia and an increase in heart rate that masks its anti-hypertensive effect. The formulation contains compounds 1 or 5 in IV SNEDDS containing 10% linoleic acid caused significant antihypertensive effect through vasodilatation and decreasing peripheral resistance without reflex tachycardia. This could be detected through the ability of this formula to reduce the elevated diastolic, pulse pressure, dicrotic notch pressure, and the systolic–dicrotic notch pressure difference and through modulation of ventricular electrical activity. The studied nano-pharmaceutical formulation suggesting them as effective medications in hypertensive emergencies, after clinical evaluation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Faculty of Pharmacy’s Research Ethics Committee, Zagazig University (#ZU-IACUC/3/F/33/2021).
Author Contributions
HMA, AME-H, and GAM performed collection extraction of plant material and isolated the active flavonoids. TAA prepared SNEDDS. AMM identified the isolated compounds, HME-B and NAH performed all biological studies. All authors read and approved the final manuscript.
Funding
This project was funded by Science and Technology Unit—King Abdulaziz University—Kingdom of Saudi Arabia—award number (UE-41-115).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abdallah, H. M., Hassan, N. A., El-Halawany, A. M., Mohamed, G. A., Safo, M. K., and El-Bassossy, H. M. (2020). Major Flavonoids from Psiadia Punctulata Produce Vasodilation via Activation of Endothelial Dependent NO Signaling. J. Adv. Res. 24, 273–279. doi:10.1016/j.jare.2020.01.002
Abdallah, H. M., Zakaria, E. M., El-Halawany, A. M., Mohamed, G. A., Safo, M. K., and El-Bassossy, H. M. (2019). Psiadia Punctulata Major Flavonoids Alleviate Exaggerated Vasoconstriction Produced by Advanced Glycation End Products. PLoS One 14, e0222101. doi:10.1371/journal.pone.0222101
Achola, K. J., Indalo, A. A., and Munenge, R. W. (1998). Pharmacological Activities of Psiadia Punculata. Pharm. Biol. 36, 88–92. doi:10.1076/phbi.36.2.88.4605
Ahmed, O. A. A., Badr-Eldin, S. M., Tawfik, M. K., Ahmed, T. A., El-Say, K. M., and Badr, J. M. (2014). Design and Optimization of Self-Nanoemulsifying Delivery System to Enhance Quercetin Hepatoprotective Activity in Paracetamol-Induced Hepatotoxicity. J. Pharm. Sci. 103, 602–612. doi:10.1002/jps.23834
Ahmed, T. A., El-Say, K. M., Hosny, K. M., and Aljaeid, B. M. (2018). Development of Optimized Self-Nanoemulsifying Lyophilized Tablets (SNELTs) to Improve Finasteride Clinical Pharmacokinetic Behavior. Drug Dev. Ind. Pharm. 44, 652–661. doi:10.1080/03639045.2017.1405977
Avadhani, S., Ihsan, M., Nunez, A., Kamran, H., Singh, S., Hasan, Z., et al. (2018). The Effect of Lower Body Positive Pressure on Left Ventricular Ejection Duration in Patients with Heart Failure. Dose-Response 16, 1559325818811543. doi:10.1177/1559325818811543
Azhar, A., and El-Bassossy, H. M. (2014). Pentoxifylline Alleviates Cardiac Ischemia and Dysfunction Following Experimental Angina in Insulin Resistance. PLoS One 9, e98281. doi:10.1371/journal.pone.0098281
Bulas, J., Potočárová, M., Murín, J., Kozlíková, K., Luha, J., and Čaprnda, M. (2017). Central Systolic Hypertension in Patients with Well-Controlled Hypertension. Biomed. Res. Int. 6, 1–10. doi:10.1155/2017/8158974
Chockalingam, A., Campbell, N. R., and George Fodor, J. (2006). Worldwide Epidemic of Hypertension. Can. J. Cardiol. 22, 553–555. doi:10.1016/s0828-282x(06)70275-6
Christensen, J. H., Schmidt, E. B., Mølenberg, D., and Toft, E. (2005). Alpha-linolenic Acid and Heart Rate Variability in Women Examined for Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 15, 345–351. doi:10.1016/j.numecd.2004.09.005
Cuthbert, J. J., Pellicori, P., and Clark, A. L. (2020). Cardiovascular Outcomes with Sacubitril-Valsartan in Heart Failure: Emerging Clinical Data. Tcrm 16, 715–726. doi:10.2147/tcrm.s234772
El-Bassossy, H. M., Al-Thubiani, W. S., Elberry, A. A., Mujallid, M. I., Ghareib, S. A., Azhar, A. S., et al. (2017a). Zingerone Alleviates the Delayed Ventricular Repolarization and AV Conduction in Diabetes: Effect on Cardiac Fibrosis and Inflammation. PLoS One 12, e0189074. doi:10.1371/journal.pone.0189074
El-Bassossy, H. M., Awan, Z., and El-Mas, M. M. (2017b). Perinatal Ciclosporin A Exposure Elicits Sex-Related Cardiac Dysfunction and Inflammation in the Rat Progeny. Toxicol. Lett. 281, 35–43. doi:10.1016/j.toxlet.2017.09.002
El-Bassossy, H. M., Banjar, Z. M., and El-Mas, M. M. (2017c). The Inflammatory State Provokes Sexual Dimorphism in Left Ventricular and Electrocardiographic Effects of Chronic Cyclosporine in Rats. Sci. Rep. 7, 42457. doi:10.1038/srep42457
El-Bassossy, H. M., Elberry, A. A., Ghareib, S. A., Azhar, A., Banjar, Z. M., and Watson, M. L. (2016). Cardioprotection by 6-gingerol in Diabetic Rats. Biochem. Biophysical Res. Commun. 477, 908–914. doi:10.1016/j.bbrc.2016.06.157
El-Bassossy, H. M., Mahmoud, M. F., and Eid, B. G. (2018). The Vasodilatory Effect of Allopurinol Mediates its Antihypertensive Effect: Effects on Calcium Movement and Cardiac Hemodynamics. Biomed. Pharmacother. 100, 381–387. doi:10.1016/j.biopha.2018.02.033
El-Bassossy, H. M., and Watson, M. L. (2015). Xanthine Oxidase Inhibition Alleviates the Cardiac Complications of Insulin Resistance: Effect on Low Grade Inflammation and the Angiotensin System. J. Transl Med. 13, 1–11. doi:10.1186/s12967-015-0445-9
El-Say, K. M., Ahmed, T. A., Badr-Eldin, S. M., Fahmy, U., Aldawsari, H., and Ahmed, O. A. A. (2015). Enhanced Permeation Parameters of Optimized Nanostructured Simvastatin Transdermal Films:ex Vivoandin Vivoevaluation. Pharm. Dev. Tech. 20, 919–926. doi:10.3109/10837450.2014.938859
Estrera, A. L., Miller, C. C., Safi, H. J., Goodrick, J. S., Keyhani, A., Porat, E. E., et al. (2006). Outcomes of Medical Management of Acute Type B Aortic Dissection. Circulation 114, I–384. doi:10.1161/circulationaha.105.001479
Gamrah, M. A., Xu, J., El Sawy, A., Aguib, H., Yacoub, M., and Parker, K. H. (2020). Mechanics of the Dicrotic Notch: An Acceleration Hypothesis. Proc. Inst. Mech. Eng. H 234, 1253–1259. doi:10.1177/0954411920921628
Gonzalez, A. A., Green, T., Luffman, C., Bourgeois, C. R. T., Gabriel Navar, L., and Prieto, M. C. (2014). Renal Medullary Cyclooxygenase-2 and (Pro)renin Receptor Expression during Angiotensin II-dependent Hypertension. Am. J. Physiology-Renal Physiol. 307, F962–F970. doi:10.1152/ajprenal.00267.2014
Hariri, L., and Patel, J. (2021). Vasodilators. StatPearls. Treasure Island, FL: StatPearls Publishing.
Irby, D., Du, C., and Li, F. (2017). Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharmaceutics 14, 1325–1338. doi:10.1021/acs.molpharmaceut.6b01027
Kester, M., Karpa, K. D., and Vrana, K. E. (2012). “Cardiovascular System,” in Elsevier's Integrated Review Pharmacology. Editors M. Kester, K. D. Karpa, and K. E. Vrana Second EditionSecond Edition (Philadelphia: W.B. Saunders), 125–151. doi:10.1016/b978-0-323-07445-2.00008-2
Kidane, Y., Bokrezion, T., Mebrahtu, J., Mehari, M., Gebreab, Y. B., Fessehaye, N., et al. (2018). Vitro Inhibition Of-Amylase And-Glucosidase by Extracts from Psiadia Punctulata and Meriandra Bengalensis. London: Evid Based Complement Alternat Med.
Kligfield, P., Okin, P., Devereux, R. B., Goldberg, H., and Borer, J. S. (1984). Duration of Ejection in Aortic Stenosis: Effect of Stroke Volume and Pressure Gradient. J. Am. Coll. Cardiol. 3, 157–161. doi:10.1016/s0735-1097(84)80443-x
Kurdi, M., and Booz, G. W. (2011). New Take on the Role of Angiotensin II in Cardiac Hypertrophy and Fibrosis. Hypertension 57, 1034–1038. doi:10.1161/hypertensionaha.111.172700
Litwin, M., Obrycki, Ł., Niemirska, A., Sarnecki, J., and Kułaga, Z. (2019). Central Systolic Blood Pressure and central Pulse Pressure Predict Left Ventricular Hypertrophy in Hypertensive Children. Pediatr. Nephrol. 34, 703–712. doi:10.1007/s00467-018-4136-7
Mallidi, J., Macías, C. G., and Lotfi, A. S. (2015). “Vasodilators and Management of Hypertensive Emergencies,” in Pathophysiology and Pharmacotherapy of Cardiovascular Disease. Editors G. Jagadeesh, P. Balakumar, and K. Maung-U. (Cham: Springer International Publishing)), 869–886. doi:10.1007/978-3-319-15961-4_40
Marangoni, F., Agostoni, C., Borghi, C., Catapano, A. L., Cena, H., Ghiselli, A., et al. (2020). Dietary Linoleic Acid and Human Health: Focus on Cardiovascular and Cardiometabolic Effects. Atherosclerosis 292, 90–98. doi:10.1016/j.atherosclerosis.2019.11.018
Micucci, M., Bolchi, C., Budriesi, R., Cevenini, M., Maroni, L., Capozza, S., et al. (2020). Antihypertensive Phytocomplexes of Proven Efficacy and Well-Established Use: Mode of Action and Individual Characterization of the Active Constituents. Phytochemistry 170, 112222. doi:10.1016/j.phytochem.2019.112222
Morelli, A., Romano, S. M., Sanfilippo, F., Santonocito, C., Frati, G., Chiostri, M., et al. (2020). Systolic-dicrotic Notch Pressure Difference Can Identify Tachycardic Patients with Septic Shock at Risk of Cardiovascular Decompensation Following Pharmacological Heart Rate Reduction. Br. J. Anaesth. 125, 1018–1024. doi:10.1016/j.bja.2020.05.058
Nilsson, N., Nezvalova-Henriksen, K., and Tho, I. (2019). Emulsion Stability of Different Intravenous Propofol Formulations in Simulated Co-administration with Remifentanil Hydrochloride. Pharm. Technol. Hosp. Pharm. 4, 77–87. doi:10.1515/pthp-2019-0014
Nirmalan, M., and Dark, P. M. (2014). Broader Applications of Arterial Pressure Wave Form Analysis. Continuing Edu. Anaesth. Crit. Care Pain 14, 285–290. doi:10.1093/bjaceaccp/mkt078
Nowak, K. L., Chonchol, M., Jovanovich, A., You, Z., Bates, J., Foy, C., et al. (2019). Serum Sodium and Pulse Pressure in SPRINT. Am. J. Hypertens. 32, 649–656. doi:10.1093/ajh/hpz055
Pharmacopeia, U. S. (2019). Generell Chapter: <729> Globule Size Distribution in Lipid Injectable Emulsions. U. S. Pharmacopeia (National Formulary) (UPS42-NF37). Berlin: Springer.
Rascher, W., and Paech, C. (2020). Arterial Hypertension in Children. in Arterial Hypertension in Children. Pediatric Pharmacotherapy. Editors W. Kiess, M. Schwab, and J. Van Den Anker (Cham: Springer International Publishing), 193–208. doi:10.1007/164_2020_359
Safar, M. E. (2018). Arterial Stiffness as a Risk Factor for Clinical Hypertension. Nat. Rev. Cardiol. 15, 97–105. doi:10.1038/nrcardio.2017.155
Senzaki, H., Masutani, S., Ishido, H., Taketazu, M., Kobayashi, T., Sasaki, N., et al. (2006). Cardiac Rest and reserve Function in Patients with Fontan Circulation. J. Am. Coll. Cardiol. 47, 2528–2535. doi:10.1016/j.jacc.2006.03.022
Singh, M., Kanoujia, J., Singh, P., Tripathi, C. B., Arya, M., Parashar, P., et al. (2016). Development of an α-linolenic Acid Containing Soft Nanocarrier for Oral Delivery: In Vitro and In Vivo Evaluation. RSC Adv. 6, 77590–77602. doi:10.1039/c6ra15166c
Taha, A. Y. (2020). Linoleic Acid-Good or Bad for the Brain?. NPJ Sci. Food 4, 1. doi:10.1038/s41538-019-0061-9
Toprak, A., Reddy, J., Chen, W., Srinivasan, S., and Berenson, G. (2009). Relation of Pulse Pressure and Arterial Stiffness to Concentric Left Ventricular Hypertrophy in Young Men (From the Bogalusa Heart Study). Am. J. Cardiol. 103, 978–984. doi:10.1016/j.amjcard.2008.12.011
Van Staden, D., Du Plessis, J., and Viljoen, J. (2020). Development of a Self-Emulsifying Drug Delivery System for Optimized Topical Delivery of Clofazimine. Pharmaceutics 12, 523. doi:10.3390/pharmaceutics12060523
Verma, T., Sinha, M., Bansal, N., Yadav, S. R., Shah, K., and Chauhan, N. S. (2020). Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 12, 1–30. doi:10.1007/s13659-020-00281-x
White, K. P., Radke-Sharpe, N. F., Kaiser, D. L., and Owens, G. K. (1989). A Dynamic Model of Angiotensin II Infusion Experiments. J. Biomed. Eng. 11, 63–71. doi:10.1016/0141-5425(89)90168-4
Yanık, H., Değirmenci, E., Büyükakıllı, B., Karpuz, D., Kılınç, O. H., and Gürgül, S. (2020). Electrocardiography (ECG) Analysis and a New Feature Extraction Method Using Wavelet Transform with Scalogram Analysis. Biomed. Tech. 1, 114. doi:10.1515/bmt-2019-0147
Keywords: methoxy flavonoids, umuhengerin, reflex tachycardia, vasodilators, SNEDDS
Citation: Abdallah HM, El-Bassossy HM, El-Halawany AM, Ahmed TA, Mohamed GA, Malebari AM and Hassan NA (2021) Self-Nanoemulsifying Drug Delivery System Loaded with Psiadia punctulata Major Metabolites for Hypertensive Emergencies: Effect on Hemodynamics and Cardiac Conductance. Front. Pharmacol. 12:681070. doi: 10.3389/fphar.2021.681070
Received: 15 March 2021; Accepted: 20 May 2021;
Published: 10 June 2021.
Edited by:
Dâmaris Silveira, University of Brasilia, BrazilReviewed by:
Hady Keita, University of the South Sierra, MexicoPaula C. Castilho, Universidade da Madeira, Portugal
Copyright © 2021 Abdallah, El-Bassossy, El-Halawany, Ahmed, Mohamed, Malebari and Hassan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossam M. Abdallah, aG1hZmlmaUBrYXUuZWR1LnNh
 Hossam M. Abdallah
Hossam M. Abdallah Hany M. El-Bassossy
Hany M. El-Bassossy Ali M. El-Halawany
Ali M. El-Halawany Tarek A. Ahmed4,5
Tarek A. Ahmed4,5 Noura A. Hassan
Noura A. Hassan