- Department of Orthopedics, Orthopedic Research Institute, West China Hospital, Sichuan University, Chengdu, China
Background: Recently, there was a series of clinical studies focusing on local injection of platelet-rich plasma (PRP) for treatment of patients with carpal tunnel syndrome (CTS). However, the safety and efficacy of PRP in these CTS patients remains controversial. Therefore, we performed a systematic review to compare PRP with other conservative treatments in treatment of CTS patients.
Methods: We systematically searched from electronic databases (Cochrane, PubMed, Web of Science, and EMBASE) up to 10 December 2021. The data of clinical results were extracted and analyzed by RevMan Manager 5.4.
Results: Finally, eight randomized controlled studies, involving 220 CTS patients undergoing local injection of PRP were enrolled in this systematic review. All enrolled trials were considered to be of high quality. In the short-term efficacy, the PRP group was significantly lower in symptom severity scale (SSS) compared with the control group (MD = −2.00; 95% CI, −3.15 to −0.85; p = 0.0007; I2 = 0%). In the mid-term efficacy, the PRP group was significantly effective than the control group in the visual analogue scale (MD = −0.63; 95% CI, −1.22 to −0.04; p = 0.04; I2 = 61%), SSS (MD = −3.56; 95% CI, −4.93 to −2.18; p < 0.00001; I2 = 0%), functional status scale (MD = −2.29; 95% CI, −3.03 to −1.56; p < 0.00001; I2 = 45%), sensory peak latency (MD = −0.39; 95% CI, −0.58 to −0.19; p = 0.0001; I2 = 0%) and cross-sectional area of median nerve (MD = -0.20; 95% CI, −0.31 to −0.10; p = 0.0002; I2 = 0%). In the mid-long-term efficacy, the PRP group was only significantly lower in SSS compared with the control group (MD = −2.71; 95% CI, −4.33 to −1.10; p = 0.001; I2 = 38%).
Conclusion: Local PRP injection is more effective than other conservative treatments in terms of mid-term efficacy in relieving pain, improving wrist function and symptoms, reducing MN swelling, and partially improving electrophysiological indicators. However, the long-term adverse side and consensus on standardization of PRP in CTS patients still need further large-scale trials.
Introduction
As a compressive peripheral nerve disease, carpal tunnel syndrome (CTS) is caused by the median nerve (MN)’s compression by increased pressure in the bone fiber tunnel formed by wrist bones, transverse carpal ligament, and digital flexor tendons (Chammas, 2014). The clinical symptom of CTS includes pain, numbness, weakness, and paresthesia in the three and a half fingers on the radial side. What’s more, as the disease progresses, muscle atrophy in the thenar area and decreased hand muscle strength will occur (Alfonso et al., 2010). The previous studies reported that the annual incidence of CTS was estimated to be 90 new cases per 100,000 men and 193–280 new cases per 100,000 women (Latinovic et al., 2006; Bongers et al., 2007).
Currently, various conservative treatments, including wrist splinting, local steroid injections, oral medications, and acupuncture are used for mild to moderate CTS. However, the results of these conservative treatments are not satisfactory to patients (Ostergaard et al., 2020). The previous study showed that about half of the CTS patients treated conservatively finally received surgery (Burton et al., 2016). Platelet-rich plasma (PRP) is a concentrated product of autologous blood, containing concentrated platelets and various growth factors (Razmara et al., 2008; Ruzafa et al., 2021a; Zhou et al., 2021). PRP not only has a good ability of anti-inflammatory and tissue repair, but also can promote the regeneration of peripheral neurons (Zheng et al., 2016; Ruzafa et al., 2021b; Negrini et al., 2021). PRP has been widely used in refractory wounds, external humeral epicondylitis, and plantar fasciitis (Chen et al., 2021a; Fei et al., 2021; Zhang et al., 2021). Recently, the application of local PRP injection has gradually applied in the treatment of CTS. Compared with other conservative managements, the clinical effect of local injection of PRP in the treatment of CTS patients is still controversial.
Therefore, we performed this systematic review and meta-analysis to evaluate the efficacy and safety of local PRP injection in treatment for CTS. The study will be evaluated from the following aspects: the visual analogue scale (VAS), Boston carpal tunnel questionnaire (BCTQ), cross-sectional area (CSA) of MN and electrodiagnostic examination parameters including distal motor latency (DML), sensory nerve conduction velocity (SNCV), sensory peak latency (SPL). BCTQ is the most commonly used self-assessment questionnaire for CTS patients, including two subscales, symptom severity scale (SSS) (11 items) and functional status scale (FSS) (8 items). The score of each item ranges from 0 to 5. The higher the score, the higher the severity and disability (Levine et al., 1993).
Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines and Quality of Reporting of Meta-analyses (QUORUM) guidelines were followed in this systematic review. In our study, we created a prospective protocol, consisting of objectives, study selection strategies, inclusion criteria, exclusion criteria, statistical analysis, and outcome measures.
Search Strategy
Two reviewers independently searched for potentially relevant published and unpublished studies using electronic databases, including Cochrane, PubMed, Web of Science, and EMBASE, from inception to 10 December 2021. The following keywords were used for the search: “Carpal Tunnel Syndrome,” “Carpal Tunnel Syndromes,” “Syndrome, Carpal Tunnel,” “Syndromes, Carpal Tunnel,” “Amyotrophy, Thenar, Of Carpal Origin,” “Median Neuropathy, Carpal Tunnel,” “Compression Neuropathy, Carpal Tunnel,” “Entrapment Neuropathy, Carpal Tunnel,” “Platelet-Rich Plasma,” “Plasma, Platelet-Rich,” “Platelet Rich Plasma,” “PRP”. After the electronic search is completed, manual searches were carried out on related literatures and references to find potential eligible studies.
Eligibility Criteria
The inclusion criteria for this study were as follows: 1) Population: patients were adults and diagnosed with CTS. 2) Intervention: patients were treated with local PRP injection. 3) Comparator: patients who were treated with other conservative management, such as wrist splint and local injection with methylprednisolone acetate, triamcinolone, hyaluronidase, dextrose and normal saline. 4) Outcomes: one of the following results was reported, VAS, BCTQ including SSS and FSS, CSA of MN and electrodiagnostic examination parameters including DML, SNCV, SPL. 5) Study design: The studies were original, randomized control trials (RCTs) only. 6) The studies report PRP’s preparations and injection procedures performed in CTS patients.
The exclusion criteria for this study were as follows: 1) Studies not published in English 2) Retrospective studies and Cohort studies. 3) Animal studies. 4) Nonoriginal research, such as reviews, technical reports. 5) Duplicated publications. 6) Single abstracts 7) Case reports. 8) Studies in which the relevant data cannot be extracted and the original author is contacted without a response. If there is a dispute between the two reviewers, it will be settled through consultation with a third reviewer.
Data Extraction
Two reviewers scanned all enrolled studies to extract data independently according to the inclusion and exclusion criteria. The demographic characteristics extracted for meta-analysis were as follows: first author, publication year, country, number of patients in different groups, male/female ratio, the average age of patients, duration of symptoms, grade of CTS, details of the comparator, outcome measures, and duration of follow-up. And extraction of related complications after local injection. In enrolled trials with more than two control groups, we only extracted the data from PRP and other conservative groups. In this study, we define the outcomes around 1 month after the intervention as short-term efficacy, 3 months as mid-term efficacy, and 6 months as mid-long-term efficacy. All data were entered into an electronic spreadsheet. Furthermore, any disagreements were resolved by discussion and consensus with a third reviewer.
Assessment of Methodological Quality
The methodological quality of trials included in this study was evaluated independently by two reviewers, according to Cochrane Collaboration for Systematic Reviews (Corbett et al., 2014). The following items were considered: random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, selective reporting, and other bias. Each item was assessed as “Low risk of bias,” “Unclear risk of bias,” or “High risk of bias.” If the item was reported incorrectly, the judgment was “High risk of bias.” If the item was reported inadequately, the judgment was “Unclear risk of bias.” If the item was reported correctly and adequately, the judgment was “Low risk of bias.” Any disagreements were resolved by discussion and consensus with a third reviewer.
Statistical Analysis
The statistical analysis was independently performed with RevMan software (Version 5.4; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) by two reviewers. The mean difference (MD) between groups of PRP and control were reported with 95% confidence interval (95% CI) and performed to evaluate continuous variables. To measure heterogeneity between studies, we used the I2 statistic. Furthermore, heterogeneity was accepted, and the randomized-effects model was performed, when I2 was>50%. Otherwise, the fixed-effects model was performed. Forest plots were used to graphically represent the difference in outcomes of groups of PRP and control and for all included studies. If p values were <0.05, the results were considered statistically significant. We did not conduct a publication bias assessment because when the number of studies included in the meta-analysis is less than 10, it is unnecessary to conduct a publication bias assessment (GS HJ., 2011).
Results
Included Study
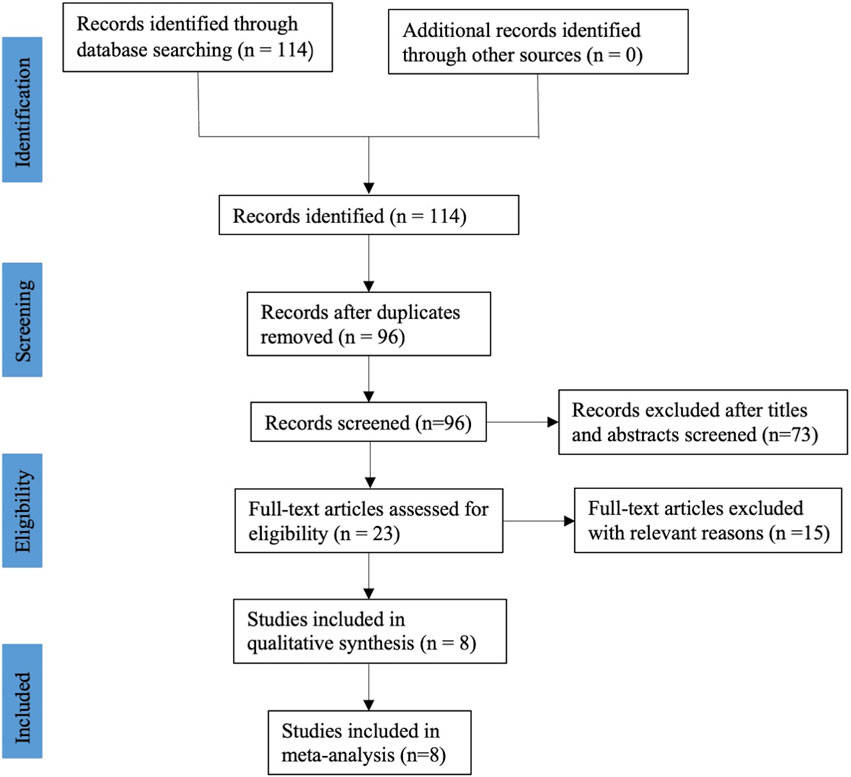
After a systematic literature search, a total of one hundred and fourteen relevant publications were retrieved. After excluded duplicate records, 96 studies were screened using their titles and abstracts, and 73 of them were removed. Through reading the full text, fifteen records were excluded because of irrelevant to our topics, no relevant data and ongoing clinical research that has not yet been published. Finally, eight RCTs (Wu et al., 2017; Malahias et al., 2018; Raeissadat et al., 2018; Senna et al., 2019; Shen et al., 2019; Eltabl et al., 2020; Hashim et al., 2020; Chen et al., 2021b), including 436 participants, met the selection criteria and were included in this meta-analysis. The flow diagram involved in the current study is shown in Figure 1.
Study Characteristics
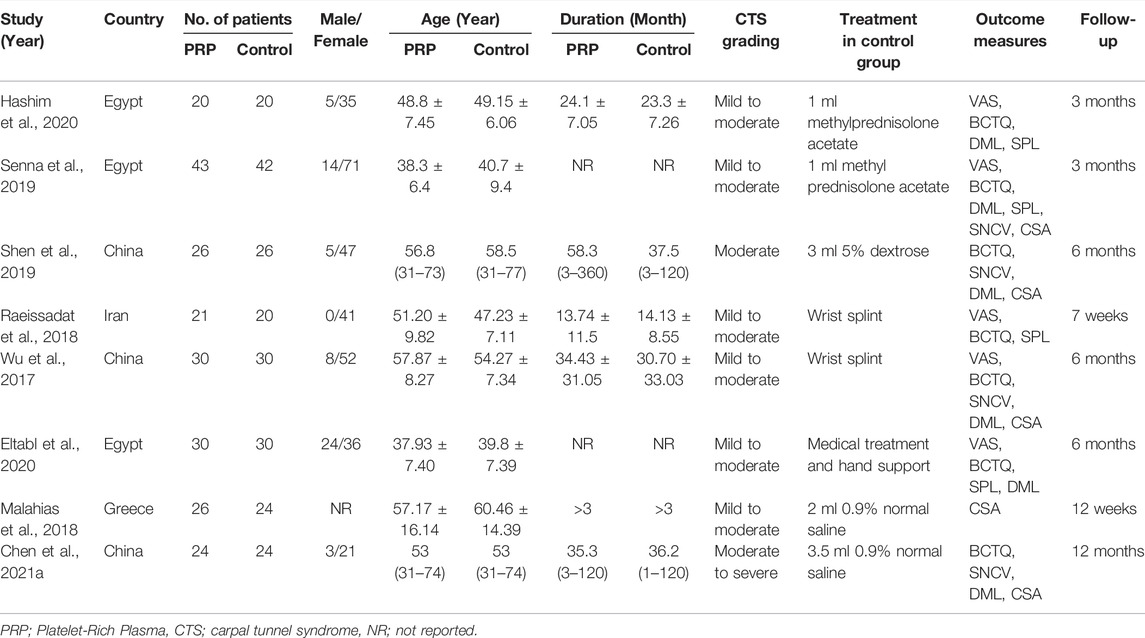
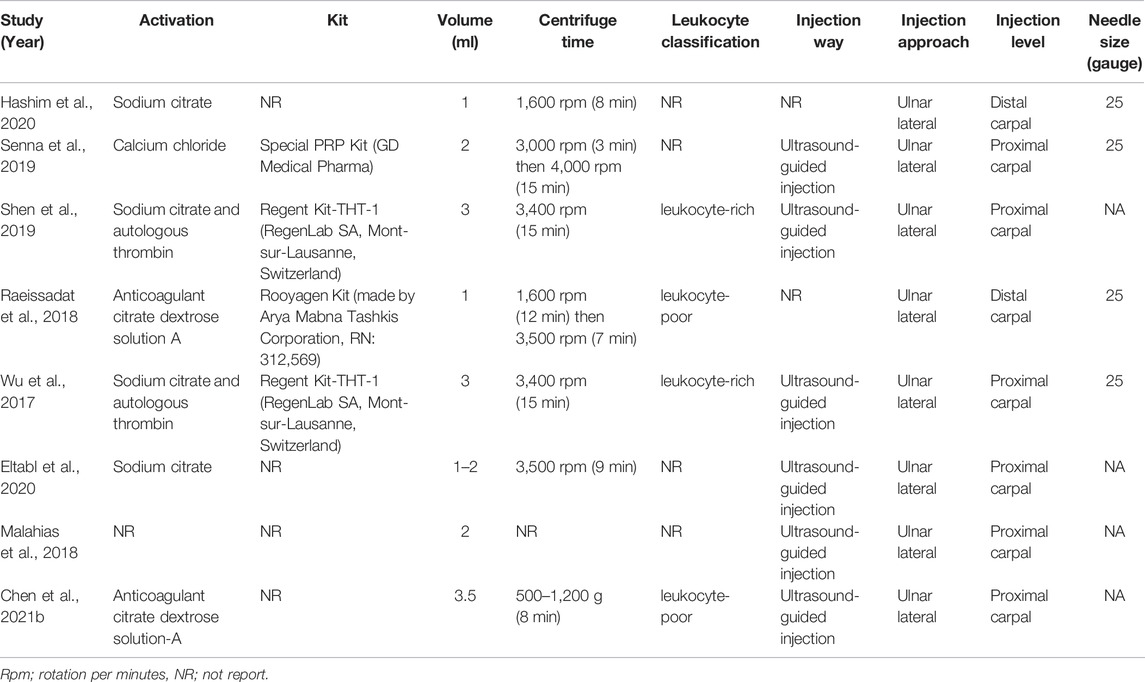
The main characteristics and preparations of PRP injection among the eight included RCTs are shown in Tables 1, 2. In these enrolled studies, three studies were conducted in Egypt (Senna et al., 2019; Eltabl et al., 2020; Hashim et al., 2020), and three studies in China (Wu et al., 2017; Shen et al., 2019; Chen et al., 2021b), each one in Iran (Raeissadat et al., 2018) and Greece (Malahias et al., 2018). In these included studies, CTS patients were treated with local autologous PRP injection in the experimental group and different types of conservative treatment in the control group. The grades of included CTS patients ranged from mild to severe. Among control groups of all enrolled studies, two studies were treated with methylprednisolone acetate (Senna et al., 2019; Hashim et al., 2020), two studies with wrist splint (Wu et al., 2017; Raeissadat et al., 2018), two studies with 0.9% normal saline (Malahias et al., 2018; Chen et al., 2021b), each one with 5% dextrose (Shen et al., 2019), medical treatment and hand support (Eltabl et al., 2020). All included studies excluded patients with hematologic disorders such as thrombocytopenia, platelet dysfunction, and coagulation disorders or patients on recent treatment with NSAIDs, antiplatelets, and anticoagulants in order to reduce the incidence of injection-related adverse events. Six studies conducted PRP injection under ultrasound guidance (Wu et al., 2017; Malahias et al., 2018; Senna et al., 2019; Shen et al., 2019; Eltabl et al., 2020; Chen et al., 2021b). All studies were conducted with ulnar in plane approach to inject superficial and deep to the median nerve. Two studies reported injections performed under the short-axis view technique (Shen et al., 2019; Chen et al., 2021b). The level of injection was distal to the carpal joint in two studies (Raeissadat et al., 2018; Hashim et al., 2020) and proximal to the carpal tunnel in the remaining studies. Four studies reported injection needle sizes were 25 gauge (Wu et al., 2017; Raeissadat et al., 2018; Senna et al., 2019; Hashim et al., 2020). In only one study, local PRP injection complications occurred in four cases of itching, one case of finger pain, and one case of burning sensation (Raeissadat et al., 2018). A single injection of PRP was performed in all enrolled studies. PRP used in all included studies was derived from autologous peripheral blood of patients. The procedures for preparing PRP were described in detail in all included studies. The local injection dose of PRP ranged from 1 to 3.5 ml in enrolled studies. Two studies reported the type of PRP was leukocyte-rich (Wu et al., 2017; Shen et al., 2019), and two studies were leukocyte-poor (Raeissadat et al., 2018; Chen et al., 2021b). Only one study reported the concentration of platelets in PRP (Hashim et al., 2020). The published years of enrolled RCTs were between 2017 and 2021.
Quality Assessment of Individual Trials
Among these enrolled studies, seven studies were performed with adequate random sequence generation (Wu et al., 2017; Malahias et al., 2018; Raeissadat et al., 2018; Senna et al., 2019; Shen et al., 2019; Hashim et al., 2020; Chen et al., 2021b). In addition, four studies were conducted with allocation concealment (Malahias et al., 2018; Raeissadat et al., 2018; Senna et al., 2019; Chen et al., 2021b), and the remaining four studies were not reported and determined to be unclear. Blinding of participants and personnel was reported in four included studies (Malahias et al., 2018; Senna et al., 2019; Hashim et al., 2020; Chen et al., 2021b). The remains cannot be blinded because of the treatment method. Blinding of outcome assessment was reported in six included studies (Wu et al., 2017; Malahias et al., 2018; Senna et al., 2019; Shen et al., 2019; Hashim et al., 2020; Chen et al., 2021b). In addition, the outcome reports and data of all studies are complete. And we did not identify other obvious sources of bias in the trials. Figures 2, 3 summarized the methodological quality of all enrolled studies.
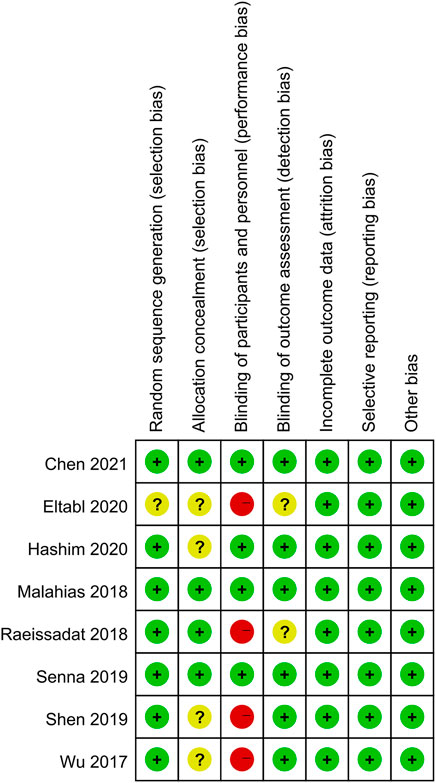
FIGURE 2. Risk of bias graph: low risk of bias in green; unclear risk of bias in yellow; high risk of bias in red.
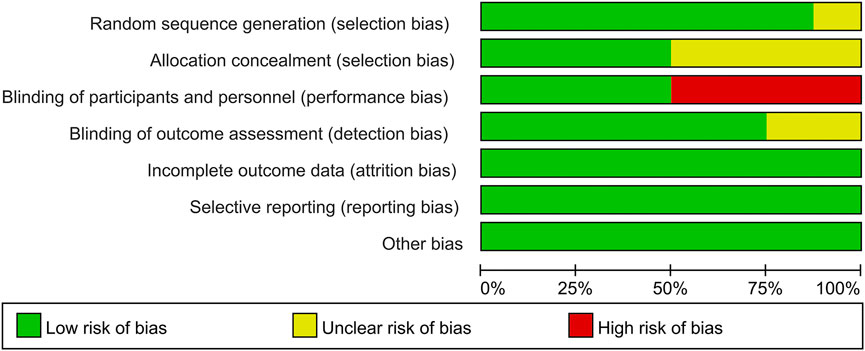
FIGURE 3. Risk of bias summary: each risk of bias item is presented as the percentage across all the included studies, which indicates the proportion of different levels of risk of bias for each item.
Meta-Analysis of Short-Term Efficacy
A total of six studies reported data on short-term efficacy after no-surgical treatment (164 and 162 patients in the PRP and control groups, respectively). The results of pooled analyses were shown in Figure 4. The results showed that the SSS of local PRP injection group were significantly lower than that of control group (MD = −2.00; 95% CI, −3.15 to −0.85; p < 0.001), However, there were no significant differences between two groups in VAS score (MD = −0.31; 95% CI, −1.03 to 0.40; p = 0.39), FSS (MD = -0.73; 95% CI, −2.00 to 0.54; p = 0.26), DML(MD = −0.24; 95% CI, −0.86 to 0.37; p = 0.44), SNCV(MD = −0.95; 95% CI, −2.93 to 1.03; p = 0.35), SPL(MD = −0.04; 95% CI, -0.37 to 0.45; p = 0.84), CSA(MD = -0.20; 95% CI, -0.74 to 0.33; p = 0.45).
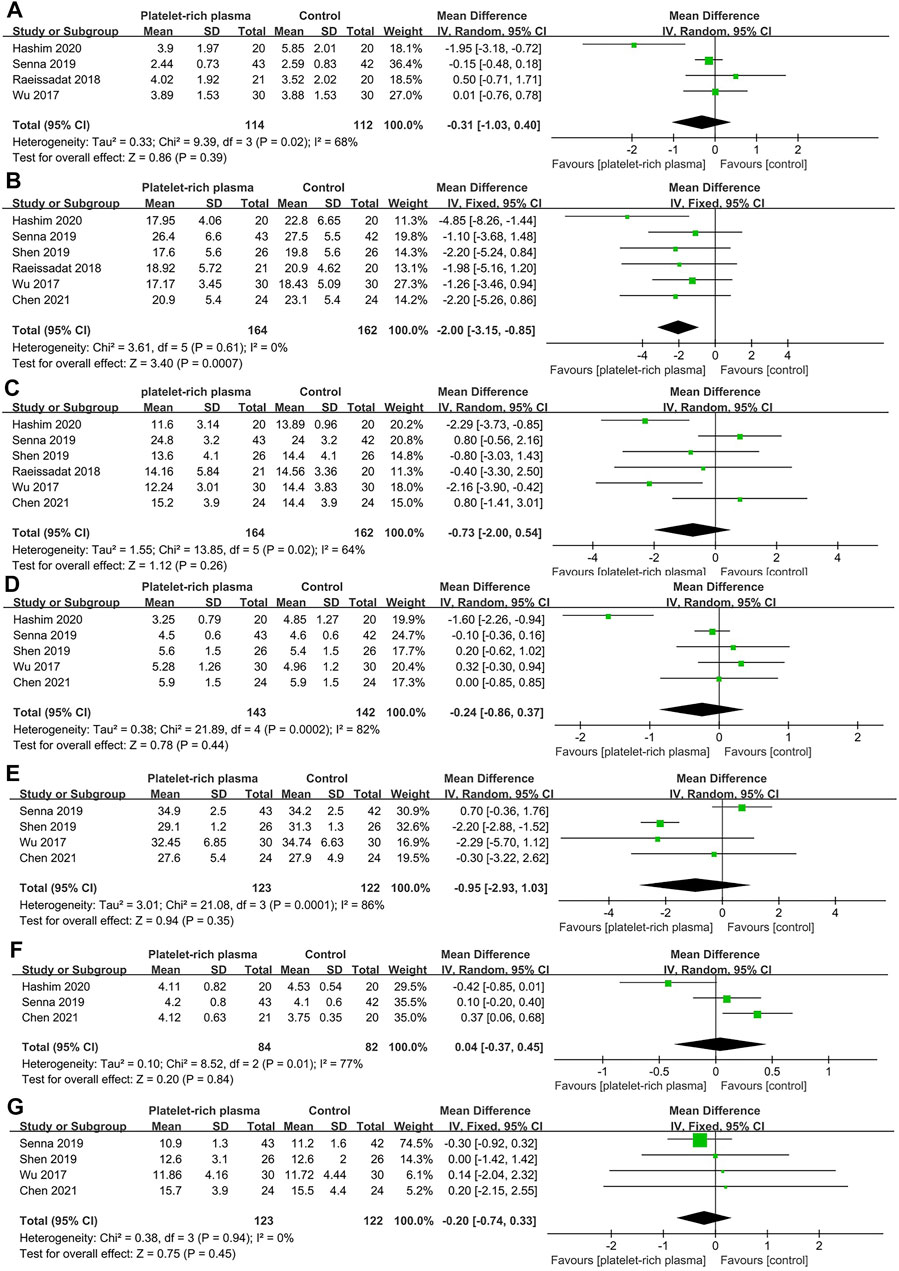
FIGURE 4. Forest plot showing short-term efficacy of local platelet-rich plasma injection versus other conservative treatments. (A) Visual analogue scale. (B) Symptom severity scale. (C) Functional status scale. (D) Distal motor latency. (E) Sensory nerve conduction velocity. (F) Sensory peak latency. (G) Cross sectional area.
Meta-Analysis of Mid-term Efficacy
A total of six studies reported data on mid-term efficacy after no-surgical treatment (169 and 166 patients in the PRP and control groups, respectively). The results of pooled analyses are shown in Figure 5. The results showed that the SSS of the local PRP injection group was significantly lower than that of the control group (MD = −0.63; 95% CI, −1.22 to −0.04; p = 0.04). Besides, the SSS and FSS of the PRP group were significantly lower than that of the control group (MD = −3.56; 95% CI, −4.93 to −2.18; p < 0.001, MD = −2.29; 95% CI, −3.03 to −1.56; p < 0.001). The CSA was significantly reduced in the PRP group than that of the control group (MD = −0.20; 95% CI, −0.31 to −0.10; p < 0.001). In terms of electrodiagnostic examination parameters, PRP group was significantly reduced in SPL (MD = −0.39; 95% CI, −0.58 to −0.19; p < 0.001). In addition, there were no significant differences between two groups in DML (MD = −0.26; 95% CI, −0.85 to 0.33; p = 0.38) and SNCV (MD = 0.57; 95% CI, −0.55 to 1.69; p = 0.32).
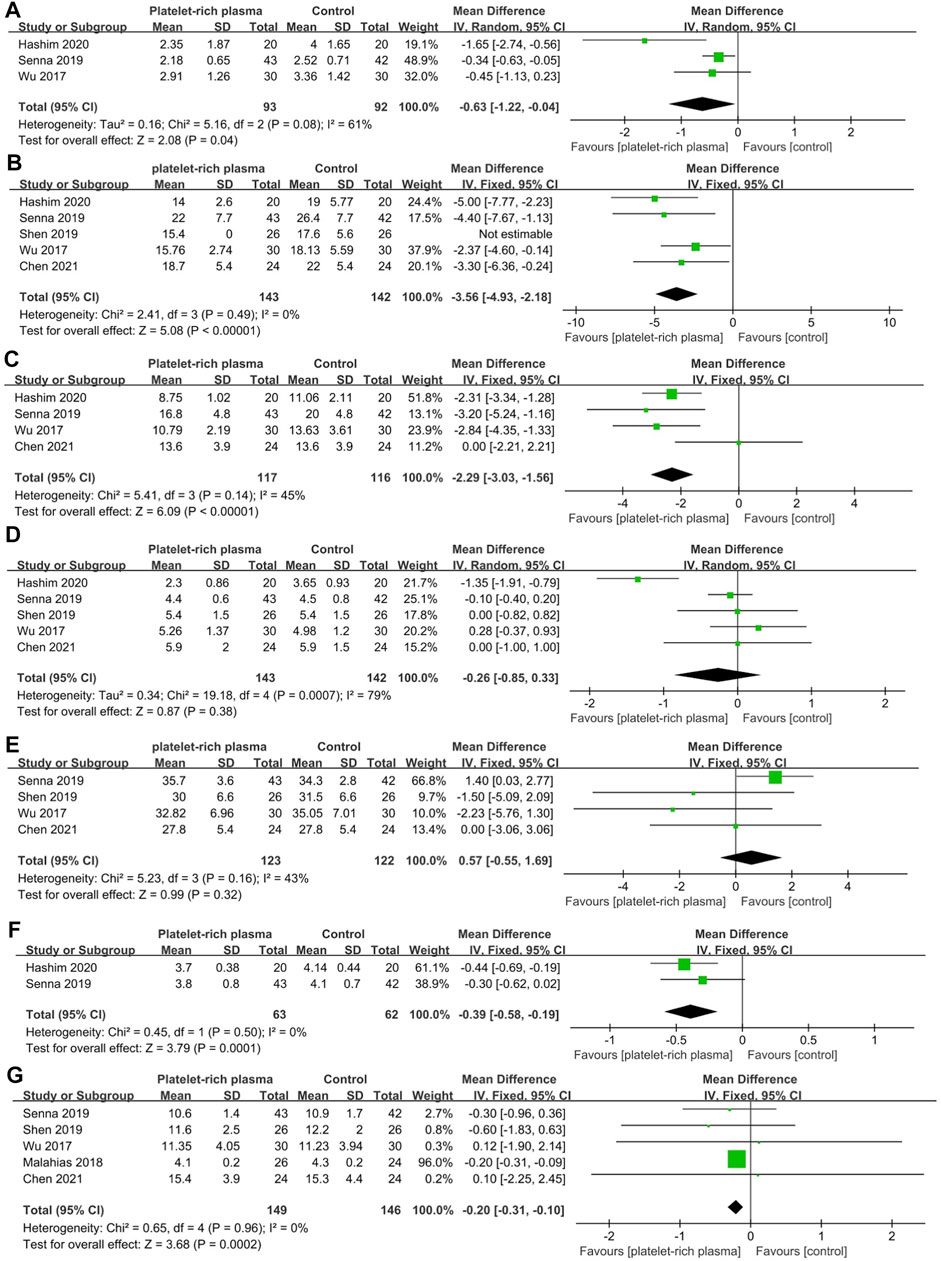
FIGURE 5. Forest plot showing mid-term efficacy of local platelet-rich plasma injection versus other conservative treatments. (A) Visual analogue scale. (B) Symptom severity scale. (C) Functional status scale. (D) Distal motor latency. (E) Sensory nerve conduction velocity. (F) Sensory peak latency. (G) Cross sectional area.
Meta-Analysis of Mid-Long-Term Efficacy
A total of four studies reported data on mid-long-term efficacy after no-surgical treatment (110 and 110 patients in the PRP and control groups, respectively). The results of pooled analyses were shown in Figure 6. The results showed that the SSS of local PRP injection group were significantly lower than that of control group (MD = −2.71; 95% CI, −4.33 to −1.10; p = 0.001), However, there were no significant differences between two groups in VAS score (MD = −3.40; 95% CI, −8.05 to 1.26; p = 0.15), FSS (MD = −5.17; 95% CI, −11.72 to 1.39; p = 0.12), DML(MD = −0.28; 95% CI, −1.41 to 0.84; p = 0.62), SNCV (MD = −0.85; 95% CI, −2.96 to 1.26; p = 0.43) , CSA(MD = −0.49; 95% CI, −1.51 to 0.52; p = 0.34).
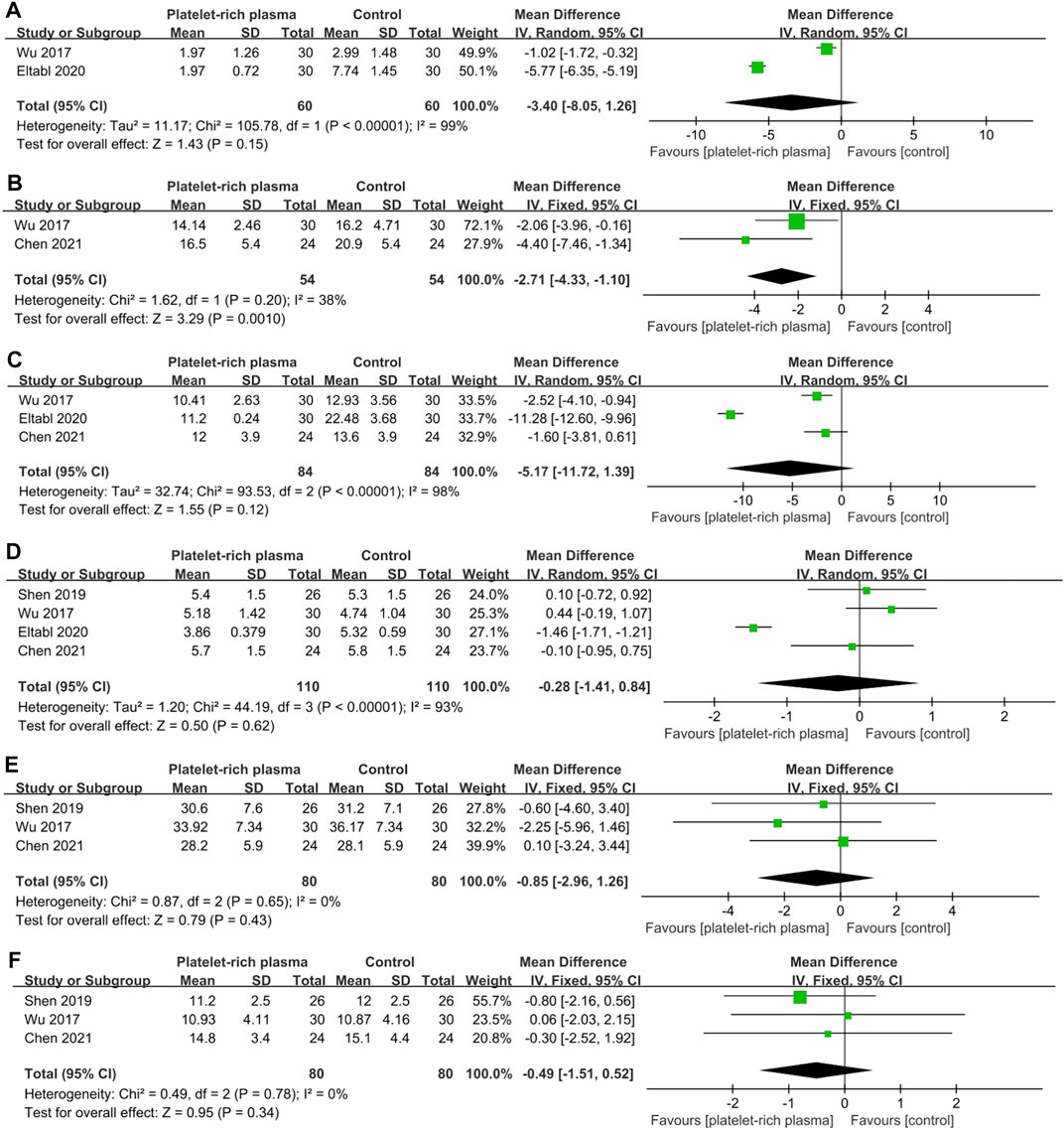
FIGURE 6. Forest plot showing mid-long-term efficacy of local platelet-rich plasma injection versus other conservative treatments. (A) Visual analogue scale. (B) Symptom severity scale. (C) Functional status scale. (D) Distal motor latency. (E) Sensory nerve conduction velocity. (F) Cross sectional area.
Sensitivity Analysis
A sensitivity analysis was performed by individually removing each study to determine whether the pooled results changed. The pooled results at different time were stable.
Discussion
In our study, we conducted a systematic review and meta-analysis to compare the clinical efficacy of PRP injection and other conservative treatments in CTS patients. The results showed that local injection of PRP is more effective than other conservative treatments in decreasing SSS score in the short-term follow-up. In the mid-term follow-up, the local PRP injection was better than other conservative treatments in terms of VAS, SSS, FSS, SPL, and CSA. In the mid-long-term follow-up, the SSS score of local injection of PRP was higher than that of control group.
The symptoms of CTS would become severe without treatment over time. Long-term compression and ischemia of the MN in the carpal tunnel may cause permanent nerve damage and aseptic inflammation. Traditional conservative treatments, including oral medications, musculoskeletal manipulation, and wrist splints, can alleviate local pain, but their repair effects on nerve function are limited (Padua et al., 2016). A local steroid injection can effectively reduce the expression of acute inflammatory factors such as CRP and IL-6 to achieve anti-inflammatory and pain relief effects (Peerbooms et al., 2019; Hashim et al., 2020). However, the application of steroids is not related to the activation of repair-related signaling pathways and will increase the risk of local soft tissue rupture and neurotoxicity (Nepple and Matava, 2009; Kim and Park, 2014). At present, many studies demonstrated that PRP had a positive effect on peripheral nerve regeneration, anti-inflammatory and regulating angiogenesis (Sánchez et al., 2012). Zheng et al. proved that PRP at a concentration of 2.5%–20% could significantly stimulate the proliferation and migration of Schwann cells in vitro and promoted the secretion of nerve growth factor and glial cell line-derived neurotrophic factor (Zheng et al., 2016). Farrag et al. proved that in the rat facial nerve axotomy model, the addition of PRP in the process of nerve suture could significantly increase the thickness of myelin sheath and the number of axons, and the speed of nerve transmission is significantly increased (Farrag et al., 2007). Therefore, injecting PRP around the diseased nerve tissue might be a promising bioremediation treatment.
At present, the biological effect mechanism of PRP is mainly through the activation of platelet concentrates on releasing a large number of cytokines, such as platelet-derived growth factor, transforming growth factor-β, insulin-like growth factor and, vascular endothelial growth factor, which have powerful effects on cell proliferation and tissue repair (Picard et al., 2015). All these cytokines can not only directly repair damaged MN, but also increase the production of α-2 collagen and type III collagen in the flexor support belt cells to reduce the pressure in the carpal tunnel (Allampallam et al., 2000)
Our meta-analysis results showed that the SSS score in the PRP group was significantly lower than that in the control group in the short-term follow-up. VAS, SSS, FSS, SPL, and CSA in the PRP group all improved significantly during the mid-term follow-up. In our opinion, we think that PRP have a certain delay effect in exerting its functions, and it takes some time to reach its maximum clinical effect. A similar phenomenon also appeared when using PRP to treat plantar fasciitis and external humeral epicondylitis (Merolla et al., 2017; Fei et al., 2021). In addition, among the studies that reported short-term efficacy, four studies conducted local PRP injections under ultrasound guidance (Wu et al., 2017; Senna et al., 2019; Shen et al., 2019; Chen et al., 2021b). During the injection process, the hydrodissection effect around the nerve sheath helps to reduce the symptoms of CTS, which refers to stripping the adhesion and compression of the related connective tissue and the flexor retinaculum around the MN to reduce the ischemic damage caused to it (Cass, 2016). A single-blind RCT showed that ultrasound-guided injection is more effective than blind injection in alleviating the symptoms of CTS in the early stage (Ustün et al., 2013). Additionally, local injection guided by ultrasound can also avoid iatrogenic MN injury and reduce complications. All these results confirmed that the close equivalent effect of PRP versus other conservative treatments in the short-term efficacy and the mid-term effects of PRP significantly improved.
During the mid-long-term follow-up, the SSS score of the PRP group was significantly lower than that of the control group. However, no significant differences were found in other outcome indicators, which is consistent with the results of previous study (Uzun et al., 2017). They believe that this temporary efficacy is attributable to the dose and frequency of PRP injections. After PRP is injected into the patient, the local repairability may decrease as platelets and growth factors are degraded. Through timely re-injection, a higher concentration of growth factors in the damaged tissues of the patient’s carpal tunnel can be maintained to achieve long-term and effective promotion of CTS patients to improve clinical symptoms and wrist function. Moreover, no correlations between the improvement of symptoms and the results of the electrodiagnostic examinations were found during follow-up periods. This inconsistency is expected because the conventional electrodiagnostic examination mainly evaluates large myelinated fibers rather than small sensory fibers, and these feeling fibers may be associated with many CTS symptoms (Soyupek et al., 2012).
The studies included in our meta-analysis included differences in the preparation methods, platelet concentration, and injection volume. There is no consensus on the preparation process and the optimal injection concentration and PRP volume for CTS patients’ treatment. The time interval between PRP preparation and injection was not explicitly described in all studies, and to our knowledge, the time from extraction to preparation to injection of PRP is relatively fast and the impact on outcome indicators is negligible. However, only one study reported platelet concentration (Hashim et al., 2020). The previous study showed that the platelet concentration of therapeutic PRP should be 4–6 times that of whole blood, cause low concentration might be ineffective in the healing process (Crane and Everts, 2008). Different types of activations in preparation of PRP could affect the release of growth factors (Harrison et al., 2011). Four studies used a 25-gauge needle for injection, the remaining studies did not specify. Local PRP injection with different needle sizes and calibres has been proved to not influence platelet functionality, so smaller-sized needles should be chosen to minimize pain during the injection (Bausset et al., 2014). In addition, the injection volume of PRP in the enrolled studies ranges from 1 to 3.5 ml. The biological effects of different injection volumes are different. The hydrodissection effect and repairability can be more noticeable when the large injection volume. PRP of rich and poor leukocytes also differ in their efficacy. However, due to limited published studies, it is necessary to conduct further research on the optimal preparation and volume of local injection of PRP in the future.
The SSS score of the PRP group was significantly lower than that of the control group, and the FSS score was significantly lower than that of the control group during the mid-term follow-up. It can be concluded that local injection of PRP in the treatment of CTS effectively relieves symptoms and improves wrist joint function, and the mid-term efficacy is the most obvious. Therefore, local PRP injection may be a better and more promising treatment compared with other conservative treatments.
As for complications after local injection of PRP, only two studies reported injection-related adverse events. The study by Raeissadat et al. showed four cases of itching, one case of finger pain and one case of burning sensation after local injection (Raeissadat et al., 2018). Senna et al. reported an increase in pain sensation in the first 48 h following injection, which was relieved by the administration of paracetamol and local ice application (Senna et al., 2019). Due to the widespread use of ultrasound guidance, serious complications such as vascular and nerve injury caused by local injection are rare. And all the studies have used the ulnar lateral approach. According to a Bayesian network meta-analysis, ultrasound-guided ulnar injection is the most effective injection method for the treatment of carpal tunnel syndrome (Chen et al., 2015). PRP prepared from autologous blood avoids immune reactions and its bioprosthetic effect also reduces the risk of tendon rupture due to local steroid injection. Therefore, local PRP injection in CTS patients is a relatively safe therapy.
There are several limitations to our study. Firstly, our study is only a summary analysis of the mid-long-term efficacy of local injection of PRP, and the follow-up time is relatively short. Secondly, this study reported more outcome indicators from short-term efficacy, mid-term efficacy, and mid-long-term efficacy, including functional and symptom scores, electrodiagnostic examinations, and CSA of MN in CTS patients. Thirdly, although all enrolled studies are RCTs, the sample size of each study is small. Despite the limitations, the current meta-analysis found that local injection of PRP is a more effective treatment for CTS.
Conclusion
This systematic review indicates that local PRP injection is more effective than other conservative treatments in terms of mid-term efficacy in relieving pain, improving wrist function and symptoms, reducing MN swelling, and partially improving electrophysiological indicators. In terms of short-term and mid-long-term efficacy, PRP can only improve wrist symptoms compared with other conservative treatments, and there is no significant difference in other aspects. Therefore, local autologous PRP injection is a safe and effective treatment in CTS patients compared with other conservative treatments. However, more research is needed in the future to explore the long-term efficacy of local PRP injection in the treatment of CTS and the unified process of PRP preparation and optimal concentration and dose.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JJ and FX contributed equally to this study. JJ contributed to search the database, extract the data, analyze the data and manuscript writing. FX helped to extract the data and review the manuscript. RL searched the database and extracted the data. ML designed this systematic review. ML is the correspondence author who responsible for the correspondence, topic identification and manuscript reviewing.
Funding
This work was financially supported by Natural Science Foundations of China (No.31870961).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Prof. Guanglin Wang and Prof. Fuguo Huang for their help in this study.
REFERENCES
Alfonso, C., Jann, S., Massa, R., and Torreggiani, A. (2010). Diagnosis, Treatment and Follow-Up of the Carpal Tunnel Syndrome: a Review. Neurol. Sci. 31 (3), 243–252. doi:10.1007/s10072-009-0213-9
Allampallam, K., Chakraborty, J., and Robinson, J. (2000). Effect of Ascorbic Acid and Growth Factors on Collagen Metabolism of Flexor Retinaculum Cells from Individuals with and without Carpal Tunnel Syndrome. J. Occup. Environ. Med. 42 (3), 251–259. doi:10.1097/00043764-200003000-00005
Bausset, O., Magalon, J., Giraudo, L., Louis, M. L., Serratrice, N., Frere, C., et al. (2014). Impact of Local Anaesthetics and Needle Calibres Used for Painless PRP Injections on Platelet Functionality. Muscles Ligaments Tendons J. 4 (1), 18–23. doi:10.11138/mltj/2014.4.1.018
Bongers, F. J., Schellevis, F. G., van den Bosch, W. J., and van der Zee, J. (2007). Carpal Tunnel Syndrome in General Practice (1987 and 2001): Incidence and the Role of Occupational and Non-occupational Factors. Br. J. Gen. Pract. 57 (534), 36–39.
Burton, C. L., Chesterton, L. S., Chen, Y., and van der Windt, D. A. (2016). Clinical Course and Prognostic Factors in Conservatively Managed Carpal Tunnel Syndrome: A Systematic Review. Arch. Phys. Med. Rehabil. 97 (5), 836–e1. doi:10.1016/j.apmr.2015.09.013
Cass, S. P. (2016). Ultrasound-Guided Nerve Hydrodissection: What Is it? A Review of the Literature. Curr. Sports Med. Rep. 15 (1), 20–22. doi:10.1249/JSR.0000000000000226
Chen, P. C., Chuang, C. H., Tu, Y. K., Bai, C. H., Chen, C. F., and Liaw, M. (2015). A Bayesian Network Meta-Analysis: Comparing the Clinical Effectiveness of Local Corticosteroid Injections Using Different Treatment Strategies for Carpal Tunnel Syndrome. BMC Musculoskelet. Disord. 16, 363. doi:10.1186/s12891-015-0815-8
Chen, S. R., Shen, Y. P., Ho, T. Y., Li, T. Y., Su, Y. C., Chou, Y. C., et al. (2021). One-Year Efficacy of Platelet-Rich Plasma for Moderate-To-Severe Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Arch. Phys. Med. Rehabil. 102 (5), 951–958. doi:10.1016/j.apmr.2020.12.025
Chen, X. T., Fang, W., Jones, I. A., Heckmann, N. D., Park, C., and Vangsness, C. T. (2021). The Efficacy of Platelet-Rich Plasma for Improving Pain and Function in Lateral Epicondylitis: A Systematic Review and Meta-Analysis with Risk-Of-Bias Assessment. Arthroscopy 37 (9), 2937–2952. doi:10.1016/j.arthro.2021.04.061
Corbett, M. S., Higgins, J. P., and Woolacott, N. F. (2014). Assessing Baseline Imbalance in Randomised Trials: Implications for the Cochrane Risk of Bias Tool. Res. Synth. Methods 5 (1), 79–85. doi:10.1002/jrsm.1090
Crane, D., and Everts, P. A. M. (2008). Platelet Rich Plasma (PRP) Matrix Grafts. Pract. Pain Manag. 8, 1–10.
Eltabl, M. A., Saif, D. S., and Alemam, S. E. (2020). Platelet-rich Plasma Injection versus Surgical and Medical Treatment of Mild-Moderate Carpal Tunnel Syndrome. Egypt. J. Neurol. Psychiatry Neurosurg. 56 (1), 88–96. doi:10.1186/s41983-020-00186-z
Farrag, T. Y., Lehar, M., Verhaegen, P., Carson, K. A., and Byrne, P. J. (2007). Effect of Platelet Rich Plasma and Fibrin Sealant on Facial Nerve Regeneration in a Rat Model. Laryngoscope 117 (1), 157–165. doi:10.1097/01.mlg.0000249726.98801.77
Fei, X., Lang, L., Lingjiao, H., Wei, C., and Zhou, X. (2021). Platelet-rich Plasma Has Better Mid-term Clinical Results Than Traditional Steroid Injection for Plantar Fasciitis: A Systematic Review and Meta-Analysis. Orthop. Traumatol. Surg. Res. 107 (6), 103007. doi:10.1016/j.otsr.2021.103007
GS HJ (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available at: http://handbook.cochrane.org/.
Harrison, S., Vavken, P., Kevy, S., Jacobson, M., Zurakowski, D., and Murray, M. M. (2011). Platelet Activation by Collagen Provides Sustained Release of Anabolic Cytokines. Am. J. Sports Med. 39 (4), 729–734. doi:10.1177/0363546511401576
Hashim, N. A., Fathy, H. A., Esawy, M. M., and Shabana, M. A. (2020). Comparison of Efficiency between Platelet Rich Plasma and Corticosteroid Injection Therapies in Patients with Carpal Tunnel Syndrome: a Prospective Randomized Controlled Study. Egypt. J. Neurol. Psychiatry Neurosurg. 56 (1), 59–67. doi:10.1186/s41983-020-00184-1
Kim, H. J., and Park, S. H. (2014). Median Nerve Injuries Caused by Carpal Tunnel Injections. Korean J. Pain 27 (2), 112–117. doi:10.3344/kjp.2014.27.2.112
Latinovic, R., Gulliford, M. C., and Hughes, R. A. (2006). Incidence of Common Compressive Neuropathies in Primary Care. J. Neurol. Neurosurg. Psychiatry 77 (2), 263–265. doi:10.1136/jnnp.2005.066696
Levine, D. W., Simmons, B. P., Koris, M. J., Daltroy, L. H., Hohl, G. G., Fossel, A. H., et al. (1993). A Self-Administered Questionnaire for the Assessment of Severity of Symptoms and Functional Status in Carpal Tunnel Syndrome. J. Bone Jt. Surg Am 75 (11), 1585–1592. doi:10.2106/00004623-199311000-00002
Malahias, M. A., Nikolaou, V. S., Johnson, E. O., Kaseta, M. K., Kazas, S. T., and Babis, G. C. (2018). Platelet-rich Plasma Ultrasound-Guided Injection in the Treatment of Carpal Tunnel Syndrome: A Placebo-Controlled Clinical Study. J. Tissue Eng. Regen. Med. 12 (3), e1480–e1488. doi:10.1002/term.2566
Merolla, G., Dellabiancia, F., Ricci, A., Mussoni, M. P., Nucci, S., Zanoli, G., et al. (2017). Arthroscopic Debridement versus Platelet-Rich Plasma Injection: A Prospective, Randomized, Comparative Study of Chronic Lateral Epicondylitis with a Nearly 2-Year Follow-Up. Arthroscopy 33 (7), 1320–1329. doi:10.1016/j.arthro.2017.02.009
Negrini, F., De Lucia, F., Negrini, S., Tornese, D., Facchini, F., Vecchio, M., et al. (2021). Case Report: Rehabilitation after Platelet-Rich Growth Factors' Intra-articular Injections for Knee Osteoarthritis: Two Case Reports of a Home-Based Protocol. Front. Pharmacol. 12, 718060. doi:10.3389/fphar.2021.718060
Nepple, J. J., and Matava, M. J. (2009). Soft Tissue Injections in the Athlete. Sports health 1 (5), 396–404. doi:10.1177/1941738109343159
Ostergaard, P. J., Meyer, M. A., and Earp, B. E. (2020). Non-operative Treatment of Carpal Tunnel Syndrome. Curr. Rev. Musculoskelet. Med. 13 (2), 141–147. doi:10.1007/s12178-020-09616-0
Padua, L., Coraci, D., Erra, C., Pazzaglia, C., Paolasso, I., Loreti, C., et al. (2016). Carpal Tunnel Syndrome: Clinical Features, Diagnosis, and Management. Lancet Neurol. 15 (12), 1273–1284. doi:10.1016/S1474-4422(16)30231-9
Peerbooms, J. C., Lodder, P., den Oudsten, B. L., Doorgeest, K., Schuller, H. M., and Gosens, T. (2019). Positive Effect of Platelet-Rich Plasma on Pain in Plantar Fasciitis: A Double-Blind Multicenter Randomized Controlled Trial. Am. J. Sports Med. 47 (13), 3238–3246. doi:10.1177/0363546519877181
Picard, F., Hersant, B., Bosc, R., and Meningaud, J. P. (2015). The Growing Evidence for the Use of Platelet-Rich Plasma on Diabetic Chronic Wounds: A Review and a Proposal for a New Standard Care. Wound Repair Regenofficial Publ. Wound Healing Soc. [and] Eur. Tissue Repair Soc. 23 (5), 638–643. doi:10.1111/wrr.12317
Raeissadat, S. A., Karimzadeh, A., Hashemi, M., and Bagherzadeh, L. (2018). Safety and Efficacy of Platelet-Rich Plasma in Treatment of Carpal Tunnel Syndrome; a Randomized Controlled Trial. BMC Musculoskelet. Disord. 19 (1), 49. doi:10.1186/s12891-018-1963-4
Razmara, M., Hjemdahl, P., Ostenson, C. G., and Li, N. (2008). Platelet Hyperprocoagulant Activity in Type 2 Diabetes Mellitus: Attenuation by Glycoprotein IIb/IIIa Inhibition. J. Thromb. Haemost. 6 (12), 2186–2192. doi:10.1111/j.1538-7836.2008.03185.x
Ruzafa, N., Pereiro, X., Fonollosa, A., Araiz, J., Acera, A., and Vecino, E. (2021). Plasma Rich in Growth Factors (PRGF) Increases the Number of Retinal Müller Glia in Culture but Not the Survival of Retinal Neurons. Front. Pharmacol. 12, 606275. doi:10.3389/fphar.2021.606275
Ruzafa, N., Pereiro, X., Fonollosa, A., Araiz, J., Acera, A., and Vecino, E. (2021). The Effect of Plasma Rich in Growth Factors on Microglial Migration, Macroglial Gliosis and Proliferation, and Neuronal Survival. Front. Pharmacol. 12, 606232. doi:10.3389/fphar.2021.606232
Sánchez, M., Guadilla, J., Fiz, N., and Andia, I. (2012). Ultrasound-guided Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis of the Hip. Rheumatology (Oxford) 51 (1), 144–150. doi:10.1093/rheumatology/ker303
Senna, M. K., Shaat, R. M., and Ali, A. A. A. (2019). Platelet-rich Plasma in Treatment of Patients with Idiopathic Carpal Tunnel Syndrome. Clin. Rheumatol. 38 (12), 3643–3654. doi:10.1007/s10067-019-04719-7
Shen, Y. P., Li, T. Y., Chou, Y. C., Ho, T. Y., Ke, M. J., Chen, L. C., et al. (2019). Comparison of Perineural Platelet-Rich Plasma and Dextrose Injections for Moderate Carpal Tunnel Syndrome: A Prospective Randomized, Single-Blind, Head-To-Head Comparative Trial. J. Tissue Eng. Regen. Med. 13 (11), 2009–2017. doi:10.1002/term.2950
Soyupek, F., Yesildag, A., Kutluhan, S., Askin, A., Ozden, A., Uslusoy, G. A., et al. (2012). Determining the Effectiveness of Various Treatment Modalities in Carpal Tunnel Syndrome by Ultrasonography and Comparing Ultrasonographic Findings with Other Outcomes. Rheumatol. Int. 32 (10), 3229–3234. doi:10.1007/s00296-011-2173-7
Ustün, N., Tok, F., Yagz, A. E., Kizil, N., Korkmaz, I., Karazincir, S., et al. (2013). Ultrasound-guided vs. Blind Steroid Injections in Carpal Tunnel Syndrome: A Single-Blind Randomized Prospective Study. Am. J. Phys. Med. Rehabil. 92 (11), 999–1004. doi:10.1097/PHM.0b013e31829b4d72
Uzun, H., Bitik, O., Uzun, Ö., Ersoy, U. S., and Aktaş, E. (2017). Platelet-rich Plasma versus Corticosteroid Injections for Carpal Tunnel Syndrome. J. Plast. Surg. Hand Surg. 51 (5), 301–305. doi:10.1080/2000656X.2016.1260025
Wu, Y. T., Ho, T. Y., Chou, Y. C., Ke, M. J., Li, T. Y., Huang, G. S., et al. (2017). Six-month Efficacy of Platelet-Rich Plasma for Carpal Tunnel Syndrome: A Prospective Randomized, Single-Blind Controlled Trial. Sci. Rep. 7 (1), 94. doi:10.1038/s41598-017-00224-6
Zhang, Y., Xing, F., Luo, R., and Duan, X. (2021). Platelet-Rich Plasma for Bone Fracture Treatment: A Systematic Review of Current Evidence in Preclinical and Clinical Studies. Front. Med. 8, 676033. doi:10.3389/fmed.2021.676033
Zheng, C., Zhu, Q., Liu, X., Huang, X., He, C., Jiang, L., et al. (2016). Effect of Platelet-Rich Plasma (PRP) Concentration on Proliferation, Neurotrophic Function and Migration of Schwann Cells In Vitro. J. Tissue Eng. Regen. Med. 10 (5), 428–436. doi:10.1002/term.1756
Keywords: carpal tunnel syndrome, local injection, systematic review, steroid, platelet-rich plasma
Citation: Jiang J, Xing F, Luo R and Liu M (2022) Effectiveness of Platelet-Rich Plasma for Patients With Carpal Tunnel Syndrome: A Systematic Review and meta-Analysis of Current Evidence in Randomized Controlled Trials. Front. Pharmacol. 13:834213. doi: 10.3389/fphar.2022.834213
Received: 13 December 2021; Accepted: 11 March 2022;
Published: 27 April 2022.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Abdallah El-Sayed Allam, Tanta University, EgyptYifei Yao, Shanghai Jiao Tong University, China
Copyright © 2022 Jiang, Xing, Luo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, bGl1bWluZ2xtMTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jiabao Jiang
Jiabao Jiang Fei Xing
Fei Xing Rong Luo
Rong Luo Ming Liu
Ming Liu