- 1Department of Critical Care Medicine, the First Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, China
- 2Department of Critical Care Medicine, the Sixth Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, China
- 3Institute of Critical Care Medicine, the Sino Russian Medical Research Center of Harbin Medical University, Harbin Medical University, Harbin, China
- 4Department of Critical Care Medicine, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, China
- 5Department of Critical Care Medicine, the Second Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, China
- 6Department of Critical Care Medicine, the Fourth Affiliated Hospital of Harbin Medical University, Harbin Medical University, Harbin, China
- 7Key Laboratory of Hepatosplenic Surgery, Ministry of Education, Harbin, China
- 8The Cell Transplantation Key Laboratory of National Health Commission, Harbin, China
In this study, we aimed to determine whether continuous renal replacement therapy (CRRT) with oXiris filter may alleviate cytokine release syndrome (CRS) in non-AKI patients with severe and critical coronavirus disease 2019 (COVID-19). A total of 17 non-AKI patients with severe and critical COVID-19 treated between February 14 and March 26, 2020 were included and randomly divided into intervention group and control group according to the random number table. Patients in the intervention group immediately received CRRT with oXiris filter plus conventional treatment, while those in the control group only received conventional treatment. Demographic data were collected and collated at admission. During ICU hospitalization, the concentrations of circulating cytokines and inflammatory chemokines, including IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, were quantitatively measured daily to reflect the degree of CRS induced by SARS-CoV-2 infection. Clinical data, including the severity of COVID-19 white blood cell count (WBC), neutrophil proportion (NEUT%), lymphocyte count (LYMPH), lymphocyte percentage (LYM%), platelet (PLT), C-reaction protein (CRP), high sensitivity C-reactive protein (hs-CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), albumin (ALB), serum creatinine (SCr), D-Dimer, fibrinogen (FIB), IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, number of hospital days and sequential organ failure assessment (SOFA) score were obtained and collated from medical records, and then compared between the two groups. Age, and SCr significantly differed between the two groups. Besides the IL-2 concentration that was significantly lower on day 2 than that on day 1 in the intervention group, and the IL-6 concentrations that were significantly higher on day 1, and day 2 in the intervention group compared to the control group, similar to the IL-10 concentration on day 5, there were no significant differences between the two groups. To sum up, CRRT with oXiris filter may not effectively alleviate CRS in non-AKI patients with severe and critical COVID-19. Thus, its application in these patients should be considered with caution to avoid increasing the unnecessary burden on society and individuals and making the already overwhelmed medical system even more strained (IRB number: IRB-AF/SC-04).
Background
Excessive and uncontrolled systemic immune responses are the real culprit of disease deterioration and death in patients with Coronavirus disease 2019 (COVID-19), and not fatal virus infection (Gao et al., 2021). Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection can trigger the excessive production and release of a series of circulating cytokines and inflammatory chemokines, especially marked by IL-6, IL-10, and TNF-α, also known as cytokine release syndrome (CRS) or cytokine storm, which have been revealed to be closely associated with organ injury, disease severity and death in patients with COVID-19 (Copaescu et al., 2020; Del Valle et al., 2020; Huang et al., 2020). Cytokines concentrations in sputum and bronchoalveolar lavage fluid (BALF) are more representative than those in serum (Wang et al., 2021). However, short-term corticosteroid treatment based on the treatment concept of alleviating excessive and uncontrolled systemic immune responses is still full of controversy in clinical practice due to significant adverse effects in the middle and late stages of SARS-CoV-2 infection (van Paassen et al., 2020; Liu et al., 2021).
The therapeutic purpose of continuous renal replacement therapy (CRRT) has evolved from the single replacement of kidney function to support of multiple organ systems via the removal of circulating cytokines and inflammatory mediators. Accordingly, it can be considered for use in acute respiratory distress syndrome (ARDS) induced by current SARS-CoV-2 infection, especially for severe and critical COVID-19 patients with CRS, regardless of the presence of acute kidney injury (AKI) complications (Gao et al., 2019; Zhang et al., 2020; Xiang et al., 2021). oXiris filter is a highly biocompatible modified hemodiafilter with a special heparin-coated design, which can be combined with CRRT in clinical practice. CRRT with oXiris filter can further enhance the clearance of circulating cytokines and inflammatory chemokines, improve clinical symptoms and laboratory indicators, reduce disease severity, and prolong the survival time in critically ill patients with COVID-19 (Cascarano et al., 2021; Premužić et al., 2021; Rosalia et al., 2021). Still, the relevant studies mainly focused on acute or chronic renal failure in patients with COVID-19, which is one of the potential indications for CRRT initiation.
In our study, we tried to explore the role of CRRT with oXiris filter on CRS in non-AKI patients with severe and critical COVID-19 by comparing serum concentrations of cytokines and inflammatory chemokines during ICU hospitalization. Our findings will provide a solid theoretical basis that can guide its clinical application.
Methods
Study Design
A total of 17 non-AKI patients with severe and critical COVID-19 treated at COVID-19 treatment center of Heilongjiang province in the First Affiliated Hospital of Harbin Medical University between February 14 and March 26, 2020, were included in this prospective non-blind randomized controlled study. These patients were randomly divided into intervention group and control group according to the random number table. After randomization, patients in the intervention group immediately received CRRT with oXiris filter plus conventional treatment, while those in the control group only received conventional treatment. Based on the Diagnosis and Treatment of New Coronavirus Pneumonia (the fifth edition), conventional treatment included antiviral, aerosol inhalation, antibiotics for confirmed infected patients, different forms of oxygen therapy for patients with decreased blood oxygen saturation, analgesia and sedation for patients with relevant needs, etc., instead of immunomodulatory therapy such as corticosteroid and IL-6 monoclonal antibody. These patients were dealt with by the same group of experienced intensivists in the ICU.
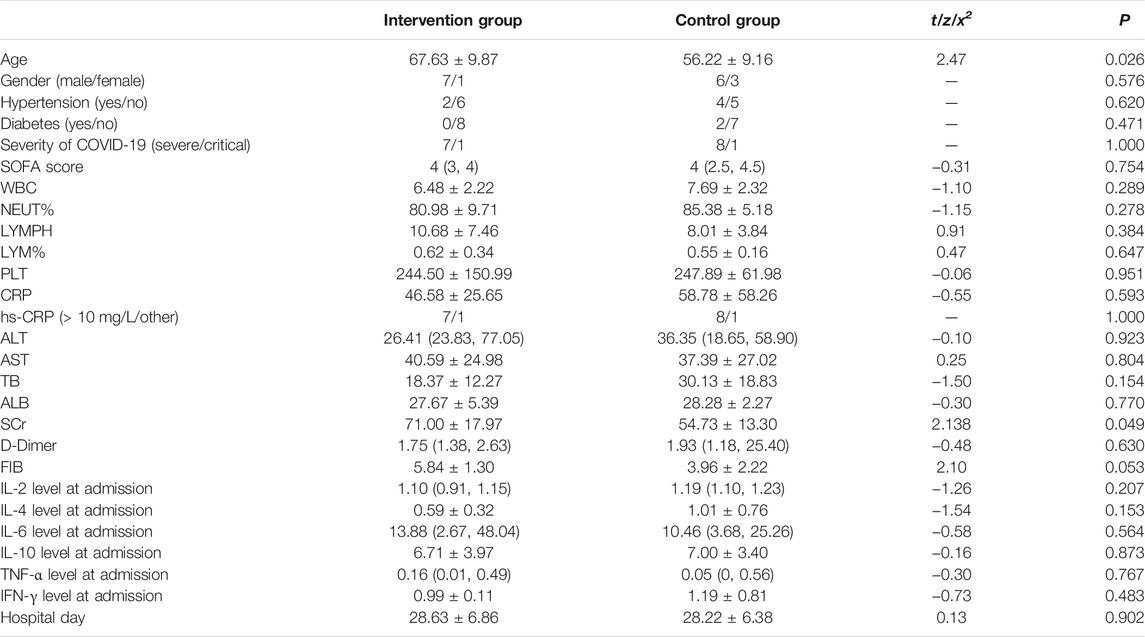
Demographic data were collected and collated at admission. Clinical data, including the severity of COVID-19 white blood cell count (WBC), neutrophil proportion (NEUT%), lymphocyte count (LYMPH), lymphocyte percentage (LYM%), platelet (PLT), C-reaction protein (CRP), high sensitivity C-reactive protein (hs-CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), albumin (ALB), serum creatinine (SCr), D-Dimer, fibrinogen (FIB), IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and number of hospital days were obtained and collated from medical records during hospitalization, and then compared between the two groups. The serum concentrations of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ were used as the primary research endpoint to reflect the degree of CRS induced by SARS-CoV-2 infection. Sequential organ failure assessment (SOFA) scores were calculated during the first 24 h clinical data after ICU admission.
The study was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University (IRB number: IRB-AF/SC-04).
Study Population
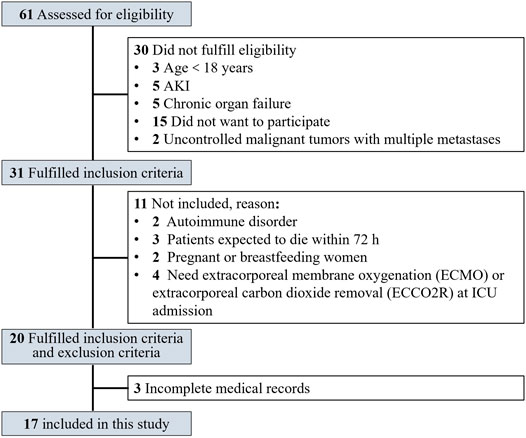
In this study, the inclusion criteria were following: admitted to ICU; age ≥18 years old; confirmed severe and critical patients with COVID-19; non-AKI; written informed consent obtained from patients or guardians; whereas COVID-19 patients who met the following criteria were excluded: uncontrolled malignant tumors with multiple metastases; leukemia; acquired immunodeficiency syndrome (AIDS); obstructive pneumonia caused by pulmonary tumors, severe pulmonary interstitial fibrosis, pulmonary alveolar proteinosis, and allergic alveolitis; chronic organ failure; immunotherapy or organ transplant within 6 months; autoimmune disorder; need extracorporeal membrane oxygenation (ECMO) or extracorporeal carbon dioxide removal (ECCO2R) at ICU admission; patients expected to die within 72 h; pregnant or breastfeeding women; incomplete medical records; any potential conditions endangering the patient’s safety. The full panel of experts was responsible for identifying potential conditions that could endanger the safety of enrolled patients.
Diagnosis of Severe and Critical COVID-19
All enrolled patients were confirmed by detection of SARS-CoV-2 nucleic acid on oropharyngeal swabs, nasopharyngeal swabs, or lower respiratory tract specimens, and then classified into severe or critical cases according to the Diagnosis and Treatment of New Coronavirus Pneumonia (the fifth edition).
Measurement of Circulating Cytokines and Inflammatory Mediators
During ICU hospitalization, the serum concentrations of cytokines and inflammatory chemokines, including IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, were quantitatively measured by cytometric bead array (CBA) on a daily basis.
Continuous Renal Replacement Therapy With oXiris Filter
After randomization, CRRT with oXiris filter was immediately applied to patients in the intervention group. In this study, a temporary double-lumen central venous catheter (11.5 F) was used to establish vascular access under ultrasound guidance. Patients in the intervention group were managed with 72 h CVVH combined oXiris filter (Baxter International, Deerfield, IL, United States), regional citrate anti-coagulation, and a pre- and post-dilution ratio of 1:1 on a Prismaflex system (Baxter International, Deerfield, IL, United States). Blood flow rates, dehydration volume, and amount of substitute fluid were individually adjusted according to the different conditions and treatment needs of each patient. Considering the saturation of membrane adsorption, Oxiris filter was changed every 12 h.
Data Collection
Demographic data, including age, gender, comorbidities, and clinical data, including the severity of COVID-19, SOFA score, WBC, NEUT%, LYMPH, LYM%, PLT, CRP, hs-CRP, ALT, AST, TB, ALB, SCr, D-Dimer, FIB, IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and number of hospital days were obtained and collated from medical records during hospitalization by dedicated personnel in our research team. None of the other members of our research team was privy to enrolled patient’s personal information beyond what was required for this study.
Statistical Analyses
SPSS 24.0 (SPSS, Inc., Chicago, IL) was used for statistical analyses. Continuous data conforming to normal distribution were described as mean ± standard deviation (SD), while continuous data with non-normal distribution were expressed as median (P25, P75). The measurement data were expressed by frequency. Independent-samples t-test was used to perform inter-group comparison for continuous data with normal distribution, while Mann-Whitney U test was employed for inter-group comparison of abnormally distributed continuous data. The Fisher’s exact test was used for comparing measurement data between the two groups. p-values <0.05 were considered to indicate statistical significance.
Results
Comparison of Demographic and Clinical Baseline Data Between the Two Groups
Flowchart of study participants was shown in Figure 1. A total of 17 non-AKI patients with severe and critical COVID-19 treated between February 14 and March 26, 2020 were included and randomly divided into intervention group and control group according to the random number table. As shown in Table 1, age and SCr were significantly different in the two groups (p = 0.026, = 0.049, respectively), despite the randomization process, while no significant difference was observed in the remaining demographic and clinical baseline data.
Comparison of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ Concentrations Between the Two Groups From Day 1 to Day 6
As shown in Table 2 and Figure 2, besides the IL-2 concentration that was significantly lower on day 2 than that on day 1 in the intervention group, and the IL-6 concentrations that were significantly higher on day 1, and day 2 in the intervention group compared to the control group, similar to the IL-10 concentration on day 5, there were no significant differences between the two groups.
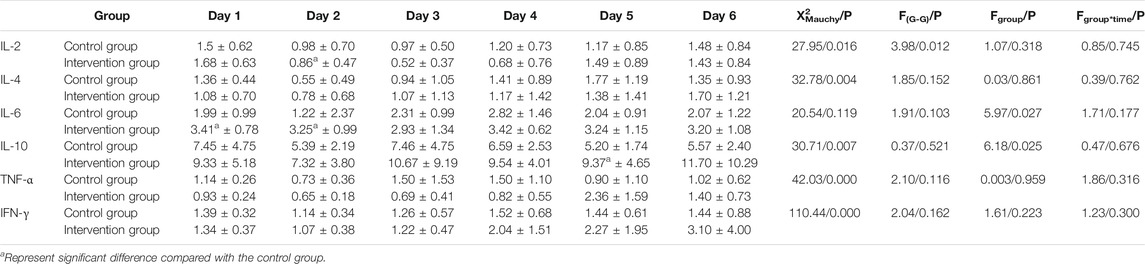
TABLE 2. Comparison of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ concentrations between the two groups from day 1 to day 6.
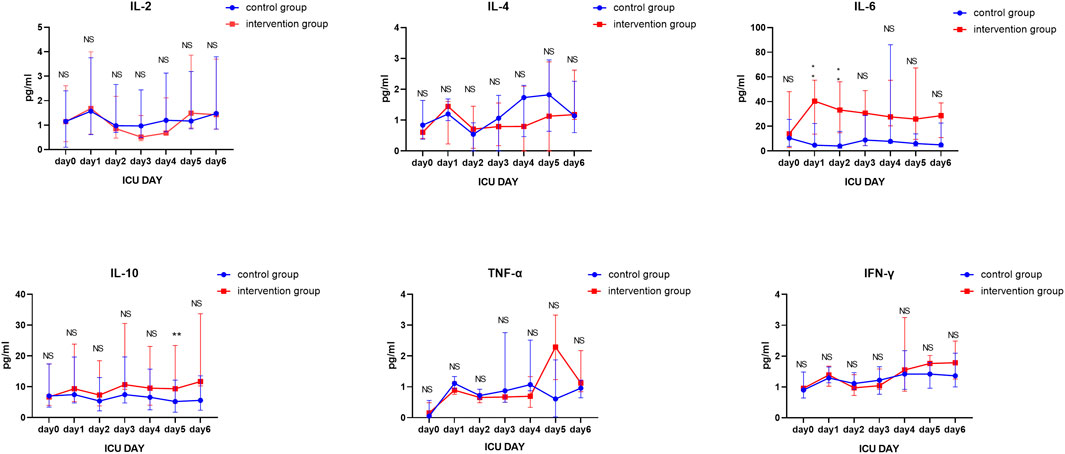
FIGURE 2. Longitudinal comparison of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ concentrations between the two groups from day 1 to day 6.
Discussion
As a novel, highly pathogenic human coronavirus (hCoV), SARS-CoV-2 may continue to pose a persistent and unprecedented threat to global public health security for a considerable time to come. Breakthrough infections caused by adaptive mutations in the SARS-COV-2 genome prevent universal vaccination from becoming the effective coping strategy against COVID-19 (Hacisuleyman et al., 2021). In the absence of available targeted interventions, there is an urgent need to explore effective treatment approaches based on an ongoing understanding of the pathogenesis of SARS-CoV-2 viral infection and disease deterioration. Among them, CRRT with oXiris filter is a highly expected and promising clinical approach (Chen et al., 2020).
Data from the Chinese Center for Disease Control and Prevention indicated that the mortality of patients with critical COVID-19 was close to 50% and even exceeded 60% in the early stages of the outbreak (Yang et al., 2020). Some of the survivors, who recovered from severe or critical COVID-19, were reported to suffer from severely impaired pulmonary diffusion capacities and abnormal chest imaging manifestations at 6-months follow-up after SARS-CoV-2 infection (Huang et al., 2021). Although the exact pathogenesis of SARS-CoV-2 infection and disease deterioration is still poorly understood, CRS characterized by an excessive and uncontrolled systemic inflammatory response has an essential role (Mehta et al., 2020). A significant increase in circulating cytokines and inflammatory chemokines burden was observed in patients with COVID-19, in association with multiple-organ dysfunction, increased disease severity, and adverse clinical outcomes (Huang et al., 2020; Zeng et al., 2020; Sims et al., 2021). Regulating inflammatory responses to restore immunological equilibrium and maintain immune homeostasis may be an entry point.
Using CRRT for immunomodulation has a long history in clinic for tapering cytokine storms and controlling the associated dysregulation of the immune system. This approach has also been proposed as adjuvant therapy in many diseases, including sepsis (Monard et al., 2019), septic AKI (Turani et al., 2019), septic shock (Schwindenhammer et al., 2019), severe Middle East Respiratory Syndrome (MERS) (Cha et al., 2015), severe acute pancreatitis (SAP) with or without ARDS (Cui et al., 2014; Gao et al., 2018), CRS induced by some immunotherapies (Constantinescu et al., 2020), severe burns (Peng et al., 2005), etc. Yet, a non-selective clearance allows harmful and beneficial substances to be simultaneously removed during CRRT (Shi et al., 2021). Moreover, the potential disadvantages of CRRT, such as technical complications of establishing vascular access, anti-coagulation-related complications, hemodynamic instability, internal environment disturbance, obstacles to the spontaneous recovery of renal function, and huge cost, should not be ignored in clinical practice. As a result, although some studies have confirmed the immunomodulation effect of CRRT, this was not sufficient to influence clinical endpoints and could even prolong the need for organ support (Park et al., 2016; Yin et al., 2020).
oXiris filter is superficially modified from an AN-69 membrane (polyacrylonitrile) with an additional positively charged polyimide ethylene layer used to enhance the cytokines-adsorbing capacity by ionic bonding and grafted with heparin (Broman et al., 2019), which was first approved and marketed in Europe in 2009. The addition of the highly adsorptive preheparinized oXiris filter can enhance the ability of CRRT to effectively remove endotoxin, circulating cytokines, and inflammatory chemokines, thus reducing lactate concentration and vasopressors infusion rate, and improving haemodynamic status, systemic perfusion, multi-organ function, and clinical outcomes without related adverse events (Broman et al., 2019; Schwindenhammer et al., 2019; Turani et al., 2019; Villa et al., 2020; Samman et al., 2021). Therefore, it gained emergency approval from US Food and Drug Administration (FDA) for the clinical treatment of COVID-19 in April 2020 to counter the CRS attack triggered by SARS-CoV-2 infection. Different from short-term corticosteroid treatment, CRRT with oXiris filter is expected to mitigate circulating cytokines and inflammatory chemokines burden and restore immune homeostasis in COVID-19 patients without a prolonged state of immunosuppression and serious secondary infection.
In our study, CRRT with oXiris filter showed no advantage in removing circulating cytokines and inflammatory chemokines in non-AKI patients with severe and critical COVID-19. The primary reason is that all the selected patients were non-AKI with normal renal clearance, which is significantly different from previous relevant studies that mainly focused on the acute or chronic renal failure patients with seriously damaged renal clearance. In such case, CRRT with or without oXiris filter becomes an important way to clear circulating cytokines and inflammatory chemokines. In addition, the concentrations of IL-6 in patients with severe COVID-19 was usually tens of pg/mL, which was similar to our results and far lower than those in patients with septic shock or CRS induced by Chimeric Antigen Receptor (CAR) T-cell infusion (Aziz et al., 2020; Monneret et al., 2021). A previous study has confirmed that the immunomodulatory treatment of Afelimomab exerts a protective effect in patients with severe sepsis only when the concentration of IL-6 exceeds the threshold of 1,000 pg/ml (Panacek et al., 2004). The concentration of IL-6 in our study, which was far from reaching or close to this threshold, may explain these negative results. Furthermore, different inflammatory subphenotypes of COVID-19 may have a certain impact on the production and release of circulating cytokines and inflammatory chemokines (Lin et al., 2020). Lastly, changes in circulating cytokines and inflammatory chemokines concentrations may not be solely influenced by extracorporeal removal, but also by SARS-CoV-2 viral load, endogenous production, innate and acquired immune defense, comorbidities and many other factors (Bermejo-Martin et al., 2020; Rajpal et al., 2020; Lumlertgul et al., 2021; Pasrija and Naime, 2021).
There are some limitations in the present study. First of all, as this was a small-size single-center study, the possible positive results may be emerge after increasing the number of cases, and the credibility and generalizability of our conclusion may weaken. Second, the duration varies from onset to ICU admission, which may be influence the production and removal of circulating cytokines and inflammatory chemokines to a certain extent, although no significant difference was observed in the clinical baseline data between the two groups except Scr. Third, the monitoring duration of circulating cytokines and inflammatory chemokines concentrations was limited to 6 days, and thus the medium and long term role of CRRT with oXiris filter in non-AKI patients with severe and critical COVID-19 was not further explored. Finally, the types of circulating cytokines and inflammatory chemokines detection were also limited.
Conclusion
CRRT with oXiris filter may not be an effective method for alleviating CRS in non-AKI patients with severe and critical COVID-19; thus, its application in these patients should be considered with caution to avoid increasing the unnecessary burden on society and individuals and making the already overwhelmed medical system even more overstretched. The findings of our study need to be further confirmed by well-designed large-sample studies.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KK, YL, YG, JZ, CW, MZ, and KY took part in the conception, literature search, study design, statistical analysis, analysis and discussion of results, and manuscript preparation, editing, and review. DF, WY, XM, MY, YG, HL, XD, YJ, JW, WX, and JW provided assistance for the conception, literature search, data acquisition and collation, statistical analysis, analysis and discussion of results, and manuscript preparation. All authors read and approved the final article.
Funding
This study was supported by the Novel Coronavirus Pneumonia Emergency Treatment and Diagnosis Technology Research Project of the Heilongjiang Provincial Science and Technology Department, the National Natural Science Foundation of China (Nos. 81772045, 81902000) and Nn10 program of Harbin Medical University Cancer Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to all colleagues who worked with them in the COVID-19 treatment center of Heilongjiang province, and all those who provided selfless advice and help for this article. We pay tribute to the medical staff who lost their lives in the national fight against the COVID-19 epidemic.
References
Aziz, M., Fatima, R., and Assaly, R. (2020). Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J. Med. Virol. 92, 2283–2285. doi:10.1002/jmv.25948
Bermejo-Martin, J. F., González-Rivera, M., Almansa, R., Micheloud, D., Tedim, A. P., Domínguez-Gil, M., et al. (2020). Viral RNA Load in Plasma Is Associated with Critical Illness and a Dysregulated Host Response in COVID-19. Crit. Care 24 (1), 691. doi:10.1186/s13054-020-03398-0
Broman, M. E., Hansson, F., Vincent, J. L., and Bodelsson, M. (2019). Endotoxin and Cytokine Reducing Properties of the oXiris Membrane in Patients with Septic Shock: A Randomized Crossover Double-Blind Study. PLoS One 14, e0220444. doi:10.1371/journal.pone.0220444
Cascarano, L., Cutuli, S. L., Pintaudi, G., Tanzarella, E. S., Carelli, S., Anzellotti, G., et al. (2021). Extracorporeal Immune Modulation in COVID-19 Induced Immune Dysfunction and Secondary Infections: the Role of oXiris® Membrane. Minerva Anestesiol 87, 384–385. doi:10.23736/S0375-9393.20.15124-1
Cha, R. H., Joh, J. S., Jeong, I., Lee, J. Y., Shin, H. S., Kim, G., et al. (2015). Renal Complications and Their Prognosis in Korean Patients with Middle East Respiratory Syndrome-Coronavirus from the Central MERS-CoV Designated Hospital. J. Korean Med. Sci. 30, 1807–1814. doi:10.3346/jkms.2015.30.12.1807
Chen, G., Zhou, Y., Ma, J., Xia, P., Qin, Y., and Li, X. (2020). Is There a Role for Blood Purification Therapies Targeting Cytokine Storm Syndrome in Critically Severe COVID-19 Patients? Ren. Fail. 42, 483–488. doi:10.1080/0886022X.2020.1764369
Constantinescu, C., Pasca, S., Tat, T., Teodorescu, P., Vlad, C., Iluta, S., et al. (2020). Continuous Renal Replacement Therapy in Cytokine Release Syndrome Following Immunotherapy or Cellular Therapies? J. Immunother. Cancer 8, e000742. doi:10.1136/jitc-2020-000742
Copaescu, A., Smibert, O., Gibson, A., Phillips, E. J., and Trubiano, J. A. (2020). The Role of IL-6 and Other Mediators in the Cytokine Storm Associated with SARS-CoV-2 Infection. J. Allergy Clin. Immunol. 146, 518–e1. doi:10.1016/j.jaci.2020.07.001
Cui, H. X., Xu, J. Y., and Li, M. Q. (2014). Efficacy of Continuous Renal Replacement Therapy in the Treatment of Severe Acute Pancreatitis Associated Acute Respiratory Distress Syndrome. Eur. Rev. Med. Pharmacol. Sci. 18, 2523–2526.
Del Valle, D. M., Kim-Schulze, S., Huang, H. H., Beckmann, N. D., Nirenberg, S., Wang, B., et al. (2020). An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 26, 1636–1643. doi:10.1038/s41591-020-1051-9
Gao, N., Yan, C., and Zhang, G. (2018). Changes of Serum Procalcitonin (PCT), C-Reactive Protein (CRP), Interleukin-17 (IL-17), Interleukin-6 (IL-6), High Mobility Group Protein-B1 (HMGB1) and D-Dimer in Patients with Severe Acute Pancreatitis Treated with Continuous Renal Replacement Therapy (CRRT) and Its Clinical Significance. Med. Sci. Monit. 24, 5881–5886. doi:10.12659/MSM.910099
Gao, Y., Qi, Z. D., Liu, R. J., Liu, H. T., Han, Q. Y., Zhang, X., et al. (2019). A Multi-center Cross-Sectional Study on Blood Purification Among Adult Patients in Intensive Care Unit in China: a Study Protocol. Chin. Med. J. (Engl) 132, 1208–1211. doi:10.1097/CM9.0000000000000180
Gao, Y., Wang, C., Kang, K., Peng, Y., Luo, Y., Liu, H., et al. (2021). Cytokine Storm May Not Be the Chief Culprit for the Deterioration of COVID-19. Viral Immunol. 34, 336–341. doi:10.1089/vim.2020.0243
Hacisuleyman, E., Hale, C., Saito, Y., Blachere, N. E., Bergh, M., Conlon, E. G., et al. (2021). Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 384, 2212–2218. doi:10.1056/NEJMoa2105000
Huang, C., Huang, L., Wang, Y., Li, X., Ren, L., Gu, X., et al. (2021). 6-month Consequences of COVID-19 in Patients Discharged from Hospital: a Cohort Study. Lancet 397, 220–232. doi:10.1016/S0140-6736(20)32656-8
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 395, 497–506. doi:10.1016/S0140-6736(20)30183-5
Lin, S. H., Zhao, Y. S., Zhou, D. X., Zhou, F. C., and Xu, F. (2020). Coronavirus Disease 2019 (COVID-19): Cytokine Storms, Hyper-Inflammatory Phenotypes, and Acute Respiratory Distress Syndrome. Genes Dis. 7, 520–527. doi:10.1016/j.gendis.2020.06.009
Liu, Z., Li, X., Fan, G., Zhou, F., Wang, Y., Huang, L., et al. (2021). Low-to-moderate Dose Corticosteroids Treatment in Hospitalized Adults with COVID-19. Clin. Microbiol. Infect. 27, 112–117. doi:10.1016/j.cmi.2020.09.045
Lumlertgul, N., Hall, A., Camporota, L., Crichton, S., and Ostermann, M. (2021). Clearance of Inflammatory Cytokines in Patients with Septic Acute Kidney Injury during Renal Replacement Therapy Using the EMiC2 Filter (Clic-AKI Study). Crit. Care 25, 39. doi:10.1186/s13054-021-03476-x
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J. (2020). COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 395, 1033–1034. doi:10.1016/S0140-6736(20)30628-0
Monard, C., Rimmelé, T., and Ronco, C. (2019). Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 47 (Suppl. 3), 1–14. doi:10.1159/000499520
Monneret, G., Benlyamani, I., Gossez, M., Bermejo-Martin, J. F., Martín-Fernandez, M., Sesques, P., et al. (2021). COVID-19: What Type of Cytokine Storm Are We Dealing with? J. Med. Virol. 93, 197–198. doi:10.1002/jmv.26317
Panacek, E. A., Marshall, J. C., Albertson, T. E., Johnson, D. H., Johnson, S., MacArthur, R. D., et al. (2004). Efficacy and Safety of the Monoclonal Anti-tumor Necrosis Factor Antibody F(ab')2 Fragment Afelimomab in Patients with Severe Sepsis and Elevated Interleukin-6 Levels. Crit. Care Med. 32, 2173–2182. doi:10.1097/01.ccm.0000145229.59014.6c
Park, J. T., Lee, H., Kee, Y. K., Park, S., Oh, H. J., Han, S. H., et al. (2016). High-Dose versus Conventional-Dose Continuous Venovenous Hemodiafiltration and Patient and Kidney Survival and Cytokine Removal in Sepsis-Associated Acute Kidney Injury: A Randomized Controlled Trial. Am. J. Kidney Dis. 68, 599–608. doi:10.1053/j.ajkd.2016.02.049
Pasrija, R., and Naime, M. (2021). The Deregulated Immune Reaction and Cytokines Release Storm (CRS) in COVID-19 Disease. Int. Immunopharmacol 90, 107225. doi:10.1016/j.intimp.2020.107225
Peng, Y., Yuan, Z., and Li, H. (2005). Removal of Inflammatory Cytokines and Endotoxin by Veno-Venous Continuous Renal Replacement Therapy for Burned Patients with Sepsis. Burns 31, 623–628. doi:10.1016/j.burns.2005.02.004
Premužić, V., Babel, J., Gardijan, D., Lapić, I., Gabelica, R., Ostojić, Z., et al. (2021). Extracorporeal Blood Purification Is Associated with Improvement in Biochemical and Clinical Variables in the Critically-Ill COVID-19 Patients. Ther. Apher. Dial. Epub ahead of print. doi:10.1111/1744-9987.13730
Rajpal, A., Rahimi, L., and Ismail-Beigi, F. (2020). Factors Leading to High Morbidity and Mortality of COVID-19 in Patients with Type 2 Diabetes. J. Diabetes 12, 895–908. doi:10.1111/1753-0407.13085
Rosalia, R. A., Ugurov, P., Neziri, D., Despotovska, S., Kostoska, E., Veljanovska-Kiridjievska, L., et al. (2021). Extracorporeal Blood Purification in Moderate and Severe COVID-19 Patients: A Prospective Cohort Study. Blood Purif., 1–10. Epub ahead of print. doi:10.1159/000515627
Samman, K. N., Baalbaki, H., Bouchard, J., and Albert, M. (2021). Continuous Renal Replacement Therapy with oXiris® Membrane in Severe Ebstein-Barr Virus-Mediated Hemophagocytic Lymphohistiocytosis: A Case Report. Blood Purif. 50, 578–581. doi:10.1159/000511724
Schwindenhammer, V., Girardot, T., Chaulier, K., Grégoire, A., Monard, C., Huriaux, L., et al. (2019). oXiris® Use in Septic Shock: Experience of Two French Centres. Blood Purif. 47 (Suppl. 3), 1–7. doi:10.1159/000499510
Shi, N., Sun, G. D., Ji, Y. Y., Wang, Y., Zhu, Y. C., Xie, W. Q., et al. (2021). Effects of Acute Kidney Injury on Acute Pancreatitis Patients' Survival Rate in Intensive Care Unit: A Retrospective Study. World J. Gastroenterol. 27, 6453–6464. doi:10.3748/wjg.v27.i38.6453
Sims, J. T., Krishnan, V., Chang, C. Y., Engle, S. M., Casalini, G., Rodgers, G. H., et al. (2021). Characterization of the Cytokine Storm Reflects Hyperinflammatory Endothelial Dysfunction in COVID-19. J. Allergy Clin. Immunol. 147, 107–111. doi:10.1016/j.jaci.2020.08.031
Turani, F., Barchetta, R., Falco, M., Busatti, S., and Weltert, L. (2019). Continuous Renal Replacement Therapy with the Adsorbing Filter oXiris in Septic Patients: A Case Series. Blood Purif. 47 (Suppl. 3), 1–5. doi:10.1159/000499589
van Paassen, J., Vos, J. S., Hoekstra, E. M., Neumann, K. M. I., Boot, P. C., and Arbous, S. M. (2020). Corticosteroid Use in COVID-19 Patients: a Systematic Review and Meta-Analysis on Clinical Outcomes. Crit. Care 24, 696. doi:10.1186/s13054-020-03400-9
Villa, G., Romagnoli, S., De Rosa, S., Greco, M., Resta, M., Pomarè Montin, D., et al. (2020). Blood Purification Therapy with a Hemodiafilter Featuring Enhanced Adsorptive Properties for Cytokine Removal in Patients Presenting COVID-19: a Pilot Study. Crit. Care 24, 605. doi:10.1186/s13054-020-03322-6
Wang, C., Kang, K., Lan, X., Fei, D., Wang, Q., Li, X., et al. (2021). Cytokine Levels in Sputum, Not Serum, May Be More Helpful for Indicating the Damage in the Lung and the Prognosis of Severe COVID-19 - A Case Series. J. Infect. 83, e6–1. doi:10.1016/j.jinf.2021.08.026
Xiang, H., Song, B., Zhang, Y., Zhang, J., and Xiong, J. (2021). The Effectiveness of Continuous Renal Replacement Therapy in Critical COVID-19 Patients with Cytokine Release Syndrome: a Retrospective, Multicenter, Descriptive Study from Wuhan, China. Aging (Albany NY) 13 (7), 9243–9252. doi:10.18632/aging.202838
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., et al. (2020). Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: a Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 8 (5), 475–481. doi:10.1016/S2213-2600(20)30079-5
Yin, F., Zhang, F., Liu, S., and Ning, B. (2020). The Therapeutic Effect of High-Volume Hemofiltration on Sepsis: a Systematic Review and Meta-Analysis. Ann. Transl Med. 8, 488. doi:10.21037/atm.2020.03.48
Zeng, F., Huang, Y., Guo, Y., Yin, M., Chen, X., Xiao, L., et al. (2020). Association of Inflammatory Markers with the Severity of COVID-19: A Meta-Analysis. Int. J. Infect. Dis. 96, 467–474. doi:10.1016/j.ijid.2020.05.055
Keywords: continuous renal replacement therapy, oXiris filter, cytokine release syndrome, cytokine storm, hyper inflammation, non-acute kidney injury, severe and critical COVID-19, SARS-CoV-2 infection
Citation: Kang K, Luo Y, Gao Y, Zhang J, Wang C, Fei D, Yang W, Meng X, Ye M, Gao Y, Liu H, Du X, Ji Y, Wei J, Xie W, Wang J, Zhao M and Yu K (2022) Continuous Renal Replacement Therapy With oXiris Filter May Not be an Effective Resolution to Alleviate Cytokine Release Syndrome in Non-AKI Patients With Severe and Critical COVID-19. Front. Pharmacol. 13:817793. doi: 10.3389/fphar.2022.817793
Received: 25 November 2021; Accepted: 12 January 2022;
Published: 04 February 2022.
Edited by:
Jean-Louis Vincent, Université libre de Bruxelles, BelgiumReviewed by:
Marcus Ewert Broman, Skåne University Hospital, SwedenIbrahim C. Haznedaroglu, Hacettepe University Hospital, Turkey
Copyright © 2022 Kang, Luo, Gao, Zhang, Wang, Fei, Yang, Meng, Ye, Gao, Liu, Du, Ji, Wei, Xie, Wang, Zhao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyan Zhao, bWluZ3lhbjE5NzBAMTI2LmNvbQ==; Kaijiang Yu, eXVrYWlqaWFuZzAwMUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Kai Kang
Kai Kang Yunpeng Luo1†
Yunpeng Luo1† Jiannan Zhang
Jiannan Zhang Changsong Wang
Changsong Wang Xianglin Meng
Xianglin Meng Jieling Wei
Jieling Wei Kaijiang Yu
Kaijiang Yu