- 1Department of Pharmacy, College of Medicine and Health Sciences, Ambo University, Ambo, Ethiopia
- 2Pharm-Biotechnology and Traditional Medicine Center of Excellence, Mbarara University of Science and Technology, Mbarara, Uganda
- 3School of Pharmacy, Faculty of Medicine, Hawassa University, Hawassa, Ethiopia
- 4Chemistry Section, Department of Applied Studies, Institute of Technology, Malawi University of Science and Technology, Limbe, Malawi
- 5School of Nursing and Midwifery, College of Medicine and Health Sciences, University of Rwanda, Butare, Rwanda
- 6Department of Biology, Faculty of Science, Mbarara University of Science and Technology, Mbarara, Uganda
Background: Viruses cause various human diseases, some of which become pandemic outbreaks. This study synthesized evidence on antiviral medicinal plants in Africa which could potentially be further studied for viral infections including Coronavirus disease 2019 (COVID-19) treatment.
Methods: PUBMED, CINAHIL, Scopus, Google Scholar, and Google databases were searched through keywords; antiviral, plant, herb, and Africa were combined using “AND” and “OR”. In-vitro studies, in-vivo studies, or clinical trials on botanical medicine used for the treatment of viruses in Africa were included.
Results: Thirty-six studies were included in the evidence synthesis. Three hundred and twenty-eight plants were screened for antiviral activities of which 127 showed noteworthy activities against 25 viral species. These, were Poliovirus (42 plants), HSV (34 plants), Coxsackievirus (16 plants), Rhinovirus (14plants), Influenza (12 plants), Astrovirus (11 plants), SARS-CoV-2 (10 plants), HIV (10 plants), Echovirus (8 plants), Parvovirus (6 plants), Semiliki forest virus (5 plants), Measles virus (5 plants), Hepatitis virus (3 plants), Canine distemper virus (3 plants), Zika virus (2 plants), Vesicular stomatitis virus T2 (2 plants). Feline herpesvirus (FHV-1), Enterovirus, Dengue virus, Ebola virus, Chikungunya virus, Yellow fever virus, Respiratory syncytial virus, Rift Valley fever virus, Human cytomegalovirus each showed sensitivities to one plant.
Conclusion: The current study provided a list of African medicinal plants which demonstrated antiviral activities and could potentially be candidates for COVID-19 treatment. However, all studies were preliminary and in vitro screening. Further in vivo studies are required for plant-based management of viral diseases.
Background
Viruses cause various human diseases of which several such as Ebola, HIV/AIDS, and Hepatitis B are hard to treat. Many pandemic outbreaks in world history were caused by a viral infection. The Spanish flu pandemic of 1918, the deadliest in history, infected an estimated 500 million people worldwide; which is about one-third of the planet’s population, and killed an estimated 20 million to 50 million people (1). In recent years, pandemics have arisen and have also been contained using various approaches. For example, Ebola virus outbreak between 2013 and 2016 with 11323 deaths (Trilla et al., 2008), Coronavirus (Severe Acute Respiratory Syndrome (SARS) with deaths of 229 (World Health Organization, 2003), Middle East respiratory syndrome (MERS) as of May 31, 2015, which had 483 (40%) mortality (Zumla et al., 2015) are some of the recorded global pandemics. Since December 2019 the world is suffering from Coronavirus disease 2019 (COVID-19) with more than 197 million people infected and more than 4, 219, 861 deaths as of August 4, 2021 (World Health Organization, 2020).
The use of natural medicinal agents dates back to human prehistory where plants formed the basis of traditional medicine (TM) systems. Traditional medicine refers to health practices, approaches, knowledge, and beliefs incorporating plant, animal, and mineral-based medicines, spiritual therapies, manual techniques, and exercises which are applied singularly or in combination to treat or to diagnose and prevent illnesses or maintain well-being (World Health Assembly, 2003). Traditional medicine has a high influence on the African health system with an estimated 80% of the population depending on TM practice for primary health care purposes (World Health Organization, 2005). The availability and affordability of the TM aligned with inherited knowledge of the practice in local communities might have contributed to their wide use (Fennell et al., 2004).
Several herbal medicines have been used to treat viral infections traditionally for a long time. Some studies have reported the inhibitory effect of medicinal plant extracts against several viruses. Some of these studies were conducted on HIV, herpes simplex virus, hepatitis B virus, and poliovirus. For example, ethnobotanical studies in Africa described the treatment of viral hepatitis with traditional medicine in Africa (Vlietinck et al., 1995; Sindambiwe et al., 1999; Cos et al., 2002a; Amenu, 2007; Abera, 2014; Traore et al., 2018). Furthermore, plants have been reported to have antiviral potential against conventional medicine-resistant strains of viruses (Serkedjieva, 2003). Nine traditional Chinese botanicals were optimized to treat the symptoms of SARS during its outbreak (Zhang et al., 2004). In another study, small molecules from natural compounds have been screened and confirmed to inhibit important proteins in SARS or MERS coronavirus (Zhang et al., 2020). Despite having lots of endemic knowledge and practice on African herbal medicine, there is a paucity of scientific evidence on their efficacy and safety. This study aimed to summarize the evidence on antiviral medicinal plants in Africa which could potentially be further studied for COVID-19 treatment.
Methods
Study Design
This review was conducted using database searches and followed statements for Reporting Systematic Reviews and Meta-Analyses (Liberati et al., 2009).
Search Strategy
Data were collected from MEDLINE/PUBMED, CINAHIL, Google Scholar, and Scopus databases. No language limitations were applied to reduce selection bias and Google was used to translate articles published in other languages than English. The search strategy used the following terms with appropriate Boolean operators; (“virus diseases” OR (“virus” AND “diseases”) OR “virus diseases” OR (“viral” AND “infection”) OR “viral infection”) OR (“poliovirus” OR “poliovirus” OR HSV OR (“simplexvirus” OR “simplexvirus” OR (“herpes” AND “simplex” AND “virus”) OR “herpes simplex virus”) OR (“enterovirus” OR “enterovirus” OR “coxsackievirus” OR (“influenza, human” OR (“influenza” AND “human”) OR “human influenza” OR “influenza”) OR (astro AND (“viruses” OR “viruses” OR “virus”)) OR (“parvovirus” OR “parvovirus”) OR (“rhinovirus” OR “rhinovirus”) OR (“enterovirus b, human” OR “human enterovirus b” OR “echovirus”) OR (“hiv"OR “hiv”) OR (“hiv”OR “hiv” OR (“human” AND “immunodeficiency” AND “virus”) OR “human immunodeficiency virus”) OR (semiliki AND (“forests”OR “forests” OR “forest”) AND (“viruses”OR “viruses” OR “virus”)) OR (“measles virus”OR (“measles” AND “virus”) OR “measles virus”) OR (“hepatitis viruses”OR (“hepatitis” AND “viruses”) OR “hepatitis viruses” OR (“hepatitis” AND “virus”) OR “hepatitis virus”) OR (“zika virus”OR (“zika” AND “virus”) OR “zika virus”) OR ((“vesicular stomatitis indiana virus”OR (“vesicular” AND “stomatitis” AND “indiana” AND “virus”) OR “vesicular stomatitis indiana virus” OR (“vesicular” AND “stomatitis” AND “virus”) OR “vesicular stomatitis virus”) AND T2) OR (“coronavirus disease 2019” OR “COVID-2019″) AND “herbal medicine” OR “traditional medicine” OR “oriental medicine” OR “Chinese medicine” OR “African medicine” OR “herbal formula” OR herb AND”) AND (“ AND (“africa"OR “africa”) AND “OR” AND ((“african continental ancestry group”OR (“african” AND “continental” AND “ancestry” AND “group”) OR “african continental ancestry group” OR “african”) AND countries).
Study Selection
We included original research articles and unpublished dissertations from their inception to 2020. The unpublished dissertations were obtained from university website (http://etd.aau.edu.et, http://erepository.uonbi.ac.ke). EndNote reference manager was used to remove the duplications of references before screening. Either in vitro studies or in vivo studies or clinical trials of herbal medicine on African medicinal plants were included. Studies were eligible for inclusion if they were conducted to determine antiviral activities using available scientific methods and conducted on medicinal plants in Africa. Studies conducted on medicinal plants outside of Africa were excluded from the study. Review articles and ethnobotanical studies were also excluded. Eligibility assessment was conducted by TB and SD independently and disagreement between authors was resolved by discussion.
Results
In this study 316 publications were retrieved of which 36 (Ferrea et al., 1993; Beuscher et al., 1994; Vlietinck et al., 1995; Nakano et al., 1997; Kitamura et al., 1998; Hussein et al., 1999; Kudi and Myint, 1999; Sindambiwe et al., 1999; Anani et al., 2000; Yoosook et al., 2000; Cos et al., 2002b; Chiang et al., 2003; Wang et al., 2004; Bessong et al., 2005; Gebre-Mariam et al., 2006; Tolo et al., 2006; Kambizi et al., 2007; Maregesi et al., 2008; Duraipandiyan and Ignacimuthu, 2009; Gyuris et al., 2009; Ojo et al., 2009; Selvarani, 2009; Sunday et al., 2010; Astani et al., 2011; Nwodo et al., 2011; Sultana, 2011; Ndhlala et al., 2013; Ogbole et al., 2013; Kwena, 2014; David et al., 2017; Clain et al., 2018; Mehrbod et al., 2018; Nasr-Eldin et al., 2018; Ogbole et al., 2018; Cambaza, 2020; Gyebi et al., 2021) were included in the qualitative synthesis, Figure 1.
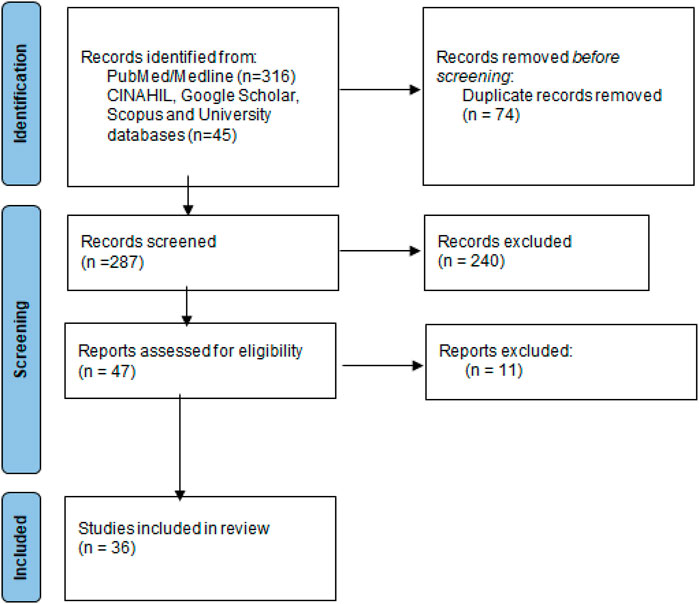
FIGURE 1. Flow diagram of included studies. Legend: The PRIMSA diagram details our search and selection process applied during the review.
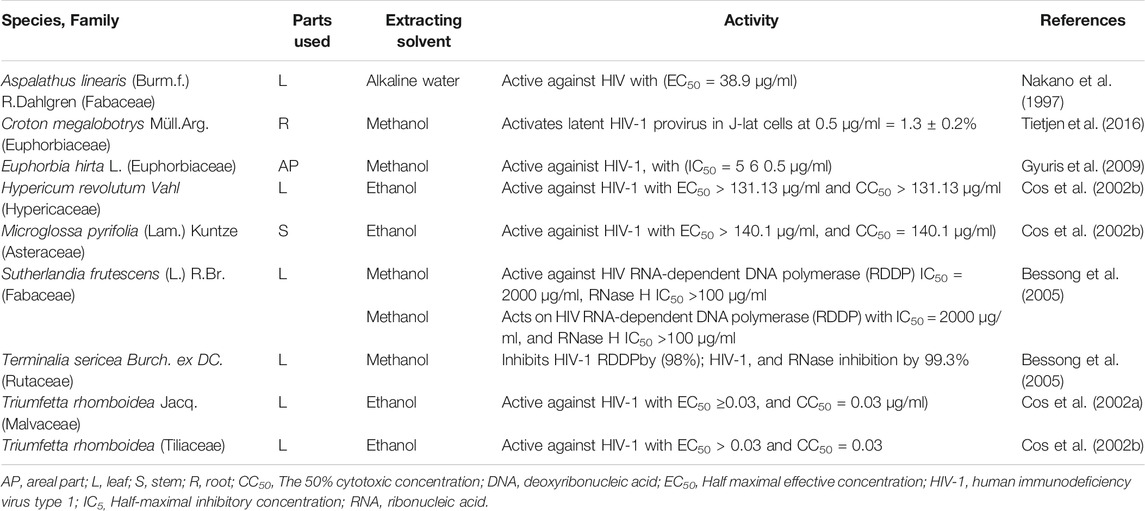
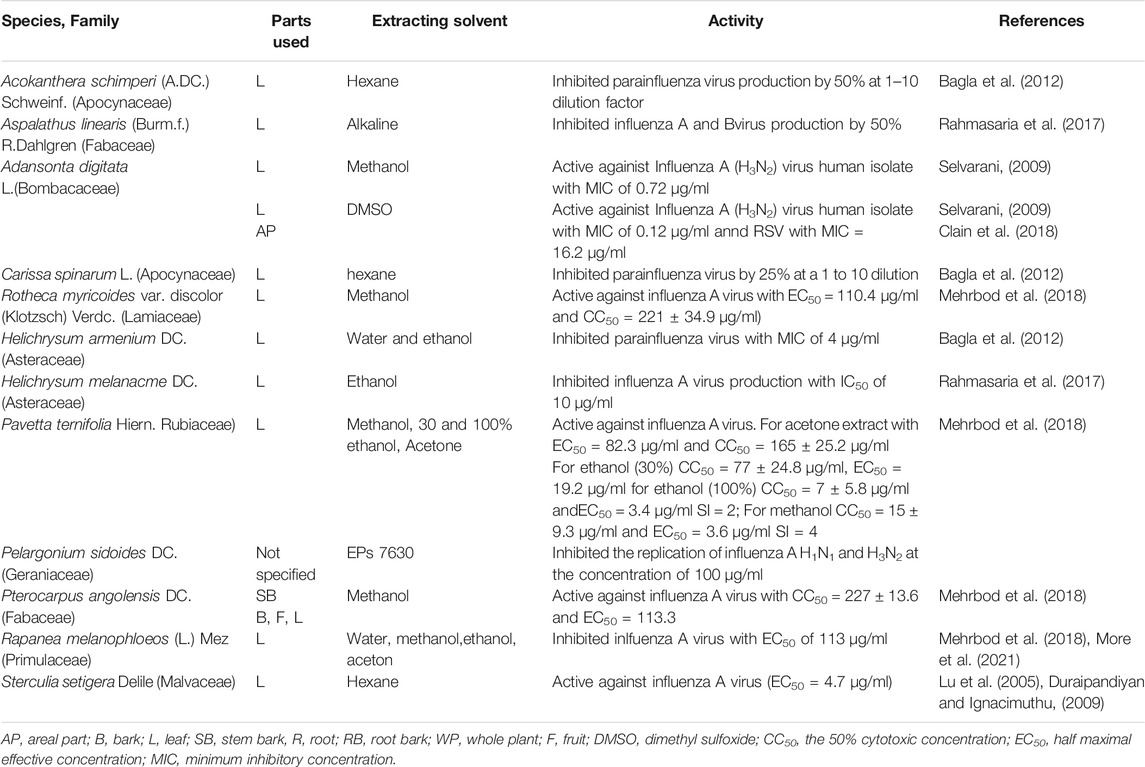
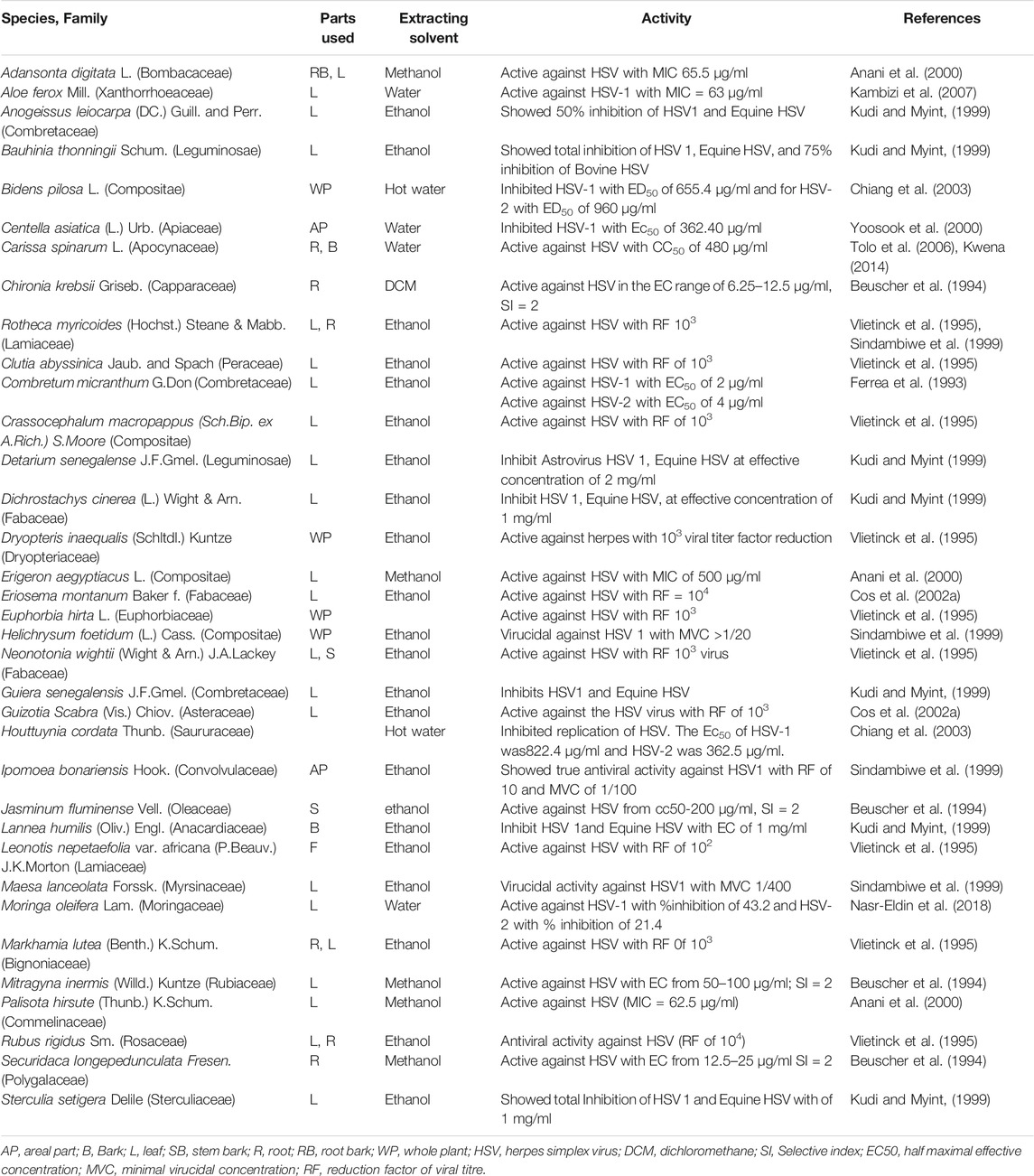
Three hundred and twenty-eight plants were screened for antiviral activities of which 127 tested showed activities against 25 viral species; Among these were Poliovirus (42 plants), HSV (34 plants), Coxsackievirus (16 plants), Rhinovirus (14plants), Influenza (12 plants), Astrovirus (11 plants), SARS-CoV-2 (10 plants), HIV (10 plants), Echovirus (8 plants), Parvovirus (6 plants, Semiliki forest virus (5 plants), Measles virus (5 plants), Hepatitis virus (3 plants), Canine distemper virus (3 plants), Zika virus (2 plants), Vesicular stomatitis virus T2 (2 plants). Feline herpes virus (FHV-1), Enterovirus, Dengue virus, Ebola virus, Chikungunya virus, Yellow fever virus, Respiratory syncytial virus, Rift Valley fever virus, Human cytomegalovirus each showed sensitivities to one plant (Tables 1–4). Isolated compounds were also identified and their activities outlined, namely alkaloids (combretine and betonicine) from Combretum micrantum (Ferrea et al., 1993), Aloin from Aloe ferox (Kambizi et al., 2007), a polysaccharide from Aspalathus. Linearis (Nakano et al., 1997), Asiaticoside from Centella asiatica (Yoosook et al., 2000), Catechin from S. frutescens (Bessong et al., 2005).
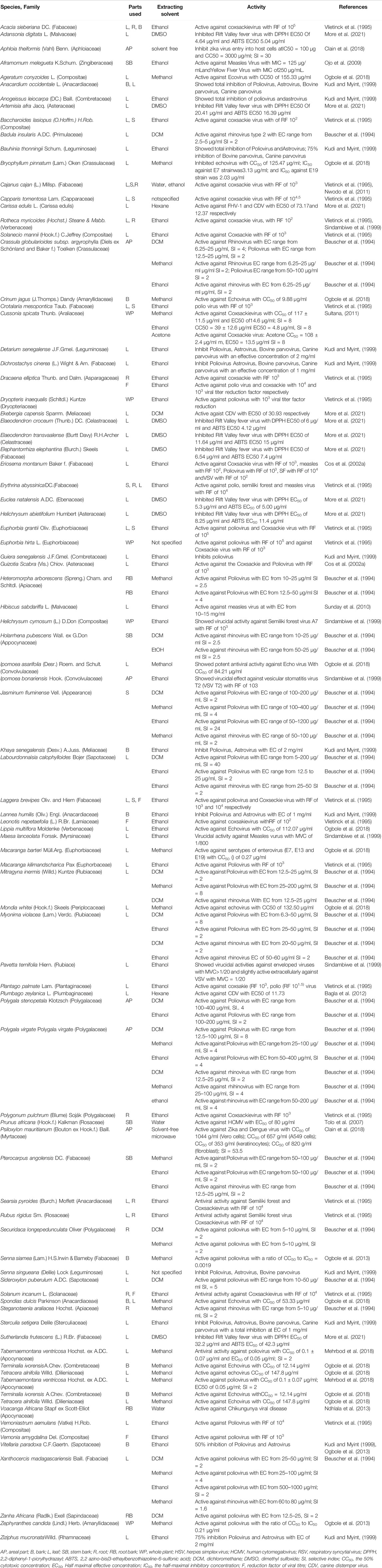
TABLE 4. Antiviral activity of African medicinal plants against poliovirus, astrovirus, coxsackievirus, Rift Valley fever virus, zika virus, measle, echovirus, yellow fiver virus, parvovirus, chikungunya virus, cytomegalovirus, CDV.
Discussion
This study summarized the antiviral activities of African medicinal plants. Forty two African medicinal plants showed noteworthy activities against poliovirus and twenty four against HSV.
Medicinal Plants Used for Severe Acute Respiratory Syndrome
Recently, 10 African medicinal plants from Morocco showed noteworthy activities against SARS-CoV-2 (58). However, there is no currently available published study on Africa medicinal plants demonstrating clinical effectiveness. In contrast, China has developed several Chinese herbal medicines (CHM) and produced numerous clinical studies and publications. There is a daring absence of published studies on herbal medicine use in Africa in comparison to the actual magnitude of its practice. Many Africans are using one or another type of African traditional medicine either for prevention or treatment of COVID-19.
For example, Madagascar produced an herbal drink from Artemisia annua called COVID Organics which was even exported abroad (Cambaza, 2020). The anecdotal use of this product resulted in exaggerated claims of their efficacies that are not evidence-based. This calls for the urgent need for further research on this as well as all other herbal formulations on their efficacy through randomized controlled trials and identify their active ingredients, develop proven formulations and dosing protocols, and define pharmacokinetics, toxicology, and safety to enable drug development. Derivatives from the herb Artemisia annua have been used for the treatment of fevers, malaria, and respiratory tract infections. The WHO has offered to support the design of a study to assess the efficacy, safety, and dosage formulation of herbal formulations that may be useful against COVID-19 (Muhammad, 2020). The WHO is currently helping the validation of some traditional medicine through clinical trials for the treatment of COVID-19 (Tih, 2020).
Studies on TM use for COVID-19 produced many publications of which four were systematic reviews and meta-analyses entirely based on CHM (Liu et al., 2006; Fan et al., 2020; Liu et al., 2020; Xiong et al., 2020) and other systematic reviews and meta-analyses were not CHM (Ang et al., 2020). Traditional medicine is being used to control coronavirus alone or in a combination with western medicine. A recent systematic review and meta-analysis of randomized controlled trials included seven randomized controlled trials and compared combined therapy of herbal medicine with Western medicine and western medicine alone (Ang et al., 2020). This demonstrated the potential role of herbal medicine in treating and/or managing COVID-19 (Ang et al., 2020). The other study which included 12 randomized controlled trials and one quasi-RCT with A total of 640 SARS-CoV-2 patients and 12 Chinese herbs did not indicate a significant difference in Chinese herbs combined with Western medicines versus Western medicines alone (Liu et al., 2006). Yet hundreds of Chinese traditional medicines had been widely used for the treatment of SARS and currently, it’s being used for SARS-CoV-2 (Shahrajabian et al., 2020). A recent review conducted by Attah et al. (2021) summarized 17 African medicinal plants studied against Covid-19 with viral protein targeted. The medicinal plants listed targeted SARS-Cov-2 3CLpro and ACE2.
An in silico screening was conducted on 62 alkaloids and 100 terpenoids from African medicinal plants against coronavirus 3-chymotrypsin-like protease (3CL pro), a highly defined hit-list of seven compounds. Furthermore, four nontoxic, druggable plant-derived alkaloids and terpenoids that bind to the receptor-binding site and catalytic dyad of SARS-CoV-2 3CLpro were identified. More than half of the selected top 20 alkaloids and terpenoids had a binding affinity for the 3CLpro of the SARS-coronaviruses that surpassed reference inhibitors. The 6-oxoisoiguesterin from Bisnorterpenes had the highest binding affinity to the 3CLpro of SARS-CoV-2 while 20-epi-isoiguesterinol from Bisnorterpenes, isoiguesterin from Bisnorterpenes, 20-epibryonolic acid from Cogniauxia podolaena was the top docked compounds to 3CLpro of SARS-CoV and MERS-CoV. The study revealed that natural agents from the alkaloids and terpenoids class of compounds are capable of inhibiting the 3CLpro with a high inhibitory pattern to both SARS-CoV-2 and SARS-CoV (Gyebi et al., 2021). Moreover, 67 compounds from Moroccan aromatic and medicinal plants were tested by molecular docking, of which 11 molecules showed good interaction with the studied enzyme [(Coronavirus (2019-nCoV) main protease] and three molecules Crocin, Digitoxigenin, b-Eudesmol had shown better interaction Coronavirus (2019-nCoV) main protease) (Aanouz et al., 2021). Crocin, a compound from Crocus Sativus, inhibited the replication of HSV (Soleymani et al., 2018). Digitoxigenin is a compound from Nerium oleander and studied for its antiviral and anticancer activity (Boff et al., 2019). Β-Eudesmol was extracted from Lauris nobilis has significant antiviral activity (Astani et al., 2011).
Medicinal Plants for Ebola Virus
Medicinal plants target viruses through various mechanisms. Garcinia kola’s A 13 components showed activity against Ebola virus probably by binding with membrane proteins, metalloproteases, and Ser/Thr Kinase through the three most featured targets; cannabinoid receptors, cyclin-dependent kinases, and matrix metalloproteinase. The components could also target cathepsin, collagenase, and another matrix metalloproteinase (King, 2000; Homsy et al., 2004; David et al., 2017). Baicalin from (Scutellariae Radix), a natural product from the plant, acts on chemokine receptors and inhibits the entry of HIV (Kitamura et al., 1998; Li et al., 2000; Wang et al., 2004). The N-butanol fraction of Bredelia micrantha showed reverse transcriptase inhibition activity. Terpenes showed an inhibitory effect against the protease enzyme (Hussein et al., 1999; Huang and Chen, 2002; Tolo et al., 2006; Yu et al., 2006).
Medicinal Plants for HIV
There are different targets for HIV drug developments. One is the viral envelope which plays a major role in infecting a cell by interacting with CD4 and chemokine receptors CCR5 and CXCR4. CV-N and Baicalin is a natural product from a plant source that acts on chemokine receptors and inhibits the entry of HIV (Kitamura et al., 1998; Li et al., 2000; Wang et al., 2004). The reverse transcriptase enzyme is also a target for drug development. The study comparing organic solvent and an aqueous fraction of various medicinal plants, and the n-butanol fraction of Bredelia micrantha showed anti-reverse transcriptase activities. Phytochemicals such as terpenes revealed inhibitory effects against protease enzyme; an important enzyme for proteolytic processing of polyprotein precursor into essential proteins for the assembly of virus particles (Hussein et al., 1999; Huang and Chen, 2002; Yu et al., 2006).
Croton megalobotrys is a plant species which showed the latent HIV-1 reversal activity. Crude extractas of the plant was comparable with known LRA prostatin which induced HIV-1 in J-lat cells. From the fraction of the crude extract, two novel phorbol esters (Namusha1 and 2) were identified. The previous study also showed that multiple phorbol esters had anti-HIV-1 activities (El-Mekkawy et al., 2000) and function as LRAs (Tietjen et al., 2018).
Medicinal Plants for Hepatitis Virus
Medicinal plants have been widely used to treat the hepatitis virus. Out of five plants examined for anti-Hepatitis B virus, three exhibited anti-hepatitis B in vitro with a CC50 value of more than 100 μg/ml. These were aqueous extracts from Carissa edulis (Apocynaceae), Prunus africana Kalkman (Rosaceae) and the methanol extract from Acacia mellifera Benth (Fabaceae). Extracts of C. edulis exhibited the highest activity; an over 12.15% inhibition rate relative to the negative control. P. africana and A. mellifera extract demonstrated 5% inhibition and 2.15% inhibition respectively, relative to controls. Further confirmation of the activity of these plants using the quantitative real-time PCR technique showed the aqueous extract of C. edulis and the methanol extract of A. mellifera exhibited sustained activity over a range of plant extract concentrations from 31.25 μg/ml to 125 μg/ml. The evaluation of the EC50 the two plant extracts exhibiting notable anti–HBV activity using this technique yielded; C. edulis’ EC50 was 331.6 μg/ml while that of A. mellifera was 295.0 μg/ml (Kwena, 2014).
African Medicinal Plants for Influenza Virus
Influenza virus infection remains a major health problem for animals and humans. Medicinal plants are becoming increasingly popular and included in primary health care in different parts of the world. A study conducted on methanol, ethanol, acetone, hot and cold aqueous extract of five plants (Pittosporum viridiflorum, Cussonia spicata, Rapanea melanophloeos, Tabernaemontana ventricosa, Clerodendrum glabrum) against influenza A virus exhibited antiviral effect. Most effective result were obtained from Rapanea melanophloeos methanol leaf extract (EC50 = 113.3 μg/ml) and Pittosporum viridiflorum methanol, 100 and 30% ethanol and acetone leaf extracts (EC50 values = 3.6, 3.4, 19.2, 82.3 μg/ml, respectively) (Mehrbod et al., 2018). Ethiopian medicinal plants like Acokanthera schimperi, Euclea schimperi, leaf extracts of Inula confertiflora prevent influenza A virus replication and those of Melilotus elegans were active against influenza A virus (Gebre-Mariam et al., 2006) (Table 2).
Medicinal Plants for Herpes Simplex Virus
In sub-Saharan Africa, high prevalence rates between 60 and 80% in young adults have been recorded in population-based studies. It is usually managed by antiviral drugs such as a nucleoside analog acyclovir. However, resistance to ACV has been reported mainly among immunocompromised patients (Morfin and Thouvenot, 2003). Medicinal plants have been considered as an alternative for the development of a new drug to overcome the resistance to the modern drug. The study was conducted on an aqueous extract from the root bark of Carissa edulis (Apocynaceae) has shown significant anti-HSV activity in vitro and in vivo (Omino and Kokwaro, 1993). The extract significantly inhibited the formation of plaques in Vero E6 cells infected with 100 PFU of the wild-type strains of HSV by 100% at 50 μg/ml in vitro with minimal cell cytotoxicity (Tolo et al., 2006). The extracts from four plants; Lannea schweinfurthii, Combretum adenogonium, Ficus sycomorus, and Terminalia mollis showed strong antiviral activity against Herpes Simplex Virus type 1. Out of 42 Egyptian medicinal plants, Ephedra alata and Moringa peregrina are found to have antiviral activity against HSV. Also, the results revealed that Capparis sinaica, Tamarix nilotica, and Cyperus rotundus are found to have a virucidal effect against HSV(Soltan and Zaki, 2009).
The current study is only a preliminary study where some studies reported naively. As all studies in vitro possible dose range, duration of action and in vivo pharmacodynamics properties cannot be established.
In conclusion, African medicinal plants pose significant antiviral activities and could potentially be candidates for viral disease treatment and/or management. It is imperative therefore that research on currently available African medicinal plants be highly recommended. Outcomes from such studies would potentially lead to breakthrough discoveries for the management and/or treatment of COVID-19 and various other viral infections upon appropriate optimization.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
PO, AW, and CT conceived the idea. TB, SD, AM, NT, NA, and BL extracted data and critically reviewed the primary studies. TB and SD analyzed the data and wrote the first draft of the manuscript. All authors reviewed and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Ambo University, Mbarara University of Science and Technology, and Hawassa University for their support of this article through providing access to the internet and databases for the review.
Abbreviations
CC50, 50% cytotoxic concentration; COVID-19, coronavirus diseases 2019; EC50, half maximal effective concentration; HIV, human immune deficiency virus; HSV, herpes simplex virus; MERS-CoV, middle east respiratory syndrome coronavirus; PRISMA, preferred reporting items for systematic reviews and meta-analysis; SARS-CoV, severe acute respiratory syndrome coronavirus; SI, selective index; CHM, chinese herbal medicine.
References
Aanouz, I., Belhassan, A., El-Khatabi, K., Lakhlifi, T., El-Ldrissi, M., and Bouachrine, M. (2021). Moroccan Medicinal Plants as Inhibitors against SARS-CoV-2 Main Protease: Computational Investigations. J. Biomol. Struct. Dyn. 39 (8), 2971–2979. doi:10.1080/07391102.2020.1758790
Abera, B. (2014). Medicinal Plants Used in Traditional Medicine by Oromo People, Ghimbi District, Southwest Ethiopia. J. Ethnobiol. Ethnomed 10, 40. doi:10.1186/1746-4269-10-40
Amenu, E. (2007). Use and Management of Medicinal Plants by Indigenous People of Ejaji Area (Chelya Woreda) West Shoa, Ethiopia: An Ethnobotanical Approach. Addis Ababa, Ethiopia: Addis Ababa University.
Anani, K., Hudson, J. B., de Souza, C., Akpagana, K., Tower, G. H., Arnason, J. T., et al. (2000). Investigation of Medicinal Plants of Togo for Antiviral and Antimicrobial Activities. Pharm. Biol. 38 (1), 40–45. doi:10.1076/1388-0209(200001)3811-BFT040
Ang, L., Song, E., Lee, H. W., and Lee, M. S. (2020). Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 9 (5), 1583. doi:10.3390/jcm9051583
Astani, A., Reichling, J., and Schnitzler, P. (2011). Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Alternat Med. 2011, 253643. doi:10.1093/ecam/nep187
Attah, A. F., Fagbemi, A. A., Olubiyi, O., Dada-Adegbola, H., Oluwadotun, A., Elujoba, A., et al. (2021). Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine with COVID-19 in Focus: A Nigerian Perspective. Front. Pharmacol. 12, 596855. doi:10.3389/fphar.2021.596855
Bagla, V. P., McGaw, L. J., and Eloff, J. N. (2012). The Antiviral Activity of Six South African Plants Traditionally Used against Infections in Ethnoveterinary Medicine. Vet. Microbiol. 155 (2-4), 198–206. Epub 2011/10/11. doi:10.1016/j.vetmic.2011.09.015
Bessong, P. O., Obi, C. L., Andréola, M. L., Rojas, L. B., Pouységu, L., Igumbor, E., et al. (2005). Evaluation of Selected South African Medicinal Plants for Inhibitory Properties against Human Immunodeficiency Virus Type 1 Reverse Transcriptase and Integrase. J. Ethnopharmacol. 99 (1), 83–91. doi:10.1016/j.jep.2005.01.056
Beuscher, N., Bodinet, C., Neumann-Haefelin, D., Marston, A., and Hostettmann, K. (1994). Antiviral Activity of African Medicinal Plants. J. Ethnopharmacol. 42 (2), 101–109. doi:10.1016/0378-8741(94)90103-1
Boff, L., Munkert, J., Ottoni, F. M., Zanchett Schneider, N. F., Ramos, G. S., Kreis, W., et al. (2019). Potential Anti-herpes and Cytotoxic Action of Novel Semisynthetic Digitoxigenin-Derivatives. Eur. J. Med. Chem. 167, 546–561. Epub 2019/02/25. doi:10.1016/j.ejmech.2019.01.076
Cambaza, E. M. (2020). The African Miracle: why COVID-19 seems to spread slowly in Sub-Saharan Africa. Revista Científica da UEM: Série Ciências Biomédicas e Saúde Pública. COVID, 1–8. preprint. doi:10.13140/RG.2.2.23824.02564
Chiang, L. C., Chang, J. S., Chen, C. C., Ng, L. T., and Lin, C. C. (2003). Anti-Herpes Simplex Virus Activity of Bidens Pilosa and Houttuynia Cordata. Am. J. Chin. Med. 31 (03), 355–362. doi:10.1142/S0192415X03001090
Clain, E., Sinigaglia, L., Koishi, A. C., Gorgette, O., Gadea, G., Viranaicken, W., et al. (2018). Extract from Aphloia Theiformis, an Edible Indigenous Plant from Reunion Island, Impairs Zika Virus Attachment to the Host Cell Surface. Sci. Rep. 8, 10856. doi:10.1038/s41598-018-29183-2
Cos, P., Hermans, N., De Bruyne, T., Apers, S., Sindambiwe, J. B., Vanden Berghe, D., et al. (2002). Further Evaluation of Rwandan Medicinal Plant Extracts for Their Antimicrobial and Antiviral Activities. J. Ethnopharmacol 79 (2), 155–163. doi:10.1016/s0378-8741(01)00362-2
Cos, P., Hermans, N., De, B. T., Apers, S., Sindambiwe, J. B., Witvrouw, M., et al. (2002). Antiviral Activity of Rwandan Medicinal Plants against Human Immunodeficiency Virus Type-1 (HIV-1). Phytomedicine 9 (1), 62–68. doi:10.1078/0944-7113-00083
David, S., Toluwase, F., and Oluwasegun, O. (2017). Bioinformatics Analysis of Garcinia Kola Active Components and Glycoproteins of Ebola Virus (Zaire Ebolavirus). J. Chem. Pharm. 9 (4), 364–370.
Duraipandiyan, V., and Ignacimuthu, S. (2009). Antibacterial and Antifungal Activity of Flindersine Isolated from the Traditional Medicinal Plant, Toddalia Asiatica (L.) Lam. J. Ethnopharmacol. 123 (3), 494–498. doi:10.1016/j.jep.2009.02.020
El-Mekkawy, S., Meselhy, M. R., Nakamura, N., Hattori, M., Kawahata, T., and Otake, T. (2000). Anti-HIV-1 Phorbol Esters from the Seeds of Croton Tiglium. Phytochemistry 53 (4), 457–464. doi:10.1016/s0031-9422(99)00556-7
Fan, A. Y., Gu, S., and Alemi, S. F. (2020). Chinese Herbal Medicine for COVID-19: Current Evidence with Systematic Review and Meta-Analysis. J. Integr. Med. 18 (5), 385–394. doi:10.1016/j.joim.2020.07.008
Fennell, C. W., Lindsey, K. L., McGaw, L. J., Sparg, S. G., Stafford, G. I., Elgorashi, E. E., et al. (2004). Assessing African Medicinal Plants for Efficacy and Safety: Pharmacological Screening and Toxicology. J. Ethnopharmacol 94 (2-3), 205–217. doi:10.1016/j.jep.2004.05.012
Ferrea, G., Canessa, A., Sampietro, F., Cruciani, M., Romussi, G., and Bassetti, D. (1993). In Vitro activity of a Combretum Micranthum Extract against Herpes Simplex Virus Types 1 and 2. Antivir. Res 21 (4), 317–325. doi:10.1016/0166-3542(93)90010-g
Gebre-Mariam, T., Neubert, R., Schmidt, P. C., Wutzler, P., and Schmidtke, M. (2006). Antiviral Activities of Some Ethiopian Medicinal Plants Used for the Treatment of Dermatological Disorders. J. Ethnopharmacol. 104 (1-2), 182–187. doi:10.1016/j.jep.2005.08.071
Gyebi, G. A., Ogunro, O. B., Adegunloye, A. P., Ogunyemi, O. M., and Afolabi, S. O. (2021). Potential Inhibitors of Coronavirus 3-chymotrypsin-like Protease (3CLpro): an In Silico Screening of Alkaloids and Terpenoids from African Medicinal Plants. J. Biomol. Struct. Dyn. 39 (9), 1–13. doi:10.1080/07391102.2020.1764868
Gyuris, A., Szlávik, L., Minárovits, J., Vasas, A., Molnár, J., and Hohmann, J. (2009). Antiviral Activities of Extracts of Euphorbia Hirta L. Against HIV-1, HIV-2 and SIVmac251. In Vivo 23 (3), 429–432.
Homsy, J., King, R., Balaba, D., and Kabatesi, D. (2004). Traditional Health Practitioners Are Key to Scaling up Comprehensive Care for HIV/AIDS in Sub-saharan Africa. Aids 18 (12), 1723–1725. doi:10.1097/01.aids.0000131380.30479.16
Huang, L., and Chen, C. H. (2002). Molecular Targets of Anti-HIV-1 Triterpenes. Curr. Drug Targets Infect. Disord. 2 (1), 33–36. doi:10.2174/1568005024605936
Hussein, G., Miyashiro, H., Nakamura, N., Hattori, M., Kawahata, T., Otake, T., et al. (1999). Inhibitory Effects of Sudanese Plant Extracts on HIV-1 Replication and HIV-1 Protease. Phytother Res. 13 (1), 31–36. doi:10.1002/(SICI)1099-1573(199902)13:1<31::AID-PTR381>3.0.CO;2-C
Kambizi, L., Goosen, B., Taylor, M., and Afolayan, A. (2007). Anti-viral Effects of Aqueous Extracts of Aloe Ferox and Withania Somnifera on Herpes Simplex Virus Type 1 in Cell Culture. S. Afr. J. Sci. 103 (9-10), 359–360.
King, R. (2000). Collaboration with Traditional Healers in HIV/AIDS Prevention and Care in Sub-saharan Africa: A Literature Review. Geneva: UNAIDS.
Kitamura, K., Honda, M., Yoshizaki, H., Yamamoto, S., Nakane, H., Fukushima, M., et al. (1998). Baicalin, an Inhibitor of HIV-1 Production In Vitro. Antivir. Res 37 (2), 131–140. doi:10.1016/s0166-3542(97)00069-7
Kudi, A. C., and Myint, S. H. (1999). Antiviral Activity of Some Nigerian Medicinal Plant Extracts. J. Ethnopharmacol. 68 (1-3), 289–294. doi:10.1016/s0378-8741(99)00049-5
Kwena, M. H. (2014). Anti-viral Activities of Selected Kenyan Medicinal Plants against the Hepatitis-B Virus. Nairobi: University of Nairobi.
Li, B. Q., Fu, T., Gong, W. H., Dunlop, N., Kung, H., Yan, Y., et al. (2000). The Flavonoid Baicalin Exhibits Anti-inflammatory Activity by Binding to Chemokines. Immunopharmacology 49 (3), 295–306. doi:10.1016/s0162-3109(00)00244-7
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. Plos Med. 6 (10), e1000100–e34. doi:10.7326/0003-4819-151-4-200908180-0013610.1371/journal.pmed.1000100
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Zhang, J., et al. (2020). Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a Systematic Review and Meta-Analysis. Pharmacol. Res. 158 (2020), 104896. doi:10.1016/j.phrs.2020.104896
Liu, X., Zhang, M., He, L., and Li, Y. (2006). Chinese Herbs Combined with Western Medicine for Severe Acute Respiratory Syndrome (SARS). Cochrane Database Syst. Rev. 10, CD004882. Published 2006 Jan 25. doi:10.1002/14651858.CD004882.pub3
Lu, S. Y., Qiao, Y. J., Xiao, P. G., and Tan, X. H. (2005). Identification of Antiviral Activity of Toddalia Asiatica against Influenza Type A Virus. Zhongguo Zhong Yao Za Zhi 30 (13), 998–1001.
Maregesi, S. M., Pieters, L., Ngassapa, O. D., Apers, S., Vingerhoets, R., Cos, P., et al. (2008). Screening of Some Tanzanian Medicinal Plants from Bunda District for Antibacterial, Antifungal and Antiviral Activities. J. Ethnopharmacol. 119 (1), 58–66. doi:10.1016/j.jep.2008.05.033
Mehrbod, P., Abdalla, M. A., Njoya, E. M., Ahmed, A. S., Fotouhi, F., Farahmand, B., et al. (2018). South African Medicinal Plant Extracts Active against Influenza A Virus. BMC Complement. Altern. Med. 18 (1), 112. doi:10.1186/s12906-018-2184-y
More, G. K., Makola, R. T., and Prinsloo, G. (2021). In Vitro Evaluation of Anti-rift Valley Fever Virus, Antioxidant and Anti-inflammatory Activity of South African Medicinal Plant Extracts. Viruses 13 (2), 221. doi:10.3390/v13020221
Morfin, F., and Thouvenot, D. (2003). Herpes Simplex Virus Resistance to Antiviral Drugs. J. Clin. Virol. 26 (1), 29–37. doi:10.1016/s1386-6532(02)00263-9
Muhammad, F. (2020). COVID-19 Pandemic: The Role of Traditional Medicine. Int. J. Infect. 7 (3), e107090. doi:10.5812/iji.107090
Nakano, M., Itoh, Y., Mizuno, T., and Nakashima, H. (1997). Polysaccharide from Aspalathus Linearis with strong Anti-HIV Activity. Biosci. Biotechnol. Biochem. 61 (2), 267–271. doi:10.1271/bbb.61.267
Nasr-Eldin, M., Abdelhamid, A., and Baraka, D. (2018). Antibiofilm and Antiviral Potential of Leaf Extracts from Moringa Oleifera and Rosemary (Rosmarinus Officinalis Lam.). Egypt. J. Microbiol. 52 (1), 129–139. doi:10.21608/ejm.2017.1439.1027
Ndhlala, A. R., Amoo, S. O., Ncube, B., Moyo, M., Nair, J. J., and Van Staden, J. (2013). Antibacterial, Antifungal, and Antiviral Activities of African Medicinal Plants. Med. Plant Res. Africa 2013, 621–659. Elsevier. doi:10.1016/b978-0-12-405927-6.00016-3
Nwodo, U. U., Ngene, A. A., Iroegbu, C. U., Onyedikachi, O. A., Chigor, V. N., and Okoh, A. I. (2011). In Vivo evaluation of the Antiviral Activity of Cajanus Cajan on Measles Virus. Arch. Virol. 156 (9), 1551–1557. doi:10.1007/s00705-011-1032-x
Ogbole, O. O., Adeniji, J. A., Ajaiyeoba, E., and Adu, D. F. (2013). Anti-polio Virus Activity of Medicinal Plants Selected from the Nigerian Ethno-Medicine. Afr. J. Biotechno 12 (24), 3878–3883. doi:10.5897/AJB12.2730
Ogbole, O. O., Akinleye, T. E., Segun, P. A., Faleye, T. C., and Adeniji, A. J. (2018). In Vitro antiviral Activity of Twenty-Seven Medicinal Plant Extracts from Southwest Nigeria against Three Serotypes of Echoviruses. Virol. J. 15 (1), 110. doi:10.1186/s12985-018-1022-7
Ojo, O., Oluyege, J., and Famurewa, O. (2009). Antiviral Properties of Two Nigerian Plants. Afr. J. Plant Sci. 3 (7), 157–159. doi:10.5897/AJPS.9000025
Omino, E. A., and Kokwaro, J. O. (1993). Ethnobotany of Apocynaceae Species in Kenya. J. Ethnopharmacol. 40 (3), 167–180. doi:10.1016/0378-8741(93)90065-d
Rahmasaria, R., Haruyama, T., Charyasriwong, S., Nishida, T., and Kobayashi, N. (2017). Antiviral Activity of Aspalathus Linearis against Human Influenza Virus. Nat. Prod. Commun. 12 (4), 599–602. doi:10.1177/1934578x1701200432
Selvarani, V. (2009). Multiple Inflammatory and Antiviral Activities in Adansonia Digitata (Baobab) Leaves, Fruits and Seeds. J. Med. Plant Res. 3 (8), 576–582. doi:10.5897/JMPR.9000918
Serkedjieva, J. (2003). Influenza Virus Variants with Reduced Susceptibility to Inhibition by a Polyphenol Extract from Geranium Sanguineum L. Pharmazie 58 (1), 53–57. Epub 2003/03/08.
Shahrajabian, M. H., Sun, W., Shen, H., and Cheng, Q. (2020). Chinese Herbal Medicine for SARS and SARS-CoV-2 Treatment and Prevention, Encouraging Using Herbal Medicine for COVID-19 Outbreak. Acta Agriculturae Scand. Section B - Soil Plant Sci. 70 (5), 437–443. doi:10.1080/09064710.2020.1763448
Sindambiwe, J. B., Calomme, M., Cos, P., Totté, J., Pieters, L., Vlietinck, A., et al. (1999). Screening of Seven Selected Rwandan Medicinal Plants for Antimicrobial and Antiviral Activities. J. Ethnopharmacol. 65 (1), 71–77. doi:10.1016/s0378-8741(98)00154-8
Soleymani, S., Zabihollahi, R., Shahbazi, S., and Bolhassani, A. (2018). Antiviral Effects of Saffron and its Major Ingredients. Curr. Drug Deliv. 15 (5), 698–704. Epub 2017/12/01. doi:10.2174/1567201814666171129210654
Soltan, M. M., and Zaki, A. K. (2009). Antiviral Screening of Forty-Two Egyptian Medicinal Plants. J. Ethnopharmacol. 126 (1), 102–107. doi:10.1016/j.jep.2009.08.001
Sultana, N. (2011). Clinically Useful Anticancer, Antitumor, and Antiwrinkle Agent, Ursolic Acid and Related Derivatives as Medicinally Important Natural Product. J. Enzyme Inhib. Med. Chem. 26 (5), 616–642. doi:10.3109/14756366.2010.546793
Sunday, O. A., Munir, A. B., Akeeb, O. O., Bolanle, A. A., and Badaru, S. (2010). Antiviral Effect of Hibiscus sabdariffa and Celosia Argentea on Measles Virus. Afr. J. Microbiol. Res. 4 (4), 293–296. doi:10.5897/AJMR.9000096
Tietjen, I., Gatonye, T., Ngwenya, B. N., Namushe, A., Simonambanga, S., Muzila, M., et al. (2016). Croton Megalobotrys Müll Arg. And Vitex Doniana (Sweet): Traditional Medicinal Plants in a Three-step Treatment Regimen that Inhibit In Vitro Replication of HIV-1. J. Ethnopharmacol. 191, 331–340. doi:10.1016/j.jep.2016.06.040
Tietjen, I., Ngwenya, B. N., Fotso, G., Williams, D. E., Simonambango, S., Ngadjui, B. T., et al. (2018). The Croton Megalobotrys Müll Arg. Traditional Medicine in HIV/AIDS Management: Documentation of Patient Use, In Vitro Activation of Latent HIV-1 Provirus, and Isolation of Active Phorbol Esters. J. Ethnopharmacol. 211, 267–277. doi:10.1016/j.jep.2017.09.038
Tih, F. (2020). WHO Holds Meeting with African Traditional Medicine Experts. Ankara, Turkey: Anadolu Agency.
Tolo, F. M., Rukunga, G. M., Muli, F. W., Njagi, E. N., Njue, W., Kumon, K., et al. (2006). Anti-viral Activity of the Extracts of a Kenyan Medicinal Plant Carissa Edulis against Herpes Simplex Virus. J. Ethnopharmacol. 104 (1-2), 92–99. doi:10.1016/j.jep.2005.08.053
Tolo, F. M., Rukunga, G. M., Muli, F. W., Ochora, J., Eizuru, Y., Muthaura, C. N., et al. (2007). In Vitro anti-viral Activity of Aqueous Extracts of Kenyan Carissa Edulis, Prunus Africana and Melia Azedarach against Human Cytomegalovirus. Afr. J. Med. Health Sci. 14 (3), 143–148.
Traore, T. K., Andre, T., Noufou, O., Ernest, S. N., Yhi-pene, N. d. J., and Pierre, G. I. (2018). Ethnopharmacological Plants Used to Treat Hepatitis and Their Anti-oxidant Activity of District of Bobo-Dioulasso (Burkina Faso). Int. J. Pharmc Res. 8 (3), 15–23. doi:10.7439/ijpr.v8i3.4643
Trilla, A., Trilla, G., and Daer, C. (2008). The 1918 “Spanish Flu” in Spain. Clin. Infect. Dis. 47 (5), 668–673. doi:10.1086/590567
Vlietinck, A. J., Van Hoof, L., Totté, J., Lasure, A., Vanden Berghe, D., Rwangabo, P. C., et al. (1995). Screening of Hundred Rwandese Medicinal Plants for Antimicrobial and Antiviral Properties. J. Ethnopharmacol 46 (1), 31–47. doi:10.1016/0378-8741(95)01226-4
Wang, Q., Wang, Y. T., Pu, S. P., and Zheng, Y. T. (2004). Zinc Coupling Potentiates Anti-HIV-1 Activity of Baicalin. Biochem. Biophys. Res. Commun. 324 (2), 605–610. doi:10.1016/j.bbrc.2004.09.093
World Health Assembly (2003). “Traditional Medicine: Report by the Secretariat,”. Fifty-sixth world health assembly, Provisional agenda item 14.10 (Geneva, Switzerland: World Health Organization). Available at: https://apps.who.int/iris/handle/10665/78244.
World Health Organization (2003). Cumulative Number of Reported Probable Cases of Severe Acute Respiratory Syndrome (SARS). Available at: http://www.who.int/csr/sars/country/2003_04_22/en/.
World Health Organization (2005). National Policy on Traditional Medicine and Regulation of Herbal Medicines: Report of a WHO Global Survey. Available at: https://apps.who.int/iris/handle/10665/43229.
World Health Organization (2020). Weekly Epidemiological Update on COVID-19 - 3 August 2021. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---3-august-2021.
Xiong, X., Wang, P., Su, K., Cho, W. C., and Xing, Y. (2020). Chinese Herbal Medicine for Coronavirus Disease 2019: a Systematic Review and Meta-Analysis. Pharmacol. Res. 160 (2020), 105056. doi:10.1016/j.phrs.2020.105056
Yoosook, C., Bunyapraphatsara, N., Boonyakiat, Y., and Kantasuk, C. (2000). Anti-herpes Simplex Virus Activities of Crude Water Extracts of Thai Medicinal Plants. Phytomedicine 6 (6), 411–419. doi:10.1016/S0944-7113(00)80068-9
Yu, D., Sakurai, Y., Chen, C. H., Chang, F. R., Huang, L., Kashiwada, Y., et al. (2006). Anti-AIDS Agents 69. Moronic Acid and Other Triterpene Derivatives as Novel Potent Anti-HIV Agents. J. Med. Chem. 49 (18), 5462–5469. doi:10.1021/jm0601912
Zhang, D. H., Wu, K. L., Zhang, X., Deng, S. Q., and Peng, B. (2020). In Silico screening of Chinese Herbal Medicines with the Potential to Directly Inhibit 2019 Novel Coronavirus. J. Integr. Med. 18 (2), 152–158. doi:10.1016/j.joim.2020.02.005
Zhang, M. M., Liu, X. M., and He, L. (2004). Effect of Integrated Traditional Chinese and Western Medicine on SARS: a Review of Clinical Evidence. World J. Gastroenterol. 10 (23), 3500–3505. doi:10.3748/wjg.v10.i23.3500
Keywords: SARS-CoV-2 (2019-nCoV), medicinal plants, viral infections, Africa, herbal mecidine
Citation: Beressa TB, Deyno S, Mtewa AG, Aidah N, Tuyiringire N, Lukubye B, Weisheit A, Tolo CU and Ogwang PE (2021) Potential Benefits of Antiviral African Medicinal Plants in the Management of Viral Infections: Systematic Review. Front. Pharmacol. 12:682794. doi: 10.3389/fphar.2021.682794
Received: 19 March 2021; Accepted: 07 December 2021;
Published: 24 December 2021.
Edited by:
Mohammed Rahmatullah, University of Development Alternative, BangladeshReviewed by:
Radu Adrian Crisan Dabija, Grigore T. Popa University of Medicine and Pharmacy, RomaniaDavid De Jong, University of São Paulo Ribeirão Preto, Brazil
Copyright © 2021 Beressa, Deyno, Mtewa, Aidah, Tuyiringire, Lukubye, Weisheit, Tolo and Ogwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serawit Deyno, ZHNlcmF3aXRAc3RkLm11c3QuYWMudWc=
 Tamirat Bekele Beressa
Tamirat Bekele Beressa Serawit Deyno
Serawit Deyno Andrew G. Mtewa
Andrew G. Mtewa Namuli Aidah2
Namuli Aidah2 Naasson Tuyiringire
Naasson Tuyiringire Ben Lukubye
Ben Lukubye Anke Weisheit
Anke Weisheit Casim Umba Tolo
Casim Umba Tolo Patrick Engeu Ogwang
Patrick Engeu Ogwang