- 1School of Medicine, Shanghai University, Shanghai, China
- 2Department of Pharmacy, The Air Force Hospital From Eastern Theater of PLA, Nanjing, China
- 3College of Pharmacology, Anhui University of Chinese Medicine, Hefei, China
- 4Joint International Research Laboratory of Metabolic and Developmental Sciences, Key Laboratory of Urban Agriculture (South) Ministry of Agriculture, Plant Biotechnology Research Center, Fudan-SJTU-Nottingham Plant Biotechnology R&D Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China
- 5Nucliec Acid Research Departement, Genetic Engineering and Biotechnological Research Institute, City of Scientific Research and Technological Applications, Alexandria, Egypt
- 6MLS Ministry of Health, Alexandria, Egypt
- 7Department of Clinical Pharmacy, Jiading Branch of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Monocyte locomotion inhibitory factor (MLIF) is a heat-stable pentapeptide from Entamoeba histolytica. Our previous study found that MLIF protects against ischemic stroke in rats and mice and exerts a neuroprotection effect in human neuroblastoma SH-SY5Y cells. Microglia/macrophage polarization has been proven to be vital in the pathology of ischemic stroke. Nevertheless, whether MLIF is able to modulate microglia/macrophage polarization remains unclear. We performed middle cerebral artery occlusion (MCAO) on C57BL/6J male mice and induced cultured BV2 microglia by oxygen-glucose deprivation (OGD), respectively. Immunfluorescence was utilized to detect the M1/2 markers, such as CD206 and CD16/32. qPCR and ELISA were used to detect the signature gene change of M1/2. The MAPK and NF-κB pathway associated proteins were measured by Western blot. To identify the protein target of MLIF, a pull-down assay was performed. We found that MLIF promoted microglia transferring from a “sick” M1 phenotype to a “healthy” M2 phenotype in vivo or in vitro. Furthermore, we proved that eukaryotic elongation factor 1A1 (eEF1A1) was involved in the modulation of microglia/macrophage polarization. Knocking down eEF1A1 by siRNA exhibited the M1 promotion effect and M2 inhibition effect. Taken together, our results demonstrated MLIF modulated microglia/macrophage polarization by targeting eEF1A1 in ischemic stroke.
Introduction
Stroke is one of the three leading causes of death and also the most common reason for adults’ disability worldwide (Campbell and Khatri, 2020). Among all types of stroke, ischemic stroke, caused by blood vessel occlusion, accounts for the majority (Barthels and Das, 2020). Mechanisms involved in the process of stroke are extremely complicated, and inflammation is an important mediator in the pathogenesis of secondary injury after ischemic stroke (Jayaraj et al., 2019; Parikh et al., 2020).
As resident immune cells in the central nervous system, microglia are one of the first responders in CNS injuries (Greenhalgh et al., 2020; Xie et al., 2020). Currently, numerous studies have suggested that activated microglia with distinct phenotypes are double-edged swords in ischemic stroke (Liu ZJ. et al., 2019; Zhang et al., 2019; Lian et al., 2020). Differential polarization of microglia could likely explain the biphasic role of microglia in brain ischemia. Induced by IFN-γ and LPS stimulation, M1 phenotype microglia display enhanced expression of CD16/32, CD86, and iNOS and production of multiple pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) (Jha et al., 2016; Subhramanyam et al., 2019). The M2 phenotype could be induced by IL-4 or IL-13 and is characterized by enhanced expression of arginase-1, Fizz1, Ym1, CD206, insulin-like growth factor (IGF-1), and anti-inflammatory cytokines (IL-10, IL-4, and IL-13) (Prinz et al., 2019; Kwon and Koh, 2020). The M1 phenotype aggravates inflammation and exacerbates neuronal death, while the M2 phenotype contributes to improving neuronal survival and tissue repair (Han et al., 2021; Zhang et al., 2021). Thus, switching microglia from the M1 phenotype toward the M2 phenotype is an effective therapeutic strategy for ischemic stroke (Jiang et al., 2020).
Monocyte locomotion inhibitory factor (MLIF), a heat-stable pentapeptide produced by Entamoeba histolytica (Utrera-Barillas et al., 2003), had been approved as an investigational new drug for potential neural protection in acute ischemic stroke (Liu et al., 2018).
Our previous study found that MLIF could significantly decrease the cerebral infarction in the MCAO mice and rats and mitigate the ICAM-1 and VCAM-1 expression in OGD- or ox-LDL–induced bEnd3 cells by targeting eEF1A1 (Zhang et al., 2012). MLIF analogs have the same effect (Yao et al., 2011). Moreover, MLIF could also inhibit OGD-induced apoptosis in SH-SY5Y neuron cells by targeting eEF1A2 (Zhu et al., 2016). However, whether MLIF could regulate microglia polarization in ischemic stroke remains unclear.
In the current research, we explored a further study on the microglia/macrophage polarization of MLIF and found that MLIF promoted microglia polarization in vivo and in vitro. Moreover, this modulation effect is related to eEF1A1, the binding protein of MLIF in BV2 cells. It has been reported that eEF1A1 mediates the secretion of inflammatory cytokines in NF-κB signaling pathways, such as IL-1β, IL-18, and IL-6 (Wei et al., 2019). Therefore, MLIF targets eEF1A1 to regulate microglia polarization, probably through inflammatory pathways. Our current study revealed a novel pharmacological mechanism of MLIF that protects against ischemic stroke via modulating microglia polarization by targeting eEF1A1.
Materials and Methods
Reagents
MLIF and biotinylated MLIF were synthesized by Chinese Peptide Company (Hangzhou, China), with a purity above 98%. MLIF was dissolved in PBS (pH 7.4) to a final concentration of 4 mg/ml and stored at −80°C. eEF1A1 antibody was purchased from Abcam (Cambridge, MA, United States), and JNK, p-JNK, ERK, p-ERK, p38, p-p38, iκB, p-iκB, p65, and p-p65 antibodies were purchased from Cell Signaling Technology (Danvers, MA, United States). IRDye 800CW secondary antibody was purchased from LI-COR (Lincoln, NE, United States). Anti-Iba1 antibody was purchased from Wako (Tokyo, Japan). CD16/32 and CD206 antibodies were purchased from R&D Systems (MN, United States).Secondary antibodies (Cy™3 AffiniPure Donkey Anti-Rabbit IgG and Alexa Fluor488 AffiniPure Donkey Anti-Goat IgG) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, United States).
Middle Cerebral Artery Occlusion
C57BL/6J male mice weighting 25–30 g were purchased from Changzhou Cavens Experimental Animals Co., Ltd. (Changzhou, China). The mice were housed in a controlled environment under a 12-h light/dark cycle and received standard rat chow and water. All animal experiments and surgical procedures were supervised by the Institutional Animal Care and Use Committee at Shanghai University and conformed to international guidelines for the ethical use of experimental animals.
Male C57BL/6J mice were randomly divided into three groups (N = 10/group): the sham group, the MCAO group, and the MLIF treatment group. For the MLIF treatment group, MLIF (1 mg/kg) was injected into the tail vein 5 min prior to reperfusion. The other groups were treated with an equal volume of saline solution.
Transient MCAO surgery was preformed as previously described (Zhang et al., 2012). Briefly, mice were anesthetized with 2% sodium pentobarbital (3 ml/kg, i.p.) during the surgery. The left common carotid artery was placed into an 11-mm silicone-coated 8–0 filament from the internal carotid artery for 2 h, and the filament was pulled out gently to accomplish 24 h of reperfusion thereafter. Body temperature was maintained at 37.5 ± 0.5°C using a heating lamp. After reperfusion for 24 h, mice were euthanized, and the brains were harvested.
Immunofluorescence
The brain slices were prepared as previously published (Zhang et al., 2016). After they were blocked with 5% goat serum in TBST, the brain slices were incubated with primary antibodies including anti-Iba1, anti-CD16/32, or anti-CD206 overnight at 4°C. After they were washed with PBS, the brain slices were incubated with Alexa Fluor488 donkey anti-mouse (1:800; Jackson ImmunoResearch Laboratories, West Grove, PA, United States) and Cy3-conjugated donkey anti-rabbit (1:800; Jackson ImmunoResearch Laboratories, West Grove, PA, United States) for 1 h at room temperature. DAPI solution (Thermo Fisher, Waltham, MA, United States) was used to stain nuclei. All images were processed using Image J (NIH Image, Bethesda, MD, United States) for counting of recognized cells. Three randomly selected microscopic fields were calculated in the peri-infarct cortex of each section. The data were expressed as the colocalization percent of CD16/32 and Iba1 or CD206 and Iba1.
Cell Culture
BV2 microglia were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, United States) with 10% fetal bovine serum (Gibco, Carlsbad, CA, United States) and 100 μg/ml penicillin–streptomycin solution (Thermo Scientific, Waltham, MA, United States). Cells were grown in 5% CO2 and 95% atmosphere at 37°C. The medium was replaced every 2 days.
Oxygen-Glucose Deprivation
The OGD insult was carried out in BV2 cells to mimic ischemic conditions in vitro as described previously (Zhu et al., 2016). The culture medium was replaced with a glucose-free DMEM before OGD, cells were then incubated in a modular hypoxia chamber (Billups-Rothenberg, Del Mar, CA, United States) flushed with 5% CO2 and 95% N2. The chamber was placed in an incubator for 4 h at 37°C. The normal medium under normoxia served as the control. MLIF (1.0 μg/ml) was given prior to the OGD insult.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from brain tissues and BV2 cells using a Total RNA Kit (Takara, Shiga, Japan). cDNAs were synthesized using 5×Primescript reverse transcription reagents (Takara, Shiga, Japan) according to the manufacturer’s instruction. qRT-PCR was performed using SYBR Premix ExTaq (Tli RnaseH Plus) (Takara, Shiga, Japan) on a 7,500 Real-Time PCR System (Applied Biosystems). The levels of all target genes were normalized to the control (GAPDH). Primers used for quantitative RT-PCR (qRT-PCR) were as follows:
Mouse CD11b Forward: CCAAGACGATCTCAGCATCA
Reverse: TTCTGGCTTGCTGAATCCTT
Mouse CD32 Forward: AATCCTGCCGTTCCTACTGATC
Reverse: GTGTCACCGTGTCTTCCTTGAG
Mouse CD86 Forward: GACCGTTGTGTGTGTTCTGG
Reverse: GATGAGCAGCATCACAAGGA
Mouse iNOS Forward: GGCAGCCTGTGAGACCTTTG
Reverse: GCATTGGAAGTGAAGCGTTTC
Mouse CD206 Forward: TTCGGTGGACTGTGGACGAGCA
Reverse: ATAAGCCACCTGCCACTCCGGT
Mouse Arg-1 Forward: GAACACGGCAGTGGCTTTAAC
Reverse: TGCTTAGCTCTGTCTGCTTTGC
Mouse Ym1 Forward: CGAGGTAATGAGTGGGTTGG
Reverse: CACGGCACCTCCTAAATTGT
Mouse Fizz1 Forward: CTGCTACTGGGTGTGCTTGT
Reverse: GCAGTGGTCCAGTCAACGAG
Mouse GAPDH Forward: CTTCACCACCATGGAGAAGGC
Reverse: GGCATGGACTGTGGTCATGAG
Enzyme-Linked Immunosorbent Assay
ELISA was used to determine the inflammatory cytokines (IL-1β, TNF-α, IL-4, and IL-10) in the ischemic brain tissue homogenates and supernatant of the BV2 cells according to the protocol of the manufacturer’s kit (R&D Systems, MN, United States). The reactions were performed in ELISA plates and were read at a wavelength of 450 nm. Concentrations of inflammatory cytokines were obtained from the standard curve.
Western Blot Analysis
BV2 cells were lysed with M-PER Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with a protease inhibitor cocktail. The total protein concentration in the lysates was measured using BCA assay (Pierce, Rockford, IL). An equal amount of the extracts was analyzed in SDS-PAGE and subsequently electro-transferred onto PVDF membranes (Millipore, Temecula, CA, United States). After blocking with 5% BSA for 2 h, the membranes were incubated with the primary antibodies overnight at 4°C. The blots were washed and incubated with appropriate IRDye 800CW secondary antibodies (LI-COR, Lincoln, Nebraska, United States) for 1 h at room temperature. After being washed, the blots were scanned using an Odyssey scanner (LI-COR, Lincoln, Nebraska, United States). GAPDH was used as a loading control. The optical densities of the protein bands were semi-quantified using Quantity One software.
Immunoprecipitation
Immunoprecipitation was performed as previously described (Zhang et al., 2012). BV2 cells were lysed with M-PER Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with a protease inhibitor cocktail. The protein supernatant was incubated with the biotinylated MLIF (40 μl, 5 mg/ml) or the control solution for 6 h. After being washed twice with 1 ml of PBS, the streptavidin–agarose beads (Invitrogen, Carlsbad, CA, United States) were added and stirred gently at 4°C for 8 h. After it was washed twice with 1 ml of PBS, the mixture was centrifugated at 12,000 g for 5 min, and the first supernatant sample was obtained (S1). Then the supernatant was repeated using the binding assay twice to obtain sample 2 (S2) and sample 3 (S3). The control groups were correspondingly named C1, C2, and C3. After protein denaturation, the samples were separated by SDS-PAGE, followed by Coomassie brilliant blue staining.
RNA Interference
eEF1A1 siRNA and control siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States). The BV2 cells were transfected with eEF1A1 siRNA or control siRNA (50 nM) using INTERFERin® Transfection Reagent (Polyplus, Illkirch, France) according to the manufacturer’s instructions. After 48 h of transfection, cells were lysed. Knockdown effect of eEF1A1 was analyzed by Western blot.
Statistical Analysis
All data were expressed as the mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA). p < 0.05 was considered as statistically significant.
Results
Monocyte Locomotion Inhibitory Factor Promoted Microglia Polarization Toward the M2 Phenotype in the Middle Cerebral Artery Occlusion Model
To evaluate the effect of MLIF on microglia polarization in ischemic stroke, co-immunostaining was performed with the representative M1 marker CD16/32 or M2 marker CD206 and the microglia/macrophage marker Iba1. As shown in Figure 1A, the percentage of the CD16/32+ Iba1+ cells was higher in MCAO mouse brain slices (p < 0.01). The colocalization was obviously less in the MLIF group (p < 0.05). In contrast, the CD206+ Iba1+ cells were more in the MLIF group than the MCAO group (p < 0.05,Figure 1B). Together, these results suggested that MLIF promoted microglia polarization to the M2 phenotype after MCAO.
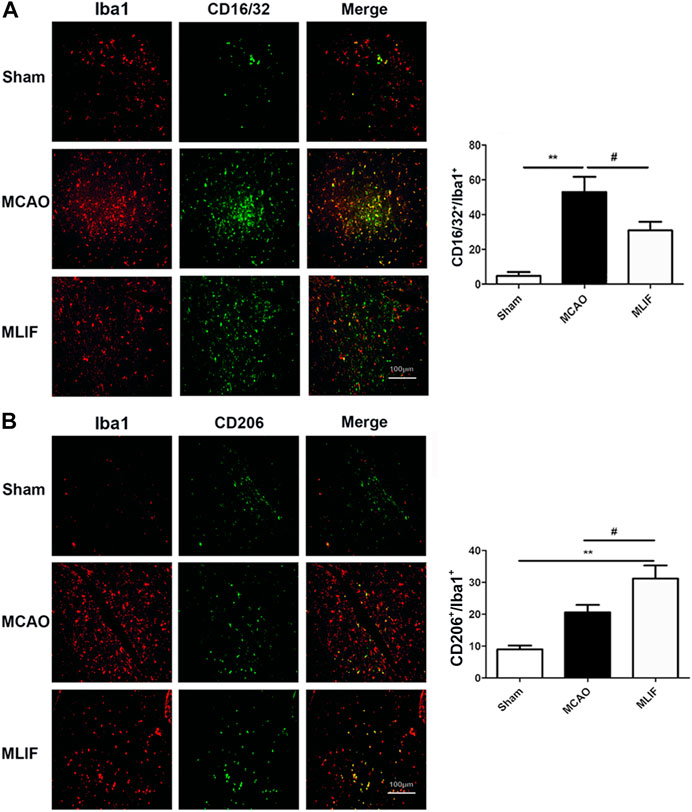
FIGURE 1. MLIF promoted microglia polarization toward the M2 phenotype in the mice MCAO model. (A,B) Double immunostaining was used to assess microglia (Iba1+) and an M1-associated marker (CD16/32+) or an M2-associated marker (CD206+) in mouse brain slices. The summarized bar graphs showed the colocalization rate of CD16/32 or CD206 with the Iba1 number in the ischemic region in the cortex. Scale bar: 200 μm. Data are expressed as the mean ± SEM. N = 6. **p < 0.01, vs Sham group, #p < 0.05 vs MCAO group.
Monocyte Locomotion Inhibitory Factor Promoted Microglia Polarization Toward the M2 Phenotype in Oxygen-Glucose Deprivation–Insulted BV2 Microglia
To further confirm the effect of MLIF on microglia polarization, we cultured BV2 microglia and established the OGD model to mimic ischemic conditions in vitro. Real-time PCR and ELISA were used to evaluate the microglia polarization effect of MLIF. As shown in Figures 2A–D, M1 signature genes including CD11b, CD32, and iNOS significantly increased compared with those in the control group (p < 0.01, p < 0.001), and MLIF significantly decreased the mRNA levels of CD11b, CD32, and iNOS (p < 0.01, p < 0.001). Meanwhile, MLIF also reduced production of inflammatory cytokines such as IL-1β and TNF-α (p < 0.01, p < 0.001,Figures 2E,F). Moreover, the MLIF-treated group exhibited higher mRNA expression of M2 signature genes including Arg-1, CD206, Ym1, and Fizz1 and more M2-related cytokines including IL-4 and IL-10 than the OGD group (Figures 2G–L). Taken together, in vitro data demonstrated that MLIF promoted M2 polarization and inhibited M1 polarization in OGD-insulted BV2 microglia.
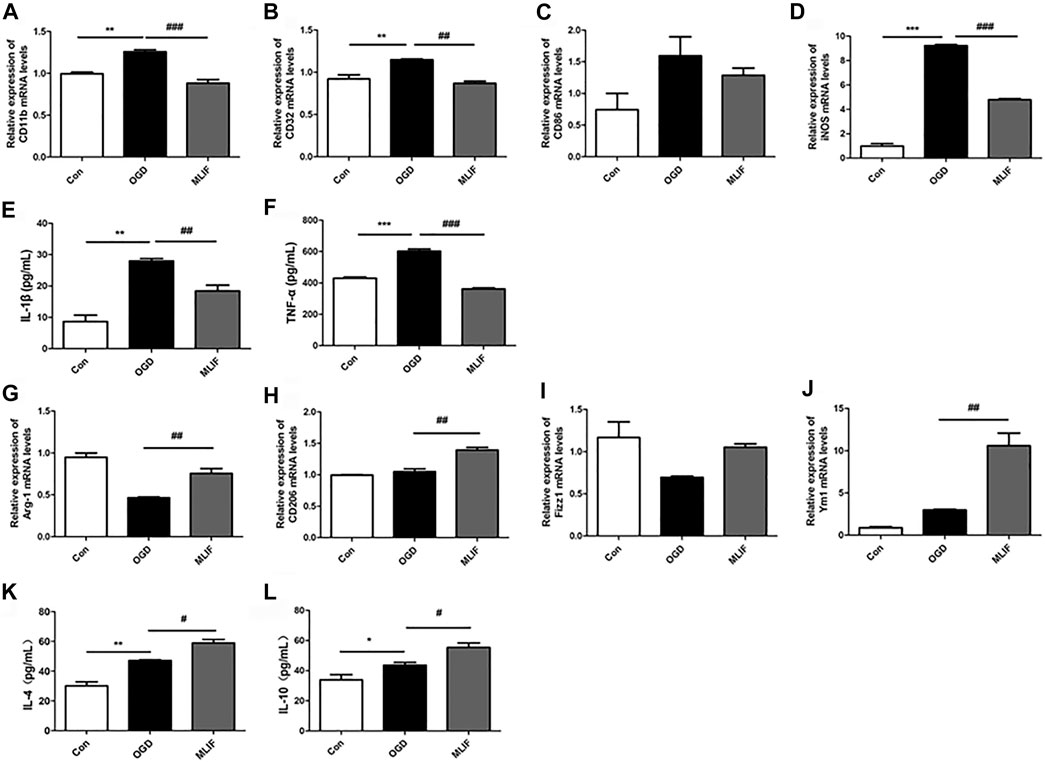
FIGURE 2. MLIF promoted microglia polarization toward M2 polarization in OGD-insulted BV2 microglia. BV2 cells were exposed to OGD for 4 h with or without MLIF (1.0 μg/ml) incubation. Real-time PCR was used to detect the M1 signature genes CD11b (A), CD32 (B), CD86 (C), and iNOS (D) and the M2 signature genes Arg-1 (G), CD206 (H), Ym1 (I), and Fizz1 (J). ELISA was used to detect M1-related inflammation cytokines IL-1β (E) and TNF-α (F) and M2-related cytokines IL-4 (K) and IL-10 (L) in the cell supernatant. Data are expressed as the mean ± SEM. N = 3. *p < 0.05, **p < 0.01, ***p < 0.001, vs Control group; #p < 0.05, ##p < 0.01, ###p < 0.001, vs OGD group.
Monocyte Locomotion Inhibitory Factor Inhibited Oxygen-Glucose Deprivation–Insulted BV2 Microglia M1 Polarization by Suppressing the NF-κB Pathway
It was reported that MLIF regulates 385 genes which are involved in the MAPK and NF-κB pathways (Silva-García et al., 2008). So we evaluated the effect of MLIF on related proteins of the MAPK and NF-κB pathways in OGD-insulted BV2 microglia. Western blot results showed that MLIF had no significant effect on the MAPK pathway (Figure 3A). However, the p-p65 and p-iκB expression was significantly increased in the OGD group (p < 0.01, p < 0.001), whereas MLIF significantly inhibited their expression (p < 0.05, p < 0.01, Figure 3B). Thus, MLIF could suppress the OGD-induced NF-κB pathway activation in OGD-insulted BV2 microglia.

FIGURE 3. Effect of MLIF on the MAPK pathway and the NF-κB pathway in OGD-insulted BV2 microglia. BV2 cells were exposed to OGD for 4 h with or without MLIF (1.0 μg/ml) incubation. (A) MAPK pathway–associated proteins p-ERK, p-JNK, and p-p38 and (B) NF-κB pathway–associated proteins p65, p-p65, iκB, and p-iκB were determined by Western blot. Data are expressed as the mean ± SEM. Results were analyzed using one-way ANOVA; N = 3. **p < 0.01, ***p < 0.001, ****p < 0.0001, vs Control group; #p < 0.05, ##p < 0.01, ####p < 0.0001, vs OGD group.
Monocyte Locomotion Inhibitory Factor Binds to Eukaryotic Translation Elongation Factor 1A1 in BV2 Microglia
The immunoprecipitation was used to find the binding protein of MLIF in BV2 microglia. As shown in Figure 4A, the specific binding protein (red arrow) appeared at ∼50 kDa in bio-MLIF groups (S1, S2, and S3) compared with control groups (C1, C2, and C3). Based on our previous study (Zhang et al., 2012), we speculated that it was eEF1A1. Then, Western blot analysis was performed with the specific anti-eEF1A1 antibody, and it was finally confirmed that the 50-kDa protein was eEF1A1 (Figure 4B).
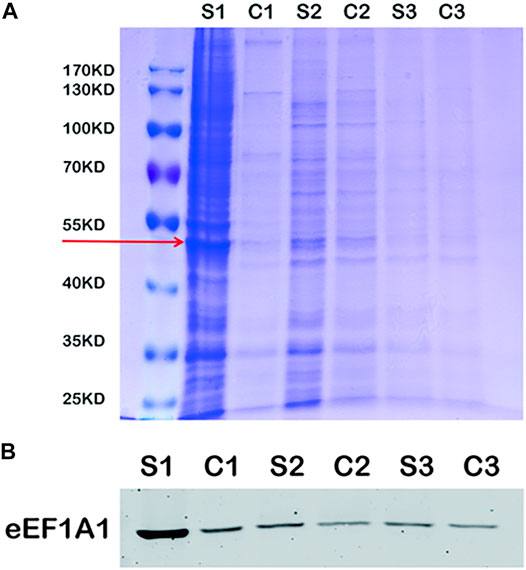
FIGURE 4. eEF1A1 is the binding protein of MLIF in BV2 microglia. (A) After immunoprecipitation, the binding proteins were separated by SDS-PAGE and stained with Coomassie brilliant blue. (B) Specific binding protein was confirmed by Western blotting in BV2 microglia.
Eukaryotic Translation Elongation Factor 1A1 Is Responsible for the Microglia Polarization Effect of Monocyte Locomotion Inhibitory Factor
To further examine the microglia polarization effect of MLIF, we used siRNA to knock down the expression of eEF1A1 in BV2 microglia. Real-time PCR and ELISA were used to determine the M1 or M2 signature genes and related cytokines. As expected, knock-down of eEF1A1 attenuated the M1 inhibitory effect (Figure 5A-E) and the M2 promotive effect of MLIF (Figures 5F–I). As shown in Figure 6, the inhibitory effect of MLIF on p-p65 and the promotive effect of MLIF on iκB were blocked by eEF1A1 siRNA. Therefore, we drew the conclusion that eEF1A1 was involved in the microglia polarization modulation of MLIF.
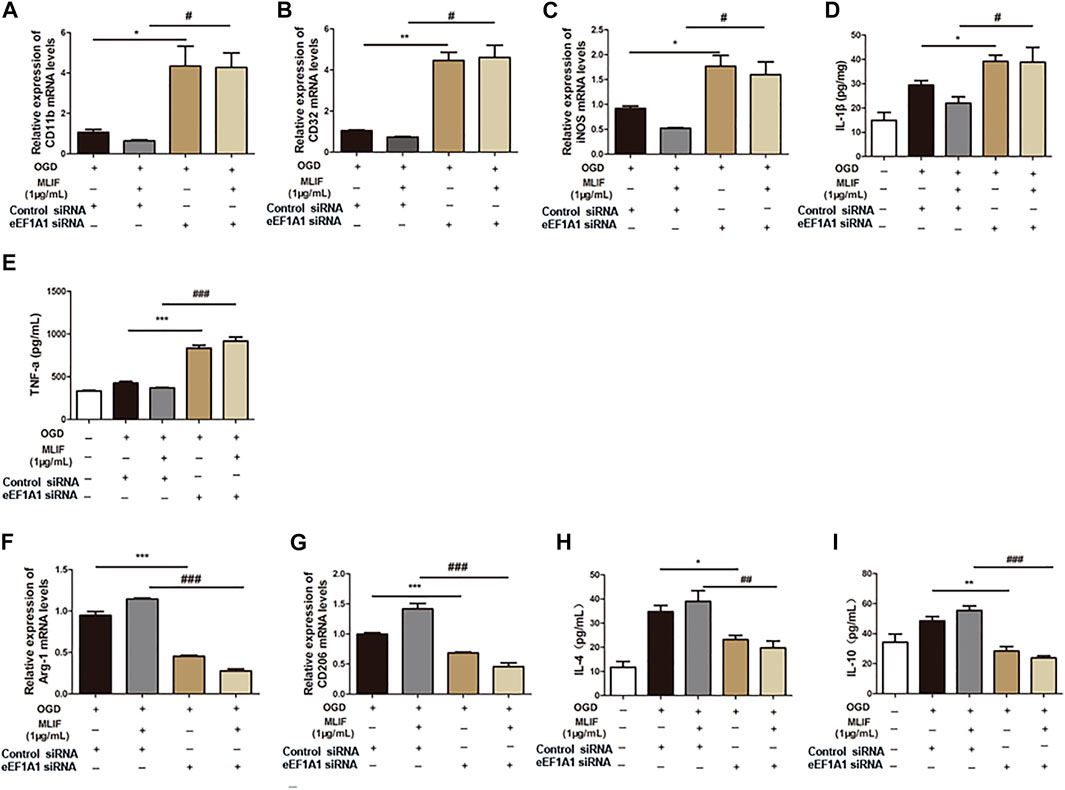
FIGURE 5. Regulation effect provided by MLIF was inhibited by eEF1A1 siRNA in BV2 microglia. OGD-insulted BV2 microglia transfected with eEF1A1 siRNA or control siRNA were treated with MLIF (1.0 μg/ml). Real-time PCR was used to detect M1 signature genes CD11b (A), CD32 (B), and iNOS (C) and M2 signature genes Arg-1 (F) and CD206 (G). ELISA was used to detect M1-related inflammation cytokines IL-1β (D) and TNF-α (E) and M2-related cytokines IL-4 (H) and IL-10 (I) in the cell supernatant. Data are expressed as the mean ± SEM. N = 3. *p < 0.05, **p < 0.01, ***p < 0.001, vs OGD + Control siRNA group; #p < 0.05, ###p < 0.001, vs MLIF + Control siRNA group.
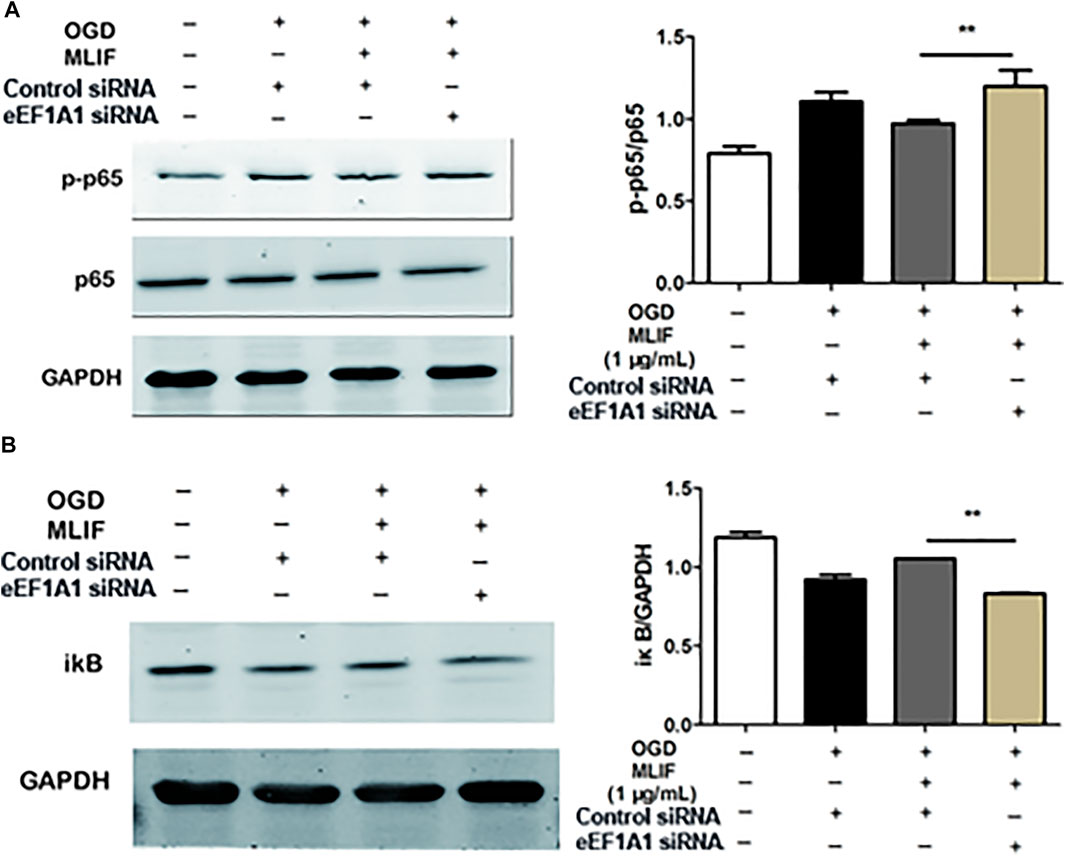
FIGURE 6. MLIF suppressed the NF-κB pathway via eEF1A1. After knock-down of eEF1A1 by using eEF1A1 siRNA or control siRNA, OGD-exposed BV2 microglia were incubated with MLIF (1.0 μg/ml). The levels of p-p65 (A) and iκB (B) were evaluated by immunoblotting. Data are expressed as the mean ± SEM. N = 3. **p < 0.01 vs Control siRNA group.
Discussion
MLIF has been proven to have protection in rat and mice ischemic stroke models in our previous study (Zhang et al., 2012). Our recent study has demonstrated that MLIF exhibits neuroprotection in OGD-insulted SH-SY5Y neuroblastoma by targeting eEF1A2 (Zhu et al., 2016). But the mechanisms underlying the anti-inflammation effects of MLIF have not been fully explored yet. As we all know, microglia are the first cells to respond to CNS injuries and are potent modulators of CNS repair and regeneration. Currently, a great deal of the literature suggests that microglia with distinct phenotypes are apparently double-edged swords in the battle for brain ischemia (Liu ZJ. et al., 2019; Zhang et al., 2019). Usually, M1 phenotype microglia accelerate the death of neurons and aggravate inflammation, while the M2 phenotype contributes to improving neuronal survival and tissue repair (Han et al., 2021). With the properties of anti-inflammation, we speculated that MLIF may protect ischemic stroke by regulating the microglia polarization.
Hu et al. (2015) reported that microglia/macrophages respond dynamically to ischemic injury, experiencing an M2 phenotype, followed by a transition to an M1 phenotype. In the current study, our immunofluorescence results have demonstrated that MLIF inhibited the expression of CD16/32+Iba1+ and promoted the expression of CD206+Iba1+ in the MCAO mice brain. We also confirm the modulation effect of MLIF in OGD-insulted BV2 microglia in vitro, and we also found that MLIF promoted the M2 phenotype and inhibited the M1 polarization of BV2 microglia, which was demonstrated by changes in the expression of M1/2 signature genes and related cytokines.
It has been reported that MLIF could regulate multitudinous gene expression in U937 cells, which were involved in the MAPK and NF-κB pathways (Silva-García et al., 2008). The mitogen-activated protein kinases (MAPKs), including three major subfamilies, the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) pathway, and p38, are a family of serine/threonine protein kinases that mediate fundamental biological processes and cellular responses to external stress signals (Kassouf and Sumara, 2020). The MAPK pathway participates in M1 macrophage polarization (He et al., 2020). Nuclear factor-κB (NF-κB) is a transcription factor that regulates the expression of genes that control cell proliferation and apoptosis, as well as genes that respond to inflammation and immune responses (Jimi et al., 2019). Inhibition of NF-κB could promote macrophages toward M2 polarization (Ye et al., 2019). Previous study has suggested that the process of macrophage M2 activation is mediated by the NF-κB and STAT3 pathways (Qian et al., 2020). Our results indicate that MLIF could suppress M1 microglia polarization by inhibiting NF-κB pathway activation in OGD-insulted BV2 microglia but has no significant effect on the MAPK pathway. To further investigate the mechanism of MLIF in regulating microglia polarization, we subsequently identify that the MLIF targeting protein was eEF1A1 by immunoprecipitation. Thus, it is presumable that eEF1A1 is involved in microglia polarization.
Eukaryotic translation elongation factor 1 alpha (eEF1A), a member of the G protein family, could interact with aminoacyl tRNA (aa-tRNA) and transfer it to the acceptor site of the ribosome during the elongation cycle in peptide synthesis (Knight et al., 2020). eEF1A has two protein isoforms in mammals, encoded by separate genes, which are 92% identical and 98% similar at the amino acid level (McLachlan et al., 2019). However, eEF1A2 is expressed in a tissue-specific manner, and eEF1A is expressed ubiquitously (Li et al., 2019). In addition to its roles in polypeptide chain elongation, the noncanonical functions of eEF1A1 have become a focus recently, such as apoptosis (Akintade and Chaudhuri, 2020), virus infection (White et al., 2021), signal transduction (Akintade and Chaudhuri, 2020), and tumorigenesis (Liu S. et al., 2019). Our previous study indicated that MLIF decreased the ICAM-1 and VCAM-1 expression and increased the eNOS expression in OGD- or ox-LDL–induced bEnd3 cells by targeting eEF1A1 (Zhang et al., 2012). It was reported that inactivation of eEF1A proteins leads to immunodeficiency and neural and muscular defects and favors apoptosis (Abbas et al., 2015). Recently, reports have suggested that the eEF1A1 protein may have therapeutic relevance in diverse conditions with altered neurite outgrowth (McLachlan et al., 2019; Duman et al., 2020). A recent study has suggested that eEF1A1 and CagA cooperated to mediate the expression of IL-6 by affecting the activity of p-STATS727 in the nucleus (Xu et al., 2020). Our previous study reported that eEF1A1/HSC70 cooperatively suppress brain endothelial cell apoptosis via regulating JNK activity (Liu et al., 2016). These studies suggested that eEF1A1 has a crucial role in neuron protection and regulation of inflammatory cytokines and adhesion molecules. Thus, we speculated that eEF1A1 may exert an important effect in regulating microglia polarization in ischemic stroke.
To confirm the importance of eEF1A1 in mediating effects of MLIF, we knocked down eEF1A1 in BV2 microglia by utilizing its siRNA. The results suggested that inhibition of eEF1A1 results in the M1 promotion effect and the M2 suppression effect on OGD-insulted BV2 microglia. What is more, the inhibition effect of MLIF on the NF-κB pathway was also attenuated. Our results suggested that the eEF1A1/NF-κB pathway plays a critical role in the microglia polarization regulated by MLIF. It also revealed the novel pharmacological effect of eEF1A1, participating in the microglia polarization in ischemic stroke.
In conclusion, the present results demonstrated that MLIF promoted microglia transition toward the M2 phenotype in the in vivo and in vitro ischemic stroke model. An NF-κB signaling pathway was involved in the pharmacological action of MLIF by targeting eEF1A1.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of Shanghai University.
Author Contributions
YL and SD carried out this study, drafted the manuscript text, and prepared the figures. YZ and JM conceived the project, interpreted the data, and revised and finalized the manuscript. ZS, QZ, YG, and YY constructed the animal model. QZ and SD performed the cell experiments and Western blot experiments. YW, TL, FM, and TA revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No.81971017) and the Innovation Fund for Interdisciplinary New Medical Graduate Students in the School of Medicine of Shanghai University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, W., Kumar, A., and Herbein, G. (2015). The eEF1A Proteins: At the Crossroads of Oncogenesis, Apoptosis, and Viral Infections. Front. Oncol. 5, 75. doi:10.3389/fonc.2015.00075
Akintade, D. D., and Chaudhuri, B. (2020). Identification of Proteins Involved in Transcription/translation (eEF 1A1) as an Inhibitor of Bax Induced Apoptosis. Mol. Biol. Rep. 47 (9), 6785–6792. doi:10.1007/s11033-020-05736-5
Barthels, D., and Das, H. (2020). Current Advances in Ischemic Stroke Research and Therapies. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (4), 165260. doi:10.1016/j.bbadis.2018.09.012
Campbell, B. C. V., and Khatri, P. (2020). Stroke. Lancet 396 (10244), 129–142. doi:10.1016/S0140-6736(20)31179-X
Duman, M., Vaquié, A., Nocera, G., Heller, M., Stumpe, M., Siva Sankar, D., et al. (2020). EEF1A1 Deacetylation Enables Transcriptional Activation of Remyelination. Nat. Commun. 11 (1), 3420. doi:10.1038/s41467-020-17243-z
Greenhalgh, A. D., David, S., and Bennett, F. C. (2020). Immune Cell Regulation of Glia during CNS Injury and Disease. Nat. Rev. Neurosci. 21 (3), 139–152. doi:10.1038/s41583-020-0263-9
Han, D., Wang, J., Wen, L., Sun, M., Liu, H., and Gao, Y. (2021). Remote Limb Ischemic Postconditioning Protects against Ischemic Stroke via Modulating Microglia/Macrophage Polarization in Mice. J. Immunol. Res. 2021, 6688053. doi:10.1155/2021/6688053
He, S., Hu, Q., Xu, X., Niu, Y., Chen, Y., Lu, Y., et al. (2020). Advanced Glycation End Products Enhance M1 Macrophage Polarization by Activating the MAPK Pathway. Biochem. Biophys. Res. Commun. 525 (2), 334–340. doi:10.1016/j.bbrc.2020.02.053
Hu, X., Leak, R. K., Shi, Y., Suenaga, J., Gao, Y., Zheng, P., et al. (2015). Microglial and Macrophage Polarization—New Prospects for Brain Repair. Nat. Rev. Neurol. 11 (1), 56–64. doi:10.1038/nrneurol.2014.207
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., and Rosenberg, G. A. (2019). Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflammation 16 (1), 142. doi:10.1186/s12974-019-1516-2
Jha, M. K., Lee, W. H., and Suk, K. (2016). Functional Polarization of Neuroglia: Implications in Neuroinflammation and Neurological Disorders. Biochem. Pharmacol. 103, 1–16. doi:10.1016/j.bcp.2015.11.003
Jiang, C. T., Wu, W. F., Deng, Y. H., and Ge, J. W. (2020). Modulators of Microglia Activation and Polarization in Ischemic Stroke (Review). Mol. Med. Rep. 21 (5), 2006–2018. doi:10.3892/mmr.2020.11003
Jimi, E., Fei, H., and Nakatomi, C. (2019). NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 20 (24). doi:10.3390/ijms20246275
Kassouf, T., and Sumara, G. (2020). Impact of Conventional and Atypical MAPKs on the Development of Metabolic Diseases. Biomolecules 10 (9). doi:10.3390/biom10091256
Knight, J. R. P., Garland, G., Pöyry, T., Mead, E., Vlahov, N., Sfakianos, A., et al. (2020). Control of Translation Elongation in Health and Disease. Dis. Model. Mech. 13 (3). doi:10.1242/dmm.043208
Kwon, H. S., and Koh, S. H. (2020). Neuroinflammation in Neurodegenerative Disorders: the Roles of Microglia and Astrocytes. Transl Neurodegener 9 (1), 42. doi:10.1186/s40035-020-00221-2
Li, D., Rawle, D. J., Wu, Z., Jin, H., Lin, M. H., Lor, M., et al. (2019). eEF1A Demonstrates Paralog Specific Effects on HIV-1 Reverse Transcription Efficiency. Virology 530, 65–74. doi:10.1016/j.virol.2019.01.023
Lian, L., Zhang, Y., Liu, L., Yang, L., Cai, Y., Zhang, J., et al. (2020). Neuroinflammation in Ischemic Stroke: Focus on MicroRNA-Mediated Polarization of Microglia. Front. Mol. Neurosci. 13, 612439. doi:10.3389/fnmol.2020.612439
Liu, S., Hausmann, S., Carlson, S. M., Fuentes, M. E., Francis, J. W., Pillai, R., et al. (2019a). METTL13 Methylation of eEF1A Increases Translational Output to Promote Tumorigenesis. Cell 176 (3), 491–e21. doi:10.1016/j.cell.2018.11.038
Liu, X., Hu, P., Wang, Y., Wang, X., Huang, J., Li, J., et al. (2018). A Validated UPLC-MS/MS Method for the Quantitation of an Unstable Peptide, Monocyte Locomotion Inhibitory Factor (MLIF) in Human Plasma and its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 157, 75–83. doi:10.1016/j.jpba.2018.04.009
Liu, Y., Jiang, S., Yang, P. Y., Zhang, Y. F., Li, T. J., and Rui, Y. C. (2016). EF1A1/HSC70 Cooperatively Suppress Brain Endothelial Cell Apoptosis via Regulating JNK Activity. CNS Neurosci. Ther. 22 (10), 836–844. doi:10.1111/cns.12581
Liu, Z. J., Ran, Y. Y., Qie, S. Y., Gong, W. J., Gao, F. H., Ding, Z. T., et al. (2019b). Melatonin Protects against Ischemic Stroke by Modulating Microglia/macrophage Polarization toward Anti-inflammatory Phenotype through STAT3 Pathway. CNS Neurosci. Ther. 25 (12), 1353–1362. doi:10.1111/cns.13261
McLachlan, F., Sires, A. M., and Abbott, C. M. (2019). The Role of Translation Elongation Factor eEF1 Subunits in Neurodevelopmental Disorders. Hum. Mutat. 40 (2), 131–141. doi:10.1002/humu.23677
Parikh, N. S., Merkler, A. E., and Iadecola, C. (2020). Inflammation, Autoimmunity, Infection, and Stroke: Epidemiology and Lessons from Therapeutic Intervention. Stroke 51 (3), 711–718. doi:10.1161/STROKEAHA.119.024157
Prinz, M., Jung, S., and Priller, J. (2019). Microglia Biology: One Century of Evolving Concepts. Cell 179 (2), 292–311. doi:10.1016/j.cell.2019.08.053
Qian, M., Wang, S., Guo, X., Wang, J., Zhang, Z., Qiu, W., et al. (2020). Hypoxic Glioma-Derived Exosomes Deliver microRNA-1246 to Induce M2 Macrophage Polarization by Targeting TERF2IP via the STAT3 and NF-Κb Pathways. Oncogene 39 (2), 428–442. doi:10.1038/s41388-019-0996-y
Silva-García, R., Estrada-García, I., Ramos-Payán, R., Torres-Salazar, A., Morales-Martínez, M. E., Arenas-Aranda, D., et al. (2008). The Effect of an Anti-inflammatory Pentapeptide Produced by Entamoeba Histolytica on Gene Expression in the U-937 Monocytic Cell Line. Inflamm. Res. 57 (4), 145–150. doi:10.1007/s00011-007-6199-y
Subhramanyam, C. S., Wang, C., Hu, Q., and Dheen, S. T. (2019). Microglia-mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev Biol 94, 112–120. doi:10.1016/j.semcdb.2019.05.004
Utrera-Barillas, D., Velazquez, J. R., Enciso, A., Cruz, S. M., Rico, G., Curiel-Quesada, E., et al. (2003). An Anti-inflammatory Oligopeptide Produced by Entamoeba Histolytica Down-Regulates the Expression of Pro-inflammatory Chemokines. Parasite Immunol. 25 (10), 475–482. doi:10.1111/j.1365-3024.2003.00657.x
Wei, S., Wang, D., Li, H., Bi, L., Deng, J., Zhu, G., et al. (2019). Fatty acylCoA Synthetase FadD13 Regulates Proinflammatory Cytokine Secretion Dependent on the NF-Κb Signalling Pathway by Binding to eEF1A1. Cell Microbiol 21 (12), e13090. doi:10.1111/cmi.13090
White, K. M., Rosales, R., Yildiz, S., Kehrer, T., Miorin, L., Moreno, E., et al. (2021). Plitidepsin Has Potent Preclinical Efficacy against SARS-CoV-2 by Targeting the Host Protein eEF1A. Science 371 (6532), 926–931. doi:10.1126/science.abf4058
Xie, S. T., Chen, A. X., Song, B., Fan, J., Li, W., Xing, Z., et al. (2020). Suppression of Microglial Activation and Monocyte Infiltration Ameliorates Cerebellar Hemorrhage Induced-Brain Injury and Ataxia. Brain Behav. Immun. 89, 400–413. doi:10.1016/j.bbi.2020.07.027
Xu, S., Wu, X., Zhang, X., Chen, C., Chen, H., and She, F. (2020). CagA Orchestrates eEF1A1 and PKCδ to Induce Interleukin-6 Expression in Helicobacter Pylori-Infected Gastric Epithelial Cells. Gut Pathog. 12, 31. doi:10.1186/s13099-020-00368-3
Yao, J., Xu, Y., Ji, F., Wang, C., Zhang, Y., Ni, J., et al. (2011). Protective Effects of MLIF Analogs on Cerebral Ischemia-Reperfusion Injury in Rats. Peptides 32 (5), 1047–1054. doi:10.1016/j.peptides.2011.03.005
Ye, Y., Jin, T., Zhang, X., Zeng, Z., Ye, B., Wang, J., et al. (2019). Meisoindigo Protects against Focal Cerebral Ischemia-Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation and Regulating Microglia/Macrophage Polarization via TLR4/NF-Κb Signaling Pathway. Front Cell Neurosci 13, 553. doi:10.3389/fncel.2019.00553
Zhang, G., Xia, F., Zhang, Y., Zhang, X., Cao, Y., Wang, L., et al. (2016). Ginsenoside Rd Is Efficacious against Acute Ischemic Stroke by Suppressing Microglial Proteasome-Mediated Inflammation. Mol. Neurobiol. 53 (4), 2529–2540. doi:10.1007/s12035-015-9261-8
Zhang, H., Lu, M., Zhang, X., Kuai, Y., Mei, Y., Tan, Q., et al. (2019). Isosteviol Sodium Protects against Ischemic Stroke by Modulating Microglia/Macrophage Polarization via Disruption of GAS5/miR-146a-5p Sponge. Sci. Rep. 9 (1), 12221. doi:10.1038/s41598-019-48759-0
Zhang, W., Tian, T., Gong, S. X., Huang, W. Q., Zhou, Q. Y., Wang, A. P., et al. (2021). Microglia-associated Neuroinflammation Is a Potential Therapeutic Target for Ischemic Stroke. Neural Regen. Res. 16 (1), 6–11. doi:10.4103/1673-5374.286954
Zhang, Y., Chen, J., Li, F., Li, D., Xiong, Q., Lin, Y., et al. (2012). A Pentapeptide Monocyte Locomotion Inhibitory Factor Protects Brain Ischemia Injury by Targeting the eEF1A1/endothelial Nitric Oxide Synthase Pathway. Stroke 43 (10), 2764–2773. doi:10.1161/STROKEAHA.112.657908
Keywords: brain ischemia, microglia polarization, inflammation, monocyte locomotion inhibitory factor (MLIF), eukaryotic translation elongation factor 1A1 (eEF1A1)
Citation: Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, Wang Y, Li T, Megahed FAK, Addissouky TA, Mao J and Zhang Y (2021) MLIF Modulates Microglia Polarization in Ischemic Stroke by Targeting eEF1A1. Front. Pharmacol. 12:725268. doi: 10.3389/fphar.2021.725268
Received: 15 June 2021; Accepted: 13 August 2021;
Published: 07 September 2021.
Edited by:
Somasundaram Arumugam, National Institute of Pharmaceutical Education and Research, Kolkata, IndiaReviewed by:
Saeideh Momtaz, Tehran University of Medical Sciences, IranHao Cheng, Fudan University, China
Copyright © 2021 Liu, Deng, Song, Zhang, Guo, Yu, Wang, Li, Megahed, Addissouky, Mao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuefan Zhang, eXVlZmFuemhhbmdAc2h1LmVkdS5jbg==; Junqin Mao, bWFvanEyMDRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yulan Liu1,2†
Yulan Liu1,2† Shanshan Deng
Shanshan Deng Qian Zhang
Qian Zhang Yuchen Guo
Yuchen Guo Yuliang Wang
Yuliang Wang Tiejun Li
Tiejun Li Fayed A. K. Megahed
Fayed A. K. Megahed Yuefan Zhang
Yuefan Zhang