- 1School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China
Colorectal cancer (CRC) has become a global public health problem because of its high incidence and mortality rate worldwide. The previous clinical treatment for CRC mainly involves conventional surgery, chemotherapy, and radiotherapy. With the development of tumor molecular targeted therapy, small molecule inhibitors present a great advantage in improving the survival of patients with advanced CRC. However, various side effects and drug resistance induced by chemotherapy are still the major obstacles to improve the clinical benefit. Thus, it is crucial to find new and alternative drugs for CRC treatment. Traditional Chinese medicines (TCMs) have been proved to have low toxicity and multi-target characteristics. In the last few decades, an increasing number of studies have demonstrated that TCMs exhibit strong anticancer effects in both experimental and clinical models and may serve as alternative chemotherapy agents for CRC treatment. Notably, Wnt/β-catenin signaling pathway plays a vital role in the initiation and progression of CRC by modulating the stability of β-catenin in the cytoplasm. Targeting Wnt/β-catenin pathway is a novel direction for developing therapies for CRC. In this review, we outlined the anti-tumor effects of small molecular inhibitors on CRC through Wnt/β-catenin pathway. More importantly, we focused on the potential role of TCMs against tumors by targeting Wnt/β-catenin signaling at different stages of CRC, including precancerous lesions, early stage of CRC and advanced CRC. Furthermore, we also discussed perspectives to develop potential new drugs from TCMs via Wnt/β-catenin pathway for the treatment of CRC.
Introduction
Colorectal cancer (CRC) is the third cause of cancer-related death worldwide according to the latest statistics of the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) (Authors Anonymous, 2021a). It estimated that there are 1.8 million new CRC cases and 880,792 CRC-related deaths in 2018 (Yang et al., 2020). Moreover, the incidence of CRC in some countries is on the rise gradually. Approximately 70% CRC cases are sporadic and develop through the adenoma-carcinoma sequence (De Filippo et al., 2002; Fodde, 2002). Tumorigenesis is usually driven by multiple genetic and molecular alterations in the different stages. The mutations of adenomatous polyposis coli (APC) gene, were first discovered as the underlying cause of the hereditary colon cancer syndrome termed familial adenomatous polyposis (FAP); in 1991 (Kinzler et al., 1991; Nishisho et al., 1991). Then some researchers found that APC gene could interact with ß-catenin and loss of APC function results in overactive T-cell factor 4 (TCF4)/β-catenin signaling. These findings establish a direct link between Wnt/β-catenin signaling pathway and human CRC. Furthermore, more than 90% of sporadic CRCs has been identified to carry mutations of one or more components of the Wnt/β-catenin signaling pathway including APC based on the genome-scale analysis (Network, 2012). Therefore, the canonical Wnt pathway plays an pivatal role in the development of CRC and may be a significant potential target for CRC treatment.
In clinical practice, standard conventional treatments for CRC are surgery, chemo-therapy and radiotherapy. Currently, with the development of tumor molecular targeted therapy, small molecule inhibitors present a great advantage in improving the survival of patients with advanced CRC. Moreover, long-term application of these therapies can lead to various side effects and toxicities, consisting of nausea, vomiting, mucositis, peripheral neuropathy, and diarrhea (Mcquade et al., 2017). Thus, it is urgent to identify new and more effective drugs for CRC treatment. TCMs have been used for more than 2000 years in China. Owing to the low toxicity and the multi-target capacity (So et al., 2019), TCMs are attracting increasing attention and acceptance for the treatment of CRC as it can alleviate chemotherapy-induced side effects and improve the quality of life of patients with CRC. Previous studies have shown that diverse TCMs exhibit excellent anti-tumor activities in both experimental models and clinical cases. In this review, we focused on ongoing strategies of TCMs used to target aberrant Wnt/β-catenin pathway compared with targeted small molecules as a novel therapeutic intervention in different stages of CRC. Taken together, TCMs will become promising alternative drugs to treat cancer with less toxicity and also be used as an adjunctive treatment together with classic drugs for improving therapeutic outcomes in CRC patients.
Wnt/β-Catenin Pathway and CRC
Wnt signaling pathway is a highly conserved signaling pathway in eukaryotes and commonly divided into canonical (β-catenin dependent) and non-canonical (β-catenin independent) pathways (Polakis, 2012). Originally, many components of the Wnt signaling were identified as key mediators of patterning decisions during embryonic development by genetic screening (Mazzotta et al., 2016). In the last decade, aberrant Wnt/β-catenin pathway activation in carcinogenesis has most prominently been described for CRC. Data from the Cancer Genome Atlas (TCGA) suggests that Wnt/β-catenin pathway is activated in 93% of nonhypermutated CRC and 97% of hypermutated CRC (Li et al., 2012; Sebio et al., 2014; Voorneveld et al., 2015). The status of Wnt/β-catenin pathway is mainly related to the stability of ß-catenin controlled by the ß-catenin destruction complex that is comprised of scaffolding proteins APC, Axin and the kinases casein kinase 1 (CK1) and Glycose synthase kinase 3β (GSK3β). Absence of Wnt ligands stimuli, the cytosolic ß-catenin is phosphorylated by GSK3β, ubiquitinated by ß-TrCP200 and targeted for proteasomal degradation. The ligand Wnt binds to the cell surface receptor Frizzled and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) to form a trimer, which recruits the Dishevelled (Dvl) protein to the plasma membrane, leading to dissociation of the destruction complex followed by cytosolic accumulation of ß-catenin. Consequently, the ß-catenin translocates to the nucleus where nuclear ß-catenin cooperates with TCF/LEF family transcription factors to active target genes such as c-myc, MMP-7, SNAIL and EGFR (Zhan et al., 2017). The activation of Wnt/β-catenin signaling is indispensable for the progression of CRC (Figure 1).

FIGURE 1. Schematic illustration of the Wnt/β-catenin signaling pathway in CRC. (A) Inactive Wnt/β-catenin pathway. In the absence of Wnt ligands, destruction complex phosphylates ß-catenin and KRAS for ubiquitination and proteolytic degradation; (B) Active ß-catenin pathway and crosstalk with KRAS/ERK pathway. In the Wnt stimuli or APC loss, GSK3β becomes inactive status, leading to the high levels of cytoplasmic ß-catenin and KRAS. While KRAS mutations have a positive feedback loop with the level of cytoplasmic ß-catenin. In addition, RNF43 mutations can relieve the degradation of fizzled protein and activate Wnt/β-catenin pathway.
The best-known mutation of APC is the major driver of Wnt pathway in colorectal tumorigenesis which functions as a negative regulator and its importance was further highlighted by several recent studies (Hankey et al., 2018). By using the CRISPR/Cas9 technique to introduce APC mutation into human intestinal organoids, the tumorigenesis of CRC could be modeled in vivo (Drost et al., 2015; Matano et al., 2015). Moreover, these studies in human and mouse models indicated that the genotypes of APC mutations are consistent with the distinct levels of canonical Wnt pathway and these alterations are associated with characteristic tumor locations within the large intestine (Buchert et al., 2010; Christie et al., 2013). Besides APC, ring finger protein 43 (RNF43) mutations and R-spondin translocations are noted in over 18 and 9% patients with CRC respectively by preventing removal of Wnt receptor. Both RNF43 and R-spondin fusion are completely opposite to APC mutations (Schatoff et al., 2017). In addition to the well-established function of Wnt/β-catenin in CRC, there is accumulating evidence indicating that the KRAS is also an important and frequently mutant gene during colorectal cancinogenesis. Up to 40% of KRAS mutations occur in patients with CRC (Arrington et al., 2012). The discovery of small-molecule RAS inhibitors or a siRNA targeting RAS displayed anti-proliferative activity on xenografts of human CRC cell line SW480 (Song et al., 2020). The mutations of KRAS result in the hyper-activation of RAS-extracellular signal-regulated kinase (ERK) pathway involving transformation of cells and tumorigenesis. Series of studies confirmed the regulation of the RAS-ERK pathway by Wnt/β-catenin signaling and its roles, such as Axin, APC, and GSK3β, and so on (Vincan and Barker, 2008). The crosstalk of RAS and Wnt/β-catenin pathways relies on the phosphorylation of RAS mediated by GSK3β. GSK3β, a key component of the ß-catenin destruction complex, is identified as a kinase inducing phosphorylations of ß-catenin and RAS at the different sites of the threonine, and subsequently recruits the ß-TrCP E3 linker for the proteasomal degradation. Inactivation of GSK3β caused by Wnt stimuli or APC loss further leads to high concentration of cytoplasmic ß-catenin and KRAS (Lee et al., 2018a). Therefore, both mutations of APC and KRAS have a positive connection with the Wnt/β-catenin pathway in colorectal tumorigenesis (Figure 1).
Metastasis is a hallmark of advanced cancer and a major challenge to clinic treatment. Epithelial-mesenchymal transition (EMT) is a crucial process by which epithelial cells lose cell polarity and cell-cell adhesion, and closely associate with invasion and metastasis in many types of malignancies including CRC (Spaderna et al., 2006; Vu and Datta, 2017). There is a complicated network involved in the regulation of EMT, containing different signaling pathways. Many investigations indicated that aberrant activation of the canonical Wnt pathway promotes EMT-associated dedifferentiation located at the invasive front of colorectal tumors. Enhanced Wnt/β-catenin signaling in CRC cells induces the action of E-cadherin repressors SNAIL and upregulation of matrix metalloproteinases (MMP) involving CRC invasion and metastasis (Gu et al., 2016). However, inactivating mutations of APC and AXIN2 can up-regulate the canonical Wnt pathway, thereby promoting EMT. Furthermore, in vitro and in vivo experiments showed that WNT3a overexpression induces SNAIL expression and promotes invasion (Qi et al., 2014).
In addition, increasing evidences suggest that cancer stem cells (CSCs) theory underlies tumor proliferation, differentiation and metastasis. Although there is still no consensus on the concept of cancer stemness, the vital role of the Wnt pathway for the function of normal and cancer stem cells is commonly accepted (Reya and Clevers, 2005). In the intestinal crypt, Wnt/β-catenin pathway exerts a crucial role in the self-renewal of CSCs in CRC (Yan K. S. et al., 2017). R-spondin receptor Lgr5, one putative mark of intestinal stem cells, is a direct target gene of the canonical Wnt signaling cascade and able to promote tumor proliferation after APC is deleted in these cells. The experiments in mouse models showed that the Lgr5+ stem cells can increase additionally the population of Lgr5-positive cells and drive adenoma expanding in colon (Barker et al., 2009). CD44v6, as another CSC marker in colorectal cancer, is promoted by Wnt/β-catenin signaling and cytokines secreted from tumor-associated cells (Todaro et al., 2014). Moreover, the tumor environment has an important effect on maintenance of cancer stemness in some studies, such as hepatocyte growth factor, which is secreted by myofibroblasts in tumor micro-environment and can induce stemness features in colorectal cancer cells by improving Wnt activity (Clara et al., 2020). Recently, several studies uncovered potential relations between Wnt pathway and non-coding microRNAs in CSCs. Scientists have discovered miR-142 can inhibit stem cell-like traits by targeting APC gene whose mutations are linked to colon cancer (Isobe et al., 2014). Taken together, these findings indicate that canonical WNT signaling plays a vital role in the maintenance and expansion of CSCs in CRC.
Small Molecules Targeting Wnt/β-Catenin Pathway for CRC Treatment
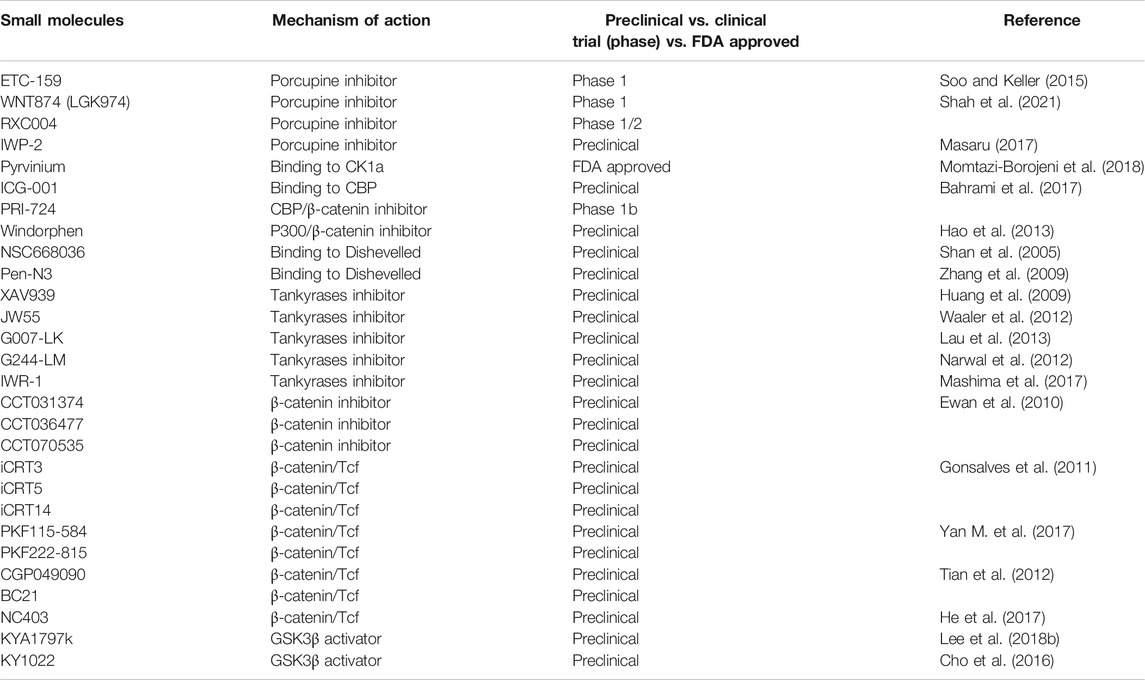
Due to the importance of canonical Wnt/β-catenin signaling in human carcinogenic development, small molecule inhibitors targeting Wnt signaling have been developed for the treatment of CRC (Table 1). Activation of Wnt signaling through ß-catenin is a critical event in CRC progression. Porcupine (PORCN) is a membrane-bound O-acyltransferase protein which regulates Wnt ligands secretion outside the cell membrane through palmitoylation. In recent years, PORCN has emerged as a molecular target for treating Wnt-driven cancers. ETC-159, WNT974 (LGK974) and Rxc004 has been identified as potent inhibitors of Wnt secretion inhibiting ß-catenin activity in preclinical studies. ETC-159 has been proven to be remarkably efficacious in treating CRCs with R-spondin translocation in vivo and in vitro experiments (Soo and Keller, 2015). During in vitro studies in RNF43 mutant and R-spondin fusion CRC cell lines, Rxc004 could potently repress the cell proliferation by arresting cell cycle at G1/S and G2/M phase (Shah et al., 2021). IWP-2 is another inhibitor of PORCN. Experiments on organoid derived from CRC patients unveiled that IWP-2 is sensitive to the cancers with loss of function RNF43 mutations (Masaru, 2017). Pyrvinium, a FDA-approved drug, has been shown to bind to CK1α and form a degradation complex with GSK-3, APC, and Axin, resulting in the inhibition of Wnt signaling. Moreover, Pyrvinium suppresses the proliferation of CRC with mutations of APC or ß-catenin in HCT116 and SW480 cell lines (Momtazi-Borojeni et al., 2018). ICG-001, a selective inhibitor of Wnt/β-catenin pathway, binds to the CREB-binding protein (CBP) and down-regulates ß-catenin/Tcf transcription. As a consequence, ICG-001 selectively induces apoptosis in colon carcinoma cells but not in normal colonic epithelial cells, which is effective in mouse with APC mutations or nude mouse xenograft models of colon cancer. PRI-724, the second generation specific CBP/catenin antagonist for oncology, has been proved to have an acceptable safety profile in early clinical trials and is now under further clinical investigation (Bahrami et al., 2017). Windorphen (WD) is an inhibitor of Wnt/β-catenin signaling by directly targeting p300 to disrupt the association of ß-catenin with p300. These findings suggest that WD can selectively kill cancer cells with aberrant activation of Wnt signaling (Hao et al., 2013). Other small molecules, such as NSC668036 and Pen-N3, block the Wnt signaling pathway through binding to the Dishevelled (Dvl) PDZ domain and interrupting the receptor Frizzled (Fz)-Dvl interaction in colon cells (Shan et al., 2005; Zhang et al., 2009).
Some studies indicate that tankyrases (TNKS) are novel targets for Wnt inhibition by regulating stabilization of Axin and hence leading to increased ß-catenin degradation. XAV939 and JW55 have been shown to target Wnt/β-catenin pathway through inhibiting the poly-ADP-ribose polymerase (PARP) domains of TNKS in DLD-1 and SW480 cell lines in vitro (Huang et al., 2009). JW55 also reduces the growth of tumor in conditional APC mutation mice (Waaler et al., 2012). G007-LK and G244-LM are two other types of small-molecule tankyrase inhibitors (Lau et al., 2013). In particular, G007-LK has greater stability and displays favorable pharmacokinetic properties to inhibit Wnt/β-catenin signaling in APC-mutant CRC xenograft tumors (Tanaka et al., 2017; Katoh, 2018). IWR-1 is another tankyrase inhibitor which interacts with PARP enzyme (Mashima et al., 2017).
β-catenin is a key mediator of Wnt signaling, regulating the stabilization of the destruction complex and consequently intracellular ß-catenin levels. Ewan K et al. revealed that three small molecule inhibitors including CCT031374, CCT036477, and CCT070535 can block the Wnt/β-catenin signaling through reducing the level of ß-catenin without altering its stability, which is different from drugs involving inhibition of TCF-dependent transcription in SW480 cells (Ewan et al., 2010). Interaction of ß-catenin with TCF binding proteins is a crucial step in the activation of target genes in response to the activation of Wnt/β-catenin pathway. A cohort of Wnt antagonists including iCRT3, iCRT5, iCRT14, PKF115-584, PKF222-815, CGP049090, and BC21 have been demonstrated to suppress the Wnt/β-catenin signaling by breaking the association between Tcf4 and ß-catenin (Gonsalves et al., 2011; Tian et al., 2012; Yan M. et al., 2017). NC043 is an inhibitor of ß-catenin/TCF4, which decreases ß-catenin/TCF4 association without affecting the cytosol-nuclear distribution of soluble ß-catenin in vivo and in vitro (He et al., 2017).
In recent years, a small molecular KYA1797K has been identified to suppress the formation of CRCs along with the mutations of APC and KRAS via activating GSK3β and subsequently reducing the level of both ß-catenin and Ras as showed both in vitro and in vivo studies. Moreover, KYA1797K can alleviate the resistant to the EGFR-targeting therapies because of KRAS mutations (Lee et al., 2018b). Whereas, KY1022 destabilizes both ß-catenin and Ras by targeting the Wnt/β-catenin signaling in the process of metastasis involving EMT, which is different from the action of KY1797K (Cho et al., 2016). As indicated above, small molecule inhibitors targeting Wnt/β-catenin pathway exhibit promising therapeutic effects on CRC. However, to the best of our knowledge, few of these small molecules has gone into clinical trials. In the future, many scientists will make great efforts to identify more small molecules targeting Wnt/β-catenin and convert them into effective therapies.
Therapeutic Mechanism of TCMs Against CRC via Wnt/β-Catenin Pathway
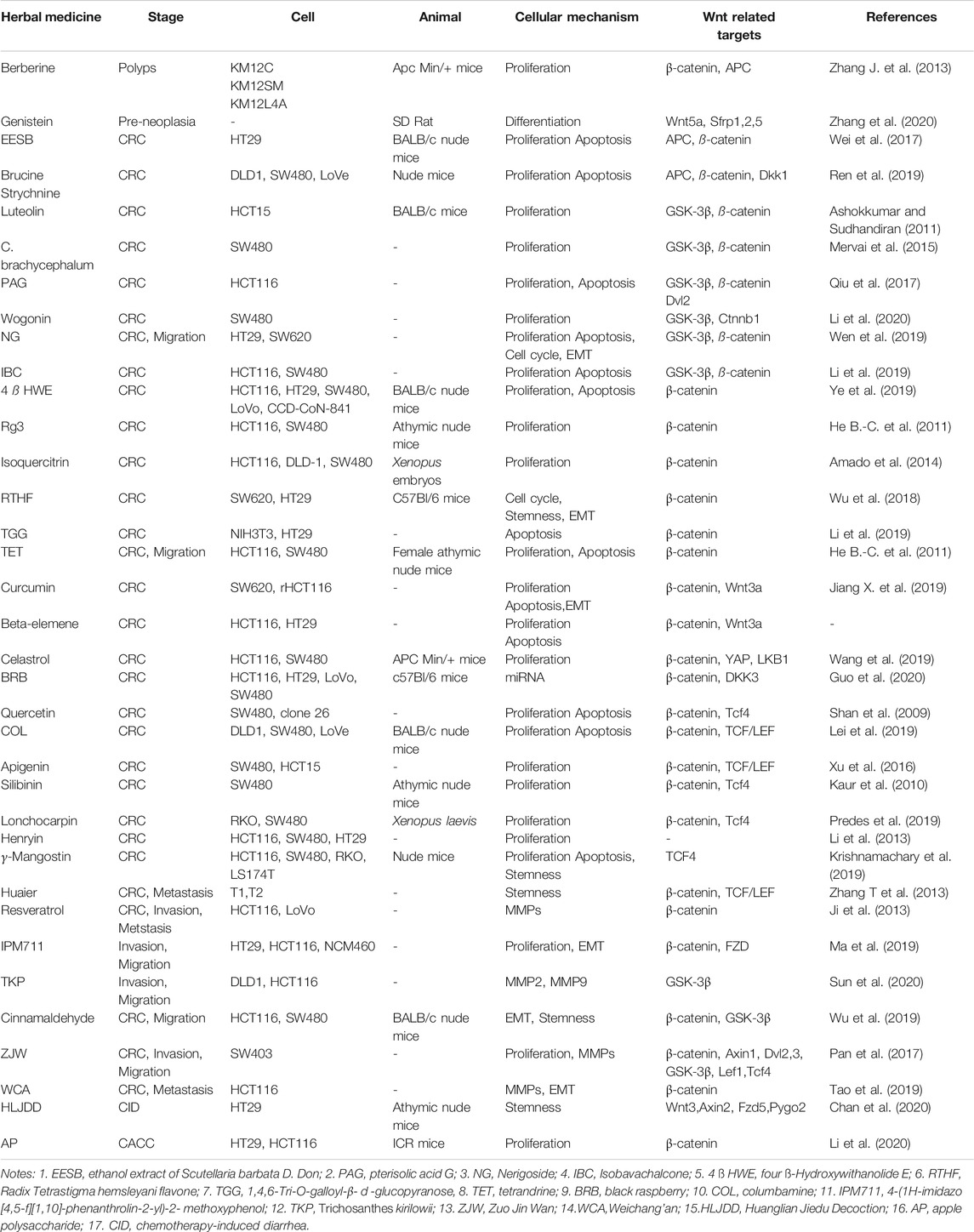
It is well documented that uncontrolled cell proliferation is a typical feature in many types tumor development, especially in CRC. The complex balance between proliferation and apoptosis is intimately connected with tissue homeostasis (Diwanji and Bergmann, 2018) and in general, increased cell proliferation along with reduced apoptosis, drives tumor formation. It has been found that many compounds or extracts from TCMs could inhibit colorectal tumorigenesis by targeting different molecules in Wnt/β-catenin pathway. Therefore, we summarized the single-herb and formula of TCM against the different stages of CRC via Wnt/β-catenin pathway (Table 2).
Effect of Active Compounds on Precancerous CRC
The presence of adenoma (polyps), is a precursor and a major risk factor for CRC (Nguyen et al., 2020). Currently, endoscopic removal is the most effective therapeutic regimen for these patients. However, TCMs also have been reported to exhibit important therapeutic effects on colon adenomas. Alkaloid berberine, which is previously used as an anti-inflammatory drug, has proximately been demonstrated to possess anti-tumor activity by reducing Wnt activity and its mechanism of action may involve inhibition of ß-catenin translocation to the nucleus by enhancing the expression of APC gene and stabilizing the complex of APC-β-catenin. Studies looking at berberine treatment in vivo have found that it gave rise to reduced formation of polyps accompanied with a decrease in cyclin D1 and c-myc expression in the intestinal adenoma model. Furthermore, oral administration of berberine has been confirmed to significantly reduce the size of polyps in patients with FAP (Zhang J. et al., 2013). In addition, the discovery of Aberrant crypt foci (ACF) in early colorectal adenomas provided new opportunities to explore the pathogenic mechanism of CRC. Genistein, a soya isoflavone, is capable of decreasing the number of total aberrant crypts in the colon cancer model with azoxymethane (AOM) injection by repressing the expressions of Wnt/β-catenin target genes, including Wnt5a, Sfrp1, Sfrp2, Sfrp5, and c-Myc. These results revealed a novel role for genistein as a suppressor of carcinogen-induced Wnt/β-catenin signaling and the prevention of early colon neoplasia (Zhang et al., 2020).
Therapeutic Mechanism of Active Compounds Against CRC in Situ
Ninety-three percent of CRC cases has at least one mutation in Wnt/β-catenin pathway genes (Pearlman et al., 2017). The most frequently mutated gene in CRC is APC which may be a promising target for drug development in CRC. The ethanol extract of Scutellaria barbata D. Don (EESB), used for the treatment of various types of cancer clinically (Wei et al., 2017; Zhang et al., 2017; Liu et al., 2018), has been found to prevent the development of human CRC via increasing APC expression with a concomitant decrease in the expression of ß-catenin, leading to inactivation of the Wnt/β-catenin pathway in a CRC xenografted mouse model and HT-29 cell line. Brucine and strychnine from nux vomic have remarkable effects in improving circulatory system and relieving arthritic and traumatic pains. Recently, Ren H et al. (2019) found both two compounds can suppress the growth significantly by inducing the apoptosis of CRCs in nude mice by enhancing the expression of APC and reducing that of ß-catenin. Meanwhile, they can greatly promote DKK1 expression, which is proved to negatively regulate Wnt/β-catenin pathway. On the other hand, some monomers derived from traditional Chinese herbs such as Luteolin, C. brachycephalum, pterisolic acid G (PAG), wogonin, nerigoside (NG) and isobavachalcone (IBC), exhibit anticancer functions by affecting the phosphorylation state of GSK-3β and ß-catenin in CRC. However, nerigoside has been found to destroy the balance of proliferation and apoptosis through the ERK/GSK3β/β-catenin signaling pathways, whereas isobavachalcone exerts its anticancer effect via the AKT/GSK-3β/β-catenin pathway in CRC (Ashokkumar and Sudhandiran, 2011; Mervai et al. (2015); Qiu et al., 2017; Li et al., 2019; Tan et al., 2019; Wen et al., 2019).
There are some compounds inhibiting CRC by mediating the core molecule of canonical Wnt pathway. Ye ZN et al. discovered that the anti-tumor effect of four ß HWE is to promote the phosphorylation and degradation of ß-catenin and the subsequent inhibition of its nuclear translocation in CRC cells (Ye et al., 2019). While, Ginsenoside Rg3 and isoquercitrin were demonstrated to inhibit Wnt/β-catenin pathway by blocking nuclear translocation of the ß-catenin protein and hence inhibiting ß-catenin/Tcf transcriptional activity (He B. et al., 2011). Moreover, some experiments in vitro showed that Radix Tetrastigma hemsleyani flavone (RTHF), 1,4,6-Tri-O-galloyl-β-d-glucopyranose (TGG) as well as tetrandrine (TET) could suppress colorectal tumor growth and downregulate target genes expression (He B.-C. et al., 2011; Wu et al., 2018; Li et al., 2019). Curcumin is another inhibitor of ß-catenin in many cancers (Deguchi, 2015). Previous studies illustrated caudal type homeobox-2 (CDX2) is a mediator of the Wnt signaling pathway, and curcumin can reduce cell proliferation and increase apoptosis by restoring CDX2 which inhibited the Wnt/β-catenin signaling pathway (Jiang X. et al., 2019). Besides, curcumin might exert anti-resistant effect of 5-FU on rHCT-116 cells by controlling WNT signal pathway to reverse the EMT progress (Lu et al., 2020). Beta-elemene, however, could elevate sensitivity to 5-FU through down-regulating miR-191 and preventing the Wnt/β-catenin pathway in CRC cells (Guo et al., 2018). Lately, accumulating evidence has strongly suggested Hippo signaling interacted with Wnt/β-catenin pathway. (Jiang Z. et al., 2019). found that celastrol, isolated from Tripterygium wilfordii plant, exerted antitumor effects by accelerating ß-catenin degradation via the HSF1–LKB1–AMPKα–YAP pathway in CRC. In addition, miRNA microarray analysis suggested that black raspberry (BRB) anthocyanins can reduce the expression of miR-483–3p accompanied by an increased level of DKK3 expression, which is one negative regulator of Wnt pathway (Guo et al., 2020).
Some studies revealed that quercetin and columbamine (inhibitors of the Wnt/ß-catenin pathway) could decrease nuclear lcatenin and downregulate the transcriptional activity of ß-catenin/Tcf, leading to inhibition of cell proliferation in SW480 cell lines (Pahlke et al., 2006; Lei et al., 2019). Similar to quercetin and columbamine, apigenin can suppress CRC proliferation by inhibiting ß-catenin nuclear entry and thereby prevented the expression of Wnt downstream target genes (Xu et al., 2016). Silibinin and lonchocarpin, also exert anticancer functions through the regulation of ß-catenin/Tcf transcriptional activity in animal and cell models (Kaur et al., 2010; Predes et al., 2019). Yet silibinin exhibited selective growth inhibitory effects on SW480 cells (human CRC cells), but not HCT116 cells, by inhibition of Wnt signaling. Henryin, used to control pain for a long time, has been reported to be capable of impairing the association of the ß-catenin/TCF4 trans-criptional complex through direct blockade of ß-catenin binding to TCF4, but not to affect the cytosol to nuclear distribution of soluble ß-catenin (Li et al., 2013). In addition, γ-mangostin, found in Mangosteen fruit, can interact with the transcription factor TCF4 at the ß-catenin binding domain, which results in the suppression of the expression of cyclin D1 and c-Myc. Furthermore, γ-mangostin treatment significantly decreased the levels of stem cell markers such as Lgr5, Dclk1 and CD44 in HCT116, LS174T and DLD1 cells, which also confirmed in vivo models (Krishnamachary et al., 2019). In the last few decades, the existence of CSCs is central to chemo-resistance and recurrence of many tumors. Some studies identified Huaier aqueous extract can take action against CRC by eradicating CSCs and the Wnt pathway may be considered as a potential target of Huaier for the treatment of CRC (Zhang T. et al., 2013).
Regulatory Mechanism of Active Compounds Against Metastatic CRC
The development of distant metastases and therefore resistance to therapy, are major clinical problems in the management of the patients with advanced cancer. Recently, medical professionals have focused on TCMs as a way to resolve these issues. Resveratrol, a natural antioxidant from Polygonum cuspidatum, inhibits the invasion and metastasis of human CRC through down-regulation of Metastasis Associated Lung Adenocarcinoma Transcript1 (MALAT1) (Xu et al., 2011; Ji et al., 2013). IPM711, a structurally modified vanillin, was reported to attenuate EMT by increasing the expression of E-cadherin (Ma et al., 2019). Furthermore, a serine protease TKP has a repressive effect on CRC cell invasion and metastasis by targeting MMP2 and MMP9, and is mediated by blockade of both Wnt/β-catenin and Hedgehog/Gli1 signaling (Sun et al., 2020). In addition, cinnamaldehyde has been certified to have potential adjuvant effect on CRC cells in combination with oxaliplatin through blocking the Wnt/β-catenin pathway and enhancing the susceptibility of oxaliplatin in the hypoxic environment (Wu et al., 2019).
Effect of TCM Formulas on CRC
As well as the monomers and extracts derived from TCMs, an increasing body of evidence suggests that TCM formulas possess anticancer properties, too. Zuo Jin Wan (ZJW) has been used in the treatment of gastrointestinal and liver diseases in China for ages (Chao et al., 2011; Sun et al., 2019), which is composed of Rhizoma Coptidis and Evodia Rutaecarpa at a ratio of 6:1. Berberine and evodiamine are two key elements of ZJW extract and possess anti-tumorigenic activity, respectively (Ayati et al., 2017; Wang et al., 2019). Over the past few decades, many clinical studies had found that some subtypes of 5-HT receptors (5-HTRs) would enhance the proliferation of CRC cells. Recent studies showed that ZJW extracts can exert anti-tumorigenic effects by suppressing the canonical Wnt/β-catenin pathway in animal and cell experiments, similar to that seen with 5-HTR antagonists (Pan et al., 2017). Weichang’ an (WCA) is a traditional Chinese medicinal formula used as an anticancer drug and the experimental data also showed the anti-metastatic function by blunting the activation of Wnt/β-catenin pathway and reducing the expression of MMP9 and the EMT-related protein ZEB1 (Tao et al., 2019). Furthermore, TCM formulations could provide an adjunct for chemotherapy in cancer patients. Huanglian Jiedu Decoction (HLJDD) has been revealed to significantly alleviate the diarrhea induced by chemotherapy in a mouse model. The experiments from the intestinal segments of 5-Fu/CPT-11-treated mice proved pre-treatment with HLJDD could activate the Wnt/β-catenin pathway by inducing the expressions of Wnt signaling components, comprised of Wnt3, Fzd5, Axin2, and Pygo2 (Chan et al., 2020). These data suggest that HLJDD could boost the regeneration of intestinal progenitor cells after chemotherapy, probably by activating Wnt/β-catenin.
In addition, TCMs can also prevent the development of colitis associated colorectal cancer (CACC) through canonical Wnt signaling. It is showed that apple polysaccharide (AP) from apple residues could affect the activation of Wnt/β-catenin signaling pathway in vivo, but not in vitro experiments (Li et al., 2020). Previous studies showed that AP treatment could effectively decrease the proliferation of Fusobacterium in AOM/DSS-induced intestinal tract. Therefore, AP may restrain the activation of Wnt/β-catenin signal pathway in CACC mice through controlling the imbalance of intestinal flora.
Development of New Drugs in Clinic
CRC is often diagnosed at an advanced stage when tumor cell dissemination has already taken place and chemotherapy was one of the major methods for the treatment of CRC in the past few decades. In clinic, it is obviously clear that fluoropyrimidines, irinotecan, and oxaliplatin have been widely applied to chemotherapeutic regimens for tumors (Gustavsson et al., 2015). The recent introduction of small molecular target agents, such as anti-EGFR (cetuximab, panitumumab) and antiangiogenic molecules (bevacizumab) have led to profound improvements in the life expectancy of patients with advanced CRC (Franke et al., 2019), but with potential lethal adverse drug events and drug resistance. Therefore, it is necessary to develop new and neo-adjuvant therapies in combination with other chemotherapeutics. TCMs and their active compounds with multi-targets was reported to prevent and treat CRC patients as promising candidates, which is distinct from small molecular inhibitors that depend on single target (Yeh et al., 2020). In addition, because of relatively lower toxicity and cheaper price, TCMs can be more accepted by patients with CRC physically and psychologically.
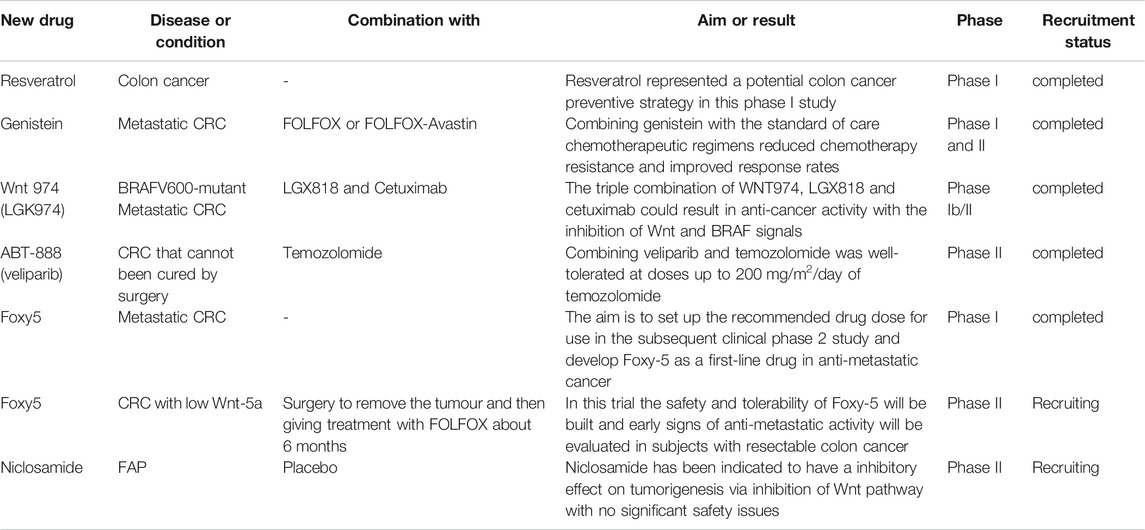
On account of the significance of Wnt/β-catenin pathway in CRC development and metastasis, some native components of TCMs was developed as novel drugs specifically targeting this signaling pathway and are already in clinical trials (Table 3). Resveratrol is a naturally occurring polyphenol with antioxidant, which has been used in many diseases involving cancers. Recently, in vitro studies suggest that resveratrol exhibited preventative colon cancer effects and this was associated with Wnt signaling (93). In this clinical trial, patients with colon cancer were randomly provided a treatment with resveratrol, and relevant studies tested its effects directly on colon cancer and normal colonic mucosa. These results showed that resveratrol could inhibit Wnt/β-catenin signaling in the normal colonic mucosa, but not in colon cancer (Nguyen et al., 2009). Thus, resveratrol represented a potential colon cancer preventive strategy in this phase I study. Genistein is also identified to block Wnt/β-catenin signaling and has a cooperative effect with chemotherapeutic agents in lab. According to pre-clinical data, investigators found that combining genistein with standard chemotherapeutic regimens could reduce chemotherapy resistance and improve patient’s response rates (Authors Anonymous, 2021b).
Besides, small molecular weight Wnt 974, a potential inhibitor of Wnt/β-catenin signaling, has been used to assess its safety and antitumor activity in combination with chemotherapeutic agents in patients with BRAF-mutant metastatic CRC and Wnt pathway mutations (Authors Anonymous, 2021c). Nevertheless, so far, the study results have not been published. ABT-888 (veliparib) has also been used in combination with chemotherapeutic drugs to inhibit the growth of metastatic CRC in phaseⅠandⅡclinical trials (Authors Anonymous, 2021d). But it has not yet been approved by the FDA for use in this cancer. Foxy5, identified by WntResearch, can prevent migration of epithelial cancer cells by mimicking the functions of Wnt-5a and thereby play the anti-metastatic role. The safety and tolerability of Foxy-5 were established and early signs of anti-metastatic activity were evaluated in subjects with resectable colon cancer. Further, researchers have already examined the maximum tolerated dose and dose-limiting toxicity of this drug (Authors Anonymous, 2021e). Interestingly, another small molecule niclosamide, an anti-helminthic drug, has been proved to have an obviously suppressive effect on colorectal tumorigenesis by attenuating Wnt/β-catenin signaling lately. In this experiment, investigators devised a double-blind randomized controlled trial to evaluate the effect of niclosamide on patients with FAP. Unfortunately, to date, this project is still in the recruitment stage (Authors Anonymous, 2021f).
Conclusions and Future Perpectives
CRC has become a global public health problem on account of its high incidence and mortality rate worldwide. The clinical treatments for CRC mainly involve surgery-based chemotherapy. In recent years, with the application of targeting small molecules against cancer, the quality of life for CRC patients has improved. Nevertheless, chemotherapy-induced side effects and drug resistance remain a major issue for clinical practice. Numerous studies have shown that TCMs can be used to exert potential anticancer activity and alleviate the side effects associated with chemotherapy. It is confirmed that various mutations in one or more members of the canonical Wnt signaling pathway take place in the progression of CRC. Therefore, in this review, we aimed to intensively explore molecular mechanisms of TCMs against cancer at the different stages in CRC progress, including precancerous lesions, early stage CRC and CRC invasion and migration based on the inhibition of the Wnt/β-catenin signaling pathway. Cell culture and animal experiments have found that TCMs play anticancer roles by regulating APC/β-catenin, GSK-3β/β-catenin, and ß-catenin/TCF4 pathways which represent the main elements of the Wnt/β-catenin pathway involved in the treatment of CRC. Thus, understanding the molecular mechanisms of action of TCMs and how they target Wnt/β-catenin may shed light on future therapies for CRC. However, it needs multi-level and multi-link comprehensive action to anti-tumor because of the complex composition of traditional Chinese medicine. This suggests that we need to investigate the crosstalk between Wnt/β-catenin signal pathway and others. In addition, there remains very few new clinical treatments under development due to lack of strict evaluation system for effectiveness and safety of TCMs. Therefore, it will hopefully pave the way for the CRC clinical treatment and may also relieve the side effects related to chemotherapy if there is a breakthrough in the study of multi-target intervention of TCM in CRC.
Author Contributions
Chang JC wrote the article; ZB and HW took part in the critical revision of this article; all of the authors had no objection to the final article.
Funding
Supported by the Guangdong Natural Science Foundation, No. 2017A030312009.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I wish to thank all members of ZB’ research team in school of Chinese medicine, Hong Kong Baptist University, as well as Jianyong Xiao.
References
Amado, N., Predes, D., Moreno, M., Carvalho, I., Mendes, F., and Abreu, J. (2014). Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies. Int. J. Mol. Sci. 15 (7), 12094–12106. doi:10.3390/ijms150712094
Arrington, A. K., Heinrich, E. L., Lee, W., Duldulao, M., Patel, S., Sanchez, J., et al. (2012). Prognostic and Predictive Roles of KRAS Mutation in Colorectal Cancer. Int. J. Mol. Sci. 13 (10), 12153–12168. doi:10.3390/ijms131012153
Ashokkumar, P., and Sudhandiran, G. (2011). Luteolin Inhibits Cell Proliferation During Azoxymethane-Induced Experimental Colon Carcinogenesis via Wnt/Beta-Catenin Pathway. Invest New Drugs 29 (2), 273–284. doi:10.1007/s10637-009-9359-9
Authors Anonymous (2021a). Cancer Today. Available at: https://gco.iarc.fr/today/fact-sheets-cancers.. (accessed 6 9 2021)
Authors Anonymous (2021b). Genistein in Treatment of Metastatic Colorectal Cancer - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01985763?term=genistein&cond=Colorectal+ Cancer&draw=2&rank=1. (accessed 6 9 2021)
Authors Anonymous (2021c). Study of WNT974 in Combination with LGX818 and Cetuximab in Patients with BRAF-Mutant Metastatic Colorectal Cancer (mCRC) and Wnt Pathway Mutations-Full Text View-ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02278133?term= wnt974& cond=colorectal+cancer&draw=2&rank=1. (accessed 6 9 2021)
Authors Anonymous (2021d). A Study of ABT-888 in Combination with Temozolomide for Colorectal Cancer - Full Text View-ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01051596?term= ABT -888&cond=colorectal+cancer&draw=2&rank=1. (accessed 6 9 2021)
Authors Anonymous (2021e). Phase I Study to Evaluate Safety, Tolerability, Anti-tumour Activity and PK Profiles of Foxy-5 in Metastatic Breast, Colon or Prostate Cancer-Full Text View-ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02020291?term=Foxy5&cond=colorectal+cancer&draw=2&rank=1. (accessed 6 9 2021)
Authors Anonymous (2021f). Drug Trial to Investigate the Safety and Efficacy of Niclosamide Tablets in Patients with Metastases of a Colorectal Cancer Progressing after Therapy - Full Text View - ClinicalTrials.Gov. Available at: https://clinicaltrials.gov/ct2/show/NCT02519582?term=niclosamide&cond = colorectal+cancer&draw=2&rank=1. (accessed 6 9 2021)
Ayati, S. H., Fazeli, B., Momtazi-Borojeni, A. A., Cicero, A. F. G., Pirro, M., and Sahebkar, A. (2017). Regulatory Effects of Berberine on microRNome in Cancer and Other Conditions. Crit. Rev. Oncology/Hematology 116, 147–158. doi:10.1016/j.critrevonc.2017.05.008
Bahrami, A., Amerizadeh, F., Shahidsales, S., Khazaei, M., Ghayour-Mobarhan, M., Sadeghnia, H. R., et al. (2017). Therapeutic Potential of Targeting Wnt/β-Catenin Pathway in Treatment of Colorectal Cancer: Rational and Progress. J. Cel. Biochem. 118 (8), 1979–1983. doi:10.1002/jcb.25903
Barker, N., Ridgway, R. A., van Es, J. H., van de Wetering, M., Begthel, H., van den Born, M., et al. (2009). Crypt Stem Cells as the Cells-Of-Origin of Intestinal Cancer. Nature 457 (7229), 608–611. doi:10.1038/nature07602
Buchert, M., Athineos, D., Abud, H. E., Burke, Z. D., Faux, M. C., Samuel, M. S., et al. (2010). Genetic Dissection of Differential Signaling Threshold Requirements for the Wnt/β-Catenin Pathway In Vivo. Plos Genet. 6 (1), e1000816. doi:10.1371/journal.pgen.1000816
Chan, Y.-T., Cheung, F., Zhang, C., Fu, B., Tan, H.-Y., Norimoto, H., et al. (2020). Ancient Chinese Medicine Herbal Formula Huanglian Jiedu Decoction as a Neoadjuvant Treatment of Chemotherapy by Improving Diarrhea and Tumor Response. Front. Pharmacol. 11. doi:10.3389/fphar.2020.00252
Chao, D.-C., Lin, L.-J., Kao, S.-T., Huang, H.-C., Chang, C.-S., Liang, J.-A., et al. (2011). Inhibitory Effects of Zuo-Jin-Wan and its Alkaloidal Ingredients on Activator Protein 1, Nuclear Factor-Κb, and Cellular Transformation in HepG2 Cells. Fitoterapia 82 (4), 696–703. doi:10.1016/j.fitote.2011.02.009
Cho, Y.-H., Cha, P.-H., Kaduwal, S., Park, J.-C., Lee, S.-K., Yoon, J.-S., et al. (2016). KY1022, a Small Molecule Destabilizing Ras via Targeting the Wnt/β-Catenin Pathway, Inhibits Development of Metastatic Colorectal Cancer. Oncotarget 7 (49), 81727–81740. doi:10.18632/oncotarget.13172
Christie, M., Jorissen, R. N., Mouradov, D., Sakthianandeswaren, A., Li, S., Day, F., et al. (2013). Different APC Genotypes in Proximal and Distal Sporadic Colorectal Cancers Suggest Distinct WNT/β-catenin Signalling Thresholds for Tumourigenesis. Oncogene 32 (39), 4675–4682. doi:10.1038/onc.2012.486
Clara, J. A., Monge, C., Yang, Y., and Takebe, N. (2020). Targeting Signalling Pathways and the Immune Microenvironment of Cancer Stem Cells - a Clinical Update. Nat. Rev. Clin. Oncol. 17 (4), 204–232. doi:10.1038/s41571-019-0293-2
Deguchi, A. (2015). Curcumin Targets in Inflammation and Cancer. Emiddt 15 (2), 88–96. doi:10.2174/1871530315666150316120458
Diwanji, N., and Bergmann, A. (2018). An Unexpected Friend − ROS in Apoptosis-Induced Compensatory Proliferation: Implications for Regeneration and Cancer. Semin. Cel Dev. Biol. 80, 74–82. doi:10.1016/j.semcdb.2017.07.004
Drost, J., van Jaarsveld, R. H., Ponsioen, B., Zimberlin, C., van Boxtel, R., Buijs, A., et al. (2015). Sequential Cancer Mutations in Cultured Human Intestinal Stem Cells. Nature 521 (7550), 43–47. doi:10.1038/nature14415
Ewan, K., Pająk, B., Stubbs, M., Todd, H., Barbeau, O., Quevedo, C., et al. (2010). A Useful Approach to Identify Novel Small-Molecule Inhibitors of Wnt-dependent Transcription. Cancer Res. 70 (14), 5963–5973. doi:10.1158/0008-5472.CAN-10-1028
Filippo, C. D., Luceri, C., Caderni, G., Pacini, M., Messerini, L., Biggeri, A., et al. (2002). Mutations of the APC Gene in Human Sporadic Colorectal Cancers. Scand. J. Gastroenterol. 37 (9), 1048–1053. doi:10.1080/003655202320378248
Fodde, R. (2002). The APC Gene in Colorectal Cancer. Eur. J. Cancer 38 (7), 867–871. doi:10.1016/s0959-8049(02)00040-0
Franke, A. J., Skelton, W. P., Starr, J. S., Parekh, H., Lee, J. J., Overman, M. J., et al. (2019). Immunotherapy for Colorectal Cancer: A Review of Current and Novel Therapeutic Approaches. J. Natl. Cancer Inst. 111 (11), 1131–1141. doi:10.1093/jnci/djz093
Gonsalves, F. C., Klein, K., Carson, B. B., Katz, S., Ekas, L. A., Evans, S., et al. (2011). An RNAi-Based Chemical Genetic Screen Identifies Three Small-Molecule Inhibitors of the Wnt/wingless Signaling Pathway. Proc. Natl. Acad. Sci. 108 (15), 5954–5963. doi:10.1073/pnas.1017496108
Gu, Y., Wang, Q., Guo, K., Qin, W., Liao, W., Wang, S., et al. (2016). TUSC3 Promotes Colorectal Cancer Progression and Epithelial-Mesenchymal Transition (EMT) through WNT/β-catenin and MAPK Signalling. J. Pathol. 239 (1), 60–71. doi:10.1002/path.4697
Guo, J., Yang, Z., Zhou, H., Yue, J., Mu, T., Zhang, Q., et al. (2020). Upregulation of DKK3 by miR‐483‐3p Plays an Important Role in the Chemoprevention of Colorectal Cancer Mediated by Black Raspberry Anthocyanins. Mol. Carcinog 59 (2), 168–178. doi:10.1002/mc.23138
Guo, Z., Liu, Z., Yue, H., and Wang, J. (2018). Retracted : Beta‐elemene Increases Chemosensitivity to 5‐fluorouracil through Down‐regulating microRNA‐191 Expression in Colorectal Carcinoma Cells. J. Cel. Biochem. 119 (8), 7032–7039. doi:10.1002/jcb.26914
Gustavsson, B., Carlsson, G., Machover, D., Petrelli, N., Roth, A., Schmoll, H.-J., et al. (2015). A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Colorectal Cancer 14 (1), 1–10. doi:10.1016/j.clcc.2014.11.002
Hankey, W., Frankel, W. L., and Groden, J. (2018). Functions of the APC Tumor Suppressor Protein Dependent and Independent of Canonical WNT Signaling: Implications for Therapeutic Targeting. Cancer Metastasis Rev. 37 (1), 159–172. doi:10.1007/s10555-017-9725-6
Hao, J., Ao, A., Zhou, L., Murphy, C. K., Frist, A. Y., Keel, J. J., et al. (2013). Selective Small Molecule Targeting β-Catenin Function Discovered by In Vivo Chemical Genetic Screen. Cel Rep. 4 (5), 898–904. doi:10.1016/j.celrep.2013.07.047
He, B., Gao, J., Luo, X., Luo, J., Shen, J., Wang, L., et al. (2011). Ginsenoside Rg3 Inhibits Colorectal Tumor Growth through the Down-Regulation of Wnt/ß-Catenin Signaling. Int. J. Oncol. 38 (2), 437. doi:10.3892/ijo.2010.858
He, B.-C., Gao, J.-L., Zhang, B.-Q., Luo, Q., Shi, Q., Kim, S. H., et al. (2011). Tetrandrine Inhibits Wnt/β-Catenin Signaling and Suppresses Tumor Growth of Human Colorectal Cancer. Mol. Pharmacol. 79 (2), 211–219. doi:10.1124/mol.110.068668
He, X., Zhang, W., Yan, C., Nie, F., Li, C., Liu, X., et al. (2017). Chemical Biology Reveals CARF as a Positive Regulator of Canonical Wnt Signaling by Promoting TCF/β-catenin Transcriptional Activity. Cell Discov 3, 17003. doi:10.1038/celldisc.2017.3
Huang, S.-M. A., Mishina, Y. M., Liu, S., Cheung, A., Stegmeier, F., Michaud, G. A., et al. (2009). Tankyrase Inhibition Stabilizes Axin and Antagonizes Wnt Signalling. Nature 461 (7264), 614–620. doi:10.1038/nature08356
Isobe, T., Hisamori, S., Hogan, D. J., Zabala, M., Hendrickson, D. G., Dalerba, P., et al. (2014). miR-142 Regulates the Tumorigenicity of Human Breast Cancer Stem Cells through the Canonical WNT Signaling Pathway. Elife 3, e01977. doi:10.7554/eLife.01977
Ji, Q., Liu, X., Fu, X., Zhang, L., Sui, H., Zhou, L., et al. (2013). Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS One 8 (11), e78700. doi:10.1371/journal.pone.0078700
Jiang, X., Li, S., Qiu, X., Cong, J., Zhou, J., and Miu, W. (2019). Curcumin Inhibits Cell Viability and Increases Apoptosis of SW620 Human Colon Adenocarcinoma Cells via the Caudal Type Homeobox-2 (CDX2)/Wnt/β-Catenin Pathway. Med. Sci. Monit. 25, 7451–7458. doi:10.12659/MSM.918364
Jiang, Z., Cao, Q., Dai, G., Wang, J., Liu, C., Lv, L., et al. (2019). Celastrol Inhibits Colorectal Cancer through TGF-β1/Smad Signaling. Onco Targets Ther. 12, 509–518. doi:10.2147/OTT.S187817
Katoh, M. (2018). Multi-layered P-revention and T-reatment of C-hronic I-nflammation, O-rgan F-ibrosis and C-ancer A-ssociated with C-anonical WNT/β-catenin S-ignaling A-ctivation (Review). Int. J. Mol. Med. 42 (2), 713–725. doi:10.3892/ijmm.2018.3689
Kaur, M., Velmurugan, B., Tyagi, A., Agarwal, C., Singh, R. P., and Agarwal, R. (2010). Silibinin Suppresses Growth of Human Colorectal Carcinoma SW480 Cells in Culture and Xenograft through Down-Regulation of β-Catenin-Dependent Signaling. Neoplasia 12 (5), 415–424. doi:10.1593/neo.10188
Kinzler, K., Nilbert, M., Su, L., Vogelstein, B., Bryan, T., Levy, D., et al. (1991). Identification of FAP Locus Genes from Chromosome 5q21. Science 253 (5020), 661–665. doi:10.1126/science.1651562
Krishnamachary, B., Subramaniam, D., Dandawate, P., Ponnurangam, S., Srinivasan, P., Ramamoorthy, P., et al. (2019). Targeting Transcription Factor TCF4 by Gamma-Mangostin, a Natural Xanthone. Oncotarget 10 (54), 5576–5591. doi:10.18632/oncotarget.27159
Lau, T., Chan, E., Callow, M., Waaler, J., Boggs, J., Blake, R. A., et al. (2013). A Novel Tankyrase Small-Molecule Inhibitor Suppresses APC Mutation-Driven Colorectal Tumor Growth. Cancer Res. 73 (10), 3132–3144. doi:10.1158/0008-5472.CAN-12-4562
Lee, S.-K., Cho, Y.-H., Cha, P.-H., Yoon, J.-S., Ro, E. J., Jeong, W.-J., et al. (2018a). A Small Molecule Approach to Degrade RAS with EGFR Repression Is a Potential Therapy for KRAS Mutation-Driven Colorectal Cancer Resistance to Cetuximab. Exp. Mol. Med. 50 (11), 1–12. doi:10.1038/s12276-018-0182-2
Lee, S.-K., Hwang, J.-H., and Choi, K.-Y. (2018b). Interaction of the Wnt/β-Catenin and RAS-ERK Pathways Involving Co-stabilization of Both β-catenin and RAS Plays Important Roles in the Colorectal Tumorigenesis. Adv. Biol. Regul. 68, 46–54. doi:10.1016/j.jbior.2018.01.001
Lei, C., Yao, Y., Shen, B., Liu, J., Pan, Q., Liu, N., et al. (2019). Columbamine Suppresses the Proliferation and Malignization of colon Cancer Cells via Abolishing Wnt/β-Catenin Signaling Pathway. Cancer Manag Res. 11, 8635–8645. doi:10.2147/CMAR.S209861
Li, V. S. W., Ng, S. S., Boersema, P. J., Low, T. Y., Karthaus, W. R., Gerlach, J. P., et al. (2012). Wnt Signaling through Inhibition of β-Catenin Degradation in an Intact Axin1 Complex. Cell 149 (6), 1245–1256. doi:10.1016/j.cell.2012.05.002
Li, W., Yang, C.-J., Wang, L.-Q., Wu, J., Dai, C., Yuan, Y.-M., et al. (2019). A Tannin Compound from Sanguisorba Officinalis Blocks Wnt/β-Catenin Signaling Pathway and Induces Apoptosis of Colorectal Cancer Cells. Chin. Med. 14, (1). 22. doi:10.1186/s13020-019-0244-y
Li, X., Pu, J., Jiang, S., Su, J., Kong, L., Mao, B., et al. (2013). Henryin, an Ent-Kaurane Diterpenoid, Inhibits Wnt Signaling through Interference with β-Catenin/TCF4 Interaction in Colorectal Cancer Cells. PLoS One 8 (7), e68525. doi:10.1371/journal.pone.0068525
Li, Y., Qin, X., Li, P., Zhang, H., Lin, T., Miao, Z., et al. (2019). Isobavachalcone Isolated From Psoralea Corylifolia Inhibits Cell Proliferation and Induces Apoptosis via Inhibiting the AKT/GSK-3beta/beta-catenin Pathway in Colorectal Cancer Cells. Drug Des. Dev. Ther. 13, 1449–1460. doi:10.2147/DDDT.S192681
Li, Y., Wang, S., Sun, Y., Xu, W., Zheng, H., Wang, Y., et al. (2020). Apple Polysaccharide Protects ICR Mice against Colitis Associated Colorectal Cancer through the Regulation of Microbial Dysbiosis. Carbohydr. Polym. 230, 115726. doi:10.1016/j.carbpol.2019.115726
Liu, J., Jiang, M., Li, Z., Zhang, X., Li, X., Hao, Y., et al. (2018). A Novel Systems Pharmacology Method to Investigate Molecular Mechanisms of Scutellaria Barbata D. Don for Non-small Cell Lung Cancer. Front. Pharmacol. 9, 1473. doi:10.3389/fphar.2018.01473
Lu, Y., Zhang, R., Zhang, X., Zhang, B., and Yao, Q. (2020). Curcumin May Reverse 5-fluorouracil Resistance on Colonic Cancer Cells by Regulating TET1-NKD-Wnt Signal Pathway to Inhibit the EMT Progress. Biomed. Pharmacother. 129, 110381. doi:10.1016/j.biopha.2020.110381
Ma, W., Li, X., Song, P., Zhang, Q., Wu, Z., Wang, J., et al. (2019). A Vanillin Derivative Suppresses the Growth of HT29 Cells through the Wnt/β-Catenin Signaling Pathway. Eur. J. Pharmacol. 849, 43–49. doi:10.1016/j.ejphar.2019.01.047
Masaru, K. (2017). Canonical and Non-canonical WNT Signaling in Cancer Stem Cells and Their Niches: Cellular Heterogeneity, Omics Reprogramming, Targeted Therapy and Tumor Plasticity (Review). Int. J. Oncol. 51 (5), 1357–1369. doi:10.3892/ijo.2017.4129
Mashima, T., Taneda, Y., Jang, M.-K., Mizutani, A., Muramatsu, Y., Yoshida, H., et al. (2017). mTOR Signaling Mediates Resistance to Tankyrase Inhibitors in Wnt-Driven Colorectal Cancer. Oncotarget 8 (29), 47902–47915. doi:10.18632/oncotarget.18146
Matano, M., Date, S., Shimokawa, M., Takano, A., Fujii, M., Ohta, Y., et al. (2015). Modeling Colorectal Cancer Using CRISPR-Cas9-Mediated Engineering of Human Intestinal Organoids. Nat. Med. 21 (3), 256–262. doi:10.1038/nm.3802
Mazzotta, S., Neves, C., Bonner, R. J., Bernardo, A. S., Docherty, K., and Hoppler, S. (2016). Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cel Rep. 7 (4), 764–776. doi:10.1016/j.stemcr.2016.08.008
Mcquade, R. M., Stojanovska, V., Bornstein, J. C., and Nurgali, K. (2017). Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr Med Chem. 24 (15), 1537–1557. doi:10.2174/0929867324666170111152436
Mervai, Z., Sólyomváry, A., Tóth, G., Noszál, B., Molnár-Perl, I., Baghy, K., et al. (2015). Endogenous Enzyme-Hydrolyzed Fruit of Cirsium Brachycephalum: Optimal Source of the Antiproliferative Lignan Trachelogenin Regulating the Wnt/β-Catenin Signaling Pathway in the SW480 Colon Adenocarcinoma Cell Line. Fitoterapia 100, 19–26. doi:10.1016/j.fitote.2014.10.017
Momtazi-Borojeni, A. A., Abdollahi, E., Ghasemi, F., Caraglia, M., and Sahebkar, A. (2018). The Novel Role of Pyrvinium in Cancer Therapy. J. Cel. Physiol. 233 (4), 2871–2881. doi:10.1002/jcp.26006
Narwal, M., Venkannagari, H., and Lehtio, L. (2012). Structural Basis of Selective Inhibition of Human Tankyrases. J. Med. Chem. 55 (3), 1360–1367. doi:10.1021/jm201510p
Network, C. G. A. (2012). Comprehensive Molecular Characterization of Human colon and Rectal Cancer. Nature 487 (7407), 330–337. doi:10.1038/nature11252
Nguyen, A. V., Martinez, M., Stamos, M. J., Moyer, M. P., Planutis, K., Hope, C., et al. (2009). Results of a Phase I Pilot Clinical Trial Examining the Effect of Plant-Derived Resveratrol and Grape Powder on Wnt Pathway Target Gene Expression in Colonic Mucosa and colon Cancer. Cancer Manag. Res. 1, 25–37.
Nguyen, L. H., Goel, A., and Chung, D. C. (2020). Pathways of Colorectal Carcinogenesis. Gastroenterology 158 (2), 291–302. doi:10.1053/j.gastro.2019.08.059
Nishisho, I., Nakamura, Y., Miyoshi, Y., Miki, Y., Ando, H., Horii, A., et al. (1991). Mutations of Chromosome 5q21 Genes in FAP and Colorectal Cancer Patients. Science 253 (5020), 665–669. doi:10.1126/science.1651563
Pahlke, G., Ngiewih, Y., Kern, M., Jakobs, S., Marko, D., and Eisenbrand, G. (2006). Impact of Quercetin and EGCG on Key Elements of the Wnt Pathway in Human Colon Carcinoma Cells. J. Agric. Food Chem. 54 (19), 7075–7082. doi:10.1021/jf0612530
Pan, J., Xu, Y., Song, H., Zhou, X., Yao, Z., and Ji, G. (2017). Extracts of Zuo Jin Wan, a Traditional Chinese Medicine, Phenocopies 5-HTR1D Antagonist in Attenuating Wnt/β-Catenin Signaling in Colorectal Cancer Cells. BMC Complement. Altern. Med. 17 (1). 506. doi:10.1186/s12906-017-2006-7
Pearlman, R., Frankel, W. L., Swanson, B., Zhao, W., Yilmaz, A., Miller, K., et al. (2017). Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients with Early-Onset Colorectal Cancer. JAMA Oncol. 3 (4), 464–471. doi:10.1001/jamaoncol.2016.5194
Polakis, P. (2012). Wnt Signaling in Cancer. Cold Spring Harbor Perspect. Biol. 4 (5), a008052. doi:10.1101/cshperspect.a008052
Predes, D., Oliveira, L., Ferreira, L., Maia, L. A., Delou, J., Faletti, A., et al. (2019). The Chalcone Lonchocarpin Inhibits Wnt/β-Catenin Signaling and Suppresses Colorectal Cancer Proliferation. Cancers 11 (12), 1968. doi:10.3390/cancers11121968
Qi, L., Sun, B., Liu, Z., Cheng, R., Li, Y., and Zhao, X. (2014). Wnt3a Expression Is Associated with Epithelial-Mesenchymal Transition and Promotes colon Cancer Progression. J. Exp. Clin. Cancer Res. 33, 107. doi:10.1186/s13046-014-0107-4
Qiu, S., Wu, X., Liao, H., Zeng, X., Zhang, S., Lu, X., et al. (2017). Pteisolic Acid G, a Novel Ent-Kaurane Diterpenoid, Inhibits Viability and Induces Apoptosis in Human Colorectal Carcinoma Cells. Oncol. Lett. 14 (5), 5540–5548. doi:10.3892/ol.2017.6889
Ren, H., Zhao, J., Fan, D., Wang, Z., Zhao, T., Li, Y., et al. (2019). Alkaloids from Nux Vomica Suppresses colon Cancer Cell Growth through Wnt/β‐catenin Signaling Pathway. Phytotherapy Res. 33 (5), 1570–1578. doi:10.1002/ptr.6347
Reya, T., and Clevers, H. (2005). Wnt Signalling in Stem Cells and Cancer. Nature 434 (7035), 843–850. doi:10.1038/nature03319
Schatoff, E. M., Leach, B. I., and Dow, L. E. (2017). Wnt Signaling and Colorectal Cancer. Curr. Colorectal Cancer Rep. 13 (2), 101–110. doi:10.1007/s11888-017-0354-9
Sebio, A., Kahn, M., and Lenz, H.-J. (2014). The Potential of Targeting Wnt/β-Catenin in colon Cancer. Expert Opin. Ther. Targets 18 (6), 611–615. doi:10.1517/14728222.2014.906580
Shah, K., Panchal, S., and Patel, B. (2021). Porcupine Inhibitors: Novel and Emerging Anti-cancer Therapeutics Targeting the Wnt Signaling Pathway. Pharmacol. Res. 167, 105532. doi:10.1016/j.phrs.2021.105532
Shan, B., Wang, M., and Li, R. (2009). Quercetin Inhibit Human SW480 Colon Cancer Growth in Association with Inhibition of Cyclin D1 and Survivin Expression through Wnt/β-Catenin Signaling Pathway. Cancer Invest. 27 (6), 604–612. doi:10.1080/07357900802337191
Shan, J., Shi, D.-L., Wang, J., and Zheng, J. (2005). Identification of a Specific Inhibitor of the Dishevelled PDZ Domain†. Biochemistry 44 (47), 15495–15503. doi:10.1021/bi0512602
So, T.-H., Chan, S.-K., Lee, V. H.-F., Chen, B.-Z., Kong, F.-M., and Lao, L.-X. (2019). Chinese Medicine in Cancer Treatment - How Is it Practised in the East and the West?. Clin. Oncol. 31 (8), 578–588. doi:10.1016/j.clon.2019.05.016
Soo, Y. H., and Keller, T. H. (2015). The Use of porcupine Inhibitors to Target Wnt-Driven Cancers. Bioorg. Med. Chem. Lett. 25 (23), 5472–5476. doi:10.1016/j.bmcl.2015.10.032
Song, J., Seo, H., Kim, M.-R., Lee, S.-J., Ahn, S., and Song, M. (2020). Active Compound of Pharbitis Semen (Pharbitis Nil Seeds) Suppressed KRAS-Driven Colorectal Cancer and Restored Muscle Cell Function during Cancer Progression. Molecules 25 (12), 2864. doi:10.3390/molecules25122864
Spaderna, S., Schmalhofer, O., Hlubek, F., Berx, G., Eger, A., Merkel, S., et al. (2006). A Transient, EMT-Linked Loss of Basement Membranes Indicates Metastasis and Poor Survival in Colorectal Cancer. Gastroenterology 131 (3), 830–840. doi:10.1053/j.gastro.2006.06.016
Sun, M.-Y., Wang, D.-D., Sun, J., Zhao, X.-H., Cai, S., Wu, Q.-X., et al. (2019). The Zuo Jin Wan Formula Increases Chemosensitivity of Human Primary Gastric Cancer Cells by AKT Mediated Mitochondrial Translocation of Cofilin-1. Chin. J. Nat. Medicines 17 (3), 198–208. doi:10.1016/S1875-5364(19)30022-6
Sun, X., Xu, X., and Song, L. (2020). TKP, a Serine Protease Extracted fromTrichosanthes Kirilowii, Inhibits the Migration and Invasion of Colorectal Adenocarcinoma Cells by Targeting Wnt/β‐catenin and Hedgehog/Gli1 Signalings. Phytotherapy Res. 34 (4), 867–878. doi:10.1002/ptr.6569
Tan, H., Li, X., Yang, W. H., and Kang, Y. (2019). A Flavone, Wogonin From Scutellaria Baicalensis Inhibits the Proliferation of Human Colorectal Cancer Cells by Inducing of Autophagy, Apoptosis and G2/M Cell Cycle Arrest via Modulating the PI3K/AKT and STAT3 Signalling Pathways. J. BUON. 24 (3), 1143–1149.
Tanaka, N., Mashima, T., Mizutani, A., Sato, A., Aoyama, A., Gong, B., et al. (2017). APC Mutations as a Potential Biomarker for Sensitivity to Tankyrase Inhibitors in Colorectal Cancer. Mol. Cancer Ther. 16 (4), 752–762. doi:10.1158/1535-7163.MCT-16-0578
Tao, L., Gu, Y., Zheng, J., Yang, J., and Zhu, Y. (2019). Weichang'an Suppressed Migration and Invasion of HCT116 Cells by Inhibiting Wnt/β‐catenin Pathway while Upregulating ARHGAP25. Biotechnol. Appl. Biochem. 66 (5), 787–793. doi:10.1002/bab.1784
Tian, W., Han, X., Yan, M., Xu, Y., Duggineni, S., Lin, N., et al. (2012). Structure-Based Discovery of a Novel Inhibitor Targeting the β-Catenin/Tcf4 Interaction. Biochemistry 51 (2), 724–731. doi:10.1021/bi201428h
Todaro, M., Gaggianesi, M., Catalano, V., Benfante, A., Iovino, F., Biffoni, M., et al. (2014). CD44v6 Is a Marker of Constitutive and Reprogrammed Cancer Stem Cells Driving colon Cancer Metastasis. Cell Stem Cell 14 (3), 342–356. doi:10.1016/j.stem.2014.01.009
Vincan, E., and Barker, N. (2008). The Upstream Components of the Wnt Signalling Pathway in the Dynamic EMT and MET Associated with Colorectal Cancer Progression. Clin. Exp. Metastasis 25 (6), 657–663. doi:10.1007/s10585-008-9156-4
Voorneveld, P. W., Kodach, L. L., Jacobs, R. J., van Noesel, C. J. M., Peppelenbosch, M. P., Korkmaz, K. S., et al. (2015). The BMP Pathway Either Enhances or Inhibits the Wnt Pathway Depending on the SMAD4 and P53 Status in CRC. Br. J. Cancer 112 (1), 122–130. doi:10.1038/bjc.2014.560
Vu, T., and Datta, P. (2017). Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 9 (12), 171. doi:10.3390/cancers9120171
Waaler, J., Machon, O., Tumova, L., Dinh, H., Korinek, V., Wilson, S. R., et al. (2012). A Novel Tankyrase Inhibitor Decreases Canonical Wnt Signaling in colon Carcinoma Cells and Reduces Tumor Growth in Conditional APC Mutant Mice. Cancer Res. 72 (11), 2822–2832. doi:10.1158/0008-5472.CAN-11-3336
Wang, D., Ge, S., Chen, Z., and Song, Y. (2019). Evodiamine Exerts Anticancer Effects via Induction of Apoptosis and Autophagy and Suppresses the Migration and Invasion of Human colon Cancer Cells. J. BUON. 24 (5), 1824–1829.
Wei, L.-H., Lin, J.-M., Chu, J.-F., Chen, H.-W., Li, Q.-Y., and Peng, J. (2017). Scutellaria Barbata D. Don Inhibits Colorectal Cancer Growth via Suppression of Wnt/β-Catenin Signaling Pathway. Chin. J. Integr. Med. 23 (11), 858–863. doi:10.1007/s11655-017-2775-3
Wen, S.-Y., Chen, Y. Y., Deng, C. M., Zhang, C. Q., and Jiang, M. M (2019). Nerigoside Suppresses Colorectal Cancer Cell Growth and Metastatic Potential Through Inhibition of ERK/GSK3beta/Beta-Catenin Signaling Pathway. Phytomedicine. 57, 352–363. doi:10.1016/j.phymed.2018.12.033
Wu, C.-E., Zhuang, Y.-W., Zhou, J.-Y., Liu, S.-l., Wang, R.-P., and Shu, P. (2019). Cinnamaldehyde Enhances Apoptotic Effect of Oxaliplatin and Reverses Epithelial-Mesenchymal Transition and Stemnness in Hypoxic Colorectal Cancer Cells. Exp. Cel Res. 383 (1), 111500. doi:10.1016/j.yexcr.2019.111500
Wu, X., Yu, N., Zhang, Y., Ye, Y., Sun, W., Ye, L., et al. (2018). Radix Tetrastigma Hemsleyani Flavone Exhibits Antitumor Activity in Colorectal Cancer via Wnt/β-Catenin Signaling Pathway. Onco Targets Ther. 11, 6437–6446. doi:10.2147/OTT.S172048
Xu, C., Yang, M., Tian, J., Wang, X., and Li, Z. (2011). MALAT-1: a Long Non-coding RNA and its Important 3' End Functional Motif in Colorectal Cancer Metastasis. Int. J. Oncol. 39 (1), 169–175. doi:10.3892/ijo.2011.1007
Xu, M., Wang, S., Song, Y., Yao, J., Huang, K., and Zhu, X. (2016). Apigenin Suppresses Colorectal Cancer Cell Proliferation, Migration and Invasion via Inhibition of the Wnt/β-Catenin Signaling Pathway. Oncol. Lett. 11 (5), 3075–3080. doi:10.3892/ol.2016.4331
Yan, K. S., Janda, C. Y., Chang, J., Zheng, G. X. Y., Larkin, K. A., Luca, V. C., et al. (2017). Non-equivalence of Wnt and R-Spondin Ligands during Lgr5+ Intestinal Stem-Cell Self-Renewal. Nature 545 (7653), 238–242. doi:10.1038/nature22313
Yan, M., Li, G., and An, J. (2017). Discovery of Small Molecule Inhibitors of the Wnt/β-Catenin Signaling Pathway by Targeting β-catenin/Tcf4 Interactions. Exp. Biol. Med. (Maywood) 242 (11), 1185–1197. doi:10.1177/1535370217708198
Yang, Y., Han, Z., Han, Z., Li, X., Huang, A., Shi, J., et al. (2020). Epidemiology and Risk Factors of Colorectal Cancer in China. Chin. J. Cancer Res. 32 (6), 729–741. doi:10.21147/j.issn.1000-9604.2020.06.06
Ye, Z.-N., Yuan, F., Liu, J.-Q., Peng, X.-R., An, T., Li, X., et al. (2019). Physalis Peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer In Vitro and In Vivo. Molecules 24 (6), 1146. doi:10.3390/molecules24061146
Yeh, M.-H., Chiu, H.-P., Wu, M.-C., Koo, M., Lin, N.-W., Liao, K.-K., et al. (2020). Integrated Chinese Herbal Medicine and Western Medicine on the Survival in Patients with Colorectal Cancer: A Retrospective Study of Medical Records. Evidence-Based Complement. Altern. Med. 2020, 1–10. doi:10.1155/2020/4561040
Zhan, T., Rindtorff, N., and Boutros, M. (2017). Wnt Signaling in Cancer. Oncogene 36 (11), 1461–1473. doi:10.1038/onc.2016.304
Zhang, J., Cao, H., Zhang, B., Cao, H., Xu, X., Ruan, H., et al. (2013). Berberine Potently Attenuates Intestinal Polyps Growth in ApcMin Mice and Familial Adenomatous Polyposis Patients through Inhibition of Wnt Signalling. J. Cel. Mol. Med. 17 (11), 1484–1493. doi:10.1111/jcmm.12119
Zhang, L., Ren, B., Zhang, J., Liu, L., Liu, J., Jiang, G., et al. (2017). Anti-tumor Effect of Scutellaria Barbata D. Don Extracts on Ovarian Cancer and its Phytochemicals Characterisation. J. Ethnopharmacology 206, 184–192. doi:10.1016/j.jep.2017.05.032
Zhang, L., Zhang, J., Gong, Y., and Lv, L. (2020). Systematic and Experimental Investigations of the Anti‐colorectal Cancer Mediated by Genistein. Biofactors 46 (6), 974–982. doi:10.1002/biof.1677
Zhang, T., Wang, K., Zhang, J., Wang, X., Chen, Z., Ni, C., et al. (2013). Huaier Aqueous Extract Inhibits Colorectal Cancer Stem Cell Growth Partially via Downregulation of the Wnt/β-Catenin Pathway. Oncol. Lett. 5 (4), 1171–1176. doi:10.3892/ol.2013.1145
Keywords: traditional Chinese medicines, colorectal cancer, Wnt/β-catenin, potential role, small molecules, therapeutic mechanism
Citation: Chang J, Xavier HW, Chen D, Liu Y, Li H and Bian Z (2021) Potential Role of Traditional Chinese Medicines by Wnt/β-Catenin Pathway Compared With Targeted Small Molecules in Colorectal Cancer Therapy. Front. Pharmacol. 12:690501. doi: 10.3389/fphar.2021.690501
Received: 03 April 2021; Accepted: 02 July 2021;
Published: 26 July 2021.
Edited by:
Cecilia Veronica Nunez, National Institute of Amazonian Research (INPA), BrazilReviewed by:
Zhaofeng Liang, Jiangsu University, ChinaMei cun Yao, Sun Yat-Sen University, China
Qiuhua Lai, Southern Medical University, China
Copyright © 2021 Chang, Xavier, Chen, Liu, Li and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoxiang Bian, Ymlhbnp4aWFuZ0BnbWFpbC5jb20=; Hui Li, bGlodWlAZ3p1Y20uZWR1LmNu
 Jinrong Chang
Jinrong Chang Hoileong Wong Xavier
Hoileong Wong Xavier Dongfeng Chen
Dongfeng Chen Yamei Liu1
Yamei Liu1 Hui Li
Hui Li Zhaoxiang Bian
Zhaoxiang Bian